Abstract
Upon receiving injury signals, neurons can activate various pathways to reduce harm, initiate neuroprotection, and repair damaged neurite without cell death. Here, we review recent progresses in the study of neurite repair focusing on neuronal cell-autonomous mechanisms, including new findings on ion channels that serve as key regulators to initiate neurite repair and intrinsic signaling pathways and transcriptional and post-transcriptional factors that facilitate neurite repair. We also touch upon reports on how dendrites may be affected upon axotomy and how the regeneration potential in injured neurites might be maximized.
Introduction
There are many types of injury or insults that can cause damages to neurons such as traumatic brain injury (TBI), spinal cord injury (SCI), neurological diseases, optic nerve injury, stroke, and ischemia [1–4]. How to respond to these insults and repair damages is a lifelong task for maintaining the mature nervous system functions. Depending on the types and the degree of insults, neurons have a variety of strategies to deal with them [2].
The process of neurite repair includes regrowth of neurites, formation of new synapses, and re-association of regenerated neurites with their synaptic partners. Unfortunately, inhibitory environment in the mature nervous system, especially in the central nervous system (CNS), normally restricts growth of differentiated neurons and makes neurite repair difficult [1–5]. Both non-cell autonomous and cell-autonomous factors can contribute to the inhibition of neurite repair. For examples, non-cell autonomous molecules released from neighboring cells such as glia cells prohibit regenerated neurite from innervating the correct targets [1,4,5]. As part of the cell-autonomous regulations, inactivations of developmental growth programs hinder neurite regrowth in mature neurons [1,3,6•,7].
In order to overcome restrictions on neurite repair, neurons need to reverse the inhibitory state to active growth in response to injury. Here, we review recent progress in elucidating mechanisms of neurite repair involving ion channels, signaling pathways, transcriptional and post-transcriptional regulations. The interactions between dendrites and axons within an injured neuron and strategies to optimize the outcome in neurite repair are also discussed in the review.
Ion channels
Ion channels are transmembrane proteins that allow ions to pass across the cell membrane. They are important for controlling the excitability of neurons and are involved in many aspects of developmental programs including neurite outgrowth and plasticity [8]. Are ion channels also involved in neurite repair? Recent studies of TRPV1, Piezo, voltage gated calcium channels (VGCCs) and potassium channels reveal the underlying mechanism for how regulation of ion flux could have impact on neurite repair upon insults.
Nociceptive ion channels transduce noxious stimuli and are important for pain sensation. Inhibition of TRPV1, a nociceptive ion channel, was found to reactivate the axon growth in sensory neurons through an increase of intracellular calcium and activation of the PKA pathway in nociceptive neurons [8]. The authors did not observe significant improvements in axon regeneration following sciatic nerve crush. It is conceivable that improvement in axon regeneration by manipulating TRPV1 may be masked by neurons without TRPV1 expression that also send their axons into the sciatic nerve, given that only a small subpopulation of sensory neurons express TRPV1. Interestingly, receptors involved in nociceptive stimulation also play roles in neurite repair. Activation or inhibition of ORL1, a nociception receptor, was reported to reduce or enhance axon regeneration, respectively, through a ROCK-dependent mechanism [9].
Mechanosensitive ion channels transduce mechanical forces and can induce downstream signaling pathways to modulate cellular responses, including neurite outgrowth. Song et al. found that mechanosensitive ion channels are involved in neurite repair. They reported that Piezo, a Ca2+ permeable mechanosensitive cation channel, inhibits axon regeneration in Drosophila sensory neurons as well as mammalian corneal sensory neurons through calcium signaling, nitric oxide (NO) synthase and cGMP-dependent kinase PKG [10•].
The voltage-gated calcium channel (VGCC) subunit Alpha2delta was identified as a negative regulator of axon growth and injury-dependent regeneration in cultured DRG neurons [11]. Systemic administration of Pregabalin (PGB), a gabapentinoid that selectively binds to VGCC Alpha2delta1/2 subunits and blocks calcium influx, could improve axon regeneration of dorsal column axons following SCI in vivo; the sooner PGB is applied, the better axons regenerate[11].
Electrical stimulation, which affects electrical signaling in neurons, has been shown to enhance axon regeneration after axon injuries [12–14]. The improvement in axon regeneration by electrical stimulation is likely through upregulation of regeneration-associated gene (RAG) expression in injured neurons [15]. The importance of electrical signaling has recently been reported for dendrite regeneration as well. The blockade in electrical activity by overexpressing the inward rectifier K+ channel Kir2.1 could inhibit dendrite regeneration following severing of dendrites in Drosophila sensory neurons [16]. It will be interesting to find out whether electrical activity also induces the RAG expression or affects other signaling pathways for dendrite regeneration.
Intrinsic signaling pathways
Intrinsic growth capacity is critical for the success in neurite repair following injury. Various signaling pathways including mammalian target of rapamycin (mTOR) pathway have been reported to promote those neurons that are not readily regenerative to re-enter the active growth state [17–19]. A recent study reported that translation of mTOR could be regulated locally in response to injury signals, which then controls the synthesis of retrograde injury signaling molecules important for intracellular communication between axons and soma [20••]. Exploring how local RNA, including mTOR mRNA, are targeted to the injury sites and identifying the targets of mTOR for local translation can provide us more insights regarding the roles of mTOR in neurite repair [21]. Using acute optic nerve injury to induce dendrite degeneration in mouse retinal ganglion cells (RGCs), Agostinone et al. found that activations of mTOR triggered by insulin can promote dendrite regeneration. They further showed that mTOR complex1 and mTOR complex2 are involved in dendrite repairs for dendrite branching and coverage area, respectively [22•]. The injured RGCs partially regain the lost electrophysiological properties with the aid of insulin [22•]. It remains to be determined whether insulin-mediated regeneration and protection can facilitate the recovery at the behavioral level as well. The requirement of mTOR in dendrite repair has also been reported in the adult zebrafish RGCs [23].
Lin28a/b are RNA-binding proteins involve in cell growth and reprograming. With their partners, let-7 microRNAs, Lin28a/b participate in sensory axon regeneration [24]. In the CNS, Lin28 can potentiate insulin-like growth factor-1 (IGF1) responsiveness in injured RGCs, which can lead to robust axon regeneration beyond the effect of Lin28 or IGF1 alone [25]. By restricting Lin28 expression in specific types of neurons in the eye, Zhang et al. found that Lin28-dependent IGF1 regulation of RGCs is non-cell autonomous. The IGF1 responsiveness of RGCs is controlled by the Lin28 expression in amacrine cells, which are inhibitory neurons innervating RGCs [25]. Study in Caenorhabditis elegans, however, showed that IGF1 is deleterious for the dendrite degeneration in adult PVD polymodal neurons while mutation in daf-2, an IGF receptor ortholog, can prevent declines in the dendrite regeneration during aging [26].
14-3-3 adaptors are hubs for cell signaling that function in neural development and axon guidance. Kaplan et al. found that stabilization of the 14-3-3 protein interactions by fusicoccin-A (FC-A) could facilitate axon outgrowth and regeneration [27]. They identified the stress response regulator GCN1 protein as one of the binding partners of 14-3-3 protein and showed that FC-A-induced neurite outgrowth requires turnover of GCN1 [27].
Besides the upstream regulators in the intrinsic growth programs, downstream effectors are also critical for neurite repair, especially those that regulate cytoskeleton dynamics [4,28]. Tedeschi et al. demonstrated that direct manipulation of actin depolymerizing factor (ADF)/cofilin can induce axon regeneration which depends on the actin-severing activity [29]. Thrombospondin-1 (Thbs1) and muscle LIM protein (MLP) are found to be RAGs [30,31]. MLP improves axon extension by cross-linking F-actin in filopodia, axonal growth cones [31]. Thbs1 enhanced regeneration is dependent on syndecan-1, a known THBS1-binding protein [30]. Neuronal-specific β-tubulin isoform Tubb3 controls the microtubule dynamic in the growth cones and has a specific role in determining the rate of peripheral axon regeneration and the functional recovery in DRG neurons after sciatic nerve crush [32]. These studies suggest that manipulations of downstream effectors is also a promising way to induce neurite repair.
Transcriptional and post-transcriptional regulations
Manipulations of transcription factors or epigenetic process could help to re-establish the growth capacity in mature neurons by regulating expression of RAGs or developmental neurite growth programs [3,4]. Sox11, a transcription factor, is one of the RAGs and can promote axon regeneration in adult RGCs through reactivating other developmental axon growth programs [33••]. Induction of axon regeneration by Sox11 is cell type specific as overexpression of Sox11 actually kills α-RGCs [33••]. In DRG neurons, Silc1, a long noncoding RNAs (lncRNA), facilitates upregulation of Sox11 upon injury and maintains Sox11 expression levels in the adult brain through cis-acting regulation [34]. Contributions of lncRNAs in regulatory programs of transcriptional responses to injury provide another handle to manipulate the expression of RAGs. Upregulation of Ascl1, also a transcription factor, promotes axon growth in DRG neurons [35].
Epigenetic processes silence gene expression by modifying the chromatin architectures including DNA methylation. Studies of dynamic changes in the chromatin modifications during development and upon physiological stimuli have begun to reveal the potential of epigenetically regulating neurite repair [3,7,36•,37]. A study from Weng et al. reported that Ten-eleven translocation methylcytosine dioxygenase-3 (Tet3), a DNA demethylation mediator that can oxidize 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) and other derivatives, is responsible for injury-induced DNA demethylation and expression of RAGs upon axotomy of adult DRG neurons [6•]. Tet signaling is also required for inducing axon regeneration in the adult CNS with the specific involvement of Tet1 but not Tet3 [6•]. Roles of PIWI-interacting RNA (piRNA) pathway in neurite repair were recently discovered. piRNA factors, PRDE-1 and PRG-1/PIWI can inhibit the axon regeneration following laser axotomy through post-transcriptionally gene silencing [36•]. It remains an open question whether piRNAs are also involved in regulations of developmental axon growth.
Roles of epitranscriptomic regulations on RNA metabolism and protein translation in the neural development and neurite repair have just begun to be elucidated [38,39•]. N6-methyladenosine (m6A) modifications of the transcripts of RAGs and some protein translation machinery components were found to change upon injury in the adult mouse DRG neurons [39•]. Moreover, loss of the epitranscriptomic regulations through m6A methylations attenuates protein translation of tagged transcripts, which in turn ameliorates axon regeneration [39•].
Beyond repair at the site of injury
Upon injury, besides the site of injury, other parts of the neuron are likely to be influenced too as a result of this intracellular communication. However, how the injured sites affect other parts of neurons or vice versa following injuries remains unexplored.
Dung and Hammarlund reported an interesting phenomenon in C. elegans DA-9 neuron, where axotomy of DA-9 neurons not only results in reduction in axon length but also induced ectopic synaptic vesicle localization to the dendrite [40••]. Aberrant release of the ectopic synaptic vesicles suppresses the behavioral recovery after axon regeneration [40••]. This study provides evidence that repair of dendritic dysregulation is also important for full functional recovery following axon injury. Dendrite degeneration and retraction accompanying axotomy in RGCs is another example of secondary injury effects and has been used as a model for dendrite degeneration and regeneration [22•]. Using adult zebrafish RGCs as a model, Beckers et al. found a counteractive interplay between axons and dendrites involving mTOR and matrix metalloproteinases (MMPs) after optic nerve crush where dendrite degeneration is a prerequisite for efficient axon regrowth [23]. Dendritic microtubule polarity was found to be dynamic following axotomy in Drosophila sensory neurons. The speed of conversion of microtubule polarity in dendrite differs depending on the distance between injury sites and soma and the microtubule polarity changes faster following proximal axotomy than after distal axotomy [41].
Communication within neurons is important for integrating the information they received and for determining what to relay onto post-synaptic neurons. While studies usually focus on repairing the part of neurite that was directly injured, it is largely unknown what is happening away from the primary injury site. Future works investigating the secondary injury effects in response to the insults and how these subsequent events could change outcomes of the primary injury or overall functional recovery will help us to uncover the missing pieces necessary to accomplish neurite repair.
Some strategies for improving neurite repair
Various repair mechanisms discovered recently are sufficient to restore at least part of the structure or functional deficiency following injury. Unbiased searches for novel molecules responsible for neurite repair continue to shed light on the mechanisms underlying neuroregeneration [42,43]. Could we maximize the benefit by combining all or several of them at once for treatment hopefully for a synergetic effect?
Anderson et al. found that manipulating a combination of three factors, including neuron intrinsic growth capacity, growth-supportive substrate, and chemoattraction, can have the most robust improvements in axon regrowth, and the chemoattraction seemed to be the key for guiding axons to regrow across complete SCI lesions in the adult CNS [44••]. The regenerated axons can form synaptic connections and show recoveries in conduction of electrical signals but not behavioral functions [44••]. Lack of myelination was proposed to be one of the limitations for functional recovery after axon regeneration [45]. It will be interesting to see if introducing the voltage-gated potassium channel blocker 4-aminopyridine (4-AP) known to further improve conduction in regenerated axons lacking myelination can further boost the improvements in the behavioral functions [45]. The rehabilitation trainings may also help to integrate the rewired circuits into the functional networks [44••,45].
The epigenetic or epitranscriptomic regulations and intrinsic signalings also function together to control neurite repair. Combinations of Tet3 knockdown or Lin28 overexpression with PTEN knockdown showed additive effects in improving axon regeneration [6•,24], while knockdown of methyltransferase like 14 (Mettl14), a component of m6A methyltransferase complex critical for epitranscriptomic regulation, reduced the PTEN deletion-induced axon regeneration [39•].
It is critical to determine how to combine factors known to improve neurite repair together for the best therapeutic outcomes. It can lead to additive or harmful effects depending on the timing, cell types, and many other factors. The case of Sox11 and PTEN is a good example.PTEN deletion alone enhances axon regeneration selectively in α-RGCs [17,18]. Combining PTEN deletion with Sox11 overexpression could unlock the restriction on PTEN deletion-dependent improvement in axon regeneration and boost the axon regeneration in non α-RGCs [33••]. However, the combination of PTEN deletion and Sox11 overexpression was not able to stop Sox11 from killing the α-RGCs [33••]. These results suggest that distinct neuron types may differ in their intrinsic capacity to regenerate upon injuries and one should be cautious when combining a set of pro-regeneration molecules in different circumstances and cell types.
Closing remarks
Understanding how neurons take on challenges from environments and recover could guide us to develop therapeutic strategies to facilitate neurite repair after injury or diseases (Figure 1). The idea to learn from the early developmental stage where neurons undergo active growth is intriguing and has driven many interesting studies to uncover potential targets as discussed in this review. We should not only focus on restorating the primary injury sites of neurons but consider to rescue both primary and secondary deficits of damaged neuron within the whole circuit. It is also important to bear in mind that the right combinations of factors for enhancing neurite repair may vary depending on the cell types. By taking all these considerations into account, we could potentially overcome difficulties to translate the success in cellular repair to functional recovery in clinical therapeutics.
Figure 1.
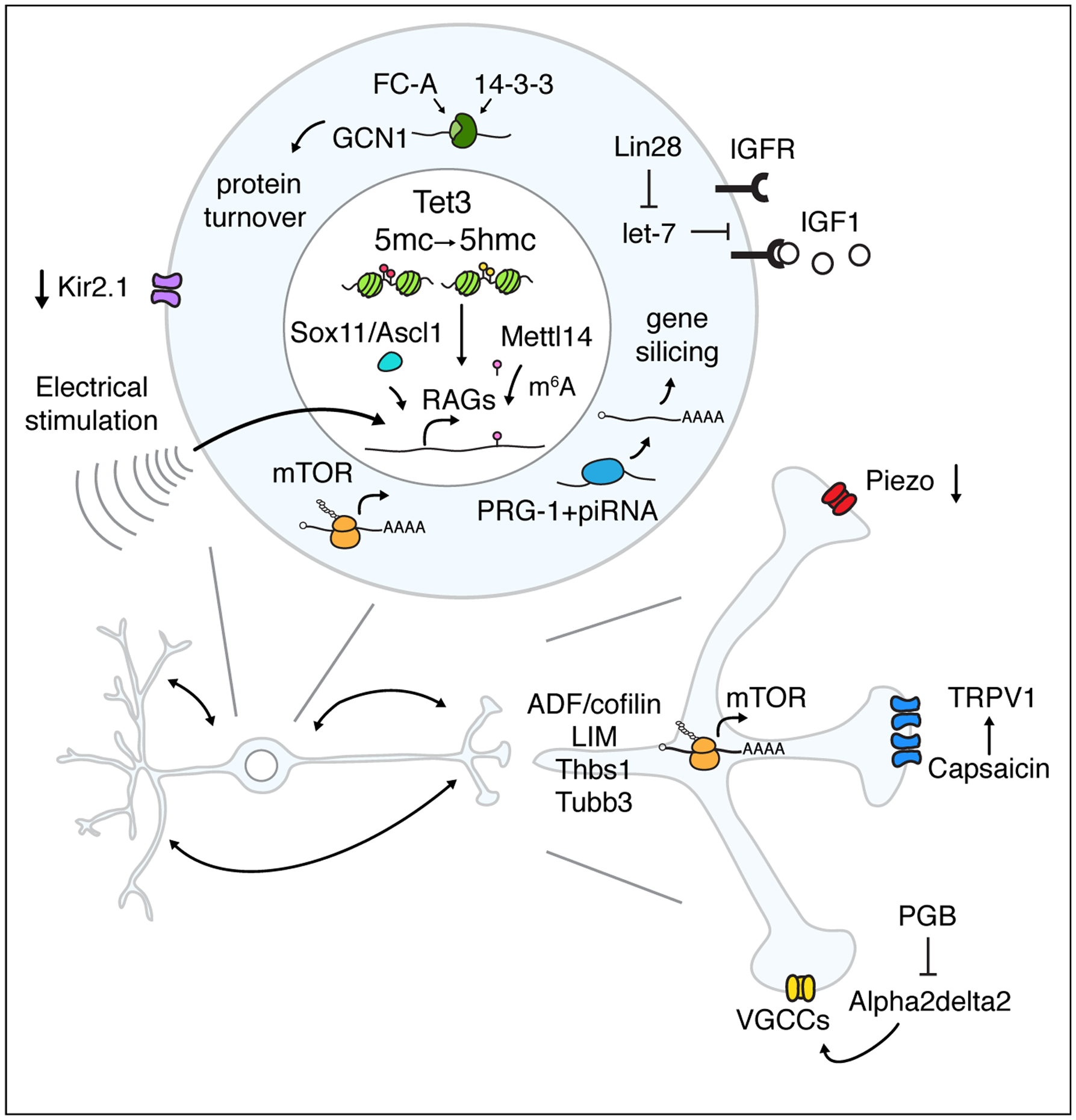
Summary of cell-autonomous regulations that can improve neurite repair.
Schematic illustration of mechanisms of neurite repair discussed in this review.
Acknowledgements
We would like to thank Dr Lily Y. Jan for critical reading of this manuscript and for insightful discussion. This work was supported by the N.I.H. grant R35NS097227 (to Y.N.J.). Y.N.J. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Laha B, Stafford BK, Huberman AD: Regenerating optic pathways from the eye to the brain. Science 2017, 356: 1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menorca RM, Fussell TS, Elfar JC: Nerve physiology: mechanisms of injury and recovery. Hand Clin 2013, 29: 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curcio M, Bradke F: Axon regeneration in the central nervous system: facing the challenges from the inside. Annu Rev Cell Dev Biol 2018, 34:495–521. [DOI] [PubMed] [Google Scholar]
- 4.Richardson CE, Shen K: Neurite development and repair in worms and flies. Annu Rev Neurosci 2019, 42:209–226. [DOI] [PubMed] [Google Scholar]
- 5.Boghdadi AG, Teo L, Bourne JA: The involvement of the myelin-associated inhibitors and their receptors in CNS plasticity and injury. Mol Neurobiol 2018, 55:1831–1846. [DOI] [PubMed] [Google Scholar]
- 6.•.Weng YL, An R, Cassin J, Joseph J, Mi R, Wang C, Zhong C, Jin SG, Pfeifer GP, Bellacosa A et al. : An intrinsic epigenetic barrier for functional axon regeneration. Neuron 2017, 94:337–346.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides evidence that RAGs expression following axotomy can be induced by activation of DNA demethylation pathway in the adult mammalian nervous system.
- 7.Mahar M, Cavalli V: Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci 2018, 19:323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey E, Karney-Grobe S, Krolak T, Milbrandt J, DiAntonio A: TRPV1 agonist, capsaicin, induces axon outgrowth after injury via Ca(2+)/PKA signaling. eNeuro 2018, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekine Y, Siegel CS, Sekine-Konno T, Cafferty WBJ, Strittmatter SM: The nociceptin receptor inhibits axonal regeneration and recovery from spinal cord injury. Sci Signal 2018, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.•.Song Y, Li D, Farrelly O, Miles L, Li F, Kim SE, Lo TY, Wang F, Li T, Thompson-Peer KL et al. : The mechanosensitive ion channel piezo inhibits axon regeneration. Neuron 2019, 102:373–389.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show that activation Piezo, an evolutionarily conserved mechanosensitive ion channel, restricts axon regeneration through calcium, NO, and PKG signaling pathways.
- 11.Tedeschi A, Dupraz S, Laskowski CJ, Xue J, Ulas T, Beyer M, Schultze JL, Bradke F: The calcium channel subunit Alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron 2016, 92:419–434. [DOI] [PubMed] [Google Scholar]
- 12.Goganau I, Sandner B, Weidner N, Fouad K, Blesch A: Depolarization and electrical stimulation enhance in vitro and in vivo sensory axon growth after spinal cord injury. Exp Neurol 2018, 300:247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senger JB, Verge VMK, Chan KM, Webber CA: The nerve conditioning lesion: a strategy to enhance nerve regeneration. Ann Neurol 2018, 83:691–702. [DOI] [PubMed] [Google Scholar]
- 14.Lim JH, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, He Z, Huberman AD: Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat Neurosci 2016, 19:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senger JLB, Verge VMK, Macandili HSJ, Olson JL, Chan KM, Webber CA: Electrical stimulation as a conditioning strategy for promoting and accelerating peripheral nerve regeneration. Exp Neurol 2018, 302:75–84. [DOI] [PubMed] [Google Scholar]
- 16.Thompson-Peer KL, DeVault L, Li T, Jan LY, Jan YN: In vivo dendrite regeneration after injury is different from dendrite development. Genes Dev 2016, 30:1776–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M et al. : Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 2008, 322:963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan X, Qiao M, Bei F, Kim IJ, He Z, Sanes JR: Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron 2015, 85:1244–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxton RA, Sabatini DM: mTOR signaling in growth, metabolism, and disease. Cell 2017, 168:960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.••.Terenzio M, Koley S, Samra N, Rishal I, Zhao Q, Sahoo PK, Urisman A, Marvaldi L, Oses-Prieto JA, Forester C et al. : Locally translated mTOR controls axonal local translation in nerve injury. Science 2018, 359:1416–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that axonal local translation following nerve injury requires local translation and targeting of mTOR mRNA in injured axon.
- 21.Riccio A: RNA targeting and translation in axons. Science 2018, 359:1331–1332. [DOI] [PubMed] [Google Scholar]
- 22.•.Agostinone J, Alarcon-Martinloaez L, Gamlin C, Yu WQ, Wong ROL, Di Polo A: Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain 2018, 141:1963–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors provide evidence that dendrite degeneration following optic nerve injury in RGCs can be protected with insulin acting via the mTOR pathway.
- 23.Beckers A, Van Dyck A, Bollaerts I, Van Houcke J, Lefevere E, Andries L, Agostinone J, Van Hove I, Di Polo A, Lemmens K et al. : An antagonistic axon-dendrite interplay enables efficient neuronal repair in the adult zebrafish central nervous system. Mol Neurobiol 2019, 56:3175–3192. [DOI] [PubMed] [Google Scholar]
- 24.Wang XW, Li Q, Liu CM, Hall PA, Jiang JJ, Katchis CD, Kang S, Dong BC, Li S, Zhou FQ: Lin28 signaling supports mammalian PNS and CNS axon regeneration. Cell Rep 2018, 24:2540–2552.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Williams PR, Jacobi A, Wang C, Goel A, Hirano AA, Brecha NC, Kerschensteiner D, He Z: Elevating growth factor responsiveness and axon regeneration by modulating presynaptic inputs. Neuron 2019, 103:39–51.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kravtsov V, Oren-Suissa M, Podbilewicz B: The fusogen AFF-1 can rejuvenate the regenerative potential of adult dendritic trees by self-fusion. Development 2017, 144:2364–2374. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan A, Morquette B, Kroner A, Leong S, Madwar C, Sanz R, Banerjee SL, Antel J, Bisson N, David S et al. : Small-molecule stabilization of 14-3-3 protein-protein interactions stimulates axon regeneration. Neuron 2017, 93:1082–1093.e5. [DOI] [PubMed] [Google Scholar]
- 28.Blanquie O, Bradke F: Cytoskeleton dynamics in axon regeneration. Curr Opin Neurobiol 2018, 51:60–69. [DOI] [PubMed] [Google Scholar]
- 29.Tedeschi A, Dupraz S, Curcio M, Laskowski CJ, Schaffran B, Flynn KC, Santos TE, Stern S, Hilton BJ, Larson MJE et al. : ADF/cofilin-mediated actin turnover promotes axon regeneration in the adult CNS. Neuron 2019, 103 1073–1085 e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bray ER, Yungher BJ, Levay K, Ribeiro M, Dvoryanchikov G, Ayupe AC, Thakor K, Marks V, Randolph M, Danzi MC et al. : Thrombospondin-1 mediates axon regeneration in retinal ganglion cells. Neuron 2019, 103:642–657.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin E, Andreadaki A, Gobrecht P, Bosse F, Fischer D: Nociceptive DRG neurons express muscle lim protein upon axonal injury. Sci Rep 2017, 7:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latremoliere A, Cheng L, DeLisle M, Wu C, Chew S, Hutchinson EB, Sheridan A, Alexandre C, Latremoliere F, Sheu SH et al. : Neuronal-specific TUBB3 is not required for normal neuronal function but is essential for timely axon regeneration. Cell Rep 2018, 24:1865–1879.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.••.Norsworthy MW, Bei F, Kawaguchi R, Wang Q, Tran NM, Li Y, Brommer B, Zhang Y, Wang C, Sanes JR et al. : Sox11 expression promotes regeneration of some retinal ganglion cell types but kills others. Neuron 2017, 94:1112–1120.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]; From a search for transcriptions factor involved in axon regeneration, this study identifies Sox11 as a key one that could enhance axon regeneration of adult RGCs in a cell-type specific manner.
- 34.Perry RB, Hezroni H, Goldrich MJ, Ulitsky I: Regulation of neuroregeneration by long noncoding RNAs. Mol Cell 2018, 72:553–567.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lisi V, Singh B, Giroux M, Guzman E, Painter MW, Cheng YC, Huebner E, Coppola G, Costigan M, Woolf CJ et al. : Enhanced neuronal regeneration in the CAST/Ei mouse strain is linked to expression of differentiation markers after injury. Cell Rep 2017, 20:1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.•.Kim KW, Tang NH, Andrusiak MG, Wu Z, Chisholm AD, Jin Y: A neuronal piRNA pathway inhibits axon regeneration in C.elegans. Neuron 2018, 97:511–519.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study discovers that piRNA pathway inhibits axon regeneration in C. elegans through post-transcriptional gene silencing.
- 37.Barker SJ, Tsai LH: MethyLock: DNA demethylation is the epigenetic key to axon regeneration. Neuron 2017, 94:221–223. [DOI] [PubMed] [Google Scholar]
- 38.Meyer KD, Jaffrey SR: Rethinking m(6)A readers, writers, and erasers. Annu Rev Cell Dev Biol 2017, 33:319–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.•.Weng YL, Wang X, An R, Cassin J, Vissers C, Liu Y, Liu Y, Xu T, Wang X, Wong SZH et al. : Epitranscriptomic m(6)A regulation of axon regeneration in the adult mammalian nervous system. Neuron 2018, 97:313–325.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate that axonal injury induce increases in m6A tagging and signaling to promote translation of RAGs and axon regeneration in adult PNS and CNS.
- 40.••.Ding C, Hammarlund M: Aberrant information transfer interferes with functional axon regeneration. eLife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that ectopic synaptic vesicles in dendrites actively suppress functional axon regeneration of adult DA9 neurons in C. elegans.
- 41.Rao KS, Rolls MM: Two Drosophila model neurons can regenerate axons from the stump or from a converted dendrite, with feedback between the two sites. Neural Dev 2017, 12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sekine Y, Lin-Moore A, Chenette DM, Wang X, Jiang Z, Cafferty WB, Hammarlund M, Strittmatter SM: Functional genome-wide screen identifies pathways restricting central nervous system axonal regeneration. Cell Rep 2018, 24:269. [DOI] [PubMed] [Google Scholar]
- 43.Kim KW, Tang NH, Piggott CA, Andrusiak MG, Park S, Zhu M, Kurup N, Cherra SJ 3rd, Wu Z, Chisholm AD et al. : Expanded genetic screening in Caenorhabditis elegans identifies new regulators and an inhibitory role for NAD(+) in axon regeneration. eLife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.••.Anderson MA, O’Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B et al. : Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 2018, 561:396–400 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that manipulating neuron intrinsic growth capacity, growth-supportive substrate and chemoattraction all together can promote robust axon regeneration in adult CNS neurons after complete SCI lesions.
- 45.Bei F, Lee HHC, Liu X, Gunner G, Jin H, Ma L, Wang C, Hou L, Hensch TK, Frank E et al. : Restoration of visual function by enhancing conduction in regenerated axons. Cell 2016, 164:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]


