Significance
Transfer of sperm-producing stem cells isolated from a donor male into testes of a recipient male has important applications for preserving germplasm and as a conduit for widespread dissemination of desirable genetics in livestock production. A key aspect is surrogate males that lack endogenous germline but are otherwise physiologically normal. Here we demonstrate that male mice, pigs, and goats rendered genetically sterile by CRISPR-Cas9 editing of the NANOS2 gene support donor-derived spermatogenesis following allogeneic stem cell transplantation. In addition, we show that CRISPR-Cas9 editing of the NANOS2 gene in cattle leads to male germline ablation. These findings represent a major advance toward surrogate sires becoming a tool for disseminating and regenerating germplasm in all mammals.
Keywords: NANOS2, spermatogonial stem cell, transplantation, surrogate sires, livestock
Abstract
Spermatogonial stem cell transplantation (SSCT) is an experimental technique for transfer of germline between donor and recipient males that could be used as a tool for biomedical research, preservation of endangered species, and dissemination of desirable genetics in food animal populations. To fully realize these potentials, recipient males must be devoid of endogenous germline but possess normal testicular architecture and somatic cell function capable of supporting allogeneic donor stem cell engraftment and regeneration of spermatogenesis. Here we show that male mice, pigs, goats, and cattle harboring knockout alleles of the NANOS2 gene generated by CRISPR-Cas9 editing have testes that are germline ablated but otherwise structurally normal. In adult pigs and goats, SSCT with allogeneic donor stem cells led to sustained donor-derived spermatogenesis. With prepubertal mice, allogeneic SSCT resulted in attainment of natural fertility. Collectively, these advancements represent a major step toward realizing the enormous potential of surrogate sires as a tool for dissemination and regeneration of germplasm in all mammalian species.
The germline provides an eternal cellular link between generations and in metazoans a male’s genetic contribution to the next generation is delivered via sperm. Thus, the genesis of sperm (i.e., spermatogenesis) is critical for the continuity and diversity of a species. In mammals, the continual sperm production required for male fertility is a stem cell-based process centered on the regenerative capacity of an undifferentiated spermatogonial population (1, 2). In addition to sustaining steady-state spermatogenesis, studies with mice have shown that a minor subset of the undifferentiated spermatogonial population is able to regenerate the spermatogenic lineage following isolation from donor tissue and transplantation into the testes of a recipient male that is depleted of endogenous germline (3, 4). These regenerative spermatogonia are often referred to as spermatogonial stem cells (SSCs).
Spermatogonial stem cell transplantation (SSCT) has many potential applications that could impact society in a major way. Autologous SSCT has been lauded as a means to restore the fertility of men who suffer from germline ablation due to collateral effects of chemo- or radiotherapy, as well as men with a genetic mutation rendering them infertile (5–7). Another potential application of SSCT is with livestock for the propagation of elite genetics within populations on a global scale (8). This accomplishment could provide an efficacious avenue for improving production characteristics and in turn enhance the capacity to provide food security for the expanding global population. In addition, SSCT in endangered species has utility for preserving unique genetic lines, thereby contributing to conservation efforts.
Application of SSCT in livestock or endangered species as a breeding tool will require deployment in an allogeneic manner and recipient males that completely lack endogenous germline so that all sperm production is donor-derived. Previous studies with rodent models have shown that recipient males rendered sterile due to deficiency of the c-Kit gene (i.e., W/Wv mice) can attain fertility following SSCT with cells from an immunologically compatible donor or from an immunologically incompatible donor (allogeneic transplant) when the recipient is subjected to an immune suppression regimen (4, 9, 10). In addition, with recipient mice that were prepared by treatment with a chemotoxic drug (i.e., busulfan) to deplete the endogenous germline and not subjected to immune suppression, donor SSC engraftment following transplantation into allogeneic recipients was reported to be compromised due to immunological rejection (10). These findings indicate that immunological compatibility between donor and recipient is required for fertility attainment by SSCT in mammals.
Sourcing immunologically compatible recipients for application of SSCT in either livestock or endangered species is improbable, given breed/species divergence and immune suppression regimens are unlikely to be desirable or feasible to apply. Preliminary SSCT studies with large animals indicate that histoincompatability between donor and recipient is tolerated (11–15), but definitive evidence of sustained donor-derived spermatogenesis and fertility is currently lacking. Moreover, to date no studies have demonstrated that donor-derived spermatogenesis can occur in livestock rendered germline ablated from genetic deficiency. Here we show unequivocally that application of SSCT in an allogeneic context is tolerated in mice, pigs, and goats lacking endogenous germline via genetic mutation of the NANOS2 gene and leads to sustain donor-derived spermatogenesis as well as attainment of fertility via natural mating.
Results
Allogeneic SSCT in Nanos2 Knockout Mice.
To date, application of SSCT in a context that will be deployable in livestock or endangered species, that is, between immunologically incompatible males where the recipient is germline ablated due to a genetic deficiency and results in propagation of the donor haplotype via natural breeding has not been demonstrated. To address this, we first designed a proof-of-concept scheme with mice that involves recipient males rendered sterile by inactivation of the evolutionarily conserved germ cell-specific gene Nanos2 for allogeneic SSCT with cells from an immunologically incompatible donor male (Fig. 1A).
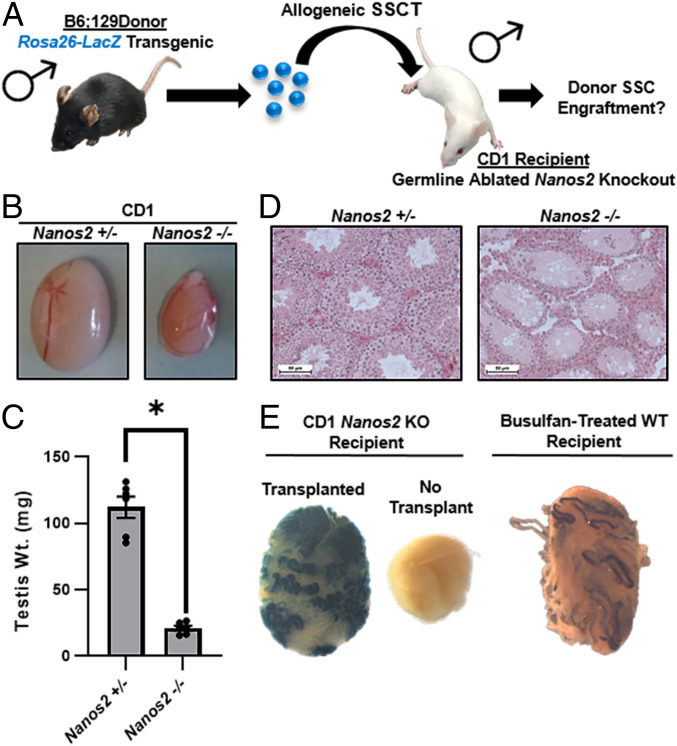
Fig. 1.
Allogeneic SSCT in Nanos2 knockout recipient mice. (A) Schematic of the experimental strategy. (B) Representative images of testes from adult mice possessing one (+/−) or two (−/−) Nanos2 alleles inactivated by CRISPR-Cas9 gene editing. (C) Quantitative comparison of testis weights between adult Nanos2 +/− and −/− mice. Data are mean ± SEM for n = 3 different males and six testes of each genotype and * denotes significantly different at P < 0.05. (D) Representative images of hematoxylin & eosin-stained testis cross-sections from adult Nanos2 +/− and −/− mice. (Scale bars, 50 μm.) (E) Representative images of X-Gal-stained testes from an adult Nanos2−/− mouse and a WT mouse treated with busulfan to deplete the endogenous germline. One testis of the Nanos2−/− mouse and the WT busulfan-treated mouse were transplanted with SSCs from an allogeneic Rosa26-LacZ;Nanos2+/+ donor mouse and the other testis was nontransplanted. Intense blue staining in the transplanted testes reflect donor SSC engraftment and rederivation of spermatogenesis.
In previous studies, Nanos2 null mouse lines were created with CD1 (i.e., ICR) outbred genetic backgrounds via targeted insertion of LacZ or CRISPR-Cas9 editing to disrupt the coding sequence (16, 17). These CD1 Nanos2−/− (knockout [KO]) males were found to be sterile in adulthood with apparent ablation of the germline, whereas males that were heterozygous for the Nanos2 mutations (i.e., +/− genotype) or KO females were of normal fertility. However, whether the testicular soma in Nanos2 KO males is of normal functionality to support spermatogenesis from allogeneic wild-type (WT) germ cells has not been determined. Here mice from a CRISPR-Cas9 Nanos2 KO line that were produced previously (16), were used as recipients for SSCT. First, we confirmed sterility of Nanos2 KO males by pairing with WT females. Over a 2-mo period, none of the Nanos2 KO males (n = 3) sired offspring but copulatory plugs were evident in all females confirming that mating had occurred. In comparison, all male Nanos2+/− littermates were of normal fertility (SI Appendix, Table S1). Also, all CRISPR-Cas9-generated Nanos2 KO females were of normal fertility (SI Appendix, Table S1). Next, at ∼2 mo of age one testis was removed from a group of Nanos2 KO males for phenotyping and the contralateral testis was transplanted with SSCs isolated from testes of immunologically incompatible Rosa26-LacZ (B6; 129s genetic background with LacZ insertion in the Rosa26 locus) donor males. As expected, the weight of nontransplanted testes from CRISPR-Cas9 Nanos2 KO males was found to be significantly (P < 0.01) reduced by >81% (mean ± SEM and n = 3) compared to age-matched heterozygous littermates (Fig. 1 B and C). In addition, examination of testicular cross-sections revealed that germline was completely lacking in testes of the Nanos2 KO males (Fig. 1D and SI Appendix, Fig. S1), confirming our previous findings (16). Next, at 2 mo posttransplantation the contralateral transplanted testis was collected and stained with X-Gal to detect colonies of donor-derived spermatogenesis. We observed extensive allogeneic LacZ-labeled donor colonization in all CD1 Nanos2 KO recipient testes with no signs of immunological rejection, e.g., inflammation or fibrosis (Fig. 1E). In addition, the degree of colonization was greater in Nanos2 KO recipient testes compared to testes of wild-type B6;129scv recipient males that had been subjected to depletion of the endogenous germline by busulfan treatment and transplanted with the same donor cell population and concentration of cells (Fig. 1E). These findings indicate that testes of Nanos2 KO mice provide an enhanced environment for engraftment by transplanted wild-type SSCs.
Restoration of Natural Fertility Following Allogeneic SSCT in Nanos2 Knockout Mice.
In previous studies, syngeneic SSCT into prepubertal (5 to 12 days of age), but not adult Wv/Wv recipient males, resulted in efficient attainment of natural fertility (9, 10). In addition, allogeneic SSCT in adult Wv/Wv recipients was able to produce long-lasting colonies of donor-derived spermatogenesis and offspring were produced by natural mating as long as the recipient was subjected to immune suppression (10). However, the efficiency of fertility attainment was quite low (∼24% of males) and the number of donor cells transplanted per testis proved to be a limiting factor (10). Here we asked whether Nanos2 KO recipients subjected to allogeneic SSCT without immune suppression could attain fertility by natural mating (Fig. 2A). To standardize the donor cell population, primary cultures of undifferentiated spermatogonia established from Rosa26-LacZ mice that are alloantigen histoincompatible with CD1 recipient mice were used. We transplanted 3 to 8 × 104 donor cells into either one or both testes of six Nanos2 KO recipient males ranging in age from 21 to 43 d at the time of transplant (SI Appendix, Table S2). At 30 d after transplantation, the recipients were paired with pubertal CD1 females to test their fertility. Copulatory plugs were observed for all of the recipients, confirming that mating occurred, and three of the six males began siring offspring between 81 and 113 d after transplant, including two recipients in which only one testis was transplanted (SI Appendix, Table S2). Over the course of a 6- to 8-mo period, the fertile recipients produced 33 litters and 111 total offspring (SI Appendix, Table S2). Based on both coat color and detection of the LacZ transgene, 100% of the offspring were determined to be of donor genetic origin (Fig. 2 B and C). Interestingly, all of the recipients that attained fertility were transplanted with donor SSCs at 21 to 28 days of age and received <1 × 105 cells per testis; none of the recipients older than 28 days of age at transplant attained fertility. Amazingly, one of the fertile recipients received only 3 × 104 cells into just one testis (SI Appendix, Table S2). Recipients that did not attain natural fertility were killed between 11 and 12 mo after transplantation; all testes contained extensive colonies of donor-derived spermatogenesis but the epididymal sperm content was surprisingly low (SI Appendix, Fig. S2). At 1 year of age (∼11 mo after transplantation), we killed the fertile recipients and similar to the infertile counterparts, testes contained extensive donor-derived spermatogenesis (Fig. 2D). In contrast to the infertile recipients, sperm content in the epididymides of fertile recipients was abundant (SI Appendix, Fig. S2). In addition, the average weight of testes for recipients that attained natural fertility was significantly greater (P < 0.05) compared to recipients that did not attain fertility (Fig. 2E). Interestingly, the attainment of natural fertility was significantly associated with a younger age at the time of transplantation (Fig. 2F). Collectively, these findings demonstrate that allogeneic donor SSC engraftment and spermatogenic regeneration in testes of prepubertal Nanos2 KO recipient male mice is robust enough to yield persistent natural fertility.
Fig. 2.
Nanos2 knockout recipient mice attain natural fertility following allogeneic SSCT. (A) Schematic of the experimental strategy. (B) Image of a CD1 Nanos2 knockout SSCT recipient and CD1 wild-type female breeder pair with litter of offspring possessing the SSCT donor haplotype. (C) Image of X-Gal-stained tail biopsies from CD1 parents (Nanos2 knockout SSCT recipient male and wild-type female) and offspring generated by natural mating for LacZ transgene activity that would be inherited from the SSCT donor. (D) Image of an X-Gal-stained testis from a CD1 Nanos2 knockout 11 mo after allogeneic SSCT with stem cells from a LacZ transgenic donor male. The Nanos2 knockout recipient had attained fertility via natural mating. (E) Quantitative comparison of testis weights for Nanos2 knockout recipients that did (+) or did not (−) attain fertility via natural mating following allogeneic SSCT. Data are mean ± SEM for n = 3 to 9 different males of each recipient outcome. (F) Comparison of age at the time of allogeneic SSCT for Nanos2 knockout recipients that did (+) or did not (−) attain natural fertility. Data are mean ± SEM for n = 3 different males of each recipient outcome. For E and F, * denotes significantly different at P < 0.05.
Donor-Derived Spermatogenesis Following Allogeneic SSCT in NANOS2 Knockout Boars.
Translation of the SSCT concept from mice to livestock has been touted for nearly two decades as a next generation breeding tool to expand the availability of sperm from genetically desirable sires (8). However, advancement from concept to reality has been limited, due in part to lack of effective approaches for preparing transplant recipient males. For SSC transplant to be implemented in livestock production, recipient males must completely lack endogenous germline to optimize SSC engraftment and ensure that all sperm production is of donor origin. In addition, somatic support cell function must be maintained in the recipient testes to provide an environment that will foster regeneration of normal spermatogenesis. As a first step in developing potentially ideal livestock recipients, we previously utilized CRISPR-Cas9-based gene editing to engineer pigs (Duroc breed) with inactivating mutations in the NANOS2 gene and found that homozygous KO males possess testes with intact seminiferous tubules and normal hormone production but complete ablation of the germline, thus mirroring the phenotype of Nanos2 KO mice (18). To explore whether a NANOS2 KO male pig could serve as a surrogate for regeneration of donor spermatogenesis following SSCT, we cloned a founder male that possessed 150-bp and 4-bp Δ NANOS2 alleles (boar #146) using somatic cell nuclear transfer. In total, six clones were generated (boars #132, 133, 134, 135, 136, and 137) and all were verified as knockouts for NANOS2 by genotyping (SI Appendix, Fig. S3). In addition, following the killing of boar #146, we examined >500 cross-sections of testicular parenchyma taken from regions throughout the testis and did not observe any seminiferous tubules with spermatogenesis (SI Appendix, Fig. S3), thus confirming the phenotype of endogenous germline ablation.
At ∼4 months of age, either one or both testes of four boars (#132, 134, 135, and 137) were injected with an unfractionated single-cell suspension from total testicular tissue of an allogeneic mixed breed wild-type donor boar. One of the boars (#133) developed osteopathic degeneration and was killed and another boar (#136) was left as a nontransplanted control. The donor cells were transferred into the NANOS2 knockout recipient testes using a variation of previously described methodology for ultrasound-guided injection into the rete testis (12) (Fig. 3A and SI Appendix, Table S3); however, successful infusion into seminiferous tubules was not observed by ultrasound imaging. Regardless, at puberty (∼6 months of age), all NANOS2 KO boars began semen collection on a regular basis to assess the presence of cells in the ejaculate. Similar to the founder and as expected due to not observing infusion of donor cell suspension into seminiferous tubules, sperm were never observed in the ejaculates of any NANOS2 KO clone over a ∼6-mo period of collection, thus confirming the azoospermic phenotype due to germline ablation and unsuccessful donor cell transplantation. Then, at 14 to 15 months of age, four of the NANOS2 KO boars (#132, 134, 135, and 137) were again transplanted with allogeneic wild-type donor cells (SI Appendix, Table S3). For the second transplantation, the donor testicular cell suspension was enriched for undifferentiated spermatogonia using a multiparameter approach which, based on immunostaining for the marker ZBTB16, consisted of ∼70% spermatogonia (SI Appendix, Fig. S4).
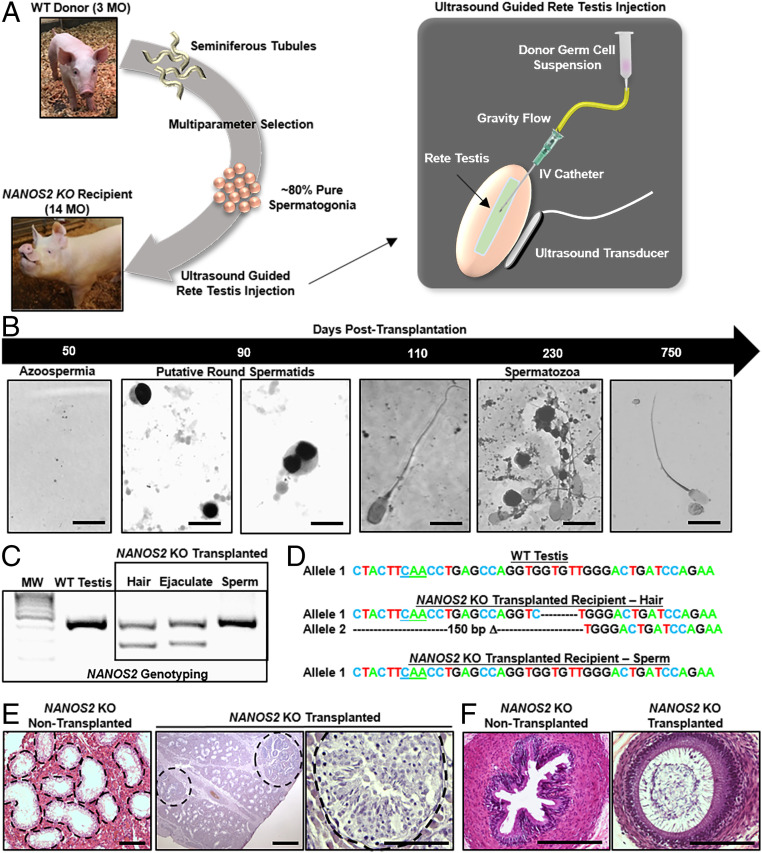
Fig. 3.
Donor-derived spermatogenesis following allogeneic SSCT in NANOS2 knockout boars. (A) Schematic of the experimental strategy. (B) Representative images portraying donor-derived spermatogenic regeneration in a NANOS2 KO recipient boar (#135) following allogeneic SSCT. (Scale bars, 10 μm.) (C) Representative image of PCR-based genotyping for NANOS2 alleles with DNA samples isolated from testicular tissue of a wild-type (NANOS2+/+) boar, as well as hair follicles, total ejaculate, and sperm collected from a NANOS2 KO recipient subjected to allogeneic SSCT with stem cells from a wild-type (NANOS2+/+) donor boar. Molecular weight (MW) is 100-bp DNA ladder. (D) Outputs from DNA sequencing analysis of PCR-based genotyping products showing detection of 4-bp and 150-bp NANOS2 deletion (Δ) alleles from hair follicle samples and intact NANOS2 allele from sperm samples both from a NANOS2 KO recipient boar subjected to allogeneic SSCT with wild-type donor stem cells. (E) Representative images of hematoxylin & eosin-stained cross-sections of testicular parenchyma from NANOS2 KO boars transplanted with donor SSCs or nontransplanted. (Scale bars, 50 μm.) (F) Representative images of hematoxylin & eosin-stained cross-sections of epididymal tubules from NANOS2 KO boars transplanted with SSCs or nontransplanted. (Scale bars, 50 μm.)
Beginning 50 d after the second transplantation procedure, the recipient boars were again subjected to semen collection. Similar to the pretransplantation collections, no cells were observed in the ejaculates (Fig. 3B). However, at 90 d posttransplantation, we began to observe round cells with a nuclear morphology similar in appearance to spermatocytes and dividing round spermatids, as described in the World Health Organization manual for human semen evaluation, in two of the transplanted animals, boars #134 and 135 (Fig. 3B). Then, beginning at ∼100 d posttransplantation, sperm appeared in the ejaculate of transplanted boar #135 (Fig. 3B). In addition, the sperm were of normal morphology, motile (Movie S1), and persisted in the ejaculate for >200 d posttransplantation (Fig. 3B). Unfortunately, the health of boar #134 deteriorated shortly after observing round cells in the ejaculate due to osteopathic degeneration which necessitated its killing. Importantly, neither round cells nor sperm were ever observed (>400-d assessment period) in the ejaculate of the nontransplanted boar #136. To confirm that the sperm produced in testes of recipient boar #135 were derived from transplanted donor SSCs, we isolated them from the ejaculate and carried out PCR genotyping for the NANOS2 gene. As expected, two different sized products were generated by PCR with DNA isolated from hair follicles of the recipient, both of which were smaller in size compared to an amplicon generated from DNA of a wild-type boar (Fig. 3C), and these were confirmed by sequencing analysis to be 4-bp and 150-bp Δ mutations (Fig. 3D). Importantly, PCR of genomic DNA from the isolated sperm generated an amplicon of identical size to wild type (Fig. 3C), which was confirmed by sequencing to be an intact NANOS2 allele (Fig. 3D). At ∼2 y after transplant, boar #135 was killed due to advanced age and sperm were detectable in the epididymal flushing (Fig. 3B). Moreover, histological assessment of testis cross-sections revealed that spermatogenesis was occurring in ∼15% of seminiferous tubules (Fig. 3E), and sperm were observable in cross-sections of epididymal tubules (Fig. 3F). As expected from lack of sperm in the ejaculate, spermatogenesis was not detected in any seminiferous tubule or epidydimal cross-section examined (n = 400 to 500) for the nontransplanted boar #136 (SI Appendix, Fig. S5). Collectively, these results demonstrate that similar to mice allogeneic donor SSC engraftment and spermatogenesis can occur in testes of NANOS2 KO recipient boars that are ablated of endogenous germline.
Generation of NANOS2 Knockout Bucks and Donor-Derived Spermatogenesis Following Allogeneic SSCT.
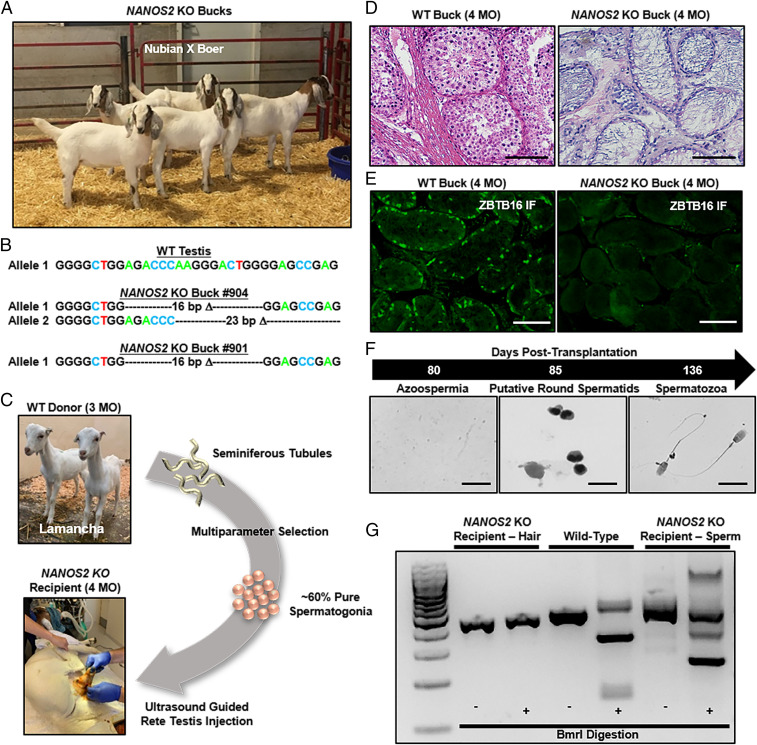
Next, we aimed to extend the SSCT concept with NANOS2 KO surrogate males to agriculturally relevant ruminant species. Because of ease in handling and a generation interval that is relatively short compared to larger ruminants like cattle, we started with goats as a model. To generate NANOS2 KO males for phenotyping and SSCT, somatic cell nuclear transfer (SCNT) was used (19) with the nuclear DNA donor deriving from XY fetal fibroblasts of Nubian × Boer crossbred male goats that were modified by CRISPR-Cas9 editing to possess 16-bp and 23-bp NANOS2 Δ alleles (SI Appendix, Fig. S6). In total, 125 one-cell-stage cloned embryos were generated and surgically transferred into seven estrous synchronized recipients. At 40 to 45 d of gestation, five recipients were confirmed pregnant by ultrasonography which yielded five NANOS2 mutant bucks being born alive (buck ID #901, 902, 904, 905, and 906) and all developed normally from birth to adulthood (Fig. 4 A and B and SI Appendix, Table S4). At the pubertal age of 4 mo, a single-cell suspension enriched for undifferentiated spermatogonia (∼60% based on immunostaining for the marker ZBTB16, SI Appendix, Fig. S4) that was isolated from testes of an age matched Lamancha breed donor male was transplanted into either one or both testes of all NANOS2 KO bucks using an ultrasound-guided rete testis injection procedure similar to that used for boars (Fig. 4C and SI Appendix, Table S5). At the time of transplant, testicular biopsies were also obtained for histological assessment. Identical to the phenotype of NANOS2 KO mice and pigs, the KO bucks contained intact seminiferous tubules but germline was not evident in testicular cross-sections (Fig. 4D), which was confirmed by undetectable immunostaining for the spermatogonial marker ZBTB16 (Fig. 4E). To assess whether donor-derived spermatogenesis was occurring in the NANOS2 KO recipients following SSCT, we began collecting ejaculates from the bucks at ∼80 d after SSCT. At ∼85 d after transplant, round cells were present in the ejaculates of three recipients (buck ID #901, 902, and 906) and sperm became evident beginning at 136 d after transplant in one buck (buck ID #902), which we confirmed was derived from transplanted donor SSCs by genotyping for an intact NANOS2 allele that was present in cells derived from the Lamancha donor males (Fig. 4 F and G). In addition, the sperm were of normal morphology and motile (Fig. 4F and Movie S2). These findings demonstrate unequivocally that germline-ablated NANOS2 KO bucks can serve as recipients for allogeneic SSCT.
Fig. 4.
Donor-derived spermatogenesis following allogeneic SSCT in germline-ablated NANOS2 knockout bucks. (A) Image of five NANOS2 KO bucks generated by somatic cell nuclear transfer of CRISPR-Cas9 gene-edited fetal goat fibroblasts. (B) Outputs from DNA sequencing analysis of PCR-based genotyping products showing 16-bp and 23-bp NANOS2 deletion (Δ) alleles. (C) Schematic for SSCT in NANOS2 KO bucks. Prepubertal Lamancha breed (dwarf ear) males were used as a source of donor SSCs. (D) Representative images of hematoxylin & eosin-stained cross-sections of testicular parenchyma from WT and NANOS2 KO bucks at 4 months of age. (Scale bars, 100 μm.) (E) Representative images of immunofluorescent staining for the spermatogonial marker ZBTB16 in cross-sections of testes from 4-mo-old wild-type and NANOS2 KO bucks. (Scale bars, 100 μm.) (F) Representative images of spermatids and sperm in the ejaculates of NANOS2 KO bucks that arose 85 to 136 d after donor SSCT. (Scale bar, 10 μm.) (G) Representative image of an agarose gel for visualizing RFLP-based genotyping for NANOS2 alleles with genomic DNA isolated from sperm in NANOS2 KO buck ejaculates and hair follicles from wild-type or NANOS2 KO bucks. The mutation generated by CRISPR-Cas9 editing of the caprine NANOS2 genomic DNA sequence removed a BmrI restriction enzyme site present in wild-type DNA, thereby rendering the sequence resistant to cleavage.
Generation of NANOS2 Knockout Cattle.
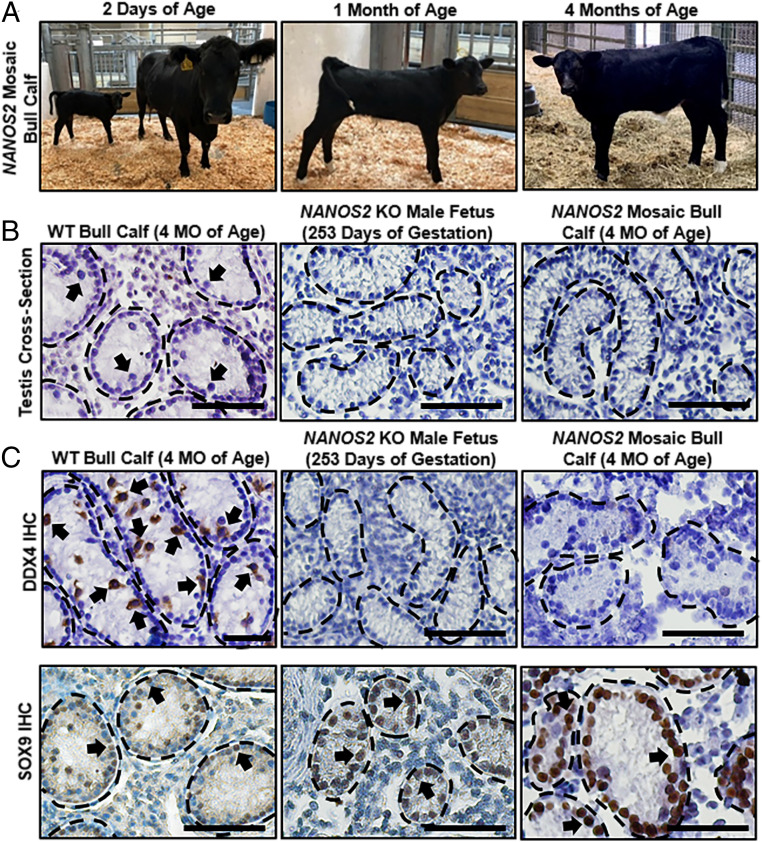
Lastly, given the success with goats, we aimed to generate NANOS2 KO cattle to determine if the phenotype of male-specific germline ablation is conserved in a larger ruminant. To achieve this, electroporation-based CRISPR-Cas9 editing of in vitro-produced embryos was carried out using methodology that we recently described (16). In total, 32 blastocyst-stage embryos were transferred into 16 estrous synchronized multiparous recipient cows, resulting in eight pregnancies, one of which ended in preterm stillbirth of a bull calf at ∼8 mo of gestation and two of which progressed to term resulting in live birth of one heifer calf and one bull calf (Fig. 5A and SI Appendix, Fig. S7 and Table S6). Both live born calves were of normal birth weight and developed in a manner consistent with possessing normal physiology (SI Appendix, Table S7); likewise, the stillborn calf was of normal weight and size for an 8-mo-old bovine fetus. Genotyping analysis revealed that the preterm stillbirth calf and live heifer calf were NANOS2 KO with both possessing biallelic editing of inactivating 11- or >300-bp Δ mutations, respectfully (SI Appendix, Fig. S7). In corroboration of the genotyping analysis, examination of cross-sections from testicular parenchyma from the stillborn NANOS2 KO calf revealed intact seminiferous cords and the presence of soma but absence of germ cells (Fig. 5B), which was confirmed by lack of immunostaining for the conserved germ cell marker DDX4 but detectable immunostaining for the somatic Sertoli cell marker SOX9 (Fig. 5C). Interestingly, genotyping of DNA isolated from different cell lineages revealed that the live born bull calf was mosaic for edited NANOS2 alleles; note that mosaicism is a common occurrence in model organisms such as mice and pigs when CRISPR-Cas9 reagents are delivered to zygotes (18, 20). For the blood cell lineage which derives from embryonic mesoderm, genomic DNA sequencing analysis of cloned PCR amplicons (n = 14) detected intact NANOS2 alleles at a frequency of 20% and INDEL mutated alleles detected at a frequency of 80% (SI Appendix, Fig. S7). In contrast, analysis of genomic DNA from hair follicle cells, which derives form embryonic ectoderm, revealed that 100% of NANOS2 amplicons (n = 16) contained INDEL mutations (SI Appendix, Fig. S7). To assess the extent of NANOS2 editing in primordial germ cells (PGCs) from which the spermatogenic lineage derives, biopsies of testicular tissue were collected from the mosaic bull calf at 4 months of age. Similar to the stillborn fetal calf, germ cells were undetectable in cross-sections of parenchyma but seminiferous tubules were intact with apparent somatic cells and these observations were confirmed by lacking immunostaining for DDX4 but detectable staining for SOX9 (Fig. 5C). Collectively, these findings solidify that NANOS2 is evolutionarily conserved as a specific regulator of male germline establishment and indicate that CRISPR-Cas9-based inactivation can produce surrogate sires for many, if not all, mammalian species.
Fig. 5.
Germline ablation in NANOS2 knockout male cattle. (A) Pictures of a NANOS2 mosaic bull calf at 2 days, 1 month, and 4 months of age that was generated by CRISPR-Cas9 editing of a bovine zygote and embryo transfer. (B) Representative images of hematoxylin & eosin-stained cross-sections of testicular parenchyma from a WT bull calf at 4 months of age, NANOS2 KO fetal bull calf at 253 d of gestation, and NANOS2 mosaic bull calf at 4 months of age. (Scale bars, 50 μm.) Arrows indicate examples of germ cells in wild-type testes. (C) Representative images of immunohistochemical staining for germ cells (DDX4+) or Sertoli cells (SOX9+) in cross-sections of testicular parenchyma from a WT bull calf at 4 months of age, NANOS2 KO fetal bull calf at 253 d of gestation, and NANOS2 mosaic bull calf at 4 months of age. (Scale bars, 50 μm.) Arrows indicate examples of DDX4-stained germ cells and SOX9-stained Sertoli cells.
Discussion
Developing applications of SSCT could impact society in several ways including the combating of male infertility, preserving endangered species, and altering the genetic makeup of livestock populations. In particular, deployment as a breeding tool in livestock—the surrogate sire concept—has the potential to dramatically enhance genetic gain on a worldwide scale to improve production characteristics for generating meat, milk, and fiber for human consumption (8, 21). Considering that broad utility of such a concept will require allogeneic transfer in diverse livestock production systems including range settings or smallholder regions of the developing world, intensive management of an SSCT recipient for immune suppression will not be feasible. Here we demonstrate unequivocally that neither allogeneic donor SSCs nor the spermatogenic lineage derived from them are immunologically rejected when transferred into the testes of NANOS2 KO recipient male mice, pigs, and goats that possess a fully functional immune system. Furthermore, using the experimentally tractable mouse model we show that NANOS2 KO recipients can attain natural fertility following SSCT and 100% of offspring possess the donor haplotype. These findings provide compelling support for feasibility of developing the surrogate sire concept in all animals including endangered species and livestock.
Although several previous studies have suggested that SSCT is possible in livestock, including pigs (11, 12), cattle (14, 15), sheep (22), and goats (13), none have reduced the approach to a context that would be applicable in an agricultural production setting. In particular, all have used cytotoxic treatments in attempts to eliminate the endogenous germline of recipients which is well known to have detrimental effects on the health of the animal and harm the somatic support structure of the testis (23–25). In addition, the cytotoxic treatment approaches devised to date are not able to produce complete ablation of the endogenous germline, with remaining resident SSCs able to reestablish spermatogenesis over time. Indeed, studies with mice have shown that all SSCT recipients treated with busulfan produce offspring from endogenous germ cells (10). Here, we demonstrate that testes of male pigs and goats with genetic deficiency in a germ cell-specific gene (NANOS2) that leads to complete germline ablation can be a surrogate host for regeneration of spermatogenesis from transplanted wild-type SSCs. This achievement is a demonstration, outside of mice, of spermatogenic regeneration in a genetically sterile male by SSCT.
To be tractable in a livestock production setting, SSCT must produce recipients with a level of donor-derived sperm production similar to that of normal males in order to achieve fertility in a natural breeding setting. Although a level comparable to that of a normal wild-type male was not achieved, we show that with a mouse model, natural fertility is attained when Nanos2 KO males are used as recipients. However, with pigs and goats, technical refinements are needed to achieve a greater level of donor-derived spermatogenesis. While a high level of donor SSC engraftment occurred in all Nanos2 KO recipient mice, only those that were less than 28 days of age at the time of transplant were able to produce offspring. This age point would be considered as prepubertal in a wild-type context, suggesting that SSCT must occur prior to when the testicular soma would normally mature in order for natural fertility to be attained. Indeed, previous studies showed that donor SSC engraftment is nearly fivefold greater in wild-type recipient mice transplanted as prepubertal pups (4 to 8 days of age) compared to adults (26). Translating from mouse to large animals, SSCT should occur prior to puberty to produce a high level of donor-derived spermatogenesis. In the current study, successful injection of donor cells into NANOS2 KO recipient pigs and goats occurred when they were past what would be consider a prime window of prepubertal development. In addition, with mice, over two decades of refinements for optimizing the several aspects of SSCT have occurred such as the volume of cell suspension transferred, number of donor cells, and route of injecting the donor cell suspension into recipient seminiferous tubules. Although we have demonstrated biological proof of concept in the current study that testes of NANOS2 KO livestock are able to harbor regeneration of spermatogenesis from a transplanted donor SSC, several simple technical refinements to the injection procedure are likely to generate surrogate males that could be used in a natural breeding scheme.
An unanticipated complication of the cohort of NANOS2 KO boars used in the current study was a relatively high incidence of osteopathic degeneration which occurred in two of the six animals. If caused by NANOS2 deficiency, this nuance could limit utility of KO males for breeding purposes. Considering that all of the boars were clones from a single nuclear transfer donor boar that had been generated by CRISPR-Cas9 editing in our previous studies who did not experience osteopathic degeneration (18), abnormalities brought about by the cloning procedure could be the cause. However, potential off-target editing by the NANOS2 CRISPR in the nuclear transfer donor boar cannot be ruled out as a contributor of the degeneration phenotype. Also, osteopathic degeneration or a similar abnormality was not observed with NANOS2 KO animals of any of the other species that we studied, thus indicating that NANOS2 deficiency was not a major contributor to the phenotype.
Beyond the utility as a next generation breeding tool in livestock production, SSCT with NANOS2 KO males could be an invaluable alternative to the current standards of busulfan and irradiation treatments to eliminate endogenous germline. Importantly, because Nanos2−/− female and Nanos2+/− male mice are fertile, KO recipients can be efficiently produced by simple cross-breeding. With mice, we found that the level of donor SSC engraftment in Nanos2 KO recipients is greater compared to conventional wild-type recipients prepared by busulfan treatment. Considering the relative ease of producing Nanos2 KO males by cross-breeding, use of cytotoxic treatments to prepare SSCT recipient males may very likely be unnecessary in all research and application settings which will also reduce welfare concerns of subjecting animals to harsh chemicals. Beyond the mouse, generating any animal model that harbors NANOS2 mutant alleles including rodents, domestic livestock, companion animals, or endangered species, is technically feasible using CRISPR-Cas9 technology. For endangered species, combining the generation of NANOS2 mutant animals with SSCT may provide a viable means for preserving unique genetics and providing an option for conservation.
For many types of stem cell transplantation, allogenecity is a major limiting factor which often results in immunological rejection of the donor cells. For example, the effectiveness of hematopoietic stem cell transplantation for rodents and humans is compromised when the donor and recipient are histoincompatible (27, 28). Previous studies with mice indicated that allogeneic SSCT was not tolerated due to recipient immunological rejection (9, 10); but, studies with pigs (11, 12), goats (13), and cattle (14, 15) have detected the presence of donor DNA in the ejaculates of recipient males following injection of a testis cell suspension from an allogeneic donor, suggesting immune tolerance. For pigs, assessment of SSCT success has relied on detection of a transgenic DNA sequence in the ejaculate of recipients that presumably integrated into donor germ cells via exposure of the mixed testicular cell suspension to a viral vector prior to transplantation and detection of the same sequence in embryos produced by in vitro fertilization with sperm of the recipient (11). For goats, a previous study showed that one out of five recipient goats transplanted with an allogeneic donor testis cell suspension generated a single offspring (out of 15 produced by the recipient) that possessed donor transgene DNA (13). For cattle, demonstration of sperm being the source of donor DNA sequence has not been definitively shown by previous studies. Although these findings are encouraging, all large animal recipients used to date have possessed endogenous germline, albeit depleted by busulfan treatment or localized testicular irradiation in some cases, which makes clear determination of donor-derived spermatogenesis challenging. In the present study, we demonstrate unequivocally that allogeneic SSCT in mice, pigs, and goats is immunologically tolerated, leading to extensive engraftment and persistent donor-derived spermatogenesis at a level sufficient to yield fertility, at least in the case of mice. Furthermore, our findings are experimental evidence of regenerating the spermatogenic lineage via SSCT in genetically sterile male livestock that lack endogenous germline. Moreover, our discovery that inactivation of NANOS2 also leads to germline ablation in male cattle opens the intriguing possibility of one day developing bull “super dads” that then can be harnessed for disseminating desirable genetics in cattle populations around the world (29). We predict that the accomplishments reported here will serve as a springboard for future refinement of methodology to establish the surrogate sire concept as a predominant breeding tool in all sectors of food animal production.
Methods
Animals.
All animal procedures were approved by the Institutional Animal Care and Use Committees of Washington State University, the University of Maryland, and Utah State University. All mouse lines were originally procured from Charles River Laboratories, CD1/ICR (stock no. 022) or The Jackson Laboratory, Rosa26-LacZ (stock no. 002073), C57BL6/J (stock no. 000664), and 129S1/svlmJ (stock no. 002448). Boars that served as a source of donor SSCs for transplantation were of mixed domestic breeds and SSC donor bucks were of the dwarf-eared Lamancha breed.
Generation of NANOS2 Gene Edited Animals.
Mice.
To produce CD1 Nanos2 knockout male mice, females that were homozygous and males that were heterozygous for inactive Nanos2 alleles, which were engineered in previous studies using CRISPR-Cas9 technology (16), were mated.
Pigs.
The production of NANOS2 knockout boars to serve as SSCT recipients involved SCNT with fibroblasts isolated from a mature boar engineered in previous studies using CRISPR-Cas9 technology to possess 4-bp and 150-bp Δ alleles (18). The SCNT procedure was as described previously (30). In total, six NANOS2 knockout cloned boars were produced and all developed normally from birth to adulthood and were azoospermic, thus mirroring the phenotype of the SCNT donor.
Goats.
Fetal fibroblasts from a Nubian × Boer donor were transfected with a Cas9/sgRNA3 plasmid vector engineered to express a sgRNA targeting the caprine NANOS2 genomic DNA sequence (SI Appendix, Fig. S6 and Cas9 mRNA as described previously) (31, 32). Gene mutation efficiency was determined by PCR/restriction fragment length polymorphism (RFLP) assays. Single-cell-derived NANOS2 knockout fibroblast colonies were isolated by limiting dilution and used for SCNT as cell donors. The SCNT procedure was performed as described previously (19). In total, five NANOS2 knockout cloned bucks were produced and developed normally to adulthood. PCR/RFLP assays and Sanger sequencing were used for the genotyping of cloned animals.
Cattle.
Bovine zygotes were generated using an in vitro production system and electroporated with CRISPR-Cas9 reagents designed to edit the NANOS2 gene, as we have described previously (16). Embryos were then cultured to the early blastocyst stage (developmental day 6) using methodology described previously (16), and those that were scored as grades 1 to 2 were transferred into uterine horns of day 7- to 9-pseudopregnant recipient cows or heifers whose estrous cycles had been synchronized using the 5-d controlled internal drug release/cosynchronization protocol. For all recipients, two embryos were transferred into the uterine horn ipsolateral to an ovary possessing a robust corpus luteum using a standard nonsurgical technique. Pregnancy diagnosis was made at 60-d postembryo transfer using ultrasonography.
Genotyping Analyses.
Genomic DNA was isolated from blood cells, hair follicle cells, skin epithelial cells, and sperm using a DNAeasy kit (Qiagen, cat. no. 69504). Genotyping for NANOS2 alleles in mouse, pig, and cattle samples was conducted using PCR and Sanger sequencing methodologies as described previously (16). For goat samples, PCR was carried out with genomic DNA and primers (forward: 5′-TGCTTAGAAGGGTCTTTGGG-3′, reverse: 5′-CATAATGCCGCAGGATGG-3′) designed to recognize the full coding sequence for caprine NANOS2. The reaction produced a wild-type amplicon of 424 bp which contained a BmrI restriction enzyme site that was removed by CRISPR-Cas9 editing which yielded GFF clones #82 and 25 that were used for SCNT. Thus, genotyping for NANOS2 mutant alleles was carried out as an RFLP assay that could be visualized with agarose gel electrophoresis; restriction enzyme digestion of the wild-type amplicon yielded fragments of ∼250 and 175 bp, whereas the CRISPR-Cas9 mutated alleles were resistant to cutting.
Donor SSC Preparation and Transplantation.
Mice.
Single-cell suspensions were generated from testes of adult Rosa26-LacZ mice and the THY1+ fraction that is enriched for SSCs was then isolated using magnetic-activated cell sorting as described previously (33, 34). Cells were either transplanted as a fresh cell suspension or used to establish primary cultures as described previously (34) and expanded for a period of 2 mo prior to collection as a single-cell suspension for transplantation. Both freshly isolated and in vitro expanded donor cell populations were suspended at a concentration of 2 to 5 × 106 cells/mL in mouse serum-free media (34) and 2 to 5 μL was microinjected into seminiferous tubules of each Nanos2 knockout recipient mouse testis as described previously (35).
Pigs and goats.
Approximately 500 mg of parenchyma was collected from prepubertal donor boar or buck testes and enzymatically disassociated using a multistep method devised originally for bovine testicular tissue (36). The resulting single-cell suspensions were then either transplanted as a mixed testicular cell suspension containing germ cells and somatic cells (pigs only) or placed in gelatin-coated plastic culture wells and incubated overnight in a serum-free media designed for culture of mouse SSCs (34). After overnight culture, the nonadherent cell suspensions, enriched for spermatogonia, were isolated by gentle swirling of the plate and collection of the cell containing media. For transplantation, the fresh mixed cell suspension or enriched spermatogonial cell suspensions were suspended in serum-free media at a concentration of 1 to 2 × 106 cells/mL. Next, NANOS2 knockout recipient boars and bucks were placed under general anesthesia and positioned in lateral recumbency for infusion of donor cells into the rete testis using a modified ultrasound-guided injection procedure described previously (12, 13, 37). Briefly, for each testis a 5-mm incision was made through the scrotum of the dorsal side, ventral to the tail of the epididymis, and parallel to the median raphe until visualization of the tunica albuginea. A stub incision was then made through the tunica albuginea and an IV catheter was inserted through the incision as parallel as possible to the rete testis which was visualized by ultrasonography using a 7.5-MHz linear transducer positioned longitudinally with respect to the long axis of the testicle. Once the tip of the catheter was visualized as being in the rete testis, the stylet was removed and surgical glue was used to fix the catheter in position. Next, an extension tubing set (Baxter; 21 inches, 2.4 mL) containing the donor cell suspension was attached to the catheter and a syringe without plunger was attached to the other end of the tubing. The plunger was then added and the solution was allowed to flow into the rete testis which could be visualized by ultrasonography. After the entire cell suspension had been infused, the catheter was removed and scrotal skin incision closed with suture. To facilitate proper positioning, the right testicle was injected with the recipient boar or buck in left lateral recumbency and vice versa.
Histological Analysis and X-Gal Staining.
For histological analysis, testicular and the epididymal tissues were fixed in Bouin’s solution for 1 h followed by dehydration in graded series of ethanol and xylenes and then embedded in paraffin. Cross-sections of 5 μm were generated with a microtome and adhered to glass slides followed by dewaxing and rehydration. For morphological assessment, cross-sections were stained with hematoxylin & eosin followed by evaluation using light microscopy and digital images were captured at 100 to 200× magnification with an Olympus IX51 and TH4-100 digital camera using CellSens Dimensions software. For mouse studies, assessment of donor-derived colonies of spermatogenesis in recipient testes was accomplished by staining with X-Gal as described previously (35).
Immunostaining Analyses.
Tissue cross-sections.
Parrafin cross-sections (5-μm thickness) adhered to glass slides were dewaxed and rehydrated. The sections were then processed for antigen retrieval by incubating in an acidic (pH 6.0) solution of 10 mM sodium citrate (Amresco, cat. no. 0101) and 0.05% Tween-20 (Sigma-Aldrich, cat no. P1379) for 20 min at 96 °C. Nonspecific antibody binding was then blocked by incubating sections in a solution containing 2% bovine serum albumin (BSA) (Thermo Fisher Scientific, cat. no. BP1600) and 0.1% Triton X-100 (Sigma-Aldrich, cat. no.T8787) in phosphate-buffered saline (PBS) at room temperature for 2 h. Sections were then incubated overnight at 4 °C with a primary antibody: rabbit anti-human ZBTB16 (Santa Cruz Biotechnology, cat. no. sc22839) at 1:200; rat anti-mouse TRA98 (Abcam cat. no. ab82527) at 1:500; rabbit anti-human DDX4 (Abcam, cat. no. ab13840) at 1:100 to 200; or rabbit anti-human SOX9 (Millipore, cat. no. AB5535) at 1:200. Sections were washed in PBS and then incubated at room temperature for 2 h with a secondary antibody: Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen, cat. no. A-11008) at 1:1,000; DyLight 594 goat anti-rat IgM (Invitrogen, cat. no. SA5-10012) at 1:1,000; horseradish peroxidase donkey anti-rabbit IgG (Vector Labs, cat. no. pk6200) at 1:1,000 followed by washing in PBS, counterstaining with hematoxylin or DAPI, and mounting of coverslips. Images were captured using an Olympus IX51 or Leica DMi8 microscope.
Cells.
Single-cell suspensions of multiparameter selected or unselected testicular populations from pigs and goats were adhered to poly-l-lysine-coated plastic dishes and subsequently fixed in 4% paraformaldehyde for 10 min at 4 °C. Cells were then incubated in PBS containing 0.1% Triton X-100 to permeabilize membranes, and nonspecific antibody binding was blocked by incubation with 10% normal serum in PBS at room temperature for 5 min. Cells were again washed with PBS and then incubated overnight at 4 °C with primary antibodies diluted in PBS containing 0.5% BSA and 0.1% Triton X-100: rabbit anti-human ZBTB16 (Santa Cruz Biotechnology, cat. no. sc22839) at 1:200 or mouse anti-human UCHL1 (Bio-Rad, cat. no. 78631004) at 1:200. On the next day, cells were washed in PBS and incubated with secondary antibody for 1 h at 4 °C: Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen, cat. no. A-11008) at 1:1,000; or Alexa Fluor 546 donkey anti-mouse IgG (Invitrogen, cat. no. A10036). Cells were again washed in PBS, then incubated with DAPI to label their nuclei, viewed by fluorescent microscopy, and digital images were captured with a DP72 camera and CellSens acquisition software. Quantification of the percentage of cells in multiparameter and unselected cell populations that were ZBTB16+ was achieved by counting fluorescently stained cells in five different fields of view for each well and dividing by the number of DAPI-stained nuclei that were present in the same fields of view.
Assessment of Germline Presence, Spermatogenesis, and Fertility.
Mice.
Presence of spermatogenic lineage in testes of Nanos2 KO mice was assessed by histomorphological examination of testis cross-sections. The fertility of all mice was assessed by pairing of one to two pubertal-aged females with a single male for periods of 1 to 6 mo and the number of pups born per month was recorded. Detection of LacZ activity by X-Gal staining was used to assess donor-derived spermatogenesis in Nanos2 KO recipient mice following SSCT. Both coat color and LacZ expression from X-Gal staining of tail tips were used to determine whether offspring produced by Nanos2 KOs following SSCT were from donor-derived sperm.
Pigs, goats, and cattle.
Histomorphological examination and immunostaining for DDX4 and SOX9 were used to determine the presence of spermatogenic lineage and Sertoli cells in cross-sections of testes, respectively. To assess the degree of spermatogenesis throughout testes of NANOS2 knockout boars subjected to SSCT or nontransplanted controls, ∼100-mg pieces of parenchyma were collected from 50 different regions in a manner that represented the entire tissue mass. For quantification, 500 to 600 cross-sections (n = 10 to 12 per tissue piece) were examined using light microscopy, and the number of tubules with or without spermatogenesis was scored. To assess the presence of sperm in the ejaculate of boars and bucks, a semen collection phantom or teaser doe in estrus was used, respectively.
Supplementary Material
Acknowledgments
We thank all members of the J.M.O., B.W., B.T., and I.A.P. laboratories for helpful discussions and constructive criticisms. We also thank staff of the Washington State University Veterinary Teaching Hospital for assistance with boar and buck transplantation surgeries, Gary Turner and Eric Lautzenheiser for husbandry of NANOS2 mutant pigs and cattle, and Fred Loiza for husbandry of the NANOS2 mutant goats. The research was supported in part by US Department of Agriculture (USDA)–National Institute of Food and Agriculture (NIFA) grant 2017-05436 awarded to B.T. and J.M.O.; USDA-NIFA grant 2018-06478 awarded to J.M.O.; intramural funds from Washington State University and Utah State University; and an industry contract from Genus plc to support the mouse, porcine, and bovine research by J.M.O.
Footnotes
Competing interest statement: J.M.O., B.W., S.L., B.T., and I.A.P. are listed as inventors on patent filings covering gene editing of livestock to generate germline ablated males.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2010102117/-/DCSupplemental.
Data Availability.
All study data are included in the article and supporting information.
References
- 1.Oatley J. M., Brinster R. L., The germline stem cell niche unit in mammalian testes. Physiol. Rev. 92, 577–595 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oatley J. M., Brinster R. L., Regulation of spermatogonial stem cell self-renewal in mammals. Annu. Rev. Cell Dev. Biol. 24, 263–286 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinster R. L., Zimmermann J. W., Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. U.S.A. 91, 11298–11302 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinster R. L., Avarbock M. R., Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. U.S.A. 91, 11303–11307 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valli H. et al., Germline stem cells: Toward the regeneration of spermatogenesis. Fertil. Steril. 101, 3–13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark A. T., Phillips B. T., Orwig K. E., Fruitful progress to fertility: Male fertility in the test tube. Nat. Med. 17, 1564–1565 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Kubota H., Brinster R. L., Technology insight: In vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat. Clin. Pract. Endocrinol. Metab. 2, 99–108 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giassetti M. I., Ciccarelli M., Oatley J. M., Spermatogonial stem cell transplantation: Insights and outlook for domestic animals. Annu. Rev. Anim. Biosci. 7, 385–401 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Kanatsu-Shinohara M. et al., Allogeneic offspring produced by male germ line stem cell transplantation into infertile mouse testis. Biol. Reprod. 68, 167–173 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Kanatsu-Shinohara M., Morimoto H., Shinohara T., Fertility of male germline stem cells following spermatogonial transplantation in infertile mouse models. Biol. Reprod. 94, 112 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Zeng W. et al., Viral transduction of male germline stem cells results in transgene transmission after germ cell transplantation in pigs. Biol. Reprod. 88, 27 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honaramooz A., Megee S. O., Dobrinski I., Germ cell transplantation in pigs. Biol. Reprod. 66, 21–28 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Honaramooz A. et al., Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol. Reprod. 69, 1260–1264 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Herrid M. et al., Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol. Reprod. 81, 898–905 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Herrid M., Vignarajan S., Davey R., Dobrinski I., Hill J. R., Successful transplantation of bovine testicular cells to heterologous recipients. Reproduction 132, 617–624 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Miao D., Giassetti M. I., Ciccarelli M., Lopez-Biladeau B., Oatley J. M., Simplified pipelines for genetic engineering of mammalian embryos by CRISPR-Cas9 electroporation. Biol. Reprod. 101, 177–187 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuda M. et al., Conserved role of nanos proteins in germ cell development. Science 301, 1239–1241 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Park K. E. et al., Generation of germline ablated male pigs by CRISPR/Cas9 editing of the NANOS2 gene. Sci. Rep. 7, 40176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang M. et al., Oocytes from small and large follicles exhibit similar development competence following goat cloning despite their differences in meiotic and cytoplasmic maturation. Theriogenology 86, 2302–2311 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Yen S. T. et al., Somatic mosaicism and allele complexity induced by CRISPR/Cas9 RNA injections in mouse zygotes. Dev. Biol. 393, 3–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottardo P. et al., A strategy to exploit surrogate sire technology in livestock breeding programs. G3 (Bethesda) 9, 203–215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stockwell S., Hill J. R., Davey R., Herrid M., Lehnert S. A., Transplanted germ cells persist long-term in irradiated ram testes. Anim. Reprod. Sci. 142, 137–140 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Kanatsu-Shinohara M. et al., Functional assessment of self-renewal activity of male germline stem cells following cytotoxic damage and serial transplantation. Biol. Reprod. 68, 1801–1807 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Bucci L. R., Meistrich M. L., Effects of busulfan on murine spermatogenesis: Cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat. Res. 176, 259–268 (1987). [DOI] [PubMed] [Google Scholar]
- 25.Ryu B. Y., Orwig K. E., Oatley J. M., Avarbock M. R., Brinster R. L., Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 24, 1505–1511 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinohara T., Orwig K. E., Avarbock M. R., Brinster R. L., Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc. Natl. Acad. Sci. U.S.A. 98, 6186–6191 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersdorf E. W., Shuler K. B., Longton G. M., Spies T., Hansen J. A., Population study of allelic diversity in the human MHC class I-related MIC-A gene. Immunogenetics 49, 605–612 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Shizuru J. A., Jerabek L., Edwards C. T., Weissman I. L., Transplantation of purified hematopoietic stem cells: Requirements for overcoming the barriers of allogeneic engraftment. Biol. Blood Marrow Transplant. 2, 3–14 (1996). [PubMed] [Google Scholar]
- 29.Ledford H., Bull “super dads” are being engineered to produce sperm from another father. Nature 567, 292–293 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Sheets T. P. et al., Somatic cell nuclear transfer followed by CRIPSR/Cas9 microinjection results in highly efficient genome editing in cloned pigs. Int. J. Mol. Sci. 17, E2031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Z., Yang M., Regouski M., Polejaeva I. A., Gene knockouts in goats using CRISPR/Cas9 system and somatic cell nuclear transfer. Methods Mol. Biol. 1874, 373–390 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Ni W. et al., Efficient gene knockout in goats using CRISPR/Cas9 system. PLoS One 9, e106718 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oatley J. M., Brinster R. L., Spermatogonial stem cells. Methods Enzymol. 419, 259–282 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Kubota H., Avarbock M. R., Brinster R. L., Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. U.S.A. 101, 16489–16494 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helsel A. R., Oatley J. M., Transplantation as a quantitative assay to study mammalian male germline stem cells. Methods Mol. Biol. 1463, 155–172 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Oatley M. J., Kaucher A. V., Yang Q. E., Waqas M. S., Oatley J. M., Conditions for long-term culture of cattle undifferentiated spermatogonia. Biol. Reprod. 95, 14 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Oatley J. M. et al., Changes in spermatogenesis and endocrine function in the ram testis due to irradiation and active immunization against luteinizing hormone-releasing hormone. J. Anim. Sci. 83, 604–612 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.