Abstract
Recognition of pathogen-derived carbohydrate constituents by antigen presenting cells is an important step in the induction of protective immunity. Here we investigated the interaction of L-SIGN (liver/lymph node specific ICAM-3-grabbing nonintegrin), a C-type lectin that functions as antigen receptor on human liver sinusoidal endothelial cells, with egg-derived glycan antigens of the parasitic trematode Schistosoma mansoni. Our data demonstrate that L-SIGN binds both schistosomal soluble egg antigens (SEA) and egg glycosphingolipids, and can mediate internalization of SEA by L-SIGN expressing cells. Binding and internalization of SEA was strongly reduced after treatment of SEA with endoglycosidase H, whereas defucosylation affected neither binding nor internalization. These data indicate that L-SIGN predominantly interacts with oligomannosidic N-glycans of SEA. In contrast, binding to egg glycosphingolipids was completely abolished after defucosylation. Our data show that L-SIGN binds to a glycosphingolipid fraction containing fucosylated species with compositions of Hex1HexNAc5−7dHex3−6Cer, as evidenced by mass spectrometry. The L-SIGN “gain of function” mutant Ser363Val, which binds fucosylated Lewis antigens, did not bind to this fucosylated egg glycosphingolipid fraction, suggesting that L-SIGN displays different modes in binding fucoses of egg glycosphingolipids and Lewis antigens, respectively. Molecular modeling studies indicate that the preferred binding mode of L-SIGN to the respective fucosylated egg glycosphingolipid oligosaccharides involves a Fucα1-3GalNAcβ1-4(Fucα1-3)GlcNAc tetrasaccharide at the nonreducing end. In conclusion, our data indicate that L-SIGN recognizes both oligomannosidic N-glycans and multiply fucosylated carbohydrate motifs within Schistosoma egg antigens, which demonstrates that L-SIGN has a broad but specific glycan recognition profile.
Keywords: C-type lectin, glycosphingolipids, L-SIGN, parasitic helminth glycans, Schistosoma mansoni
Introduction
Schistosomiasis is a human parasitic disease caused by helminths of the genus Schistosoma that affects more than 200 million people worldwide (Pearce and MacDonald 2002). Infection starts when cercariae released by the intermediate host, a snail of the genus Biomphalaria, penetrates the skin of its vertebrate host and transforms into schistosomula. They migrate to the portal system and mature to adult worms that produce large amounts of eggs. Eggs that become lodged within host tissues, mainly liver and intestine, are primarily responsible for the development of a strong anti-inflammatory Th2 response that enables parasite survival and induces granuloma formation around the eggs, which is a major cause of pathology (Pearce and MacDonald 2002).
Schistosoma mansoni synthesizes a multitude of complex carbohydrates, which include both parasite-specific glycan antigens, as well as glycan antigens that are shared with the host. One example for a host-like glycan is the Lewis X (LeX) epitope Galβ1-4(Fucα1-3)GlcNAc, which is expressed in all schistosomal life stages (Cummings and Nyame 1996; Robijn et al. 2005), but also on human leukocytes as CD15 (Fukuda et al. 1986). Glycan antigens expressed by schistosomes induce strong humoral and cellular immune responses in their host (Cummings and Nyame 1999). Especially soluble egg antigens (SEA) have been shown to be highly immunogenic in mice and humans due to the presence of carbohydrate epitopes such as GalNAcβ1-4GlcNAc- (LDN), GalNAcβ1-4(Fucα1-3)GlcNAc- (LDN-F), and GalNAcβ1-4(Fucα1-2Fucα1-3)GlcNAc- (LDN-DF) (see Table I) (van Die and Cummings 2006).
Table I.
Carbohydrate epitopes mentioned in this study
| Carbohydrate epitope | Structure | Structure plot |
|---|---|---|
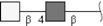
| LacdiNAc (LDN) | GalNAcβ1-4GlcNAc | |
|
||
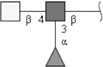
| Terminally fucosylated LDN (F-LDN) | Fucα1-3GalNAcβ1-4GlcNAc | |
|
||
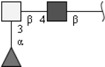
| Fucosylated LDN (LDN-F) | GalNAcβ1-4(Fucα1-3)GlcNAc | |
|
||
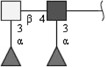
| Fucosylated LDN-F (F-LDN-F) | Fucα1-3GalNAcβ1-4(Fucα1-3)GlcNAc | |
|
||
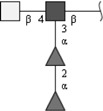
| Difucosylated LDN (LDN-DF) | GalNAcβ1-4(Fucα1-2Fucα1-3)GlcNAc | |
|
||
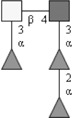
| Fucosylated LDN-DF (F-LDN-DF) | Fucα1-3GalNAcβ1-4(Fucα1-2Fucα1-3)GlcNAc | |
|
||
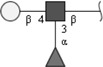
| Lewis X (LeX) | Galβ1-4(Fucα1-3)GlcNAc | |
|
||
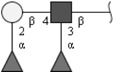
| Lewis Y (LeY) | Fucα1-2Galβ1-4(Fucα1-3)GlcNAc | |
|
||
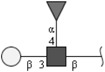
| Lewis A (LeA) | Galβ1-3(Fucα1-4)GlcNAc | |
|
||
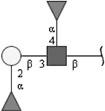
| Lewis B (LeB) | Fucα1-2Galβ1-3(Fucα1-4)GlcNAc | |
|
Respective abbreviations are given in parentheses. Structure plots were generated in the notation of the Consortium for Functional Glycomics (http://www.functionalglycomics.org) using the visual editor of “GlycoWorkbench”. This software application is developed and available as part of the EUROCarbDB project (http://www.eurocarbdb.org/applications/ms-tools). Light grey square, N-acetylgalactosamine; dark grey square, N-acetylglucosamine; triangle, fucose; circle, galactose.
While much remains to be understood about the immunological events triggered by schistosomal glycoconjugates, it is increasingly acknowledged that these biological effects depend on the recognition of these glycans by specific receptors on immune cells. The recognition of carbohydrates is mediated by a family of receptors called lectins, which bind glycan antigens via one or more carbohydrate recognition domains (CRD). We have recently described that both the C-type lectin DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3 (ICAM-3)-grabbing nonintegrin; CD209) as well as its homologue L-SIGN (liver/lymph node-specific ICAM-3-grabbing nonintegrin; CD209L; DC-SIGN-R) bind to glycans of S. mansoni soluble egg antigens (SEA) (van Die et al. 2003; van Liempt et al. 2004; Meyer et al. 2005). The role of DC-SIGN as a broad pathogen receptor has been well established (Geijtenbeek et al. 2000; Alvarez et al. 2002; Colmenares et al. 2002; Cambi et al. 2003; Geijtenbeek et al. 2003; Lozach et al. 2003). In addition, DC-SIGN functions as a cell adhesion receptor mediating the interaction between dendritic cells (DCs) and resting T cells by binding to ICAM-3, and the transendothelial migration of DCs by binding to ICAM-2 (Geijtenbeek et al. 2000).
L-SIGN displays 77% amino acid identity with DC-SIGN, and is expressed on endothelial cells in lymph node sinuses, capillary endothelial cells in the placenta and on liver sinusoidal cells (LSECs) (Soilleux et al. 2000; Bashirova et al. 2001; Pohlmann et al. 2001; Engering et al. 2004). In the liver LSECs function as liver-resident antigen presenting cells (Knolle and Gerken 2000) and are important in tolerance induction (Limmer et al. 2000; Knolle and Limmer 2001). LSECs may mediate the clearance of antigens from the circulation in the same manner as DCs do (Bashirova et al. 2001; Karrar et al. 2007). In addition to L-SIGN, LSECs express lectins like the mannose receptor, high levels of adhesion molecules and costimulatory molecules such as MHC class II, CD40, CD80, and CD86 (Adams et al. 1989; McNab et al. 1996). It has been suggested that LSECs can potentially function as cells that are capable of trapping CD4+ and CD8+ T cells (Karrar et al. 2007). DC-SIGN and L-SIGN share a di-leucine motif and a cluster of three acidic amino acids in their cytoplasmic tails, which are known to be essential for antigen uptake (Soilleux et al. 2000; Bashirova et al. 2001; Engering et al. 2002). Recent studies with Ebola virus, Severe Acute Respiratory Syndrome (SARS) virus or antibodies against L-SIGN, clearly demonstrated that L-SIGN indeed is able to internalize antigens (Alvarez et al. 2002; Jeffers et al. 2004; Ludwig et al. 2004; Dakappagari et al. 2006). Likewise, Ludwig et al. monitored the internalization of Hepatitis C virus (HCV) envelope glycoproteins E1 and E2 by L-SIGN and the intracellular localization of these glycoproteins in LSECs by confocal microscopy (Ludwig et al. 2004). Similar to DC-SIGN, L-SIGN can recognize high-mannose type N-glycans and the fucosylated glycan epitopes Lewis A (LeA, Galβ1-3(Fucα1-4)GlcNAc-), Lewis B (LeB, Fucα1-2Galβ1-3(Fucα1-4)GlcNAc-) and Lewis Y (LeY, Fucα1-2Galβ1-4(Fucα1-3)GlcNAc-) (Geijtenbeek et al. 2003; Guo et al. 2004; van Liempt et al. 2004). L-SIGN, however, does not bind to the LeX epitope, which is one of the major schistosome ligands of DC-SIGN, although the formation of crystals between L-SIGN and LeX indicates that a weak interaction is possible (Guo et al. 2004). The inability of L-SIGN to bind to LeX epitopes is mainly due to the presence of a single amino acid in the CRD of L-SIGN, Ser363 that prevents interaction with the Fuc(α1-3)GlcNAc unit in LeX, but supports binding of the Fucα1-4GlcNAc moiety present in LeA and LeB antigens. The equivalent amino acid residue Val351 in DC-SIGN creates a hydrophobic pocket that strongly interacts with the Fuc(α1-3/4)GlcNAc moiety of LeX, other Lewis antigens, and probably LDN-F (Guo et al. 2004; van Liempt et al. 2004; van Liempt et al. 2006).
The interaction of L-SIGN with S. mansoni egg glycoproteins and its location on liver endothelial cells suggest that L-SIGN may function in the recognition of glycan antigens of eggs that are trapped in the liver, thus contributing to glycan-specific immune responses and/or the immunopathology of schistosomiasis. To increase our understanding of the role of L-SIGN we investigated the binding properties of L-SIGN to both schistosomal egg glycoproteins and glycosphingolipids. Our data revealed that L-SIGN interacts predominantly with oligomannosidic N-glycans of SEA. Remarkably, recognition of schistosomal egg glycosphingolipids by L-SIGN was mediated via fucosylated carbohydrate entities, utilizing a binding mode that may be different from the way it binds to Lewis antigens.
Results
Recognition of S. mansoni SEA by L-SIGN
Previous studies have shown that L-SIGN can recognize both high-mannose type N-glycans as well as particular fucosylated structures within Lewis antigens (Guo et al. 2004; van Liempt et al. 2004) and may also interact with SEA (van Liempt et al. 2004). Furthermore, we have demonstrated that L-SIGN does not bind to LeX, a major glycan antigen of SEA (van Liempt et al. 2004). To identify the carbohydrate ligands of SEA that are recognized by L-SIGN, SEA was treated with endo H to remove oligomannosidic N-glycans. In parallel, SEA was subjected to HF-treatment to remove fucose residues. Our data show that HF-treated SEA has lost its ability to react with antibodies directed against the fucose-containing epitopes LeX and LDN-DF (Figure 1A), whereas binding of these antibodies to endo H-treated SEA was hardly affected (results not shown). Hence, it can be concluded that the HF-treatment resulted in a nearly complete removal of fucose residues from SEA. In addition, the reactivity of HF-treated SEA with antibodies recognizing LDN glycan antigens was increased after defucosylation, which shows that the remaining glycan is intact and may expose an increased amount of terminal LDN units.
Fig. 1.
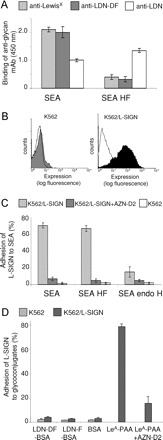
Binding of S. mansoni SEA by L-SIGN transfected cells. (A) ELISA was performed to characterize the glycan epitopes of SEA and defucosylated SEA (SEA HF). Similar amounts of SEA (5 μg/mL) were applied in each case. Using antibodies recognizing the fucose-containing glycan epitopes LeX and LDN-DF the efficacy of HF-treatment was determined. In parallel, an anti-LDN mAb was employed to evaluate the integrity of the remaining glycans. Data represent a typical result out of three experiments performed in duplicate, with error bars indicating standard deviation. (B) The expression of L-SIGN on transfected (K562/L-SIGN) and nontransfected K562 cells was determined by flow cytometry using the mAb AZN-D2 that recognizes L-SIGN. The isotype control is shown as lines. (C) Binding of L-SIGN to soluble egg antigen (SEA) of S. mansoni, defucosylated SEA (SEA HF) and endo H-treated SEA (SEA endo H) was determined by cell adhesion assays using K562/L-SIGN transfected cells in the absence (light grey bars) or presence (dark grey bars) of a blocking mAb to L-SIGN (AZN-D2). All results are representative of three independent experiments, performed in triplicate, with error bars indicating standard deviation. (D) Adhesion of K562/L-SIGN transfected cells to neoglycoconjugates carrying LDN-F (LDN-F-BSA) and LDN-DF (LDN-DF-BSA). The neoglycoconjugate LeA-PAA was used as a positive control. One representative experiment out of three is shown.
Subsequent cellular adhesion assays using K562 cells stably transfected with L-SIGN (K562/L-SIGN), revealed that binding of L-SIGN to SEA was apparently not affected by defucosylation, whereas treatment with endo H almost completely abolished SEA recognition by L-SIGN (Figure 1B and C). These data indicate that the binding of L-SIGN to S. mansoni SEA is predominantly mediated by oligomannosidic N-glycans, whereas the fucose-containing glycan epitopes present on SEA are obviously not or much less involved in this type of interaction. In agreement with this assumption, direct binding assays showed that K562 cells stably transfected with L-SIGN did not bind to neoglycoproteins carrying LDN-F or LDN-DF (Figure 1D), indicating that these glycan antigens, which in addition to LeX are major fucosylated glycan antigens on SEA, are not ligands of L-SIGN.
Internalization of SEA by L-SIGN expressing cells
To further characterize the interaction between SEA and L-SIGN, we investigated whether SEA is internalized from the cell surface of L-SIGN transfected K562 cells. We found that 64% of the biotinylated SEA disappeared from the cell surface within an incubation time of 15 min at 37°C (Figure 2A), whereas no detectable loss of SEA was observed in the case of paraformaldehyde fixed cells, in which membrane transport had been blocked. These data suggest that SEA is rapidly internalized from the cell surface. The internalization could be significantly inhibited by preincubation of the cells with the L-SIGN specific mAb AZN-D2, thus demonstrating that this process is L-SIGN dependent (Figure 2A). Defucosylation of biotinylated SEA by HF-treatment led only to a minimal reduction of binding and a similar rate of internalization as compared to untreated SEA (Figure 2B). In contrast, binding and internalization of endo H-treated SEA to L-SIGN transfected cells was hardly detectable by flow cytometry analysis (data not shown), in agreement with the previously observed binding properties of L-SIGN to SEA (see Figure 1B).
Fig. 2.
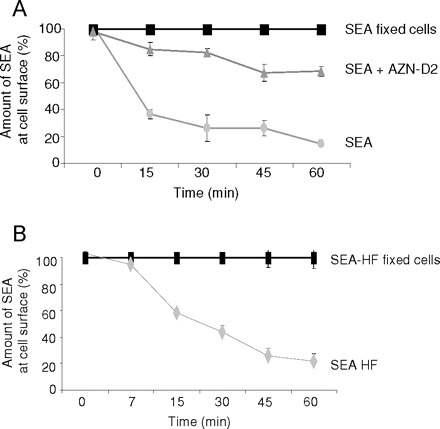
Internalization of SEA in L-SIGN-transfected cells. (A) Internalization of biotinylated SEA (grey circles) from the cell surface of K562 cells stably transfected with L-SIGN was analyzed after different time intervals of incubation at 37°C using flow cytometry. Fixed cells (black squares) were used to correct for the off-rate of SEA at 37°C. Internalization of biotinylated SEA in the presence of mAb AZN-D2 (grey triangles) is clearly reduced. Data represent mean values of duplicate determinations. The representative result of one out of three independent experiments is shown. (B) Internalization of defucosylated, biotinylated SEA (SEA-HF, grey diamonds) bound to the cell surface of L-SIGN transfected cells was analyzed at different time points of incubation at 37°C using flow cytometry. Fixed cells (black squares) were used to correct for the off-rate of biotinylated SEA-HF at 37°C. Values represent means of duplicates. A representative result out of two independent experiments is shown.
Recognition of S. mansoni egg glycosphingolipids by L-SIGN
To establish whether L-SIGN binds authentic schistosomal glycosphingolipids, we performed cellular adhesion assays using K562 cells stably transfected with L-SIGN. Glycosphingolipids from S. mansoni cercariae, adults and eggs were isolated by organic solvent extraction and quantified by compositional analysis with regard to their carbohydrate content to ensure the application of similar, defined amounts of glycosphingolipids in all experiments. The results (Figure 3A) revealed that L-SIGN predominantly recognized egg glycosphingolipids, and to a significantly weaker extent glycosphingolipids from adult worms or cercariae. The blocking antibody AZN-D2 completely inhibited L-SIGN binding of egg glycosphingolipids, indicating that recognition is mediated through the CRD of L-SIGN (Figure 3A). The interaction of L-SIGN transfected cells with egg glycosphingolipids was abolished by adding EDTA (data not shown), thus demonstrating that the binding of egg glycosphingolipids to L-SIGN is calcium-dependent. To investigate whether the recognition of egg glycosphingolipids by L-SIGN is fucose-dependent, egg glycosphingolipids were treated with HF, which resulted in a removal of fucose residues from the glycan moieties, as demonstrated by ELISA using antibodies against the fucose-containing epitopes F-LDN and LDN-DF (Figure 3C). Integrity of the remaining glycan moieties of these glycosphingolipids was demonstrated
Fig. 3.
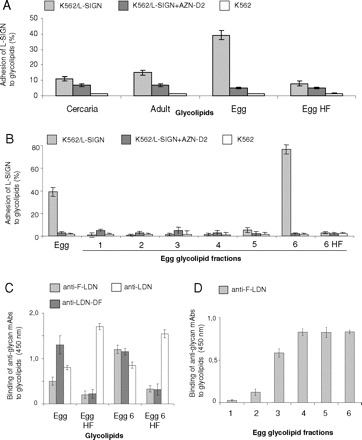
Binding of L-SIGN-transfected cells to glycosphingolipids of S. mansoni. Similar amounts of glycolipids (9 ng/well) were applied in each case. All results are representative of three independent experiments, performed in triplicate (A, B) or duplicate (C, D), with error bars indicating standard deviation. (A) Binding of L-SIGN transfected cells (K562/L-SIGN) to glycosphingolipids obtained from cercariae (Cercaria), adult worms (Adult), eggs (Egg) and defucosylated egg glycosphingolipids (Egg HF) was determined by cell adhesion assay in the absence (light grey bars) or presence (dark grey bars) of a blocking mAb to L-SIGN (AZN-D2). (B) The binding of L-SIGN transfected cells (K562/L-SIGN) to fractionated egg glycosphingolipids was investigated by cell adhesion assay. Total egg glycosphingolipids (Egg), glycosphingolipid fractions 1 to 6 obtained by silica cartridge fractionation (1–6) and defucosylated fraction 6 egg glycosphingolipids (6 HF) were assayed for their binding to L-SIGN-transfected cells in the absence (light grey bars) or presence of AZN-D2 (dark grey bars) as blocking antibody. (C) Characterization of the glycan epitopes of schistosomal total egg glycosphingolipids (Egg), defucosylated total egg glycosphingolipids (Egg HF) and egg glycosphingolipid fraction 6 (Egg 6) and defucosylated egg glycosphingolipid fraction 6 (Egg 6 HF) by ELISA. Antibodies recognizing the fucose-containing glycan epitopes F-LDN and LDN-DF were tested to determine the efficacy of HF-treatment. In parallel, an anti-LDN mAb was employed to evaluate the integrity of the remaining glycans. (D) Reactivity of the monoclonal antibody M2D3H, recognizing F-LDN glycan epitopes, with the schistosomal egg glycosphingolipid fractions 1 to 6, monitored by ELISA.
by ELISA using an anti-LDN monoclonal antibody (Figure 3C), and by MALDI-TOF-MS (Figure 4). Intriguingly, defucosylation of the egg glycosphingolipids resulted in an almost complete loss of L-SIGN binding (Figure 3A). Hence, these studies demonstrate for the first time that L-SIGN binds to authentic carbohydrate structures expressed by S. mansoni egg glycosphingolipids and that this binding is fucose-dependent.
Fig. 4.
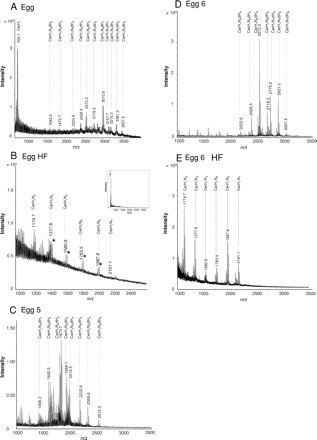
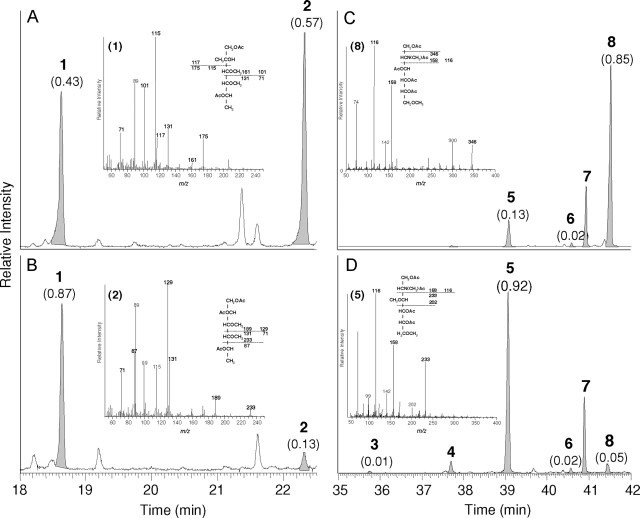
MALDI-TOF-MS analysis of isolated glycosphingolipids from S. mansoni eggs. Total egg glycosphingolipids (A), defucosylated egg glycosphingolipids obtained after HF-treatment (B) and egg glycosphingolipid fractions 5 (C) and 6 (D), as well as defucosylated fraction 6 glycosphingolipids (E) from S. mansoni were analyzed by MALDI-TOF-MS. Relevant mass ranges of the recorded spectra are displayed. Inset in (B): Entire mass spectrum of total egg glycosphingolipids after HF-treatment. Deduced compositions are assigned to major pseudomolecular ions ([M+Na]+). Corresponding potassium ions ([M+K]+) are marked by asterisks. The complex pattern of registered signals is due to carbohydrate and ceramide heterogeneity. H: hexose; N: N-acetylhexosamine; dH: deoxyhexose (fucose); Cer: ceramide.
Binding of L-SIGN to individual S. mansoni egg glycosphingolipid fractions
To further characterize the glycans that mediate binding of L-SIGN to egg glycosphingolipids, we have size-fractionated these glycosphingolipids using silica cartridges. The monoclonal antibody M2DH3 reacted with most of these fractions, indicating the presence of terminal F-LDN epitopes on the glycosphingolipids (Figure 3D). L-SIGN, however, showed a very specific binding pattern in binding fraction 6 only (Figure 3B). Glycosphingolipid recognition could be completely inhibited using AZN-D2 as a blocking antibody, thus indicating L-SIGN specificity. A weak binding was observed in the case of the preceding fraction 5, which may be due to an overlap of related components. Fractions containing larger glycolipid species (Figure 4A) have not been recovered by this fractionation procedure and have, therefore, not been tested. Treatment of the fraction 6 glycosphingolipids with HF resulted in the removal of the fucose residues, as evidenced by ELISA (Figure 3D) and MALDI-TOF-MS (Figure 4). Defucosylation clearly abolished the binding of L-SIGN to these glycosphingolipids (Figure 3B), as already observed for total egg glycosphingolipids. These data indicated that L-SIGN specifically interacts with fraction 6 egg glycosphingolipid species in a fucose-dependent manner.
Characterization of total and fractionated S. mansoni egg glycosphingolipids
To allow a structural characterization of the glycosphingolipid species that are bound by L-SIGN, isolated egg glycosphingolipids were analyzed by MALDI-TOF-MS (Figure 4A). In agreement with previous studies (Kantelhardt et al. 2002; Wuhrer et al. 2002), the major signal observed for total egg glycosphingolipids corresponded to ceramide monohexoside (Hex1Cer), with a mass of m/z 769.1 [M+Na]+. In addition, a complex pattern of glycosphingolipids was registered, mainly due to the high heterogeneity of the respective carbohydrate and ceramide moieties. Prevailing species with masses of m/z 2572.0 [M+Na]+ and m/z 3010.8 [M+Na]+ represented monosaccharide compositions of Hex1HexNAc6dHex4 and Hex1HexNAc6dHex7. As a striking feature, a high number of multiply fucosylated species was observed comprising up to nine dHex units and a mass of m/z 3507.5 [M+Na]+. To remove all fucose residues, egg glycosphingolipids were treated with HF and the resulting products were similarly analyzed by mass spectrometry (Figure 4B). Again, Hex1Cer (m/z 769.1 [M+Na]+) could be observed as the major species (Figure 4B; inset). A more detailed view of the spectrum, however, displayed the additional presence of complex glycosphingolipids with compositions of Hex1HexNAc2−7Cer (Figure 4B). Remaining fucosylated glycosphingolipid species were not observed.
Glycosphingolipid fractions that showed binding to L-SIGN were similarly analyzed by MALDI-TOF-MS. The results obtained for fraction 5 and fraction 6 are shown in Fig- ures 4C and D, respectively. The major compound of fraction 5 comprised a mass of m/z 1873.7 [M+Na]+ representing a Hex1HexNAc4dHex2Cer glycosphingolipid. The highest mass registered at m/z 2572.0 [M+Na]+ corresponded to a glycosphingolipid with a carbohydrate composition of Hex1HexNAc6dHex4, thus reflecting an overall composition of Hex1HexNAc3−6dHex1−4Cer of the glycosphingolipids in this fraction. In contrast, fraction 6 (Figure 4D) comprised more complex glycosphingolipids with higher masses. The main compound was registered at a mass of m/z 2572.0 [M+Na]+ in agreement with a composition of Hex1HexNAc6dHex4Cer, whereas the overall composition of the major signals observed in this fraction was Hex1HexNAc5−7dHex3−6Cer. MALDI-TOF-MS analysis of fraction 6 glycosphingolipids treated with HF revealed a complete lack of fucose and the presence of glycosphingolipids with a composition of Hex1HexNAc2−7Cer (Figure 4E). The higher abundance of compounds with two or three HexNAc residues as compared to the starting material indicated that some degradation of the carbohydrate chains has occurred during chemical defucosylation. But as already evidenced by ELISA (Figure 3D) HF-treated fraction 6 glycosphingolipids still carried intact LDN epitopes, which is in agreement with MALDI-TOF-MS data.
MALDI-TOF-MS (/MS) of S. mansoni egg glycans
To simplify compositional and linkage analyses, egg glycosphingolipid fraction 6 was treated with endoglycoceramidase and the resulting oligosaccharides were analyzed by MALDI-TOF-MS (Figure 5A). As already shown for intact egg glycosphingolipids (Figure 3D) the glycan moieties in fraction 6 are multiply fucosylated (dHex2−5). The major oligosaccharide consisted of Hex1HexNAc6dHex4. Treatment of these glycans with HF yielded defucosylated glycans with the overall compositions of Hex1HexNAc5−7 (see inset in Figure 5A). The major glycan in this fraction 6 with the mass of m/z 2005.6 was further analyzed by MALDI-TOF-MS/MS (LID) (Figure 5B). Obtained data underline the composition of Hex1HexNAc6dHex4. Moreover, the characteristic fragments registered in the MS/MS spectrum clearly demonstrated the presence of HexNAc1dHex2 (m/z 350.0; fragment B2α) and HexNAc2dHex2 unit (m/z 699.0; fragment B3α) as terminal epitopes and simultaneously excluded the occurrence of HexNAc1dHex2 and HexNAc2dHex3 structural elements, thus ruling out the presence of F-LDN-DF moieties in this compound. Hence, these data identify the F-LDN-F unit as major terminal epitope in fraction 6 egg glycosphingolipids.
Fig. 5.
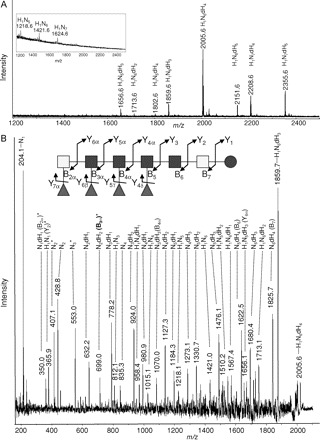
MALDI-TOF-MS and MS/MS of glycans released by endoglycoceramidase from egg glycosphingolipid fraction 6. Oligosaccharides were released from fraction 6 egg glycosphingolipids by treatment with endoglycoceramidase and analyzed by MALDI-TOF-MS (A) and MS/MS (B). (A) MALDI-MS spectrum of glycans liberated from fraction 6 glycosphingolipids. Monoisotopic masses of pseudomolecular ions ([M+Na]+) and deduced monosaccharide compositions are assigned. H: hexose; N: N-acetylhexosamine; dH: deoxyhexose (Fucose). Inset in (A): MALDI-TOF-MS of oligosaccharides released from fraction 6 egg glycosphingolipids after subsequent incubation with HF to release fucose residues. (B) Sodiated pseudomolecular ions of the glycan species m/z 2005.6 [M+Na]+ with a composition of Hex1HexNAc6dHex4, obtained by endoglycoceramidase treatment of fraction 6 egg glycosphingolipids, were analyzed by MALDI-TOF-MS/MS in the LID mode. Composition is given in symbols according to the Consortium for Functional Glycomics (see Table I for details); light grey square: N-acetylgalactosamine; dark square: N-acetylglucosamine; circle: glucose; triangle: deoxyhexose (fucose). Fragments in the structure are assigned using the nomenclature of Domon and Costello (Domon and Costello 1988). For the sake of clarity only the composition and not the putative origin of each fragment is assigned in the spectrum. H: hexose; N: N-acetylhexose; dH: deoxyhexose. Protonated ions are marked with an asterisk. Signals resulting from single cleavages are specified. Fragments verifying the presence of a terminal HexNAc2dHex2 unit are presented in bold-type.
Linkage analyses of S. mansoni egg glycans
To further corroborate the presence of the F-LDN-F epitope, linkage analysis of the glycan moieties, obtained from the total egg glycosphingolipids and glycosphingolipid fraction 6 was performed (Figure 6 A and 6B). This analysis revealed the presence of two different fucose derivatives (terminal fucose and 2-substituted fucose). The identity of these residues was confirmed by the electron impact mass spectrometry (see insets in Figure 6A and 6B). The linkage data strongly support our hypothesis that the glycosphingolipids in fraction 6 are multiply fucosylated, as already demonstrated by MALDI-TOF-MS. The amount of disubstituted fucose, however, has clearly decreased in fraction 6, compared to the total egg glycosphingolipids. Whereas the ratio of terminal fucose: 2-substituted fucose amounted to 0.43:0.57, in the case of total glycosphingolipid-derived glycans, it was found to be 0.87:0.13 in fraction 6. These data suggest that the terminal α1-3-fucose moieties in fraction 6 are of major importance for recognition by L-SIGN.
Fig. 6.
Linkage analyses of glycans released by endoglycoceramidase from total egg glycosphingolipids and egg glycosphingolipid fraction 6. (A–D), linkage analyses of oligosaccharides released from both total and fraction 6 glycosphingolipids before and after HF-treatment. Partially methylated alditol acetates obtained were separated by gas chromatography and registered in the positive ion mode after electron impact ionization. To facilitate understanding, only parts of the entire chromatograms are shown. (A and B), detection of fucose derivatives obtained from total (A) and fraction 6 (B) glycans. Peak areas normalized to the sum of terminal fucose (1) and 2-substituted fucose (2) set to 1.0 are shown in parentheses. Inset in (A): Electron impact mass spectrum of 1,5-di-O-acetyl-2,3,4-tri- O-methyl-fucitol (terminal fucose); Inset in (B): 1,2,5-tri-O-acetyl-3,4-di-O-methyl-fucitol (2-substituted fucose). Characteristic primary and some secondary fragment ions are assigned. (C and D), detection of partially methylated HexNAc-derivatives obtained from fraction 6 glycans before (C) and after HF-treatment (D). The ratios of terminal GlcNAc (3), 4-substituted GlcNAc (5), 3-substituted GlcNAc (6) and 3,4-disubstituted GlcNAc (8) set to 1.0 are shown in parentheses to underline the clear loss of 3,4-disubstituted GlcNAc after incubation of fraction 6 glycans with HF. Inset in (C): Mass spectrum of 2-deoxy-2-(N-methyl) acetamido-1,3,4,5,-tetra-O-acetyl-6-O-methylglucitol. Inset in (D): 2-deoxy-2-(N-methyl)acetamido-1,4,5,-tri-O-acetyl-3,6-di-O-methylglucitol. (1) terminal fucose; (2) 2-substituted fucose; (3) terminal GlcNAc; (4) terminal GalNAc; (5) 4-substituted GlcNAc; (6) 3-substituted GlcNAc; (7) 3-substituted GalNAc; (8) 3,4-disubstituted GlcNAc.
Linkage analyses of fraction 6 glycans (Figure 6C) further revealed five differently substituted HexNAc residues (terminal GalNAc; 4-substituted GlcNAc; 3-substituted GlcNAc; 3-substituted GalNAc and 3,4-disubstituted GlcNAc). The ratio of mono-substituted GlcNAc residues to 3,4-disubstituted GlcNAc clearly differed before and after HF-treatment. HF-treated fraction 6 glycan moieties which are not recognized by L-SIGN comprised only trace amounts of 3,4-disubstituted GlcNAc, whereas this type of branched monosaccharide unit represented a major constituent of untreated fraction 6 carbohydrate chains. Hence, linkage data clearly revealed a high branching of fraction 6 glycan species due to multiple fucosylation in agreement with MALDI-TOF-MS. On the basis of MALDI-TOF-MS and linkage data we propose a summarized structure of fraction 6 egg glycosphingolipids, which is shown in Figure 8A.
Fig. 8.
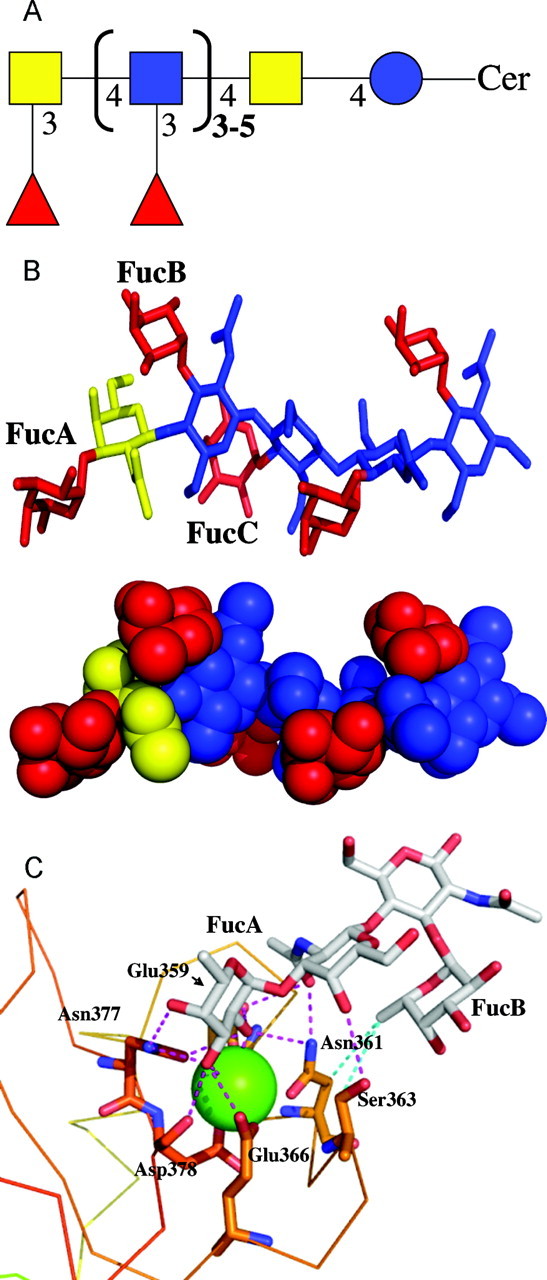
Modeling of FDLNF and L-SIGN. (A) The carbohydrate epitope F-LDN-F, recognized by L-SIGN and present in schistosomal egg glycosphingolipids, is given as a schematic structure, to summarize the data obtained by ELISA, MALDI-TOF-MS and linkage analyses. The nomenclature as used by the EuroCarbDB is utilized (see Table I for details). blue circle: Glucose; yellow square: N-acetylgalactosamine; blue square: N-acetylglucosamine; red triangle: fucose. (B) Stick and space-fill representation of the lowest energy conformation of the terminal decasaccharide of the highly fucosylated glycosphingolipid. Fucose residues are colored in red, GalNAc in yellow and GlcNAc in blue. (C) Docking mode of the terminal tetrasaccharide of the highly fucosylated glycosphingolipid in the binding site of L-SIGN. Protein is represented by a line, oligosaccharide and amino acids of interest by stick and calcium ion by green sphere. Carbohydrate atoms are marked in green [C], red [O] and blue [N]. Hydrogen bonds are represented by magenta dot lines, and hydrophobic contacts by blue ones. Drawing has been performed with Pymol software (DeLano Scientific LCC, South San Francisco, CA) and hydrogen atoms have been omitted for clarity.
Binding of the L-SIGN mutant Ser363Val to egg glycosphingolipids and SEA
To characterize in more detail the binding properties of the carbohydrate recognition domain from L-SIGN involved in the interaction with S. mansoni SEA and egg glycosphingolipids, we tested the binding capacity of two L-SIGN mutants (Figure 7), in which Ser363 has been replaced by Gly (S363G), or Val (S363V), the latter of which is present in DC-SIGN at the equivalent position. The S363G mutant recognized neither SEA nor egg glycosphingolipids, indicating the importance of Ser at this position for both types of recognition. Remarkably, L-SIGN S363V did not interact with the multiply fucosylated egg glycosphingolipid fraction 6 species. As described previously, the single amino acid replacement of Ser by Val (S363V) allows L-SIGN to recognize LeX (van Liempt et al. 2004). In agreement with these previous findings, we have demonstrated in this study that the L-SIGN mutant S363V recognizes SEA (Figure 7). In contrast to wild-type L-SIGN, L-SIGN S363V also showed a clear binding to endo H-treated SEA and a strongly reduced recognition of the corresponding defucosylated antigens. These data most likely reflect the gained capacity of L-SIGN S363V to bind to LeX -epitope of SEA.
Fig. 7.
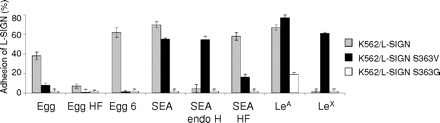
Binding characteristics of wild-type L-SIGN and L-SIGN mutants. The binding of K562 cells stably expressing wild-type L-SIGN, as well as K562 cells stably expressing the L-SIGN mutants S363V and S363G, to S. mansoni egg glycosphingolipids and SEA is shown using cell adhesion assays. Total egg glycosphingolipids (Egg), defucosylated egg glycosphingolipids (Egg HF) and fraction 6 egg glycosphingolipids (Egg 6) as well as SEA, endo H-treated SEA (SEA endo H) and defucosylated SEA (SEA HF) were coated. LeA-PAA (LeA) and LeX-PAA (LeX) were used as controls. The experiment shown is a representative of three independent experiments, all performed in triplicate, with error bars indicating standard deviation.
Conformational analyses of the major fucosylated oligosaccharide from egg glycosphingolipid fraction 6
The initial conformation of the decasaccharide bearing F-LDN-F at its nonreducing end, i.e., Fucα1-3GalNAcβ1-4[(Fucα1-3)GlcNAcβ1-4]4 (Figure 8A), was built using its structural similarity with LeX. For this trisaccharide, NMR and conformational studies (Lemieux et al. 1980; Imberty et al. 1999) demonstrated that due to the presence of adjacent branching, the fucose and galactose rings stack one on each other, resulting in a rigid conformation for both the Galβ1-4GlcNAc and Fucα1-3GlcNAc glycosidic linkages, and indeed this corresponds to the conformation observed in the crystal structure of the L-SIGN/LeX complex (Guo et al. 2004). The situation is similar in the decasaccharide and all Fucα1-3GlcNAc glycosidic linkages can only adopt the conformation that brings the branched fucose above the next GlcNAc (or GalNAc) of the main chain (Figure 8B). Only the fucose located at the nonreducing end, i.e., the one at position 3 of GalNAc appears to be more flexible and conformational analysis indicates that the Ψ torsion angle can vary up to 60°. The conformation displayed in Figure 8B is the lowest energy one.
Docking of glycosphingolipid oligosaccharides in the binding site of L-SIGN
A first docking approach was undertaken by fitting fucose residues of fucosylated oligosaccharides in the binding site of L-SIGN with the orientation observed in the crystal structure of the lectin complexed with LeX (Guo et al. 2004), which validated the binding mode that we proposed previously by modeling (van Liempt et al. 2004). Three different orientations of the decasaccharide were tested, with either fucose A (α1-3 linked to GalNAc), B or C (α1-3 linked to external or internal GlcNAc), respectively. In all cases, the binding appears possible, with no major steric conflict, but not very favorable, due to the close position of Ser363 to the methyl-group of the N-acetyl residue of the adjacent GalNAc or GlcNAc. Only the fucose in the primary binding site establishes hydrogen bonds with the protein. It is therefore proposed that when possible, this binding mode will not result in sufficient affinity to be detected experimentally (by analogy to the nondetection of LeX binding by L-SIGN).
Another orientation of fucose can be proposed, since it has been observed that the fucoses in several sialyl- and sulfo-LeX derivatives are bound to mannose-binding protein (MBP) with O-2 and O-3 of fucose involved in calcium coordination (Ng et al. 1996; Ng and Weis 1997; Ng et al. 2002), and not O-3 and O-4 as in the first binding mode described above. Again three different fucose residues of the oligosaccharides were docked in this orientation. Docking of fucose A resulted in a stable binding mode. Geometric optimization of amino acid side chains and ligand yielded to the complex displayed in Figure 8C. In addition to the contact between the fucose, the calcium ion and the protein hydroxyl group in the main binding site, the GalNAc residue directly hydrogen bonds to Ser363 by its O-4 hydroxyl group and to Asn361 and Glu359 by the N-acetyl carbonyl. The adjacent GlcNAc does not interact directly with the protein but the fucose residue that it carries (Fucose B) establishes hydrophobic contact with the same region (CH2 of carbon Cβ of Asn361 and Ser363). The remaining part of the decasaccharide (reducing end) does not interact with the protein and, therefore, has not been displayed in Figure 8C. Docking of the other fucose residues (i.e., the ones linked to GlcNAc) is also possible but, in the absence of an axial group at O-4 in the adjacent residue, the strong stabilisation that involves Ser363 does not occur.
Discussion
The human C-type lectin L-SIGN (liver/lymph node specific ICAM-3-grabbing nonintegrin) is expressed on liver sinusoidal endothelial cells (LSECs), which have a function as antigen-presenting cells in the liver (Bashirova et al. 2001). Since the liver is one of the main organs that is heavily affected during schistosomiasis as a result of the granuloma formation around trapped parasitic eggs (Bashirova et al. 2001; Wynn et al. 2004), L-SIGN is in the perfect position to function as an adhesion and internalization receptor for schistosome egg antigens. We therefore hypothesized that L-SIGN might be involved in the immunobiology and/or liver pathology of schistosomiasis, which implicates that L-SIGN should be able to recognize schistosomal antigens. Here we show that L-SIGN indeed recognizes egg antigens of the human helminth parasite S. mansoni, on glycoproteins such as soluble egg antigens (SEA), and egg-derived glycosphingolipids. Remarkably, L-SIGN recognizes completely different glycan entities on egg glycoproteins and glycosphingolipids.
Our data demonstrate that within egg glycoproteins, L-SIGN recognizes primarily oligomannosidic N-glycans and shows little interaction with fucose residues. In addition to complex-type N-glycans, SEA contains hybride-type N-glycans and high-mannose type glycans, but data regarding the precise structures of the glycoprotein-glycans expressed in the egg stage or on SEA are still incomplete (Wuhrer and Geyer 2006).
Here we demonstrate for the first time that L-SIGN is also able to interact with pathogens via recognition of fucosylated glycans. Whereas L-SIGN did not bind to glycosphingolipids from cercarial or adult schistosomal stages, it specifically recognized a multiply fucosylated fraction within egg glycosphingolipids in a fucose dependent manner. This leads to the surprising conclusion that L-SIGN displays a completely different binding pattern to schistosome glycan antigens than DC-SIGN, both in its binding to SEA, as well as in its binding profile to schistosomal glycosphingolipids. It would be of interest to further characterize the relative binding affinities of L-SIGN to high-mannose type glycans and glycan epitopes on egg glycosphingolipids, but such experiments could not be performed so far due to limited amounts of parasite material. DC-SIGN, which contains a CRD domain that is highly similar to the one of L-SIGN, hardly interacts with the total egg glycosphingolipids (Meyer et al. 2005), or with the specific egg glycosphingolipid fraction that is bound by L-SIGN (data not shown). By contrast, DC-SIGN strongly binds to fucosylated cercarial and adult glycosphingolipids (Meyer et al. 2005), which are hardly recognized by L-SIGN. This differential binding mode of L-SIGN and DC-SIGN to fucosylated glycans is also reflected by the results of cell adhesion assays using a L-SIGN S363V mutant, which contains a Val present at a similar position as in DC-SIGN (van Liempt et al. 2004). In contrast to the wild-type L-SIGN, this mutant is able to recognize the fucosylated LeX carbohydrate epitope in analogy to DC-SIGN. Intriguingly, this mutant is not able to recognize the multiply fucosylated glycan moieties of fraction 6 egg glycosphingolipids. Hence it may be suggested that L-SIGN can recognize fucose-containing Lewis antigens, such as LeA and LeB, in a binding mode essentially similar to DC-SIGN, but recognizes multiply-fucosylated glycosphingolipid glycans via an alternative binding-mode. It is remarkable that L-SIGN is able to interact with both oligomannosidic glycans, and differently fucosylated oligosaccharide ligands although comprising only one CRD. It has been reported, however, that the binding pocket of a lectin may change depending on the structural features of the glycan bound (Mitchell et al. 2001; Meyer et al. 2005; Karrar et al. 2007).
Structural data obtained by MALDI-TOF-MS and linkage analysis revealed that S. mansoni egg glycosphingolipids consist of a backbone of N-acetylhexosamine residues which may be heavily decorated with fucosyl- and oligofucosyl side chains (Khoo et al. 1997; Wuhrer et al. 2002). Our present analysis provided evidence that the minimum requirement for recognition of egg glycosphingolipids by L-SIGN is the presence of terminal Fucα1-3GalNAcβ1-4(Fucα1-3)GlcNAcβ (F-LDN-F) tetrasaccharide. To increase our insight in the structural parameters that determine the recognition of multiply fucosylated egg glycans by L-SIGN, we performed molecular modeling studies in which the docking of different fucosylated entities of the characterized egg glycosphingolipid glycan into the CRD of L-SIGN was investigated. From the present modeling study, it is not possible to state that L-SIGN binds to F-LDN-F or other fucosylated oligosaccharides in only one single way. Different binding modes are possible, involving either fucose on the chito-oligosacccharide backbone, or the one present at the nonreducing end. Nevertheless, based on the number of hydrogen bonds and hydrophobic contacts, one binding mode appears to be strongly preferred. This binding mode involves the terminal Fucα1-3GalNAc unit, and has the particularity that O-2 and O-3 hydroxyl groups of the fucose are coordinated by the calcium ion present in the binding site, instead of O-3 and O-4 as observed in L-SIGN/LeX crystal structure (Guo et al. 2004).
The binding of the fucose residue linked to position 3 of GalNAc is not only favored in terms of energy, but is also validated by the experimental data since it allows for rationalizing some observations. The mutant Ser363Val, which strongly binds Lewis X, does not bind to the multiply fucosylated glycosphingolipid, which is in agreement with Ser363 being involved in a crucial hydrogen bond with O-4 of GalNAc. In the same manner, this O-4 hydroxyl group has to be in an axial configuration for optimal binding (i.e., Gal configuration over Glc), which would explain the strong observed preference for Fucα1-3GalNAc over Fucα1-3GlcNAc. The N-acetyl group of this GalNAc also plays a crucial role since its carbonyl atom is involved in two hydrogen bonds with protein side chains (Glu359 and Asn361). This prediction is in agreement with the absence of binding of pseudo-LeY (Fucα1-3Galβ1-4(Fucα1-3)GlcNAc-) that lacks this specific N-acetyl group (Wuhrer et al. 2000).
The finding that L-SIGN hardly interacts with multiply fucosylated glycoproteins within SEA is surprising. Many of the fucosylated epitopes in egg glycolipids are also found in SEA glycoproteins (Robijn et al. 2005). We have shown that the SEA glycoprotein preparation that we used in this study reacts with monoclonal antibodies that recognize Lewis X, LDN-F, LDN-DF, and F-LDN epitopes (Figure 1A, and results not shown). We could show that neoglycocojugates carrying Lewis X, LDN-F, and LDN-DF glycan antigens are not recognized by L-SIGN (van Liempt et al. 2004) and this study). Since the anti-F-LDN monoclonal antibody M2DH3 strongly recognizes SEA, cercarial glycolipids, and fraction 3 to fraction 6 egg glycolipid (Figure 3D; results not shown; (Kantelhardt et al. 2002)), it is unlikely that L-SIGN displays more than a weak interaction with F-LDN. In addition, our modeling data show that the GlcNAc of the F-LDN-F moiety does not interact directly with the protein. The α1-3fucose residue that it carries (Fucose B, see Figure 8C), however, establishes hydrophobic contact with the CH2 of carbon Cβ of Asn361 and Ser363, thus contributing to the binding. Interestingly, from this model it can be deduced that an additional fucose α1-2-linked to fucose B, resulting in the epitope F-LDN-DF, would not sterically interfere with the binding of L-SIGN to the F-LDN-F unit. Thus, although we do not have experimental data to support this type of binding, we cannnot exclude the possibility that the glycan F-LDN-DF can be bound by L-SIGN. The minor binding that we observed for L-SIGN to fucosylated species within SEA may be due to the presence of small amounts of F-LDN-(D)F epitopes within SEA, or to the binding to unknown fucosylated species.
Several studies have demonstrated that glycosylation of schistosome antigens plays an important role in immunological processes during schistosome infection (Faveeuw et al. 2002, 2003), such as the induction of hepatic granuloma formation by SEA or schistosome eggs that leads to severe fibrosis, hepatosplenomegaly and portal hypertension usually accompanying schistosomiasis (Okano et al. 1999; Pearce and MacDonald 2002; Sneller 2002). Schistosomal fucosylated glycoproteins and glycosphingolipids can be highly antigenic and act as potent immunomodulators (Velupillai et al. 2000; Okano et al. 2001; Van der Kleij et al. 2002) during parasite infection, indicating a major role of schistosomal egg carbohydrates in the initiation and homeostasis of the inflammatory response (Dyatlovitskaya and Bergelson 1987; Ziegler-Heitbrock et al. 1992; Lochnit et al. 1998; Eberl et al. 2001; Van der Kleij et al. 2002; van Die and Cummings 2006).
Although the involvement of glycans in immunomodulation is clearly established in schistosome infection, not much is known about the receptors involved in these processes. The present study is the first description of a lectin binding the stage-specific multiply fucosylated glycosphingolipids from schistosome eggs. In addition, our data demonstrate that L-SIGN has the potential to rapidly internalize egg glycoproteins. This may indicate that L-SIGN may capture and present antigens that are secreted by the eggs during schistosome infection, which in turn, may lead to the stimulation of T-cells. It is attractive to speculate that the uptake and the presentation of schistosome egg antigens by L-SIGN contributes to the high levels of glycan-specific antibodies found after egg-laying in schistosome infection.
Alternatively, or in addition to a proposed function in the presentation of schistosome egg antigens to T cells, L-SIGN may play a role in the actual trapping of schistosome eggs in the liver. LSECs, on which L-SIGN is constitutively expressed, are in permanent contact with the blood stream in the liver. Eggs, released from schistosome couples located in the liver portal veins, circulate through the bloodstream and L-SIGN is in the ideal position to interact with passing eggs. Whereas such eggs are obviously much too large to be internalized, they could be trapped by L-SIGN.
It will be important to establish whether the differential interaction of L-SIGN with either oligomannose-type N-glycans, and/or multiply fucosylated egg glycosphingolipids, controls the functional activity of L-SIGN in schistosome infections. Understanding of the molecular mechanisms by which the interaction between human lectins and parasitic glycans modulate the host immune response, and contribute to the severe pathology that is observed in schistosomiasis, may open novel ways to develop improved treatment of this infection.
Materials and methods
Cell lines, antibodies and neoglycoconjugates
Human K562 cells stably expressing L-SIGN (K562/L-SIGN) have been described by Bashirova et al. (Bashirova et al. 2001) and K562 cells stably expressing the L-SIGN mutant S363V, in which Ser363 has been replaced by Val, by van Liempt et al. (van Liempt et al. 2004). The following antibodies were used: AZN-D2 (anti-DC-SIGN/anti-L-SIGN) (Bashirova et al. 2001), anti-Lewis X (G8G12) (Bickle and Andrews 1988), anti-LDN-DF (mAb 114-5B1-A) (van Remoortere et al. 2000), anti FLDN (mAb M2D3H) (Bickle and Andrews 1988), anti-LDN-F (SmLDNF1) (Nyame et al. 2000) and anti-LDN (mAb SMLDN1.1) (Nyame et al. 1999). Neoglycoconjugates containing LeX or LeA multivalently coupled to biotinylated polyacrylamide (PAA) were from Lectinity (Lappeenranta, Finland). Neoglycolipids containing the LNFP III-epitope were synthesized as described before (Meyer et al. 2005). Neoglycoproteins consisting of BSA carrying LDN-DF and LDN-F antigens, respectively, were synthesized as described previously (van Remoortere et al. 2000).
Preparation of schistosome SEA and glycosphingolipid fractions
Crude S. mansoni SEA extract was centrifuged at 100,000 × g for 90 min at 4°C and sterilized by passing through a 0.2 μm filter (Nyame et al. 2003). Purified glycosphingolipids were isolated from lyophilized S. mansoni adult worms, eggs and cercariae by organic solvent extraction, saponification, desalting and anion-exchange chromatography as described previously (Wuhrer et al. 2000).
Neutral glycosphingolipids were fractionated chromatographically. Samples were dissolved in chloroform and applied after sonication on a 1 mL or 5 mL, depending on the amount of sample, silica cartridge (Waters, Eschborn, Germany), equilibrated with chloroform. Subsequent elution was achieved with chloroform:methanol (CM) and chloroform:methanol:water (CMW). Eluents used were CM 90:10 (v/v); CM 60:40 (v/v); CM 50:50 (v/v); CM 40:60 (v/v), CMW 65:25:4 (v/v/v) and CMW 10:70:20 (v/v/v). Resulting fractions (named 1–6) were analyzed by MALDI-TOF-MS and their carbohydrate content was quantified by compositional analyses using derivatization with anthranilic acid (Anumula 1994).
Matrix-assisted laser-desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF-MS and MS/MS)
MALDI-TOF-MS and MS/MS analysis was performed on an Ultraflex instrument (Bruker-Daltonik, Bremen, Germany) equipped with a nitrogen laser and a LIFT-MS/MS facility as described previously (Geyer et al. 2005; Lehr et al. 2007). The instrument was operated in the positive-ion reflector mode using 6-aza-2-thiothymine (Sigma-Aldrich, München, Germany) as matrix throughout. About 100 to 500 individual spectra were summarized in each case.
Release and linkage analysis of schistosomal glycans
Carbohydrate moieties were liberated from egg glycosphingolipids by treatment with recombinant endoglycoceramidase II (from Rhodococcus spp., Takara Shuzu Co., Otsu, Shiga, Japan). Released glycans were separated from ceramide residues by reverse-phase (RP-) chromatography as described previously (Wuhrer et al. 2000). For linkage analysis oligosaccharides were permethylated with methyl iodide after deprotonation with lithium methylsulfinyl carbanion and hydrolyzed (4 M aqueous trifluoroacetic acid, 100°C, 4 h). Partially methylated alditol acetates obtained after sodium borohydride reduction and peracetylation were analyzed by capillary gas–liquid chromatography followed by electron impact ionization mode (single ion monitoring), using a PTV injector, fused-silica bonded-phase capillary columns of different polarity (60 m VF5MS and 30 m VF200MS; Varian Inc., CA) and helium as carrier gas as described elsewhere (Geyer and Geyer 1994).
Deglycosylation of schistosome egg glycoconjugates
To remove fucose residues from the egg glycoconjugates, dried samples of SEA or egg glycosphingolipids were treated with 48% (v/v) fluoric acid (HF) (Merck, Darmstadt, Germany) at 4°C for 48 h. HF was removed by a stream of nitrogen and the resulting pellet washed twice with methanol as described previously (Wuhrer et al. 2002). The degree of defucosylation and the integrity of the remaining glycan antigens, were assessed by MALDI-TOF-MS analysis and ELISA using monoclonal antibodies recognizing specific glycan epitopes.
To remove oligomannosidic N-glycans, SEA was treated with endoglycosidase H (endo H from Streptomyces plicatus, recombinant, E. coli; Calbiochem, Merck Darmstadt, Germany), which cleaves the chitobiose units of asparagine-linked oligomannose and hybrid, but not complex-type oligosaccharides of glycoproteins, as described by the manufacturer. In brief, SEA (100 μg) was denaturated by 5 min heating at 100°C in denaturating solvent (1% SDS, 2M β-mercaptoethanol). After cooling down, the enzyme (10 milliunits) was added and incubated at 37°C for 3 h.
Enzyme-linked immunosorbent assay (ELISA) with antiglycan antibodies
Solutions of total egg or HF-treated glycosphingolipids were diluted with ethanol, applied (6 or 9 ng/well) to NUNC maxisorb plates (Roskilde, Denmark) and incubated for 60 min at 37°C to coat the glycosphingolipids to the plate. SEA was diluted to 5 μg/mL in coating buffer (50 mM NaHCO3) and coated 1 h at 37°C or at 4°C overnight. Plates were blocked with 1% ELISA grade BSA (Fraction V, fatty acid free; CalBiochem, San Diego, CA) in phosphate-buffered saline (PBS; 0.14 M NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.1 mM Na2HPO4) and incubated with anti-glycan antibodies recognizing LeX, LDN or LDN-DF (see Table I). Binding was registered using a horseradish peroxidase-labeled goat-anti-mouse antibody (Dako, Glostrup, Denmark). After coloring (4.5 mL 0.2 M Na2HPO4, 4.5 mL 0.1 M Citric acid, 1 mL TMB-solution (3,3′,5,5′-tetramethylbenzidine; 1 mg/mL H2O), 10 μL H2O2) the optical density was measured at a wavelength of 450 nm using an ELISA reader (BioRad, Hercules, CA).
Enzyme-linked immunosorbent assay (ELISA) with DC-SIGN-Fc
Total cercarial and egg glycosphingolipids, as well as separated egg glycosphingolipid fractions 5 and 6 (9 ng) were diluted in ethanol on NUNC maxisorb plates (Roskilde, Denmark), and incubated for 60 min at 37°C to coat the glycosphingolipids to the plate. Plates were blocked with 1% ELISA grade BSA (Fraction V, Fatty acid free; Calbiochem, San Diego, CA) in TSM (20 mM Tris–HCl pH 7.4, 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2) and incubated with DC-SIGN-Fc (3 μg/mL) (Geijtenbeek et al. 2002). Binding was detected using a peroxidase labeled goat-anti-human IgG-Fc (Jackson, West Grove, PA). EDTA (10 mM, Roth, Karlsruhe, Germany) was added when indicated to investigate whether the binding was calcium dependent.
Cell adhesion assays with L-SIGN transfected cells
Ninety-six-well plates (NUNC maxisorb) were coated overnight at 4°C with S. mansoni SEA or neoglycoconjugates (5 μg/mL in 50 mM NaHCO3) or schistosomal glycosphingolipids (6 or 9 ng/well in ethanol, dried at 37°C). Blocking (30 min at 37°C) was performed with 1% BSA in TSM. Cells labeled with Calceine AM (25 μL/7 × 106 cells; Molecular Probes, Eugene, OR), were added for 1.5 h at 37 °C in the presence or absence of mAb AZN-D2 (20 μg/mL). Nonadherent cells were removed by gently washing with TSM. Adherent cells were lysed with 50 mM Tris–HCl, pH 7.4, 0.1% SDS and fluorescence was quantified using a Fluostar spectrofluorimeter (BMG Labtech, Offenburg, Germany) at 485/520 nm. Results are expressed as the mean percentage of adhesion of triplicate wells. All experiments are performed at least three times.
Internalization of SEA in L-SIGN transfected cells
Internalization of SEA was assessed as described previously (van Liempt et al. 2007). Shortly, K562/L-SIGN transfected cells (0.5 × 106 per sample) were incubated with biotinylated-SEA, or endo H-treated or HF-treated biotinylated-SEA (10 μg/mL) in TSA (TSM with 1% BSA) for 1 h on ice. Unbound ligand was washed off twice with ice-cold TSA. Specificity of the binding was established using nontransfected K562 cells, or by inhibition with the mAb AZN-D2 that recognizes L-SIGN. To this end K562/L-SIGN transfected cells were preincubated with the mAb for 30 min at 37°C. To control the off-rate of SEA at 37°C, cells were fixed with paraformaldehyde (2% in PBS) for 20 min at room temperature, prior to SEA binding. All cells were then incubated at 37°C with slight horizontal shaking (500 rpm) to enable internalization. At different time points (15–60 min) aliquots were taken and stored on ice. After washing with TSA, cells were incubated with Alexa 488-labeled avidin (Molecular Probes, Eugene, OR) for 30 min at 4°C, washed and analyzed by flow cytometry on a BD FACS Calibur or BD FACSScan (Beckton Dickinson, San Jose, CA). The relative differences in mean fluorescence intensity were determined in relation to the fluorescence observed in the case of fixed cells.
Molecular modeling
The coordinates of the crystal structure of human L-SIGN interacting with LeX containing trisaccharide (Guo et al. 2004) (code 1SL6) were taken from the Protein Data Bank. The structure was edited using the Sybyl software (Tripos Inc., St Louis, MO), in order to contain only one protein monomer together with calcium ions and the LeX trisaccharide. Protein hydrogen atoms were added, the peptide atoms partial charges were calculated using the Pullman procedure and the calcium ions were given a charge of two.
The tetrasaccharide Fucα1-3GalNAcβ1-4(Fucα1-3) GlcNAcβ was built by graphically editing the galactose residue of LeX extracted from the above mentioned crystal structure into a GalNAc residue and adding a fucose residue on its 3 position. A decasaccharide with three additional Fucα1-3GlcNAcβ units on the reducing end was also built. Atom types and charges for oligosaccharides were defined using the PIM parameters developed for carbohydrates. A conformational search with the TRIPOS force-field allowed for defining the preferred conformations of the oligosaccharides.
Docking studies were performed by homology with L-SIGN/LeX complex or with other C-type lectin interacting with fucose (see results). In all cases, one of the fucose residues of the oligosaccharide of interest was superimposed on the fucose in the crystal structure and the structures were merged. When needed, subsequent energy minimizations were performed using the Tripos force-field (Clark et al. 1989) with geometric optimization of the sugar and the side chains of amino acids in the binding sites. A distance-dependent dielectric constant was used in the calculations. Energy minimizations were carried out using the Powell procedure until a gradient deviation of 0.05 kcal/mol/Å was attained.
Funding
Deutsche Forschungsgemeinschaft (SFB 535, A15 to S.M. and R.G.); Technology Foundation STW (VDG 6502) of the Netherlands Organization for Scientific Research (NWO) to B.T. and I.v.D.
Acknowledgments
We gratefully acknowledge Dr. Yvette van Kooyk and Dr. Theo Geijtenbeek (VUmc, Amsterdam, The Netherlands) for supplying us with K562/L-SIGN expressing cells and AZN-D2 antibody, and Sandra van Vliet, Caroline van Stijn, Marloes van den Broek and Lynn Meurs (VUmc, Amsterdam, The Netherlands) for helpful advice and technical assistance in part of the experiments. We thank Peter Kaese (Institute of Biochemistry, Justus-Liebig-University Giessen, Germany) for performing linkage analyses. We thank Dr. Michael Doenhoff (School of Biological Science, University of Wales, Bangor, UK) and Dr. Fred Lewis (Biomedical Research Institute, Rockville, MD, USA) for S. mansoni stages and SEA and Dr. Richard Cummings (Department of Biochemistry, Emory University School of Medicine, Atlanta, GA, USA) and Dr. Quentin Bickle (London School of Hygiene and Tropical Medicine, London, UK) for providing us with anti-glycan antibodies.
Glossary
Abbreviations
- CD
cluster of differentiation
- CRD
carbohydrate recognition domain
- DC
dendritic cell
- DC-SIGN
dendritic cell-specific intercellular adhesion molecule-3 (ICAM-3)-grabbing nonintegrindHex, deoxyhexose
- Egg 6
silica gel fraction 6 of S. mansoni egg glycolipids
- Egg 6 HF
HF-treated silica gel fraction 6 of S. mansoni egg glycolipids
- EI
electron impact
- FA
Forssman Antigen
- FLDN-F (Fucα1-3)
GalNAcβ1-4(Fucα1-3)GlcNAc
- Gb3
globotriaosylceramide
- HCV
Hepatitis C Virus
- Hex
hexose
- HexNAc
N-acetylhexosamine
- ICAM
intercellular adhesion molecule
- LDN
GalNAcβ1-4GlcNAc
- LDN-DF
GalNAcβ1-4(Fucα1-2Fucα1-3)GlcNAc
- LDN-F
GalNAcβ1-4(Fucα1-3)GlcNAc
- LID
laser-induced dissociation
- LSECs
liver sinusoidal cells
- L-SIGN
liver/lymph node-specific ICAM-3-grabbing nonintegrin
- mAb
monoclonal antibody
- MALDI-TOF-MS
matrix-assisted laser-desorption/ ionization-time-of-flight mass spectrometry
- PAA
polyacrylamide
- SARS
severe acute respiratory syndrome
- SEA
soluble egg antigens
Conflict of interest statement
None declared.
References
- Adams DH, Hubscher SG, Shaw J, Rothlein R, Neuberger JM. Intercellular adhesion molecule 1 on liver allografts during rejection. Lancet. 1989;2:1122–1125. doi: 10.1016/s0140-6736(89)91489-x. [DOI] [PubMed] [Google Scholar]
- Alvarez CP, Lasala F, Carrillo J, Muniz O, Corbi AL, Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol. 2002;76:6841–6844. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumula KR. Quantitative determination of monosaccharides in glycoproteins by high-performance liquid chromatography with highly sensitive fluorescence detection. Anal Biochem. 1994;220:275–283. doi: 10.1006/abio.1994.1338. [DOI] [PubMed] [Google Scholar]
- Bashirova AA, Geijtenbeek TB, van Duijnhoven GC, van Vliet SJ, Eilering JB, Martin MP, Wu L, Martin TD, Viebig N, Knolle PA, KewalRamani VN, van Kooyk Y, Carrington M. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J Exp Med. 2001;193:671–678. doi: 10.1084/jem.193.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickle QD, Andrews BJ. Characterisation of Schistosoma mansoni monoclonal antibodies which block in-vitro killing: Failure to demonstrate blockage of immunity in vivo. Parasite Immunol. 1988;10:151–168. doi: 10.1111/j.1365-3024.1988.tb00211.x. [DOI] [PubMed] [Google Scholar]
- Cambi A, Gijzen K, de Vries JM, Torensma R, Joosten B, Adema GJ, Netea MG, Kullberg BJ, Romani L, Figdor CG. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol. 2003;33:532–538. doi: 10.1002/immu.200310029. [DOI] [PubMed] [Google Scholar]
- Clark M, Cramer RDI, Van Den Opdenbosch N. Validation of the general purpose Tripos 5.2 force field. J Comput Chem. 1989;10:982–1012. [Google Scholar]
- Colmenares M, Puig-Kroger A, Pello OM, Corbi AL, Rivas L. Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, CD209), a C-type surface lectin in human DCs, is a receptor for Leishmania amastigotes. J Biol Chem. 2002;277:36766–36769. doi: 10.1074/jbc.M205270200. [DOI] [PubMed] [Google Scholar]
- Cummings RD, Nyame AK. Glycobiology of schistosomiasis. FASEB J. 1996;10:838–848. doi: 10.1096/fasebj.10.8.8666160. [DOI] [PubMed] [Google Scholar]
- Cummings RD, Nyame AK. Schistosome glycoconjugates. Biochim Biophys Acta. 1999;1455:363–374. doi: 10.1016/s0925-4439(99)00063-0. [DOI] [PubMed] [Google Scholar]
- Dakappagari N, Maruyama T, Renshaw M, Tacken P, Figdor C, Torensma R, Wild MA, Wu D, Bowdish K, Kretz-Rommel A. Internalizing antibodies to the C-type lectins, L-SIGN and DC-SIGN, inhibit viral glycoprotein binding and deliver antigen to human dendritic cells for the induction of T cell responses. J Immunol. 2006;176:426–440. doi: 10.4049/jimmunol.176.1.426. [DOI] [PubMed] [Google Scholar]
- Domon B, Costello C. A systematic nomenclature for carbohydrate fragmentation in FAB-MS/MS spectra of glycoconjugates. Glycoconjugate J. 1988;5:253–257. [Google Scholar]
- Dyatlovitskaya EV, Bergelson LD. Glycosphingolipids and antitumor immunity. Biochim Biophys Acta. 1987;907:125–143. doi: 10.1016/0304-419x(87)90002-3. [DOI] [PubMed] [Google Scholar]
- Eberl M, Langermans JA, Vervenne RA, Nyame AK, Cummings RD, Thomas AW, Coulson PS, Wilson RA. Antibodies to glycans dominate the host response to schistosome larvae and eggs: Is their role protective or subversive? J Infect Dis. 2001;183:1238–1247. doi: 10.1086/319691. [DOI] [PubMed] [Google Scholar]
- Engering A, Geijtenbeek TB, van Vliet S J, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- Engering A, van Vliet SJ, Hebeda K, Jackson DG, Prevo R, Singh SK, Geijtenbeek TB, van Krieken H, van Kooyk Y. Dynamic populations of dendritic cell-specific ICAM-3 grabbing nonintegrin-positive immature dendritic cells and liver/lymph node-specific ICAM-3 grabbing nonintegrin-positive endothelial cells in the outer zones of the paracortex of human lymph nodes. Am J Pathol. 2004;164:1587–1595. doi: 10.1016/S0002-9440(10)63717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faveeuw C, Angeli V, Fontaine J, Maliszewski C, Capron A, Van Kaer L, Moser M, Capron M, Trottein F. Antigen presentation by CD1d contributes to the amplification of Th2 responses to Schistosoma mansoni glycoconjugates in mice. J Immunol. 2002;169:906–912. doi: 10.4049/jimmunol.169.2.906. [DOI] [PubMed] [Google Scholar]
- Faveeuw C, Mallevaey T, Paschinger K, Wilson IB, Fontaine J, Mollicone R, Oriol R, Altmann F, Lerouge P, Capron M, Trottein F. Schistosome N-glycans containing core a3-fucose and core b2-xylose epitopes are strong inducers of Th2 responses in mice. Eur J Immunol. 2003;33:1271–1281. doi: 10.1002/eji.200323717. [DOI] [PubMed] [Google Scholar]
- Fukuda MN, Dell A, Tiller PR, Varki A, Klock JC, Fukuda M. Structure of a novel sialylated fucosyl lacto-N-norhexaosylceramide isolated from chronic myelogenous leukemia cells. J Biol Chem. 1986;261:2376–2383. [PubMed] [Google Scholar]
- Geijtenbeek TB, Krooshoop DJ, Bleijs DA, van Vliet SJ, van Duijnhoven GC, Grabovsky V, Alon R, Figdor CG, van Kooyk Y. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol. 2000;1:353–357. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, van Duijnhoven GC, van Vliet SJ, Krieger E, Vriend G, Figdor CG, van Kooyk Y. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J Biol Chem. 2002;277:11314–11320. doi: 10.1074/jbc.M111532200. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer H, Wuhrer M, Resemann A, Geyer R. Identification and characterization of keyhole limpet hemocyanin N-glycans mediating cross-reactivity with Schistosoma mansoni. J Biol Chem. 2005;280:40731–40748. doi: 10.1074/jbc.M505985200. [DOI] [PubMed] [Google Scholar]
- Geyer R, Geyer H. Saccharide linkage analysis using methylation and other techniques. Methods Enzymol. 1994;230:86–107. doi: 10.1016/0076-6879(94)30009-7. [DOI] [PubMed] [Google Scholar]
- Guo Y, Feinberg H, Conroy E, Mitchell DA, Alvarez R, Blixt O, Taylor ME, Weis WI, Drickamer K. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat Struct Mol Biol. 2004;11:591–598. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- Imberty A, Bettler E, Karababa M, Mazeau K, Petrova P, Pérez S. Building sugars: The sweet part of structural biology. In: Vijayan M., Yathindra N., et al., editors. Perspectives in Structural Biology. Hyderabad: Indian Academy of Sciences and Universities Press; 1999. pp. 392–409. [Google Scholar]
- Jeffers SA, Tusell SM, Gillim-Ross L, Hemmila EM, Achenbach JE, Babcock GJ, Thomas WD, Jr., Thackray LB, Young MD, Mason RJ, Ambrosino DM, Wentworth DE, Demartini JC, Holmes KV. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci USA. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantelhardt SR, Wuhrer M, Dennis RD, Doenhoff MJ, Bickle Q, Geyer R. Fucα1-3GalNAc-: The major antigenic motif of Schistosoma mansoni glycolipids implicated in infection sera and keyhole-limpet haemocyanin cross-reactivity. Biochem J. 2002;366:217–223. doi: 10.1042/BJ20011678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrar A, Broome U, Uzunel M, Qureshi AR, Sumitran-Holgersson S. Human liver sinusoidal endothelial cells induce apoptosis in activated T cells: A role in tolerance induction. Gut. 2007;56:243–252. doi: 10.1136/gut.2006.093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo KH, Chatterjee D, Caulfield JP, Morris HR, Dell A. Structural characterization of glycosphingolipids from the eggs of Schistosoma mansoni and Schistosoma japonicum. Glycobiology. 1997;7:653–661. doi: 10.1093/glycob/7.5.653. [DOI] [PubMed] [Google Scholar]
- Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- Knolle PA, Limmer A. Neighborhood politics: The immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 2001;22:432–437. doi: 10.1016/s1471-4906(01)01957-3. [DOI] [PubMed] [Google Scholar]
- Lehr T, Geyer H, Maass K, Doenhoff MJ, Geyer R. Structural characterization of N-glycans from the freshwater snail Biomphalaria glabrata cross-reacting with Schistosoma mansoni glycoconjugates. Glycobiology. 2007;17:82–103. doi: 10.1093/glycob/cwl048. [DOI] [PubMed] [Google Scholar]
- Lemieux RU, Bock K, Delbaere LTJ, Koto S, Rao VSR. The conformations of oligosaccharides related to the ABH and Lewis human blood group determinants. Can J Chem. 1980;58:631–653. [Google Scholar]
- Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle PA. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nature Medicine. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- Lochnit G, Nispel S, Dennis RD, Geyer R. Structural analysis and immunohistochemical localization of two acidic glycosphingolipids from the porcine, parasitic nematode, Ascaris suum. Glycobiology. 1998;8:891–899. doi: 10.1093/glycob/8.9.891. [DOI] [PubMed] [Google Scholar]
- Lozach PY, Lortat-Jacob H, de Lacroix de Lavalette A, Staropoli I, Foung S, Amara A, Houles C, Fieschi F, Schwartz O, Virelizier JL, Arenzana-Seisdedos F, Altmeyer R. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J Biol Chem. 2003;278:20358–20366. doi: 10.1074/jbc.M301284200. [DOI] [PubMed] [Google Scholar]
- Ludwig IS, Lekkerkerker AN, Depla E, Bosman F, Musters RJ, Depraetere S, van Kooyk Y, Geijtenbeek TB. Hepatitis C virus targets DC-SIGN and L-SIGN to escape lysosomal degradation. J Virol. 2004;78:8322–8332. doi: 10.1128/JVI.78.15.8322-8332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab G, Reeves JL, Salmi M, Hubscher S, Jalkanen S, Adams DH. Vascular adhesion protein 1 mediates binding of T cells to human hepatic endothelium. Gastroenterology. 1996;110:522–528. doi: 10.1053/gast.1996.v110.pm8566600. [DOI] [PubMed] [Google Scholar]
- Meyer S, van Liempt E, Imberty A, van Kooyk Y, Geyer H, Geyer R, van Die I. DC-SIGN mediates binding of dendritic cells to authentic pseudo-LewisY glycolipids of Schistosoma mansoni cercariae, the first parasite-specific ligand of DC-SIGN. J Biol Chem. 2005;280:37349–37359. doi: 10.1074/jbc.M507100200. [DOI] [PubMed] [Google Scholar]
- Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- Ng KK, Drickamer K, Weis WI. Structural analysis of monosaccharide recognition by rat liver mannose-binding protein. J Biol Chem. 1996;271:663–674. doi: 10.1074/jbc.271.2.663. [DOI] [PubMed] [Google Scholar]
- Ng KK, Kolatkar AR, Park-Snyder S, Feinberg H, Clark DA, Drickamer K, Weis WI. Orientation of bound ligands in mannose-binding proteins. Implications for multivalent ligand recognition. J Biol Chem. 2002;277:16088–16095. doi: 10.1074/jbc.M200493200. [DOI] [PubMed] [Google Scholar]
- Ng KK, Weis WI. Structure of a selectin-like mutant of mannose-binding protein complexed with sialylated and sulfated Lewis(x) oligosaccharides. Biochemistry. 1997;36:979–988. doi: 10.1021/bi962564e. [DOI] [PubMed] [Google Scholar]
- Nyame AK, Leppanen AM, Bogitsh BJ, Cummings RD. Antibody responses to the fucosylated LacdiNAc glycan antigen in Schistosoma mansoni-infected mice and expression of the glycan among schistosomes. Exp Parasitol. 2000;96:202–212. doi: 10.1006/expr.2000.4573. [DOI] [PubMed] [Google Scholar]
- Nyame AK, Leppanen AM, DeBose-Boyd R, Cummings RD. Mice infected with Schistosoma mansoni generate antibodies to LacdiNAc (GalNAcβ1-4GlcNAc) determinants. Glycobiology. 1999;9:1029–1035. doi: 10.1093/glycob/9.10.1029. [DOI] [PubMed] [Google Scholar]
- Nyame AK, Lewis FA, Doughty BL, Correa-Oliveira R, Cummings RD. Immunity to schistosomiasis: Glycans are potential antigenic targets for immune intervention. Exp Parasitol. 2003;104:1–13. doi: 10.1016/s0014-4894(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Okano M, Satoskar AR, Nishizaki K, Abe M, Harn DA, Jr. Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J Immunol. 1999;163:6712–6717. [PubMed] [Google Scholar]
- Okano M, Satoskar AR, Nishizaki K, Harn DA, Jr. Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J Immunol. 2001;167:442–450. doi: 10.4049/jimmunol.167.1.442. [DOI] [PubMed] [Google Scholar]
- Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- Pohlmann S, Soilleux EJ, Baribaud F, Leslie GJ, Morris LS, Trowsdale J, Lee B, Coleman N, Doms RW. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc Natl Acad Sci USA. 2001;98:2670–2675. doi: 10.1073/pnas.051631398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robijn ML, Wuhrer M, Kornelis D, Deelder AM, Geyer R, Hokke CH. Mapping fucosylated epitopes on glycoproteins and glycolipids of Schistosoma mansoni cercariae, adult worms and eggs. Parasitology. 2005;130:67–77. doi: 10.1017/s0031182004006390. [DOI] [PubMed] [Google Scholar]
- Sneller MC. Granuloma formation, implications for the pathogenesis of vasculitis. Cleveland Clinic J Med. 2002;69(Suppl 2):S1140–S1143. doi: 10.3949/ccjm.69.suppl_2.sii40. [DOI] [PubMed] [Google Scholar]
- Soilleux EJ, Barten R, Trowsdale J. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J Immunol. 2000;165:2937–2942. doi: 10.4049/jimmunol.165.6.2937. [DOI] [PubMed] [Google Scholar]
- Van der Kleij D, van Remoortere A, Schuitemaker JH, Kapsenberg ML, Deelder AM, Tielens AG, Hokke CH, Yazdanbakhsh M. Triggering of innate immune responses by schistosome egg glycolipids and their carbohydrate epitope GalNAcβ1-4(Fucα1-2Fucα1-3)GlcNAc. J Infect Dis. 2002;185:531–539. doi: 10.1086/338574. [DOI] [PubMed] [Google Scholar]
- van Die I, Cummings RD. Glycans modulate immune responses in helminth infections and allergy. Chem Immunol Allergy. 2006;90:91–112. doi: 10.1159/000088883. [DOI] [PubMed] [Google Scholar]
- van Die I, van Vliet SJ, Nyame AK, Cummings RD, Bank CM, Appelmelk B, Geijtenbeek TB, van Kooyk Y. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13:471–478. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- van Liempt E, Bank CM, Mehta P, Garcia Vallejo JJ, Kawar ZS, Geyer R, Alvarez RA, Cummings RD, van Kooyk Y, van Die I. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580:6123–6131. doi: 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- van Liempt E, Imberty A, Bank CM, van Vliet SJ, van Kooyk Y, Geijtenbeek TB, van Die I. Molecular basis of the differences in binding properties of the highly related C-type lectins DC-SIGN and L-SIGN to Lewis X trisaccharide and Schistosoma mansoni egg antigens. J Biol Chem. 2004;279:33161–33167. doi: 10.1074/jbc.M404988200. [DOI] [PubMed] [Google Scholar]
- van Liempt E, van Vliet SJ, Engering A, Garcia Vallejo JJ, Bank CM, Sanchez-Hernandez M, van Kooyk Y, van Die I. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol Immunol. 2007;44:2605–2615. doi: 10.1016/j.molimm.2006.12.012. [DOI] [PubMed] [Google Scholar]
- van Remoortere A, Hokke CH, van Dam GJ, van Die I, Deelder AM, Van Den Eijnden DH. Various stages of schistosoma express Lewis(x), LacdiNAc, GalNAcβ1-4 (Fucα1-3)GlcNAc and GalNAcβ1-4(Fucα1-2Fucα1-3)GlcNAc carbohydrate epitopes: Detection with monoclonal antibodies that are characterized by enzymatically synthesized neoglycoproteins. Glycobiology. 2000;10:601–609. doi: 10.1093/glycob/10.6.601. [DOI] [PubMed] [Google Scholar]
- Velupillai P, dos Reis EA, dos Reis MG, Harn DA. Lewis (x)-containing oligosaccharide attenuates schistosome egg antigen- induced immune depression in human schistosomiasis. Hum Immunol. 2000;61:225–232. doi: 10.1016/s0198-8859(99)00136-6. [DOI] [PubMed] [Google Scholar]
- Weiss JB, Aronstein WS, Strand M. Schistosoma mansoni: Stimulation of artificial granuloma formation in vivo by carbohydrate determinants. Exp Parasitol. 1987;64:228–236. doi: 10.1016/0014-4894(87)90147-0. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Dennis RD, Doenhoff MJ, Lochnit G, Geyer R. Schistosoma mansoni cercarial glycolipids are dominated by Lewis X and pseudo-Lewis Y structures. Glycobiology. 2000;10:89–101. doi: 10.1093/glycob/10.1.89. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Geyer R. Glycoconjugate Structures. In: In Male A, editor., editor. PARASIRIC FLATWORMS - Molecular Biology, Biochemistry, Immunology and Parasitology. Wallingford, UK: CAB International; 2006. [Google Scholar]
- Wuhrer M, Kantelhardt SR, Dennis RD, Doenhoff MJ, Lochnit G, Geyer R. Characterization of glycosphingolipids from Schistosoma mansoni eggs carrying Fucα1-3GalNAc-, GalNAcβ1-4[Fucα1-3]GlcNAc- and Galβ1-4[Fucα1-3]GlcNAc- (Lewis X) terminal structures. Eur J Biochem. 2002;269:481–493. doi: 10.1046/j.0014-2956.2001.02673.x. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201:156–167. doi: 10.1111/j.0105-2896.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW, Kafferlein E, Haas JG, Meyer N, Strobel M, Weber C, Flieger D. Gangliosides suppress tumor necrosis factor production in human monocytes. J Immunol. 1992;148:1753–1758. [PubMed] [Google Scholar]