Abstract
The possibility is examined that immunomodulatory pharmacotherapy may be clinically useful in managing the pandemic coronavirus disease 2019 (COVID-19), known to result from infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a positive-sense single-stranded RNA virus. The dominant route of cell entry of the coronavirus is via phagocytosis, with ensconcement in endosomes thereafter proceeding via the endosomal pathway, involving transfer from early (EEs) to late endosomes (LEs) and ultimately into lysosomes via endolysosomal fusion. EE to LE transportation is a rate-limiting step for coronaviruses. Hence inhibition or dysregulation of endosomal trafficking could potentially inhibit SARS-CoV-2 replication. Furthermore, the acidic luminal pH of the endolysosomal system is critical for the activity of numerous pH-sensitive hydrolytic enzymes. Golgi sub-compartments and Golgi-derived secretory vesicles also depend on being mildly acidic for optimal function and structure. Activation of endosomal toll-like receptors by viral RNA can upregulate inflammatory mediators and contribute to a systemic inflammatory cytokine storm, associated with a worsened clinical outcome in COVID-19. Such endosomal toll-like receptors could be inhibited by the use of pharmacological agents which increase endosomal pH, thereby reducing the activity of acid-dependent endosomal proteases required for their activity and/or assembly, leading to suppression of antigen-presenting cell activity, decreased autoantibody secretion, decreased nuclear factor-kappa B activity and decreased pro-inflammatory cytokine production. It is also noteworthy that SARS-CoV-2 inhibits autophagy, predisposing infected cells to apoptosis. It is therefore also suggested that further pharmacological inhibition of autophagy might encourage the apoptotic clearance of SARS-CoV-2-infected cells.
Keywords: Antiviral treatment, COVID-19, SARS-CoV-2, Immunomodulation, Macrolide
Graphical abstract
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a positive-sense single-stranded RNA virus pathogen which is the cause of severe and sometimes fatal respiratory illness, named coronavirus disease 2019 (COVID-19) [[1], [2], [3]].
In general, COVID-19 is a mild illness; symptoms include pyrexia, cough, shortness of breath and, less commonly, diarrhoea. Yet many people infected with the virus remain asymptomatic throughout its course and unknowingly infect others [4]. However, the weight of evidence suggests that some 20% of patients develop symptoms of a severity that requires hospitalisation. Approximately 40% of these patients develop severe pneumonia and acute respiratory distress syndrome (ARDS) and require prolonged ventilation [5,6]. Once ventilated, the prognosis is poor. Massive alveolar damage leading to a pattern of progressive respiratory failure commonly results in death in over 50% of cases [5,6].
From a histological perspective patients with COVID-19 ARDS display evidence of diffuse alveolar damage, increased permeability of epithelial and endothelial cells, fibrin-rich hyaline membranes, significant leakage of fluid into the pulmonary interstitium, gross disruption of gas exchange and signs of hypoxia [5,[7], [8], [9]]. Commonly reported pulmonary immune abnormalities include activated alveolar and bone-derived macrophages carrying markers of pyroptosis [7,10,11] and high levels of neutrophil extracellular trap-producing neutrophils [8,12]. Unsurprisingly, these abnormalities in the pulmonary immune cells are accompanied by severe hypercytokinaemia in the lungs [10,13,14].
The most commonly reported peripheral pro-inflammatory cytokine (PIC) and chemokine profiles of COVID-19 patients includes elevated levels of tumour necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), IL-6, IL-10, monocyte chemoattractant protein-1 (MCP-1) and C-X-C motif chemokine 10 (CXCL10) [10,[15], [16], [17], [18], [19]]. Importantly, the levels of immune activation and dysregulation of cytokine and chemokine levels increases with disease severity [10,[15], [16], [17], [18], [19]] and may be 10-fold higher in patients with severe pneumonia and individuals requiring mechanical ventilation than in patients whose symptoms are relatively mild [20].
Systemic inflammation is associated with mortality, with high neutrophil lymphocyte ratios and levels of IL-6 being predictive of a poor outcome [[20], [21], [22], [23]]. Other markers of excessive systemic inflammation such as lymphopaenia, T and NK cell exhaustion and an elevated T helper 17 cell (Th17) to regulatory T cell (Treg) ratio, and the presence of large numbers of TNF-α- and Il-6-secreting macrophages are also all frequently observed in patients with severe COVID-19 [[24], [25], [26], [27]]. There is also evidence of increased NLR family pyrin domain containing 3 (NLRP3) activity and widespread tissue pyroptosis and/or necroptosis in lymph nodes, spleen, liver, heart and the kidney [28,29] reviewed by [26].This pattern of cellular damage is also observed in epithelial and endothelial cells [30,31]. Such data combined with evidence of a hypercoagulative state [32,33] and the existence of microthrombi in many COVID-19 patients are suggestive of widespread endotheliopathy [34,35].
This pattern of immune and histological abnormalities seen in patients with severe COVID-19 is also seen in sepsis and septic shock [[36], [37], [38], [39], [40]]. Indeed, there is a growing consensus that severe COVID-19 infection may be a distinct form of viral sepsis [1,41,42] which has considerable long-term implications for post-discharge as well as in hospital pathology, as discussed below.
The authors of large observational studies involving over 80,000 patients have concluded that the long-term prognosis for sepsis survivors is poor, with a death rate estimated to be 15% in the first year and 6 to 8% per annum over the next five years [43,44]. In addition, several studies have reported persistent and profound long-term physical disability [45,46] and serious psychiatric sequalae [47,48], reviewed by [49], in survivors of SARS and Middle East Respiratory Syndrome (MERS) infection. Crucially, there is accumulating evidence to suggest that this may be true of survivors of severe COVID-19, with many patients experiencing grossly reduced exercise capacity, extreme fatigue as well as serious neurological sequelae and neuropsychiatric sequelae [50], reviewed by [51].
In addition, while severe pneumonia progressing to ARDS is the main cause of mortality [10,33,[52], [53], [54]], serious multi-organ pathology is also commonplace and is a cause of significant levels of morbidity and mortality [5]. For example, acute myocardial injury, often resulting in fatal or non- fatal myocardial infarction, occurs in 20 to 40% of patients [5,[54], [55], [56]] reviewed by [57]. Furthermore, acute renal injury, hepatic dysfunction and various dimensions of ocular damage occur in approximately 30% of patients [6,58,59] reviewed by [57].
Given the numbers of individuals infected and estimates that approximately 20% require hospitalisation, the current pandemic is likely to lead to a considerable burden on health care systems as well as in the medium and longer term. Hence the development of a therapeutic approach which may reduce the numbers of patients progressing to severe disease is of prime importance from an individual and a societal perspective.
This represents a formidable challenge; however, as the weight of evidence suggests that the main driver of symptoms and severe pathology is the development of a cytokine storm [13,16,60]. In addition, the contribution of the virus and the host immune response to pathology appears to vary over the course of the illness. In particular, the weight of evidence suggests that SARS-CoV-2 and the host immune response are involved in the earliest stages of the illness and thereafter a dysregulated immune response plays the predominant role (2020). Hence an agent or agents with antiviral and immunomodulatory properties would appear to be desirable. Furthermore, the therapeutic window for early intervention appears to be very narrow. The weight of evidence suggests that symptom onset occurs on average four to five days post-infection with peak viral load occurring some five to six days later [[61], [62], [63]]. In addition, patients with severe disease may progress to ARDS in eight or nine days [64]. Hence the therapeutic effects of any approach aimed at preventing the development of severe disease would need to be of relatively rapid onset.
Currently, therapeutic options in the treatment of COVID-19 are extremely limited. Thankfully, there is now evidence that dexamethasone reduces mortality in patients requiring mechanical ventilation [65]. However, the results achieved using remdesivir and tocilizumab have thus far been disappointing [66,67]. In addition, the early hope that hydroxychloroquine might reduce mortality in patients with severe COVID-19 appears to be fading. Despite promising results from small open-label observational studies and randomised controlled trials (RCTs) suggesting that hydroxychloroquine might increase rates of viral clearance and reduce mortality [[68], [69], [70]], a much larger prospective open-label RCT found no evidence of improved mortality [71]. It should be noted that this is consistent with the results from large retrospective studies which also reported no improvement in mortality when administered to hospitalised patients with COVID-19 pneumonia patients requiring oxygen [72,73]. However, other large retrospective analyses suggest that combination therapy involving hydroxychloroquine and azithromycin may reduce mortality in COVID-19 patients if administered early in the course of the illness and may prevent the development of severe disease [[74], [75], [76]].
This is potentially of major therapeutic relevance as there is considerable evidence from observational studies and RCTs that hydroxychloroquine and azithromycin possess immunomodulatory and antiviral activities [[77], [78], [79], [80], [81]]. Moreover, several retrospective analyses and RCTs suggest that fears over potential cardiovascular complications with hydroxychloroquine and/or azithromycin may also be unfounded; large retrospective studies have reported no evidence of increased cardiovascular mortality or life-threatening arrhythmias in COVID-19 patients treated with hydroxychloroquine or azithromycin, whether prescribed singly or in combination, compared with untreated controls [[74], [75], [76]].
In light of the above, the remainder of this paper has three aims. First, to review the evidence relating to the immunomodulatory and antiviral effects of hydroxychloroquine and azithromycin while discussing their respective modes of action. Second, to discuss evidence of safety and the likelihood of serious and minor side effects. Third, to come to a view on the merits of a RCT of a combination of hydroxychloroquine and azithromycin with a particular focus on the dose and duration of therapy as well as patient selection.
2. Hydroxychloroquine
2.1. Immunomodulator in rheumatic diseases
Many research teams have reported significant reductions in mortality in patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) following prolonged ingestion of hydroxychloroquine at doses of 6.5 mg/kg compared with being untreated [82,83] reviewed by [84]. Several authors have also reported large and significant reductions in organ damage [[85], [86], [87]] and a reduced number of fatal and non-fatal cardiovascular events [[88], [89], [90]]. A recent meta-analysis of 19 RCTs and case-controlled observational studies concluded that hydroxychloroquine administration is associated with a 28% reduction in cardiovascular disease (CVD) in treated SLE patients compared with untreated controls [91]. There is also some evidence to suggest that patients treated with hydroxychloroquine may experience less neurological damage after 20 years of therapy compared with their untreated counterparts [92]. Furthermore, the results from prospective observational studies suggest that use of hydroxychloroquine may lead to a significant reduction in renal damage compared with non-users [[93], [94], [95]]. There may also be less pulmonary damage in SLE patients treated with hydroxychloroquine compared with untreated patients or patients treated with alternative therapies [96].
These are important data, as multiple end-organ damage is relatively common in SLE and is a major determinant of morbidity and mortality in this illness [97] (reviewed in [98]). For example, it is estimated that up to 50% of SLE patients have evidence of cardiac pathology and the incidence of myocarditis may be as high as 15% (reviewed by [99]). In addition, several authors have estimated that as many as 90% of SLE patients display evidence of neurological and neuropsychiatric abnormalities [100,101]. Approximately 50% of SLE patients have underlying renal pathology, and lupus nephritis is a major driver of mortality [102]. Finally, a recent review concluded that there is a six-fold increase in pulmonary disease in SLE patients compared with age- and sex-matched controls [103] and a recent meta-analysis concluded that pulmonary pathology is seen in the majority of patients [104].
There is also considerable observational evidence of reduced levels of mortality in SLE patients treated with hydroxychloroquine compared with age- and sex-matched controls or patients who have discontinued hydroxychloroquine therapy [90,[105], [106], [107]]. The greatest improvement in mortality recorded thus far involved patients enrolled in the LUMINA (Lupus in Minorities; Nature vs. nurture) study. A mortality rate of 5% was reported for hydroxychloroquine adherents and 17% for non-adherents or historically untreated controls [105]. Increased mortality levels in patients who have discontinued hydroxychloroquine have also been highlighted in a recent study. A fourfold increase in mortality was observed in non-adherent patients compared with patients who continued on hydroxychloroquine therapy [108].
However, while the results reported above are encouraging, it should be noted that the majority of the studies discussed are observational or quasi-experimental in nature. In addition, while many such studies are prospective, many are retrospective. These study designs suffer many well-documented limitations owing to lack of randomisation and difficulty in establishing cause and effect associations [109]. Readers interested in detailed treatments regarding the limitations and utility of observational, quasi-experimental and retrospective studies are referred to excellent reviews by [[110], [111], [112]]. However, having emphasised the level of uncertainty in the data discussed above, it should be noted that several meta-analyses of observational studies and RCTs report broadly consistent findings [79,84].
Hydroxychloroquine has also been used in an attempt to alleviate the persistent immune activation experienced by HIV-positive patients without access to antiretroviral (ARV) therapies and in patients well controlled on such regimes [[113], [114], [115], [116], [117]]. However, thus far the evidence in this domain is limited to observational studies and hence these results await confirmation via well designed RCTs.
2.2. Mode of action as an immunomodulator
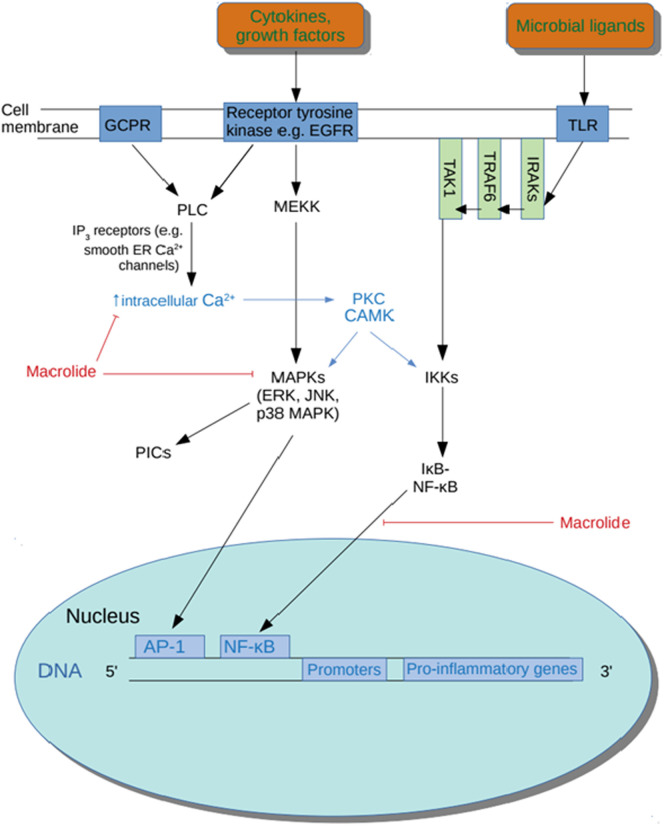
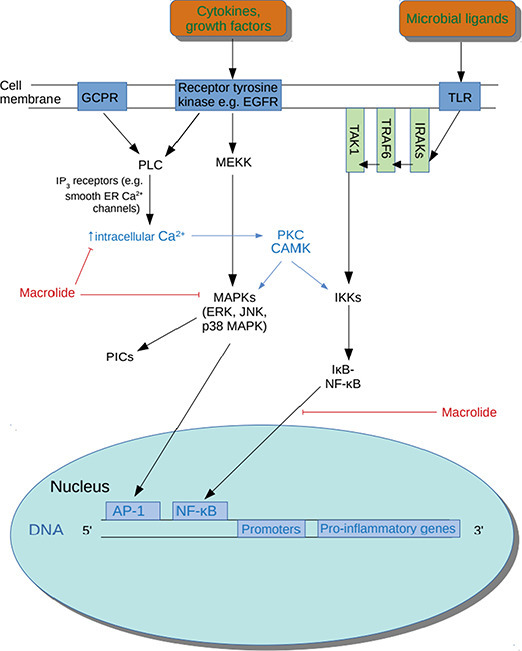
The weight of evidence suggests that hydroxychloroquine and chloroquine mainly exert their immunomodulatory effects by inhibiting endosomal toll-like receptor (TLR)-3, 7, 8 and 9 activation by increasing endosomal pH, thereby reducing the activity of the acid-dependent endosomal proteases required for their activity and/or assembly [[118], [119], [120], [121], [122]]. The net effect of such inhibition is the suppression of antigen-presenting cell activity, decreased autoantibody secretion and decreased activity of NF-κB and levels of PIC production [115,120,121,123,124], (reviewed by [125]). Characteristic changes in immune and inflammatory profiles induced by hydroxychloroquine therapy typically involve reductions in IL-1β, IL-6, TNF-α, CXCL-10, sCD40L and interferon-α (IFN-α2) [[115], [116], [117],123,124,[126], [127], [128]]. Hydroxychloroquine therapy may also reduce B cell activity [129] and Th17 cell differentiation [130,131], while promoting a Th1 to Th2 transition [130] and inhibiting the production of the PICs CCL2 and CXCL10 [132].
2.3. Antiviral action
Several authors have reported inhibition of human coronavirus (HCoV) 229E, HCoV OC43, MERS-CoV, SARS-CoV and SARS-CoV-2 replication following the administration of chloroquine or hydroxychloroquine in vitro in a range of cell types including Vero cells and lung epithelial cells [[133], [134], [135], [136], [137], [138], [139]]. The proposed mechanism underpinning these observations is deacidification of the endolysosomal system and the endoplasmic reticulum (ER) and Golgi apparatus, which is explained below [77].
A hallmark feature of the endolysosome system is its acidic luminal pH [[140], [141], [142]] that is critical for the activity of up to 60 different pH-sensitive hydrolytic enzymes, including proteases, lipases and nucleases [143,144]. The Golgi sub-compartments [145] and Golgi-derived secretory vesicles [140,146] are also mildly acidic and dependent on these conditions for optimal function and structure [145]. Compromised Golgi functions stemming from deacidification include dysregulated processing of proteins and defects in posttranslational modifications, which play a vital role in the function of mature proteins [147,148]. Importantly, raising pH inhibits enzymes involved in glycosylation [[148], [149], [150]]. Other impairments stemming from deacidification include dysregulated or inhibited transfer of proteins from the ER to the Golgi [151] and reverse transport from Golgi to ER [152,153]. Unsurprisingly, deacidification of the Golgi also leads to detrimental effects on protein sorting, transport from the Golgi to secretory vesicles and subsequent transport to the plasma [154].
Chloroquine and hydroxychloroquine are classified as weak diprotic bases [155,156]. In physiological conditions these compounds readily transverse plasma membranes into the cytosol and into the endolysosomal and ER Golgi compartment [157,158]. However, once ensconced within these compartments, they become protonated and thereafter are unable to escape into the cytosol. Over time, the preferential increase in concentration of chloroquine or hydroxychloroquine raises the pH in the lumen of these organelles resulting in a wide range of adverse functional consequences [159].
For example, chloroquine-mediated deacidification compromises endolysosomal trafficking by inducing mis-localisation of endosomes towards the periphery rather than the normal perinuclear region [160]. Other adverse effects include impaired endolysosomal fusion and increased lysosome exocytosis [161,162]. There are also data to suggest that chloroquine administration results in enhanced release of exosomes [163]. Several functional impairments also result from chloroquine administration, including undegraded substrates and atypical cleavages that lead to the generation of toxic intermediates, which are to be expected given the pH-dependent activity of endolysosomal enzymes discussed above [164,165]. Significant enlargement of endosomes and lysosomes also appears to be a characteristic consequence of chloroquine exposure, although the functional consequences of this phenomenon are currently unknown [166,167].
Chloroquine also concentrates in, and raises the pH of, the normally acidic Golgi [145], disrupting cisternal structure [168], impairing protein glycosylation [148] and formation and release of transport vesicles [169,170]. It should also be noted that prolonged chloroquine administration may ultimately result in lysosome membrane permeabilisation [171,172] resulting in the translocation of lysosomal contents into the cytosol, in turn leading to extreme mitochondrial damage and cell death [164,173]. The therapeutic implications of this phenomenon are discussed below, highlighting the role of the endolysosomal system and the Golgi apparatus in the replication of SARS-CoV-2.
In the case of SARS-CoV-2, initial entry into cells is enabled by engagement of the viral spike (S) protein with membrane-bound angiotensin-converting enzyme 2 (ACE-2) and subsequent proteolytic cleavage of the latter by a range of host cell enzymes [174,175]. The relative importance of these enzymes in enabling and propagating infection remains a matter of debate. Some research teams have proposed that the presence of the type II transmembrane serine protease TMPRSS2 is the sole determining factor [176] while others have suggested that the presence of TMPRSS2 and furin is required [177]. More recently, another research team has suggested that the presence of furin alone is sufficient [178]. This area is not just of academic importance as evidence suggests that furin activity is pH-dependent, while that does not appear to be the case for activity of TMPRSS2 [176,177,179,180]. Hence, if the presence of TMPRSS2 is the sole requirement for viral entry, then the argument that the use of hydroxychloroquine could prevent SARS-CoV-2 entry would appear to be weak. If, on the other hand, the optimal activity of furin is an indispensable element in viral entry and replication, then the theoretical argument for the prophylactic use of hydroxychloroquine becomes stronger. The matter of SARS-CoV-2 entry into cells may also be more complex than suggested in the evidence discussed above, as it has been proposed that the presence or otherwise of these enzymes determines whether the virus enters via fusion with the plasma membrane or via endocytosis and eventual occupancy within endosomes (reviewed by [181]).
However, the weight of evidence suggests that the dominant route of cell entry is via phagocytosis, with ensconcement in endosomes thereafter proceeding via the endosomal pathway [[182], [183], [184]]. This mode of transport involves transfer between early (EEs) to late endosomes (LEs) and ultimately into lysosomes via endolysosomal fusion [[182], [183], [184]]. This is important as transport from EEs to LEs is a rate-limiting step for coronaviruses [183,185]. Hence inhibition or dysregulation of endosomal trafficking could potentially have a major role in inhibiting the replication of SARS-CoV-2.
The next stage in the replication cycle involves the fusion of the virion with the lysosomal membrane enabled by pH-sensitive lysosomal furins and cathepsins releasing viral RNA into the cytoplasm [178]. Replication in the cytosol is initiated by the translation of the virally encoded replicase protein, leading to the production of a polyprotein subsequently cleaved by a virally encoded protease to produce 16 non-structural proteins (nsps) and the accessory proteins ORF3a, 3b, 6, 7a, 7b, 8, 9b, 9c and 10 [186] [187,188].
In the next stage, the three nsps 3 4 and 6 stimulate the production of double-membrane vesicles from ER membrane and establish replication transcription complexes (RTCs) [187,188]. Coronavirus RTCs are predominantly an assembly by RNA-dependent RNA polymerase (RdRp) and helicase-containing subunits and are a source of negative-sense RNA, subgenomic and genomic messenger RNAs (mRNAs). The structural viral nucleocapsid (N) protein is translated in the cytoplasm, thereafter binding with genomic mRNAs [[189], [190], [191]]. The other structural viral proteins (M, S and E) are translated in the ER and then migrate to the ER-Golgi intermediate compartment (ERGIC) [192].
All human coronaviruses form virions by budding into the lumen of ERGICs [193,194]. Viral proteins are thereafter glycosylated in the Golgi via pH-sensitive enzymes [[195], [196], [197], [198], [199]]. This is an important point, as the weight of evidence suggests that the glycosylation of proteins plays an indispensable role in enabling the optimal functioning of proteins responsible for suppressing host immune responses, initiating the formation of RCTs and the subversion of the immune response [[200], [201], [202], [203], [204]]. Finally, coronaviruses, including SARS-CoV-2, exit the Golgi in secretory vesicles and are transported to the plasma before exiting into the intercellular environment via exocytosis [205,206]. Hence, the deacidification of the trans-Golgi network is highly likely to result in dysfunctional virions and impeded exit of such virions into the extracellular compartment. Thus when the data are considered as a whole, the use of hydroxychloroquine or chloroquine would appear to be a rational antiviral therapy so far as SARS-CoV-2 is concerned. In addition, there may be other factors associated with coronavirus replication which may also be targeted via the use of hydroxychloroquine or chloroquine, which are discussed briefly below.
Inducing ER stress and the activation of the unfolded protein response (UPR) is a conserved replication strategy among coronaviruses aimed at inhibiting host proteins involved in metabolism and the immune response to viral invasion [207,208]. Examples of coronavirus proteins which have the capacity to induce ER stress include 3a and ORF-8b of SARS-CoV [195,209]. Broadly, the translation of host proteins is dependent on unphosphorylated eukaryotic initiation factor 2 (eIF2) [207,208] and activation of the UPR leads to phosphorylation of eIF2 by protein kinase R-like ER kinase (PERK or EIF2AK3), thereby inhibiting or terminating host protein synthesis [210]. Importantly, prolonged UPR activation is a common cause of apoptosis in coronavirus-infected cells [208].
Furthermore, there is accumulating evidence that MERS-CoV, SARS-CoV and SARS-CoV-2 inhibit autophagy [[211], [212], [213], [214]] reviewed by [215]. This state of affairs also has deleterious consequences for cell survival by sensitising such cells to apoptosis [216,217]. These data are intriguing given the relative success of hydroxychloroquine and chloroquine as adjuvants to chemotherapy achieved via the inhibition of autophagy and the promotion of ER stress leading to increased rates of apoptosis [218,219]. This offers the tantalising possibility that it might be possible to encourage the apoptotic clearance of SARS-CoV-2-infected cells already predisposed to apoptosis by the administration of hydroxychloroquine at the doses used in the treatment of cancer patients, which appear to be well tolerated and free of serious side effects [218,219].
The replication of viruses such as Ebola, dengue, Zika, HIV and SARS-CoV-2 is heavily dependent on the activity of endosomal and lysosomal proteases, glycosylating enzymes and glycosyltransferases, which in turn is dependent on an acidic pH within these vesicles [118,138,220,221]. The ability to induce endosomal acidification via mechanisms described above has led to research into the use of hydroxychloroquine in the treatment of Ebola, dengue and Zika viral infections, as the replication of these enzymes appears to be inhibited in an environment of decreased endosomal pH [77,222]. These matters are considered in more detail in an excellent review by [223].
There is in vivo evidence, albeit from murine models, that hydroxychloroquine administration does indeed lead to the inhibition of SARS-CoV, dengue, Ebola and hand, foot and mouth disease viruses suggesting that the drug at least has the potential to inhibit the replication of these viruses in humans at the correct dosage and duration of therapy [134,222,224,225]. It is also reassuring to note that hydroxychloroquine has demonstrated success in inhibiting the replication of HIV in patients naïve to ARV therapy [226].
2.4. Potential side effects
2.4.1. Cardiovascular complications
Hydroxychloroquine inhibits the action of cardiac potassium channels, which may inhibit myocyte repolarisation and potentially lead to prolongation of the QT interval and arrhythmias such as the life-threatening torsade de pointes (TdP) [227,228] However, several large retrospective studies investigating the use of hydroxychloroquine in the treatment of COVID-19 found no evidence of increased cardiovascular mortality or life-threatening arrhythmias in patients treated with the drug and reported that increased QT intervals were rarely clinically significant and warranting the discontinuation of therapy [[73], [74], [75], [76]]. Perhaps most importantly, these results are supported by data reported by authors of a recent large RCT who also concluded that the administration of hydroxychloroquine was not associated with any significant cardiovascular complications [71]. This latter finding is of particular note as the authors of the study used a loading dose of 2500 mg followed by 800 mg/day for a maximum of 10 days [71]. This is considerably higher than the maximum dose of 600 mg/day for 10 days which has been used by authors investigating the use of hydroxychloroquine in early COVID-19 [69,75].
In addition, two narrative reviews have concluded that the occurrence of hydroxychloroquine-associated cardiomyopathy is limited to seven reported cases over many thousands of patient-years of usage in the treatment of SLE and RA [229]. Furthermore, there is evidence to suggest that the phenomenon is influenced by dose and duration of therapy. A slightly higher incidence has been observed in those using hydroxychloroquine over 20 years of therapy (cumulative dose 2419 g) compared with 10 years' duration (cumulative dose 1542 g) [230]. In addition, large meta-analyses, involving almost 50,000 patients, have concluded that there is no evidence of increased instances of arrhythmia following the use of any quinoline derivative in the treatment of malaria [231,232].
2.4.2. Retinopathy
Recent narrative and systematic reviews have concluded that the risk of developing retinopathy with hydroxychloroquine at 5 mg/kg/day is considerably less than 1% irrespective of length of treatment [233,234]. Moreover, the risk of developing this side effect at the more conventional dose of 6 mg/kg/day is related to cumulative dose and duration [235]. In particular, the risk of developing retinopathy at a dose of 6 mg/kg/day over five years is approximately four in 1000, while the risk increases to approximately 1% after five to seven years of continuous therapy, as reviewed in [235]. Hence the risk of developing retinopathy over a relatively short course of treatment would appear to be minimal.
2.5. Consideration of dosage and duration of therapy
The full effect of hydroxychloroquine as an immunomodulator may take between three to six months to develop [236,237]. This is primarily because of the pharmacokinetics of the drug and the time required to saturate lysosomes [157,238], which would be expected given its mode of action and the need to accumulate in deep tissue [239]. The extensive concentration in tissues following hydroxychloroquine administration is reflected in an extremely large volume of distribution (44,257 L) and a very long elimination half-life of between 40 and 96 days [158,240]. Clearly these data suggest that achieving steady-state levels may take several months at a daily dose of 5 to 6.5 mg/kg [240].
In addition, a consideration of other pharmacokinetic parameters suggests that establishing an optimum dose likely to achieve therapeutic levels in most patients enrolled in a clinical trial is at best a complex exercise. For example, while there appears to be a modest correlation between dose and drug plateau levels [241], there is an extremely large interindividual variation in plasma levels following oral ingestion at any trialled dose, which may be as large as 100% [242,243]. This is problematic, as levels of undissociated drug in the plasma determine the distribution of hydroxychloroquine between this compartment and deep tissues which is the dominant factor in the performance of the drug [158]. This unpredictable relationship between dose and therapeutic levels is of major concern in relation to determining an effective dose for a trial as there is evidence to suggest that this feature is associated with the failure of malaria prophylaxis despite apparently adequate dosage and compliance [244].
However, it is encouraging to note that data from animal studies suggest that the relationship between dose and tissue uptake is not linear and that a threefold increase in the dose may lead to a twenty-fold increase in tissue levels during acute and chronic administration [157]. In addition, there is also evidence to suggest that increasing the dose of hydroxychloroquine to 10 mg/kg could reduce the half-life to two weeks [157]. It is also encouraging to note that the rate of hydroxychloroquine distribution into deep tissues may be increased significantly via the use of a loading dose [245]. Clearly the weight of evidence argues for a high dose, a prolonged course of therapy and the use of a loading dose.
There is evidence to suggest that the efficacy of hydroxychloroquine as an immunomodulator and antiviral agent is dose and duration related. For example, a dose of 800 mg for eight weeks has been shown to reduce viral load in ARV-naïve HIV patients, while a dose of 200 mg appears to produce no effect [226] (reviewed in [246]).
Several research teams have attempted to predict the optimum dose and duration and the results of such studies are elegantly reviewed in [137]. Given the experience with ARV-naïve HIV patients, it is interesting to note that a recommended dose of at least 800 mg per day appears to be the view of the majority of these researchers [139,247]. Indeed, it has been suggested that a dose below that level is unlikely to have any effect in inhibiting the replication of SARS-CoV-2 [248]. In addition, a synthesis of evidence from these in vivo studies suggests that the optimal duration may be as long as 10 days [139,249]. It is also noteworthy that one research team concluded that the time needed to achieve steady-state in pulmonary tissue may be considerably longer [139].
However, establishing therapeutic levels in vivo is a different challenge from viral inhibition in vitro. This would seem to be particularly so in the case of hydroxychloroquine as a treatment of COVID-19 as there is evidence to suggest that uptake of the drug into lysosomes is inhibited in an environment of tissue and cellular inflammation and acidosis [250,251]. This is problematic in the course of a viral infection as both states are present in the intracellular and intercellular environment [[252], [253], [254]]. In addition, it is also worthy of note that the pH of endolysosomes and the ER-Golgi apparatus is maintained within homeostatic norms via the activity of the Na+-H+ exchanger isoform-1 (NHE-1) membrane pump [255]. This system may take some time to overcome and hence the pH-increasing capacity of hydroxychloroquine may be delayed and/or require a higher dose than predicted from in vitro experimentation. These factors further argue against the success of relatively low-dose and/or short courses. In fact, recent evidence from animal studies suggests that short courses of a few days are ineffective with regard to the inhibition of SARS-CoV-2 replication even at doses of 50 mg/kg [256,257]. Given a consideration of the factors discussed above and the presence of these data it would seem reasonable to suggest that any future planned trials using short courses of hydroxychloroquine should not take place.
3. Azithromycin
3.1. Immunomodulator
Several quasi-experimental studies and open-label RCTS have established that the macrolide azithromycin accumulates in macrophages, monocytes and neutrophils and rapidly reaches levels in these immune cells which are up to 2000 times greater than levels seen in plasma [[258], [259], [260], [261]]. Importantly, there is in vivo evidence to suggest that azithromycin is an effective NF-κB inhibitor (see Fig. 1 ) and hence this property of accumulation within macrophages and neutrophils offers the prospect of highly localised immune modulation and minimising the risk of systemic immune suppression [262,263]. From the perspective of treating COVID-19 this is potentially a major therapeutic benefit as there is ample evidence that coronavirus infection results in activation of NF-κB [[264], [265], [266], [267], [268]].
Fig. 1.
Actions of macrolides.
The weight of evidence obtained from observational studies and RCTs suggests that the use of azithromycin therapy in a range of pulmonary and autoimmune illnesses results in increased M2 polarisation of activated alveolar and peripheral macrophages [[269], [270], [271], [272], [273]]. This is accompanied by improved efferocytosis [274] and phagocytosis [[275], [276], [277]]. These findings are of potential therapeutic relevance assuming that many of the elements driving the pathophysiology of sepsis-induced ARDS apply to COVID-19 as M1 polarisation-compromised phagocytosis and efferocytosis of alveolar macrophages play a pivotal role in the pathophysiology of that condition [36,38,278].
Prolonged azithromycin treatment also exerts a range of positive effects on neutrophils leading to improved function and decreased survival resulting in large decreases in the synthesis and release of PICs [[279], [280], [281], [282]]. One mechanism involved in decreased neutrophil survival following azithromycin therapy involves the inhibition of granulocyte-macrophage-colony stimulating factor (GM-CSF) levels [283,284]. This may be a very important therapeutic benefit as elevated levels of GM-CSF are a major driver of extreme lung damage and increased mortality in many illnesses including ARDS and severe pneumonia [285,286]. In addition, several authors have reported reduced neutrophil infiltration into the lungs and the amelioration of pre-existing neutrophilia in study participants following prolonged azithromycin therapy [[287], [288], [289], [290]]. When viewed as a whole, the effects of azithromycin on neutrophils may be of major importance as increased neutrophil recruitment and impaired neutrophil apoptosis also play a major role in the pathogenesis of ARDS [278,291,292]. Finally, there is evidence to suggest that azithromycin inhibits the activity of the NLRP3 inflammasome and the subsequent release of IL-1β and IL-18 [259,293]. This may also be a major advantage in a therapy aimed at the treatment of COVID-19 as the activation of this complex and cellular pyroptosis appears to play a major role in the early and later stages of COVID-19 and in the development of ARDS [38,294]. The main elements believed to be involved in the pathophysiology of ARDS are represented in Fig. 2 .
Fig. 2.
Elements involved in the pathophysiology of ARDS.
There is also some evidence, albeit from retrospective studies, to suggest that azithromycin may reduce intensive care unit stay and 60-day mortality in patients suffering from sepsis [[295], [296], [297]]. These findings are broadly supported by prospective studies examining the effects of azithromycin on survival using animal models of sepsis [[298], [299], [300]]. Moreover, the results from the latter studies strongly suggest that the improvements in mortality following azithromycin administration are related to immunomodulation rather than antibacterial effects [298,300].
3.2. Antiviral action
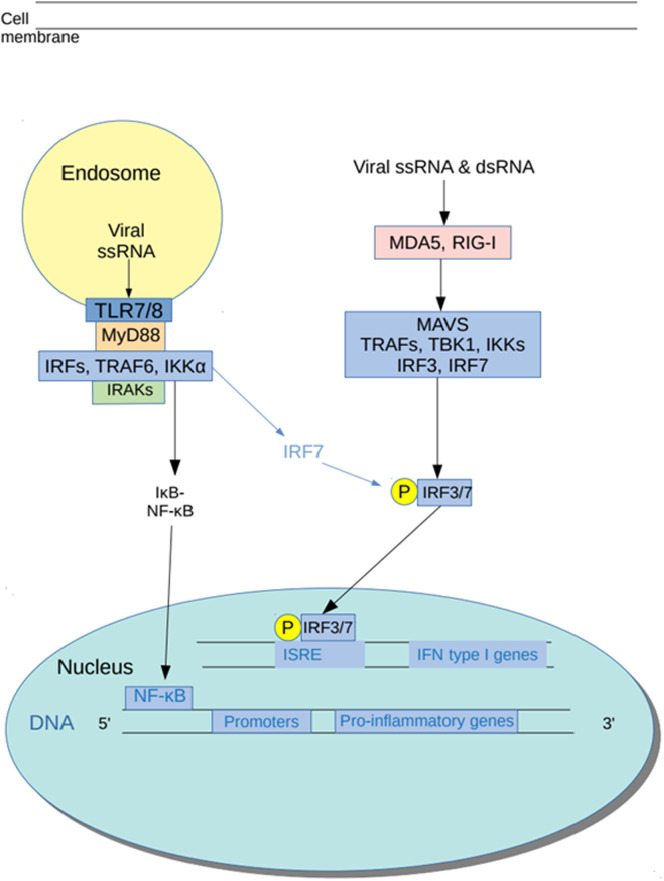
There is copious, albeit in vitro, evidence of direct antiviral effects of azithromycin (reviewed in [301]). Importantly, these effects have been observed in upper airway cells [78,302,303] and appear to be achieved via the upregulation of type I and type III INF responses [78,304]. These are significant data as recent evidence suggests that interferon production is grossly suppressed in patients with severe COVID-19 [305]. In addition, the mechanisms underpinning these effects seem to involve increased transcription and activity of melanoma differentiation-associated protein 5 (MDA5) and retinoic acid-inducible gene I (RIG-1) [78,304]. This is of particular importance in suggesting an antiviral treatment for early COVID-19 as, in line with other pathogenic human coronaviruses, the truncated SARS-CoV-2 endonuclease inhibits MDA5 activity; MDA5 is the main pattern recognition receptor for coronavirus and thus this action delays INFγ signalling [[306], [307], [308]]. There is also evidence of significant inhibition of autophagy in bronchial epithelial cells, suggesting another potential vehicle for synergy between azithromycin and hydroxychloroquine as antiviral agents [[309], [310], [311]]. Finally, it is encouraging to note that in vitro data suggest that a combination of azithromycin and hydroxychloroquine displays higher antiviral activity than either agent alone [312].
3.3. Safety considerations in the treatment of COVID-19
A large observational study by Ray and fellow workers reported that the use of azithromycin was associated with an approximately threefold increase in the risk of a fatal myocardial infarction compared with amoxicillin or placebo in patients with a high risk of developing CVD [313]. However, a meta-analysis of 12 RCTs involving the long-term use of azithromycin in a range of chronic conditions involving 15,558 patients concluded that the use of azithromycin was not associated with increased cardiovascular events [314]. These conclusions have been supported by a more recent meta-analysis of 33 observational studies and RCTs and data on 22,601,032 patients [315].
As previously discussed, large retrospective studies involving the combination of azithromycin and hydroxychloroquine for the treatment of COVID-19 reported no increase in cardiovascular complications in treated patients compared with untreated controls [[74], [75], [76]]. These findings are consistent with smaller observational studies examining the potential increased cardiovascular risk in patients prescribed a combination of azithromycin and hydroxychloroquine for the treatment of COVID-19 [316,317]. However, thus far there is no information regarding the safety of hydroxychloroquine and azithromycin in the cardiovascular arena so far as RCTs are concerned and hence there is a clear need for caution. In this regard, it should be noted that there are several published guidelines detailing protocols regarding matters such as baseline electrocardiography and cardiac monitoring for selecting appropriate patients and their subsequent management, from the perspective of mitigating against any potential cardiac pathology and maximising patient safety; these have been developed in anticipation of cardiovascular complications resulting from the use of hydroxychloroquine. Excellent examples of this approach have been produced by [227,318].
3.4. Consideration of dosage and duration of therapy
Azithromycin is unique within the macrolide class of antibiotics in being a weak diprotic base and a potent lysosomotropic compound [319,320]. The “trapping” of the drug within acidic lysosomes results in a massive increase in concentration in intracellular compartments in a similar manner to hydroxychloroquine and chloroquine [321,322], largely explaining the rapid accumulation into macrophages and neutrophils discussed above [323,324]. This property also leads to sustained drug levels in tissues [325], a comparatively large volume of distribution of 31 L/kg [326,327] and an elimination half-life of approximately five days [326]. In addition, high tissue levels persist for days following cessation of therapy [328,329].
The absorption and distribution of azithromycin into tissues are far more rapid than is the case for hydroxychloroquine and concentrations in tissues may be some 300-fold higher than in plasma [261]. Doubling the dose from 500 mg to 1000 mg shortens the half-life and produces a dramatic increase in the concentration of the drug in the lung [[330], [331], [332]].
The weight of evidence gleaned from animal and human studies strongly suggests that the immunomodulatory effects of azithromycin are related to dose and duration of therapy and would seem to be maximal at a dose of 100 mg/kg, although 20 mg/kg also results in immunomodulatory activity [[298], [299], [300]]. A synthesis of the data provided by these authors suggests that intravenous administration results in more rapid effects than oral administration [298,300].
Importantly, the results of an in vivo quasi-experimental prospective study investigating the effects of azithromycin on MDA5 activity suggest that the effective dose is 50 mg/kg, which in humans of average body mass would correspond to approximately 4 g daily [78]. More generally, there is in vivo evidence reporting that an inhibitory effect of azithromycin on influenza is achieved at a dose of 20 mg/kg [333]. In addition, the results of human RCTs examining the antiviral effects of azithromycin suggest that early administration is vital [334] and the macrolide may two weeks to display antiviral, or indeed immunomodulatory, activity [335].
Finally, it should be emphasised that the potential therapeutic window for the delivery of an effective therapy aimed at inhibiting the replication of SARS-CoV-2 would appear to be extremely narrow. For example, thus far no research team has detected viable virus in any patient after nine days post-infection [336]. In addition, there is evidence to suggest that peak viral levels may occur within five days of infection [336,337] and potentially while many individuals are yet to display symptoms [338,339]. Moreover, a recent study reported viral levels in hospitalised COVID-19 patients were almost two orders of magnitude lower than those in COVID-19 patients in the community, further emphasising the need for early treatment [340].
4. Conclusion
It is concluded that the safety profiles of hydroxychloroquine and azithromycin are acceptable, either alone or in combination, as therapeutic agents aimed at preventing the development of severe COVID-19, although routine cardiac monitoring is clearly indicated. It is further concluded that hydroxychloroquine is a highly effective immune modulator in chronic usage but its slow onset of action may limit its usefulness as an immunomodulator in the treatment of COVID-19. Azithromycin, on the other hand, displays potential as an immunomodulator targeting many elements involved in the pathogenesis of COVID-19 if administered at a high dose early in the course of the illness. The potential synergy of these molecules as antiviral agents is intriguing and offers a rational therapeutic intervention. However, issues remain regarding optimal dosage and duration of treatment, but short courses and/or low doses are very unlikely to succeed. Finally, the narrow window of opportunity for therapeutic intervention must be emphasised and the greatest chance of success is probably confined to patients in early disease; future trials should be focused on patients in a community setting.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgements
MB is supported by a NHMRC Senior Principal Research Fellowship 1059660.
References
- 1.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 5.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac Injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L., et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carsana L., Sonzogni A., Nasr A., Rossi R., Pellegrinelli A., Zerbi P., et al. 2020. Pulmonary Post-mortem Findings in a Large Series of COVID-19 Cases From Northern Italy. medRxiv. (2020.04.19.20054262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. 2020. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series From New Orleans. medRxiv. (2020.04.06.20050575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.-Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., et al. 2020. The Landscape of Lung Bronchoalveolar Immune Cells in COVID-19 Revealed by Single-cell RNA Sequencing. medRxiv. (2020.02.23.20026690) [Google Scholar]
- 11.Salomé B., Magen A. Dysregulation of lung myeloid cells in COVID-19. Nat. Rev. Immunol. 2020;20:277. doi: 10.1038/s41577-020-0303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 13.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang D., Guo R., Lei L., Liu H., Wang Y., Wang Y., et al. 2020. COVID-19 Infection Induces Readily Detectable Morphological and Inflammation-Related Phenotypic Changes in Peripheral Blood Monocytes, the Severity of Which Correlate With Patient Outcome. medRxiv. (2020.03.24.20042655) [Google Scholar]
- 15.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C., Cai J., Chen R., Shi Z., Bian X., Xie J., et al. Aveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID-19. Research Square. 2020 doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., et al. 2020. Detectable Serum SARS-CoV-2 Viral Load (RNAaemia) Is Closely Associated With Drastically Elevated Interleukin 6 (IL-6) Level in Critically Ill COVID-19 Patients. medRxiv. (2020.02.29.20029520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W., Lan Y., Yuan X., Deng X., Li Y., Cai X., et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerging Microbes & Infections. 2020;9:469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X., et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020;81:e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang A.-P., Liu J., Tao W., Li H.-M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharmacol. 2020:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. 2020. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). medRxiv. (2020.02.18.20024364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamilloux Y., Henry T., Belot A., Viel S., Fauter M., El Jammal T., et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England) 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M. Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection. SSRN Electron. J. 2020 [Google Scholar]
- 32.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang X., Du R., Wang R., Cao T., Guan L., Yang C., et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158:195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J. Thromb. Haemost. 2020;18:786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han S., Mallampalli R.K. The acute respiratory distress syndrome: from mechanism to translation. Journal of Immunology (Baltimore, Md: 1950) 2015;194:855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang M., Cai S., Su J. The pathogenesis of sepsis and potential therapeutic targets. Int. J. Mol. Sci. 2019;20:5376. doi: 10.3390/ijms20215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang X., Xiu H., Zhang S., Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediat. Inflamm. 2018;2018 doi: 10.1155/2018/1264913. (1264913-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nedeva C., Menassa J., Puthalakath H. Sepsis: inflammation is a necessary evil. Frontiers in Cell and Developmental Biology. 2019;7 doi: 10.3389/fcell.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Poll T., van de Veerdonk F.L., Scicluna B.P., Netea M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017;17:407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 41.Poston J.T., Patel B.K., Davis A.M. Management of critically ill adults with COVID-19. JAMA. 2020;323:1839–1841. doi: 10.1001/jama.2020.4914. [DOI] [PubMed] [Google Scholar]
- 42.Wujtewicz M., Dylczyk-Sommer A., Aszkiełowicz A., Zdanowski S., Piwowarczyk S., Owczuk R. COVID-19 – what should anaethesiologists and intensivists know about it? Anaesthesiology intensive therapy. 2020;52:34–41. doi: 10.5114/ait.2020.93756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahmel T., Schmitz S., Nowak H., Schepanek K., Bergmann L., Halberstadt P., et al. Long-term mortality and outcome in hospital survivors of septic shock, sepsis, and severe infections: the importance of aftercare. PLoS One. 2020;15:e0228952–e. doi: 10.1371/journal.pone.0228952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shankar-Hari M., Harrison D.A., Ferrando-Vivas P., Rubenfeld G.D., Rowan K. Risk factors at index hospitalization associated with longer-term mortality in adult sepsis survivors. JAMA Netw. Open. 2019;2:e194900–e. doi: 10.1001/jamanetworkopen.2019.4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed H., Patel K., Greenwood D.C., Halpin S., Lewthwaite P., Salawu A., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J. Rehabil. Med. 2020;52:jrm00063. doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 46.Liheng Guo1, Yun Han1,2, Jian Li1,2, Quanfu Chen1, Yi Ren3, Qiaomei Wu1, Jian Zhang1, Yuzhi Chen2, Minzhou Zhang. Long-term outcomes in patients with severe acute respiratory syndrome treated with oseltamivir: a 12-year longitudinal study Int. J. Clin. Exp. Med. 2019;12(10):12464-12471www.ijcem.com/ISSN:1940-5901/.
- 47.Davydow D.S., Desai S.V., Needham D.M., Bienvenu O.J. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom. Med. 2008;70:512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mak I.W.C., Chu C.M., Pan P.C., Yiu M.G.C., Chan V.L. Long-term psychiatric morbidities among SARS survivors. Gen. Hosp. Psychiatry. 2009;31:318–326. doi: 10.1016/j.genhosppsych.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Connor C.M. COVID-19 fatigue: not so fast. JACC Heart failure. 2020;8:592–594. doi: 10.1016/j.jchf.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 56.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020:cvaa106. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang X., Yu Y., Xu J., Shu H., Ja Xia, Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. S2213-600(20)30079-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., et al. 2020. Effect of Dexamethasone in Hospitalized Patients With COVID-19: Preliminary Report. medRxiv. (2020.06.22.20137273) [Google Scholar]
- 66.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet (London, England) 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., et al. 2020. Efficacy of Hydroxychloroquine in Patients With COVID-19: Results of a Randomized Clinical Trial. medRxiv. (2020.03.22.20040758) [Google Scholar]
- 69.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu B., Wang D.W., Li C. 2020. Hydroxychloroquine Application Is Associated With a Decreased Mortality in Critically Ill Patients With COVID-19. medRxiv. (2020.04.27.20073379) [Google Scholar]
- 71.Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., et al. 2020. Effect of Hydroxychloroquine in Hospitalized Patients With COVID-19: Preliminary Results From a Multi-Centre, Randomized, Controlled Trial. medRxiv. (2020.07.15.20151852) [Google Scholar]
- 72.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., et al. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N. Engl. J. Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J., et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K., et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. International Journal of Infectious Diseases. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lagier J.-C., Million M., Gautret P., Colson P., Cortaredona S., Giraud-Gatineau A., et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Travel Med. Infect. Dis. 2020:101791. doi: 10.1016/j.tmaid.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Million M., Lagier J.-C., Gautret P., Colson P., Fournier P.-E., Amrane S., et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101738. (101738-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Bari M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017;5 doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Menzel M., Akbarshahi H., Tufvesson E., Persson C., Bjermer L., Uller L. Azithromycin augments rhinovirus-induced IFNβ via cytosolic MDA5 in experimental models of asthma exacerbation. Oncotarget. 2017;8:31601–31611. doi: 10.18632/oncotarget.16364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rempenault C., Combe B., Barnetche T., Gaujoux-Viala C., Lukas C., Morel J., et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann. Rheum. Dis. 2018;77:98–103. doi: 10.1136/annrheumdis-2017-211836. [DOI] [PubMed] [Google Scholar]
- 80.Soeltoft K., Hallas J., Wasko M.C.M., Pedersen A.B., Ulrichsen S.P., Ellingsen T. OP0191 all-cause mortality and cardiovascular death in hydroxychloroquine users in rheumatoid arthritis patients – a population based danish cohort study. Ann. Rheum. Dis. 2018;77:144–145. [Google Scholar]
- 81.Zimmermann P., Ziesenitz V.C., Curtis N., Ritz N. The immunomodulatory effects of macrolides—a systematic review of the underlying mechanisms. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Floris A., Piga M., Mangoni A.A., Bortoluzzi A., Erre G.L., Cauli A. Protective effects of hydroxychloroquine against accelerated atherosclerosis in systemic lupus erythematosus. Mediat. Inflamm. 2018;2018 doi: 10.1155/2018/3424136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Legault K., Cuello-García C.A. Hydroxychloroquine for systemic lupus erythematosus in adults. Cochrane Database Syst. Rev. 2016 [Google Scholar]
- 84.Ruiz-Irastorza G., Ramos-Casals M., Brito-Zeron P., Khamashta M.A. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann. Rheum. Dis. 2010;69:20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 85.Akhavan P.S., Su J., Lou W., Gladman D.D., Urowitz M.B., Fortin P.R. The early protective effect of hydroxychloroquine on the risk of cumulative damage in patients with systemic lupus erythematosus. J. Rheumatol. 2013;40:831–841. doi: 10.3899/jrheum.120572. [DOI] [PubMed] [Google Scholar]
- 86.Fessler B.J., Alarcón G.S., McGwin G., Roseman J., Bastian H.M., Friedman A.W., et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis & Rheumatism. 2005;52:1473–1480. doi: 10.1002/art.21039. [DOI] [PubMed] [Google Scholar]
- 87.Petri M., Purvey S., Fang H., Magder L.S. Predictors of organ damage in systemic lupus erythematosus: the Hopkins Lupus Cohort. Arthritis Rheum. 2012;64:4021–4028. doi: 10.1002/art.34672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fasano S., Pierro L., Pantano I., Iudici M., Valentini G. Longterm hydroxychloroquine therapy and low-dose aspirin may have an additive effectiveness in the primary prevention of cardiovascular events in patients with systemic lupus erythematosus. J. Rheumatol. 2017;44:1032–1038. doi: 10.3899/jrheum.161351. [DOI] [PubMed] [Google Scholar]
- 89.Jung H., Bobba R., Su J., Shariati-Sarabi Z., Gladman D.D., Urowitz M., et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis & Rheumatism. 2010;62:863–868. doi: 10.1002/art.27289. [DOI] [PubMed] [Google Scholar]
- 90.Ruiz-Irastorza G., Egurbide M.V., Pijoan J.I., Garmendia M., Villar I., Martinez-Berriotxoa A., et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus. 2006;15:577–583. doi: 10.1177/0961203306071872. [DOI] [PubMed] [Google Scholar]
- 91.Liu D., Li X., Zhang Y., Kwong J.S., Li L., Zhang Y., et al. Chloroquine and hydroxychloroquine are associated with reduced cardiovascular risk: a systematic review and meta-analysis. Drug design, development and therapy. 2018;12:1685–1695. doi: 10.2147/DDDT.S166893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Piga M., Peltz M.T., Montaldo C., Perra D., Sanna G., Cauli A., et al. Twenty-year brain magnetic resonance imaging follow-up study in Systemic Lupus Erythematosus: factors associated with accrual of damage and central nervous system involvement. Autoimmun. Rev. 2015;14:510–516. doi: 10.1016/j.autrev.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 93.Beck L., Bomback A.S., Choi M.J., Holzman L.B., Langford C., Mariani L.H., et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am. J. Kidney Dis. 2013;62:403–441. doi: 10.1053/j.ajkd.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Pons-Estel G.J., Alarcon G.S., McGwin G., Jr., Danila M.I., Zhang J., Bastian H.M., et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum. 2009;61:830–839. doi: 10.1002/art.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu C.-L., Chang C.-C., Kor C.-T., Yang T.-H., Chiu P.-F., Tarng D.-C., et al. Hydroxychloroquine use and risk of CKD in patients with rheumatoid arthritis. Clin. J. Am. Soc. Nephrol. 2018;13:702–709. doi: 10.2215/CJN.11781017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yeo K.-J., Chen H.-H., Chen Y.-M., Lin C.-H., Chen D.-Y., Lai C.-M., et al. Hydroxychloroquine may reduce risk of Pneumocystis pneumonia in lupus patients: a Nationwide, population-based case-control study. BMC Infect. Dis. 2020;20 doi: 10.1186/s12879-020-4826-1. (112-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kyttaris V.C. Systemic lupus erythematosus: from genes to organ damage. Methods in molecular biology (Clifton, NJ) 2010;662:265–283. doi: 10.1007/978-1-60761-800-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hammond E.R., Murimi I.B., Lin D.H., Nab H., Kan H., Onasanya O., et al. FRI0356 organ damage in systemic lupus erythematosus is consistently associated with increased mortality: a meta-analysis. Ann. Rheum. Dis. 2018;77 (713-) [Google Scholar]
- 99.Tincani A., Rebaioli C.B., Taglietti M., Shoenfeld Y. Heart involvement in systemic lupus erythematosus, anti-phospholipid syndrome and neonatal lupus. Rheumatology. 2006;45:iv8–iv13. doi: 10.1093/rheumatology/kel308. [DOI] [PubMed] [Google Scholar]
- 100.Kampylafka E.I., Alexopoulos H., Kosmidis M.L., Panagiotakos D.B., Vlachoyiannopoulos P.G., Dalakas M.C., et al. Incidence and prevalence of major central nervous system involvement in systemic lupus erythematosus: a 3-year prospective study of 370 patients. PLoS One. 2013;8:e55843–e. doi: 10.1371/journal.pone.0055843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muscal E., Brey R.L. Neurologic manifestations of systemic lupus erythematosus in children and adults. Neurol. Clin. 2010;28:61–73. doi: 10.1016/j.ncl.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Almaani S., Meara A., Rovin B.H. Update on lupus nephritis. Clin. J. Am. Soc. Nephrol. 2017;12:825–835. doi: 10.2215/CJN.05780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Forbess L.J., Rossides M., Weisman M.H., Simard J.F. New-onset non-infectious pulmonary manifestations among patients with systemic lupus erythematosus in Sweden. Arthritis Research & Therapy. 2019;21:48. doi: 10.1186/s13075-018-1804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Medlin J.L., Hansen K.E., McCoy S.S., Bartels C.M. Pulmonary manifestations in late versus early systemic lupus erythematosus: a systematic review and meta-analysis. Semin. Arthritis Rheum. 2018;48:198–204. doi: 10.1016/j.semarthrit.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alarcón G.S., McGwin G., Bertoli A.M., Fessler B.J., Calvo-Alén J., Bastian H.M., et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L) Ann. Rheum. Dis. 2007;66:1168–1172. doi: 10.1136/ard.2006.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hsu C.Y., Lin Y.S., Cheng T.T., Syu Y.J., Lin M.S., Lin H.F., et al. Adherence to hydroxychloroquine improves long-term survival of patients with systemic lupus erythematosus. Rheumatology (Oxford) 2018;57:1743–1751. doi: 10.1093/rheumatology/key167. [DOI] [PubMed] [Google Scholar]
- 107.Shinjo S.K., Bonfá E., Wojdyla D., Borba E.F., Ramirez L.A., Scherbarth H.R., et al. Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis & Rheumatism. 2010;62:855–862. doi: 10.1002/art.27300. [DOI] [PubMed] [Google Scholar]
- 108.Avina-Zubieta A., Jorge A., DeVera M.A., Lu N., Esdaile J., Choi H. 299 increased mortality among patients with systemic lupus erythematosus after hydroxychloroquine discontinuation. Lupus Science & Medicine. 2019;6:A217–A218. [Google Scholar]
- 109.Thiese M.S. Observational and interventional study design types; an overview. Biochem Med (Zagreb) 2014;24:199–210. doi: 10.11613/BM.2014.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boyko E.J. Observational research—opportunities and limitations. J. Diabetes Complicat. 2013;27:642–648. doi: 10.1016/j.jdiacomp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schweizer M.L., Braun B.I., Milstone A.M. Research methods in healthcare epidemiology and antimicrobial stewardship-quasi-experimental designs. Infect. Control Hosp. Epidemiol. 2016;37:1135–1140. doi: 10.1017/ice.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tofthagen C. Threats to validity in retrospective studies. J Adv Pract Oncol. 2012;3:181–183. [PMC free article] [PubMed] [Google Scholar]
- 113.Paton N.I., Aboulhab J. Hydroxychloroquine, hydroxyurea and didanosine as initial therapy for HIV-infected patients with low viral load: safety, efficacy and resistance profile after 144 weeks. HIV Medicine. 2005;6:13–20. doi: 10.1111/j.1468-1293.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 114.Paton N.I., Aboulhab J., Karim F. Hydroxychloroquine, hydroxycarbamide, and didanosine as economic treatment for HIV-1. Lancet (London, England) 2002;359:1667–1668. doi: 10.1016/S0140-6736(02)08557-4. [DOI] [PubMed] [Google Scholar]
- 115.Piconi S., Parisotto S., Rizzardini G., Passerini S., Terzi R., Argenteri B., et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy–treated immunologic nonresponders. Blood. 2011;118:3263–3272. doi: 10.1182/blood-2011-01-329060. [DOI] [PubMed] [Google Scholar]
- 116.Sperber K., Chiang G., Chen H., Ross W., Chusid E., Gonchar M., et al. Comparison of hydroxychloroquine with zidovudine in asymptomatic patients infected with human immunodeficiency virus type 1. Clin. Ther. 1997;19:913–923. doi: 10.1016/s0149-2918(97)80045-8. [DOI] [PubMed] [Google Scholar]
- 117.Sperber K., Louie M., Kraus T., Proner J., Sapira E., Lin S., et al. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin. Ther. 1995;17:622–636. doi: 10.1016/0149-2918(95)80039-5. [DOI] [PubMed] [Google Scholar]
- 118.Al-Bari M.A. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015;70:1608–1621. doi: 10.1093/jac/dkv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Diebold S., Brencicova E. Nucleic acids and endosomal pattern recognition: how to tell friend from foe? Front. Cell. Infect. Microbiol. 2013;3 doi: 10.3389/fcimb.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kužnik A., Benčina M., Švajger U., Jeras M., Rozman B., Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 2011;186:4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 121.Lee S.-J., Silverman E., Bargman J.M. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat. Rev. Nephrol. 2011;7:718–729. doi: 10.1038/nrneph.2011.150. [DOI] [PubMed] [Google Scholar]
- 122.Tatematsu M., Funami K., Seya T., Matsumoto M. Extracellular RNA sensing by pattern recognition receptors. Journal of Innate Immunity. 2018;10:398–406. doi: 10.1159/000494034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Monzavi S.M., Alirezaei A., Shariati-Sarabi Z., Tavakol Afshari J., Mahmoudi M., Dormanesh B., et al. Efficacy analysis of hydroxychloroquine therapy in systemic lupus erythematosus: a study on disease activity and immunological biomarkers. Inflammopharmacology. 2018;26:1175–1182. doi: 10.1007/s10787-018-0512-y. [DOI] [PubMed] [Google Scholar]
- 124.Willis R., Seif A.M., McGwin G., Jr., Martinez-Martinez L.A., Gonzalez E.B., Dang N., et al. Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus. 2012;21:830–835. doi: 10.1177/0961203312437270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao W., Xiong Y., Li Q., Yang H. Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bodewes I.L.A., Gottenberg J.-E., van Helden-Meeuwsen C.G., Mariette X., Versnel M.A. Hydroxychloroquine treatment downregulates systemic interferon activation in primary Sjögren's syndrome in the JOQUER randomized trial. Rheumatology. 2019;59:107–111. doi: 10.1093/rheumatology/kez242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Misra D.P., Negi V.S. Interferon targeted therapies in systemic lupus erythematosus. Mediterranean Journal of Rheumatology. 2017;28:13–19. doi: 10.31138/mjr.28.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sacre K., Criswell L.A., McCune J.M. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther. 2012;14:R155. doi: 10.1186/ar3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mahdy A.A., Raafat H.A., El-Fishawy H.S., Gheita T.A. Therapeutic potential of hydroxychloroquine on serum B-cell activating factor belonging to the tumor necrosis factor family (BAFF) in rheumatoid arthritis patients. Bulletin of Faculty of Pharmacy, Cairo University. 2014;52:37–43. [Google Scholar]
- 130.Ghasemnejad-Berenji H., Ghaffari Novin M., Hajshafiha M., Nazarian H., Hashemi S.M., Ilkhanizadeh B., et al. Immunomodulatory effects of hydroxychloroquine on Th1/Th2 balance in women with repeated implantation failure. Biomedicine & Pharmacotherapy. 2018;107:1277–1285. doi: 10.1016/j.biopha.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 131.Yang J., Yang X., Yang J., Li M. Hydroxychloroquine inhibits the differentiation of Th17 cells in systemic lupus erythematosus. J. Rheumatol. 2018;45:818–826. doi: 10.3899/jrheum.170737. [DOI] [PubMed] [Google Scholar]
- 132.Dominguez-Gutierrez P.R., Ceribelli A., Satoh M., Sobel E.S., Reeves W.H., Chan E.K. Reduced levels of CCL2 and CXCL10 in systemic lupus erythematosus patients under treatment with prednisone, mycophenolate mofetil, or hydroxychloroquine, except in a high STAT1 subset. Arthritis Res Ther. 2014;16:R23. doi: 10.1186/ar4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Keyaerts E., Li S., Vijgen L., Rysman E., Verbeeck J., Van Ranst M., et al. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob. Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kono M., Tatsumi K., Imai A.M., Saito K., Kuriyama T., Shirasawa H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antivir. Res. 2008;77:150–152. doi: 10.1016/j.antiviral.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morrisette T., Lodise T.P., Scheetz M.H., Goswami S., Pogue J.M., Rybak M.J. The pharmacokinetic and pharmacodynamic properties of hydroxychloroquine and dose selection for COVID-19: putting the cart before the horse. Infect. Dis. Ther. 2020:1–12. doi: 10.1007/s40121-020-00325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Demaurex N. pH homeostasis of cellular organelles. Physiology. 2002;17:1–5. doi: 10.1152/physiologyonline.2002.17.1.1. [DOI] [PubMed] [Google Scholar]
- 141.Huotari J., Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mindell J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- 143.de Duve C. The lysosome turns fifty. Nat. Cell Biol. 2005;7:847–849. doi: 10.1038/ncb0905-847. [DOI] [PubMed] [Google Scholar]
- 144.Koh J.-Y., Kim H.N., Hwang J.J., Kim Y.-H., Park S.E. Lysosomal dysfunction in proteinopathic neurodegenerative disorders: possible therapeutic roles of cAMP and zinc. Molecular Brain. 2019;12:18. doi: 10.1186/s13041-019-0439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kellokumpu S. Golgi pH, ion and redox homeostasis: how much do they really matter? Frontiers in Cell and Developmental Biology. 2019;7 doi: 10.3389/fcell.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Paroutis P., Touret N., Grinstein S. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology. 2004;19:207–215. doi: 10.1152/physiol.00005.2004. [DOI] [PubMed] [Google Scholar]
- 147.Axelsson M.A.B., Karlsson N.G., Steel D.M., Ouwendijk J., Nilsson T., Hansson G.C. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology. 2001;11:633–644. doi: 10.1093/glycob/11.8.633. [DOI] [PubMed] [Google Scholar]
- 148.Rivinoja A., Kokkonen N., Kellokumpu I., Kellokumpu S. Elevated Golgi pH in breast and colorectal cancer cells correlates with the expression of oncofetal carbohydrate T-antigen. J. Cell. Physiol. 2006;208:167–174. doi: 10.1002/jcp.20653. [DOI] [PubMed] [Google Scholar]
- 149.Hassinen A., Kellokumpu S. Organizational interplay of Golgi N-glycosyltransferases involves organelle microenvironment-dependent transitions between enzyme homo- and heteromers. J. Biol. Chem. 2014;289:26937–26948. doi: 10.1074/jbc.M114.595058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rivinoja A., Hassinen A., Kokkonen N., Kauppila A., Kellokumpu S. Elevated Golgi pH impairs terminalN-glycosylation by inducing mislocalization of Golgi glycosyltransferases. J. Cell. Physiol. 2009;220:144–154. doi: 10.1002/jcp.21744. [DOI] [PubMed] [Google Scholar]
- 151.Appenzeller-Herzog C. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J. Cell Sci. 2006;119:2173–2183. doi: 10.1242/jcs.03019. [DOI] [PubMed] [Google Scholar]
- 152.Bräuer P., Parker J.L., Gerondopoulos A., Zimmermann I., Seeger M.A., Barr F.A., et al. Structural basis for pH-dependent retrieval of ER proteins from the Golgi by the KDEL receptor. Science. 2019;363:1103–1107. doi: 10.1126/science.aaw2859. [DOI] [PubMed] [Google Scholar]
- 153.Cancino J., Capalbo A., Di Campli A., Giannotta M., Rizzo R., Jung Juan E., et al. Control systems of membrane transport at the interface between the endoplasmic reticulum and the Golgi. Dev. Cell. 2014;30:280–294. doi: 10.1016/j.devcel.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 154.Palokangas H., Ying M., Väänänen K., Saraste J. Retrograde transport from the pre-Golgi intermediate compartment and the Golgi complex is affected by the vacuolar H+-ATPase inhibitor bafilomycin A1. Mol. Biol. Cell. 1998;9:3561–3578. doi: 10.1091/mbc.9.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kuhn Y., Rohrbach P., Lanzer M. Quantitative pH measurements in Plasmodium falciparum-infected erythrocytes using pHluorin. Cell. Microbiol. 2007;9:1004–1013. doi: 10.1111/j.1462-5822.2006.00847.x. [DOI] [PubMed] [Google Scholar]
- 156.Skinner-Adams T.S., Stack C.M., Trenholme K.R., Brown C.L., Grembecka J., Lowther J., et al. Plasmodium falciparum neutral aminopeptidases: new targets for anti-malarials. Trends Biochem. Sci. 2010;35:53–61. doi: 10.1016/j.tibs.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 157.Browning D.J. Hydroxychloroquine and Chloroquine Retinopathy. 2014. Pharmacology of chloroquine and hydroxychloroquine; pp. 35–63. [Google Scholar]
- 158.Collins K.P., Jackson K.M., Gustafson D.L. Hydroxychloroquine: a physiologically-based pharmacokinetic model in the context of cancer-related autophagy modulation. J. Pharmacol. Exp. Ther. 2018;365:447–459. doi: 10.1124/jpet.117.245639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Martin R.E., Marchetti R.V., Cowan A.I., Howitt S.M., Broer S., Kirk K. Chloroquine transport via the malaria parasite's chloroquine resistance transporter. Science. 2009;325:1680–1682. doi: 10.1126/science.1175667. [DOI] [PubMed] [Google Scholar]
- 160.Johnson D.E., Ostrowski P., Jaumouillé V., Grinstein S. The position of lysosomes within the cell determines their luminal pH. J. Cell Biol. 2016;212:677–692. doi: 10.1083/jcb.201507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Circu M., Cardelli J., Barr M.P., O'Byrne K., Mills G., El-Osta H. Modulating lysosomal function through lysosome membrane permeabilization or autophagy suppression restores sensitivity to cisplatin in refractory non-small-cell lung cancer cells. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kokkonen N., Rivinoja A., Kauppila A., Suokas M., Kellokumpu I., Kellokumpu S. Defective acidification of intracellular organelles results in aberrant secretion of cathepsin D in cancer cells. J. Biol. Chem. 2004;279:39982–39988. doi: 10.1074/jbc.M406698200. [DOI] [PubMed] [Google Scholar]
- 163.Ortega F.G., Roefs M.T., de Miguel Perez D., Kooijmans S.A., de Jong O.G., Sluijter J.P., et al. Interfering with endolysosomal trafficking enhances release of bioactive exosomes. Nanomedicine. 2019;20:102014. doi: 10.1016/j.nano.2019.102014. [DOI] [PubMed] [Google Scholar]
- 164.Boya P. Lysosomal function and dysfunction: mechanism and disease. Antioxid. Redox Signal. 2012;17:766–774. doi: 10.1089/ars.2011.4405. [DOI] [PubMed] [Google Scholar]
- 165.Caporaso G.L., Gandy S.E., Buxbaum J.D., Greengard P. Chloroquine inhibits intracellular degradation but not secretion of Alzheimer beta/A4 amyloid precursor protein. Proc. Natl. Acad. Sci. U. S. A. 1992;89:2252–2256. doi: 10.1073/pnas.89.6.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mauthe M., Orhon I., Rocchi C., Zhou X., Luhr M., Hijlkema K.-J., et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435–1455. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yoon Y.H., Cho K.S., Hwang J.J., Lee S.-J., Choi J.A., Koh J.-Y. Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells. Investigative Opthalmology & Visual Science. 2010;51:6030. doi: 10.1167/iovs.10-5278. [DOI] [PubMed] [Google Scholar]
- 168.Thorens B., Vassalli P. Chloroquine and ammonium chloride prevent terminal glycosylation of immunoglobulins in plasma cells without affecting secretion. Nature. 1986;321:618–620. doi: 10.1038/321618a0. [DOI] [PubMed] [Google Scholar]
- 169.Hiebsch R.R., Wattenberg B.W. Springer Berlin Heidelberg; 1993. Fusion rapidly follows vesicle transport to the target membrane in protein transport through the Golgi apparatus in vitro. A re-evaluation of transport kinetics based on the finding that the glycosylation used to mark transport, and not transport itself, is rate limiting in the assay. Molecular Mechanisms of Membrane Traffic; pp. 45–51. [Google Scholar]
- 170.Oda K., Ikehara Y. Weakly basic amines inhibit the proteolytic conversion of proalbumin to serum albumin in cultured rat hepatocytes. Eur. J. Biochem. 1985;152:605–609. doi: 10.1111/j.1432-1033.1985.tb09238.x. [DOI] [PubMed] [Google Scholar]
- 171.Khan N., Halcrow P.W., Lakpa K.L., Afghah Z., Miller N.M., Dowdy S.F., et al. Two-pore channels regulate Tat endolysosome escape and Tat-mediated HIV-1 LTR transactivation. FASEB J. 2020;34:4147–4162. doi: 10.1096/fj.201902534R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sironi J., Aranda E., Nordstrøm L.U., Schwartz E.L. Lysosome membrane permeabilization and disruption of the molecular target of rapamycin (mTOR)-lysosome interaction are associated with the inhibition of lung cancer cell proliferation by a chloroquinoline analog. Mol. Pharmacol. 2019;95:127–138. doi: 10.1124/mol.118.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang F., Gómez-Sintes R., Boya P. Lysosomal membrane permeabilization and cell death. Traffic. 2018;19:918–931. doi: 10.1111/tra.12613. [DOI] [PubMed] [Google Scholar]
- 174.Jaimes J.A., Millet J.K., Whittaker G.R. Proteolytic cleavage of the SARS-CoV-2 spike protein and the role of the novel S1/S2 site. iScience. 2020;23 doi: 10.1016/j.isci.2020.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Poduri R., Joshi G., Jagadeesh G. Drugs targeting various stages of the SARS-CoV-2 life cycle: exploring promising drugs for the treatment of Covid-19. Cell. Signal. 2020;74 doi: 10.1016/j.cellsig.2020.109721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hoffmann M., Kleine-Weber H., Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell. 2020;78(e5):779–784. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bestle D., Heindl M.R., Limburg H., Van Lam van T., Pilgram O., Moulton H., et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020;3 doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Feliciangeli S.F., Thomas L., Scott G.K., Subbian E., Hung C.-H., Molloy S.S., et al. Identification of a pH sensor in the furin propeptide that regulates enzyme activation. J. Biol. Chem. 2006;281:16108–16116. doi: 10.1074/jbc.M600760200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L., et al. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10:e1004502–e. doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stadler K., Ha H.R., Ciminale V., Spirli C., Saletti G., Schiavon M., et al. Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level. Am. J. Respir. Cell Mol. Biol. 2008;39:142–149. doi: 10.1165/rcmb.2007-0217OC. [DOI] [PubMed] [Google Scholar]
- 184.Yang N., Shen H.-M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int. J. Biol. Sci. 2020;16:1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mingo R.M., Simmons J.A., Shoemaker C.J., Nelson E.A., Schornberg K.L., D'Souza R.S., et al. Ebola virus and severe acute respiratory syndrome coronavirus display late cell entry kinetics: evidence that transport to NPC1+ endolysosomes is a rate-defining step. J. Virol. 2015;89:2931–2943. doi: 10.1128/JVI.03398-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sa Ribero M., Jouvenet N., Dreux M., Nisole S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020;16:e1008737–e. doi: 10.1371/journal.ppat.1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hagemeijer M.C., Monastyrska I., Griffith J., van der Sluijs P., Voortman J., van Bergen en Henegouwen P.M., et al. Membrane rearrangements mediated by coronavirus nonstructural proteins 3 and 4. Virology. 2014;458-459:125–135. doi: 10.1016/j.virol.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wolff G., Limpens R.W.A.L., Zevenhoven-Dobbe J.C., Laugks U., Zheng S., de Jong A.W.M., et al. A molecular pore spans the double membrane of the coronavirus replication organelle. Science. 2020:eabd3629. doi: 10.1126/science.abd3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Adams M.J., Lefkowitz E.J., King A.M.Q., Harrach B., Harrison R.L., Knowles N.J., et al. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2016) Arch. Virol. 2016;161:2921–2949. doi: 10.1007/s00705-016-2977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Drosten C., Preiser W., Günther S., Schmitz H., Doerr H.W. Severe acute respiratory syndrome: identification of the etiological agent. Trends Mol. Med. 2003;9:325–327. doi: 10.1016/S1471-4914(03)00133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gorbalenya A.E., Enjuanes L., Ziebuhr J., Snijder E.J. Nidovirales: evolving the largest RNA virus genome. Virus Res. 2006;117:17–37. doi: 10.1016/j.virusres.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Astuti I., Ysrafil Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes & metabolic syndrome. 2020;14:407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Stertz S., Reichelt M., Spiegel M., Kuri T., Martínez-Sobrido L., García-Sastre A., et al. The intracellular sites of early replication and budding of SARS-coronavirus. Virology. 2007;361:304–315. doi: 10.1016/j.virol.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Venkatagopalan P., Daskalova S.M., Lopez L.A., Dolezal K.A., Hogue B.G. Coronavirus envelope (E) protein remains at the site of assembly. Virology. 2015;478:75–85. doi: 10.1016/j.virol.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Minakshi R., Padhan K. The YXXΦ motif within the severe acute respiratory syndrome coronavirus (SARS-CoV) 3a protein is crucial for its intracellular transport. Virol. J. 2014;11:75. doi: 10.1186/1743-422X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ritchie G., Harvey D.J., Feldmann F., Stroeher U., Feldmann H., Royle L., et al. Identification of N-linked carbohydrates from severe acute respiratory syndrome (SARS) spike glycoprotein. Virology. 2010;399:257–269. doi: 10.1016/j.virol.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Siu K.-L., Chan C.-P., Kok K.-H., Chiu-Yat Woo P., Jin D.-Y. Suppression of innate antiviral response by severe acute respiratory syndrome coronavirus M protein is mediated through the first transmembrane domain. Cellular & Molecular Immunology. 2014;11:141–149. doi: 10.1038/cmi.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Walls A.C., Xiong X., Park Y.-J., Tortorici M.A., Snijder J., Quispe J., et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176(e15):1026–1039. doi: 10.1016/j.cell.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yuan X., Li J., Shan Y., Yang Z., Zhao Z., Chen B., et al. Subcellular localization and membrane association of SARS-CoV 3a protein. Virus Res. 2005;109:191–202. doi: 10.1016/j.virusres.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Davies J.P., Almasy K.M., McDonald E.F., Plate L. 2020. Comparative Multiplexed Interactomics of SARS-CoV-2 and Homologous Coronavirus Non-structural Proteins Identifies Unique and Shared Host-cell Dependencies. bioRxiv: The Preprint Server for Biology. (2020.07.13.201517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Masters P.S. Academic Press; 2006. The Molecular Biology of Coronaviruses. Advances in Virus Research; pp. 193–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Oostra M., de Haan C.A.M., de Groot R.J., Rottier P.J.M. Glycosylation of the severe acute respiratory syndrome coronavirus triple-spanning membrane proteins 3a and M. J. Virol. 2006;80:2326–2336. doi: 10.1128/JVI.80.5.2326-2336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Watanabe Y., Allen J.D., Wrapp D., McLellan J.S., Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369:330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang T.-J., Chang Y.-C., Ko T.-P., Draczkowski P., Chien Y.-C., Chang Y.-C., et al. Cryo-EM analysis of a feline coronavirus spike protein reveals a unique structure and camouflaging glycans. Proc. Natl. Acad. Sci. 2020;117:1438–1446. doi: 10.1073/pnas.1908898117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hassanpour M., Rezaie J., Nouri M., Panahi Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhu N., Wang W., Liu Z., Liang C., Wang W., Ye F., et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat. Commun. 2020;11:3910. doi: 10.1038/s41467-020-17796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Host factors in coronavirus replication. Curr. Top. Microbiol. Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fung T.S., Liu D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shi C.-S., Nabar N.R., Huang N.-N., Kehrl J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discovery. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Morris G., Puri B.K., Walder K., Berk M., Stubbs B., Maes M., et al. The endoplasmic reticulum stress response in neuroprogressive diseases: emerging pathophysiological role and translational implications. Mol. Neurobiol. 2018;55:8765–8787. doi: 10.1007/s12035-018-1028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cottam E.M., Maier H.J., Manifava M., Vaux L.C., Chandra-Schoenfelder P., Gerner W., et al. Coronavirus nsp6 proteins generate autophagosomes from the endoplasmic reticulum via an omegasome intermediate. Autophagy. 2011;7:1335–1347. doi: 10.4161/auto.7.11.16642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cottam E.M., Whelband M.C., Wileman T. Coronavirus NSP6 restricts autophagosome expansion. Autophagy. 2014;10:1426–1441. doi: 10.4161/auto.29309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gassen N.C., Papies J., Bajaj T., Dethloff F., Emanuel J., Weckmann K., et al. 2020. Analysis of SARS-CoV-2-Controlled Autophagy Reveals Spermidine, MK-2206, and Niclosamide as Putative Antiviral Therapeutics. bioRxiv. (2020.04.15.997254) [Google Scholar]
- 214.Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B., et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2014;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Carmona-Gutierrez D., Bauer M.A., Zimmermann A., Kainz K., Hofer S.J., Kroemer G., et al. Digesting the crisis: autophagy and coronaviruses. Microb Cell. 2020;7:119–128. doi: 10.15698/mic2020.05.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fitzwalter B.E., Towers C.G., Sullivan K.D., Andrysik Z., Hoh M., Ludwig M., et al. Autophagy inhibition mediates apoptosis sensitization in cancer therapy by relieving FOXO3a turnover. Dev. Cell. 2018;44(e3):555–565. doi: 10.1016/j.devcel.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tompkins K.D., Thorburn A. Regulation of apoptosis by autophagy to enhance cancer therapy. The Yale Journal of Biology and Medicine. 2019;92:707–718. [PMC free article] [PubMed] [Google Scholar]
- 218.Chude C.I., Amaravadi R.K. Targeting autophagy in cancer: update on clinical trials and novel inhibitors. Int. J. Mol. Sci. 2017;18:1279. doi: 10.3390/ijms18061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zeh H.J., Bahary N., Boone B.A., Singhi A.D., Miller-Ocuin J.L., Normolle D.P., et al. A randomized phase II preoperative study of autophagy inhibition with high-dose hydroxychloroquine and gemcitabine/Nab-paclitaxel in pancreatic cancer patients. Clin. Cancer Res. 2020;26:3126–3134. doi: 10.1158/1078-0432.CCR-19-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Naarding M.A., Baan E., Pollakis G., Paxton W.A. Effect of chloroquine on reducing HIV-1 replication in vitro and the DC-SIGN mediated transfer of virus to CD4+ T-lymphocytes. Retrovirology. 2007;4:6. doi: 10.1186/1742-4690-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Akpovwa H. Chloroquine could be used for the treatment of filoviral infections and other viral infections that emerge or emerged from viruses requiring an acidic pH for infectivity. Cell Biochem. Funct. 2016;34:191–196. doi: 10.1002/cbf.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.D'Alessandro S., Scaccabarozzi D., Signorini L., Perego F., Ilboudo D.P., Ferrante P., et al. The use of antimalarial drugs against viral infection. Microorganisms. 2020;8 doi: 10.3390/microorganisms8010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Falzarano D., de Wit E., Feldmann F., Rasmussen A.L., Okumura A., Peng X., et al. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tan Y.W., Yam W.K., Sun J., Chu J.J.H. An evaluation of chloroquine as a broad-acting antiviral against Hand. Foot and Mouth Disease. Antiviral research. 2018;149:143–149. doi: 10.1016/j.antiviral.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 226.Paton N.I., Goodall R.L., Dunn D.T., Franzen S., Collaco-Moraes Y., Gazzard B.G., et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA. 2012;308:353–361. doi: 10.1001/jama.2012.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bauman J.L., Tisdale J.E. Chloroquine and hydroxychloroquine in the era of SARS - CoV2: caution on their cardiac toxicity. Pharmacotherapy. 2020;40:387–388. doi: 10.1002/phar.2387. [DOI] [PubMed] [Google Scholar]
- 228.Jeevaratnam K., Chadda K.R., Huang C.L.H., Camm A.J. Cardiac potassium channels: physiological insights for targeted therapy. J. Cardiovasc. Pharmacol. Ther. 2018;23:119–129. doi: 10.1177/1074248417729880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Joyce E., Fabre A., Mahon N. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur. Heart J. Acute Cardiovasc. Care. 2013;2:77–83. doi: 10.1177/2048872612471215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tselios K., Deeb M., Gladman D.D., Harvey P., Akhtari S., Mak S., et al. Antimalarial-induced cardiomyopathy in systemic lupus erythematosus: as rare as considered? J. Rheumatol. 2019;46:391–396. doi: 10.3899/jrheum.180124. [DOI] [PubMed] [Google Scholar]
- 231.Chan X.H.S., Win Y.N., Haeusler I.L., Tan J.Y., Loganathan S., Saralamba S., et al. Factors affecting the electrocardiographic QT interval in malaria: a systematic review and meta-analysis of individual patient data. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Haeusler I.L., Chan X.H.S., Guerin P.J., White N.J. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med. 2018;16:200. doi: 10.1186/s12916-018-1188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bok S., Kim Y.-E., Woo Y., Kim S., Kang S.-J., Lee Y., et al. Hypoxia-inducible factor-1α regulates microglial functions affecting neuronal survival in the acute phase of ischemic stroke in mice. Oncotarget. 2017;8:111508–111521. doi: 10.18632/oncotarget.22851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Fanouriakis A., Kostopoulou M., Alunno A., Aringer M., Bajema I., Boletis J.N., et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019;78:736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 235.Geamănu Pancă A., Popa-Cherecheanu A., Marinescu B., Geamănu C.D., Voinea L.M. Retinal toxicity associated with chronic exposure to hydroxychloroquine and its ocular screening. Review. J Med Life. 2014;7:322–326. [PMC free article] [PubMed] [Google Scholar]
- 236.Abarientos C., Sperber K., Shapiro D.L., Aronow W.S., Chao C.P., Ash J.Y. Hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis and its safety in pregnancy. Expert Opin. Drug Saf. 2011;10:705–714. doi: 10.1517/14740338.2011.566555. [DOI] [PubMed] [Google Scholar]
- 237.Kalia S., Dutz J.P. New concepts in antimalarial use and mode of action in dermatology. Dermatol. Ther. 2007;20:160–174. doi: 10.1111/j.1529-8019.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kaufmann A.M., Krise J.P. Lysosomal sequestration of amine-containing drugs: analysis and therapeutic implications. J. Pharm. Sci. 2007;96:729–746. doi: 10.1002/jps.20792. [DOI] [PubMed] [Google Scholar]
- 239.Chen X., Geiger J.D. Janus sword actions of chloroquine and hydroxychloroquine against COVID-19. Cell. Signal. 2020;73 doi: 10.1016/j.cellsig.2020.109706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 241.Munster T., Gibbs J.P., Shen D., Baethge B.A., Botstein G.R., Caldwell J., et al. Hydroxychloroquine concentration-response relationships in patients with rheumatoid arthritis. Arthritis & Rheumatism. 2002;46:1460–1469. doi: 10.1002/art.10307. [DOI] [PubMed] [Google Scholar]
- 242.Fan H.-W., Ma Z.-X., Chen J., Yang X.-Y., Cheng J.-L., Li Y.-B. Pharmacokinetics and bioequivalence study of hydroxychloroquine sulfate tablets in Chinese healthy volunteers by LC-MS/MS. Rheumatol Ther. 2015;2:183–195. doi: 10.1007/s40744-015-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kauv J., Lê M.P., Veyrier M., Le Hingrat Q., Visseaux B., Massias L., et al. Failure of hydroxychloroquine pre-exposure prophylaxis in COVID-19 infection? A case report. J. Antimicrob. Chemother. 2020:dkaa213. doi: 10.1093/jac/dkaa213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lim H.-S., Im J.-S., Cho J.-Y., Bae K.-S., Klein T.A., Yeom J.-S., et al. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by <em>Plasmodium vivax. </em>. Antimicrob Agents Chemother. 2009;53:1468–1475. doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Furst D.E., Lindsley H., Baethge B., Botstein G.R., Caldwell J., Dietz F., et al. Dose-loading with hydroxychloroquine improves the rate of response in early, active rheumatoid arthritis: a randomized, double-blind six-week trial with eighteen-week extension. Arthritis Rheum. 1999;42:357–365. doi: 10.1002/1529-0131(199902)42:2<357::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 246.Savarino A., Shytaj I.L. Chloroquine and beyond: exploring anti-rheumatic drugs to reduce immune hyperactivation in HIV/AIDS. Retrovirology. 2015;12:51. doi: 10.1186/s12977-015-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kang C.K., Seong M.W., Choi S.J., Kim T.S., Choe P.G., Song S.H., et al. In vitro activity of lopinavir/ritonavir and hydroxychloroquine against severe acute respiratory syndrome coronavirus 2 at concentrations achievable by usual doses. Korean J. Intern. Med. 2020;35:782–787. doi: 10.3904/kjim.2020.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Garcia-Cremades M., Solans B.P., Hughes E., Ernest J.P., Wallender E., Aweeka F., et al. Optimizing hydroxychloroquine dosing for patients with COVID-19: an integrative modeling approach for effective drug repurposing. Clin. Pharmacol. Ther. 2020;108:253–263. doi: 10.1002/cpt.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pellegrini P., Strambi A., Zipoli C., Hägg-Olofsson M., Buoncervello M., Linder S., et al. Acidic extracellular pH neutralizes the autophagy-inhibiting activity of chloroquine: implications for cancer therapies. Autophagy. 2014;10:562–571. doi: 10.4161/auto.27901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhitomirsky B., Assaraf Y.G. Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance. Oncotarget. 2015;6:1143–1156. doi: 10.18632/oncotarget.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Erra Díaz F., Dantas E., Geffner J. Unravelling the interplay between extracellular acidosis and immune cells. Mediat. Inflamm. 2018;2018 doi: 10.1155/2018/1218297. (1218297-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Martínez D., Vermeulen M., von Euw E., Sabatté J., Maggíni J., Ceballos A., et al. Extracellular acidosis triggers the maturation of human dendritic cells and the production of IL-12. J. Immunol. 2007;179:1950–1959. doi: 10.4049/jimmunol.179.3.1950. [DOI] [PubMed] [Google Scholar]
- 254.Rajamäki K., Nordström T., Nurmi K., Åkerman K.E., Kovanen P.T., Öörni K., et al. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J. Biol. Chem. 2013;288:13410–13419. doi: 10.1074/jbc.M112.426254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Xiong J., Zhu M.X. Regulation of lysosomal ion homeostasis by channels and transporters. Sci. China Life Sci. 2016;59:777–791. doi: 10.1007/s11427-016-5090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R., et al. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 257.Rosenke K., Jarvis M.A., Feldmann F., Schwarz B., Okumura A., Lovaglio J., et al. 2020. Hydroxychloroquine Proves Ineffective in Hamsters and Macaques Infected with SARS-CoV-2. bioRxiv: The Preprint Server for Biology. (2020.06.10.145144) [Google Scholar]
- 258.Amsden G.W., Gray C.L. Serum and WBC pharmacokinetics of 1500 mg of azithromycin when given either as a single dose or over a 3 day period in healthy volunteers. J. Antimicrob. Chemother. 2001;47:61–66. doi: 10.1093/jac/47.1.61. [DOI] [PubMed] [Google Scholar]
- 259.Gualdoni G.A., Lingscheid T., Schmetterer K.G., Hennig A., Steinberger P., Zlabinger G.J. Azithromycin inhibits IL-1 secretion and non-canonical inflammasome activation. Sci. Rep. 2015;5 doi: 10.1038/srep12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Idris S.F., Chilvers E.R., Haworth C., McKeon D., Condliffe A.M. Azithromycin therapy for neutrophilic airways disease: myth or magic? Thorax. 2009;64:186–189. doi: 10.1136/thx.2008.103192. [DOI] [PubMed] [Google Scholar]
- 261.Liu P., Allaudeen H., Chandra R., Phillips K., Jungnik A., Breen J.D., et al. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimen. Antimicrob. Agents Chemother. 2007;51:103–109. doi: 10.1128/AAC.00852-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Aghai Z.H., Kode A., Saslow J.G., Nakhla T., Farhath S., Stahl G.E., et al. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr. Res. 2007;62:483–488. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 263.Stellari F.F., Sala A., Donofrio G., Ruscitti F., Caruso P., Topini T.M., et al. Azithromycin inhibits nuclear factor-κB activation during lung inflammation: an in vivo imaging study. Pharmacol. Res. Perspect. 2014;2:e00058–e. doi: 10.1002/prp2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-Guardeño J.M., Fernandez-Delgado R., Fett C., et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014;88:913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Eleouet J-Fo, Chilmonczyk S., Besnardeau L., Laude H. Transmissible gastroenteritis coronavirus induces programmed cell death in infected cells through a caspase-dependent pathway. J. Virol. 1998;72:4918–4924. doi: 10.1128/jvi.72.6.4918-4924.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kanzawa N., Nishigaki K., Hayashi T., Ishii Y., Furukawa S., Niiro A., et al. Augmentation of chemokine production by severe acute respiratory syndrome coronavirus 3a/X1 and 7a/X4 proteins through NF-kappaB activation. FEBS Lett. 2006;580:6807–6812. doi: 10.1016/j.febslet.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li J., Liu Y., Zhang X. Murine coronavirus induces type I interferon in oligodendrocytes through recognition by RIG-I and MDA5. J. Virol. 2010;84:6472–6482. doi: 10.1128/JVI.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yang Y., Zhang L., Geng H., Deng Y., Huang B., Guo Y., et al. The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists. Protein & Cell. 2013;4:951–961. doi: 10.1007/s13238-013-3096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cory T.J., Birket S.E., Murphy B.S., Hayes D., Jr., Anstead M.I., Kanga J.F., et al. Impact of azithromycin treatment on macrophage gene expression in subjects with cystic fibrosis. J. Cyst. Fibros. 2014;13:164–171. doi: 10.1016/j.jcf.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Feola D.J., Garvy B.A., Cory T.J., Birket S.E., Hoy H., Hayes D., Jr., et al. Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas. Antimicrob. Agents Chemother. 2010;54:2437–2447. doi: 10.1128/AAC.01424-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Haydar D., Cory T.J., Birket S.E., Murphy B.S., Pennypacker K.R., Sinai A.P., et al. Azithromycin polarizes macrophages to an M2 phenotype via inhibition of the STAT1 and NF-κB signaling pathways. J. Immunol. 2019;203:1021–1030. doi: 10.4049/jimmunol.1801228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Murphy B.S., Sundareshan V., Cory T.J., Hayes D., Anstead M.I., Feola D.J. Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother. 2008;61:554–560. doi: 10.1093/jac/dkn007. [DOI] [PubMed] [Google Scholar]
- 273.Wang J., Xie L., Wang S., Lin J., Liang J., Xu J. Azithromycin promotes alternatively activated macrophage phenotype in systematic lupus erythematosus via PI3K/Akt signaling pathway. Cell Death Dis. 2018;9:1080. doi: 10.1038/s41419-018-1097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sharif O., Brunner J.S., Vogel A., Schabbauer G. Macrophage rewiring by nutrient associated PI3K dependent pathways. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hodge S., Hodge G., Brozyna S., Jersmann H., Holmes M., Reynolds P.N. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur. Respir. J. 2006;28:486–495. doi: 10.1183/09031936.06.00001506. [DOI] [PubMed] [Google Scholar]
- 276.Hodge S., Hodge G., Jersmann H., Matthews G., Ahern J., Holmes M., et al. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;178:139–148. doi: 10.1164/rccm.200711-1666OC. [DOI] [PubMed] [Google Scholar]
- 277.Hodge S., Reynolds P.N. Low-dose azithromycin improves phagocytosis of bacteria by both alveolar and monocyte-derived macrophagesin chronic obstructive pulmonary disease subjects. Respirology. 2012;17:802–807. doi: 10.1111/j.1440-1843.2012.02135.x. [DOI] [PubMed] [Google Scholar]
- 278.Fan E.K.Y., Fan J. Regulation of alveolar macrophage death in acute lung inflammation. Respir. Res. 2018;19:50. doi: 10.1186/s12931-018-0756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Koch C.C. Apoptosis, oxidative metabolism and interleukin-8 production in human neutrophils exposed to azithromycin: effects of Streptococcus pneumoniae. J. Antimicrob. Chemother. 2000;46:19–26. doi: 10.1093/jac/46.1.19. [DOI] [PubMed] [Google Scholar]
- 280.Legssyer R., Huaux F., Lebacq J., Delos M., Marbaix E., Lebecque P., et al. Azithromycin reduces spontaneous and induced inflammation in ΔF508 cystic fibrosis mice. Respir. Res. 2006;7 doi: 10.1186/1465-9921-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Parnham M.J., Čulić O., Eraković V., Munić V., Popović-Grle S., Barišić K., et al. Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short-term azithromycin treatment. Eur. J. Pharmacol. 2005;517:132–143. doi: 10.1016/j.ejphar.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 282.Silvestri M., Oddera S., Eftimiadi C., Rossi G.A. Azithromycin induces in vitro a time-dependent increase in the intracellular killing of Staphylococcus aureus by human polymorphonuclear leucocytes without damaging phagocytes. J. Antimicrob. Chemother. 1995;36:941–950. doi: 10.1093/jac/36.6.941. [DOI] [PubMed] [Google Scholar]
- 283.Castellani S., D'Oria S., Diana A., Polizzi A.M., Di Gioia S., Mariggiò M.A., et al. G-CSF and GM-CSF modify neutrophil functions at concentrations found in cystic fibrosis. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Yamasawa H., Oshikawa K., Ohno S., Sugiyama Y. Macrolides inhibit epithelial cell–mediated neutrophil survival by modulating granulocyte macrophage colony–stimulating factor release. Am. J. Respir. Cell Mol. Biol. 2004;30:569–575. doi: 10.1165/rcmb.2003-0105OC. [DOI] [PubMed] [Google Scholar]
- 285.Choi J.C., Jung J.W., Kwak H.W., Song J.H., Jeon E.J., Shin J.W., et al. Granulocyte macrophage-colony stimulating factor (GM-CSF) augments acute lung injury via its neutrophil priming effects. J. Korean Med. Sci. 2008;23:288–295. doi: 10.3346/jkms.2008.23.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Puljic R., Benediktus E., Plater-Zyberk C., Baeuerle P.A., Szelenyi S., Brune K., et al. Lipopolysaccharide-induced lung inflammation is inhibited by neutralization of GM-CSF. Eur. J. Pharmacol. 2007;557:230–235. doi: 10.1016/j.ejphar.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 287.Luu N.V., Yang J., Qu X.J., Guo M., Wang X., Xian Q.Y., et al. Azithromycin inhibits neutrophil accumulation in airways by affecting interleukin-17 downstream signals. Chin. Med. J. 2012;125:491–495. [PubMed] [Google Scholar]
- 288.Piacentini G.L., Peroni D.G., Bodini A., Pigozzi R., Costella S., Loiacono A., et al. Azithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: a preliminary report. Allergy and Asthma Proceedings. 2007;28:194–198. doi: 10.2500/aap.2007.28.2958. [DOI] [PubMed] [Google Scholar]
- 289.Tsai W.C., Rodriguez M.L., Young K.S., Deng J.C., Thannickal V.J., Tateda K., et al. Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infection. Am. J. Respir. Crit. Care Med. 2004;170:1331–1339. doi: 10.1164/rccm.200402-200OC. [DOI] [PubMed] [Google Scholar]
- 290.Verleden G.M., Vanaudenaerde B.M., Dupont L.J., Van Raemdonck D.E. Azithromycin reduces airway neutrophilia and interleukin-8 in patients with bronchiolitis obliterans syndrome. Am. J. Respir. Crit. Care Med. 2006;174:566–570. doi: 10.1164/rccm.200601-071OC. [DOI] [PubMed] [Google Scholar]
- 291.Potey P.M., Rossi A.G., Lucas C.D., Dorward D.A. Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding biological function and therapeutic potential. J. Pathol. 2019;247:672–685. doi: 10.1002/path.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zemans R.L., Matthay M.A. What drives neutrophils to the alveoli in ARDS? Thorax. 2017;72:1–3. doi: 10.1136/thoraxjnl-2016-209170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Lendermon E.A., Coon T.A., Bednash J.S., Weathington N.M., McDyer J.F., Mallampalli R.K. Azithromycin decreases NALP3 mRNA stability in monocytes to limit inflammasome-dependent inflammation. Respir. Res. 2017;18 doi: 10.1186/s12931-017-0608-8. (131-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Yap J.K.Y., Moriyama M., Iwasaki A. Inflammasomes and pyroptosis as therapeutic targets for COVID-19. J. Immunol. 2020:ji2000513. doi: 10.4049/jimmunol.2000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Afshar M., Foster C.L., Layden J.E., Burnham E.L. Azithromycin use and outcomes in severe sepsis patients with and without pneumonia. J. Crit. Care. 2016;32:120–125. doi: 10.1016/j.jcrc.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kawamura K., Ichikado K., Takaki M., Eguchi Y., Anan K., Suga M. Adjunctive therapy with azithromycin for moderate and severe acute respiratory distress syndrome: a retrospective, propensity score-matching analysis of prospectively collected data at a single center. Int. J. Antimicrob. Agents. 2018;51:918–924. doi: 10.1016/j.ijantimicag.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 297.Kawamura K., Ichikado K., Takaki M., Sakata Y., Yasuda Y., Shingu N., et al. Efficacy of azithromycin in sepsis-associated acute respiratory distress syndrome: a retrospective study and propensity score analysis. Springerplus. 2016;5 doi: 10.1186/s40064-016-2866-1. (1193-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ivetić Tkalčević V., Bošnjak B., Hrvačić B., Bosnar M., Marjanović N., Ferenčić Ž., et al. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. Eur. J. Pharmacol. 2006;539:131–138. doi: 10.1016/j.ejphar.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 299.Nicolau D.P., Banevicius M.A., Marangos M.N., Klepser M.E., Quintiliani R., Nightingale C.H. Influence of adjunct azithromycin on the mortality of experimental Pseudomonas aeruginosa sepsis. Int. J. Antimicrob. Agents. 1997;8:239–241. doi: 10.1016/s0924-8579(97)00017-4. [DOI] [PubMed] [Google Scholar]
- 300.Patel A., Joseph J., Periasamy H., Mokale S. Azithromycin in combination with ceftriaxone reduces systemic inflammation and provides survival benefit in a murine model of polymicrobial sepsis. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.00752-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Bleyzac N., Goutelle S., Bourguignon L., Tod M. Azithromycin for COVID-19: more than just an antimicrobial? Clinical drug investigation. 2020;40:683–686. doi: 10.1007/s40261-020-00933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 2010;36:646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 303.Schögler A., Kopf B.S., Edwards M.R., Johnston S.L., Casaulta C., Kieninger E., et al. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur. Respir. J. 2015;45:428–439. doi: 10.1183/09031936.00102014. [DOI] [PubMed] [Google Scholar]
- 304.Li C., Zu S., Deng Y.-Q., Li D., Parvatiyar K., Quanquin N., et al. Azithromycin protects against zika virus infection by upregulating virus-induced type I and III interferon responses. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.00394-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Uhl S., Hoagland D., Møller R., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hackbart M., Deng X., Baker S.C. Coronavirus endoribonuclease targets viral polyuridine sequences to evade activating host sensors. Proc. Natl. Acad. Sci. 2020;117:8094–8103. doi: 10.1073/pnas.1921485117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Konno Y., Kimura I., Uriu K., Fukushi M., Irie T., Koyanagi Y., et al. 2020. SARS-CoV-2 ORF3b Is a Potent Interferon Antagonist Whose Activity Is Further Increased by a Naturally Occurring Elongation Variant. bioRxiv. (2020.05.11.088179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., et al. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Moriya S., Che X.-F., Komatsu S., Abe A., Kawaguchi T., Gotoh A., et al. Macrolide antibiotics block autophagy flux and sensitize to bortezomib via endoplasmic reticulum stress-mediated CHOP induction in myeloma cells. Int. J. Oncol. 2013;42:1541–1550. doi: 10.3892/ijo.2013.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Qiao X., Wang X., Shang Y., Li Y., S-z Chen. Azithromycin enhances anticancer activity of TRAIL by inhibiting autophagy and up-regulating the protein levels of DR4/5 in colon cancer cells in vitro and in vivo. Cancer Communications. 2018;38:43. doi: 10.1186/s40880-018-0309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Renna M., Schaffner C., Brown K., Shang S., Tamayo M.H., Hegyi K., et al. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J. Clin. Invest. 2011;121:3554–3563. doi: 10.1172/JCI46095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M., et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020;145 doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M. Azithromycin and the risk of cardiovascular death. N. Engl. J. Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Almalki Z.S., Guo J.J. Cardiovascular events and safety outcomes associated with azithromycin therapy: a meta-analysis of randomized controlled trials. Am Health Drug Benefits. 2014;7:318–328. [PMC free article] [PubMed] [Google Scholar]
- 315.Gorelik E., Masarwa R., Perlman A., Rotshild V., Muszkat M., Matok I. Systematic review, meta-analysis, and network meta-analysis of the cardiovascular safety of macrolides. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.00438-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Saleh M., Gabriels J., Chang D., Soo Kim B., Mansoor A., Mahmood E., et al. Effect of chloroquine, hydroxychloroquine, and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ. Arrhythm. Electrophysiol. 2020;13:e008662. doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sarayani A., Cicali B., Henriksen C.H., Brown J.D. Safety signals for QT prolongation or Torsades de Pointes associated with azithromycin with or without chloroquine or hydroxychloroquine. Res. Soc. Adm. Pharm. 2020 doi: 10.1016/j.sapharm.2020.04.016. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin. Proc. 2020;95:1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Funk R.S., Krise J.P. Cationic amphiphilic drugs cause a marked expansion of apparent lysosomal volume: implications for an intracellular distribution-based drug interaction. Mol. Pharm. 2012;9:1384–1395. doi: 10.1021/mp200641e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Poschet J.F., Perkett E.A., Timmins G.S., Deretic V. 2020. Azithromycin and Ciprofloxacin Have a Chloroquine-like Effect on Respiratory Epithelial Cells. bioRxiv. (2020.03.29.008631) [Google Scholar]
- 321.Munić V., Kelnerić Ž., Mikac L., Eraković Haber V. Differences in assessment of macrolide interaction with human MDR1 (ABCB1, P-gp) using rhodamine-123 efflux, ATPase activity and cellular accumulation assays. Eur. J. Pharm. Sci. 2010;41:86–95. doi: 10.1016/j.ejps.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 322.Togami K., Chono S., Morimoto K. Subcellular distribution of azithromycin and clarithromycin in rat alveolar macrophages (NR8383) in vitro. Biol. Pharm. Bull. 2013;36:1494–1499. doi: 10.1248/bpb.b13-00423. [DOI] [PubMed] [Google Scholar]
- 323.Hand W.L., Hand D.L. Characteristics and mechanisms of azithromycin accumulation and efflux in human polymorphonuclear leukocytes. Int. J. Antimicrob. Agents. 2001;18:419–425. doi: 10.1016/s0924-8579(01)00430-7. [DOI] [PubMed] [Google Scholar]
- 324.McDonald P.J., Pruul H. Phagocyte uptake and transport of azithromycin. Eur. J. Clin. Microbiol. Infect. Dis. 1991;10:828–833. doi: 10.1007/BF01975835. [DOI] [PubMed] [Google Scholar]
- 325.Amsden G.W. Advanced-generation macrolides: tissue-directed antibiotics. Int. J. Antimicrob. Agents. 2001;18(Suppl. 1):S11–S15. doi: 10.1016/s0924-8579(01)00410-1. [DOI] [PubMed] [Google Scholar]
- 326.Drew R.H., Gallis H.A. Azithromycin—spectrum of activity, pharmacokinetics, and clinical applications. Pharmacotherapy. 1992;12:161–173. [PubMed] [Google Scholar]
- 327.Rapp R.P. Pharmacokinetics and pharmacodynamics of intravenous and oral azithromycin: enhanced tissue activity and minimal drug interactions. Ann. Pharmacother. 1998;32:785–793. doi: 10.1345/aph.17299. [DOI] [PubMed] [Google Scholar]
- 328.Foulds G., Shepard R.M., Johnson R.B. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 1990;25(Suppl A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- 329.Lode H., Borner K., Koeppe P., Schaberg T. Azithromycin—review of key chemical, pharmacokinetic and microbiological features. J. Antimicrob. Chemother. 1996;37(Suppl C):1–8. doi: 10.1093/jac/37.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- 330.Danesi R., Lupetti A., Barbara C., Ghelardi E., Chella A., Malizia T., et al. Comparative distribution of azithromycin in lung tissue of patients given oral daily doses of 500 and 1000 mg. J. Antimicrob. Chemother. 2003;51:939–945. doi: 10.1093/jac/dkg138. [DOI] [PubMed] [Google Scholar]
- 331.Di Paolo A., Barbara C., Chella A., Angeletti C.A., Del Tacca M. Pharmacokinetics of azithromycin in lung tissue, bronchial washing, and plasma in patients given multiple oral doses of 500 and 1000 mg daily. Pharmacol. Res. 2002;46:545–550. doi: 10.1016/s1043661802002384. [DOI] [PubMed] [Google Scholar]
- 332.Lucchi M., Damle B., Fang A., de Caprariis P.J., Mussi A., Sanchez S.P., et al. Pharmacokinetics of azithromycin in serum, bronchial washings, alveolar macrophages and lung tissue following a single oral dose of extended or immediate release formulations of azithromycin. J. Antimicrob. Chemother. 2008;61:884–891. doi: 10.1093/jac/dkn032. [DOI] [PubMed] [Google Scholar]
- 333.Tran D.H., Sugamata R., Hirose T., Suzuki S., Noguchi Y., Sugawara A., et al. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. The Journal of Antibiotics. 2019;72:759–768. doi: 10.1038/s41429-019-0204-x. [DOI] [PubMed] [Google Scholar]
- 334.Bacharier L.B., Guilbert T.W., Mauger D.T., Boehmer S., Beigelman A., Fitzpatrick A.M., et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034–2044. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Beigelman A., Isaacson-Schmid M., Sajol G., Baty J., Rodriguez O.M., Leege E., et al. Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 2015;135(e1):1171–1178. doi: 10.1016/j.jaci.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Cevik M., Tate M., Lloyd O., Maraolo A.E., Schafers J., Ho A. 2020. SARS-CoV-2, SARS-CoV-1 and MERS-CoV Viral Load Dynamics, Duration of Viral Shedding and Infectiousness: A Living Systematic Review and Meta-analysis. medRxiv. (2020.07.25.20162107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Liu Y., Yan L.-M., Wan L., Xiang T.-X., Le A., Liu J.-M., et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect. Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 340.Argyropoulos K.V., Serrano A., Hu J., Black M., Feng X., Shen G., et al. Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with outcome and symptoms. Am. J. Pathol. 2020;190:1881–1887. doi: 10.1016/j.ajpath.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]