Abstract
Alzheimer Disease (AD) is a pathology suffered by millions of people worldwide and it has a great social and economic impact. Previous studies reported a relationship between alterations in different amino acids and derivatives involved in neurotransmission systems and cognitive impairment. Therefore, in this study the neurotransmission impairment associated to early AD has been evaluated. For this purpose, different amino acids and derivatives were determined in saliva samples from AD patients and healthy subjects, by means of an analytical method based on chromatography coupled to tandem mass spectrometry. Results showed statistically significant differences in salivary levels for the compounds myo-inositol, creatine and acetylcholine; and other compounds (myo-inositol, glutamine, creatine, acetylcholine) showed significant correlations with some cognitive tests scores. Therefore, these compounds were included in a multivariate analysis and the corresponding diagnosis model showed promising indices (AUC 0.806, sensitivity 61%, specificity 92%). In conclusion, some amino acids and derivatives involved in neurotransmission impairment could be potential biomarkers in early and non-invasive AD detection.
Subject terms: Neurochemistry, Biochemistry, Biomarkers, Diseases, Alzheimer's disease
Introduction
Alzheimer Disease (AD) is a pathology suffered by millions of people worldwide and the increasing number of cases in recent years generates a great concern for the economic and social effects produced1,2. In addition to this, there is a concern about the still little knowledge about the mechanisms involved in the onset and development of the disease, besides there is a lack of early diagnostic methods, as well as effective treatments to fight against the disease3–5.
AD is a long neurodegenerative disease that produces different anatomical and physiological changes not only in brain but also in periphery6–9. In the course of the disease, some peptides aggregates appear in the brain, they are i) amyloid plaques, generated by the accumulation of β-amyloid peptide10 and ii) neurofibrillary tangles, generated by tau hyperphosphorylation or acetylation11,12. Physiologically, a reduction of synapsis occurs13, and neurotransmission is altered. In this sense, the study of different compounds involved in neurotransmission systems could be useful14. Previous studies have reported relationship between different neurotransmitters and AD pathology15, most of them used plasma or CSF samples16–18, while only a few used non-invasive samples, such as urine from murine animal models19,20.
Specifically, acetylcholine (Ach) is the most studied neurotransmitter in AD. In fact, its impairment constitutes one of the most studied therapeutic targets21. In addition, myo-inositol has been evaluated in AD brain by means of Magnetic Resonance Spectroscopy (MRS)22. Moreover, Kuzyk et al. found creatine accumulations in AD mouse model23. In addition, CSF glutamate and glutamine were found elevated in probable AD patients24. Definitely, different amino acids such as glutamate, serine and alanine seem to play an important role in AD cognition decline25. However, this is the first study that evaluate the salivary levels of different related-neurotransmission compounds in AD patients. In general, saliva sampling and processing involve some preanalytical variables that should be taken into account to obtain reproducible results among studies26.
The aim of this study is to evaluate some amino acids and derivatives related to neurotransmission alterations under AD conditions as potential biomarkers for early and non-invasive AD detection.
Material and methods
Participants selection and samples collection
The study participants were patients from the Neurology Service in the University and Polytechnic Hospital La Fe (Valencia, Spain) who previously signed the informed consent. The experimental protocol for the study was approved by the Ethics Committee (CEIC) of the Health Research Institute La Fe (Valencia, Spain), and it is in accordance with the appropriate guidelines (Declaration of Helsinki).
Participants were classified into AD (including mild cognitive impairment (MCI) due to AD (n = 17) and mild to moderate dementia due to AD (n = 14) and healthy control (HC, (n = 12)) groups. For this, they were subjected to neuropsychological tests (Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), Clinical Dementia Rating (CDR), Minimental State Examination (MMSE), Functional Activities Questionnaire (FAQ))27–30, structural neuroimaging by means of nuclear magnetic resonance (NMR) or computerized axial tomography (CAT)31, and cerebrospinal fluid (CSF) biomarkers (β-amyloid peptide (Aβ), total Tau (t-Tau), phosphorylated Tau (p-Tau))32,33. Specifically, in the AD group, the MCI-AD participants showed cognitive complaints without daily living activities impairment, while mild dementia-AD participants showed minor daily living activities impairment. This classification was carried out following the National Institute on Aging-Alzheimer’s Association (NIA-AA) recommendations34–36. Also, all AD participants showed positive levels of CSF biomarkers (Aβ < 700 pg mL-1, t-Tau > 380 pg mL-1, p-Tau > 70 pg mL-1) and altered neuropsychological evaluation (CDR > 0, RBANS.DM < 65, MMSE < 27). However, the HC group was characterized by negative CSF biomarkers, and normal neuropsychological evaluation. Regarding exclusion criteria, patients with a history of structural brain disease (tumour, stroke, etc.), major head trauma, epilepsy, multiple sclerosis and major psychiatric disorders were excluded, as well as patients not able to undergo neuropsychological evaluations.
Saliva samples were obtained from all the participants. They were whole-mouth saliva and collected by spitting into sterile bottles (without additives) between 10 and 12 a.m. (minimum 30 min after breakfast). Participants rinsed their mouth before saliva collection, between 1–2 mL could be collected across subjects with minor differences between control and case subjects. Then, the samples were aliquoted into 2 mL tubes, and those with visible blood contamination were excluded from the study. Finally, samples were stored at -80ºC until the analysis.
Standards
Glutamate, glutamine, γ-aminobutyric (GABA), taurine (Taur), aspartic acid, myo-inositol (MI), N-Acetyl-L-aspartic acid (NAA), aspartic acid (AA), creatine (Cr), acetylcholine (Ach) and acetonitrile were obtained from Sigma-Aldrich (St. Louis, MO, USA). Deuterated phenylalanine (Phe-D5) with a 98% atom D enrichment was purchased from CDN Isotopes (Pointe-Claire, Canada).
Sample treatment
The saliva sample (150 µL) was added to 300 µl of acetonitrile and centrifuged at 1200 g, 5 min at 4ºC. Then, 5µL of the internal standard (IS) solution (Phe-D5, 10 mmol L-1) were added to 95 µL of supernatant. The IS is an amino acid added as corrector of injection volume and ionization procedure. The range of calibration to quantify the analytes in samples was 0.3–5000 µmol L-1. Finally, the samples were injected in the Ultra Performance Liquid Chromatography–tandem Mass Spectrometry (UPLC-MS/MS) system. Simultaneously, salivary total proteins were determined in each sample (5 µL) using a colorimetric protein assay kit (Pierce BCA) to standardize the analytes concentrations results. In general, these concentrations vary depending on saliva flow. So, the use of total proteins as corrective index approximates salivary flow rate adjustment37,38.
UPLC-MS/MS analysis
The chromatographic system was a Waters Acquity UPLC-XevoTQ system (Milford, MA, USA) with triple quadrupole as mass analyzer. The instrumental conditions were positive electrospray ionization (ESI), capillary voltage 3.50 kV, extractor 5.00 V, source temperature 120 °C, desolvation temperature 350 °C, nitrogen cone flow 50 L/h and desolvation gas flow 750 L/h.
Separation conditions were selected to achieve appropriate chromatographic retention and resolution by using an HILIC column (100 × 2.1 mm, 7 µm, 100 Å) from Phenomenex. Mobile phase used was CH3OH (5 mmol L-1 NH4HCO2): H2O (5 mmol L-1 NH4HCO2), (70:30) with isocratic gradient 10 min. The flow rate, column temperature and injection volume were set at 0.4 mL/min, 30°, and 5 µL, respectively.
During batch analysis, samples were kept at 4 °C. Mass spectrometric detection was carried out by multiple reaction monitoring (MRM) (see Table 1) (all 10 compounds analysed in a single MRM run).
Table 1.
MS/MS acquisition parameters.
| Analyte | Parent ion (M/Z) | Cone (V) | Daughter ion (M/Z) | Confirmation ion | Collision energy (Ev) |
|---|---|---|---|---|---|
| Glutamate | 148 | 20 | 84 | 102 | 15 |
| Glutamine | 147 | 10 | 84 | 130 | 10 |
| GABA | 104 | 20 | 69 | 87 | 10 |
| Aspartic acid | 134 | 20 | 74 | 88 | 10 |
| Acetylcholine | 146 | 20 | 87 | – | 10 |
| Creatine | 132 | 20 | 90 | – | 10 |
| NAA | 176 | 15 | 134 | 158 | 10 |
| Myo-inositol | 179 | 20 | 161 | – | 10 |
| Taurine | 126 | 15 | 108 | – | 10 |
| Phe-D5 | 171.1 | 35 | 125 | – | 10 |
NAA: N-Acetyl-L-aspartic acid.
The data were acquired and processed using the MassLynx 4.1 and QuanLynx 4.1 (Waters) software, respectively. Peak area integration was used, and analytes responses were expressed as ratios to the Phe-D5 (IS) in all standards and samples.
Method validation
The validation procedure consisted of the assessment of some analytical characteristics (linearity, precision, accuracy, limit of detection (LOD), limit of quantification (LOQ), stability). The individual standards were prepared in H2O, and the calibration standards in CH3CN:H2O (50:50, v/v). Standards were run in triplicate. The linearity was evaluated constructing a calibration curve (0.1–5000 nmol L-1) for each analyte (n = 9). The precision was estimated from standards at mid concentration level (1000 nmol L-1 for each analyte) within one validation batch (intra-day) and among validation batches (inter-day, along 1 month) (by triplicate). The accuracy was evaluated by means of the recovery test. For that, saliva samples were spiked at three concentration levels (low, mid, high), and they were analyzed each of the three validation days. The LODs and LOQs were established, as the concentrations generating a signal-to-noise ratio of 3and 10, respectively. Analytes stability after three freeze–thaw cycles was assessed by means of a spiked saliva sample (1000 nmol L-1, each analyte).
Statistical analysis
Univariant analysis was carried out using SPSS software version 20.0 (SPSS, Inc., Chicago, IL, USA), and for the multivariant analysis Unscrambler software v7.6 (Norway) was used. Differences between AD and healthy control participants were evaluated by means of t-test for numerical variables with normal distribution (Kolmogorov–Smirnov test), expressing results as mean ± standard deviation. While, Mann–Whitney and Chi-square non-parametric tests were used for numerical and categorical variables without normal distribution, expressing results as median and interquartile range (IQR). In addition, correlations between salivary analytes and standard neuropsychological scales, as well as CSF biomarkers were analysed by Pearson Correlation. In all the cases, statistical significance was fixed in a p value of 0.05.
Multivariate analysis was carried out by means of Partial Least Square (PLS) using some dependent variables (metabolites) and one independent variable (participant’s group). Then, the Receiver operating characteristic curve (ROC) of the model was obtained.
Results
Analytical performance data
The analytical method was validated by employing standards containing all the analytes in the 0.1–5000 nmol L-1 concentrations range. Results are summarized in Supplementary Material (Table S1). The method provided an adequate linearity for all the analytes (R2 between 0.996 and 0.999), and satisfactory sensitivity (LODs between 0.09 and 1.4 nmol L-1). Also, suitable precision was obtained with intra-day and inter-day coefficients of variation of 0.8–3.2% (n = 3) and 2.9–10.2% (n = 6), respectively (at a concentration of 1000 nmol L-1). The accuracy of the method was evaluated by analysing spiked saliva samples containing analytes at different concentrations (low, medium and high) within the tested concentration ranges. The results obtained are listed at Table S2 (Supplementary Material), quantitative recoveries were achieved for all the analytes. The analytes stability was assayed after three freeze thaw cycles, determining the concentrations in spiked saliva samples (1000 nmol L-1, each analyte) by triplicate. The recoveries were between 88 and 106%. So, no significant deterioration of the analytes was observed.
Demographic and clinic participants description
Participants’ demographic and clinical data are shown in Table 2. As it is expected, statistically significant differences were observed between participants groups for neuropsychological variables (RBANS, CDR, FAQ, MMSE) and CSF biomarkers (β-amyloid, Tau, p-Tau).
Table 2.
Demographic and clinical variables of the study participants.
| HC (n = 12) | AD (n = 31) | p Value | |
|---|---|---|---|
| Age (years (median, IQR)) | 69 (60, 70) | 69 (67, 74) | 0.072 |
| Gender (female, n (%)) | 4 (33%) | 18 (58%) | 0.146 |
| CSF β-Amyloid (pg mL-1) (median (IQR)) | 1178 (1031, 1414) | 552 (465, 678) | < 0.001* |
| CSF total Tau (pg mL-1) (median (IQR)) | 274 (167, 368) | 641 (387, 1043) | 0.002* |
| CSF phosphorylated Tau (pg mL-1) (median (IQR)) | 44 (36, 57) | 101 (71, 146) | < 0.001* |
| CDR (median, IQR) | 0 (0, 0) | 0.5 (0.5, 1) | < 0.001* |
| MMSE (median, IQR) | 30 (28, 30) | 24 (18, 26) | < 0.001* |
| RBANS.MI (median, IQR) | 86 (81, 95) | 53 (40, 73) | < 0.001* |
| RBANS.VC (median, IQR) | 201 (81, 116) | 72 (56, 89) | 0.001* |
| RBANS.L (median, IQR) | 92 (83, 95) | 60 (51, 82) | < 0.001* |
| RBANS.A (median, IQR) | 97 (84, 100) | 60 (56, 79) | < 0.001* |
| RBANS.DM (median, IQR) | 98 (89, 105) | 48 (40, 60) | < 0.001* |
| FAQ (median, IQR) | 0 (0, 1) | 6 (1, 13) | < 0.001* |
*p < 0.05.
IQR: Interquartile range, CDR, clinical dementia rating, RBANS: repeatable battery for the assessment of neuropsychological status (immediate memory [RBANS.IM], visuospatial/constructional [RBANS.V/C], language [RBANS.L], attention [RBANS.A], delayed memory [RBANS.DM]). FAQ: Functional Activities Questionnaire.
Amino acids and derivatives impairment in Alzheimer Disease
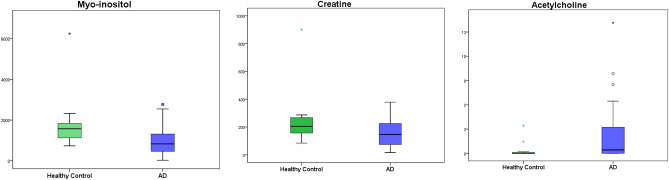
Different amino acids and derivatives (aspartic acid, glutamic acid, glutamine, GABA, creatine, taurine, N-acetil aspartate, myo-inositol, acetylcholine) were determined in saliva samples from AD patients at early stages of the pathology (MCI and mild dementia), and from HC participants (Table 3). As result, salivary myo-inositol (p = 0.018) and creatine (p = 0.049) showed lower levels in AD compared to control group, while acetylcholine showed higher levels in AD patients (p = 0.015) (Fig. 1). Not statistically significant differences were found for the other compounds. Also, the compounds levels were analysed in function of gender, observing that only glutamine showed statistically significant differences (p = 0.031).
Table 3.
Concentrations of neurotransmitters in saliva samples.
| Healthy control (n = 12)a | AD (n = 31)a | p Value | |
|---|---|---|---|
| Taurine (ng mg protein -1) | 3453.71 (1248.69) | 3834.07 (2409.42) | 0.607 |
| NAA (ng mg protein -1) | 95.38 (60.86) | 80.70 (64.73) | 0.502 |
| Myo-inositol (ng mg protein -1) | 1867.83 (1451.67) | 1027.38 (776.03) | 0.018* |
| Aspartic Acid (ng mg protein -1) | 124.18 (77.57) | 169.38 (138.75) | 0.186 |
| Glutamic Acid (ng mg protein -1) | 953.14 (392.68) | 1349.75 (1072.05) | 0.083 |
| Glutamine (ng mg protein -1) | 1525.73 (1518.57) | 3582.30 (3544.51) | 0.060 |
| Creatine (ng mg protein -1) | 255.17 (213.58) | 157.47 (103.85) | 0.049* |
| GABA (ng mg protein -1) | 8.94 (4.91) | 12.41 (18.30) | 0.524 |
| Acetilcholine (ng mg protein -1) | 0.02 (0.03) | 1.91 (3.13) | 0.015* |
*p < 0.05.
aMean (standard deviation).
Figure 1.
Box plot representing the myo-inositol creatine and acetylcholine salivary levels found for each participant group.
After that, some correlations were observed between some salivary analytes and standard neuropsychological scales in AD. Specifically, delayed memory domain from RBANS scale (RBANS.DM) correlated with myo-inositol (PCC = 0.327, p = 0.032) and acetylcholine levels (PCC = -0.304, p = 0.047); as well as MMSE score correlated with myo-inositol (PCC = 0.437, p = 0.003), glutamine (PCC = -0.337, p = 0.027) and creatine (PCC = 0.342, p = 0.025) levels (see Figure S1 in Supplementary Material). Moreover, we found that salivary myo-inositol levels correlated with CSF β-amyloid (PCC = 0.351, p = 0.028).
Then, different diagnosis models were developed to compare individual accuracy of the selected compounds, as they showed statistically significant differences between AD and HC groups, as well as correlations with some relevant clinical variables used in AD diagnosis. Results indicated that acetylcholine (AUC-ROC 0.660 (CI 95%, 0.492–0.828) and glutamine (AUC-ROC 0.777 (CI 95%, 0.619–0.935) showed satisfactory accuracy; while creatine (AUC-ROC 0.331 (CI 95%, 0.167–0.494) and myo-inositol (AUC-ROC 0.261 (CI 95%, 0.113–0.408) showed poor accuracy. Finally, a multivariate model was performed including all these compounds (myo-inositol, creatine, glutamine, acetylcholine) (p ≤ 0.06).
Multivariate analysis.
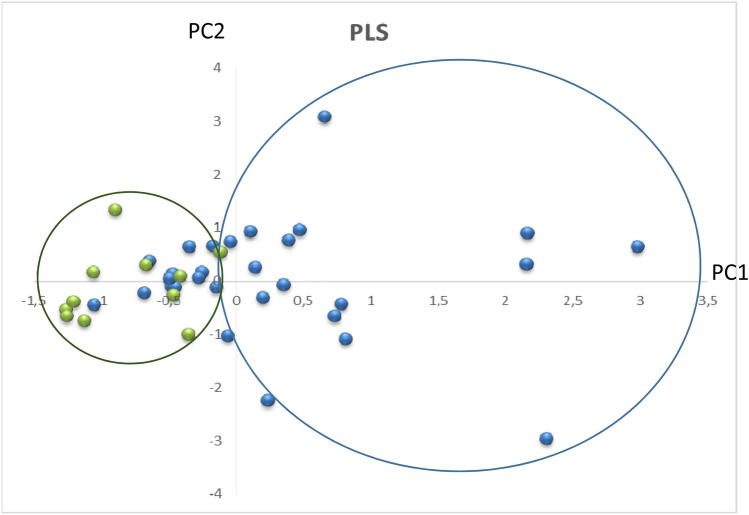
Different combinations of the previously selected compounds (myo-inositol, creatine, glutamine, acetylcholine) were used to carry out multivariate analysis. In general, the developed models showed AUC-ROC values between 0.382 and 0.806 (see Table 4). However, model 1 (myo-inositol, creatine, glutamine, acetylcholine) showed best performance. The results from this multivariate analysis model are depicted in Fig. 2. As can be seen the salivary levels of myo-inositol, creatine, glutamine and acetylcholine could discriminate between AD and HC groups, except for a 30% of doubtful cases. Most of these not clearly classified cases showed the lowest CDR scores (0–0.5). The model showed an AUC-ROC of 0.806 (CI 95%, 0.674–0.939). Regarding the predictive power of this model it is important to highlight the specificity of 92%, while the sensitivity was 61%.
Table 4.
Diagnosis indices for the developed multivariate analysis.
| Model | Index (CI 95%) | ||||||
|---|---|---|---|---|---|---|---|
| AUC-ROC | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Positive odds ratio | Negative odds ratio | |
| 1 | 0.806 (0.674, 0.939) | 61.3 (42.3, 77.6) | 91.7 (59.7, 99.6) | 95.0 (73.1, 99.7) | 47.8 (27.4, 68.9) | 7.35 (1.10, 49.04) | 0.42 (0.26, 0.68) |
| 2 | 0.632 (0.445, 0.818) | 69.7 (52.7, 82.6) | 75.0 (46.8, 91.1) | 88.5 (71.0, 96.0) | 47.4 (27.3, 68.3) | 2.79 (1.02, 7.62) | 0.40 (0.23, 0.71) |
| 3 | 0.602 (0.405, 0.800) | 73.3 (55.6, 85.8) | 66.7 (39.1, 86.2) | 84.6 (66.5, 93.9) | 50.0 (28.0, 72.0) | 2.20 (0.96, 5.04) | 0.40 (0.21, 0.77) |
| 4 | 0.386 (0.221, 0.551) | 32.3 (18.6, 49.9) | 83.3 (55.2, 95.3) | 83.3 (55.2, 95.3) | 32.3 (18.9, 49.9) | 1.94 (0.49, 7.57) | 0.81 (0.57, 1.15) |
| 5 | 0.767 (0.606, 0.929) | 83.9 (67.4, 92.9) | 66.7 (39.1, 86.2) | 86.7 (70.3, 94.7) | 61.5 (35.5, 82.3) | 2.52 (1.11, 5.68) | 0.24 (0.10, 0.56) |
| 6 | 0.762 (0.604, 0.920) | 71.0 (53.4, 83.9) | 75.0 (46.8, 91.1) | 88.0 (70.0, 95.8) | 50.0 (29.0, 71.0) | 2.84 (1.04, 7.76) | 0.39 (0.21, 0.70) |
| 7 | 0.571 (0.386, 0.756) | 25.8 (13.7, 43.2) | 91.7 (64.6, 98.5) | 88.9 (56.5, 98.0) | 32.4 (19.1, 49.2) | 3.10 (0.43, 22.19) | 0.81 (0.61, 1.08) |
| 8 | 0.676 (0.489, 0.863) | 80.6 (63.7, 90.8) | 58.3 (32.0, 80.7) | 83.3 (66.4, 92.7) | 53.8 (29.1, 76.8) | 1.94 (0.97, 3.86) | 0.33 (0.15, 0.72) |
| 9 | 0.548 (0.357, 0.740) | 29.0 (16.1, 46.6) | 91.7 (64.6, 98.5) | 90.0 (59.6, 98.2) | 33.3 (19.8, 50.4) | 3.48 (0.49, 24.62) | 0.77 (0.58, 1.04) |
| 10 | 0.692 (0.515, 0.869) | 58.1 (40.8, 73.6) | 75.0 (46.8, 91.1) | 85.7 (65.4, 95.0) | 40.9 (23.3, 61.3) | 2.32 (0.83, 6.47) | 0.56 (0.35, 0.91) |
| 11 | 0.738 (0.583, 0.892) | 58.1 (40.8, 73.6) | 91.7 (64.6, 98.5) | 94.7 (75.4, 99.1) | 45.8 (27.9, 64.9) | 6.97 (1.04, 46.60) | 0.46 (0.30, 0.71) |
(Model 1) Myo-inositol, glutamine, creatine, acetylcholine; (Model 2) Myo-inositol, creatine; (Model 3) Myo-inositol, glutamine; (Model 4) Myo-inositol, acetylcholine; (Model 5) Glutamine, creatine; (Model 6) Glutamine, acetylcholine; (Model 7) Creatine, acetylcholine; (Model 8) Myo-inositol, creatine, glutamine; (Model 9) Myo-inositol, creatine, acetylcholine; (Model 10) Myo-inositol, glutamine, acetylcholine; (Model 11) Glutamine, creatine, acetylcholine.
CI: confidence interval, AUC-ROC: Area Under Curve- Receiver Operating Curve.
Figure 2.
PLS score plot represents differential distribution between AD and healthy control groups. As visual approach, blue circle englobes AD patients, green circle englobes healthy and some AD participants. PC: principal component.
Discussion
Saliva sample has the advantage of simple collection, being convenient and acceptable for all patients, and achieving high participant recruitment. In addition, several studies have found that saliva levels were comparable to blood levels, adding some clinical value to these salivary determinations39. The salivary levels of neurotransmitters were evaluated from two different approaches. First, univariate analysis showed significant differences between AD and HC participants for some compounds (myo-inositol, creatine, acetylcholine), as well as, correlations with clinical AD variables (neuropsychological scales, CSF biomarkers) for other compounds (myo-inositol, glutamine, creatine). Second, multivariate analysis, including the previously selected compounds (myo-inositol, glutamine, creatine, acetylcholine), showed satisfactory accuracy discriminating between AD and healthy control individuals.
Regarding univariate analysis, glutamine showed negative correlation with MMSE score. Similarly, previous studies showed increased CSF levels of glutamate and glutamine in patients with probable AD24. In addition, the glutamate/glutamine ratio showed a reduction in ageing and AD40. It could be explained by the glutamine neuroprotector effect observed in cell culture, protecting against amyloid-β peptide41. Also, in the present study myo-inositol levels were lower in AD patients. Specifically, salivary myo-inositol showed lower levels in saliva samples from patients suffering from important cognitive impairment, while higher salivary levels corresponded to higher neuropsychological tests scores (RBANS, MMSE). In this sense, Shinno et al. found lower levels of myo-inositol in brain, specifically in the anterior cingulate gyros, and it could be associated with AD development42. In contrast, a previous study found that urinary myo-inositol excretion could be used as cognitive impairment biomarker43. In definitive, myo-inositol levels could be impaired under AD conditions, making difficult the transport of substances between brain and other biofluids44. In addition, salivary myo-inositol showed correlation with CSF β-amyloid levels, considered the gold standard in AD diagnosis. Similarly, previous studies showed that brain myo-inositol measured by magnetic resonance spectroscopy correlated with β-amyloid even before the cognitive impairment appearance45. In general, these findings could constitute a relevant aspect in non-invasive diagnosis development, since these amino acids and derivates measured in non-invasive samples (saliva, urine) could replace CSF sampling in AD current diagnosis. Moreover, other inositol stereoisomers, such as scyllo-inositol, had been tested as potential therapy because of its anti-oligomer activity in cell culture46. In this sense, an increase in myo-inositol levels could be protective against amyloid-β plaques formation and so AD development.
For acetylcholine, the present study showed higher levels in patients with lower scores for RBANS and higher scores for CDR and FAQ. Of note, CDR and FAQ lower scores show better cognitive status, while higher punctuations in RBANS show better cognitive state. Therefore, high salivary acetylcholine levels corresponded to patients with cognitive impairment. Nevertheless, opposite results were obtained in a previous study carried out in plasma samples47.
For creatine, lower salivary levels were obtained in AD patients, as well as positive correlation with MMSE. In this sense, a few studies in literature have focused on this metabolite as neurodegeneration biomarker48,49.
Regarding multivariate analysis, the studied neurotransmitters could be useful as AD biomarkers. Specifically, the simultaneous determination of a panel of 4 neurotransmitters (myo-inositol, acetylcholine, creatine, glutamine) could improve the accuracy differentiating between AD and healthy participants. In general, these results would explain an alteration in neurotransmission under AD conditions. A previous study in AD showed an imbalance in neurotransmitters levels in brain50. In addition, neurotransmitters’ transporters showed alterations in the pathology51. Therefore, neurotransmitters were accumulated or reduced in brain and different biofluids depending on their transport, and an alteration in their distribution could lead to the typical manifestations of AD. In this sense, a previous AD study determining glutamate in CSF samples showed sensitivity 95.2%, specificity 100%, and AUC = 0.9924. Also, a study determining 10 amino acids as potential blood biomarkers in AD showed an AUC = 0.94852. Despite obtaining satisfactory diagnosis indexes, these works used invasive samples. Nevertheless, the present study could allow a first screening approach in early AD diagnosis, using non-invasive samples. In fact, this new tool, showing high positive predictive value, could reduce invasive and expensive diagnostic methods (CSF biomarkers, neuroimaging techniques) only to doubtful cases, avoiding conventional diagnosis methods in positive screening cases.
Regarding specificity, some studies found neurotransmitter impairment in other dementias (Parkinson disease, vascular dementia, dementia with Lewy bodies, fronto-temporal dementia)53,54, or pathologies55. Nevertheless, there is a lack of studies focusing on differential AD diagnosis based on neurotransmitters.
Among the study limitations, it is important to highlight the small sample size and so the reduced statistical power. Also, a single internal standard has been used to correct 10 different analytes which could impact in quantitative accuracy. In addition, some saliva collection variables have not been standardised, and potential biomarkers specificity has not been evaluated.
Conclusions
Some neurotransmitters levels in saliva could be impaired under AD conditions. Among the evaluated compounds, it is important to highlight myo-inositol, acetylcholine, creatine and glutamine, since these compounds correlated with cognitive impairment associated to AD, and also myo-inositol showed correlation with CSF amyloid-β levels. These neurotransmitters could be used as promising non-invasive biomarkers for AD. From them, an optimum multivariate model was developed constituting a potential tool in the early screening of AD patients and reducing invasive and expensive diagnostic methods, which would be applied to doubtful cases. Nevertheless, further work is required, increasing sample size to clinically validate these preliminary results, as well as to evaluate their specificity.
Supplementary information
Acknowledgements
CC-P acknowledges a post-doctoral “Miguel Servet I” Grant (CP16/00082) from the Health Research Institute Carlos III (Spanish Ministry of Economy and Competitiveness). CP-B acknowledges a pre-doctoral Grant (associated to “Miguel Servet” project CP16/00082) from the Health Research Institute Carlos III (Spanish Ministry of Economy, Industry and Competitiveness).
Author contributions
C.C.-P. and M.B. designed the study. C.P-B, and I.T-C. carried out the experimental and samples analysis. I.F. and L.G. carried out the neuropsychological tests. C.P-B. and C.C-P. wrote the main manuscript text. M.B. and M.V. supervised the clinical issues. All authors reviewed the manuscript.
Funding
This work was supported by the Instituto de Salud Carlos III (Miguel Servet I Project (CP16/00082) (Spanish Ministry of Economy and Competitiveness).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-73362-z.
References
- 1.Fiest, K. M. et al. The prevalence and incidence of dementia due to Alzheimer’s disease: a systematic review and meta-analysis. Can. J. Neurol. Sci. / J. Can. des Sci. Neurol.43, S51–S82 (2016). [DOI] [PubMed]
- 2.Marešová P, Mohelská H, Dolejš J, Kuča K. Socio-economic aspects of Alzheimer’s disease. Curr. Alzheimer Res. 2015;12:903–911. doi: 10.2174/156720501209151019111448. [DOI] [PubMed] [Google Scholar]
- 3.Niu H, Álvarez-Álvarez I, Guillén-Grima F, Aguinaga-Ontoso I. Prevalencia e incidencia de la enfermedad de Alzheimer en Europa: metaanálisis. Neurología. 2017;32:523–532. doi: 10.1016/j.nrl.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Epperly T, Dunay MA, Boice JL. Alzheimer disease: pharmacologic and nonpharmacologic therapies for cognitive and functional symptoms. Am. Fam. Physician. 2017;95:771–778. [PubMed] [Google Scholar]
- 5.Alberici A, Benussi A, Premi E, Borroni B, Padovani A. Clinical, genetic, and neuroimaging features of early onset Alzheimer disease: the challenges of diagnosis and treatment. Curr. Alzheimer Res. 2014;11:909–917. doi: 10.2174/1567205011666141107151606. [DOI] [PubMed] [Google Scholar]
- 6.Ashford JW, Furst AJ. Advancing brain imaging for Alzheimer’s disease: integrating anatomic and physiologic measures. J. Alzheimer’s Dis. 2014;41:759–763. doi: 10.3233/JAD-141100. [DOI] [PubMed] [Google Scholar]
- 7.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011;1:a006189–a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tramutola A, et al. Protein nitration profile of CD3 + lymphocytes from Alzheimer disease patients: novel hints on immunosenescence and biomarker detection. Free Radic. Biol. Med. 2018;129:430–439. doi: 10.1016/j.freeradbiomed.2018.10.414. [DOI] [PubMed] [Google Scholar]
- 9.Wang, J., J Glu, B., L Masters, C. & Wang, Y.-J. A Systemic view of alzheimer disease - insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol.13, 703 (2017). [DOI] [PubMed]
- 10.Barage SH, Sonawane KD. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides. 2015;52:1–18. doi: 10.1016/j.npep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Carlomagno Y, et al. An acetylation–phosphorylation switch that regulates tau aggregation propensity and function. J. Biol. Chem. 2017;292:15277–15286. doi: 10.1074/jbc.M117.794602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar P, Jha NK, Jha SK, Ramani K, Ambasta RK. Tau phosphorylation, molecular chaperones, and ubiquitin E3 ligase: clinical relevance in Alzheimer’s disease. J. Alzheimer’s Dis. 2014;43:341–361. doi: 10.3233/JAD-140933. [DOI] [PubMed] [Google Scholar]
- 13.Sheng M, Sabatini BL, Sudhof TC. Synapses and Alzheimer’s disease. Cold Spring Harb. Perspect. Biol. 2012;4:a005777–a005777. doi: 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin C-H, Huang Y-J, Lin C-J, Lane H-Y, Tsai GE. NMDA neurotransmission dysfunction in mild cognitive impairment and Alzheimer’s disease. Curr. Pharm. Des. 2014;20:5169–5179. doi: 10.2174/1381612819666140110115603. [DOI] [PubMed] [Google Scholar]
- 15.Kandimalla R, Reddy PH. Therapeutics of neurotransmitters in Alzheimer’s disease. J. Alzheimer’s Dis. 2017;57:1049–1069. doi: 10.3233/JAD-161118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strac DS, Muck-Seler D, Pivac N. Neurotransmitter measures in the cerebrospinal fluid of patients with Alzheimer’s disease: a review. Psychiatr. Danub. 2015;27:14–24. [PubMed] [Google Scholar]
- 17.van der Cammen TJM, Tiemeier H, Engelhart MJ, Fekkes D. Abnormal neurotransmitter metabolite levels in Alzheimer patients with a delirium. Int. J. Geriatr. Psychiatry. 2006;21:838–843. doi: 10.1002/gps.1569. [DOI] [PubMed] [Google Scholar]
- 18.Trushina E, Dutta T, Persson X-MT, Mielke MM, Petersen RC. Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer’s disease using metabolomics. PLoS ONE. 2013;8:e63644. doi: 10.1371/journal.pone.0063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X-E, et al. Analysis of amino acid and monoamine neurotransmitters and their metabolites in rat urine of Alzheimer’s disease using in situ ultrasound-assisted derivatization dispersive liquid-liquid microextraction with UHPLC–MS/MS. J. Pharm. Biomed. Anal. 2017;135:186–198. doi: 10.1016/j.jpba.2016.11.056. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, et al. Study on urine metabolic profile of Aβ25–35-induced Alzheimer’s disease using UHPLC-Q-TOF-MS. Neuroscience. 2018;394:30–43. doi: 10.1016/j.neuroscience.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: targeting the cholinergic system. Curr. Neuropharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oeltzschner G, et al. Neurometabolites and associations with cognitive deficits in mild cognitive impairment: a magnetic resonance spectroscopy study at 7 Tesla. Neurobiol. Aging. 2019;73:211–218. doi: 10.1016/j.neurobiolaging.2018.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuzyk A, et al. Association among amyloid plaque, lipid, and creatine in hippocampus of TgCRND8 mouse model for alzheimer disease. J. Biol. Chem. 2010;285:31202–31207. doi: 10.1074/jbc.M110.142174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madeira, C. et al. Elevated glutamate and glutamine levels in the cerebrospinal fluid of patients with probable Alzheimer’s disease and depression. Front. Psychiatry9, (2018). [DOI] [PMC free article] [PubMed]
- 25.Lin C-H, Yang H-T, Lane H-Y. D-glutamate, D-serine, and D-alanine differ in their roles in cognitive decline in patients with Alzheimer’s disease or mild cognitive impairment. Pharmacol. Biochem. Behav. 2019;185:172760. doi: 10.1016/j.pbb.2019.172760. [DOI] [PubMed] [Google Scholar]
- 26.Gleerup, H. S., Hasselbalch, S. G. & Simonsen, A. H. Biomarkers for Alzheimer’s disease in saliva: a systematic review. Dis. Markers2019, (2019). [DOI] [PMC free article] [PubMed]
- 27.Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J. Gerontol. 1982 doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 29.Hughes CP, Berg L, Danziger WL, Coben LA, M. R. A new clinical scale for the staging of dementia. Br. J. Psychiatry140, 566–572 (1982). [DOI] [PubMed]
- 30.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Frisoni GB, Fox NC, Jack CR, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anoop A, Singh PK, Jacob RS, Maji SK. CSF biomarkers for Alzheimer’s disease diagnosis. Int. J. Alzheimers. Dis. 2010;2010:1–12. doi: 10.4061/2010/606802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blennow K, et al. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimer’s Dement. 2015;11:58–69. doi: 10.1016/j.jalz.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKhann GM, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albert MS, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jack CR, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh NP, Montague JC, Callow N, Rowlands AV. Saliva flow rate, total protein concentration and osmolality as potential markers of whole body hydration status during progressive acute dehydration in humans. Arch. Oral Biol. 2004;49:149–154. doi: 10.1016/j.archoralbio.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Justino AB, Teixeira RR, Peixoto LG, Jaramillo OLB, Espindola FS. Effect of saliva collection methods and oral hygiene on salivary biomarkers. Scand. J. Clin. Lab. Invest. 2017;77:415–422. doi: 10.1080/00365513.2017.1334261. [DOI] [PubMed] [Google Scholar]
- 39.Williamson S, Munro C, Pickler R, Grap MJ, Elswick RK. Comparison of biomarkers in blood and saliva in healthy adults. Nurs. Res. Pract. 2012;2012:1–4. doi: 10.1155/2012/246178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang D, et al. Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur. Radiol. 2017;27:2698–2705. doi: 10.1007/s00330-016-4669-8. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Herrup K. Glutamine acts as a neuroprotectant against DNA damage, beta-amyloid and H2O2-induced stress. PLoS ONE. 2012;7:e33177. doi: 10.1371/journal.pone.0033177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shinno H, et al. A decrease in N-acetylaspartate and an increase in myoinositol in the anterior cingulate gyrus are associated with behavioral and psychological symptoms in Alzheimer’s disease. J. Neurol. Sci. 2014;343:233–234. doi: 10.1016/j.jns.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 43.Maeba, R., Araki, A. & Fujiwara, Y. Serum ethanolamine plasmalogen and urine myo-inositol as cognitive decline markers. in 69–111 (2018). doi:10.1016/bs.acc.2018.08.001 [DOI] [PubMed]
- 44.Teranishi Y, et al. Proton myo-inositol cotransporter is a novel γ-secretase associated protein that regulates Aβ production without affecting Notch cleavage. FEBS J. 2015;282:3438–3451. doi: 10.1111/febs.13353. [DOI] [PubMed] [Google Scholar]
- 45.Voevodskaya O, et al. Myo-inositol changes precede amyloid pathology and relate to APOE genotype in Alzheimer disease. Neurology. 2016;86:1754–1761. doi: 10.1212/WNL.0000000000002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin M, Selkoe DJ. Systematic analysis of time-dependent neural effects of soluble amyloid β oligomers in culture and in vivo: Prevention by scyllo-inositol. Neurobiol. Dis. 2015;82:152–163. doi: 10.1016/j.nbd.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peña-Bautista C, et al. Plasma alterations in cholinergic and serotonergic systems in early Alzheimer Disease: diagnosis utility. Clin. Chim. Acta. 2020;500:233–240. doi: 10.1016/j.cca.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 48.Kori M, Aydın B, Unal S, Arga KY, Kazan D. Metabolic biomarkers and neurodegeneration: a pathway enrichment analysis of Alzheimer’s disease, parkinson’s disease, and amyotrophic lateral sclerosis. Omi. A J. Integr. Biol. 2016;20:645–661. doi: 10.1089/omi.2016.0106. [DOI] [PubMed] [Google Scholar]
- 49.Bürklen TS, et al. The creatine kinase/creatine connection to Alzheimer’s disease: CK inactivation, APP-CK complexes and focal creatine deposits. J. Biomed. Biotechnol. 2006;2006:1–11. doi: 10.1155/JBB/2006/35936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snowden SG, et al. neurotransmitter imbalance in the brain and Alzheimer’s disease pathology. J. Alzheimer’s Dis. 2019;72:35–43. doi: 10.3233/JAD-190577. [DOI] [PubMed] [Google Scholar]
- 51.Chen KH, Reese EA, Kim H-W, Rapoport SI, Rao JS. Disturbed neurotransmitter transporter expression in Alzheimer’s disease brain. J. Alzheimer’s Dis. 2011;26:755–766. doi: 10.3233/JAD-2011-110002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Corso G, et al. Serum amino acid profiles in normal subjects and in patients with or at risk of Alzheimer dementia. Dement. Geriatr. Cogn. Dis. Extra. 2017;7:143–159. doi: 10.1159/000466688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klietz, M. et al. Altered Neurometabolic profile in early parkinson’s disease: a study with short echo-time whole brain MR spectroscopic imaging. Front. Neurol.10, (2019). [DOI] [PMC free article] [PubMed]
- 54.Kantarci K, et al. 1H MR spectroscopy in common dementias. Neurology. 2004;63:1393–1398. doi: 10.1212/01.WNL.0000141849.21256.AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gardner A, Carpenter G, So P-W. Salivary metabolomics: from diagnostic biomarker discovery to investigating biological function. Metabolites. 2020;10:47. doi: 10.3390/metabo10020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.