Significance
Wolbachia (wMel strain)-infected Aedes aegypti mosquitoes are refractory to disseminated arboviral infections. Yet previous studies into the mechanism behind Wolbachia-mediated virus blocking have not considered the involvement of lipids, apart from cholesterol, during superinfection. We used liquid chromatography mass spectrometry to study the lipidome in mosquito cells infected with virus, wMel, or superinfected with both virus and wMel. Interestingly, a class of lipids, acyl-carnitines increased during virus infection but remained low with wMel. These findings uncover a previously unknown role for acyl-carnitines in the interaction among virus, wMel, and cells, suggesting a mechanism underlying Wolbachia-mediated pathogen blocking. Importantly, this study supports Wolbachia introgression into A. Aegypti populations as a biocontrol method to reduce arboviral (e.g., DENV) transmission.
Keywords: Wolbachia, lipids, flavivirus
Abstract
Wolbachia-infected mosquitoes are refractory to flavivirus infections, but the role of lipids in Wolbachia-mediated virus blocking remains to be elucidated. Here, we use liquid chromatography mass spectrometry to provide a comprehensive picture of the lipidome of Aedes aegypti (Aag2) cells infected with Wolbachia only, either dengue or Zika virus only, and Wolbachia-infected Aag2 cells superinfected with either dengue or Zika virus. This approach identifies a class of lipids, acyl-carnitines, as being down-regulated during Wolbachia infection. Furthermore, treatment with an acyl-carnitine inhibitor assigns a crucial role for acyl-carnitines in the replication of dengue and Zika viruses. In contrast, depletion of acyl-carnitines increases Wolbachia density while addition of commercially available acyl-carnitines impairs Wolbachia production. Finally, we show an increase in flavivirus infection of Wolbachia-infected cells with the addition of acyl-carnitines. This study uncovers a previously unknown role for acyl-carnitines in this tripartite interaction that suggests an important and broad mechanism that underpins Wolbachia-mediated pathogen blocking.
The discovery that introduction of Wolbachia, an endosymbiotic bacterium, into Aedes aegypti (A. aegypti) interferes with RNA virus replication has led to the deployment of Wolbachia as a tool for mosquito biocontrol (1, 2). Studies have shown that Wolbachia blocks the replication of arboviruses such as dengue virus (DENV), Zika virus (ZIKV), and chikungunya virus (1, 3, 4). With field trials demonstrating that Wolbachia are also capable of spreading through wild A. aegypti populations, Wolbachia biocontrol holds much promise in eliminating arbovirus transmission (1, 2). Multiple Wolbachia strains exist but only certain variants (wMel, wAlbB) block virus transmission without greatly impacting mosquito fitness (5–8). Currently, the release of wMel A. aegypti mosquitoes is being evaluated for its potential to control DENV transmission in several countries (including Brazil, Indonesia, Vietnam, Columbia, Australia) (9–12).
One mystery that remains unsolved is the mechanism behind Wolbachia-mediated virus blocking. Possible contributory factors include immune activation through the up-regulation of the reactive oxygen species (ROS)-dependent activation of Toll pathway genes and host microRNAs in A. aegypti (13–17). A comparative analysis of Wolbachia strains suggested the presence of a single mechanism of virus protection with a broad spectrum of virus inhibition (14). It has also been inferred that the degree of blocking conferred by different Wolbachia strains correlates with the density of those strains in key tissues, leading to the hypothesis that competition for host intracellular molecules underpins the virus blocking mechanism (6, 14, 18). Indeed, supplementation with cholesterol in Drosophila and mosquito cell lines containing Wolbachia increased virus replication, implying that both virus and Wolbachia may be competing for cholesterol (19, 20). Other groups have shown that Wolbachia-infected mosquito cells had perturbed cellular lipid profiles and up-regulation of the fatty acid synthase (FAS) and Acyl-CoA thioesterase (ACOT) enzymes, resulting in increased complex lipid synthesis and fatty acyl-CoAs (FA-CoA) catabolism (16, 20–22).Together these studies suggest that the lipidome is significantly altered with Wolbachia infection and may consequently affect the capacity for viral infection.
Flaviviridae such as DENV, West Nile virus (WNV), and hepatitis C virus rely heavily on cellular lipids during replication (23–25). Not only are the lipids integrated into virus-induced structures, but virus replication has been shown to alter cellular metabolism profoundly, including increases in fatty acid and cholesterol biosynthesis and up-regulation of the host FAS enzyme (26–29). Lipidomic studies on DENV-infected mosquitoes, mosquito cells, and whole WNV virions have identified discrete alterations of the lipidome during infection (25, 30, 31). Any Wolbachia-mediated alterations to the cellular lipidome that are not favorable would undeniably affect virus replication.
To gain insights into the Wolbachia-mediated virus blocking mechanism, we used liquid chromatography mass spectrometry (LCMS) to examine changes in the cellular lipidome upon combinations of infection with wMel, DENV, and ZIKV. Our results demonstrated significant fluctuations in various lipids that function as membrane building blocks, energy intermediates, and lipids involved in biosynthesis and lipolysis pathways. One prominent observation involves a class of lipids known as acyl-carnitines. Our results demonstrate that wMel modulates acyl-carnitine levels in mosquito cells, which is highly detrimental to both ZIKV and DENV replication. In conclusion, our findings provide evidence that a Wolbachia-modulated lipidome contributes toward flavivirus resistance. Additionally, we uncover a previously unknown role of acyl-carnitines in this tripartite interaction that suggests an important and broad mechanism that underpins Wolbachia-mediated pathogen blocking.
Results
The Mosquito Cell Lipidome Is Significantly Altered by wMel and Virus Infection.
To investigate if the mosquito lipidome is involved in the Wolbachia-mediated virus blocking mechanism, we undertook a whole-cell lipidomics approach. Briefly, Aag2.wMel and Aag2.TET cells were infected with DENV-1 and two different strains of ZIKV (Zika Asian and Zika African) and extracted for LCMS analysis 48 h postinfection (48 h.p.i.) (SI Appendix, Figs. S1 and S2). LCMS targeted lipidomic analysis resulted in the identification of a total of 335 lipids across five major lipid classes: phospholipids, sphingolipids, glycerolipids, sterols, and carnitines (Dataset S1). The abundance of these lipids under the various conditions (wMel infection only, wMel/virus infection, virus infection only, and the no-infection control) is shown in Dataset S1 (SI Appendix). These features were subjected to statistical analysis to simultaneously compare the differences in metabolite abundance among three datasets: wMel-infected and uninfected cells, virus-infected and uninfected cells, and wMel/virus-infected cells and uninfected cells. Lipids showing statistically significant differences in abundance (P < 0.05) were identified (SI Appendix, Fig. S3 and Dataset S2).
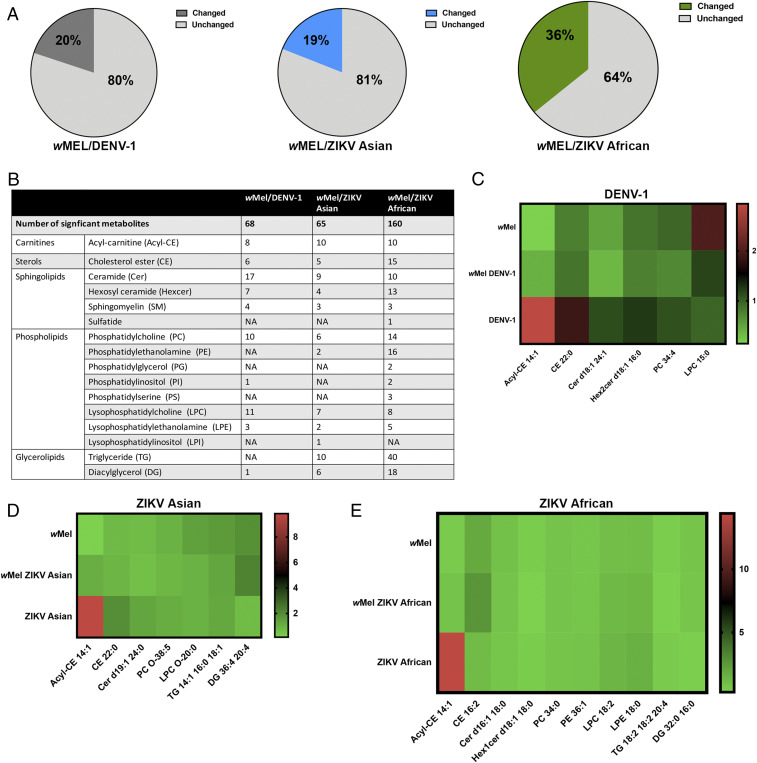
Relative to uninfected cells, 20%, 19%, and 36% of the total lipids were altered in abundance in wMel/DENV-1, wMel/ZIKV Asian, and wMel/ZIKV African conditions, respectively (Fig. 1A). There were significant changes in various lipid classes, but a majority of the changes were in phospholipids and sphingolipids (Fig. 1B). Data from the heatmap analysis showed that acyl-carnitines, cholesterol esters, and sphingolipids were generally up-regulated during virus infection but decreased in abundance with wMel infection (Fig. 1 C–E). The exception was ZIKV African, which resulted in an increase in acyl-carnitines but not cholesterol esters or sphingolipids (Fig. 1E). There were no distinct patterns of change in phospholipid abundance (Fig. 1 C–E). Both Zika viruses caused a decrease in DGs, but coinfection with wMel increased the DG levels (Fig. 1 D and E). In contrast, DENV-1 infection did not modulate the glycerolipids significantly (Fig. 1B). In summary, acyl-carnitines, cholesterol esters, sphingolipids, and DGs are modulated differently during virus infection as compared to wMel infection, thus suggesting that these lipid classes may be implicated in the mechanism of virus blocking.
Fig. 1.
Results from LCMS analysis of the mosquito cellular lipidome upon infection wMel/arbovirus infection. (A) Pie charts showing the percentages of the cellular lipidome changed upon superinfection of Aag2.wMel cells with Dengue-1 (DENV-1), Zika African (ZIKV African) and Zika Asian (ZIKV Asian) viruses in comparison to uninfected cells. (B) Table listing the number of metabolites significantly modulated under the three experimental conditions (wMel/DENV-1, wMel/ZIKV Asian, wMel/ZIKV African) and the various lipid classes they belong to. (C–E) Heatmaps illustrating normalized metabolites, each representative of its lipid class under three conditions: wMel infection only, virus infection only, and wMel/virus infection. Briefly, metabolites that were significantly modulated during superinfection with virus/wMel were grouped into lipid classes (Dataset S2). Lipid classes with <5 significant metabolites were excluded. An example was then selected to depict the pattern of abundance of each lipid class under each infection condition. After selection, fold changes were calculated based on measures of the specific metabolite during infection expressed as a ratio against that metabolite in uninfected cells. This data were applied to heatmaps for easy interpretation of the common changes seen in the lipidome. DENV-1 (C), ZIKV Asian (D), ZIKV African (E).
Acyl-Carnitines Increase Significantly upon Infection with Three Viruses but Remain Down-Regulated during wMel Infection.
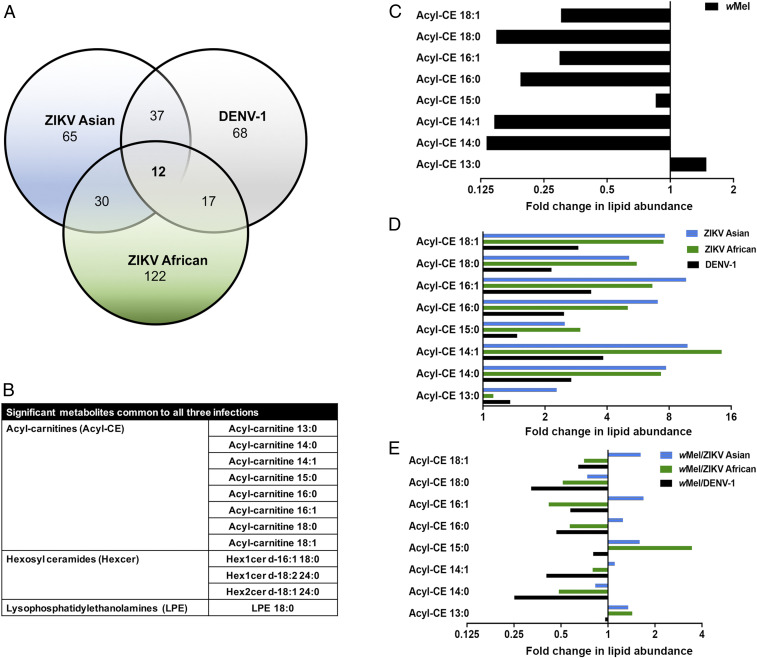
We tested for significant lipids that were common to infection by all three viruses in Aag2.wMel cells. Remarkably, we discovered that only 12 lipid moieties were significantly altered in Aag2.wMel cells during infection with DENV-1, ZIKV African, and ZIKV Asian as compared to uninfected cells (Fig. 2 A and B). Significantly modulated lipids comprised of a lysophospholipid LPE 18:0, three sphingolipids, (Hex1cer d-16:1 18:0, Hex1cer d-18:2 24:0, and Hex2cer d18:1 24:0) and eight acyl-carnitines. Acyl-carnitines are intermediate molecules that shuttle fatty acyl-CoA from the cytoplasm into the mitochondria for β-oxidation and subsequent energy (ATP) production.
Fig. 2.
Role of acyl-carnitines in virus superinfection of Aag2.wMel cells. (A) Venn diagram displaying the number of metabolites significantly altered (P < 0.05; in comparison to uninfected cells) in Aag2.wMel cells superinfected with DENV-1 or ZIKV Asian or ZIKV African. (B) Table showing metabolites common to superinfection with all three viruses in wMel-infected cells. (C–E) Bar charts showing the fold changes in acyl-carnitines during infection with wMel only (C), virus only (D), and wMel/virus (E). Fold changes shown are measures of the specific metabolite during infection expressed as a ratio against that metabolite in uninfected cells.
To further investigate the changes in acyl-carnitines, we compared wMel-infected, virus-infected, and wMel/virus-infected cells to uninfected cells. We observed that the levels of most acyl-carnitines were consistently lower (below basal fold change of 1) upon wMel infection as compared to uninfected cells (Fig. 2C). On the contrary, infection with all three viruses results in elevated levels of most acyl-carnitines, with fold changes ranging from 1.1 to 14 (Fig. 2D). However, upon superinfection with wMel and virus, we observed a drastic reduction in a majority of the acyl-carnitines, with the average fold change below the basal level of 1 (Fig. 2E). This provided further evidence that Wolbachia might be modulating levels of acyl-carnitines, which might impact virus replication.
Inhibiting Acyl-Carnitines Results in Abolished Virus Replication in Human Cells.
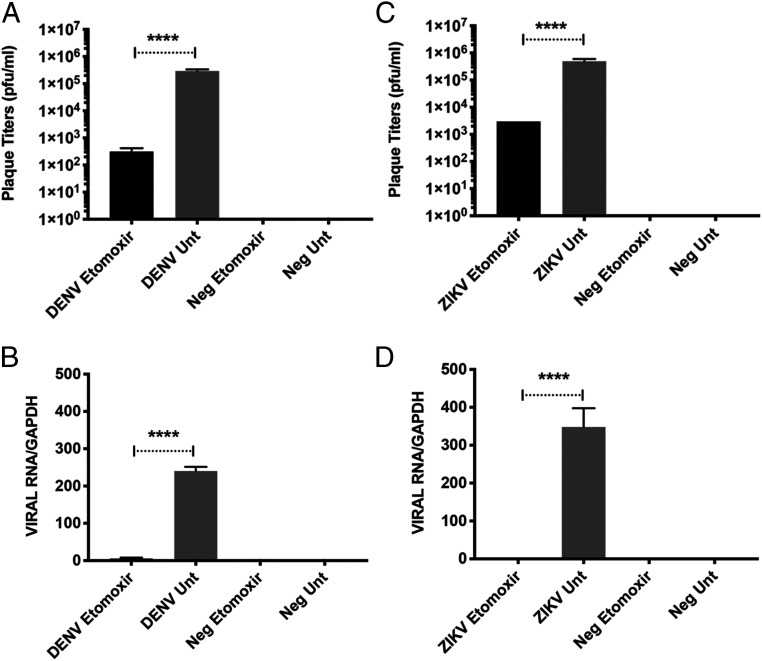
To test if elevated acyl-carnitine levels are required during virus infection, we next examined the impact of a widely used acyl-carnitine inhibitor, etomoxir, on infection with DENV-1 and ZIKV Asian (the more epidemiologically relevant ZIKV strain). Etomoxir is a small molecule inhibitor that irreversibly inhibits carnitine palmitoyl-transferase 1a (CPT1a), the mitochondrial enzyme responsible for the formation of acyl-carnitines (32, 33). Through LCMS analysis, we first confirmed that etomoxir treatment reduces levels of most acyl-carnitines but also causes global changes in the cellular lipidome (SI Appendix, Figs. S4 and S5). As compared to untreated cells, dramatically reduced viral RNA and plaque titers were observed in etomoxir-treated human cells for both ZIKV Asian and DENV-1 (Fig. 3). In fact, replication was almost abolished in treated cells, suggesting that acyl-carnitines are crucial in the virus lifecycle (SI Appendix, Figs. S3 and S4).
Fig. 3.
Effects of etomoxir, an irreversible acyl-carnitine inhibitor, on replication of dengue and Zika virus. HuH-7 cells were treated with 200 µM etomoxir, a widely used irreversible inhibitor of acyl-carnitines, for 48 h after which virus was added at MOI 5. Virus supernatant and cellular RNA were harvested at 24 h.p.i. for plaque assays and qRT-PCR. All results from qRT-PCR were normalized against GAPDH. (A) Plaque titers following DENV infection. (B) Expression levels of DENV RNA using primers targeting 3′ UTR. (C) Plaque titers following ZIKV Asian infection. (D) Expression levels of ZIKV Asian RNA using primers targeting NS5. Data are represented as mean ± SEM; ****P < 0.0001, two-tailed Student’s t test.
Etomoxir Treatment of A. aegypti Elevates wMel Levels but Restricts Virus Replication.
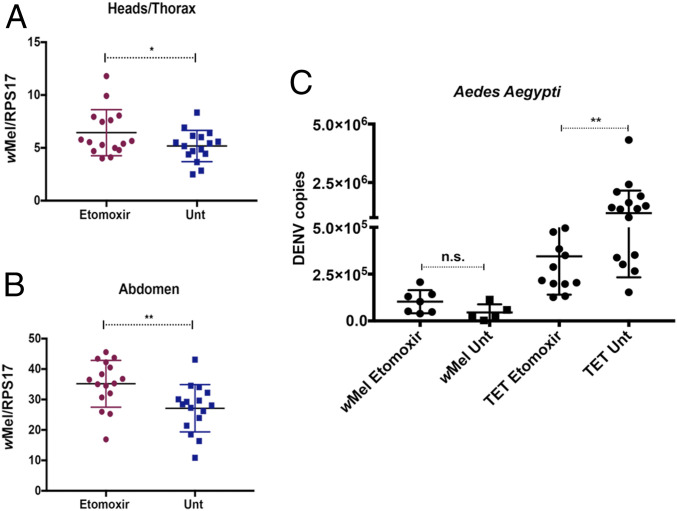
Our findings suggested that acyl-carnitines are important for virus replication. To test if wMel might be limiting virus replication in mosquitoes by affecting acyl-carnitine levels, A. aegypti mosquitoes reared with etomoxir were tested for wMel density and DENV blocking. Surprisingly, wMel densities in both heads/thoraxes and abdomens were significantly elevated upon etomoxir treatment (Fig. 4 A and B). The difference in levels was more evident in the abdomen than in the heads/thoraxes (Fig. 4 A and B) (34). These results collectively suggest that wMel thrives in an environment with reduced acyl-carnitines. There was no difference in DENV-2 RNA levels between etomoxir-treated and untreated wMel A. aegypti: both groups restricted DENV replication similarly (Fig. 4C). However, there was a significant 3.5-fold reduction of DENV copies in etomoxir-treated Tet-control mosquitoes as compared to untreated Tet-control mosquitoes (Fig. 4C). This reduction, although significant, was not as high as the DENV restriction observed in wMel mosquitoes (Fig. 4C). Taken together, this confirmed that the effect on acyl-carnitines by wMel could be one contributory factor that blocks virus replication in wMel-infected mosquitoes.
Fig. 4.
Effects of acyl-carnitine depletion on wMel and superinfection with dengue in A. aegypti mosquitoes. wMel A. aegypti mosquito larvae were reared in water alone or in water containing 100 µM etomoxir. Three days postemergence, female mosquitoes were collected for testing of Wolbachia density. Individual, female mosquitoes from each sample group were dissected to separate the abdomens from the heads and thoraxes. DNA was extracted and subjected to qPCR. Primers used targeted the Wolbachia surface protein, and results were normalized against the single-copy mosquito rps17 gene. Blue squares represent untreated mosquitoes, and red dots represent treated mosquitoes. (A) Wolbachia densities in the heads/thoraxes of treated versus untreated mosquitoes. (B) Wolbachia densities in abdomens of treated versus untreated mosquitoes. (C) Treated and untreated wMel and Tet-control adult female mosquitoes (7 d old) were injected with 3.4 × 105 TCID50/mL of DENV-2. Mosquitoes were incubated for 7 d before total RNA extraction was performed on entire mosquitoes, followed by DENV qRT-PCR. DENV absolute quantities were determined by extrapolation from an internal standard curve generated from plasmid DNA encoding the conserved 3′ UTR sequence. TET: mosquitoes from a tetracycline-treated line to deplete wMel completely; Unt: untreated; wMel: mosquitoes with wMel infection. Data are represented as mean ± SEM; *P < 0.05; **P < 0.01; n.s.: not significant, two-tailed Student’s t test.
Provision of Acyl-Carnitines to Aag2.wMel Cells Increased Virus Replication.
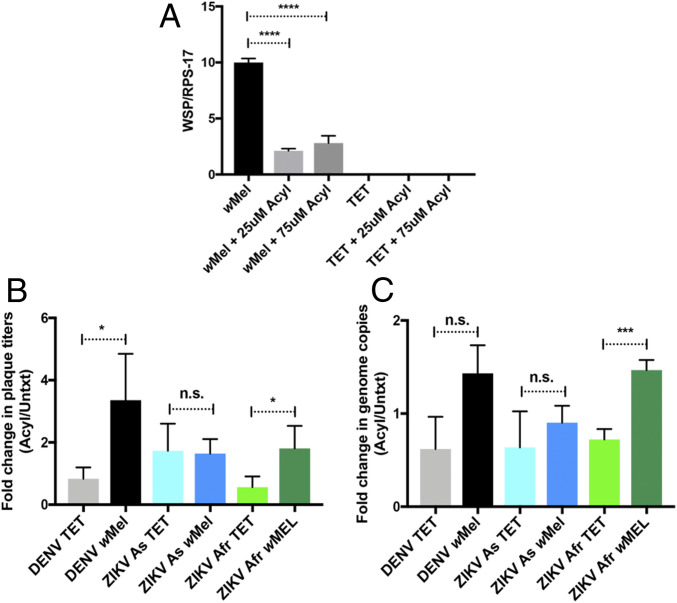
To further test if Wolbachia-mediated virus blocking is dependent on acyl-carnitines, we used commercially available acyl-carnitines to study wMel density and virus replication in Aag2.wMel and Aag2.TET cells. The relative wMel density significantly dropped from 10 to 2.5, with the addition of exogenous acyl-carnitines to Aag2.wMel cells (Fig. 5A). This further confirmed that wMel prefers an environment with reduced acyl-carnitines. We next measured virus replication in Aag2.wMel cells with the provision of exogenous acyl-carnitines. Our results revealed that DENV-1, ZIKV Asian, and ZIKV African plaque titers increased at least twofold and viral RNA levels in Aag2.wMel cells increased about onefold to twofold with the addition of exogenous acyl-carnitines (Fig. 5 B and C). As expected, the plaque titers and viral RNA levels increased in treated cells as compared to untreated cells even in the absence of wMel, further confirming that acyl-carnitines are crucial in virus infection (Fig. 5 B and C). Taken collectively, these data indicate that virus replication in wMel-infected cells can be increased by addition of acyl-carnitines, further supporting the hypothesis that acyl-carnitines are directly involved in the Wolbachia-mediated virus blocking mechanism.
Fig. 5.
Effects of addition of commercially available acyl-carnitines on flavivirus superinfection of wMel-infected cells. (A) Aag2.wMel and Aag2.TET cells were seeded onto plates and treated with a combination of commercially available acyl-carnitines or untreated. Concentrations shown (in micromolars) are the concentration of each acyl-carnitine added per well. Relative density of wMel is displayed, 3 d posttreatment. Primers used targeted the Wolbachia surface protein (WSP), and results were normalized against the single-copy mosquito rps17 gene. (B and C) Aag2.wMel and Aag2.TET cells were infected with DENV (MOI 1), ZIKV Asian (MOI 0.1), and ZIKV African (MOI 0.2) viruses. At 24 h.p.i., three commercially available carnitines, each at a concentration of 25 μM, were mixed and added to infected cells (49). Untreated cells (no added acyl-carnitines) were used as controls. Virus supernatant and cellular RNA were harvested at 96 h.p.i. Abbreviation “Acyl” represents the mixture of acyl-carnitines added. “Untxt” represents no treatment. TET: cells from a tetracycline-treated line to deplete wMel completely; wMel: cells with wMel infection. Results from at least three independent experiments are represented. Data are represented as mean ± SEM; *P < 0.05; ***P < 0.001; ****P < 0.0001; n.s.: not significant. (B) Fold change of plaque titers in the treated samples (Acyl) versus the untreated samples (Untxt) are shown. (C) Fold change of the relative expression of viral RNA levels in the treated samples (Acyl) versus the untreated samples (Untxt) are shown. All viral RNA levels were normalized against the mosquito housekeeping gene (rps17). Specific primers were used for each virus: Primers for DENV targeted the 3′ UTR, and primers for ZIKV Asian and ZIKV African targeted NS5.
Proposed Model Depicting the Mechanism behind Wolbachia-Mediated Blocking.
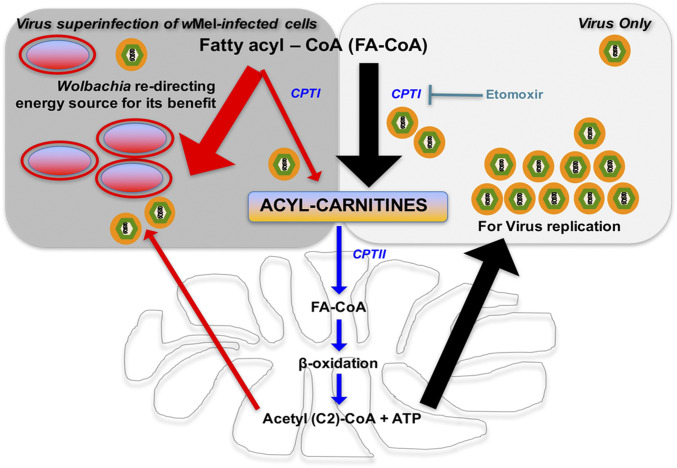
Based on our findings, we propose a model for the mechanism behind Wolbachia-mediated blocking (Fig. 6). During flavivirus infection, FA-CoAs (activated fatty acids) are converted to acyl-carnitines for transport into the mitochondria for β-oxidation and subsequent energy production (Fig. 6). Mitochondrial β-oxidation results in the production of ATP and acetyl (C2)-CoA (Fig. 6). The resulting ATP might be used as the energy source to produce more virions, and acetyl (C2)-CoA can be used for de novo synthesis of longer chain FA-CoA molecules at the site of virus replication (27, 30, 35). Indeed, several studies have shown that increased β-oxidation is vital for flavivirus replication (35–37). Such elevated energetic demands during virus infection might lead to large amounts of FA-CoAs entering the mitochondria but only partially being broken down by β-oxidation, which becomes a bottleneck for this reaction. This mitochondrial overload results in an accumulation of acyl-carnitines (Fig. 6). Thus, the increased levels of acyl-carnitines observed are important and necessary for efficient flavivirus replication.
Fig. 6.
Proposed model depicting the mechanism behind Wolbachia-mediated blocking. Flavivirus infection results in FA-CoAs (activated fatty acids) being converted to acyl-carnitines for transport into the mitochondria for β-oxidation. Mitochondrial β-oxidation results in production of ATP (energy that might be redirected to produce novel virions) and acetyl (C2)-CoA (that can be recycled to synthesize longer chain FA-CoA at site of viral replication). Such elevated energetic demands might lead to the accumulation of acyl-carnitines due to mitochondrial overload. Increased levels of acyl-carnitines are important and necessary for efficient virus replication. When Wolbachia is present, acyl-carnitine levels are modulated, driving the metabolic force in the opposite direction. This is likely due to Wolbachia redirecting the energy source for either the production of bacteria-specific lipids or Wolbachia-driven energy production. This immense reduction in acyl-carnitines caused by wMel renders virus replication highly inefficient in the presence of Wolbachia as downstream β-oxidation is dramatically reduced. Black arrows depict the scenario during virus infection, and red arrows depict the pathway altered in the presence of Wolbachia.
However, when Wolbachia is present, it modulates acyl-carnitine levels by driving the metabolic force in the opposite direction (Fig. 6). This is likely due to Wolbachia redirecting the energy source for either the production of bacteria-specific lipids or Wolbachia-driven energy production. The immense reduction in acyl-carnitines caused by wMel renders flavivirus replication highly inefficient in wMel-infected mosquito cells as downstream β-oxidation is dramatically reduced (Fig. 6). Reducing and sustaining the low acyl-carnitine levels could thus be detrimental to many viruses, as it interferes with FA-CoA metabolism, the primary hub integrating multiple lipid metabolic pathways. We suggest that these opposing metabolic driving forces result in competition between the endosymbiont wMel and virus and encompass a broad mechanism of Wolbachia-mediated virus blocking.
Discussion
Wolbachia has been introduced into mosquitoes to successfully prevent the spread of clinically important arboviruses, yet the mechanisms by which Wolbachia block virus transmission are not fully understood. Using a whole-cell lipidomics approach, here we report the involvement of acyl-carnitines, a class of lipids previously unknown to play a crucial role in Wolbachia-mediated virus blocking. Acyl-carnitines are important intermediate molecules that transport FA-CoA from the cytoplasm to the mitochondria for β-oxidation and, consequently, ATP production.
We observed a significant increase in acyl-carnitine levels upon ZIKV and DENV infection of Aag2.TET cells. Using an acyl-carnitine inhibitor, etomoxir, we also showed significant reduction in flavivirus replication in human cells. We found that etomoxir causes global changes in the lipidome, which could be attributed to off-target effects or overstimulation of compensatory lipid pathways caused by the irreversible effect on CPT1a (38, 39). As the lipidome and cellular metabolism consists of elaborate networks of closely interconnected pathways, it is impossible to disrupt acyl-carnitine levels without affecting downstream lipid profiles or energy production. We recognize that this is a drawback of working on the lipidome. However, considering that we have already studied other global changes in the lipidome before with the superinfection experiment (Figs. 1 and 2) and identified acyl-carnitines to be of interest, it is unlikely that other changes caused in the lipidome by the use of etomoxir would be relevant to the ultimate aim of this study. Since our goal was to directly block the formation of acyl-carnitines and as etomoxir treatment did significantly deplete most acyl-carnitines (SI Appendix, Fig. S5), we concluded that etomoxir treatment did provide valuable insights into the behavior of wMel and the viruses when acyl-carnitine levels are disrupted.
Consistent with our findings, a recent study demonstrated a remarkable accumulation of acyl-carnitines in mosquito midguts upon DENV infection (30). Elevated acyl-carnitine levels during virus infection could be attributed to mitochondrial overload. Due to elevated energetic demands during virus infection, an increased amount of FA-CoA continuously enter the mitochondria but are only partially broken down by β-oxidation, which becomes a bottleneck in this pathway. This results in a corresponding increase in acyl-carnitines, upstream of β-oxidation (30).
Our data revealed that depleting acyl-carnitines in mosquitoes resulted in significantly higher levels of wMel. Interestingly, it has been previously demonstrated that Wolbachia can increase FA-CoA (activated fatty acid) catabolism by increasing the expression of ACOT enzymes (22). Taken together, this suggests that Wolbachia drives the conversion of FA-CoA to free fatty acids, thereby resulting in decreased acyl-carnitine levels. As acyl-carnitines are involved in ATP/energy generation from lipids, this depletion might result in a general decline in β-oxidation and ATP production. Indeed, measurement of cellular ATP generation showed a significant reduction of ATP levels in the presence of wMel (SI Appendix, Fig. S6). Reduced ATP levels would affect virus replication too, as virus replication is energetically demanding. Although it remains unclear why Wolbachia redirects this pathway, it might be for the production of bacteria-specific lipids or Wolbachia-driven energy production. It is noteworthy that the wMel-induced reduction in acyl-carnitine levels does not lead to a massive fitness cost in mosquitoes. A recent study showed that Wolbachia subverts the endoplasmic reticulum to acquire their vacuolar membrane and colonize the host cell at high density without triggering endoplasmic reticulum stress or compromising host cell viability (40). This suggests that the presence of Wolbachia, while causing changes, does not perturb the tightly regulated mechanisms of cell homeostasis.
Our model suggests that modulation of acyl-carnitines by Wolbachia is an important and broad mechanism behind virus blocking. Indeed, provision of exogenous acyl-carnitines increased the infection of all three viruses in wMel cell, although not to the same extent. Unfortunately, these increases were not substantial as there is no commercially available general acyl-carnitine compound that can be used in place of the eight identified acyl-carnitines in Fig. 2. The supplemented acyl-carnitines may not be important to virus replication but were used since they were the only commercially available compounds. It is also unclear which stage of virus replication acyl-carnitines are needed for. To elucidate the “extent” of this increased replication in wMel cells was difficult too, given that viruses depend on acyl-carnitines even in the absence of wMel.
To date, there has been one other lipidomic study of Wolbachia-infected cells published (21). This study was conducted on Aedes albopictus Aa23 cells and compared cellular lipidomic profiles between Wolbachia-infected and uninfected cells (21). Virus infection was not performed (21). Their data showed substantial shifts in the cellular lipid profile, a depletion of sphingolipids, some reductions in diacylglycerols, and phosphatidylcholines in Wolbachia-infected cells (21). Consistent with this study, we observed depletion of some sphingolipids, particularly ceramides during wMel infection (Dataset S1). However, there were no notable reductions in diacylglycerols or phosphatidylcholines (Dataset S1).
Additional mechanisms for the pathogen blocking phenotype of Wolbachia have been proposed. The down-regulation of insulin receptor kinase activity in Wolbachia-positive cells was recently shown to contribute to blocking of virus replication (41). Inhibition of the insulin receptor thus impaired insulin signaling, leading to reduced virus replication (41). Interestingly, acyl-carnitines have been associated with insulin resistance (42). During metabolic overload, when there is incomplete β-oxidation, the intramitochondrial accumulation of intermediates such as acyl-carnitines could result in impaired insulin signaling (42). The modulation of cholesterol and lipid homeostasis in the presence of Wolbachia may also contribute toward the pathogen-blocking effect as virus would have limited access to these metabolites essential for replication (19–21). Interestingly, several hits from our lipidomic study pertain to signaling pathways that may be highly relevant to at least some of these proposed mechanisms.
In brief, our study suggests that the down-regulation of acyl-carnitines in the presence of Wolbachia is one mechanism contributing to its pathogen blocking “syndrome” and provides initial insights into the importance of the metabolic environment during this tripartite interaction. Along with recent reports, this finding provides further reassurance that Wolbachia biocontrol may be highly effective in controlling arbovirus transmission in future as arboviruses would not be able to easily evolve to escape this phenomenon of reduced cellular resources (2, 43).
Materials and Methods
Cells, Viruses, and Infections.
HuH-7, C6/36, and BHK-21 cells were obtained from the American Type Culture Collection and cultured accordingly. Aag2.wMel cells, infected with the wMel strain of Wolbachia, and Aag2.TET cells were gifts from the McGraw laboratory, Monash University, Australia. Tetracycline treatment was used to abolish wMel from the Aag2.wMel cells to prepare Aag2.TET cells (44). All A. aegypti mosquitoes (wMel and TET lines) were reared and maintained as described previously (6).
Zika virus strain stocks were gifts from Julian Druce, Victorian Infectious Diseases Reference Laboratory, Melbourne, Australia. Zika African strain (GenBank accession no. KU963573.2) was isolated from Uganda while the Asian strain was isolated from a local patient who had just returned from the Pacific. The DENV-1 (GenBank accession no. FJ432734) and the DENV-2 Cosmopolitan strains were gifts from Oxford University Clinical Research Unit, Vietnam. All viruses were expanded by inoculation into C6/36 cells with a multiplicity of infection (MOI) of 0.1 and collection of culture supernatant 7 d postinfection following filtration. All viral isolates included in this study were passaged less than six times. Infectious titers were determined by plaque assay. Infectivity was measured using plaque assay and qRT-PCR analysis of viral genomes.
Liquid Chromatography–Mass Spectrometry.
For LCMS analysis, metabolites in both polar and nonpolar phase extracts were analyzed by LCMS in positive ionization mode to obtain the most comprehensive coverage. The levels were then compared to internal standard controls and ratios between infected and uninfected cells determined.
To ensure that the lipidomic profile of the cells accurately represented the infected environment, supernatant from the infected cells was evaluated by plaque assay for virus titers to confirm infection, before proceeding with LCMS. Cells were washed with 1× PBS and quenched by adding liquid nitrogen to completely cover the cells (45). Upon evaporation of the liquid nitrogen, 600 μL of 1:9 chloroform:methanol (vol/vol) containing 10 µg/mL of each internal standard (PC19:0/19:0 [part 850367], PE-d31 [part 860374], PG17:0/17:0 [part 830456], DG-d5 1,3-15:0/15:0 [part 110536] and TG-d5 19:0/12:0/19:0 [part 8609040], Avanti Polar Lipids) was added and incubated for 10 min at room temperature (45). Cells were scraped, and the extract along with the cell debris was transferred to microfuge tubes and stored at −80 °C until further processing (45). Samples were centrifuged at 16,100 × g (Beckman Coulter Microfuge 22R Refrigerated Microcentrifuge) for 10 min and the supernatant transferred to fresh tube (45). The pellet was further extracted with 200 μL of 1:1 chilled chloroform:methanol (vol/vol) with vortexing for 30 min at room temperature and sonication for 10 min followed by centrifugation and the supernatant combined with the first extract (45). The combined supernatants were transferred into glass inserts and dried in a vacuum concentrator (Christ RVC 2-33) (45). Sample were reconstituted with 200 μL of methanol:water-saturated butanol (1.2:8.8, vol/vol) mixture (45). Pooled biological quality control samples were prepared by pooling aliquots of the extracts from each sample and ran after every five samples (45).
Lipids were analyzed according to ref. 45 using an Agilent 1290 LC system and Agilent Triple Quadrupole 6490 mass spectrometer (MS). Briefly, lipids were separated by injecting 1 µL of the sample onto a 100 mm × 2.1 mm × 1.8 µm Zorbax Eclipse Plus C-18 column (Agilent Technologies) followed by elution at flow rate of 0.4 mL/min using a solvent system of A: water/acetonitrile/isopropanol (50:30:20, vol/vol/v) and B: water/acetonitrile/isopropanol (1:9:90, vol/vol/v), with a total run time of 14 min. Lipids were quantified by electrospray ionization-mass spectrometry using multiple reaction monitoring with capillary voltage 3,500 V, fragmentor voltage 380 V, collision energy 15–60 V, and collision gas (nitrogen) at 17 L/min. Quantitation was based on relative changes in peak areas. Data processing was performed using Agilent’s Mass Hunter Quantitative Analysis (QQQ) software.
Plaque Assay.
Serial dilutions (10-fold) of virus were added to BHK-21 cells in 24-well plates and incubated for 1 h at 37 °C. Media was aspirated and replaced with 0.8% methyl-cellulose in maintenance medium (RPMI-1640, 2% fetal calf serum (FCS), 25 mM Hepes, penicillin, and streptomycin). After 6 d at 37 °C, cells were fixed with 20% formaldehyde at room temperature for 20 min and washed with water, and 1 mL of 1% crystal violet was added for 20 min. The plates were washed and dried, and the plaque-forming units per milliliter were calculated.
Quantification of RNA.
Total RNA from virus-infected cells was extracted using the RNeasy kit (Qiagen). Superscript III reverse transcriptase and random hexamers (Invitrogen) were used in the reverse transcription reactions to generate cDNA. qRT-PCR was performed using the iQ SYBR Green Supermix Kit (Bio-Rad) and LightCycler 480 SYBR Green I Master mix, according to the manufacturer's instructions. Quantification of DENV levels was performed as previously described (46). Primers used for Zika virus detection are as follows: Zika4481 F (CTGTGGCATGAACCCAATAG) and Zika4552c R (ATCCCAKAGRGCACCACTCC). They bind universally to both strains of Zika virus used in this study. All PCRs were normalized against GAPDH. Reactions were run on a Roche Lightcycler 480 (Roche), and data analysis was carried out with the Lightcycler 480 software. qRT-PCR conditions to detect viral RNA: 95 °C for 5 min, followed by 45 cycles of 95 °C for 10 s, 55 °C for 5 s, and 72 °C for 10 s. Cycling conditions for the quantification of immune genes: 95 °C for 5 min, followed by 45 cycles of 95 °C for 5 s, 53 °C for 10 s, and 72 °C for 10 s.
Calculation of Wolbachia Density.
Wolbachia density in cells was determined by comparing the abundance of Wolbachia TM513 gene to that of the single-copy mosquito rps17 gene using relative qPCR. Aag2.wMel and Aag2.TET cells were first lysed with squash extraction buffer (10 mM Tris Buffer, 1 mM ethylenediamine tetraacetic acid [EDTA], 50 mM NaCl in milli-Q water, 30 uL of Proteinase K). Cell lysates were then incubated at 56 °C for 5 min, followed by 98 °C for 5 min and spun down at full speed for 1 min to remove the supernatant. The qPCR was performed with 1 μL of the supernatant using the LightCycler 480 SYBR Green I Master mix, according to the manufacturer’s instructions. Probes and primers used are as described: For wMel detection, TM513 F (5′-CAAATTGCTCTTGTCCTGTGG-3′), TM 513 R (5′-GGGTGTTAAGCAGAGTTACGG-3′), and TM513 Cy5 probe (5′-TGAAATGGAAAAATTGGCGAGGTGTAGG-3′) were used. To detect the housekeeping gene, RPS 17 F (5′-TCCGTGGTATCTCCATCAAGCT-3′), RPS 17 R (5′-CACTTCCGGCACGTAGTTGTC-3′), and RPS 17 FAM probe (5′-CAGGAGGAGGAACGTGAGCGCAG-3′) were utilized. PCR cycling conditions were 95 °C for 5 min, 45 cycles of 95 °C for 10 s, 60 °C for 15 s, 72 °C for 1 s, followed by cooling at 40 °C for 10 s.
Wolbachia density was quantified for the A. aegypti mosquitoes used in the Etomoxir-treatment experiment. At 3 d postemergence, 16 individual females from each sample group were dissected to separate the heads from the abdomens/thoraxes. DNA was then extracted using the QIAcube HT purification kit (Qiagen), according to manufacturer’s instructions. Wolbachia density was then determined by qRT-PCR by comparing the abundance of wsp (Wolbachia surface protein) gene to that of the single-copy mosquito rps17 gene. Rps17 primers were as above. Wsp primers were wsp F (5′-CTGGTGTTAGTTATGATGTAAC-3′ and wsp R (5′- AAAAATTAAACGCTACTCCA-3′). For each sample, qPCR amplification of DNA was performed in duplicate with a LightCycler 480 II Instrument (Roche) using LightCycler Multiplex RNA Virus Master (Roche) according to the manufacturer’s protocol. The temperature profile of the qPCR was 10 min of preincubation at 95 °C, 45 cycles of 95 °C for 10 s, 60 °C for 15 s, 72 °C for 10 s. wsp:rps17 ratios were obtained for each biological replicate using the LightCycler 480 II software (Roche) and then compared independently for each sample.
Mosquito Microinjections.
Adult microinjections were performed as previously described using DENV-2 Cosmopolitan strain, 3.4 × 105 TCID50/mL (6). Quantification of DENV-2 genomic copies was done on whole mosquitoes using the DENV 3′UTR primer/probe set, as previously described (6).
Fluorescent In Situ Hybridization.
Aag-2 cells were seeded in an eight-well chamber slide (Lab-Tek) and incubated overnight at 25 °C. Fluorescent in situ hybridization was carried out using wMel-specific 16S 22 rRNA probes as described previously (4). The Zeiss LSM710 confocal microscope was used for imaging.
Pharmacological Agent Studies.
Etomoxir (Sigma).
A widely used inhibitor, etomoxir is an irreversible inhibitor of the enzyme carnitine palmitoyltransferase-1 (CPT-1) that converts fatty acyl-CoAs in the cytoplasm into acyl-carnitines (32, 33). This prevents the formation of acyl-carnitines, thus strongly inhibiting mitochondrial β-oxidation. Cytotoxicity was assessed by serial dilution of etomoxir on HuH-7 cells and viability assessed with the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega). Nile red staining was performed as described to confirm that the drug is effective (47, 48). For the experiment, Huh-7 cells were treated with media containing 200 µM etomoxir for 48 h, after which virus was added at MOI 5. Inoculum was removed after 2 h, and fresh media was added. Both supernatant and infected cells were removed after 24 h for plaque assay and qRT-PCR, respectively. Treatment experiments were performed in mosquitoes by rearing the larvae in 100 µM etomoxir-treated water. Wolbachia density was measured at 3 d postemergence of mosquitoes.
Acyl-carnitines 12:0, 16:0, and 18:1 (Avanti Polar Lipids).
Rescue experiments were conducted by mixing all three commercially available carnitines, C12:0 carnitine (part 870850), C16:0 carnitine (part 870851), and C18:1 carnitine (part 870852) each at a concentration of 25 μM as described, and adding them to infected cells 24 h.p.i (49). Samples were harvested at 96 h.p.i. and virus titers/RNA levels determined.
Western blot analysis.
Mouse anti-Wolbachia WSP antibody was obtained from Bei Resources. Mouse anti-dengue-E (clone 4G2) and mouse anti-flavivirus NS1 (clone 4G4) were generously provided by Ooi Eng Eong (Duke-National University of Singapore Medical School, Singapore) and Roy Hall (University of Queensland, Brisbane, Australia) respectively. Briefly, infected cells were lysed with Nonidet P-40 lysis buffer containing protease inhibitors and loaded on a Bis/Tris polyacrylamide gel. Proteins were transferred to a Hi-Bond enhanced chemiluminescence nitrocellulose membrane and incubated with primary antibodies overnight at 4 °C. Membranes were incubated with species-specific secondary antibodies conjugated to either Alexa Fluor 488 or 647, and proteins were detected with the Bio-Rad Pharos FX system.
Cellular ATP assay.
Measurement of cellular ATP levels were performed using the Luminescent ATP Detection Assay kit (Abcam) as per manufacturer’s instructions.
Statistical Analysis.
For LCMS, each metabolite was first normalized to the median of the sample. The interaction between bacteria and virus (i.e., all four datasets: bacteria+/− and virus+/−) were analyzed using a two-way ANOVA test. Results of the ANOVA tests were controlled for false positives using the Benjamini–Hochberg method (50). All results are presented as mean ± SD of at least three independent experiments, unless otherwise indicated. Data were analyzed using unpaired, two-tailed Student’s t test or two-way ANOVA and considered significant if P < 0.05 (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Supplementary Material
Acknowledgments
This work was supported by the Wellcome Trust (UK) and the National Health and Medical Research Council (Australia). We thank Dr. Elizabeth A. McGraw for providing the A. aegypti cells and protocols used in maintaining the wMel cell lines. They also thank Drs. Brunda Nijagal and Kirsty McPherson for their invaluable advice throughout this work.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. R.J.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914814117/-/DCSupplemental.
Data Availability.
The authors declare that the data supporting the findings of this study are available within the SI Appendix files.
References
- 1.Dutra H. L. et al., Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 19, 771–774 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neill S. L. et al., Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res. 2, 36 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aliota M. T., Peinado S. A., Velez I. D., Osorio J. E., The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci. Rep. 6, 28792 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreira L. A. et al., A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Ant T. H., Herd C. S., Geoghegan V., Hoffmann A. A., Sinkins S. P., The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 14, e1006815 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser J. E. et al., Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog. 13, e1006751 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann A. A., Ross P. A., Rašić G., Wolbachia strains for disease control: Ecological and evolutionary considerations. Evol. Appl. 8, 751–768 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joubert D. A. et al., Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathog. 12, e1005434 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anders K. L. et al., The AWED trial (Applying Wolbachia to Eliminate Dengue) to assess the efficacy of Wolbachia-infected mosquito deployments to reduce dengue incidence in Yogyakarta, Indonesia: Study protocol for a cluster randomised controlled trial. Trials 19, 302 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrington L. B. et al., Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 115, 361–366 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacidônio E. C., Caragata E. P., Alves D. M., Marques J. T., Moreira L. A., The impact of Wolbachia infection on the rate of vertical transmission of dengue virus in Brazilian Aedes aegypti. Parasit. Vectors 10, 296 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Servick K., Winged warriors. Science 354, 164–167 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Hussain M., Frentiu F. D., Moreira L. A., O’Neill S. L., Asgari S., Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 108, 9250–9255 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez J. et al., Symbionts commonly provide broad spectrum resistance to viruses in insects: A comparative analysis of Wolbachia strains. PLoS Pathog. 10, e1004369 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan X. et al., Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 109, E23–E31 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rancès E., Ye Y. H., Woolfit M., McGraw E. A., O’Neill S. L., The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 8, e1002548 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang G., Hussain M., O’Neill S. L., Asgari S., Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 110, 10276–10281 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu P., Bian G., Pan X., Xi Z., Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl. Trop. Dis. 6, e1754 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caragata E. P. et al., Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 9, e1003459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geoghegan V. et al., Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nat. Commun. 8, 526 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molloy J. C., Sommer U., Viant M. R., Sinkins S. P., Wolbachia modulates lipid metabolism in Aedes albopictus mosquito cells. Appl. Environ. Microbiol. 82, 3109–3120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye Y. H., Woolfit M., Rancès E., O’Neill S. L., McGraw E. A., Wolbachia-associated bacterial protection in the mosquito Aedes aegypti. PLoS Negl. Trop. Dis. 7, e2362 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liebscher S. et al., Phospholipase A2 activity during the replication cycle of the flavivirus West Nile virus. PLoS Pathog. 14, e1007029 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasheri N. et al., Modulation of fatty acid synthase enzyme activity and expression during hepatitis C virus replication. Chem. Biol. 20, 570–582 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Perera R. et al., Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog. 8, e1002584 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aktepe T. E., Liebscher S., Prier J. E., Simmons C. P., Mackenzie J. M., The host protein reticulon 3.1A is utilized by flaviviruses to facilitate membrane remodelling. Cell Rep. 21, 1639–1654 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Heaton N. S. et al., Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc. Natl. Acad. Sci. U.S.A. 107, 17345–17350 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackenzie J. M., Khromykh A. A., Parton R. G., Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell Host Microbe 2, 229–239 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Rothwell C. et al., Cholesterol biosynthesis modulation regulates dengue viral replication. Virology 389, 8–19 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Chotiwan N. et al., Dynamic remodeling of lipids coincides with dengue virus replication in the midgut of Aedes aegypti mosquitoes. PLoS Pathog. 14, e1006853 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martín-Acebes M. A. et al., The composition of West Nile virus lipid envelope unveils a role of sphingolipid metabolism in flavivirus biogenesis. J. Virol. 88, 12041–12054 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pike L. S., Smift A. L., Croteau N. J., Ferrick D. A., Wu M., Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim. Biophys. Acta 1807, 726–734 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Wong B. W. et al., The role of fatty acid β-oxidation in lymphangiogenesis. Nature 542, 49–54 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Genty L. M., Bouchon D., Raimond M., Bertaux J., Wolbachia infect ovaries in the course of their maturation: Last minute passengers and priority travellers? PLoS One 9, e94577 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heaton N. S., Randall G., Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 8, 422–432 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandes-Siqueira L. O., Zeidler J. D., Sousa B. G., Ferreira T., Da Poian A. T., Anaplerotic role of glucose in the oxidation of endogenous fatty acids during Dengue virus infection. MSphere 3, e00458-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tongluan N. et al., Involvement of fatty acid synthase in dengue virus infection. Virol. J. 14, 28 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein Geltink R. I. et al., Mitochondrial priming by CD28. Cell 171, 385–397.e11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuzarte-Luís V. et al., Dietary alterations modulate susceptibility to Plasmodium infection. Nat. Microbiol. 2, 1600–1607 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Fattouh N., Cazevieille C., Landmann F., Wolbachia endosymbionts subvert the endoplasmic reticulum to acquire host membranes without triggering ER stress. PLoS Negl. Trop. Dis. 13, e0007218 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haqshenas G. et al., A role for the insulin receptor in the suppression of Dengue virus and Zika virus in Wolbachia-infected mosquito cells. Cell Rep. 26, 529–535.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Schooneman M. G., Vaz F. M., Houten S. M., Soeters M. R., Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes 62, 1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritchie S. A., Wolbachia and the near cessation of dengue outbreaks in Northern Australia despite continued dengue importations via travellers. J. Travel Med. 25, tay084 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Frentiu F. D., Robinson J., Young P. R., McGraw E. A., O’Neill S. L., Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS One 5, e13398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huynh K. et al., Lipidomic profiling of murine macrophages treated with fatty acids of varying chain length and saturation status. Metabolites 8, E29 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manokaran G. et al., Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science 350, 217–221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herms A. et al., Cell-to-cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity. Curr. Biol. 23, 1489–1496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herms A. et al., AMPK activation promotes lipid droplet dispersion on detyrosinated microtubules to increase mitochondrial fatty acid oxidation. Nat. Commun. 6, 7176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deguchi H. et al., Acylcarnitines are anticoagulants that inhibit factor Xa and are reduced in venous thrombosis, based on metabolomics data. Blood 126, 1595–1600 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benjamini Y., Hochberg Y., Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. A Stat. Soc. 57, 289–300 (1995). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the SI Appendix files.