Abstract
Cryptococcosis has become a major global health problem since the advent of the HIV pandemic in 1980s. Although its molecular epidemiology is well-defined, using isolates recovered since then, no pre-HIV-pandemic era epidemiological data exist. We conducted a molecular epidemiological study using 228 isolates of the C. neoformans/C. gattii species complexes isolated before 1975. Genotypes were determined by URA5 restriction fragment length polymorphism analysis and multi-locus sequence typing. Population genetics were defined by nucleotide diversity measurements, neutrality tests, and recombination analysis. Growth at 37°C, melanin synthesis, capsule production, and urease activity as virulence factors were quantified. The pre-HIV-pandemic isolates consisted of 186 (81.5%) clinical, 35 (15.4%) environmental, and 7 (3.1%) veterinary isolates. Of those, 204 (89.5%) belonged to C. neoformans VNI (64.0%), VNII (14.9%) and VNIV (10.5%) while 24 (10.5%) belonged to C. gattii VGIII (7.5%), VGI (2.6%) and VGII (0.5%). Among the 47 sequence types (STs) identified, one of VNII and 8 of VNIV were novel. ST5/VNI (23.0%) in C. neoformans and ST75/VGIII (25.0%) in C. gattii were the most common STs in both species complexes. Among C. neoformans, VNIV had the highest genetic diversity (Hd = 0.926) and the minimum recombination events (Rm = 10), and clinical isolates had less genetic diversity (Hd = 0.866) than environmental (Hd = 0.889) and veterinary isolates (Hd = 0.900). Among C. gattii, VGI had a higher nucleotide diversity (π = 0.01436) than in VGIII (π = 0.00328). The high-virulence genotypes (ST5/VNI and VGIIIa/serotype B) did not produce higher virulence factors levels than other genotypes. Overall, high genetic variability and recombination rates were found for the pre-HIV-pandemic era among strains of the C. neoformans/C. gattii species complexes. Whole genome analysis and in vivo virulence studies would clarify the evolution of the genetic diversity and/or virulence of isolates of the C. neoformans/C. gattii species complexes during the pre- and post-HIV-pandemic eras.
Author summary
Since the beginning of the HIV pandemic in 1980, infections due to isolates of the Cryptococcus neoformans/C. gattii species complexes have caused many deaths worldwide, especially in the HIV-infected population. Annually, approximately one-third, of all AIDS-related deaths,—representing more than 1,000,000 cases,—are caused by cryptococcosis. Since 1980, extensive molecular epidemiological surveys have been conducted, and the VNI molecular type has been found to be responsible for more than 90% of cryptococcosis in HIV patients. Whether the high VNI prevalence is associated with the HIV pandemic remains controversial as information on the isolates of the pre-HIV pandemic era is lacking. Therefore, this study of the molecular epidemiology and in vitro characteristics of the strains from the pre-HIV-pandemic era was undertaken. We found that only 64% of cryptococcosis was caused by VNI, and 9 sequence types existed only in the pre-HIV pandemic era. Unlike what was already known about the strains collected during the HIV pandemic era, ST5 and VGIIIa,—supposedly high virulence genotypes,—did not express higher virulence factors than other genotypes. These results implied that the HIV pandemic altered both the molecular epidemiology and virulence of Cryptococcus neoformans/C. gattii species complexes have been altered during HIV pandemic. However, detailed mechanism of these alteration remains to be deciphered further.
Introduction
C. neoformans and C. gattii species complexes are the etiologic agents of human and animal cryptococcosis. C. neoformans is known to infect mainly immunocompromised hosts, whereas C. gattii infects previously healthy individuals more often than those with known immunosuppression [1, 2]. Cryptococcosis is initiated by the inhalation of infectious propagules (basidiospores or dehydrated yeasts), which colonize the lungs and hematogenously disseminate to the central nervous system [3]. PCR-fingerprinting, restriction fragment length polymorphism analysis of the orotidine monophosphate pyrophosphorylase gene (URA5), multi-locus microsatellite typing (MLMT), and multi-locus sequence typing (MLST) analysis have been used to classify C. neoformans and C. gattii into eight major molecular types. They are C. neoformans VNI (var. grubii, serotype A); VNII (var. grubii, serotype A); VNIII (serotype AD); VNIV (var. neoformans, serotype D) and C. gattii, VGI (serotype B); VGII (serotype B); VGIII (serotype B and C); and VGIV (C. gattii, serotypes B and C). Among the global isolates from clinical, veterinary, and environmental sources, the molecular types VNI and VGII have been the most common ones identified [4–6]. MLST has become the most widely employed method for cryptococcal molecular epidemiology due to its high discriminatory power and reproducibility as well as the availability of a large online database which allows accurate interlaboratory comparisons between isolates collected world-wide [7, 8]. Studies on the genetic structure of C. neoformans VNI and VNII using MLST in Asia, Europe, South Africa, and South America have revealed that the majority of isolates belong to sequence types (STs) 4, 5, 6, 23, 63 and 93 [9–12].
It remains unclear whether the rise in the prevalence of cryptococcosis following the advent of the HIV pandemic in the 1980s was caused solely by the deficient immune status of AIDS patients or in combination with the evolution of highly virulent cryptococcal strains. C. neoformans ST5/VNI and C. gattii ST20/VGIIa were proposed to be high-virulence genotypes causing outbreaks in many countries [13–17]. Moreover, although C. gattii was previously thought to be mainly an opportunistic pathogen, VGIII infections have been increasingly reported among immunocompetent patients and animals in North and South America [18–21]. A recent study showed that VGIIIa (VGIII/serotype B) was more virulent in mice than its counterpart, VGIIIb (VGIII/ serotype C) [18, 22]. It has been speculated that natural evolution had resulted in the emergence of virulence difference between the two VGIII subgroups. Extensive molecular epidemiological studies using isolates from various regions around the world have been conducted to verify the evolution of different traits among molecular types [4, 23–25].
However, no data obtained by using sequencing-based methods are available on the molecular epidemiology and population structure of the C. neoformans/C. gattii species complexes isolates in the pre-HIV pandemic era. With this in mind, we investigated the genetic diversity and in vitro virulence factors of the C. neoformans/C. gattii species complexes in 228 clinical, environmental, and veterinary isolates recovered during the pre-HIV-pandemic era.
Materials and methods
Study isolates
A total of 228 cryptococcal strains from the pre-HIV-pandemic era were obtained from the National Institutes of Health in Bethesda, Maryland and were maintained in a 20% glycerol stock (-80°C) at Siriraj Hospital, Mahidol University. Each isolate was removed from glycerol stock and cultured on Sabouraud dextrose agar (4% dextrose, 1% peptone, 1.5% agar, and final pH 5.6 ± 0.2; Oxoid Ltd, Basingstoke, UK) at 30°C for 48–72 hours, and its species status was confirmed with the RapID Yeast Plus System (Thermo Fisher Scientific, Waltham, MA, USA). URA5 restriction fragment length polymorphism was performed to differentiate between C. neoformans and C. gattii. This study was carried out with the prior approval of the Siriraj Institutional Ethics Committee (Si 091/2016). Only one representative strain per patient or source was used for all genetic diversity analyses. For patient B, all sequential strains were used for the genetic diversity analysis as the sequential strains of this patient belonged to different ST (S1 Table).
Genotype and MLST analysis
DNA was extracted using chemical lysis solutions with heating according to a previous protocol, but with minor modifications [26]. The URA5 gene was amplified with the following primers, URA5 (5’ATGTCCTCCCAAGCCCTCGACTCCG3’) and SJ01 (5’TTAAGACC TCTGAACACCGTACTC3’). The genotypes were determined with a restriction fragment length polymorphism analysis (RFLP) of the URA5 gene digested with restriction enzymes HhaI and Sau96I (Thermo Fisher Scientific, MA, USA) [3]. A set of standard laboratory reference strains representing each of the eight major molecular types were used for the molecular typing: WM148 (VNI), WM626 (VNII), WM 628 (VNIII), WM 629 (VNIV), WM 179 (VGI), WM 178 (VGII), WM 175 (VGIII), and WM 779 (VGIV) [3].
MLST analysis of the C. neoformans/C. gattii species complexes isolates was performed using the International Society for Human and Animal Mycology (ISHAM) consensus scheme of seven unlinked loci (CAP59, GPD1, IGS1, LAC1, PLB1, SOD1, and URA5). The allele types and sequence types (STs) were defined according to the ISHAM-MLST database for C. neoformans and C. gattii (http://mlst.mycologylab.org) [2]. All sequences are deposited in GenBank, and their accession numbers are described in S1 Table.
Phylogenetic analysis
The generated sequences were manually edited and aligned with Clustal W using the program MEGA, version 6.06 (http://www.megasoftware.net) [27]. The concatenated alignments were then imported and analyzed using the neighbor-joining method with the p-distance. Bootstrap analysis, using 1,000 replicates with pairwise deletion, was employed to estimate the support for clades of the concatenate dataset.
Genetic diversity analysis
The intra- and inter-population genetic variabilities were estimated by the number of polymorphic sites, number of haplotypes, haplotype diversity, nucleotide diversity, and average number of nucleotide differences. The number of polymorphic sites (S) is analogous to the number of alleles among sequences of genes [28]. The number of haplotypes (h) is a set of DNA variations, or polymorphisms, that tend to be inherited together, while haplotype diversity (Hd; also called gene diversity) is the probability of a difference between two randomly sampled alleles. Nucleotide diversity (π; also termed average pairwise difference) represents the average number of nucleotide differences (k) per site in pairwise comparisons of DNA sequences. To perform the analyses, isolates were stratified by different categories, including molecular types and source of isolations, and their DNA sequences were analyzed using DnaSP, version 6.12.01 (Universitat de Barcelona, Barcelona, Spain) [29, 30].
In addition, the selective neutrality of mutations was measured by Tajima’s D (D) tests for neutrality. This test distinguishes between the neutral and non-neutral evolution of a DNA sequence. The neutral evolution includes mutation-drift equilibrium, while the non-neutral evolution represents sequences evolving by directional or balancing selection, and demographic expansion or contraction. The Tajima’s D method (D) compares the average number and the estimated number of nucleotide differences from the number of segregating sites in the studied population [31]. Thus, the value of these tests would be close to zero under the neutrality. A negative or positive result suggests evidence of purifying (deleterious change) or balancing (the Darwinian or beneficial change) selection, respectively. P-values were generated using 1,000 simulations under a model of selective neutrality implemented in the DnaSP program [31].
Linkage disequilibrium and recombination analysis
The presence of recombination within each population was performed using the percentage of phylogenetically compatible pairs of loci (PcP), and the index of association (IA), while the rBarD values of the different C. neoformans/C. gattii species complexes subpopulations were calculated using a clone-corrected dataset in order to avoid the bias of “high frequency” sequence types in the analysis in the software Multilocus version 1.3 using 1,000 randomizations. The absence of a difference between both datasets (p > 0.05) supports the null hypotheses of linkage equilibrium and sexual recombination, whereas significant differences supports linkage disequilibrium (LD) and clonality [32].The minimum number of recombination events (Rm) per gene and per population were calculated for each orthologous gene using the four-gamete test, which located pairs of the closest polymorphic sites within the 4 haplotypes likely to be generated by recombination between them; the DnaSP program was used [33]. The pairwise homoplasy index (PHI) test was used to infer if there was a statistical significance for a recombination by using SplitsTree, version 4.15.1 (http://www.splitstree.org) [34].
Genetic differentiation based on allelic profile
A hierarchical analysis of molecular variance (AMOVA) was performed in GenAlEx 6.503 for Excel in order to examine the distribution of genetic variation, and determine the extent of connectivity among populations based on allelic profiles [35, 36]. AMOVA is a statistical technique that estimates the extent of genetic differentiation between individuals and populations directly from molecular data. The technique treats the raw molecular data as a pairwise matrix of genetic distances between all the possible combinations of isolates, with sub-matrices corresponding to the different hierarchical data-partitions (here, the genetic differences between different sources of isolation and geographical regions). In addition, the population differentiation test (FST) from an AMOVA, assuming that the isolates were all haploids or homozygous diploids, was used to test the null hypotheses (H0) of no population differentiation. Values of FST can range from 0, which implies that the two populations are interbreeding freely (in these scenario we accept H0 and the p > 0.05), to 1, where all genetic variation is explained by the population structure and the two populations do not share any genetic diversity [37].
In vitro analysis of virulence factors
In vitro analyses of important virulence factors of C. neoformans/C. gattii species complex were performed; these including the, growth rate at 37°C, melanin production, urease activity, and capsule formation. Each of these tests on the isolates was performed in triplicate.
Growth rate at 37°C
The analysis of the cryptococcal growth dynamics based on cell numbers was performed according to previously published reports [38, 39], with minor modifications. Briefly, cryptococcal overnight cultures in a yeast-peptone-dextrose medium were washed twice and resuspended in fresh medium. The concentration of yeast cells was adjusted to optical density (OD) 600 at 0.1 in 10 ml of the medium. The culture was incubated at 37°C in a shaking incubator and the OD 600 was monitored at 0, 2, 4, 6, 8, 10, 12, and 24 h. The growth rate at 37°C was calculated by the population doubling time between the exponential and stationary growth phases.
Melanin production
Each cryptococcal strain was grown in Sabouraud dextrose agar for 48 h at 30°C. Approximately 107 yeast cells were suspended on phosphate-buffered saline before adding 10 ml of melanin induction medium (0.1% peptone, 0.2% dextrose and 10 mM dopamine hydrochloride) and incubated at 37°C in a shaking incubator. The initial cell concentration of each strain was determined by plate counts in duplicate. Supernatants of the culture were taken at 48 h, and their ODs at 475 nm were measured by spectrophotometer [40, 41].
Urease activity
Each cryptococcal strain was grown in Sabouraud dextrose agar for 48 h at 30°C. The urease activity was determined according to a previous study, but with a minor modification [42]. Approximately 107 yeast cells were suspended on phosphate-buffered saline and 50 μl of the suspension was added in each well of a 96-well plate containing a urea-broth base with a 2% urea solution (Thermo Fisher Scientific, Waltham, MA, USA). The plates were incubated at 37°C for 48 h and the ODs were measured at 550 nm to infer urease activity of each strain.
Capsule formation
To investigate the capsule formation, stationary-phase fungal cultures were washed and resuspended in phosphate-buffered saline. Approximately 107 yeast cells were then placed in 6-well plates containing 2 ml Dulbecco’s Modified Eagle Medium (DMEM) with 10% bovine serum albumin and they were incubated at 37°C with 5% CO2 for 48 h. Cells of each isolate were mounted in India ink to visualize the size of the polysaccharide capsule under a light microscope [15, 43]. A capsule size of at least 20 yeast cells per isolate was determined by the ImageJ program (NIH, Bethesda, MD, USA) and a calculation was made of the diameter ratio of capsule to capsule(from the cell wall to cell-wall boundary of each cell [15].
Statistical analysis
Comparisons of the Hd data of different molecular types, or of the virulence characteristic data of different molecular types and sources, were performed with a two-tailed unpaired t-test using GraphPad Prism version 8.0.2 (GraphPad Software, California, USA). P-values of < 0.05 were considered statistically significant.
Results
Demographic data
Of the 228 isolates of the C. neoformans/C. gattii species complexes recovered during the pre-HIV-pandemic era (S1 Table), 204 (89.5%) and 24 (10.5%) strains were C. neoformans and C. gattii, respectively. Most isolates (186 strains; 81.5%) were recovered from clinical samples of 151 patients, followed by 35 environmental strains (15.4%) (isolated from animal dropping [74.29%], soil [14.29%], and tree hollows [5.71%]), and 7 veterinary strains (3.1%) (isolated from wound [57.14%], pus or exudate [28.57%], and animal lesions [14.29%]). In terms of their geographical distribution, 196 strains (86.0%) were recovered from the USA, followed by Thailand (14 strains; 6.1%), Denmark (10 strains; 4.4%), Italy (7 strains; 3.1%), and Canada (1 strain; 0.4%). The significant differences in genotype distributions between the pre- and during HIV pandemic eras based on geographic origin were shown in Table 1 [4, 44, 45].
Table 1. Comparison of genotype distributions of the pre- and during HIV pandemic eras based on geographic origin.
| Molecular type | USA* (%) | Thailand* (%) | Denmark* (%) | Italy* (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-HIV | During HIV | Pre-HIV | During HIV | Pre-HIV | During HIV | Pre-HIV | During HIV | |
| VNI | 131 (61.21%) |
710 (73.05%) |
13 (92.86%) |
899 (95.84%) |
2 (20%) |
61 (58.65%) |
0 | 231 (54.35%) |
| VNII | 34 (15.89%) |
5 (0.51%) |
0 | 12 (1.28%) |
0 | 5 (4.81%) |
1 (11.11%) |
0 |
| VNIII | 3 (1.40%) |
55 (5.66%) |
0 | 1 (0.11%) |
0 | 13 (12.50%) |
2 (22.22%) |
79 (18.59%) |
| VNIV | 24 (11.21%) |
59 (6.07%) |
0 | 1 (0.11%) |
8 (80%) |
21 (20.19%) |
6 (66.67%) |
112 (26.35%) |
| VGI | 5 (2.34%) |
6 (0.62%) |
1 (7.14%) |
4 (0.43%) |
0 | 2 (1.92%) |
0 | 3 (0.71%) |
| VGII | 1 (0.47%) |
108 (11.11%) |
0 | 21 (2.24%) |
0 | 2 (1.92%) |
0 | 0 |
| VGIII | 16 (7.48%) |
29 (2.98%) |
0 | 0 | 0 | 0 | 0 | 0 |
| VGIV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 214 (100%) |
972 (100%) |
14 (100%) |
938 (100%) |
10 (100%) |
104 (100%) |
9 (100%) |
425 (100%) |
| Reference | This study | [44] | This study | [4, 44] | This study | [44, 45] | This study | [44] |
| P-value** | <0.0001 | 0.042 | 0.0001 | <0.0001 | ||||
Note:
*Limited number of isolates and non-systematic strain collection
**Fisher’s exact test was performed by http://www.quantitativeskills.com/sisa/statistics/table2xr.htm
The VNI/ST5, high-virulence genotype, was most prevalent among C. neoformans
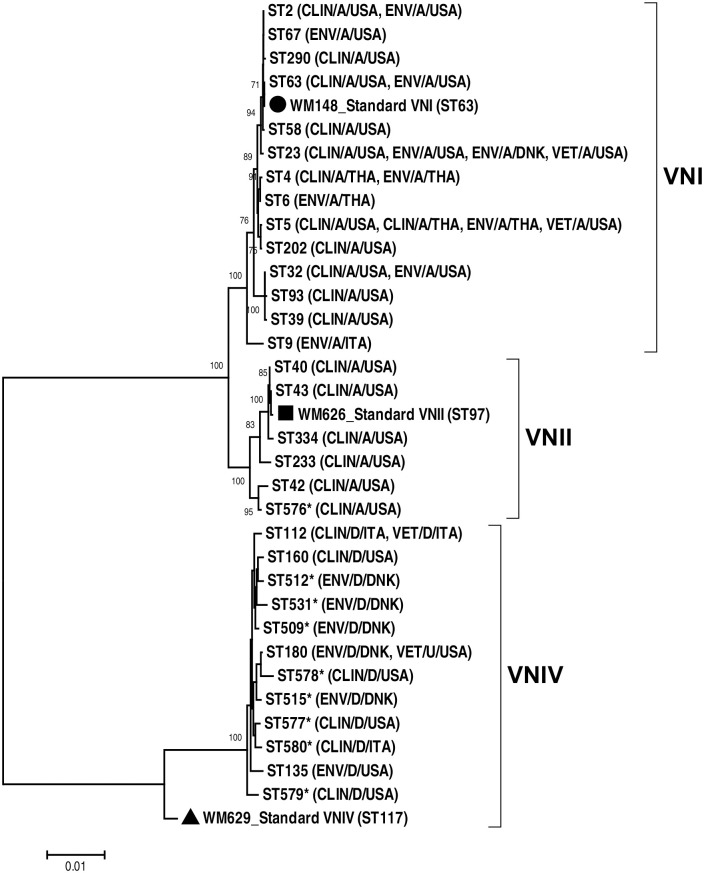
Overall, most isolates were identified as VNI (146 strains; 64.0%), followed by VNII (34 strains; 14.9%), VNIV (24 strains; 10.5%), VGIII (17 strains; 7.5%), VGI (6 strains; 2.6%), and VGII (1 strain; 0.5%). The most common allele type of the CAP59, GPD1, IGS1, LAC1, PLB1, SOD1, and URA5 gene in C. neoformans was allele type AT1 (39.2%), AT1 (44.6%), AT1 (61.8%), AT5 (23.5%), AT1 (33.3%), AT1 (71.1%), and AT1 (42.6%), respectively (S1 Table). The MLST analysis divided the 204 C. neoformans isolates into 32 STs, comprised of ST5 (47 strains; 23.0%), ST2 (27 strains; 13.2%), ST63 (20 strains; 9.8%), ST40 (20 strains; 9.8%), and other STs (90 strains; 44.1%). Nine novel STs were identified in this study, specifically, ST576, ST509, ST512, ST515, ST531, ST577, ST578, ST579, and ST580. Most isolates were mating type alpha (197 strains, 96.9%) and 7 strains (3.4%) were mating type a, which all belonged to VNIV (S1 Table and Fig 1). When considering only the representative strains from each patient/source, the ST5 genotype was the most prevalent (42/170 strains; 24.7%; Table 2).
Fig 1. Neighbor-joining phylogenetic tree inferred using the concatenated sequences of the seven MLST loci (CAP59, GPD1, IGS1, LAC1, PLB1, SOD1, and the URA5) of the C. neoformans sequence types (STs) investigated in the present study.
Bootstrap values, based on 1,000 replicates, are reported at each branch node and only bootstrap above 70% are presented in phylogenetic tree. (Abbreviations; CLIN: clinical, ENV: environmental, VET: veterinary, A: serotype A, D: serotype D, U: Unknown, USA: United State of America, THA: Thailand, DNK: Denmark, ITA: Italy, * represents a novel ST).
Table 2. Sequence type frequency of representative C. neoformans/C. gattii species complexes isolates.
| Species | Molecular type | Sequence typea | Number of isolates | |||
|---|---|---|---|---|---|---|
| CLIN | ENV | VET | Total (%) | |||
| C. neoformans | VNI | 2 | 23 | 3 | 26 (15.3%) | |
| 4 | 1 | 8 | 9 (5.3%) | |||
| 5 | 38 | 2 | 2 | 42 (24.7%) | ||
| 6 | 1 | 1 (0.6%) | ||||
| 23 | 10 | 5 | 1 | 16 (9.4%) | ||
| 32 | 7 | 3 | 10 (5.9%) | |||
| 39 | 2 | 2 (1.2%) | ||||
| 58 | 1 | 1 (0.6%) | ||||
| 63 | 11 | 1 | 12 (7.1%) | |||
| 67 | 1 | 1 (0.6%) | ||||
| 93 | 4 | 4 (2.4%) | ||||
| 202 | 1 | 1 (0.6%) | ||||
| 290 | 1 | 1 (0.6%) | ||||
| VNII | 9 | 1 | 1 (0.6%) | |||
| 40 | 10 | 10 (5.9%) | ||||
| 42 | 4 | 4 (2.4%) | ||||
| 43 | 4 | 4 (2.4%) | ||||
| 233 | 1 | 1 (0.6%) | ||||
| 334 | 1 | 1 (0.6%) | ||||
| 576 | 1 | 1 (0.6%) | ||||
| VNIV | 112 | 4 | 1 | 5 (2.9%) | ||
| 135 | 1 | 1 (0.6%) | ||||
| 160 | 1 | 1 (0.6%) | ||||
| 180 | 4 | 1 | 5 (2.9%) | |||
| 509 | 1 | 1 (0.6%) | ||||
| 512 | 1 | 1 (0.6%) | ||||
| 515 | 1 | 1 (0.6%) | ||||
| 531 | 1 | 1 (0.6%) | ||||
| 577 | 2 | 2 (1.2%) | ||||
| 578 | 2 | 2 (1.2%) | ||||
| 579 | 1 | 1 (0.6%) | ||||
| 580 | 1 | 1 (0.6%) | ||||
| Total | 131 | 34 | 5 | 170 (100.0%) | ||
| C. gattii | VGI | 51 | 1 | 1 | 2 (8.7%) | |
| 106 | 1 | 1 (4.3%) | ||||
| 162 | 1 | 1 (4.3%) | ||||
| 208 | 1 | 1 (4.3%) | ||||
| VGII | 20 (VGIIb) | 1 | 1 (4.3%) | |||
| VGIII | 68 (VGIIIb) | 2 | 2 (8.7%) | |||
| 75 (VGIIIa) | 5 | 1 | 6 (26.1%) | |||
| 84 (VGIIIb) | 1 | 1 (4.3%) | ||||
| 86 (VGIIIb) | 1 | 1 (4.3%) | ||||
| 89 (VGIIIa) | 1 | 1 (4.3%) | ||||
| 93 (VGIIIa) | 1 | 1 (4.3%) | ||||
| 142 (VGIIIb) | 2 | 2 (8.7%) | ||||
| 146 (VGIIIa) | 1 | 1 (4.3%) | ||||
| 164 (VGIIIb) | 1 | 1 (4.3%) | ||||
| 209 (VGIIIb) | 1 | 1 (4.3%) | ||||
| Total | 21 | 2 | 23 (100.0%) | |||
Abbreviations: CLIN, clinical; ENV, environment; VET, veterinary
aBold with underline signifies the novel sequence types found in this study
High-virulence VGIIIa/serotype B clade was most prevalent among C. gattii
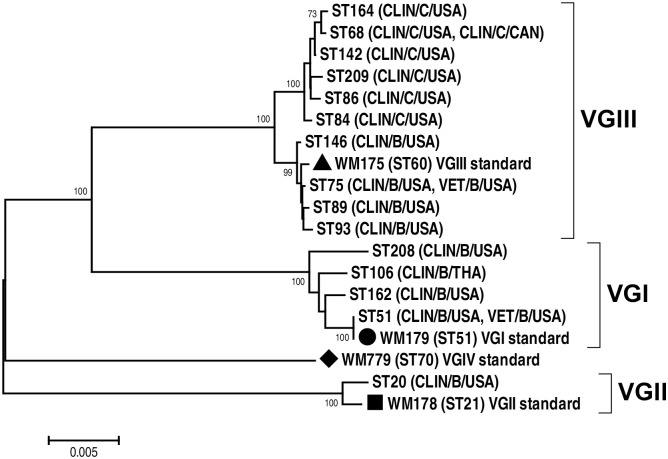
For C. gattii, the most common allele type of the CAP59, GPD1, IGS1, LAC1, PLB1, SOD1, and URA5 genes was allele type AT18 (37.5%), AT9 (37.5%), AT1 (37.5%), AT3 (29.2%), AT6 (29.2%), AT28 (37.5%), and AT19 (29.2%), respectively (S1 Table). Of the 24 C. gattii isolates, 15 STs were identified: VGIII/ST75 (6 strains; 25.0%), VGIII/ST142 (2 strains; 8.3%), VGI/ST208 (2 strains; 8.3%), and other STs (14 strains; 58.4%). Most isolates were mating type alpha (19 strains, 79.2%), while 5 strains (20.8%) were mating type a, one in VGI and four in VGIII (S1 Table and Fig 2). When considering only the representative strains from each patient/source, the high-virulence VGIIIa/serotype B clade was the most common (9/23 isolates; 39.1% of all C. gattii isolates). The high-virulence VGIIa/ST20 genotype was the only one isolated, and the less virulent VGIIb/ST7 genotype was not present among the herein studied pre-HIV pandemic isolates (Table 2).
Fig 2. Neighbor-joining phylogenetic tree inferred using the concatenated sequences of the seven MLST loci (CAP59, GPD1, IGS1, LAC1, PLB1, SOD1, and the URA5) of the C. gattii sequence types (STs) investigated in the present study.
Bootstrap values, based on 1,000 replicates, are reported at each branch node and only bootstrap above 70% are presented in phylogenetic tree. (Abbreviations; CLIN: clinical, VET: veterinary, B: serotype B, C: serotype C, USA: United State of America, THA: Thailand, CAN: Canada).
VNIV had the highest genetic diversity among the C. neoformans species complex
Significant differences in the Hd values were observed in comparisons between the isolates of each molecular type (VNI vs VNIV, p = 0.040 and VNII vs VNIV, p = 0.003). VNIV isolates demonstrated the highest genetic diversity, having the highest Hd value (0.926) and π (0.00308; Table 3).
Table 3. Polymorphism summary and neutrality test for groups of isolates of C. neoformans species complex according to molecular types and sources of isolation.
| Locus | pb | S | h | Hd | k | π | D | |
|---|---|---|---|---|---|---|---|---|
| VNI (n = 126) | CAP59 | 560 | 1 | 2 | 0.498 | 0.498 | 0.00089 | 1.860 |
| GPD1 | 538 | 2 | 4 | 0.526 | 13.586 | 0.02525 | -2.456* | |
| IGS1 | 721 | 11 | 3 | 0.236 | 2.353 | 0.00326 | 0.404 | |
| LAC1 | 470 | 5 | 5 | 0.745 | 1.691 | 0.00360 | 1.708 | |
| PLB1 | 533 | 2 | 3 | 0.610 | 0.989 | 0.00185 | 2.471* | |
| SOD1 | 536 | 1 | 2 | 0.016 | 0.016 | 0.00003 | -1.000 | |
| URA5 | 637 | 2 | 3 | 0.549 | 0.605 | 0.00095 | 0.941 | |
| Concatenated | 4,001 | 24 | 13 | 0.815 | 6.659 | 0.00166 | 1.457 | |
| VNII (n = 21) | CAP59 | 560 | 1 | 2 | 0.381 | 0.381 | 0.00068 | 0.656 |
| GPD1 | 544 | 1 | 2 | 0.381 | 0.381 | 0.00070 | 0.656 | |
| IGS1 | 720 | 21 | 4 | 0.471 | 3.371 | 0.00468 | -1.594 | |
| LAC1 | 471 | 3 | 2 | 0.095 | 0.286 | 0.00061 | -1.727 | |
| PLB1 | 533 | 3 | 2 | 0.381 | 1.143 | 0.00214 | 0.973 | |
| SOD1 | 529 | 6 | 3 | 0.610 | 2.229 | 0.00421 | 1.056 | |
| URA5 | 637 | 4 | 3 | 0.452 | 1.238 | 0.00194 | 0.324 | |
| Concatenated | 3,994 | 38 | 6 | 0.729 | 8.933 | 0.00224 | -0.606 | |
| VNIV (n = 22) | CAP59 | 560 | 5 | 5 | 0.616 | 1.353 | 0.00242 | -0.123 |
| GPD1 | 546 | 4 | 4 | 0.574 | 1.174 | 0.00215 | 0.119 | |
| IGS1 | 685 | 9 | 9 | 0.858 | 3.174 | 0.00463 | 0.863 | |
| LAC1 | 473 | 12 | 8 | 0.874 | 2.763 | 0.00584 | -0.657 | |
| PLB1 | 517 | 2 | 3 | 0.668 | 0.816 | 0.00158 | 1.048 | |
| SOD1 | 525 | 4 | 5 | 0.511 | 0.921 | 0.00175 | -0.530 | |
| URA5 | 639 | 9 | 7 | 0.837 | 2.421 | 0.00379 | -0.157 | |
| Concatenated | 3,940 | 44 | 12 | 0.926 | 12.147 | 0.00308 | -0.082 | |
| Clinical (n = 131) | CAP59 | 560 | 44 | 6 | 0.692 | 7.489 | 0.01337 | -0.206 |
| GPD1 | 537 | 58 | 7 | 0.709 | 9.961 | 0.01855 | -0.242 | |
| IGS1 | 678 | 107 | 11 | 0.542 | 17.641 | 0.02602 | -0.372 | |
| LAC1 | 467 | 52 | 10 | 0.800 | 8.946 | 0.01916 | -0.186 | |
| PLB1 | 517 | 42 | 7 | 0.756 | 7.712 | 0.01492 | 0.010 | |
| SOD1 | 518 | 69 | 8 | 0.423 | 11.692 | 0.02257 | -0.316 | |
| URA5 | 637 | 45 | 12 | 0.685 | 7.274 | 0.01142 | -0.358 | |
| Concatenated | 3,913 | 417 | 23 | 0.866 | 70.307 | 0.01797 | -0.289 | |
| Environment (n = 34) | CAP59 | 560 | 42 | 7 | 0.733 | 15.927 | 0.02844 | 1.991 |
| GPD1 | 537 | 55 | 7 | 0.560 | 21.160 | 0.03940 | 1.903 | |
| IGS1 | 682 | 100 | 8 | 0.638 | 36.367 | 0.05332 | 1.825 | |
| LAC1 | 467 | 54 | 11 | 0.820 | 19.642 | 0.04206 | 1.786 | |
| PLB1 | 517 | 39 | 6 | 0.802 | 15.269 | 0.02953 | 2.164* | |
| SOD1 | 527 | 64 | 4 | 0.437 | 25.194 | 0.04781 | 2.251* | |
| URA5 | 636 | 40 | 8 | 0.832 | 14.214 | 0.02235 | 1.634 | |
| Concatenated | 3,926 | 393 | 14 | 0.889 | 146.540 | 0.03733 | 1.962 | |
| Veterinary (n = 5) | CAP59 | 560 | 40 | 3 | 0.833 | 26.333 | 0.04702 | 2.148 |
| GPD1 | 537 | 52 | 3 | 0.833 | 34.333 | 0.06394 | 2.191 | |
| IGS1 | 683 | 90 | 3 | 0.700 | 53.000 | 0.07760 | 1.723 | |
| LAC1 | 468 | 42 | 4 | 0.900 | 24.600 | 0.05256 | 1.658 | |
| PLB1 | 517 | 38 | 4 | 0.900 | 22.400 | 0.04333 | 1.714 | |
| SOD1 | 527 | 63 | 2 | 0.600 | 37.800 | 0.07173 | 1.892* | |
| URA5 | 637 | 35 | 4 | 0.900 | 20.200 | 0.03171 | 1.518 | |
| Concatenated | 3,929 | 362 | 4 | 0.900 | 213.600 | 0.05436 | 1.726 |
Abbreviations: pb, total number of sites in alignments—excluding indels and missing data; S, polymorphic sites; h, number of haplotypes; Hd, haplotype diversity; k, average number of nucleotide difference; π, nucleotide diversity; D, Tajima’s D
*P-value < 0.05 as the significance level
The independent MLST loci analysis for the Hd of each molecular type showed that the LAC1 locus was the most variable in the isolates of VNI (Hd = 0.745) and VNIV (Hd = 0.874), while least variable in the VNII (Hd = 0.095) isolates. The SOD1 locus was the most variable in the VNII (Hd = 0.610) isolates, whereas it was the least variable in the isolates of VNI (Hd = 0.016) and VNIV (Hd = 0.511; Table 3). The neutrality test, Tajima’s D (D), showed evidence of balancing selection or expansion of rare polymorphisms for most loci for the overall C. neoformans species complex (p > 0.05; Table 3).
Lowest genetic diversity was found among the clinical isolates of the C. neoformans species complex
When isolates were stratified according to their isolation sources, the Hd value was lowest among the clinical isolates (0.866) compared to those from other sources (ENV: 0.889, VET: 0.900), especially with those isolates from a veterinary source (CLIN vs VET, p-value = 0.041). The π value was lowest among the clinical isolates (0.01797) compared to the environment (0.03733) and veterinary (0.05436) isolates (CLIN vs ENV, p = 0.001; CLIN vs VET, p < 0.001; ENV vs VET, p = 0.035; Table 3).
When each locus was considered individually, the most variable one in the isolates from clinical, environmental, and veterinary source was LAC1 (Hd = 0.800; π = 0.01916), LAC1 (Hd = 0.820; π = 0.04206), and LAC1/PLB1/URA5 (Hd = 0.900; π = 0.05256/0.04333/0.03171), respectively (Table 3). On the other hand, the least variable locus in the isolates from the clinical, environmental, and veterinary sources was SOD1 with Hd = 0.423; π = 0.02257, Hd = 0.437; π = 0.04781, and Hd = 0.600; π = 0.07173, respectively (Table 3). No significant difference in neutrality test among each source of isolation was observed (p > 0.05; Table 3).
The nucleotide diversity was higher among the VGI than VGIII isolates in C. gattii
The VGI and VGIII isolates showed no significant differences in their Hd values (0.867 and 0.875, respectively; p = 0.110), and the π values of the VGI isolates were not significantly different from those of the VGIII isolates (0.01436 and 0.00328, respectively; p = 0.305). No group was found to be under significant selective pressure according to the neutrality test (p > 0.05; Table 4). A comparison of the different sources among the C. gattii isolates was not pertinent as there were no environmental isolates available prior to HIV epidemic [46] and the number of veterinary isolates was too low (2 isolates; Table 4).
Table 4. Polymorphism summary and neutrality test for groups of isolates of C. gattii species complex according to molecular types and sources of isolation.
| Locus | pb | S | h | Hd | k | π | D | |
|---|---|---|---|---|---|---|---|---|
| VGI (n = 5) | CAP59 | 557 | 5 | 3 | 0.733 | 2.267 | 0.00407 | 0.197 |
| GPD1 | 547 | 5 | 3 | 0.800 | 2.667 | 0.00488 | 1.219 | |
| IGS1 | 690 | 6 | 4 | 0.867 | 2.867 | 0.00415 | 0.520 | |
| LAC1 | 473 | 7 | 3 | 0.733 | 3.733 | 0.00789 | 1.267 | |
| PLB1 | 532 | 2 | 3 | 0.733 | 0.867 | 0.00163 | -0.050 | |
| SOD1 | 701 | 10 | 4 | 0.867 | 4.333 | 0.00618 | -0.063 | |
| URA5 | 638 | 7 | 4 | 0.867 | 2.933 | 0.00460 | -0.251 | |
| Concatenated | 4,133 | 158 | 4 | 0.867 | 59.333 | 0.01436 | -0.924 | |
| VGIII (n = 17) | CAP59 | 557 | 5 | 2 | 0.529 | 2.647 | 0.00475 | 2.548* |
| GPD1 | 547 | 4 | 4 | 0.669 | 1.471 | 0.00269 | 0.743 | |
| IGS1 | 692 | 4 | 4 | 0.654 | 1.721 | 0.00249 | 1.390 | |
| LAC1 | 472 | 10 | 6 | 0.787 | 3.956 | 0.00838 | 1.235 | |
| PLB1 | 535 | 4 | 4 | 0.676 | 1.397 | 0.00261 | 0.553 | |
| SOD1 | 711 | 2 | 3 | 0.581 | 0.632 | 0.00089 | 0.172 | |
| URA5 | 638 | 6 | 6 | 0.779 | 1.794 | 0.00281 | 0.037 | |
| Concatenated | 4,152 | 35 | 10 | 0.875 | 13.618 | 0.00328 | 1.296 |
Abbreviations: pb, total number of sites in alignments—excluding indels and missing data; S, polymorphic sites; h, number of haplotypes; Hd, haplotype diversity; k, average number of nucleotide difference; π, nucleotide diversity, D; Tajima’s D
*P-value < 0.05 as the significance level
An independent analysis of each locus showed the least variability in the CAP59, LAC1, and PLB1 loci in VGI (Hd = 0.733; π = 0.00407, 0.00789, and 0.00163, respectively) and in the CAP59 locus in the VGIII group (Hd = 0.529; π = 0.00475). The most variable loci in the VGI isolates were the IGS1, SOD1, and URA5 loci (Hd = 0.867; π = 0.00415, 0.00618, and 0.00460, respectively), while the URA5 locus was the most variable of the VGIII isolates (Hd = 0.779; π = 0.00281;Table 4).
Linkage disequilibrium were detected among C. neoformans/C. gattii species complexes populations
Among the different molecular types of the C. neoformans species complex, all molecular types values for PcP, IA, and rBarD were strongly rejected (p < 0.05) the null hypothesis of linkage equilibrium and free recombination (Table 5). The minimal number of recombination events (Rm) showed that the VNIV group had a higher Rm (10) than the VNI (2) and VNII groups (5), and the VNII and VNIV groups showed evidence for recombination (p-values for PHI test of < 0.001 and 0.019, respectively; Table 5). The independent loci analysis showed that two recombination events were identified in the IGS1 gene in the VNII population, one in the CAP59, LAC 1, and URA5 genes, and three in the IGS1 gene of the VNIV population (Table 5).
Table 5. Multilocus linkage disequilibrium and recombination analyses amongst C.neoformans/C. gattii species complexes according to different molecular type and source of isolate.
| Population | PcPa | IAb | rBarDc | Rmd | Rm per gene | PHI test |
|---|---|---|---|---|---|---|
| Molecular type | ||||||
| VNI (n = 126) | 0.809*** | 1.699*** | 0.307*** | 2 | 0.617 | |
| VNII (n = 21) | 1.000*** | 4.078*** | 0.692*** | 5 | IGS1 = 2 | < 0.001 |
| VNIV (n = 22) | 0.714*** | 1.551*** | 0.267*** | 10 | CAP59, LAC 1, URA5 = 1/ IGS1 = 3 | 0.019 |
| VGI (n = 5) | 1.000* | 3.854*** | 0.653*** | 4 | 0.568 | |
| VGIII (n = 17) | 0.952*** | 3.375*** | 0.566*** | 4 | 0.094 | |
| Sources of C. neoformans species complex | ||||||
| Clinical (n = 131) | 0.809*** | 3.361*** | 0.566*** | 12 | URA5 = 1/ IGS1, SOD1 = 2/ LAC1 = 4 | < 0.001 |
| Environmental (n = 34) | 0.952*** | 3.033*** | 0.521*** | 13 | GPD1, IGS1 = 2/ CAP59, LAC1 = 3 | 0.509 |
| Veterinary (n = 5) | 1.000ns | 0.615ns | 0.632ns | 0 | > 0.999 | |
| Sources of C. gattii species complex | ||||||
| Clinical (n = 21) | 0.952*** | 3.577*** | 0.605*** | 11 | URA5 = 1/ IGS = 2/ LAC1, SOD = 3 | 0.016 |
| Veterinary (n = 2)e | ND | ND | ND | ND | ND | ND |
apercentage of phylogenetically compatible pairs (PcP) of loci;
bindex of association;
cscaled index of association (IA) by the number of loci (m– 1);
dminimal number of recombination based on each population;
enot determined—there were not enough samples for analysis
As to the C. gattii species complex, all molecular types showed values for PcP, IA, and rBarD that rejected (p < 0.05) the null hypothesis of linkage equilibrium and free recombination (Table 5). The PHI test did not show evidence of recombination among the molecular types of the C. gattii species complex, VGI (p = 0.568) and VGIII (p = 0.094; Table 5).
Based on the sources of the isolates, the C. neoformans species complex showed values for PcP, IA and, rBarD that strongly rejected (p < 0.05) the null hypothesis of linkage equilibrium and free recombination for the clinical and environmental sources, but, this hypothesis was not rejected for the veterinary population (Table 5). The environmental (13) and clinical (12) isolates had a higher Rm than the veterinary isolates (0). Interestingly, the concatenated dataset showed evidence for recombination only in the clinical isolates (p-value of the PHI test < 0.001; Table 5). Taken together, the results from the different sources showed that, in the case of the clinical population, there was one recombination event in the URA5 gene, two each in the IGS1 and SOD1 genes, and four in the LAC1 gene. As to the environmental population, there was two recombination events each in the GPD1 and IGS1 genes, and three each in the CAP59 and LAC1 genes (Table 5).
The C. gattii species complex showed values for PcP, IA and rBarD that rejected (p < 0.05) the null hypothesis of linkage equilibrium and free recombination (Table 5). The minimal number of recombination events (Rm) revealed that the clinical group had high Rm (10) and showed evidence for recombination (p-value for the PHI test of 0.016; Table 5). The independent loci analysis showed that in the case of the clinical population, there was one recombination event in the URA5 gene, two in the IGS1 gene, and three each in the LAC 1 and SOD1 genes (Table 5).
Genetic differentiation among C. neoformans species complex
Among the different genotype, AMOVA showed that the proportion of variance components within populations of the C. neoformans (51%) and C. gattii (67%) species complexes were higher than the proportions of variance components found among populations. The pairwise FST tests showed that both species complexes had significantly distinct subpopulations (p < 0.001) (Table 6).
Table 6. Hierarchical analysis of molecular variance (AMOVA) of different populations of C. neoformans/C. gattii species complexes.
| d.f.a | Sum of squares | Variance components | Percentage of variations | FSTb | |
|---|---|---|---|---|---|
| All C. neoformans isolates: VNI (126), VNII (21), and VNIV (22) | |||||
| Among populations | 2 | 220.928 | 1.660 | 49% | 0.493 (p < 0.001) |
| Within populations | 307 | 518.311 | 1.705 | 51% | |
| Total | 309 | 739.239 | 3.365 | 100% | |
| All C. gattii isolates: clinical (22) and veterinary (2) | |||||
| Among populations | 1 | 19.748 | 1.164 | 33% | 0.332 (p < 0.001) |
| Within populations | 40 | 88.824 | 2.337 | 67% | |
| Total | 41 | 108.571 | 3.501 | 100% | |
| All C. neoformans isolates: clinical (131), environmental (34), and veterinary (5) | |||||
| Among populations | 2 | 24.258 | 0.147 | 6% | 0.058 (p < 0.001) |
| Within populations | 405 | 971.408 | 2.399 | 94% | |
| Total | 407 | 995.667 | 2.546 | 100% | |
| All C. gattii isolates: clinical (22) and veterinary (2) | |||||
| Among populations | 1 | 4.189 | 0.190 | 6% | 0.063 (p = 0.172) |
| Within populations | 46 | 128.727 | 2.798 | 94% | |
| Total | 47 | 132.917 | 2.988 | 100% | |
adegrees of freedom;
b the population differentiation test
Based on the sources of isolation, AMOVA showed that almost all variance components were found within populations rather than among populations in both the C. neoformans species complex (94%) and C. gattii species complex (94%). The pairwise FST tests were calculated among populations, and they showed that the C. neoformans species complex had significantly distinct populations (p < 0.001); in contrast, the C. gattii species complex did not have statistical support for differentiation (p = 0.172; Table 6).
Sequential clinical strains showed less evidence of sequence type change
Of the 16 patients with sequential strains (S3 Table), 11 patients (68.8%) were identified as VNI, 3 patients (18.8%) as VNII, 1 patient (6.2%) as VNIV, and 1 patient (6.2%) as VGI. Majority of patients were infected by mating type alpha (15/16 patients, 93.8%) and one patient was infected by mating type a.
When each patient was considered individually, 15 patients (93.8%) were infected with the same sequence type including ST2/VNI (1 patient), ST5/VNI (3 patients), ST40/VNII (1 patient), ST42/VNII (1 patient), ST43/VNII (1 patient), ST58/VNI (1 patient), ST63/VNI (3 patients), ST93/VNI (1 patient), ST290/VNI (1 patient), ST578/VNIV (1 patient), and ST208/VGI (1 patient). One patient (patient B) was infected with a different sequence type: ST32/VNI and ST2/VNI (isolated after long-term treatment with a relapsed infection).
High-virulence genotypes did not express more virulence factors than low-virulence genotypes
The values of the in vitro expression of the virulence factors among C. neoformans/C. gattii are presented in S2 Table. Based on the sequence type groups, the ST5 strains—recognized as a high prevalence and virulence genotype in the VNI molecular type [47, 48]—produced significantly smaller capsules than the strains in other STs in VNI (2.04 and 2.26, respectively; p = 0.001). Furthermore, there was no difference in the in vitro expressions of the virulence factors of the isolates of VNI and the other molecular types in C. neoformans (Table 7).
Table 7. Comparison of in vitro virulence characteristics of C. neoformans in ST5 and non-ST5 strains in the VNI group.
| Characteristics | Group | ST5 (n = 47) | Non-ST5 (n = 99) | P-valuea |
|---|---|---|---|---|
| Mean ± SD O.D. value of urease activity | 0.382 ± 0.13 | 0.380 ± 0.15 | 0.938 | |
| Mean ± SD O.D. value of melanin production | 0.180 ± 0.02 | 0.178 ± 0.02 | 0.565 | |
| Mean ± SD of population doubling time | 114.28 ± 12.65 | 113.40 ± 11.01 | 0.831 | |
| Mean ± SD ratio of capsule production | 2.04 ± 0.36 | 2.26 ± 0.39 | 0.001 | |
Abbreviation: O.D., optical density
aAnalyzed associations of different sequence-type groups by two-tailed unpaired t-test, with a p-value of < 0.05 as significance level
Likewise, there was no significant difference in the in vitro expressions of the virulence factors of VGIIIa/serotype B and VGIIIb/serotype C (Table 8).
Table 8. Comparison of in vitro virulence characteristics of C. gattii in VGIIIa/serotype B and VGIIIb/serotype C strains.
| Characteristics | Group | VGIIIa/serotype B (n = 9) | VGIIIb/serotype C (n = 8) | P-valuea |
|---|---|---|---|---|
| Mean ± SD O.D. value of urease activity | 0.316 ± 0.12 | 0.281 ± 0.12 | 0.552 | |
| Mean ± SD O.D. value of melanin production | 0.183 ± 0.02 | 0.184 ± 0.01 | 0.931 | |
| Mean ± SD of population doubling time | 137.90 ± 26.17 | 136.90 ± 22.03 | 0.934 | |
| Mean ± SD ratio of capsule production | 2.88 ± 0.36 | 2.99 ± 0.39 | 0.685 | |
Abbreviation: O.D., optical density
aAnalyzed associations of different sequence-type groups by two-tailed unpaired t-test, with a p-value of < 0.05 as significance level
Discussion
Before the standard molecular typing system had been established for the C. neoformans/C. gattii species complexes by Meyer et al in 1999 [49], our understanding of the cryptococcal molecular epidemiology was extremely limited. Since then, significant progress has been made in molecular typing as well as in our understanding of the genotype-specific characteristics of the two species complexes [13, 15, 22, 38, 50]. Still, the association of cryptococci with immunocompromised humans became clear only after the advent of the HIV-pandemic in 1980s. Since the populations of the two species complexes prior to the HIV pandemic has not been studied, we conducted a molecular epidemiological study of the isolates from the pre-HIV era and analyzed the in vitro expressions of the known virulence factors. As is the case with the isolates of the post-HIV era, the most prevalent molecular type of C. neoformans was VNI (146/204 isolates; 71.6%), indicating that VNI is a major molecular type of C. neoformans in both the pre-HIV- and HIV-pandemic eras (S4 Table). Interestingly, we found that VGIII was the predominant molecular type of the C. gattii species complex in our collection during the pre-HIV-pandemic era (17/24 isolates; 70.8%). This finding contrasts with the fact that the VGI and VGII molecular types are currently known as the most frequent types among the C. gattii species complex [44, 51]. This might be due to the geographic distribution that VGIII was commonly reported in the USA whereas the isolates were mostly recovered in the USA (Table 1 and S4 Table) [22, 51].
Further genotype analysis of C. neoformans by MLST showed that ST5/VNI was the most common genotype (43/164 isolates; 26.2%) among the clinical isolates of C. neoformans. This result is similar to the findings of a recent systematic review of Asian cryptococcosis in which ST5/VNI was reported to be the most common genotype among non-HIV patients in both East Asian (87.9%) and other Asian (39.3%) countries [52]. Our findings is also similar to what has been reported for Vietnam (83.7%) [47] and Laos (25%) [53]. Three other common genotypes—namely, ST2/VNI (13.2%), ST63/VNI (9.8%), and ST40/VNII (9.8%)—were reported in Asia, Africa, Europe, and the United States [4, 10, 11, 23, 54]. Interestingly, the most common genotype among the environmental isolates, ST4/VNI (25%), was also the most common (32.6%) sequence type isolated from HIV patients outside of the East Asian countries [52]. This result concurs with the proposal that HIV patients contracted cryptococcosis from their environment as the diversity of the genotypes in the environment is reflected in the clinical isolates [55]. Similarly, ST4/VNI has been found to be very rare among clinical isolates from Europe, the Mediterranean area (1%) [54], and Brazil (0%) [56]; predictably, they have rarely been isolated from the environment in those geographic areas.
Among the isolates from both clinical and environmental sources, 9 novel STs were identified—one in VNII (ST576), and 8 in VNIV (ST509, ST512, ST515, ST531, ST577, ST578, ST579, and ST580. This suggests, that these STs may have been suppressed during the post-pandemic era. As the response to stressful conditions, including the host immune response, were different for each serotype, survival of these 9 STs may have been affected during the post-HIV era since HIV patients are the major source for cryptococcal isolates [57–59]. On the other hand, this might simply have been caused by a sampling bias during the HIV pandemic. Therefore, further sampling of more isolates during this present time would be beneficial.
Among the C. gattii strains, the ST75/VGIII (6/24 strains; 25.0%) genotype was the most common ST isolated during the pre-HIV-pandemic era. The prevalence of this ST is actually consistent with the prevalence of ST isolated during the HIV-pandemic era as ST75/VGIII was the most common causative agent of human and animal cryptococcosis due to C. gattii in North and South America [20, 22, 60]. Moreover, a recent study reported that the VGIIIa/serotype B was more virulent than VGIIIb/serotype C [18]. These data could support the predominance of this ST in North America. Surprisingly, the absence of the high-virulence Vancouver outbreak genotype, ST20/VGIIa during the pre-HIV-pandemic era supports the hypothesis that this genotype was a result of a recent Cryptococcus evolution/recombination [14, 61].
Previous population genetic analyses of HIV-pandemic C. neoformans isolates have shown that the VNIV isolates are genetically more diverse and have a higher recombination rate than the VNI and VNII isolates [9, 54, 62–64]. Our study showed the same results suggesting that the manner of VNIV propagation is different from those of VNI and VNII (primarily clonal expansion in VNI and VNII vs recombinational events in VNIV). This was confirmed by our result showing the highest Rm in VNIV. Moreover, we found that the clinical isolates of C. neoformans had a lower genetic diversity (Hd = 0.866, π = 0.01797) than the environmental isolates (Hd = 0.889, π = 0.03733). This finding is similar to that of a European study, in which a higher diversity was observed among the environmental isolates than the clinical isolates [54]. However, the contradiction between a high genetic diversity (Hd = 0.900, π = 0.05436) for the veterinary isolates remains disputable; more isolates should be investigated since only 5 were analyzed in the current study. As for the C. gattii species complex, the degree of genetic diversity between the different molecular types which was similar except for the higher π value was higher for VGI compared than VGIII (0.01436 vs 0.00328). This value could have been affected by the number of polymorphic sites because VGI had approximately 5 times as many polymorphic sites as VGIII (158 vs 35) [65]. Moreover, there has been a report of a low genetic flow in the VGIII molecular type [18].
The linkage equilibrium revealed significant disequilibrium and the genetic differentiation analysis detected a significantly low genetic differentiation in almost all subpopulations of the C. neoformans/C. gattii species complex with different molecular types and sources of isolation These finding suggest limited genetic exchange among each population, as proposed by previous studies [5, 9, 12, 66, 67]. This low genetic exchange or recombination rate might increase the risk of extinction due to reductions in the genetic diversity and a loss of population fitness, as suggested by a recent study [68]. Therefore, some populations in the pre-HIV pandemic strains might have been suppressed during the HIV pandemic, as in the cases of the novel pre-HIV pandemic genotypes.
As to the polymorphism of each locus of the housekeeping genes, the LAC1 gene possessed the highest number of haplotypes, a higher π, and a higher Rm compared with those of the other genes in both C. neoformans species complex. In addition, the LAC1 gene in environmental isolates had a higher π value than the clinical isolates for both VNI and VNIV; this can be explained by the fact that the fungus has to adapt itself to the substrate in order to survive in the environment [54]. In fact, a recent study on the LAC1 gene showed that polymorphism within the LAC1 gene, whose protein product catalyzes melanin synthesis, to be associated with variable melanin levels, suggesting a correlation between gene polymorphism and melanin production levels [69].
One of the 16 patients with sequential strains was infected with different cryptococcal strains. Though this has rarely occurred, mixed and/or sequential infections by different cryptococcal strains in a single patient have been reported [70–72]. One study reported that a patient was infected with two different strains after 11 days in hospital, and that the second strain prove to be less susceptible to antifungal treatment than the first [70]. Another study reported that 4 patients had relapsed infections caused by isolates that were genetically different from the initial etiologic agents [71]. Similar circumstances were observed in our case of sequential infections in which a relapsed infection caused by a different isolate after long-term treatment. In another study, some HIV patients in the Ivory Coast were suspected of having been infected by mixed strains, but only one strain was isolated at diagnosis; and the second strain, which was more resistant to antifungal drug treatment, emerged later in a fungal culture [73].
Although a higher virulence has been documented for the ST5/VNI and VGIIIa/serotype B [18, 47], our in vitro analysis of the virulence factors in vitro did not correlate with these strain types. There were no significant differences between the ST5/VNI and non-ST5/VNI strains, except that there was a smaller capsule production in the less virulent ST5/VNI strains. While the difference in capsule size was significant, it was minimal (2.04 vs 2.26); it is also known that the in vitro capsule size does not correlate with the in vivo virulence of C. neoformans [74]. Only the absence or presence of the capsule has been directly correlated with cryptococcal virulence [75–77], and this finding has been supported by pathogenesis studies that showed the capsule accumulation in vivo was higher than in vitro [78, 79]. A similar picture was seen with the C. gattii in vitro expression of virulence factors: no significant difference was observed between the high-virulence VGIIIa/serotype B and the low-virulence VGIIIb/serotype C strains.
In conclusion, these data marked high genetic variability and recombination events in the pre-HIV-pandemic strains of the C. neoformans/C. gattii species complexes. The identification of novel STs in this study suggests that some STs were either suppressed or disappeared during the HIV pandemic. The difference in the virulence of the high- and low-virulence genotypes might not have developed until after the start of the pandemic. However, to gain in-depth information on the evolution of these historical strains, analysis of amino acid changes and specific indels with a specifically targeted whole gene sequencing is needed. Moreover, due to a low number of C. gattii species complex strains used in the current study, additional C. gattii species complex strains need to be collected for use in further investigations.
Supporting information
(XLSX)
(DOCX)
(XLSX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The study was supported by the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (grant number PHD/0056/2561, to PN and SP). PN was also supported by Siriraj Research Development Fund, Faculty of Medicine Siriraj Hospital, Mahidol University (grant number (IO) R016133012) and SP by the Siriraj Graduate Scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dou HT, Xu YC, Wang HZ, Li TS. Molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii in China between 2007 and 2013 using multilocus sequence typing and the DiversiLab system. Eur J Clin Microbiol Infect Dis. 2015;34(4):753–62. Epub 2014/12/05. 10.1007/s10096-014-2289-2 [DOI] [PubMed] [Google Scholar]
- 2.Fang W, Fa Z, Liao W. Epidemiology of Cryptococcus and cryptococcosis in China. Fungal Genet Biol. 2015;78:7–15. Epub 2014/12/03. 10.1016/j.fgb.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 3.Meyer W, Castaneda A, Jackson S, Huynh M, Castaneda E, IberoAmerican Cryptococcal Study G. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 2003;9(2):189–95. 10.3201/eid0902.020246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaocharoen S, Ngamskulrungroj P, Firacative C, Trilles L, Piyabongkarn D, Banlunara W, et al. Molecular epidemiology reveals genetic diversity amongst isolates of the Cryptococcus neoformans/C. gattii species complex in Thailand. PLoS Negl Trop Dis. 2013;7(7):e2297 Epub 2013/07/19. 10.1371/journal.pntd.0002297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simwami SP, Khayhan K, Henk DA, Aanensen DM, Boekhout T, Hagen F, et al. Low diversity Cryptococcus neoformans variety grubii multilocus sequence types from Thailand are consistent with an ancestral African origin. PLoS Pathog. 2011;7(4):e1001343 10.1371/journal.ppat.1001343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer W, Gilgado F, Ngamskulrungroj P, Trilles L, Hagen F, Castañeda E, et al. Molecular Typing of the Cryptococcus neoformans/Cryptococcus gattii Species Complex In: Heitman JKT, Kwon-Chung K, Perfect J, Casadevall A, editor. Cryptococcus. Washington, DC: American Society of Microbiology; 2011. p. 327–57. [Google Scholar]
- 7.Enright MC, Spratt BG. Multilocus sequence typing. Trends Microbiol. 1999;7(12):482–7. Epub 1999/12/22. 10.1016/s0966-842x(99)01609-1 [DOI] [PubMed] [Google Scholar]
- 8.Urwin R, Maiden MC. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 2003;11(10):479–87. Epub 2003/10/15. 10.1016/j.tim.2003.08.006 [DOI] [PubMed] [Google Scholar]
- 9.Khayhan K, Hagen F, Pan W, Simwami S, Fisher MC, Wahyuningsih R, et al. Geographically structured populations of Cryptococcus neoformans Variety grubii in Asia correlate with HIV status and show a clonal population structure. PLoS One. 2013;8(9):e72222 Epub 2013/09/11. 10.1371/journal.pone.0072222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchini A, Smith IM, Sedlacek L, Schwarz R, Tintelnot K, Rickerts V. Molecular typing of clinical Cryptococcus neoformans isolates collected in Germany from 2004 to 2010. Med Microbiol Immunol. 2014;203(5):333–40. Epub 2014/05/20. 10.1007/s00430-014-0341-6 [DOI] [PubMed] [Google Scholar]
- 11.Beale MA, Sabiiti W, Robertson EJ, Fuentes-Cabrejo KM, O'Hanlon SJ, Jarvis JN, et al. Genotypic Diversity Is Associated with Clinical Outcome and Phenotype in Cryptococcal Meningitis across Southern Africa. PLoS Negl Trop Dis. 2015;9(6):e0003847 Epub 2015/06/26. 10.1371/journal.pntd.0003847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira-Paim K, Andrade-Silva L, Fonseca FM, Ferreira TB, Mora DJ, Andrade-Silva J, et al. MLST-Based Population Genetic Analysis in a Global Context Reveals Clonality amongst Cryptococcus neoformans var. grubii VNI Isolates from HIV Patients in Southeastern Brazil. PLoS Negl Trop Dis. 2017;11(1):e0005223 Epub 2017/01/19. 10.1371/journal.pntd.0005223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Varma A, Diaz MR, Litvintseva AP, Wollenberg KK, Kwon-Chung KJ. Cryptococcus neoformans strains and infection in apparently immunocompetent patients, China. Emerg Infect Dis. 2008;14(5):755–62. 10.3201/eid1405.071312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci U S A. 2004;101(49):17258–63. 10.1073/pnas.0402981101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngamskulrungroj P, Serena C, Gilgado F, Malik R, Meyer W. Global VGIIa isolates are of comparable virulence to the major fatal Cryptococcus gattii Vancouver Island outbreak genotype. Clin Microbiol Infect. 2011;17(2):251–8. Epub 2010/03/25. 10.1111/j.1469-0691.2010.03222.x [DOI] [PubMed] [Google Scholar]
- 16.Choi YH, Ngamskulrungroj P, Varma A, Sionov E, Hwang SM, Carriconde F, et al. Prevalence of the VNIc genotype of Cryptococcus neoformans in non-HIV-associated cryptococcosis in the Republic of Korea. FEMS Yeast Res. 2010;10(6):769–78. 10.1111/j.1567-1364.2010.00648.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mihara T, Izumikawa K, Kakeya H, Ngamskulrungroj P, Umeyama T, Takazono T, et al. Multilocus sequence typing of Cryptococcus neoformans in non-HIV associated cryptococcosis in Nagasaki, Japan. Med Mycol. 2013;51(3):252–60. Epub 2012/08/21. 10.3109/13693786.2012.708883 [DOI] [PubMed] [Google Scholar]
- 18.Firacative C, Roe CC, Malik R, Ferreira-Paim K, Escandon P, Sykes JE, et al. MLST and Whole-Genome-Based Population Analysis of Cryptococcus gattii VGIII Links Clinical, Veterinary and Environmental Strains, and Reveals Divergent Serotype Specific Sub-populations and Distant Ancestors. PLoS Negl Trop Dis. 2016;10(8):e0004861 Epub 2016/08/06. 10.1371/journal.pntd.0004861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lizarazo J, Escandon P, Agudelo CI, Firacative C, Meyer W, Castaneda E. Retrospective study of the epidemiology and clinical manifestations of Cryptococcus gattii infections in Colombia from 1997–2011. PLoS Negl Trop Dis. 2014;8(11):e3272 Epub 2014/11/21. 10.1371/journal.pntd.0003272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockhart SR, Iqbal N, Harris JR, Grossman NT, DeBess E, Wohrle R, et al. Cryptococcus gattii in the United States: genotypic diversity of human and veterinary isolates. PLoS One. 2013;8(9):e74737 Epub 2013/09/11. 10.1371/journal.pone.0074737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trilles L, Lazera Mdos S, Wanke B, Oliveira RV, Barbosa GG, Nishikawa MM, et al. Regional pattern of the molecular types of Cryptococcus neoformans and Cryptococcus gattii in Brazil. Mem Inst Oswaldo Cruz. 2008;103(5):455–62. 10.1590/s0074-02762008000500008 [DOI] [PubMed] [Google Scholar]
- 22.Byrnes EJ 3rd, Li W, Ren P, Lewit Y, Voelz K, Fraser JA, et al. A diverse population of Cryptococcus gattii molecular type VGIII in southern Californian HIV/AIDS patients. PLoS Pathog. 2011;7(9):e1002205 Epub 2011/09/13. 10.1371/journal.ppat.1002205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litvintseva AP, Thakur R, Vilgalys R, Mitchell TG. Multilocus sequence typing reveals three genetic subpopulations of Cryptococcus neoformans var. grubii (serotype A), including a unique population in Botswana. Genetics. 2006;172(4):2223–38. Epub 2005/12/03. 10.1534/genetics.105.046672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser JA, Giles SS, Wenink EC, Geunes-Boyer SG, Wright JR, Diezmann S, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature. 2005;437(7063):1360–4. Epub 2005/10/14. 10.1038/nature04220 [DOI] [PubMed] [Google Scholar]
- 25.Umeyama T, Ohno H, Minamoto F, Takagi T, Tanamachi C, Tanabe K, et al. Determination of epidemiology of clinically isolated Cryptococcus neoformans strains in Japan by multilocus sequence typing. Jpn J Infect Dis. 2013;66(1):51–5. Epub 2013/02/23. 10.7883/yoken.66.51 [DOI] [PubMed] [Google Scholar]
- 26.Danesi P, Firacative C, Cogliati M, Otranto D, Capelli G, Meyer W. Multilocus sequence typing (MLST) and M13 PCR fingerprinting revealed heterogeneity amongst Cryptococcus species obtained from Italian veterinary isolates. FEMS Yeast Res. 2014;14(6):897–909. Epub 2014/07/02. 10.1111/1567-1364.12178 [DOI] [PubMed] [Google Scholar]
- 27.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–9. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu YX. Statistical properties of segregating sites. Theor Popul Biol. 1995;48(2):172–97. Epub 1995/10/01. 10.1006/tpbi.1995.1025 [DOI] [PubMed] [Google Scholar]
- 29.Strobeck C. Average number of nucleotide differences in a sample from a single subpopulation: a test for population subdivision. Genetics. 1987;117(1):149–53. Epub 1987/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Depaulis F, Veuille M. Neutrality tests based on the distribution of haplotypes under an infinite-site model. Mol Biol Evol. 1998;15(12):1788–90. Epub 1999/01/23. 10.1093/oxfordjournals.molbev.a025905 [DOI] [PubMed] [Google Scholar]
- 31.de Jong MA, Wahlberg N, van Eijk M, Brakefield PM, Zwaan BJ. Mitochondrial DNA signature for range-wide populations of Bicyclus anynana suggests a rapid expansion from recent refugia. PLoS One. 2011;6(6):e21385 10.1371/journal.pone.0021385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agapow P-M, Burt A. Indices of multilocus linkage disequilibrium. Molecular Ecology Notes. 2001;1(1‐2):101–2. 10.1046/j.1471-8278.2000.00014.x [DOI] [Google Scholar]
- 33.Hudson RR, Kaplan NL. Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics. 1985;111(1):147–64. Epub 1985/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23(2):254–67. Epub 2005/10/14. 10.1093/molbev/msj030 [DOI] [PubMed] [Google Scholar]
- 35.Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics (Oxford, England). 2012;28(19):2537–9. Epub 2012/07/20. 10.1093/bioinformatics/bts460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131(2):479–91. Epub 1992/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michalakis Y, Excoffier L. A generic estimation of population subdivision using distances between alleles with special reference for microsatellite loci. Genetics. 1996;142(3):1061–4. Epub 1996/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang YC, Khanal Lamichhane A, Bradley J, Rodgers L, Ngamskulrungroj P, Kwon-Chung KJ. Differences between Cryptococcus neoformans and Cryptococcus gattii in the Molecular Mechanisms Governing Utilization of D-Amino Acids as the Sole Nitrogen Source. PLoS One. 2015;10(7):e0131865 Epub 2015/07/02. 10.1371/journal.pone.0131865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang YC, Lamichhane AK, Kwon-Chung KJ. Role of actin-bundling protein Sac6 in growth of Cryptococcus neoformans at low oxygen concentration. Eukaryot Cell. 2012;11(7):943–51. Epub 2012/05/09. 10.1128/EC.00120-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pukkila-Worley R, Gerrald QD, Kraus PR, Boily MJ, Davis MJ, Giles SS, et al. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot Cell. 2005;4(1):190–201. Epub 2005/01/12. 10.1128/EC.4.1.190-201.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ngamskulrungroj P, Meyer W. Melanin production at 37 °C is linked to the high virulent Cryptococcus gattii Vancouver Island outbreak genotype VGIIa. Aust Mycol. 2009;28:19–22. [Google Scholar]
- 42.Torres-Rodriguez JM, Alvarado-Ramirez E, Gutierrez-Gallego R. Urease activity in Cryptococcus neoformans and Cryptococcus gattii. Rev Iberoam Micol. 2008;25(1):27–31. Epub 2008/03/15. 10.1016/s1130-1406(08)70007-x [DOI] [PubMed] [Google Scholar]
- 43.Zaragoza O, Fries BC, Casadevall A. Induction of capsule growth in Cryptococcus neoformans by mammalian serum and CO(2). Infection & Immunity. 2003;71(11):6155–64. Epub 2003/10/24. 10.1128/IAI.71.11.6155-6164.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cogliati M. Global Molecular Epidemiology of Cryptococcus neoformans and Cryptococcus gattii: An Atlas of the Molecular Types. Scientifica (Cairo). 2013;2013:675213 Epub 2013/11/28. 10.1155/2013/675213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagen F, Hare Jensen R, Meis JF, Arendrup MC. Molecular epidemiology and in vitro antifungal susceptibility testing of 108 clinical Cryptococcus neoformans sensu lato and Cryptococcus gattii sensu lato isolates from Denmark. Mycoses. 2016;59(9):576–84. Epub 2016/04/12. 10.1111/myc.12507 [DOI] [PubMed] [Google Scholar]
- 46.Ellis DH, Pfeiffer TJ. Natural habitat of Cryptococcus neoformans var. gattii. J Clin Microbiol. 1990;28(7):1642–4. Epub 1990/07/01. 10.1128/JCM.28.7.1642-1644.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Day JN, Qihui S, Thanh LT, Trieu PH, Van AD, Thu NH, et al. Comparative genomics of Cryptococcus neoformans var. grubii associated with meningitis in HIV infected and uninfected patients in Vietnam. PLoS Negl Trop Dis. 2017;11(6):e0005628 Epub 2017/06/15. 10.1371/journal.pntd.0005628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ngamskulrungroj P. A Non-HIV Specific ST5 Genotype of Cryptococcus neoformans-gattii Species Complex. Siriraj Med J. 2015;67(6):301–5. [Google Scholar]
- 49.Meyer W, Marszewska K, Amirmostofian M, Igreja RP, Hardtke C, Methling K, et al. Molecular typing of global isolates of Cryptococcus neoformans var. neoformans by polymerase chain reaction fingerprinting and randomly amplified polymorphic DNA-a pilot study to standardize techniques on which to base a detailed epidemiological survey. Electrophoresis. 1999;20(8):1790–9. Epub 1999/08/06. [DOI] [PubMed] [Google Scholar]
- 50.Hatthakaroon C, Pharkjaksu S, Chongtrakool P, Suwannakarn K, Kiratisin P, Ngamskulrungroj P. Molecular epidemiology of cryptococcal genotype VNIc/ST5 in Siriraj Hospital, Thailand. PLoS One. 2017;12(3):e0173744 10.1371/journal.pone.0173744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev. 2014;27(4):980–1024. Epub 2014/10/04. 10.1128/CMR.00126-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ngamskulrungroj P, Kammarnjassadakul P, Khayhan K, Pitak-Arnnop P, Ungprasert P. An Association of Cryptococcus neoformans/C. gattii Genotype and HIV Status in Asia: A Systematic Review. Siriraj Med J. 2019;71(1):158–64. [Google Scholar]
- 53.Thanh LT, Phan TH, Rattanavong S, Nguyen TM, Duong AV, Dacon C, et al. Multilocus sequence typing of Cryptococcus neoformans var. grubii from Laos in a regional and global context. Med Mycol. 2018. 10.1093/mmy/myy105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cogliati M, Desnos-Ollivier M, McCormick-Smith I, Rickerts V, Ferreira-Paim K, Meyer W, et al. Genotypes and population genetics of cryptococcus neoformans and cryptococcus gattii species complexes in Europe and the mediterranean area. Fungal Genet Biol. 2019;129:16–29. Epub 2019/04/07. 10.1016/j.fgb.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 55.Kwon-Chung KJ, Fraser JA, Doering TL, Wang Z, Janbon G, Idnurm A, et al. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb Perspect Med. 2014;4(7):a019760 10.1101/cshperspect.a019760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rocha DFS, Cruz KS, Santos C, Menescal LSF, Neto J, Pinheiro SB, et al. MLST reveals a clonal population structure for Cryptococcus neoformans molecular type VNI isolates from clinical sources in Amazonas, Northern-Brazil. PLoS One. 2018;13(6):e0197841 Epub 2018/06/09. 10.1371/journal.pone.0197841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiesner DL, Moskalenko O, Corcoran JM, McDonald T, Rolfes MA, Meya DB, et al. Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. MBio. 2012;3(5). Epub 2012/09/28. 10.1128/mBio.00196-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andrade-Silva LE, Ferreira-Paim K, Ferreira TB, Vilas-Boas A, Mora DJ, Manzato VM, et al. Genotypic analysis of clinical and environmental Cryptococcus neoformans isolates from Brazil reveals the presence of VNB isolates and a correlation with biological factors. PLoS One. 2018;13(3):e0193237 Epub 2018/03/06. 10.1371/journal.pone.0193237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Velez N, Alvarado M, Parra-Giraldo CM, Sanchez-Quitian ZA, Escandon P, Castaneda E. Genotypic Diversity Is Independent of Pathogenicity in Colombian Strains of Cryptococcus neoformans and Cryptococcus gattii in Galleria mellonella. J Fungi (Basel). 2018;4(3). Epub 2018/07/07. 10.3390/jof4030082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singer LM, Meyer W, Firacative C, Thompson GR 3rd, Samitz E, Sykes JE. Antifungal drug susceptibility and phylogenetic diversity among Cryptococcus isolates from dogs and cats in North America. J Clin Microbiol. 2014;52(6):2061–70. Epub 2014/04/04. 10.1128/JCM.03392-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Byrnes EJ 3rd, Bildfell RJ, Frank SA, Mitchell TG, Marr KA, Heitman J. Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. J Infect Dis. 2009;199(7):1081–6. Epub 2009/02/18. 10.1086/597306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li W, Averette AF, Desnos-Ollivier M, Ni M, Dromer F, Heitman J. Genetic Diversity and Genomic Plasticity of Cryptococcus neoformans AD Hybrid Strains. G3 (Bethesda). 2012;2(1):83–97. Epub 2012/03/03. 10.1534/g3.111.001255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Desnos-Ollivier M, Patel S, Raoux-Barbot D, Heitman J, Dromer F. Cryptococcosis Serotypes Impact Outcome and Provide Evidence of Cryptococcus neoformans Speciation. MBio. 2015;6(3):e00311 Epub 2015/06/11. 10.1128/mBio.00311-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cogliati M, Zani A, Rickerts V, McCormick I, Desnos-Ollivier M, Velegraki A, et al. Multilocus sequence typing analysis reveals that Cryptococcus neoformans var. neoformans is a recombinant population. Fungal Genet Biol. 2016;87:22–9. Epub 2016/01/16. 10.1016/j.fgb.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nei M, Li WH. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979;76(10):5269–73. Epub 1979/10/01. 10.1073/pnas.76.10.5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campbell LT, Currie BJ, Krockenberger M, Malik R, Meyer W, Heitman J, et al. Clonality and recombination in genetically differentiated subgroups of Cryptococcus gattii. Eukaryot Cell. 2005;4(8):1403–9. Epub 2005/08/10. 10.1128/EC.4.8.1403-1409.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, Litvintseva AP, Frazzitta AE, Haverkamp MR, Wang L, Fang C, et al. Comparative analyses of clinical and environmental populations of Cryptococcus neoformans in Botswana. Mol Ecol. 2015;24(14):3559–71. Epub 2015/06/09. 10.1111/mec.13260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weeks AR, Stoklosa J, Hoffmann AA. Conservation of genetic uniqueness of populations may increase extinction likelihood of endangered species: the case of Australian mammals. Front Zool. 2016;13:31 Epub 2016/07/12. 10.1186/s12983-016-0163-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samarasinghe H, Aceituno-Caicedo D, Cogliati M, Kwon-Chung KJ, Rickerts V, Velegraki A, et al. Genetic Factors and Genotype-Environment Interactions Contribute to Variation in Melanin Production in the Fungal Pathogen Cryptococcus neoformans. Sci Rep. 2018;8(1):9824 Epub 2018/07/01. 10.1038/s41598-018-27813-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mlinaric-Missoni E, Hagen F, Chew WH, Vazic-Babic V, Boekhout T, Begovac J. In vitro antifungal susceptibilities and molecular typing of sequentially isolated clinical Cryptococcus neoformans strains from Croatia. J Med Microbiol. 2011;60(Pt 10):1487–95. 10.1099/jmm.0.031344-0 [DOI] [PubMed] [Google Scholar]
- 71.Illnait-Zaragozi MT, Martinez-Machin GF, Fernandez-Andreu CM, Hagen F, Boekhout T, Klaassen CH, et al. Microsatellite typing and susceptibilities of serial Cryptococcus neoformans isolates from Cuban patients with recurrent cryptococcal meningitis. BMC Infect Dis. 2010;10:289 Epub 2010/10/06. 10.1186/1471-2334-10-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Wyk M, Govender NP, Mitchell TG, Litvintseva AP. Multilocus sequence typing of serially collected isolates of Cryptococcus from HIV-infected patients in South Africa. J Clin Microbiol. 2014;52(6):1921–31. Epub 2014/03/22. 10.1128/JCM.03177-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kassi FK, Drakulovski P, Bellet V, Roger F, Chabrol A, Krasteva D, et al. Cryptococcus genetic diversity and mixed infections in Ivorian HIV patients: A follow up study. PLoS Negl Trop Dis. 2019;13(11):e0007812 Epub 2019/11/19. 10.1371/journal.pntd.0007812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dykstra MA, Friedman L, Murphy JW. Capsule size of Cryptococcus neoformans: control and relationship to virulence. Infect Immun. 1977;16(1):129–35. Epub 1977/04/01. 10.1128/IAI.16.1.129-135.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang YC, Penoyer LA, Kwon-Chung KJ. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect Immun. 1996;64(6):1977–83. Epub 1996/06/01. 10.1128/IAI.64.6.1977-1983.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang YC, Kwon-Chung KJ. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect Immun. 1998;66(5):2230–6. Epub 1998/05/09. 10.1128/IAI.66.5.2230-2236.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Rivera J, Chang YC, Kwon-Chung KJ, Casadevall A. Cryptococcus neoformans CAP59 (or Cap59p) is involved in the extracellular trafficking of capsular glucuronoxylomannan. Eukaryot Cell. 2004;3(2):385–92. Epub 2004/04/13. 10.1128/ec.3.2.385-392.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clancy CJ, Nguyen MH, Alandoerffer R, Cheng S, Iczkowski K, Richardson M, et al. Cryptococcus neoformans var. grubii isolates recovered from persons with AIDS demonstrate a wide range of virulence during murine meningoencephalitis that correlates with the expression of certain virulence factors. Microbiology. 2006;152(Pt 8):2247–55. Epub 2006/07/20. 10.1099/mic.0.28798-0 [DOI] [PubMed] [Google Scholar]
- 79.Jain N, Cook E, Xess I, Hasan F, Fries D, Fries BC. Isolation and characterization of senescent Cryptococcus neoformans and implications for phenotypic switching and pathogenesis in chronic cryptococcosis. Eukaryot Cell. 2009;8(6):858–66. Epub 2009/05/05. 10.1128/EC.00017-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.