Abstract
Patient: Male, 47-year-old
Final Diagnosis: Acute respiratory distress syndrome (ARDS) • COVID-19
Symptoms: Chills • dyspnea • fever • respiratory distress
Medication: —
Clinical Procedure: Central venous catheterization • endotracheal Intubation
Specialty: Critical Care Medicine
Objective:
Unusual clinical course
Background:
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARSCoV-2), which originated in Wuhan, China, in late 2019 and has led to an ongoing pandemic. COVID-19 typically affects the respiratory tract and mucous membranes, leading to pathological involvement of various organ systems. Although patients usually present with fever, cough, and fatigue, less common manifestations have been reported including symptoms arising from thrombosis and thromboembolism. A spectrum of dermato-logic changes is becoming recognized in patients with COVID-19 who initially present with respiratory symptoms. The mechanism behind these manifestations remains unclear. This report presents the case of a 47-year-old Hispanic man who developed cutaneous vasculitic lesions and gangrene of the toes following admission to hospital with COVID-19 pneumonia.
Case Report:
COVID-19 has been associated with cardiovascular disease entities including stroke, acute coronary syndrome, venous thromboembolism, and peripheral vascular disease. We present a case in which a 47-year-old Hispanic man arrived at the Emergency Department with COVID-19 and was admitted for respiratory failure. Despite anticoagulation initiated on admission in the presence of an elevated D-dimer, the patient developed gangrene of all his toes, which required bilateral transmetatarsal amputation.
Conclusions:
This case shows that dermatologic manifestations may develop in patients who initially present with COVID-19 pneumonia. These symptoms may be due to venous thrombosis following SARS-CoV-2 vasculitis, leading to challenging decisions regarding anticoagulation therapy. Randomized controlled trials are needed to evaluate the efficacy of anticoagulation, to choose appropriate anticoagulants and dosing, and to assess bleeding risk.
MeSH Keywords: Anticoagulants, Gangrene, Microvessels, Thrombophilia, Venous Thrombosis
Background
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first identified in Wuhan, China, in late 2019 and has led to an ongoing global pandemic. According to the Johns Hopkins University of Medicine Coronavirus Resource Center, as of July 31, 2020, there have been 17 516 264 confirmed cases of COVID-19 and 678 226 deaths worldwide [1]. The virus affects all age groups, but disease severity increases with advancing age and in individuals with underlying medical conditions such as hypertension, diabetes, heart disease, lung disease, and cancer. SARS-CoV-2 is usually spread via droplets or contact with surfaces that have been contaminated. The virus typically invades the respiratory tract and mucous membranes, leading to pathological involvement of different organ systems. Patients usually present with fever, cough, and fatigue, but less common manifestations have been reported, including focal neurological deficits as a result of strokes arising from thrombosis and thromboembolism. Cutaneous manifestations with an array of morphologies have also been documented, which are suggestive of immune or inflammatory pathways in the patho-physiology of COVID-19 [2–4]. Chilblain-like lesions have been observed in patients with COVID-19. Chilblains are cold-induced lesions resulting in redness, pruritis, and inflammation of the toes due to damage of skin capillary beds; however, the mechanism of such lesions with COVID-19 remains unclear. This report presents the case of a 47-year-old Hispanic man who developed cutaneous vasculitic lesions and gangrene of the toes following admission to hospital with COVID-19 pneumonia.
Case Report
A 47-year-old Hispanic nonsmoking man with a medical history of diabetes mellitus and chronic back pain presented to the Emergency Department with progressive dyspnea associated with fever, chills, and a productive cough with rusty brown sputum for 10 days. He also reported fatigue, myalgia, and diffuse headache; he denied sick contacts, nausea, chest pain, leg pain, leg swelling, recent travel, exposure to cold, or history of toe abnormalities. He received the influenza vaccine for the current season and reported not taking any medications. On physical examination, he had an oral temperature of 40.1°C (104.1°F), blood pressure of 148/80 mmHg, heart rate of 135 beats per minute, respiratory rate of 38 cycles per minute, and an oxygen saturation of 60% on ambient air and 70% on 6 L of oxygen by nasal cannula. His weight was 86 kg, his height was 177.8 cm, and his body mass index was 27.2 kg/m2. The patient was alert and oriented to person, place, time, and situation. Cardiovascular exam revealed tachycardia, regular rhythm, no heart murmurs, no jugular venous distension, and normal peripheral pulses. Auscultation of the lungs demonstrated diffusely diminished breath sound bilaterally, and there was no lower extremity edema. Abdominal examination was benign. The patient was anicteric and had moist oral mucosa, and his feet had no skin abnormalities.
Laboratory values at admission revealed white blood cell count of 18×109/L; lactic acid, 3.0 mmol/L; C-reactive protein, 171 mg/L; D-dimer, >4.3 mg/L; and ferritin, 567 ng/mL. Creatinine and troponin values were normal. Basic natriuretic peptide, platelets, liver function transaminases, and the coagulation profile were within normal limits. An influenza test was negative, and he was tested for COVID-19 as well. An arterial blood gas demonstrated respiratory acidosis with an abnormal alveolar-arterial gradient. Electrocardiogram showed sinus tachycardia with no ST-T wave changes and a QTc interval of 483 ms. A chest X-ray revealed diffuse bilateral infiltrates, while a lower extremity venous Doppler revealed an extensive deep venous thrombosis (DVT) in the right leg, extending from the superficial femoral vein through the posterior tibial vein, and a left-sided popliteal DVT. The patient was admitted to the Intensive Care Unit with an initial diagnosis of acute hypoxic respiratory failure due to community-acquired pneumonia suggestive of COVID-19 pneumonia. He was placed on mechanical ventilation and started on azithromycin at 500 mg daily; ceftriaxone, 1 g daily; weight-based, full-dose enoxaparin, 80 mg every 12 h; hydroxychloroquine, 400 mg every 12 h for 2 doses (followed by 200 mg every 12 h for 4 days); vitamin C, 500 mg twice daily; zinc sulfate, 220 mg daily; and methylprednisolone, 40 mg every 12 h.
Over the next few days, the patient had a waxing and waning course; on the third day of hospitalization, positive results for SAR-CoV-2 infection were received for the patient’s reverse transcription-polymerase chain reaction (RT-PCR) test from a nasopharyngeal swab using the Abbott RealTime SARS-CoV-2 assay. On the fourth day of hospitalization, he developed a painless bluish-purple discoloration of his toes bilaterally (Figures 1, 2). His feet were warm to the touch and nontender and had 3+ palpable dorsalis pedis pulses bilaterally; there were no sensory deficits of the toes or lower extremities. A computed tomography (CT) angiogram of the aorta and lower extremity arteries bilaterally revealed patent arteries in the lower extremities. The initial purplish-discoloration of his toes continued to worsen as the toes became progressively gangrenous and stone-like; D-dimer remained elevated and the coagulation profile remained unremarkable during the time he was on full-dose anticoagulation.
Figure 1.
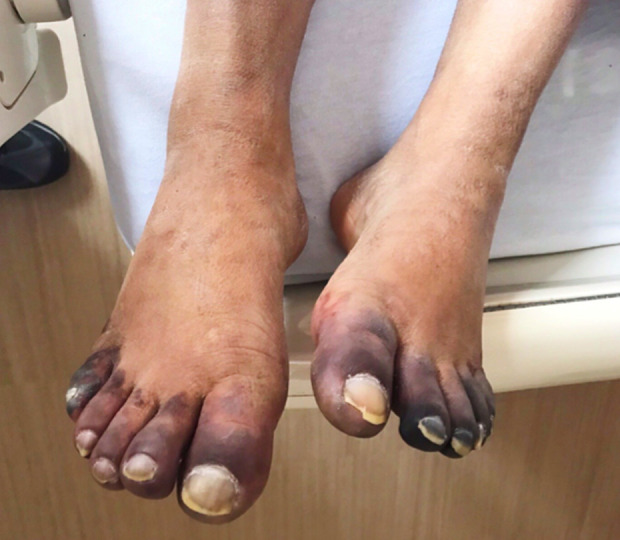
Dermatologic manifestations on the dorsal aspect of the patient’s feet on day 11 of hospitalization in the setting of coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2.
Figure 2.
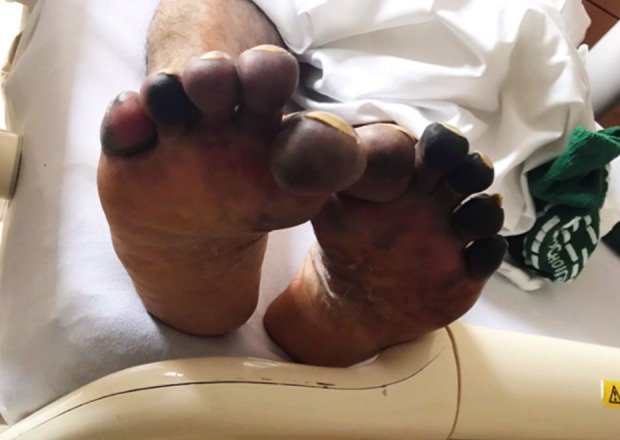
Dermatologic manifestations on the plantar aspect of the patient’s feet on day 11 of hospitalization in the setting of coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2.
The patient’s respiratory condition improved; he was extubated on day 9 of hospitalization and gradually required less supplemental oxygen over the subsequent days. He was also managed for the pain of the toes with oxycodone 20 mg every 12 h as needed. Results from RT-PCR testing from a nasopharyngeal swab using the Abbott RealTime SARS-CoV-2 assay were negative for SAR-CoV-2 infection twice (on day 20 and 22). His anticoagulation was transitioned from enoxaparin to apixaban 5 mg twice a day. However, his toes continued to be gangrenous; on day 26 of hospitalization, he underwent a bilateral transmetatarsal amputation after there was demarcation of the gangrene. He performed well postoperatively and was subsequently discharged to a subacute rehabilitation facility.
Discussion
COVID-19 is a rapidly evolving disease, and the incidence and epidemiology of complications such as COVID toes remain uncertain at the time of this writing. Several proposed mechanisms may account for the dermatological and vascular changes seen in the toes of a subset of patients with COVID-19. In one study, findings supported endothelial destruction induced by viral particles [5]. Affected patients in that study had negative test results for COVD-19 based on throats swabs and measurements for circulating antibodies; however, SARS-CoV-2 virus was present in the skin’s blood vessel endothelial cells of patients with symptoms of COVID toes. Such vascular damage could explain the findings seen in the patient discussed in our case.
Outside direct viral endothelial damage in COVID-19, there is a state of severe inflammation with high plasma levels of pro-inflammatory cytokines including interleukin (IL)-2, IL-6, and IL-7 [6]. Another hypothesis is that this inflammatory process leads to endotheliitis and hence endothelial injury [7]. Evidence for elevated D-dimer and thrombocytopenia in COVID-19 patients supports this hypothesis [6,8,9], suggesting a coagulopathic process. Because these factors play a key role in the Virchow’s triad, it is reasonable to assume that this process can lead to small vessel thrombosis, as described in postmortem findings in COVID-19 patients [7]. Diffuse microthrombosis in the digits, which possess end arterioles, may lead to ischemia and finally gangrene of distal tissues (Figure 3). Although various authors have described the use of D-dimers in evaluating for the presence of coagulopathy, no randomized controlled trials have shown that it influences the course of COVID-19.
Figure 3.
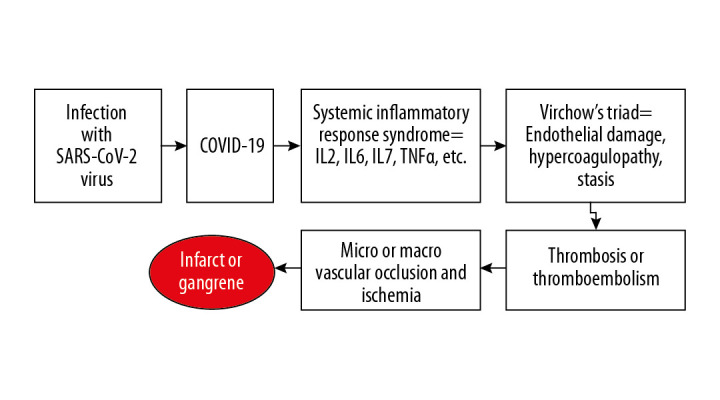
Graphical abstract displaying the proposed vasculopathic sequence of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) resulting in gangrenous toes. IL – interleukin; TNFα – tumor necrosis factor α.
Another theory regarding the development of COVID toes and the progression to gangrene involves venous thrombosis and its rare association with venous gangrene. Based on data from the National Hospital Discharge Survey from 1979 to 2006, only 0.43% of patients had gangrene from deep vein thrombosis [10]. This phenomenon was implicated mainly in patients with an underlying malignancy. Considering the above clinical evidence, we believe that the pathophysiology underlying the gangrenous toes in our patient was either microvascular thrombosis (from system inflammation or direct viral endothelial damage) or related to a rare complication of venous thrombosis manifesting as venous gangrene. However, knowledge of COVID-19 pathophysiology is only based on small series and limited data.
Risk factors for COVID toes presumably include elements of a patient’s clinical profile that would elevate components of Virchow’s triad, as well as complications of chronic conditions that result in peripheral vasculopathy, such as diabetes mellitus. Although the patient had diabetes, which is associated with microvascular complications [11], it cannot solely explain the rapid progression of gangrene of his toes. Because COVID toes developed within 4 days of presentation and progressed to gangrene within 10 days, it is unlikely that diabetes alone could have played a role the rapid progression.
The clinical presentation of COVID toes is progressive. Usually, mild discoloration occurs early in the course and evolves to gangrene within a relatively short timeframe, depending on the severity of the disease. The dermatological changes of the toes are often associated with pain, given that damage is likely mainly vascular and not neuronal as in diabetics. Some cases present with pruritis as well as vesicular lesions. Laboratory tests typically demonstrate elevated D-dimer and acute phase reactants, mainly secondary to the systemic hyperinflammatory state.
Given the potential for coagulopathy in COVID-19 and the evidence of benefits from anticoagulation in a prior observational study on influenza H1N1 disease [12], several clinicians recommend the use of intermediate or full-dose anticoagulation therapy for the routine care of hospitalized COVID-19 patients. This recommendation is based on a hypothesis that the therapy can alter the course of microvascular thrombosis in these patients [13]. The observational study on influenza H1N1 disease involved critically ill patients with acute respiratory distress syndrome and risk factors for venous thromboembolism (VTE) [12]. It showed that empirical anticoagulation therapy reduced VTE incidence without increased risk of hemorrhage. In a recently published observational study, the use of anticoagulation was associated with better outcomes in severely ill patients with COVID-19 who had elevated D-dimer [14]. Unfractionated-heparin has been suggested as a better choice for anticoagulation compared with low-molecular-weight heparin [15]. Our patient was placed on full-dose, weight-based enoxaparin on day 1 of hospitalization after testing revealed an elevated D-dimer level. Although there have been reports of severe arterial thrombosis associated with COVID-19, notwithstanding anticoagulation or antiplatelet therapy [16], throughout the course of our patient’s hospitalization, he was found to have sufficient lower extremity macrovascular circulation as evidenced by palpable peripheral pulses and a CT angiogram that did not find any macrovascular occlusion.
Overall prognosis of COVID toes is variable and is highly dependent on the extent of the underlying systemic inflammation and any concurrent venous thrombosis. Failure of anticoagulation leads to higher morbidity and eventual demarcation of the gangrenous toes leading to amputation, and it is also associated with higher mortality.
This case demonstrates that dermatologic manifestations may develop in patients who initially present with COVID-19 pneumonia. These symptoms can be due to venous thrombosis following SARS-CoV-2 vasculitis, resulting in challenging decisions regarding anticoagulation therapy. Randomized controlled clinical trials are needed to evaluate the efficacy of anticoagulation to enable choosing appropriate anticoagulants and dosing, as well as assessing bleeding risk.
Conclusions
Clinicians should monitor patients with pneumonia secondary to COVID-19 for cutaneous vasculitic lesions, such as COVID toes, and should initiate anticoagulation promptly.
Footnotes
Department and Institution where work was done
Critical Care Department/Long Island Community Hospital (formerly Brookhaven Memorial Hospital), Patchogue, NY, U.S.A.
Conflicts of interest
None.
References:
- 1.World Health Organization (WHO) Situation report – 46. Geneva: WHO; 2020. Coronavirus disease 2019 (COVID-19) https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200306-sitrep-46-covid-19.pdf?sfvrsn=96b04adf_4. [Google Scholar]
- 2.Noakes A, Majoe S. Understanding the role that ‘COVID toe’ has in recognizing the potential extent of COVID-19 infections: A case study. Pathog Glob Health. 2020;114(6):283–84. doi: 10.1080/20477724.2020.1785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: Lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16682. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: An international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83(4):1118–29. doi: 10.1016/j.jaad.2020.06.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: Histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020 doi: 10.1111/bjd.19327. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang T, Sun LX, Feng RE. [Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(6):496–502. doi: 10.3760/cma.j.cn112147-20200311-00312. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 7.Oudkerk M, Büller HR, Kuijpers D, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020 doi: 10.1148/radiol.2020201629. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–73. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippi G, Favaloro EJ. D-dimer is associated with severity of coronavirus disease 2019: A pooled analysis. Thromb Haemost. 2020;120(5):876–78. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musani MH, Musani MA, Verardi MA. Venous gangrene a rare but dreadful complication of deep venous thrombosis. Clin Appl Thromb Hemost. 2011;17(6):E1–3. doi: 10.1177/1076029610376629. [DOI] [PubMed] [Google Scholar]
- 11.Lippi G, Franchini M, Targher G, et al. Epidemiological association between fasting plasma glucose and shortened APTT. Clin Biochem. 2009;42:118–20. doi: 10.1016/j.clinbiochem.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Obi AT, Tignanelli CJ, Jacobs BN, et al. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg Venous Lymphat Disord. 2019;7:317–24. doi: 10.1016/j.jvsv.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–47. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–99. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turshudzhyan A. Anticoagulation options for coronavirus disease 2019 (COVID-19)-induced coagulopathy. Cureus. 2020;12(5):e8150. doi: 10.7759/cureus.8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashi M, Jacquin A, Dakhil B, et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


