Abstract
Background
Perihilar cholangiocarcinoma (PHC) often requires extensive surgery which is associated with substantial morbidity and mortality. This study aimed to compare an Eastern and Western PHC cohort in terms of patient characteristics, treatment strategies and outcomes including a propensity score matched analysis.
Methods
All consecutive patients who underwent combined biliary and liver resection for PHC between 2005 and 2016 at two Western and one Eastern center were included. The overall perioperative and long-term outcomes of the cohorts were compared and a propensity score matched analysis was performed to compare perioperative outcomes.
Results
A total of 210 Western patients were compared to 164 Eastern patients. Western patients had inferior survival compared to the East (hazard-ratio 1.72 (1–23-2.40) P < 0.001) corrected for age, ASA score, tumor stage and margin status. After propensity score matching, liver failure rate, morbidity, and mortality were similar. There was more biliary leakage (38% versus 13%, p=0.015) in the West
Discussion
There were major differences in patient characteristics, treatment strategies, perioperative outcomes and survival between Eastern and Western PHC cohorts. Future studies should focus whether these findings are due to the differences in the treatment or the disease itself.
Introduction
Perihilar cholangiocarcinoma (PHC) is a rare biliary malignancy that arises in the proximal ducts between the segmental bile ducts and cystic duct.(1) Due to this location, obstructive cholestasis is frequent in these patients, as is involvement of the portal vein (branches) or hepatic artery.(1, 2) These factors, among others, render PHC resectable in only 10–20% of patients, and radical resection mostly necessitates combined biliary and extended hepatic resection.(1, 2) These procedures are associated with high morbidity and mortality rates, but offer the only chance for long term survival.(1)
What stands out in literature are the differences in reported treatment and outcomes between Western (e.g. Europe, Northern America) and Eastern centers (e.g. China, Japan). The incidence of cholangiocarcinoma in Asian countries exceeds that in Western countries.(3, 4) When considering differences in treatment, preoperative nasobiliary drainage and preoperative portal vein embolization are widely used in the East, but not or only sparsely in the West.(5–8) Despite the different use of PVE, the incidence of postoperative liver failure seems to be similar in Eastern and Western centers with incidences of 22–33%.(7–9) The disease-associated and overall mortality is however usually higher in the West with reported 90-day mortality of 10–18%,(10–13) as compared to 0–5% in the East.(14–17) These large differences hamper the comparability of the series in terms of outcomes.
Besides these variable outcomes, patients’ and disease characteristics, inclusion criteria as well as the applied treatment strategies vary widely between Eastern and Western centers.
The aim of this study was to compare all aspects of a major Western series of patients who underwent resection for PHC in two units with a major cohort from a specialized Eastern center. Baseline and treatment characteristics were compared. Secondly, the differences in short term postoperative outcomes were compared in a propensity score matched analysis. Finally, an overall survival comparison between the Eastern and Western cohorts was performed.
Methods
All consecutive patients who underwent combined biliary and liver resection for perihilar cholangiocarcinoma between 2005 and 2016 were included at three major hepatobiliary centers. The participating centers were Memorial Sloan Kettering Cancer Center, New York, NY and the Academic Medical Center in Amsterdam the Netherlands, which were defined as Western centers. The third center was Hokkaido University Graduate School of Medicine in Sapporo, Japan and was defined as the Eastern center.
Inclusion criteria were curative intent resection of presumed perihilar malignancy defined as a biliary mass or stricture originating from the bile ducts between the segmental bile ducts and cystic duct. Patients who had undergone extrahepatic bile duct resection without liver resection were excluded.
All data was retrospectively collected from a prospectively maintained database. Additional data was collected from the electronic medical records. The need for ethical approval was waived by the respective institutional review boards, as was the need for individual informed consent.
Variables
Preoperative cholangitis was defined as an episode of fever, leukocytosis or elevated C-reactive protein, and malaise requiring additional biliary drainage before resection.(18, 19) All complications within 30 days after surgery were scored and graded according to Dindo et al, with all events classified as grade IIIa or higher defined as major complications.(20) The incidence of postoperative liver failure, biliary leakage and postoperative hemorrhage were scored and graded according to the respective criteria of the International Study Group of Liver Surgery (ISGLS).(21–23) Mortality was defined as death within 90 days after surgery. Negative resection margin was defined as no residual tumor at the margin as reported by the pathologist at final pathology, regardless of the tumor distance to the surgical margin.
Patient workup – West
In patients with obstructive jaundice preoperative biliary drainage of the FLR was performed using either endoscopic or percutaneous biliary drainage. Additional drainage was performed in the presence of persistent cholestasis or following cholangitis. Portal vein embolization was considered if FLR volume share was below 40%. No ICG retention testing was performed, however hepatobiliary scintigraphy as functional test has become part of standard practice in Amsterdam.(24) The CUSA was used for dissection of the parenchyma, with or without intermittent Pringle maneuver at the discretion of the attending surgeon using ‘20 min on – 10 min off’ intervals.
Patient workup – East
Biliary drainage was performed in jaundiced patients and following cholangitis with the aim to reduce bilirubin below 34 μmol/L. Endoscopic nasobiliary drainage was the usual initial drainage technique for the FLR. Portal vein embolization was considered in patients scheduled for (extended) right or extended left hepatectomy. Following PVE patients proceeded to hepatectomy when FLR volume exceeded 30% and the indocyanine green retention rate (at 15 min) was below 15%. Routine lymphadenectomy of the hepatoduodenal ligament was performed in all patients. When required, portal vein reconstruction was completed before hepatic transection. Forceps clamp crushing was used for parenchymal transection using intermittent Pringle maneuver of ‘15 min on – 5 min off’ intervals.(25)
Statistical analysis
Categorical variables were displayed as number with percentages and differences between categorical variables were tested using chi-square or Fisher’s exact tests. Continuous variables were displayed as median with inter-quartile-range (IQR) and differences between these variables were tested using Mann-Whitney tests. Propensity score matching was performed using the psmatching plugin for SPSS using the essential for R extension. Nearest neighbor matching (1:1) was performed (caliper 0.2) using the variables age, sex, ASA score, jaundice at presentation, biliary drainage (dichotomized to yes/no), preoperative cholangitis, preoperative bilirubin level, type of liver resection, portal vein reconstruction, hepatic artery reconstruction, and FLR volume share. Survival curves were generated using the Kaplan Meier method. Kaplan Meier curves were compared using the log-rank test. Multivariable analysis for survival was performed using Cox proportional hazards regression analysis. Full case analysis was used in the multivariable analysis, alternatively multiple imputations was used which yielded similar results. All statistical analysis were performed using SPSS (version 23.0, IBM, Chicago, IL).
Results
Overall comparison of cohorts
A total of 164 consecutive patients underwent resection of perihilar cholangiocarcinoma at Hokkaido University in Sapporo, Japan, between January 2005 and December 2016 (East). In the same period 210 patients underwent resection of perihilar cholangiocarcinoma at the Academic Medical Center in Amsterdam, the Netherlands and Memorial Sloan Kettering Cancer Center in New York, US (West). The differences between the two Western centers are illustrated in table S1 and S2.
Baseline characteristics of the Eastern and Western series are provided in table 1. The more frequent biliary drainage in unjaundiced patients in the East (78% 64/82 versus 28% 27/56, P < 0.001), likely accounts for the increased rate of cholangitis observed in these patients in the East (32% 26/82 versus 13% 7/54, P = 0.014). Consequently, the increased cholangitis rate likely accounts for the higher incidence of liver failure in jaundiced patients in the East (23% 19/82 versus 7% 4/55, P = 0.0188)
Table 1:
Baseline and operative characteristics
| West (n=210) | East (n=164) | P-value | |
|---|---|---|---|
| Age, median (IQR) | 65 (56–71) | 68 (64–73) | < 0.001 |
| Male gender, n (%) | 141 (67) | 116 (71) | 0.501 |
| BMI, kg/m2, median (IQR) | 25 (23–28) | 22 (21–24) | < 0.001 |
|
ASA score,
n (%)
1 2 3 4 |
32 (15) 116 (55) 60 (29) 2 (1) |
28 (17) 127 (77) 9 (6) 0 (0) |
< 0.001 |
| Bilirubin at presentation, μmol/L, median (IQR) | 68 (19–197) | 57 (27–144) | 0.962 |
| Jaundice at presentation, n (%) | 154 (73) | 82 (50) | < 0.001 |
|
Directly preoperative bilirubin, μmol/L, median
(IQR) |
15 (9–32) | 14 (10–21) | 0.170 |
|
Preoperative biliary drainage,
n (%)
None Percutaneous Endoscopic Both |
40 (19) 29 (14) 84 (40) 57 (27) |
20 (12) 14 (9) 115 (70) 15 (9) |
< 0.001 |
| Preoperative cholangitis, n (%) | 68 (32) | 65 (40) | 0.193 |
| Total liver volume, mL, median (IQR) | 1863 (1539–2112) | 1147 (1016–1310) | < 0.001 |
| Future liver remnant volume, mL, median (IQR) | 956 (650–1318) | 583 (490–723) | < 0.001 |
| Future liver remnant share, %, median (IQR) | 50 (35–74) | 49 (43–63) | 0.823 |
| Portal vein embolization, n (%) | 14 (7) | 90 (55) | < 0.001 |
|
Type of resection,
n (%)
Left hemihepatectomy Extended left hemihepatectomy Right hemihepatectomy Extended right hemihepatectomy Central hepatectomy or segmentectomy |
81 (39) 20 (10) 43 (21) 60 (29) 6 (3) |
54 (33) 12 (7) 91 (56) 5 (3) 2 (1) |
< 0.001 |
| Portal vein reconstruction, n (%) | 42 (20) | 93 (57) | < 0.001 |
| Hepatic artery reconstruction, n (%) | 0 (0) | 18 (11) | < 0.001 |
Operative outcomes of the Eastern and Western cohorts are shown in table 2.
Table 2:
Outcomes
| West (n=210) | East (n=164) | P-value | |
|---|---|---|---|
| Estimated blood loss, mL, median (IQR) | 1150 (600–2225) | 1795 (1302–2654) | < 0.001 |
| Margin, R0, n (%) | 164 (78) | 135 (82) | 0.363 |
|
Final pathology,
n (%)
Perihilar cholangiocarcinoma Other malignancy IPNB Benign |
184 (88) 10 (5) 8 (4) 8 (4) |
155 (95) 9 (6) 0 (0) 0 (0) |
0.004 |
|
Harvested lymph nodes,
median (IQR)
Total Tumor positive |
5 (3–7) 0 (0–1) |
12 (9–16) 0 (0–2) |
< 0.001 0.012 |
| Tumor size, cm, median (IQR) | 3.0 (1.9–4.0) | 2.5 (2.0–3.5) | 0.390 |
| Perineural invasion, n (%) | 113 (66) | 116 (82) | < 0.001 |
| Lymfvascular invasion, n (%) | 50 (29) | 61 (43) | <0.001 |
|
AJCC 7th
edition stage
(PHC only)
0 I II IIIA IIIB IVA IVB |
(n=181)
14 (8) 8 (4) 59 (33) 21 (12) 46 (25) 29 (16) 4 (2) |
(n=131)
1 (1) 9 (7) 45 (34) 8 (6) 38 (29) 24 (18) 6 (5) |
0.043 |
| Morbidity, Dindo grade III or higher, n (%) | 113 (54) | 79 (48) | 0.297 |
| Liver failure, ISGLS grade B/C, n (%) | 43 (21) | 43 (26) | 0.217 |
|
Biliary leakage, ISGLS grade B/C, n (%)
Grade A Grade B Grade C |
79 (38) 18 (7) 72 (34) 7 (3) |
30 (18) 6 (4) 28 (17) 2 (1) |
< 0.001 |
| Hemorrhage, ISGLS grade B/C, n (%) | 14 (7) | 23 (14) | 0.023 |
| 90-day mortality | 28 (13) | 12 (7) | 0.066 |
Overall and recurrence-free survival comparison of patients with perihilar cholangiocarcinoma
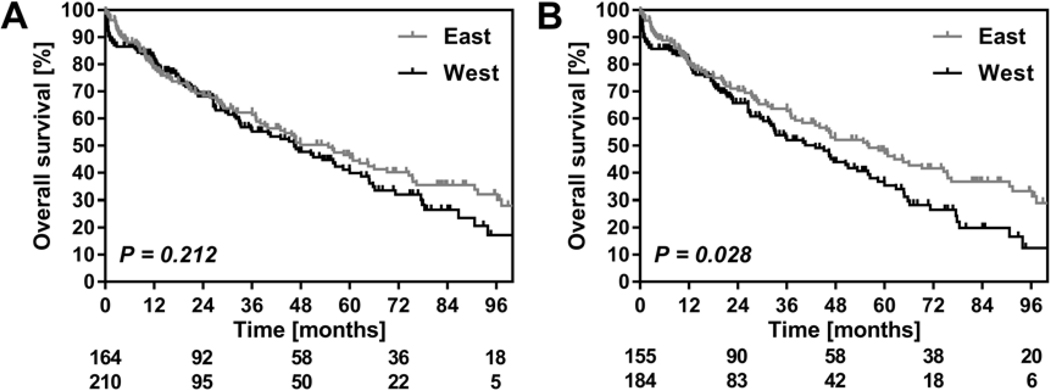
Median follow up was 55 (95%CI 48–62) months in the entire cohort. Median overall survival in the entire Western cohort was 46 (95%CI 33–60) months and 5-year survival was 41% (Figure 1A). In the East, median overall survival was 54 (95%CI 41–69) months, with 47% 5-year survival (P = 0.212). In patients with confirmed diagnosis of PHC, median overall survival was 43 (95%CI 32–53) months in the West versus 56 (42–70) months in the East (P = 0.028, Figure 1B). A multivariable analysis to identify factors associated with survival was performed in patients with a definitive pathological diagnosis of PHC as is shown in table 3.
Figure 1:
(A) Overall survival comparison in the all-inclusive Eastern and Western cohorts. Curves were generated using the Kaplan-Meier methods. Depicted below the graph is the number of patients at risk with the Eastern cohort above the Western cohort. (B) Overall survival in patients with confirmed perihilar cholangiocarcinoma at final pathology and depicted below the graph is the number of patients at risk with the Eastern cohort above the Western cohort. All curves were compared using the Log-Rank test.
Table 3:
Multivariable analysis for survival in patients with perihilar cholangiocarcinoma at pathology
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Hazard ratio (95%CI) | P-Value | Hazard ratio (95%CI | P-Value | |
| Age (continuous) | 0.99 (0.98–1.01) | 0.291 | 1.00 (0.99–1.02) | 0.718 |
| ASA ≥ 2 | 1.00 (0.67–1.51) | 0.990 | 1.16 (0.74–1.82) | 0.517 |
|
AJCC stage
7th edition
0-I II-IIIA IIIB IVA-IVB |
indicator 2.46 (1.06–5.70) 6.06 (2.60–12.14) 5.74 (2.44–13.51) |
0.036 < 0.001 < 0.001 |
indicator 2.50 (1.07–5.81) 6.48 (2.77–15.20) 5.87 (2.44–14/07) |
0.033 < 0.001 < 0.001 |
| Positive margin | 1.25 (0.87–1.78) | 0.223 | 1.09 (0.73–1.62) | 0.670 |
| Western Center | 1.40 (1.03–1.88) | 0.029 | 1.72 (1.23–2.40) | 0.001 |
Propensity score matched analysis – no differences in perioperative outcomes
After propensity score matching two homogenous cohorts of 45 patients were generated (Table 4).
Table 4:
Propensity score matched comparison of perioperative outcomes
| West (n=45) | East (n=45) | ||
|---|---|---|---|
| Age, median (IQR) | 69 (60–76) | 66 (64–73) | 0.824 |
| Male gender, n | 28 | 34 | 0.255 |
|
ASA score,
n
1 2 3 |
8 33 4 |
6 33 6 |
0.710 |
| Jaundice at presentation, n | 17 | 15 | 0.826 |
| Directly preoperative bilirubin, μmol/L, median (IQR) | 12 (9–21) | 15 (10–22) | 0.445 |
|
Preoperative biliary drainage, n
None Percutaneous Endoscopic Both |
11 8 14 12 |
9 2 32 2 |
< 0.001 |
| Preoperative cholangitis, n | 14 | 17 | 0.658 |
| Future live remnant share, %, median (IQR) | 64 (40–76) | 60 (48–69) | 0.939 |
| Portal vein embolization, n | 6 | 8 | 0.772 |
|
Type of resection, n
Left hemihepatectomy Extended left hemihepatectomy Right hemihepatectomy Extended right hemihepatectomy |
26 3 13 3 |
27 2 13 3 |
0.974 |
| Portal vein reconstruction, n | 13 | 12 | 1.000 |
| Operative time, min, median (IQR) | 447 (394–564) | 639 (553–749) | < 0.001 |
| Estimated blood loss, mL, median (IQR) | 1280 (500–2422) | 1610 (1310–2470) | 0.013 |
| Margin, R0, n | 29 | 37 | 0.094 |
|
Final pathology,
n
Perihilar cholangiocarcinoma IPNB Benign |
41 3 1 |
45 - - |
0.123 |
| Morbidity, Dindo grade III or higher, n | 25 | 19 | 0.292 |
| Liver failure, ISGLS grade B/C, n | 7 | 10 | 0.591 |
| Biliary leakage, ISGLS grade B/C, n | 17 | 6 | 0.015 |
| Hemorrhage, ISGLS grade B/C, n | 5 | 0.714 | |
| 90-day mortality, n | 3 | 4 | 1.000 |
Discussion
This study illustrates the large overall differences in baseline characteristics, treatment, and clinical outcomes between an Eastern and Western cohort of patients undergoing combined hilar and liver resections for PHC. In the present multivariable analysis, patients in the Western cohort had inferior survival compared to Eastern patients when corrected for age, ASA score, AJCC stage, and resection margin. In a propensity score matched comparison correcting for factors that influence short-term postoperative outcomes overall morbidity was similar in both cohorts, as was the incidence of liver failure and 90-day mortality.
One of the most remarkable differences in the preoperative workup between the cohorts was the difference in the usage of PVE, which was applied in 55% of the Eastern patients while only in 7% of Western patients.(24) Notably, preoperative FLR volume shares were ultimately comparable between cohorts, despite more extended resections in the West. The incidence of postoperative liver failure was also comparable between cohorts. The greater number of vascular resections in the Eastern cohort, a known risk factor for local complications and liver failure, might partially account for these contradictory results, along with the greater blood loss in the Eastern center which has also been identified as a risk-factor for liver failure.(26) This hypothesis is however not supported by the comparable incidence of liver failure in the propensity matched cohort, where vascular resections were similar between groups, although blood loss remained greater In the Eastern center.
The second, repeatedly renewed point of discussion is the different approach to biliary drainage in the Eastern and the Western centers. Several studies support selective drainage policies as the benefits of biliary drainage likely not outweigh the drawbacks in patients with large remnant livers (>50%).(11, 27) Despite less jaundiced patients in the East (73% vs. 50%), biliary drainage was performed in similar proportions (88% vs 81%) associated with similar cholangitis rates (40% vs. 32%). The statistics are further complicated by the different policies for biliary drainage: In the East, primary endoscopic nasobiliary drainage is performed whereas in the West, endoscopic stenting is usually undertaken first with larger rates of additional percutaneous drainage. As reported previously,(27) biliary drainage should likely be avoided in unjaundiced patients and patients with a large FLR (>50%) in order to avoid cholangitis.
There was more (grade B/C) biliary leakage in the Western cohort, and since grade A leakage was not more frequent in the East, this is not likely due to the used definition or classification of biliary leakage. In the West the biliodigestive anastomoses were only drained in case of an indwelling preoperative PTBD catheter. In the East, the anastomoses were routinely drained which might also account for the difference in biliary leakage rates although it remains uncertain if transanastomic drainage really protects the hepaticojejunostomy. On the other hand, external biliary drainage likely increases the risk of liver failure.(7)
Perhaps one of the most relevant outcome parameters is perioperative mortality which was 13% in the West compared to 7% in the East in the unmatched cohort. However, in the propensity score matched comparison, perioperative mortality was similar between cohorts (i.e. 7% vs 9%). Notably, the procedures in the East took longer time with more blood loss, which are both known factors associated with adverse outcomes. However, this did not translate into more frequent adverse outcomes.(28)
Besides the differences in patient characteristics and management, the higher incidence of cholangiocarcinoma in the East raises the question whether the disease in the Eastern population can be compared to the disease in the West. The present multivariable analysis for survival identified a survival benefit for Eastern over Western patients, corrected for age, ASA score, cancer stage, and margin status. These results suggest that the disease entity in the West might be more aggressive compared to its Eastern counterpart. A previously reported, smaller comparative study did not find any survival difference between a Western and Eastern cohort of resectable PHC, however, median follow-up in that report was only 20 months compared to the current 55 months in the present series. Furthermore, only disease specific analyses were performed in the former study with cohorts containing major differences in tumor characteristics and margin status which all potentially confound the result and even the tumor biology of PHC may be different across the different parts of the world. In addition, lymph node dissection was more extended in Eastern patients. While the proportion of positive nodes was similar, it cannot be excluded that more extended lymph node dissection in the West would result in more patients with lymph node positive tumor stage, which could alter the overall survival comparison. Although more elaborate dissection might improve prognostication, a benefit in survival itself is unlikely.(29)
The current study has limitations, first of all the restrospective design is subject to selection bias. However, all consecutive patients were included since 2005, resulting in a more homogenous cohort compared to most reports including patients over a longer period while treatment strategy had continued to evolve over time. Also the median follow-up in the current study was substantially longer compared to previous reports.(15) Only one Eastern center was included and ideally this report should be followed-up by a larger multicenter effort across regions. Although a prospective design would be ideal, the very low incidence of cholangiocarcinoma renders a retrospective design more feasible. Also, there are some differences between the Westen centers, however, these are less pronounced compared to the differences with the Eastern cohort. In addition, this collaborative Westen cohort has been previously used in multiple reports and risk-score analyses.(9, 27, 30–32) Adjuvant chemotherapy was not included in the analyses since only 4% of patients received adjuvant therapy, not allowing for any valid conclusions.
In conclusion, the current report identified major differences in almost all aspects of management of PHC between patient cohorts accrued in two Western and one Eastern center specialized in hepatobiliary surgery. The cohorts were different in patient characteristics while in the preoperative work-up, distinct strategies were employed. In the matched cohorts, outcomes were comparable except for a higher incidence of postoperative biliary leakage in the West. The higher mortality often reported in the West over the East might be due to the more extended resections unertaken in the West. The Eastern cohort of patients with PHC showed a survival benefit over their Western counterparts on multivariable analysis. This appears not to be due to inferior oncologic resections in the West, since parameters such as margin status were similar. Future studies should confirm whether all reported differences between Eastern and Western centers are due to treatment, or whether there might be differences in tumor types or disease biology. The current report further highlights the importance of external validation of PHC research results worldwide.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005. October 8;366(9493):1303–14. [DOI] [PubMed] [Google Scholar]
- 2.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996. October;224(4):463–73; discussion 73–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015. April;29(2):221–32. [DOI] [PubMed] [Google Scholar]
- 4.Kirstein MM, Vogel A. Epidemiology and Risk Factors of Cholangiocarcinoma. Visc Med. 2016. December;32(6):395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakami H, Kuwatani M, Onodera M, Haba S, Eto K, Ehira N, et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol. 2011. February;46(2):242–8. [DOI] [PubMed] [Google Scholar]
- 6.Kawashima H, Itoh A, Ohno E, Itoh Y, Ebata T, Nagino M, et al. Preoperative endoscopic nasobiliary drainage in 164 consecutive patients with suspected perihilar cholangiocarcinoma: a retrospective study of efficacy and risk factors related to complications. Ann Surg. 2013. January;257(1):121–7. [DOI] [PubMed] [Google Scholar]
- 7.Olthof PB, Coelen RJ, Wiggers JK, Besselink MG, Busch OR, van Gulik TM. External biliary drainage following major liver resection for perihilar cholangiocarcinoma: impact on development of liver failure and biliary leakage. HPB (Oxford). 2016. April;18(4):348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, Yamaguchi J, et al. The Predictive Value of Indocyanine Green Clearance in Future Liver Remnant for Posthepatectomy Liver Failure Following Hepatectomy with Extrahepatic Bile Duct Resection. World J Surg. 2016. June;40(6):1440–7. [DOI] [PubMed] [Google Scholar]
- 9.Olthof PB, Wiggers JK, Groot Koerkamp B, Coelen RJ, Allen PJ, Besselink MG, et al. Postoperative Liver Failure Risk Score: Identifying Patients with Resectable Perihilar Cholangiocarcinoma Who Can Benefit from Portal Vein Embolization. J Am Coll Surg. 2017. September;225(3):387–94. [DOI] [PubMed] [Google Scholar]
- 10.Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012. January;147(1):26–34. [DOI] [PubMed] [Google Scholar]
- 11.Farges O, Regimbeau JM, Fuks D, Le Treut YP, Cherqui D, Bachellier P, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013. January;100(2):274–83. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser GM, Paul A, Sgourakis G, Molmenti EP, Dechene A, Trarbach T, et al. Novel prognostic scoring system after surgery for Klatskin tumor. Am Surg. 2013. January;79(1):90–5. [PubMed] [Google Scholar]
- 13.Coelen RJ, Olthof PB, van Dieren S, Besselink MG, Busch OR, van Gulik TM. External Validation of the Estimation of Physiologic Ability and Surgical Stress (E-PASS) Risk Model to Predict Operative Risk in Perihilar Cholangiocarcinoma. JAMA Surg. 2016. December 1;151(12):1132–8. [DOI] [PubMed] [Google Scholar]
- 14.Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013. July;258(1):129–40. [DOI] [PubMed] [Google Scholar]
- 15.Kimura N, Young AL, Toyoki Y, Wyatt JI, Toogood GJ, Hidalgo E, et al. Radical operation for hilar cholangiocarcinoma in comparable Eastern and Western centers: Outcome analysis and prognostic factors. Surgery. 2017. September;162(3):500–14. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003. July;238(1):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006. August;244(2):240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Gaag NA, de Castro SM, Rauws EA, Bruno MJ, van Eijck CH, Kuipers EJ, et al. Preoperative biliary drainage for periampullary tumors causing obstructive jaundice; DRainage vs. (direct) OPeration (DROP-trial). BMC Surg. 2007. March 12;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiggers JK, Coelen RJ, Rauws EA, van Delden OM, van Eijck CH, de Jonge J, et al. Preoperative endoscopic versus percutaneous transhepatic biliary drainage in potentially resectable perihilar cholangiocarcinoma (DRAINAGE trial): design and rationale of a randomized controlled trial. BMC Gastroenterol. 2015. February 14;15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. August;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011. May;149(5):713–24. [DOI] [PubMed] [Google Scholar]
- 22.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011. May;149(5):680–8. [DOI] [PubMed] [Google Scholar]
- 23.Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford). 2011. August;13(8):528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olthof PB, Coelen RJS, Bennink RJ, Heger M, Lam MF, Besselink MG, et al. (99m)Tc-mebrofenin hepatobiliary scintigraphy predicts liver failure following major liver resection for perihilar cholangiocarcinoma. HPB (Oxford). 2017. October;19(10):850–8. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi Y, Tsuchikawa T, Okamura K, Nakamura T, Tamoto E, Noji T, et al. Risk factors for a high Comprehensive Complication Index score after major hepatectomy for biliary cancer: a study of 229 patients at a single institution. HPB (Oxford). 2016. September;18(9):735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sultana A, Brooke-Smith M, Ullah S, Figueras J, Rees M, Vauthey JN, et al. Prospective evaluation of the International Study Group for Liver Surgery definition of post hepatectomy liver failure after liver resection: an international multicentre study. HPB (Oxford). 2017. December 26. [DOI] [PubMed] [Google Scholar]
- 27.Wiggers JK, Groot Koerkamp B, Cieslak KP, Doussot A, van Klaveren D, Allen PJ, et al. Postoperative Mortality after Liver Resection for Perihilar Cholangiocarcinoma: Development of a Risk Score and Importance of Biliary Drainage of the Future Liver Remnant. J Am Coll Surg. 2016. August;223(2):321–31 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virani S, Michaelson JS, Hutter MM, Lancaster RT, Warshaw AL, Henderson WG, et al. Morbidity and mortality after liver resection: results of the patient safety in surgery study. J Am Coll Surg. 2007. June;204(6):1284–92. [DOI] [PubMed] [Google Scholar]
- 29.Kambakamba P, Linecker M, Slankamenac K, DeOliveira ML. Lymph node dissection in resectable perihilar cholangiocarcinoma: a systematic review. Am J Surg. 2015. October;210(4):694–701. [DOI] [PubMed] [Google Scholar]
- 30.Groot Koerkamp B, Wiggers JK, Gonen M, Doussot A, Allen PJ, Besselink MG, et al. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol. 2015. September;26(9):1930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groot Koerkamp B, Wiggers JK, Allen PJ, Besselink MG, Blumgart LH, Busch OR, et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J Am Coll Surg. 2015. December;221(6):1041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiggers JK, Groot Koerkamp B, Coelen RJ, Doussot A, van Dieren S, Rauws EA, et al. Percutaneous Preoperative Biliary Drainage for Resectable Perihilar Cholangiocarcinoma: No Association with Survival and No Increase in Seeding Metastases. Ann Surg Oncol. 2015. December;22 Suppl 3:S1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.