Abstract
For the last two decades, researchers have placed hopes in a new era in which a combination of reperfusion and neuroprotection would revolutionize the treatment of stroke. Nevertheless, despite the thousands of papers available in the literature showing positive results in preclinical stroke models, randomized clinical trials have failed to show efficacy. It seems clear now that the existing data obtained in preclinical research have depicted an incomplete picture of stroke pathophysiology. In order to ameliorate bench-to-bed translation, in this review we first describe the main actors on stroke inflammatory and immune responses based on the available preclinical data, highlighting the fact that the link between leukocyte infiltration, lesion volume and neurological outcome remains unclear. We then describe what is known on neuroinflammation and immune responses in stroke patients, and summarize the results of the clinical trials on immunomodulatory drugs. In order to understand the gap between clinical trials and preclinical results on stroke, we discuss in detail the experimental results that served as the basis for the summarized clinical trials on immunomodulatory drugs, focusing on (i) experimental stroke models, (ii) the timing and selection of outcome measuring, (iii) alternative entry routes for leukocytes into the ischemic region, and (iv) factors affecting stroke outcome such as gender differences, ageing, comorbidities like hypertension and diabetes, obesity, tobacco, alcohol consumption and previous infections like Covid-19.
We can do better for stroke treatment, especially when targeting inflammation following stroke. We need to re-think the design of stroke experimental setups, notably by (i) using clinically relevant models of stroke, (ii) including both radiological and neurological outcomes, (iii) performing long-term follow-up studies, (iv) conducting large-scale preclinical stroke trials, and (v) including stroke comorbidities in preclinical research.
Keywords: Ischemic stroke, Inflammation, Immune response, Clinical trials, Translational research, Experimental models
1. Introduction
Stroke is the second leading cause of death for people older than 60 years and the first cause of disability. It is estimated that by 2050, more than 1.5 billion people will be over 65, increasing therefore stroke prevalence (Krishnamurthi et al., 2010). Taking into account the tremendous socio-economic impact of this fact, the better understanding of the pathophysiology in order to finally develop new treatments for patients is crucial. This is especially important considering that for the moment there are only two approved treatments for acute ischemic stroke (AIS): (i) clot thrombolysis through the intravenous administration of tissue plasminogen activator (tPA, Actilyse), which is limited in clinical practice due to a short therapeutic window (4.5 h) and a risk of intracerebral hemorrhage; and (ii) clot removal by endovascular thrombectomy up to 24 h after stroke onset depending on imaging criteria (Casetta et al., 2020, Thomalla and Gerloff, 2019). Additional agents to combine with tPA administration and/or thrombectomy to enlarge the therapeutic window of the current therapies, enhance their efficacy and improve outcomes associated with stroke are needed.
Insights into the consequences of glucose and oxygen deprivation concerning oxidative, excitotoxic and microvasculature injuries, blood brain barrier (BBB) disruption, oedema and neuronal death have been possible thanks to the efforts of hundreds of research groups that have dedicated the past decades to the better understanding of the mechanisms underlying stroke. However, there are two more recently described key players, which will be the focus of this review: neuroinflammation and immune responses to stroke. Neuroinflammation is defined as an inflammatory response within the central nervous system (CNS), thus, the brain or spinal cord. This process is due to the production of different mediators such as cytokines, chemokines, reactive oxygen species, and secondary messengers by resident CNS glia, endothelial cells, and peripherally derived immune cells. On the other hand, immune response combines the terms of innate and adaptive immune responses, involved in regular brain development but also in different pathologic conditions, such as neurodegenerative diseases or stroke (Ransohoff et al., 2015). Briefly, immune cells classically involved in innate responses include natural killer cells, neutrophils, dendritic cells and macrophages that participate in the selective recognition and the clearance of pathogens and toxic cell debris during infection or tissue injury (Medzhitov and Janeway, 2002). However, it is now evident that resident cells, glial cells, ependymal cells and even neurons are capable of mounting innate immune responses on their own (Nguyen et al., 2002, Ransohoff et al., 2015).
Although many aspects of postischemic inflammation, that is, the response of the immune system to disruption of tissue homeostasis, manifest themselves days and weeks after the event, the inflammatory cascade is activated immediately after the vessel occlusion (Anrather and Iadecola, 2016). A secondary huge cascade of inflammatory responses is orchestrated within minutes and persists among days or even weeks after. This process, especially during the first 48 h hours after stroke onset, has been deeply studied and reviewed during the last decades (Anrather and Iadecola, 2016, Shi et al., 2019, Chamorro et al., 2016, Drieu et al., 2018). The immune response is initiated immediately after the cell injury, by the release of different damage-associated molecular patterns (DAMPS), such as heat shock proteins, high mobility group box 1 and interleukin-33, purines (ATP, UTP, and their catabolites), peroxiredoxins, and mitochondrial-derived N-formyl peptides. These molecules activate pattern recognition receptors on microglia, the brain resident immune cells, and astrocytes. Later on, activation of endothelial cells aggravates the BBB breakdown allowing peripheral leukocytes to arrive to the injured area (Gadani et al., 2015, Shi et al., 2019). Due to the disruption of the BBB, cytokines and DAMPs enter the circulation and induce a systemic immune response, with an increase in pro-inflammatory cytokines and circulating leukocytes during the first hours after stroke (Anrather and Iadecola, 2016). However, after this early activation of the immune system, a state of systemic immunodepression that predisposes to poststroke infections occurs. Indeed, complications from pulmonary or urinary tract infections have been observed in ~ 20% of patients with stroke (Meisel et al., 2005, Prass et al., 2003, Langhorne et al., 2000). Thus, an ambition to understand the role of the immune system and the potential of immunomodulatory drugs to act as a treatment for the stroke, have been a driving force for the extensive research in the past decade.
After a brief description of the main actors of the inflammatory/immune responses after stroke, in this review we will first focus on clinical data, obtained in stroke patients and randomized clinical trials (RCT), and we will then compare and summarize the preclinical findings that served as a basis support for clinical trials. Next, we will discuss aspects that are often neglected yet important to be considered in preclinical research, in order to give light to the unfortunate bench-to-bed translational gap in ischemic stroke research concerning immunomodulatory drugs. In particular, we will discuss about (i) the selection of the best experimental model(s; in terms of the nature of arterial occlusion and its potential recanalization) for a given experimental design, (ii) the timing and selection of outcome measuring, (iii) the existence of alternative entry routes for leukocytes into the ischemic region, and (iv) factors affecting stroke outcome (gender differences, ageing, comorbidities like hypertension diabetes and previous infections, and external factors related to daily life like obesity, tobacco and alcohol consumption).
2. Main actors on stroke inflammatory and immune responses
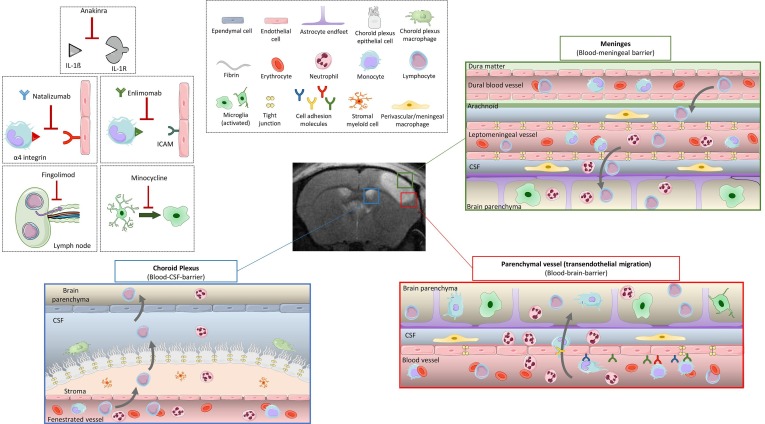
A schematized, non-exhaustive overview of the inflammatory and immune responses after stroke is depicted in Fig. 1 . Microglial response is one of the first steps before the innate immune responses triggered after stroke. These cells can adopt a large spectrum of phenotypes ranging from pro- to anti-inflammatory and are known to rapidly respond to neuronal injury in the boundary zone of the infarct after stroke (Szalay et al., 2016). Due to the lack of nutrients, differences in ion gradients and altered neuronal activity, microglial responses start minutes after stroke in both the core and the penumbra regions. Microglial cells detect with their processes purinergic metabolites and changes in ionic gradients in surrounding suffering cells (Cserep, 2020). At the acute phase after stroke onset, microglial cells at the core of the lesion detect DAMPs and HMGB1 via their receptors -mainly belonging to the Toll-like Receptor family (TLR). This ligand-receptor link leads to the activation of the NF-κB pathway and thus, to the activation of microglial cells. Once activated after stroke onset, microglial cells increase their expression of CD11b, CD45 and CD68 corresponding to a phagocytic phenotype responsible for the clearance of cellular debris, as well as TNF and the pro-inflammatory interleukins IL-1 and IL-6 that exacerbate astrocyte and endothelial cell (EC) activation (Xu et al., 2020). Afterwards, DAMPs and cytokines expressed during the early phase of ischemic injury can access to the systemic circulation through the disrupted BBB or the cerebrospinal fluid drainage system that includes venous and lymphatic outflows (Anrather and Iadecola, 2016).
Fig. 1.
Inflammatory/immune responses after ischemic stroke, and targets of the immunomodulatory drugs tested on clinical trials. In the healthy brain, three main barriers protect the parenchyma from external pathogens: the blood–brain barrier (BBB) around the cerebral vessels, the blood-meningeal barrier in the meninges, and the blood-CSF barrier of the choroid plexus. Immune cells circulate freely in the blood, and a few lymphocytes patrol the CSF to do immunosurveillance. In the brain parenchyma resting microglia survey the environment with their processes. After stroke, microglia switches from a resting form to an activated state, adopting a phagocytic phenotype and secreting pro-inflammatory factors. The BBB is disrupted, local ECs are activated and express CAMs. The tight junctions between ECs disappear. This allows leukocyte rolling and adhesion at the luminal side of the blood vessel and then transmigration from the vascular compartment to the brain parenchyma. Leukocytes can also invade the brain through blood-meningeal and blood-CSF barriers. Once infiltrated in the tissue, neutrophils secrete pro-inflammatory factors that will recruit monocytes/macrophages, and later lymphocytes to the parenchyma. Immunomodulatory drugs tested on clinical trials and discussed in this review include (i) Anakinra, an antagonist of the proinflammatory cytokine interleukin-1, (ii) Natalizumab, which acts by blocking the binding of integrin α4 to the adhesion molecule VCAM to reduce leukocyte infiltration. (iii) Enlimomab is an antibody targeting the adhesion molecule ICAM. (iv) Minocycline inhibits microglial activation among other anti-inflammatory properties. (v) Fingolimod is a high-affinity agonist for several of the sphingosine-1-phosphate receptors that prevents the egress of lymphocytes from lymph nodes, thus limiting the infiltration of lymphocytes to the brain. BBB, blood–brain barrier; CAM, cellular adhesion molecule; CSF: cerebrospinal fluid; EC, endothelial cell.
Following ischemia and lasting from hours to weeks after, astrocytes are other key players. It is well-known that cytokines from neurons and glial cells lead to astrocyte reactivity hyperplasia. Astrocytes endfeet are in close interaction with brain capillary endothelial cells and pericytes that form BBB. During ischemia, MMP-9 disrupts the connection between astrocyte endfeet and endothelial cells by degrading basal lamina (del Zoppo, 2010). Astrocyte proliferation results in the synthesis of inflammatory factors such as monocyte chemotactic protein-1, IL-1β, glial fibrillary acidic protein (GFAP), vimentin, and nestin that can lead to reactive gliosis and later scar formation (Jayaraj et al., 2019). Hence, it has been classically assumed that the ruptured BBB acts as a major gateway for the invasion of peripheral leukocytes through their transendothelial migration. As discussed afterwards, alternative entry routes for leukocytes have been described in recent years.
Activated cerebral microvessels become more permeable to molecules that are normally prevented from crossing the blood–brain barrier. In particular, the immunological BBB is substantially altered after ischemia. Together with the secretion of chemokines, the successive entry of systemic leukocytes including neutrophils, macrophages, and lymphocytes is promoted (Veltkamp and Gill, 2016). Corresponding to the upregulation of adhesion molecules on EC, integrins exhibit similar functions on activated leukocytes. Leukocytes start to roll on the vessel wall with the help of selectins expressed by activated EC. After rolling, leukocytes adhere to the vessel wall by strong links with intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Similar to other inflammatory cells, leukocytes release proinflammatory factors in the ischemic region of the brain. Leukocytosis has been described as a marker of inflammatory response after stroke. Neutrophils are the first bloodborne immune cells to invade the ischemic tissue, followed by monocytes. The sequence of leukocyte recruitment into the brain after experimental stroke has been well characterized (Gelderblom et al., 2009, Drieu et al., 2020a, Drieu et al., 2020b), whereas the temporal and spatial profile of the immune cell recruitment after stroke in humans requires better characterization (Veltkamp and Gill, 2016).
Neutrophils enhance leukocyte recruitment by degranulation of their content, rich in cytokines/chemokines, proteolytic enzymes and activated-complement system (Mayadas et al., 2014). In addition to this, neutrophils could contribute to brain injury after ischemic stroke by obstructing microvessel circulation, damaging EC and extracellular matrix by hydrolytic enzymes and free radicals, promoting intravascular thrombus formation together with platelet activation, and releasing cytokines and chemotactic factors that could promote extension of the inflammatory response. Moreover, neutrophil extracellular traps (NETs; networks of DNA, histones and proteolytic enzymes) secreted by neutrophils are capable of activating platelets and contribute to the thrombotic processes (Rayasam et al., 2018). Clinical data and experimental models of stroke have shown that neutrophils infiltrate the brain parenchyma within hours of stroke onset (Gelderblom et al., 2009, Drieu et al., 2020a, Drieu et al., 2020b, Cai et al., 2020). Furthermore, clinical studies demonstrated that the neutrophil-to-lymphocyte ratio might be a strong prognostic marker in acute ischemic stroke (Xue et al., 2017) and that the NETs are increased in patients with stroke (Vallés et al., 2017).
The consequences of the adaptive immune response on ischemic stroke are still debated, as both beneficial and deleterious results have been reported depending on the type of lymphocyte subpopulation. Infiltrating γδ T, CD8+ T, and NK cells contribute to the acute brain injury after stroke onset, while regulatory T and B cells are reported to be protective (Feng et al., 2017). Experimental models of stroke have shown that lymphocyte infiltration into the ischemic tissue comes later after stroke onset, starting at day 3 (Drieu et al., 2020a, Drieu et al., 2020b). Postmortem human samples have shown that lymphocyte infiltration into the ischemic area occurs from day 3 and can be present up to 53 years after stroke (Mena et al., 2004).
Neuroinflammation is also considered necessary for the reparation phase that persists after the initial brain insult. Initial inflammation is self-limiting and ultimately gives a way to structural remodeling and functional reorganization. After the acute phase of ischemic stroke, the inflammatory response gradually decreases, and ischemic stroke enters another stage during which tissue repair dominates the infarction region (Xu et al., 2020). The end of the acute phase is characterized by removal of dead cells/tissue debris, creation of an anti-inflammatory milieu and production of prosurvival factors (Malone et al., 2019). This phase aims to restore tissue integrity and involves matrix remodeling, neurogenesis, axon sprouting, dendritogenesis and oligodendrogenesis (Peruzzotti-Jametti et al., 2014) carried out in concert by many cell types, ranging from immune cells to neurons and astrocytes. Together, these cells produce growth factors and proteases, allowing the remodeling of the ischemic site (Iadecola and Anrather, 2011).
3. What do we know about neuroinflammation and immune responses in stroke patients?
Unfortunately, due to the lack of specificity of biomarkers for imaging patients in vivo and the reduced number of post-mortem analyses, studying inflammation/immune responses in humans is limited. As aforementioned, glial cells are one of the main key players in the inflammatory responses triggered after ischemic stroke. Long-term imaging or additional post-mortem pathological studies would be crucial to definitely confirm whether global glial activation after stroke is persistent in humans (Shi et al., 2019). However, different groups have been able to perform short term studies by using in vivo imaging techniques. Focusing on microglial cells, by using the PK11195-PET radioactive ligand of the translocator protein, highly expressed by activated microglia, and diffusion tensor imaging MRI, the involvement of this cell-type is clearer. In this manner, activated microglia have demonstrated to accumulate focally within a week after stroke and can be detected in distal regions several months after (Gerhard et al., 2005, Thiel et al., 2010, Radlinska et al., 2009). In addition, in another study positive signals have been observed in the subacute phase (6 to 21 weeks after the lesion) in non-infarcted ipsilateral areas (Morris et al., 2018).
However, it is worth to mention that translocator protein is also expressed by monocytes or macrophages and to a lesser extent, by astrocytes, reducing the specificity of the technique (Dupont et al., 2017). Furthermore, the absence of a specific astrocyte biomarker feasible for in vivo imaging in patients leads to a tremendous gap in clinical evidence supporting a role for astrocytes in brain inflammation after stroke. Therefore, it becomes crucial to improve and to develop these techniques for the future.
In addition, clinical evidences regarding peripheral immune cells mobilization and vascular inflammation are scarce (Shi et al., 2019). We encourage researchers within the field to increase the clinical research, so the prompting results obtained in preclinical models aforementioned can be confirmed and better therapeutic targets/manipulations can be suggested.
4. Results of the clinical trials on immunomodulatory agents
Given that mechanisms of immune-mediated neuronal injury have relatively recently gained increasing attention, few drugs with the primary aim of modulating inflammatory/immune pathways have reached the Phase II or Phase III RCT stage in acute stroke. In this review, we will focus on RCT aiming to address neuroinflammation/immunomodulation, setting aside any RCT involving tPA or thrombectomy by itself (see (Chamorro et al., 2016, Smith et al., 2015). A schematic view of the immunomodulatory drug targets tested in clinical trials is shown in Fig. 1.
There have been several RCT pursuing the modulation of proinflammatory cytokine interleukin-1, by the use of recombinant interleukin-1 receptor antagonist (IL-1Ra) (Anakinra). This compound, historically used to treat rheumatoid arthritis and similar inflammatory diseases, showed a good safety profile. Besides the promising results obtained in preclinical studies, in human studies, a phase II trial in 2005 showed Anakinra to be safe and well tolerated in AIS patients (Emsley et al., 2005). With the replacement of the intravenous formulation by a subcutaneous injection, the effects of the drughave been re-investigated at a twice-daily dose since (Sobowale et al., 2016). However, even though Anakinra significantly decreased plasma levels of pro-inflammatory IL-6 and C-reactive protein, patients did not show a reduction in disability levels at 3 months compared to placebo. A possible negative interaction between IL and 1Ra and tPA has been revealed (Smith et al., 2015) and has been suggested to mask the potential beneficial effect of Anakinra. Thus, further preclinical and clinical studies are required to confirm its use as stroke treatment.
Minocycline, a tetracycline derivative, is a well-documented protective compound in preclinical stroke models. It exerts anti-inflammatory properties by inhibiting the expression of polyadenosine diphosphate ribose polymerase 1 and matrix metalloproteinases, and inhibiting microglial activation. It has shown an acceptable safety profile in an open-label, dose-escalation study in patients treated within 6 h after the symptoms onset, including a subset of patients who also received alteplase. A small pilot study confirmed the safety of intravenous minocycline administered within 24 h of stroke onset, but did not show efficacy of the drug by improving the proportion of patients free of disability at 90 days (Kohler et al., 2013). However, the meta-analysis of previous RCT demonstrated the efficacy and minocycline has been suggested as a promising neuroprotective agent in AIS patients (Malhotra et al., 2018).
Several options have been followed pursuing the avoidance of invasion of peripheral cells to the CNS. Among them, we can find Natalizumab, a humanized CD49d antibody that blocks α4-integrin, attenuating leucocytic infiltration. Promising results were obtained in animal and in a first clinical trial in which Natalizumab did not have an effect on the infarct volume growth on brain MRI at day 5 (primary outcome), but the therapy was safe and provided global clinical gains on the Stroke Clinical Impact-16 scale and in cognitive function at 90 days (Elkins et al., 2017). Disappointingly, the follow-up phase IIb trial (“ACTION2”) did not meet its primary or secondary endpoints. As a result, further development of Natalizumab in AIS will not be pursued (Malone et al., 2019).
A role of the endothelial β2-integrin ligand ICAM-1 in neutrophil recruitment across the BBB in stroke was proposed as an alternative pharmacological target. Although positive results were obtained in preclinical research, a phase III clinical trial of anti-ICAM-1 antibody Enlimomab, failed to replicate these results, with the antibody-treated group reporting higher mortality (Enlimomab Acute Stroke Trial Investigators, 2001).
Fingolimod is a high-affinity agonist for several of the sphingosine-1-phosphate receptors that prevents the egress of lymphocytes from lymph nodes, limiting the infiltration of lymphocytes into the brain, and inhibiting local activation of microglia and macrophages. Preclinical studies using several rodent models of brain ischemia have shown that Fingolimod can reduce infarct size, neurological deficit, oedema, and the number of dying cells in the core and peri-infarct area (Liu et al., 2013). In stroke patients, when administered within 72 h after stroke onset, oral Fingolimod caused no severe adverse effects and had efficacy in limiting secondary tissue injury, in decreasing microvascular permeability, attenuating neurological deficits, and in promoting recovery (Zhang et al., 2017). In addition to this, the combination of Fingolimod with alteplase resulted in fewer circulating lymphocytes, smaller lesion volumes, less hemorrhages, and attenuated neurological deficits (Zhu et al., 2015, Zhang et al., 2017). However, while Fngolimod remains one of the most compelling stroke immunotherapies, further large-scale clinical trials are required (Malone et al., 2019).
5. Experimental basis for the clinical trials on immunomodulatory drugs: Translational gaps on stroke.
The existing preclinical data on immunomodulatory drugs discussed in this review is summarized in Table 1 . As aforementioned, in spite of the beneficial effects shown in preclinical research, RCT have shown disappointing results. To overcome this gap, the Stroke Therapy Academic Industry Roundtable (STAIR) recommendations and guidelines have been proposed. However, there are still some important factors which are often neglected, that could explain -at least in part- the bench to bedside translational failure. We will particularly focus here on (i) the characteristics of the arterial recanalization process and its derived inflammatory responses, which are extremely dependent on the experimental model used, (ii) alternative entry routes for leucocytes to the brain parenchyma, (iii) the appropriate timing of measurements in preclinical models and (iv) the vital importance of concomitant factors affecting stroke that needs to be taken into account.
-
i)
Characteristics of the arterial occlusion and recanalization processes: the importance of choosing the appropriate experimental model
Table 1.
Existing preclinical data on the immunomodulatory drugs discussed in this review: Anakinra, Natalizumab, Enlimomab, Minocycline and Fingolimod.
| Drug | Experimental model | Animal species (*) | Administration route/timing | Lesion volume | Neuromotor deficit | Main results | Ref. |
|---|---|---|---|---|---|---|---|
| Anakinra | pMCAO | Rat | Central, 30′ before and 10′ after | 24 h | No | Reduced infarct volume. | (Relton and Rothwell, 1992) |
| pMCAO | Rat | Subcut., before and up to 7 days | 24 h, 7 days | Neurological score at 24 h and 7 days | Decreased the number of necrotic neurons and the number of PMN leukocytes. Improved neurological scores. | (Garcia et al., 1995) | |
| pMCAO | Rat | ICV, 30′ after | 24 h, 7 days | No | Reduced total and cortical infarct volume 24 h and 7 days post. | (Loddick and Rothwell, 1996) | |
| tMCAO (60′) | Rat | ICV, 1,2 or 3 h after | 24 h and 48 h | No | Reduced brain damage, when administered 3 h after. | (Mulcahy et al., 2003) | |
| tMCAO (60′) | Rat | Subcut, just after and 24 h after | 24,48 h and 28 days | Gross neurological score and functional recovery, motor tasks, days 6–9 and 25–28 | Enhanced functional recovery and protected against sociability defect and depression. | (Girard et al., 2014) | |
| tMCAO (70 or 90′) | Rats, young and aged and Corpulent | Subcut, 3 and 6 h after | 24 h and 7 days | Motor, behavioral and cylinder tests, 24 h and 7 days | Improved stroke outcome and promotes neurogenesis in both young and aged/co-morbid rats. | (Pradillo et al., 2017) | |
| tMCAO (45′) | Mouse | Subc.,30′ and 3 h after | 24 h and 7 days | Bederson and Corner tets, 24 h and 7 days | Preclinical cross-laboratory stroke trial supporting the therapeutic potential of interleukin 1 receptor antagonist. | (Maysami et al., 2016) | |
| Permanent (electrocoag.) | Mouse (10 months) | Subc., 30′ and 3 h after | 3 and 28 days | Catwalk test 3,14,21,18 days | |||
| Permanent (electrocoag.) | Mouse | Subc., 30′ and 3 h after | 24 h and 7 days | Hunter and corner tests, 24 h, 2, 7 and 28 days | |||
| tMCAO (30′) | Mouse | Subc., 30′ and 3 h after | 24 h | Neurological Score, 24 h | |||
| tMCAO (Thrombin) | Mouse | Subc., 30′ and 3 h after | 7 days | No | |||
| Permanent (Electrocoag.) | Mouse | Subc., 30′ and 3 h after | 7 days | Corner test, 1,2 and 7 days | |||
| Minocycline | tMCAO (90′) | Rat | I.P, every day, up to 3 | 24 h | No | Decreased infarct volume and inflammation. | (Yrjänheikki et al., 1999) |
| tMCAO (90′) | Rat | IV, 4,5, and 6 h after | 24 h | Neurological Score, 24 h | Decreased infarct volume and improved neurological deficits. | (Xu et al., 2004) | |
| pBCCAO | Rat | I.P, just after, twice a day, up to 14 days | No | No | Decreased neuronal and myelin damage and inflammation. | (Cho et al., 2006) | |
| tBCCAO(30′) | Rat | I.P, 12 h before, just after and every 24 h up to 3 days | No | No | Enhanced neuronal viability, decreased inflammation. | (Cai et al., 2006) | |
| tMCAO (60′) | Rat | I.P, 4 days after up to 3 weeks | No | Motor function and learning and memory tests, 6 weeks after | Promoted neurogenesis, improved motor function and memory. | (Liu et al., 2007) | |
| tMCAO (60′) | Rat | I.P, 30′ and 2 h after up to 3 days | 3 days | Neurological deficit scores, 3 days | Improved neurological deficits, decreased infarct volume. | (Chu et al., 2007) | |
| pBCCAO | Female rat | Oral, once a day up to 16 weeks | No | Morris and openfield tests,4, 8, 12 and 16 weeks | Improved memory, decreased infarct volume, iNOS and inflammation. | (Cai et al., 2008) | |
| tMCAO(30′) | Rat | I.V, 60′ after | 3 days | Body swing and Bederson test,3 days | Improved neurological deficits and decreased infarct volume. | (Matsukawa et al., 2009) | |
| tMCAO (2 h) | Rat | I.V, 1 h after, once per day up to 6 | 7 days | No | Decreased infarct volume and inflammation. | (Martín et al., 2011) | |
| pBCCAO | Female Wistar rat | Oral, once per day up to 16 weeks | No | No | Decreased neuroinflammation. | (Cai et al., 2010) | |
| tBCCAO | Rat | I.P, just after and every 12 h up to 3 days | 24 h and 3 days | Water maze 24 h and 3 days | Improved memory, decreased infarct volume. | (Zheng et al., 2013) | |
| tMCAO (2 h) | Rat | I.V, just after and up to 14 days | No | Neurological severity scores and the staircase test, 2 days, 1, 2 and 4 weeks | Reduced BBB permeability and improved sensorimotor deficits. | (Tao et al., 2013) | |
| Endothelin | Rat | I.P, twice a day up to 48 h | No | Modified sticky-tape test,24 h, 3 and 7 days | Improved sensorimotor deficits, inflammation and neuronal viability. | (Cardoso et al., 2013) | |
| tBCCAO (20′) | Rat | Oral, 48,24 h and 1 h before | No | No | Reduced neuronal degeneration. | (Aras et al., 2013) | |
| pBCCAO | Female rat | Oral, 4 days after ischemia, daily up to 4 weeks | No | Water maze 1,2,3,4 and 5 days | Improved memory, enhanced neuronal plasticity. | (Zhao et al., 2015) | |
| tMCAO (90′) | Hypertens. rat | I.V, just after | 48 h, 1,2 and 4 weeks | No | Decreased infarct volume, inflammation, improved BBB permeability. | (Yang et al., 2015) | |
| tMCAO (90′) | Rat | I.V, just after | 14 days | Bederson, beam walk, rotarod performance and grip test, 1, 3, 7 and 14 days | Decreased infarct volume, improved neurobehavioural and motor functions. | (Soliman et al., 2015) | |
| tMCAO (30′) | Rat | I.V, 30′ before | 7 days | Adhesive removal test, 1 and 7 days | Decreased infarct volume, improved neurobehavioral functions. | (Park et al., 2015) | |
| tBCCAO (20′) | Rat | I.P, once per day up to 7 | No | Morris water-maze 7 days | Improved memory, enhanced neuronal viability. | (Naderi et al., 2017) | |
| Photothromb. | Rat | I.P, 1 h, 12,24,36 and 48 h after | 3 and 24 h, 2,3 and 7 days | The forelimb placing response and cylinder tests, 3 and 24 h, 2,3 and 7 days | Improved motor function and decreased phagocityc cells. | (Yew et al., 2019) | |
| tMCAO (60′) | Rat | I.V, just after | 24 h | No | Reduced infarct size, microglial activation and white matter injury. | (Faheem et al., 2019) | |
| pMCAO | Mouse | I.P, 60′ before, 30′ and 4 h after | 24 h | Neurological score, 24 h | Decreased infarct volume, oxidative stress and improved neurological deficits. | (Morimoto et al., 2005) | |
| pMCAO | Mouse | I.P.,12 h before or 2 h after | 24 h and 72 h | No | Decreased infarct volume. | (Koistinaho et al., 2005) | |
| tMCAO (45′) | Mouse | I.P, 30′ and 12 h after, twice per day up to 7 | 1, 3, 7 and 30 days | Corner and ladder tests, 3, 7 and 30 days | Decreased infarct volume and improved sensorimotor deficits. | (Tang et al., 2007) | |
| tMCAO (4 h) | Mouse | I.P, once per day up to 14 | 24 h | Neurologic score 4 h and 1, 7, and 14 days after | Decreased infarct volume and improved neurological deficits. | (Hayakawa et al., 2008) | |
| Photothromb. | Mouse | Subcut., 30′ before and 2 h after | 24 h | No | Decreased infarct volume. | (Park et al., 2011) | |
| Thromboemb. | Male and female mouse | 60′ after | 48 h | Neurological deficit score and adhesive tape test, 48 h | Decreased infarct volume and improved neurological deficits. | (Hoda et al., 2014) | |
| tMCAO (2 h) | Mouse | I.P, 12 h before or after | 48 h | Bederson test, 48 h | Decreased infarct volume and inflammation and improved neurological deficits. | (Jin et al., 2015) | |
| tMCAO (1 h) | Mouse | I.P, 1 h after, once per day up to 3 days | 72 h | Neurological score, 72 h | Decreased infarct volume and inflammation and improved neurological deficits. | (Lu et al., 2016) | |
| pMCAO | ICR mouse | I.P, 60′ before | 24 h | No | Decreased infarct volume. | (Tanaka et al., 2018) | |
| Tromboembol. (Thrombin) | Mouse | I.P, 1 h or 48 h after | 24 h and 5 days | Grip test, 24 h and 5 days | Early treatment reduced infarct volume and improved behavior | (Drieu et al., 2020a) | |
| Natalizumab | tMCAO (3 h) | Rat | I.P, 2 h after | 48 h after | Bederson test, 2, 3, 4, 5, 6, 24, and 48 h | peripheral leukocytosis, improves neurological outcome, and decreases infarct volume. | (Becker et al., 2001) |
| tMCAO (90′) | Rat | I.V, just after | 24 h after | No | Improved functional outcome and decreased infarct volume. | (Relton, 2001) | |
| tMCAO (60′) | Hypertens. Rat | I.V, 24 h before | 24 h after | No | Decreased infarct volume. | (Relton et al., 2001) | |
| tMCAO (30′ and 60′) and pMCAO | Mouse | I.P, 24 h before and 3 h after | 1,3 and 7 days | Corner test, 1,3 and 7 days | Improved functional outcome and decreased infarct volume. | (Liesz et al., 2011b) | |
| tMCAO (30′) and pMCAO (electrocoag.) | Mouse | I.P, 24 h before and 3 h after | 1 and 7 days | Bederson, grip and corner test 1 and 7 days. | No effect | (Langhauser et al., 2014) | |
| tMCAO (60′) and pMCAO (electrocoag.) | Mouse | I.P, 3 h after | 7 days or 4 days | Rotarod and adhesive removal tests 1,3 and 7 days; or 2 and 4 days. | Reduced leukocyte invasion and infarct volume after pMCAO, no effect after tMCAO. | (Llovera et al., 2015) | |
| tMCAO (45′) and pMCAO (electrocoag.) | LysM-eGFP and CXCR1-eGFP mouse | I.V, just after and 24 h after | 4 and 7 days | No | Reduced infarct volume and improved functional outcome. | (Llovera et al., 2015) | |
| Tromboembol. (Thrombin) | Mouse | I.P, 1 h or 48 h after | 24 h and 5 days | Grip test 24 h and 5 days | No effect on lesion volume or neurological deficit. | (Drieu et al., 2020a) | |
| Fingolimod | tMCAO (2 h) | Rat | I.P, just after | 1 and 3 days | Neurological score, 1 and 3 days | Reduction in infarct volume and functional deficits. | (Hasegawa et al., 2010) |
| tMCAO (1 h) | Rat | I.P, 24 h before and once daily every 2 days | 7 days after | Passive avoidance test and neurological score (24 h,3 and 7 days) | Reduced oedema and neurological score 24 h, 3 and 7 days after. | (Nazari et al., 2016) | |
| tMCAO (90′) | Mouse | I.P before | 24 h | Neurological test, 24 h | Reduction in stroke volume and functional deficits | (Czech et al., 2009) | |
| tMCAO (60′) | Swiss-Webster ND4 mouse | I.P 30′ or 48 h before | 24 h | Neurological test, 24 h | Reduction in infarct volume and functional deficits. | (Wacker et al., 2009) | |
| tMCAO (60′) | Mouse | I.P, 5′ before and once per day for 3 days | 24 h and 4 days | Neurological test, 24 h and 4 days | Reduction infarct volume. | (Shichita et al., 2009) | |
| tMCAO (90′ and 3 h) | Mouse | I.P 2 h after | 24 h | Neurological test, 24 h | Reduced infarct volume | (Pfeilschifter et al., 2011) | |
| tMCAO (60′) and pMCAO | Mouse | Oral, 48 h before and 3 h after; and I.P once daily 48 h before | 24 h | Croner test, 1, 3 and 7 days | No differences. | (Liesz et al., 2011a) | |
| tMCAO (90′ and 2 h) and pMCAO | Mouse and rats | I.P 30′ after, 1 h or 2, 24 and 48 h after. 2 h or 4 h after in pMCAO | 48 h | Wire grip test, 1, 3, 7, 10 and 14 days | Reduced infarct volume; improved neurological function. | (Wei et al., 2011) | |
| tMCAO (30′) | Mouse | I.P, before and after2 days | 1 and 7 days | Ladder rung walking test, 1 and 7 days | Reduced infarct volume and improved neurological function 1 day after. | (Schuhmann et al., 2016) | |
| tMCAO (60 and 90′) | C57 and Rag 1-/- mouse | I.P, just before | 24 h and 3 days | Bederson and grip test, 1 day | Reduced infarct size and functional improvement 1 day after | (Kraft et al., 2013) | |
| Thromboemb. (thrombin) | Mouse | I.P 30′, 24 and 48 h after; I.V, 3 h, 24 and 48 h after | 3 days | Grid and cylinder test, 3 days | Reduced infarct volume and improved functional outcome. | (Campos et al., 2013) | |
| tMCAO (180′) | Mouse | I.P, before | No | Neurological score 1 day | Higher mortality. Association with tPA performed. | (Cai et al., 2013) | |
| Photothromb. | Mouse | I.P, twice per day beginning 3 days after up to 5 days | No | Grid and cylinder test, 7,14,21 and 31 days | Improvement in functional outcome on day 7 and day 31. | (Brunkhorst et al., 2013) | |
| Photothromb. | Mouse | I.P, 2 h after and daily | 1 and 3 days | Modified neurological severity scores, 7 days | Decreased infarct volume, improved neurological deficits and attenuates autophagic activity. | (Li et al., 2017) | |
| tMCAO (45′) | Mouse | I.P, after | No | Neurological test, 48 h | Attenuated haemorrhagic transformation after ischemia | (Salas-Perdomo et al., 2019) | |
| Photothromb. | Mouse | I.P, 24 h after and 1, 7 or 14 days | No | Modified neurological severity score up to 14 days | Promoted angiogenesis via microglial M2 polarization and exerted neuroprotection. | (Shang et al., 2020) | |
(*) Unless specified, animals were young and healthy males.
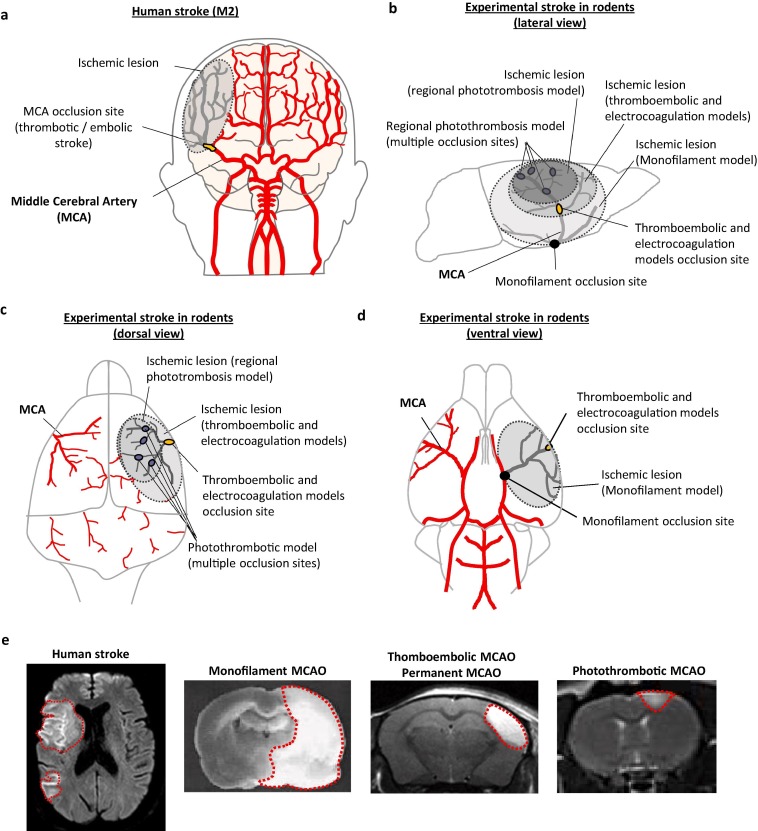
Preclinical models of ischemic stroke are necessary for investigating pathogenic processes triggered after the ischemic insult, as well as to screen for candidate therapeutic strategies. This section aims to highlight the importance of an element that could partially explain the translational gap in stroke research in general and in the neuroinflammation domain in particular, as is the choice of clinically relevant experimental models. We will briefly discuss the main models of middle cerebral artery occlusion (MCAO) used in preclinical research (mechanical occlusion by an intraluminal filament, permanent electrocoagulation, photothrombosis and thromboembolic models). Attention should be paid on the selection of the most adapted experimental model and the best combination of models for future studies, not only when testing new anti-inflammatory/immunomodulatory drugs, but also in the description of the cellular and molecular mechanisms involved in the pathogenesis of stroke. Each stroke model has experimental advantages and disadvantages, and shows more or less resemblance to the clinical reality, which have been resumed in Table 2 . A descriptive scheme comparing the blockade of the MCA in patients (the MCA being the most common occlusion site in AIS patients) and in experimental models of stroke discussed in this review, as well as the comparison of the corresponding ischemic lesions visualized by MRI, are shown in Fig. 2 . In our opinion, and following the STAIR recommendations, the use of several clinically relevant experimental models for a given drug is one of the critical improvements that researchers can implement to ameliorate the translation between preclinical and clinical stroke research.
Table 2.
Comparison of the different experimental models of stroke in terms of (i) resemblance to human stroke usually included in randomized clinical trials on stroke, and (ii) experimental advantages and disadvantages.
| Similarities to stroke patients included in RCT* | Differences with stroke patients included in RCT* | Experimental advantages | Experimental disadvantages | |
|---|---|---|---|---|
| Intraluminal monofilament MCAO |
|
|
|
|
| Electrocoagulation MCAO |
|
|
|
|
| ThromboembolicMCAO- Thrombin injection- FeCl3 contact |
|
|
|
|
*RCT (randomized clinical trials) on immunomodulatory/anti-inflammatory drugs; MCAO, Middle Cerebral Artery Occlusion
Fig. 2.
Comparison of human stroke and experimental models of stroke in rodents. a) Schematic view of the human brain vasculature with one of the most frequent stroke subtypes, a thrombotic/embolic occlusion of the M2 segment of the middle cerebral artery (MCA), usually included in randomized clinical trials (RCT) on immunomodulatory/anti-inflammatory drugs for stroke treatment. b) Schematic view of the rodent brain vasculature (lateral view). In thromboembolic and electrocoagulation experimental stroke models, there is only one site of MCA occlusion, whereas in regional photothrombotic stroke there are multiple occlusion sites within the area illuminated by the laser. In both cases, lesions are limited to the brain cortex. The occlusion site in the monofilament experimental model is located at the origin of the MCA, leading to a bigger ischemic volume including both cortical and subcortical brain regions. c) Schematic view of the rodent brain vasculature (dorsal view). d) Schematic view of the rodent brain vasculature (ventral view). e) Comparison of ischemic lesions (delimitated area) visualized by MRI in human stroke (M1-M2 occlusion) and different experimental models of stroke.
5.1. Mechanical MCAO by an intraluminal filament
The most frequently used experimental model of ischemic stroke in rodents is the intraluminal filament model (Sommer, 2017). To induce MCAO, a nylon filament is introduced into the internal carotid artery and advanced until the origin of the MCA, occluding the blood flow. The filament model does not require craniotomy and can be used to either model permanent ischemia (Chu et al., 2014, Pedragosa et al., 2018) or transient ischemia by withdrawal of the filament, allowing reperfusion at different time points, from 30 min to 120 min (Yan et al., 2015, Buscemi et al., 2019). This model is characterized by a large infarct volume, and longer durations of occlusion usually resulting in larger infarcts involving both the cortex and striatum (see Fig. 2), and may be associated with some mortality (Hata et al., 2000). It often induces hypothalamus damage that rarely occurs in human stroke (Uzdensky, 2018). While researchers see fewer infiltrating leukocytes in general after transient MCAO in compared with some permanent models of occlusion (Zhou et al., 2013), the inflammatory response, especially once BBB injury occurs is substantial in this model and includes excessive leukocyte recruitment and production of inflammatory cytokines. Thousands of anti-inflammatory molecules have been tested in this model and have shown beneficial effects on ischemic volume and/or neurological outcome. Unfortunately, very few, if any, have so far shown real clinical benefit so far.
This model typically shows a prompt recovery of a primary core, whereas a sudden and rapid reperfusion results in a secondary, delayed injury, known as “ischemia–reperfusion injury”. Indeed, it has been shown that 70% of the lesion volume in this experimental model is in fact due to the formation of microthrombi after to the withdrawal of the filament (Gauberti et al., 2014a, Gauberti et al., 2014b). This delayed injury evolves after a free interval of as long as 6 to 12 h, which confers to this experimental setup a longer therapeutic window compared to the human pathophysiology or to other experimental stroke models.
The prompt reperfusion of the MCA after the removal of the filament contrasts with the gradual reperfusion occurs in many non-treated human ischemic stroke (Hossmann, 2012); however, even after mechanical thrombectomy the secondary damage has not been observed in patients. Using the data from one randomized trial, Gauberti et al. (Gauberti et al., 2018) measured the impact of abrupt reperfusion on infarct growth in patients that benefited from mechanical thrombectomy. No growth in ischemic lesion after abrupt reperfusion was observed. Moreover, in most of the patients the impact of ischemia–reperfusion injury on the lesion growth was limited during the first 24 h. Considering the fact that reperfusion injury plays a prominent role in the mechanical MCAO models, this discovery questions the relevance of the filament model as well as the current understanding of the human stroke pathophysiology.
5.2. MCAO by electrocoagulation
The MCAO by electrocoagulation is another of the most frequently used stroke models (Howells et al., 2010). This model consists of the permanent occlusion of the MCA using electrical stimulation, which induces the coagulation of the artery. It is usually followed by the dissection of the artery to avoid any risk of recanalization. A craniotomy is necessary in order to access the MCA. This experimental model does not induce the presence of a penumbra area. The lesion is smaller than in the monofilament model and is confined to the cortical area (see Fig. 2). The resulting infarct volume and localization after permanent MCA coagulation corresponds to ischemic brain lesions in the majority of human strokes in proportion to brain size (Llovera et al., 2014).
The inflammatory reaction described in this model is greater than in the filament model, although it could be overestimated due to the confounding effects of electrocoagulation. The microglial activation is more important, as is leukocyte infiltration. This inflammatory response is also exacerbated in terms of pro-inflammatory cytokines and adhesion molecules expression (Zhou et al., 2013). However, only minor behavioral deficits can be detected in behavioral tests and functional recovery appears within the first days after stroke. That makes it difficult to assess long-term functional outcome in this model (Llovera et al., 2014).
5.3. Photothrombosis model
Photothrombosis stroke model involves the focal illumination of cerebral vessels through the skull after intravenous injection of a photosensitive dye (Watson et al., 1985). The photosensitive dye (Rose Bengal or erythrosin B) is first administered intraperitoneally in mice or intravenously in rats. Then, photo activation of the dye leads to formation of oxygen free radicals, such as singlet oxygen and superoxide, resulting in endothelial damage and platelet activation and aggregation in all the vessels within the illuminated area (Kim et al., 2000). This model does not require a craniotomy, since the light source can be applied directly and passes through the skull. It produces a small-size and well-defined cortical lesion (Labat-gest and Tomasi, 2013) (Fig. 2). Moreover, it does not require mechanical manipulations with blood vessel such as ligation or filament insertion, which carry the risk of side lesions.
It was shown in this model microglial activation, lymphocyte infiltration and an increase in the level of pro-inflammatory cytokines up to 14 days after the occurrence of ischemic stroke (Feng et al., 2017). Moreover, this model induces long-term sensorimotor deficits with high survival (Lunardi Baccetto and Lehmann, 2019), which may be useful to study late inflammatory process involved in recovery and regeneration after stroke.
However, disadvantages of this stroke model are major differences from the ischemic stroke pathology. The injury caused by photothrombosis presents simultaneously early acute cytotoxic and vasogenic edema, microvascular injury and blood–brain barrier breakdown (Lee et al., 1996). Additionally, this model causes well-defined ischemic lesion without the penumbra area typically present in stroke pathology.
5.4. Thromboembolic models
Several thromboembolic models exist. A previously formed clot (by autologous or heterologous blood) can be directed to the base of the MCA (Ansar et al., 2014). A clot can also be created by the application of iron or aluminum chloride to the artery (Bonnard and Hagemeyer, 2015). Another model of thromboembolic stroke consists in injecting thrombin directly into the MCA by using a micropipette, which causes the in situ formation of a fibrin-rich clot (Orset et al., 2007, Le Behot et al., 2014). These fibrin-rich clots may be spontaneously lysed (partially or completely), as occurs in non-treated patients (Drieu et al., 2020a, Drieu et al., 2020b). Infarct volume and localization after both ferric chloride and thrombin models correspond to ischemic brain lesions in the majority of human strokes in proportion to brain size. As in humans, the lesion is mostly cortical and well defined (see Fig. 2), with the presence of a peri-lesional area that can be saved by early-tPA fibrinolysis in the thrombin-induced thromboembolic model (Orset et al., 2007; Macrez et al., 2011; Orset et al., 2016). The thrombin model is the only one to have shown a similar profile of time-to-treatment-related benefit after tPA administration (Orset et al., 2007; Macrez, 2011; Orset et al., 2016). By contrast, clots formed by the ferric chloride thromboembolic model are platelet-rich and cannot be lysed by tPA (Martinez de Lizarrondo et al., 2017).
We have recently provided a detailed longitudinal description of the inflammatory response following the thrombin-induced stroke experimental model consisting in an early myeloid response peaking at 48 h after stroke onset and still present (although to a lesser extent) 5 days after stroke onset. T-lymphocytes infiltrate later on (from 48 h after stroke onset). These cellular responses are accompanied by EC activation at 24 h-48 h that is not present later on (5 days after stroke onset). Whereas ischemic lesions are maximal at 24–48 h after stroke onset, BBB leakage progressively increases with time, being maximal at 5 days after stroke (Drieu et al., 2020a, Drieu et al., 2020b).
Regarding translational issues, thromboembolic models are, in many aspects, closer to the human pathophysiology (see table 2) and may thus provide an opportunity to investigate not only fibrinolytic drugs but also strategies targeting inflammation and immune responses triggered after stroke onset. In this regard, the thrombin-induced stroke model has shown similarities with the results obtained in several RCT. In addition to the aforementioned results on tPA, Natalizumab, which has shown its effectiveness in several other models before failing in the clinic, is not beneficial in the thrombin model of stroke (Drieu et al., 2020a, Drieu et al., 2020b), but minocycline has shown promising results (Drieu et al., 2020a, Drieu et al., 2020b) as in humans (Malhotra et al., 2018). Apart from this, there is not hindsight enough on the use of the thrombin model to demonstrate a better translation into the clinic compared to other experimental models. For this reason, and as recommended by the STAIR roundtable, several stroke models should be tested in multicenter studies for a given drug before its translation into randomized clinical trials.
Similar to the electrocoagulation model, one drawback of these models is the low behavioral deficits. Only minor behavioral deficits can be detected in the first days after stroke with behavioral tests and functional recovery appears after few days. This currently makes it difficult to assess long-term functional results. It would be necessary to further characterize the functional deficits of this model over the long term, especially in the context of the study of late inflammatory response and recovery.
-
ii)
Timing and selection of outcome measuring
The timing of outcome assessment is another factor that contributes to contradictory results between mouse and human studies. While in most clinical studies the neurological outcome is measured in patients up to several months after the stroke (usually 90 days), a vast majority of preclinical studies in mice focus only on the first days after the stroke (Veltkamp and Gill, 2016, Dreikorn et al., 2018).
The inflammatory reaction participates in each stage of stroke pathophysiology. It takes place from the first minutes after occlusion and lasts until the late phase, including the recovery and regeneration processes. This is why we should use behavioral tests, which measure functional recovery at longer time points, if we want preclinical studies to be more transposable to the clinic. As aforementioned, the main obstacle is that in mice it is difficult to obtain observable neurological deficits for a long time, in models with young animals with cortical well-standardized lesions. Furthermore, while recovery processes in rodents can be completed 4 weeks post-stroke, in humans they occur within about 3 months and may continue for years (Sommer, 2017, Cassidy and Cramer, 2017).
In spite of these difficulties, recent studies have shown encouraging results for mouse to human translation. For example, Sadler and collaborators (Sadler et al., 2020) have demonstrated the impact of short-chain fatty acids on microglial activation, which depended on the recruitment of T cells to the infarcted brain. Using an automated task for the training and assessment of distal forelimb function (Becker et al., 2016) in a mouse model of photothrombotic stroke, differences in motor deficit were observed up to 56 days after stroke onset. Another study has recently shown that targeting integrin alpha-9-beta-1 (highly expressed on activated neutrophils during ischemic stroke) significantly improved short and long-term functional outcomes (up to 4 weeks) in two different stroke models with pre-existing comorbidity (Dhanesha et al., 2020).
This being said, the STAIR X recommendations have highlighted that the time point of the standard 90-day functional outcome may be reconsidered for different reasons. First, it may result in enrollment bias, as socioeconomic and other factors may involve recruitment of only certain individuals into prospective trials. Furthermore, evaluation at later time points exclude patients that might succumb due to the other illnesses. Also, the impact of acute stroke interventions may be more efficiently revealed by early improvements in clinical deficits. Early National Institutes of Health Stroke Scale improvement or the serial change in neurological deficits from baseline to 24 h provides an early indication of what might ensue at 90 days, although early evaluation may raise challenges in some cases, as intubated or other patients may not be evaluable (Liebeskind et al., 2018).
An additional issue on the translation between preclinical and clinical studies is to deal with the discrepancies between the very specific and sensitive outcome measurements (histological, different imaging techniques and/or specific and sophisticated behavior tests) in preclinical research versus the well-established but yet less sensitive standardized clinical scores (composite scores, mRS, etc). In fact, it is possible that some of the positive results found in preclinical research are not strong enough to be detected in the neurological or functional outcome measurements in clinical practice. This is why it is important, in our opinion, to perform several outcome measurements for every drug tested. In addition, multicenter studies may help to increase the relevance of the preclinical results.
-
iii)
Entry routes for leukocytes into the ischemic region.
In the past few years, research on anti-inflammatory strategies for stroke has focused on limiting the transendothelial migration of peripheral immune cells into the brain parenchyma, aiming to reduce stroke severity. These studies are based on the idea of endothelial transmigration of peripheral immune cells through the BBB, but new evidence suggests that there are other pathways at least just as important (if not more): migration through the meninges and choroid plexus (ChP), (for a complete review, see (Benakis et al., 2018).
The ChP is originally known as the main producer of CSF, which fills all brain ventricles, subarachnoid spaces and perivascular spaces and thereby reaches a large surface area of the CNS. This highly vascularized brain structure resides in the brain ventricles and consists of an epithelial layer forming a tight blood-cerebrospinal fluid barrier (BCSFB), which surrounds a core of fenestrated capillaries and connective tissue. However, data is accumulating that CNS specific immune processes like immune surveillance are regulated by the ChP (Ghersi-Egea et al., 2018). First, it has been demonstrated by Ge and collaborators that ChP responds to a cortical ischemic lesion by elevated adhesion molecules and chemokines, and that monocytes-derived macrophages can invade ischemic hemisphere through ChP and CSF (Ge et al., 2017). Furthermore, it has been shown the ChP is a key invasion route for T-cell to the cortex in a permanent electrocoagulation stroke model, via a CCR2-ligand dependent mechanism (Llovera et al., 2017).
In addition to this, several recent studies have shown that the meninges could be a major player in leukocyte infiltration into the brain parenchyma. Immunohistochemistry and flow cytometry analysis revealed that γδT cells increased in the meninges early after stroke onset (Benakis et al., 2016) and preceded their accumulation in the ischemic area (Gelderblom et al., 2014). Moreover, ischemic injury can induce the growth of meningeal lymphatic vessels (LVs) and the absence of these LVs impact post-stroke outcomes. Interestingly, only photothrombotic stroke (and not transient MCAO) induce lymphangiogenesis. However, removal of meningeal lymphatics exacerbates severity of stroke, only in the transient MCAO model (Yanev et al., 2020). These data indicate that the meninges could orchestrate leukocyte infiltration into the brain parenchyma.
Taken together, these recent studies reveal the previously unrecognized diversity of selective invasion routes for leukocyte subpopulations after stroke. Understanding how immune cells migrate to the brain via these alternative pathways may help us to develop more effective approaches for anti-inflammatory/ immunomodulatory stroke therapies. As discussed above, several clinical trials have been initiated using Enlimomab (anti-ICAM-1 antibody), Natalizumab (anti-CD49d) or Fingolimod (FTY720). While Fingolimod (FTY720) could reduce the infarct volume and improve recovery, Natalizumab and enlimomab failed to show an effect on their primary endpoints (Fu et al., 2015, Elkins et al., 2017, Enlimomab Acute Stroke Trial Investigators, 2001). One of the reasons for these discrepancies might be due to the incomplete concept that lymphocytes infiltrate the brain mainly via the transendothelial route of parenchymal capillaries, without considering the meninges and ChP. Natalizumab and Enlimomab aimed to block the lymphocyte entrance to the CNS by blocking specific adhesion molecules required for transendothelial migration across parenchymal vessels. However, VCAM as well as ICAM-1 are not expressed on ChP EC (Steffen et al., 1996). Hence, lymphocytes in the ChP vasculature have no access to these adhesion molecules and blocking of these will not affect the ChP infiltration route (Benakis et al., 2018). In contrast, Fingolimod works by promoting lymphocyte retention in the thymus and lymph nodes (Mandala et al., 2002) and thus reduces the number of circulating lymphocytes independently of adhesion molecules expression at the various migration routes, which might explain why currently the only positive results on treatment efficacy are obtained with this drug in patients with stroke (Benakis et al., 2018). This could also explain the promising results obtained with minocycline treatment. Indeed, minocycline exerts anti-inflammatory effects on several targets, notably microglial activation, which do not depend on the migration route of peripheral immune cells (Malhotra et al., 2018). Thus, therapeutic targets should then be identified by focusing on compounds which act directly on circulating immune cells rather than on the different adhesion molecules, to maximize effectiveness across all of the entry pathways.
-
iv)
Factors affecting stroke outcome
Last, but no less important, several factors affecting stroke outcome are usually neglected in stroke preclinical research from an inflammatory perspective. In this review, we are going to summarize some of them: gender differences, ageing, pre-stroke infections, comorbidities such as hypertension and diabetes, and external factors related to daily life e.g. obesity, tobacco and alcohol consumption. In our opinion, inclusion of these comorbidities in preclinical research would increase the value, robustness and translational potential of the results.
As can be seen in the literature reviewed in this paper, the vast majority of the preclinical data has been obtained in young male mice/rats. However, while stroke in general is more common in men than women within the young and middle-aged population, women have a higher lifetime risk of stroke than men (20–21% versus 14–17%) with poorer functional outcomes (Guzik and Bushnell, 2017). Furthermore, the risk of stroke in women is correlated with ageing, particularly after 65 years compared to men (Appelros et al., 2009). This could partially explain women having more severe ischemic stroke in addition to age- related factors such as atrial fibrillation (Phan et al., 2019, Marzona et al., 2018).
Regarding the preclinical data, it has been shown in rodents, that premenopausal females exhibit smaller infarct sizes than age-matched males. Estrogen, the primary ovarian hormone has been described as a key element as the treatment with this hormone in the male or ovariectomized animals has shown to reduce infarct and neuronal death following ischemia and to decrease proinflammatory cytokines (Wise et al., 2001, Liu et al., 2012). Thus, besides the hormonal changes observed with estrus cycle, evidence as well as the STAIR recommendations (Fisher, 2003), indicate gender as an important biological variable to be addressed in stroke research.
As aforementioned, most of the preclinical stroke data has been obtained in young and healthy animals and to date, studies on the effectiveness of anti-inflammatory/immunomodulatory drugs in co-morbid animals are limited. Concerning the drugs reviewed here (see Table 1), there is only one study on the effects of Anakinra in young vs aged and Corpulent rats (a model of atherosclerosis, obesity and insulin resistance) (Pradillo et al., 2017), and another one on Minocycline in hypertensive rats (Yang et al., 2015), and one on Natalizumab in hypertensive rats (Relton et al., 2001). These studies have been performed on the transient MCAO model of stroke and have shown beneficial effects of the tested drug in these co-morbid animals.
With the life expectancy increase of the population, ageing has become a principal risk factor for ischemic stroke, with incidence rates accelerating exponentially above 70 years (Sandu et al., 2017). Interestingly, the neuroprotection has been related to anti-inflammation in young brain but related to proinflammation in the aged brain, with reduced microglial proliferation, attenuated macrophage cytokine production, and recruitment of macrophages in ischemic injury (Kim and Cho, 2016). Ageing has impact on immune cells; it is well known that aging is associated with an increase in basal inflammation in the CNS, overall decline in cognitive function, and poorer recovery following injury. Aged rodents had increased brain injury and increased CD8 + T cells in the aged CNS were associated with compromised proinflammatory functions in microglia. The primed microglia increased the inflammation and leukocyte recruitment in the brain following stroke in a gender independent manner (Ritzel et al., 2016). Consistent with this evidence, several in vivo studies have shown that brain of aged mice have increased proinflammatory and decreased anti-inflammatory profiles (Tang et al., 2014) or increased numbers of M2 macrophages in lymph nodes, bone marrow and spleen (Jackaman et al., 2013, Kim and Cho, 2016).
Another risk factor related to stroke is infection preceding stroke. Thromboinflammatory (platelet-mediated) processes beyond infection are likely to be an important contributor to stroke pathophysiology. Although several infections are documented to increase stroke risk, such as Herpex simplex 1 and 2, cytomegalovirus, Epstein-Barr virus, Hemophilus influenza; or Mycoplasma pneuomoniae and Helicobacter pylori, controversial results are also found. For instance, by the results obtained in the Northern Manhattan Study, it is described that patients with high chronic infectious burden showed 1.4-fold increased risk of stroke (Elkind et al., 2010). However, others have not replicated the results (Palm et al., 2016, Parikh et al., 2020). More recently, although additional research is required, Covid-19 infection has been shown to increase the risk (or probability) of stroke as well. Several studies have been conducted in China. For instance, a retrospective study of data from the Covid-19 outbreak in Wuhan, China, showed that the incidence of stroke among hospitalized Covid-19 patients was approximately 5%. Another retrospective study has shown that among 221 patients with Covid-19, 13 (5.9%) developed cerebrovascular diseases after the infection. Out of these patients, 11 (84.6%) were diagnosed with ischemic stroke. Another study has reported that cryptogenic stroke was confirmed in 32 of 3556 hospitalized patients with positive Covid-19 test. (Qin et al., 2020, Oxley et al., 2020, Mao et al., 2020). Other countries have shown evidences on this line as well, like the observational study conducted in New York, in which 32 (0.9%) ,out of 3556 hospitalized patients with diagnosis of Covid-19 infection, had imaging-proven ischemic stroke (Yaghi et al., 2020). A retrospective cohort study from two New York City academic hospitals, approximately 1.6% of adults with COVID-19 who visited the emergency department or were hospitalized experienced ischemic stroke (Merkler et al., 2020). Other study conducted in Italy has shown that among 388 patients with confirmed COVID-19, 9 patients experienced ischemic stroke (Lodigiani et al., 2020). Data from a Spanish hospital has shown that the incidence of CVD among the patients with COVID-19 infection was 1.4% of which the 73.9% were classified as cerebral ischemia (Hernández-Fernández et al., 2020). Thus, even if more research in the field is needed, the presence of infectious diseases seems to be a factor to be taken into account as well, while working in stroke therapies.
Besides gender and aging, there are several modifiable comorbidities related to stroke that should also be considered. For instance, hypertension is the most common modifiable risk factor for stroke, affecting about one-third of US adults over 20 years. Although most of them are aware, only about half have blood pressure controlled regularly (Guzik and Bushnell, 2017). Hypertension increases endothelial oxidative stress and inflammation, and it leads to worse stroke outcomes and higher mortality. Studies investigating the underlying pathophysiology of hypertension indicate the involvement of neuroimmune interactions (Kim and Cho, 2016), but mechanisms linking the immune responses to hypertension are still vague. The inclusion of rodent models of hypertension, such as spontaneously hypertensive rats (SHR), salt-induced hypertension model, or Angiotensin II-induced hypertension model (Lopez Gelston and Mitchell, 2017), in stroke preclinical research would help to correct translation of the data to RCT.
Similarly, disorders of glucose metabolism are major risk factors for stroke, including type 1 and type 2 diabetes mellitus (Guzik and Bushnell, 2017). These disorders are highly prevalent in patients with stroke: 25–45% of the patients have diabetes mellitus (Kernan et al., 2014). Data on diabetic patients in stroke clinical trials are missing. Meta-analysis of prospective studies showed a hazard ratio of 2.27 for ischemic stroke in diabetics compared to non-diabetics (Emerging Risk Factors Collaboration et al., 2010). Furthermore, diabetes has also shown a Hazard Ratio of 1.59 for stroke recurrence (Kaplan et al., 2005). In animal models, acute hyperglycemia immediately before or during ischemia exacerbates the ischemic brain injury. Elevated proinflammatory cytokines; tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), and interferon-γ (IFN-γ), as well as altered activation of macrophages, T cells, natural killer cells, and other immune cell populations are associated with major comorbidities (including diabetes) for stroke (Shukla et al., 2017).
Regarding the life style of the actual society, there are several habits that influence on stroke risk from the inflammatory point of view, which cannot be neglected. Obesity, characterized by a body mass index (BMI) of at least 30 kg/m2, is largely a consequence of fat-rich diet and physical inactivity and is associated with several risk factors including inflammation, metabolic syndrome, hypertension, diabetes and hypercholesterolaemia (Sandu et al., 2017). It has been demonstrated that obesity increases levels of pro-inflammatory mediators such as Interleukin 6 or C-reactive protein, causing a chronic inflammation, which is linked to a higher stroke risk and worse outcome (Haley and Lawrence, 2016). Efforts are being done to improve the stroke translational research. For instance, Pradillo et al (Pradillo et al., 2017) reported the improvement of stroke outcome through the administration of the IL-1Ra after stroke in control and overweight Wistar rats.
Other life-style risk factors are tobacco and alcohol consumption. It is well known that current smokers have more than doubled risk of stroke, with an apparent dose–response relationship seen (Guzik and Bushnell, 2017). Focusing on alcohol consumption, alcohol has emerged as a potentially important factor with a J- or U-shaped association to stroke risk. Epidemiological studies suggest that low-moderate ethanol intake lowers the incidence of ischemic stroke, reduces mortality and infarct volume from ischemic stroke, whereas heavy ethanol consumption increases the incidence of ischemic stroke and worsens the prognosis of ischemic stroke. Chronic ethanol consumption dose-dependently affects both incidence and prognosis of stroke (Xu et al., 2019). Although the association between alcohol and cardiovascular diseases has been a point of interest since3 decades ago, there are still considerable gaps in this area. Heavy alcohol consumption >2 drinks/day on average in midlife increases stroke risk, especially shortly after baseline (on average 50 years) until the age of 75 years, and may shorten time to stroke by ≈5 years regardless of familial and other common confounds. Although often neglected as a risk factor, heavy drinking in midlife is at least comparable with well-known risk factors of stroke such as diabetes mellitus or hypertension (Kadlecová et al., 2015). Studies in animal models have shown that ethanol may affect both basal and post-ischemic inflammatory profile in the brain (Drieu et al., 2020a, Drieu et al., 2020b). In a recent translational study, the chronic exposure to alcohol in both humans and mice has shown to exacerbate ischemic lesions (Drieu et al., 2020a, Drieu et al., 2020b). The preclinical results of this study have shown that alcohol consumption itself provokes a neurovascular inflammatory priming. The term “priming” is used to describe the propensity of a particular cell type to make an exaggerated response to a secondary stimulus at the parenchymal, perivascular and vascular levels whose consequence could be the exacerbated response to the ischemic injuries. In humans, chronic alcohol drinking (≥6 drinks/day in the last 5 years) has been independently associated (a) to stroke severity baseline, (b) to early neurological deterioration (END, defined as the increase of NIHSS in ≥ 4 in the first 48 h after admission), and (c) to higher infarct volume in stroke patients. Beyond the significance of these results on alcohol consumption and stroke outcome, these results highlight the decisive impact of the “basal” inflammatory state preceding stroke.
6. Conclusions
We must acknowledge that we are still far from the complete understanding of the role of inflammation and immune responses to stroke. Several studies have described the inflammatory and immune responses after stroke in different experimental models (Gelderblom et al., 2009, Zhou et al., 2013, Drieu et al., 2020a, Drieu et al., 2020b) and humans and it is undeniable that neuroinflammation and immune responses are present minutes after stroke onset and last for months or even years. Moreover, anti-inflammatory and immunomodulatory therapies have shown beneficial effects in some of the experimental models (in particular, in the intraluminal filament model). Some of these molecules have been tested in RCT but have failed to show positive results in stroke patients. This fact suggests that the mechanisms through which inflammatory processes contribute to injury after stroke are not well understood and could reflect a mismatch between the burden of inflammation in these particular experimental models and in stroke patients. It is also possible that the different experimental approaches actually model different aspects of the disease, which highlights the importance of testing drugs in several clinically relevant experimental models before their translation to clinical trials. In the past years, several studies have followed this guideline (Martinez de Lizarrondo et al., 2017, Llovera et al., 2015). Furthermore, the development of multicenter preclinical studies would help to definitely confirm the potential preclinical positive results before proceeding to clinical trials. Moreover, the burden of comorbidities (ageing, inflammatory states preceding stroke, etc) is often neglected in experimental studies and, thus, far from the clinical reality of stroke patients. Therefore, we encourage scientists to increase the knowledge in this field, before going ahead to clinical research.
We need to better understand the involvement of inflammation/ immune system in stroke with the final aim of modulating it and improving the pharmacological approaches available. For this, the choice of clinically relevant experimental models of stroke; the inclusion of measurements including acute but also long-term quantification of infarct volume and behavioral outcome; performing multicenter studies; and the inclusion of coexisting risk factors, are mandatory before considering the translation of a new stroke therapy into randomized clinical trials. In conclusion, in our opinion, as well as other authors’, (Chamorro et al., 2016, Shi et al., 2019), all the above-mentioned parameters should be considered when working in the stroke preclinical and clinical research field. This will improve the liability and translational strength of the results.
Acknowledgements
MG is a PhD student from the European Uniońs Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 813294. DL and AL are PhD students from the Region Normandie.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2020.09.025.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Anrather J., Iadecola C. Inflammation and stroke: an overview. Neurother. J. Am. Soc. Exp. Neurother. 2016;13:661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansar S., Chatzikonstantinou E., Wistuba-Schier A., Mirau-Weber S., Fatar M., Hennerici M.G., Meairs S. Characterization of a new model of thromboembolic stroke in C57 black/6J mice. Transl. Stroke Res. 2014;5:526–533. doi: 10.1007/s12975-013-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelros P., Stegmayr B., Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- Aras M., Urfalı B., Serarslan Y., Ozgür T., Ulutaş K.T., Urfalı S., Altaş M., Yılmaz N. Protective effects of minocycline against short-term ischemia-reperfusion injury in rat brain. Pediatr. Neurosurg. 2013;49:172–178. doi: 10.1159/000362202. [DOI] [PubMed] [Google Scholar]
- Becker A.M., Meyers E., Sloan A., Rennaker R., Kilgard M., Goldberg M.P. An automated task for the training and assessment of distal forelimb function in a mouse model of ischemic stroke. J. Neurosci. Methods. 2016;258:16–23. doi: 10.1016/j.jneumeth.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K., Kindrick D., Relton J., Harlan J., Winn R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32:206–211. doi: 10.1161/01.str.32.1.206. [DOI] [PubMed] [Google Scholar]
- Benakis C., Brea D., Caballero S., Faraco G., Moore J., Murphy M., Sita G., Racchumi G., Ling L., Pamer E.G., Iadecola C., Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C., Llovera G., Liesz A. The meningeal and choroidal infiltration routes for leukocytes in stroke. Ther. Adv. Neurol. Disord. 2018;11 doi: 10.1177/1756286418783708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnard T., Hagemeyer C.E. Ferric chloride-induced thrombosis mouse model on carotid artery and mesentery vessel. J. Vis. Exp. JoVE. 2015;e52838 doi: 10.3791/52838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkhorst R., Kanaan N., Koch A., Ferreirós N., Mirceska A., Zeiner P., Mittelbronn M., Derouiche A., Steinmetz H., Foerch C., Pfeilschifter J., Pfeilschifter W. FTY720 treatment in the convalescence period improves functional recovery and reduces reactive astrogliosis in photothrombotic stroke. PloS One. 2013;8 doi: 10.1371/journal.pone.0070124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscemi L., Price M., Bezzi P., Hirt L. Spatio-temporal overview of neuroinflammation in an experimental mouse stroke model. Sci. Rep. 2019;9:507. doi: 10.1038/s41598-018-36598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai A., Schlunk F., Bohmann F., Kashefiolasl S., Brunkhorst R., Foerch C., Pfeilschifter W. Coadministration of FTY720 and rt-PA in an experimental model of large hemispheric stroke-no influence on functional outcome and blood-brain barrier disruption. Exp. Transl. Stroke Med. 2013;5:11. doi: 10.1186/2040-7378-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., Liu S., Hu M., Huang F., Zhu Q., Qiu W., Hu X., Colello J., Zheng S.G., Lu Z. Functional dynamics of neutrophils after ischemic stroke. Transl. Stroke Res. 2020;11:108–121. doi: 10.1007/s12975-019-00694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z., Lin S., Fan L.-W., Pang Y., Rhodes P.G. Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience. 2006;137:425–435. doi: 10.1016/j.neuroscience.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Cai Z.-Y., Yan Y., Chen R. Minocycline reduces astrocytic reactivation and neuroinflammation in the hippocampus of a vascular cognitive impairment rat model. Neurosci. Bull. 2010;26:28–36. doi: 10.1007/s12264-010-0818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z.-Y., Yan Y., Sun S.-Q., Zhang J., Huang L.-G., Yan N., Wu F., Li J.-Y. Minocycline attenuates cognitive impairment and restrains oxidative stress in the hippocampus of rats with chronic cerebral hypoperfusion. Neurosci. Bull. 2008;24:305–313. doi: 10.1007/s12264-008-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos F., Qin T., Castillo J., Seo J.H., Arai K., Lo E.H., Waeber C. Fingolimod reduces hemorrhagic transformation associated with delayed tissue plasminogen activator treatment in a mouse thromboembolic model. Stroke. 2013;44:505–511. doi: 10.1161/STROKEAHA.112.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso M.M., Franco E.C.S., de Souza C.C., da Silva M.C., Gouveia A., Gomes-Leal W. Minocycline treatment and bone marrow mononuclear cell transplantation after endothelin-1 induced striatal ischemia. Inflammation. 2013;36:197–205. doi: 10.1007/s10753-012-9535-5. [DOI] [PubMed] [Google Scholar]
- Casetta I., Fainardi E., Saia V., Pracucci G., Padroni M., Renieri L., Nencini P., Inzitari D., Morosetti D., Sallustio F., Vallone S., Bigliardi G., Zini A., Longo M., Francalanza I., Bracco S., Vallone I.M., Tassi R., Bergui M., Naldi A., Saletti A., De Vito A., Gasparotti R., Magoni M., Castellan L., Serrati C., Menozzi R., Scoditti U., Causin F., Pieroni A., Puglielli E., Casalena A., Sanna A., Ruggiero M., Cordici F., Di Maggio L., Duc E., Cosottini M., Giannini N., Sanfilippo G., Zappoli F., Cavallini A., Cavasin N., Critelli A., Ciceri E., Plebani M., Cappellari M., Chiumarulo L., Petruzzellis M., Terrana A., Cariddi L.P., Burdi N., Tinelli A., Auteri W., Silvagni U., Biraschi F., Nicolini E., Padolecchia R., Tassinari T., Filauri P., Sacco S., Pavia M., Invernizzi P., Nuzzi N.P., Marcheselli S., Amistà P., Russo M., Gallesio I., Craparo G., Mannino M., Mangiafico S., Toni D., Registryof Endovascular Treatment in Acute Stroke, I. Endovascular thrombectomy for acute ischemic stroke beyond 6 hours from onset: a real-world experience. Stroke. 2020;51:2051–2057. doi: 10.1161/STROKEAHA.119.027974. [DOI] [PubMed] [Google Scholar]
- Cassidy J.M., Cramer S.C. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl. Stroke Res. 2017;8:33–46. doi: 10.1007/s12975-016-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro Á., Dirnagl U., Urra X., Planas A.M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- Cho K.-O., La H.O., Cho Y.-J., Sung K.-W., Kim S.Y. Minocycline attenuates white matter damage in a rat model of chronic cerebral hypoperfusion. J. Neurosci. Res. 2006;83:285–291. doi: 10.1002/jnr.20727. [DOI] [PubMed] [Google Scholar]
- Chu H.X., Kim H.A., Lee S., Moore J.P., Chan C.T., Vinh A., Gelderblom M., Arumugam T.V., Broughton B.R.S., Drummond G.R., Sobey C.G. Immune cell infiltration in malignant middle cerebral artery infarction: comparison with transient cerebral ischemia. J. Cereb. Blood Flow Metab Off. J. Int. Soc. Cereb. Blood Flow Metab. 2014;34:450–459. doi: 10.1038/jcbfm.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L.-S., Fang S.-H., Zhou Y., Yu G.-L., Wang M.-L., Zhang W.-P., Wei E.-Q. Minocycline inhibits 5-lipoxygenase activation and brain inflammation after focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2007;28:763–772. doi: 10.1111/j.1745-7254.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- Cserep Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science. 2020 doi: 10.1126/science.aax6752. [DOI] [PubMed] [Google Scholar]
- Czech B., Pfeilschifter W., Mazaheri-Omrani N., Strobel M.A., Kahles T., Neumann-Haefelin T., Rami A., Huwiler A., Pfeilschifter J. The immunomodulatory sphingosine 1-phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochem. Biophys. Res. Commun. 2009;389:251–256. doi: 10.1016/j.bbrc.2009.08.142. [DOI] [PubMed] [Google Scholar]
- del Zoppo G.J. The neurovascular unit in the setting of stroke. J. Intern. Med. 2010;267:156–171. doi: 10.1111/j.1365-2796.2009.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanesha, N., Jain, M., Tripathi, A., Doddapattar, P., Chorawala, M., Bathla, G., Nayak, M.K., Ghatge, M., Lentz, S.R., Kon, S., Chauhan, A.K., 2020. Targeting Myeloid-Specific Integrin α9β1 Improves Short and Long-Term Stroke Outcomes in Murine Models With Preexisting Comorbidities by Limiting Thrombosis And Inflammation. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.120.316659. [DOI] [PMC free article] [PubMed]
- Dreikorn M., Milacic Z., Pavlovic V., Meuth S.G., Kleinschnitz C., Kraft P. Immunotherapy of experimental and human stroke with agents approved for multiple sclerosis: a systematic review. Ther. Adv. Neurol. Disord. 2018;11 doi: 10.1177/1756286418770626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drieu A., Buendia I., Levard D., Hélie P., Brodin C., Vivien D., Rubio M. Immune responses and anti-inflammatory strategies in a clinically relevant model of thromboembolic ischemic stroke with reperfusion. Transl. Stroke Res. 2020;11:481–495. doi: 10.1007/s12975-019-00733-8. [DOI] [PubMed] [Google Scholar]
- Drieu A., Lanquetin A., Levard D., Glavan M., Campos F., Quenault A., Lemarchand E., Naveau M., Pitel A.L., Castillo J., Vivien D., Rubio M. Alcohol exposure-induced neurovascular inflammatory priming impacts ischemic stroke and is linked with brain perivascular macrophages. JCI Insight. 2020;5 doi: 10.1172/jci.insight.129226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drieu A., Levard D., Vivien D., Rubio M. Anti-inflammatory treatments for stroke: from bench to bedside. Ther. Adv. Neurol. Disord. 2018;11 doi: 10.1177/1756286418789854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont A.-C., Largeau B., Santiago Ribeiro M.J., Guilloteau D., Tronel C., Arlicot N. Translocator protein-18 kDa (TSPO) positron emission tomography (PET) imaging and its clinical impact in neurodegenerative diseases. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18040785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind M.S.V., Ramakrishnan P., Moon Y.P., Boden-Albala B., Liu K.M., Spitalnik S.L., Rundek T., Sacco R.L., Paik M.C. Infectious burden and risk of stroke: the northern Manhattan study. Arch. Neurol. 2010;67:33–38. doi: 10.1001/archneurol.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins J., Veltkamp R., Montaner J., Johnston S.C., Singhal A.B., Becker K., Lansberg M.G., Tang W., Chang I., Muralidharan K., Gheuens S., Mehta L., Elkind M.S.V. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017;16:217–226. doi: 10.1016/S1474-4422(16)30357-X. [DOI] [PubMed] [Google Scholar]
- Collaboration Emerging Risk Factors, Sarwar N., Gao P., Seshasai S.R.K., Gobin R., Kaptoge S., Di Angelantonio E., Ingelsson E., Lawlor D.A., Selvin E., Stampfer M., Stehouwer C.D.A., Lewington S., Pennells L., Thompson A., Sattar N., White I.R., Ray K.K., Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet Lond. Engl. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley H.C.A., Smith C.J., Georgiou R.F., Vail A., Hopkins S.J., Rothwell N.J., Tyrrell P.J., Investigators Acute Stroke. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J. Neurol. Neurosurg. Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlimomab Acute Stroke Trial Investigators Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- Faheem H., Mansour A., Elkordy A., Rashad S., Shebl M., Madi M., Elwy S., Niizuma K., Tominaga T. Neuroprotective effects of minocycline and progesterone on white matter injury after focal cerebral ischemia. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2019;64:206–213. doi: 10.1016/j.jocn.2019.04.012. [DOI] [PubMed] [Google Scholar]
- Feng Y., Liao S., Wei C., Jia D., Wood K., Liu Q., Wang X., Shi F.-D., Jin W.-N. Infiltration and persistence of lymphocytes during late-stage cerebral ischemia in middle cerebral artery occlusion and photothrombotic stroke models. J. Neuroinflammation. 2017;14:248. doi: 10.1186/s12974-017-1017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, Stroke Therapy Academic Industry Roundtable Recommendations for advancing development of acute stroke therapies: Stroke Therapy Academic Industry Roundtable 3. Stroke. 2003 doi: 10.1161/01.STR.0000072983.64326.53. [DOI] [PubMed] [Google Scholar]
- Fu Y., Liu Q., Anrather J., Shi F.-D. Immune interventions in stroke. Nat. Rev. Neurol. 2015;11:524–535. doi: 10.1038/nrneurol.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani S.P., Walsh J.T., Lukens J.R., Kipnis J. Dealing with danger in the CNS: the response of the immune system to injury. Neuron. 2015;87:47–62. doi: 10.1016/j.neuron.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J.H., Liu K.F., Relton J.K. Interleukin-1 receptor antagonist decreases the number of necrotic neurons in rats with middle cerebral artery occlusion. Am. J. Pathol. 1995;147:1477–1486. [PMC free article] [PubMed] [Google Scholar]
- Gauberti M., Lapergue B., Martinez de Lizarrondo S., Vivien D., Richard S., Bracard S., Piotin M., Gory B. Ischemia-reperfusion injury after endovascular thrombectomy for ischemic stroke. Stroke. 2018;49:3071–3074. doi: 10.1161/STROKEAHA.118.022015. [DOI] [PubMed] [Google Scholar]
- Gauberti M., Martinez de Lizarrondo S., Orset C., Vivien D. Lack of secondary microthrombosis after thrombin-induced stroke in mice and non-human primates. J. Thromb. Haemost. JTH. 2014;12:409–414. doi: 10.1111/jth.12487. [DOI] [PubMed] [Google Scholar]
- Gauberti Maxime, Montagne A., Quenault A., Vivien D. Molecular magnetic resonance imaging of brain-immune interactions. Front. Cell. Neurosci. 2014;8:389. doi: 10.3389/fncel.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R., Tornero D., Hirota M., Monni E., Laterza C., Lindvall O., Kokaia Z. Choroid plexus-cerebrospinal fluid route for monocyte-derived macrophages after stroke. J. Neuroinflamm. 2017;14:153. doi: 10.1186/s12974-017-0909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M., Arunachalam P., Magnus T. γδ T cells as early sensors of tissue damage and mediators of secondary neurodegeneration. Front. Cell. Neurosci. 2014;8:368. doi: 10.3389/fncel.2014.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M., Leypoldt F., Steinbach K., Behrens D., Choe C.-U., Siler D.A., Arumugam T.V., Orthey E., Gerloff C., Tolosa E., Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Gerhard A., Schwarz J., Myers R., Wise R., Banati R.B. Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. NeuroImage. 2005;24:591–595. doi: 10.1016/j.neuroimage.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Ghersi-Egea J.-F., Strazielle N., Catala M., Silva-Vargas V., Doetsch F., Engelhardt B. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. (Berl.) 2018;135:337–361. doi: 10.1007/s00401-018-1807-1. [DOI] [PubMed] [Google Scholar]
- Girard S., Murray K.N., Rothwell N.J., Metz G.A.S., Allan S.M. Long-term functional recovery and compensation after cerebral ischemia in rats. Behav. Brain Res. 2014;270:18–28. doi: 10.1016/j.bbr.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik A., Bushnell C. Stroke epidemiology and risk factor management. Contin. Minneap. Minn. 2017;23:15–39. doi: 10.1212/CON.0000000000000416. [DOI] [PubMed] [Google Scholar]
- Haley M.J., Lawrence C.B. Obesity and stroke: Can we translate from rodents to patients? J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016;36:2007–2021. doi: 10.1177/0271678X16670411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y., Suzuki H., Sozen T., Rolland W., Zhang J.H. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–374. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata R., Maeda K., Hermann D., Mies G., Hossmann K.A. Evolution of brain infarction after transient focal cerebral ischemia in mice. J. Cereb. Blood Flow Metab Off. J. Int. Soc. Cereb. Blood Flow Metab. 2000;20:937–946. doi: 10.1097/00004647-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Mishima K., Nozako M., Hazekawa M., Mishima S., Fujioka M., Orito K., Egashira N., Iwasaki K., Fujiwara M. Delayed treatment with minocycline ameliorates neurologic impairment through activated microglia expressing a high-mobility group box1-inhibiting mechanism. Stroke. 2008;39:951–958. doi: 10.1161/STROKEAHA.107.495820. [DOI] [PubMed] [Google Scholar]
- Hernández-Fernández, F., Valencia, H.S., Barbella-Aponte, R.A., Collado-Jiménez, R., Ayo-Martín, Ó., Barrena, C., Molina-Nuevo, J.D., García-García, J., Lozano-Setién, E., Alcahut-Rodriguez, C., Martínez-Martín, Á., Sánchez-López, A., Segura, T., 2020. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain J. Neurol. https://doi.org/10.1093/brain/awaa239. [DOI] [PMC free article] [PubMed]
- Hoda M.N., Fagan S.C., Khan M.B., Vaibhav K., Chaudhary A., Wang P., Dhandapani K.M., Waller J.L., Hess D.C. A 2 × 2 factorial design for the combination therapy of minocycline and remote ischemic perconditioning: efficacy in a preclinical trial in murine thromboembolic stroke model. Exp. Transl. Stroke Med. 2014;6:10. doi: 10.1186/2040-7378-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann K.-A. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J. Cereb. Blood Flow Metab Off. J. Int. Soc. Cereb. Blood Flow Metab. 2012;32:1310–1316. doi: 10.1038/jcbfm.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells D.W., Porritt M.J., Rewell S.S.J., O’Collins V., Sena E.S., van der Worp H.B., Traystman R.J., Macleod M.R. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J. Cereb. Blood Flow Metab Off. J. Int. Soc. Cereb. Blood Flow Metab. 2010;30:1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nat. Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackaman C., Radley-Crabb H.G., Soffe Z., Shavlakadze T., Grounds M.D., Nelson D.J. Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice. Aging Cell. 2013;12:345–357. doi: 10.1111/acel.12062. [DOI] [PubMed] [Google Scholar]
- Jayaraj R.L., Azimullah S., Beiram R., Jalal F.Y., Rosenberg G.A. Neuroinflammation: friend and foe for ischemic stroke. J. Neuroinflamm. 2019;16:142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Liang J., Wang J., Kolattukudy P.E. MCP-induced protein 1 mediates the minocycline-induced neuroprotection against cerebral ischemia/reperfusion injury in vitro and in vivo. J. Neuroinflamm. 2015;12:39. doi: 10.1186/s12974-015-0264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlecová P., Andel R., Mikulík R., Handing E.P., Pedersen N.L. Alcohol consumption at midlife and risk of stroke during 43 years of follow-up: cohort and twin analyses. Stroke. 2015;46:627–633. doi: 10.1161/STROKEAHA.114.006724. [DOI] [PubMed] [Google Scholar]
- Kaplan R.C., Tirschwell D.L., Longstreth W.T., Manolio T.A., Heckbert S.R., Lefkowitz D., El-Saed A., Psaty B.M. Vascular events, mortality, and preventive therapy following ischemic stroke in the elderly. Neurology. 2005;65:835–842. doi: 10.1212/01.wnl.0000176058.09848.bb. [DOI] [PubMed] [Google Scholar]
- Kernan W.N., Ovbiagele B., Black H.R., Bravata D.M., Chimowitz M.I., Ezekowitz M.D., Fang M.C., Fisher M., Furie K.L., Heck D.V., Johnston S.C.C., Kasner S.E., Kittner S.J., Mitchell P.H., Rich M.W., Richardson D., Schwamm L.H., Wilson J.A., CouncilCouncil on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease, American Heart Association Stroke. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- Kim E., Cho S. Microglia and monocyte-derived macrophages in stroke. Neurother. J. Am. Soc. Exp. Neurother. 2016;13:702–718. doi: 10.1007/s13311-016-0463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.W., Sugawara T., Chan P.H. Involvement of oxidative stress and caspase-3 in cortical infarction after photothrombotic ischemia in mice. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2000;20:1690–1701. doi: 10.1097/00004647-200012000-00008. [DOI] [PubMed] [Google Scholar]
- Kohler E., Prentice D.A., Bates T.R., Hankey G.J., Claxton A., van Heerden J., Blacker D. Intravenous minocycline in acute stroke: a randomized, controlled pilot study and meta-analysis. Stroke. 2013;44:2493–2499. doi: 10.1161/STROKEAHA.113.000780. [DOI] [PubMed] [Google Scholar]
- Koistinaho M., Malm T.M., Kettunen M.I., Goldsteins G., Starckx S., Kauppinen R.A., Opdenakker G., Koistinaho J. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J. Cereb. Blood Flow Metab Off. J. Int. Soc. Cereb. Blood Flow Metab. 2005;25:460–467. doi: 10.1038/sj.jcbfm.9600040. [DOI] [PubMed] [Google Scholar]
- Kraft P., Göb E., Schuhmann M.K., Göbel K., Deppermann C., Thielmann I., Herrmann A.M., Lorenz K., Brede M., Stoll G., Meuth S.G., Nieswandt B., Pfeilschifter W., Kleinschnitz C. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke. 2013;44:3202–3210. doi: 10.1161/STROKEAHA.113.002880. [DOI] [PubMed] [Google Scholar]
- Krishnamurthi, R.V., Feigin, V.L., Forouzanfar, M.H., Mensah, G.A., Connor, M., Bennett, D.A., Moran, A.E., Sacco, R.L., Anderson, L.M., Truelsen, T., O’Donnell, M., Venketasubramanian, N., Barker-Collo, S., Lawes, C.M.M., Wang, W., Shinohara, Y., Witt, E., Ezzati, M., Naghavi, M., Murray, C., Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010), GBD Stroke Experts Group, 2013. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob. Health 1, e259-281. https://doi.org/10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed]
- Labat-gest V., Tomasi S. Photothrombotic ischemia: a minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J. Vis. Exp. JoVE. 2013 doi: 10.3791/50370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhauser F., Kraft P., Göb E., Leinweber J., Schuhmann M.K., Lorenz K., Gelderblom M., Bittner S., Meuth S.G., Wiendl H., Magnus T., Kleinschnitz C. Blocking of α4 integrin does not protect from acute ischemic stroke in mice. Stroke. 2014;45:1799–1806. doi: 10.1161/STROKEAHA.114.005000. [DOI] [PubMed] [Google Scholar]
- Langhorne P., Stott D.J., Robertson L., MacDonald J., Jones L., McAlpine C., Dick F., Taylor G.S., Murray G. Medical complications after stroke: a multicenter study. Stroke. 2000;31:1223–1229. doi: 10.1161/01.str.31.6.1223. [DOI] [PubMed] [Google Scholar]
- Le Behot A., Gauberti M., Martinez De Lizarrondo S., Montagne A., Lemarchand E., Repesse Y., Guillou S., Denis C.V., Maubert E., Orset C., Vivien D. GpIbα-VWF blockade restores vessel patency by dissolving platelet aggregates formed under very high shear rate in mice. Blood. 2014;123:3354–3363. doi: 10.1182/blood-2013-12-543074. [DOI] [PubMed] [Google Scholar]
- Lee, V.M., Burdett, N.G., Carpenter, A., Hall, L.D., Pambakian, P.S., Patel, S., Wood, N.I., James, M.F., 1996. Evolution of photochemically induced focal cerebral ischemia in the rat. Magnetic resonance imaging and histology. Stroke 27, 2110–2118; discussion 2118-2119. https://doi.org/10.1161/01.str.27.11.2110. [DOI] [PubMed]
- Li X., Wang M.-H., Qin C., Fan W.-H., Tian D.-S., Liu J.-L. Fingolimod suppresses neuronal autophagy through the mTOR/p70S6K pathway and alleviates ischemic brain damage in mice. PloS One. 2017;12 doi: 10.1371/journal.pone.0188748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeskind, D.S., Derdeyn, C.P., Wechsler, L.R., STAIR X Consortium, 2018. STAIR X: Emerging Considerations in Developing and Evaluating New Stroke Therapies. Stroke 49, 2241–2247. https://doi.org/10.1161/STROKEAHA.118.021424. [DOI] [PubMed]
- Liesz A., Sun L., Zhou W., Schwarting S., Mracsko E., Zorn M., Bauer H., Sommer C., Veltkamp R. FTY720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PloS ONE. 2011;6 doi: 10.1371/journal.pone.0021312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A., Zhou W., Mracskó É., Karcher S., Bauer H., Schwarting S., Sun L., Bruder D., Stegemann S., Cerwenka A., Sommer C., Dalpke A.H., Veltkamp R. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain J. Neurol. 2011;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- Liu F., Benashski S.E., Xu Y., Siegel M., McCullough L.D. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. J. Neuroendocrinol. 2012;24:319–330. doi: 10.1111/j.1365-2826.2011.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhang C., Tao W., Liu M. Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (S1P) receptor agonist FTY720 (Fingolimod) in animal models of stroke. Int. J. Neurosci. 2013;123:163–169. doi: 10.3109/00207454.2012.749255. [DOI] [PubMed] [Google Scholar]
- Liu Z., Fan Y., Won S.J., Neumann M., Hu D., Zhou L., Weinstein P.R., Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Llovera G., Benakis C., Enzmann G., Cai R., Arzberger T., Ghasemigharagoz A., Mao X., Malik R., Lazarevic I., Liebscher S., Ertürk A., Meissner L., Vivien D., Haffner C., Plesnila N., Montaner J., Engelhardt B., Liesz A. The choroid plexus is a key cerebral invasion route for T cells after stroke. Acta Neuropathol. (Berl.) 2017;134:851–868. doi: 10.1007/s00401-017-1758-y. [DOI] [PubMed] [Google Scholar]
- Llovera G., Hofmann K., Roth S., Salas-Pérdomo A., Ferrer-Ferrer M., Perego C., Zanier E.R., Mamrak U., Rex A., Party H., Agin V., Fauchon C., Orset C., Haelewyn B., De Simoni M.-G., Dirnagl U., Grittner U., Planas A.M., Plesnila N., Vivien D., Liesz A. Results of a preclinical randomized controlled multicenter trial (pRCT): Anti-CD49d treatment for acute brain ischemia. Sci. Transl. Med. 2015;7:299ra121. doi: 10.1126/scitranslmed.aaa9853. [DOI] [PubMed] [Google Scholar]
- Llovera G., Roth S., Plesnila N., Veltkamp R., Liesz A. Modeling stroke in mice: permanent coagulation of the distal middle cerebral artery. J. Vis. Exp. JoVE. 2014:e51729. doi: 10.3791/51729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddick S.A., Rothwell N.J. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J. Cereb. Blood Flow Metab Off. J. Int. Soc. Cereb. Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.-D., Sacco C., Alexia B., Sandri M.T., BarcoHumanitas COVID-19 Task Force S. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Gelston C.A., Mitchell B.M. Recent advances in immunity and hypertension. Am. J. Hypertens. 2017;30:643–652. doi: 10.1093/ajh/hpx011. [DOI] [PubMed] [Google Scholar]
- Lu Y., Xiao G., Luo W. Minocycline suppresses NLRP3 inflammasome activation in experimental ischemic stroke. Neuroimmunomodulation. 2016;23:230–238. doi: 10.1159/000452172. [DOI] [PubMed] [Google Scholar]
- Lunardi Baccetto S., Lehmann C. Microcirculatory changes in experimental models of stroke and CNS-injury induced immunodepression. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrez Richard, et al. Antibodies preventing the interaction of tissue-type plasminogen activator with N-methyl-D-aspartate receptors reduce stroke damages and extend the therapeutic window of thrombolysis. Stroke. 2011 doi: 10.1161/STROKEAHA.110.606293. [DOI] [PubMed] [Google Scholar]
- Malhotra K., Chang J.J., Khunger A., Blacker D., Switzer J.A., Goyal N., Hernandez A.V., Pasupuleti V., Alexandrov A.V., Tsivgoulis G. Minocycline for acute stroke treatment: a systematic review and meta-analysis of randomized clinical trials. J. Neurol. 2018;265:1871–1879. doi: 10.1007/s00415-018-8935-3. [DOI] [PubMed] [Google Scholar]
- Malone K., Amu S., Moore A.C., Waeber C. The immune system and stroke: from current targets to future therapy. Immunol. Cell Biol. 2019;97:5–16. doi: 10.1111/imcb.12191. [DOI] [PubMed] [Google Scholar]
- Mandala S., Hajdu R., Bergstrom J., Quackenbush E., Xie J., Milligan J., Thornton R., Shei G.-J., Card D., Keohane C., Rosenbach M., Hale J., Lynch C.L., Rupprecht K., Parsons W., Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., He, Q., Chang, J., Hong, C., Zhou, Y., Wang, D., Miao, X., Li, Y., Hu, B., 2020. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed]
- Martín A., Boisgard R., Kassiou M., Dollé F., Tavitian B. Reduced PBR/TSPO expression after minocycline treatment in a rat model of focal cerebral ischemia: a PET study using [(18)F]DPA-714. Mol. Imaging Biol. 2011;13:10–15. doi: 10.1007/s11307-010-0324-y. [DOI] [PubMed] [Google Scholar]
- Martinez de Lizarrondo S., Gakuba C., Herbig B.A., Repessé Y., Ali C., Denis C.V., Lenting P.J., Touzé E., Diamond S.L., Vivien D., Gauberti M. Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi. Circulation. 2017;136:646–660. doi: 10.1161/CIRCULATIONAHA.117.027290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzona I., Proietti M., Farcomeni A., Romiti G.F., Romanazzi I., Raparelli V., Basili S., Lip G.Y.H., Nobili A., Roncaglioni M.C. Sex differences in stroke and major adverse clinical events in patients with atrial fibrillation: a systematic review and meta-analysis of 993,600 patients. Int. J. Cardiol. 2018;269:182–191. doi: 10.1016/j.ijcard.2018.07.044. [DOI] [PubMed] [Google Scholar]
- Matsukawa N., Yasuhara T., Hara K., Xu L., Maki M., Yu G., Kaneko Y., Ojika K., Hess D.C., Borlongan C.V. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci. 2009;10:126. doi: 10.1186/1471-2202-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayadas T.N., Cullere X., Lowell C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maysami S., Wong R., Pradillo J.M., Denes A., Dhungana H., Malm T., Koistinaho J., Orset C., Rahman M., Rubio M., Schwaninger M., Vivien D., Bath P.M., Rothwell N.J., Allan S.M. A cross-laboratory preclinical study on the effectiveness of interleukin-1 receptor antagonist in stroke. J. Cereb. Blood Flow Metab Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016;36:596–605. doi: 10.1177/0271678X15606714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- Meisel C., Schwab J.M., Prass K., Meisel A., Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- Mena H., Cadavid D., Rushing E.J. Human cerebral infarct: a proposed histopathologic classification based on 137 cases. Acta Neuropathol. (Berl.) 2004;108:524–530. doi: 10.1007/s00401-004-0918-z. [DOI] [PubMed] [Google Scholar]
- Merkler A.E., Parikh N.S., Mir S., Gupta A., Kamel H., Lin E., Lantos J., Schenck E.J., Goyal P., Bruce S.S., Kahan J., Lansdale K.N., LeMoss N.M., Murthy S.B., Stieg P.E., Fink M.E., Iadecola C., Segal A.Z., Cusick M., Campion T.R., Diaz I., Zhang C., Navi B.B. Risk of Ischemic Stroke in Patients With Coronavirus Disease 2019 (COVID-19) vs Patients With Influenza. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto N., Shimazawa M., Yamashima T., Nagai H., Hara H. Minocycline inhibits oxidative stress and decreases in vitro and in vivo ischemic neuronal damage. Brain Res. 2005;1044:8–15. doi: 10.1016/j.brainres.2005.02.062. [DOI] [PubMed] [Google Scholar]
- Morris R.S., Simon Jones P., Alawneh J.A., Hong Y.T., Fryer T.D., Aigbirhio F.I., Warburton E.A., Baron J.-C. Relationships between selective neuronal loss and microglial activation after ischaemic stroke in man. Brain J. Neurol. 2018;141:2098–2111. doi: 10.1093/brain/awy121. [DOI] [PubMed] [Google Scholar]
- Mulcahy N.J., Ross J., Rothwell N.J., Loddick S.A. Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br. J. Pharmacol. 2003;140:471–476. doi: 10.1038/sj.bjp.0705462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi Y., Sabetkasaei M., Parvardeh S., Moini Zanjani T. Neuroprotective effects of pretreatment with minocycline on memory impairment following cerebral ischemia in rats. Behav. Pharmacol. 2017;28:214–222. doi: 10.1097/FBP.0000000000000297. [DOI] [PubMed] [Google Scholar]
- Nazari M., Keshavarz S., Rafati A., Namavar M.R., Haghani M. Fingolimod (FTY720) improves hippocampal synaptic plasticity and memory deficit in rats following focal cerebral ischemia. Brain Res. Bull. 2016;124:95–102. doi: 10.1016/j.brainresbull.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Nguyen M.D., Julien J.-P., Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat. Rev. Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Orset C., Haelewyn B., Allan S.M., Ansar S., Campos F., Cho T.H., Durand A., El Amki M., Fatar M., Garcia-Yébenes I., Gauberti M., Grudzenski S., Lizasoain I., Lo E., Macrez R., Margaill I., Maysami S., Meairs S., Nighoghossian N., Orbe J., Paramo J.A., Parienti J.-J., Rothwell N.J., Rubio M., Waeber C., Young A.R., Touzé E., Vivien D. Efficacy of alteplase in a mouse model of acute ischemic stroke: a retrospective pooled analysis. Stroke. 2016;47:1312–1318. doi: 10.1161/STROKEAHA.116.012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orset C., Macrez R., Young A.R., Panthou D., Angles-Cano E., Maubert E., Agin V., Vivien D. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38:2771–2778. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-vessel stroke as a presenting feature of covid-19 in the young. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm F., Pussinen P.J., Aigner A., Becher H., Buggle F., Bauer M.F., Grond-Ginsbach C., Safer A., Urbanek C., Grau A.J. Association between infectious burden, socioeconomic status, and ischemic stroke. Atherosclerosis. 2016;254:117–123. doi: 10.1016/j.atherosclerosis.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Parikh N.S., Merkler A.E., Iadecola C. Inflammation, autoimmunity, infection, and stroke: epidemiology and lessons from therapeutic intervention. Stroke. 2020;51:711–718. doi: 10.1161/STROKEAHA.119.024157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.H., Shin T.K., Lee H.Y., Kim S.J., Lee W.S. Matrix metalloproteinase inhibitors attenuate neuroinflammation following focal cerebral ischemia in mice. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2011;15:115–122. doi: 10.4196/kjpp.2011.15.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.I., Park S.K., Jang K.S., Han Y.M., Kim C.H., Oh S.J. Preischemic neuroprotective effect of minocycline and sodium ozagrel on transient cerebral ischemic rat model. Brain Res. 2015;1599:85–92. doi: 10.1016/j.brainres.2014.12.051. [DOI] [PubMed] [Google Scholar]
- Pedragosa J., Salas-Perdomo A., Gallizioli M., Cugota R., Miró-Mur F., Briansó F., Justicia C., Pérez-Asensio F., Marquez-Kisinousky L., Urra X., Gieryng A., Kaminska B., Chamorro A., Planas A.M. CNS-border associated macrophages respond to acute ischemic stroke attracting granulocytes and promoting vascular leakage. Acta Neuropathol. Commun. 2018;6:76. doi: 10.1186/s40478-018-0581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzotti-Jametti L., Donegá M., Giusto E., Mallucci G., Marchetti B., Pluchino S. The role of the immune system in central nervous system plasticity after acute injury. Neuroscience. 2014;283:210–221. doi: 10.1016/j.neuroscience.2014.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter W., Czech-Zechmeister B., Sujak M., Foerch C., Wichelhaus T.A., Pfeilschifter J. Treatment with the immunomodulator FTY720 does not promote spontaneous bacterial infections after experimental stroke in mice. Exp. Transl. Stroke Med. 2011;3:2. doi: 10.1186/2040-7378-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan H.T., Reeves M.J., Blizzard C.L., Thrift A.G., Cadilhac D.A., Sturm J., Otahal P., Rothwell P., Bejot Y., Cabral N.L., Appelros P., Kõrv J., Vibo R., Minelli C., Gall S.L. Sex differences in severity of stroke in the INSTRUCT study: a meta-analysis of individual participant data. J. Am. Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradillo J.M., Murray K.N., Coutts G.A., Moraga A., Oroz-Gonjar F., Boutin H., Moro M.A., Lizasoain I., Rothwell N.J., Allan S.M. Reparative effects of interleukin-1 receptor antagonist in young and aged/co-morbid rodents after cerebral ischemia. Brain. Behav. Immun. 2017;61:117–126. doi: 10.1016/j.bbi.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prass K., Meisel C., Höflich C., Braun J., Halle E., Wolf T., Ruscher K., Victorov I.V., Priller J., Dirnagl U., Volk H.-D., Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J. Exp. Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Yang S., Zhang S., Chen M., Yu H., Tian D.-S., Wang W. Clinical Characteristics and Outcomes of COVID-19 Patients With a History of Stroke in Wuhan, China. Stroke. 2020;51:2219–2223. doi: 10.1161/STROKEAHA.120.030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radlinska B.A., Ghinani S.A., Lyon P., Jolly D., Soucy J.-P., Minuk J., Schirrmacher R., Thiel A. Multimodal microglia imaging of fiber tracts in acute subcortical stroke. Ann. Neurol. 2009;66:825–832. doi: 10.1002/ana.21796. [DOI] [PubMed] [Google Scholar]
- Ransohoff R.M., Schafer D., Vincent A., Blachère N.E., Bar-Or A. Neuroinflammation: ways in which the immune system affects the brain. Neurother. J. Am. Soc. Exp. Neurother. 2015;12:896–909. doi: 10.1007/s13311-015-0385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayasam A., Hsu M., Kijak J.A., Kissel L., Hernandez G., Sandor M., Fabry Z. Immune responses in stroke: how the immune system contributes to damage and healing after stroke and how this knowledge could be translated to better cures? Immunology. 2018;154:363–376. doi: 10.1111/imm.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relton J. Inhibition of alpha4 integrin to protect the brain against ischemic injury. Drug News Perspect. 2001;14:346–352. [PubMed] [Google Scholar]
- Relton J.K., Rothwell N.J. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res. Bull. 1992;29:243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- Relton J.K., Sloan K.E., Frew E.M., Whalley E.T., Adams S.P., Lobb R.R. Inhibition of alpha4 integrin protects against transient focal cerebral ischemia in normotensive and hypertensive rats. Stroke. 2001;32:199–205. doi: 10.1161/01.str.32.1.199. [DOI] [PubMed] [Google Scholar]
- Ritzel R.M., Crapser J., Patel A.R., Verma R., Grenier J.M., Chauhan A., Jellison E.R., McCullough L.D. Age-associated resident memory CD8 T cells in the central nervous system are primed to potentiate inflammation after ischemic brain injury. J. Immunol. Baltim. Md. 2016;1950(196):3318–3330. doi: 10.4049/jimmunol.1502021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler R., Cramer J.V., Heindl S., Kostidis S., Betz D., Zuurbier K.R., Northoff B.H., Heijink M., Goldberg M.P., Plautz E.J., Roth S., Malik R., Dichgans M., Holdt L.M., Benakis C., Giera M., Stowe A.M., Liesz A. Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J. Neurosci. Off. J. Soc. Neurosci. 2020;40:1162–1173. doi: 10.1523/JNEUROSCI.1359-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Perdomo A., Miró-Mur F., Gallizioli M., Brait V.H., Justicia C., Meissner A., Urra X., Chamorro A., Planas A.M. Role of the S1P pathway and inhibition by Fingolimod in preventing hemorrhagic transformation after stroke. Sci. Rep. 2019;9:8309. doi: 10.1038/s41598-019-44845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandu R.E., Dumbrava D., Surugiu R., Glavan D.-G., Gresita A., Petcu E.B. Cellular and molecular mechanisms underlying non-pharmaceutical ischemic stroke therapy in aged subjects. Int. J. Mol. Sci. 2017;19 doi: 10.3390/ijms19010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann M.K., Krstic M., Kleinschnitz C., Fluri F. Fingolimod (FTY720) reduces cortical infarction and neurological deficits during ischemic stroke through potential maintenance of microvascular patency. Curr. Neurovasc. Res. 2016;13:277–282. doi: 10.2174/1567202613666160823152446. [DOI] [PubMed] [Google Scholar]
- Shang K., He J., Zou J., Qin C., Lin L., Zhou L.-Q., Yang L.-L., Wu L.-J., Wang W., Zhan K.-B., Tian D.-S. Fingolimod promotes angiogenesis and attenuates ischemic brain damage via modulating microglial polarization. Brain Res. 2020;1726 doi: 10.1016/j.brainres.2019.146509. [DOI] [PubMed] [Google Scholar]
- Shi K., Tian D.-C., Li Z.-G., Ducruet A.F., Lawton M.T., Shi F.-D. Global brain inflammation in stroke. Lancet Neurol. 2019;18:1058–1066. doi: 10.1016/S1474-4422(19)30078-X. [DOI] [PubMed] [Google Scholar]
- Shichita T., Sugiyama Y., Ooboshi H., Sugimori H., Nakagawa R., Takada I., Iwaki T., Okada Y., Iida M., Cua D.J., Iwakura Y., Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat. Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- Shukla V., Shakya A.K., Perez-Pinzon M.A., Dave K.R. Cerebral ischemic damage in diabetes: an inflammatory perspective. J. Neuroinflamm. 2017;14:21. doi: 10.1186/s12974-016-0774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.J., Denes A., Tyrrell P.J., Di Napoli M. Phase II anti-inflammatory and immune-modulating drugs for acute ischaemic stroke. Expert Opin. Investig. Drugs. 2015;24:623–643. doi: 10.1517/13543784.2015.1020110. [DOI] [PubMed] [Google Scholar]
- Sobowale O.A., Parry-Jones A.R., Smith C.J., Tyrrell P.J., Rothwell N.J., Allan S.M. Interleukin-1 in stroke: from bench to bedside. Stroke. 2016;47:2160–2167. doi: 10.1161/STROKEAHA.115.010001. [DOI] [PubMed] [Google Scholar]
- Soliman S., Ishrat T., Fouda A.Y., Patel A., Pillai B., Fagan S.C. Sequential therapy with minocycline and candesartan improves long-term recovery after experimental stroke. Transl. Stroke Res. 2015;6:309–322. doi: 10.1007/s12975-015-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C.J. Ischemic stroke: experimental models and reality. Acta Neuropathol. (Berl.) 2017;133:245–261. doi: 10.1007/s00401-017-1667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen B.J., Breier G., Butcher E.C., Schulz M., Engelhardt B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am. J. Pathol. 1996;148:1819–1838. [PMC free article] [PubMed] [Google Scholar]
- Szalay G., Martinecz B., Lénárt N., Környei Z., Orsolits B., Judák L., Császár E., Fekete R., West B.L., Katona G., Rózsa B., Dénes Á. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 2016;7:11499. doi: 10.1038/ncomms11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Ishihara Y., Mizuno S., Ishida A., Vogel C.F., Tsuji M., Yamazaki T., Itoh K. Progression of vasogenic edema induced by activated microglia under permanent middle cerebral artery occlusion. Biochem. Biophys. Res. Commun. 2018;496:582–587. doi: 10.1016/j.bbrc.2018.01.094. [DOI] [PubMed] [Google Scholar]
- Tang X.N., Wang Q., Koike M.A., Cheng D., Goris M.L., Blankenberg F.G., Yenari M.A. Monitoring the protective effects of minocycline treatment with radiolabeled annexin V in an experimental model of focal cerebral ischemia. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2007;48:1822–1828. doi: 10.2967/jnumed.107.041335. [DOI] [PubMed] [Google Scholar]
- Tang Y., Li T., Li J., Yang J., Liu H., Zhang X.J., Le W. Jmjd3 is essential for the epigenetic modulation of microglia phenotypes in the immune pathogenesis of Parkinson’s disease. Cell Death Differ. 2014;21:369–380. doi: 10.1038/cdd.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T., Xu G., Si Chen C., Feng J., Kong Y., Qin X. Minocycline promotes axonal regeneration through suppression of RGMa in rat MCAO/reperfusion model. Synap. N. Y. N. 2013;67:189–198. doi: 10.1002/syn.21629. [DOI] [PubMed] [Google Scholar]
- Thiel A., Radlinska B.A., Paquette C., Sidel M., Soucy J.-P., Schirrmacher R., Minuk J. The temporal dynamics of poststroke neuroinflammation: a longitudinal diffusion tensor imaging-guided PET study with 11C-PK11195 in acute subcortical stroke. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2010;51:1404–1412. doi: 10.2967/jnumed.110.076612. [DOI] [PubMed] [Google Scholar]
- Thomalla G., Gerloff C. Acute imaging for evidence-based treatment of ischemic stroke. Curr. Opin. Neurol. 2019;32:521–529. doi: 10.1097/WCO.0000000000000716. [DOI] [PubMed] [Google Scholar]
- Uzdensky A.B. Photothrombotic stroke as a model of ischemic stroke. Transl. Stroke Res. 2018;9:437–451. doi: 10.1007/s12975-017-0593-8. [DOI] [PubMed] [Google Scholar]
- Vallés J., Lago A., Santos M.T., Latorre A.M., Tembl J.I., Salom J.B., Nieves C., Moscardó A. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: prognostic significance. Thromb. Haemost. 2017;117:1919–1929. doi: 10.1160/TH17-02-0130. [DOI] [PubMed] [Google Scholar]
- Veltkamp R., Gill D. Clinical trials of immunomodulation in ischemic stroke. Neurother. J. Am. Soc. Exp. Neurother. 2016;13:791–800. doi: 10.1007/s13311-016-0458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker B.K., Park T.S., Gidday J.M. Hypoxic preconditioning-induced cerebral ischemic tolerance: role of microvascular sphingosine kinase 2. Stroke. 2009;40:3342–3348. doi: 10.1161/STROKEAHA.109.560714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B.D., Dietrich W.D., Busto R., Wachtel M.S., Ginsberg M.D. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann. Neurol. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- Wei Y., Yemisci M., Kim H.-H., Yung L.M., Shin H.K., Hwang S.-K., Guo S., Qin T., Alsharif N., Brinkmann V., Liao J.K., Lo E.H., Waeber C. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann. Neurol. 2011;69:119–129. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise P.M., Dubal D.B., Wilson M.E., Rau S.W., Böttner M., Rosewell K.L. Estradiol is a protective factor in the adult and aging brain: understanding of mechanisms derived from in vivo and in vitro studies. Brain Res. Brain Res. Rev. 2001;37:313–319. doi: 10.1016/s0165-0173(01)00136-9. [DOI] [PubMed] [Google Scholar]
- Xu G., Li C., Parsiola A.L., Li J., McCarter K.D., Shi R., Mayhan W.G., Sun H. Dose-dependent influences of ethanol on ischemic stroke: role of inflammation. Front. Cell. Neurosci. 2019;13:6. doi: 10.3389/fncel.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Fagan S.C., Waller J.L., Edwards D., Borlongan C.V., Zheng J., Hill W.D., Feuerstein G., Hess D.C. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol. 2004;4:7. doi: 10.1186/1471-2377-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Lu J., Shao A., Zhang J.H., Zhang J. Glial cells: role of the immune response in ischemic stroke. Front. Immunol. 2020;11:294. doi: 10.3389/fimmu.2020.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J., Huang W., Chen X., Li Q., Cai Z., Yu T., Shao B. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J. Stroke Cerebrovasc Dis. Off. J. Natl. Stroke Assoc. 2017;26:650–657. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E., Humbert K., Henninger N., Trivedi T., Lillemoe K., Alam S., Sanger M., Kim S., Scher E., Dehkharghani S., Wachs M., Tanweer O., Volpicelli F., Bosworth B., Lord A., Frontera J. SARS-CoV-2 and Stroke IN a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T., Chopp M., Chen J. Experimental animal models and inflammatory cellular changes in cerebral ischemic and hemorrhagic stroke. Neurosci. Bull. 2015;31:717–734. doi: 10.1007/s12264-015-1567-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanev P., Poinsatte K., Hominick D., Khurana N., Zuurbier K.R., Berndt M., Plautz E.J., Dellinger M.T., Stowe A.M. Impaired meningeal lymphatic vessel development worsens stroke outcome. J. Cereb. Blood Flow Metab Off. J. Int. Soc. Cereb. Blood Flow Metab. 2020;40:263–275. doi: 10.1177/0271678X18822921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Yirong, Salayandia V.M., Thompson J.F., Yang L.Y., Estrada E.Y., Yang Yi. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J. Neuroinflamm. 2015;12:26. doi: 10.1186/s12974-015-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew W.P., Djukic N.D., Jayaseelan J.S.P., Walker F.R., Roos K.A.A., Chataway T.K., Muyderman H., Sims N.R. Early treatment with minocycline following stroke in rats improves functional recovery and differentially modifies responses of peri-infarct microglia and astrocytes. J. Neuroinflamm. 2019;16:6. doi: 10.1186/s12974-018-1379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjänheikki J., Tikka T., Keinänen R., Goldsteins G., Chan P.H., Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Zhou Y., Zhang R., Zhang M., Campbell B., Lin L., Shi F.-D., Lou M. Rationale and design of combination of an immune modulator fingolimod with alteplase bridging with mechanical thrombectomy in acute ischemic stroke (FAMTAIS) trial. Int. J. Stroke Off. J. Int. Stroke Soc. 2017;12:906–909. doi: 10.1177/1747493017710340. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Xiao M., He W., Cai Z. Minocycline upregulates cyclic AMP response element binding protein and brain-derived neurotrophic factor in the hippocampus of cerebral ischemia rats and improves behavioral deficits. Neuropsychiatr. Dis. Treat. 2015;11:507–516. doi: 10.2147/NDT.S73836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Xu L., Yin J., Zhong Z., Fan H., Li X., Chang Q. Effect of minocycline on cerebral ischemia-reperfusion injury. Neural Regen. Res. 2013;8:900–908. doi: 10.3969/j.issn.1673-5374.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Liesz A., Bauer H., Sommer C., Lahrmann B., Valous N., Grabe N., Veltkamp R. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol. Zurich Switz. 2013;23:34–44. doi: 10.1111/j.1750-3639.2012.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Fu Y., Tian D., Sun N., Han W., Chang G., Dong Y., Xu X., Liu Q., Huang D., Shi F.-D. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation. 2015;132:1104–1112. doi: 10.1161/CIRCULATIONAHA.115.016371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.