Abstract
Portal hypertension is the main consequence of liver cirrhosis, leading to severe complications such as variceal hemorrhage, ascites or hepatic encephalopathy. As an attempt to decompress the portal venous system, portal flow is derived into the systemic venous system through spontaneous portosystemic shunts (SPSSs), bypassing the liver. In this review, we aim to provide an overview of the published reports in relation to the prevalence and physiopathology behind the appearance of SPSS in liver cirrhosis, as well as the complications derived from its formation and its management. The role of SPSS embolization is specifically discussed, as SPSSs have been assessed as a therapeutic target, mainly for patients with recurrent/persistent hepatic encephalopathy and preserved liver function. Furthermore, different aspects of the role of SPSS in liver transplantation, as well as in candidates for transjugular intrahepatic portosystemic shunt are reviewed. In these settings, SPSS occlusion has been proposed to minimize possible deleterious effects, but results are so far inconclusive.
Keywords: collaterals, embolization, hepatic encephalopathy, liver transplant, portal hypertension, review, TIPS
Introduction
Spontaneous portosystemic shunts (SPSSs) are communications among the venous portal system and the venous systemic circulation that bypass the liver.1 Their presence has been related to portal hypertension (PH).2 However, many questions regarding their prevalence, pathophysiology and repercussion in liver disease have not been completely elucidated.
Hepatic encephalopathy (HE) has been strongly linked with SPSS.3–5 This severe complication implies hospitalizations, high morbidity and mortality and detriment in quality of life among patients and their relatives.6,7 The generation of nitrogenous-containing products in the gut, rich in ammonia, and the decrease in the cleaning function in liver cirrhosis are involved in its pathophysiology.8 With the presence of SPSSs, a direct bypass effect is added, amplifying the pass of toxins and the accumulation of ammonia.9
The close relation between SPSSs and HE is also reflected in the definition of HE: “Hepatic encephalopathy is a brain dysfunction caused by liver insufficiency and/or portosystemic shunt”.10,11 Either persistent or recurrent HE has been linked to the presence of shunts in many studies. Moreover, the acquired experience with surgical shunts during the last century and lately with transjugular intrahepatic portosystemic shunts (TIPS) has allowed achieving important knowledge in HE pathophysiology that is also applicable to SPSSs.12
In recent years, patients with cirrhosis have an easier and widespread access to noninvasive imaging techniques, not only ultrasound, but also abdominal contrast-enhanced computerized tomography (CT) or magnetic resonance (MR) imaging, which have facilitated the diagnosis of SPSSs.13 At the same time, SPSSs have been considered as a therapeutic target to reverse difficult cases of HE.14 More recently, special situations, as liver transplant (LT) or a broader TIPS indication, have posed new challenges, in which the role of SPSSs will have to be defined.
The aim of this review is to provide a comprehensive revision of the role of SPSSs in cirrhotic patients, focused on clinical aspects and current therapeutic management.
SPSS prevalence in liver cirrhosis
How the prevalence of SPSSs has been evaluated has changed over time. The initial postmortem studies15,16 were followed by diagnostic techniques, such as splenoportography, angiography or percutaneous transhepatic portography.17 These procedures were replaced by current noninvasive imaging techniques, such as Doppler ultrasound, contrast-enhanced CT or MR imaging.13
However, assessing the true prevalence of SPSSs is still a matter of discussion; most studies are retrospective and include small samples of patients, not always comparable in liver function or even basal liver disease. The different diagnostic methods used over time or technical improvements in a specific method make comparisons among studies difficult.18 Doppler ultrasound provides useful information about the presence and direction of portal flow or flow within a shunt.19 Nevertheless, it is operator-dependent and may under-diagnose deeper collaterals.20 By contrast, CT and MR provide cross-sectional imaging, which allows observing the whole portal system.13 Currently, contrast-enhanced abdominal CT seems the most appropriate imaging technique for searching shunts, considering availability, costs, information provided and the possibility of performing a three-dimensional reconstruction.21 MR imaging is as accurate as CT22 but is more expensive and might be less available.23
Even the definition of a SPSS is not always comparable, as some studies considered gastroesophageal varices (GEVs) as a type of SPSS24 and others exclude varices from the classification.25,26 With these limitations, the largest series available performed by ultrasound estimate the global percentage of SPSSs between 34% and 42%.27–31 There are fewer studies carried out with enhanced CT or MR imaging with data about general prevalence (Table 1).25,32,33 Aucejo et al. identified 12% of SPSSs in a cohort of 127 patients with cirrhosis evaluated for LT.32 A more recent study, conducted by the Baveno VI Cooperation Group, consisted of an international multicenter collaboration that evaluated 1729 patients with liver cirrhosis.25 SPSSs were present in 60% of the sample and half of them (488 patients, 28% from the total) were classified as large SPSSs, with a pre-established cutoff of 8 mm. This value was chosen considering the smallest symptomatic embolized shunt reported in the literature.34 In this broad cohort, more than one-third of patients with large SPSSs had also small SPSSs, and 9% had more than one large SPSS.25 Other authors have also identified more than one SPSS in 22–25% of their sample.27,31 Rodriguez et al. provided recent data from a cohort of 326 patients with cirrhosis and candidates for LT in which a high rate of SPSS was found (almost 80%), with a slight predominance of small SPSSs (46% from the total) over large SPSSs (35%).33
Table 1.
Prevalence and types of SPSSs.
| Reference | Imaging test | Number of patients evaluated | Prevalence of SPSS number (percentage) | Type of SPSS number (percentage from the total sample, unless specified) |
|---|---|---|---|---|
| Cho et al.35 | CT | 60 | NA | - Coronary venous: 48 (80) - Paraumbilical: 26 (43) - Abdominal wall: 18 (30) - Perisplenic: 18 (30) - Mesenteric: 6 (10) - Splenorenal: 6 (10) - Gastrorenal: 4 (7) |
| Sacerdoti et al.36 | DUS | 184 | NA | - Paraumbilical: 62 (33.7) |
| Von Herbay et al.27 | DUS | 109 | 41 (38) | - Splenorenal: 16 (15) - Paraumbilical: 8 (9) - Gastric: 2 (2) - Combinations (>1 SPSS): 9 (8) - Others: 5 (5) - Without SPSS: 68 (62) |
| Dömland et al.37 | DUS | 70 | NA | - Paraumbilical: 16 (23) |
| Chen et al.38 | DUS | 254 | NA | - Paraumbilical: 28 (11.1) |
| Del Piccolo et al.39 | DUS | 95 | NA | - Paraumbilical: 56 (59) - Alone: 31 (33) - Combination: 25 (26) |
| Berzigotti et al.30 | DUS | 126 | 42 (33.3) | - Paraumbilical: 23 (18.2) - Splenorenal: 13 (10.3) - Left Gastric Vein: 11 (8.7) - Others: 5 (3.9) |
| Aucejo et al.32 | CT | 127 | 16 (12.6) | - Splenorenal: 12 (9.4) - Coronary: 2 (1.6) - IMV cava: 1 (0.8) - Others: 1 (0.8) |
| Zardi et al.28 | DUS | 326 | 130 (39.9) | - Splenorenal: 45 (13.8) - Left gastric vein: 36 (11) - Combination: 25 (7.7) - Paraumbilical: 24 (7.4) - Without SPSS: 196 (60.1) |
| Tarantino et al.40 | DUS | 81 | NA | - Splenorenal: 15 (18.5) |
| Berzigotti et al.31 | DUS | 86 | 36 (42) | - Paraumbilical: 17 (19.8) - Left gastric vein: 15 (17.4) - Splenorenal: 6 (10.0) - Short gastric vein: 5 (5.8) |
| Kondo et al.41 | DUS | 181 | NA | - Paraumbilical: 47 (26) |
| Maruyama et al.42 | DUS | 162 | NA | - Splenorenal: 30 (18.5) - Short gastric vein: 17 (10.5) |
| Achiwa et al.43 | CT | 451 | NA | - Splenorenal: 50 (11.1) - Gastrorenal: 23 (5.8) - Paraumbilical: 8 (1.8) - Mesocaval: 3 (0.7) - Others: 2 (0.4) |
| Simón-Talero et al.25 | CT (1630) MRI (99) | 1729 | 1036 (60) - Large SPSS: 488 (28%) - Small SPSS: 548 (32%) - Without SPSS: 693 (40%) |
Large SPSS: - Splenorenal: 224 (46)a - Paraumbilical: 132 (27)a - Gastrorenal: 44 (9)a - Mesocaval: 25 (5)a - IMV cava: 20 (4)a - Others: 35 (7)a Small SPSS: - Paraumbilical: 296 (54)b - Splenorenal: 99 (18)b - Gastrorenal: 83 (15)b - Mesocaval: 44 (8)b - IMV cava: 3 (0.5)b - Others: 20 (3.5)b |
| Lipinski et al.29 | DUS | 982 | 338 (34) | - Paraumbilical: 232 (68.6)c
- Splenorenal: 55 (16)c - Mesenteric: 24 (7)c - Combination: 27 (8)c |
| Saks et al.44 | CT | 741 | NA | - Splenorenal: 173 (23) |
| Gómez-Gavara et al.45 | CT MRI |
429 | 75 (17.5)d | - Splenorenal: 40 (60.6)c
- Left gastric: 16 (24.2)c - Mesenterico-iliac: 10 (15.1)c |
| Rodríguez et al.33 | CT/MRI | 326 | 263 (80.7%) - Large SPSS: 113 (35%) - Small SPSS: 150 (46%) - Without SPSS: 63 (19%) |
CT, computed tomography; DUS, Doppler ultrasound; IMV, inferior mesenteric vein; MRI, magnetic resonance imaging; NA, data not available and not possible to calculate with the data provided; PVT, portal vein thrombosis; SPSS, spontaneous portosystemic shunt.
Percentage from the total of Large SPSS.
Percentage from the total of Small SPSS.
Percentage from the total of SPSS.
9 patients with SPSS were excluded due to extent PVT and type of SPSS is not mentioned.
Many studies have evaluated the type of shunt detected,25,27–29,32,35,43 and most of them have focused on the identification of a specific type of SPSS (Table 1).30,31,36–42,44,46 Paraumbilical vein shunt was the most frequently reported SPSS, found in up to 43% of patients30,31,35–38,47 and even 59% when combined with other SPSSs.39 Despite the high prevalence, its classical advanced form as caput medusa is rarely seen.48 The left renal vein is frequently involved in portosystemic collateral drainage: splenorenal (10–23%) and gastrorenal shunts (5–11%) were also frequently described.28,32,40,42–44 By contrast, splenorenal shunt was the most frequent large SPSS found in the Baveno VI Cooperation Group cohort.25 Mesenteric collaterals from superior and inferior mesenteric veins are also present in many series in a lower percentage.25,32,35,43 Intrahepatic SPSSs are rare and very infrequently reported49 (Figures 1 and 2).
Figure 1.
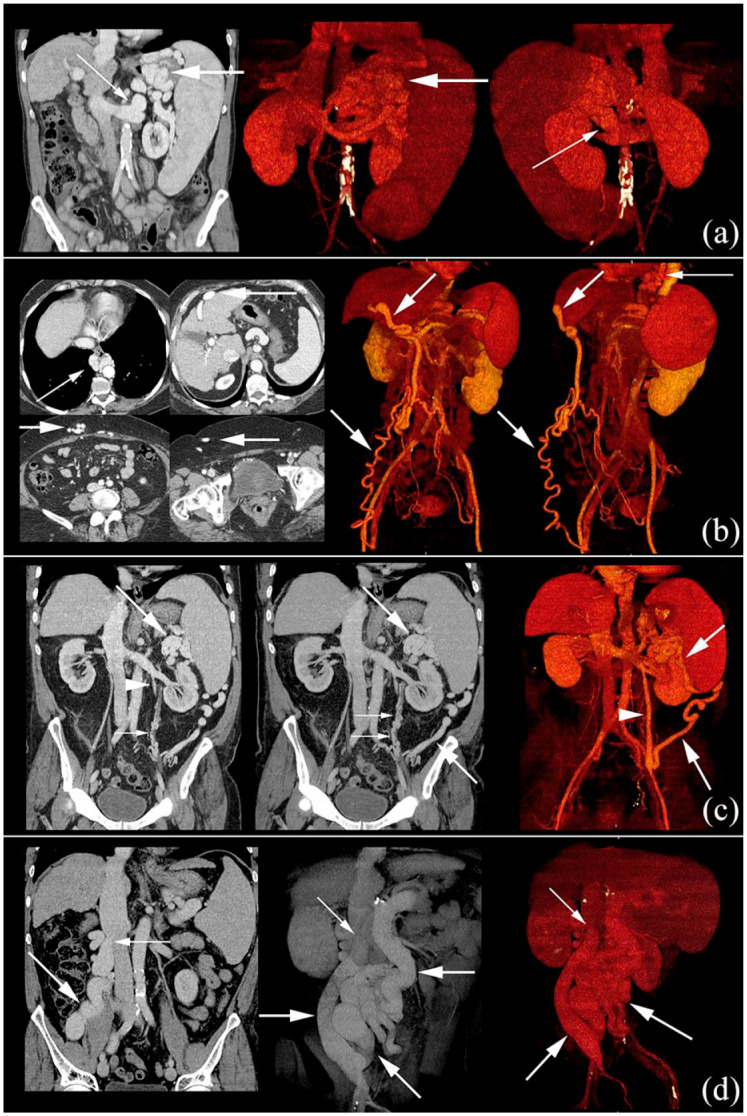
Contrast-enhanced CTs of different SPSS.
(a) Gastrorenal shunt (thin arrows). Thick arrows: varices of coronary vein. Coronal image and two volume rendering images. (b) Paraumbilical shunt that drain through collaterals (thick arrows) to the right common femoral vein. Thin arrows: paraesophageal varices. Four axial images and two volume rendering images. (c) Splenorenal shunt (thick arrows) that communicates with left renal vein through left gonadal vein (arrowhead). Secondary peri-ureteral collaterals (thin arrows). Coronal image, maximum intensity projection coronal image and volume rendering image. (d) Mesocaval shunt (thick arrows), from SMV to IVC (thin arrows) through right gonadal vein. Coronal image and two volume rendering images.
CT: computed tomography; IVC, inferior vena cava; SMV, superior mesenteric vein; SPSS, spontaneous portosystemic shunt.
Figure 2.
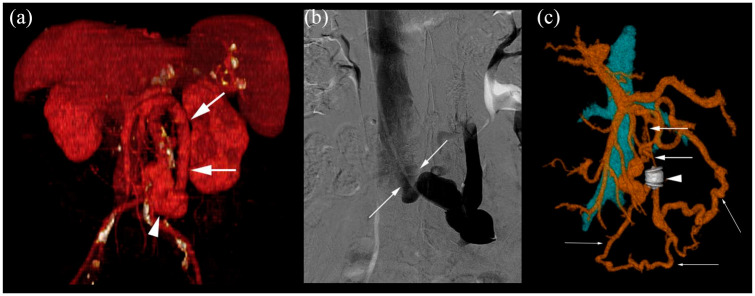
Embolization of SPSS from IMV to ICV.
(a) Dilated IMV (arrows) and large shunt (arrowhead), that drains to ICV. Volume rendering image. (b) Contrast-enhanced shunt during the angiographic procedure, showing the drainage of the collateral from IMV to IVC. Angiography image (digital subtraction ). (c) After the embolization with Amplatzer (arrowhead), distal diameter of IMV was reduced (thick arrows). New collaterals through left colic vein (thin arrows) to IVC were developed. Volume rendering image.
CT: computed tomography; IVC, inferior vena cava; IMV, inferior mesenteric vein; SPSS, spontaneous portosystemic shunt.
According to the anatomical drainage, different theoretical classifications have been proposed1,43,50 depending on its situation (left-sided shunt, including gastrorenal and splenorenal shunt, and right- or central-sided shunt, which includes paraumbilical shunts),17,51 or according to the manner the shunt reaches its drainage in the renal vein (named direct splenorenal shunts in the left subfrenic compartment or indirect splenorenal shunt, also called gastrorenal shunts).47 This information has special interest in the surgical approach of LT.
A specific relationship between etiology and the type of shunt has been suggested. In some series, it was observed than paraumbilical veins were more frequent among alcohol-related cirrhosis than in viral hepatitis.25,29,38 These results, however, are not consistent in all studies and should be confirmed. Hepatocellular carcinoma has also been related to SPSSs with inconclusive and contradictory results.29,33,40
Pathophysiology
The development of collaterals is directly linked to the presence of PH; SPSSs are formed as a compensatory mechanism, in an attempt to decompress the portal venous system. Collaterals appear after reopening closed embryonic venous channels that communicate venous portal flow with the systemic circulation, bypassing the liver. Moreover, there are evidences supporting that SPSSs are not only developed from pre-existing vascular channels, but also as new vessels formed in a process of neoangiogenesis.52,53
An explanatory model for the compensatory mechanism and development of SPSSs has been proposed, equating it to an electrical circuit.54 The authors rely on Ohm’s law to propose two variables involved. The increase in portal venous pressure (PVP) and the decrease in shunt resistance (SR), defining the shunt flow as PVP/SR. When PVP has a significant increase as a consequence of liver cirrhosis, shunt flow rises. In the same way, if SR decreases, shunt flow would increment, as it occurs in aneurysmal dilations of the vascular channels. Secondarily to these events, PVP and portal blood flow would be reduced, due to the circuit bypass created by the shunts. This model has therapeutic implications; the increase of PVP after SPSS occlusion should be taken into consideration when an embolization is been planned, as PVP could increase enough to open new shunts.14
The development of SPSS seems also to have implications on liver function. Kumamoto et al. proposed the term “portosystemic shunt syndrome” referring to a significant reduction of hepatic reserve (reflected by progression of Child–Pugh score) over 5 years, as compared with patients with cirrhosis and PH without gastrorenal shunts.55 Saad et al. based on this concept, described a complete syndrome with clinical manifestations and imaging findings56 that developed in three phases: (1) early stage, characterized by few episodes of HE and relatively well-preserved liver function; (2) late stage, in which overt HE is more frequent and liver function starts to deteriorate; radiological signs include incipient liver atrophy, disappearance of portal branches and the possibility of portal thrombosis; in the main portal vein, the hepatopetal flow becomes sluggish; and (3) end stage, in which HE is markedly disabling, the patient has advanced liver failure and portal thrombosis or liver atrophy are easier to find; portal flow can reverse and become hepatofugal. Thus, despite the fact that a SPSS is initially a compensatory mechanism, as PH progresses, the shunt increases in size, worsening the bypass effect and contributing to the deterioration of liver function.57
Paraumbilical shunts deserve a special mention. Their development occurs due to the expansion of paraumbilical veins, normally collapsed, located at the falciform ligament. When they are the only SPSS present, significant blood volume circulates through the portal vein with hepatopetal direction.37,57 However, these characteristics do not imply an effective portal perfusion and do not confer a protective role: as other SPSSs, paraumbilical veins are specially identified as liver function deteriorates.36–38
Clinical studies and complications related to SPSS
The relation between PH complications and SPSS has been considered in many clinical studies, with different conclusions (Table 2). On one hand, the presence of SPSS has been linked to an increase of PH-related complications, such as ascites or GEVs.3,25,30,31,58 By contrast, a protective effect has also been proposed. A decrease in the rate of ascites, varices and gastrointestinal bleeding (GIB) in patients with large SPSSs has been described, especially in those cases with HE.59,60 Onishi et al. showed that patients with splenorenal or gastrorenal shunts and HE presented fewer esophageal varices (EVs) and a reduced incidence of episodes of GIB.59 Takashi et al. also found a protective effect, with a low percentage of EVs in patients with shunts and HE.60 In a case–control study performed by Riggio et al., patients with SPSSs had fewer EVs and ascites, supporting a protective and compensatory mechanism.5 Tarantino et al. showed that patients without SPSSs had a higher rate of large EVs.40 Finally, Saks et al. identified a higher percentage of EVs, but a lower probability of ascites.44
Table 2.
Relation between SPSS and decompensating events (GEV, ascites and HE), and liver function. Studies in which percentage of GEV, GIB, presence of ascites, HE or liver function are mentioned, are detailed on the table.
| REF | Gastroesophageal varices | Gastrointestinal Bleeding | Ascites | Hepatic Encephalopathy | Liver Function |
|---|---|---|---|---|---|
| Lam3
Dig Dis Sci 1981 |
SPSS: 95% No-SPSS: NA | No differences | No differences | ||
| Takashi4
J Hepatol 1985 |
Lower % in SPSS + HE vs SPSS without HE (14% vs 92%)** |
Not available (case- control study) | No differences | ||
| Onishi59
Am J Gastroenterol 1986 |
EV: Lower % in SPSS + HE vs SPSS without HE or no-SPSS (45%; 87%; 97%)*** GV: Lower % in SPSS without HE (27%; 7%, 30%)$ |
No differences | No differences | SPSS: 46% No-SPSS: Not valuable (pre-defined inclusion criteria) |
SPSS + HE: Worse albumin and bilirubin than no-SPSS* |
| Sacerdoti36
Hepatology 1995 |
No differences | Higher % in SPSS vs no-SPSS (61.3% vs 37.7%)* |
SPSS: Worse Child-Pugh (8.6 ±2.2 vs 7.4±1.7)*** |
||
| Von Herbay27
J Clin Ultrasound 2000 |
Higher % in SPSS vs no-SPSS (93% vs 66%)** |
Higher % in SPSS vs no-SPSS (61% vs 32%)** |
SPSS: Lower % in Child-Pugh A vs B/C (27%; 46%; 44%)* |
||
| Dömland37
Ultraschall Med 2000 |
SPSS: Lower % in Child-Pugh A vs B* vs C** (6.3%;25.9%;33.3%) | ||||
| Chen39
AJR 2002 |
SPSS: Lower % in Child-Pugh A vs B*** vs C*** (2.4%;11.3%;22.6%) | ||||
| Del Piccolo39
Metab Brain Dis 2002 |
Higher risk with low effective portal flow: - altered neuropsychological test (60% vs 40%)*** - altered EEG (63% vs 37%)*** |
||||
| Riggio5
Hepatology 2005 |
Lower % of large EV among HE vs no-HE (7% vs 42%)*** |
Lower % of ascites among HE vs no-HE (21% vs 78%)** | |||
| Berzigotti30
Digest Liver Dis 2008 |
No differences at baseline. Higher formation of VE in new SPSS over time (56.2% vs 22.2%)* Higher progression in new SPSS (52.9% vs 30.6%)* |
SPSS: Higher % of ascites (35.7% vs 14.3%)** |
SPSS: Higher Child-Pugh vs no-SPSS (7.6±1.8 vs 6.0±1.3)*** |
||
| Zardi28
J Gastroenterol 2009 |
Higher % in SPSS vs no-SPSS (64% vs 53%)* |
SPSS: Lower % in Child-Pugh A vs Child B/C*** | |||
| Tarantino40
BMC Gastroenterology 2009 |
Lower % of large EV in SPSS vs no-SPSS (7% vs 45%)** |
No differences | No differences | No differences | |
| Berzigotti31
J Gastroenterol 2011 |
Higher % in SPSS vs no-SPSS (52% vs 9%)** |
||||
| Kondo41
J Clin Gastroenterol 2014 |
Higher % in SPSS vs no-SPSS (75% vs 46%)** |
No differences at baseline Higher % in SPSS over time (33.3% vs 2.9%)* |
No differences | No differences | |
| Maruyama42
Scand J Gastroenterol 2015 |
EV: No differences GV: Higher % in SGV vs SRS and no-SPSS (100%; 0%; 14.8%) |
No differences | No differences | No differences | No differences |
| Qi46
Med Sci Monit 2017 |
No differences | No differences | No differences | SPSS: Worse Child-Pugh and MELD | |
| Simón-Talero25
Gastroenterology 2018 |
GEV: Higher % in large and small SPSS vs no-SPSS (EV:71%;71%;59%)*** (GV: 10%; 7%;4%)* |
Higher GIB in large and small SPSS vs no-SPSS (25%;26%,11%)*** |
Higher % in large and small SPSS vs no-SPSS (57%; 55%; 32%)*** |
Higher % in Large-SPSS > Small SPSS > no-SPSS (32%,19%;8%)*** | SPSS: Worse Child-Pugh and MELD (Large-SPSS > Small SPSS > no-SPSS (13;11;9)*** |
| Lipinski29
Scand J Gastroenterol 2018 |
Higher % in SPSS (60-65% vs 50-55%)** |
No differences | No differences | Higher % in SPSS (25-30% vs 10-15%)*** | SPSS: Worse Child-Pugh*** and MELD vs no-SPSS (15; 13)*** |
| Saks44
Hepatology Communications 2018 |
No differences | Lower % in SPSS vs no-SPSS (43% vs 59%) ** | No differences | No differences in MELD score |
<0.05; ** <0.01; *** <0.001; $: unknown-p value; EV: Esophageal varices; GV: Gastric varices; GEV: Gastroesophageal varices; HE: Hepatic encephalopathy; MELD: Model for End Stage Liver Disease; SGV: Short Gastric Veins; SPSS: Spontaneous portosystemic shunt; SRS: Splenorenal shunt.
These results, however, contrast with other studies,3,25,27–31,36,41,42,58 in which patients with SPSSs presented, in addition to HE, signs of clinically significant portal hypertension as varices or ascites. In the cohort provided by Aseni et al. the whole group with SPSSs had GIVs, with a rate of bleeding of 60%.58 Berzigotti et al. showed that the appearance of SPSSs had a correlation with the development or worsening of GEVs.30 In another study performed by the same group, 89% of patients with cirrhosis and SPSSs had an hepatic venous pressure gradient (HVPG) higher than 16 mmHg, which was linked to an increased risk of complications and death.31 Park et al. also found high HVPG in patients with GEVs and SPSSs (gastrorenal and/or splenorenal shunts), without detecting differences with the group without SPSSs (18.3 ± 5.8 versus 17.0 ± 8.1 mmHg, respectively).61 In the large clinical study driven by the Baveno VI Cooperation Group, patients with SPSSs more often had HE, variceal bleeding, ascites, spontaneous bacterial peritonitis and hepatorenal syndrome.25 These differences were especially significant in patients with preserved liver function, that is, patients with a Model for End-stage Liver Disease (MELD) score of 6–9 or Child–Pugh A.
Some reports have highlighted an association between a specific type of collateral and different complications. For varices, different drainage has been proposed according to GEVs (or EVs) or gastric varices (GVs) alone: EVs/GEVs are commonly supplied from the coronary/left gastric vein; GVs alone are frequently supplied from the short or posterior gastric veins, and are closely linked to gastrorenal shunts,62 also suggesting a PH-related mechanism. Paraumbilical veins have been related to a lower risk of GIB, considering its hemodynamical effect (potentially less splenomegaly, smaller portal diameter, hepatopetal portal vein flow with high velocity);28 however, these results have not been sustained by other groups.25,58 Lipinski et al. found a specific relationship between paraumbilical shunt and ascites,29 not described in other cohorts.
These contradictory findings are probably explained due to the cross-sectional nature of most studies, which has hindered the interpretation of the results. Liver cirrhosis is a dynamic disease with different stages and compensatory mechanisms, difficult to understand in a static moment. As explained, SPSSs participate as part of the compensatory measures, initially reducing PVP and decreasing the number of PH-related complications. As the disease advances, SPSSs may be insufficient, and not only fail to decrease PVP, but also contribute to reduce liver perfusion, worsening liver failure.57,63
This hypothesis is reinforced by the fact that patients with SPSSs showed worse liver function in many studies (assessed both Child–Pugh class and MELD score).25,27–29,36–38,42 In the largest published cohort previously mentioned, patients with large SPSSs had higher Child–Pugh and MELD score than those with small collaterals, and both had worse liver function than patients without shunts.25
Hepatic encephalopathy
The relation between HE and the presence of shunts is well known. Moreover, in recent decades, surgical shunts and, more recently, TIPS placement have allowed acquiring a wide experience in HE.64,65 Previous reports have observed that 46–71% of patients with recurrent and/or persistent HE showed large SPSSs at radiological examination.5,59 In the case–control study performed by Riggio et al. with 28 patients, large SPSSs were identified in 71% of patients with chronic HE, while only 14% of the group without HE presented SPSSs.5 Similarly to TIPSs, a relation between SPSS size and HE has been observed.25,33,66 In the recent work by Praktiknjo et al. large SPSSs, classified according to the total shunt area, had higher risk of developing HE and higher ammonia levels.66
In addition to size, the presence of hepatofugal blood flow in SPSSs is also an important component for developing HE.59 Both size and hepatofugal flow support the bypass mechanism in which blood flow circulating through SPSSs, carrying neurotoxins from intestine, bypasses the liver.67 Some studies have suggested that paraumbilical shunts, responsible for hepatopetal flow in the portal vein, do not influence HE.4,28 However, larger series have provided evidence against this hypothesis, with the same rate of HE complications than other SPSSs,25,68 supporting the concept of “ineffective portal flow”.39
As a result of these mechanisms, patients with cirrhosis and SPSSs can develop HE with a relatively preserved liver function and have less identifiable precipitating events.3 In this setting, the SPSS would act as a facilitating factor. Nevertheless, the presence of SPSSs alone is not enough for explaining the development of HE; in patients with noncirrhotic portal hypertension who develop portosystemic collaterals, in whom liver function is preserved, HE is rarely seen.69 HE is driven by the accumulation of those neurotoxins due to liver disease and aggravated by SPSSs.67,70
Therefore, large SPSSs should be investigated in patients with HE, especially in recurrent or persistent episodes despite relatively well-preserved liver function.10,11 Also, a high rate of covert HE has recently been reported among these patients, associated with a significant risk of developing overt HE.71 For the diagnosis of SPSS, a high index of suspicion is needed, as abdominal CT is not routinely performed in cirrhotic patients and abdominal ultrasound could not identify deep SPSSs.72
There is a special form of HE with cerebellar and extrapyramidal symptoms called hepatocerebral syndrome or cirrhosis-related Parkinsonism, in which large SPSSs are frequently identified.73 In this condition, T1 hyperintensity in basal ganglia due to manganese deposition is commonly described in cerebral MR imaging, suggesting a mechanism of toxicity.74 This clinical presentation does not respond to classical ammonia-lowering treatments,75 and neither has the effect of levodopa been well established.76 Nevertheless, shunt occlusion (discussed in next section) and LT have been suggested as therapeutic options,77 provided that other etiologies are excluded. Hepatic myelopathy is a very infrequent type of HE, characterized by progressive spastic paraparesis and hyper-reflexia.10,11 that has been related to long-standing shunts in most of the cases, up to 85%.78 Its management includes, with limited experience, LT, which can achieve an improvement in symptoms especially in earlier stages.79,80
The importance of identifying SPSSs rests in the possibility of improving measures against HE.10,11 These patients may benefit from intensive management and closer follow-up, optimizing treatment (with nonabsorbable disaccharides and also considering the addition of antibiotics as rifaximin) and providing recommendations about avoiding precipitating events (as constipation, use of sedative drugs, diuretic treatment overuse, and early identification and treatment of infections).70,81 If despite these measures, recurrent or persistent HE is maintained, second-line more-invasive management should be considered.82 These approaches will be developed in the next section.
Embolization
Interventional radiology embolization is a useful technique for the management of PH-related complications of SPSSs, especially gastrorenal shunts with associated GV.1 Balloon-occluded retrograde transvenous obliteration (B-RTO) is an effective method to control GV bleeding, frequently used in Asia.13 It has also showed effectiveness as treatment of HE associated with GVs.83 However, the secondary increase in PVP can worsen other PH-related complications, as EVs or ascites. Moreover, the sclerosing material could also produce secondary effects, as pulmonary edema or portal vein thrombosis.51 Other techniques as coil-assisted retrograde transvenous obliteration (CARTO) or vascular plug-assisted transvenous obliteration (PARTO) have been developed, providing the same results with less secondary effects.84,85
Regarding HE, the presence of shunts not only justifies the refractivity to treatment but also provides a therapeutic target.86 However, the possibility of increasing PH after embolization, as well as causing procedure-related complications, should be carefully considered.
In the last 40 years many publications have provided experience in SPSS embolization in HE. Initially, the level of evidence was limited to case reports and short series, even with contradictory results. However, in the past decade, larger series have been reported, and have allowed obtaining homogeneous conclusions.68,87–91 It has been proved that embolizing shunts to treat refractory HE is both efficient and safe, in well-selected patients (Table 3). Three months after embolization, around 60% of patients remain free of neurological symptoms68 and a high percentage remain free of HE at 1–2 years (49–55%).68,87,88 Late recurrences are probably related to development of new collaterals or recanalization of previously closed ones.14
Table 3.
Efficacy and safety in SPSS embolization. Main results from recent studies that evaluate efficacy and safety of embolization.
| Reference | n | SPSS | Embolization (access, material and technique) | Efficacy/Safety |
|---|---|---|---|---|
| Laleman et al.68 | 37 | Splenorenal: 20 Paraumbilical: 9 Mesocaval: 7 Mesorenal: 1 |
Percutaneous (paraumbilical); transhepatic (the others) Material: coils (59%), Amplatzer plugs (35%), matrix or a combination |
% free of HE: - Short-term (100 days): 59.4%* - Long-term (2 years): 48.6%*- Procedure-related complications: 7 mild/1 capsular bleeding - Long-term: De novo EV: 2 (1 small, 1 large) GEV: no significant increase EV bleeding: 1 nonfatal at 55 months Ascites: no significant differences PVT: 4 (11%; 1 in PV, 3 in one branch) |
| Lynn et al.87 | 20 | Splenorenal: 12 | Transhepatic (25%), also right femoral, internal jugular, paraumbilical, right axillary. Material: coils (75%), Amplatzer plugs (20%) or combination |
% HE with sustained improvement - Short-term (1–4 months): 100%** - Long-term (6–12 months): 92%**- Procedure-related complications: 10% 1 mild/1 bacterial cholangitis - Long-term (12 months): De novo EV: 1 (small) Ascites (new or worsening): 6 (4 paracentesis) |
| An et al.88 | 17 | Splenorenal: 14 Paraumbilical: 3 | Percutaneous (paraumbilical); femoral (splenorenal) Material: Amplatzer plugs, coils combined with gelatin sponges |
Recurrence of OHE for 2 years: 39.9% (embolized) versus 79.9% (control)*- No serious procedure-related complications - Long-term: MELD ⩽ 15 and no HCC Ascites: mild 3 (18%) EV (small-sized new or worsened): 3 (18%) No GIB No PVT |
| Naeshiro et al.89 | 14 | Splenorenal: 3 Gastrorenal: 4 Mesocaval: 5 Portocaval: 2 |
Percutaneous Material: EOI, coils and NBCA (B-RTO or CARTO) |
HE disappearance in 1–2 weeks: 93%- No serious procedure-related complications - Long-term: EV: worsening at 3 months (21%) EV: worsening at 24 months (29%) GIB: 14% |
| Inoue et al.90 | 19 | Splenorenal: 19 | EOI, coil and NBCA (B-RTO) |
HE improvement: 100%*- No serious procedure-related complications - Long term: ascites: 21% |
| Philips et al.91 | 21 | Splenorenal: 17 Mesocaval: 7 plus others |
Transjugular (71%), transhepatic (19%), transfemoral (4.8%) CARTO, PARTO, B-RTO or a combination |
HE improvement: - Short-term follow up: 71%* - Long-term: 23%Serious procedure-related complications: 1 hemoperitoneum with multiple organ failure - Long-term: EV: No significant increase GIB: 1 nonfatal, controlled with band ligation (122 days post-occlusion). Ascites: no significant increase |
B-RTO, balloon-occluded retrograde transvenous obliteration; CARTO, coil-assisted retrograde transvenous obliteration; EOI, ethanolamine oleate with iopaminol; EV, esophageal varices; GEV, gastroesophageal varices; GIB, gastrointestinal bleeding; HE, hepatic encephalopathy; HCC, hepatocellular carcinoma; NBCA, N-butyl cyanoacrylate; MELD, Model for End-stage Liver Disease; OHE, Overt hepatic encephalopathy; PARTO, vascular plug-assisted transvenous obliteration; PVT, portal vein thrombosis; SPSS, spontaneous portosystemic shunt.
p < 0.05. **Per-protocol analysis.
Severe procedure-related complications, including thrombosis, or aggravation of PH-related features such as GIB or ascites, have not been observed in a significant proportion (Table 3). In the different series published, patients were carefully selected before the procedure. Patients with severe/refractory ascites or large GEVs were not considered as candidates to embolization. MELD score pre-embolization has been identified as a good predictor of outcomes, with a range of cutoffs from 11 to 15; patients with higher MELD will probably not benefit from embolization68,87,88 and show worse outcomes and more complications. In a recent work, low liver stiffness values measured by transient elastography, were linked with better outcomes; the cutoff used was 21.6 kPa, which correlates with clinically significant portal hypertension.92
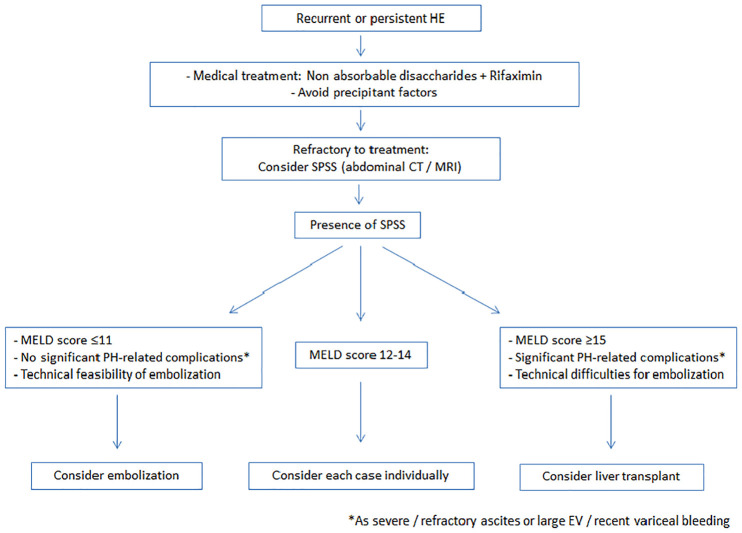
Therefore, patients with recurrent or persistent HE secondary to SPSSs with deteriorated liver function and PH-related complications should be considered as candidates for LT. By contrast, when liver function is preserved, shunt embolization would be a recommended and less-invasive approach.82 (Figure 3) Shunt embolization would avoid continuous episodes of HE in patients with good liver function that would remain for a long time on the waiting list.86
Figure 3.
Algorithm including persistent or recurrent HE suggested management, considering SPSS embolization or liver transplant.
HE, hepatic encephalopathy; SPSS, spontaneous portosystemic shunt.
With regard to cirrhosis-related Parkinsonism, the experience is very limited, but in the same direction; Parkinsonism and HE symptoms can improve significantly after shunt embolization in carefully selected patients.91 As in the previously mentioned studies, deteriorated liver function is a contraindication for this therapeutic option, with lack of benefit and more mortality after shunt occlusion.14 The limited evidence available establishes a Child–Pugh score >11 as a cutoff. In case of hepatic myelopathy, the usefulness of this technique has not been determined, due to its extremely low frequency. Currently, the experience is limited to isolated case reports, in which this alternative approach is also recommended to patients with preserved liver function.93
Portopulmonary hypertension
SPSS are associated to other less frequent complications, such as portopulmonary hypertension (POPH). POPH is defined by hemodynamic criteria assessed by right heart catheterization in the presence of portal hypertension, with or without cirrhosis. The association between SPSSs and POPH is not completely understood. SPSS may probably increase the pulmonary flow but also facilitate the transit of substances, such as vasoactive factors, which are produced in the splanchnic circulation.94 These factors skip the liver and produce vasoconstriction in the pulmonary vascular bed.95 It has been shown that the frequency of shunts is similar between patients without and with POPH, including mild and moderate/severe POPH. Nevertheless, large SPSSs are more frequently seen among the latter. Moreover, the presence of large collaterals is significantly associated with treatment failure.96 In many cases, the appearance of SPSSs precedes the diagnose of POPH, therefore identifying a subgroup of patients at risk of both developing POPH and treatment failure, whom may benefit from a closer follow-up, screening and prompt treatment.
Survival
SPSSs have been suggested as an aggravating factor for liver disease, worsening hepatic failure by decreasing blood supply.55 This effect is well known in surgical and radiological shunts, and has been previously discussed as the “portosystemic shunt syndrome”.97,98 In this sense, embolization of SPSSs in selected patients could offer extra benefit. Some studies indeed have pointed out a protective role of the procedure, improving liver function and even reducing mortality.55,88,90,99 Ishikawa et al. found that patients with low basal stiffness (up to 21.6 KPa) showed an improvement of MELD sodium score and higher survival after embolization.92
In the study conducted by the Baveno VI Cooperation Group, a relevant relation between SPSSs and mortality was identified.25 SPSSs were independently associated with mortality or LT, with significant differences in the group of patients with preserved liver function (MELD score of 6–9). No relation between mortality and SPSS size or anatomical type was identified. Nevertheless, Praktiknjo et al. recently used the sum of the cross-sectional areas of all SPSSs identified, finding that a large SPSS area (>83 mm2) was associated with worse survival.66 The main interpretation of these results is that the area allows to magnify the differences between patients, and that the sum of the area is a more reliable information about the attempt to compensate PH, reflecting more clearly the real hemodynamic situation and clinical course of patients.
SPSS and outcomes after TIPS
TIPS placement has become an established therapy for PH-related complications, including refractory ascites and acute or recurrent GEV bleeding.100–102 Its main limitation is the risk of liver dysfunction and the development of HE after the procedure.64,65,98 That is the reason that TIPS placement is preferably performed in selected patients with enough hepatic reservation and if possible, without previous episodes of HE, with chronic HE being a relative contraindication.103
Saad and Darcy compared the experience of TIPS placement and B-RTO in the management of GV bleeding by evaluating the hemodynamic and liver function consequences of both techniques.104 As previously mentioned, GVs alone are frequently supplied from posterior or short gastric veins through a gastrorenal SPSSs. The authors suggest that the amount of portal blood flow diverted through the gastric shunt should be taken into account as a predictor of response. Following this argument, the possible consequences of TIPS placement in case of gastrorenal shunt were analyzed around the so-called “throughput theory”, according to which a significant gastrorenal shunt could act as a competing shunt. Also, the influence of the anatomical situation was proposed as the “proximity theory”, in which the location of the posterior or short gastric veins, closer to gastrorenal shunts than to TIPSs, was introduced as another competing factor. In contrast, coronary/left gastric veins, which usually drained to EVs, have their origin in the right side of the portal circulation, closer to the portal system; this anatomical differences could explain the more effective role of TIPS in EVs.104
Although recent data suggest that rifaximin has a protective effect, reducing the risk of HE after TIPS when administered 15 days before the procedure and maintained 6 months after,105 it can be insufficient in some cases. Some studies suggest that pre-existing large SPSSs increase the risk of post-TIPS HE, which diminishes when these shunts are embolized.106–109 After TIPS placement, nearly one-third of SPSSs remained unchanged, although with a reduction in portal pressure. He et al. proposed prophylactic embolization of SPSS during TIPS, and showed that the risk of HE was similar to those without shunts [hazard ratio (HR) for HE in 5 years of 1 (TIPS) versus 1.38 (1.08–1.77) (TIPS + SPSS) versus 0.82 (0.49–1.37) (TIPS + embolization); p = 0.029].106 They also showed that these patients did not have a higher risk of rebleeding, recurrence of ascites, TIPS dysfunction or death of any cause. Similar results were obtained by Leng et al. in a recent study that evaluated the efficacy of TIPS combined with SPSS embolization in variceal bleeding, comparing the results and risks with patients without SPSSs.107 In this study, a control group submitted to TIPS with nonembolized SPSSs was not included. By contrast, in terms of survival, Borentain et al. found that concomitant SPSSs were associated not only with the appearance of HE, but also with an increased risk of early mortality after TIPS.108 However, the follow-up was limited to the first 30 days after TIPS placement. More evidence is needed to obtained solid conclusions.
SPSSs and influence after liver transplantation
The clinical impact of SPSSs in LT is still a matter of debate. Previous studies have reported that large SPSSs are associated with increased rate of complications after LT when left untouched, including primary nonfunction and disfunction of the graft, higher risk of portal vein thrombosis and reappearance of HE.110–112 These complications are thought to be driven by the diminished irrigation to the graft, as part of the flow circulates through the shunt, causing a “portal steal” phenomenon.113 Thereby, SPSS ligation during LT surgery has been proposed and successful short-term outcomes reported.114 However, there are concerns about procedure-related complications, such as bleeding or inferior vena cava thrombosis. In the study performed by Gómez-Gavara et al. consecutive patients with splenorenal shunt >1 cm were included and approximately half of them had the shunt ligated during surgery.45 In the remaining patients the shunt was left in place, according to a clamping test performed during the surgery, which consisted of checking whether the hepatic portal flow improved or not after clamping the shunt. Interestingly, SPSS ligation during LT was associated not only with less postoperative morbidity, HE and portal vein thrombosis, but also with better patient and graft long-term survival during a mean follow-up of 25 months. Recently, Alland et al. have observed in recipients of living-donor LT that portal vein thrombosis and size of splenorenal shunt, previous to LT, were predictors of portal complications (defined as portal stenosis, thrombosis or hepatofugal flow, requiring surgical, percutaneous or medical management); in particular, portal vein thrombosis and splenorenal shunt diameter <8 mm led to a risk of portal complications of 8.3%, which increased to 16.7% when shunt diameter was 8–15 mm and to 38.5% with diameters >15 mm. The authors proposed to consider intraoperative intervention in these cases.115
Nevertheless, an association between SPSSs and more complications after LT has not always been observed. Saks et al. evaluated retrospectively the outcomes of patients undergoing LT with nonligated splenorenal shunts, finding that their presence was not associated with post-LT mortality or graft failure, compared with cirrhotic patients without shunts.44 Despite no ligation being performed, almost half of the evaluated shunts spontaneously decreased in size after LT. In the recent study performed by Rodrígez et al., in which the majority of SPSSs were not ligated (only five large SPSSs from a cohort of 263 shunts), SPSSs did not influence graft survival or patient survival, regardless of the size of the collateral and the type of graft used (cardiac-death donation or brain-death donation).33
To summarize, the management of SPSSs in LT is still controversial. Current recommendations suggest considering the ligation of SPSSs in high-risk patients with low portal venous flow or very large shunts, to avoid graft hypoperfusion, portal complications or HE. However, in small-sized grafts and technically difficult scenarios, SPSSs should not be ligated. During long-term follow-up, in case of persistence of symptomatic large SPSSs, shunt embolization could be considered, although the experience in embolization after LT is extremely limited, documented as isolated case reports.87,116
Conclusion
In conclusion, SPSSs are very frequent in patients with cirrhosis their prevalence increases as liver function deteriorates, and represent an indirect indicator of severe PH. Furthermore, their presence has been related to worse prognosis, especially in patients with preserved liver function. SPSSs may identify a subgroup of patients with good liver function but advanced PH, thus more likely to develop complications. Large SPSSs are frequently found in patients with persistent and recurrent HE, being a useful therapeutic target in selected cases. Similarly, patients with liver cirrhosis candidates for TIPS with concomitant large SPSSs, may benefit from simultaneous embolization to avoid post-TIPS HE. Finally, the management of SPSSs in LT is still controversial, considering the ligation of large SPSSs to avoid graft hypoperfusion a possible intervention.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Judit Vidal-González is a recipient of a PFIS grant (FI19/00330) from Instituto de Salud Carlos III, Spain. Macarena Simón-Talero is a recipient of a Juan Rodés grant JR 17/00029 from Instituto de Salud Carlos III, Spain. Joan Genescà is a recipient of a Research Intensification grant from Instituto de Salud Carlos III, Spain. The study was partially funded by grants PI18/00947 from Instituto de Salud Carlos III and co-funded by European Union (ERDF/ESF, “Investing in your future”). Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas is supported by Instituto de Salud Carlos III, Spain.
ORCID iD: Macarena Simón-Talero  https://orcid.org/0000-0002-1409-3936
https://orcid.org/0000-0002-1409-3936
Contributor Information
Judit Vidal-González, Liver Unit, Department of Internal Medicine, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Vall d’Hebron Barcelona Hospital Campus, Universitat Autònoma de Barcelona, Barcelona, Spain.
Sergi Quiroga, Radiology Department, Hospital Universitari Vall d’Hebron, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain.
Macarena Simón-Talero, Liver Unit, Department of Internal Medicine, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Vall d’Hebron Barcelona Hospital Campus, Universitat Autònoma de Barcelona, Pg. Vall d’Hebron, 119-129, Barcelona, 08035, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, CIBERehd, Instituto de Salud Carlos III, Madrid, Spain.
Joan Genescà, Liver Unit, Department of Internal Medicine, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Vall d’Hebron Barcelona Hospital Campus, Universitat Autònoma de Barcelona, Barcelona, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, CIBERehd, Instituto de Salud Carlos III, Madrid, Spain.
References
- 1. Nardelli S, Riggio O, Gioia S, et al. Spontaneous porto-systemic shunts in liver cirrhosis: clinical and therapeutical aspects. World J Gastroenterol 2020; 26: 1726–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017; 65: 310–335. [DOI] [PubMed] [Google Scholar]
- 3. Lam KC, Juttner HU, Reynolds TB. Spontaneous portosystemic shunt: relationship to spontaneous encephalopathy and gastrointestinal hemorrhage. Dig Dis Sci 1981; 26: 346–352. [DOI] [PubMed] [Google Scholar]
- 4. Takashi M, Igarashi M, Hino S, et al. Portal hemodynamics in chronic portal-systemic encephalopathy. Angiographic study in seven cases. J Hepatol 1985; 1: 467–476. [DOI] [PubMed] [Google Scholar]
- 5. Riggio O, Efrati C, Catalano C, et al. High prevalence of spontaneous portal-systemic shunts in persistent hepatic encephalopathy: a case-control study. Hepatology 2005; 42: 1158–1165. [DOI] [PubMed] [Google Scholar]
- 6. Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther 2007; 25(Suppl. 1): 3–9. [DOI] [PubMed] [Google Scholar]
- 7. Montagnese S, Bajaj JS. Impact of hepatic encephalopathy in cirrhosis on quality-of-life issues. Drugs 2019; 79(Suppl. 1): 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Acharya C, Bajaj JS. Altered microbiome in patients with cirrhosis and complications. Clin Gastroenterol Hepatol 2019; 17: 307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tarantino G, Citro V, Esposito P, et al. Blood ammonia levels in liver cirrhosis: a clue for the presence of portosystemic collateral veins. BMC Gastroenterol 2009; 9: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver diseases and the European Association for the Study of the Liver. Hepatology 2014; 60: 715–735. [DOI] [PubMed] [Google Scholar]
- 11. American Association for the Study of Liver Diseases and European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol 2014; 61: 642–659. [DOI] [PubMed] [Google Scholar]
- 12. Rössle M. TIPS: 25 years later. J Hepatol 2013; 59: 1081–1093. [DOI] [PubMed] [Google Scholar]
- 13. Bandali MF, Mirakhur A, Lee EW, et al. Portal hypertension: imaging of portosystemic collateral pathways and associated image-guided therapy. World J Gastroenterol 2017; 23: 1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Philips CA, Rajesh S, Augustine P, et al. Portosystemic shunts and refractory hepatic encephalopathy: patient selection and current options. Hepat Med 2019; 11: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruysch F. Curae posteriores, seu thesaurus anatomicus omnium praecedentium maximus. Jaussonio-Waesbergios. Amsterdam: Jansson-Waesberge, 1738, p.48. [Google Scholar]
- 16. Doehner GA, Ruzicka FF, Jr, Rousselot LM, et al. The portal venous system: on its pathological Roentgen anatomy. Radiology 1956; 66: 206–217. [DOI] [PubMed] [Google Scholar]
- 17. Philips CA, Arora A, Shetty R, et al. A comprehensive review of portosystemic collaterals in cirrhosis: historical aspects, anatomy, and classifications. Int J Hepatol 2016; 2016: 6170243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCain AH, Bernardino ME, Sones PJ, Jr, et al. Varices from portal hypertension: correlation of CT and angiography. Radiology 1985; 154: 63–69. [DOI] [PubMed] [Google Scholar]
- 19. Wachsberg RH, Bahramipour P, Sofocleous CT, et al. Hepatofugal flow in the portal venous system: pathophysiology, imaging findings, and diagnostic pitfalls. Radiographics 2002; 22: 123–140. [DOI] [PubMed] [Google Scholar]
- 20. Bagheri M, Hajati A, Hosseini M, et al. Comparison of findings of spontaneous splenorenal shunt in color Doppler sonography with multislice CT scan (64 slices) in liver transplant candidates. Eur J Radiol 2012; 81: 2027–2036. [DOI] [PubMed] [Google Scholar]
- 21. Henseler KP, Pozniak MA, Lee FT, Jr, et al. Three-dimensional CT angiography of spontaneous portosystemic shunts. Radiographics 2001; 21: 691–704. [DOI] [PubMed] [Google Scholar]
- 22. Kim M, Mitchell DG, Ito K. Portosystemic collaterals of the upper abdomen: review of anatomy and demonstration on MR imaging. Abdom Imaging 2000; 25: 462–470. [DOI] [PubMed] [Google Scholar]
- 23. Kang HK, Jeong YY, Choi JH, et al. Three-dimensional multi-detector row CT portal venography in the evaluation of portosystemic collateral vessels in liver cirrhosis. Radiographics 2002; 22: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 24. Nicoară-Farcău O, Wang X, Luo X. Definition of SPSS: we need to speak the same language. J Hepatol 2020; 73: 463–464. [DOI] [PubMed] [Google Scholar]
- 25. Simón-Talero M, Roccarina D, Martínez J, et al. Association between portosystemic shunts and increased complications and mortality in patients with cirrhosis. Gastroenterology 2018; 154: 1694–1705. [DOI] [PubMed] [Google Scholar]
- 26. Praktiknjo M, Torner J, Simón-Talero M, et al. Reply to: “Definition of SPSS: we need to speak the same language”: computer-assisted image processing for better quantification. J Hepatol 2020; 73: 464–465. [DOI] [PubMed] [Google Scholar]
- 27. von Herbay A, Frieling T, Häussinger D. Color Doppler sonographic evaluation of spontaneous portosystemic shunts and inversion of portal venous flow in patients with cirrhosis. J Clin Ultrasound 2000; 28: 332–339. [DOI] [PubMed] [Google Scholar]
- 28. Zardi EM, Uwechie V, Caccavo D, et al. Portosystemic shunts in a large cohort of patients with liver cirrhosis: detection rate and clinical relevance. J Gastroenterol 2009; 44: 76–83. [DOI] [PubMed] [Google Scholar]
- 29. Lipinski M, Saborowski M, Heidrich B, et al. Clinical characteristics of patients with liver cirrhosis and spontaneous portosystemic shunts detected by ultrasound in a tertiary care and transplantation centre. Scand J Gastroenterol 2018; 53: 1107–1113. [DOI] [PubMed] [Google Scholar]
- 30. Berzigotti A, Merkel C, Magalotti D, et al. New abdominal collaterals at ultrasound: a clue of progression of portal hypertension. Dig Liver Dis 2008; 40: 62–67. [DOI] [PubMed] [Google Scholar]
- 31. Berzigotti A, Rossi V, Tiani C, et al. Prognostic value of a single HVPG measurement and Doppler-ultrasound evaluation in patients with cirrhosis and portal hypertension. J Gastroenterol 2011; 46: 687–695. [DOI] [PubMed] [Google Scholar]
- 32. Aucejo FN, Hashimoto K, Quintini C, et al. Triple-phase computed tomography and intraoperative flow measurements improve the management of portosystemic shunts during liver transplantation. Liver Transpl 2008; 14: 96–99. [DOI] [PubMed] [Google Scholar]
- 33. Rodriguez EA, Perez R, Zhang N, et al. Clinical outcomes of portosystemic shunts on the outcome of liver transplantation. Liver Transpl 2020; 26: 693–701. [DOI] [PubMed] [Google Scholar]
- 34. Sakurabayashi S, Sezai S, Yamamoto Y, et al. Embolization of portal-systemic shunts in cirrhotic patients with chronic recurrent hepatic encephalopathy. Cardiovasc Intervent Radiol 1997; 20: 120–124. [DOI] [PubMed] [Google Scholar]
- 35. Cho KC, Patel YD, Wachsberg RH, et al. Varices in portal hypertension: evaluation with CT. Radiographics 1995; 15: 609–622. [DOI] [PubMed] [Google Scholar]
- 36. Sacerdoti D, Bolognesi M, Bombonato G, et al. Paraumbilical vein patency in cirrhosis: effects on hepatic hemodynamics evaluated by Doppler sonography. Hepatology 1995; 22: 1689–1694. [PubMed] [Google Scholar]
- 37. Dömland M, Gebel M, Caselitz M, et al. Comparison of portal venous flow in cirrhotic patients with and without paraumbilical vein patency using duplex-sonography. Ultraschall Med 2000; 21: 165–169. [DOI] [PubMed] [Google Scholar]
- 38. Chen CH, Wang JH, Lu SN, et al. Comparison of prevalence for paraumbilical vein patency in patients with viral and alcoholic liver cirrhosis. Am J Gastroenterol 2002; 97: 2415–2418. [DOI] [PubMed] [Google Scholar]
- 39. Del Piccolo F, Sacerdoti D, Amodio P, et al. Central nervous system alterations in liver cirrhosis: the role of portal-systemic shunt and portal hypoperfusion. Metab Brain Dis 2002; 17: 347–358. [DOI] [PubMed] [Google Scholar]
- 40. Tarantino G, Citro V, Conca P, et al. What are the implications of the spontaneous spleno-renal shunts in liver cirrhosis? BMC Gastroenterol 2009; 9: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kondo T, Maruyama H, Sekimoto T, et al. Influence of paraumbilical vein patency on the portal hemodynamics of patients with cirrhosis. J Clin Gastroenterol 2014; 48: 178–183. [DOI] [PubMed] [Google Scholar]
- 42. Maruyama H, Kondo T, Kiyono S, et al. Influence of splenorenal shunt on long-term outcomes in cirrhosis. Scand J Gastroenterol 2015; 50: 593–600. [DOI] [PubMed] [Google Scholar]
- 43. Achiwa S, Hirota S, Kako Y, et al. Radiological anatomy of spontaneous splenorenal shunts in patients with chronic liver disease. Jpn J Radiol 2017; 35: 206–214. [DOI] [PubMed] [Google Scholar]
- 44. Saks K, Jensen KK, McLouth J, et al. Influence of spontaneous splenorenal shunts on clinical outcomes in decompensated cirrhosis and after liver transplantation. Hepatol Commun 2018; 2: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gómez-Gavara CG, Bhangui P, Salloum C, et al. Ligation versus no ligation of spontaneous portosystemic shunts during liver transplantation: audit of a prospective series of 66 consecutive patients. Liver Transpl 2018; 24: 505–515. [DOI] [PubMed] [Google Scholar]
- 46. Qi X, Qi X, Zhang Y, et al. Prevalence and clinical characteristics of spontaneous splenorenal shunt in liver cirrhosis: a retrospective observational study based on contrast-enhanced Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) scans. Med Sci Monit 2017; 23: 2527–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wind P, Alves A, Chevallier JM, et al. Anatomy of spontaneous splenorenal and gastrorenal venous anastomoses. Review of the literature. Surg Radiol Anat 1998; 20: 129–134. [PubMed] [Google Scholar]
- 48. Johns TN, Evans BB. Collateral pathways in portal hypertension. Ann Surg 1962; 155: 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tan YW, Sheng JH, Tan HY, et al. Rare spontaneous intrahepatic portosystemic shunt in hepatitis B-induced cirrhosis: a case report. World J Clin Cases 2019; 7: 2573–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moubarak E, Bouvier A, Boursier J, et al. Portosystemic collateral vessels in liver cirrhosis: a three-dimensional MDCT pictorial review. Abdom Imaging 2012; 37: 746–766. [DOI] [PubMed] [Google Scholar]
- 51. Nardelli S, Gioia S, Ridola L, et al. Radiological intervention for shunt related encephalopathy. J Clin Exp Hepatol 2018; 8: 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fernández M, Semela D, Bruix J, et al. Angiogenesis in liver disease. J Hepatol 2009; 50: 604–620. [DOI] [PubMed] [Google Scholar]
- 53. Bosch J, Abraldes JG, Fernández M, et al. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol 2010; 53: 558–567. [DOI] [PubMed] [Google Scholar]
- 54. Kim M, Lee KY. Understanding the pathophysiology of portosystemic shunt by simulation using an electric circuit. Biomed Res Int 2016; 2016: 2097363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kumamoto M, Toyonaga A, Inoue H, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric fundal varices: hepatic deterioration links to portosystemic shunt syndrome. J Gastroenterol Hepatol 2010; 25: 1129–1135. [DOI] [PubMed] [Google Scholar]
- 56. Saad WE, Lippert A, Saad NE, et al. Ectopic varices: anatomical classification, hemodynamic classification, and hemodynamic-based management. Tech Vasc Interv Radiol 2013; 16: 158–175. [DOI] [PubMed] [Google Scholar]
- 57. Saad WE. Portosystemic shunt syndrome and endovascular management of hepatic encephalopathy. Semin Intervent Radiol 2014; 31: 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aseni P, Beati C, Brambilla G, et al. Does large spontaneous portal systemic shunt in cirrhosis protect from the risk of gastroesophageal bleeding? J Clin Gastroenterol 1986; 8(3 Pt 1): 235–238. [DOI] [PubMed] [Google Scholar]
- 59. Ohnishi K, Sato S, Saito M, et al. Clinical and portal hemodynamic features in cirrhotic patients having a large spontaneous splenorenal and/or gastrorenal shunt. Am J Gastroenterol 1986; 81: 450–455. [PubMed] [Google Scholar]
- 60. Takashi M, Igarashi M, Hino S, et al. Esophageal varices: correlation of left gastric venography and endoscopy in patients with portal hypertension. Radiology 1985; 155: 327–331. [DOI] [PubMed] [Google Scholar]
- 61. Park EJ, Jang JY, Lee JE, et al. The risk factors for bleeding of fundal varices in patients with liver cirrhosis. Gut Liver 2013; 7: 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Watanabe K, Kimura K, Matsutani S, et al. Portal hemodynamics in patients with gastric varices. A study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology 1988; 95: 434–440. [DOI] [PubMed] [Google Scholar]
- 63. Guillaume M, Bureau C. Should the presence of spontaneous portosystemic shunts be implemented to the model for end-stage liver disease score for a better prediction of outcome? Gastroenterology 2018; 154: 1569–1571. [DOI] [PubMed] [Google Scholar]
- 64. Riggio O, Angeloni S, Salvatori FM, et al. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol 2008; 103: 2738–2746. [DOI] [PubMed] [Google Scholar]
- 65. Pereira K, Carrion AF, Martin P, et al. Current diagnosis and management of post-transjugular intrahepatic portosystemic shunt refractory hepatic encephalopathy. Liver Int 2015; 35: 2487–2494. [DOI] [PubMed] [Google Scholar]
- 66. Praktiknjo M, Simón-Talero M, Römer J, et al. Total area of spontaneous portosystemic shunts independently predicts hepatic encephalopathy and mortality in liver cirrhosis. J Hepatol 2020; 72: 1140–1150. [DOI] [PubMed] [Google Scholar]
- 67. Hadjihambi A, Arias N, Sheikh M, et al. Hepatic encephalopathy: a critical current review. Hepatol Int 2018; 12(Suppl. 1): 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Laleman W, Simon-Talero M, Maleux G, et al. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology 2013; 57: 2448–2457. [DOI] [PubMed] [Google Scholar]
- 69. Nicoletti V, Gioia S, Lucatelli P, et al. Hepatic encephalopathy in patients with non-cirrhotic portal hypertension: description, prevalence and risk factors. Dig Liver Dis 2016; 48: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 70. Amodio P. Hepatic encephalopathy: diagnosis and management. Liver Int 2018; 38: 966–975. [DOI] [PubMed] [Google Scholar]
- 71. Greinert R, Zipprich A, Simon-Talero M, et al. Covert hepatic encephalopathy and spontaneous portosystemic shunts increase the risk of developing overt hepatic encephalopathy. Liver Int. Epub ahead of print 5 September 2020. DOI:10.1111/liv.14660. [DOI] [PubMed] [Google Scholar]
- 72. Córdoba J. New assessment of hepatic encephalopathy. J Hepatol 2011; 54: 1030–1040. [DOI] [PubMed] [Google Scholar]
- 73. Tryc AB, Goldbecker A, Berding G, et al. Cirrhosis-related Parkinsonism: prevalence, mechanisms and response to treatments. J Hepatol 2013; 58: 698–705. [DOI] [PubMed] [Google Scholar]
- 74. Rose C, Butterworth RF, Zayed J, et al. Manganese deposition in basal ganglia structures results from both portal-systemic shunting and liver dysfunction. Gastroenterology 1999; 117: 640–644. [DOI] [PubMed] [Google Scholar]
- 75. Weissenborn K. Hepatic encephalopathy: definition, clinical grading and diagnostic principles. Drugs 2019; 79(Suppl. 1): 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Butterworth RF. Parkinsonism in cirrhosis: pathogenesis and current therapeutic options. Metab Brain Dis 2013; 28: 261–267. [DOI] [PubMed] [Google Scholar]
- 77. Maffeo E, Montuschi A, Stura G, et al. Chronic acquired hepatocerebral degeneration, pallidal T1 MRI hyperintensity and manganese in a series of cirrhotic patients. Neurol Sci 2014; 35: 523–530. [DOI] [PubMed] [Google Scholar]
- 78. Conn HO, Rössle M, Levy L, et al. Portosystemic myelopathy: spastic paraparesis after portosystemic shunting. Scand J Gastroenterol 2006; 41: 619–625. [DOI] [PubMed] [Google Scholar]
- 79. Pinarbasi B, Kaymakoglu S, Matur Z, et al. Are acquired hepatocerebral degeneration and hepatic myelopathy reversible? J Clin Gastroenterol 2009; 43: 176–181. [DOI] [PubMed] [Google Scholar]
- 80. Caldwell C, Werdiger N, Jakab S, et al. Use of model for end-stage liver disease exception points for early liver transplantation and successful reversal of hepatic myelopathy with a review of the literature. Liver Transpl 2010; 16: 818–826. [DOI] [PubMed] [Google Scholar]
- 81. Leise MD, Poterucha JJ, Kamath PS, et al. Management of hepatic encephalopathy in the hospital. Mayo Clin Proc 2014; 89: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Takenaga S, Aizawa Y. Efficacy and safety of transcatheter embolization for hepatic encephalopathy caused by spontaneous portosystemic shunts. Interv Radiol 2017; 2: 51–58. [Google Scholar]
- 83. Ishikawa T, Sasaki R, Nishimura T, et al. Comparison of patients with hepatic encephalopathy and those with gastric varices before and after balloon-occluded retrograde transvenous obliteration. Hepatol Res 2018; 48: 1020–1030. [DOI] [PubMed] [Google Scholar]
- 84. Lee EW, Saab S, Kaldas F, et al. Coil-Assisted Retrograde Transvenous Obliteration (CARTO): an alternative treatment option for refractory hepatic encephalopathy. Am J Gastroenterol 2018; 113: 1187–1196. [DOI] [PubMed] [Google Scholar]
- 85. Gwon DI, Kim YH, Ko GY, et al. Vascular plug-assisted retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy: a prospective multicenter study. J Vasc Interv Radiol 2015; 26: 1589–1595. [DOI] [PubMed] [Google Scholar]
- 86. Temmerman F, Laleman W, Maleux G, et al. Treatment of recurrent severe hepatic encephalopathy in patients with large porto-collaterals shunts or transjugular portosystemic shunt. Acta Gastroenterol Belg 2020; 83: 67–71. [PubMed] [Google Scholar]
- 87. Lynn AM, Singh S, Congly SE, et al. Embolization of portosystemic shunts for treatment of medically refractory hepatic encephalopathy. Liver Transpl 2016; 22: 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. An J, Kim KW, Han S, et al. Improvement in survival associated with embolisation of spontaneous portosystemic shunt in patients with recurrent hepatic encephalopathy. Aliment Pharmacol Ther 2014; 39: 1418–1426. [DOI] [PubMed] [Google Scholar]
- 89. Naeshiro N, Kakizawa H, Aikata H, et al. Percutaneous transvenous embolization for portosystemic shunts associated with encephalopathy: long-term outcomes in 14 patients. Hepatol Res 2014; 44: 740–749. [DOI] [PubMed] [Google Scholar]
- 90. Inoue H, Emori K, Toyonaga A, et al. Long term results of balloon-occluded retrograde transvenous obliteration for portosystemic shunt encephalopathy in patients with liver cirrhosis and portal hypertension. Kurume Med J 2014; 61: 1–8. [DOI] [PubMed] [Google Scholar]
- 91. Philips CA, Kumar L, Augustine P. Shunt occlusion for portosystemic shunt syndrome related refractory hepatic encephalopathy-a single-center experience in 21 patients from Kerala. Indian J Gastroenterol 2017; 36: 411–419. [DOI] [PubMed] [Google Scholar]
- 92. Ishikawa T, Sasaki R, Nishimura T, et al. Liver stiffness measured by transient elastography as predictor of prognoses following portosystemic shunt occlusion. J Gastroenterol Hepatol 2019; 34: 215–223. [DOI] [PubMed] [Google Scholar]
- 93. Wang MQ, Liu FY, Duan F. Management of surgical splenorenal shunt-related hepatic myelopathy with endovascular interventional techniques. World J Gastroenterol 2012; 18: 7104–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Koulava A, Sannani A, Levine A, et al. Diagnosis, treatment, and management of orthotopic liver transplant candidates with portopulmonary hypertension. Cardiol Rev 2018; 26: 169–176. [DOI] [PubMed] [Google Scholar]
- 95. Zardi EM, Zardi DM, Giorgi C, et al. Portopulmonary hypertension and hepatorenal syndrome. Two faces of the same coin. Eur J Intern Med 2017; 43: 22–27. [DOI] [PubMed] [Google Scholar]
- 96. Talwalkar JA, Swanson KL, Krowka MJ, et al. Prevalence of spontaneous portosystemic shunts in patients with portopulmonary hypertension and effect on treatment. Gastroenterology 2011; 141: 1673–1679. [DOI] [PubMed] [Google Scholar]
- 97. Spina GP, Santambrogio R, Opocher, et al. Factors predicting chronic hepatic encephalopathy after distal splenorenal shunt: a multivariate analysis of clinical and hemodynamic variables. Surgery 1993; 114: 519–526. [PubMed] [Google Scholar]
- 98. Riggio O, Merlli M, Pedretti G, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Incidence and risk factors. Dig Dis Sci 1996; 41: 578–584. [DOI] [PubMed] [Google Scholar]
- 99. Nakazawa M, Imai Y, Uchiya H, et al. Balloon-occluded retrograde transvenous obliteration as a procedure to improve liver function in patients with decompensated cirrhosis. JGH Open 2017; 1: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017; 152: 157–163. [DOI] [PubMed] [Google Scholar]
- 101. Hernández-Gea V, Berbel C, Baiges A, et al. Acute variceal bleeding: risk stratification and management (including TIPS). Hepatol Int 2018; 12(Suppl. 1): 81–90. [DOI] [PubMed] [Google Scholar]
- 102. Brunner F, Berzigotti A, Bosch J. Prevention and treatment of variceal haemorrhage in 2017. Liver Int 2017; 37(Suppl. 1): 104–115. [DOI] [PubMed] [Google Scholar]
- 103. Wang Q, Lv Y, Bai M, et al. Eight millimetre covered TIPS does not compromise shunt function but reduces hepatic encephalopathy in preventing variceal rebleeding. J Hepatol 2017; 67: 508–516. [DOI] [PubMed] [Google Scholar]
- 104. Saad WE, Darcy MD. Transjugular Intrahepatic Portosystemic Shunt (TIPS) versus Balloon-occluded Retrograde Transvenous Obliteration (BRTO) for the management of gastric varices. Semin Intervent Radiol 2011; 28: 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bureau C, Jezequel C, Archambault I, et al. Rifaximin for the prevention of hepatic encephalopathy in patients treated by TIPS: a multicenter randomized placebo-controlled trial. Hepatology 2019; 70(Suppl. 1): 10A. [Google Scholar]
- 106. He C, Lv Y, Wang Z, et al. Association between non-variceal spontaneous portosystemic shunt and outcomes after TIPS in cirrhosis. Dig Liver Dis 2018; 50: 1315–1323. [DOI] [PubMed] [Google Scholar]
- 107. Leng X, Zhang F, Zhang M, et al. Comparison of transjugular intrahepatic portosystemic shunt for treatment of variceal bleeding in patients with cirrhosis with or without spontaneous portosystemic shunt. Eur J Gastroenterol Hepatol 2019; 31: 853–858. [DOI] [PubMed] [Google Scholar]
- 108. Borentain P, Soussan J, Resseguier N, et al. The presence of spontaneous portosystemic shunts increases the risk of complications after transjugular intrahepatic portosystemic shunt (TIPS) placement. Diagn Interv Imaging 2016; 97: 643–650. [DOI] [PubMed] [Google Scholar]
- 109. Vidal-González J, Simón-Talero M, Genescà J. Should prophylactic embolization of spontaneous portosystemic shunts be routinely performed during transjugular intrahepatic portosystemic shunt placement? Dig Liver Dis 2018; 50: 1324–1326. [DOI] [PubMed] [Google Scholar]
- 110. De Carlis L, Del Favero E, Rondinara G, et al. The role of spontaneous portosystemic shunts in the course of orthotopic liver transplantation. Transpl Int 1992; 5: 9–14. [DOI] [PubMed] [Google Scholar]
- 111. Margarit C, de Cenarruzabeitia IL, Lázaro JL, et al. Portacaval shunt and inferior vena cava preservation in orthotopic liver transplantation. Transplant Proc 2005; 37: 3896–3898. [DOI] [PubMed] [Google Scholar]
- 112. Mueller AR, Platz KP, Kremer B. Early postoperative complications following liver transplantation. Best Pract Res Clin Gastroenterol 2004; 18: 881–900. [DOI] [PubMed] [Google Scholar]
- 113. Horrow MM, Phares MA, Viswanadhan N, et al. Vascular steal of the portal vein after orthotopic liver transplant: intraoperative sonographic diagnosis. J Ultrasound Med 2010; 29: 125–128. [DOI] [PubMed] [Google Scholar]
- 114. Golse N, Bucur PO, Faitot F, et al. Spontaneous splenorenal shunt in liver transplantation: results of left renal vein ligation versus renoportal snastomosis. Transplantation 2015; 99: 2576–2585. [DOI] [PubMed] [Google Scholar]
- 115. Allard MA, Akamatsu N, Kokudo T, et al. Clinical significance of spontaneous porto-systemic shunts in living donor liver transplantation. Liver Transpl. Epub ahead of print 16 May 2020. DOI: 10.1002/lt.25798. [DOI] [PubMed] [Google Scholar]
- 116. Al Hajjaj A, Bonatti H, Krishna M, et al. Percutaneous transfemoral embolization of a spontaneous splenorenal shunt presenting with ischemic graft dysfunction 18 months post-transplant. Transpl Int 2008; 21: 816–819. [DOI] [PubMed] [Google Scholar]