Rehabilitative exercise after spinal cord injury aims to strengthen spared neural networks. Jo and Perez show that combining exercise with non-invasive stimulation targeting corticospinal-motoneuronal synapses enhances functional recovery in patients with chronic incomplete spinal cord injury.
Keywords: exercise training, maximal voluntary contraction, neuromodulation, spinal plasticity, motor evoked potentials
Abstract
Rehabilitative exercise in humans with spinal cord injury aims to engage residual neural networks to improve functional recovery. We hypothesized that exercise combined with non-invasive stimulation targeting spinal synapses further promotes functional recovery. Twenty-five individuals with chronic incomplete cervical, thoracic, and lumbar spinal cord injury were randomly assigned to 10 sessions of exercise combined with paired corticospinal-motor neuronal stimulation (PCMS) or sham-PCMS. In an additional experiment, we tested the effect of PCMS without exercise in 13 individuals with spinal cord injury with similar characteristics. During PCMS, 180 pairs of stimuli were timed to have corticospinal volleys evoked by transcranial magnetic stimulation over the primary motor cortex arrive at corticospinal-motor neuronal synapses of upper- or lower-limb muscles (depending on the injury level), 1–2 ms before antidromic potentials were elicited in motor neurons by electrical stimulation of a peripheral nerve. Participants exercised for 45 min after all protocols. We found that the time to complete subcomponents of the Graded and Redefined Assessment of Strength, Sensibility and Prehension (GRASSP) and the 10-m walk test decreased on average by 20% after all protocols. However, the amplitude of corticospinal responses elicited by transcranial magnetic stimulation and the magnitude of maximal voluntary contractions in targeted muscles increased on overage by 40–50% after PCMS combined or not with exercise but not after sham-PCMS combined with exercise. Notably, behavioural and physiological effects were preserved 6 months after the intervention in the group receiving exercise with PCMS but not in the group receiving exercise combined with sham-PCMS, suggesting that the stimulation contributed to preserve exercise gains. Our findings indicate that targeted non-invasive stimulation of spinal synapses might represent an effective strategy to facilitate exercise-mediated recovery in humans with different degrees of paralysis and levels of spinal cord injury.
Introduction
Rehabilitation strategies in humans with spinal cord injury (SCI) rely on the use of exercise (Harkema et al., 2012; Behrman et al., 2017). Exercise training aims to drive neural networks in an activity-dependent manner to elicit plasticity and facilitate functionally relevant muscle activity below the level of injury (Knikou, 2010). Studies in animals (Courtine et al., 2009; Hill et al., 2009; McPherson et al., 2015) and humans (Rejc et al., 2017; Gad et al., 2018; Gill et al., 2018) agree that physiological and functional effects of exercise can be augmented by the use of neural stimulation, which is thought to increase the likelihood of activating spared neural pathways. Even though these approaches have facilitated exercise-mediated recovery, the overall effects remain limited. Clearly, there is a need to develop interventions that can more effectively engage spared neural connections to further improve functional recovery in humans with SCI.
A strategy that engages residual neuronal networks in humans with SCI is paired corticospinal-motor neuronal stimulation (PCMS) (Bunday and Perez, 2012; Urbin et al., 2017; Bunday et al., 2018). During PCMS, corticospinal volleys evoked by transcranial magnetic stimulation (TMS) over the primary motor cortex are timed to arrive at corticospinal-motor neuronal synapses of limb muscles before or after antidromic potentials elicited in motor neurons by electrical stimulation of a peripheral nerve (Taylor and Martin, 2009). PCMS likely elicits spike-timing dependent plasticity (STDP) changes at spinal synapses of somatic motor neurons. In animals, STDP-like plasticity is thought to engage long-term potentiation (LTP)-like mechanisms that depend on N-methyl-d-aspartate (NMDA) (Bi and Poo, 1998; Feldman, 2012). In humans, STDP-like plasticity elicited by PCMS are blocked by the NMDA antagonist dextromethorphan (Donges et al., 2018). NMDA receptors in the spinal cord can influence the output of motor neurons (Manuel et al., 2012) and voluntary activity facilitates PCMS-mediated plasticity in animals (McPherson et al., 2015) and humans (Bunday et al., 2018) with SCI. Indeed, the number of individuals with SCI responding to PCMS increased when PCMS was applied during voluntary activity compared with rest (Bunday et al., 2018). Exercise combined with invasive (Harkema et al., 2011; Angeli et al., 2014; Gill et al., 2018) and non-invasive (Gerasimenko et al., 2015; Sayenko et al., 2015; Gad et al., 2018) electrical stimulation of the spinal cord was found to potentiate the beneficial effects of induced-plasticity protocols in humans with SCI. Thus, we questioned if we could augment changes in corticospinal transmission and motor output by combining exercise with PCMS. We hypothesized that PCMS enhances exercise-related recovery by enhancing transmission in the corticospinal pathway at the spinal level.
To test our hypothesis, individuals with chronic incomplete SCI were randomly assigned to 10 sessions of exercise training combined with PCMS or sham-PCMS. In an additional experiment, we tested the effect of PCMS without exercise in individuals with SCI with similar characteristics as in the other groups. We found that corticospinal drive and maximal voluntary contraction in targeted muscles increased after PCMS with or without exercise but not after sham-PCMS with exercise. Behavioural effects were preserved for 6 months in the group receiving PCMS with exercise but not sham-PCMS. We argue that targeting spinal synapses is an effective strategy to enhance recovery in humans with SCI.
Materials and methods
Participants
Thirty-eight individuals with SCI (mean age 44.2 ± 14.8 years, nine female; Table 1) participated in the study. Written informed consent was obtained from all subjects for study participation and in two subjects to publish their images in an online open-access publication. All procedures were approved by the local ethics committee at the University of Miami in accordance with the guidelines established in the Declaration of Helsinki. Participants with SCI had a chronic (≥1 year) injury between C2–L3 (Table 1). Twelve of 38 individuals were categorized by the American Spinal Cord Injury Impairment Scale (AIS) as AIS A (complete injury) due to the lack of sacral sparing (Marino et al., 2003), despite being able to elicit voluntary force with the targeted muscle. The other 26 individuals were classified as incomplete AIS B (n = 6), C (n = 11), and D (n = 9) (Table 1).
Table 1.
Spinal cord injury participants
| Targeted muscle | Age, years | Sex | Injury level | AIS | Aetiology | Time after injury, years | |
|---|---|---|---|---|---|---|---|
| PCMS+exercise | |||||||
| 1 | Deltoid | 52 | M | C3 | A | T | 33 |
| 2 | Deltoid | 46 | M | C3 | B | T | 25 |
| 3 | Biceps brachii | 50 | F | C4 | A | T | 23 |
| 4 | Biceps brachii | 25 | M | C4 | A | T | 3 |
| 5 | Biceps brachii | 58 | M | C5 | B | T | 37 |
| 6 | First dorsal interosseous | 44 | M | C4 | C | T | 10 |
| 7 | First dorsal interosseous | 49 | M | C3 | A | T | 1 |
| 8 | First dorsal interosseous | 39 | M | C5 | D | T | 12 |
| 9 | First dorsal interosseous | 28 | F | C5 | D | T | 2 |
| 10 | Abductor pollicis brevis | 23 | M | C5 | A | T | 5 |
| 11 | Abductor pollicis brevis | 28 | F | C5 | B | T | 2 |
| 12 | Tibialis anterior | 69 | F | L3 | C | T | 3 |
| 13 | Tibialis anterior | 82 | M | T8 | D | NT | 9 |
| sham-PCMS+exercise | |||||||
| 1 | Deltoid | 43 | M | C2 | A | T | 7 |
| 2 | Deltoid | 54 | M | C4 | B | T | 17 |
| 3 | Biceps brachii | 30 | M | C6 | A | T | 11 |
| 4 | Biceps brachii | 22 | M | C5 | A | T | 5 |
| 5 | First dorsal interosseous | 37 | M | C5 | C | T | 2 |
| 6 | First dorsal interosseous | 54 | F | C5 | D | T | 16 |
| 7 | First dorsal interosseous | 38 | M | C4 | C | T | 16 |
| 8 | First dorsal interosseous | 43 | M | C4 | C | T | 12 |
| 9 | Abductor pollicis brevis | 59 | M | C5 | D | T | 15 |
| 10 | Abductor pollicis brevis | 31 | M | C6 | A | T | 5 |
| 11 | Tibialis anterior | 39 | M | T10 | C | T | 1 |
| 12 | Tibialis anterior | 52 | M | T5 | D | T | 1 |
| PCMS | |||||||
| 1 | Deltoid | 64 | M | C4 | A | T | 7 |
| 2 | Deltoid | 27 | M | C4 | A | T | 11 |
| 3 | Biceps brachii | 47 | M | C5 | B | T | 3 |
| 4 | Biceps brachii | 21 | M | C3 | A | T | 2 |
| 5 | First dorsal interosseous | 37 | M | C6 | C | T | 5 |
| 6 | First dorsal interosseous | 27 | F | C5 | D | T | 5 |
| 7 | First dorsal interosseous | 47 | M | C4 | D | T | 13 |
| 8 | First dorsal interosseous | 33 | F | C4 | B | T | 16 |
| 9 | Abductor pollicis brevis | 50 | M | C4 | C | T | 2 |
| 10 | Abductor pollicis brevis | 60 | M | C5 | D | T | 16 |
| 11 | Tibialis anterior | 57 | F | L1 | C | T | 1 |
| 12 | Tibialis anterior | 71 | F | L2 | C | T | 2 |
| 13 | Tibialis anterior | 45 | M | L2 | C | T | 11 |
AIS = American Spinal Injury Association Impairment Scale; C = cervical; F = female; L = lumbar; M = male; T = thoracic; T/NT = traumatic/non-traumatic.
Experimental set-up
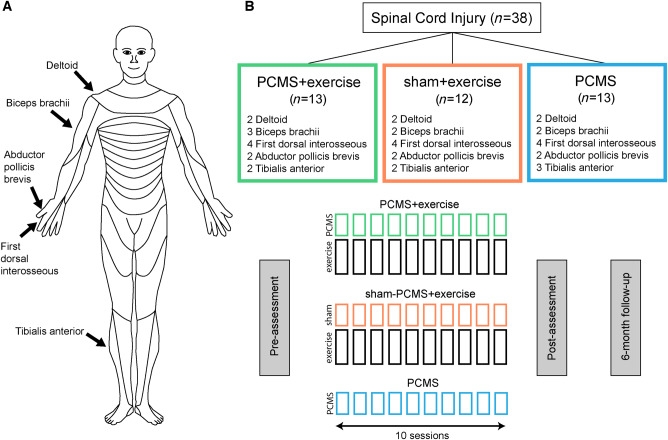
Individuals were randomly assigned to 10 sessions of exercise combined with PCMS (PCMS+exercise, n = 13) or sham-PCMS (sham-PCMS+exercise, n = 12) completed in 2–3 weeks. We previously showed that the facilitatory effects of PCMS on corticospinal excitability were present 30 min after the stimulation and returned to baseline ∼60–80 min after the end of the stimulation (Bunday and Perez, 2012; Urbin et al., 2017; Bunday et al., 2018). Thus, the exercise training lasted for 45 min and began immediately after PCMS+exercise or sham-PCMS+exercise. We used a randomized complete block design to include individuals with different levels of SCI. This design allowed us to stratify subjects to have a better representation of participants across interventions (Piantadosi, 2017). In each intervention, individuals were drawn from three different blocks: (i) shoulder block, including individuals who did not show residual voluntary contraction and the presence of a motor evoked potential (MEP) in hand muscles (e.g. first dorsal interosseous and/or abductor pollicis brevis) but showed residual voluntary contraction and a MEP in deltoid and/or biceps brachii; (ii) hand block, including individuals who showed residual voluntary contraction and a MEP in the first dorsal interosseous and/or abductor pollicis brevis; and (iii) leg block, in this group, participants who showed residual voluntary contraction and the presence of a MEP in the tibialis anterior were included (Fig. 1). In an additional experiment, we tested the effect of PCMS without exercise (hereafter referred to as ‘PCMS') in 13 individuals with similar characteristics as in the other groups. Subjects in each block across interventions had similar age (P = 0.7), time post-injury (P = 0.4), level of maximal voluntary contraction (MVC) measured by EMG activity (P = 0.6), maximal motor response (M-max, P = 0.7), and the presence of an MEP in each muscle tested. Note that during MVC testing subjects were asked to perform three brief MVCs for 3–5 s with each of the muscles tested, separated by at least 30 s of rest. The maximal mean EMG activity measure over a period of 1 s on the rectified response generated during each MVC was analysed and the highest value of the three trials was used. Note that for these measurements, we subtracted the mean background resting EMG activity obtained on each day (1 s before the MVC) to facilitate comparisons of EMG amplitudes across different days. Because measurements in each subject were compared with their own baseline (pre-intervention measure), we were able to include MEPs or MVCs from different muscles for comparisons across groups and therefore test individuals with different injury levels. These are important considerations when examining the efficacy of translational approaches across individual SCI (Jones et al., 2018).
Figure 1.
Experimental set-up. Diagrams showing muscles tested (A) and the study design (B). Thirty-eight individuals with chronic incomplete SCI were randomly assigned to 10 sessions of exercise combined with PCMS or sham-PCMS and PCMS.
In all subjects, we tested the following measurements before and after each intervention: MEPs and MVCs. A subset of subjects completed clinically relevant functional tasks (PCMS+exercise: n = 6; sham-PCMS+exercise: n = 8; PCMS: n = 8; see details below and Supplementary material). We tested the more affected side of individuals with SCI. However, if MEPs were not elicited in the more affected side, the other side was tested. A subgroup of subjects returned for a 6-month follow-up session to examine the same measurements (PCMS+exercise: n = 5, sham-PCMS+exercise: n = 5). Note that individuals in the PCMS group were not able to return for a 6-month follow-up session.
EMG recordings
EMGs were recorded from the targeted muscles, which included deltoid, biceps brachii, first dorsal interosseous, abductor pollicis brevis, and tibialis anterior through surface electrodes secured to the skin over the belly of each muscle (Ag-AgCl, 10 mm diameter). The signals were amplified, filtered (20–1000 Hz), and sampled at 10 kHz for offline analysis (CED 1401 with Signal software, Cambridge Electronic Design, Cambridge, UK).
PCMS and sham-PCMS
During PCMS, 180 pairs of stimuli were delivered every 10 s (∼30 min, 0.1 Hz) where corticospinal volleys evoked by TMS over the primary motor cortex were timed to arrive at corticospinal-motor neuronal synapses of each muscle ∼1–2 ms before antidromic potentials evoked in motor neurons by peripheral nerve stimulation. TMS stimuli were delivered at an intensity of 100% of the maximum stimulator output during PCMS with and without exercise. During sham-PCMS, a coil was placed ∼10 cm behind the subject’s head and triggered every 10 s for 180 stimuli and peripheral nerve stimulation electrodes were placed in the same position as for PCMS but no stimulation was provided. Note that this approach was used for sham-PCMS instead of having antidromic potentials evoked in motor neurons to arrive before corticospinal volleys evoked by TMS at spinal synapses to avoid possible inhibitory effects that might have a detrimental effect on motor output (Taylor and Martin, 2009). Although closer times between the arrivals of antidromic potentials in motor neurons prior to corticospinal volleys evoked by TMS at spinal synapses might not have detrimental effects (Bunday and Perez, 2012) these effects over multiple sessions remain unknown.
Transcranial magnetic stimulation
Transcranial magnetic stimuli were delivered from a Magstim® 200 stimulator through either a figure-of-eight coil (loop diameter, 7 cm; type number SP15560) or a double-cone coil (used for the tibialis anterior muscle; type number 9902-00) with a monophasic current waveform. TMS was delivered to the optimal scalp position for activation of the targeted muscle. The optimal scalp position was determined by moving the coil in small steps along the hand/arm/leg representation of the primary motor cortex to find the region where the largest MEP could be evoked with the minimum intensity in the targeted muscle. This scalp position was saved using a stereotaxic neuro-navigation system (Brainsight 2, Rogue Research). The TMS coil was held to the head of the subject with a custom coil holder, while the head was firmly secured to a headrest by straps to limit head movements. TMS stimuli were delivered at an intensity of 100% of the maximum stimulator output.
Peripheral nerve stimulation
Supra-maximum electrical stimulation (200 μs pulse duration, model DS7AH, Digitimer) was delivered to the ulnar nerve at the wrist for the first dorsal interosseous, median nerve at the wrist for abductor pollicis brevis, brachial plexus at the erb’s point for the deltoid and biceps brachii, and common peroneal nerve under the head of the fibula for the tibialis anterior. The anode and cathode were 3-cm apart and 1 cm in diameter with the cathode positioned proximally. The stimuli were delivered at an intensity of 120% of the M-max for each muscle.
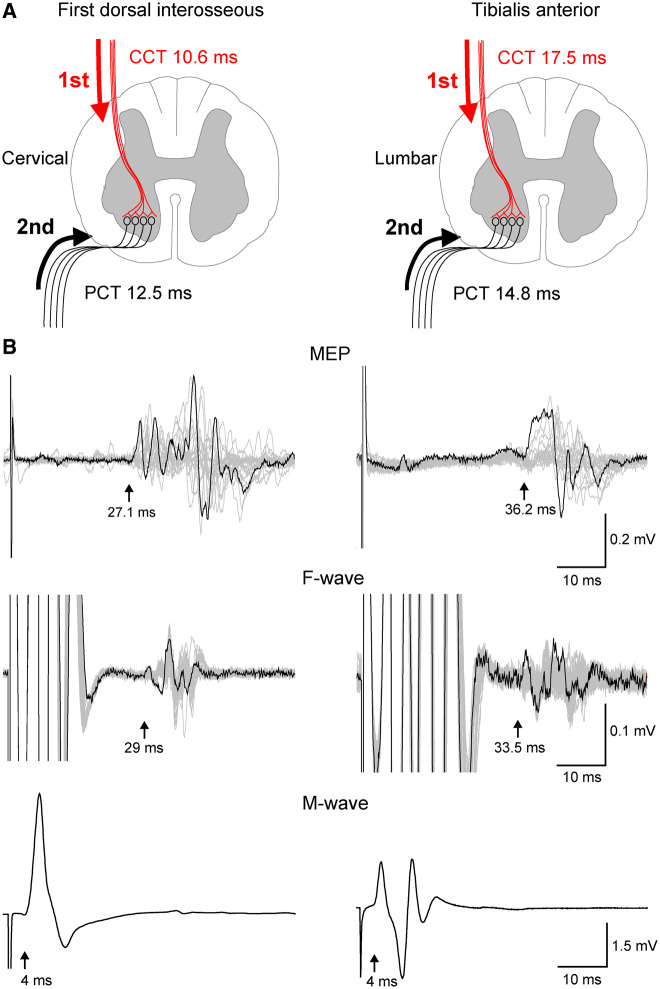
Transcranial magnetic and peripheral nerve stimulation interstimulus interval
The interstimulus interval (ISI) between TMS and peripheral nerve stimulation was set to allow descending volleys elicited by TMS to arrive at the presynaptic terminal of corticospinal neurons ∼1–2 ms before antidromic peripheral nerve stimulation volleys reached the motor neurons reached during PCMS (Fig. 2A). The methods for timing the arrival of volleys at the spinal cord have been described previously (Bunday and Perez, 2012; Urbin et al., 2017; Bunday et al., 2018). Briefly, the ISI was tailored to individual subjects based on conduction times calculated from latencies of MEPs, F-waves, and M-waves (Fig. 2B). MEP latencies were recorded during isometric ∼10% of MVC of the target muscle to determine the shortest and clearest response for our estimations. The onset latency was defined as the time when each response exceeded 2 standard deviations (SD) of the mean rectified prestimulus activity (100 ms) in the averaged waveform. Peripheral conduction time (PCT) was calculated as:
| (1) |
Figure 2.
Central (CCT) and peripheral (PCT) conduction time. Stimuli were timed to arrive at corticospinal-motor neuronal synapses by calculating CCT and PPT (A) using latencies from MEPs, F-waves, and M-waves (B). Traces shown from first dorsal interosseous and tibialis anterior muscles with latencies indicated by arrows.
Central conduction time (CCT) was calculated as:
| (2) |
When it was not possible to record F-waves (i.e. deltoid and biceps brachii) we stimulated C-roots with TMS at cervical spinous processes C3–4 or C5–6, respectively (Table 2 and Supplementary material).
Table 2.
Response latencies and conduction times
| PCMS+exercise | sham-PCMS+exercise | PCMS | |
|---|---|---|---|
| Deltoid/biceps brachii | |||
| MEP | 13.4 ± 1.2 | 12.5 ± 0.8 | 13.0 ± 1.2 |
| C-root | 6.4 ± 0.6 | 6.0 ± 0.1 | 7.0 ± 0.7 |
| M-max | 5.1 ± 0.5 | 4.7 ± 0.9 | 5.4 ± 1.1 |
| CCT | 5.5 ± 1.8 | 4.8 ± 0.6 | 4.5 ± 0.9 |
| PCT | 1.8 ± 0.3 | 1.8 ± 1.0 | 2.1 ± 0.5 |
| First dorsal interosseous/abductor pollicis brevis | |||
| MEP | 28.3 ± 2.1 | 27.1 ± 3.3 | 29.9 ± 3.8 |
| F-wave | 29.7 ± 1.5 | 29.6 ± 3.8 | 32.3 ± 3.9 |
| M-max | 3.4 ± 0.3 | 3.7 ± 0.4 | 3.7 ± 0.6 |
| CCT | 11.7 ± 2.4 | 10.4 ± 3.2 | 11.9 ± 5.3 |
| PCT | 13.2 ± 0.8 | 13.0 ± 1.8 | 14.3 ± 1.9 |
| Tibialis anterior | |||
| MEP | 37.4 ± 3.8 | 38.1 ± 2.1 | 36.7 ± 6.3 |
| F-wave | 34.8 ± 9.3 | 33.8 ± 5.6 | 36.2 ± 1.9 |
| M-max | 3.6 ± 0.8 | 3.2 ± 1.0 | 3.6 ± 0.6 |
| CCT | 18.3 ± 0.4 | 19.6 ± 1.2 | 17.0 ± 1.8 |
| PCT | 15.6 ± 5.0 | 15.3 ± 2.3 | 15.9 ± 1.1 |
Conduction times are given in milliseconds.
CCT = central conduction time; PCT = peripheral conduction time.
Exercise training
All participants exercised for 45 min immediately after PCMS+exercise or sham-PCMS+exercise (Supplementary Fig. 1). Upper-limb exercises involved gross grasping, fine grasping, and hand cycle using an arm ergometer (Supplementary material). During fine grasping, participants were asked to reach and grasp smaller objects (peg, bead, pinch pin, cube). During gross grasping, participants were asked to reach and grasp larger objects (cylinder, block, cup, lid). During the hand cycle, the arm ergometer was used for 15 min and grasping gloves were used as needed. Note that a few participants required some assistance to grasp an object during the upper-limb training (PCMS+exercise: n = 3; sham-PCMS+exercise: n = 2) and although all participants were ambulatory, a few were not able to walk without an assistive device (PCMS+exercise: n = 1; sham-PCMS+exercise: n = 1; PCMS: n = 1). Note that lower-limb exercises involved overground walking, treadmill walking, and stair climbing. During walking, subjects used a body-weight support system (ZeroG, Aretech LLC) with 0–70% of body-weight support. The system was mounted over an overhead track and used an active trolley system that automatically followed the patient as he or she walked. During treadmill walking, subjects walked at a speed of 0.1–0.3 m/s for 15 min using the ZeroG system. During stair climbing, subjects climbed up and down four steps with three full repetitions.
Motor evoked potentials
The maximal MEP amplitude (MEP-max) was found in each subject for each muscle tested. The MEP-max was defined in all participants at rest by increasing stimulus intensities in 5% steps of maximal device output until the MEP amplitude did not show additional increases. During measurements before and after each intervention, TMS intensity was set to elicit an MEP of ∼50% of the MEP-max amplitude on each muscle tested. The same intensity was used during pre- and post-assessments. TMS pulses were delivered at 4-s intervals (0.25 Hz). Thirty MEPs were recorded for each assessment and peak-to-peak MEP amplitude was measured in each trial and averaged. To compare MEPs with similar background EMG activity between interventions, trials in which the background EMG activity (100 ms before the TMS stimulus artefact) was 2 SD above the mean resting background EMG activity were excluded from the analysis (Bunday et al., 2014). A total of 5.6 ± 5.0% of trials were excluded.
Functional outcomes
In the group receiving upper-limb exercises, we tested gross (i.e. jar opening and water bottle tests) and fine (i.e. keys and nine-hole peg tests) grasping functions using subcomponents of the Graded and Redefined Assessment of Strength, Sensibility and Prehension (GRASSP) test that all participants could complete (Supplementary material). In the group receiving lower-limb exercises, we used the 10-m walk test while subjects were connected to the ZeroG system to assess walking speed in metres per second. The same percentage of body-weight support was used during pre- and post-assessments within each subject. Overall, a stopwatch was used to measure the time to execute each task. Each task was repeated three times and the average time was used.
Data analysis
Normal distribution was tested by the Shapiro-Wilk’s test and homogeneity of variances by the Mauchly’s test of sphericity. When sphericity could not be assumed, the Greenhouse-Geisser correction statistic was used. Repeated-measures ANOVA was performed to determine the effect of Intervention (PCMS+exercise, sham-PCMS+exercise, PCMS) and Time (pre-assessment, post-assessment) on MEP amplitude, background EMG activity before MEP stimulus artefact, MVCs, and functional outcomes. Bonferroni post hoc tests were used to test significant comparisons. One-way ANOVA was used to compare differences across Intervention groups in age, time post-injury, M-max, MEP amplitude, MVCs, and functional outcomes measured at pre-assessment. Paired t-tests were used to compare 6-month assessments for MEP amplitude, MVCs, and functional outcomes. Significance was set at P < 0.05. Group data are presented as mean ± SD.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.
Results
Motor evoked potentials
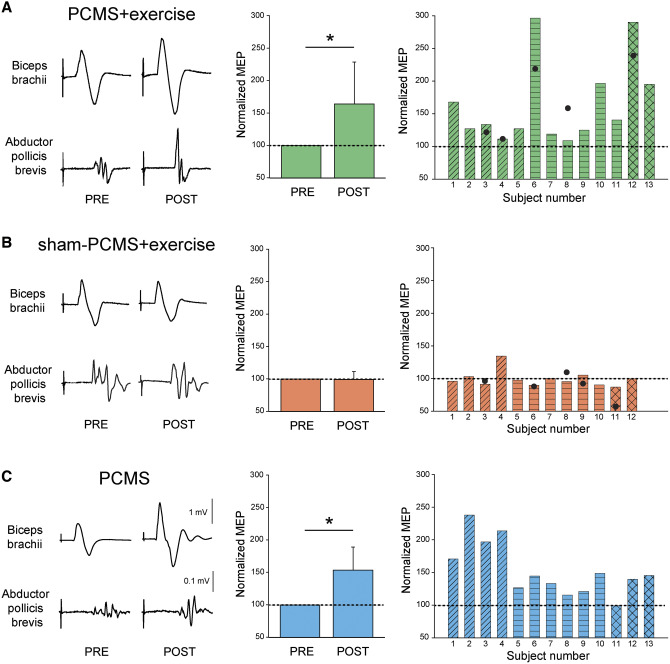
Figure 3 shows raw MEP traces from six representative participants in whom PCMS+exercise and sham-PCMS+exercise and PCMS targeted the biceps brachii (PCMS+exercise: Subject 5, sham-PCMS+exercise: Subject 3, PCMS: Subject 4; Table 1) and abductor pollicis brevis (PCMS+exercise: Subject 10, sham-PCMS+exercise: Subject 10, PCMS: Subject 10; Table 1) muscles. Note that the amplitude of MEPs increased in all subjects tested after 10 sessions of PCMS combined or not with exercise. No changes were observed in MEP amplitude after sham-PCMS combined with exercise.
Figure 3.
MEPs. Raw MEP traces from six representative participants from biceps brachii and abductor pollicis brevis muscles before and after 10 sessions. Waveforms represent the average of 30 MEPs. Graphs show group (left) and individual (right) data for PCMS+exercise (A; n = 13) and sham-PCMS+exercise (B; n = 12), and PCMS (C; n = 13) groups. The x-axes of the left graphs show the time of measurements (PRE = pre-assessment; POST = post-assessment) and the y-axis shows the amplitude of MEPs as percentage of MEPs at pre-assessment. The x-axes of the right graphs show individual subjects and filled circles indicate the 6-month follow-up results. Data of participants included in the shoulder (transverse lines), hand (horizontal lines), and leg (crossed lines) blocks are shown for each intervention. Filled circles show individual MEP data in a subset of subjects at the 6-month follow-up after PCMS+exercise (n = 5) and sham-PCMS+exercise (n = 5). Scale bars shown for biceps brachii and first dorsal interosseous muscles are the same across participants. Error bars indicate SD, *P < 0.05.
Repeated-measures ANOVA showed an effect of Intervention [F(2,35) = 7.5, P < 0.01], Time [F(1,35) = 28.5, P < 0.001] and in their interaction [F(2,35) = 7.5, P < 0.01] on MEP amplitude. Post hoc analysis revealed that MEP size increased after 10 sessions of PCMS+exercise (64.6 ± 65.0%, P < 0.01) or PCMS (53.2 ± 40.7%, P < 0.01) but not after sham-PCMS+exercise (−0.9 ± 12.4%, P = 0.8) compared with baseline. Note that all participants in the PCMS+exercise group (13 of 13) and the majority in the PCMS group (12 of 13) showed increases in MEP amplitude during post- compared with pre-assessment measurements in all muscles tested (Fig. 3A and C). Specifically, MEP amplitude increased in the group that underwent PCMS+exercise by 33.5 ± 21.0% in the deltoid/biceps brachii (n = 5), by 64.5 ± 71.8% in the first dorsal interosseous/abductor pollicis brevis (n = 6) and by 142.7 ± 67.2% in the tibialis anterior (n = 2). Similarly, MEP amplitude increased in the group that underwent PCMS by 104.7 ± 28.3% in the deltoid/biceps brachii (n = 4), by 31.5 ± 13.1% in the first dorsal interosseous/abductor pollicis brevis (n = 6) and by 28.0 ± 25.4% in the tibialis anterior (n = 3). In contrast, no differences were observed in any of the muscles tested after the group that did sham-PCMS+exercise [by 6.2 ± 19.4% in the deltoid/biceps brachii (n = 4), by −3.7 ± 5.9% in the first dorsal interosseous/abductor pollicis brevis (n = 6) and by −6.6 ± 0.9% in the tibialis anterior (n = 2)]. We found no differences among the three groups in MEP amplitudes measured in all muscles at pre-assessment (P = 0.9). Also, we found no effect of Intervention [F(2,35) = 2.4, P = 0.2], Time [F(1,35) = 0.3, P = 0.6] nor in their interaction [F(2,35) = 1.5, P = 0.2] on mean background EMG activity measured prior to the TMS stimulus artefact in all muscles tested. Filled circles show individual MEP data in a subset of subjects at the 6-month follow-up after PCMS+exercise (Fig. 3A, n = 5) and sham-PCMS+exercise (Fig. 3B, n = 5).
Maximal voluntary contraction
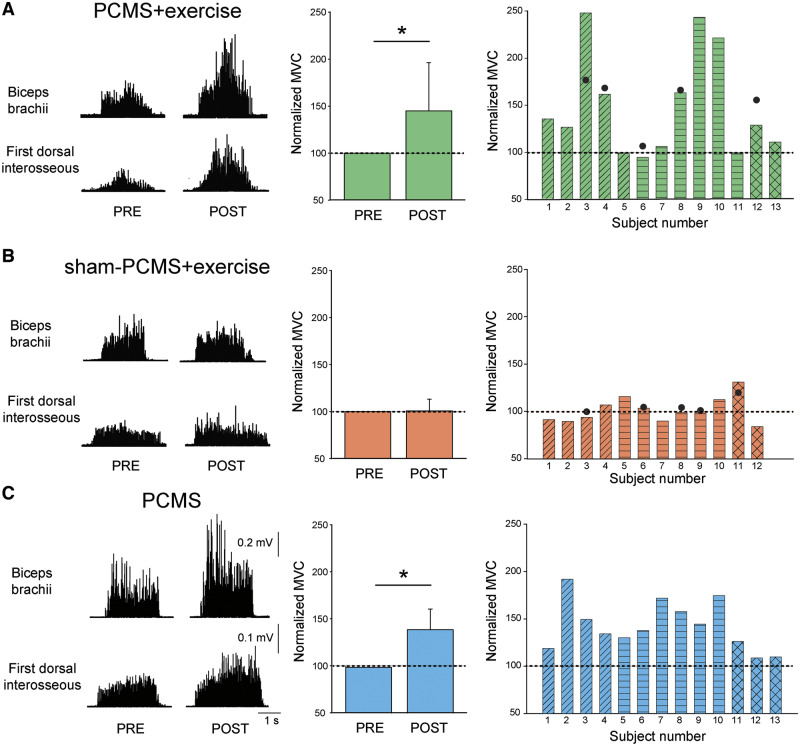
Figure 4 shows raw EMG traces during MVC of six representative participants in whom PCMS targeted the biceps brachii (PCMS+exercise: Subject 3, sham-PCMS+exercise: Subject 4, and PCMS: Subject 3; Table 1) and first dorsal interosseous (PCMS+exercise: Subject 9, sham-PCMS+exercise: Subject 5, and PCMS: Subject 7; Table 1) muscles. MVC values increased only in participants with SCI in whom PCMS was combined or not with exercise, but not sham-PCMS combined with exercise, regardless of the muscle tested.
Figure 4.
MVCs. Rectified EMG traces during MVCs from six representative participants from biceps brachii and first dorsal interosseous muscles before and after 10 sessions. Graphs show group (left) and individual (right) data for PCMS+exercise (A; n = 13), sham-PCMS+exercise (B; n = 12), and PCMS (C; n = 13) groups. The x-axes of the left graphs show the time of measurements (PRE = pre-assessment; POST = post-assessment) and the y-axis shows the size of MVCs as percentage of MVCs at pre-assessment. The x-axes of the right graphs show individual subjects and filled circles indicate the 6-month follow-up results. Data of participants included in shoulder (transverse lines), hand (horizontal lines), and leg (crossed lines) block are shown for each intervention. Scale bars shown for biceps brachii and first dorsal interosseous muscles are the same across participants. Error bars indicate SDs, *P < 0.05.
Repeated-measures ANOVA showed an effect of Intervention [F(2,35) = 6.1, P < 0.01], Time [F(1,35) = 27.1, P < 0.001] and in their interaction [F(2,35) = 6.1, P < 0.01] on MVC. Post hoc analysis revealed that MVC increased after 10 sessions of PCMS+exercise (48.0 ± 54.9%, P < 0.01) and PCMS (42.8 ± 25.7%, P < 0.001) but not after sham-PCMS+exercise (1.3 ± 13.5%, P = 0.7) compared with baseline. Note that the majority of participants in the PCMS+exercise group (9 of 13; Fig. 4A) and all participants in the PCMS group (13 of 13 Fig. 4C), but not in the sham-PCMS+exercise group (3 of 12; Fig 4B) showed increases in MVC ≥10% during post- compared with pre-assessment measurements in all muscles tested. Specifically, MVCs increased in the group that underwent PCMS+exercise by 53.0 ± 56.6% in the deltoid/biceps brachii (n = 5), by 53.6 ± 65.0% in the first dorsal interosseous/abductor pollicis brevis (n = 6), and by 18.9 ± 12.5% in the tibialis anterior (n = 2). Similarly, MVC increased in the group that underwent PCMS without exercise by 48.6 ± 31.5% in the deltoid/biceps brachii (n = 4), by 52.8 ± 18.3% in the first dorsal interosseous/abductor pollicis brevis (n = 6) and by 15.0 ± 9.8% in the tibialis anterior (n = 3). In contrast, no differences were observed in any of the muscles tested after the group that received sham-PCMS+exercise [by −4.8 ± 7.9% in the deltoid/biceps brachii (n = 4), by 3.3 ± 9.5% in the first dorsal interosseous/abductor pollicis brevis (n = 6) and by 7.4 ± 33.3% in the tibialis anterior (n = 2)]. No differences were found in MVC in all muscles tested between groups in the pre-assessment measurement (P = 0.9). MVC values remained increased for 6 months in a subgroup of participants that received PCMS+exercise (Fig. 4A, filled circles, n = 5) but not sham-PCMS+exercise (Fig. 4B, filled circles, n = 5).
Functional outcomes
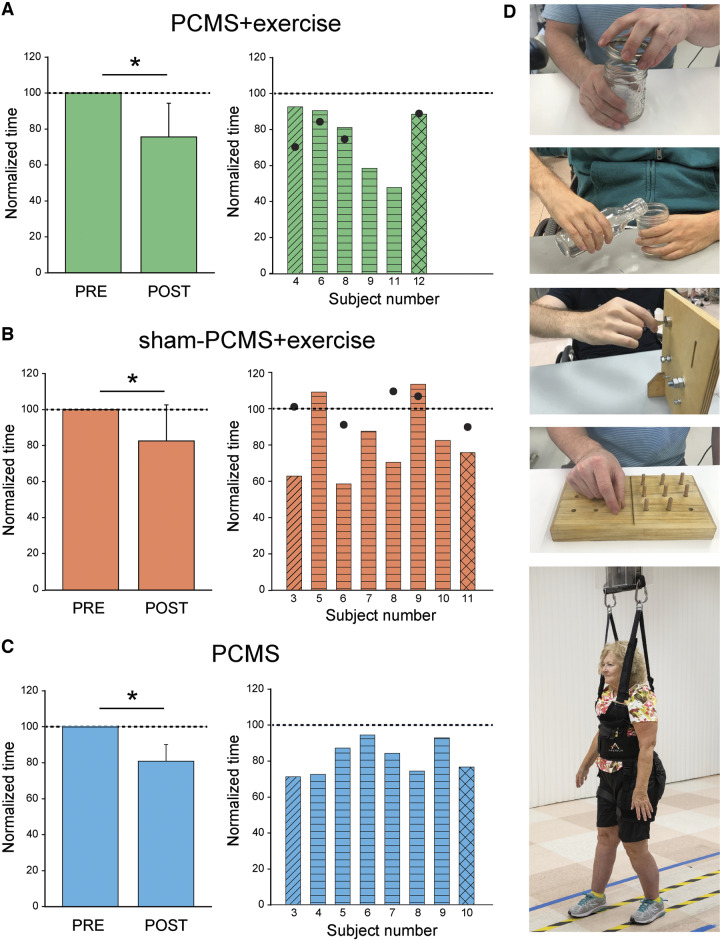
Repeated-measures ANOVA showed an effect of Time [F(1,19) = 32.8, P < 0.001] but not Intervention [F(2,19) = 0.3, P = 0.7] or in their interaction [F(2,19) = 0.3, P = 0.7] on functional outcomes. Post hoc analysis revealed that the time to perform functional tests decreased after 10 sessions in all groups; PCMS+exercise (by 24.4 ± 18.6%, P < 0.05), PCMS (by 19.5 ± 9.1%, P < 0.05) and sham-PCMS+exercise (by 17.4 ± 20.1%, P < 0.05). We found no differences between groups in the time to complete functional outcome tested in the pre-assessment session (P = 0.4). Note that all participants in the group receiving PCMS+exercise (Fig. 5A), the majority of participants in sham-PCMS+exercise group (six of eight; Fig. 5B), and all participants in the group receiving PCMS (Fig. 5C) showed decreases in the time to complete functional tests in the post- compared with the pre-assessment measurements. The time to complete subcomponents of the GRASSP and walking time (Fig. 5D) decreased after PCMS+exercise (GRASSP = 26.7%, walking speed = 12.4%), with sham-PCMS+exercise (GRASSP = 16.5%, walking speed = 24.1%) and PCMS (GRASSP = 18.7%, walking speed = 24.5%). Notably, functional outcomes remained increased at the 6-month follow-up in a subgroup of participants that received PCMS+exercise (n = 4; Fig. 5A, filled circles) but not sham-PCMS+exercise (n = 5; Fig. 5B, filled circles).
Figure 5.
Functional outcomes. Graphs show group (left) and individual (right) data for PCMS+exercise (A; n = 6), and sham-PCMS+exercise (B; n = 8), and PCMS (C; n = 8) groups. The x-axes show the time of measurements (PRE = pre-assessment; POST = post-assessment) and the y-axes show the time to perform tasks as percentage of the time at pre-assessment. The x-axes of the right graphs show individual subjects and filled circles indicate the 6-month follow-up results. (D) Tests involved subcomponents of the GRASSP and the 10-m walk tests. Data of participants included in the shoulder (transverse lines), hand (horizontal lines), and leg (crossed lines) block are shown for each intervention. Filled circles show individual functional outcomes in a subset of subjects at the 6-month follow-up after PCMS+exercise and sham-PCMS+exercise. Error bars indicate SDs, *P < 0.05.
Six-month follow-up
In a subset of subjects who completed the 6-month follow-up visit, we found that MEP amplitude increased after 10 sessions (by 88.2 ± 96.5%; Fig. 6A) and 6 months (by 72.0 ± 57.7%; P < 0.05; Fig. 6A) in the PCMS+exercise group compared with baseline. However, no differences were observed in MEP amplitude at any time in subjects receiving sham-PCMS+exercise (by −6.3 ± 7.1%; P = 0.2 after 10 sessions and by −11.0 ± 22.1%; P = 0.3 after 6 months compared with baseline; Fig. 6A). MVCs increased after 10 sessions (by 57.4 ± 57.5%, P < 0.05; Fig. 6B) and remained increased for 6 months (by 54.5 ± 25.0%, P < 0.05; Fig. 6B) compared with baseline after PCMS+exercise but not after sham-PCMS+exercise (by 4.6 ± 12.5%; P = 0.5 after 10 sessions and 8.8 ± 7.8%; P = 0.1 after 6 months compared with baseline). In addition, functional outcomes increased after 10 sessions of PCMS+exercise (by 12.9 ± 4.9%, P < 0.05; Fig. 6C) and remained increased for 6 months (by 21.6 ± 9.6%, P < 0.05; Fig. 6C) compared with baseline whereas the increase present after 10 sessions of sham-PCMS+exercise (23.6 ± 21.7%, P < 0.05; Fig. 6C) did not persist 6 months later (0.6 ± 10.5%, P = 0.3; Fig. 6C).
Figure 6.
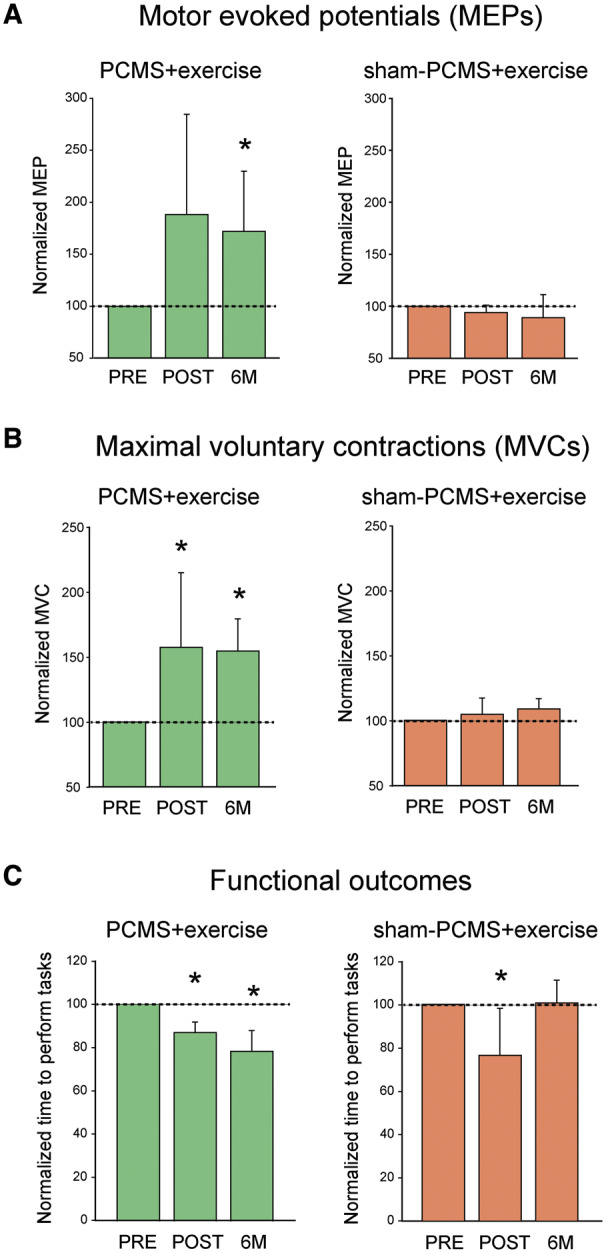
Six-month follow-up results. Graphs show results after 10 sessions of PCMS+exercise (green bars) and sham-PCMS+exercise (orange bars) at the 6-month follow-up assessment. (A) MEPs (n = 5 for PCMS+exercise and 5 for sham-PCMS+exercise). The x-axis shows the time of assessments (PRE = pre-assessment; POST = post-assessment; 6M = 6-month follow-up assessment) and the y-axis shows the amplitude of MEPs as percentage of MEPs at pre-assessment (A), the MVCs as percentage of MVCs at pre-assessment (B), and the time to perform tasks as percentage of time at pre-assessment (C). Note that MEPs and MVCs increased after 10 sessions in the PCMS+exercise group and remained increased for 6 months compared with baseline but not in the sham-PCMS+exercise group. However, functional outcomes improved after 10 sessions of PCMS+exercise but did not persist 6 months later. Error bars indicate SDs, *P < 0.05.
Discussion
Our findings suggest that PCMS is an effective strategy to facilitate exercise-mediated recovery in humans with SCI. Note that we customized PCMS to target muscles with residual voluntary drive below the level of the injury in individuals with cervical, thoracic, and lumbar SCI, highlighting the clinical potential of this approach. We found that clinical functional outcomes improved to a similar extent after all protocols. However, the amplitude of corticospinal responses elicited by TMS and the magnitude of MVCs in targeted muscles increased after PCMS combined or not with exercise, but not after sham-PCMS combined with exercise. Behavioural and physiological effects were preserved for 6 months in the group receiving exercise combined with PCMS but not sham-PCMS. We argue that PCMS represents a strategy to boost residual corticospinal connections and preserve exercise-mediated recovery in humans with different degrees of paralysis and levels of SCI.
Exercise is one of the most common strategies used to rehabilitate humans with SCI (Harkema et al., 2012; Behrman et al., 2017). Here, we found that 10 sessions of upper- and lower-limb exercise improved fine and gross hand function and walking ability in individuals with chronic incomplete SCI. This is consistent with previous reports showing that a similar number of sessions of upper-limb (Yozbatiran et al., 2012; Francisco et al., 2017; Potter-Baker et al., 2018) and lower-limb (Benito et al., 2012; Jayaraman et al., 2013; Stone et al., 2018) exercise improved functional outcomes following SCI. Although it is difficult to compare the magnitude of our effects with those found in previous studies, because the duration, types of training, and characteristics of participants might differ, we were still able to identify some similarities. For example, our participants with incomplete cervical SCI improved gross and fine hand function scores by ∼20%, which is consistent with what has been reported after ∼10 sessions of robotic upper-limb training of a similar population (Francisco et al., 2017). Our participants with thoracic SCI decreased their walking speed by ∼18%, which is similar to what was found after ∼15 sessions of walking training in a similar population (Benito et al., 2012). Importantly, we found that 10 sessions of exercise were not sufficient to elicit changes in MEP amplitude and MVCs in any of the muscles tested. Previous studies have reported either changes (Hoffman and Field-Fote, 2007) or no changes (Belci et al., 2004) in electrophysiological outcomes after similar periods of training. One might expect that electrophysiological outcomes are more sensitive to detect changes after an intervention than clinical functional examinations, which use nominal scales. Indeed, studies agreed that electrophysiological outcomes are needed to increase the sensitivity of clinical examinations following SCI (Steeves et al., 2007; Ellaway et al., 2011; Macklin et al., 2016). Then, what is the origin of the changes in motor performance that we observed in the clinical examinations? Evidence shows that individuals with CNS damage, including those with SCI (Ardestani et al., 2019), improve movements through compensation or the use of alternative movements or effectors to accomplish the same goal (Kitago and Krakauer, 2013; Farris et al., 2015), which could have contributed to our results. The complexity of multi-joint movements during the hand motor tasks (Maier and Hepp-Reymond, 1995) tested in our clinical examinations makes it difficult to exclude the possibility that compensation contributed to our findings. Tasks involving grasping are complex because they include making contact with an object, requiring anticipation of the task constraints and control of the hand to position the fingers on specific locations for subsequent manipulations (Fu and Santello, 2011). The optimal control theory could be used to explain the ability of the human sensorimotor system to compensate for variability and uncertainty (Franklin and Wolpert, 2011), which could occur even though strict standardization procedures are used during functional exams. We favour the possibility that MEPs and MVCs did not change when exercise was combined with sham-PCMS because the exercise did not effectively engage the corticospinal pathway. This is supported by our results showing that when PCMS was combined with exercise both MEP and MVC outcomes increased after 10 sessions. Studies in animals (Courtine et al., 2009; Hill et al., 2009; McPherson et al., 2015) and humans (Rejc et al., 2017; Gad et al., 2018; Gill et al., 2018) suggest that functional effects of exercise can be augmented by increasing the likelihood of activating spared neural pathways by neural stimulation. We also found that clinical outcomes improved in the group receiving PCMS and sham-PCMS combined with exercise but the ability to perform maximal efforts or MVCs improved in the group only receiving PCMS. This agrees with evidence showing that the relationship between muscle strength and performance during daily life-like activities is not always linear in controls (Buchner et al., 1996) and patients with motor disorders (Eng, 2004; Kim, 2016). It is also possible that improvements in MVCs are not relevant when the functional task tested required less than maximal efforts. Note that during MVCs we measured EMG but not force. Concerns about comparing EMG amplitudes across days could have been overcome by reporting force values but the complex relationship between EMG and force output (Paquin and Power, 2018) highlights the need for future studies to include both outcomes. Importantly, changes in MVCs were preserved for 6 months in the group receiving exercise with PCMS but not sham-PCMS. This is consistent with results in stroke patients showing that neurostimulation can help to maintain the effects gained by exercise training (Levy et al., 2016). Although it is unclear if similar results would be found in the group receiving PCMS (these participants could not return for the follow-up session) the lack of these data does not affect our interpretation that PCMS contributed to augment exercise-mediated recovery. Because of the small number of subjects tested in the 6-month follow-up session these extended results need to be interpreted with caution. The use of a randomized complete block design allowed us to stratify subjects to have a better representation of participants across interventions (Piantadosi, 2017). Stratification procedures are recommended when there is a concern regarding the possibility of unequal distribution of important covariates between groups receiving an intervention (Lammertse et al., 2007). We acknowledge that the group receiving PCMS was not randomized (as the other groups) and hence may be susceptible to unobserved confounding variables. However, the fact that subjects across groups had similar demographics and residual function suggests that this confounder may contribute less to our effects.
Note that 10 sessions of PCMS with and without exercise resulted in similar increases in MEPs and MVCs. This is consistent with previous results supporting the use of PCMS to potentiate voluntary output after SCI (Bunday and Perez, 2012; Urbin et al., 2017). Consistent with recent results (Donges et al., 2019), we did not find an increase in MVCs after a single session of PCMS in any of the muscles tested (see Supplementary material) but we found that repeated PCMS sessions increased this outcome. PCMS likely engages LTP-like mechanisms that depend on NMDA receptor activity (Bi and Poo, 1998; Feldman, 2012). PCMS effects on corticospinal transmission in humans can be blocked by an NMDA antagonist (Donges et al., 2018) and potentiated by applying PCMS during tonic voluntary activity (Bunday et al., 2018). Thus, targeting spinal synapses by PCMS might represent a mechanism to engage residual corticospinal connections after the injury to improve functional recovery, consistent with our previous results (Bunday and Perez, 2012; Urbin et al., 2017).
Non-invasive neuromodulatory strategies used in humans with SCI in combination with exercise include electrical stimulation of the spinal cord (Harkema et al., 2011; Angeli et al., 2014; Gerasimenko et al., 2015) or a peripheral nerve (Beekhuizen and Field-Fote, 2008; Hoffman and Field-Fote, 2010, 2013; Gomes-Osman and Field-Fote, 2015a, b; Gad et al., 2018), operant conditioning (Thompson et al., 2013), and stimulation of the motor cortex (Belci et al., 2004). We used PCMS prior to exercise with the goal to facilitate and engage neuronal networks. This agrees with results showing that priming hand training with different forms of neuromodulation enhances motor performance in controls (Lotze et al., 2017) and in patients with motor disorders (Iyer et al., 2003). Nevertheless, the extent to which some of these other neuromodulatory approaches potentiate the effects of exercise still remains incompletely understood because only a few studies included a group that received exercise with sham neurostimulation (Belci et al., 2004; Gomes-Osman and Field-Fote, 2015,b). Another factor that makes the comparison across studies using neuromodulation difficult is the stimulus intensity. We used suprathreshold intensity for the peripheral nerve stimulation because we aimed to antidromically activate all motor neurons. The TMS intensity was 100% of the maximum stimulator output because higher TMS intensities result in larger descending volleys (Di Lazzaro et al., 2004). These parameters might increase the ability for eliciting spinal plasticity. This is consistent with results showing that increased amplitude and number of descending volleys by voluntary activity combined with supramaximal activation of motor neurons increased the after-effects of PCMS in humans with and without SCI (Bunday et al., 2018). Others have suggested that ‘priming’ of spinal networks to be more responsive to training can be done using subthreshold stimulation before and/or during the exercise (Taccola et al., 2018). The above threshold stimulation parameters used in our protocol make it difficult to implement PCMS while subjects performed exercise without perturbing the movement. Future studies need to consider different stimulation intensities used to elicit plasticity, which might vary according to the pathways targeted and the goal of the plasticity protocol. We argue that PCMS strengthens the connections between corticospinal neurons and motor neurons and increases motor output by enhancing synaptic plasticity (Taylor and Martin, 2009; Bunday and Perez, 2012). In contrast to other protocols, PCMS relies on the existence of a descending synaptic connection with motor neurons for its estimation.
Funding
National Institute of Neurological Disorders and Stroke (Grant Number: R01 NS090622-01), Department of Veterans Affairs (Grant Numbers: I01BX007080, I01RX002474).
Competing interests
The authors report no competing interests.
Supplementary Material
Glossary
- GRASSP =
Graded and Redefined Assessment of Strength, Sensibility and Prehension
- MEP =
motor evoked potential
- MVC =
maximal voluntary contraction
- PCMS =
paired corticospinal-motor neuronal stimulation
- SCI =
spinal cord injury
- TMS =
transcranial magnetic stimulation
References
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ.. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014; 137: 1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardestani MM, Henderson CE, Salehi SH, Mahtani GB, Schmit BD, Hornby TG.. Kinematic and neuromuscular adaptations in incomplete spinal cord injury after high- versus low-intensity locomotor training. J Neurotrauma 2019; 36: 2036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekhuizen KS, Field-Fote EC.. Sensory stimulation augments the effects of massed practice training in persons with tetraplegia. Arch Phys Med Rehabil 2008; 89: 602–8. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Ardolino EM, Harkema SJ.. Activity-based therapy: from basic science to clinical application for recovery after spinal cord injury. J Neurol Phys Ther 2017; 41 (Suppl 3): S39–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belci M, Catley M, Husain M, Frankel HL, Davey NJ.. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord 2004; 42: 417–9. [DOI] [PubMed] [Google Scholar]
- Benito J, Kumru H, Murillo N, Costa U, Medina J, Tormos JM, et al. Motor and gait improvement in patients with incomplete spinal cord injury induced by high-frequency repetitive transcranial magnetic stimulation. Top Spinal Cord Inj Rehabil 2012; 18: 106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GQ, Poo MM.. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 1998; 18: 10464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner DM, Larson EB, Wagner EH, Koepsell TD, de Lateur BJ.. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing 1996; 25: 386–91. [DOI] [PubMed] [Google Scholar]
- Bunday KL, Tazoe T, Rothwell JC, Perez MA.. Subcortical control of precision grip after human spinal cord injury. J Neurosci 2014; 34: 7341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Perez MA.. Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr Biol 2012; 22: 2355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Urbin MA, Perez MA.. Potentiating paired corticospinal-motoneuronal plasticity after spinal cord injury. Brain Stimul 2018; 11: 1083–92. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 2009; 12: 1333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, . et al. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 2004; 115: 255–66. [DOI] [PubMed] [Google Scholar]
- Donges SC, Boswell-Ruys CL, Butler JE, Taylor JL.. The effect of paired corticospinal-motoneuronal stimulation on maximal voluntary elbow flexion in cervical spinal cord injury: an experimental study. Spinal Cord 2019; 57: 796–804. [DOI] [PubMed] [Google Scholar]
- Donges SC, D’Amico JM, Butler JE, Taylor JL.. Involvement of N-methyl-d-aspartate receptors in plasticity induced by paired corticospinal-motoneuronal stimulation in humans. J Neurophysiol 2018; 119: 652–61. [DOI] [PubMed] [Google Scholar]
- Ellaway PH, Kuppuswamy A, Balasubramaniam AV, Maksimovic R, Gall A, Craggs MD, et al. Development of quantitative and sensitive assessments of physiological and functional outcome during recovery from spinal cord injury: a clinical initiative. Brain Res Bull 2011; 84: 343–57. [DOI] [PubMed] [Google Scholar]
- Eng JJ. Strength training in individuals with stroke. Physiother Can 2004; 56: 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris DJ, Hampton A, Lewek MD, Sawicki GS.. Revisiting the mechanics and energetics of walking in individuals with chronic hemiparesis following stroke: from individual limbs to lower limb joints. J Neuroeng Rehabil 2015; 12: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron 2012; 75: 556–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco GE, Yozbatiran N, Berliner J, O’Malley MK, Pehlivan AU, Kadivar Z, et al. Robot-assisted training of arm and hand movement shows functional improvements for incomplete cervical spinal cord injury. Am J Phys Med Rehabil 2017; 96(10 Suppl 1): S171–S7. [DOI] [PubMed] [Google Scholar]
- Franklin DW, Wolpert DM.. Computational mechanisms of sensorimotor control. Neuron 2011; 72: 425–42. [DOI] [PubMed] [Google Scholar]
- Fu Q, Santello M.. Towards a complete description of grasping kinematics: a framework for quantifying human grasping and manipulation. Conf Proc IEEE Eng Med Biol Soc 2011; 2011: 8247–50. [DOI] [PubMed] [Google Scholar]
- Gad P, Lee S, Terrafranca N, Zhong H, Turner A, Gerasimenko Y, et al. Non-invasive activation of cervical spinal networks after severe paralysis. J Neurotrauma 2018; 35: 2145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Lu DC, Modaber M, Zdunowski S, Gad P, Sayenko DG, et al. Noninvasive reactivation of motor descending control after paralysis. J Neurotrauma 2015; 32: 1968–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med 2018; 24: 1677–82. [DOI] [PubMed] [Google Scholar]
- Gomes-Osman J, Field-Fote EC.. Cortical vs. afferent stimulation as an adjunct to functional task practice training: a randomized, comparative pilot study in people with cervical spinal cord injury. Clin Rehabil 2015a; 29: 771–82. [DOI] [PubMed] [Google Scholar]
- Gomes-Osman J, Field-Fote EC.. Improvements in hand function in adults with chronic tetraplegia following a multiday 10-Hz repetitive transcranial magnetic stimulation intervention combined with repetitive task practice. J Neurol Phys Ther 2015b; 39: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 2011; 377: 1938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL.. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil 2012; 93: 1508–17. [DOI] [PubMed] [Google Scholar]
- Hill RL, Zhang YP, Burke DA, Devries WH, Zhang Y, Magnuson DS, et al. Anatomical and functional outcomes following a precise, graded, dorsal laceration spinal cord injury in C57BL/6 mice. J Neurotrauma 2009; 26: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L, Field-Fote E.. Effects of practice combined with somatosensory or motor stimulation on hand function in persons with spinal cord injury. Top Spinal Cord Inj Rehabil 2013; 19: 288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman LR, Field-Fote EC.. Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: a case report. Phys Ther 2007; 87: 208–23. [DOI] [PubMed] [Google Scholar]
- Hoffman LR, Field-Fote EC.. Functional and corticomotor changes in individuals with tetraplegia following unimanual or bimanual massed practice training with somatosensory stimulation: a pilot study. J Neurol Phys Ther 2010; 34: 193–201. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Schleper N, Wassermann EM.. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci 2003; 23: 10867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman A, Thompson CK, Rymer WZ, Hornby TG.. Short-term maximal-intensity resistance training increases volitional function and strength in chronic incomplete spinal cord injury: a pilot study. J Neurol Phys Ther 2013; 37: 112–7. [DOI] [PubMed] [Google Scholar]
- Jones LAT, Bryden A, Wheeler TL, Tansey KE, Anderson KD, Beattie MS, et al. Considerations and recommendations for selection and utilization of upper extremity clinical outcome assessments in human spinal cord injury trials. Spinal Cord 2018; 56: 414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. The effects of hand strength on upper extremity function and activities of daily living in stroke patients, with a focus on right hemiplegia. J Phys Ther Sci 2016; 28: 2565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitago T, Krakauer JW.. Motor learning principles for neurorehabilitation. Handb Clin Neurol 2013; 110: 93–103. [DOI] [PubMed] [Google Scholar]
- Knikou M. Neural control of locomotion and training-induced plasticity after spinal and cerebral lesions. Clin Neurophysiol 2010; 121: 1655–68. [DOI] [PubMed] [Google Scholar]
- Lammertse DTuszynski MH, Steeves JD, Curt A, Fawcett JW, Rask C, . et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord 2007; 45: 232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RM, Harvey RL, Kissela BM, Winstein CJ, Lutsep HL, Parrish TB, et al. Epidural electrical stimulation for stroke rehabilitation: results of the prospective, multicenter, randomized, single-blinded everest trial. Neurorehabil Neural Repair 2016; 30: 107–19. [DOI] [PubMed] [Google Scholar]
- Lotze M, Ladda AM, Roschka S, Platz T, Dinse HR.. Priming hand motor training with repetitive stimulation of the fingertips; performance gain and functional imaging of training effects. Brain Stimul 2017; 10: 139–46. [DOI] [PubMed] [Google Scholar]
- Macklin RA, Brooke VJ, Calabro FJ, Ellaway PH, Perez MA.. Discrepancies between clinical assessments of sensory function and electrical perceptual thresholds after incomplete chronic cervical spinal cord injury. Spinal Cord 2016; 54: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier MA, Hepp-Reymond MC.. EMG activation patterns during force production in precision grip. I. Contribution of 15 finger muscles to isometric force. Exp Brain Res 1995; 103: 108–22. [DOI] [PubMed] [Google Scholar]
- Manuel M, Li Y, Elbasiouny SM, Murray K, Griener A, Heckman CJ, et al. NMDA induces persistent inward and outward currents that cause rhythmic bursting in adult rodent motoneurons. J Neurophysiol 2012; 108: 2991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 2003; 26 (Suppl 1): S50–6. [DOI] [PubMed] [Google Scholar]
- McPherson JG, Miller RR, Perlmutter SI.. Targeted, activity-dependent spinal stimulation produces long-lasting motor recovery in chronic cervical spinal cord injury. Proc Natl Acad Sci USA 2015; 112: 12193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin J, Power GA.. History dependence of the EMG-torque relationship. J Electromyogr Kinesiol 2018; 41: 109–15. [DOI] [PubMed] [Google Scholar]
- Piantadosi S. Clinical trials: a methodologic perspective. 3rd edn. Hoboken, NJ: Wiley; 2017. [Google Scholar]
- Potter-Baker KA, Janini DP, Lin YL, Sankarasubramanian V, Cunningham DA, Varnerin NM, et al. Transcranial direct current stimulation (tDCS) paired with massed practice training to promote adaptive plasticity and motor recovery in chronic incomplete tetraplegia: a pilot study. J Spinal Cord Med 2018; 41: 503–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejc E, Angeli CA, Atkinson D, Harkema SJ.. Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci Rep 2017; 7: 13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayenko DG, Atkinson DA, Floyd TC, Gorodnichev RM, Moshonkina TR, Harkema SJ, et al. Effects of paired transcutaneous electrical stimulation delivered at single and dual sites over lumbosacral spinal cord. Neurosci Lett 2015; 609: 229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 2007; 45: 206–21. [DOI] [PubMed] [Google Scholar]
- Stone WJ, Stevens SL, Fuller DK, Caputo JL.. Strength and step activity after eccentric resistance training in those with incomplete spinal cord injuries. Top Spinal Cord Inj Rehabil 2018; 24: 343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccola GSayenko D, Gad P, Gerasimenko Y, Edgerton VR. . And yet it moves: Recovery of volitional control after spinal cord injury. Prog Neurobiol 2018; 160: 64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Martin PG.. Voluntary motor output is altered by spike-timing-dependent changes in the human corticospinal pathway. J Neurosci 2009; 29: 11708–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Pomerantz FR, Wolpaw JR.. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci 2013; 33: 2365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbin MA, Ozdemir RA, Tazoe T, Perez MA.. Spike-timing-dependent plasticity in lower-limb motoneurons after human spinal cord injury. J Neurophysiol 2017; 118: 2171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yozbatiran N, Berliner J, O’Malley MK, Pehlivan AU, Kadivar Z, Boake C, et al. Robotic training and clinical assessment of upper extremity movements after spinal cord injury: a single case report. J Rehabil Med 2012; 44: 186–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.