Abstract
The rapid spread of the SARS‐CoV‐2 pandemic poses particular challenges to the management of persons with chronic disease. Reports of a possible neuroinvasiveness of SARS‐CoV‐2 as well as pathophysiological mechanisms and indirect consequences in severe COVID‐19 cases raise the question of whether the infection can be associated with an increased risk of seizure recurrence or the development of new onset and acute symptomatic seizures. Although the literature does not provide relevant evidence for seizure worsening in persons with epilepsy during the course of a SARS‐CoV‐2 infection, there are theoretical risks, for example, seizures triggered by fever. Moreover, a severe disease course and advanced disease stages can, for instance, result in hypoxic encephalopathy, cerebrovascular events, and cytokine storm, which may trigger the development of acute seizures. This is further confirmed by reports of occasional seizures in COVID‐19 patients. Although the low number of reports so far suggests that the risk may be relatively low, the reports indicate that an early neurological manifestation with seizures should not be ruled out. In the context of these cases, we discuss possible pathophysiological mechanisms that may trigger ictogenesis in patients with SARS‐CoV‐2 infection.
Keywords: CNS, COVID‐19, SARS‐CoV‐2, seizures
Key Points.
Occasional cases with new onset seizures reported in COVID‐19 patients are reviewed
The possible neuroinvasiveness and the probable mechanisms linking SARS‐CoV‐2 and seizures are described
Literature and social media search revealed no clinical evidence for a risk of seizure worsening in persons with epilepsy
The possibility of seizures due to nonspecific mechanisms triggered by fever, hypoxia, cytokine storm, or cerebrovascular events is discussed
Seizure risk must therefore be considered in COVID‐19 patients, particularly in a subgroup with severe disease course and risk factors
1. INTRODUCTION
In December 2019, a high number of patients suffering from pneumonia were admitted to hospitals of the city of Wuhan in the Hubei province of China. The cause of this sudden outbreak of pneumonia cases was identified in January 2020 by the Chinese Center for Disease Control and Prevention to be a novel coronavirus, 1 which was first named 2019‐nCoV by the World Health Organization (WHO). 2 The novel coronavirus belongs to the genus Betacoronavirus, which is an enveloped nonsegmented positive‐sense RNA virus. 3 Depending on the subgenus and species, betacoronaviruses can proliferate in cells from different animal species and humans. The first zoonotic transmission of a coronavirus was observed during the outbreak of another betacoronavirus, severe acute respiratory syndrome coronavirus (SARS‐CoV). 4 About 10 years later, the Middle East respiratory syndrome coronavirus (MERS‐CoV) was identified as another zoonotic betacoronavirus that caused an epidemic. 5
The genetic sequencing of the novel coronavirus isolated from patients in Wuhan resulted in the development of reverse transcription polymerase chain reaction diagnostic test kits based on full genomic sequence data. The viral genome showed a 77% similarity with the genome of SARS‐CoV. 6 Therefore, the virus has been renamed SARS‐CoV‐2, 7 and the infectious disease caused by it is known as coronavirus disease 2019 (COVID‐19). 8
During the epidemic and its rapid development into a pandemic, it became evident that the rate of SARS‐CoV‐2 human‐to‐human transmission is much higher than for SARS‐CoV. 9 , 10 The novel coronavirus primarily affects the respiratory system. Frequent clinical symptoms observed worldwide comprise fever (>90%), cough (75%), and dyspnea (50%). Other major symptoms include fatigue, with small but significant numbers of patients also reporting gastrointestinal discomfort (diarrhea and emesis). 11
Some respiratory viruses can also spread and affect other organ systems, including the central nervous system (CNS). Previously, it was believed that only neurotropic viruses affect the CNS, but later respiratory viruses including human respiratory syncytial virus, the influenza A virus, the Nipah virus, the human coronavirus (SARS‐CoV), and the human metapneumovirus were also classified as pathological factors for CNS infections. 12 The clinical manifestations that have been reported in subgroups of patients affected by these viruses include acute encephalitis with febrile or afebrile seizures and status epilepticus as well as chronic encephalopathies as a long‐term consequence. 13 These reports of different human respiratory viruses that can be associated with a risk for CNS infection and neuropathological consequences raise the question of whether SARS‐CoV‐2 also possesses neuroinvasive capacities and may cause neurological symptoms. This question is of particular relevance for the management of patients with epilepsy.
In this context, we would like to point out that the International League Against Epilepsy (ILAE) immediately established a COVID‐19 Task Force with renowned international experts, which makes up‐to‐date information about the implications of the SARS‐CoV‐2 pandemic and recommendations for patients, clinicians, and researchers available (https://www.ilae.org/about‐ilae/committees‐task‐forces‐and‐advisory‐commissions/ilae‐covid‐task‐force/amp;/amp%3B). This task force is providing comprehensive information at the ILAE website concerning various issues and challenges related to the COVID‐19 pandemic, such as the need for efficient telemedicine approaches (https://www.ilae.org/patient‐care/covid‐19‐and‐epilepsy). The ILAE has also provided a brief summary of the current state of knowledge regarding seizures in COVID‐19 patients. For patients with epilepsy, it is emphasized that infections and fever in general can increase the risk for increase in frequency or severity of seizures (https://www.ilae.org/patient‐care/covid‐19‐and‐epilepsy/for‐patients). Regarding new onset seizures, three case reports with COVID‐19–associated seizures are listed in the respective ILAE document, and it is concluded that the evidence for the risk of new onset seizures has been limited up to now (https://www.ilae.org/files/dmfile/Seizures‐in‐COVID‐19.pdf). In addition, a group of international clinical experts published consensus statements on the impact of the COVID‐19 pandemic on patients with epilepsy. 14
Although it is evident from the very low number of reported cases with more severe neurological manifestations that COVID‐19–associated neurological disease seems to be a very rare event affecting only a small subgroup of patients, it is nevertheless of interest to review the characteristics of available cases. In this context, we will discuss proven and potential mechanisms, by which an infection with respiratory viruses and with SARS‐CoV‐2 in particular may affect seizure thresholds. Respective information can provide guidance for future research avenues.
2. MATERIALS AND METHODS
An extensive database search was completed to identify original and review articles including clinical observational studies, case reports, and/or case series in PubMed/MEDLINE and other databases such as ScienceDirect, Semantic Scholar, and Google Scholar. The key search words included “SARS‐CoV‐2” OR “COVID‐19” AND “epilepsy” OR “seizures” OR “central nervous system” OR “brain” OR “neurological findings” OR “cerebrovascular disease” OR “CNS infection” OR “neurological disorder”. After analyzing the identified articles and excluding the repetitions, we selected 14 articles that included studies indicating clinical relevance of more severe neurological symptoms in patients with COVID‐19. The selected articles are either published or available in preprint format. In addition, we have also picked up news or social media posts reporting seizures in persons diagnosed with COVID‐19. Moreover, we considered guidelines and recommendations for epileptologists and patients with epilepsy provided by the ILAE and national epilepsy societies (Box 1).
Box 1. Search strategy profile of the review.
| Search items | “SARS‐CoV‐2” OR “COVID‐19” AND “epilepsy” OR “seizures” OR “central nervous system” OR “brain” OR “neurological findings” OR “cerebrovascular disease” OR “CNS infection” OR “neurological disorder” |
| Databases | PubMed/MEDLINE, ScienceDirect, Semantic Scholar, Google Scholar, and Google Search |
| Other websites/webinars |
Websites:
Webinars:
|
3. NEUROLOGICAL CLINICAL FINDINGS ASSOCIATED WITH CORONAVIRUS INFECTIONS
3.1. SARS‐CoV and MERS‐CoV
The betacoronaviruses SARS‐CoV and MERS‐CoV have been associated with occasional neuropathological alterations and neurological symptoms. Various case reports focusing on SARS‐CoV infections have suggested a neuroinvasive potential, with occurrence of neurological symptoms such as generalized tonic‐clonic seizures, loss of consciousness, headache, and dizziness. Histopathological evaluation with techniques such as immunohistochemistry have provided evidence that SARS‐CoV can be detected in brain tissue associated with necrotic neuronal cells. 15 , 16 , 17 Neuronal degeneration and edema have been reported in few cases. 18 The studies suggested a critical involvement of the CNS, with an upregulation of proinflammatory cytokines during the disease course following an initial induction of a viral pneumonia. 18 , 19
Similarly, there are various case reports of patients who suffered from MERS‐CoV infection and exhibited neurological symptoms. The neurological manifestation was in most cases associated with a severe disease course leading to death in patients with underlying comorbid conditions, namely, diabetes mellitus, chronic renal failure, and hypertension. The clinical neurological findings comprised ataxia, nausea, coma, encephalitis, motor deficits, loss of consciousness, irreversible brain stem dysfunction, axonal polyneuropathy, and acute sensory neuropathy induced by infection. 20 , 21 Few cases developed Guillain‐Barré syndrome and Bickerstaff encephalitis. 22 Neuropathological alterations have been discussed as a potential contributor to worsening of the condition of patients with comorbidities resulting in multiple organ failure and ultimately leading to death. 18 Moreover, brain damage specific to the thalamus and brain stem was also reported in a transgenic mouse model with MERS‐CoV infection. 23
3.2. SARS‐CoV‐2
Considering this evidence for infections with SARS‐CoV and MERS‐CoV, it is reasonable to have scientific curiosity regarding whether the novel SARS‐CoV‐2 may also have a neuroinvasive potential based on its similarities with SARS‐CoV. It was recently reported that, extrapolating from epidemiological data for both SARS‐CoV and MERS‐CoV to the number of positive cases for SARS‐CoV‐2, a total of 11,476 and 10,144 patients were estimated to have CNS and peripheral nervous system complications, respectively. 24
According to the WHO, SARS‐CoV‐2 has a median latency period of about 14 days based on epidemiological data. 25 Thus, the latency phase and further disease course would theoretically allow ample time for the virus to spread and reach brain tissues and affect neuronal cells.
In this context, it is of interest that SARS‐CoV‐2 has already been detected in cerebrospinal fluid (CSF) of patients later diagnosed with COVID‐19–associated viral encephalitis. 26 , 27 This finding seems to be in accordance with the reported neuroinvasiveness of other betacoronaviruses. 13 However, it needs to be considered that detection of virus in the CSF can also be an indirect consequence of hypoxia‐, sepsis‐, and inflammation‐associated damage of biological barriers during the disease course. Moreover, there are several studies that report neurological dysfunction even in the absence of virus in the CSF, and rather argue against direct viral CNS penetration. 28 , 29 , 30 , 31 Thus, further research seems to be necessary to assess the relevance of a possible neuroinvasiveness of SARS‐CoV‐2, and if further confirmed to explore the circumstances under which brain penetration occurs in subgroups of patients.
There have been reports of COVID‐19 patients exhibiting atypical neurological signs such as headache, dizziness, and nausea. 32 A recent study has also demonstrated that a considerable number of patients with severe COVID‐19 had neurological manifestations including acute cerebrovascular events, skeletal muscle injury, and loss of consciousness, along with a few minor neurological symptoms including headache, dizziness, hyposmia/anosmia, hypogeusia/dysgeusia, impaired vision, and neuralgia. 33 Hence, the propensity of neuroinvasion needs to be explored to provide better guidance to prevent the proliferation of SARS‐CoV‐2 in vulnerable patients. This in particular applies to patients with a weak immune system or underlying neurological disorders such as epilepsy.
Table 1 provides a summary of the clinical evidence for neurological symptoms from the 14 identified publications. So far, there are only very few reports of new onset or acute seizures in patients with COVID‐19. The first case report described seizures in a COVID‐19 patient from Japan. 26 A 24‐year‐old male presented with generalized seizures. Further diagnostic workup detected SARS‐CoV‐2 RNA in CSF and abnormal findings in medial temporal lobe including hippocampus, suggesting encephalitis. 26 Another publication from Iran reported generalized tonic‐clonic seizures in a 30‐year‐old SARS‐CoV‐2–positive, otherwise healthy female. 28 Both cases presented with repeated seizures on hospitalization and had no history of epilepsy or previous seizures. In the USA, social media posts from Colorado and Florida described seizures during the course of COVID‐19, with febrile seizures in a 1‐year‐old infant 34 and another case with seizures in a 74‐year‐old patient with Parkinson disease. 35 A 75‐year‐old male in Vancouver, after returning from Vegas, reported two seizures and tested positive for SARS‐CoV‐2. 36 Although these social media and press reports suggest that seizures can occur in association with COVID‐19, the information needs to be considered with extreme care, as these are reports based on description by lay persons.
TABLE 1.
Neurological symptoms reported in patients infected with SARS‐CoV‐2
| Name of study | Country | Type of study | Sample patient characteristics | Confirmation for SARS‐CoV‐2 | Primary clinical symptoms | Neurological findings | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples, n | Age/mean age | Gender | Comorbidities | Nasopharyngeal swab (RT‐PCR) | In CSF (gene sequencing/RT‐PCR) | ||||||
| Neurological manifestations of hospitalized patients with COVID‐19 in Wuhan, China: a retrospective case series study | China | Retrospective case series study | 214 | 52.7 ± 15.5 y | 59.3% female | Hypertension (23.8%), diabetes mellitus (14.0%), cardiovascular disease (7.0%), malignancy (6.1%) | Positive | NA | Fever (61.7%), dry cough (50.0%), anorexia (31.8%), diarrhea (19.2%), pharyngalgia (14.5%), abdominal pain (4.7%) | Dizziness (16.8%), headache (13.1%), impaired consciousness (14.8%), acute cerebrovascular disease/ischemic stroke (5.7%), seizure (0.5%) | 33 |
| Frequent convulsive seizures in an adult patient with COVID‐19: a case report | Iran | Case report | 1 | 30 y | Female | None | Positive | Negative | Dry cough, fever, fatigue | Generalized tonic‐clonic seizure | 28 |
| Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study | China | Cohort study | 8 | 2 mo‐15 y | Female, 2; male, 6 | Acute lymphocytic leukemia (12.5%), lacrimal sac dredge (12.5%), pharyngitis (12.5%) | Positive | NA | Fever (75%), cough (75%), polypnea (100%) | Headache (12.5%), nausea/vomiting (50%), status epilepticus (12.5%), toxic encephalopathy (12.5%) | 39 |
| COVID‐19 and intracerebral haemorrhage: causative or coincidental | Iran | Case report | 1 | 79 y | Male | NA | Positive | NA | Fever, dry cough | Acute loss of consciousness, intracerebral hemorrhage | 40 |
| Neurological complications of coronavirus disease (COVID‐19): encephalopathy | USA | Case report | 1 | 74 y | Male | Atrial fibrillation, cardioembolic stroke, Parkinson disease, chronic obstructive pulmonary disease, recent cellulitis | Positive | Negative | Fever, cough |
Encephalopathy, headache, altered mental status |
30 |
| COVID‐19–associated Acute haemorrhagic necrotizing encephalopathy: CT and MRI features | USA | Case report | 1 | >50 y | Female | NA | Positive | NA | Cough, fever, altered mental status | Acute necrotizing hemorrhagic encephalopathy | 41 |
| A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2 | Japan | Case report | 1 | 24 y | Male | NA | Negative | Positive | Headache, fatigue, fever, sore throat | Meningitis, encephalitis, generalized seizures, neck stiffness | 26 |
| SARS‐CoV‐2: underestimated damage to nervous system | China | Review | 1 | 56 y | NA | NA | Positive | Positive | Severe symptoms associated with COVID‐19 | Viral encephalitis | 27 |
| Neurologic features in severe SARS‐CoV‐2 infection | France | Case series report | 58 | 63 y | NA | Transient ischemic attack (12%), partial epilepsy (12%), mild cognitive impairment (12%) | Positive | Negative | Acute respiratory distress syndrome | Agitation (69%), confusion (45%; based on CAM‐ICU score), dysexecutive syndrome (24%), diffused corticospinal tract signs (67%), leptomeningeal enhancement (14%), frontotemporal hypoperfusion (19%), ischemic stroke (5%) | 29 |
| Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019 | China | Case report | 1 | 64 y | Male | None | Positive | Negative | Fever, mild cough, insomnia, muscle soreness | Poor mental state, altered consciousness, lethargy, irritability, dissociated speech, neck stiffness, positive ankle clonus | 31 |
| Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID‐19): a retrospective study | China | Retrospective cohort study |
97 Severe group, 25 Mild group, 72 |
Median age = 39 y Severe group, 58 y Mild group, 37 y |
64.9% female |
Severe group: hypertension (40%), diabetes (4.2%), cardiovascular disease (0%), cerebrovascular disease (1.4%) Mild group: hypertension (6.9%), diabetes (8%), cardiovascular disease (8%), cerebrovascular disease (8%) |
Positive | NA | Fever (58.8%), cough (55.7%), fatigue (33%) | Dizziness and headache (7.2%) | 42 |
| New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study | China | Retrospective multicenter study | 304 | 44 y | 59.9% male | NA | Positive | NA | Fever, pneumonialike symptoms, respiratory distress | Acute cerebrovascular disease (1%), traumatic brain injury (0.3%), hypoxia (77), seizurelike symptoms (0.6%), encephalopathic condition (2.6%) | 38 |
| Focal status epilepticus as unique clinical feature of COVID‐19: a case report | Italy | Case report | 1 | 78 y | Female | Hypertension, postencephalitic epilepsy | Positive | NA | Focal status epilepticus |
Focal status epilepticus, fluent aphasia, right central facial nerve palsy, extensive gliosis and atrophy in left temporoparietal lobe |
37 |
| Cerebrovascular complications in patients with SARS‐CoV‐2 infection: case series | Italy | Case series | 6 | 69 y | Male, 5; female, 1 | Smoking (16%), myocardial infarction (16%), diabetes mellitus (50%), arterial hypertension/hypertension (50%), hypertensive cardiomyopathy (16%), aortic valve regurgitation (16%), transitory ischemic attack (16%), aortic valve replacement (16%), cerebellar stroke (16%), thrombocytosis (16%) | Positive | NA | Cough (100%), fever (100%), dyspnea (66%) | Ischemic stroke (67%), hemorrhagic stroke (33%), loss of consciousness (33%), confusion (16%), focal seizures (16%) | 43 |
Retrospective analysis of a more comprehensive data set focused on neurological manifestations in 214 hospitalized patients in Wuhan reported seizures in one of the patients. 33 In a recent case report, a 78‐year‐old female patient, later confirmed with SARS‐CoV‐2 infection, exhibited focal status epilepticus as the initial presenting symptom for COVID‐19. 37
Although these reports may suggest a possible mechanistic link between the onset of seizures and COVID‐19, in total only a relatively low number of cases with new onset or acute seizures have been reported so far. This has also been supported by a recent observational study, which did not detect new onset or acute seizures in a retrospective multicenter cohort study of >300 patients diagnosed with COVID‐19, despite a significant proportion of patients with various risk factors such as hypoxia, acute cerebrovascular events, sepsis, imipenem use, and metabolic disturbances. 38 As recently summarized by an international group of epileptologists, no case or study has been reported yet to justify any direct relation between the potentiation of epileptic seizures and COVID‐19. 14 In this context, it also needs to be taken into consideration that seizure onset during an infection can also be only a coincidence, considering the extreme ratio between reported COVID‐19 cases with seizures and the overall number of reported COVID‐19 cases, as well as that epilepsy is a neurological disease with a high prevalence. This might apply to at least some of the cases that have been reported.
Taken together, the number of reports with new onset or acute seizures in patients with COVID‐19 in relation to the overall number of patients suggests that a relevant lowering in seizure thresholds is a rare event. Seizures may rather occur in patients with a severe condition and terminal stage patients, in whom an increased seizure susceptibility can be a consequence of fever and can be secondary to blood‐brain barrier damage due to hypoxia or sepsis. On the other hand, available data indicate that one should not completely rule out seizures as an initial symptom of a SARS‐CoV‐2 infection. As mentioned above, this would be in line with previous findings for the other betacoronaviruses SARS‐CoV and MERS‐CoV.
Furthermore, some of the above reports of the onset of seizures reported in COVID‐19 patients did not mention sufficient details to arrive at a conclusion. From the 14 articles identified in the present review, eight do not report on testing the presence of SARS‐CoV‐2 infection in the CSF of patients exhibiting neurologic dysfunction. 33 , 37 , 38 , 39 , 40 , 41 , 42 , 43 In addition, few reports do not mention whether the patient exhibiting seizures had a previous history of epilepsy or seizures. 27 , 40 , 41 There are other methodological limitations, including data collected only during the acute phase with lack of follow‐ups, 38 the lack of diagnosis and reporting of mild neurological symptoms, 33 limited resources to treat critically ill patients, 42 and absence of electroencephalographic recordings 38 or advanced neuroimaging techniques such as magnetic resonance imaging (MRI) and other diagnostic techniques. 33 , 42 Hence, the reporting of neurological symptoms was often subjective based on patients’ descriptions, thereby being inconclusive regarding a link between neurologic dysfunction and a possible neuroinvasive potential of SARS‐CoV‐2.
In view of the lack of comprehensive information about the patients’ anamnesis and the lack of a detailed neurological diagnostic workup in the majority of reports, it remains impossible to conclude whether seizures described in the context of COVID‐19 were unprovoked seizures occurring regardless of a causal association in patients with an increased seizure susceptibility, acute symptomatic seizures occurring as an indirect consequence during a severe disease course, or acute symptomatic seizures linked with an actual neuroinvasion of the virus. It is emphasized that seizures occurring in patients with SARS‐CoV‐2 infection do not necessarily imply that seizures have been directly or indirectly caused by the viral infection. For instance, seizures reported in patients with SARS‐CoV‐2 infection may at least in some patients also merely reflect unprovoked seizures in patients with a history of epilepsy that occur regardless of a true causal association.
Nevertheless, reports of seizures in patients with SARS‐CoV‐2 infection also raise general concerns regarding whether seizure worsening might be an issue in patients with epilepsy and a SARS‐CoV‐2 infection. As mentioned above, a risk for a more severe disease course has been discussed for patient subgroups including patients with compromised lung function related to tuberous sclerosis complex (TSC) 44 or patients with exposure to steroids or immunosuppressive drugs used to treat autoimmune encephalitis, TSC, infantile spasms, or electrical status epilepticus during slow‐wave sleep. 14 , 45 In addition, concerns may exist regarding possible worsening of seizures. 14 This may in particular apply to patients with a risk for hyperthermia‐ and fever‐associated seizures, for example, patients with Dravet syndrome. Moreover, it is worth noting that the association between pulmonary disorders and epilepsy is, in general, bidirectional. Although seizures can cause respiratory abnormalities, respiratory dysfunction and associated hypoxia may precipitate seizures. 46 , 47 Although no evidence for such a bidirectional relationship has been confirmed for COVID‐19 yet, one needs to further consider specific risks in susceptible patient populations. However, so far, no clinical evidence exists for increases in seizure severity or frequency in people with epilepsy (PWE).
Taken together, the current state of knowledge, with a lack of relevant evidence for seizure worsening and with evidence for a low rate of new onset seizures, indicates that it is likely that the occurrence of acute symptomatic new onset seizures in the reported cases was rather related to nonspecific mechanisms such as hypoxia, cytokine storm, or cerebrovascular events. A seizure risk should in general be considered in patients with COVID‐19, in particular applying to the subgroup with a severe course. However, conclusions about a direct link with COVID‐19 require an individual assessment of the patient's history and various risk factors. Moreover, more research is necessary to provide more detailed epidemiological data and to explore mechanisms of a possible direct or indirect impact of SARS‐CoV‐2 infection on the brain.
4. POSSIBLE MECHANISMS
4.1. Mechanisms for neurological effects in COVID‐19 patients
Respiratory viruses can damage the nasal epithelium and can penetrate through the endothelium and reach the CNS via bloodstream or lymph nodes. 48 Human coronaviruses such as SARS‐CoV can disrupt innate immunity by infecting myeloid cells and can accumulate in infected leukocytes, thereby disseminating to the CNS. 12 Another route of transmission of coronavirus is through the olfactory bulb leading to transneuronal proliferation to the CNS, as observed in a transgenic mouse model following intranasal infection with SARS‐CoV. 49
An indirect mechanism may be related to an interaction with angiotensin‐converting enzyme 2 (ACE2). SARS‐CoV binds to ACE2, which is expressed at the surface of lung alveolar epithelial cells, enterocytes, endothelial cells, and arterial smooth muscle cells. 50 Further studies analyzing tissue distribution of ACE2 revealed its expression in neuronal and glial cells in the brain. 51 The ACE2 receptor has also been identified to act as a receptor‐binding domain for viral entry into the host cell. 52 A recent study concluded that SARS‐CoV‐2, identical to SARS‐CoV, uses a densely glycosylated spike protein (S1) that catalyzes the interaction of the viral membrane with the host cell membrane regulated by the binding of the S1 subunit to membrane‐associated ACE2 of the host cell. 9 Concomitantly, it has also been confirmed that SARS‐CoV‐2 has a higher affinity for the ACE2 receptor than SARS‐CoV. 9 For SARS‐CoV, it has been reported that the interaction with ACE2 can result in its downregulation. 53 This may cause an imbalance between the protective function of ACE2 versus detrimental effects mediated by ACE. 53 Although ACE2 mediates antioxidant, anti‐inflammatory, and vasodilatory effects, ACE can mediate oxidative stress, proinflammatory effects, vasoconstriction, and vascular leakage. A respective imbalance may not only have direct consequences for lung function, but may also result in high blood pressure and an increased risk for intracranial hemorrhage. 53 There is initial evidence that SARS‐CoV2 infection may also result in a downregulation of ACE2. 54 , 55 It would be highly interesting to further explore the consequences of SARS‐COV2 infection for balance in the functional ACE:ACE2 ratio. Cerebrovascular consequences of a possible imbalance may contribute to intracranial hemorrhage, which may be one potential cause of acute brain‐insult–associated seizures in patients with a severe COVID‐19 disease course.
In addition to the neurological findings, recent reports also describe loss of smell and/or taste experienced by patients with COVID‐19. 56 , 57 It has been postulated that the anosmia and the partial loss of taste could be a result of neuronal viral infection that targets the olfactory system. 58 This may cause damage or destruction of the olfactory receptor neurons that are present in the olfactory epithelium. The expression of ACE2 receptors is also reported in the olfactory epithelium; however, it is so far unknown whether it is expressed in neuronal or nonneuronal cells in this location. If ACE2 expression is neuronal, then the receptor‐bound SARS‐CoV‐2 may reach the brain through the olfactory nerve via axonal transport. 58 This hypothesis is based on previous experiments, which demonstrated that the structurally similar SARS‐CoV can cause brain infection in a human‐ACE2–expressing transgenic mouse through transsynaptic axonal transport via olfactory receptor neurons. 49 An alternate route of viral entry to the brain has been for instance reported for human herpes‐6 virus. 59 The experiments indicated a dissemination to the brain by circulating through the CSF adjacent to the olfactory nerve bundles, after crossing the nonneuronal epithelium cells. This route of brain infection resulted in a distribution through the brain, including the medulla oblongata, with the control center for cardiorespiratory function. 59 Future studies exploring possible routes of SARS‐CoV‐2 brain infection would be of particular interest in view of reports of neurological symptoms in patients with COVID‐19. In this context, it needs to be considered that biological barriers including the blood‐brain barrier can be compromised in critically ill patients with hypoxia, sepsis, and/or secondary bacterial infections. Thus, a direct entry of a virus might occur during a severe disease course and in terminal disease stages. Therefore, any postmortem evidence for virus detection in brain tissues needs to be interpreted with extreme care considering the disease course and stage. 60 Respective findings may not necessarily imply a relevant neuroinvasiveness of the virus during milder disease courses.
Cytokine storm is the dramatic and detrimental consequence due to the rapid release of inflammatory polypeptide mediators generating systemic responses. These polypeptides, also known as cytokines, can cross the blood‐brain barrier, causing neuroinflammation, which can contribute to development of poststroke syndrome, encephalopathies, and chronic neurodegenerative diseases. 61 Evidence for a cytokine storm has also been reported in subgroups of patients with severe COVID‐19. 42 , 62 , 63 Thus, cytokine storm must be considered as another possible cause of neuronal damage in patients with a severe COVID‐19 disease course. The cytokines released during a COVID‐19–associated cytokine storm include interleukins such as IL‐6 and IL‐1β, granulocyte colony‐stimulating factor, granulocyte‐macrophage colony‐stimulating factor, and tumor necrosis factor. 42 , 62 , 63 , 64 , 65 Among these, increased levels of IL‐6 have been found to be a common marker in COVID‐19 patients with severe conditions. To overcome this, Chinese researchers conducted a pilot clinical trial (ChiCTR2000029765) to assess treatment of COVID‐19 patients with tocilizumab, an antibody targeting IL‐6 receptor. In this small group of treated patients, signs of improvement in their respiratory functioning with reduced fever were reported. 66 Acute necrotizing encephalopathy, a rare disorder causing brain dysfunction due to virus infections associated with a cytokine storm, has been recently observed in a COVID‐19 patient with an MRI displaying clear evidence of hemorrhage. 60
4.2. Probable mechanisms linking SARS‐CoV‐2 and seizures
Among the severe neurological symptoms mentioned in the various case reports/series and articles selected as per Table 1, seizures reached the highest prevalence in COVID‐19 cases (Figure 1). As discussed above, seizure occurrence in COVID‐19 patients may be merely coincidence. Nevertheless, occasional cases with new onset seizures raise the question of whether in addition to nonspecific mechanisms related to hypoxia or sepsis, there could be specific mechanisms associated with SARS‐CoV‐2 infection and induction of seizures. Respective knowledge may also guide the future identification of individual risk factors.
FIGURE 1.
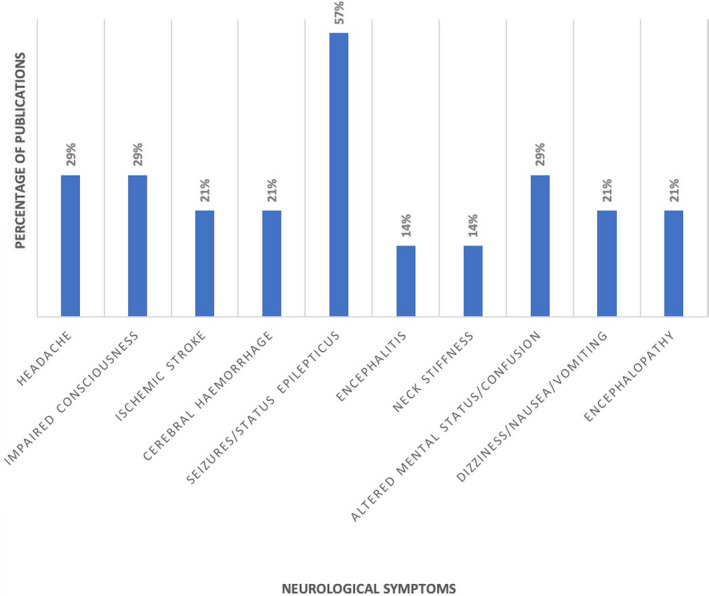
Percentage of articles published during the period January 1, 2020 through April 30, 2020 reporting the respective neurological symptoms in patients with SARS‐CoV‐2. The publications considered comprise original and review articles, including clinical observational studies, case reports, and/or case series, and were identified after an extensive database search in PubMed/MEDLINE and other databases including ScienceDirect, Semantic Scholar, and Google Scholar. The search string included “SARS‐CoV‐2” OR “COVID‐19” AND “epilepsy” OR “seizures” OR “central nervous system” OR “brain” OR “neurological findings” OR “cerebrovascular disease” OR “CNS infection” OR “neurological disorder”
As discussed above, SARS‐CoV‐2 binds to ACE2, which in turn mediates host cell entry. In this context, it is of interest that there are reports suggesting that an upregulation of components of the renin‐angiotensin system can occur in the hippocampus of patients with temporal lobe epilepsy (TLE). 67 The study provided evidence for an induction of the angiotensin II receptors AT1 and AT2 in selected brain regions. 67 AT1 receptor activation is known to mediate proinflammatory and vasoconstrictive effects. 68 ACE function also affects the kallikrein‐kinin system that regulates B1 and B2 kinins. 69 Receptors of these kinins also proved to be overexpressed in the hippocampus of TLE patients. 69 A preclinical study reported reduction in the severity of seizures in response to clinically relevant doses of the ACE inhibitor enalapril in a rat epilepsy model. 70 In view of these findings pointing to a proconvulsant effect of angiotensin II, a possible downregulation of ACE2 during a betacoronavirus infection may theoretically contribute to an increased seizure susceptibility related to a shift of angiotensin processing by ACE rather than ACE2. It is emphasized that so far, neither experimental nor clinical data are available to support this hypothesis in the context of SARS‐CoV‐2 expression. Moreover, again it needs to be considered that a respective pathogenetic mechanism would only be of relevance if the virus enters brain tissue and reaches brain regions relevant for seizure generation in a significant manner. Studies would be necessary to explore a possible infection‐associated regulation of ACE2, its consequences on the balance in the renin‐angiotensin system in different tissues, and its functional consequences.
Circulatory IL‐6 serves as a pyrogen that contributes to a fever response during infections. 71 Previous clinical reports have associated elevated levels of IL‐6 with the occurrence of febrile seizures. 72 , 73 IL‐6 is a proinflammatory cytokine, which can play a role in neuroinflammation and seems to contribute to the impact of inflammatory signaling on seizure thresholds. A preclinical study reported an intensification of seizures in response to intranasal IL‐6 administration in a seizure model in rats. 74 In another study, mice infected with Theiler murine encephalomyelitis virus exhibited acute seizure induction with a parallel increase in IL‐6 levels. 75 Various clinical reports also link escalated levels of IL‐6 with the onset of seizures. 76 , 77 , 78 These studies provide further evidence for the development of seizures associated with elevated levels of IL‐6. Considering that viral infections with viruses such as SARS‐CoV‐2 are frequently associated with fever and elevated IL‐6 levels, there is the risk that seizure thresholds are lowered in affected patients. However, again there have been no findings yet supporting a respective concern.
Thus, in conclusion, there are several possible mechanisms by which COVID‐19 may theoretically increase seizure susceptibility. However, in this context, one also needs to consider that hypoxic encephalopathy was reported in 20% of 113 deceased patients with COVID‐19. 79 Therefore, hypoxia should be considered as another factor that may trigger seizures in COVID‐19 cases with pneumonia and a severe disease course.
5. CONCLUSION
Literature and social media search did not reveal clinical evidence for a risk of seizure worsening in patients with epilepsy. Nevertheless, different pathophysiological mechanisms associated with COVID‐19 may theoretically lower seizure thresholds and increase seizure susceptibility in patient subgroups. Although a final conclusion regarding a possible neuroinvasiveness of SARS‐CoV‐2 seems to require further research and scientific confirmation, other mechanisms and potential pathological consequences of COVID‐19 that may trigger seizure activity, such as fever, hypoxic damage, cerebrovascular events, and cytokine storm, should be carefully considered for management of patients with an increased risk. This in particular applies in situations with capacity limitations in medical care often associated with recommendations not to immediately visit a medical practice when only developing mild to moderate respiratory symptoms, and with telemedicine approaches replacing many face‐to‐face doctor's appointments. It is highly recommended that PWE developing COVID‐19–associated symptoms be closely supported and that further data be collected about a possible influence of COVID‐19 on seizure control.
The necessity for great care and respective measures is confirmed by occasional reports of new onset and acute seizures in patients with COVID‐19 without a history of seizures or a previous epilepsy diagnosis. Considering the low number of reports in relation to the overall number of patients with COVID‐19 worldwide, the risk for COVID‐19–associated seizure development may be relatively low except for critically ill and terminal stage patients. However, available information also suggests that an early neurological manifestation is possible and that seizures should not be ruled out as an initial symptom.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
ETHICAL APPROVAL
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
Research focused on pathophysiology and therapeutic management of epilepsy in H.P.'s group is currently supported by Deutsche Forschungsgemeinschaft PO 681/8‐1. The research on epilepsy by D.V. and her group is supported by University Grants Commission Special Assistance Program, Phase 2 and Department of Science and Technology, Government of India under the Fund for Improvement of Science & Technology (S&T) infrastructure in universities & higher educational institutions program.
Vohora D, Jain S, Tripathi M, Potschka H. COVID‐19 and seizures: Is there a link?. Epilepsia. 2020;61:1840–1853. 10.1111/epi.16656
Divya Vohora and Shreshta Jain contributed equally.
Correction added on October 6, 2020, after first online publication: email address and affiliation updated for Heidrun Potschka under correspondence.
Contributor Information
Divya Vohora, Email: dvohora@jamiahamdard.ac.in.
Heidrun Potschka, Email: potschka@pharmtox.vetmed.uni-muenchen.de.
REFERENCES
- 1. Zhu NA, Zhang D, Wang W, Li X, Yang BO, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance, 11 January 2020. Available at: https://www.who.int/publications‐detail/clinical‐management‐of‐severe‐acute‐respiratory‐infection‐when‐novel‐coronavirus‐(ncov)‐infection‐is‐suspected. Accessed May 30, 2020.
- 3. Richman DD, Whitley RJ, Hayden FG. Clinical Virology. Hoboken, NJ: John Wiley & Sons; 2016. [Google Scholar]
- 4. Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. [DOI] [PubMed] [Google Scholar]
- 5. Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. [DOI] [PubMed] [Google Scholar]
- 6. Wu F, Zhao SU, Yu B, Chen Y‐M, Wang W, Song Z‐G, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Coronavirus. Available at: https://www.who.int/health‐topics/coronavirus#tab=tab_1. Accessed April 30, 2020.
- 9. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C‐L, Abiona O, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;13(367):1260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahase E. Covid‐19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368:m1036. [DOI] [PubMed] [Google Scholar]
- 11. Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID‐19). J Gen Intern Med. 2020;35(5):1545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. French JA, Brodie MJ, Caraballo R, Devinsky O, Ding D, Jehi L, et al. Keeping people with epilepsy safe during the COVID‐19 pandemic. Neurology. 2020;94(23):1032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang QL, Ding YQ, Hou JL, He L, Huang ZX, Wang HJ, et al. Detection of severe acute respiratory syndrome (SARS)‐associated coronavirus RNA in autopsy tissues with in situ hybridization. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:1125–7. [PubMed] [Google Scholar]
- 17. Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10:342–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zegarra JA, Chino B, Munive V, Tairo T, Lastarria PC. Neurological component in coronaviruses induced disease: systematic review of SARS‐CoV, MERS‐CoV, AND SARS‐CoV‐2 [published online ahead of print April 7, 2020] OSF Preprints. 10.31219/osf.io/2fqtz [DOI] [Google Scholar]
- 19. Cheng VCC, Lau SKP, Woo PCY, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20:660–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Algahtani H, Subahi A, Shirah B. Neurological complications of Middle East respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep Neurol Med. 2016;2016:3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS‐CoV). Infection. 2015;43:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim J‐E, Heo J‐H, Kim H‐O, Song S‐H, Park S‐S, Park T‐H, et al. Neurological complications during treatment of Middle East respiratory syndrome. J Clin Neurol. 2017;13(3):227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li K, Wohlford‐Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR, et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213:712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID‐19. Lancet Neurol. 2020;19(9):767–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang D, Hu BO, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. J Infect Dis. 2020;94:55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou L, Zhang M, Wang J, Gao J. Sars‐Cov‐2: Underestimated damage to nervous system. Travel Med Infect Dis. 2020;101642. 10.1016/j.tmaid.2020.101642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karimi N, Sharifi Razavi A, Rouhani N. Frequent convulsive seizures in an adult patient with COVID‐19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. [Google Scholar]
- 29. Helms J, Kremer S, Merdji H, Clere‐Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. 2020;382(23):2268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID‐19): encephalopathy. Cureus. 2020;12:e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yin R, Feng W, Wang T, Chen G, Wu T, Wu D, et al. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J Med Virol. 2020. 10.1002/jmv.25888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92(6):552–5. 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurology. 2020;77(6):683. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holohan M.Nearly a week after COVID‐19 diagnosis, 1‐year‐old is recovering, 'happy'. Available at: https://www.today.com/health/kids‐coronavirus‐1‐year‐old‐recovering‐after‐covid‐19‐diagnosis‐t177203. Accessed April 25, 2020.
- 35. Rabin RC.Some coronavirus patients show signs of brain ailments. Available at: https://www.nytimes.com/2020/04/01/health/coronavirus‐stroke‐seizures‐confusion.html. Accessed April 30, 2020.
- 36. Mangione KBC.B.C. Senior initially denied COVID‐19 test found out he had it after 2 seizures, family says. Available at: https://bc.ctvnews.ca/b‐c‐senior‐initially‐denied‐covid‐19‐test‐found‐out‐he‐had‐it‐after‐2‐seizures‐family‐says‐1.4874166. Accessed April 25, 2020.
- 37. Vollono C, Rollo E, Romozzi M, Frisullo G, Servidei S, Borghetti A, et al. Focal status epilepticus as unique clinical feature of COVID‐19: a case report. Seizure. 2020;78:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu L, Xiong W, Liu D, Liu J, Yang D, Li N, et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61(6):e49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sun D, Li H, Lu X‐X, Xiao H, Ren J, Zhang F‐R, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr. 2020;16(3):251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharifi‐Razavi A, Karimi N, Rouhani N. COVID‐19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID‐19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;296(2):201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nie S, Zhao X, Zhao K, Zhang Z, Zhang Z, Zhang Z. Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID‐19): a retrospective study. medRxiv. 2020. 10.1101/2020.03.24.20042283 [DOI] [Google Scholar]
- 43. Morassi M, Bagatto D, Cobelli M, D’Agostini S, Gigli GL, Bnà C, et al. Cerebrovascular complications in patients with SARS‐CoV‐2 infection: case series. J Neurol. 2020;267(8):2185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. EpiCARE . COVID‐19/General advice for persons with epilepsy. Available at: https://epi‐care.eu/wp‐content/uploads/2020/04/COVID‐19‐and‐Epilepsy_ERN‐EpiCARE.Recommendations_ENGLISH.pdf. Accessed April 30, 2020.
- 46. De Reuck J, Proot P, Van Maele G. Chronic obstructive pulmonary disease as a risk factor for stroke‐related seizures. Eur J Neurol. 2007;14:989–92. [DOI] [PubMed] [Google Scholar]
- 47. Pourshahid S, Dedhia S, Hakim S, Barakat M, Genin D. Seizure and pulmonary embolism: a differential that can save a life. Case Rep Pulmonol. 2017;2017:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Desforges M, Le Coupanec A, Brison E, Meessen‐Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host‐virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–8. [DOI] [PubMed] [Google Scholar]
- 52. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guzzi PH, Mercatelli D, Ceraolo C, Giorgi FM. Master regulator analysis of the SARS‐CoV‐2/human interactome J Clin Med. 2020;9(4):982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS‐CoV‐2 infection. Eur J Intern Med. 2020;76:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self‐reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross‐sectional study. Clin Infect Dis. 2020;71(15):889–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xydakis MS, Dehgani‐Mobaraki P, Holbrook EH, Geisthoff UW, Bauer C, Hautefort C, et al. Smell and taste dysfunction in patients with COVID‐19. Lancet Infect Dis. 2020;20(9:)1015–1016. 10.1016/s1473-3099(20)30293-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Butowt R, Bilinska K. SARS‐CoV‐2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci. 2020;11(9):1200–3. [DOI] [PubMed] [Google Scholar]
- 59. Harberts E, Yao K, Wohler JE, Maric D, Ohayon J, Henkin R, et al. Human herpesvirus‐6 entry into the central nervous system through the olfactory pathway. Proc Natl Acad Sci U S A. 2011;108:13734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Das G, Mukherjee N, Ghosh S. Neurological insights of COVID‐19 pandemic. ACS Chem Neurosci. 2020;11(9):1206–9. [DOI] [PubMed] [Google Scholar]
- 61. Clark IA, Vissel B. The meteorology of cytokine storms, and the clinical usefulness of this knowledge. Semin Immunopathol. 2017;39:505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu YI, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tan M, Liu Y, Zhou R, Deng X, Li F, Liang K, et al. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160(3):261–268. 10.1111/imm.13223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;28(395):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Argañaraz GA, Konno AC, Perosa SR, Santiago JFC, Boim MA, Vidotti DB, et al. The renin‐angiotensin system is upregulated in the cortex and hippocampus of patients with temporal lobe epilepsy related to mesial temporal sclerosis. Epilepsia. 2008;49(8):1348–57. [DOI] [PubMed] [Google Scholar]
- 68. Dandona P, Dhindsa S, Ghanim H, Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007;21(1):20–7. [DOI] [PubMed] [Google Scholar]
- 69. Perosa SR, Argañaraz GA, Goto EM, Costa LGP, Konno AC, Varella PPV, et al. Kinin B1 and B2 receptors are overexpressed in the hippocampus of humans with temporal lobe epilepsy. Hippocampus. 2007;17:26–33. [DOI] [PubMed] [Google Scholar]
- 70. Pereira MG, Becari C, Oliveira JA, Salgado MCO, Garcia‐Cairasco N, Costa‐Neto CM. Inhibition of the renin–angiotensin system prevents seizures in a rat model of epilepsy. Clin Sci. 2010;119:477–82. [DOI] [PubMed] [Google Scholar]
- 71. Rummel C, Sachot C, Poole S, Luheshi GN. Circulating interleukin‐6 induces fever through a STAT3‐linked activation of COX‐2 in the brain. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1316–26. [DOI] [PubMed] [Google Scholar]
- 72. Chen Q, Li M, Zhang X, Zhang X, Zhong R, Lin W. Association between interleukin‐6 gene polymorphisms and febrile seizure risk: a meta‐analysis. Medicine (Baltimore). 2019;98:e17167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Azab SF, Abdalhady MA, Almalky MAA, Amin EK, Sarhan DT, Elhindawy EM, et al. Serum and CSF adiponectin, leptin, and interleukin 6 levels as adipocytokines in Egyptian children with febrile seizures: a cross‐sectional study Ital. J Pediatr. 2016;42(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kalueff AV, Lehtimaki KA, Ylinen A, Honkaniemi J, Peltola J. Intranasal administration of human IL‐6 increases the severity of chemically induced seizures in rats. Neurosci Lett. 2004;22(365):106–10. [DOI] [PubMed] [Google Scholar]
- 75. Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Uludag IF, Duksal T, Tiftikcioglu BI, Zorlu Y, Ozkaya F, Kirkali G. IL‐1β, IL‐6 and IL1Ra levels in temporal lobe epilepsy. Seizure. 2015;26:22–5. [DOI] [PubMed] [Google Scholar]
- 77. de Vries EE, van den Munckhof B, Braun KP, van Royen‐Kerkhof A, de Jager W, Jansen FE. Inflammatory mediators in human epilepsy: a systematic review and meta‐analysis. Neurosci Biobehav Rev. 2016;63:177–90. [DOI] [PubMed] [Google Scholar]
- 78. Alapirtti T, Lehtimäki K, Nieminen R, Mäkinen R, Raitanen J, Moilanen E, et al. The production of IL‐6 in acute epileptic seizure: a video‐EEG study. J Neuroimmunol. 2018;15(316):50–5. [DOI] [PubMed] [Google Scholar]
- 79. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]


