Significance
The CD8+ T cell response is crucial in protecting the organism against infections and cancer. This protective response relies on the production of effector molecules and the generation of long-lived memory cells. Understanding the processes governing CD8+ T cell responses is essential for the development of better vaccine strategies and immune cell therapies. Here, we show that deletion of the transcription factor NR4A3 leads to increased memory generation and effector functions. This results from an early impact on the CD8+ T cell transcriptional memory program and on chromatin accessibility for bZIP transcription factors. Thus, we identify NR4A3 as a key regulator of CD8+ T cell function and differentiation, which could have therapeutic implications.
Keywords: early CD8+ T cell response, nuclear receptors, T cell memory, NR4A3, T cell function
Abstract
Enhancing long-term persistence while simultaneously potentiating the effector response of CD8+ T cells has been a long-standing goal in immunology to produce better vaccines and adoptive cell therapy products. NR4A3 is a transcription factor of the orphan nuclear receptor family. While it is rapidly and transiently expressed following T cell activation, its role in the early stages of T cell response is unknown. We show that NR4A3-deficient murine CD8+ T cells differentiate preferentially into memory precursor and central memory cells, but also produce more cytokines. This is explained by an early influence of NR4A3 deficiency on the memory transcriptional program and on accessibility of chromatin regions with motifs for bZIP transcription factors, which impacts the transcription of Fos/Jun target genes. Our results reveal a unique and early role for NR4A3 in programming CD8+ T cell differentiation and function. Manipulating NR4A3 activity may represent a promising strategy to improve vaccination and T cell therapy.
CD8+ T lymphocytes undergo massive expansion and differentiation following T cell receptor (TCR) recognition of foreign antigens presented as peptide fragments on MHC class I molecules. At the peak of the CD8+ T cell response to acute infection, two main populations of effectors are generated: short-lived effector cells (SLECs; KLRG1hiCD127lo) and memory precursor effector cells (MPECs; KLRG1loCD127hi) (1–3). Both populations have effector functions but most of the SLECs will die during the contraction phase while MPECs will further differentiate into long-lived CD8+ memory T cells to confer long-term protection. The SLEC and MPEC differentiation choice is influenced by inflammatory signals perceived by the T cells and requires the induction of a complex transcriptional program (2, 3). As such, several transcription factors were shown to contribute to this differentiation event. SLEC differentiation is dependent on T-bet, Blimp-1, Id2, Rbpjk, and Zeb2 while Eomes, Bcl-6, Id3, Bach2, and TCF-1 are involved in MPEC and memory T cell differentiation (2, 4–15). Evidence supports that this differentiation choice is determined very early in the response, as early as the first T cell division (16). Furthermore, the chromatin landscape changes rapidly following naive CD8+ T cell activation with massive opening of chromatin regions. Interestingly, the differentially accessible regions (DARs) of chromatin between naive and recently activated CD8+ T cells contain regions that are later preferentially found in either MPECs or SLECs (17–19). This suggests that early events of T cell activation poise the cells for differentiation and that further events fix the cellular differentiation choice. The DARs associated with early CD8+ T cell activation are enriched for motifs recognized by several transcription factors, including some that are known to influence CD8+ T cell differentiation (17–19).
Among early events that occur following TCR stimulation of naive CD8+ T cells is the rapid expression of the nuclear orphan receptor NR4A family members (NR4A1, NR4A2, and NR4A3) (20). These orphan nuclear receptors act as transcription factors by binding to the NBRE consensus sequence motif on DNA (reviewed in ref. 21). Their activity is regulated at the level of their expression and they are induced very rapidly by a variety of extracellular signals. They are thus considered immediate early genes. They control proliferation, differentiation, survival, and metabolism in several cell types (21). In the immune system, they play crucial roles in the differentiation and response of myeloid cells (22–26). In T cells, the deletion of the three members during thymocyte differentiation abrogates regulatory T cell differentiation while single deletion results in an increase in these cells (27–29). Their roles in the peripheral mature T cell compartment have not been extensively studied but NR4As are highly expressed by exhausted T cells (30–34) and recent studies have found roles for these in contexts of recurrent antigen stimulation (33, 35). However, very little is known about the role of these members during CD8+ T cell response to acute infection and on the specific action of NR4A3 in CD8+ T cells. The rapid and transient up-regulation of NR4A1-3 expression following CD8+ T cell activation suggests that these transcription factors will have an early transcriptional influence on CD8+ T cell response to acute infection. This possibility is further supported by the enrichment of the NBRE motif in DARs that are more open at 2 to 24 h postnaive CD8+ T cell activation while they are not enriched in more differentiated effector CD8+ T cells (17–19).
In this study, we have found an important and unique role for NR4A3 in the early stages of an acute CD8+ T cell response. We demonstrate that NR4A3 limits the generation of MPECs and memory CD8+ T cells, particularly central memory CD8+ T cells, while restraining cytokine production. Early in the CD8+ T cell response, NR4A3 decreases chromatin accessibility of regions containing bZIP transcription factor motifs and reduces the expression of transcription factors involved in memory differentiation while enhancing expression of transcription factors controlling SLEC differentiation. This highlights the crucial early role for the NR4A3 family member in acute CD8+ T cell responses.
Results
NR4A3 Deficiency Promotes MPEC Differentiation and Enhances Effector Functions.
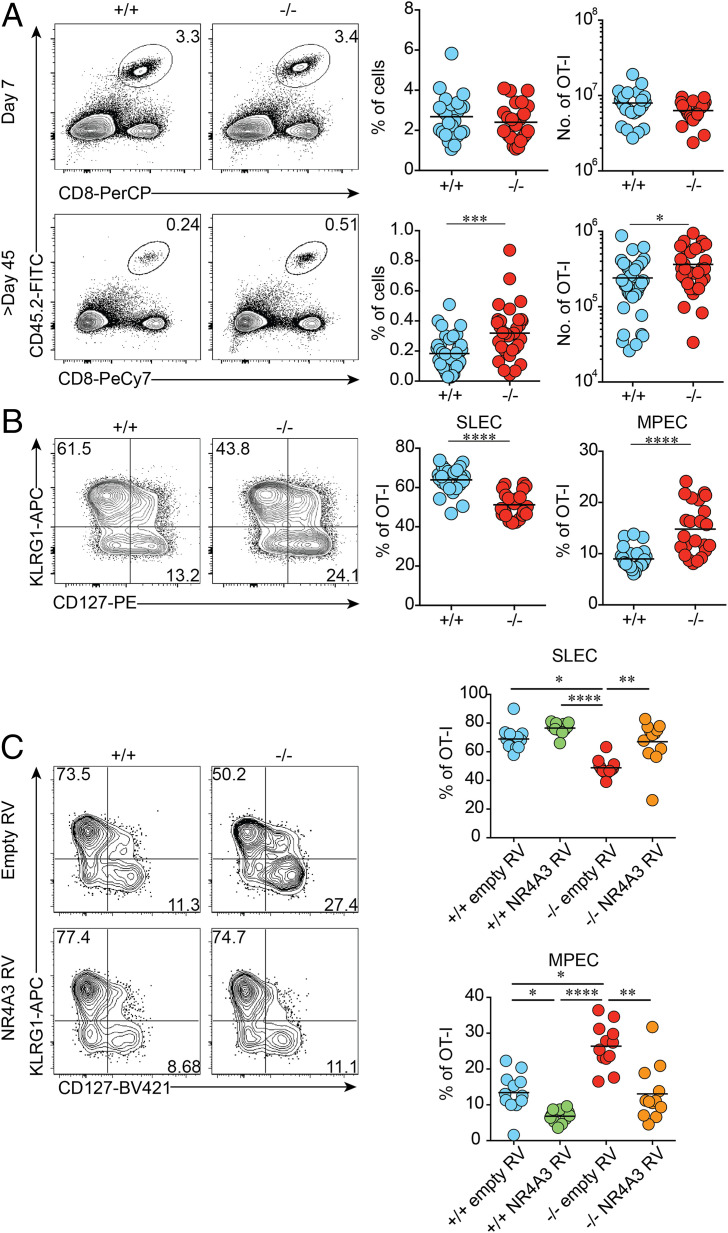
To evaluate the intrinsic role of NR4A3 during CD8+ T cell response, we crossed Nr4a3−/− mice with OT-I mice, expressing a transgenic TCR specific for the ovalbumin (OVA) peptide 257 to 264 in the context of the MHC class I molecules Kb (36). A total of 5 × 103 Nr4a3+/+ or Nr4a3−/− OT-I T cells were adoptively transferred into wild-type (WT) congenic B6.SJL mice 1 d prior to infection with a sublethal dose of Listeria monocytogenes encoding OVA (Lm-OVA). As shown in Fig. 1A, NR4A3 deficiency did not affect CD8+ T cell expansion at the peak of the response (day 7) or the kinetic of the CD8+ T cell response (SI Appendix, Fig. S1A). We did, however, consistently observe the generation of twofold more memory CD8+ T cells in the absence of NR4A3 (Fig. 1A and SI Appendix, Fig. S1A). This increased memory generation was also observed following lymphocytic choriomeningitis virus encoding OVA (LCMV-OVA) infection when OT-I Nr4a3−/− T cells were adoptively transferred in competition with Nr4a3+/+ OT-I T cells (SI Appendix, Fig. S1B). The enhanced generation of memory CD8+ T cells in absence of NR4A3 led us to evaluate whether the proportion of SLECs and MPECs was affected. At day 7 postinfection, we observed an increased proportion of MPECs while fewer SLECs were present in the spleen (Fig. 1B) and blood (SI Appendix, Fig. S1C). Similar effects on MPEC/SLEC differentiation were obtained following dendritic cell vaccination, LCMV-OVA infection, and polyclonal CD8+ T cell response (SI Appendix, Fig. S1 D–F). Although, we did not observe differences in thymic differentiation and peripheral OT-I T cells (SI Appendix, Fig. S2 A–E), we confirmed our results using sorted CD44lo naive OT-I T cells (SI Appendix, Fig. S2F). Finally, to test whether overexpression of NR4A3 promoted the generation of SLECs, we stimulated Nr4a3+/+ and Nr4a3−/− OT-I CD8+ T cells with OVA-pulsed splenocytes in vitro for 24 h, spin transduced them with defective retrovirus encoding Nr4a3, and adoptively transferred the transduced OT-I cells into B6.SJL mice infected with Lm-OVA the day before. As shown in Fig. 1C, overexpression of NR4A3 in Nr4a3+/+ or Nr4a3−/− OT-I T cells led to increased SLEC differentiation while MPEC generation was decreased. Therefore, NR4A3 modulates the generation of CD8+ memory T cells by influencing the generation of MPECs at the peak of the CD8+ T cell response.
Fig. 1.
NR4A3 restrains CD8+ T cell memory generation. (A) Effector and memory responses in the spleen of mice adoptively transferred with CD45.2+ Nr4a3+/+ or Nr4a3−/− OT-I cells prior to Lm-OVA infection. (B) Proportions of MPECs (CD127+KLRG1−) and SLECs (CD127−KLRG1+) within OT-I effectors at day 7 postinfection. (C) Nr4a3+/+ and Nr4a3−/− OT-I cells transduced with empty- or Nr4a3-encoding retrovirus (RV) were transferred into Lm-OVA-infected mice. At day 7 postinfection, MPEC and SLEC distribution in GFP+-transduced cells was evaluated. +/+: Nr4a3+/+ cells; −/−: Nr4a3−/− cells. Each dot represents one mouse. Data are from at least three independent experiments. Unpaired Student’s t test, with a Welch’s correction when applied, was used for two-group comparison and Kruskal–Wallis ANOVA with Dunn’s multiple comparison for multiple group comparison: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The generation of fewer SLECs in absence of NR4A3 raises the possibility that effectors are less differentiated and as such might be less functional. However, after a short in vitro restimulation with the OVA peptide, more Nr4a3−/− effectors produced cytokines (IFN-γ, TNF-α, and IL-2) than their WT counterpart and they produced them in higher amounts (Fig. 2A). The effect of NR4A3 deficiency on cytokine production was also observed in the polyclonal T cell repertoire setting (SI Appendix, Fig. S3A). This enhanced cytokine production is observed in both MPECs and SLECs (SI Appendix, Fig. S3B). Overexpression of NR4A3 in Nr4a3+/+ and Nr4a3−/− OT-I T cells led to a reduction of cytokine production (Fig. 2B). Therefore, NR4A3 deficiency promotes both memory generation and functional capacity of CD8+ T cells, two processes that are usually not coregulated.
Fig. 2.
NR4A3 restrains cytokine production by CD8+ T cells. (A) Day 7 postinfection with Lm-OVA, OT-I effectors were restimulated for 5 h with OVA peptide and assessed for cytokine secretion. The percentage of positive OT-I cells for each cytokine and mean fluorescence intensity (MFI) on cells positive for the measured cytokines are shown. (B) Retrovirally transduced GFP+ cells, as in Fig. 1C, were sorted prior to restimulation in order to measure cytokine production. +/+: Nr4a3+/+ cells; −/−: Nr4a3−/− cells. Data are from two (B) or at least three independent experiments. Unpaired Student’s t test, with a Welch’s correction when applied, was used for two-group comparison and Kruskal–Wallis ANOVA with Dunn’s multiple comparison for multiple group comparison: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
NR4A3 Deficiency Promotes the Differentiation of Central Memory CD8+ T Cells.
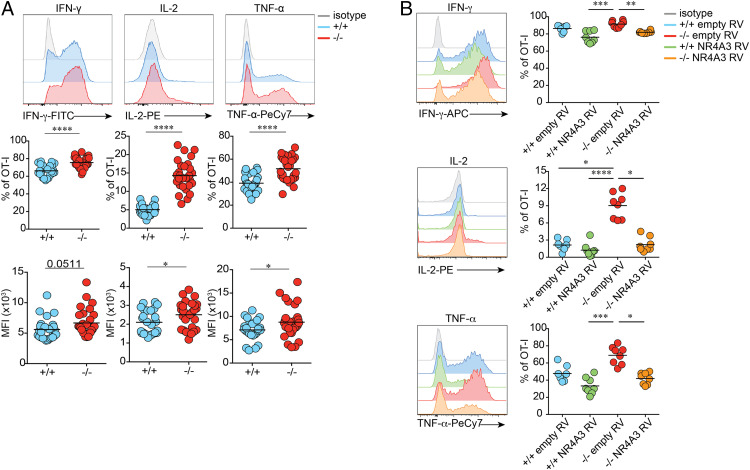
To test whether enhanced memory generation was a consequence of an increased proportion of MPECs at the peak of the T cell response, we sorted Nr4a3+/+ and Nr4a3−/− OT-I MPECs from the spleen of mice infected with Lm-OVA (day 5) and adoptively transferred the same numbers in competition in WT recipients (Fig. 3A). The rate of memory generation from Nr4a3+/+ and Nr4a3−/− MPECs was slightly higher for NR4A3-deficient MPECs, indicating that increased memory CD8+ T cell generation was the consequence of both an increased MPEC generation and enhanced differentiation into memory T cells (Fig. 3A). Greater differentiation of MPECs into long-lived memory CD8+ T cells is probably not the consequence of improved survival during the contraction phase but may result from enhanced sensitivity of Nr4a3−/− OT-I memory T cells to IL-15 (SI Appendix, Fig. S4 A and B).
Fig. 3.
NR4A3 deficiency promotes MPEC differentiation into memory cells and increases central memory T cell generation. (A, Top) Experimental design assessing the potential of Nr4a3+/+ and Nr4a3−/− OT-I MPECs for the formation of memory cells. (A, Bottom) The percentage of adoptively transferred CD45.2+ memory cells, either +/+ or −/−, from spleen and LNs 20 d posttransfer, was normalized to the percentage of CD45.1+ cells in order to obtain a ratio of memory generation (representative flow plots are from spleen). (B) Memory responses in the LN and bone marrow (BM) of mice adoptively transferred with CD45.2+ Nr4a3+/+ or Nr4a3−/− OT-I cells prior to Lm-OVA infection. CD62L expression (C) and proportion of IL-2 and IFN-γ coproducing (D) OT-I memory Nr4a3+/+ or Nr4a3−/− cells in the spleen. (E) Experimental design to test the secondary response of Nr4a3+/+ or Nr4a3−/− memory cells. Quantification in the spleen of the response (F) and cytokine production (G) of secondary effectors Nr4a3+/+ or Nr4a3−/−. (H) Nr4a3+/+ and Nr4a3−/− OT-I memory cells were generated in response to Lm-OVA infection. At least 40 d postinfection, ∼2 × 105 Nr4a3+/+ or Nr4a3−/− OT-I memory cells were adoptively transferred into B16-OVA bearing mice (7 d postimplantation). Mouse survival and tumor growth were monitored every 2 d. Each dot represents one mouse. Endo: total endogenous CD8+CD45.2− cells. Survival curves were compared using log-rank Mantel–Cox test (H). Data are from two (A, +/+/CD45.1+ competitive group and H), or at least three independent experiments. Unpaired Student’s t test, with a Welch’s correction when applied, was used: *P < 0.05, **P < 0.01, ***P < 0.001.
The characterization of CD8+ memory T cell generation shows that the number of memory CD8+ T cells is increased in the spleen (Fig. 1A), lymph nodes, and bone marrow in the absence of NR4A3 (Fig. 3B) with a higher proportion of cells bearing a CD62Lhi central memory phenotype (Fig. 3C). As a consequence, a greater proportion of Nr4a3−/− OT-I CD8+ memory T cells coproduce IL-2 and IFN-γ (Fig. 3D). Similar results were obtained using LCMV-OVA infection in a competitive setting (SI Appendix, Fig. S4 C and D). Finally, we did not observe any differences in homeostatic proliferation of Nr4a3+/+ and Nr4a3−/− OT-I memory T cells (SI Appendix, Fig. S4 E and F)
Following adoptive transfer and challenge with a high dose of Lm-OVA, Nr4a3−/− OT-I memory T cells expand as efficiently as Nr4a3+/+ OT-I memory cells (Fig. 3 E and F) and the generated secondary CD8+ T effectors were still more polyfunctional after rechallenge (Fig. 3G) and differentiate more into MPECs and CD62Lhi cells (SI Appendix, Fig. S4G). To analyze the capacity of Nr4a3+/+ and Nr4a3−/− OT-I memory T cells to control a bacterial challenge, we generated OT-I memory CD8+ T cells following LCMV-OVA infection and challenge the recipient mice with 5 × 105 colony-forming units (CFUs) of Lm-OVA. We did not observe a better control of bacterial burdens in mice containing Nr4a3−/− OT-I memory T cells (SI Appendix, Fig. S4H). This might be the consequence of a very potent control of bacteria in the WT setting or may reflect a lack of better control of Lm-OVA by central memory T cells. The enrichment for memory T cells with a central memory phenotype and the enhanced functionality of Nr4a3−/− CD8+ T cells led us to evaluate whether they would better control tumor growth. To do so, 2 × 105 Nr4a3+/+ or Nr4a3−/− memory OT-I T cells generated following Lm-OVA infection were adoptively transferred into mice bearing B16-OVA melanomas. In this setting, only NR4A3-deficient memory CD8+ T cells were able to provide prolonged mice survival and reduced tumor growth (Fig. 3H and SI Appendix, Fig. S4I). Therefore, NR4A3 deficiency favors the generation of more central memory CD8+ T cells that are endowed with enhanced functions and ability to control tumor growth.
NR4A3 Deficiency Influences Early Expression of Transcription Factors Important for MPEC and Memory CD8+ T Cell Generation.
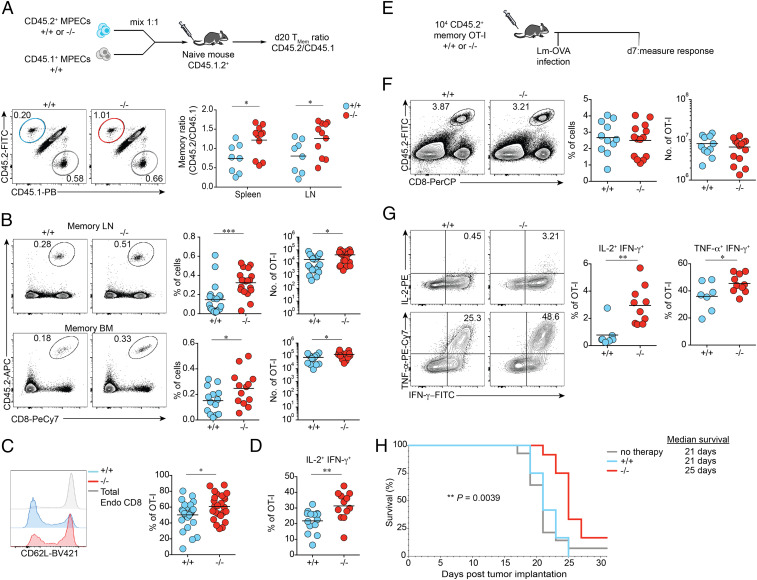
To understand how NR4A3 deficiency promotes enhanced MPEC and memory CD8+ T cell generation while at the same time enhancing effector functions, we performed the analysis of the transcriptome of Nr4a3+/+ and Nr4a3−/− OT-I T cells early after in vivo T cell activation, before MPEC/SLEC differentiation (SI Appendix, Fig. S5A), as Nr4a3 expression is induced early and transiently following T cell stimulation in vivo (peak at 24 h postinfection with Lm-OVA; SI Appendix, Fig. S5B). At early time points, day 2 and day 3 postinfection, we did not observe any defect in proliferation and acquisition of the activation markers CD69 and CD44 by Nr4a3+/+ and Nr4a3−/− OT-I T cells (SI Appendix, Fig. S5 C and D) further validating the choice of day 3 for the RNA sequencing (RNA-seq) analysis. At day 3 postinfection with Lm-OVA, 355 genes were differentially expressed in Nr4a3+/+ and Nr4a3−/− OT-I T cells (fold change [FC] >|1.5| and P adjusted [adj] <0.05), among them 274 genes show higher expression in absence of NR4A3 while 81 genes were down-regulated (Fig. 4A and Dataset S1). Gene set enrichment analysis (GSEA) showed enrichment for several gene signatures associated with immune memory (Fig. 4B). In agreement with this, at day 3 of the response, the expression of transcription factors controlling MPEC and memory generation (Tcf7, Eomes, Id3, Bcl6, and Bach2) was increased while the expression of many transcription factors controlling SLEC differentiation (Id2, Prdm1, Zeb2, and Rbpj) was down-regulated in the absence of NR4A3 (Fig. 4C). We validated by fluorescence-activated cell sorting (FACS) and RT-qPCR analysis that the expression of these transcription factors was modulated by NR4A3 in OT-I effector T cells at day 3 postinfection with Lm-OVA (SI Appendix, Fig. S5 E and F) and the changes in the expression of these transcription factors were maintained in day-7 effectors (Fig. 4 D and E), except for T-bet. Our data also reveal a role for NR4A3 in promoting SLEC differentiation via the regulation of the Il2ra gene (37, 38). Il2ra gene transcription and CD25 protein expression are significantly down-regulated in day-3 Nr4a3−/− OT-I effector T cells (Fig. 4 A, F, and G). Moreover, Nr4a3−/− OT-I T cells at day 3 postinfection are enriched for the CD25loCD62Lhi subset when compared to Nr4a3+/+ OT-I T cells (SI Appendix, Fig. S5G), a subset identified by others to be committed to differentiate into memory cells (39), further supporting the idea that NR4A3 deficiency has an early impact on the acquisition of the memory program. Altogether, these data show that NR4A3 deficiency has an immediate impact on the central molecular events that regulate CD8+ T cell fate.
Fig. 4.
NR4A3 is an early regulator of the CD8+ T cell memory gene signature. (A) Volcano plot of genes differentially expressed in Nr4a3+/+ and Nr4a3−/− OT-I cells assessed by RNA-seq at day 3 postinfection with Lm-OVA. Indicated in black, genes P adj <0.05. Indicated in red, genes P adj <0.05 and |FC|>1.5. (B) GSEA of the NR4A3-regulated transcriptome reveals enrichment of memory signatures. (C) Heat map illustrating the relative expression in Nr4a3+/+ and Nr4a3−/− OT-I cells of genes encoding for selected transcription factors controlling CD8+ T cell differentiation. At day 7 post-Lm-OVA infection, the expression of transcription factors known to be involved in CD8+ T cell memory generation was measured by FACS (D) or by qRT-PCR (E) in spleens. (F) Il2ra transcription by day-3 OT-I effectors (data from RNA-seq). (G) At day 3 post-Lm-OVA infection, expression of CD25 on Nr4a3+/+ and Nr4a3−/− OT-I cells was measured by flow cytometry. Endo: total CD8+CD45.2− endogenous cells. Each dot represents one mouse. Data are from two (A, C, E, and G) or at least three independent experiments (D). A Mann–Whitney unpaired t test (F), when a low number of experimental samples were available, was used and an unpaired Student’s t test, with a Welch’s correction when applied, was used for the other two-group comparison: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
NR4A3 Influences Chromatin Accessibility Early during T Cell Activation.
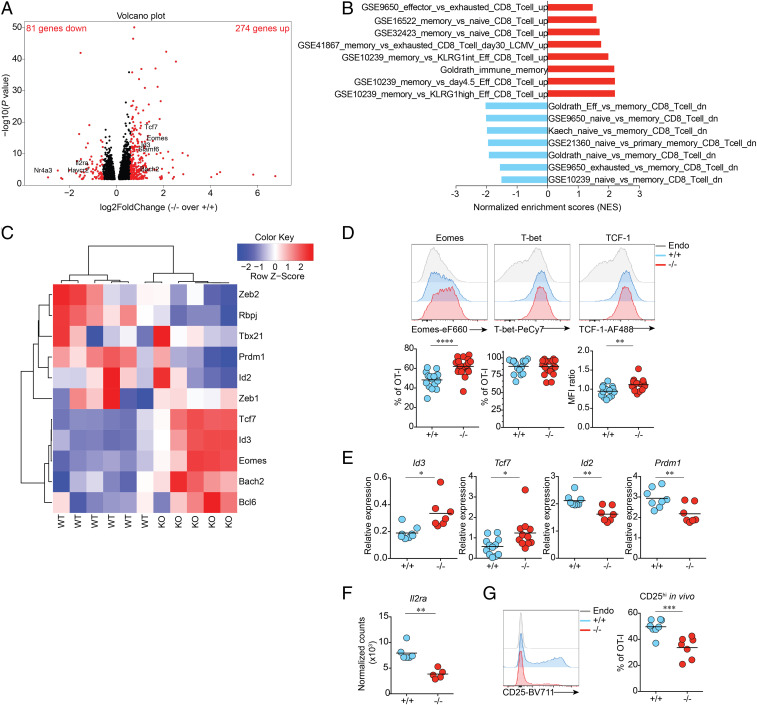
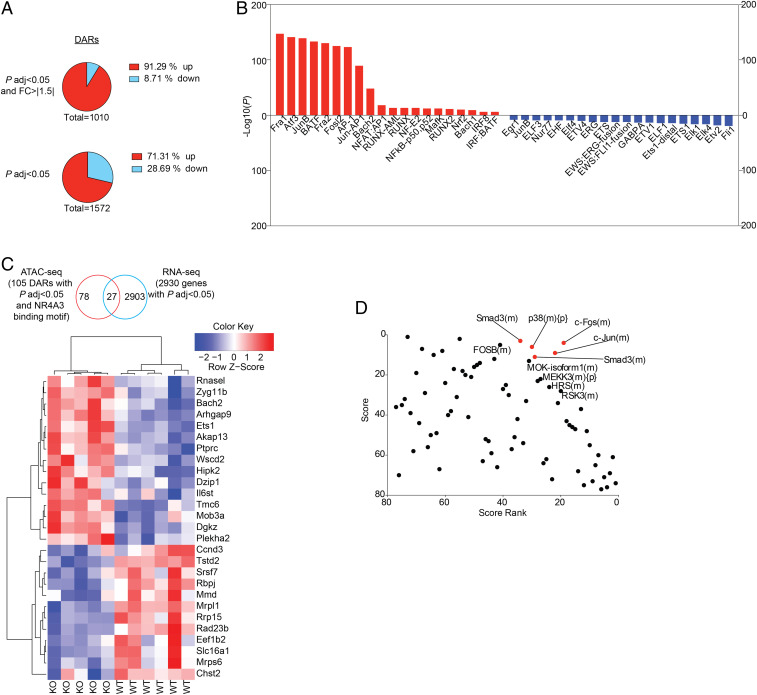
As NR4A3 is rapidly and transiently induced in antigen-stimulated CD8+ T cells and because it has an early impact on the programming of effector differentiation, we decided to determine the effect of NR4A3 deficiency on chromatin accessibility using assay for transposase-accessible chromatin sequencing (ATAC-seq). To do so, we first validated that Nr4a3−/− OT-I T cells stimulated in vitro for 3 d showed similar changes in expression of selected transcription factors and enhanced cytokine production (SI Appendix, Fig. S6A). In this in vitro setting, Nr4a3 transcription peaks at 12 h poststimulation (SI Appendix, Fig. S6B). Therefore, we extracted nuclei from Nr4a3+/+ and Nr4a3−/− OT-I T cells stimulated for 12 h to perform the ATAC-seq. As shown in Fig. 5A and Dataset S2, a total of 1,572 DARs of chromatin were identified (P adj <0.05) with 1,010 having a fold change of >|1.5| and a P adj <0.05 with most of them (922; 91%) being more open in the absence of NR4A3. The more opened DARs in Nr4a3−/− cells were enriched for regions containing consensus binding sites for the bZIP family of transcription factors (AP-1, Jun, Fos, and BATF) while the less opened DARs were enriched for NR4A3 and its family members consensus site (NBRE; identified as Nur77 in the figure) and Fli1 (Fig. 5B). Among the DARs (with a cutoff of P adj <0.05), 105 of them contained NBRE motifs (Dataset S3) and 25% of them show differential transcription by Nr4a3−/− OT-I day-3 effectors (Fig. 5C). Among all of the DARs (P adj <0.05) at 12 h poststimulation of Nr4a3+/+ and Nr4a3−/− OT-I T cells, 20% of the genes associated with them are differentially transcribed by day-3 in vivo effectors (P adj <0.05; SI Appendix, Fig. S4C). This demonstrates that NR4A3 has an early influence on the transcriptional program of CD8+ T cells with a major impact on genes that are regulated by bZIP transcription factors, which share a similar consensus binding sequence on DNA. The enhanced opening of chromatin regions containing bZIP consensus binding sites is in line with recent reports suggesting that NR4A family members affect the binding of Jun to target genes (33, 35).
Fig. 5.
Early NR4A3 modulation of chromatin accessibility during T cell activation. (A) Number of DARs in the chromatin of Nr4a3−/− versus Nr4a3+/+ OT-I T cells stimulated for 12 h with anti-CD3/CD28 assessed by ATAC-seq (Top chart: cutoff of P adj <0.05 and |FC|>1.5; Bottom chart: cutoff P adj <0.05 only). “Up” indicates the proportion of DARs that are more open in Nr4a3−/− T cells while “down” indicates the proportion of DARs less open in Nr4a3−/− T cells, compared to Nr4a3+/+ T cells. (B) Transcription factor motif enrichment analysis of DARs that are more open in Nr4a3−/− cells (red bars) or less open in Nr4a3−/− cells (blue bars). (C) Analysis of the genes found to be regulated by Nr4a3 at day 3 postinfection by RNA-seq analysis and linked to NR4A motif containing DARs between Nr4a3+/+ and Nr4a3−/− T cells determined by ATAC-seq. (D) RNA-seq data comparing Nr4a3+/+ and Nr4a3−/− T cells expression profiles were submitted to upstream master regulator analysis in the GeneXplain platform. Top-ranked upstream regulators have the lowest rank sum value, obtained by adding the score and score rank. The top five results are highlighted in red. ATAC-seq experiment was performed once on two biological samples of each genotype.
Analysis of our RNA-seq data using the GeneXplain platform (40) identifies Fos and Jun as the top upstream master regulators of the differentially expressed genes (Fig. 5D) further supporting a role for NR4A3 in modulating the action of the AP-1 transcription factor. Altogether this indicates that the absence of NR4A3 favors the binding of AP-1 and/or other bZIP transcription factors to chromatin to promote the transcription of target genes. Interestingly, AP-1 and Jun are known to promote the transcription of effector genes (14, 41), thus providing a possible explanation for the enhanced effector functions of Nr4a3−/− CD8+ T cells.
Discussion
Our results identify that NR4A3 restrains memory potential and effector functions of CD8+ T cells in acute infection. In this context, NR4A3-deficient CD8+ T cells generate more MPECs and central memory CD8+ T cells while having enhanced capacity to produce cytokines.
At the molecular level, our results demonstrate that NR4A3 influences CD8+ T cell differentiation by affecting the chromatin accessibility of certain genes early following CD8+ T cell activation, which then leads to a transcriptional program that favors SLEC generation while repressing effector functions. In the absence of NR4A3, most of the DARs are more open and these are significantly enriched for the bZIP (Jun, Fos, AP-1, etc.) family transcription factor motif. This is similar to what has been recently reported for NR4A1 in CD4+ T cells and for NR4A triple-deficient CD8+ tumor-infiltrating lymphocytes (TILs) (33, 35). Interestingly, NR4A1-deficient CD4+ T cells and NR4A triple-deficient CD8+ TILs are also more functional than their WT counterpart. This suggests that all three NR4A family members have the ability to influence effector functions and that they do so using a similar molecular mechanism. It is therefore intriguing that loss of only one member is sufficient to affect T cell functionality. Further studies should reveal whether each member affects a different set of genes controlling T cell functions and that these are possibly regulated by different bZIP transcription factors.
Our molecular analysis also reveals that NR4A3 affects MPEC/SLEC differentiation by controlling the early induction of the SLEC molecular program. It could be considered surprising that NR4A3 restrains both effector functions and memory generation. There are several members of the bZIP transcription factor family and their individual expression varies at different stages of the immune response (17). In the course of an immune response, bZIP transcription factor binding motif is enriched in open chromatin regions of SLECs, MPECs, and memory CD8+ T cells when compared to naive cells (17–19). Also, members of this family have been shown to target promemory genes, such as Bcl6, Tcf7, and Eomes, as well as genes more traditionally associated with effector functions (41). Finally, it is possible that some bZIPs contribute to the acquisition of effector function while others promote memory generation. As a whole, these elements underline the complexity of this family of transcription factors and suggest that, while perhaps counterintuitive at face value, the fact that we observed an enrichment for bZIP motifs in opened chromatin regions of Nr4a3−/− CD8+ T cells early in the response is compatible with both an increase in effector function and in memory potential. This interpretation is supported by a recent report demonstrating that CAR T cells overexpressing c-Jun are more functional and undergo less terminal differentiation (42).
Intriguingly, NR4A1, which does compete with c-Jun, was reported to have either no effect on MPEC/SLEC differentiation or to limit SLEC formation, which would be the opposite effect of NR4A3 (35, 43). These observations strengthen the notion that the different NR4A family members have nonoverlapping functions during the CD8+ T cell response. While NR4A3 could have a dominant effect over NR4A1 in affecting the binding of bZIP transcription factors to their target genes or could selectively affect the binding of certain bZIP transcription factor family members, it is also possible that the increased MPEC formation in Nr4a3−/− mice was not solely the consequence of enhanced accessibility of bZIP transcription factors to DNA. NR4A3’s transcriptional effects could be mediated by a direct regulation of NR4A3 via binding on genes containing an NBRE motif in conjunction with the reported ability of NR4As to modulate chromatin remodeling (44). Among the differentially accessible chromatin regions between Nr4a3+/+ and Nr4a3−/− activated OT-I T cells, 105 of them contain a putative NBRE motif (Dataset S3). Very few of these regions are in proximity of genes known to influence SLEC/MPEC differentiation and effector functions. These include Rbpj and Bach2 (8, 9, 14). While we found DARs in proximity to genes related to CD8+ T cell differentiation or function, such as Il2ra, Irf4, and Myc, these regions did not contain classical NBRE motifs (37, 45–48). In other studies looking at early in vitro T cell activation or in TILs, NBRE motifs were found close to Foxo1, Havcr2, Kit, Tcf7, Tbx21, Id3, and Bcl6, but we did not detect a difference in chromatin opening at these sites in our study (19, 33). Because the expression of some of these genes was differential in our RNA-seq, NR4A3 could regulate their expression, but not by influencing the accessibility of chromatin. Alternatively, we might have missed these important sites because we elected to perform our ATAC-seq study at 12 h poststimulation. This decision was motivated by the desire to uncover the early events mediated by NR4A3 whose peak of TCR-induced mRNA expression was identified at 12 h. However, NR4A3 protein expression could be maintained for a longer period of time and there could be NR4A3-regulated targets of importance at later time points. Another possibility is the reported ability of NR4A family members to regulate transcription without directly binding to DNA. This process of transrepression was shown in myeloid cells and is the result of interaction of NR4A2 with the p65 subunit of NFκB, which favors the recruitment of CoREST that actively removes p65 from the promoters of inflammatory genes (49). Relevant to this, note that NFκB binding motif is also enriched in our ATAC-seq data. Overall, this highlights the complexity of the NR4A family and underlies the need for further mechanistic studies.
The NR4A family members are induced by TCR signaling, and may thus be important during thymic T cell differentiation or play a role in the maintenance of memory cells following tonic signaling. Lack of NR4A3 expression during thymic differentiation could impact the response of peripheral mature CD8+ T cells. We think that this is an unlikely possibility as overexpression of NR4A3 after activation of naive Nr4a3−/− CD8+ T cells was able to recapitulate the WT phenotype for MPEC/SLEC differentiation and cytokine production. In support of this, thymic differentiation and peripheral T cell phenotype was not affected by NR4A3 deficiency. We have also not formally excluded a role for NR4A3 in regulating CD8+ T cell memory in response to tonic signaling. However, as expected for an immediate early gene, during an acute response Nr4a3 transcription is already abolished at day 2 in vivo (SI Appendix, Fig. S5 and ref. 20). In addition, data suggest that in both T cells and B cells, NR4A3 is not induced following tonic signaling, contrary to what was observed for NR4A1 (50, 51). This, coupled to our sequencing data obtained early following TCR stimulation support that the impact of NR4A3 on CD8+ T cell differentiation is not likely to be via a role in the response of memory T cells to tonic signaling. It would be of interest to confirm this using an inducible cre/lox system. However, growing evidence demonstrates that in tertiary tissue, NR4A family members are reexpressed and important for resident memory T cells, suggesting that the biological role of NR4As could be dependent on tissue microenvironment (52–55).
Altogether, our results suggest that the modulation of NR4A3 activity represents a promising avenue for enhancing the generation of memory CD8+ T cell following vaccination and for the production of better T cells for adoptive cell therapy of cancer patients.
Materials and Methods
Mice.
B6.SJL, C57BL/6, CD45.1.2 (F1 of B6.SJL × C57BL/6), Nr4a3−/− and Nr4a3+/+, OT-I (36) (Rag1−/− CD45.2) mice were all bred at the Maisonneuve-Rosemont Hospital Research Center facility. Nr4a3−/− mice were a kind gift from Orla M. Conneely (Baylor College of Medicine, Houston, TX; 56). OT-I/B6.SJL mice were obtained by crossing OT-I (Rag1−/− CD45.2) mouse with B6.SJL (CD45.1). Nr4a3−/− were backcrossed for at least 10 generations to C57BL/6 and then crossed to OT-I mouse to obtain OT-I Nr4a3+/+ (Rag1−/− CD45.2) and OT-I Nr4a3−/− (Rag1−/− CD45.2) mice. Mice were housed in a pathogen-free environment and treated in accordance with the Canadian Council on Animal Care guidelines.
Cell Lines.
B16-OVA cells were kindly provided by A. Lamarre, Institut National de la Recherche Scientifique-Institut Armand-Frappier, Laval, QC, Canada. B16-OVA cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% of Nu serum, sodium pyruvate (1 mM), penicillin/streptomycin in presence of 5 mg/mL G418 to select for OVA expressing cells. HEK293T cells were kindly provided by H. Melichar, University of Montreal, Montreal, QC, Canada and cultured in DMEM supplemented with 10% of Nu serum and penicillin/streptomycin.
Adoptive Transfer and Immune Responses.
For the study of effector and memory CD8+ T cell generation, 5 × 103 to 104 Nr4a3+/+ or Nr4a3−/− OT-I CD8+ T cells (CD45.2+) isolated from lymph nodes (LNs) were adoptively transferred into recipients by i.v. injection. The following day, mice were infected or vaccinated as described below. For the study of the early in vivo immune response (days 1 to 3), 2 × 106 OT-I T cells were adoptively transferred. For competitive experiments, 104 Nr4a3+/+ or Nr4a3−/− OT-I T cells (CD45.2+) were cotransferred with 104 WT B6.SJL OT-I T cells (CD45.1+) into CD45.1.2 recipients.
Lm-OVA Infection.
A total of 2 × 103 CFU of L. monocytogenes genetically modified to express OVA (Lm-OVA) [grown as previously described (57, 58)] was injected i.v. For analysis of secondary response, 104 Nr4a3+/+ or Nr4a3−/− (CD45.2+), generated in vivo following Lm-OVA infection, were adoptively transferred into a B6.SJL (CD45.1+). On the following day, mice were infected with 104 CFUs of Lm-OVA. The secondary response was evaluated at day 7 postinfection.
LCMV-OVA Infection.
The strain of LCMV Armstrong encoding OVA (LCMV-OVA) was kindly provided by Juan C. de la Torre, The Scripps Research Institute, La Jolla, CA (59). The virus was produced by infection of the L929 fibroblast cell line, cultured in Minimum Essential Medium (MEM) containing 5% heat inactivated Nu serum, followed by harvesting of the produced virus in the supernatant. The virus titer was determined using MC57G fibroblasts as previously described (60). Mice were infected with 2 × 104 PFUs of LCMV-OVA by i.v. injection.
Dendritic Cell Immunization.
Dendritic cell (DC) immunization was performed as previously described (38). Bone marrow cells were cultured at 0.25 × 106 cells/mL in six-well plates in complete media supplemented with recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF; 25 ng/mL) and IL-4 (from P815-IL-4 cell culture supernatant). On days 2 and 3 of the differentiation culture, half of the media was removed and replaced with a fresh IL-4 and rGM-CSF supplemented complete media. On day 6, lipopolysaccharides (LPS; 0.1 µg/mL) was added to mature DCs and OVA peptide was loaded overnight (2 µg/mL). For immunization, 5 × 105 OVA peptide loaded DCs were injected i.v.
Analysis of Polyclonal CD8+ T Cell Response following Lm-OVA Infection.
At day 7 postinfection with Lm-OVA (2 × 103 CFUs, i.v.), the response of OVA-specific CD8+ T cells in the spleen was evaluated using Kb-OVA tetramer staining as previously described (9).
Retroviral Supernatant Production.
pMSCV IRES GFP (plasmid of murine stem cell virus with internal ribosome entry site element with GFP also called pMIG) was a kind gift from Guy Sauvageau (Institut de recherche en immunologie et en cancérologie IRIC, Montreal, QC, Canada, 61) and pMIG-Nr4a3 has been previously described (23). Retroviral supernatants were produced by collecting the cell culture media at days 2 and 3 after transfection of HEK293T cells with pMIG or pMIG-Nr4a3.
Nr4a3 Retroviral Overexpression.
Nr4a3+/+ or Nr4a3−/− OT-I T cells (CD45.2+) were isolated from LNs and mixed with B6.SJL spleen (CD45.1+) cells in a 2:3 ratio. Cells were cultured at 5 × 106 cells/mL in the presence of 0.1 µg/mL of OVA peptide. At 24 h poststimulation, cells were harvested and spin transduced with retroviral supernatant (pMIG retrovirus [RV] or pMIG Nr4a3 containing RV) in the presence of polybrene (8 µg/mL) for 1 h at 2,500 rpm and 37 °C. The cells were incubated for an additional 1 to 2 h at 37 °C and then injected (3 × 105 OT-I cells) in mice infected 24 h earlier with Lm-OVA (2 × 103 CFU/mouse).
MPEC Competitive Transfer.
A total of 106 Nr4a3+/+ or Nr4a3−/− OT-I T cells (CD45.2+) were adoptively transferred in competition with 106 OT-I T cells (CD45.1+) from B6.SJL mice into CD45.1+CD45.2+ mice. The following day, recipient mice were infected with 2 × 103 CFUs of Lm-OVA. At day 5 postinfection, spleens were harvested to sort Nr4a3+/+ or Nr4a3−/− OT-I MPECs (CD8+CD45.2+CD127+KLRG1−) and WT OT-I MPECs (CD8+CD45.1+ CD127+ KLRG1−). A total of 1 to 2 × 105 MPECs of each genotype were mixed in a 1:1 ratio and transferred into CD45.1+CD45.2+ recipient mice. Twenty days later, spleens and LNs were harvested to track OT-I T cell differentiation.
Stimulation of Memory T Cells with rhIL-15.
Splenocytes (106/mL) from mice containing OT-I memory T cells were labeled with CellTrace Violet (CTV) and cultured with 50 ng/mL of rhIL-15 (R&D) for 3 d.
Adoptive Cell Therapy with In Vivo Generated Memory T Cells.
B6.SJL mice were injected s.c. with 5 × 105 B16-OVA cells into the right flank. Seven days posttumor implantation, tumor-bearing mice were treated with 1.35–2 × 105 in vivo generated Nr4a3+/+ or Nr4a3−/− OT-I memory T cells obtained following Lm-OVA infection. Tumor growth was followed until experimental endpoint (200 mm2 or tumor ulceration) was reached.
Samples Preparation for Flow Cytometry Analysis.
Blood was collected in phosphate buffered saline (PBS) containing 2 mM ethylenediaminetetraacetic acid (EDTA) and lymphocytes were isolated with lymphocyte separation medium. Spleens and LNs were dissociated using frosted glass slides. Red blood cell lysis was performed on spleens and bone marrow using 0.83% NH4Cl for 5 min at room temperature (RT). Extracellular staining was performed for 20 min at 4 °C as in a previously described FACS wash (FW) buffer (62). Intranuclear staining for different transcription factors was performed using the Foxp3/Transcription Factor Staining Buffer Set according to manufacturer (Invitrogen) instructions.
Lm-OVA Challenge.
Mice that were previously adoptively transferred with OT-I T cells and infected with LCMV-OVA (as described above) were challenged at day 43 with 5 × 105 CFUs of Lm-OVA. On day 3 postchallenge, spleen and liver were collected for analysis of bacterial burdens as previously described (57).
Flow Cytometry and Cell Sorting.
Flow cytometry was performed on cellular suspensions of spleen, LN, bone marrow, and blood. Extracellular and intranuclear stainings were performed as previously described (57, 62, 63). CTV staining was performed according to the manufacturer (Thermo Fisher) recommendations. Apoptosis was quantified using AnnexinV and 7-amino-actinomycin D (7-AAD) staining as previously described (38). Ki67 staining was performed by using FoxP3 Transcription Factor Staining Kit following manufacturer recommendations. At 2 wk following bromodeoxyuridine (BrdU) treatment (64), BrdU staining was performed as previously described (38). Cell sorting of OT-I Nr4a3+/+ or Nr4a3−/− cells was performed on in vitro stimulated or ex vivo splenocytes. Briefly, cell suspensions were incubated with viability dye and Fc-block antibodies for 10 min at RT. Cell suspensions were then stained with extracellular antibodies 20 min at 4 °C in sorting buffer (PBS, 1% Nu serum, 1 mM EDTA, 25 mM Hepes). Viable cells (Zombie-dyenegCD8+CD45.2+) were then sorted (BD FACSAria III) directly in TRIzol (Life Technologies) reagent and stored at −80 °C until RNA extraction or sorted in serum-containing media (20% Nu serum containing RPMI) for subsequent use. For cytokine production by retrovirally transduced OT-I T cells, CD8+CD45.2+GFP+ or CD8+CD45.2+GFP− cells were sorted and then restimulated as described below. Flow cytometry analysis was performed on a BD LSR II or BD LSRFortessa X-20 from BD Biosciences and data were analyzed using FlowJo software (Tree Star). A list of antibodies and flow cytometry reagents is provided in SI Appendix, Table S1.
Analysis of Cytokine Production.
Ex vivo splenocytes were collected and stimulated with the OVA peptide (2 µg/mL) in the presence of brefeldin A (BFA, 10 µg/mL) for 5 h at 37 °C while in vitro activated OT-I effectors were restimulated with OVA-loaded splenocytes. Following stimulation, cells were fixed, permeabilized, and stained as previously described (63). Briefly, restimulated cells were fixed 20 min with 2% paraformaldehyde at RT. Fixed cells were permeabilized with saponin (0.5%) FW for 10 min at RT. Permeabilized cells were stained with anti-cytokine antibodies followed by cell surface staining.
Anti-CD3/CD28 Stimulation.
A total of 106 cells/mL from LNs of OT-I mice were stimulated in 24-well plates with 1 µg of plate-bound anti-CD3 (clone 145-2C11) antibody and 5 µg/mL soluble anti-CD28 (clone 37.51) antibody for 72 h.
Quantitative Real-Time PCR.
Total RNA from cells sorted in TRIzol was extracted using manufacturer recommendations. RNA was reverse-transcribed into cDNA and then used for the qPCR reaction with Power SYBR Green reagents (Life Technologies) on a 7500 Real-Time PCR System (Applied Biosystems). Each sample was normalized to a reference gene expression (Hprt) and a calibrator (reference sample) as previously described (57). ΔCT was calculated as the difference between the CT value of the target gene and the CT value of the reference gene (Hprt). The ΔΔCT was then calculated by subtracting the mean of ΔCT value of the sample from the ΔCT value of a reference sample. The relative level of target gene expression was calculated using 2−ΔΔCT. The sequences of the Id2, Id3, Tcf7, Prdm1, Il2, Tnfa, and Ifng primers are listed in SI Appendix, Table S1.
RNA-Seq Analysis.
Mice were adoptively transferred with naive OT-I T cells purified with the EasySep Isolation Kit and infected with Lm-OVA. At 72 h postinfection, viable and activated (Zombie-dyeneg CD44hi) Nr4a3+/+ or Nr4a3−/− OT-I T cells were sorted directly into TRIzol reagent. A total of 5 to 6 individual biological samples (2 to 3 mice per group of two independent experiments) were collected for each genotype with 105 cells per sample. RNA extraction, library preparation, sequencing, and bioinformatical analysis were done at the IRIC Genomics Platform (University of Montreal). Briefly, for cDNA libraries preparation, 100 ng of RNA was used and the cDNA library sequencing was performed on Illumina Nextseq500 (75 cycles single-end reads). Sequenced reads were trimmed for sequencing adapters and low-quality 3′ bases using Trimmomatic version 0.35 (65) and then aligned to the reference mouse genome version GRCh38 (gene annotation from Gencode version M13, based on Ensembl 88) using STAR version 2.5.1b (66). Gene expressions were obtained both as readcount directly from STAR as well as computed using RSEM (67) in order to obtain gene and transcript-level expression, either in transcripts per kilobase million or fragments per kilo base per million mapped reads values, for these stranded RNA libraries. DESeq2 version 1.22.2 (68) was then used to normalize gene readcounts and compute differential expression between the different experimental conditions.
ATAC-Seq Analysis.
Nr4a3+/+ or Nr4a3−/− OT-I T cells were isolated from LNs and purified using the naive CD8+ T cell isolation kit of EasySep according to the manufacturer instructions. Isolated naive OT-I T cells were stimulated as described in Materials and Methods with anti-CD3/CD28 antibodies. At 12 h poststimulation, cells were harvested and nuclei isolated as previously described (69). Briefly, the stimulated OT-I cells were harvested on ice by washing the stimulation plates with ice-cold PBS. The collected cells were centrifuged 5 min at 1,300 rpm and then resuspended to 20 × 106 cells per mL with ice-cold PBS. The cell lysis was performed for 10 min on ice by adding to one volume of ice-cold PBS resuspended cells four volumes of lysis buffer (12.5 mM Tris, pH 7.4, 45 mM KCl, 6.25 mM MgCl2, 375 mM sucrose, 0.125% Nonidet P-40, and tablet of complete [EDTA free] protease inhibitor mixture [Roche]/50 mL). Lysed cells were then centrifuged at 500 × g for 7 min at 4 °C in a precooled centrifuge with swinging buckets. Obtained nuclei pellet was resuspended by pipetting in 1/10 of the original lysis volume in wash buffer (10 mM Tris, pH 7.4, 60 mM KCl, 15 mM NaCl, 5 mM MgCl2, 300 mM sucrose) and then the wash buffer was added to 1/2 of the lysis volume. The resuspended nuclei were centrifuged at 500 × g 7 min at 4 °C in a precooled centrifuge with swinging buckets and the washed nuclei were counted and used for the transposase reaction. ATAC-seq was performed as previously described (70) with some modifications (Tn5 tagmentation, DNA purification, library preparation, and sequencing and bioinformatical analysis were performed at the Montreal Clinical Research Institute Genomics Platform at the University of Montreal). Briefly, 5 × 104 nuclei were directly treated with Tn5 transposase at 37 °C for 30 min. After the enzymatic reaction, the DNA was purified by Mini-Elute PCR Purification columns (Qiagen) and enriched by 12 cycles of PCR. The library was recovered from PCR by GeneRead Purification columns (Qiagen) and then it was double-strand sequenced on Illumina (HiSeq4000 PE50). A total of 47.3 to 75 × 106 reads/sample were obtained. These reads were assessed for quality control with FASTQC (0.11.8) and combined with MultiQC. The adapters were removed with Trimmomatic (0.36) (71) and reads were aligned with Bowtie2 (2.2.6) (72) to mm10 mouse genome. Mitochondrial reads and PCR duplicates were removed with Picard’s MarkDuplicates tools (2.4.1) and shift reads of Tn5 insertion were determined with Deeptools (73). Peaks calling was done with MACS2 (2.0.10) (74) and their filtering with ENCODE blacklisted regions. Comparison of DARs between 12-h stimulated OT-I Nr4a3−/− and Nr4a3+/+ samples was performed with DESeq2 (1.22.2) (75). The log2FC was calculated between Nr4a3−/− and Nr4a3+/+ conditions; a positive DAR value means that the chromatin is more open in the Nr4a3−/− sample and a negative DAR value means that the chromatin is more open in the Nr4a3+/+ sample. DARs were annotated and different transcription binding motifs identified with HOMER (4.8.0). Tn5 tagmentation, DNA purification, library preparation, and sequencing and bioinformatical analysis were performed at the Montreal Clinical Research Institute (IRCM) Genomics Platform (University of Montreal).
Statistical Analyses.
Statistical analyses were performed using an unpaired (two tailed) Student’s t test, with a Welch’s correction when applied, or using a Mann–Whitney test when a low number of samples (lower than seven samples) was available. For multiple group comparisons, the one-way ANOVA (Kruskal–Wallis) analysis with Dunn’s multiple comparisons was performed. For survival curve comparison a log-rank Mantel–Cox test was used. The statistical analysis was performed using Prism software (GraphPad Software). Data are presented as individual samples with mean and *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank all laboratory members for helpful discussion. We thank S. Lesage, J.-S. Delisle, and H. Melichar for critical reading of the manuscript. We thank Dr O. Conneely for providing Nr4a3−/− mice, A. Lamarre for the B16-OVA cell line, J. C. de la Torre for LCMV-OVA, S. P. Schoenberger for Lm-OVA, and M.-È. Lebel for advice on the B16-OVA tumor model. We thank M. Dupuis for cell sorting and the animal care technicians for mice husbandry. We are grateful to S. Boissel and P. Gendron from the IRIC genomic platform for help with the RNA-seq experiment and bioinformatical analysis and to V. Calderon, C. Grou, and O. Neyret from IRCM for help with the ATAC-seq experiment. This work was supported by a grant from the Canadian Institutes of Health Research (MOP 142333) and by a discovery grant from the Natural Sciences and Engineering Research Council of Canada (RGPIN-2015-06645) to N.L. L.O. was supported by a studentship from the Fonds de la Recherche Québec-Santé.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2007224117/-/DCSupplemental.
Data and Materials Availability.
The raw RNA-seq and ATAC-seq data are available in the Gene Expression Omnibus (GSE143513) (76) and the analyzed results are presented in the supplemental materials. All other data needed to evaluate the conclusions of this paper are presented in the figures or the supplementary materials.
References
- 1.Kaech S. M. et al., Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4, 1191–1198 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Joshi N. S. et al., Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarkar S. et al., Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 205, 625–640 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutishauser R. L. et al., Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31, 296–308 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallies A., Xin A., Belz G. T., Nutt S. L., Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity 31, 283–295 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Yang C. Y. et al., The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat. Immunol. 12, 1221–1229 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji Y. et al., Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat. Immunol. 12, 1230–1237 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backer R. A. et al., A central role for Notch in effector CD8(+) T cell differentiation. Nat. Immunol. 15, 1143–1151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathieu M., Duval F., Daudelin J. F., Labrecque N., The Notch signaling pathway controls short-lived effector CD8+ T cell differentiation but is dispensable for memory generation. J. Immunol. 194, 5654–5662 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Omilusik K. D. et al., Transcriptional repressor ZEB2 promotes terminal differentiation of CD8+ effector and memory T cell populations during infection. J. Exp. Med. 212, 2027–2039 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominguez C. X. et al., The transcription factors ZEB2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J. Exp. Med. 212, 2041–2056 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Intlekofer A. M. et al., Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Ichii H. et al., Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat. Immunol. 3, 558–563 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Roychoudhuri R. et al., BACH2 regulates CD8(+) T cell differentiation by controlling access of AP-1 factors to enhancers. Nat. Immunol. 17, 851–860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X. et al., Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity 33, 229–240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang J. T. et al., Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315, 1687–1691 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Scott-Browne J. P. et al., Dynamic changes in chromatin accessibility occur in CD8+ T cells responding to viral infection. Immunity 45, 1327–1340 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu B. et al., Epigenetic landscapes reveal transcription factors that regulate CD8+ T cell differentiation. Nat. Immunol. 18, 573–582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D. et al., The transcription factor Runx3 establishes chromatin accessibility of cis-regulatory landscapes that drive memory cytotoxic T lymphocyte formation. Immunity 48, 659–674.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Best J. A. et al.; Immunological Genome Project Consortium , Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nat. Immunol. 14, 404–412 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearen M. A., Muscat G. E. O., Minireview: Nuclear hormone receptor 4A signaling: Implications for metabolic disease. Mol. Endocrinol. 24, 1891–1903 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanna R. N. et al., The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat. Immunol. 12, 778–785 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulet S. et al., The orphan nuclear receptor NR4A3 controls the differentiation of monocyte-derived dendritic cells following microbial stimulation. Proc. Natl. Acad. Sci. U.S.A. 116, 15150–15159 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullican S. E. et al., Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat. Med. 13, 730–735 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Carlin L. M. et al., Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 153, 362–375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanna R. N. et al., NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ. Res. 110, 416–427 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekiya T. et al., Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 14, 230–237 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Fassett M. S., Jiang W., D’Alise A. M., Mathis D., Benoist C., Nuclear receptor Nr4a1 modulates both regulatory T-cell (Treg) differentiation and clonal deletion. Proc. Natl. Acad. Sci. U.S.A. 109, 3891–3896 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Q. N., Suen A. Y. W., Henao Caviedes L. M., Baldwin T. A., Nur77 regulates nondeletional mechanisms of tolerance in T cells. J. Immunol. 199, 3147–3157 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Mognol G. P. et al., Exhaustion-associated regulatory regions in CD8+ tumor-infiltrating T cells. Proc. Natl. Acad. Sci. U.S.A. 114, E2776–E2785 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pauken K. E. et al., Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez G. J. et al., The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity 42, 265–278 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J. et al., NR4A transcription factors limit CAR T cell function in solid tumours. Nature 567, 530–534 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo H. et al., TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc. Natl. Acad. Sci. U.S.A. 116, 12410–12415 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X. et al., Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature 567, 525–529 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hogquist K. A. et al., T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994). [DOI] [PubMed] [Google Scholar]
- 37.Kalia V. et al., Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32, 91–103 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Boulet S., Daudelin J.-F., Labrecque N., IL-2 induction of Blimp-1 is a key in vivo signal for CD8+ short-lived effector T cell differentiation. J. Immunol. 193, 1847–1854 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Arsenio J. et al., Early specification of CD8+ T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nat. Immunol. 15, 365–372 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wingender E., Kel A., geneXplain–Eine integrierte Bioinformatik-Plattform. BIOspektrum (Heidelb.) 18, 554–556 (2012). [Google Scholar]
- 41.Kurachi M. et al., The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat. Immunol. 15, 373–383 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynn R. C. et al., c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature 576, 293–300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowyhed H. N., Huynh T. R., Thomas G. D., Blatchley A., Hedrick C. C., Cutting edge: The orphan nuclear receptor Nr4a1 regulates CD8+ T cell expansion and effector function through direct repression of Irf4. J. Immunol. 195, 3515–3519 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duren R. P., Boudreaux S. P., Conneely O. M., Genome wide mapping of NR4A binding reveals cooperativity with ETS factors to promote epigenetic activation of distal enhancers in acute myeloid leukemia cells. PLoS One 11, e0150450 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Man K. et al., The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat. Immunol. 14, 1155–1165 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Raczkowski F. et al., The transcription factor Interferon Regulatory Factor 4 is required for the generation of protective effector CD8+ T cells. Proc. Natl. Acad. Sci. U.S.A. 110, 15019–15024 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang R. et al., The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chou C. et al., c-Myc-induced transcription factor AP4 is required for host protection mediated by CD8+ T cells. Nat. Immunol. 15, 884–893 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saijo K. et al., A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137, 47–59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moran A. E. et al., T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jennings E., et al. , Differential Nr4a1 and Nr4a3 expression discriminates tonic from activated TCR signalling events in vivo. bioRxiv:10.1101/767566v2 (15 January 2020). [Google Scholar]
- 52.Milner J. J. et al., Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 552, 253–257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beura L. K. et al., T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity 48, 327–338.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boddupalli C. S. et al., ABC transporters and NR4A1 identify a quiescent subset of tissue-resident memory T cells. J. Clin. Invest. 126, 3905–3916 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurd N. S. et al., Early precursors and molecular determinants of tissue-resident memory CD8+ T lymphocytes revealed by single-cell RNA sequencing. Sci. Immunol. 5, eaaz6894 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ponnio T., Burton Q., Pereira F. A., Wu D. K., Conneely O. M., The nuclear receptor Nor-1 is essential for proliferation of the semicircular canals of the mouse inner ear. Mol. Cell. Biol. 22, 935–945 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathieu M. et al., CD40-activated B cells can efficiently prime antigen-specific naïve CD8+ T cells to generate effector but not memory T cells. PLoS One 7, e30139 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bahjat K. S. et al., Cytosolic entry controls CD8+-T-cell potency during bacterial infection. Infect. Immun. 74, 6387–6397 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerlach C. et al., The chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity 45, 1270–1284 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Battegay M. et al., Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33, 191–198 (1991). [DOI] [PubMed] [Google Scholar]
- 61.Antonchuk J., Sauvageau G., Humphries R. K., HOXB4 overexpression mediates very rapid stem cell regeneration and competitive hematopoietic repopulation. Exp. Hematol. 29, 1125–1134 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Ostiguy V., Allard E.-L., Marquis M., Leignadier J., Labrecque N., IL-21 promotes T lymphocyte survival by activating the phosphatidylinositol-3 kinase signaling cascade. J. Leukoc. Biol. 82, 645–656 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Lacombe M.-H., Hardy M.-P., Rooney J., Labrecque N., IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J. Immunol. 175, 4400–4407 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Chaix J. et al., Cutting edge: CXCR4 is critical for CD8+ memory T cell homeostatic self-renewal but not rechallenge self-renewal. J. Immunol. 193, 1013–1016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bolger A. M., Lohse M., Usadel B., Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dobin A. et al., STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li B., Dewey C. N., RSEM: Accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pipkin M. E., Lichtenheld M. G., A reliable method to display authentic DNase I hypersensitive sites at long-ranges in single-copy genes from large genomes. Nucleic Acids Res. 34, e34 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buenrostro J. D., Giresi P. G., Zaba L. C., Chang H. Y., Greenleaf W. J., Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lohse M. et al., RobiNA: A user-friendly, integrated software solution for RNA-seq-based transcriptomics. Nucleic Acids Res. 40, W622-7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langmead B., Salzberg S. L., Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramírez F. et al., deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160-5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y. et al., Model-based analysis of ChIP-seq (MACS). Genome Biol. 9, R137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anders S., Huber W., Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Odagiu L., Labrecque N., Early programming of CD8+ T cell response by the orphan nuclear receptor NR4A3. Gene expression omnibus (GEO). https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE143513. Deposited 13 January 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw RNA-seq and ATAC-seq data are available in the Gene Expression Omnibus (GSE143513) (76) and the analyzed results are presented in the supplemental materials. All other data needed to evaluate the conclusions of this paper are presented in the figures or the supplementary materials.