Activity-based protein profiling indicates the role of serine hydrolases in rice germination and lipid mobilization.
Abstract
Elucidating proteolipidome dynamics is crucial for understanding the roles of these molecules in plant physiology and disease. Sequence-based functional annotation of the protein is inadequate, since protein activities depend on posttranslational modification. In this study, we applied a gel-free activity-based protein profiling approach to unravel the active lipases, including other Serine hydrolases (SHs), expressed during seed germination in rice (Oryza sativa). We successfully mapped the active sites of 43 active SHs encompassing lipases/esterases, GDSL lipases, proteases, Ser carboxypeptidases, ABHD protein, pectin acetylesterase, and other SHs. The mRNA expression levels of those genes encoding the identified SHs were monitored using microarray analysis. The lipidome analysis revealed distinct patterns of molecular species distribution in individual lipid classes and displayed the metabolic connections between lipid mobilization and rice seedling growth. Changes in the mobilization of storage lipids and their molecular species remodeling were correlated with the expression of the identified lipases and their lipase activity in a time-dependent manner. The physiological significance of the identified SHs was explored during biotic stress with Fusarium verticillioides infection. The fungal infection significantly reduced lipase activity and lipid mobilization, thus impairing the rice seedling. Collectively, our data demonstrate application of the functional proteome strategy along with the shotgun lipidome approach for the identification of active SHs, and thus for deciphering the role of lipid homeostasis during rice seed germination.
Germination is one of the most important physiological events in the life cycle of plants, and it is crucial for the transition from seed to seedling. During this period, various hydrolytic enzymes play a vital role in the mobilization of stored reserves like lipid, carbohydrate, and protein. In germinating seeds, the lipolytic enzyme activities play a crucial role in proper seedling growth and development (Li et al., 2007; Müller and Ischebeck, 2018). Lipases and esterases belong to the Serine hydrolase (SH) superfamily, members of which contain a conserved Ser nucleophile and catalyze versatile hydrolytic reactions. Most SHs share the common α/β hydrolase fold and possess a Ser-His-Asp catalytic triad (Tripathi and Sowdhamini, 2006). Although there are reports of lipolytic activities during the germination of rice (Oryza sativa; Li et al., 2007; Dolui et al., 2020), comprehensive details and a functional annotation of the active SHs, specifically lipases, are still lacking. The advances in genomic and proteomic approaches have provided extensive information about the presence and expressional profile of rice enzymes (Ma et al., 2005; Yamakawa et al., 2007; Chepyshko et al., 2012; He and Yang, 2013). However, they fail to provide insights into the functional status of enzymes. The major limitation of this abundance-based method is that the level of mRNA partially/poorly correlates with protein function (Birner-Gruenberger and Hermetter, 2007). To bridge this gap, activity-based protein profiling (ABPP) is a suitable and powerful analytical platform that can be used for functional annotation of enzymes (Evans and Cravatt, 2006; Paulick and Bogyo 2008). In principle, ABPP uses an active site-directed probe to monitor the functional state of enzymes, and it enables the detection as well as affinity purification of the target enzymes (Liu et al., 1999; Evans and Cravatt, 2006). The application of ABPP, in combination with mass spectrometry (MS), can deliver the identification of enzyme functions irrespective of their abundance or their existing annotation in an unbiased manner (Ortega et al., 2016). Since its inception, ABPP has been used for various applications, including target identification, high-throughput screening, discovery of enzyme activities in pathologic states, and characterization of enzyme active sites (Adam et al., 2004; Jessani et al., 2004; Bachovchin et al., 2009). SHs have been exploited in all three of the kingdoms of life through ABPP (Cravatt et al., 2008; Morimoto and van der Hoorn, 2016; Zweerink, et al., 2017). However, the application of ABPP in plants is still emerging, and the majority of the studies have been carried out in the model plant Arabidopsis (Arabidopsis thaliana) or limited to tomato (Solanum lycopersicum; van der Hoorn et al., 2004; Tian et al., 2007). These reported studies have already revealed regulatory mechanisms in plant physiological processes such as germination, senescence, and disease resistance/plant immunity (Morimoto and van der Hoorn 2016). ABPP has also been exploited to study plant-pathogen interactions in plants like Arabidopsis, tomato, and saffron (Crocus sativus; van der Hoorn et al., 2004; Tian et al., 2007; Kaschani et al., 2009; Husaini et al., 2018). Among cereal crops, rice is a vital staple food for half of the world population, with nutritional and economic importance. However, functional validation of the rice genome and enzyme families by ABPP has not yet been explored. Rice is vulnerable to fungal infection, which compromises the production and quality of grains. Fusarium verticillioides is one such fungus that causes bakanae (foolish seedling) in rice. This disease is transmitted through seeds (Desjardins et al., 1997) and is a potential threat to rice production due to the production of mycotoxins, namely fumonisins B1 (FB1) and B2 (FB2; Ross et al., 1990). The SHs play crucial roles in plant processes ranging from normal plant physiology to pathogenesis. The rice genome sequence project revealed the presence of several SHs; however, their functional characterization is lacking. Here, we report comprehensive profiling of active SHs expressed during rice germination by the ABPP approach with mRNA analysis. The correlation between lipase activity and lipid mobilization was monitored by shotgun lipidome analysis using high-resolution MS (HRMS). Further, we investigated the physiological influence of the identified SHs on plant lipid metabolism and its energy mobilization under biotic stress conditions induced by F. verticillioides.
RESULTS
Detection of Active SHs in Rice by Gel-Based ABPP
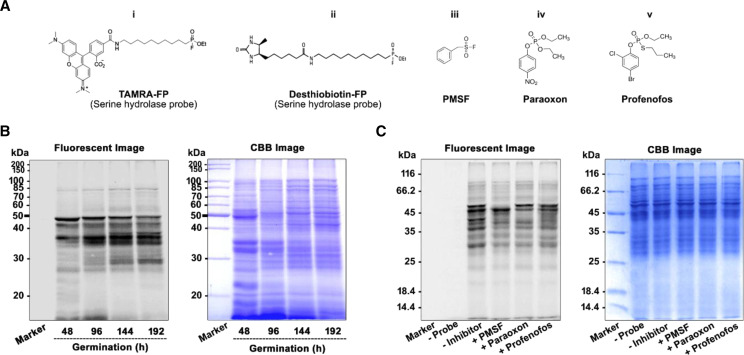
We performed in-gel fluorescence ABPP to get insight into the active SHs present in the target rice proteome. It is the most basic and standard format of ABPP for the visualization and monitoring of probe labeling in the proteome, and it can be performed in a facile manner (Patricelli et al., 2001). In this study, the labeling of the proteome for SHs during germination was done using a fluorophosphonate-based SH probe (Fig. 1, A and B). For ABPP labeling, samples from germinated rice seed and seedlings up to the two-leaf stage were collected every 24 h for protein extraction. SH activity was detected every 24 h by ABPP assay using TAMRA-fluorophosphonate (TAMRA-FP; Supplemental Fig. S1A), and the level of lipase activity was monitored by in vitro lipase assay using triacylglycerol (TAG) as a substrate (Supplemental Fig. S1B). The in-gel fluorescence scanning profile reveals the time-dependent increase of distinct signals with varying intensity (Fig. 1B), and there were two significant signals, observed at ∼50 and ∼27 kD from 48 h. Overall, it gives a clear glimpse of the temporal trend of emergence and reduction of active SHs with the progression of germination. The intensity of the signal depends on the availability and accessibility of SH active sites. We observed different patterns in the SH activity profile with the emergence of many higher- as well as lower-Mr SHs that are predominantly present up to 192 h (Fig. 1B, lanes 3 and 4).
Figure 1.
Detection and competitive labeling of SHs by in-gel ABPP assay. A, Structures of the FP probe and the reported SH inhibitors: Rhodamine conjugated fluorescent FP probes (TAMRA-FP), for detection of SHs (i); Desthiobiotin conjugated fluorophosphonate probe for enrichment of SHs (ii); and SH inhibitors PMSF (iii), Paraoxon (iv), and Profenofos (v). B, In-gel fluorescence labeling of rice SHs during germination. O. sativa proteome was incubated with 2 μm FP-SH probe at 37°C for 1 h followed by separation on 12% SDS-PAGE. The labeled SHs were detected by fluorescence scanning, and the fluorescence intensity represents the abundance of labeled protein present in the sample. The total protein profile was visualized by Coomassie brilliant blue (CBB) staining of the gel (right). C, In vitro competitive ABPP. O. sativa protein from 192 h was preincubated with 100 μm of inhibitor or DMSO (control) at 37°C for 30 min, followed by incubation with an FP-SH probe for 1 h. SH inhibitors compete with FP probes for active-site binding, thus leading to a loss of fluorescence intensity. After fluorescence scanning, the total protein profile was monitored by CBB staining (right). All experiments were performed for a minimum of three biological replicates. The images and results presented were generally derived from one representative experiment.
SH Inhibitors Selectively Block the Active-Site Labeling of Rice SHs
The competitive ABPP technique involves preincubation of native proteins with inhibitors followed by labeling with an ABPP probe. Comparison of the labeling patterns of the control (untreated) proteome with the inhibitor-pretreated proteome detected various inhibitory activities. For competitive ABPP, we used a TAMRA-FP probe (Fig. 1A, i) that reacts covalently and irreversibly with the conserved active-site Ser. Here, we performed an in vitro and gel-based competitive ABPP assay for inhibition of the rice proteome at the 192-h time point using the reported SH inhibitor phenylmethylsulfonyl fluoride (PMSF; Fig. 1A, iii), a covalent Ser protease inhibitor (Zhao et al., 2019), as well as the reported agrochemicals paraoxon and profenofos (Fig. 1A, iv and v). This competitive ABPP experiment was also performed with the proteome at the 144-h time point (Supplemental Fig. S2). As expected, PMSF was able to inhibit and prevent labeling of most of the SHs, as evidenced by the overall reduction in signals in Figure 1C (lane 2). The pesticide paraoxon also inhibited many signals in this study, and a similar observation was found with profenofos (Fig. 1C, lanes 3 and 4, respectively). Thus, this competitive ABPP assay revealed that the signals displayed by the labeling of SH probe are potent SH targets.
Identification of Active SHs by Active-Site Peptide Profiling
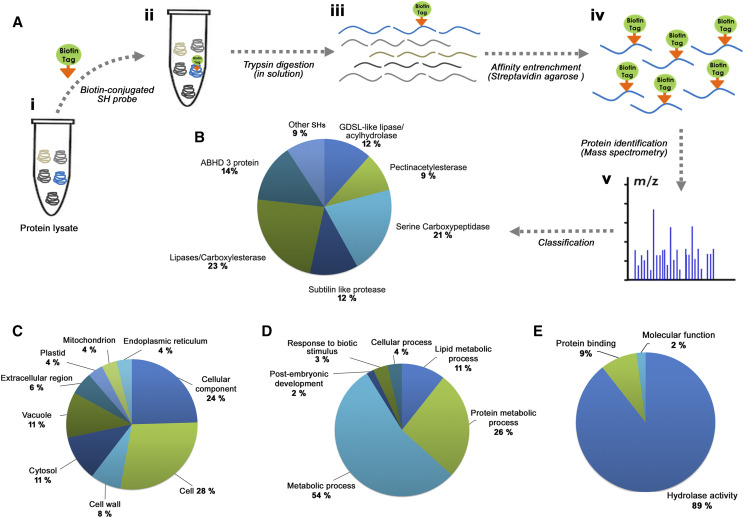
The gel-based ABPP does not reveal the biological identity of the labeled protein and cannot assign a molecular function to the detected signals. This limitation has led researchers to develop a gel-free liquid chromatography (LC)-MS-coupled format of ABPP called active-site peptide profiling, which is more robust, reliable, and informative (Evans and Cravatt, 2006; Paulick and Bogyo, 2008). In this approach, both the identification of target enzymes and the precise mapping of specific residues (active sites) labeled by the probe are possible (Adam et al., 2004). To reveal the biological identity of those SHs detected upon in-gel fluorescence ABPP, we carried out active-site-directed peptide profiling. In brief, the target proteome was labeled with the desthiobiotin-conjugated probe (Fig. 1A, ii) followed by trypsin digestion of the proteome. The peptides with labeled active sites were affinity captured on a streptavidin agarose matrix (Fig. 2). The number of active-site-labeled peptides present in the sample logically equals the number of functional SHs present in the proteome (Okerberg et al., 2005). Through this platform, we mapped the active SHs in rice, which was temporally active during different stages of germination. In this study, we identified 43 active SHs, including lipases/esterases, GDSL lipases, proteases including subtilisin-like proteases, Ser carboxypeptidases, and other uncharacterized SHs (Fig. 2B).
Figure 2.
Enrichment of SHs by active-site peptide profiling and GO analysis. A, Schematic representation depicts the active-site peptide profiling workflow for the enrichment and identification of SH present in the sample. Protein lysates were incubated with biotin-conjugated SH probe followed by trypsin digestion in solution (i-iii). Subsequently, the probe-labeled target peptide was captured by the streptavidin agarose affinity matrix (iv). Finally, the active-site peptides were eluted and analyzed by MS (v). B, Distribution of the 43 identified SHs under various classes. The identified protein portfolio represents different classes of SHs (lipases/esterase, GDSL lipases, pectin acetylesterases, Ser protease/peptidase, Ser carboxypeptidases, Subtilisin like protease, ABHD protein, and other SHs). C to E, GO enrichment analysis of ABPP-identified SHs was carried out and classified under the GO categories Cellular component (C), Biological process (D), and Molecular function (E). All experiments were performed for a minimum of three biological replicates.
Further, to understand the biological functions of the identified SHs, we performed Gene Ontology (GO) enrichment analysis (Fig. 2; Supplemental Dataset S1). The GO analysis was grouped into three different categories: cellular components (Fig. 2C), biological process (Fig. 2D), and molecular function (Fig. 2E). The cellular component analysis of these identified SHs showed their ubiquitous distribution (Fig. 2C). GO annotation of biological processes confirms the involvement of SHs in various metabolic processes. As shown in Figure 2D, a major portion of the proteins were associated with the lipid metabolic process, which is understandable since there are many active lipases expressed during germination. The annotation of “molecular function” for the identified proteins falls under three major categories: hydrolase activity, catalytic activity, and transferase activities (Fig. 2E). The complete portfolio of the identified SHs is depicted in Figure 3 (see Supplemental Dataset 2). Analysis of active-site peptides and their Pfam structure reveals the presence of the conserved motif and domain in each class of SHs (Fig. 3, A and B). The microarray analysis of the identified SHs showed differential expression at three different stages of germination (Fig. 3C). We performed active-site peptide enrichment in rice germination at four different time points. As evidenced from Figure 3D, SHs are consistently present throughout all the selected stages of germination, and many among them were active and labeled by the probe at more than two or three time points (Fig. 3C).
Figure 3.
Analysis of identified SHs. A, List of SHs identified by gel-free ABPP using NanoLC-MS/MS analysis and their corresponding active-site peptide sequences. The SHs are clustered under various classes based on structural features. The active site Ser residue is highlighted in red. B, PFAM structure of each identified SH. C, Heat map displaying mRNA expression of the genes encoding the identified SHs. Values were obtained from microarray analysis, and data were calculated from the mean ratios of the comparison between the zero time point and other stages. D, Venn diagrams display the numbers of identified SHs at each time point. SHs are consistently present across different time points, and many are present throughout the selected stages of germination. All experiments were performed for a minimum of three biological replicates.
Level of mRNA Expression of Genes Encoding Lipases during Rice Germination
Based on their canonical and conserved sequence elements, lipases were grouped into GxSxG and GDSL families. The presence of GxSxG motif-containing lipases are called “classical” lipases, and they carry an active-site Ser residue in their catalytic triad Ser, His, Asp/Glu. The GDSL family of lipases also possesses a Ser-containing motif (GxSxxxxG), which is located closer to the N terminus of the protein. GDSL lipase family members show broad substrate specificity and regiospecificity (Akoh et al., 2004). We investigated the expression profile of lipolytic enzymes expressed during rice germination through microarray analysis. For this, we selected 67 lipases, 36 phospholipases, and 110 GDSL lipases (with unique Os identifier [ID]) from the Rice Genome Annotation Project database based on their existing annotations and putative functions. Among the phospholipases, the expression of 17 genes was confirmed by analyzing their mRNA transcript level (Fig. 4A). We performed clustering of those selected genes based on their mRNA transcript level over three different time points, and significantly higher expression was observed in nine phospholipases (Supplemental Fig. S3A). Among the putative lipase genes, we detected mRNA expression for 28 genes. Differential expression was observed in 14 putative lipases (Fig. 4B). However, maximum expression was observed for seven genes during germination compared to control (Supplemental Fig. S3B). We also investigated the mRNA level of the 110 GDSL lipase genes. The majority of these genes were found to be expressed during germination (Fig. 4C), and 37 of them were highly expressed throughout all three stages of germination, i.e. at 48 to 192 h of germination (Fig. 4C).
Figure 4.
mRNA levels of genes encoding various lipases during rice germination. We selected the different groups of lipase enzymes based on their existing annotations and putative functions from the Rice Genome Annotation Project database. A, Expression pattern phospholipase genes. Based on their domain and motif organization, phospholipases were clustered into four different subclasses: patatin-like phospholipase, phospholipase A, phospholipase C, and phospholipase D. B, Expression pattern of putative lipases along with unique Os ID. The mRNA expression level of putative lipase genes depicts a gradual increase in their abundance as germination progresses. C, Heat map of GDSL lipases along with unique Os ID are represented. The mRNA expression level of the GDSL lipase genes shows that they are predominantly expressed during germination. All the experiments were performed for a minimum of three biological replicates.
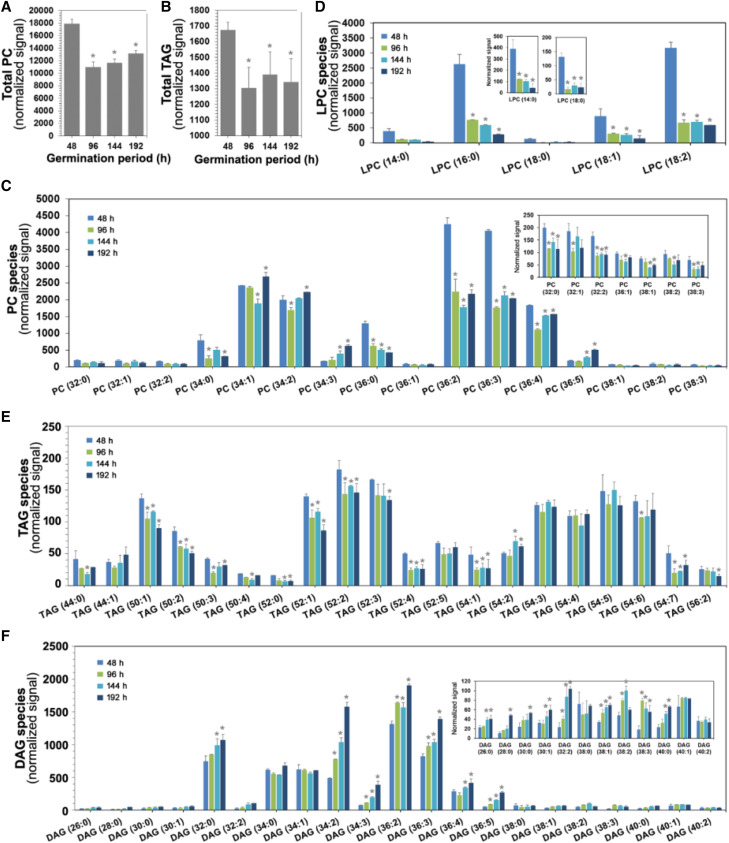
Lipidome Analysis of Rice Seedlings by HRMS
In plants, high-throughput lipid profiling is being used to follow metabolic changes in response to developmental, environmental, and stress-induced physiological changes. The plant lipidome includes storage lipids and structural lipids (phospholipids and sphingolipids) and has an essential role in growth and development. TAG is the primary form of storage lipid in seeds followed by other minor lipids. The glycerophospholipids are the major membrane lipids, which include phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidyl-Ser, phosphatidic acid (PA), and phosphatidylglycerol. We performed profiling and molecular species analysis of storage lipids as well as phospholipids at different stages of rice germination. The total lipid species of PC and TAG are represented in Figure 5, A and B. First, we analyzed the phospholipid content, particularly that of PC, since hydrolysis of the phospholipid monolayer is an important event in the mobilization of stored lipids for seed germination. We observed a significant reduction of PC(36:0), PC(36:2), and PC(36:3) compared with other PC species during germination at 48 h (Fig. 5C). However, from 144 h onward, there was a gradual increase in these PC molecular species (Fig. 5C) as a result of membrane reorganization. Further, there was a constant increase in the level of PC(34:3) and PC(36:5) and a gradual decrease of PC(36:0) species with the advancement of germination. The level of lysophosphatidylcholine (LPC) was reduced during germination (Fig. 5D).
Figure 5.
Lipidome analysis of rice during germination by HRMS. Total lipid was extracted from different time points of rice seedling germination, and the level of lipid class and its molecular species were analyzed by precursor ion or neutral loss scanning. In each class, the extracted lipids were analyzed by MS/MSALL. The identity of individual lipid molecular species was determined based on their m/z signals from the spectra. A, Level of total PC content during germination. B, Level of total TAG content at different time intervals of germination. C to F, Individual lipid molecular species analysis of PC (C), LPC (D), TAG (E), and DAG (F) across the four different stages of germination. The lipid nomenclature was given as total number of carbon atoms:total number of double bonds in the fatty acyl groups. Asterisks indicate significance (*P < 0.05) as compared with the 48 h time point. Data were represented as the mean ± sd of three biological replicates.
For other phospholipid species, we observed a gradual increase of PI as germination progressed, as a result of membrane remodeling (Supplemental Fig. S4A). In the case of storage lipid (TAG), there was a clear trend of mobilization/utilization of TAG species, with shorter fatty acyl chain [TAG(50:0), TAG(52:0), and TAG(52:1)] or saturated (or not highly unsaturated) species decreased during germination. However, TAG species with longer fatty acyl chains and a higher degree of unsaturation [TAG(54:3), TAG(54:4), and TAG(54:5)] are not utilized preferentially or consistently by the endogenous lipases, since their relative abundances do not change significantly, as shown in Figure 5E. In agreement with this phenomenon, we also observed a gradual increase in the level of diacylglycerol (DAG) species containing short-chain fatty acids with less unsaturation [DAG(32:0), DAG(34:2), DAG(36:2), and DAG(36:3)] with the gradual progression of germination, as a result of TAG mobilization by lipases (Fig. 5F). The temporal trends of other phospholipid species (PI, LPI, lysophosphatidylethanolamine, and lysophosphatidylglycerol) were analyzed similarly (Supplemental Fig. S4)
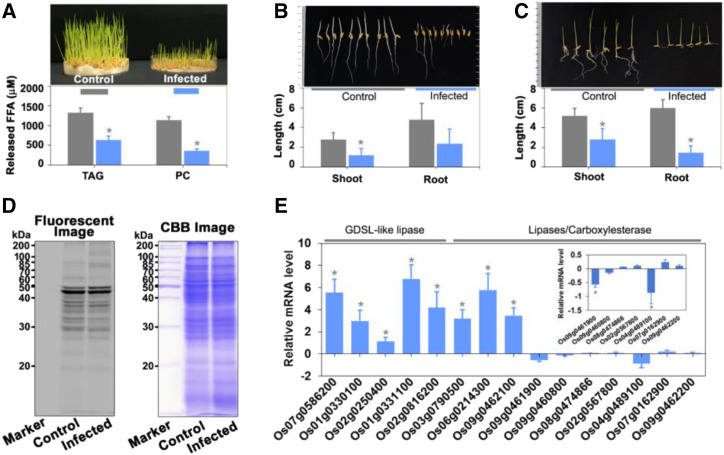
SH Activities and Lipidome Profiling Following F. verticillioides Infection
In general, agricultural crops bear microbes on or within their seed. Physiological changes occurring due to pathogen infection are manifested in terms of morphological features. In this study, we documented the morphological changes that occurred due to F. verticillioides infection in rice seedlings during germination. The control plants were healthy and exhibited normal shoots and roots after 7 d of germination (Fig. 6A). Following F. verticillioides infection, seedling growth was stunted, with overall morphological changes in shoots and roots (Fig. 6A, top). In the infected plants, there was a significant reduction in root length, whereas shoot length was reduced by 50% (Fig. 6, B and C). ABPP is an excellent method of choice for plant-pathogen interaction studies because it is a kind of diagnostic tool for recording changes in the activity of proteins in proteomes rather than the abundance of transcripts and proteins. In the gel-based fluorescence ABPP, we observed the loss of some signals as well as the appearance of newer signals around 70 kD in the infected sample (Fig. 6D). There was a noticeable reduction in signal intensity around 40 and 20 kD (Fig. 6D). These data reveal that SHs may be direct mediators of the stress condition and regulate the process either positively or negatively. In addition, we also analyzed the mRNA level of genes encoding each of the 43 identified SHs (Fig. 6E; Supplemental Fig. S5) upon infection by F. verticillioides. Among the different groups of SHs, expression levels for each of the five GDSL lipases/acylhydrolases were upregulated in the infected plant (Fig. 6E). In contrast, the mRNA level corresponding to lipases/carboxylesterases was downregulated (Fig. 6E). As a result of this, the overall lipase (glycerolipase and phospholipase) activity was reduced in the infested condition (Fig. 6A, bottom). Further, the mRNA levels corresponding to rice bran-specific lipases (reported in our previous study A.K. Dolui, A.K. Vijayakumar, R. Rajasekharan, P. Vijayaraj, unpublished data) were monitored (Supplemental Fig. S5F).
Figure 6.
F. verticillioides infection during rice germination. A, In vitro lipase activity of control and infected plants. The assay was performed for 30 min at 37°C in the presence of 50 μg total protein from control and infected plants (as an enzyme source) with either 50 μm PC or 50 μm TAG. Lipase activity was quantified by measuring released fatty acids. Phenotypes of control and infected plants are shown at top. Plant shoot and root length establishment, as well as the morphological changes upon F. verticillioides infection were examined by the paper towel (B) and petri-dish (C) methods. D, Detection, and profiling of SHs in control and infected plants by in-gel fluorescence labeling. Rice seeds were inoculated or not with F. verticillioides, and protein was extracted after 1 week. After delipidation, the proteins were labeled for 1 h with a 2 μm SH probe and separated on 12% SDS-PAGE followed by in-gel fluorescence scanning at 532 nm. After fluorescence scanning, the protein profile was monitored by Coomassie brilliant blue (CBB) staining. E, Level of mRNA expression of the identified SHs in F. verticillioides-infected plants, with representative data for GDSLs and lipases/carboxylesterases. Actin was used as an internal control. All the experiments were performed for a minimum of three biological replicates. Asterisks indicate significance (*P < 0.05) for control versus infected plants. Data are represented as the mean ± sd of three biological replicates.
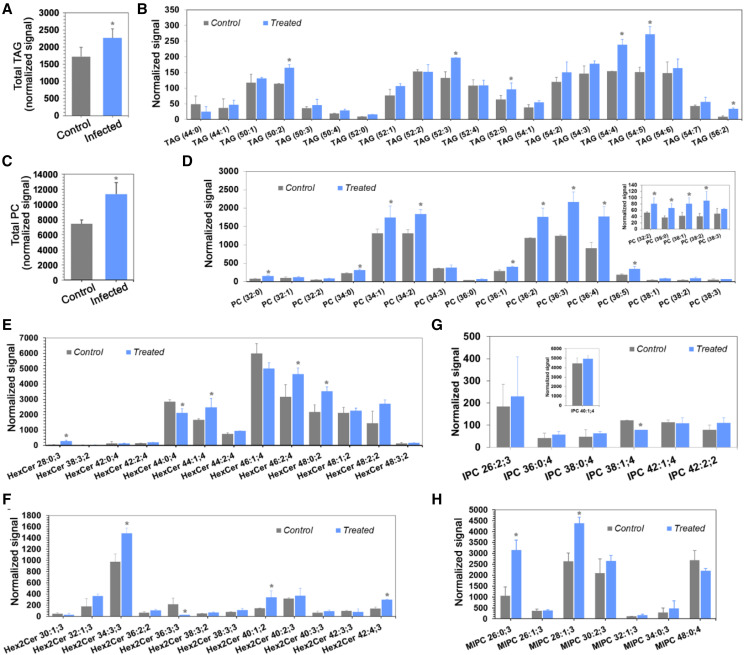
Lipids play a crucial role in plant immunity, and alteration in the lipid molecular species can facilitate or prevent pathogenesis. Lipidome analysis of control and infected plants revealed impaired mobilization of lipids. In infected plants, the levels of PC (Fig. 7, C and D) and LPC (Supplemental Fig. S6A) were increased significantly compared with control plants (Fig. 7), and similar results were observed with TAG (Fig. 7, A and B) and DAG (Supplemental Fig. S6B). Sphingolipids play an essential role in controlling the host-pathogen interaction (Ishikawa et al., 2016). We analyzed the major classes of plant sphingolipids, such as ceramide, glucosylceramide (GlcCer), and phosphoceramide (GIPC), in this study. We observed an overall increase in the level of different species of hexaceramide (HexCer; Fig. 7E), dihexaceramide (Hex2Cer; Fig. 7F), and trihexoceramide (Supplemental Fig. S6C), except for HexCer(46:1,40) and HexCer(44:0,4), which showed the opposite trend in infected plants compared to control plants. There were significant differences among the phosphoceramide species (inositol phosphorylceramide [IPC] and mannosylinositol phosphorylceramide [MIPC]) in the infected plants compared to the control sample (Fig. 7, G and H). A similar trend was observed in different species of ceramides (Cer, CerP, and CerPE; Supplemental Fig. S6, D–F). The interaction of biotic-agent fungi with plants triggers changes in host lipid homeostasis (Shah, 2005). In order to establish a favorable energy balance for defense, the upregulation of primary metabolic pathways is compensated by the downregulation of other metabolic pathways, such as starch and lipid metabolism (Less et al., 2011). Impaired plant growth might be due to inhibition of SH activity, in particular lipases, by toxins secreted by F. verticillioides, which in turn affects lipid hydrolysis/mobilization.
Figure 7.
F. verticillioides infection reduces lipid mobilization during rice germination. The total lipid was extracted from control, and F. verticillioides-infected rice seedlings. The level of each lipid class, such as phospholipids, neutral lipids, sphingolipids, and its molecular species was analyzed by precursor ion or neutral loss scanning. In each class, the extracted lipids were analyzed by MS/MSALL. For relative quantification of the total lipids, the amount of each molecular species was determined. The intensity of individual lipid molecular species was determined based on their m/z signals from the spectra. A, Level of total TAG content in control and infected plants. B, TAG molecular species in control and infected plants. C, Level of total PC content in control and infected plants. D, PC molecular species in control and infected plants. F to H, Levels of sphingolipid species for hexaceramide (E), dihexaceramide (F), IPC (G), and MIPC (H) in control and infected plants were also analyzed. Lipid nomenclature is given as total number of carbon atoms:total number of double bonds in the fatty acyl groups. For sphingolipids, the nomenclature is given as the number of total carbon atoms: the total number of double bonds in the fatty acyl groups;total number of hydroxyl groups. Data were represented as the mean ± sd of three biological replicates (n = 3). Asterisks indicate significance (*P < 0.05) as comparison of control with the infected plant.
DISCUSSION
Temporal Activity of SHs Displayed by Gel-Based ABPP during Germination
Hydrolysis of stored TAG by lipases plays a crucial role in plant growth and development, particularly during the initial stage of seed germination. In rice, the lipase activity follows a distinct pattern in different parts of the grain during germination. In the endosperm, the activity of lipases was reported to increase notably on day 4 of germination. By contrast, activity progressed with growth in the radicle (Dolui et al., 2020). Since the rice embryo contains most of the storage lipids, significant lipase activity was reported in the radical, which emerges from the embryo (Guzmán-Ortiz et al., 2018). In addition to lipases, Ser proteases actively participate in seed germination by hydrolysis of nonfunctional proteins. They follow two distinct patterns of activity in the course of plant seed development (van der Hoorn and Kaiser, 2012). During seed development, these enzymes participate in the build-up of storage proteins, whereas in germination, they are responsible for the mobilization of these stored reserves (Tan-Wilson and Wilson, 2012). The involvement of SCP46 Ser carboxypeptidase activity was reported in seed filling during the maturation stage as well as in seed germination upon hormonal induction (Li et al., 2016). In this study, the differential pattern of many fluorescent signals at different time points (Fig. 1B) reflects their (lipases, proteases) functional status at a particular stage.
Competitive ABPP Reveals Functional Readout of Inhibitor Selectivity
Competitive ABPP is a highly efficient tool to determine target identification and selective labeling of enzyme inhibitors in complex proteomes. Competitive ABPP takes advantage of the ability of activity-based probes (ABPs) to bind selectively and covalently in the active site of targeted enzymes, which in turn enables measurement of the binding affinity of competitive inhibitors at these active sites (Baggelaar and Van der Stelt, 2017). The competitive ABPP method was used for the safety assessment of agrochemicals in terms of potential off-targets, as demonstrated in the mouse brain proteome, and each inhibitor displayed differential sensitivity toward the SHs (Nomura and Casida, 2011). Lipases are sensitive to organophosphorus inhibitors, and each lipase has a diverse spectrum of inhibitor sensitivity (Quistad et al., 2006). SHs are potent targets of various commercial agrochemicals, such as herbicides, insecticides, and fungicides, which are used extensively on agricultural crops. In general, the majority of agrochemicals are organophosphate- or phosphonate-based compounds (Kaschani et al., 2012). In comparison to the conventional substrate-based assays, this competitive ABPP holds several advantages.
First and foremost, ABPP can be used in complex proteomes against native enzymes rather than in recombinantly expressed and purified proteins. The method provides functional information to predict the substrate or catalytic properties of the putative enzymes (Baggelaar and Van der Stelt, 2017). We used the commercial esterase inhibitor paraoxon (Ross et al., 2016), which significantly reduced the fluorescent signal corresponding to the possible presence of SHs in the sample (Fig. 1C, lane 5). A similar result was observed with profenofos, a potent esterase (acetylcholinesterase) inhibitor (Nomura and Casida, 2011). The loss of protein signals upon pretreatment with paraoxon or profenofos (Fig. 1C, lane 6) corresponds to possible SHs, specifically Ser esterases.
Mapping of SH Active Sites Displays Various Enzyme Activity
ABPP is an unbiased method for investigating dynamics in the expression as well as function of an entire enzyme family (Liu et al., 1999). The range of SHs identified and functionally annotated in this study illustrates the robustness of this method. The majority of the identified proteins are categorized as typical SHs, which share the α/β-hydrolase fold, possess a Ser-His-Asp catalytic triad, and are in accord with their existing annotation. However, the active-site Ser residue was reported in pectin acetylesterases, which are noncanonical SHs. The enzyme superfamilies are groups of enzymes that are separated/created by the process of divergent evolution but are united by both similar protein folds and related mechanistic strategies for minimizing the free energies of the rate-limiting transition states in the reactions they catalyze (Babbitt and Gerlt, 1997). Most strikingly, these identified proteins are actively present throughout germination and are detected in more than one stage (Fig. 3D), which reflects the fact that they are potential SHs. The abundance of these proteins was monitored by microarray analysis followed by reverse transcription quantitative PCR (qPCR) validation (Supplemental Fig. S3C). As a typical case, the Ser lipase gene (Os04g0489100), which was highly expressed at the 96-h time point (microarray data) during germination, is also labeled and detected by ABPP at that particular stage. The advantage of this method over the gel-based ABPP is that even peptides arising from a low amount of protein can be labeled and identified by MS. Most importantly, it reveals active-site information of the target proteins, which is invaluable to the investigator (Okerberg et al., 2005). However, when we compared the ABPP results to the mRNA level of lipases (Fig. 4), it was apparent that the identified proteins are a subset of the expressed lipase genes. The absence of many of these expressed lipases in our active-site-based protein profiling study indicates that these enzymes were (1) not active under our experimental conditions, or (2) not able to react with the probe (Kaschani et al., 2009). In addition, labeling of protein extracts in vitro is limited by exposure of proteins to unnatural conditions due to the loss of native compartmentalization and cellular structure, which may affect protein activity (van der Hoorn et al., 2011).
Lipases and Their mRNA Transcripts Are Abundant in Seeds
The quintessential characteristic of germination reflects the abundant levels of transcripts of hydrolytic enzymes, which are either synthesized during seed maturation and subsequently stored or newly synthesized during germination (Sreenivasulu et al., 2008). TAG is a preferred form of storage lipid because of its nonpolar and anhydrous property to confer resistance during seed maturation. Moreover, on complete oxidation, it can yield more than twice as much energy as protein or carbohydrate hydrolysis on a per unit volume basis (Quettier and Eastmond, 2009). In plants, TAG is stored in the oleosome, which consists of a phospholipid monolayer associated with proteins. We observed higher expression of phospholipase genes such as phospholipases A, C, and D in the early stage of rice germination (Fig. 4A). During this period, the increased expression of phospholipases and their subsequent hydrolytic actions on the phospholipid monolayer lead to TAG degradation (May et al., 1998). In our earlier study, we functionally characterized a PC-phospholipase enzyme that was highly expressed during rice seed germination (Dolui et al., 2020).
Further, the mRNA expression levels of the many classical lipase genes (class-3 lipases and other lipases) were gradually increased (Fig. 4B), and which subsequently may have a role in hydrolyzing TAG. In Arabidopsis, the SUGAR-DEPENDENT 1 (SDP1) gene encodes a TAG lipase with a patatin-like acyl-hydrolase domain that actively mobilizes TAG during germination (Eastmond, 2006). The mRNA expression profile of all 110 genes was represented in the form of a heat map followed by their respective Os ID and signature GDSL motif (Fig. 4C). Jiang et al. (2012) investigated the expressional status of lipase genes during different developmental stages of rice. They also found a similar trend of expression of GDSL lipase genes during germination. These GDSL family lipases are primarily involved in the regulation process of plant development, morphogenesis, and seedling establishment (Pringle and Dickstein 2004; Ling et al., 2006).
Lipid Turnover and Its Remodeling during Rice Seedling Establishment
Recent advancements in MS-based methods allow comprehensive metabolome analysis in a facile manner. Plant lipidomics approaches rely on tandem MS using a triple quadrupole mass spectrometer, where precursor ion and neutral loss scanning are monitored for the identification of lipid species (Welti and Wang, 2004). In general, the activation of phospholipases and subsequent hydrolysis of the phospholipid monolayer, particularly PC, is an essential event for the initiation of energy mobilization through TAG degradation during the initial period of seed germination, which is crucial for the seedling (Dolui et al., 2020). The stored TAG is hydrolyzed to DAG followed by the release of free fatty acids catalyzed by lipases. The released free fatty acids are subsequently utilized for energy production by β-oxidation. We observed similar results, and there were distinct patterns in the mobilization of TAG molecular species (Fig. 5E). These differences in mobilization of different fatty acids in TAGs are due to a preference by seed lipases for certain TAG molecular species (Shrestha et al., 2016). Oleic, palmitic, and linoleic acids are the predominant fatty acids present in rice lipids (Denev et al., 2009). Moreover, shorter-chain fatty acids are preferential substrates of rice lipases and are hydrolyzed faster than long-chain fatty acids (Aizono et al., 1973; Kim, 2004). Reported lipase activity showed that the lipase specifically hydrolyzes oleic acid-, linoleic acid-, and palmitic acid-containing lipids in Arabidopsis (Rietz et al., 2010), as well as in the oilseed plant castor (Ricinus communis; Bayon et al., 2015). The dynamic remodeling of membrane lipids is an essential event during seed imbibition (Yu et al., 2015). Lipid remodeling can be elucidated by the analysis of lipid molecular species with different headgroup structures, fatty acid lengths, and degrees of unsaturation (Higashi et al., 2015). In this study, higher levels of phospholipid species, particularly PC(34:3), PC(36:5), and PI(36:3), were observed throughout germination, and there was also an increase in the level of PC(36:4) in the latter phase of germination (144–192 h). PC helps to maintain membrane fluidity with optimum cell membrane function (Helmreich, 2003). However, we could not observe any detectable galactolipid species during the seedling developmental stage of rice, because seed membranes have low levels of monogalactosyldiacylglycerol and digalactosyldiacylglycerol. Further, these two lipid species are plastidic in nature, explaining their absence in seeds (Crowe and Crowe, 1992).
Differential SHs Activity during F. verticillioides Infection in Rice
Plant-pathogen interaction is multilayered, whereby proteins secreted by the pathogen, as well as proteins produced by the host in response, play a decisive role in mediating the process (Kaschani et al., 2012). Kaschani et al. (2009) reported the expression pattern of SHs during the infection of Arabidopsis with the necrotrophic fungus Botrytis cinerea. In this study, we monitored the changes in the SH activity upon infection with F. verticillioides by ABPP as well as mRNA expression analysis. The reduction in the fluorescent signal around 60 to 70 kD upon infection might be due to the depletion of SH protein (Fig. 6D). The reduction of protein intensity might be due to the suppression of SH activity by F. verticillioides infection or to inhibition by secreted toxins. There was an appearance of more reliable fluorescent signals around 85 kD in the infected sample that could be an expression of SH in response to or defense against the infection (Fig. 6D). Plant GDSL lipases play a defensive role in both biotic and abiotic stresses. However, the specific functions of this group of lipases in plant-pathogen interaction vary depending on the plant species (Lee et al., 2009; Ding et al., 2019). For example, in our study, we monitored the differential expression of SHs following fungal infection. The mRNA level of genes encoding each of the five GDSL lipases was upregulated in the infected sample, whereas the mRNA levels for lipases/carboxylesterases showed the opposite trend (Fig. 6E). These GDSL lipases may play a decisive role in defense against pathogens. The differential activity profile displayed by the gel-based ABPP could be used to explore the role of SHs in plant pathogenesis. Further, it will pave the way for better management of crop development. Similarly, there was a differential glycosidase activity profile observed upon Fusarium oxysporum infection in saffron (Husaini et al., 2018). Besides, Cys protease labeling was demonstrated in Arabidopsis leaf proteomes using a Cys protease-specific probe (van der Hoorn et al., 2004). Tian et al. (2007) reported the inhibition of tomato apoplastic Cys protease and its role in fungal resistance.
Reduction of Storage Lipid Mobilization Impairs Seed Germination during Plant-Pathogen Interaction
Infestation by biotic agents such as fungi causes an alteration in the metabolome profile of the host plant and also directly regulates plant physiology. Sphingolipids play an essential role in controlling the host-pathogen interaction. There is reported evidence that lipids and lipid-associated metabolites play defensive roles in plant-microbe interactions (Shah, 2005). In this study, we analyzed the sphingolipid profile in both control and infected plants by shotgun lipidome using HRMS. There was an overall increase in sphingolipid species, particularly ceramides (including ceramide, HexCer, and Hex2Cer), and phosphoryl ceramide (IPC and MIPC) upon fungal infection (Fig. 7). The elevation of sphingolipids in infected plants may be due to perturbation of normal lipid metabolism under the stress condition or to the defensive response of the plant toward the infestation. Reported evidence indicates that some plant necrotrophic pathogens, such as F. verticillioides and Alternaria alternata f. sp. lycopersici, control the host ceramide synthase through mycotoxins (Abbas et al., 1994; Takahashi et al., 2009). F. verticillioides and Fusarium proliferatum secrete FB1, a mycotoxin. FB1, a structural analog of long-chain bases, is reported to influence plant sphingolipid metabolism. Hence, FB1 inhibits ceramide synthase, eventually increasing the intracellular levels of phytosphingosine and dihydrosphingosine (Abbas et al., 1994). The accumulation of these long-chain base metabolites is known to trigger subsequent plant cell death (Stockmann-Juvala and Savolainen, 2008). In addition to sphingolipids, PA was also reported to function in biotic and abiotic stress. The biological crosstalk between PA and sphingolipids remains poorly understood (Guo and Wang, 2012); however, in this study, no significant difference was observed in PA level between control and infected plants.
CONCLUSION
In this functional proteolipidomics study, we characterized the active SHs expressed during rice seed germination and their role in lipid mobilization. Lipases play a central role in the turnover of seed oil and modulate plant immunity through lipid homeostasis. We observed correlation between the expression levels of the identified SHs, particularly lipases with altered lipidome profiles. Further, F. verticillioides infection significantly reduced lipase activity and lipid mobilization, thus impairing the rice seedling. Collectively, the comprehensive knowledge generated from this study reveals the functional SHs expressed during germination and their physiological significance in plant growth and development.
MATERIALS AND METHODS
Chemicals for ABPP and Inhibition Study
FP SH and Desthiobiotin-FP SH probes, Zeba spin desalting columns (5 mL), and high-capacity streptavidin agarose were purchased from Thermo Scientific. Dimethyl sulfoxide (DMSO), dithiothreitol (DTT), iodoacetamide, MS-grade trypsin from porcine pancreas, and trifluoroacetic acid were purchased from Sigma Aldrich. Chemicals like PMSF, Paraoxon, and Profenofos for inhibition study were purchased from Sigma Aldrich. Liquid chromatography MS-grade acetonitrile and water were procured from JT Baker Avantor. Urea was ordered from MP Biomedicals. Fusarium verticillioides was a kind gift from Dr. Chandra Nayak, Department of Applied Botany, University of Mysore.
Plant Growth Conditions and Pathogen Infection
Freshly harvested rice (Oryza sativa ‘IR64’) seeds were procured from the Grain Science and Technology department of our institute. Seeds were surface sterilized with 0.1% (w/v) mercuric chloride solution for 5 min followed by five washes with profuse water. Next, seeds were immersed in sterile water for 24 h for inhibition. Afterward, seeds were carefully kept on moist cotton placed in sterile petri plates. The plates were kept in the plant growth chamber at 23°C for 1 week (192 h), and sampling was done every 24 h for protein extraction and ABPP assay. The F. verticillioides strain was obtained from the Department of Applied Botany, University of Mysore. F. verticillioides were cultured into potato dextrose agar media. One-week-old culture was then used for infection study. For this purpose, the inoculum was collected by making a suspension of conidia in sterile distilled water. The seeds were treated with the suspension and kept on moist cotton placed in sterile petri plates. Another set of seeds was kept in sterile distilled water as a control. ABPP assay was performed with a protein sample extracted after 1 week.
Protein Extraction from Rice Seeds
Germinated seeds and seedlings were ground in liquid nitrogen using pestle and mortar, making a fine powder. Immediately after grinding, the sample was used for protein extraction. One gram of sample was dissolved in 10.0 mL of prechilled equilibration buffer (50 mm Tris-HCl [pH 8.0], 150 mm NaCl, 1 mm MgCl2, 1 mm KCl, and 10% [v/v] glycerol. Protein was extracted for 30 min by vortexing with intermittent cooling at 4°C in ice, followed by centrifugation at 3,000g for 5 min at 4°C. The cell-free extract was then ultracentrifuged at 100,000g for 90 min at 4°C (using a Type 90 Ti Fixed-Angle Titanium Rotor, Beckman Coulter optima xpn-100 ultracentrifuge). The supernatant was collected. Protein estimation was done by the Bradford method (Bradford, 1976).
Activity-Based Protein Lbeling and Detection
Protein was labeled with the probe according to the manufacturer’s instructions and protocol. One hundred micrograms of protein was transferred to a 0.5-mL microcentrifuge tube, and volume was made up to 50 μL with extraction buffer. One microliter (2 μm) of the SH probe was added and incubated for 1 h at 37°C in the dark. Reaction was stopped by adding 4× loading buffer (200 mm Tris-HCl [pH 6.8], 400 mm DTT, 8% [w/v] SDS, 0.04% [w/v] bromophenol blue, and 40% [v/v] glycerol) to a 1× final concentration and boiled for 7 min. The labeled proteins were resolved by 12% (v/v) SDS-PAGE, followed by detection using fluorescent scanning at 532 nm using a Typhoon FLA9500 phosphor imager. Subsequent to fluorescence detection, the gels were stained with Coomassie brilliant blue R-250.
Competitive ABPP Study
For this study, protein extracts were diluted to 1.0 mg mL−1 concentration in the extraction buffer. Samples (50-μL aliquots) were then preincubated with either 100 μm inhibitor (PMSF, Paraoxon, or Profenofos) or DMSO as a control for 30 min at 37°C. Next, 2 μm of the probe was added, followed by incubation in the dark for 1 h at 37°C. The reaction was stopped by adding 4× loading buffer and boiled for 7 min. Finally, the labeled proteins were resolved by 12% SDS-PAGE, followed by detection using fluorescent scanning at 532 nm with the Typhoon FLA9500 phosphor imager.
Activity Site Peptide Enrichment
One milligram of protein was transferred to a 1.5-mL microcentrifuge tube, and the volume was made up to 500 μL with extraction buffer. Ten microliters of desthiobiotin-FP SH probe or DMSO was added as a control and incubated for 1 h at 37°C. The reaction was stopped by adding 500 μL of 10 m urea. Ten microliters of 500 mm DTT was added and kept at 65°C for 30 min. The sample was cooled down to room temperature, and then 40 μL of 1 m iodoacetamide was added and incubated for 30 min protected from light. Following this, the desalting of protein was done using Zeba spin desalting column to reduce the urea to a suitable concentration for trypsin digestion. Desalted protein was digested with 20 μg of MS-grade trypsin (from Sigma) at 37°C for 16 h with constant shaking. Labeled peptides were affinity captured by incubating the trypsin-digested sample with 50 μL of 50% (v/v) high-capacity streptavidin agarose for 1 h at room temperature. The resin was pelleted by centrifugation at 1,000g for 1 min and washed successively with lysis buffer, phosphate-buffered saline, and LC-MS grade water (each three times). Captured peptides were eluted by adding 75 μl of elution buffer (50% [v/v] acetonitrile and 0.1% [v/v] of trifluoroacetic acid) by incubating the sample for 3 min. This step was repeated for two additional times. Eluate fractions were pooled together and lyophilized in a vacuum concentrator. Finally, samples were resuspended in 0.1% (v/v) TFA and injected directly onto an LC tandem MS (LC-MS/MS) for analysis.
LC-MS/MS Analysis of Enriched Probe Targets
The LC-MS/MS analysis of peptide was carried out in Thermo LTQ Orbitrap, which was coupled with an EASY nano LC1200 system (Agilent). The LC analysis was performed with a PepMap 100 (75 µm × 2 cm, with nanoViper C18 fitting, 3 µm particle size, and 100 Å pore size; Thermo Fisher Scientific). The nanoViper fitting is capable of withstanding pressure up to 1,000 bar and provides virtually zero dead volume. The LC analytical column used for this study was an EASY SPRAY PREAMP (RSLC C18; 50 cm × 75 µm; 3 µm particle size, 100 Å pore size; Thermo Fisher Scientific) silica column, which was tailored for high-resolution separation of peptides. The column temperature was set to 35°C. The LC solvent system consisted of two mobile phases: Solvent A (0.1% [v/v] formic acid in HPLC-grade water) and Solvent B (80% [v/v] acetonitrile and 0.1% [v/v] formic acid in HPLC-grade water). The desalted peptides were directly loaded onto the analytical column. Next, the peptides were separated on the analytical column by running a 180-min gradient of solvents A and B (start with 10% [v/v] solvent B for 2 min, gradient 10% to 45% [v/v] solvent B for 166 min, gradient 45% to 95% [v/v] up to 172 min). The flow rate was limited to 250 nL min−1. MS was performed in positive ion mode. Precursor ion scanning was done in an Orbitrap analyzer in the scan range 375 to 1,700 at a resolution of 120,000 with a maximum ion injection time of 50 ms. Product ion spectra were monitored in an ion-type analyzer. The ionization potential voltage was set to 1.8 kV. For fragmentation, high collision energy (CID) was set to 30% for the generation of MS/MS spectra, which facilitated peptide sequencing and identification. For the MS/MS events, an ion trap detector was used. MS/MS scan range (m/z) was set to 100 to 2,000, with a maximum ion injection time of 35 ms.
Peptide and Protein Identification
Raw data files were converted to Mascot Generic File files. For peptide and protein identification, the MS/MS spectra data were searched using a Mascot search engine (version 2.6.2) against the O. sativa taxonomy filter. For the Mascot search, the following parameters were included: fixed modification of carbamidomethyl; variable modifications of acetyl (protein N-term), biotin: Thermo-88317 (Ser), and oxidation of Met. Enzyme specificity was set to Trypsin/P, and the maximum missed cleavage allowed was 2. The peptide mass tolerance was set to 10 ppm, and fragment mass tolerance was set to 0.6 D. Ion score cutoff was set to 30.
Microarray Analysis
RNA was isolated according to Vicient and Delseny (1999)'s phenol-chloroform extraction method, with modifications. The quality of isolated RNA was evaluated by Nanodrop Biospectrometer (Eppendorf), and the integrity of RNA was checked by agarose gel (1.5% [w/v]) electrophoresis and Agilent BioAnalyzer. All the samples used for gene expression were labeled using an Agilent Quick-Amp labeling kit (p/n5190-0442). Total RNA was reverse transcribed using an oligo(dT) primer tagged to a T7 polymerase promoter and converted to double-stranded complementary DNA (cDNA; One-Color Microarray-Based Gene Expression Analysis). Complementary RNA (cRNA) was generated and labeled with Cy3 CTP dye (Agilent). Labeled cRNA was cleaned up using Qiagen’s RNeasy Minikit (catalog no. 74106). The cRNA sample was hybridized onto genotypic designed custom Rice (Rice_GXP_8X60K AMADID: 084057) arrays. Hybridization was carried out using an In situ Hybridization kit (5190-6420, Agilent). Raw data were analyzed using GeneSpring GX software (Agilent).
RNA Isolation, cDNA Synthesis, and qPCR Analysis
One microgram of high-quality RNA was used for cDNA synthesis using the Maxima First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. The cDNAs were diluted 20 fold, and 1 μL of the diluted cDNA was used for qPCR analysis. The qPCR assay was done in triplicate in a CFX Connect Real-Time PCR Detection System in 10 μL reaction mixture using the cDNAs, gene-specific primers (listed in Supplemental Table S1), and iTaq Universal SYBR Green Supermix (Bio-Rad). For normalization of gene expression, actin was used as an internal control. The qPCR data were calculated as the fold change of the control value, which was set to 1.
Lipidome Analysis by HRMS
For lipid extraction, a 100-mg sample was used, which was finely ground with liquid nitrogen. Lipid was extracted according to the Bligh and Dyer (1959) method. Following extraction, lipids were lyophilized using a vacuum concentrator. The lipids were dissolved in 500 μL of solvent (chloroform:methanol [1:2] containing 7.5 mm ammonium acetate). All samples were analyzed by direct infusion using MS/MSALL on a TripleTOF 5600 System (Sciex) as described by Yadav and Rajasekharan (2016). The samples were loaded in both positive and negative mode at a flow rate of 7 μL min−1. For the MS/MSALL technique, the time-of-flight MS experiment was set to scan m/z from 100 to 1,200, which was followed by product ion analyses (mass range 100 to 1,500 in MS/MS experiments). The following parameters were included for the time-of-flight MS experiment: ion source gas 1 at 15 psi, ion source gas 2 at 20 psi, curtain gas at 25 psi, a temperature of 200°C, ion spray voltage floating at +5,100 V. A declustering potential of ±80, collision energy of ±50 eV, and collision energy spread of 30 were used. A washing step was included between samples to wash away any carryover from the previous sample. SPLASH LIPIDOMIX Mass Spec Standard (catalog no. 330707-1EA, Avanti Lipids) was used as an internal standard. In each class, the extracted lipids were analyzed by MS/MSALL. The intensity of individual lipid molecular species was determined based on their m/z signals from the spectra. For the identification of different molecular species of lipids, peak view software (Sciex) was used. Data were further processed by LipidView software (Sciex).
In Vitro Lipase Assay
The lipase activity was performed as described previously (Dolui et al., 2020). Briefly, the assay was performed for 30 min at 37°C in the presence of 50 µg total protein (from either a different time point of germination or the infected plant) as an enzyme source. The assay mixture consisted of 50 mm Tris-HCl (pH 8.0), 1 mm MgCl2, 1 mm KCl, and 10% (v/v) glycerol in the presence of 50 μm substrate. For phospholipase activity, 10 mm CaCl2 was incorporated into the assay buffer. Further, activity was quantified by measuring the released free fatty acids using the fluorescent kit method (catalog no. 700310, Cayman Chemical).
FFA Estimation
The released free fatty acids (FFAs) from in vitro lipase assays were monitored by the fluorescent‐based FFA estimation method, as described previously (Dolui and Vijayaraj, 2020). Briefly, the released FFA was coupled through a consecutive enzymatic reaction to produce an H2O2-dependent generation of resorufin. The fluorescence was measured at excitation/emission 530/590 using a fluorescent plate reader. Each experiment was carried out in triplicate with appropriate controls (no enzyme, no substrate, and blank).
Data Reproducibility and Statistical Analyses
The images and results presented were generally derived from one representative experiment. Data were represented as the mean ± sd from multiple biological replicates from different plants. Fungal infection study values originated from three or more biological replicates. The lipidome analysis, gene expression, and lipase assay values were derived from a minimum of three biological replicates. For the statistical evaluation of the data, one‐way ANOVA with Tukey’s honestly significant difference (HSD) mean-separation test or Student’s t test was carried out using IBM SPSS Statistics software. The reported trends were generally observed in at least three independent experiments.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. In-gel fluorescence labeling of SHs and in vitro lipase assay.
Supplemental Figure S2. In-gel competitive ABPP assay.
Supplemental Figure S3. Analysis of relative mRNA expression of lipase, phospholipase, and identified SHs during rice seed germination
Supplemental Figure S4. Lipidome analysis of rice during germination.
Supplemental Figure S5. Analysis of relative mRNA levels of genes encoding identified SHs under F. verticillioides infection
Supplemental Figure S6. Lipidome analysis of rice under F. verticillioides infection.
Supplemental Table S1. List of primers used in this study.
Supplemental Dataset S1. Gene ontology.
Supplemental Dataset S2. Active-site peptide profiling.
Acknowledgments
We are grateful to Ram Rajasekharan (CSIR-CFTRI and School of Life Sciences, Central University of Tamil Nadu) for his constant support and encouragement, to Dr. Appukuttan Jayadeep (CSIR-CFTRI) for providing the fresh rice (IR64) seed throughout the study, and to Dr. Chandra Nayak (Department of Applied Botany, University of Mysore) for providing the F. verticillioides culture. The authors are grateful to C-CAMP for use of their Mass Spectrometry Facility and to Genotypic Technology for microarray analysis.
Footnotes
This work was supported by the Department of Science and Technology, Ministry of Science and Technology under the DST-INSPIRE Faculty Scheme (grant no. IFA14–LSPA28), and by the Council of Scientific and Industrial Research-University Grants Commission (Junior Research Fellowship to A.K.D.).
References
- Abbas HK, Tanaka T, Duke SO, Porter JK, Wray EM, Hodges L, Sessions AE, Wang E, Merrill AH Jr., Riley RT(1994) Fumonisin- and AAL-toxin-induced disruption of sphingolipid metabolism with accumulation of free sphingoid bases. Plant Physiol 106: 1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam GC, Burbaum J, Kozarich JW, Patricelli MP, Cravatt BF(2004) Mapping enzyme active sites in complex proteomes. J Am Chem Soc 126: 1363–1368 [DOI] [PubMed] [Google Scholar]
- Aizono Y, Funatsu M, Sugano M, Hayashi K, Fujiki Y(1973) Enzymatic properties of rice bran lipase. Agric Biol Chem 37: 2031–2036 [Google Scholar]
- Akoh CC, Lee GC, Liaw YC, Huang TH, Shaw JF(2004) GDSL family of serine esterases/lipases. Prog Lipid Res 43: 534–552 [DOI] [PubMed] [Google Scholar]
- Babbitt PC, Gerlt JA(1997) Understanding enzyme superfamilies. Chemistry as the fundamental determinant in the evolution of new catalytic activities. J Biol Chem 272: 30591–30594 [DOI] [PubMed] [Google Scholar]
- Bachovchin DA, Brown SJ, Rosen H, Cravatt BF(2009) Identification of selective inhibitors of uncharacterized enzymes by high-throughput screening with fluorescent activity-based probes. Nat Biotechnol 27: 387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggelaar MP, Van der Stelt M (2017) Competitive ABPP of serine hydrolases: A case study on DAGL-alpha. In Overkleeft HS, Florea BI, eds, Activity-Based Proteomics: Methods and Protocols, Methods in Molecular Biology 1491 Springer, New York, pp 161–169 [DOI] [PubMed] [Google Scholar]
- Bayon S, Chen G, Weselake RJ, Browse J(2015) A small phospholipase A2-α from castor catalyzes the removal of hydroxy fatty acids from phosphatidylcholine in transgenic Arabidopsis seeds. Plant Physiol 167: 1259–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birner-Gruenberger R, Hermetter A(2007) Activity-based proteomics of lipolytic enzymes. Curr Drug Discov Technol 4: 1–11 [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJA(1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Bradford MM.(1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Chepyshko H, Lai CP, Huang LM, Liu JH, Shaw JF(2012) Multifunctionality and diversity of GDSL esterase/lipase gene family in rice (Oryza sativa L. japonica) genome: New insights from bioinformatics analysis. BMC Genomics 13: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Wright AT, Kozarich JW(2008) Activity-based protein profiling: From enzyme chemistry to proteomic chemistry. Annu Rev Biochem 77: 383–414 [DOI] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM(1992) Membrane integrity in anhydrobiotic organisms: Toward a mechanism for stabilizing dry seeds In Somero GN, Osmond CB, and Bolis CL, eds, Water and Life. Spinger-Verlag, Berlin, pp 87–103 [Google Scholar]
- Denev R, Kuzmanova I, Panayotova S, Momchilova S, Kancheva V, Lokesh BR(2009) Lipid composition of Indian rice bran oil. C R Acad Bulg Sci 62: 709–716 [Google Scholar]
- Desjardins AE, Plattner RD, Nelson PE(1997) Production of fumonisin B1 and moniliformin by Gibberella fujikuroi from rice from various geographic areas. Appl Environ Microbiol 63: 1838–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding LN, Li M, Wang WJ, Cao J, Wang Z, Zhu KM, Yang YH, Li YL, Tan XL(2019) Advances in plant GDSL lipases: From sequences to functional mechanisms. Acta Physiol Plant 41: 151 [Google Scholar]
- Dolui AK, Latha M, Vijayaraj P(2020) OsPLB gene expressed during seed germination encodes a phospholipase in rice. 3 Biotech 10: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolui AK, Vijayaraj P(2020) A solvent-free delipidation method for functional validation of lipases. 3 Biotech 10: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ.(2006) SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell 18: 665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Cravatt BF(2006) Mechanism-based profiling of enzyme families. Chem Rev 106: 3279–3301 [DOI] [PubMed] [Google Scholar]
- Guo L, Wang X(2012) Crosstalk between phospholipase D and sphingosine kinase in plant stress signaling. Front Plant Sci 3: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Ortiz FA, Castro-Rosas J, Gómez-Aldapa CA, Mora-Escobedo R, Rojas-León A, Rodríguez-Marín ML, Román-Gutiérrez AD(2018) Enzyme activity during germination of different cereals: A review. Food Rev Int 35: 1–24 [Google Scholar]
- He D, Yang P(2013) Proteomics of rice seed germination. Front Plant Sci 4: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmreich EJM.(2003) Environmental influences on signal transduction through membranes: A retrospective mini-review. Biophys Chem 100: 519–534 [DOI] [PubMed] [Google Scholar]
- Higashi Y, Okazaki Y, Myouga F, Shinozaki K, Saito K(2015) Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci Rep 5: 10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husaini AM, Morimoto K, Chandrasekar B, Kelly S, Kaschani F, Palmero D, Jiang J, Kaiser M, Ahrazem O, Overkleeft HS, et al. (2018) Multiplex, fluorescent activity-based protein profiling identifies active α-glycosidases and other hydrolases in plants. Plant Physiol 177: 24–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Ito Y, Kawai-Yamada M(2016) Molecular characterization and targeted quantitative profiling of the sphingolipidome in rice. Plant J 88: 681–693 [DOI] [PubMed] [Google Scholar]
- Jessani N, Humphrey M, McDonald WH, Niessen S, Masuda K, Gangadharan B, Yates JR III, Mueller BM, Cravatt BF(2004) Carcinoma and stromal enzyme activity profiles associated with breast tumor growth in vivo. Proc Natl Acad Sci USA 101: 13756–13761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Chen R, Dong J, Xu Z, Gao X(2012) Analysis of GDSL lipase (GLIP) family genes in rice (Oryza sativa). Plant Omics 5: 351–358 [Google Scholar]
- Kaschani F, Gu C, Niessen S, Hoover H, Cravatt BF, van der Hoorn RAL(2009) Diversity of serine hydrolase activities of unchallenged and botrytis-infected Arabidopsis thaliana. Mol Cell Proteomics 8: 1082–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschani F, Gu C, van der Hoorn RAL (2012) Activity-based protein profiling of infected plants. In Bolton MD, Thomma BPHJ, eds, Plant Fungal Pathogens: Methods and Protocols. Methods in Molecular Biology 835 Springer, New York, pp 47–59 [DOI] [PubMed] [Google Scholar]
- Kim Y.(2004) Cloning and expression of a lipase gene from rice (Oryza sativa cv. Dongjin). Mol Cells 18: 40–45 [PubMed] [Google Scholar]
- Lee DS, Kim BK, Kwon SJ, Jin HC, Park OK(2009) Arabidopsis GDSL lipase 2 plays a role in pathogen defense via negative regulation of auxin signaling. Biochem Biophys Res Commun 379: 1038–1042 [DOI] [PubMed] [Google Scholar]
- Less H, Angelovici R, Tzin V, Galili G(2011) Coordinated gene networks regulating Arabidopsis plant metabolism in response to various stresses and nutritional cues. Plant Cell 23: 1264–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Lin F, Xue HW(2007) Genome-wide analysis of the phospholipase D family in Oryza sativa and functional characterization of PLD β 1 in seed germination. Cell Res 17: 881–894 [DOI] [PubMed] [Google Scholar]
- Li Z, Tang L, Qiu J, Zhang W, Wang Y, Tong X, Wei X, Hou Y, Zhang J(2016) Serine carboxypeptidase 46 regulates grain filling and seed germination in rice (Oryza sativa L.). PLoS One 11: e0159737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, Zhao J, Zuo K, Qiu C, Yao H, Qin J, Sun X, Tang K(2006) Isolation and expression analysis of a GDSL-like lipase gene from Brassica napus L. J Biochem Mol Biol 39: 297–303 [DOI] [PubMed] [Google Scholar]
- Liu Y, Patricelli MP, Cravatt BF(1999) Activity-based protein profiling: The serine hydrolases. Proc Natl Acad Sci USA 96: 14694–14699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Chen C, Liu X, Jiao Y, Su N, Li L, Wang X, Cao M, Sun N, Zhang X, et al. (2005) A microarray analysis of the rice transcriptome and its comparison to Arabidopsis. Genome Res 15: 1274–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C, Preisig-Müller R, Höhne M, Gnau P, Kindl H(1998) A phospholipase A2 is transiently synthesized during seed germination and localized to lipid bodies. Biochim Biophys Acta 1393: 267–276 [DOI] [PubMed] [Google Scholar]
- Morimoto K, van der Hoorn RA(2016) The increasing impact of activity-based protein profiling in plant science. Plant Cell Physiol 57: 446–461 [DOI] [PubMed] [Google Scholar]
- Müller AO, Ischebeck T(2018) Characterization of the enzymatic activity and physiological function of the lipid droplet-associated triacylglycerol lipase AtOBL1. New Phytol 217: 1062–1076 [DOI] [PubMed] [Google Scholar]
- Nomura DK, Casida JE(2011) Activity-based protein profiling of organophosphorus and thiocarbamate pesticides reveals multiple serine hydrolase targets in mouse brain. J Agric Food Chem 59: 2808–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okerberg ES, Wu J, Zhang B, Samii B, Blackford K, Winn DT, Shreder KR, Burbaum JJ, Patricelli MP(2005) High-resolution functional proteomics by active-site peptide profiling. Proc Natl Acad Sci USA 102: 4996–5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega C, Anderson LN, Frando A, Sadler NC, Brown RW, Smith RD, Wright AT, Grundner C(2016) Systematic survey of serine hydrolase activity in mycobacterium tuberculosis defines changes associated with persistence. Cell Chem Biol 23: 290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricelli MP, Giang DK, Stamp LM, Burbaum JJ(2001) Direct visualization of serine hydrolase activities in complex proteomes using fluorescent active site-directed probes. Proteomics 1: 1067–1071 [DOI] [PubMed] [Google Scholar]
- Paulick MG, Bogyo M(2008) Application of activity-based probes to the study of enzymes involved in cancer progression. Curr Opin Genet Dev 18: 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle D, Dickstein R(2004) Purification of ENOD8 proteins from Medicago sativa root nodules and their characterization as esterases. Plant Physiol Biochem 42: 73–79 [DOI] [PubMed] [Google Scholar]
- Quettier AL, Eastmond PJ(2009) Storage oil hydrolysis during early seedling growth. Plant Physiol Biochem 47: 485–490 [DOI] [PubMed] [Google Scholar]
- Quistad GB, Liang SN, Fisher KJ, Nomura DK, Casida JE(2006) Each lipase has a unique sensitivity profile for organophosphorus inhibitors. Toxicol Sci 91: 166–172 [DOI] [PubMed] [Google Scholar]
- Rietz S, Dermendjiev G, Oppermann E, Tafesse FG, Effendi Y, Holk A, Parker JE, Teige M, Scherer GF(2010) Roles of Arabidopsis patatin-related phospholipases a in root development are related to auxin responses and phosphate deficiency. Mol Plant 3: 524–538 [DOI] [PubMed] [Google Scholar]
- Ross MK, Pluta K, Bittles V, Borazjani A, Allen Crow J(2016) Interaction of the serine hydrolase KIAA1363 with organophosphorus agents: Evaluation of potency and kinetics. Arch Biochem Biophys 590: 72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PF, Nelson PE, Richard JL, Osweiler GD, Rice LG, Plattner RD, Wilson TM(1990) Production of fumonisins by Fusarium moniliforme and Fusarium proliferatum isolates associated with equine leukoencephalomalacia and a pulmonary edema syndrome in swine. Appl Environ Microbiol 56: 3225–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J.(2005) Lipids, lipases, and lipid-modifying enzymes in plant disease resistance. Annu Rev Phytopathol 43: 229–260 [DOI] [PubMed] [Google Scholar]
- Shrestha P, Callahan DL, Singh SP, Petrie JR, Zhou XR(2016) Reduced triacylglycerol mobilization during seed germination and early seedling growth in Arabidopsis containing nutritionally important polyunsaturated fatty acids. Front Plant Sci 7: 1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasulu N, Usadel B, Winter A, Radchuk V, Scholz U, Stein N, Weschke W, Strickert M, Close TJ, Stitt M, et al. (2008) Barley grain maturation and germination: Metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiol 146: 1738–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmann-Juvala H, Savolainen K(2008) A review of the toxic effects and mechanisms of action of fumonisin B1. Hum Exp Toxicol 27: 799–809 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Berberich T, Kanzaki H, Matsumura H, Saitoh H, Kusano T, Terauchi R(2009) Unraveling the roles of sphingolipids in plant innate immunity. Plant Signal Behav 4: 536–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan-Wilson AL, Wilson KA(2012) Mobilization of seed protein reserves. Physiol Plant 145: 140–153 [DOI] [PubMed] [Google Scholar]
- Tian M, Win J, Song J, van der Hoorn R, van der Knaap E, Kamoun S(2007) A Phytophthora infestans cystatin-like protein targets a novel tomato papain-like apoplastic protease. Plant Physiol 143: 364–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi LP, Sowdhamini R(2006) Cross genome comparisons of serine proteases in Arabidopsis and rice. BMC Genomics 7: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoorn RAL, Colby T, Nickel S, Richau KH, Schmidt J, Kaiser M(2011) Mining the active proteome of Arabidopsis thaliana. Front Plant Sci 2: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoorn RAL, Leeuwenburgh MA, Bogyo M, Joosten MHAJ, Peck SC(2004) Activity profiling of papain-like cysteine proteases in plants. Plant Physiol 135: 1170–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoorn RAL, Kaiser M(2012) Probes for activity-based profiling of plant proteases. Physiol Plant 145: 18–27 [DOI] [PubMed] [Google Scholar]
- Vicient CM, Delseny M(1999) Isolation of total RNA from Arabidopsis thaliana seeds. Anal Biochem 268: 412–413 [DOI] [PubMed] [Google Scholar]
- Welti R, Wang X(2004) Lipid species profiling: A high-throughput approach to identify lipid compositional changes and determine the function of genes involved in lipid metabolism and signaling. Curr Opin Plant Biol 7: 337–344 [DOI] [PubMed] [Google Scholar]
- Yadav PK, Rajasekharan R(2016) Misregulation of a DDHD domain-containing lipase causes mitochondrial dysfunction in yeast. J Biol Chem 291: 18562–18581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa H, Hirose T, Kuroda M, Yamaguchi T(2007) Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray. Plant Physiol 144: 258–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Li A, Li W(2015) How membranes organize during seed germination: Three patterns of dynamic lipid remodelling define chilling resistance and affect plastid biogenesis. Plant Cell Environ 38: 1391–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A, Li Y, Leng C, Wang P, Li Y(2019) Inhibitory effect of protease inhibitors on larval midgut protease activities and the performance of Plutella xylostella (Lepidoptera: Plutellidae). Front Physiol 9: 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink S, Kallnik V, Ninck S, Nickel S, Verheyen J, Blum M, Wagner A, Feldmann I, Sickmann A, Albers SV, et al. (2017) Activity-based protein profiling as a robust method for enzyme identification and screening in extremophilic Archaea. Nat Commun 8: 15352. [DOI] [PMC free article] [PubMed] [Google Scholar]