Abstract
Background:
Women who experience pregnancy loss are especially prone to high stress, though the effects of stress on reproductive outcomes in this vulnerable population are unknown. We assessed relationships between perceived stress and hormones, anovulation, and fecundability among women with prior loss.
Methods:
One thousand two hundred fourteen women with 1–2 prior losses were followed for ≤6 cycles while attempting pregnancy and completed end-of-cycle stress assessments. For cycles 1 and 2, women also collected daily urine and completed daily perceived stress assessments. We assessed anovulation via. an algorithm based on human chorionic gonadotropin (hCG), pregnanediol-3-glucuronide (PdG), luteinizing hormone (LH), and fertility monitor readings. Pregnancy was determined via. hCG. Adjusted weighted linear mixed models estimated the effect of prospective phase-varying (menses, follicular, periovulatory, and luteal) perceived stress quartiles on estrone-1-glucuronide (E1G), PdG, and LH concentrations. Marginal structural models accounted for time-varying confounding by hormones and lifestyle factors affected by prior stress. Poisson and Cox regression estimated risk ratios and fecundability odds ratios of cycle-varying stress quartiles on anovulation and fecundability. Models were adjusted for age, race, body mass index (BMI), parity, and time-varying caffeine, alcohol, smoking, intercourse, and pelvic pain.
Results:
Women in the highest versus lowest stress quartile had lower E1G and PdG concentrations, a marginally higher risk of anovulation [1.28; 95% confidence interval (CI) = 1.00, 1.63], and lower fecundability (0.71; 95% CI = 0.55, 0.90).
Conclusion:
Preconception perceived stress appears to adversely affect sex steroid synthesis and time to pregnancy. Mechanisms likely include the effects of stress on ovulatory function, but additional mechanisms, potentially during implantation, may also exist.
According to the most recent American Psychological Association poll, more Americans report symptoms of stress than ever before, with women consistently reporting higher levels of stress than men.1 In the wake of an emerging epidemic, it is essential to understand the associated effects of stress on health outcomes. Reproduction is an especially vulnerable time period where stress may play a critical role. Moreover, women who experience pregnancy loss are especially prone to high stress,2–4 and given the high prevalence of loss among reproductive-age women, estimated to be 15% of clinically recognized pregnancies and up to 30% of all conceptions,5,6 it is important to understand the role of stress in this susceptible population.
Stress is a broad concept ranging from induced physical stress used in experimental studies to better understand pathophysiological mechanisms of stress to recording of life stressors in observational studies to better understand how human health is affected by various stress types. The effect of perceived stressors, defined as challenges that individuals view as taxing or exceeding their coping abilities,1 on risk of adverse health outcomes is particularly useful as it can account for an individual’s biological vulnerability, psychosocial resources, and learned patterns of coping.7 While animal models show links between severe stress and adverse effects on female reproductive function,8–10 human studies are less clear11–14 and have focused on healthy populations. There are no studies evaluating the role of stress among women with prior pregnancy loss, and the mechanisms remain elusive. Moreover, given that stress may change on a daily basis and influence fecundity and that a longer time to conception may in turn increase stress levels, approaches to account for time-dependent confounding are needed to disentangle these effects.
Accordingly, the objective of this study was to assess the effect of daily preconception stress on reproductive hormones, ovulatory function, and time to pregnancy in a prospective cohort of women experiencing prior pregnancy loss.
METHODS
Study Population
The Effects of Aspirin in Gestation and Reproduction (EAGeR) trial (2006–2012) was a multicenter, block-randomized, double-blinded, placebo-controlled trial to evaluate the effect of preconception-initiated daily low-dose aspirin on reproductive outcomes in 1228 women, 18–40 years of age, with 1–2 prior pregnancy losses. Fourteen women withdrew on the day of randomization leaving a total of 1214 women. Details of the study protocol have been published previously.15 Briefly, women were included if they had regular menstrual cycles (21–42 days), no known history of infertility, and intended to conceive. The study was approved by the Institutional Review Board at each site. All participants gave written informed consent before randomization.
Participant Follow-up
Participants were followed for up to 6 menstrual cycles while attempting pregnancy. During the preconception period, women underwent active follow-up during the first 2 menstrual cycles, which included daily completion of a diary, fertility monitor (Clearblue; SPD Swiss Precision Diagnostics GmbH, Geneva, Switzerland), and urine samples. Follow-up clinic visits occurred twice per cycle during active follow-up. If women did not achieve pregnancy in the first 2 menstrual cycles, they entered passive follow-up. During passive follow-up (cycles 3–6), women continued to use fertility monitors and completed end-of-cycle clinic visits including providing a urine and blood sample.
Perceived Stress Assessment
At study enrollment, participants were provided with a daily diary and instructed in its use. As part of the daily diary recording during active preconception follow-up (cycles 1 and 2), participants were asked to record their average daily stress levels via. a Likert scale: 0 = no stress, 1 = little stress, 2 = moderate stress, and 3 = a lot of stress. Additionally, at the end-of-cycle clinic visits during passive preconception follow-up (i.e., cycles 3–6), participants were asked to rate their average daily perceived stress during their last menstrual cycle on a scale from 0 (no stress) to 10 (maximum stress). Among the total cohort of 1214 women, 1034 (85%) women recorded perceived stress in the first 2 menstrual cycles, while 1135 (93%) women recorded perceived stress in the first 6 menstrual cycles.
Reproductive Outcomes
Reproductive Hormones
Urinary reproductive hormones [estrone-1-glucuronide (E1G), estradiol (E2), pregnanediol-3-glucuronide (PdG), follicle-stimulating hormone (FSH), and luteinizing hormone (LH)] were assayed at four time points during each of the first 2 preconception cycles. We selected urine specimens corresponding to relevant phases of the menstrual cycle including menses (day 2), estimated day of ovulation (LH peak + 1 day), peri-implantation (LH peak + 9 days), and one random luteal sample between 4 days after LH peak and 2 days before the start of the next menses. Urinary E1G, E2, and PdG were measured by competitive chemiluminescence duplex assay. Limits of detection were 0.40 ng/ml for E1G, 0.04 ng/ml for E2, and 45 ng/ml for PdG (Quansys Biosciences, Logan, UT). The interassay laboratory coefficients of variability (CVs) were 17% at 36.3 ng/ml and 20% at 1.9 ng/ml for E1G, 14% at 2.0 ng/ml and 19% at 0.3 ng/ml for E2, and 23% at 4060 ng/ ml and 20% at 1604 ng/ml for PdG, using an in-house urine control. Urinary LH and FSH were measured via. reagent/ sandwich immunoassay (Roche Diagnostics, Indianapolis, IN) with an interassay CV of 1.6% and 1.8%, respectively, and intra-assay CVs of <10%. Hormones were not adjusted for creatinine given our hypothesis.16
Anovulation
Ovulation was detected using fertility monitors for all 6 cycles of preconception follow-up, with cycles with a lack of a peak reading or daily luteinizing hormone concentrations <2.5 times the average of the previous 5 days considered anovulatory. Urinary luteal PdG measurements were used to improve the sensitivity of ovulation detection in the first 2 cycles of study participation, with cycles with PdG <5 μg/ml considered anovulatory.17–19 Any cycle with a verified conception was defined as ovulatory. For fertility monitor criteria to apply, data must have been available on the 15th day before the end of the cycle to classify a lack of peak, and for the moving average method, tests on at least two of the previous five days with at least one in the immediate two previous days; otherwise the cycle was unable to be categorized by the fertility monitor criteria. Among cycles with complete data (n = 3,785), 496 (13%) were deemed anovulatory.
Pregnancy
For this analysis, we defined pregnancy as a human chorionic gonadotropin (hCG)-detected pregnancy, determined by two methods.20 The first was a positive result on a “real-time” urine pregnancy test (Quidel Quickvue; Quidel Corporation, San Diego, CA), sensitive to 25 mIU/ml hCG, conducted each time participants reported missing menses on any end-cycle visit. Additionally, batched augmented daily urine hCG testing was performed later in the laboratory on the last 10 days of each woman’s first and second cycle of study participation (using daily first morning urine collected at home) and on spot urine samples collected at all end-cycle visits.20
Covariate Assessment
Baseline data collection and randomization occurred at a study visit on menstrual cycle days 2–4. Women completed questionnaires capturing self and partner demographic background including age and race, education, marital status, income, employment, physical activity (International Physical Activity Questionnaire),21 and reproductive history including whether they had ever tried for >12 months to conceive (subfertility), prior use of oral contraceptives, and age of menarche.15 Physical measurements including height and weight [used to calculate body mass index (BMI)] were also captured during the baseline visit.15 Time-varying covariates including intercourse (times/day); pelvic pain/cramping (0 = none, 1 = mild, 2 = moderate, 3 = severe); and alcohol (drinks/day), caffeine (drinks/day), and tobacco (yes/no) consumption were captured via. the preconception daily diaries.
Statistical Analysis
Descriptive statistics (including daily diary compliance, preconception perceived stress central tendency and variability, and participant characteristics by stress quartile) were calculated. We used linear mixed models to evaluate the prospective association between the first two menstrual cycle’s time-varying perceived stress quartile and phase-specific geometric mean urine concentrations of E1G, E2, PdG, FSH, and LH. There were 1033 and 773 women who completed daily diary stress assessment for cycles 1 and 2, respectively (307 women became pregnant in the first cycle and moved to pregnancy follow-up protocol). As has been done by prior studies,14,22 perceived stress was allowed to vary by phase, including “menses” (daily stress averaged up to the day of each woman’s hormone assessment during menses); “follicular” (average from “menses” through 1 day after fertility monitor peak); “periovulatory” (day after fertility monitor peak); and “luteal” (average from day after ovulation through start of next menses).23,24 Stress quartiles were calculated for each menstrual cycle phase. Results were adjusted for age, race, BMI, and parity. Race was considered because previous research has shown that reproductive hormone metabolism and perceived stress vary by race.25,26 In addition, because hormone concentrations change over the cycle in response to complex feedback mechanisms with other hormones, and because that fluctuation may change stress vulnerability,27 we also conducted analyses using marginal structural models with stabilized inverse-probability-of-exposure weights28,29 to appropriately account for time-varying confounding by hormones and lifestyle factors affected by prior stress levels (eFigure 1; http://links.lww.com/EDE/B572). Weighted linear mixed-effects models with random intercepts were used to estimate the parameters of the marginal structural models adjusting for age, race, BMI, parity, and time-varying, phase-average caffeine, alcohol, smoking, intercourse frequency, and pelvic pain/cramping. Tests for trend were calculated, to inform specific patterns,30 by specifying a continuous variable for the median of each stress quartile.
Relative risks (RRs) were calculated to assess the risk of anovulation per menstrual cycle by perceived stress quartile and also by high (quartile 4) versus low (quartile 1–3) stress for comparability with other studies.22 The menstrual cycle was the unit of analysis, with 1–6 menstrual cycles per participant, and multiple cycles addressed by using log-binomial Poisson regression models with generalized estimating equations.18 Models were adjusted for age, race, BMI, parity, and cycle-average caffeine, alcohol, smoking, intercourse frequency, and pelvic pain, and they were weighted to account for the number of contributed cycles as women who are more fertile have fewer observed cycles during the study.
We used discrete Cox proportional hazard regression models, accounting for right censoring, to estimate the impact of cycle-averaged, time-varying perceived stress on time to pregnancy.31 Fecundability odds ratios denote the cycle-specific odds of pregnancy in the exposed relative to the unexposed, and a fecundability odds ratio <1.0 corresponds to a longer time to pregnancy among the exposed group. Results were adjusted for age, race, BMI, parity, and time-varying cycle-average caffeine, alcohol, smoking, intercourse frequency, and pelvic pain/cramping.
Several sensitivity analyses were conducted to test the robustness of our findings. In regards to anovulation, we conducted an analysis restricted to cycles with outcome determined by PdG or pregnancy (hereafter, referred to as restricted anovulation algorithm) given that luteal progesterone via. biomarker is considered to have better diagnostic accuracy compared with a fertility monitor.32 To address residual confounding, additional covariates (including partner’s age, study site, education, marital status, income, employment status, treatment arm, subfertility, prior oral contraceptive use, and age of menarche) were evaluated in our adjusted models. Also, to address potential unmeasured residual time-fixed confounding, such as by underlying genetics or certain environmental exposures, we conducted a case-crossover analysis restricted to PdG or pregnancy in the first 2 cycles among women experiencing both an ovulatory cycle and an anovulatory cycle.22
Finally, given the potential for selection bias by excluding women with missing perceived stress data or missing pregnancy outcome data (due to early withdrawal), we performed several sensitivity analyses on our primary outcome of time to pregnancy. We compared complete case results (N = 1,135) to a multiple imputation analysis to address missing exposure and covariate data under the rationale that our data were missing at random (i.e., probability of missing data depends on observed data). Additionally, because 128 women did not have complete outcome information due to withdrawal from the study, we performed several sensitivity analyses to investigate potential selection bias. Potential pregnancy outcome of women who withdrew was imputed using the following three strategies: (1) the survival probability of no pregnancy achieved derived from the complete cases using Kaplan-Meier multiple imputation (600 imputations), (2) achievement of pregnancy in 1 cycle after withdrawal, and (3) no pregnancy achieved after 6 cycles. The Kaplan-Meier-based imputation is a missing at random-like principled approach that is plausible and considers covariates,33 while the latter two methods are extreme possibilities of the influence of potential unobserved outcomes. All analyses were conducted using SAS 9.4. (SAS Institute Inc., Cary, NC).
RESULTS
Study participants completed daily diaries for an average of 49.0 days during the first 2 cycles of follow-up [standard deviation = 15 days; median = 52 days; interquartile range (IQR): 38, 58 days]. The 230 women who achieved pregnancy in the first cycle completed on average 28.6 ± 5.6 days (median = 29; IQR: 27, 32) of perceived stress diary entries for cycle 1. In addition, 171 women achieved pregnancy in the second cycle and completed on average 27.9 ± 6.0 days (median = 27; IQR: 25, 30) of perceived stress diary entries for cycle 1 and 30.7 ± 7.1 days (median = 31; IQR: 28, 34) for cycle 2. Women on average had a stress level of 0.82 ± 0.53 (on the 0 “no stress” to 3 “a lot of stress” scale) for the first 2 cycles (median = 0.77; IQR: 0.37, 1.19; range: 0.0–2.8) with no appreciable difference between cycle 1 (mean = 0.84; median = 0.80) and cycle 2 (mean = 0.81; median = 0.77). There also appeared to be no appreciable difference in perceived stress during passive preconception follow-up (cycle 3: mean = 4.6 ± 2.4, median = 4, IQR: 2, 6, range: 1–10; cycle 4: mean = 4.9 ± 2.6, median = 5, IQR: 3, 7, range 1–10; cycle 5: mean = 4.9 ± 2.5, median = 5, IQR: 3, 7, range: 1–10; and cycle 6: mean = 5.1 ± 2.5, median = 5, IQR: 3, 7, range: 1–10).
Based on quartiles of average stress captured in the daily diaries, women had on average 0.20 ± 0.11 for Q1, 0.59 ± 0.12 for Q2, 0.97 ± 0.11 for Q3, and 1.53 ± 0.31 for Q4. Women with higher stress levels tended to have a higher level of education, were married, have increased pelvic pain, and lower intakes of alcohol and caffeine (Table 1).
TABLE 1.
Participant Characteristics by Preconception Perceived Stress Quartile During Active Follow-upa
| Perceived Stress Quartile |
|||||
|---|---|---|---|---|---|
| Characteristic | Total (N = 1,034) |
1 0.00–0.37 (n = 260) |
2 0.38–0.77 (n = 257) |
3 0.78–1.19 (n = 259) |
4 1.19–2.77 (n = 258) |
| Demographics | |||||
| Age (yrs); mean ± SD | 29.0 ± 4.8 | 28.6 ± 4.6 | 29.2 ± 4.9 | 29.3 ± 4.6 | 29.0 ± 5.0 |
| Partner’s age, mean ± SD | 30.4 ± 5.6 | 30.0 ± 5.3 | 30.8 ± 5.8 | 30.4 ± 5.0 | 30.6 ± 6.1 |
| Race, n (%) | |||||
| White | 983 (95) | 240 (92) | 245 (95) | 249 (96) | 249 (97) |
| Non-white | 51 (5) | 20 (8) | 12 (5) | 10 (4) | 9 (4) |
| BMI (kg/m2); mean ± SD | 26.4 ± 6.6 | 26.1 ± 7.0 | 26.5 ± 6.1 | 26.8 ± 7.0 | 26.2 ± 6.4 |
| Education, n (%) | |||||
| >High school graduate | 903 (87) | 213 (82) | 221 (86) | 237 (92) | 232 (90) |
| ≤High school graduate | 130 (13) | 46 (18) | 36 (14) | 22 (9) | 26 (10) |
| Marital status, n (%) | |||||
| Living with partner | 57 (6) | 20 (8) | 16 (6) | 11 (4) | 10 (4) |
| Married | 952 (92) | 225 (88) | 238 (91) | 245 (95) | 244 (95) |
| Other | 25 (2) | 12 (5) | 7 (3) | 2 (1) | 4 (1) |
| Income (annual), n (%) | |||||
| ≥$100,000 | 412 (40) | 99 (39) | 118 (45) | 108 (42) | 87 (34) |
| $75,000–$99,999 | 139 (13) | 34 (13) | 35 (13) | 28 (11) | 42 (16) |
| $40,000–$74,999 | 159 (15) | 35 (14) | 40 (15) | 42 (16) | 42 (16) |
| $20,000–$39,999 | 252 (24) | 67 (26) | 56 (22) | 67 (26) | 62 (24) |
| ≤$19,999 | 71 (7) | 22 (9) | 12 (5) | 13 (5) | 24 (9) |
| Employed, n (%) | |||||
| Employed | 768 (74) | 183 (70) | 201 (78) | 197 (76) | 187 (73) |
| Unemployed | 247 (24) | 69 (27) | 54 (21) | 58 (22) | 66 (26) |
| Treatment arm, n (%) | |||||
| Treatment | 516 (50) | 122 (50) | 132 (51) | 129 (50) | 133 (52) |
| Placebo | 518 (50) | 138 (53) | 125 (49) | 130 (50) | 125 (49) |
| Subfertilityb, n (%) | |||||
| Yes | 83 (8) | 14 (6) | 21 (8) | 26 (10) | 22 (9) |
| No | 931 (92) | 236 (94) | 235 (92) | 229 (90) | 231 (91) |
| Prior oral contraceptives, n (%) | |||||
| Yes | 181 (18) | 57 (23) | 36 (14) | 47 (18) | 41 (16) |
| No | 828 (82) | 194 (77) | 215 (86) | 208 (82) | 211 (84) |
| Age of menarche (yrs); mean ± SD | 12.8 ± 3.1 | 12.7 ± 1.7 | 12.7 ± 1.5 | 13.2 ± 5.6 | 12.6 ± 1.4 |
| Reproductive history | |||||
| No. previous live births, n (%) | |||||
| 0 | 483 (47) | 115 (44) | 117 (46) | 119 (46) | 132 (51) |
| 1 | 372 (36) | 106 (41) | 88 (34) | 100 (39) | 78 (30) |
| 2 | 179 (17) | 39 (15) | 52 (20) | 40 (15) | 48 (19) |
| Sexual intercoursec, (median, IQR) | 0.30 (0.21, 0.42) | 0.32 (0.22, 0.42) | 0.29 (0.21, 0.42) | 0.29 (0.20, 0.40) | 0.30 (0.20, 0.44) |
| Pelvic paind, (median, IQR) | 0.15 (0.06, 0.29) | 0.10 (0.03, 0.20) | 0.17 (0.08, 0.30) | 0.16 (0.06, 0.29) | 0.19 (0.07, 0.37) |
| Alcohol consumere, n (%) | 400 (39) | 114 (44) | 108 (42) | 96 (37) | 80 (31) |
| Smokerf, n (%) | 277 (27) | 72 (28) | 69 (27) | 69 (27) | 65 (25) |
| Caffeinated drinksg, (median, IQR) | 0.38 (0.04, 1.11) | 0.31 (0.04, 0.98) | 0.56 (0.11, 1.13) | 0.34 (0.02, 1.09) | 0.28 (0.04, 1.23) |
| Perceived stressh, (median, IQR) | 0.77 (0.38, 1.19) | 0.20 (0.10, 0.30) | 0.60 (0.49, 0.68) | 0.97 (0.88, 1.07) | 1.45 (1.30, 1.67) |
| Physical activityi, n (%) | |||||
| Low | 291 (28) | 76 (30) | 75 (31) | 80 (31) | 60 (23) |
| Moderate | 430 (42) | 112 (43) | 103 (40) | 105 (41) | 110 (43) |
| High | 312 (30) | 72 (28) | 79 (31) | 73 (28) | 88 (34) |
Included women who recorded perceived stress in the first 2 menstrual cycles (N = 1034 women). Among the included women, there were no missing values for women’s age, race, marital status, treatment arm, previous live births, sexual intercourse, and pelvic pain. There were 16 observations missing partner’s age, 11 missing BMI, 1 missing education, 1 missing income, 19 missing employed, 20 missing subfertility, 25 missing prior oral contraceptive use, 22 missing age of menarche, 2 missing alcohol, 2 missing caffeine, and 1 missing physical activity.
Women who reported to ever having tried for >12 months to conceive were considered subfertile.
Women reported acts of intercourse per day.
Women reported information regarding pelvic pain and cramping daily on scale of 0 = none, 1 = mild, 2 = moderate, and 3 = severe.
Women reported alcohol drinks consumed per day, which was dichotomized to any alcohol or none (yes/no).
Women reported tobacco smoking per day (yes/no).
Women reported caffeinated drinks consumed per day.
Women recorded their daily stress levels via. a Likert scale with 0 = no stress, 1 = little stress, 2 = moderate stress, and 3 = a lot of stress.
At baseline, women completed the short form of the International Physical Activity Questionnaire, from which low, moderate, and high physical activity was categorized via. standard protocol.
Women in the highest daily stress quartile had lower E1G concentrations compared with women in lower stress quartiles [Q4 = 27.3 ng/ml (95% confidence interval [CI] = 25.3, 29.4) versus Q1 = 32.3 (95% CI = 30.0, 34.7), Q2 = 34.8 (95% CI = 32.4, 37.4), Q3 = 29.4 (95% CI = 27.5, 31.6), P trend <0.001] after adjusting for relevant confounders (Table 2). Similar adjusted lower concentrations of PdG for high versus lower stress were found: [Q4 = 5.59 µg/ml (95% CI = 5.15, 6.06) versus Q1 = 6.83 (95% CI = 6.32, 7.36), Q2 = 7.00 (95% CI = 6.51, 7.53), Q3 = 5.81 (95% CI = 5.39, 6.27) P trend <0.001].
TABLE 2.
Geometric Mean Concentrations of Reproductive Hormones According to Time-Varying Perceived Stress Quartile Over First 2 Menstrual Cycles (Active Follow-up)a
| Q1 | Q2 | Q3 | Q4 | P Trend | |
|---|---|---|---|---|---|
| Estradiol (ng/ml) | |||||
| Model 1 | 1.24 (1.18, 1.3) | 1.31 (1.25, 1.38) | 1.26 (1.21, 1.32) | 1.18 (1.12, 1.24) | 0.09 |
| Model 2 | 1.17 (1.12, 1.23) | 1.27 (1.23, 1.36) | 1.21 (1.15, 1.27) | 1.11 (1.05, 1.17) | 0.05 |
| Estrone-1-glucuronide (ng/ml) | |||||
| Model 1 | 32.3 (30.0, 34.7) | 34.8 (32.4, 37.4) | 29.4 (27.5, 31.6) | 27.3 (25.3, 29.4) | <0.001 |
| Model 2 | 31.6 (29.6, 33.9) | 36.7 (34.3, 39.2) | 33.3 (31.1, 35.7) | 26.3 (24.4, 28.2) | <0.001 |
| Pregnanediol-3-glucuronide (µg/ml) | |||||
| Model 1 | 6.14 (5.64, 6.69) | 6.13 (5.65, 6.65) | 4.69 (4.33, 5.08) | 4.70 (4.31, 5.12) | <0.001 |
| Model 2 | 6.83 (6.32, 7.36) | 7.00 (6.51, 7.53) | 5.81 (5.39, 6.27) | 5.59 (5.15, 6.06) | <0.001 |
| Follicle-stimulating Hormone (mIU/ml) | |||||
| Model 1 | 1.90 (1.76, 2.04) | 2.01 (1.87, 2.15) | 1.95 (1.82, 2.09) | 2.00 (1.86, 2.15) | 0.40 |
| Model 2 | 1.75 (1.64, 1.87) | 2.09 (1.96, 2.23) | 1.88 (1.76, 2.01) | 1.74 (1.62, 1.87) | 0.54 |
| Luteinizing hormone (mIU/ml) | |||||
| Model 1 | 0.63 (0.57, 0.69) | 0.75 (0.68, 0.82) | 0.66 (0.60, 0.73) | 0.64 (0.58, 0.71) | 0.92 |
| Model 2 | 0.57 (0.52, 0.62) | 0.77 (0.70, 0.84) | 0.66 (0.60, 0.73) | 0.55 (0.50, 0.61) | 0.38 |
Model 1: Linear mixed model; adjusted for age, race, BMI, and parity. Model 2: Marginal structural model. Adjusted for age, race, BMI, and parity, and time-varying, phase-average caffeine, alcohol, smoking, intercourse frequency, and pelvic pain/cramping.
Included women who recorded perceived stress in the first 2 menstrual cycles (N = 1034 women).
There was a 28% higher risk of anovulation between the highest (Q4) versus lower (Q1–Q3) perceived stress quartiles when using the full anovulation algorithm [adjusted Risk Ratio (aRR) = 1.28 (95% CI = 1.00, 1.63)] after full adjustment (Table 3). Sensitivity analyses using the restricted anovulation algorithm based on PdG and hCG pregnancy did not appreciably alter the estimates [aRR = 1.39 (95% CI = 1.00, 1.92)]. Among the 24 women included in the case-crossover sensitivity analysis, a similar estimate, albeit less precise, for anovulation was found [aRR = 1.40 (95% CI = 0.62, 3.15)].
TABLE 3.
Associations Between Preconception Perceived Stress and the Risk of Anovulation
| Perceived Stress | Model 1 RR (95% CI) |
Model 2 RR (95% CI) |
|---|---|---|
| Anovulation determined using full algorithm (N = 1135 womena, 3418 cycles) | ||
| Quartile | ||
| Q1 | 1.00 | 1.00 |
| Q2 | 1.13 (0.84, 1.53) | 1.10 (0.81, 1.51) |
| Q3 | 0.98 (0.71, 1.35) | 0.94 (0.67, 1.32) |
| Q4 | 1.31 (0.95, 1.79) | 1.30 (0.94, 1.78) |
| High versus not high (Q4 versus Q1–3) | 1.26 (0.99, 1.62) | 1.28 (1.00, 1.63) |
| Anovulation determined using pregnanediol-3-glucuronide < 5 μg/ml and human chorionic gonadotropin pregnancy (N = 1035 womenb; 1698 cycles) | ||
| Quartile | ||
| Q1 | 1.00 | 1.00 |
| Q2 | 1.05 (0.68, 1.62) | 1.01 (0.65, 1.56) |
| Q3 | 0.96 (0.61, 1.52) | 0.94 (0.59, 1.50) |
| Q4 | 1.41 (0.92, 2.16) | 1.36 (0.89, 2.07) |
| High versus not high (Q4 versus Q1–3) | 1.41 (1.00, 1.98) | 1.39 (1.00, 1.92) |
Model 1: Adjusted for number of cycles a participant contributed to the analysis, age, race, BMI, and parity. Model 2: Adjusted for number of cycles a participant contributed to the analysis, age, race, BMI, parity, and cycle-average caffeine, alcohol, smoking, intercourse frequency, and pelvic pain/cramping.
Included women who recorded perceived stress in the first 6 menstrual cycles (N= 1135 women).
Included women who recorded perceived stress in the first 2 menstrual cycles (N= 1034 women).
Time to pregnancy models by cycle-specific stress quartile (i.e., averaging daily stress for cycles 1 and 2 and using end-of-cycle stress for cycles 3–6) was consistent across various adjustment for potential confounding factors. We found that time to pregnancy was longer among women in the highest versus lowest perceived stress quartile in our complete case analysis (fecundability odds ratios = 0.67; 95% CI = 0.53, 0.85) after adjusting for age, race, BMI, and parity and after additionally adjusting for time-varying cycle-average caffeine, alcohol, smoking, intercourse frequency, and pelvic pain/cramping (fecundability odds ratios = 0.71; 95% CI = 0.55, 0.90) (Figure). Additional adjustment for other potential confounding factors did not appreciably alter estimates.
FIGURE.
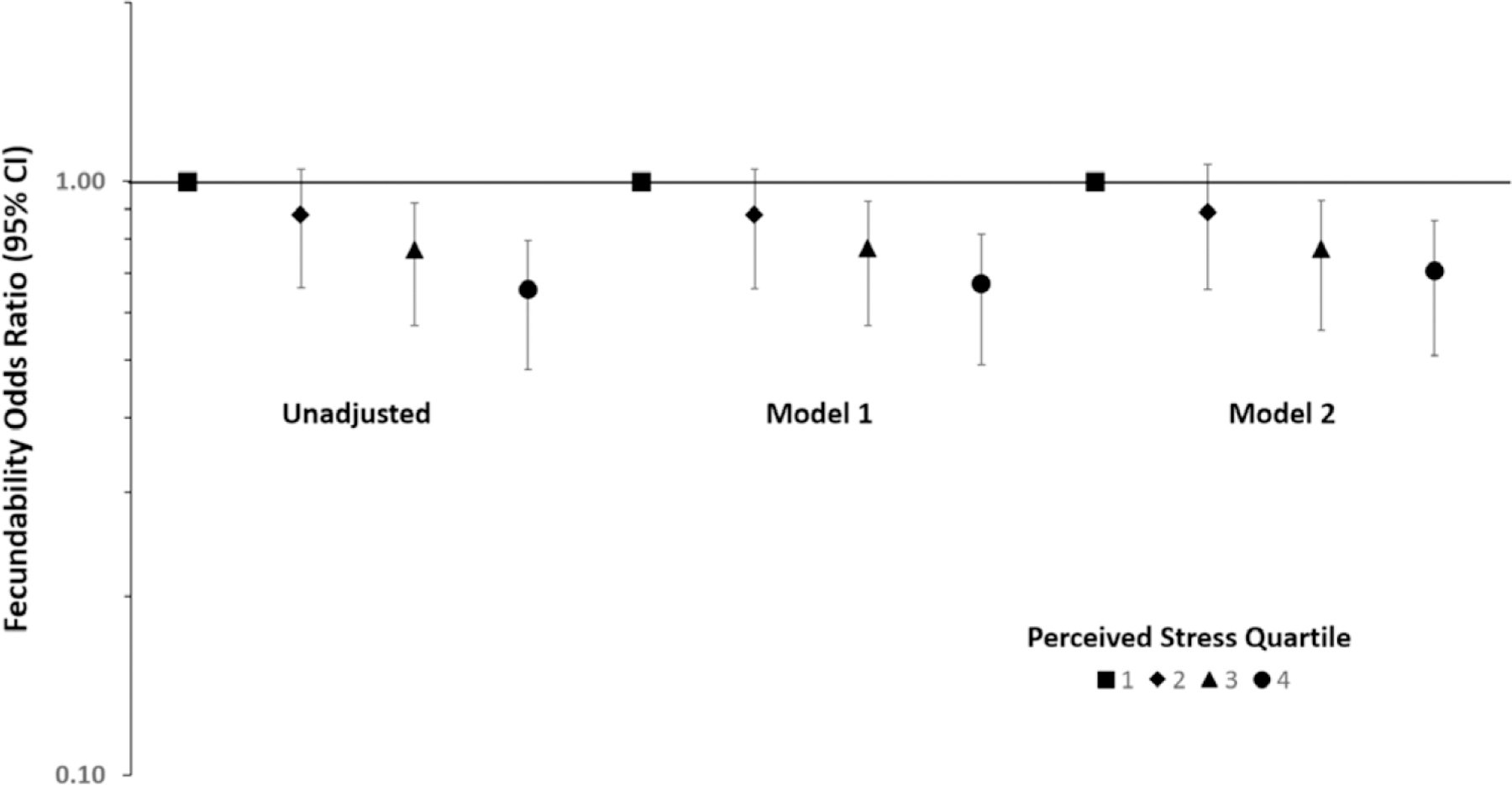
Fecundability odds ratio by preconception (cycles 1 to ≤ 6) perceived stress quartile (Q2–Q4), relative to first quartile (Q1). Model 1 adjusted for age, race, BMI, and parity. Model 2 adjusted for age, race, BMI, parity, and time-varying cycle-average caffeine, alcohol, smoking, intercourse frequency, and pelvic pain/cramping. Complete case analysis (N = 1135 women, 3418 cycles).
Results of our sensitivity analysis, whereby we imputed missing values for perceived stress and covariates in addition to using three different methods to impute pregnancy during our 6 cycles of follow-up, are shown in eFigure 2; http://links.lww.com/EDE/B573. Multiple imputation to address missing exposure and covariate data revealed no substantial changes for the fully adjusted model (fecundability odds ratios = 0.71; 95% CI = 0.56, 0.91). Additionally, multiple imputation to address missing exposure, covariate, and outcome data also did not appreciably alter estimates. The results of the Kaplan-Meier-based imputation (fecundability odds ratios = 0.76; 95% CI = 0.60, 0.97) are similar to the complete case results for perceived stress implying that plausible outcomes of withdrawals are unlikely to have substantively changed our results. Moreover, the two extreme cases for potential outcomes of withdrawals also resulted in largely similar results (pregnancy in the next cycle after withdrawal fecundability odds ratios = 0.79; 95% CI = 0.62, 1.00; and no pregnancy achieved after 6 cycles fecundability odds ratios = 0.70; 95% CI = 0.55, 0.90), further supporting the robustness of our findings.
DISCUSSION
We found that higher preconception perceived stress was associated with lower E1G and PdG, a higher risk of anovulation, and a longer time to pregnancy. While severe stress under experimental conditions or among individuals sharing specific stressors has been well documented to adversely affect female reproductive function,10 our study indicates that everyday stress among women with proven fertility but who have experienced a prior pregnancy loss is linked to alterations in reproductive hormones, ovulatory function, and longer time to pregnancy.
Our finding of reduced urinary estrogen and progesterone metabolites with higher perceived stress supports the hypothesis that stress activates the hypothalamic-pituitary adrenal axis, leading to a suppression of the hypothalamic-pituitary gonadal axis, and reduces sex steroid synthesis.34,35 Given prior evidence for a reduction in fecundability with higher urine α-amylase concentrations,11 the effect of stress on time to pregnancy may additionally operate via. the sympathetic adrenal medullary axis leading to implantation failure due to a potentially direct effect on the endometrium or on the trophoblast-endometrium interface. Only one prior study has assessed the effects of perceived stress on reproductive hormones among premenopausal women.22 Similar to our current findings, we previously found higher daily perceived stress to be associated with lower total and free serum E2 among 259 healthy, premenopausal US women in the BioCycle Study, with stress measured similarly via. a 3-point Likert scale ranging from not stressful to very stressful.22 The overall lower progesterone concentrations found in both studies in relation to higher stress levels are also consistent.22
Even fewer studies have assessed the effect of perceived stress on ovulatory function. In the BioCycle Study, we found a 2.3-times higher odds of sporadic anovulation (95% CI = 1.0, 4.7) among women with high versus low perceived stress after adjustment for age, race, percent body fat, daily vigorous exercise, and depression.22 This finding is consistent with the 1.28 increased risk (95% CI = 1.00, 1.63) we found in the EAGeR trial. The lower effect estimates in the EAGeR trial compared with the BioCycle Study may be the result of different populations given that the women enrolled in the EAGeR trial had proven fertility but one to two prior pregnancy losses, whereas women in the BioCycle Study were eumenorrheic but included both nulliparous and parous women. Another study assessing perceived stress and ovulatory function also found a positive, albeit imprecise, relationship between psychological stress in the workplace and anovulation (odds ratio = 1.34; 95% CI, 0.35, 4.28) among 276 healthy, premenopausal US women.36 The major limitation of this study was a single measure of stress at an unspecified time point via. telephone interview.36 While we did not have a single measure of stress in the EAGeR trial to compare our results to those from the Kaiser study, our prior research within the BioCycle Study has shown poor agreement between a baseline assessment of stress via the Perceived Stress Scale and prospectively measured perceived stress over the menstrual cycle.22
Regarding time to pregnancy, our findings of a positive association between preconception stress and time to pregnancy are in agreement with two prior prospective studies. Among 274 women with no known infertility participating in the Oxford Conception Study, day 6 α-amylase was associated with reduced fecundability in the first cycle attempting pregnancy (highest posterior density: −0.284; 95% CI = −0.540, −0.029) for highest versus lowest α-amylase quartile.12 Similarly, in the Longitudinal Investigation of Fertility and the Environment Study of 401 couples who had no known infertility diagnosis, the highest versus lowest tertile of menstrual cycle day 2 salivary α-amylase concentrations was associated with lower fecundability (fecundability odds ratios: 0.71; 95% CI = 0.51, 1.00).11 In contrast, there were no clear relationships between psychosocial measures of stress, captured once every cycle, and time to pregnancy in the Oxford Conception Study.13 Comparisons between the EAGeR trial and the Oxford Conception Study in regards to perceived stress are difficult given our use of a daily diary, versus once-permenstrual-cycle questionnaire via. Cohen’s Perceived Stress Scale, to capture preconception stress during the first 2 menstrual cycles. Backed by prior research,22,37,38 the assessment of everyday psychosocial stress captured daily over the course of the entire menstrual cycle may more accurately reflect how a woman reacts physically to daily stressors compared with a single baseline or cycle measurement. Additionally, prospective assessment of stress throughout the study period is preferable to retrospective assessment, as was done at the end of each cycle for cycles 3–6 of this secondary data analysis, with the caveat that overly burdensome study protocol can in itself cause stress. There is the potential for reverse causality. However, we observed little change in stress over cycles 3–6, suggesting that its impact on our findings would be small.
While we did not find perceived stress to vary over the cycle (eFigure 3; http://links.lww.com/EDE/B574) nor did we find differences in phase-specific stress and fecundability within the EAGeR trial, a recent analysis of the Mount Sinai Study of Women Office Workers did find that perceived stress that occurred during the follicular phase was associated with lower fecundability, while stress occurring during the luteal phase was associated with higher fecundability.14 Due to the paucity of research and inconsistent findings, more research is needed to look at the effects of perceived stress on fecundability during critical windows of exposure.
Our study had many strengths. Enrolling women before conception and capturing daily perceived stress along with important confounding factors, both fixed and time-varying, for up to 6 menstrual cycles, using gold standard assessments for identifying chemical pregnancies, provided an improvement over prior studies. Additionally, our assessment of daily perceived stress and up to four timed measurements of reproductive hormones in the first 2 menstrual cycles helped inform potential biological pathways. Our assessment of cycle-average stress and use of fertility monitors during passive follow-up (cycles 3–6) allowed us to extend our analyses of the effect of stress on ovulatory function across more consecutive menstrual cycles than prior studies.36
Nevertheless, this study was limited in our ability to clearly identify the mechanisms of stress on fertility due to our sole reliance on self-reported stress versus a combination of stress biomarkers and perceived stress as used in prior studies.11–13 Also, this study was conducted in a rather homogenous sample of women, all with one to two prior pregnancy losses, which may limit generalizability. The effects of perceived stress on non-white women of lower socioeconomic status are still yet to be fully explored and may show increased effect estimates for more extreme chronic stress. Additionally, while there was no clear relationship between women who withdrew or were lost to follow-up in either our primary exposure or outcome, to minimize concern regarding selection bias, we performed several sensitivity analyses to account for missingness of our exposure of perceived stress, confounding factors, and outcome of pregnancy, with no appreciable alterations in our estimates. Finally, while we were able to assess phase-specific perceived stress levels in relation to reproductive hormone concentrations, our study was limited in looking at phase-specific stress in relation to fecundability over the full follow-up period of 6 cycles.
In summary, among women with proven fertility but one to two prior losses, higher perceived stress was associated with lower urinary concentrations of sex steroids and a longer time to pregnancy. Our findings are in agreement with prior adequately powered, prospective studies.11,12,22 The degree to which perceived stress reduces fecundability via. ovulatory dysfunction versus implantation failure, or other means, has yet to be clearly outlined.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD (contract nos. HHSN267200603423, HHSN267200603424, and HHSN267200603426).
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
The data and the computer code are available upon request, with completion of appropriate National Institute of Child Health and Human Development data sharing agreement.
REFERENCES
- 1.American Psychological Association. Stress In America: Coping with Change. Stress In America Survey 2017. Available at: https://www.apa.org/news/press/releases/stress/2016/coping-with-change.pdf. Accessed August 8, 2018.
- 2.Neugebauer R, Kline J, Stein Z, et al. Association of stressful life events with chromosomally normal spontaneous abortion. Am J Epidemiol. 1996;143:588–596. [DOI] [PubMed] [Google Scholar]
- 3.Graignic-Philippe R, Dayan J, Chokron S, et al. Effects of prenatal stress on fetal and child development: a critical literature review. Neurosci Biobehav Rev. 2014;43:137–162. [DOI] [PubMed] [Google Scholar]
- 4.Coughlan C, Walters S, Ledger W, et al. A comparison of psychological stress among women with and without reproductive failure. Int J Gynaecol Obstet. 2014;124:143–147. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. [DOI] [PubMed] [Google Scholar]
- 6.Marriott L, Zinaman M, Abrams KR, et al. Analysis of urinary human chorionic gonadotrophin concentrations in normal and failing pregnancies using longitudinal, cox proportional hazards and two-stage modelling. Ann Clin Biochem. 2017;54:548–557. [DOI] [PubMed] [Google Scholar]
- 7.Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura K, Sheps S, Arck PC. Stress and reproductive failure: past notions, present insights and future directions. J Assist Reprod Genet. 2008;25:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferin M Clinical review 105: stress and the reproductive cycle. J Clin Endocrinol Metab. 1999;84:1768–1774. [DOI] [PubMed] [Google Scholar]
- 10.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. [DOI] [PubMed] [Google Scholar]
- 11.Lynch CD, Sundaram R, Maisog JM, et al. Preconception stress increases the risk of infertility: results from a couple-based prospective cohort study–the LIFE study. Hum Reprod. 2014;29:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis GM, Lum KJ, Sundaram R, et al. Stress reduces conception probabilities across the fertile window: evidence in support of relaxation. Fertil Steril. 2011;95:2184–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch CD, Sundaram R, Buck Louis GM, et al. Are increased levels of self-reported psychosocial stress, anxiety, and depression associated with fecundity? Fertil Steril. 2012;98:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhter S, Marcus M, Kerber RA, et al. The impact of periconceptional maternal stress on fecundability. Ann Epidemiol. 2016;26:710.e7–716.e7. [DOI] [PubMed] [Google Scholar]
- 15.Schisterman EF, Silver RM, Perkins NJ, et al. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: design and baseline characteristics. Paediatr Perinat Epidemiol. 2013;27:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zacur H, Kaufman SC, Smith B, et al. Does creatinine adjustment of urinary pregnanediol glucuronide reduce or introduce measurement error? Gynecol Endocrinol. 1997;11:29–33. [DOI] [PubMed] [Google Scholar]
- 17.Johnson S, Weddell S, Godbert S, et al. Development of the first urinary reproductive hormone ranges referenced to independently determined ovulation day. Clin Chem Lab Med. 2015;53:1099–1108. [DOI] [PubMed] [Google Scholar]
- 18.Park SJ, Goldsmith LT, Skurnick JH, et al. Characteristics of the urinary luteinizing hormone surge in young ovulatory women. Fertil Steril. 2007;88:684–690. [DOI] [PubMed] [Google Scholar]
- 19.Behre HM, Kuhlage J, Gassner C, et al. Prediction of ovulation by urinary hormone measurements with the home use clearPlan fertility monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15:2478–2482. [DOI] [PubMed] [Google Scholar]
- 20.Schisterman EF, Mumford SL, Schliep KC, et al. Preconception low dose aspirin and time to pregnancy: findings from the effects of aspirin in gestation and reproduction randomized trial. J Clin Endocrinol Metab. 2015;100:1785–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 22.Schliep KC, Mumford SL, Vladutiu CJ, et al. Perceived stress, reproductive hormones, and ovulatory function: a prospective cohort study. Epidemiology. 2015;26:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norman G Likert scales, levels of measurement and the “laws” of statistics. Adv Health Sci Educ Theory Pract. 2010;15:625–632. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan GM, Artino AR Jr. Analyzing and interpreting data from Likert-type scales. J Grad Med Educ. 2013;5:541–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9:625–637. [DOI] [PubMed] [Google Scholar]
- 26.Giurgescu C, Zenk SN, Dancy BL, et al. Relationships among neighborhood environment, racial discrimination, psychological distress, and preterm birth in African American women. J Obstet Gynecol Neonatal Nurs. 2012;41:E51–E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ossewaarde L, Hermans EJ, van Wingen GA, et al. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35:47–55. [DOI] [PubMed] [Google Scholar]
- 28.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 30.Savitz DA. Reconciling theory and practice regarding p values. Epidemiology. 2013;24:781–782. [DOI] [PubMed] [Google Scholar]
- 31.Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch KE, Mumford SL, Schliep KC, et al. Assessment of anovulation in eumenorrheic women: comparison of ovulation detection algorithms. Fertil Steril. 2014;102:511.e2–518.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Herring AH, Zhou H, et al. A multiple imputation method for sensitivity analyses of time-to-event data with possibly informative censoring. J Biopharm Stat. 2014;24:229–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nepomnaschy PA, Welch K, McConnell D, et al. Stress and female reproductive function: a study of daily variations in cortisol, gonadotrophins, and gonadal steroids in a rural Mayan population. Am J Hum Biol. 2004;16:523–532. [DOI] [PubMed] [Google Scholar]
- 35.Vrekoussis T, Kalantaridou SN, Mastorakos G, et al. The role of stress in female reproduction and pregnancy: an update. Ann N Y Acad Sci. 2010;1205:69–75. [DOI] [PubMed] [Google Scholar]
- 36.Fenster L, Waller K, Chen J, et al. Psychological stress in the workplace and menstrual function. Am J Epidemiol. 1999;149:127–134. [DOI] [PubMed] [Google Scholar]
- 37.Piazza JR, Almeida DM, Dmitrieva NO, et al. Frontiers in the use of biomarkers of health in research on stress and aging. J Gerontol B Psychol Sci Soc Sci. 2010;65:513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolger N, Davis A, Rafaeli E. Diary methods: capturing life as it is lived. Annu Rev Psychol. 2003;54:579–616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


