Background.
Hemochromatosis (HC) is an autosomal recessive disease characterized by impaired iron metabolism and a rare indication for orthotopic liver transplantation (LT). Data about iron reaccumulation and remodeling of the liver graft after LT are limited. Therefore, we performed an evaluation of the histopathologic changes during long-term follow-up in patients with HC.
Methods.
A retrospective analysis of patients undergoing LT at our center between 1990 and 2016 identified 29 patients with HC. End points were the evaluation of post-LT iron reaccumulation and the stage of fibrosis as well as the degree of inflammation of the liver graft. Secondary end points were patient survival and postoperative complications.
Results.
The median age was 52.7 y, and there were more male (82.8%) than female patients (17.2%). Post-LT serum ferritin values (>1000 μg/L) were only temporarily elevated in 2 patients. The median estimated survival after LT was 45.5 mo (0.1–285.9 mo). Twenty patients (69%) died during follow-up of 10 y. The survival of patients with HC was significantly worse (P = 0.001) when compared with the overall cohort of patients undergoing LT because of to other causes.
Conclusions.
There was no significant iron overload detected in patients with HC after LT, and only minimal iron deposits were described in liver biopsies. Nevertheless, patients suffering from HC show a lower post-LT survival when compared with patients without iron storage disease but mostly because of extrahepatic causes.
Hereditary hemochromatosis (HC) is a rare autosomal recessive disease characterized by impaired iron metabolism. It is the most frequent genetic defect in Caucasians, with an incidence of about 1 in 1000 people. Men have a 24-fold increased risk compared with women.1,2 A mutation of the HFE gene can be identified in >80%, and it is expressed through the replacement of cysteine by tyrosine (C282Y).3 Increased iron uptake, impaired hepcidin-ferroportin interaction, and increased iron deposition in several organs such as liver, pancreas, skin, joints, pituitary, and heart are the main features of the disease.4,5 The typical clinical triad consists of liver cirrhosis, diabetes mellitus, and hyperpigmentation.6
Indeed, patients with HC approximately accumulate 80 mg iron in the hepatocytes, which are the main iron deposits in the human body. The increased iron content affects the liver parenchyma by damaging the DNA and promoting the formation of radicals and collagen fibers in the extracellular space.7 The most effective treatment is the elimination of excessive iron through phlebotomy or iron chelators avoiding iron deposition and subsequent organ damage.8 If administered before the development of liver cirrhosis or diabetes mellitus, this therapy is associated with good prognosis and normal life expectancy.9
However, severe clinical courses may lead to end-stage liver disease requiring further treatment. HC remains a rare indication for orthotopic liver transplantation (LT). Because of poor outcome and the unclear influence of preoperative iron overload, surgery had been discussed controversially. There are little data about development of the graft, post-LT complications, and iron reaccumulation to strengthen current knowledge on that topic.10
Our study aims to extend the currently existing data. We performed a retrospective evaluation of the histopathologic changes in patients after LT due to HC during long-term follow-up to define iron reaccumulation as well as inflammatory or fibrotic remodeling of the liver graft based on protocol biopsies.
MATERIALS AND METHODS
We performed a retrospective analysis of patients undergoing LT at our center between 1990 and 2016. In total, 2590 patients received LT during this period and 29 patients could be identified undergoing LT due to HC. Data were extracted from our digital patient documentation system and collected from routine examinations of patients during long-term follow-up. Examinations were scheduled at 3, 5, 7, 10, 13, 15, 17, 20, and 23 y after the LT. Data on gender, time since LT, age at LT, time of death, cause and diagnosis of death, serum iron (μmol/L), serum transferrin (g/L), serum ferritin (μg/L), hemoglobin (g/dL), and MCV values (fl).
To classify the degrees of inflammation of the biopsies, grading according to Desmet et al (0 = no signs of inflammation, 1 = minimal, 2 = low grade, 3 = moderate, 4 = high grade) and staging and scoring according to Desmet et al were performed (0 = no fibrosis, 1 = low-grade fibrosis, 2 = moderate fibrosis, 3 = high-grade fibrosis, 4 = cirrhosis).11 In order to assess siderosis of the liver, grading according to Schirmacher et al11 was used (0 = no iron loading, 1 = low iron loading, 2 = moderate iron loading, 3 = heavy iron loading, 4 = excessive iron loading).12
Primary end points were iron reaccumulation as well as the determination of stage of fibrosis and the degree of inflammation of the liver graft. Secondary end points were the evaluation of patient survival and postoperative complications.
Statistical analysis was performed by using IBM SPSS Statistics Version 25 for MAC (IBM, Armonk, NY). P values <0.05 were considered statistically significant. Only biopsies and laboratory values of the first 10 y after LT were taken into account, as the number of cases for the follow-up examinations 13 to 23 y after LT dropped below 5. To compare the liver pathologies and laboratory values at the different time points of the follow-up examinations, a 2-factor ANOVA was performed (log rank). Survival was estimated by a Kaplan-Meier calculation.
All procedures were based on the principles of the Charter of Charité-Universitätsmedizin Berlin to ensure good scientific practice. The study was performed according to the principles of good scientific practice and the Declaration of Helsinki.
RESULTS
Twenty-nine patients could be included for analysis with a prevalence of 1.1% of HC in our total transplant cohort. Demographic data are given in Table 1. The median age of the patients was 52.7 y, with the youngest patient being 3 mo and the oldest 68.7 y old. Five (17.2%) of 29 patients were female and 24 (82.8%) male. The median follow-up was 3.8 y (0–24 years). Twenty-seven (93.1%) were diagnosed with hereditary HC and 2 (6.9%) with neonatal HC. Eight patients (27.5%) were already suffering from hepatocellular carcinoma (HCC) due to liver cirrhosis at the time of LT. Among patients without HCC, we found 1 (3.5%) patient with a coexisting viral hepatitis C and 6 (20.7%) patients with a history of severe alcohol abuse before LT and histopathologically confirmed components of alcohol-induced steatohepatitis. One (3.5%) patient additionally suffered from nonalcoholic steatohepatitis. After LT, none of those patients suffered from other comorbidities such as alcohol-induced or nonalcoholic steatohepatitis as well as viral hepatitis except for 1 patient who experienced recurrent HCV infection that was successfully treated with pegylated interferon-α2a and ribavirin in 2010 rather soon after LT without relevant fibrosis progression.
TABLE 1.
Demographic characteristics of patient cohort (n = 29) on age, sex, diseases at LT, immunosuppression protocol, episodes of rejection, and overall mortality and causes
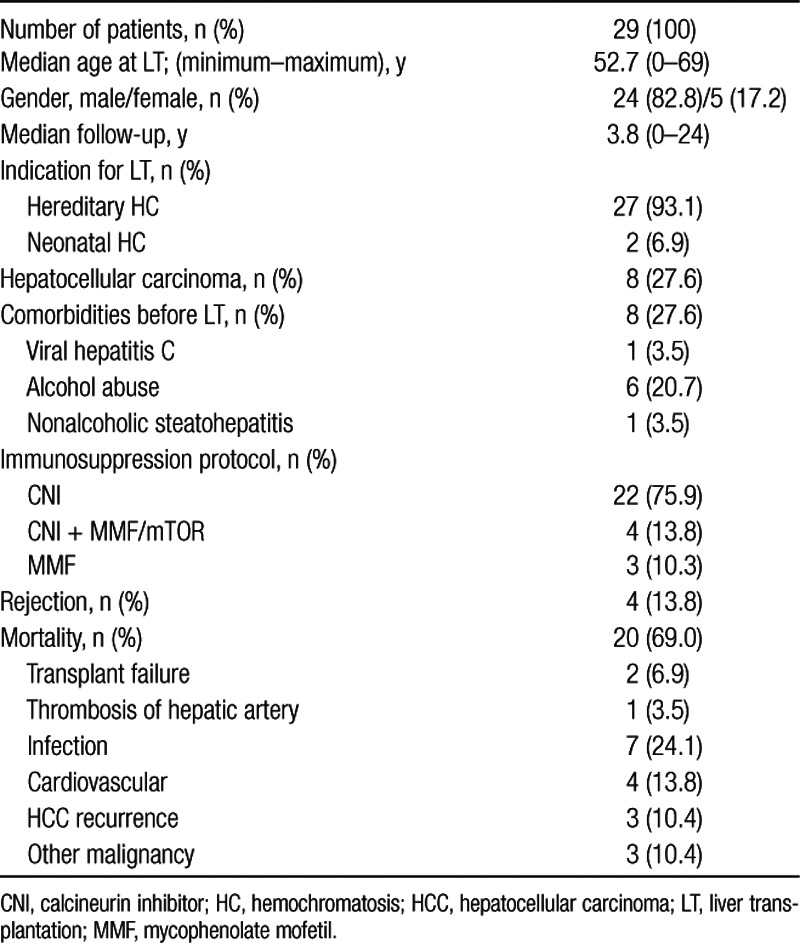
During the whole follow-up, 20 patients (69%) died. The majority of patients were on calcineurin inhibitor-based monotherapy (cyclosporine or tacrolimus; n = 22 [75.9%]). Four patients (13.8%) received a combination therapy with calcineurin inhibitor and either mycophenolate mofetil or in case of a former HCC an mTOR inhibitor (everolimus, sirolimus). Mycophenolate mofetil monotherapy at the end of the observation was applied in 3 patients (10.8%). Graft rejection was described in 4 patients (13.8%), 1 (n = 2), 3, and 5 y post-LT. Late acute cellular rejections were not observed.
As shown in Figure 1, the median hemoglobin values were inside the reference range at all times (m: 13–17 g/dL, w: 12–16 g/dL). For 10 y, a statistically insignificant tendency toward a slight increase of the values was evident (P = 0.416). The median serum iron values decreased after LT and remained within the respective reference range (9–29 μmol/L) and constant over the period of 10 y (Figure 1). The values for serum ferritin also decreased from a higher level before LT and were relatively constant afterward (Figure 1). Strongly elevated serum ferritin values (>1000 μg/L) were only measured in 2 patients. One patient showed temporary elevated values at 1-, 3-, and 5-y follow-up examinations, which subsequently normalized. One patient showed a strongly increased ferritin and iron value only at 3 y after LT (Figure 1). At the same time point, iron deposits in the liver biopsy were noticeable. However, 5 y after LT, the patient showed normalized iron and ferritin values and no iron deposits could be detected in the biopsy. The median serum transferrin values were below the reference range (2.0–3.6 g/L) at all times with a slight but not significantly relevant increase after LT and remained constant over 10 y (Figure 1).
FIGURE 1.
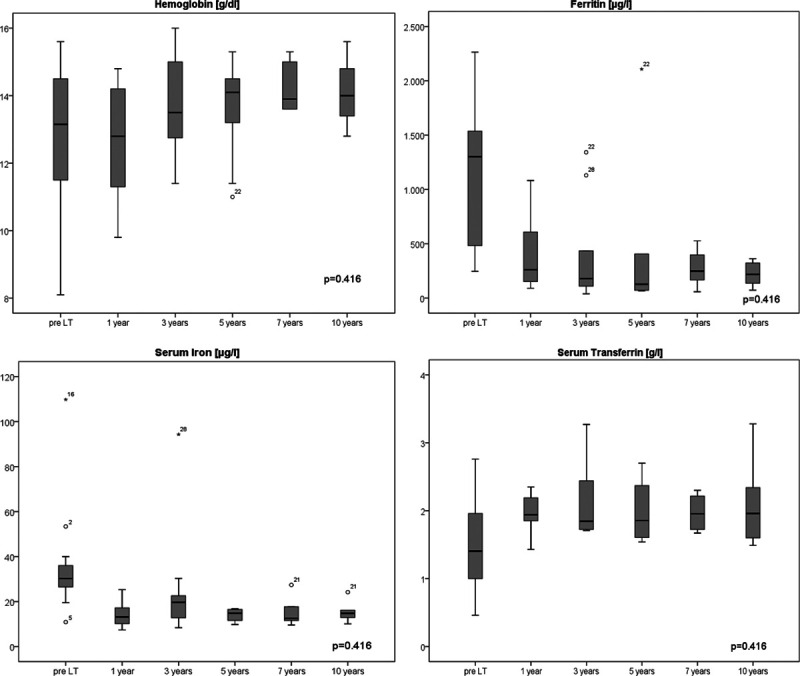
Box plots show the laboratory results of hemoglobin (g/dL), serum iron (µg/L), ferritin (µg/L), and serum transferrin (g/L) pretransplantation and at the check examination after 1, 3, 5, 7, and 10 y after liver transplantation (LT). There were no significant differences over the y (N = 29).
The proportion of patients showing inflammation of the liver graft in the biopsy declined over the years (P = 0.323, Figure 2). Indeed, although 1 y after LT 73% of the patients showed inflammation (grade 1 or higher), only 40% of them did after 10 y. Seven years post-LT, only 20% of the patients were diagnosed with inflammation of the liver in the biopsy at all.
FIGURE 2.
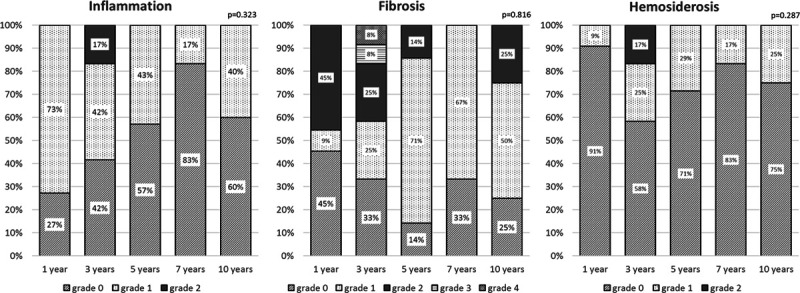
Bar graphs show laboratory results of inflammation, fibrosis, and hemosiderosis of the graft according to the routine biopsies at the check examination after 1, 3, 5, 7, and 10 y after liver transplantation. There were no significant difference over the y (N = 29).
Fibrosis stage 2 was detected at 1, 3, 5, and 10 y after LT (P = 0.816, Figure 2). After 3 y, 1 patient was diagnosed with severe fibrosis (stage 3) and another with liver cirrhosis (stage 4, Figure 2). The last one had to undergo retransplantation due to graft failure and died 3 mo later of postoperative complications.
The percentage of patients with iron overload in the liver biopsy was the highest after 3 y (42%) and declined afterward but without significance (P = 0.287, Figure 2). After 1 y, only 9% of the patients showed iron deposits in the graft. Severe or excessive iron deposition in the liver parenchyma was not seen in any of the patients at any of the follow-up time points.
The median survival after LT was 45.5 mo (0.1–285.9 mo). The 1-y survival rate in liver transplant patients with HC was 69%. After 3 y, 59% of the patients were still alive, after 5 y 51%, and after 10 y 39% (Figure 3). The estimated survival in patients with HC was significantly worse (P = 0.001) when compared with the overall cohort of patients undergoing LT at our center between 1990 and 2016.
FIGURE 3.
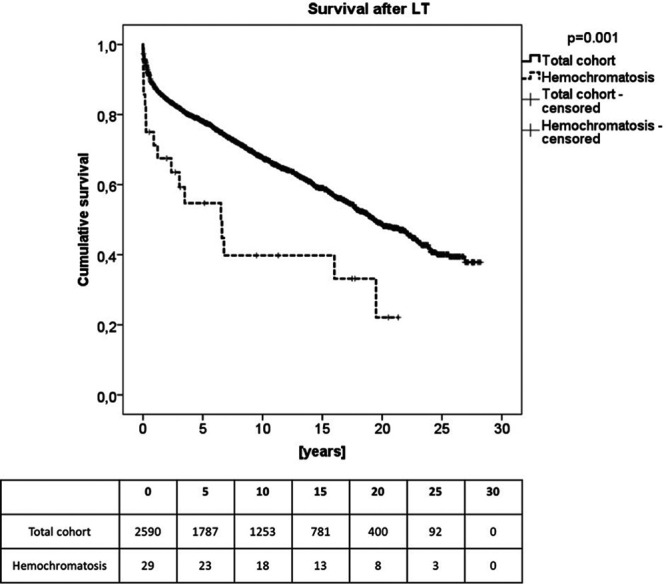
Kaplan-Mayer analysis shows the estimated overall survival of patients with hemochromatosis after liver transplantation (LT) compared with the total cohort of our center. Patients with hemochromatosis showed a significant worse survival compared with the control (N = 29).
Twenty of the 29 patients died. Seven patients (24.2%) died of infection and subsequent septic multiorgan failure. Three of these patients (10.4%) suffered from Aspergillus fumigatus infection. Four patients died (13.8%) because of cardiovascular reasons, 3 because of cardiovascular failure, and 1 of pulmonary embolism during an orthopedic operation. Three patients (10.4%) succumbed to HCC recurrence and another 6 (20.7%) other malignancies. Two patients died because of transplant failure (6.8%), one 3.4 y and the other 19.4 y after LT. One of the neonatal HC patients died of hepatic artery thrombosis that was developed in a left lateral postmortal split 3 mo after LT.
DISCUSSION
HC has an incidence of 0.1% and is a rare indication for LT.1,2 Deficient diagnosis and reduced patient survival post-LT are important reasons for restrictive LT in those patients. However, LT remains the only therapeutic option to end-stage liver failure. Therefore, long-term studies of HC patients are crucial to further our knowledge on prognosis, survival, and postoperative complications after LT. We performed a retrospective analysis of patients with HC undergoing LT to add our 30-y experience at a high-volume center to previous consisting data.
Significantly reduced postoperative survival is a major concern in this specific patient group. In our study, the 1-, 3-, 5-, and 10-y survival rates of patients undergoing LT due to HC were significantly worse when compared with the average survival expectations of the overall cohort of all LT patients at our center. Indeed, 20 of the 29 patients included in the study died within the 10-y follow-up period. These results are confirmatory to those of similar other studies.13-15 Moreover, a review article by Brandhagen10 cited data gathered from United Network for Organ Sharing, including only 1% HC patients (n = 30 000). Patient survival was significantly decreased in this patient group, with 1- and 5-y survival rates declining from 83% and 70% to 75% and 64%.10
Main causes of patient mortality are fatal infections or cardiovascular complications. Patients suffering from an iron overload have an increased susceptibility to infections. Iron alters the functionality of the immune system and serves as an essential nutrient to a lot of organisms. Thus, the assumption remains that the patients continue to be at an increased risk of infections or that this risk may be further increased by necessary immunosuppression after LT. Especially fungal infections more frequently occur in patients with iron excess (24%) compared with patients with a physiological iron balance (7%) and are the most frequent cause of death in patients with HC after LT.13 Brandhagen10 and Brandhagen et al13 reported about 29% death due to infectious diseases, 23% due to circulatory complications, and 10% due to malignant disease. In our study, 24.2% died because of infections and 10.4% particularly because of aspergillus fumigatus. Preoperative iron depletion through phlebotomy might reduce the risk of infectious complications. Furthermore, the second frequent cause of death was malignancy (31.0%) and third cardiovascular events (13.8%). Three patients died because of circulatory failure and 1 due to pulmonary embolism; both of these pathologies were also seen in other studies as common causes of mortality in LT patients with HC.16 It is important to correlate these findings with the preoperative circulatory evaluation of patients, to distinguish preexisting conditions from conditions related to the surgery. However, we lost only 2 patients (6.8%) because of transplant failure during long-term follow-up and 1 newborn because of a thrombosis of the hepatic artery (n = 1; 3.5%), being in the range of previously reported prevalence of hepatic artery thrombosis in children (up to 8.3%).17
Therefore, transplant-related death remains rare even in a group of HC patients, but they tend to pretend their higher risk for severe infectious and cardiovascular complications even after successful LT.
A reason might be a further disturbed iron metabolism, which should be evaluated and addressed by this analysis. However, the laboratory results were generally inconspicuous over the course of 10 y. Only the median serum transferrin values were below the reference range at all times, which might be a sign of possible iron overload. As serum ferritin is an acute-phase protein, its value cannot accurately estimate the iron content of the organism and may be falsified by possible hidden infections. Nevertheless, most of the patients did not show clinical signs of infections for the main period of follow-up. On the other hand, median serum iron levels within the reference range speak against significant iron overload after LT. The evaluation of liver biopsies showed that pathological abnormalities such as high-degree inflammation, fibrosis, and hemosiderosis tend to occur more frequently and most severely 3 y post-LT. These pathological abnormalities no longer appear in subsequent follow-up examinations. In total, we had 8 patients presenting coexisting hepatic pathologies such as viral hepatitis, alcohol-induced, and nonalcoholic steatohepatitis before LT. Recurrence of the underlying disease was not documented during monitoring except for 1 case with successfully treated HCV recurrence without further complications on or histopathologic changes of the graft. Without a proven relapse of alcohol abuse, weight gain, or uncontrolled recurrent viral hepatitis, the influence of those comorbidities on the histopathologic change of the graft may be low during the long-term follow-up. Former studies performed in the 1990s with few numbers of patients could not prove positive or negative hepatic iron accumulation or ferritin elevation after LT as there were contrary results and very heterogenous patients’ groups.10,16,18-24 However, the fact that only 2 of the 20 deceased patients succumbed to transplant failure speaks against a causal relationship.
It is still unknown whether the main source of pathology for HC is in the liver or in the small intestine. Considering that if the latter were the case an LT would be the ultimate cure to the disease, knowledge on iron reaccumulation in the liver and the organism post-LT is crucial. Data on this topic resulting from different studies with varying follow-up periods are controversial and strayed by different confounding factors such as the administration of perioperative blood transfusions.24,25 We performed a long-term follow-up of 10 y and detected no significant iron overload in the laboratory analysis and biopsies. Only in 2 patients, strongly elevated serum ferritin values >1000 μg/L could temporarily be measured but normalized again. Only at 3 y post-LT, moderate iron deposits were described in the biopsies of 2 patients. It should be postulated that multicenter long-term studies are necessary to evaluate this aspect more clearly, because the possibility that the follow-up was not long enough for iron to accumulate exists.
The possible limitation of the present study mainly results from the small patient cohort. However, we could show a prevalence of our transplant cohort of 1.1% after 30 y of experience, and due to the rare incidence for HC in general, higher patient numbers could only be achieved in large multicenter studies. Thus, this analysis should serve as a contribution from a high-volume center to the already existing literature, with the special feature that routine biopsies in our long-term follow-up allow an initial assessment of the histopathologic changes.
No further significant iron overload could be detected after LT in patients with HC, and even in the liver biopsies, minimal iron deposits were described in only a portion of them. Nevertheless, patients suffering from HC have a lower estimated survival than patients without iron storage disease after LT. However, the main cause of death was due to infectious diseases, followed by malignancies and cardiovascular reasons but not due to severe transplant dysfunction or failure.
ACKNOWLEDGMENTS
We would like to thank all employees of our outpatient’s department for supporting our work.
Footnotes
Published online 28 May, 2020.
The authors declare no funding or conflicts of interest.
E.M.D. participated in the research design and the writing of the paper. E.K. participated in the writing of the paper. J.N. participated in the performance of the research. W.S. participated in the writing of the paper. R.Ö. participated in the writing of the paper. J.P. participated in the research design. D.E. participated in the research design and the writing of the paper.
REFERENCES
- 1.Kim P, Weiskirchen S, Uerlings R, et al. Quantification of liver iron overload disease with laser ablation inductively coupled plasma mass spectrometry. BMC Med Imaging. 2018; 18:51.doi:10.1186/s12880-018-0291-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013; 87:183–190 [PubMed] [Google Scholar]
- 3.Wrede CE, Hutzler S, Bollheimer LC, et al. Correlation between iron status and genetic hemochromatosis (codon C282Y) in a large German population. Isr Med Assoc J. 2004; 6:30–33 [PubMed] [Google Scholar]
- 4.Feder JN, Penny DM, Irrinki A, et al. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc Natl Acad Sci U S A. 1998; 95:1472–1477. doi:10.1073/pnas.95.4.1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brissot P, Pietrangelo A, Adams PC, et al. Haemochromatosis. Nat Rev Dis Primers. 2018; 4:18016 doi:10.1038/nrdp.2018.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podzolkov VI, Pokrovskaya AE, Vargina TS, et al. Diagnostic difficulties of primary hemochromatosis in a patient with posthemorrhagic anemia. Ter Arkh. 2019; 91:118–121. doi:10.26442/00403660.2019.04.000170 [DOI] [PubMed] [Google Scholar]
- 7.Kumar V, Abbas A, Fausto N, et al. Robbins and Cotran - Pathologic Basis of Disease 2005Philadelphia, PA: Saunders; 908–911 [Google Scholar]
- 8.Oppl B, Zwerina J. [Hemochromatosis]. Z Rheumatol. 2015; 74:609–616; quiz 617. doi:10.1007/s00393-015-1583-4 [DOI] [PubMed] [Google Scholar]
- 9.Niederau C, Fischer R, Sonnenberg A, et al. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med. 1985; 313:1256–1262. doi:10.1056/NEJM198511143132004 [DOI] [PubMed] [Google Scholar]
- 10.Brandhagen DJ. Liver transplantation for hereditary hemochromatosis. Liver Transpl. 2001; 7:663–672. doi:10.1053/jlts.2001.25359 [DOI] [PubMed] [Google Scholar]
- 11.Schirmacher P, Fleig WE, Dienes HP; Deutsche Gesellschaft fur Pathologie (DGP), Deutsche Gesellschaft fur Verdauungs- und Stoffwechselkrankheiten (DGVS), Kompetenznetz Hepatitis (HepNet) Deutsche Gesellschaft fur Pathologie (DGP), Deutsche Gesellschaft fur Verdauungs- und Stoffwechselkrankheiten (DGVS), Kompetenznetz Hepatitis (HepNet). [Biopsy diagnosis of chronic hepatitis]. Z Gastroenterol. 2004; 42:175–185. doi:10.1055/s-2004-812728 [DOI] [PubMed] [Google Scholar]
- 12.Riemann J, Fischbach W, Galle P, et al. Band 2: II Leber: 8.3 Pathologien. In: Gastroenterologie in Klinik und Praxis. 2008Stuttgart, Germany: Thieme Verlag; 1206 [Google Scholar]
- 13.Brandhagen DJ, Alvarez W, Therneau TM, et al. Iron overload in cirrhosis-HFE genotypes and outcome after liver transplantation. Hepatology. 2000; 31:456–460. doi:10.1002/hep.510310227 [DOI] [PubMed] [Google Scholar]
- 14.Kowdley KV, Brandhagen DJ, Gish RG, et al. ; National Hemochromatosis Transplant Registry National Hemochromatosis Transplant Registry. Survival after liver transplantation in patients with hepatic iron overload: the National Hemochromatosis Transplant Registry. Gastroenterology. 2005; 129:494–503. doi:10.1016/j.gastro.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 15.Crawford DH, Fletcher LM, Hubscher SG, et al. Patient and graft survival after liver transplantation for hereditary hemochromatosis: implications for pathogenesis. Hepatology. 2004; 39:1655–1662. doi:10.1002/hep.20242 [DOI] [PubMed] [Google Scholar]
- 16.Pillay P, Tzoracoleftherakis E, Tzakis AG, et al. Orthotopic liver transplantation for hemochromatosis. Transplant Proc. 1991; 23:1888–1889 [PMC free article] [PubMed] [Google Scholar]
- 17.Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009; 9:746–757. doi:10.1111/j.1600-6143.2008.02541.x [DOI] [PubMed] [Google Scholar]
- 18.Poulos JE, Bacon BR. Liver transplantation for hereditary hemochromatosis. Dig Dis. 1996; 14:316–322. doi:10.1159/000171562 [DOI] [PubMed] [Google Scholar]
- 19.Dietze O, Vogel W, Braunsperger B, et al. Liver transplantation in idiopathic hemochromatosis. Transplant Proc. 1990; 22:1512–1513 [PubMed] [Google Scholar]
- 20.Dabkowski PL, Angus PW, Smallwood RA, et al. Site of principal metabolic defect in idiopathic haemochromatosis: insights from transplantation of an affected organ. BMJ. 1993; 306:1726 doi:10.1136/bmj.306.6894.1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams PC, Ghent CN, Grant DR, et al. Transplantation of a donor liver with haemochromatosis: evidence against an inherited intrahepatic defect. Gut. 1991; 32:1082–1083. doi:10.1136/gut.32.9.1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams PC, Jeffrey G, Alanen K, et al. Transplantation of haemochromatosis liver and intestine into a normal recipient. Gut. 1999; 45:783 doi:10.1136/gut.45.5.783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koskinas J, Portmann B, Lombard M, et al. Persistent iron overload 4 years after inadvertent transplantation of a haemochromatotic liver in a patient with primary biliary cirrhosis. J Hepatol. 1992; 16:351–354. doi:10.1016/s0168-8278(05)80668-3 [DOI] [PubMed] [Google Scholar]
- 24.Powell LW. Does transplantation of the liver cure genetic hemochromatosis? J Hepatol. 1992; 16:259–261. doi:10.1016/S0168-8278(05)80654-3 [DOI] [PubMed] [Google Scholar]
- 25.Farrell FJ, Nguyen M, Woodley S, et al. Outcome of liver transplantation in patients with hemochromatosis. Hepatology. 1994; 20:404–410 [PubMed] [Google Scholar]


