ABSTRACT
The morphogenesis of the mammalian secondary plate is a series of highly dynamic developmental process, including the palate shelves vertical outgrowth, elevation to the horizontal plane and complete fusion in the midline. Extracellular matrix (ECM) proteins not only form the basic infrastructure for palatal mesenchymal cells to adhere via integrins but also interact with cells to regulate their functions such as proliferation and differentiation. ECM remodeling is essential for palatal outgrowth, expansion, elevation, and fusion. Multiple signaling pathways important for palatogenesis such as FGF, TGF β, BMP, and SHH remodels ECM dynamics. Dysregulation of ECM such as HA synthesis or ECM breakdown enzymes MMPs or ADAMTS causes cleft palate in mouse models. A better understanding of ECM remodeling will contribute to revealing the pathogenesis of cleft palate.
KEYWORDS: ECM, cleft palate, morphogenesis, remodeling, development
Introduction
The morphogenesis of the mammalian secondary palate begins with the outgrow of two palatal shelves from the maxillary processes on both sides of the tongue on an embryonic day (E) 12.1 The two vertically oriented palatal shelves soon elevate horizontally and opposite each other on E 14–15.1 Then, the palatal shelves epithelia disintegrate in the midline and their mesenchymal compartment fuse completely to form an intact palatal roof.1 Cells in the palatal shelves originate from three sources of embryonic tissue/structures: the superficial palatal epithelium is derived from the embryonic ectoderm, the underlying palatal mesenchyme mainly from the neural crest.1,2 Supporting these cells is the infrastructure composed by complex extracellular matrix network.
The extracellular matrix (ECM) is a three-dimensional, highly dynamic non-cellular architectural scaffold present in all tissues. In mammals, the ECM is composed of a complex protein network including collagens (Col), proteoglycans (PGs), glycoproteins, and Proteoglycans (PGs).3 ECM not only support the tissue integrity and elasticity but also control tissue homeostasis.4 ECM remodeling is an important process in the morphogenesis of many organs such as lungs, intestine, and mammary glands.5 During development, ECM is undergoing dynamic deposition, degradation by growth factors-controlled synthesis and proteolysis by matrix-degrading enzymes.4 Abnormal ECM remodeling can lead to embryonic lethality or abnormal morphogenesis or pathological conditions such as fibrosis and cancer.5
In the palate, ECM not only forms the basic infrastructure where cells adhere via integrins but also play important roles in integrating and regulating growth factors network. They store and release growth factor, therefore controlling the bioavailability of active growth factors such as Tgf-βs, which in turn remodel ECM dynamics and palatal cell differentiation.6-8 They accumulate water, bind other ECM molecules, mediating palatal shelf growth, expansion, and elevation.9-14 Multiple signaling pathways important for palatogenesis such as FGF, TGF β, BMP, and SHH regulate ECM dynamics during palate development (Figure 1).6-8,14-18 In this review, we will summarize the dynamic deposition and degradation of ECM during palate development.
Figure 1.
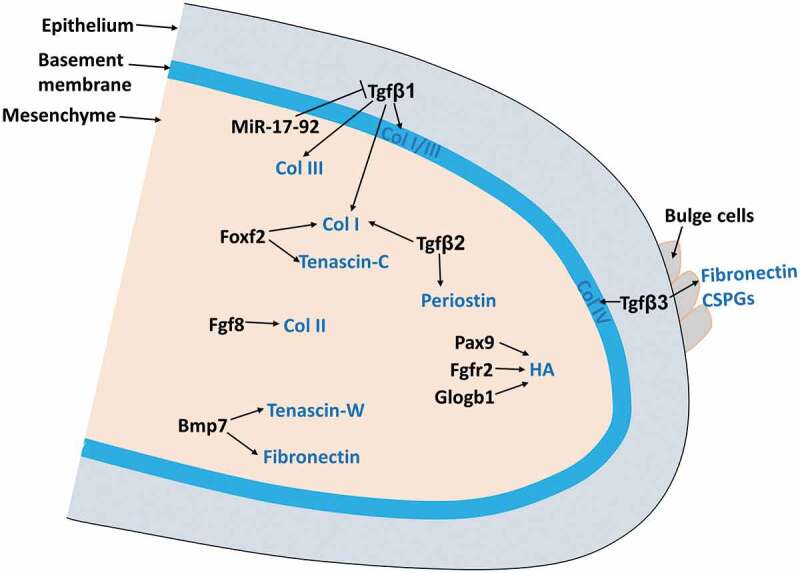
ECM remodeling during palate development.
ECM molecules remodeling in the palate
Collagens (Col)
Collagens are the major components of ECM in connective tissues. There are 28 distinct collagens composed of α1, α2, α3 subunits combination and classified into fibrillar collagens (Collagen I–III, V, and XI) and non-fibrillar forms (Collagen VI, IX, IV, etc.).5,19 Fibrillar collagens form strong and stable fibrils and organize the fibrils into three-dimensional network, for example, Collagen I fibrils for bones and Collagen II fibrils for cartilages.5 Non-fibrillar forms of collagens include Fibril-Associated Collagens and basement collagens. Fibril-Associated Collagens, such as Collagen IX, associate with collagen fibrils and bind them together to form thicker collagen fibers. Basement collagens are sheet-forming collagens such as Collagen IV, which form the two-dimensional network for all basal laminae.5,19 A variety of collagens are highly expressed in the palate and dynamically remodeled during palatogenesis (Tables 1 and 2).
Table 1.
Cleft palate-related ECM gene mutation in human and mice.
| Gene | Protein | Human Disease | Cleft palate related Clinal features | References | Mouse mutation | Mouse mutation phenotypes | References |
|---|---|---|---|---|---|---|---|
| COL2A1 | Col II | Stickler syndrome type 1 (OMIM 108300) | Cleft palate or bifid uvula, | 26,109-111 | Transgenic Del1 mice carrying six copies of with COL2A1 (Del) mutation with a 150-bp deletion containing the 45-bp exon 7 and intro7: | Craniofacial ossification retarded; reduced cartilage and bone growth; cleft palate; | 38 |
| Kniest dysplasia (OMIM 156550) | Cleft palate | 111 | Transgenic mice with targeted inactivation of the COL2A1 gene | No endochondral bone or epiphyseal growth plate in long bones with normal membranous and periosteal skeleton | 34 | ||
| Platyspondyly lethal skeletal dysplasia (OMIM 151210) | No cleft palate | 111 | ENU induced mutation has a G to A transition at Chromosome 15:97815207, which causes a premature stop codon at amino acid 645 | Cleft palate; shortened nose | 112 | ||
| COL11A1 | Col XI | Stickler syndrome type 2 (OMIM 604841) |
Cleft palate | 110 | Deletion of a cytidine residue about 570 nt downstream of the translation initiation codon in COL11A1 (cho) mRNA causes a reading frame shift and introduces a premature stop codon | Cleft palate, shortened head and mandible, short limbs, protruding tongue | 39,40 |
| Marshall syndrome (OMIM 154780) |
Cleft palate | 26 | |||||
| COL11A2 | Col XI | Stickler syndrome type 3: (OMIM 184480) |
Cleft palate | 110 | Full-length Col11a2 chain was unable to occur because of the presence of premature termination codons | NO cleft palate; Hearing loss, smaller size, shorter snout, skeletal abnormalities including abnormal cranium morphology and long bone epiphyseal plate |
41,42 |
| Nance-Insley syndrome (OMIM 215150) |
Cleft palate | 41 | |||||
| FN1 | Fibronectin | NR | Conditional knock out FN1 in cranial neural crest cells | Cleft palate, abnormal cardiac morphogenesis, thymus development defects | 48 | ||
| Conditional knock out FN1 in mesoderm cells | Cleft palate, abnormal cardiac morphogenesis, thymus development defects, edema, etc. | 47 | |||||
| TNXB | Tenascin-X | Tenascin-X deficiency (OMIM 606408) |
Bifid uvula | 55,56,113,114 | NR | ||
| ACAN | Aggrecan | NR | Gene mutation in ACAN: cmd/cmd (cartilage matrix defieciency), 7-bp deletion in exon 5; cmd-Bc, spontaneous mutation, complete loss of exons 2-18 |
Cleft palate, short limbs, tail, and snout | 115,116 | ||
| VCAN | Versican | NR | Haploinsufficiendy of Versican in Adamts20 mutant mice (Vcanhdf/+;Adamts20bt/bt) | 65% mutant exhibited cleft palate | 12 |
NR, not reported.
Table 2.
ECM molecules expression, function, and remodeling pattern during palatogenesis.
| Expression pattern in the palate |
Functional roles during palatogenesis |
Remodeling during palatogenesis |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ECM component |
Subtype | Location | Detection methods | Species/References | Potential mechanism | Potential function | References | Abnormal remodeling | Detection methods | References |
| Collagens (Col) | Col I | Before palatal elevation: Epithelial basement membrane and palatal mesenchyme (stronger on the nasal side than the oral side in the middle and posterior palate) After palatal elevation: Mesenchyme around the bone | IHC | Mouse7,20-23,117 | Main structure protein | Build up palate infrastructure | 7 | Col I is downregulated in the palatal shelves of Foxf2−/- mouse embryos | IHC | 15 |
| Col I and its degrading enzyme MMP 13 are downregulated in T1KO mice | qRT-PCR, WB, IHC | 14 | ||||||||
| qRT-PCR, dot blot | Human:24 | Col I synthesis by palatal mesenchymal cells can be induced by TGFβ1 and inhibited by MiR-17-92 clusters | qRT-PCR, WB | 16 | ||||||
| Col I synthesis by palatal mesenchymal cells can be induced by TGFβ2 | IHC | 7 | ||||||||
| Col II | After the palatal shelf elevation: A few palatal mesenchymal cells | IHC | Mouse:8 | Main cartilage ECM | Palatal mesenchyme osteogenic/chondrogenic fate determination | 8 | Enhanced FGF8 signaling causes strong expression of Col II in palatal mesenchymal cells | IHC | 8 | |
| Col III | Before palatal elevation: Epithelial basement membrane and palatal mesenchyme | IHC | Mouse:23,25 | Main structure protein | Build up palate infrastructure | 23 | Col III synthesis by palatal mesenchymal cells can be induced by TGFβ1 and inhibited by MiR-17-92 clusters | qRT-PCR, WB | 16 | |
| qRT-PCR, dot blot | Human:24 | |||||||||
| Col IV | Epithelial basement membrane | IHC | 10,17,20,25 | NR | Col IV expression in the palate is reduced in the Tgf-β3 mutant and can be re-induced by Tgf-β3 | IHC | 17 | |||
| Fibronectin | Before palate elevation: Palatal mesenchyme and around MEE, with strong expression around the bulging MEE cells After palate elevation: MES | IHC, ISH | 6,17,20-23 | NR | Absent in MEE cells and apical surface in the Tgf-β3 mutant palate and can be re-induced by Tgf-β3; Downregulated in BMP 7 homozygous palate; EDA domain reduced in Foxf2−/- palatal shelves; Inhibited by retinoid acid in a dose-dependent manner. | IHC, qRT-PCR, WB | 6,15,17,49 | |||
| Fibrillins | Fibrillin-1 | Weakly expressed in the palatal mesenchyme only before palatal shelf elevation | IHC | 6 | Compose microfibirls and activate Tgf-β | Palatal fusion | 6 | NR | ||
| Fibrillin-2 | Before palatal shelf elevation: enriched in the nasal side of palatal mesenchyme After palatal elevation: increased and expanded around MEE cells and in the oral side of palatal mesenchyme | IHC | 6 | Inhibited by retinoid acid in a dose-dependent manner | qRT-PCR | 49 | ||||
| Tenascins | Tenascin-C | Before palatal elevation: in the mesenchyme close to the nasal and distal surface of the shelf; After palatal elevation: accumulated in the mesenchyme close to the MES | IHC, ISH | 1,6,53 | NR | Reduced in Foxf2−/- mutant palatal shelves Inhibited by retinoid acid in a dose-dependent manner | qRT-PCR | 15,49 | ||
| Tenascin-W | Before palatal elevation: weekly expressed in the proximal-nasal quadrant of the vertical shelves; After palatal elevation: restricted to the dorsal mesenchyme around the MES, corresponding to the future osteogenic domains of hard palate | IHC, ISH | 1,6,53 | NR | Diminished in the palatal shelves of BMP7−/- palatal shelves; BMP7 induce Tenascin-W production in embryonic fibroblasts in vitro | ISH, WB | 6 | |||
| Periostin | In the anterior palate (hard palate), periostin is expressed in the mesenchyme on the oral side and part of basal membrane; Intensified around MEE cells in palate fusing process; highly expressed in the entire posterior palate (soft palate) | IHC, ISH | 7,53,62 | May help to determine soft palate formation | Soft palate formation | 7 | TGFβ2 induce periostin production in cultured palate shelves. | IHC | 7 | |
| Laminin | Laminin 5 | Discontinuously in the basement membrane and intercellularly in MEE cells | IHC | 17,25 | NR | Becomes continuous in the basement membrane under MEE cells in the Tgf-β3 mutant palate | IHC | 17 | ||
| Proteoglycans | CSPGs | CS chains Before the palatal shelf elevation: palatal mesenchyme; During the palatal shelf elevation and the palatal shelf fusion: palatal mesenchyme, transiently upregulated on apical surface of MEE surface; After the palatal shelf fusion: Palatal mesenchyme around the bone | IHC | Mouse:11,14,18 | Hold up water; Bind other ECM molecules; Modify TGFβ signaling | Palatal growth, expansion, elevation and adhesion | 12-14,118 | Reduced in TGFβ3−/- mutant the palatal shelf mesenchyme and disappeared in mutant MEE surface; TGFβ3 induce CSPGs production in cultured palate shelf MEE cells. | IHC | 18 |
| Versican Before the palatal shelf elevation: Palatal mesenchyme (stronger in nasal/medial and tip than oral/lateral palate) During the palatal shelf elevation and the palatal shelf fusion: Palatal mesenchyme (stronger in nasal/medial and tip than oral/lateral palate, although increased in oral/lateral part), transiently upregulated on apical surface of MEE surface; After the palatal shelf fusion: Decreased in palatal mesenchyme | IHC | 11,12 | Cleaved versican decreased in Adamts9;±Adamts20bt/bt palatal shelves | IHC | 12 | |||||
| Biglycan/Decorin palatal mesenchymal cells at different palate development stages (Decorin restricted to the nasal side); peaked in MEE cells as the palatal shelf adhered | IHC, ISH, qRT-PCR | 13,76,77 | Disappeared in the MES of TGFβrI kinase inhibitor (SB431542)–treated palates; Decorin are unable to downregulate in the mesenchyme when palatal shelves are elevating; Decorin are downregulated in ectopic Hh signaling palatal shelves | IHC, ISH | 13,76,77 | |||||
| HSPGs | Before the palatal shelf elevation: Epithelial basement membrane and anterior palatal mesenchyme; During the palatal shelf elevation and the palatal shelf fusion: Epithelial basement membrane (stronger in the oral/lateral side than the nasal/medial side) and the tip of the whole palatal mesenchyme; After the palatal shelf fusion: Epithelial basement membrane (stronger in the oral side than the nasal side); gradually disappear from the palatal mesenchyme; Around vessel basement membrane at all stages | IHC | 11,25 | |||||||
| KSPGs | Lumican Restricted to the nasal mesenchyme of the palate | ISH | 77 | Lumican are downregulated in ectopic Hh signaling palatal shelves | ISH | 77 | ||||
| HA | Before the palatal shelf elevation: palatal mesenchyme (anterior/mid-part stronger than anterior-most and posterior palate; stronger in the nasal/medial side than the oral/lateral side) | IHC | 9,11,78 | Retain water | Palatal shelf elevation | 1,9-11 | Reduced in Fgfr2C342Y, Pax9 and Golgb1 mutant palatal shelves; HA synthase Has 1, 2, and 3 disrupted in Tgf-β3 mutant shelves. | IHC | 9,49,88,89 | |
Col I and III are widely expressed in the palatal mesenchyme before and after palate shelf elevation.7,20-25 During palatogenesis, their degradation is highly regulated. For example, Col I is downregulated in the palatal shelves of Foxf2−/- embryos which failed to elevate palate shelves.15 Interestingly, in human palatal fibroblasts derived from orofacial cleft patients, COL I and III mRNA levels are strongly decreased, in contrast, the protein levels are increased compared to the control sample possibly contributing to decreased collagen degradation by MMPs and increased collagen cross-links.24 COL I mRNA and protein levels in the palate are also downregulated in chondroitin sulfate proteoglycan defective mutants which have thinner palate due to abnormal bone and cartilage development.14 TGF-β1, one of the most important growth factors during palate development, can induce palatal mesenchymal cells proliferation and Col I and III synthesis, which can be inhibited by MiR-17-92 clusters.16
Col IV is expressed in the epithelial cell basement membrane.10,17,20,25 Col IV expression is reduced in the Tgf-β3 mutant in which palatal shelves failed to fuse.17 Addition of Tgf-β3 in the palate culture increases Col IV deposition in the basement membrane of MEE cells.17
In human, mutation of COL2A1, COL11A1, COL11A2, COL9A1, and COL9A2 cause a group of hereditary conditions known as Stickler syndrome I–V, respectively, characterized by high myopia, retinal detachment, hearing loss, midfacial underdevelopment, and cleft palate is only described in Stickler syndrome I–III (Table 1).26-32
Type II collagen is the major extracellular matrix component of cartilage and essential for endochondral bone formation.5 In the palate mesenchyme, only a few osteoblast precursors express Col II in the palatal mesenchyme.8 Inactivate Type II collagen in mouse develops short bones and cleft palate.33-38 In these mutants, both chondrocyte differentiation and intramembranous ossification are disrupted. Augmented fibroblast growth factor 8 (FGF8) signaling in the anterior hard palate by using Shox2Cre causes a subset of palatal mesenchymal cells differentiating into Col II+ chondrogenic cells at the expense of osteogenic cell fate.8 These results indicate that appropriate level of Type II collagen is necessary for palate mesenchymal cell fate determination.
Mutation of COL11A1 in human causes Marshall syndrome and Stickler syndrome type. 226 Col11a1 homozygote mice have craniofacial abnormalities including cleft palate, shortened head, and mandible, short limbs, protruding tongue et.al.39,40 The tongue protrusion is possible to obstruct palatal shelf elevation, contact, and fusion. However, the Col11a1 mutant palatal shelves can make contact and fuse when placed close to each other in organ culture.39 It indicates that Col11a1 may play a role in palate growth. Mutation of COL11A2 in human cause Stickler syndrome type 3 and Nance-Insley syndrome.26,41 However, inactivation of Col11a2 in mice does not lead to cleft palate, although other clinical features such as hearing loss and abnormal skeleton development replicate the human phenotypes.41,42
Glycoproteins
Fibronectins (FN)
Fibronectin (FN) is a glycoprotein with a high molecular weight of 230–270 KD. As one of the most widely expressed ECM in the vertebrate, FN is composed of types I, II, and III repeating units.4,43 FN existing in multiple isoforms is encoded by a single FN gene, located in the human chromosome 2 and rodent chromosome 1.44 Alternative splicing occurs at three regions, EIIIA/EDA and EIIIB/EDB and V region. FN has multiple sites for self-assembly and ligand binding for integrins, heparin, fibrin, collagen/gelatin, and growth factors, mediating biological processes such as cell adhesion, migration, differentiation.4
FN is one of the most abundant ECM components in the palate (Table 2). Although FN null embryos are embryonic lethal45,46, conditional knockout FN in cranial neural crest cells and mesodermal cells leads to cleft palate.47,48 Before palate shelve elevation, FN locates in the palatal mesenchyme20-23 and around MEE, with strong expression around the bulging MEE cells.17,20 FN expression is totally absent in MEE cells and apical surface in Tgf-β3 mutant, and the addition of Tgf-β3 in the palate culture increase FN deposition on the MEE apical surface (Martinez-Sanz et al., 2008). FN production by the human fetal palatal mesenchymal cells also can be inhibited by retinoid acid, a known cleft palate inducer, in a dose-dependent manner.49 Strong Fibronectin mRNA expression is also observed at the midline epithelial seam (MES) in E14.5 wildtype or BMP heterozygous embryos.6 While the same stage of BMP homozygous embryos has delayed palatal shelf elevation, only little Fibronectin mRNA expression was found in their still vertical palatal shelves. Besides, anti-fibronectin antibody can block palate shelve adhesion.17 Recently, fibronectin splice-isoform ED-A domain, essential for Tgfβ latency complex formation, is shown reduced in the palatal shelves of Foxf2−/- embryos which failed to elevate.15 These results indicate fibronectin is important during palate shelve elevation and fusion, downstream of Foxf2 and TGF/BMP signaling.
Tenascins
Tenascins are a family of polymorphic glycoproteins with a molecular weight of 150–380 KD, including tenascin-C, -R, -W, -X and -Y.43 Tenascins are composed of repeated domains including type III domains, EGF-like repeats, and a C-terminal globular domain.43 Tenascin-R is mainly found in the central nervous system and Tenascin-X and -Y in skeletal muscles.43 Tenascin-C and -W are widely expressed in developing tissues and play important roles in tissue morphogenesis and tumor growth.43 The expression of tenascins is regulated by mechanical loading both during development and in adulthood.50 Unlike most ECM proteins mediating cell adhesion and cytoskeletal organization, Tenascins modulate cell-matrix interactions and function as adaptors.51,52
Tenascin-C and Tenascin-W showed distinct spatial and temporal expression patterns during palatogenesis (Table 2).1,6,53 At E13.5, before palatal shelves elevated, Tenascin-C expression was found in the mesenchyme close to the nasal and distal surface of the shelf; After elevation, Tenascin-C expression accumulated in the mesenchyme close to the MES. In contrast, before elevation, Tenascin-W weekly expressed in the proximal-nasal quadrant of the vertical shelves; After elevation, Tenascin-W was restricted to the dorsal mesenchyme around the MES, corresponding to the future osteogenic domains of hard palate. Tenascin-W, not Tenascin-C diminished in the palatal shelves of BMP7−/- embryos can be induced by Bmp7 in embryonic cranial fibroblasts in vitro.6 While Tenascin-C expression is reduced in Foxf2−/- mutant palatal shelves15 and retinoid acid overexpressed human palatal mesenchymal cells49, it is possible that they are involved in different pathways regulating palate development.
Although Tenascin-X expression has not reported in the palate, Tenascin-X deficiency (CAH-X syndrome) exhibits a bifid uvula, a mildest form of cleft palate.54,55 Interestingly, three proteins important in palate development, TGF-β2, TGF-β3, MMP13, are all increased in Tenascin-X deficiency patient fibroblast and tissues.56
Periostin
Periostin is a secreted 90KD glycoprotein identified from a mouse MC3T3-E1 osteoblastic cell line and originally named as osteoblast-specific factor 2.57 Periostin promote cell motility via integrin-dependent cell adhesion.58 Periostin also plays an essential role in bone and tooth development.59 Periostin null mice are growth retarded, showing incisor enamel defects indicating important roles for tooth and bone development.59-61
Periostin protein and mRNA are spatiotemporally expressed in the palate (Table 2).7,53,62,63 In the anterior palate (hard palate), periostin is expressed by in the mesenchyme on the oral side and part of basal membrane.7,62 In contrast, periostin is highly expressed in the entire posterior palate (soft palate).7,62
Periostin intensified around MEE when they are undergoing EMT and transdifferentiating into MES.62 In contrast, laminin and Type IVcollagen, two major ECM in MEE basement membrane, are degraded earlier than periostin.62 This indicates that periostin is involved regulating MEE fate during the palate fusion process.62
Both TGF-β2, Col I and periostin expression are detected in the palatine aponeurosis region of the soft palate.7 Exogenous TGF-β2 can induce periostin and Col I expression in the palate tissue in organ culture which indicates that TGF signaling might regulate soft palate development by mediating periostin expression.7
Laminins
Laminins are a group of heterotrimeric glycoproteins composed of α, β, γ polypeptide chains and contribute to the assembly of the basement membrane.64 Laminins can bind to and interact with other ECMs such as Col IV and nidogen and activate cell receptors such as integrins, glycolipids, proteoglycans, and glycoproteins65, therefore mediate cell adhesion, migration, and differentiation.64 Before palatal shelve elevation, laminin is present discontinuously in the basement membrane and intercellularly in MEE cells (Table 2).17,66 However, in the Tgf-β3 mutant which failed to fuse, laminins are upregulated and become continuous in the basement membrane under MEE cells.17 It indicates that dynamic assembly of laminins in the basement membrane is regulated by the growth factors.
Fibrillins
Fibrillins are a group of large extracellular glycoproteins including three isoforms, Fibrillin-1, −2, −3.43 They compose the core microfibrils in the ECM of elastic and non-elastic tissues, and interact with integrins directly43 or bind and activate Tgf-β.67 Fibrillin-1 mRNA is weakly expressed in the palatal mesenchyme only before palatal shelf elevation (Table 2).6 In contrast, Fibrillin-2 mRNA is enriched in the nasal side of palatal mesenchyme before palatal shelf elevation, then increased and expanded around Tgf-β3+MEE cells and in the oral side of palatal mesenchyme.6 The close relationship of strong Fibrillin-2+mesenchyme cells and Tgf-β3+MEE cells indicates that Fibrillin-2 may be important for Tgf-β mediated palatal fusion. Retinoid acid, an important regulator during embryogenesis, dose-dependently inhibit fibrillin-2 production in human fetal palatal mesenchymal cells in vitro.49
Proteoglycans (PGs)
Proteoglycans are a group of complex protein families characterized by anionic glycosaminoglycan (GAG) chains covalently bounding to core proteins.68 GAG chains can be classified into the below 5 classes: chondroitin sulfate (CS), heparan sulfate (HS), keratan sulfate (KS), dermatan sulfate (DS), and hyaluronan (also called hyaluronic acid, HA). Hyaluronan is a non-sulfated glycosaminoglycan and not attached to a protein core. CS, HS, KS, and DS attaching to core proteins form CSPGs, HSPGs, KSPGs, and DSPGs, respectively. PGs are classified based on their cellular and subcellular location, overall gene/protein homology and protein modules within the respective protein cores.68 Only pericellular and extracellular proteoglycans regarded as ECM proteins will be discussed in this review. Pericellular proteoglycans such as perlecan (HSPGs) located in the basement membrane interact with each other and participate in modulating growth factors.68 Extracellular proteoglycans constitute the major structural complex, provide viscoelastic properties, retain water, and keep osmotic pressure and regulate cell migration, proliferation, apoptosis, and angiogenesis by interacting with several receptor tyrosine kinases.68 During palate development, CSPGs, HSPGs, KSPGs, and DSPGs are all enriched in palatal shelves (Table 2).11,25,69,70 For a long time, GAGs accumulation and hydration were regarded as the main source of the intrinsic force for palatal shelf elevation, as cleft palate is induced after GAG biosynthesis suppression.9,71,72 But evidence has emerged that proteoglycans are also essential for palatal adhesion and osteogenesis.
CSPGs
CSPGs are highly expressed in the palatal mesenchyme during the palate development (Table 2).11,14,18 Interestingly, CS chains are transiently upregulated on the apical surface of palatal medial edge epithelial (MEE) cells when they become closer and make contact.11,18 Alteration of CS chain synthesis or its specific digestion disrupts palatal shelves adhesion in vitro, indicating CSPGs play a functional role at palatal adhesion.18 The expression of CS chains is shown absent in the MEE cells of TGF-β3 null mutant mice, whose palatal shelves are unable to fuse in the midline.18 However, the expression of CS chains, together with palatal shelf adhesion, can be re-induced by the addition of TGF-β3 in palate shelf organ culture.18,73 Besides CS chains, core proteins such as biglycan, decorin, versican are also significantly increased in MEE cells as the palatal shelf adhering.12,13 Inhibitor of TGFβ signaling with SB431542, a TGFβrI kinase inhibitor74, caused the failure of palate shelf fusion together with the downregulation of biglycan and decorin protein from the MES. These studies indicate that biglycan and decorin are involved in palatal shelve adhesion downstream of TGFβ signaling. Although biglycan and decorin single or double knockout transgenic mice have no cleft palate75, other factors might compensate their roles during palatal shelve adhesion.13 All these results indicate that remodeling of proteoglycans by TGFβ signaling are important for the palatal adhesion process.
The protein and mRNA levels of biglycan and decorin are also found in the palatal mesenchymal cells at different palate development stages, although their expression is transiently downregulated when palatal shelves are elevating and closing.13,76 Interestingly, in retinoic acid included mice cleft palate, decorin positive area, not biglycan, is unable to downregulate in the mesenchyme when palatal shelves are elevating, indicating that decorin is more important in palatal shelf elevation than biglycan during palatogenesis.76
Ectopic Hh signaling in the palatal mesenchyme leads to the defective palatine formation and fully penetrant cleft palate and defective osteogenesis.77 In these mutants, significantly downregulation of the mRNA of decorin (Dcn) and lumican (Lum, a major KSPGs) in the palatal mesenchyme indicate that decorin and lumican also play roles in the palatal cell fate determination. Recently, another study showed that reducing half the abundance of CSPGs by knocking out a key CS biosynthesis glycosyltransferase caused malocclusion, skin hyperextension, severe intramembranous ossification, and cartilage formation defects in the craniofacial development.14 These mutants exhibited significant thinner palate (5% mutant has a cleft palate), where Col I, Wnt3a, β-catenin are all downregulated.14 Therefore, CSPGs in palatal mesenchyme probably mediate palatal mesenchyme osteogenesis by regulating the biosynthesis of collagen type 1 and deposition of CS-binding molecules Wnt3a during palate development.
HSPGs
Heparan sulfate (HS), a sulfated GAG, is dynamically expressed in the developing palate (Table 2).11,70,78,79 Before palate shelf elevation, HS expressed in the basement membrane of the whole palate and in the mesenchyme of the anterior palate.11 The expression of HS in the mesenchyme become evident in the mesenchyme at the tip of whole palatal shelf when palatal shelf is elevating and gradually disappears when palatal shelf fused together.11 The expression of HS in the basement membrane is stronger in the oral/lateral side than the nasal/medial side after palatal shelf elevation.11 HS directly or indirectly regulates SHH and FGF signaling80-83, two key signaling pathways during palate development.1,84 HSPGs bind both FGFs and FGFRs directly, stable their ternary complex, remain FGFs concentration in the local area, and regulate signaling activation.81,82,85 HSPGs can act as Shh co-receptors activating Shh signaling and promote cell proliferation.80 Genetically abolish heparan sulfate in the lung epithelial cells leads to reduced SHH production in the epithelial and expanded Fgf10 expression in the underlying mesenchyme in the lung development.83 Shh secreted by the palatal epithelium signals to the underlying palatal mesenchyme and regulate Fgfs expression.1 Fgfs, in return, can either positively or negatively regulate SHH expression in the epithelium.1,84,86,87 But additional studies need to clarify if HSPGs coordinate SHH and FGF signaling transduction in the epithelial–mesenchymal interactions during palate development.
HA
Hyaluronic acid (HA) is a high molecular mass GAG, which helps to retain a large amount of water in the mesenchyme. As a major component of palatal mesenchyme, HA is shown accumulating in the nasal side and in the hinge region of the palatal mesenchyme with higher levers in the anterior/mid-part than anterior-most and posterior palate (Table 2).9,11,78 Regionally specific accumulation of extracellular GAGs, predominantly HA, is proposed to be the intrinsic force to drive palatal shelf elevation.10,49 Mice homozygous for Fgfr2C342, Pax9 and Golgb1 mutation, which have a palatal shelf elevation defect, exhibit reduced HA accumulation in the palatal shelves.9,88,89 HA synthase Has 1, 2, and 3, which synthesize HA at the plasma membrane, are disrupted in TGF-β3 mutant palatal shelves which failed to fuse in the midline, indicating that HA remodeling in palate is highly regulated by Tgf-β signaling pathways.49
Integrin signaling
Integrins are a family of heterodimeric transmembrane receptors facilitating cell-ECM adhesion and signal transduction. By the combination of 18 α-subunits and 8 β-subunits, 24 distinct integrin heterodimers form and bind to different ECM proteins, such as α1β1, α2β1, α10β1, α11β1 for collagen; α3β4, α6β4, α7β4, α9β4 for laminin; α5β1, α8β1, αvβ1, αvβ6, etc., for RGD (a tripeptide sequence, present in ECM such as fibronectin and vitronectin).90 Several integrin subunits are present in the palate. Integrins α5 is expressed by the palatal mesenchyme and apical side of MEE cells at E13.5, and its expression around MEE is absent in the Tgf-β3 mutant.17 In contrast, Integrins β1 is absent from palatal mesenchymal cells but highly expressed by MEE cells at E13.5.17,62 Although the expression of Integrins β1 is no change in the Tgf-β3 mutant.17 The addition of Tgf-β3 in the palate culture induces both Integrins α5 and Integrins β1 expression on the MEE apical surface.17 Anti-Integrins α5 antibody blocks palate shelve adhesion in organ culture.17 Inactivation of Integrins α5 from either palatal neural crest cells with TFAP2αIRESCre or from mesodermal cells with Mesp1Cre can cause cleft palate.47,48 But further studies are needed to clarify how Integrins α5 and Integrins β1 are involved in ECM remodeling and signaling transduction during palatal shelf elevation and adhesion. Integrin αV, β3, β5 are also highly expressed by MEE cells.62 But their functions during palatal adhesion need to be further investigated. Loss of both Integrins α5 and αV from palatal neural crest cells with Wnt1Cre leads to cleft palate, where palatal shelves still remain small at E17.591, indicating that Integrins α5 and αV are essential for palatal shelve expansion. Only a small population of Integrin β8 heterozygous and homozygous embryos developed cleft palate92, indicating other α subunits are required for cleft palate phenotype.
Talin (Tln) is one of the important intracellular proteins which activates integrins by binding to its β subunit.93,94 Two Tln isoforms are present in most vertebrates95 and three in zebrafish.96 In zebrafish, tln1 is required for the cranial neural crest cell proliferation during palate morphogenesis.97 In mice, global loss of Tln1 leads to embryonic lethality during gastrulation98, while Tln2 null mice are viable and fertile.99 Conditional mouse models would provide more evidence on how Talin engages in Integrin signal transduction during palate development.
ECM remodeling by Extracellular metalloproteinases
ECM is dynamically remodeled by extracellular metalloproteinases, including Matrix metalloproteinases (MMPs) and their endogenous tissue inhibitors (TIMPs), a disintegrin and metalloproteinases (ADAMs), and ADAMs with thrombospondin motifs (ADAMTS). During palate development, MMPs and TIMPs are spatiotemporally expressed in the mouse embryos, correlating to their ECM substrates (Table 3).100
Table 3.
MMP associated with palate development.
| Expression pattern in the palate |
Mouse mutant associated with cleft palate |
|||||||
|---|---|---|---|---|---|---|---|---|
| Extracellular metalloproteinases | Subtype/alternative name | Location | Detection methods | References | ECM substrates during palate development | Mouse mutants | Defects | References |
| MMPs | MMP-1/Collagenase-1 | Unknown | qRT-PCR | 24 | NR | NR | ||
| MMP-2/Gelatinase A | Before palate shelf elevation: in the palatal mesenchyme and basement membrane, intensified gradually in the nasal-medial part. During the palatal shelf elevation and the palatal shelf fusion: intensified gradually in the tip and nasal-medial part and MES After the palatal shelf fusion: strong in palatal mesenchyme around MES. |
qRT-PCR, IHC, ISH | 1-3,24,100,105 | Cleaves type I collagen | NR | |||
| MMP-3 | Extensively expressed in palatal mesenchyme, transiently upregulated in a subset of nasal palatal epithelial cells. | IHC | 100,102,106 | NR | NR | |||
| MMP-9/Gelatinase B | Extensively expressed in palatal mesenchyme, transiently upregulated while palatal shelves elevating and fusing. | qRT-PCR, IHC, ISH | Human and mouse:24,102,104 | Cleaves laminin | NR | |||
| MMP13 | Before palate shelf elevation: in the palatal mesenchyme and basement membrane, intensified gradually in the nasal-medial part. During the palatal shelf elevation and the palatal shelf fusion: intensified gradually in the tip and nasal-medial part and MES After the palatal shelf fusion: still strong in the nasal-medial mesenchyme and MES but decreased in other area. |
IHC, ISH | Mouse100,105,119 | NR | NR | |||
| MMP-14/Membrane Type 1-MMP (MT1-MMP) | Highly in MEE | IHC, ISH | 105 | NR | Double knockout of MMP-14 and −16 | 80% of double null of MMP-14 and −16 have a cleft palate | 107 | |
| MMP-16/Membrane Type 3-MMP (MT3-MMP) | Unknown | NR | Double knockout of MMP-14 and −16 | 80% of double null of MMP-14 and −16 have a cleft palate | 107 | |||
| MMP-25 | The tips of palatal epithelium and mesenchyme | IHC, ISH | 108 | NR | NR | |||
| TIMPs | TIMP-1 | Before palate shelf elevation: in the central and maxillary region of palatal mesenchyme During the palatal shelf elevation and the palatal shelf fusion: intensified gradually in the tip and nasal-medial part and MES; palatal basement membrane After the palatal shelf fusion: strong in the nasal-medial mesenchyme and MES but decreased in other area. |
IHC | 100 | NR | NR | ||
| TIMP-2 | Before palate shelf elevation: in the central and maxillary region of palatal mesenchyme During the palatal shelf elevation and the palatal shelf fusion: intensified within palatal mesenchyme; palatal basement membrane After the palatal shelf fusion: strong in the nasal-medial mesenchyme and future osteogenic sites. |
IHC | 100 | NR | NR | |||
| TIMP-3 | Palatal epithelium, transiently expressed in mid-oral and ventral-medial mesenchyme. | IHC | 100 | NR | NR | |||
| TIMP-4 | Oral mucosa | IHC | 104 | NR | NR | |||
| ADAMTS | ADAMTS9 | Palatal capillary endothelium | ISH | 12 | Versican | Haploinsufficiendy of Adamts9 in Adamts20 mutant mice (Adamts9±;Adamts20bt/bt) | Complete cleft palate | 12 |
| ADAMTS20 | Palatal mesenchyme | ISH | 12 | |||||
NR, not reported.
IHC, immunohistochemical staining.
ISH, in situ hybridization.
MMPs 2, 3, 9, 13, 14, and 25 and TIMPs 1, 2, and 3 are spatiotemporally expressed in the mouse embryonic palate and MMP-9 and TIMP 4 are detected in the newborn human palate tissue suggesting ECM remodeling by MMPs and TIMPs are essential for palate development (Table 3).100-104 More importantly, MMP-13 and TIMP-2 are transiently highly upregulated in the MEE while palatal shelves are elevating and fusing.100-103 Their expression patterns preceded the decreases of their substrates, fibronectin, collagen I, and III.100,101,103 In Tgf-β mutant mouse, which failed to fuse palate shelves in the midline, MMP-13 and TIMP-2 are significantly reduced or totally absent in the MEE.105 And inhibition of MMP-13 synthesis and excessive TIMP-2 in palatal organ culture phenocopies Tgf-β mutant phenotype105,106, indicating that ECM remodeling in the midline is essential for palatal fusion.
Almost 80% of double null of MMP-14 and −16 have a cleft palate.107 Neither MMP-14 nor MMP-16 single mutant exhibits a cleft palate, indicating that MMP-14 and MMP-16 have overlapping roles on ECM remodeling during palate development.107
MMP-25 protein and mRNA are increased in the tips of palatal shelve while palatal growing, where it significantly decreased when neutralize TGF-β3108 Knockdown of MMP-25 in palatal organ culture impairs palate shelf fusion and persistent MES, indicating MMP-25 is a direct transcriptional target for Tgf-β3 in the palate development.108
Adamts 20 was found universally in the palatal shelf mesenchyme, while Adamts 9 mainly in the palate microvascular endothelium.12 Although Adamts 9 and 20 single mutant did not show cleft palate, haploinsufficiency of Adamts9 in Adamts 20 mutant mice (Adamts 9±;Adamts20bt/bt) showed a secondary cleft palate with 100% penetrance.12 These mice had defects in palatal mesenchymal cell proliferation and versican cleaving.12 Haploinsufficiency of versican (VCAN) in Adamts 20 mutant mice (Vcanhdf/+;Adamts20bt/bt) phenocopied the phenotype of Adamts 9±;Adamts20bt/bt mice.12 The collectively versican proteolysis by ADAMTS 9 and 20 in the palate are important for regulating palatal mesenchyme cell proliferation.
Taken together, these studies indicate an important role of ECM remodeling by extracellular metalloproteinases for palatal shelve expansion and fusion. But the corresponding ECM substrates of most extracellular metalloproteinases during palatogenesis are still unknown. It will be interesting to further explore how breakdown of other ECM such as collagen, proteoglycans, fibronectin, etc., by extracellular metalloproteinases facilitate palatogenesis.
Conclusion and future direction
In summary, many ECM and related genes are found to be involved in the palate development. ECM not only form the basic infrastructure of palatal shelves, but also play pivotal roles regulating cell proliferation, adhesion, cell fate determination in the morphogenesis of the secondary palate. However, the complex ECM functions and remodeling for palatal shelf expansion, elevation, and fusion has not yet been identified. We still know only little about the interaction of ECM themselves, and ECM and growth factors at different stages of palate development. Although a variety of ECM proteins expressed temporospatial during palatogenesis, it is still unknown how their dynamical expression patter might contribute to the distinct anterior-posterior palatal shelf elevation behavior. Besides, multiple signaling pathway regulates palate ECM elasticity and stiffness. But the contribution of mechanical transduction of ECM stiffness to palate elevation remains largely unknown. Future genetic studies will help us further understand the function of ECM remodeling during palatogenesis.
Funding Statement
This work was supported by the shenzhen Fundamental research fund of Science and Technology Foundation of Shenzhen city under [Grant JCYJ20170818101216838].
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article. The authors report no conflict interest.
References
- 1.Li C, Lan Y, Jiang R.. Molecular and cellular mechanisms of palate development. J Dent Res. 2017;96:1184–91. doi: 10.1177/0022034517703580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lan Y, Xu J, Jiang R. Cellular and molecular mechanisms of palatogenesis. Curr Top Dev Biol. 2015;115:59–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hynes RO, Naba A. Overview of the matrisome–an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hynes RO. The extracellular matrix: not just pretty fibrils. Science (New York, NY). 2009;326:1216–19. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.d’Amaro R, Scheidegger R, Blumer S, Pazera P, Katsaros C, Graf D, Chiquet M.. Putative functions of extracellular matrix glycoproteins in secondary palate morphogenesis. Front Physiol. 2012;3:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oka K, Honda MJ, Tsuruga E, Hatakeyama Y, Isokawa K, Sawa Y. Roles of collagen and periostin expression by cranial neural crest cells during soft palate development. J Histochem Cytochem. 2012;60:57–68. doi: 10.1369/0022155411427059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Huang Z, Wang W, Tan X, Li H, Zhang Y, Tian W, Hu T, Chen YP. FGF8 signaling alters the osteogenic cell fate in the hard palate. J Dent Res. 2018;97:589–96. doi: 10.1177/0022034517750141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan Y, Zhang N, Liu H, Xu J, Jiang R. Golgb1 regulates protein glycosylation and is crucial for mammalian palate development. Development. 2016;143:2344–55. doi: 10.1242/dev.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson MW. Palate development. Development. 1988;103:Suppl:41–60. [DOI] [PubMed] [Google Scholar]
- 11.Vaziri Sani F, Kaartinen V, El Shahawy M, Linde A, Gritli-Linde A. Developmental changes in cellular and extracellular structural macromolecules in the secondary palate and in the nasal cavity of the mouse. Eur J Oral Sci. 2010;118:221–36. doi: 10.1111/j.1600-0722.2010.00732.x. [DOI] [PubMed] [Google Scholar]
- 12.Enomoto H, Nelson CM, Somerville RP, Mielke K, Dixon LJ, Powell K, Apte SS. Cooperation of two ADAMTS metalloproteases in closure of the mouse palate identifies a requirement for versican proteolysis in regulating palatal mesenchyme proliferation. Development. 2010;137:4029–38. doi: 10.1242/dev.050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim I, Serrano MJ, Ruest LB, Svoboda KKH. Biglycan and decorin expression and distribution in palatal adhesion. J Dent Res. 2017;96:1445–50. doi: 10.1177/0022034517722783. [DOI] [PubMed] [Google Scholar]
- 14.Ida-Yonemochi H, Morita W, Sugiura N, Kawakami R, Morioka Y, Takeuchi Y, Sato T, Shibata S, Watanabe H, Imamura T, et al. Craniofacial abnormality with skeletal dysplasia in mice lacking chondroitin sulfate N-acetylgalactosaminyltransferase-1. Sci Rep. 2018;8:17134. doi: 10.1038/s41598-018-35412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nik AM, Johansson JA, Ghiami M, Reyahi A, Carlsson P. Foxf2 is required for secondary palate development and Tgfbeta signaling in palatal shelf mesenchyme. Dev Biol. 2016;415:14–23. doi: 10.1016/j.ydbio.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Shi JY, Zhu GQ, Shi B. MiR-17-92 cluster regulates cell proliferation and collagen synthesis by targeting TGFB pathway in mouse palatal mesenchymal cells. J Cell Biochem. 2012;113:1235–44. doi: 10.1002/jcb.23457. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Sanz E, Del Rio A, Barrio C, Murillo J, Maldonado E, Garcillan B, Amorós M, Fuerte T, Fernández Á, Trinidad E, et al. Alteration of medial-edge epithelium cell adhesion in two Tgf-beta3 null mouse strains. Differentiation. 2008;76:417–30. doi: 10.1111/j.1432-0436.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gato A, Martinez ML, Tudela C, Alonso I, Moro JA, Formoso MA, Ferguson MWJ, Martínez-Álvarez C. TGF-beta(3)-induced chondroitin sulphate proteoglycan mediates palatal shelf adhesion. Dev Biol. 2002;250:393–405. doi: 10.1006/dbio.2002.0792. [DOI] [PubMed] [Google Scholar]
- 19.Mienaltowski MJ, Birk DE. Structure, physiology, and biochemistry of collagens. Adv Exp Med Biol. 2014;802:5–29. [DOI] [PubMed] [Google Scholar]
- 20.Silver MH, Foidart JM, Pratt RM. Distribution of fibronectin and collagen during mouse limb and palate development. Differentiation. 1981;18:141–49. doi: 10.1111/j.1432-0436.1981.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 21.Kurisu K, Ohsaki Y, Nagata K, Kukita T, Yoshikawa H, Inai T. Immunocytochemical demonstration of simultaneous synthesis of types I, III and V collagen and fibronectin in mouse embryonic palatal mesenchymal cells in vitro. Coll Relat Res. 1987;7:333–40. doi: 10.1016/S0174-173X(87)80026-2. [DOI] [PubMed] [Google Scholar]
- 22.Kurisu K, Ohsaki Y, Nagata K, Inai T, Kukita T. Heterogeneous distribution of the precursor of type I and type III collagen and fibronectin in the rough endoplasmic reticulum of palatal mesenchymal cells of the mouse embryo cultured in ascorbate-depleted medium. Cell Tissue Res. 1992;267:429–35. doi: 10.1007/BF00319365. [DOI] [PubMed] [Google Scholar]
- 23.Ohsaki Y, Nagata K, Kurisu K. Localization of types I and III collagen and fibronectin in the developing mouse palatal shelves. Acta Anat. 1995;153:161–67. doi: 10.1159/000147696. [DOI] [PubMed] [Google Scholar]
- 24.Gagliano N, Carinci F, Moscheni C, Torri C, Pezzetti F, Scapoli L, Martinelli M, Gioia M, Stabellini G. New insights in collagen turnover in orofacial cleft patients. Cleft Palate-Craniofacial J. 2010;47:393–99. doi: 10.1597/07-196.1. [DOI] [PubMed] [Google Scholar]
- 25.Fantauzzo KA, Soriano P. PDGFRbeta regulates craniofacial development through homodimers and functional heterodimers with PDGFRalpha. Genes Dev. 2016;30:2443–58. doi: 10.1101/gad.288746.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higuchi Y, Hasegawa K, Yamashita M, Tanaka H, Tsukahara H. A novel mutation in the COL2A1 gene in a patient with Stickler syndrome type 1: a case report and review of the literature. J Med Case Rep. 2017;11:237. doi: 10.1186/s13256-017-1396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robin NH, Moran RT, Ala-Kokko L. Stickler syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, editors. GeneReviews((R)). Seattle: University of Washington, Seattle University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle; 1993. (All rights reserved). [Google Scholar]
- 28.Melkoniemi M, Koillinen H, Mannikko M, Warman ML, Pihlajamaa T, Kaariainen H, Rautio J, Hukki J, Stofko JA, Cisneros GJ, et al. Collagen XI sequence variations in nonsyndromic cleft palate, Robin sequence and micrognathia. Eur J Hum Genet. 2003;11:265–70. doi: 10.1038/sj.ejhg.5200950. [DOI] [PubMed] [Google Scholar]
- 29.Nikopensius T, Jagomagi T, Krjutskov K, Tammekivi V, Saag M, Prane I, Piekuse L, Akota I, Barkane B, Krumina A, et al. Genetic variants in COL2A1, COL11A2, and IRF6 contribute risk to nonsyndromic cleft palate. Birth Defects Res Part A Clin Mol Teratol. 2010;88:748–56. doi: 10.1002/bdra.v88:9. [DOI] [PubMed] [Google Scholar]
- 30.Hoornaert KP, Vereecke I, Dewinter C, Rosenberg T, Beemer FA, Leroy JG, Bendix L, Björck E, Bonduelle M, Boute O, et al. Stickler syndrome caused by COL2A1 mutations: genotype-phenotype correlation in a series of 100 patients. Eur J Hum Genet. 2010;18:872–80. doi: 10.1038/ejhg.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards AJ, Laidlaw M, Meredith SP, Shankar P, Poulson AV, Scott JD, Snead MP. Missense and silent mutations in COL2A1 result in Stickler syndrome but via different molecular mechanisms. Hum Mutat. 2007;28:639. doi: 10.1002/()1098-1004. [DOI] [PubMed] [Google Scholar]
- 32.Richards AJ, Laidlaw M, Whittaker J, Treacy B, Rai H, Bearcroft P, Baguley DM, Poulson A, Ang A, Scott JD, et al. High efficiency of mutation detection in type 1 stickler syndrome using a two-stage approach: vitreoretinal assessment coupled with exon sequencing for screening COL2A1. Hum Mutat. 2006;27:696–704. doi: 10.1002/humu.v27:7. [DOI] [PubMed] [Google Scholar]
- 33.Barbieri O, Astigiano S, Morini M, Tavella S, Schito A, Corsi A, Martino DD, Bianco P, Cancedda R, Garofalo S, et al. Depletion of cartilage collagen fibrils in mice carrying a dominant negative Col2a1 transgene affects chondrocyte differentiation. Am J Physiol Cell Physiol. 2003;285(6):C1504–12. doi: 10.1152/ajpcell.00579.2002. [DOI] [PubMed] [Google Scholar]
- 34.Li SW, Prockop DJ, Helminen H, Fassler R, Lapvetelainen T, Kiraly K, Peltarri A, Arokoski J, Lui H, Arita M, et al. Transgenic mice with targeted inactivation of the Col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995;9(22):2821–30. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- 35.Vandenberg P, Khillan JS, Prockop DJ, Helminen H, Kontusaari S, Ala-Kokko L. Expression of a partially deleted gene of human type II procollagen (COL2A1) in transgenic mice produces a chondrodysplasia. Proc Natl Acad Sci U S A. 1991;88(17):7640–44. doi: 10.1073/pnas.88.17.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metsaranta M, Garofalo S, Decker G, Rintala M, de Crombrugghe B, Vuorio E. Chondrodysplasia in transgenic mice harboring a 15-amino acid deletion in the triple helical domain of pro alpha 1(II) collagen chain. J Cell Biol. 1992;118:203–12. doi: 10.1083/jcb.118.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garofalo S, Vuorio E, Metsaranta M, Rosati R, Toman D, Vaughan J, Lozano G, Mayne R, Ellard J, Horton W, et al. Reduced amounts of cartilage collagen fibrils and growth plate anomalies in transgenic mice harboring a glycine-to-cysteine mutation in the mouse type II procollagen alpha 1-chain gene. Proc Natl Acad Sci U S A. 1991;88:9648–52. doi: 10.1073/pnas.88.21.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savontaus M, Rintala-Jamsa M, Morko J, Ronning O, Metsaranta M, Vuorio E. Abnormal craniofacial development and expression patterns of extracellular matrix components in transgenic Del1 mice harboring a deletion mutation in the type II collagen gene. Orthod Craniofac Res. 2004;7:216–26. doi: 10.1111/j.1601-6343.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 39.Lavrin IO, McLean W, Seegmiller RE, Olsen BR, Hay ED. The mechanism of palatal clefting in the Col11a1 mutant mouse. Arch Oral Biol. 2001;46:865–69. doi: 10.1016/S0003-9969(01)00044-9. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Lacerda DA, Warman ML, Beier DR, Yoshioka H, Ninomiya Y, Oxford JT, Morris NP, Andrikopoulos K, Ramirez F, et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell. 1995;80(3):423–30. doi: 10.1016/0092-8674(95)90492-1. [DOI] [PubMed] [Google Scholar]
- 41.McGuirt WT, Prasad SD, Griffith AJ, Kunst HP, Green GE, Shpargel KB, Runge C, Huybrechts C, Mueller RF, Lynch E, et al. Mutations in COL11A2 cause non-syndromic hearing loss (DFNA13). Nat Genet. 1999;23(4):413–19. doi: 10.1038/70516. [DOI] [PubMed] [Google Scholar]
- 42.Li SW, Takanosu M, Arita M, Bao Y, Ren ZX, Maier A, Prockop DJ, Mayne R. Targeted disruption of Col11a2 produces a mild cartilage phenotype in transgenic mice: comparison with the human disorder otospondylomegaepiphyseal dysplasia (OSMED). Dev Dyn. 2001;222:141–52. doi: 10.1002/()1097-0177. [DOI] [PubMed] [Google Scholar]
- 43.Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol. 2014;802:31–47. [DOI] [PubMed] [Google Scholar]
- 44.Zollinger AJ, Smith ML. Fibronectin, the extracellular glue. Matrix Biol. 2017;60–61:27–37. doi: 10.1016/j.matbio.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Astrof S, Crowley D, Hynes RO. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev Biol. 2007;311:11–24. doi: 10.1016/j.ydbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Astrof S, Hynes RO. Fibronectins in vascular morphogenesis. Angiogenesis. 2009;12:165–75. doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang D, Wang X, Mittal A, Dhiman S, Hou SY, Degenhardt K, Astrof S. Mesodermal expression of integrin alpha5beta1 regulates neural crest development and cardiovascular morphogenesis. Dev Biol. 2014;395:232–44. doi: 10.1016/j.ydbio.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Astrof S. Neural crest cell-autonomous roles of fibronectin in cardiovascular development. Development. 2016;143:88–100. doi: 10.1242/dev.125286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galloway JL, Jones SJ, Mossey PA, Ellis IR. The control and importance of hyaluronan synthase expression in palatogenesis. Front Physiol. 2013;4:10. doi: 10.3389/fphys.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jarvinen TA, Jozsa L, Kannus P, Jarvinen TL, Hurme T, Kvist M, Pelto-Huikko M, Kalimo H, Järvinen M.. Mechanical loading regulates the expression of tenascin-C in the myotendinous junction and tendon but does not induce de novo synthesis in the skeletal muscle. J Cell Sci. 2003;116:857–66. doi: 10.1242/jcs.00303. [DOI] [PubMed] [Google Scholar]
- 51.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–90. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sage EH. Regulation of interactions between cells and extracellular matrix: a command performance on several stages. J Clin Invest. 2001;107:781–83. doi: 10.1172/JCI12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiquet M, Blumer S, Angelini M, Mitsiadis TA, Katsaros C. Mesenchymal remodeling during palatal shelf elevation revealed by extracellular matrix and F-actin expression patterns. Front Physiol. 2016;7:392. doi: 10.3389/fphys.2016.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen W, Kim MS, Shanbhag S, Arai A, VanRyzin C, McDonnell NB, Merke DP. The phenotypic spectrum of contiguous deletion of CYP21A2 and tenascin XB: quadricuspid aortic valve and other midline defects. Am J Med Genet Part A. 2009;149a:2803–08. doi: 10.1002/ajmg.a.v149a:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merke DP, Chen W, Morissette R, Xu Z, Van Ryzin C, Sachdev V, Hannoush H, Shanbhag SM, Acevedo AT, Nishitani M, et al. Tenascin-X haploinsufficiency associated with Ehlers-Danlos syndrome in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2013;98(2):E379–87. doi: 10.1210/jc.2012-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morissette R, Merke DP, McDonnell NB. Transforming growth factor-beta (TGF-beta) pathway abnormalities in tenascin-X deficiency associated with CAH-X syndrome. Eur J Med Genet. 2014;57:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294(Pt 1):271–78. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62:5358–64. [PubMed] [Google Scholar]
- 59.Kii I, Amizuka N, Minqi L, Kitajima S, Saga Y, Kudo A. Periostin is an extracellular matrix protein required for eruption of incisors in mice. Biochem Biophys Res Commun. 2006;342:766–72. doi: 10.1016/j.bbrc.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 60.Rios HF, Ma D, Xie Y, Giannobile WV, Bonewald LF, Conway SJ, Feng JQ. Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J Periodontol. 2008;79(8):1480–90. doi: 10.1902/jop.2008.070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, et al. periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005;25:11131–44. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitase Y, Yamashiro K, Fu K, Richman JM, Shuler CF. Spatiotemporal localization of periostin and its potential role in epithelial-mesenchymal transition during palatal fusion. Cells Tissues Organs. 2011;193:53–63. doi: 10.1159/000320178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kruzynska-Frejtag A, Wang J, Maeda M, Rogers R, Krug E, Hoffman S, Markwald RR, Conway SJ. Periostin is expressed within the developing teeth at the sites of epithelial-mesenchymal interaction. Dev Dyn. 2004;229(4):857–68. doi: 10.1002/()1097-0177. [DOI] [PubMed] [Google Scholar]
- 64.Yao Y. Laminin: loss-of-function studies. Cell Mol Life Sci. 2017;74:1095–115. doi: 10.1007/s00018-016-2381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 66.Yamada T, Hirata A, Sasabe E, Yoshimura T, Ohno S, Kitamura N, Yamamoto T. TCDD disrupts posterior palatogenesis and causes cleft palate. J Cranio-maxillo-facial Surg. 2014;42:1–6. doi: 10.1016/j.jcms.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 67.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–11. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 68.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jakobsen LP, Borup R, Vestergaard J, Larsen LA, Lage K, Maroun LL, Kjaer I, Niemann CU, Andersen M, Knudsen MA, et al. Expression analyses of human cleft palate tissue suggest a role for osteopontin and immune related factors in palatal development. Exp Mol Med. 2009;41(2):77–85. doi: 10.3858/emm.2009.41.2.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh GD, Moxham BJ, Langley MS, Waddington RJ, Embery G. Changes in the composition of glycosaminoglycans during normal palatogenesis in the rat. Arch Oral Biol. 1994;39:401–07. doi: 10.1016/0003-9969(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 71.Brinkley LL, Morris-Wiman J. The role of extracellular matrices in palatal shelf closure. Curr Top Dev Biol. 1984;19:17–36. [DOI] [PubMed] [Google Scholar]
- 72.Singh GD, Moxham BJ, Langley MS, Embery G. Glycosaminoglycan biosynthesis during 5-fluoro-2-deoxyuridine-induced palatal clefts in the rat. Arch Oral Biol. 1997;42:355–63. doi: 10.1016/S0003-9969(97)00031-9. [DOI] [PubMed] [Google Scholar]
- 73.Martinez-Alvarez C, Tudela C, Perez-Miguelsanz J, O’Kane S, Puerta J, Ferguson MW. Medial edge epithelial cell fate during palatal fusion. Dev Biol. 2000;220:343–57. doi: 10.1006/dbio.2000.9644. [DOI] [PubMed] [Google Scholar]
- 74.Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 75.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–89. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 76.Zhang YX, Mori T, Iseki K, Hagino S, Takaki H, Takeuchi M, Hikake T, Tase C, Murakawa M, Yokoya S, et al. Differential expression of decorin and biglycan genes during palatogenesis in normal and retinoic acid-treated mice. Dev Dyn. 2003;226(4):618–26. doi: 10.1002/dvdy.10267. [DOI] [PubMed] [Google Scholar]
- 77.Hammond NL, Brookes KJ, Dixon MJ. Ectopic hedgehog signaling causes cleft palate and defective osteogenesis. J Dent Res. 2018;97:1485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Knudsen TB, Bulleit RF, Zimmerman EF. Histochemical localization of glycosaminoglycans during morphogenesis of the secondary palate in mice. Anat Embryol (Berl). 1985;173:137–42. doi: 10.1007/BF00707312. [DOI] [PubMed] [Google Scholar]
- 79.Larsson KS. Studies on the closure of the secondary palate. IV. Autoradiographic and histochemical studies of mouse embryos from cortisone-treated mothers. Acta Morphol Neerl Scand. 1962;4:369–86. [PubMed] [Google Scholar]
- 80.Witt RM, Hecht ML, Pazyra-Murphy MF, Cohen SM, Noti C, van Kuppevelt TH, Fuller M, Chan JA, Hopwood JJ, Seeberger PH, et al. Heparan sulfate proteoglycans containing a glypican 5 core and 2-O-sulfo-iduronic acid function as sonic hedgehog co-receptors to promote proliferation. J Biol Chem. 2013;288:26275–88. doi: 10.1074/jbc.M112.438937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science (New York, NY). 1991;252:1705–08. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 82.Matsuo I, Kimura-Yoshida C. Extracellular modulation of fibroblast growth factor signaling through heparan sulfate proteoglycans in mammalian development. Curr Opin Genet Dev. 2013;23:399–407. doi: 10.1016/j.gde.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 83.He H, Huang M, Sun S, Wu Y, Lin X. Epithelial heparan sulfate regulates sonic hedgehog signaling in lung development. PLoS Genet. 2017;13:e1006992. doi: 10.1371/journal.pgen.1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu J, Liu H, Lan Y, Aronow BJ, Kalinichenko VV, Jiang R. A Shh-Foxf-Fgf18-Shh molecular circuit regulating palate development. PLoS Genet. 2016;12:e1005769. doi: 10.1371/journal.pgen.1005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–48. doi: 10.1016/0092-8674(91)90512-W. [DOI] [PubMed] [Google Scholar]
- 86.Rice R, Spencer-Dene B, Connor EC, Gritli-Linde A, McMahon AP, Dickson C, Thesleff I, Rice DPC. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J Clin Invest. 2004;113:1692–700. doi: 10.1172/JCI20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han J, Mayo J, Xu X, Li J, Bringas P Jr., Maas RL, Rubenstein JLR, Chai Y. Indirect modulation of Shh signaling by Dlx5 affects the oral-nasal patterning of palate and rescues cleft palate in Msx1-null mice. Development. 2009;136(24):4225–33. doi: 10.1242/dev.036723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Snyder-Warwick AK, Perlyn CA, Pan J, Yu K, Zhang L, Ornitz DM. Analysis of a gain-of-function FGFR2 crouzon mutation provides evidence of loss of function activity in the etiology of cleft palate. Proc Natl Acad Sci U S A. 2010;107:2515–20. doi: 10.1073/pnas.0913985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li C, Lan Y, Krumlauf R, Jiang R. Modulating Wnt signaling rescues palate morphogenesis in pax9 mutant mice. J Dent Res. 2017;96:1273–81. doi: 10.1177/0022034517719865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 91.Turner CJ, Badu-Nkansah K, Crowley D, van der Flier A, Hynes RO. alpha5 and alphav integrins cooperate to regulate vascular smooth muscle and neural crest functions in vivo. Development. 2015;142:797–808. doi: 10.1242/dev.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA.. Talin binding to integrin beta tails: a final common step in integrin activation. Science (New York, NY). 2003;302:103–06. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 94.Calderwood DA, Fujioka Y, de Pereda JM, Garcia-Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, Ginsberg MH. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci U S A. 2003;100:2272–77. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Senetar MA, McCann RO. Gene duplication and functional divergence during evolution of the cytoskeletal linker protein talin. Gene. 2005;362:141–52. doi: 10.1016/j.gene.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 96.Wu Q, Zhang J, Koh W, Yu Q, Zhu X, Amsterdam A, Davis GE, Arnaout MA, Xiong JW.. Talin1 is required for cardiac Z-disk stabilization and endothelial integrity in zebrafish. FASEB J. 2015;29:4989–5005. doi: 10.1096/fj.15-273409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ishii K, Mukherjee K, Okada T, Liao EC. Genetic requirement of talin1 for proliferation of cranial neural crest cells during palate development. Prs-Glob Open. 2018;6:e1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Monkley SJ, Zhou XH, Kinston SJ, Giblett SM, Hemmings L, Priddle H, Brown JE, Pritchard CA, Critchley DR, Fässler R. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev Dyn. 2000;219:560–74. doi:. [DOI] [PubMed] [Google Scholar]
- 99.Debrand E, Conti FJ, Bate N, Spence L, Mazzeo D, Pritchard CA, Monkley SJ, Critchley DR. Mice carrying a complete deletion of the talin2 coding sequence are viable and fertile. Biochem Biophys Res Commun. 2012;426(2):190–95. doi: 10.1016/j.bbrc.2012.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morris-Wiman J, Burch H, Basco E. Temporospatial distribution of matrix metalloproteinase and tissue inhibitors of matrix metalloproteinases during murine secondary palate morphogenesis. Anat Embryol (Berl). 2000;202:129–41. doi: 10.1007/s004290000098. [DOI] [PubMed] [Google Scholar]
- 101.Morris-Wiman J, Du Y, Brinkley L. Occurrence and temporal variation in matrix metalloproteinases and their inhibitors during murine secondary palatal morphogenesis. J Craniofac Genet Dev Biol. 1999;19:201–12. [PubMed] [Google Scholar]
- 102.de Oliveira Demarchi AC, Zambuzzi WF, Paiva KB, da Silva-valenzuela M, Nunes FD, de Cassia Savio Figueira R, Sasahara RM, Demasi MAA, Winnischofer SMB, Sogayar MC, et al. Development of secondary palate requires strict regulation of ECM remodeling: sequential distribution of RECK, MMP-2, MMP-3, and MMP-9. Cell Tissue Res. 2010;340:61–69. doi: 10.1007/s00441-010-0931-6. [DOI] [PubMed] [Google Scholar]
- 103.Mansell JP, Kerrigan J, McGill J, Bailey J, TeKoppele J, Sandy JR. Temporal changes in collagen composition and metabolism during rodent palatogenesis. Mech Ageing Dev. 2000;119:49–62. doi: 10.1016/S0047-6374(00)00168-8. [DOI] [PubMed] [Google Scholar]
- 104.Smane-Filipova L, Pilmane M, Akota I. MMPs and TIMPs expression in facial tissue of children with cleft lip and palate. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:538–42. doi: 10.5507/bp.2016.055. [DOI] [PubMed] [Google Scholar]
- 105.Blavier L, Lazaryev A, Groffen J, Heisterkamp N, DeClerck YA, Kaartinen V. TGF-beta3-induced palatogenesis requires matrix metalloproteinases. Mol Biol Cell. 2001;12:1457–66. doi: 10.1091/mbc.12.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown NL, Yarram SJ, Mansell JP, Sandy JR. Matrix metalloproteinases have a role in palatogenesis. J Dent Res. 2002;81:826–30. doi: 10.1177/154405910208101206. [DOI] [PubMed] [Google Scholar]
- 107.Shi J, Son MY, Yamada S, Szabova L, Kahan S, Chrysovergis K, Wolf L, Surmak A, Holmbeck K. Membrane-type MMPs enable extracellular matrix permissiveness and mesenchymal cell proliferation during embryogenesis. Dev Biol. 2008;313:196–209. doi: 10.1016/j.ydbio.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown GD, Nazarali AJ. Matrix metalloproteinase-25 has a functional role in mouse secondary palate development and is a downstream target of TGF-beta3. BMC Dev Biol. 2010;10:93. doi: 10.1186/1471-213X-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kondo H, Matsushita I, Nagata T, Hayashi T, Kakinoki M, Uchio E, Kondo M, Ohji M, Kusaka S. Novel mutations in the COL2A1 gene in Japanese patients with Stickler syndrome. Hum Genome Var. 2016;3(1):16018. doi: 10.1038/hgv.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dixon MJ, Marazita ML, Beaty TH, Murray JC. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 2011;12:167–78. doi: 10.1038/nrg2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kannu P, Bateman J, Savarirayan R. Clinical phenotypes associated with type II collagen mutations. J Paediatr Child Health. 2012;48:E38–43. doi: 10.1111/j.1440-1754.2010.01979.x. [DOI] [PubMed] [Google Scholar]
- 112.Fairfield H, Gilbert GJ, Barter M, Corrigan RR, Curtain M, Ding Y, D’Ascenzo M, Gerhardt DJ, He C, Huang W, et al. Mutation discovery in mice by whole exome sequencing. Genome Biol. 2011;12:R86. doi: 10.1186/gb-2011-12-9-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miller WL, Merke DP. Tenascin-X, congenital adrenal hyperplasia, and the CAH-X syndrome. Horm Res Paediatr. 2018;89:352–61. doi: 10.1159/000481911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O’Connell M, Burrows NP, van Vlijmen-willems MJ, Clark SM, Schalkwijk J. Tenascin-X deficiency and Ehlers-Danlos syndrome: a case report and review of the literature. Br J Dermatol. 2010;163:1340–45. doi: 10.1111/j.1365-2133.2010.09949.x. [DOI] [PubMed] [Google Scholar]
- 115.Kimata K, Barrach HJ, Brown KS, Pennypacker JP. Absence of proteoglycan core protein in cartilage from the cmd/cmd (Cartilage matrix deficiency) mouse. J Biol Chem. 1981;256:6961–68. [PubMed] [Google Scholar]
- 116.Krueger RC Jr., Kurima K, Schwartz NB. Completion of the mouse aggrecan gene structure and identification of the defect in the cmd-Bc mouse as a near complete deletion of the murine aggrecan gene. Mamm Genome. 1999;10:1119–25. doi: 10.1007/s003359901176. [DOI] [PubMed] [Google Scholar]
- 117.Sasaki S, Kurisu K. Effect of triamcinolone acetonide on proliferation and collagen and glycosaminoglycan syntheses in palatal mesenchymal cells from the mouse fetus. J Craniofac Genet Dev Biol. 1983;3:351–69. [PubMed] [Google Scholar]
- 118.Foreman DM, Sharpe PM, Ferguson MW. Comparative biochemistry of mouse and chick secondary-palate development in vivo and in vitro with particular emphasis on extracellular matrix molecules and the effects of growth factors on their synthesis. Arch Oral Biol. 1991;36:457–71. doi: 10.1016/j.ejmg.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 119.Jin JZ, Tan M, Warner DR, Darling DS, Higashi Y, Gridley T, Ding J. Mesenchymal cell remodeling during mouse secondary palate reorientation. Dev Dyn. 2010;239:2110–17. doi: 10.1002/dvdy.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]


