Abstract
Ammonia and ammonium have received less attention than other forms of air pollution, with limited progress in controlling emissions at UK, European and global scales. By contrast, these compounds have been of significant past interest to science and society, the recollection of which can inform future strategies. Sal ammoniac (nūshādir, nao sha) is found to have been extremely valuable in long-distance trade (ca AD 600–1150) from Egypt and China, where 6–8 kg N could purchase a human life, while air pollution associated with nūshādir collection was attributed to this nitrogen form. Ammonia was one of the keys to alchemy—seen as an early experimental mesocosm to understand the world—and later became of interest as ‘alkaline air’ within the eighteenth century development of pneumatic chemistry. The same economic, chemical and environmental properties are found to make ammonia and ammonium of huge relevance today. Successful control of acidifying SO2 and NOx emissions leaves atmospheric NH3 in excess in many areas, contributing to particulate matter (PM2.5) formation, while leading to a new significance of alkaline air, with adverse impacts on natural ecosystems. Investigations of epiphytic lichens and bog ecosystems show how the alkalinity effect of NH3 may explain its having three to five times the adverse effect of ammonium and nitrate, respectively. It is concluded that future air pollution policy should no longer neglect ammonia. Progress is likely to be mobilized by emphasizing the lost economic value of global N emissions ($200 billion yr−1), as part of developing the circular economy for sustainable nitrogen management.
This article is part of a discussion meeting issue ‘Air quality, past present and future’.
Keywords: alkaline air, nitrogen, nūshādir, lichens, ecosystem recovery, circular economy
1. Introduction
Over recent decades ammonia (NH3) has often seemed like the Cinderella of air pollution, as it has been given much less attention than other pollutants, such as sulfur dioxide (SO2), nitrogen oxides (NOx), ozone (O3) and particulate matter (PM). In the 1980s, research focused on ‘acid rain’, especially in the light of SO2 and NOx emissions [1–3] with only a few researchers at that time examining the possible effects of NH3 and ammonium on the environment, including threats to soils, biodiversity and forest health [4–6]. The same can be said for European air pollution policy, with successive international protocols on SO2 and NOx emissions [7,8], preceding the multi-pollutant, multi-effect Gothenburg Protocol [9], which included NH3 for the first time. Even then, the commitments for NH3 were much less ambitious than for other air pollutants, requiring that little action be taken by most countries. The situation is similar with the 2020 ceilings of the revised Gothenburg Protocol of 2012. With insufficient measures implemented, several countries are unlikely to meet their legally binding NH3 ceilings for 2020, while overall Europe-wide NH3 emissions have actually been increasing since 2013 [10]. The barriers appear to be primarily political, as The Netherlands and Denmark have shown that it is possible to reduce NH3 emissions substantially.
With this perspective in mind, it is appropriate to take stock of what ammonia has meant to people in the past, what it means today, and what it might mean in the future. We rapidly discover that NH3 and were historically far from insignificant, fulfilling several important roles. Whereas recent efforts have focused on reducing NH3 emissions from agriculture, with the main sources being livestock excreta and fertilizers, the historical picture helps to raise awareness of the multi-dimensional relevance of ammonia for environment and society.
Considering the present, across much of Europe and North America we now inherit a world where substantial emission controls have already been achieved for SO2 and NOx. We consider in detail the implications of the changed ratio of NH3 to the acid gases, especially for some of the most sensitive ecological receptors. Instead of acid rain, we now face challenges from ‘alkaline air’, which was the original name given by Joseph Priestley [11] for gaseous ammonia. Today, we may also define alkaline air more generally as air where alkaline gases (primarily NH3, but in principle also including volatile amines) dominate over those that are acidic in nature.
Finally, we consider what might be expected for the future. What are the implications of current legislation, of the slightly more ambitious emission reductions for 2030 under the revised EU National Emissions Ceilings Directive (2016/2284/EU)? We conclude by placing NH3 mitigation in the context of the circular economy for nitrogen and United Nations actions on nitrogen to help meet multiple Sustainable Development Goals (SDGs).
In the following sections, we show how a broad approach linking past, present and future could help raise awareness about the importance of ammonia and nitrogen as a contribution to catalysing action on the SDGs. We juxtapose the historical value of ammonium in international trade and alchemy with current development of the nitrogen circular economy. The analysis is underpinned with a more detailed examination of ecological datasets for epiphytic lichens and bog ecosystems which together emphasize the emerging importance of alkaline air.
2. Ammonia in the past and implications for the present
While the popular historical narrative ascribes the discovery of ammonia to Priestley [11], his achievement needs to be set in the context of at least two millennia of human exploration and investigation into ammonia and ammonium.
(a). Ammonia in ancient times
By the start of the Tang Dynasty (AD 618–907), ammonium salts for use in metallurgy, medicine and food were already being traded as a luxury product along the Silk Road in Central Asia [12]. Spontaneous combustion of near-surface coal deposits explains the development of fire caves, some of which burn for hundreds of years. Nitrogen (N) in the burnt coal volatilizes as NH3, reacting with co-emitted hydrochloric acid (HCl), sulfuric acid (H2SO4) and nitric acid (HNO3) to form a mix of ammonium chloride, sulfate and nitrate salts [13]. Ammonium chloride tends to dominate in the collected sublimate (also known as nūshādir, nao sha, sal ammoniac, the Eagle (nasr) and a wealth of other names), presumably because it is more volatile than ammonium sulfate, while ammonium nitrate formation may be limited by low HNO3 concentrations relative to NOx (ammonium nitrate is also decomposed to N2, N2O and water at high temperatures). Along with many other point sources, NH3 emissions from such fire caves can now be detected from space [14], such as at Jharia in India [10] (figure 1).
Figure 1.
Fire cave at Jharia, India, where spontaneous combustion of surface coal deposits has resulted in burning at this location for over a century. Sites such as this across Central Asia, together with volcanic fumaroles, represent the earliest recorded sources of traded ammonium salts (Photo © Johnny Haglund). (Online version in colour.)
The historical collection of the sal ammoniac sublimate around the cooler edge of fire caves, as well as from a range of volcanic fumaroles (from Etna in Sicily to Mount Damavand in Iran), allowed it to become a key commodity of long-distance trade up to the early nineteenth century [12]. The importance and the stability of the sal ammoniac market can be illustrated by comparing prices from AD 620 (Central Asia) with those from AD 1000–1140 (Mediterranean trade) as shown in table 1. The estimates for Central Asia are based on transactions recorded in tax records discovered near Turfan, in present-day Xinjiang province of China [15]. These values are compared with documentary records recovered from a geniza or document repository, as uniquely preserved in Cairo [16].
Table 1.
Comparison of sal ammoniac prices with spice, silk and slaves for Central Asian and Mediterranean trade during the seventh and eleventh to twelveth centuries. For calculations, see electronic supplementary material, §1.
| prices in nuqra dirhams (pure silver dirhams, d)a |
||||||
|---|---|---|---|---|---|---|
| location (main trade locations) | date | sal ammoniac (d/kg) | spice (d/kg) | silk (d/kg) | slaves (d/slave) | N cost of a human (kg N/slave)b |
| Turfan (China, Central Asia) | ca 620 | 6c | 5 | 17 | 120 | 6 |
| Egypt (Sicily, Tunisia) | 1000–1140 | 7.6 (5.9–10.6)d |
5 | 59 | 243 (208–278)d | 8.4 (5.7–11.1)d |
bConverted based on N content of sal ammoniac of 26.2%.
cThe estimates for Turfan draw on six transactions for sal ammoniac, of which one includes the amount of tax paid, with a second combined transaction of sal ammoniac and spice that agrees within 10%.
d95% confidence limits with n = 12 and 19 for sal ammoniac and slave price, respectively.
Table 1 shows impressive similarity for the prices of sal ammoniac and spice from these independent datasets, while the price of silk and slaves had increased substantially in Cairo compared with Central Asia. With today's perspective, it is shocking to note that just 6–8 kg N would purchase a human being. This reflects both a high price of nitrogen and a low value of human life compared with the present. Relative to changing gold and silver prices (electronic supplementary material, §2), N compounds are today around three orders of magnitude cheaper, with prices decreasing rapidly during the twentieth century as large-scale manufacture, mainly through the Haber–Bosch Process [17], has increased their availability.
The burning coal caves of Central Asia also provide the first recorded example of ammoniacal air pollution. It appears that locals would encourage the natural coal burning specifically to harvest sal ammoniac, as recorded by ibn-Hauqal:
Over the spot whence the vapour issues, they have erected a house, the doors and windows of which are kept so closely shut and plastered over with clay that none of the vapour can escape. On the upper part of this house the nūshādir rests. When the doors are to be opened, a swiftly running man is chosen, who having his body covered over with clay, opens the door; takes as much as he can of the nūshādir, and runs off; if he should delay, he would be burnt (translated by Ouseley [18], p. 264, who renders nūshādir as ‘copperas’).
Further details of the pollution threat are given by al-Mas'ūdī:
Travellers in summer take their road from Khorāsān to China by this mountain; for there is a valley through it, which is forty or fifty miles long. At the entrance of the valley wait some men who offer themselves to carry the baggage, if they are well paid. They use sticks to drive the passengers on their journey; for any stoppage or rest would be fatal to the traveller, in consequence of the irritation which the ammoniacal vapours of this valley produce on the brain, and on account of the heat. The way becomes more and more narrow till the travellers come to the end of their perilous passage. Here are pits with water, in which they throw themselves, to obtain relief … When travellers arrive in the Chinese territories, they are beaten as in passing (to counteract the congestion of blood in the brain) (translated by Sprenger [19], pp. 359–360).
Caution is needed with regard to the comment of al-Mas’ūdī about effects of nūshādir on the brain. This may reflect the fact that the Chinese term, nao sha, includes a component referring to the brain, so that nao sha was sometimes termed brain salt (see [20], pp. 446–447), for which there are several possible explanations.
(b). Early ammonia science and philosophy
While the above examples illustrate the historic importance of ammonium in trade and air pollution, these were probably not the earliest applications. Pliny the Elder (Natural History 28: 19, 149) was already familiar with use of the fumes of deer horn and hair to make people breathe naturally when choking with hysteria. This use is directly analogous to the eighteenth century popularity of ammonium carbonate as ‘smelling salts’; these liberate gaseous NH3, which acts as a vasodilator in the airways. Ammonia and ammonium were also known in scientific circles, if not always openly. In particular, they were at the heart of alchemy, well-known as a ‘reserved’ science (i.e. unspoken, secret, limited to the few), making it extremely difficult to trace how they were used.
One of the most clear alchemical writers on nūshādir was the Persian physician al-Rāzī. It has often been stated that earlier Greek alchemy used exclusively metals and other minerals, while Islamic alchemy introduced the use of organic materials (e.g. [20], p. 435, [21]). The following illustrates the methods of al-Rāzī:
Take of black cleaned hair, distil its water and oil and calcine its residue according to what is [explained] further above, and put away each part of it separately … Then tie it [the solidified oil] up in a linen cloth and hang it into distilled urine in a clay container on the hook of the blind [cucurbit]. Place it on a small oven under which burns a fire of a lamp. Leave it for 24 h, that the urine becomes red. Then pour it off and renew the urine. Repeat this operation until all the colour is extracted. Then gather all and distil. Distil white urine, but its redness remains. Then mix that what remained from the oil in a batch with the distilled juice of a lemon and treat it with the urine with the help of the operation…
Then convert it into a hard state in a blind [cucurbit]; it solidifies it into white nuqra like crystal … But if you want, that it [transmutes] into the red [into gold], thus put in it before it solidifies, the red [residue] … it solidifies, transmuting into red nuqra, a dirham of which transmutes 1800 dirham of any metal whichever you want into pure gold (trans. by G. Fischer from [22], pp. 109–110).
Special caution is needed here, as al-Rāzī uses so-called ‘cover names’ (Decknamen), referring to the ammoniacal distillates as ‘urine’ (because of how it comes out of the alembic) or ‘lemon juice’ (because of its sharpness). Considering these processes, the Arab/Persian alchemist Jābir refers to alchemy as a mesocosm or middle-world, which links understanding of the macrocosm (universe) with the microcosm (humans) (cf. [23], p. 74). In experiments like this, ammonia and ammonium were key to early experimental philosophy. As to the gold, even more caution is needed. In the margin of one manuscript, one of al-Rāzī's readers commented: ‘Truly I have looked into this book … Do not occupy yourself with them [the essences of Arsenic and Sulphur] unless you already know the secret of the process … Only if you know the secret, God willing, will you accomplish the work’ (translated by Heym [24], p. 191).
In fact, it looks likely that earlier Greek alchemists were already familiar with ammonia and ammonium salts. For example, characteristic steps from the al-Rāzī process given above can be found in writings of the Greek alchemist Zosimus (e.g. [25], pp. 30–33; [26], pp. 486–492) and in those attributed to Democritus (e.g. [27], p. S91; electronic supplementary material, §3). There is also a question about the oldest name for sal ammoniac. The term nūshādir appears earliest in its Chinese rendering as nao sha, but has a well-established Iranian etymology, meaning ‘immortal fire’ [12]. It is a name that matches just as well to the macrocosmic fire caves as to the processing of ‘elements’ in the mesocosmic analysis of earlier Greek alchemy, leaving open the question of its origin.
Obscure as these beginnings may seem, they form the foundations on which modern science was built. This is no more apparent than with Isaac Newton, who experimented and wrote extensively on alchemy, but deliberately kept his findings secret (e.g. [28], p. 159) and encouraged others to do so. Newton thus wrote to Henry Oldenburg, the Secretary of the Royal Society, encouraging Robert Boyle not to reveal alchemical secrets:
[It] may possibly be an inlet to something more noble, not to be communicated without immense dammage to ye world if there should be any verity in ye Hermetick writers, therefore I question not but that ye great wisdom of ye noble Authour [Boyle] will sway him to high silence till he shall be resolved of what consequence ye thing may be … there being other things beside ye transmutation of metals … which none but they [the alchemists] understand … but pray keep this letter private to your self [29].
The message was the traditional one of many alchemists over the centuries: not to reveal the secrets of alchemy, which could otherwise lead to the destruction of society (cf. al-Jildakī [30], p. 49). While Boyle may have engaged in the practice of advertising secrecy [31], Newton appears to have recognized the ethical dilemma concerning open explanation of alchemy.
(c). The discovery of ‘alkaline air’
Ultimately, the scientific community turned away from the secrecy of alchemy, pushing towards openness of scientific publication for practical benefit. As the experimentalist Stephen Hales wrote in the year that Newton died:
If those who unhappily spent their time and substance in search after an imaginary production, that was to reduce all things to gold, had, instead of that fruitless pursuit, bestowed their labour in searching after this much neglected volatile Hermes, who has so often escaped thro’ their burst receivers, in the disguise of a subtile spirit, a mere explosive matter; they would then instead of reaping vanity, have found their researches rewarded with very considerable and useful discoveries ([32], p. 180).
Hales’ experiments were to be decisive as a prelude to the scientific discovery of ammonia. His work introduced the idea of ‘pneumatic chemistry’, distilling all sorts of products and then collecting the resulting gases in an inverted vessel over a trough of water. In the case of ammonia distilled from blood or harts-horn, this first filled the vessel, but then gradually dissolved in the water, leaving Hales with no ammonia to collect (e.g. [32], p. 95, Experiment XLIX). Continuing these kinds of experiments 50 years later, Joseph Priestly instead filled his pneumatic trough with mercury in which the ammonia would not dissolve. This enabled him to isolate and characterize pure ammonia gas [11]. Priestley's first report was in a private letter to Benjamin Franklin in September 1773, later presenting his findings to the Royal Society ([33], pp. 93–99).
It was only in the 1790s that Priestley's alkaline air started to become known as ‘ammonia pura’, given its relationship to sal ammoniac. Subsequent chemical discoveries came quickly, with Scheele [34] showing that it was present in the atmosphere, and Berthellot [35] demonstrating that it consisted of one part nitrogen to three parts hydrogen.
3. Ammonia and present-day changes in air pollution climate
The reminder of ammonia as alkaline air is highly relevant to the present, as emissions of SO2 and NOx have decreased greatly over the last 30 years, leaving European and North American atmospheres increasingly rich in NH3. This can be illustrated by the temporal evolution of emissions, gas and aerosol concentrations and rainfall acidity across the UK. While SO2 emissions have been almost entirely abated (97% reduction since 1970) and NOx emissions reduced by 70%, estimated NH3 emissions increased substantially up to 1990, decreased by 18% (1990–2013), and then increased 9% (2013–2017; figure 2a). National mean NH3 concentrations have not changed significantly since the National Ammonia Monitoring Network [37] was started in 1997 (though increasing in remote areas), while aerosol concentrations have decreased significantly, consistent with declining SO2 and HNO3 (figure 2b). This has led to less formation of ammonium sulfate and ammonium nitrate, which will have also helped maintain gaseous NH3 levels [38,39].
Figure 2.
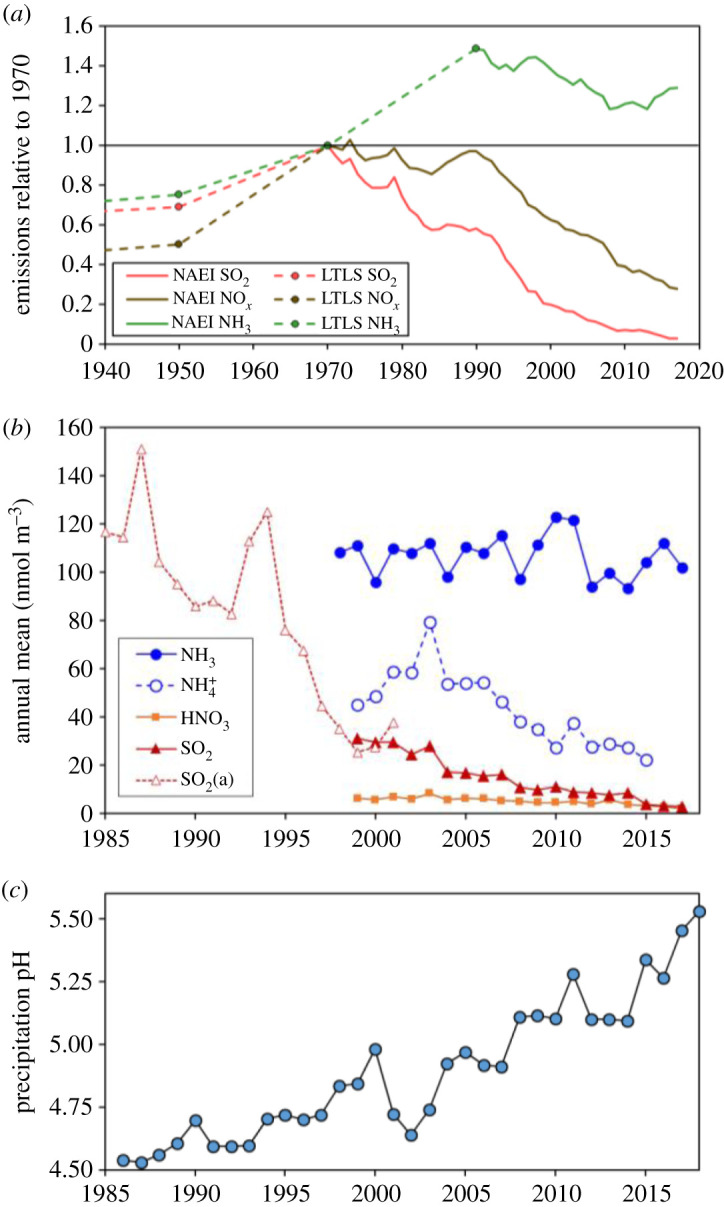
(a) Emissions of SO2, NOx and NH3 from the UK relative to 1970, comparing the Defra National Atmospheric Emissions Inventory (NAEI) including estimates from the Long-Term Large-Scale (LTLS) model for earlier years [36]. (b) Annual mean concentrations of gaseous NH3, SO2 and HNO3 and of aerosol NH4+ (for 12 sites), from the UK monitoring network (for further details and error analysis, see Tang et al. [37,38], compared with the earlier trend for five sites (SO2(a)), normalized to the UK mean for 1999–2001. (c) Volume-weighted mean pH of precipitation across the UK based on spatial interpolation of measured values.
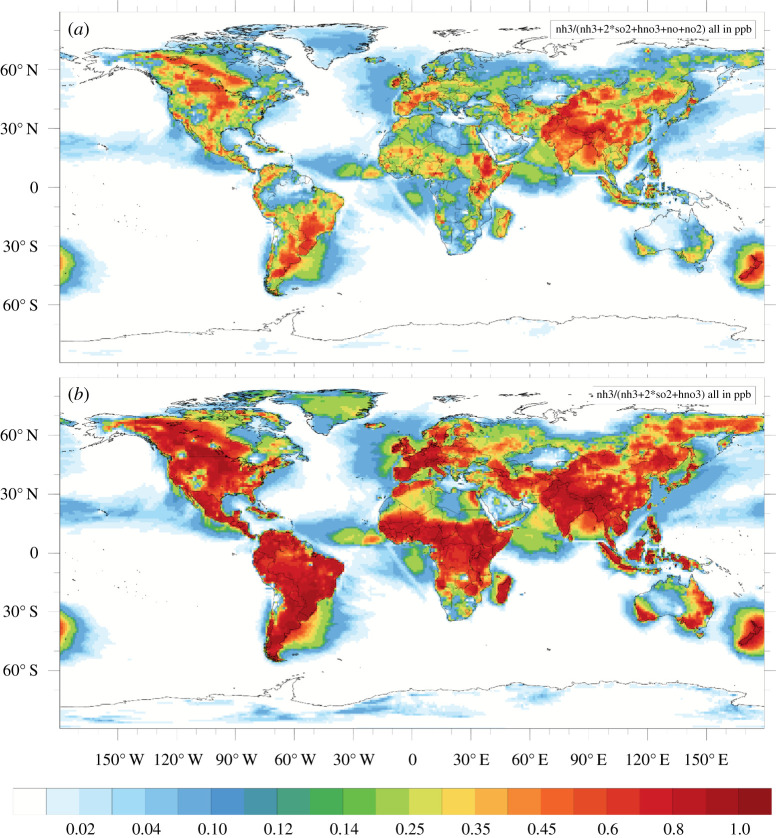
As a consequence, acid rain is now a thing of the past for UK conditions. Since 1986, volume-weighted rain pH has increased from 4.62 to 5.48 (figure 2c), now being close to the value of 5.6 due to dissolution of atmospheric CO2. Together these changes demonstrate how alkaline air is becoming increasingly important across the UK countryside, in a pattern that is reflected across much of Europe and North America [40,41]. A corresponding trend is now occurring in China, following implementation of SO2 emission controls from 2012 [42], while in India, NOx emissions have been increasing even faster than NH3 emissions [43]. The gaseous alkaline fraction (expressed as NH3 divided by the sum of NH3, 2SO2, HNO3 and HCl) is now at 88% in the UK (electronic supplementary material, §4), while estimated global variation is shown in figure 3. In many areas of the world, the gaseous alkaline fraction is over 60% (including NOx) or 80% (excluding NOx).
Figure 3.
Global distribution of the gaseous alkaline fraction for 2010 as estimated by the EMEP-WRF global model [44], here calculated based on surface atmosphere mixing ratios (ppbv/ppbv) as NH3 / (NH3 + HNO3 + 2SO2 + NOx): (a) including NOx, (b) excluding NOx, since it is unclear to what extent NOx concentrations influence leaf surface acidity (see electronic supplementary material, §4).
The net result of these changes is that is now making an increasing relative contribution to the composition of airborne particulate matter, relevant for effects on human health [45]. In parallel, the increasingly alkaline, NH3-rich atmosphere is having substantial consequences for the natural environment, as examined in detail below for lichens and other sensitive plants.
(a). Response of lichens to atmospheric ammonia
While lichens are well known to be sensitive to SO2 concentrations, here we emphasize that NH3 is now the primary air pollution driver of lichen distributions in many areas of Europe. To understand the dynamics, we first consider a local-scale transect from Scotland [46] that shows how lichens can change in the vicinity of a poultry farm emitting NH3. Lichens on tree trunks of both Scots pine (Pinus sylvestris) and Sitka spruce (Picea sitchensis), and on branches of birch (Betula pubescens), which are all naturally acid-barked trees, were scored according to a standard methodology [47,48]. In this approach, lichen species are categorized as ‘acidophytes’ (e.g. Usnea, Hypogymnia, Pseudevernia, Bryoria), preferring naturally acidic bark, and ‘nitrophytes’ (e.g. Xanthoria, Physcia), favouring higher levels of nitrogen air pollution (electronic supplementary material, §3) [49]. Using this approach, frequency-based lichen indices for acidophytes (LA) and nitrophytes (LN) were calculated (see electronic supplementary material, §5), where the difference (LAN = LA–LN) distinguishes bark dominated by acidophytes (+ value) or nitrophytes (− value).
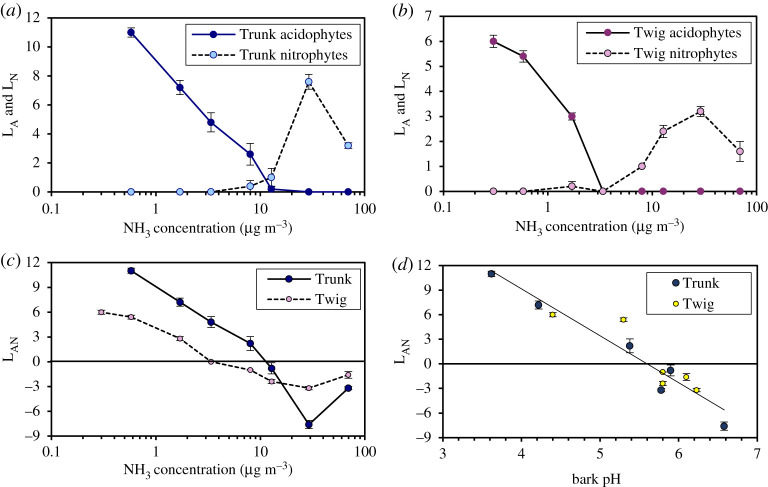
Findings from the local transect are summarized in figure 4, showing how acidophyte species were gradually eradicated between mean NH3 concentrations of 1 and 12 µg m−3, with acidophytes on twigs being more sensitive to NH3 than those on trunks. Acidophytes on both twigs and trunks were already significantly reduced at the third cleanest location (approximately 1.7 µg m−3, two-sample t-test, two-tail assuming unequal variance, trunks: p < 0.001; twigs: p < 0.01), where the first nitrophytes on twigs were also recorded. Highest nitrophyte occurrence was recorded at 30 µg m−3, with a significant reduction at 70 µg m−3 for both trunks (p < 0.001) and twigs (p = 0.01). Figure 4d shows that there was also a significant relationship between LAN and measured bark pH. This effect can be largely explained by NH3 increasing bark pH nearer the farm (see electronic supplementary material, §5). It is notable that there is no significant difference in the relationship between LAN and bark pH for twigs versus trunks (figure 4d). This indicates that the greater sensitivity of acidophyte lichens on twigs is consistent with the differences in bark chemistry between twigs and trunks. One of the advantages of the local study shown in figure 4 is that it covers a wide range of pollution levels from 0.3 to 70 µg m−3 demonstrating its wide relevance for different pollution conditions.
Figure 4.
Response of epiphytic lichens on trunks and twigs of Betula pubescens to increasing NH3 concentrations in a farm transect in South Scotland. (a) trunks and (b) twigs, for a cover index of lichen acidophytes (LA) and nitrophytes (LN). (c) Results for the joint index LAN = LA – LN. (d) Combined relationship between LAN and bark pH for twigs and trunks: LAN = − 5.73 (bark pH) + 32.1, with R2 = 0.91. Error bars are ±1 standard error for five replicate trees at each location. (Online version in colour.)
The lichen methodology was subsequently applied at 30 sites across the UK [50]. It must be recognized that different tree species also have naturally different bark pH, and therefore the analysis distinguished lichen communities on naturally acid-barked oak (Quercus robor, Q. petraea, recorded where available) from communities on other tree species. LAN was generally not found to be correlated with SO2 concentrations (except for a weak relationship for oak trunks, p = 0.04, n = 11), with a lack of relationship with SO2 also found in a later survey [51].
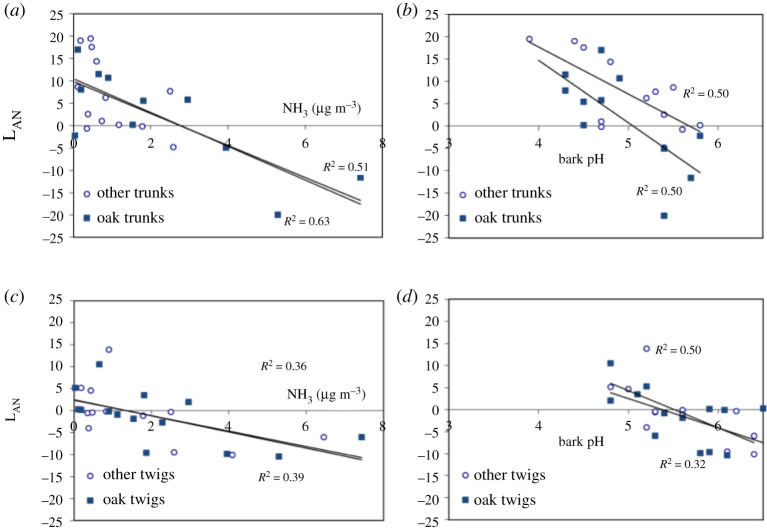
At the UK-scale, trunks and twigs both show reducing LAN score with higher NH3 and with higher bark pH, demonstrating the broad relevance of these relationships (figure 5). Substantial scatter can be seen between NH3 concentration and LAN score, which is expected based on natural variation in bark pH, even under clean conditions. In addition, variations with climate may have introduced some scatter. For example, precipitation was found to have a weakly significant effect for lichens on twigs (p < 0.05: R2 = 0.15 (all data), R2 = 0.35 (oak); electronic supplementary material, figure S4), but was not significant for lichens on trunks.
Figure 5.
Relationship between epiphytic lichens on trunks and twigs of oak and other tree species to ambient NH3 concentrations (a,c) and bark pH (b,d) from 30 sites across the UK for trunks (a,b) and twigs (c,d). Results are shown for the joint index LAN = LA – LN, where LA is the cover score for acidophyte lichens and LN the score for nitrophytes. (Online version in colour.)
However, an even higher correlation was found for the UK by relating the LAN scores to a combined index of NH3 (μg m−3) + 4 (bark pH) (figure 6). This points to NH3 as potentially having two effects. Firstly, NH3 has an alkaline effect, shown by its increasing bark pH (electronic supplementary material, figure S3A). Secondly, there appears to be an effect that is not explained by the changes in bark pH. If NH3 only had its effect by altering bark pH, then this would not explain why a combined index of NH3 and bark pH gives an improved relationship with LAN than with bark pH alone. Other diversity and nitrogen indicators are illustrated in electronic supplementary material, §5 (electronic supplementary material, figure S3), with further statistical comparisons and the full UK dataset given in electronic supplementary material, figure S4 and table S6, respectively.
Figure 6.
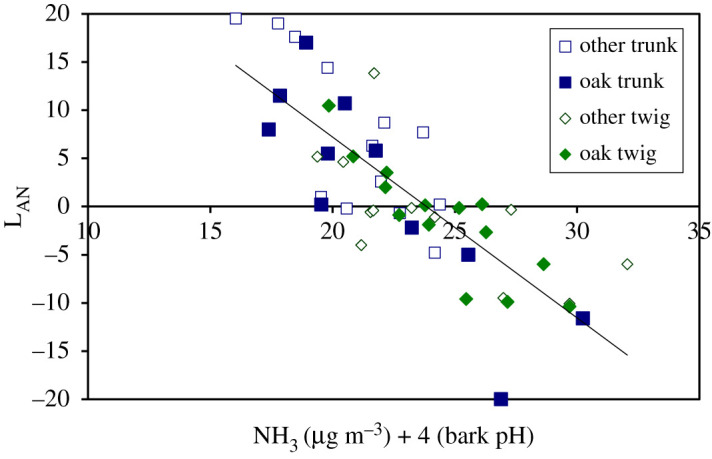
Relationship between the lichen index (LAN) and a combination of NH3 concentration and bark pH. LAN = LA – LN, where LA and LN are abundance indices for acidophyte and nitrophyte lichen species, respectively. Combining the trunk and twig data, the R2 values are 0.72 for lichens on oak (n = 25, p < 0.001), 0.56 for other trees (n = 25, p = 0.001) and 0.64 for all data (n = 50, p < 0.001), where LAN = − 1.8771 [NH3 + 4 (bark pH)] + 44.752. (For further comparisons see electronic supplementary material, figure S4.) (Online version in colour.)
Comparable results have been recorded for The Netherlands [52], showing in particular a long-term decline of acidophyte lichen species from 1991 to 2016, consistent with differences in bark pH [49]. Since these datasets focus on the principles of lichen responses to acid and alkaline gases, similar relationships can be expected in other parts of the world. For example, extremely high levels of NH3 in the Indo-Gangetic Plain [14] can be expected to be adversely affecting acidophyte epiphytic lichens in the oak forests of the Himalayan foothills, which have significant economic importance, being traded to Arabic speaking countries to make valued-added products like perfumes [53]. While data on NH3 responses have not been available until now, first analysis as part of the GCRF South Asian Nitrogen Hub [54] shows major gradients in modelled NH3 and N wet deposition both N-S and W-E across sub-Himalayan forests and at levels that greatly exceed the known impact thresholds for lichens in temperate biomes.
(b). Ecological effects of ammonia versus ammonium and nitrate
Differential sensitivity of vegetation according to the form of N air pollution is also indicated by the results of a long-term pollution manipulation experiment at Whim Bog in Southern Scotland [55,56]. In this globally unique experiment, the effects of gaseous NH3, assessed using free-air enrichment from a line-source of NH3, are compared with the effects of wet deposited and , as delivered to replicated mesocosms (12.8 m2; four replicate plots for each N level/form combination) through a misting system (see electronic supplementary material, §6). Treatments at this site have been continuing for 18 years since 2002, allowing examination of the long-term effects of different N forms. Background deposition to the site is estimated at 8 kg N ha−1 yr−1, with treatments achieving total inputs of 16, 32 and 64 kg N ha−1 yr−1. Assessment of plant species composition is based on recording at three permanent quadrats for each experimental plot, with each quadrat divided into 16 sub-quadrats (see electronic supplementary material, §6).
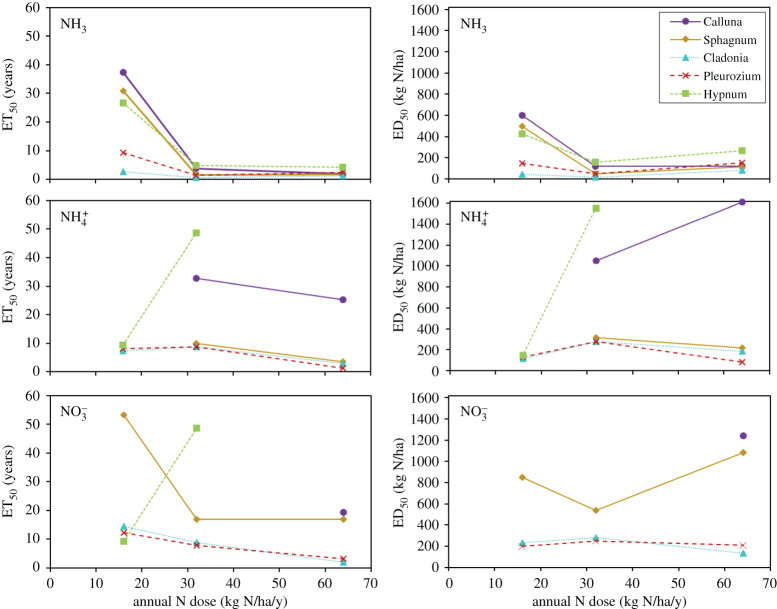
The outcomes for changes in plant cover of the main species sensitive to N pollution are summarized in figure 7. This provides an analysis of ‘Eradication Time 50’ (ET50), which is defined here as the time taken to reduce species cover by 50% relative to cover at the start of the experiment. This is shown together with the ‘Eradication Dose 50’ (ED50), the cumulative N deposition over the period associated with a relative 50% reduction in cover of each species. The values of ED50 are calculated as the product of ET50 and the annual nitrogen inputs for 2002–2019 (see electronic supplementary material, figure S5 and table S6). Changes for other species included hare's-tail cotton grass (Eriophorum vaginatum), which benefited from NH3 relative to other, more-sensitive species [56].
Figure 7.
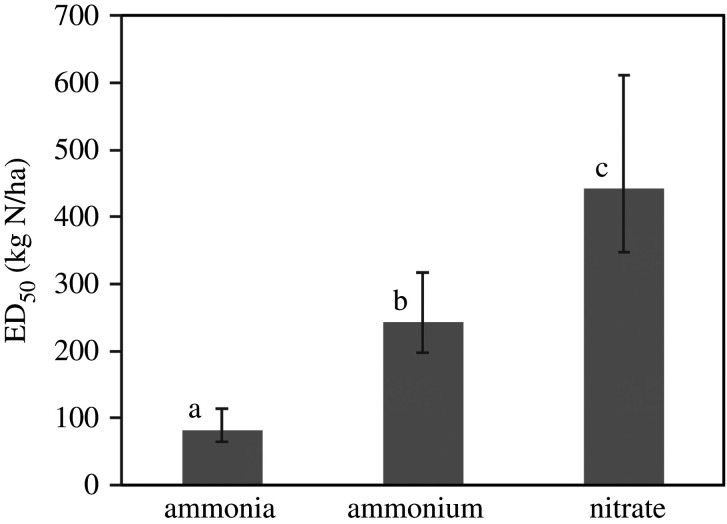
Response of bog vegetation to exposure of gaseous NH3 and wet deposited and expressed as the time taken to reduce cover of each plant species by 50% of initial values (Eradication Time 50%, ET50), and the Eradication Dose 50% (ED50) representing the total accumulated N dose that led to a halving of cover. The smallest values are most robust, with large values most uncertain, since these depend on extrapolation. (Online version in colour.)
Considering the species shown in figure 7, reindeer lichen (Cladonia portentosa) was found to be overall most sensitive, followed by red-stemmed feather-moss (Pleurozium schreberi) and red bog-moss (Sphagnum capillifolium). ET50 for NH3 at 32 kg N ha−1 yr−1 for these species were all estimated in the range of 0.6–1.6 years. By contrast, common heather (Calluna vulgaris) and heath plait-moss (Hypnum jutlandicum) were less sensitive to NH3, with ET50 values of 3.8–4.9 years, for the same N dose. Expressed as ED50, the most sensitive species had values of 19–51, while Calluna and Hypnum had values of 122 and 157 kg N ha−1 (electronic supplementary material, table S7).
Overall, it is expected that ET50 values are larger at lower annual N dose rates, as shown by the left side of figure 7. By contrast, it is expected that ED50 should be independent of annual N dose rate, so the extent to which this expectation is not met indicates that the ecological response is not directly proportional to accumulated N inputs.
As expected, ET50 values were larger at low N inputs and smaller at high N inputs. This is shown for impacts of NH3 (all species) and for impacts of wet deposited and for the most sensitive species (Cladonia, Pleurozium). By contrast, significant scatter is seen for Calluna and Hypnum in response to wet deposited N, reflective of longer and more uncertain ET50 estimates, with values greater than 17 years based on extrapolation of linear fits (electronic supplementary material, figure S5). Data for Cladonia, Pleurozium and to some extent Sphagnum give the best evidence for the utility of the ED50 indicator, as the plots for these species all show little difference between N dose rate, as expected compared with the ET50 indicator. By comparison, lower ED50 values for Hypnum, Sphagnum and Calluna for the NH3 treatments at 32 and 64 kg N ha−1 yr−1, as compared with the 16 kg N ha−1 yr−1 NH3 treatment, suggest that these species are more-than-proportionately vulnerable at the higher N rates. This could point to a toxic effect as a result of higher NH3 concentrations rather than dose.
The most important observation from this dataset for the present analysis is that it further demonstrates the higher sensitivity to NH3 compared with wet deposited and . This is most clearly seen by calculating statistics based on normalizing the data as 1/ED50, which also allows inclusion of all treatments, with the data then plotted as ED50 (figure 8). Overall, it can be seen that the reductions in cover of these five species occur three and five times faster for gaseous NH3 than for the same N dose of wet deposited and , respectively. Comparable differences were also revealed by an independent assessment of physiological response for lichen transplants (electronic supplementary material, figure S6).
Figure 8.
Differential sensitivity of bog vegetation to gaseous NH3 and wet deposited and , expressed as the average Eradication Dose 50 (ED50). Mean and standard errors for five plant species at three N input levels (n = 15, figure 7 and electronic supplementary material, table S7). Different letters show statistical significance with p < 0.05 (two-tail); (a,c) are significantly different at p < 0.01, based on paired t-tests of the reciprocal values.
Although it is widely assumed that effects on plant nutrition are governed by total N inputs, our data thus show that reality is more complex, otherwise there would be no difference between the wet and treatments, as well as between the wet N and dry NH3 treatments. Other experimental studies have mainly been conducted using increased wet deposited N or fertilizer addition [57,58]. Had such studies included NH3 enhancement, they may therefore have found even larger adverse effects per unit N added. This is not to suggest that effects of wet deposited N are unimportant, but rather to emphasize the need to consider N form. For example, in agricultural areas subject to high NH3 concentrations, our results show that remaining bog habitats will be more than proportionately at risk (based on estimated N deposition). Conversely, wet deposited N may lead to larger effects on an area basis, since the largest areas of bogs are far from agricultural sources, where wet N deposition dominates total N inputs [56].
(c). Recovery following reduction in ammonia concentrations
The preceding examples, showing higher sensitivity of plants to NH3 compared with and , raise the question of whether there are also different rates of recovery following reductions in N pollution. It has been suggested that recovery in species composition and certain N pools may take several decades after a reduction in wet deposition of N [59]. This may be especially the case in slow-growing naturally acidic ecosystems, where N pools change slowly due to lack of removal by harvests and inhibition of denitrification [60,61].
There are no known experimental studies of the simultaneous reduction in NH3, and pollution for any ecosystem. However, field data from a site in Northern Ireland illustrate the potential for surprisingly rapid recovery following a reduction in gaseous NH3 concentrations. In this case study, a poultry farm 50 m west of Moninea Bog Special Area of Conservation (SAC) led to greatly increased concentrations of NH3 (10–40 µg m−3) compared with local and regional background values of 1.5 and 0.5 µg m−3. The lichens Cladonia portentosa, C. uncialis and Sphagnum species were largely eradicated on the bog within 400 m of the farm, with excessive growth of algae on trunks of Betula pubescens (50–100 m from the farm) replacing the natural acidophyte lichen flora [62].
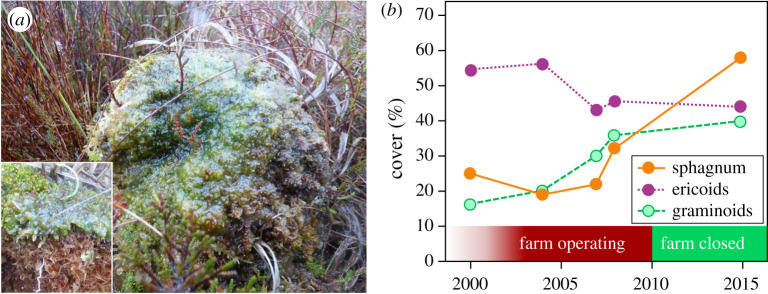
As a consequence of legal requirements for the protection of Moninea Bog, the poultry farm ceased operation in 2010, allowing examination of ecosystem recovery. Observations in 2017 showed mean atmospheric NH3 concentrations of 1.5 µg m−3, with substantial recovery of Cladonia portentosa and Sphagnum spp. As all large clumps (ca 200–400 mm diameter) of C. portentosa had been eradicated, only uniformly small specimens (ca 50–70 mm) were found in 2017 in the zone where they had been previously eradicated. The extent of Cladonia and Sphagnum growth indicated that recovery must have started within 2–4 years of the reduction in NH3 concentrations. Conversely, it was not obvious whether the condition (i.e. overall health) of Calluna had improved, while residual algae levels on Betula less than 100 m from the farm indicated only partial recovery. An illustration of a potential ‘alternative stable state’ [59] was also found (figure 9a). This showed continued colonization of a Sphagnum hummock by algae, 7 years after NH3 concentrations reduced. It suggests ongoing competition, where a coating of gelatinous algal slime restricts gas exchange and growth of the Sphagnum until the latter can manage to grow over the algae. Monitoring across Moninea Bog by the Northern Ireland Environment Agency confirmed the recovery of Sphagnum after 2010, with no evidence of any recovery in Calluna (figure 9b). The latter effect may be age-dependent, where recovery of old Calluna plants (weakened or dead) is limited, while ultimately conditions may favour recolonization by young Calluna plants.
Figure 9.
(a) Hummock of Sphagnum moss on Moninea Bog photographed in 2017, seven years after reduction in NH3 concentrations, showing a hummock still covered by algal slime characteristic of high NH3 levels (insert: partial cross-section). (b) Statutory monitoring showed an overall tendency for recovery in Sphagnum populations at Moninea Bog, but not yet of Calluna nor a return to previously lower levels of graminoids. These data confirm independent expert examination of the site by the authors in 2007 and 2017. (Online version in colour.)
4. Discussion
(a). Mechanisms of ammonia impacts on vegetation
The examples presented highlight the increased sensitivity of lichens and bog vegetation to gaseous NH3 compared with wet deposited and , which appears to be at least partly related to the alkaline effect of NH3. In this way, higher correlations were found between LAN and NH3 in the UK-scale lichen survey than with total N deposition. In fact, the highest single-factor correlation was found with the ratio of N to S deposition (electronic supplementary material, figure S4). This indicator is also closely correlated with NH3 concentrations (R2 = 0.87), and, like NH3, also provides an indication of acid-base balance.
Both the national and local-scale lichen surveys showed that bark pH is positively correlated with NH3 concentration, while LAN score is negatively correlated with bark pH. Hence, one of the ways in which NH3 appears to affect epiphytic lichens is by increasing substrate pH. That this is one of the driving variables is also shown by the differences between tree species, with nitrophytes found to be more prevalent on trees with naturally higher bark pH. Natural differences in bark pH can similarly explain differences in lichen communities between twigs and trunks (figures 4d and 6). This raises the question of whether the NH3 effect on lichens is entirely mediated by its effect on surface pH.
Examination of the UK-scale data suggests a more complex interaction. If NH3 has its effect solely through changing bark pH, then differences in pH should fully explain the variation in LAN with NH3. This would therefore not explain why a combined indicator of NH3 + 4 (bark pH) gives a better relationship with LAN than with bark pH alone (figure 6; electronic supplementary material, figure S4). It suggests that NH3 may be affecting lichens by both a pH effect and another effect, such as that related to nutrient N (as more usually considered to drive N effects on ecosystems [57]).
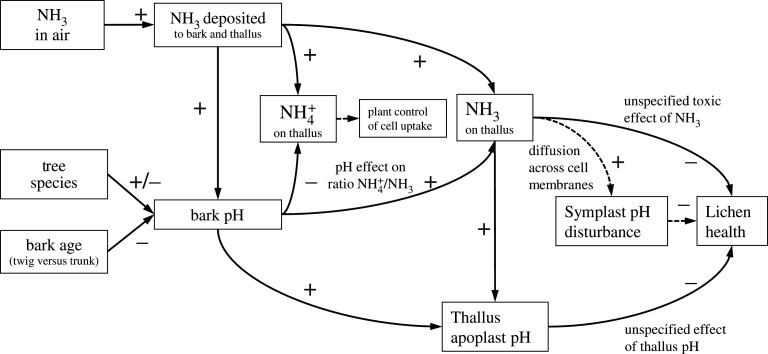
Possible relationships are summarized in figure 10: NH3 deposited on bark increases on the lichen thallus, uptake of which will be under control of cell membranes. Such altered nutrient supply may affect competition between species. In parallel, the deposition adds NH3 to the bark/thallus surface, which increases bark pH because of the alkaline nature of NH3. The bark pH is also affected by tree species and bark age (twig versus trunk). With NH3 increasing bark pH, the chemical equilibrium favours NH3 rather than , which further increases NH3 levels on the thallus. Two subsequent effects may then be expected. Firstly, that changed bark pH affects apoplast pH of the lichen thallus (cf. electronic supplementary material, figure S7), which could affect lichen health (e.g. by affecting the buffering systems of lichen acids characteristic of different species). Secondly, NH3 may have a direct toxic effect, including that mediated by the passive diffusion of NH3 across cell membranes, leading to disturbance of symplastic pH. That plant sensitivity to NH3 and other forms of N deposition is partly due to different abilities of species to manage cell pH homeostasis has been argued by Pearson & Soares [63], who elsewhere demonstrated a positive correlation between leaf buffering capacity index and nitrate reductase (NR) activity across 18 plant species [64]. This would offer another reason why acidophyte lichens, adapted to nutrition (with low NR activity expected), would be more vulnerable to atmospheric NH3.
Figure 10.
Possible mechanisms by which atmospheric NH3 pollution affects epiphytic lichens, including both positive (+) and negative (−) effects. Solid lines indicate observed relationships or those directly implied by physico-chemistry. Dashed lined indicate hypothesized relationships. The toxic and pH effects apply especially to acidophyte lichens, but may also apply to nitrophyte lichens at high levels of NH3 exposure (figure 4).
Effects of NH3 on lichen pH are also seen at Whim Bog. Electronic supplementary material, figure S8 shows that NH3 increased the pH of transplanted Cladonia portentosa thalli, confirming the thallus pH effect (figure 10), with responses seen within one month of transplantation. By contrast, the surface pH of live Sphagnum capillifolium growing in-situ remained unaffected, which may reflect a greater water-holding and buffering capacity of Sphagnum.
The importance of such pH effects may also explain the rapid recovery of Cladonia portentosa and Sphagnum spp. following reduction in NH3 levels at Moninea Bog. Even though the peat might still contain high N levels, reduced alkalinity from less NH3 would be expected to allow rather rapid re-adjustment of surfaces, allowing colonization of acidophyte species.
While uncertainties remain over the exact mechanisms, the higher sensitivity to NH3 compared with wet deposited observed at Whim Bog tends to support this picture. Based on the values of ED50 (with NH3 being three times more damaging than NH4+), this suggests that ¾ of the NH3 effect on peatland vegetation could be related to pH effects, while ¼ of the NH3 effect is attributable to the common effect of increased nutrient N supply. One of the implications of our findings is therefore to pay more attention to the ‘critical level’ for NH3 concentrations for which the UNECE has adopted a value of 1 µg m−3 for lichens, bryophytes and associated habitats [65,66].
The extent to which such relationships can be generalized between species, habitats and world regions remains an important question for further work. Each species responds individually according to its nitrogen and pH preferences, sensitivity to NH3 toxicity and ability to compete with other species for light and other resources. For example, investigations on Cladonia portentosa from Whim Bog showed that different N forms affect different metabolic pathways [67,68], which may have varying importance between species. It is also possible to identify useful functional groups, as illustrated by the nitrophyte/acidophyte lichen groupings. Calluna vulgaris offers another illustration as this is found to be more sensitive to NH3 at Whim Bog than Cross-Leaved Heath (Erica tetralix) [56]. If it could be shown (according to [64]) that this reflects a lower apoplastic buffering capacity of Calluna than Erica, then this would encourage further use of buffering capacity as a predictive indicator. In the same way, species/group differences in characteristic lichen acids may also point towards predictive capability with global relevance, which may be tested by the GCRF South Asian Nitrogen Hub.
(b). The future of alkaline air and nitrogen policy
The higher sensitivity of vegetation to gaseous NH3 compared with wet deposited NH4+ and has direct implications for the success of past SO2 and NOx emission reductions in protecting ecosystems. While the acid rain problem has now been addressed in the UK and most of Europe, the modest reductions in NH3 emissions mean that alkaline air is emerging as a new ecological challenge. The data presented here focus on naturally acidophyte species, which appear to be especially vulnerable to alkaline air. It remains to be tested whether naturally basic habitats, such as chalk grasslands, would be less vulnerable to ammonia.
Already there are indications that NH3 concentrations are actually increasing in some parts of Europe rather than decreasing. While this is partly related to reduced SO2 and NOx concentrations leading to increased NH3 lifetimes, as reflected in NH3 monitoring for remote areas [38], there is also concern about climate change impacts on NH3 concentrations. As most NH3 globally results from volatilization processes, climate warming will increase NH3 emissions [69,70]. Strategies to address alkaline air therefore need to include measures that both reduce NH3 emissions directly [71] and minimize climate change drivers. In addition to control of CO2 and CH4 emissions, decreasing losses of all N compounds (including N2O, NO and N2 to air, and losses to water) becomes critical to increasing economy-wide nitrogen use efficiency, with multiple benefits for climate, air quality, water quality, biodiversity and stratospheric ozone protection [54,72]. Such a perspective could help transform current efforts to meet the EU National Emission Ceilings commitments for 2030, as well as many other policy goals.
This takes us closer to developing the big idea whereby ammonia becomes a key focus in an emerging international strategy to manage the global nitrogen cycle. This is why the historical perspective of §2 is so important, in raising awareness about ammonia. One of the lessons of history is that ammonia has always been of significant societal importance. From its role as part of the alchemists' objective to prepare Gold and the Elixir of Life, to its economically high value as a luxury product of international trade, ammonia continues today to be important in sustaining humanity through nitrogen fertilizers and biological nitrogen fixation. If society is to learn to manage nitrogen better, then these stories can help by raising wider awareness.
Ultimately, it may be the economic value of nitrogen that counts most. It has been estimated that global N losses to the environment amount to around 200 million tonnes [73,74]. This means that at a nominal market price of US$1 per kg N, a goal to ‘halve nitrogen waste’ from all sources by 2030 would offer a circular economy opportunity worth US$100 billion per year, amounting to an annual saving of approximately 12 kg N per person (cf. §2a). These issues have recently been recognized in the first Resolution on Sustainable Nitrogen Management adopted at the UN Environment Assembly (UNEP/EA.4/Res.14), with the ambition to halve nitrogen waste adopted in the Colombo Declaration [75]. The follow-up to these activities is bringing ammonia and air pollution together as part of the global nitrogen challenge, by working to establish an Interconvention Nitrogen Coordination Mechanism (INCOM), with targeted science support through the International Nitrogen Management System (INMS) [54,72]. Together these activities can be expected to emphasize how ammonia and the wider nitrogen cycle must be at the heart of the solutions needed for both environment and economy in working towards the UN Sustainable Development Goals.
Supplementary Material
Acknowledgements
We gratefully acknowledge funding from the UK Natural Environment Research Council (NERC, including NE/R016429/1 and NE/R000131/1 as part of the UK-SCAPE and SUNRISE programmes delivering National Capability), the Department for Environment Food and Rural Affairs, the Northern Ireland Environment Agency (NIEA), the UK Joint Nature Conservation Committee, the NEWS India-UK Virtual Joint Centre on Agricultural Nitrogen (supported through the Newton-Bhabha Fund, by the UKRI and the Indian Department of Biotechnology), the UKRI Global Challenges Research Fund (South Asian Nitrogen Hub), the EU NitroPortugal project and the ‘Towards INMS’ project of the Global Environment Facility (GEF) and UNEP. We thank Kate Mason for literature support, Geertje Fischer for translations from Karimov (1957), Tony Simcock of the History of Science Museum, Oxford, and UK site operators, including those listed in electronic supplementary material, table S6.
Data accessibility
Data associated with this paper are included in the electronic supplementary material.
Authors' contributions
The article was conceived and written by M.A.S. with text contributions from N.v.D., M.R.J., L.J.S., D.F., M.I.N., S.M. and P.A.W. The air quality measurements were made by Y.S.T., A.S., S.L., I.D.L. and N.v.D. and coordinated by C.F.B. with input from M.A.S. and D.F. Emission data were prepared by U.D., while M.V. performed the global analysis of gaseous alkaline fraction. Measurements at Whim Bog were made by M.R.J., N.v.D., S.L., I.D.L., L.J.S., M.I.N., S.M., with data analysis and interpretation by P.E.L., N.v.D., L.J.S., S.M. and M.A.S. The South Asian element is contributed by M.V., S.C., C.J.E., M.J., M.I.N., A.M., C.E.S., M.A.S. and other authors. The lichen surveys were coordinated by M.A.S., I.D.L., N.v.D. and P.A.W.; the analysis at Moninea Bog was led by M.A.S., I.D.L., N.v.D. and P.C., with input from S.M. and Y.S.T. The historical perspective was prepared by M.A.S. and the policy/future perspective prepared with input from M.A.S., C.M.H. and D.F.
Competing interests
We declare we have no competing interests.
Funding
This study supported by the UK Natural Environment Research Council (NERC, including grant no. NE/R016429/1 and NE/R000131/1 as part of the UK-SCAPE and SUNRISE programmes delivering National Capability), the Department for Environment Food and Rural Affairs, the Northern Ireland Environment Agency (NIEA), the UK Joint Nature Conservation Committee, the NEWS India-UK Virtual Joint Centre on Agricultural Nitrogen (supported through the Newton-Bhabha Fund, by the UKRI and the Indian Department of Biotechnology), the UKRI Global Challenges Research Fund (South Asian Nitrogen Hub), the EU NitroPortugal project and the ‘Towards INMS’ project of the Global Environment Facility (GEF) and UNEP.
References
- 1.Fowler D, Cape JN, Leith ID, Paterson IS, Kinnaird JW, Nicholson IA. 1982. Rainfall acidity in northern Britain. Nature 297, 383–385. ( 10.1038/297383a0) [DOI] [Google Scholar]
- 2.Fowler D, et al. 2020. A chronology of global air quality. Phil. Trans. R. Soc. A 378, 20190314 ( 10.1098/rsta.2019.0314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irwin JG, et al. 1997. Acid deposition in the United Kingdom 1986–1995. Fourth report of the review group on acid rain. London, UK: Department of Environment. [Google Scholar]
- 4.Van Breemen N, Burrough PA, Velthorst EJ, van Dobben HF, de Wit T, Ridder TB, Reijnders HFR. 1982. Soil acidification from atmospheric ammonium sulphate in forest canopy throughfall. Nature 299, 548–550. ( 10.1038/299548a0) [DOI] [Google Scholar]
- 5.Heil GW, Diemont WH. 1983. Raised nutrient levels change heathland into grassland. Vegetatio 53, 113–120. ( 10.1007/BF00043031) [DOI] [Google Scholar]
- 6.Nihlgård B. 1985. The ammonium hypothesis—an additional explanation to the forest dieback in Europe. Ambio 14, 2–8. [Google Scholar]
- 7.UNECE. 1985. Protocol on the reduction of sulphur emissions or their transboundary fluxes by at least 30 per cent. Geneva, Switzerland: United Nations Economic Commission for Europe. [Google Scholar]
- 8.UNECE. 1988. The Sofia protocol concerning the control of emissions of nitrogen oxides or their transboundary fluxes. Geneva, Switzerland: United Nations Economic Commission for Europe. [Google Scholar]
- 9.UNECE. 1999. Protocol to abate acidification, eutrophication and ground-level ozone (Gothenburg Protocol). Geneva, Switzerland: United Nations Economic Commission for Europe; (Protocol revised 2012). [Google Scholar]
- 10.Sutton MA, Howard CM. 2018. Ammonia maps make history. Nature 564, 49–50. ( 10.1038/d41586-018-07584-7) [DOI] [PubMed] [Google Scholar]
- 11.Priestley J. Experiments and observations on different kinds of air, 411 pp 1st Vol. (1774) 324 pp., 2nd Vol. (1775) 399 pp., 3rd vol. (1777), London, UK: J. Johnson. [Google Scholar]
- 12.Sutton MA, Erisman JW, Dentener F, Moeller D. 2008. Ammonia in the environment: from ancient times to the present. Environ. Pollut 156, 583–604. ( 10.1016/j.envpol.2008.03.013) [DOI] [PubMed] [Google Scholar]
- 13.Belakovski D. 1990. Die Mineralien der brennenden Kohlefloze von Ravat in Tadshikistan. Lapis 15, 21–26. [Google Scholar]
- 14.Van Damme M, Clarisse L, Whitburn S, Hadji-Lazaro J, Hurtmans D, Clerbaux C, Coheur PF. 2018. Industrial and agricultural ammonia point sources exposed. Nature 564, 99–103. ( 10.1038/s41586-018-0747-1) [DOI] [PubMed] [Google Scholar]
- 15.Skaff JK. 1998. The Sasanian and Arab-Sasanian Silver Coins from Turfan: their relationship to International Trade and the Local Economy. Asia Major 3rd Series 11, 67–116. [Google Scholar]
- 16.Goitein SD. 1999. A Mediterranean society. The Jewish communities of the world as portrayed in the documents of the Cairo geniza. Vol. 1, economic foundations. Berkeley, CA: University of California Press. [Google Scholar]
- 17.Erisman JW, Sutton MA, Galloway JN, Klimont Z, Winiwarter W. 2008. How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636–639. ( 10.1038/ngeo325) [DOI] [Google Scholar]
- 18.Ouseley W. 1800. The oriental geography of Ebn haukal an Arabian traveller of the tenth century. London, UK: Oriental Press/T. Cadell & W. Davies; [Kitāb al-Masālik wa-al-Mamālik] [Google Scholar]
- 19.Sprenger A. 1841. El-Mas'ūdī's historical encyclopaedia entitled ‘meadows of gold and mines of gems’ translated from the Arabic. London, UK: Oriental Translation Fund of Great Britain and Ireland. [Google Scholar]
- 20.Needham J, Ho RY, Lu GD, Sivin N. 1980. Part 4: spagyrical discovery and invention: apparatus, theories and gifts. In Science and civilisation in China (ed. C Cullen), vol. 5 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 21.Holmyard EJ. 1957. Alchemy. Middlesex, UK: Penguin Books. [Google Scholar]
- 22.Karimov UI. 1957. Неизвестное Сочинение Ар-Рази ‘Книга Тайны Тайн’. Tashkent: Academy of Sciences of the Uzbek SSR; [An unknown work of ar-Razi ‘Book of the Secret of Secrets', in Russian]. [Google Scholar]
- 23.Zirnis P. 1979. Kitāb Usṭuqus al-Uss of Jābir ibn Ḥayyān. PhD thesis, New York University, New York. [Google Scholar]
- 24.Heym G. 1938. Al-Rāzī and alchemy. Ambix 1, 184–191. ( 10.1179/amb.1938.1.3.184) [DOI] [Google Scholar]
- 25.Mertens M. 1995. Les Alchimistes Grecs. Zosime de Panopolis. Mémoires Authentiques. Paris: Les Belles Lettres. [Google Scholar]
- 26.Abt T. (ed.) 2011. The Book of Pictures Muṣ̣ḥaf aṣ-ṣuwar by Zosimos of Panopolis. (trans. S. Fuad and T. Abt) Zurich: Living Human Heritage Publications. [Google Scholar]
- 27.Martelli M. 2013. The four books of pseudo-Democritus. In Ambix, vol. 60(Supplement 1) Leeds: Maney Publishing. [Google Scholar]
- 28.Dobbs BJT. 1975. The foundations of Newton's alchemy. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 29.Newton Project. 2013. Letter from Newton to Henry Oldenburg, dated 26 April 1676. MS Add. 9597/2/18/53-54, Cambridge University Library. www.newtonproject.ox.ac.uk/catalogue/record/NATP00268 (accessed 1 March 2020).
- 30.Taslimi M. 1954. An examination of the ‘Nihāyat al-Ṭalab’ and the determination of its place and value in the history of Islamic chemistry. PhD thesis University College, London. [Google Scholar]
- 31.Principe LM. 2003. Boyle's alchemical pursuits. In Robert Boyle reconsidered (ed. Hunter M.), pp. 91–105. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 32.Hales S. 1727. Vegetable statics, (republished 1961 London, UK: Scientific Book Guild. [Google Scholar]
- 33.Schofield RE. 2004. The enlightened Joseph Priestley: A study of his life and work from 1773 to 1804. Harrisburg, PA: Penn State Press. [Google Scholar]
- 34.Scheele C-W. 1977. Chemische Abhandlungen über Luft und Feuer, Leipzig, Germany. [Google Scholar]
- 35.Berthollet CL. 1785. Analyse de l'Alcali Volatil. Mémoires de l'Académie Royale des Sciences 1788, 316–326. [Google Scholar]
- 36.Tipping E, et al. 2017. Long-term increases in soil carbon due to ecosystem fertilization by atmospheric nitrogen deposition demonstrated by regional-scale modelling and observations. Nat. Sci. Rep. 7, 1890 ( 10.1038/s41598-017-02002-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutton MA, Tang YS, Dragosits U, Fournier N, Dore T, Smith RI, Weston KJ, Fowler D. 2001. A spatial analysis of atmospheric ammonia and ammonium in the UK. The Scientific World 1, 275–286. ( 10.1100/tsw.2001.313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang YS, et al. 2018. Drivers for spatial, temporal and long-term trends in atmospheric ammonia and ammonium in the UK. Atmos. Chem. Phys. 18, 705–733. ( 10.5194/acp-18-705-2018) [DOI] [Google Scholar]
- 39.Tang YS, et al. 2018. Acid gases and aerosol measurements in the UK (1999–2015): regional distributions and trends. Atmos. Chem. Phys. 18, 16 293–16 324. ( 10.5194/acp-18-16293-2018) [DOI] [Google Scholar]
- 40.Braban CF, Aas W, Colette A, Banin L, Ferm M, González Ortiz A, Pandolfi M, Putaud JP, Spindler G, with contributions from 33 others. 2016. Sulfur and nitrogen compounds and Particulate Matter, ch. 3. In Air pollution trends in the EMEP region between 1990 and 2012. (Collette A. and 55 others), pp. 22–42. EMEP Co-operative Programme for Monitoring and Evaluation of the Long-range Transmission of Air Pollutants in Europe. EMEP/CCC-Report 1/2016. [Google Scholar]
- 41.Butler T, Vermeylen F, Lehmann CM, Likens GE, Puchalski N. 2016. Increasing ammonia concentration trends in large regions of the USA derived from the NADP/AMoN network. Atmos. Environ. 146, 132–140. ( 10.1016/j.atmosenv.2016.06.033) [DOI] [Google Scholar]
- 42.Liu XJ, et al. 2020. Environmental impacts of nitrogen emissions in China and the role of policies in emission reduction. Phil. Trans. R. Soc. A 378, 20190324 ( 10.1098/rsta.2019.0324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutton MA, et al. 2017. The Indian nitrogen challenge in a global perspectives. Chapter 1. In The Indian nitrogen assessment: sources of reactive nitrogen, environmental and climate effects, management options, and policies (eds Abrol YP, Adhya TK, Aneja VP, Raghuram N, Pathak H, Kulshrestha U, Sharma C, Singh B). Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 44.Vieno M, et al. 2016. The UK particulate matter air pollution episode of March–April 2014: more than Saharan dust. Environ. Res. Lett. 11, 044004 ( 10.1088/1748-9326/11/4/044004) [DOI] [Google Scholar]
- 45.Brunekreef B, Harrison RM, Künzli N, Querol X, Sutton MA, Heederik DJJ, Sigsgaard T. 2015. Reducing the health effect of particles from agriculture. Lancet Respiratory Med. 3, 831–832. ( 10.1016/S2213-2600(15)00413-0) [DOI] [PubMed] [Google Scholar]
- 46.Sutton MA, Wolseley PA, Leith ID van Dijk N, Tang YS, James PW, Theobald MR, Whitfield CP. 2009. Estimation of the ammonia critical level for epiphytic lichens based on observations at farm, landscape and national scales, ch. 6. In Atmospheric ammonia: detecting emission changes and environmental impacts (eds Sutton MA, Reis S, Baker SMH), pp. 71–86. Berlin, Germany: Springer. [Google Scholar]
- 47.Wolseley PA, James PW, Theobald MR, Sutton MA. 2006. Detecting changes in epiphytic lichen communities at sites affected by atmospheric ammonia from agricultural sources. The Lichenologist 38, 161–176. ( 10.1017/S0024282905005487) [DOI] [Google Scholar]
- 48.Wolseley PA, Leith ID, Sheppard LJ, Lewis JEJ, Crittenden P, Sutton MA.2013. Guide to using a lichen based index to nitrogen air quality. Field Studies Council.
- 49.Van Herk CM. 2001. Bark pH and susceptibility to toxic air pollutants as independent causes of changes in epiphytic lichen composition in space and time. Lichenologist 33, 419–441. ( 10.1006/lich.2001.0337) [DOI] [Google Scholar]
- 50.Wolseley P, Sutton M, Leith I, van Dijk N. 2010. Epiphytic lichens as indicators of ammonia concentrations across the UK. Bibliotheca Lichenologica 105, 75–85. [Google Scholar]
- 51.Lewis J. 2012. Bio-monitoring for atmospheric nitrogen pollution using epiphytic lichens and bryophytes. PhD thesis, University of Nottingham. [Google Scholar]
- 52.Van Herk CM. 2017. Monitoring van korstmossen in de provincie drenthe 1991–2016. Soest (The Netherlands): Lichenologisch Onderzoekbureau Nederland (LON). [Google Scholar]
- 53.Chatterjee S, Acharya A, Jha AB, Singh J, Prasad R. 2011. Setting standards for sustainable harvest of wild medicinal plants in Uttarakhand: a case study of lichens. In Community-based biodiversity conservation in the Himalayas (eds Gokhale Y, Nege AK), pp. 101–123. New Delhi: The Energy Resources Institute (TERI). [Google Scholar]
- 54.Sutton MA, et al. 2019. Nitrogen - grasping the challenge. A manifesto for science-in-action through the international nitrogen management system. Summary report. Edinburgh: Centre for Ecology & Hydrology; See https://papersmart.unon.org/resolution/sustainable-nitrogen-management (UNEP-SL/UNGC/ Res.L.14/INF/6). [Google Scholar]
- 55.Sheppard LJ, Leith ID, Mizunuma T, Cape JN, Crossley A, Leeson S, Sutton MA, van Dijk N, Fowler D. 2011. Dry deposition of ammonia gas drives species change faster than wet deposition of ammonium ions: evidence from a long-term field manipulation. Glob. Change Biol. 17, 3589–3607. ( 10.1111/j.1365-2486.2011.02478.x) [DOI] [Google Scholar]
- 56.Levy P, van Dijk N, Gray A, Sutton M, Jones M, Leeson S, Dise N, Leith ID, Sheppard LJ. 2019. Response of a peat bog vegetation community to long-term experimental addition of nitrogen. J. Ecol. 107, 1167–1186. ( 10.1111/1365-2745.13107) [DOI] [Google Scholar]
- 57.Stevens CJ, Bell JNB, Brimblecombe P, Clark CM, Dise NB, Fowler D, Lovett GM, Wolseley PA. 2020. The impact of air pollution on terrestrial managed and natural vegetation. Phil. Trans. R. Soc. A 378, 20190317 ( 10.1098/rsta.2019.0317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hautier Y, Niklaus PA, Hector A. 2009. Competition for light causes plant biodiversity loss after eutrophication. Science 324, 636–638. ( 10.1126/science.1169640) [DOI] [PubMed] [Google Scholar]
- 59.Stevens CJ. 2016. How long do ecosystems take to recover from atmospheric nitrogen deposition? Biol. Conserv. 200, 160–167. ( 10.1016/j.biocon.2016.06.005) [DOI] [Google Scholar]
- 60.Power SA, Green ER, Barker CG, Bell JNB, Ashmore MR. 2006. Ecosystem recovery: heathland response to a reduction in nitrogen deposition. Glob. Change Biol. 12, 1241–1252. ( 10.1111/j.1365-2486.2006.01161.x) [DOI] [Google Scholar]
- 61.Guerrieri R, Mencuccini M, Sheppard LJ, Saurer M, Perks M, Levy P, Sutton MA, Borghetti M, Grace J. 2011. The legacy of enhanced N and S deposition as revealed by the combined analysis of delta 13C, delta 18O and delta 15N in tree rings. Glob. Change Biol. 17, 1946–1962. ( 10.1111/j.1365-2486.2010.02362.x) [DOI] [Google Scholar]
- 62.Sutton MA, Leith ID, Bealey WJ, van Dijk N, Tang YS. 2011. Moninea Bog: A case study of atmospheric ammonia impacts on a Special Area of Conservation. In Nitrogen deposition and natura 2000: science & practice in determining environmental impacts (eds Hicks WK, Whitfield CP, Bealey WJ, Sutton MA), pp. 59–71. Brussels: COST Office. [Google Scholar]
- 63.Pearson J, Soares A. 1998. Physiological responses of plant leaves to atmospheric ammonia and ammonium. Atmos. Environ. 32, 533–538. ( 10.1016/S1352-2310(97)00008-3) [DOI] [Google Scholar]
- 64.Pearson J, Soares A. 1995. A hypothesis of plant susceptibility to atmospheric pollution based on intrinsic nitrogen metabolism: why acidity really is the problem. Water, Air and Soil Pollution 85, 1227–1232. ( 10.1007/BF00477149) [DOI] [Google Scholar]
- 65.Sutton MA, Reis S, Baker SMH (eds). 2009. Atmospheric ammonia: detecting emission changes and environmental impacts. 464 pp. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 66.Cape JN, van der Eerden LJ, Sheppard LJ, Leith ID, Sutton MA. 2009. Evidence for changing the critical level for ammonia. Environ. Pollut. 157, 1033–1037. ( 10.1016/j.envpol.2008.09.049) [DOI] [PubMed] [Google Scholar]
- 67.Munzi S, Cruz C, Branquinho C, Pinho P, Leith ID, Sheppard LJ. 2014. Can ammonia tolerance amongst lichen functional groups be explained by physiological responses? Environ. Pollut 187, 206–209. ( 10.1016/j.envpol.2014.01.009) [DOI] [PubMed] [Google Scholar]
- 68.Munzi S, Sheppard LJ, Leith ID, Cruz C, Branquinho C, Bini L, Gagliardi A, Cai G, Parrotta L. 2017. The cost of surviving nitrogen excess: energy and protein demand in the lichen Cladonia portentosa as revealed by proteomic analysis. Planta 245, 819–833. ( 10.1007/s00425-017-2647-2) [DOI] [PubMed] [Google Scholar]
- 69.Sutton MA, et al. 2013. Towards a climate-dependent paradigm of ammonia emission and deposition. Phil. Trans. R. Soc. B 368, 20130166 ( 10.1098/rstb.2013.0166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riddick SN, et al. 2018. Global assessment of the effect of climate change on ammonia emissions from seabirds. Atmos. Environ. 184, 212–223. ( 10.1016/j.atmosenv.2018.04.038) [DOI] [Google Scholar]
- 71.Bittman S, Dedina M, Howard CM, Oenema O, Sutton MA (eds). 2014. Options for ammonia mitigation: guidance from the UNECE task force on reactive nitrogen. Edinburgh: TFRN-CLRTAP / Centre for Ecology and Hydrology, UK. [Google Scholar]
- 72.Sutton M, et al. 2019. The nitrogen fix: from nitrogen cycle pollution to nitrogen circular economy. Frontiers 2018/2019. In Emerging issues of environmental concern, pp 52–65. Nairobi, Kenya: United Nations Environment Programme. [Google Scholar]
- 73.Fowler D, Pyle JA, Raven JA, Sutton MA. 2013. The global nitrogen cycle in the twenty-first century: introduction. Phil. Trans. R. Soc. B 368, 20130165 ( 10.1098/rstb.2013.0165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sutton MA, et al. 2013. Our Nutrient World: The challenge to produce more food and energy with less pollution. Edinburgh, United Kingdom: Centre for Ecology and Hydrology, on behalf of the Global Partnership on Nutrient Management and the International Nitrogen Initiative. [Google Scholar]
- 75.UNEP. 2019. Launch of United Nations global campaign on sustainable nitrogen management, 23–24 October 2019, Colombo, Sri Lanka: Colombo Declaration on Sustainable Nitrogen Management; See https://papersmart.unon.org/resolution/sustainable-nitrogen-management (accessed: 1 March 2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this paper are included in the electronic supplementary material.