Abstract
Patients with well-controlled low-density lipoprotein cholesterol levels, but persistent high triglycerides, remain at increased risk for cardiovascular events as evidenced by multiple genetic and epidemiologic studies, as well as recent clinical outcome trials. While many trials of low-dose ω3-polyunsaturated fatty acids (ω3-PUFAs), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) have shown mixed results to reduce cardiovascular events, recent trials with high-dose ω3-PUFAs have reignited interest in ω3-PUFAs, particularly EPA, in cardiovascular disease (CVD). REDUCE-IT demonstrated that high-dose EPA (4 g/day icosapent-ethyl) reduced a composite of clinical events by 25% in statin-treated patients with established CVD or diabetes and other cardiovascular risk factors. Outcome trials in similar statin-treated patients using DHA-containing high-dose ω3 formulations have not yet shown the benefits of EPA alone. However, there are data to show that high-dose ω3-PUFAs in patients with acute myocardial infarction had reduced left ventricular remodelling, non-infarct myocardial fibrosis, and systemic inflammation. ω3-polyunsaturated fatty acids, along with their metabolites, such as oxylipins and other lipid mediators, have complex effects on the cardiovascular system. Together they target free fatty acid receptors and peroxisome proliferator-activated receptors in various tissues to modulate inflammation and lipid metabolism. Here, we review these multifactorial mechanisms of ω3-PUFAs in view of recent clinical findings. These findings indicate physico-chemical and biological diversity among ω3-PUFAs that influence tissue distributions as well as disparate effects on membrane organization, rates of lipid oxidation, as well as various receptor-mediated signal transduction pathways and effects on gene expression.
Keywords: Eicosapentaenoic acid (EPA), Docosahexaenoic acid (DHA), Oxylipin Free fatty acid receptor (FFAR), Peroxisome proliferator- activated receptor (PPAR)
Introduction
The recent success of clinical studies with high-dose omega-3 polyunsaturated fatty acids (ω3-PUFAs), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and specifically EPA, has reignited interest in the potential mechanism of action of ω3-PUFA therapy in cardiovascular disease (CVD). Reporting in 2018, the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) was the largest randomized trial to date to employ high-dose EPA (4 g/day). Results from REDUCE-IT indicated that EPA significantly reduced risk of ischaemic events, including death from cardiovascular causes, in patients with high triglycerides (TG) despite statin use.1 In addition, OMEGA-REMODEL, a smaller randomized trial that examined high-dose ω3-PUFAs (EPA + DHA, 4 g/day) on remodelling for 6 months post-myocardial infarction (MI), reported significant reductions in ventricular remodelling and inflammation.2 In stark contrast, the Vitamin D and Omega-3 Trial (VITAL), also reporting in 2018, found no improvement in primary cardiovascular outcomes with low-dose ω3-therapy (1 g/day).3 However, despite the positive outcomes from REDUCE-IT and OMEGA-REMODEL, there remains controversy regarding the efficacy of ω3-PUFAs in CVD. This controversy stems from a history of clinical trials with low-dose ω3-PUFAs that have failed to show efficacy.3–9 More recently, the STRENGTH trial using high-dose mixed ω3-PUFAs was stopped for futility.10 Additionally, there is ongoing debate regarding the mechanistic basis for the cardioprotective effects of ω3-PUFAs, which has also hampered their wider use in CVD. While there has long been interest in how the effects of ω3-PUFAs differ from ω6-PUFAs, recent results have highlighted the potential that EPA has unique benefits. Therefore, the goals of this review are to outline the mechanistic basis for EPA cardioprotective effects in CVD, and finally to suggest new directions in understanding EPA in CVD. ω3-polyunsaturated fatty acids have multiple biological activities including: (i) distinct physiochemical properties; (ii) as a precursor to oxylipins and other lipid mediators; and as direct receptor agonists at (iii) free fatty acid receptors (Ffars) or (iv) peroxisome proliferator-activated receptors (PPARs).
Distinct effects of eicosapentaenoic acid on membrane structure, lipid oxidation, biomarkers, plaque composition, and endothelial function
ω3-polyunsaturated fatty acids modify lipid composition of caveolae and cellular localization of nitric oxide synthase
Rather than diffusing in an unrestricted fashion, membrane constituents are organized into a complex ‘mosaic’ of specific lipids that create distinct environments or microdomains for various proteins involved in signal transduction and receptor binding.11,12 Such microdomains or ‘lipid rafts’ are typically detergent-resistant and enriched with unesterified cholesterol and sphingolipids. These sphingolipids contain saturated fatty acids (SFAs) that provide a highly ordered structure and sequester specific proteins.13 Proteins that preferentially localize to these domains have a certain tertiary structure that supports their function as an integral membrane protein. Lipid rafts move or ‘float’ as a coherent structural unit within the surrounding liquid-disordered lipid bilayer and can also cluster with other rafts to form larger platforms. The basis for differences in fluidity between rafts and the surrounding membrane is the differences in the degree of hydrocarbon chain saturation of constituent sphingolipids that associate strongly with cholesterol.11 Treatment of cells with ω3-PUFAs like EPA modifies the acyl chain composition of membrane phospholipids and thereby alters the properties and protein function of various lipid domains.14
Caveolae are a lipid raft subtype that appear as microscopic, flask-shaped invaginations along the membrane surface and are commonly found in endothelial cells, adipocytes, and smooth muscle cells. The principal protein component of caveolae is caveolin, a scaffolding protein that binds cholesterol efficiently and interacts with various signalling macromolecules, including G proteins and calcium regulating proteins.15 Caveolin modulates endothelial nitric oxide synthase (eNOS) activity as it binds directly to the enzyme, thereby blocking access of the co-factor, calcium/calmodulin.16 The expression of caveolin is markedly elevated under conditions of hypercholesterolaemia because of enrichment of plasma membrane cholesterol levels, resulting in reduced eNOS activation in a manner that can be reversed with statin treatment.17 In cultured human endothelial cells, EPA treatment modifies the subcellular distribution of eNOS and dramatically changes the lipid composition of caveolae.14 As a result of its incorporation into phospholipid acyl chains, EPA changes the fluid dynamics of the caveolae by increasing acyl chain unsaturation. Eicosapentaenoic acid treatment also displaces caveolin-1 from caveolae and causes the translocation of eNOS from caveolae fractions to soluble fractions where it can be activated. Finally, EPA changes the overall distribution of caveolin-1 and eNOS in plasma membranes.
Distinct membrane interactions of eicosapentaenoic acid and docosahexaenoic acid
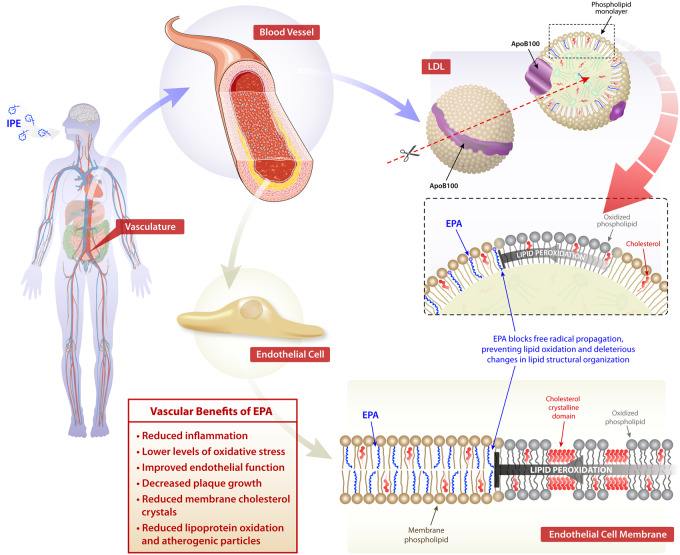
ω3-polyunsaturated fatty acids and their bioactive lipid metabolites incorporate into cellular membranes and lipoproteins associated with vascular tissues, along with the atherosclerotic plaque, where they interfere with inflammation, oxidative stress, and endothelial dysfunction.18–22 Treatment with EPA, at pharmacologic doses, affects lipid oxidation rates, membrane organization, and signal transduction.18–21 Eicosapentaenoic acid and DHA have disparate effects on membrane lipid dynamics due to differences in their carbon length and number of double bonds. Eicosapentaenoic acid inserts into lipoprotein particles and cellular membranes where it scavenges free radicals through electron stabilization mechanisms associated with its multiple double bonds (Figure 1). The antioxidant effects of EPA were not reproduced with DHA over time or other FDA-approved TG-lowering agents such as gemfibrozil, as well as fenofibric and nicotinic acids.18,23,24 The antioxidant effects of EPA are also observed in membranes under conditions of hyperglycaemia.23 Unlike EPA, other TG-lowering agents like the fibrates are relatively hydrophilic and do not possess chemical moieties capable of stabilizing unpaired free electrons in the membrane hydrocarbon core, while EPA has an extended and stable orientation in the membrane as determined by small-angle X-ray diffraction approaches.25 In contrast, DHA interacts with the phospholipid head group region with concomitant changes in hydrocarbon core electron density consistent with increased membrane disorder. These differences in membrane interactions with DHA are consistent with the rapid isomerization or conformational changes over a nanoscale time frame.18,19,21,24,26 In a more recent study, the antioxidant effects of EPA in small dense low density lipoprotein (sdLDL) and membranes were compared to other long-chain fatty acids. The results showed that hydrocarbon length and number of double bonds for fatty acids are important predictors of antioxidant activity in lipoproteins and membranes, along with membrane cholesterol domain formation.27 Eicosapentaenoic acid appears to have the optimal combination of chain length and unsaturation for these antioxidant properties, followed by other ω3-PUFAs. Fatty acids with two or fewer double bonds and the ω6-PUFA arachidonic acid (AA) failed to exhibit antioxidant activity as compared to ω3-PUFAs.
Figure 1.
Molecular lipid interactions of eicosapentaenoic acid. Schematic illustration of the proposed location of phospholipid-linked eicosapentaenoic acid in cellular membranes and lipoproteins. Eicosapentaenoic acid is rapidly esterified and incorporated into lipoproteins and membrane phospholipids. Biophysical studies indicate eicosapentaenoic acid has an extended orientation that preserves membrane fluidity and inhibition of lipid oxidation and membrane cholesterol domain formation.
Effects of ω3-polyunsaturated fatty acids on membrane cholesterol crystal formation
A characteristic feature of an atherosclerotic plaque is the formation of cholesterol crystals that originate from cholesterol-enriched membranes of various cells, including macrophages and smooth muscle cells.28,29 Over time, these toxic cholesterol crystals destabilize the plaque as they continue to form or precipitate from membrane domains.30 Using various imaging approaches,31 cholesterol crystals have been observed in vascular tissue where they associated with the plaque’s fibrous cap.32 In patients that have experienced myocardial infarction, there is abundant evidence of cholesterol crystals in the diseased plaque in a proportional manner.33 In addition to excess cholesterol accumulation, membrane cholesterol domains are also stimulated by oxidative stress and high glucose.34 Along with oxidized LDL, cholesterol crystals activate nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasomes, which regulates the processing of pro-interleukin 1 beta (IL-1β) into an active cytokine.35
Due to its potent antioxidant activity and lipophilicity, EPA significantly inhibits glucose-induced cholesterol crystalline domain formation in membrane lipid vesicles at pharmacologically relevant concentrations.23,36 The inhibition of cholesterol domain formation by EPA is not reproduced with other TG-lowering agents or vitamin E due to differences in membrane distribution and antioxidant activities.23 These findings suggest that EPA preferentially intercalates into the hydrocarbon core of the membrane bilayer where it can trap free radicals with its multiple double bonds, leading to reduced cholesterol domain formation. The effects of EPA on cholesterol domain formation was not observed with DHA as it has different interactions with the highly planar steroid ring structure of cholesterol that is oriented parallel to surrounding phospholipid acyl chains.18,37,38
Effects of ω3-polyunsaturated fatty acids on cardiovascular biomarkers, plaque biology, and high density lipoprotein (HDL) function
Eicosapentaenoic acid and other ω3-PUFAs have been shown to favourably influence circulating levels of biomarkers associated with cardiovascular risk in patients with dyslipidaemia. Prescription EPA (2–4 g/day) in patients with high or very high triglyceride (TG) levels reduces high-sensitivity C-reactive protein (hsCRP), lipoprotein-associated phospholipase A2 (Lp-PLA2), and oxidized LDL-cholesterol (oxLDL) levels, as well as the arachidonic acid (AA)-to-EPA ratio, as compared to placebo.39–44 The ability of EPA to reduce hsCRP is actually enhanced in combination with a statin in proportion to its intensity.40 Similar effects on hsCRP were not observed with DHA in a similar patient population even with 4 g/day but, like EPA, treatment was associated with reduced Apolipoprotein CIII.45 Eicosapentaenoic acid treatment also down-regulates cAMP responsive element protein 1 (CREB1) and hypoxia inducible factor 1 (HIF1) gene expression.46 In cellular studies, EPA reduces production of inflammatory mediators in macrophages, including TNF-α and IL-1β, compared to DHA.47
Eicosapentaenoic acid also has direct vascular effects that impact the progression of atherosclerosis as compared to other ω3-PUFAs. In mice that lack apolipoprotein E (Apoe−/−) fed a Western diet supplemented with EPA (1%, w/w) or DHA (1%, w/w) for 3 weeks, EPA treatment reduces plaque volume as compared to DHA.48 Eicosapentaenoic acid and its metabolites, especially 12-hydroxyeicospentaenoic acid (12-HEPE), preferentially associate with thin-cap plaques and accumulation of anti-inflammatory M2 macrophages, while DHA associates with plaques of various sizes. In the aortic root, total EPA and 12-HEPE levels follow a concentration gradient from the vascular endothelium to the media. In patients with coronary artery disease (CAD), treatment with EPA improves high-density lipoprotein mediated cholesterol efflux along with antioxidant and anti-inflammatory effects.49 In human endothelial cells, EPA-enriched HDL increases resolvin E3 production while reducing cytokine-stimulated vascular cell adhesion molecule 1 (VCAM-1) expression.50 As observed in Apo-B containing particles, the lipophilic properties and molecular dimensions of EPA allow it to insert more efficiently into the HDL particle, with improved antioxidant function, as compared to DHA.51
Effects of ω3-polyunsaturated fatty acids and statins on endothelial function
Atherosclerosis is causally related to endothelial cell (EC) dysfunction characterized by a loss of normal nitric oxide (NO) bioavailability that results in abnormal vasodilation and plaque development.52–54 In isolated tissue, EPA reverses endothelial dysfunction triggered by either oxLDL or high glucose in a manner that is enhanced in combination with a statin.55 This improvement in function is evidenced by pronounced increases in the release ratio of NO to peroxynitrite (ONOO–) in the endothelial cells. The combined benefit of EPA with a statin on endothelial function and NO synthase activity was not reproduced with DHA under identical conditions. By reducing LDL oxidation, EPA also attenuated the effects of oxLDL on endothelial function unlike DHA over the same time course. Similar effects of EPA vs. DHA were seen ex vivo in arterial segments from a rodent model.55
The endothelial benefits of EPA are not caused by differences in expression of endothelial nitric oxide synthase (eNOS) but are likely due to improved eNOS coupling.55 In the absence of eNOS coupling, excessive rather than NO is generated by eNOS, which leads to increased levels of highly reactive ONOO– molecules that cause cellular and lipoprotein oxidation (oxLDL). EPA treatment of human ECs, along with a statin, reduces the effects of oxLDL on endothelial dysfunction and preserves NO bioavailability. The favourable effects of EPA and the statin may be due to shared antioxidant properties and a common molecular distribution in cellular membranes.23 There are also benefits of EPA in an ex vivo rodent system.55 The combined treatment of EPA and a statin produces an additional improvement with respect to NO bioavailability following exposure to hyperglycaemia.
Summary
Based on its unique molecular structure, EPA has several significant effects on cellular and lipoprotein membrane structure and dynamics, including:
Eicosapentaenoic acid incorporated in phospholipid acyl chains modifies membrane dynamics of caveolae (lipid rafts), displaces caveolin-1, the structural backbone of caveolae, and displaces signalling proteins localized to caveolae, like eNOS, allowing for increased activation.
Eicosapentaenoic acid inserts into lipoprotein particles and cellular membranes where it scavenges free radicals through electron stabilization due to its multiple double bonds. Eicosapentaenoic acid, as opposed to DHA, appears to have the optimal combination of chain length and unsaturation for these antioxidant properties.
Eicosapentaenoic acid, as opposed to DHA, significantly inhibits glucose-induced cholesterol crystalline domain formation in membrane lipid vesicles, which is not reproduced with other TG-lowering agents.
Eicosapentaenoic acid and other ω3-PUFAs reduce circulating levels of biomarkers associated with cardiovascular risk in patients with dyslipidaemia.
Eicosapentaenoic acid, in combination with statins, reverses endothelial dysfunction as evidenced by increased nitric oxide bioavailability due to shared antioxidant properties and a common molecular distribution in cellular membranes.
Oxylipins as signalling metabolites derived from eicosapentaenoic acid
Oxylipins are the subclass of lipid mediators derived by enzymatic oxygenation of PUFAs that have specific biological activities.56 At the molecular level, PUFAs serve as a reservoir of precursors from which a broad array of lipid mediators are synthesized through oxygenation,57 amidation,58 nitrosylation,59 and more. Oxylipins are produced by enzymes in multiple signalling pathways, including cyclooxygenase (COX) and lipoxygenase families (5-LOX, 12-LOX, and 15-LOX), as well as a broad cross section of cytochrome p450s with epoxygenase (CYPepox), hydroxylase (CYPhx), or ω/ω-1 hydroxylase (CYPω-hx) activities. In many cases, CYPs exhibit these activities simultaneously. While COX enzymes are selective for arachidonic acid (AA), LOX, and CYP have activity on multiple PUFAs including AA and EPA and the pleiotropic activity forms complex webs of overlapping signalling pathways. Table 1 summarizes the most common AA and EPA oxylipins in LOX and CYP signalling. The summary exemplifies the complexity of signalling matrices generated because: (i) the same signalling molecule can be produced from distinct enzymes—e.g. 12-HEPE is produced by 12-LOX and by CYPhx; and (ii) the same enzymes can produce oxylipins from multiple PUFAs—e.g. 12-LOX produces 12-hydroxyeicotetraenoic (12-HETE) from AA and 12-HEPE from EPA.
Table 1.
Examples of arachidonic acid vs. eicosapentaenoic acid oxylipin activitiesa
| Family | Specific enzyme | PUFA | ||||
|---|---|---|---|---|---|---|
| LOX | AA | Activities | EPA | Activities | Direct comparisons | |
| 5-LOX | 5-HpETE |
Inflammatory: Inhibits platelet aggregation65 |
5-HpEPE |
Inflammatory: Inhibits platelet aggregation65 |
5-HpETE < 12-HpETE 5-HpEPE < 12-HpEPE |
|
| 5-HETE |
Inflammatory: Chemotaxis, immune cell activation66–68 Cardiovascular: Inhibits PGI2 synthesis |
5-HEPE |
Inflammatory: Chemotaxis67 Metabolic: Glucose-dependent insulin secretion69 |
Inflammatory: AA > EPA |
||
| 5-KETE |
Inflammatory: Eosinophil and neutrophil migration70 |
5-KEPE |
Inflammatory: Eosinophil and neutrophil migration70 |
Inflammatory: AA ≫ EPA |
||
| LtB4 |
Inflammatory: Neutrophil aggregation71 Polymorphonuclear cell activation72 |
LtB5 |
Inflammatory: Neutrophil aggregation71 |
Inflammatory: EPA < AA |
||
| LtC4 |
Inflammatory: Block coronary anaphylaxis-induced vascoconstriction73 |
LtC5 |
Inflammatory: Block coronary anaphylaxis-induced vascoconstriction73 |
Inflammatory: EPA < AA |
||
| LxB4 |
Inflammatory: Immune cell migration and activation74 Cardiovascular: Aortic vasodilation74 |
LxB5 |
Cardiovascular: No activity74 |
Inflammatory: AA > EPA |
||
| 12-LOX | 12-HpETE |
Inflammatory: Inhibits platelet aggregation65 |
12-HpEPE |
Inflammatory: Inhibits platelet aggregation65 |
Inflammatory: 12-HpETE > 5/15-HpETE 12-HpEPE > 5/15-HpEPE |
|
| 12-HETE |
Inflammatory: Inhibits platelet aggregation65 Increased monocyte adhesion to fatty streaks75,76 Cardiovascular: Increased mitochondrial Ca2+/nitric oxide77 |
12-HEPE |
Inflammatory: Inhibits platelet aggregation65 |
Inflammatory: AA = EPA HETEs < HpETEs HEPEs < HpEPEs |
||
| 15-LOX | 15-HpETE |
Inflammatory: Inhibits platelet aggregation65 Cardiovascular: Loss of cardiomyocyte membrane integrity78 Species/context dependent vasodilation/vaso-constriction79 |
15-HpEPE |
Inflammatory: Inhibits platelet aggregation65 |
Inflammatory: AA = EPA Peroxides > alcohols 12-LOX > 15-LOX |
|
| 15-HETE |
Inflammatory: Inhibit platelet aggregation65 Cardiovascular: Species/context dependent vasodilation and vasoconstriction79 |
15-HEPE |
Inflammatory: Inhibition of 5-LOX80 |
Inflammatory: AA = EPA 15-HpETE < 12-HpETE 15-HpEPE < 12-HpEPE |
||
|
| ||||||
| CYP | AA | Activities | EPA | Activities | Direct comparisons | |
|
| ||||||
| CYPhx | HETEsb | HEPEsa | ||||
| CYPω/ω-1 hx | 18-HETE c | 18-HEPE |
Inflammatory: Decreased TNFα secretion by macrophages81 Cardiovascular: Protects heart from pressure Overload fibrosis; blocks cardiac Fibroblast inflammation82 |
|||
| CYPepox | 5(6)-EpETrE |
Cardiovascular: Vasodilatory83–85 |
5(6)-EpETE | |||
| 8(9)-EpETrE |
Cardiovascular: Protects against reperfusion injury86 |
8(9)-EpETE |
Inflammatory: Inhibit platelet aggregation87 Cardiovascular: Vasodilatory88 |
EpETE > EpETrE87 EpETrE = EpETE88 |
||
| 11(12)-EpETrE |
Inflammatory: Inhibition of NF-κB89 Cardiovascular: Protection against reperfusion injury86 |
11(12)-EpETE |
Inflammatory: Inhibit platelet aggregation87 Cardiovascular: Vasodilatory88 |
EpETE > EpETrE87 EpETrE = EpETE88 |
||
| 14(15)-EpETrE |
Cardiovascular: Vasodilatory, >14,15-DiHETrE90 Vasodilatory Protects against reperfusion injury86 |
14(15)-EpETE |
Inflammatory: Inhibit platelet aggregation87 Cardiovascular: Vasodilatory88 |
EpETE > EpETrE87 EpETrE = EpETE88 |
||
| 17(18)-EpETE |
Inflammatory: Inhibit platelet aggregation EpETE87 Anti-inflammation91 Cardiovascular: Vasodilatory92 |
|||||
| 5,6-DiHETrE |
Cardiovascular: Vasodilatory; >5(6)-EpETrE83 Coronary artery hyperpolarizing (BKc)93 |
5,6-DiHETE | ||||
| 8,9-DiHETrE |
Cardiovascular: Vasodilatory; Coronary artery hyperpolarizing (BKc)93 |
8,9-DiHETE |
Inflammatory: Inhibit platelet aggregation ≪ EpETE87 Cardiovascular: |
|||
| 11,12-DiHETrE |
Cardiovascular: Vasodilatory; Coronary artery hyperpolarizing (BKc)93 |
11,12-DiETE | ||||
| 14,15-DiHETrE |
Cardiovascular: Vasodilatory; Coronary artery hyperpolarizing (BKc)93 |
14,15-DiETE | ||||
| 17,18-DiHETE | NA | |||||
As summarized from Gabbs et al.64 with modifications and emphasis on CVD-outcomes.
CYPs have generalized oxygenation activity, both at double bonds (similar to LOX) and at saturated positions. For simplicity, only a few unique activities are shown.
18-HEPE is produced distinctly from 18-HETE. The former is oxygenated at the ω3-double bond (Δ18) of EPA, the latter is produced at the saturated Δ18 position of AA.
Our understanding of the initiation of oxylipin synthesis and generation of signalling activity is guided by the common model depicted in Figure 2. Precursor PUFAs stored in membrane phospholipids are made available to enzymes by the activity of phospholipase A2 (PLA2), which releases PUFAs from the sn-2 position into the cytosol where COX, LOX, and CYP divert them into each pathway.60–63 Over 70 metabolites of AA are known to exist, and since EPA has an additional double bond (five compared to four in AA), even more metabolites of EPA are likely to exist, and almost 50 have been described to date.64
Figure 2.
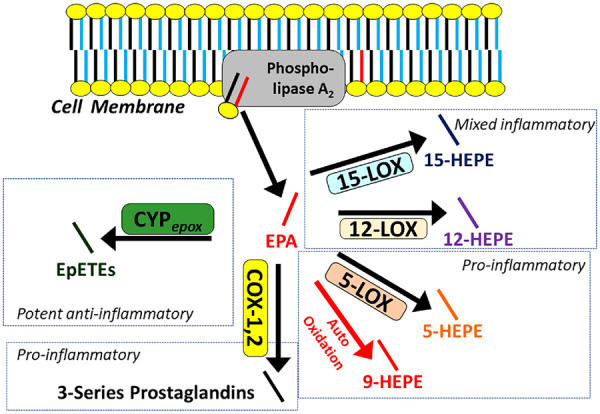
The common model of oxylipin production by oxylipin producing enzymes, including various lipoxygenase, cyclooxygenases, and cytochrome p450 epoxygenases. Polyunsaturated fatty acids are released by phospholipase A2 from the membrane, making them available to enzymes for downstream signalling by oxylipins. Eicosapentaenoic acid is depicted in red, other polyunsaturated fatty acids are depicted in blue. The relative production of eicosapentaenoic acid-derived oxylipins is a function of the relative provision of eicosapentaenoic acid to enzymes by phospholipase A2, which in turn, reflects their relative abundance in the phospholipid membrane. Generalized pathway activities are summarized. For details, see Table 1. COX, cyclooxygenase; CYPepox, cytochrome P450 expoxygenases; LOX, lipoxygenase.
Eicosapentaenoic acid administration changes the amount and types of oxylipins in plasma
Pharmaceutical EPA as icosapent ethyl at 4 g/day increases membrane EPA content by nearly 8-fold but reduces membrane AA content by 27%.94 1.86 g/day EPA as omega-3 acid ethyl esters (in conjunction with 1.5 g/day DHA) not only induces a 540% increase in %EPA but also induces an 11% decrease in %AA.95 This shift in membrane substrate availability is reflected in treatment-dependent changes in tissue oxylipins, with a general pattern of increasing EPA membrane content, at the expense of AA. Like fatty acids, oxylipins are distributed through plasma and the majority circulate esterified in the glycerolipids of lipoproteins (VLDL, LDL, and HDL). A small portion (<5%) circulate unesterified and bound to albumin in a manner similar to non-esterified fatty acids (often termed ‘free’ oxylipins, which are most often reported). As a general rule, treatments that increase tissue EPA result in concurrent increases in total (esterified + unesterified) plasma alcohols and epoxides derived from EPA: 4 weeks of 3.4 g/day omega-3 acid ethyl esters induced an 8.4-fold increase in plasma %EPA and concurrently increased plasma esterified HEPEs by 5.7-fold, epoxytetraenoic acids (EpETEs) by 4.7-fold, and dihydroxyeicostetraenoic acids (DiHETEs) by 4.1-fold.96 Treatment reduced %AA to 0.88-fold of baseline and similarly reduced oxylipins derived from AA: HETEs to 0.8-fold of baseline and dihydroxytrienoic acids (DiHETrEs) to 0.81-fold of baseline; epoxyeicosatrienoic acids (EpETrEs) were not significantly reduced, but the data were most consistent with a reduction to 0.9-fold of baseline. For both EPA- and AA-oxylipins, changes in plasma concentration were reflective of the abundance their PUFA precursors. Eicosapentaenoic acid treatment is not only effective at increasing steady-state abundance of EPA oxylipins, but it also decreases the abundance of AA oxylipins in proportion to decreases in AA availability. However, a more detailed examination in the randomized clinical trial (RCT) setting reveals pool-specific changes. In subjects with metabolic syndrome responding to oral EPA (combined with DHA) at 3.4 g/day ω3-acid-ethyl esters, the reduction in AA-oxylipins was restricted to two HETEs, oxoeicosatetraenoic acids (KETEs), and leukotriene B4 (LTB4) derivatives in LDL and HDL respectively, but the same VLDL oxylipins were unchanged. In addition, AA-oxylipins including EpETrEs and DiHETrEs were unchanged for all lipoproteins,95 indicating that after treatment CYPepox signalling is sustained but LOX and CYPhx signalling are limited by AA.
Eicosapentaenoic acid oxylipins share ligand activity with oxylipins from other polyunsaturated fatty acids
In addition to enzymes that can produce oxylipins from multiple PUFAs, oxylipins also have overlapping activities as receptor ligands, and the classic model of a unique ligand-receptor pair does not hold. This expectation follows from Burr’s original findings that linoleic acid97 and alpha-linolenic acid98 are both effective in rescuing rats with essential fatty acid deficiency,99 which was explained in 1930 as complementary PUFA activities. Unfortunately, few studies systematically compare the bioactivity of ω6 and ω3 homologs. AA and EPA are both effective substrates for CYPepox activity, producing EpETrEs and EpETEs from AA and EPA, respectively. Both series potently induce dilation of rat coronary microvessels by activation of BKCa channels.88 EpETEs appear to be moderately more potent than their EpETrE homologs, but all homologs bind and activate the channel. Hence, the most likely benefit of EPA is to add the benefit of EpETEs to that of EpETrEs. Additive instances are common. Among 12-LOX pathway metabolites, 12-hydroperoxides (12-HpETE for AA, 12-HpEPE for EPA) equivalently suppress collagen-mediated platelet aggregation and serotonin release.65
Instances in which the effect of EPA-oxylipins are different from the AA-homolog are also reported, with examples both where the EPA-oxylipin is more potent than the AA-homolog, and also where it is less potent. Among 5-LOX pathway metabolites, the EPA-derived 5-KEPE is 10-fold less potent than the AA-derived 5-KETE in mobilizing cytosolic Ca2+ in human neutrophils, and it is less potent in stimulating neutrophil or eosinophil migration.70 Another EPA-oxylipin derived from 5-LOX (5-HEPE) acts on GPR119 to produce an insulinogenic effect but the AA homolog (5-HETE) has only half the effect.69 Hence, different GPR119-dependent insulinogenic responses among individuals could potentially be explained by availability of cell membrane AA and EPA, independent of genetic polymorphisms. By considering the interactions of PUFAs with each other, a better assessment of how the bioactivities of mixtures are established can be derived.
Finally, ω3-PUFAs can produce distinct oxylipins with no direct homolog. With EPA, this occurs because ω3-PUFAs have one more double bond compared to AA, and hence an additional point for oxygenation not available on AA. For mid-chain alcohols, this metabolite is 18-HEPE, which is a target of much investigative interest, in part because of its role as a precursor to specialized pro-resolving mediators such as Resolvin E1. Interestingly, 18-HEPE protects against pressure overload-induced heart failure.82 For fatty acid epoxides, the unique metabolite is 17(18)-EpETE, and it is thought to have potent anti-inflammatory activity and to direct macrophage polarization towards the M2 phenotype.91
Finally, pathway diversion is a potential mechanism. The phenomenon has been demonstrated in the context of heart failure, where activation of mitochondrial iPLA2γ diverts oxylipin production away from epoxides (EpETrEs) towards mid-chain alcohols (HETEs) which promotes pathology by inducing necrosis and apoptosis,100 and such a shift could amplify cases where EPA is a neutral antagonist to an oxylipin producing enzyme as is the case with COX.101
Summary
There are at least four ways increasing EPA availability can alter the oxylipin signalling pathways and affect downstream physiologic function:
Eicosapentaenoic acid-oxylipins can add to the activity of AA-oxylipins.
Eicosapentaenoic acid-oxylipins can have effects distinct from their AA-homologs.
Eicosapentaenoic acid-oxylipins can neutralize the activity of their AA-homologs.
Eicosapentaenoic acid could divert activity from one oxylipin pathway to another.
As the results from STRENGTH are clarified, if evidence for differences between EPA and DHA emerges, the same molecular rationale that accounts for differences between AA and EPA can similarly explain differences between EPA and DHA oxylipins.
Free fatty acid receptors
While ω3-PUFA incorporation into cellular membranes has profound effects on membrane structure and the organization of membrane-associated signalling complexes, mechanistically, it fails to explain activation of intracellular signalling pathways associated with EPA-mediated cardioprotection. In the early 2000s, a subgroup of orphan receptors, G-protein coupled receptor 40 (GPR40), GPR43, GPR41, and GPR120, were defined as receptors for endogenous free fatty acids and were subsequently renamed free fatty acid receptor 1 (Ffar1), Ffar2, Ffar3, and Ffar4, respectively. Ffar2 and Ffar3 are receptors for short chain FA (≤8 carbons)102 and will not be discussed further, whereas Ffar1 and Ffar4 are receptors for medium and long-chain FA (≥10 carbons), including, but not limited to, ω3-PUFAs.102 The discovery of GPRs that bind to and are activated by endogenous free fatty acids establishes and entirely novel paradigm whereby fatty acids function as signalling molecules, not simply as an energy source.
Free fatty acid receptor 1 expression and physiology
In 2003, Ffar1 was the first identified receptor for free FAs and is activated by medium and long-chain FAs.103–105 It is located at chromosome 19q13.1 in humans and is expressed as a single-gene product. Ffar1 is highly expressed in the pancreas, GI-tract, brain, and lung, with lower levels of expression in skeletal muscle, heart, liver, bone, and brain.102 In the pancreas, Ffar1 is highly expressed in the pancreatic islets, including β-cells, where long-chain PUFA stimulation enhances glucose-stimulated insulin secretion,103,104 and α-cells, where it modulates glucagon release. In the gastrointestinal (GI) tract, Ffar1 is expressed in enteroendocrine cells and regulates the release of glucagon like peptide-1 (GLP-1) and peptide hormone YY (PYY) from intestinal L-cells,106 cholecystokinin (CCK) from intestinal I cells,107 and gastric inhibitory peptide (GIP) from intestinal K-cells.108 Additionally, Ffar1 and other Ffars play an important role producing anti-inflammatory signals in the gut to regulate metabolism.109 Ffar1 is also expressed in several brain regions including the hypothalamus, hippocampus, medulla oblongata, olfactory bulb, cerebellum, striatum, and cerebral cortex, and might have a role in neurodevelopment, inflammation and pain, and neurodegenerative diseases.110 Finally, Ffar1 is expressed at low levels in mouse heart, 30-fold lower than Ffar4,111 although relative expression levels of Ffar1 in human heart might be higher (unpublished observation). However, there are no studies of which we are aware that address Ffar1 function in the heart.
Free fatty acid receptor 4 expression and physiology
In 2005, Ffar4 was identified as another receptor for long-chain FAs.112 Ffar4, which shows little structural similarity to Ffar1, is located at chromosome 10q23.33 in humans, and two splice variants are expressed in humans, a short isoform (Ffar4S, analogous to rodent Ffar4) and long isoform (Ffar4L) that is unique to humans and differs from Ffar4S in a 16 amino acid insertion in the third intracellular loop.113,114 Ffar4 expression is detected in several tissues with similar expression patterns in mouse and human,112 with high levels of expression in lung and GI-tract, and lower levels of expression in other tissues including brain, pancreas, small intestine, adipose, taste buds, muscle, heart, and liver.112,115,116 Like Ffar1, Ffar4 is expressed in intestinal L-cells and regulates the release of glucagon like peptide-1 (GLP-1) in STC-1 mouse enteroendocrine cells in vitro112 and using α-linolenic acid infusion in rats in vivo.115 However, this was not confirmed in Ffar4 knockout mice, suggesting this effect might be mediated by Ffar1.117 In the pancreas, Ffar4 is expressed in pancreatic islet α cells where activation induces glucagon release,118 and ∂ cells where activation induces somatostatin release.119 Ffar4 might also be expressed in pancreatic, β cells, where Ffar4 was shown to induce insulin release BRINBD11 cells, isolated islets, and in vivo,120 although this was not observed in Ffar4 knockout mice.118 Ffar4 is also expressed in adipose tissue, and increases surface expression of GLUT4 to attenuate insulin resistance.121 Ffar4 is expressed in macrophages and induces a more anti-inflammatory phenotype, characterized by increased expression of anti-inflammatory genes including arginase-1, IL-10, MCL1, Ym-1, Clcc7a, and MMR, with decreased expression of pro-inflammatory genes including IL-6, TNFα, MCP-1, IL-1β, iNOS, adiponectin, and CD11c.121 Furthermore, activation of Ffar4 in macrophages reduced inflammation in adipose tissue and improved insulin sensitivity in mice.121 The combined insulin-sensitizing and anti-inflammatory effects of Ffar4 in pancreatic islet cells, adipocytes, and macrophages suggest Ffar4 as a potential therapeutic target in the management of type-II diabetes. Finally, Ffar4 is expressed in mouse heart at 30-fold higher levels than Ffar1, while Ffar2/3 were significantly lower levels.111 Further, Ffar4 is expressed in isolated mouse cardiac myocytes and fibroblasts.111 Because Ffar4 is the main Ffar expressed in heart, the remainder of the section will focus on Ffar4 in CVD.
Free fatty acid receptor 4 signalling
Although commonly referred to as a receptor for ω3-PUFAs, Ffar4 binds to and is activated by a wide array of saturated, monounsaturated, and polyunsaturated, medium and long-chain FAs with potencies in the low µM range, including ω3-PUFAs, α-linolenic acid (αLA), EPA, and DHA.122 However, SFAs tend to show lower efficacy for activation of downstream signalling pathways, suggesting a potential functional bias towards PUFAs that likely affects biological activity in vivo.122 Additionally, a novel class of branched chain fatty acids, fatty acid esters of hydroxy fatty acids (FAHFAs), were identified as potential ligands for Ffar4 and have anti-diabetic and anti-inflammatory properties.123
Ffar4 signals through both Gαq and β-arrestin-2 (not β-arrestin-1), although there are cell-type specific effects and differences between the Ffar4S and Ffar4L isoforms.112,114,121 The original description of Ffar4 indicated that activation of the receptor increased intracellular Ca2+ and activation of ERK in STC-1 enteroendocrine tumour cells suggesting signalling through Gq/11,112 which was confirmed in subsequent studies.114,124 Alternatively, Ffar4 signals through βarrestin2 to activate TAB1 which inhibits TAK1, preventing NFκB and JNK pro-inflammatory signalling in macrophages.121 Interestingly, Ffar4 signals through both Gq and βarrestin2 to activate cytoplasmic phospholipase A2 (cPLA2), resulting in activation of cyclooxygenase 2 (COX2) and production of prostaglandin E2 (PGE2) in macrophages.125 However, in adipocytes, Ffar4 signals through Gq to increase cell surface expression of Glut4.121 Recent studies also suggest Ffar4 might signal through Gi to induce ghrelin release in the stomach126 and somatostatin release in pancreatic δ-cells.119 Adding to the complexity of Ffar4 signalling, the human Ffar4S and Ffar4L isoforms differentially activate Gq and β-arrestin, with the Ffar4S activating both, whereas the Ffar4L only signals through β-arrestin.114 However, Ffar4L expression appears rather limited, and the physiological significance of different isoform signalling is not entirely clear.127,128 Collectively, these studies indicate the importance of deciphering Ffar4 cell-specific signalling.
Free fatty acid receptor 4 as a mechanistic basis for ω3-polyunsaturated fatty acid-mediated cardioprotection
There are very few studies that directly evaluate Ffar4 as a mechanistic basis for EPA-mediated cardioprotection, but evidence suggests EPA-Ffar4 signalling might explain at least some of the protective effects of EPA in heart failure (HF) and atherosclerosis. In the heart, EPA supplementation prevents interstitial fibrosis and contractile dysfunction in a mouse model of pressure overload heart failure (transverse aortic constriction, TAC).111,129 In fact, EPA-mediated prevention of fibrosis has now been replicated in many models including: mice,82,130,131 rats,132–134 dogs,135,136 rabbits,137 and humans.2 However, dietary supplementation with EPA fails to increase EPA incorporation into cardiac myocytes or fibroblasts, suggesting a mechanism independent of membrane incorporation accounts for EPA-mediated anti-fibrotic and cardioprotective effects.111,129 Interestingly, Ffar4 is expressed in the mouse heart, at 30-fold higher levels of expression than Ffar1, and is expressed in both cardiac myocytes and fibroblasts.111 Ffar4 is also detected in human hearts, although Ffar1 is expressed at proportionally higher levels in human vs. mouse heart (unpublished observation). More importantly, in primary cultures of mouse cardiac fibroblasts, Ffar4 is both sufficient and necessary to prevent TGFβ1-induced fibrosis,111 but whether Ffar4 mediates EPA-mediated anti-fibrotic and cardioprotective effects in vivo remains to be determined. In a model of vascular injury induced by FeCl3, endogenously produced ω3-PUFAs in fat-1 mice prevents neointimal hyperplasia and vascular inflammation in the common carotid artery, which required Ffar4, as protection is lost in fat-1/Ffar4 knockout mice.138 Interestingly, Ffar4 is anti-inflammatory, reducing macrophage infiltration into adipose tissue in mice fed a high-fat diet and inducing macrophage phenotype switching from pro-inflammatory M1 to anti-inflammatory M2.121 Given the important role of inflammation in the pathology of CVD, it will be important to understand the interaction between Ffar4-mediated anti-inflammatory signals and direct Ffar4 actions in the cardiovascular system.
Summary
Ffar1 and Ffar4 signalling is the newest mechanism proposed to explain EPA-cardioprotective signalling. In summary:
Ffar1 and Ffar4 are G-protein-coupled receptors for medium and long-chain FAs (C10–C24), which include but are not limited to ω3-PUFAs.
Ffar1 regulates metabolism and is highly expressed in pancreatic islets, regulating insulin release in β cells and glucagon release from α cells, and the GI tract, regulating gut hormone release including, GLP-1, PPY, CCK, and GIP, as well as inflammatory signalling in the gut.
Ffar4 also regulates metabolism and is expressed in the pancreatic islets, regulating glucagon release from α cells and somatostatin release from ∂ cells, and in the GI tract, regulating GLP-1 release.
Ffar4 regulates inflammation, and in macrophages, ω3-PUFAs induce an M2-like anti-inflammatory phenotype. Ffar4 anti-inflammatory signalling in macrophages reduces inflammation in adipose tissue in obesity.
Ffar4 is expressed in the mouse heart, in both cardiac myocytes and fibroblasts, at much higher levels than Ffar1. Ffar4 attenuates pro-fibrotic signalling in cultured fibroblasts, and might mediate at least some of the cardioprotective functions of EPA, but this remains to be tested.
Peroxisome proliferator-activated receptors
Peroxisome proliferator-activated receptor structure, expression, and pharmacology
The PPARs are a subfamily of ligand-activated nuclear receptors/transcription factors that belong to the superfamily of nuclear receptors.139,140 Structurally, PPARs contain an N-terminal transactivating domain, a highly conserved DNA-binding domain that recognizes PPAR-specific DNA binding domains, or PPAR-response elements (PPREs), and a C-terminal ligand-binding domain.141,142 Ligand binding to PPARs facilitates heterodimerization with the retinoid-X-receptor (RXR), binding to PPREs, a six nucleotide dimeric repeat separated by a single nucleotide (AGGTCA-N-AGGTCA), and activation of transcription.141,142 Of note, the binding pocket of PPARs is relatively large compared to other nuclear receptors, facilitating binding of endogenous FAs and greater overall flexibility in ligand binding.
There are three PPAR isoforms PPARα, PPARβ/∂, and PPARγ that differ in tissue distribution, ligand binding profiles, and regulation of distinct target genes/physiologic functions.143,144 Peroxisome proliferator-activated receptor α, the first cloned PPAR, is expressed in metabolically active tissues including, liver, heart, skeletal muscle, and kidney, and regulates genes associated with fatty acid uptake, intracellular transport, and β-oxidation.144–146 Peroxisome proliferator-activated receptor γ is highly expressed in adipocytes and is a master regulator of adipogenesis, energy balance, and lipid biosynthesis; however, PPARγ is also expressed in several other tissues including skeletal and cardiac muscle where it regulates fatty acid oxidation.144–146 PPARβ/δ is widely expressed, with higher expression in the large and small intestine, heart, brain, and adipose and regulates fatty acid oxidation, improves lipid profiles, and attenuates adiposity.144–146
While several synthetic PPAR agonists are in clinical use, including thiazolidinediones (TZDs, PPARγ agonists) and fibrates (PPARα agonists),143,147 all three PPARs also bind to and are activated by endogenous FAs, typically in the low µM range, although with differing specificity for each PPAR isoform. While all PPARs bind PUFAs with similar affinity, PPARα also shows high affinity for MUFAs and SFAs, PPARβ/δ shows lower affinity for MUFAs and SFAs, and PPARγ is the most selective for PUFAs showing little or no affinity for MUFAs and SFAs.148 Of note, EPA is one of the most potent PUFAs at all three PPARs.148 Additionally, PPARs can also bind to certain oxylipins derived from AA,142,147 and it seems likely oxylipins derived from other FAs would likely also bind to PPARs, although this has not been rigorously examined.
Peroxisome proliferator-activated receptors in atherosclerosis
Peroxisome proliferator-activated receptors have received significant attention as potential therapeutic targets for the management of atherosclerosis.143 As discussed above, clinical trials indicate that EPA, one of the more potent endogenous ligands for PPARs, lowers plasma TG without raising LDL cholesterol and can reduce atherosclerotic plaques.149 Furthermore, REDUCE-IT demonstrated that EPA (icosapent ethyl) reduced residual atherosclerotic cardiovascular disease (ASCVD) risk beyond statins in patients with ASCVD but also in diabetic patients with multiple risk factors.150 However, most of the current mechanistic understanding of PPARs in vascular disease is based on studies using high-affinity synthetic PPAR agonists, including fibrates (PPARα) and TZDs (PPARγ).147
Functionally, all three PPARs isoforms are expressed in vascular endothelial cells, smooth muscle cells, and multiple inflammatory cells including macrophages and foam cells in atherosclerotic plaques.143,144 In vascular endothelial cells, PPARα activation inhibits NFκB activation and suppresses inflammation, reducing expression of VCAM-1 and leucocyte adhesion,151–153 whereas PPARγ and PPARδ may increase nitric oxide (NO) and reduce oxidative stress in diabetes.154–157 In vascular smooth muscle cells, PPARα attenuates inflammation,158 whereas PPARγ down-regulates matrix metalloproteinase (MMP) expression, such as MMP9 that might be involved in plaque rupture.152 In macrophages and foam cells within atherosclerotic lesions, PPARγ generally attenuates inflammation by regulating inflammatory gene expression.152,159,160
However, mouse models with global deletion of PPARα (PPARαKO) display slower development of atherosclerosis,161 conversely PPARα deletion in macrophages increases atherosclerosis,162 suggesting cell-type specific effects of PPARα signalling are crucial to atherogenesis. Furthermore, endothelial cell-specific deletion of PPARγ induces dyslipidaemia, suggesting that endothelial PPARγ is also a potentially important regulator of atherogenesis.163 In total, PPARs in the vascular system are important in atherogenesis, and while synthetic PPAR agonists show some promise in therapeutic treatment of atherosclerosis, far fewer studies have examined the benefits of endogenous FAs, such as EPA. However, EPA activation of PPARγ attenuates pro-inflammatory signalling and inhibits endothelial lipase expression in macrophages.164 Interestingly, DHA signalling through PPARα increases phagocytosis and induces an M2-like phenotype in macrophages,165 while maresins, a class of oxylipins derived from DHA, signalling through PPARα inhibit inflammation in endothelial cells.166 In summary, mechanistic studies designed to explain a potential for a clinical benefit of EPA acting through PPARs in atherosclerosis are somewhat lacking.
Peroxisome proliferator-activated receptors in the liver
In the liver, PPARα activation modulates the expression of multiple target genes involved in TG metabolism. Clinically, PPARα agonists (gemfibrozil, clofibrate, fenofibrate, and fenofibric acid) decrease plasma TG levels and increase HDL levels. Despite their ability to effectively lower TGs, clinical trials have failed to show that their use was associated with reduced risk for cardiovascular events when added to statins as compared to statin therapy alone.167–170 These findings suggest that TG-lowering alone through this mechanism does not lead to reduced cardiovascular risk. As an endogenous ligand for PPARs, EPA may have additional effects through this pathway that limit atherosclerosis and vascular inflammation. Differences between natural and synthetic PPARα ligands are currently being explored.
Peroxisome proliferator-activated receptors in heart failure
Peroxisome proliferator-activated receptor regulation of metabolism in the heart is another potential therapeutic target for the management of HF. Under basal conditions, mitochondrial FA oxidation is the major source of energy production in the myocardium, but in HF, mitochondrial FA oxidation often declines and glycolytic pathways become more predominant, ultimately contributing to contractile dysfunction.171 Mechanistically, PPARα-RXRα complex formation is decreased in hypertrophic cardiac myocytes,172 while PPARα in turn might complex with silent information regulator 1 (Sirt1) in HF, contributing to the down-regulation of genes regulating FA oxidation.173 In PPARαKO mice, chronic pressure overload reduces FA oxidation and worsens pathologic remodelling.174,175 Conversely, cardiac myocyte-specific overexpression of PPARα induces hypertrophy, cardiac lipotoxicity, and insulin resistance similar to diabetic cardiomyopathy.176 Cardiac myocyte-specific knockout of PPARγ induces cardiac hypertrophy,177,178 while cardiac myocyte-specific overexpression of PPARγ and rosiglitazone (PPARγ agonist) also induce hypertrophy.178,179 Cardiac myocyte-specific deletion of PPARδ disrupts FA oxidation and induces cardiomyopathy.180 In summary, the data from PPAR knockout and transgenic mice is sometimes conflicting, but suggests that fine regulation of PPAR signalling in cardiac myocytes is essential to maintain cardiac homeostasis.
Eicosapentaenoic acid and mixed ω3-PUFAs demonstrated improved HF outcomes in GISSI-HF and OMEGA-REMODEL.2,181 However, much less is known about EPA-PPAR signalling in cardiac myocytes. In cultured neonatal cardiac myocytes, EPA mediated activation of PPARα attenuates endothelin-1 induced hypertrophy.182 In rats fed a standard or high-fat diet, the PPARα agonist WY14643 increased monounsaturated FA and ω3-PUFA replacement with ω6-PUFA.183 In models of cardiac allografts, EPA reduced allograft rejection, which was abrogated by a PPPAγ antagonist.184,185 In humans, ω3-PUFA supplementation (3.4 g/day) 2–3 weeks before elective cardiac surgery increased nuclear transactivation of PPARγ, increased FA metabolic, anti-inflammatory, and antioxidant gene expression, and increased mitochondrial respiration rate in atrial biopsies.186 These studies do suggest that EPA signalling through PPARs have significant biological effects in the heart, but whether EPA signalling through PPARs prevents HF is still an open question.
Summary
Peroxisome proliferator-activated receptors are a second and distinct receptor-mediated mechanism proposed to explain EPA-cardioprotective signalling. In summary:
Peroxisome proliferator-activated receptors are nuclear receptors/transcription factors, and upon ligand binding, PPARs heterodimerize with RXR, bind to PPAR-response elements, and activate transcription.
There are three PPAR isoforms, PPARα, PPARβ/δ, and PPARγ, and synthetic PPAR agonists are in clinical use, including thiazolidinediones (TZDs, PPARγ agonists) and fibrates (PPARα agonists), to regulate diabetes.
Clinical trials suggest that EPA attenuates atherosclerosis. Further, EPA is one of the most potent FA activators of PPARs, suggesting a potential mechanistic explanation for EPA-mediated protection in atherosclerosis, but this remains to be tested.
PPARs are expressed in the heart and regulate FA oxidation. While EPA and mixed ω3-PUFAs demonstrated improved HF outcomes, whether this is mediated by PPARs remains to be tested.
Final summary and future directions
The recent success of high-dose ω3-PUFAs, particularly EPA administered as icosapent ethyl, in a clinical outcome trial has brought its role in CVD back into the spotlight. However, questions still remain about ω3-PUFAs, as the benefit of mixed and low-dose ω3-PUFAs has been equivocal at best. Undoubtably, understanding the underlying mechanisms of ω3-PUFAs in the cardiovascular system will lead to improved formulations that produce greater efficacy in those patient populations most likely to benefit from such an intervention. Potential mechanisms to explain the cardioprotective benefit of EPA, in particular, include its unique physiochemical interactions in membranes and lipoproteins, the production of EPA-derived cardioprotective oxylipins, activation of Ffar4 signalling, and induction of favourable PPAR-mediated gene expression. A major challenge going forward is how to systematically elucidate the key biological mechanism(s) underpinning the emerging cardioprotective effects of EPA. In light of multiple potential mechanisms to explain such cardioprotection, traditional reductionist experimental approaches might prove inadequate to address what is essentially a systems biological challenge where multiple mechanisms are involved that vary among different cell and tissue types. New experimental paradigms will likely be needed to address this important question.
Funding
This work was supported by National Institutes of Health and National Heart Lung Blood Institutes [R01 HL130099 and R01 HL152215 to T.D.O. and G.C.S., K01 HL133416 to A.M.N.]. This paper was published as part of a supplement supported by an unrestricted educational grant from Amarin Pharma, Inc.
Conflict of interest: T.D.O., R.P.M., M.J.B., A.M.N., and G.C.S. have received honoraria for consultation and instruction, and T.D.O., R.P.M., A.M.N., and M.J.B. have received grant/research support from Amarin Pharma Inc. R.P.M. has also received support from Pfizer Inc., Amgen Inc., ARCA Biopharma, and Novartis AG. R.P.M. is also a paid speaker and consultant for Amarin Pharma Inc., Pfizer Inc., and Novartis AG. A.M.N. has received funding for research to her institution from Amgen, Janssen, Amarin, Sanofi, Regeneron, and honoraria and consulting fees from Amgen, AstraZeneca, Janssen, Esperion, Amarin, Sanofi, Regeneron, NovoNordisk, Novartis, The Medicines Company, New Amsterdam, and Pfizer.
References
- 1. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 2. Heydari B, Abdullah S, Pottala JV, Shah R, Abbasi S, Mandry D, Francis SA, Lumish H, Ghoshhajra BB, Hoffmann U, Appelbaum E, Feng JH, Blankstein R, Steigner M, McConnell JP, Harris W, Antman EM, Jerosch-Herold M, Kwong RY.. Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction: the OMEGA-REMODEL randomized clinical trial. Circulation 2016;134:378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D'Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE; VITAL Research Group. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, Zotos PC, Haboubi NA, Elwood PC.. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr 2003;57:193–200. [DOI] [PubMed] [Google Scholar]
- 5. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–2026. [DOI] [PubMed] [Google Scholar]
- 6. Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J; for the OMEGA Study Group. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010;122:2152–2159. [DOI] [PubMed] [Google Scholar]
- 7. Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S; for the SU.FOL.OM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Group ASC, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J.. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018;379:1540–1550. [DOI] [PubMed] [Google Scholar]
- 9.Risk Prevention Study Collaborative Group. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med 2013;368:1800–1808. [DOI] [PubMed] [Google Scholar]
- 10. Ferrieres J. The return of triglycerides and revival of omega-3 fatty acids! Arch Cardiovasc Dis 2020;113:369–373. [DOI] [PubMed] [Google Scholar]
- 11. Brown DA. Seeing is believing: visualization of rafts in model membranes. Proc Natl Acad Sci USA 2001;98:10517–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mason RP, Jacob RF.. Membrane microdomains and vascular biology: emerging role in atherogenesis. Circulation 2003;107:2270–2273. [DOI] [PubMed] [Google Scholar]
- 13. Brown DA, London E.. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 2000;275:17221–17224. [DOI] [PubMed] [Google Scholar]
- 14. Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J.. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie 2007;89:169–177. [DOI] [PubMed] [Google Scholar]
- 15. Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP.. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol 1999;19:7289–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T.. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem 1996;271:22810–22814. [DOI] [PubMed] [Google Scholar]
- 17. Feron O, Dessy C, Desager JP, Balligand JL.. Hydroxy-methylgluataryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation 2001;103:113–118. [DOI] [PubMed] [Google Scholar]
- 18. Mason RP, Jacob RF, Shrivastava S, Sherratt SCR, Chattopadhyay A.. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim Biophys Acta 2016;1858:3131–3140. [DOI] [PubMed] [Google Scholar]
- 19. Shaikh SR. Biophysical and biochemical mechanisms by which dietary N-3 polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. J Nutr Biochem 2012;23:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaikh SR, Wassall SR, Brown DA, Kosaraju R.. n-3 polyunsaturated fatty acids, lipid microclusters, and vitamin E. Curr Top Membr 2015;75:209–231. [DOI] [PubMed] [Google Scholar]
- 21. Williams JA, Batten SE, Harris M, Rockett BD, Shaikh SR, Stillwell W, Wassall SR.. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys J 2012;103:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mason RP, Libby P, Bhatt DL.. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol 2020;40:1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mason RP, Jacob RF.. Eicosapentaenoic acid inhibits glucose-induced membrane cholesterol crystalline domain formation through a potent antioxidant mechanism. Biochim Biophys Acta 2015;1848:502–509. [DOI] [PubMed] [Google Scholar]
- 24. Mason RP, Sherratt SCR, Jacob RF.. Eicosapentaenoic acid inhibits oxidation of ApoB-containing lipoprotein particles of different size in vitro when administered alone or in combination with atorvastatin active metabolite compared with other triglyceride-lowering agents. J Cardiovasc Pharmacol 2016;68:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sherratt SCR, Mason RP.. Eicosapentaenoic acid and docosahexaenoic acid have distinct membrane locations and lipid interactions as determined by X-ray diffraction. Chem Phys Lipids 2018;212:73–79. [DOI] [PubMed] [Google Scholar]
- 26. Shaikh SR, Kinnun JJ, Leng X, Williams JA, Wassall SR.. How polyunsaturated fatty acids modify molecular organization in membranes: insight from NMR studies of model systems. Biochim Biophys Acta 2015;1848:211–219. [DOI] [PubMed] [Google Scholar]
- 27. Sherratt SCR, Juliano RA, Mason RP.. Eicosapentaenoic acid (EPA) has optimal chain length and degree of unsaturation to inhibit oxidation of small dense LDL and membrane cholesterol domains as compared to related fatty acids in vitro. Biochim Biophys Acta 2020;1862:183254. [DOI] [PubMed] [Google Scholar]
- 28. Mason RP, Walter MF, Day CA, Jacob RF.. Active metabolite of atorvastatin inhibits membrane cholesterol domain formation by an antioxidant mechanism. J Biol Chem 2006;281:9337–9345. [DOI] [PubMed] [Google Scholar]
- 29. Tulenko TN, Chen M, Mason PE, Mason RP.. Physical effects of cholesterol on arterial smooth muscle membranes: evidence of immiscible cholesterol domains and alterations in bilayer width during atherogenesis. J Lipid Res 1998;39:947–956. [PubMed] [Google Scholar]
- 30. Grebe A, Latz E.. Cholesterol crystals and inflammation. Curr Rheumatol Rep 2013;15:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kellner-Weibel G, Yancey PG, Jerome WG, Walser T, Mason RP, Phillips MC, Rothblat GH.. Crystallization of free cholesterol in model macrophage foam cells. Arterioscler Thromb Vasc Biol 1999;19:1891–1898. [DOI] [PubMed] [Google Scholar]
- 32. Abela GS, Aziz K.. Cholesterol crystals cause mechanical damage to biological membranes: a proposed mechanism of plaque rupture and erosion leading to arterial thrombosis. Clin Cardiol 2005;28:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dai J, Tian J, Hou J, Xing L, Liu S, Ma L, Yu H, Ren X, Dong N, Yu B.. Association between cholesterol crystals and culprit lesion vulnerability in patients with acute coronary syndrome: an optical coherence tomography study. Atherosclerosis 2016;247:111–117. [DOI] [PubMed] [Google Scholar]
- 34. Self-Medlin Y, Byun J, Jacob RF, Mizuno Y, Mason RP.. Glucose promotes membrane cholesterol crystalline domain formation by lipid peroxidation. Biochim Biophys Acta 2009;1788:1398–1403. [DOI] [PubMed] [Google Scholar]
- 35. Karasawa T, Takahashi M.. Role of NLRP3 inflammasomes in atherosclerosis. J Atheroscler Thromb 2017;24:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Braeckman RA, Stirtan WG, Soni PN.. Pharmacokinetics of eicosapentaenoic acid in plasma and red blood cells after multiple oral dosing with icosapent ethyl in healthy subjects. Clin Pharmacol Drug Develop 2014;3:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soni SP, LoCascio DS, Liu Y, Williams JA, Bittman R, Stillwell W, Wassall SR.. Docosahexaenoic acid enhances segregation of lipids between: 2H-NMR study. Biophys J 2008;95:203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wassall SR, Stillwell W.. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem Phys Lipids 2008;153:57–63. [DOI] [PubMed] [Google Scholar]
- 39. Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA, Soni PN.. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol 2012;110:984–992. [DOI] [PubMed] [Google Scholar]
- 40. Bays HE, Ballantyne CM, Braeckman RA, Stirtan WG, Soni PN.. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs 2013;13:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN.. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol 2011;108:682–690. [DOI] [PubMed] [Google Scholar]
- 42. Braeckman RA, Manku MS, Bays HE, Stirtan WG, Soni PN.. Icosapent ethyl, a pure EPA omega-3 fatty acid: effects on plasma and red blood cell fatty acids in patients with very high triglyceride levels (results from the MARINE study). Prostag Leukotr Ess 2013;89:195–201. [DOI] [PubMed] [Google Scholar]
- 43. Satoh N, Shimatsu A, Kotani K, Sakane N, Yamada K, Suganami T, Kuzuya H, Ogawa Y.. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care 2007;30:144–146. [DOI] [PubMed] [Google Scholar]
- 44. Satoh-Asahara N, Shimatsu A, Sasaki Y, Nakaoka H, Himeno A, Tochiya M, Kono S, Takaya T, Ono K, Wada H, Suganami T, Hasegawa K, Ogawa Y.. Highly purified eicosapentaenoic acid increases interleukin-10 levels of peripheral blood monocytes in obese patients with dyslipidemia. Diabetes Care 2012;35:2631–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dunbar RL, Nicholls SJ, Maki KC, Roth EM, Orloff DG, Curcio D, Johnson J, Kling D, Davidson MH.. Effects of omega-3 carboxylic acids on lipoprotein particles and other cardiovascular risk markers in high-risk statin-treated patients with residual hypertriglyceridemia: a randomized, controlled, double-blind trial. Lipids Health Dis 2015;14:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsunoda F, Lamon-Fava S, Asztalos BF, Iyer LK, Richardson K, Schaefer EJ.. Effects of oral eicosapentaenoic acid versus docosahexaenoic acid on human peripheral blood mononuclear cell gene expression. Atherosclerosis 2015;241:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mickleborough TD, Tecklenburg SL, Montgomery GS, Lindley MR.. Eicosapentaenoic acid is more effective than docosahexaenoic acid in inhibiting proinflammatory mediator production and transcription from LPS-induced human asthmatic alveolar macrophage cells. Clin Nutr 2009;28:71–77. [DOI] [PubMed] [Google Scholar]
- 48. Sato T, Horikawa M, Takei S, Yamazaki F, Ito TK, Kondo T, Sakurai T, Kahyo T, Ikegami K, Sato S, Sato R, Jinno Y, Kawano H, Naoe S, Arita M, Kashiwagi Y, Setou M.. Preferential incorporation of administered eicosapentaenoic acid into thin-cap atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2019;39:1802–1816. [DOI] [PubMed] [Google Scholar]
- 49. Tanaka N, Ishida T, Nagao M, Mori T, Monguchi T, Sasaki M, Mori K, Kondo K, Nakajima H, Honjo T, Irino Y, Toh R, Shinohara M, Hirata K.. Administration of high dose eicosapentaenoic acid enhances anti-inflammatory properties of high-density lipoprotein in Japanese patients with dyslipidemia. Atherosclerosis 2014;237:577–583. [DOI] [PubMed] [Google Scholar]
- 50. Tanaka N, Irino Y, Shinohara M, Tsuda S, Mori T, Nagao M, Oshita T, Mori K, Hara T, Toh R, Ishida T, Hirata KI.. Eicosapentaenoic acid-enriched high-density lipoproteins exhibit anti-atherogenic properties. Circ J 2018;82:596–601. [DOI] [PubMed] [Google Scholar]
- 51. Sherratt SCR, Mason RP.. Eicosapentaenoic acid inhibits oxidation of high density lipoprotein particles in a manner distinct from docosahexaenoic acid. Biochem Biophys Res Commun 2018;496:335–338. [DOI] [PubMed] [Google Scholar]
- 52. Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G.. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 1987;84:9265–9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kojda G, Harrison D.. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 1999;43:562–571. [DOI] [PubMed] [Google Scholar]
- 54. Rees DD, Palmer RM, Moncada S.. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci USA 1989;86:3375–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mason RP, Dawoud H, Jacob RF, Sherratt SCR, Malinski T.. Eicosapentaenoic acid improves endothelial function and nitric oxide bioavailability in a manner that is enhanced in combination with a statin. Biomed Pharmacother 2018;103:1231–1237. [DOI] [PubMed] [Google Scholar]
- 56. Shearer GC, Newman JW.. Impact of circulating esterified eicosanoids and other oxylipins on endothelial function. Curr Atheroscler Rep 2009;11:403–410. [DOI] [PubMed] [Google Scholar]
- 57. Capdevila J, Pramanik B, Napoli JL, Manna S, Falck JR.. Arachidonic acid epoxidation: epoxyeicosatrienoic acids are endogenous constituents of rat liver. Arch Biochem Biophys 1984;231:511–517. [DOI] [PubMed] [Google Scholar]
- 58. Fowler CJ, Doherty P, Alexander SPH.. Endocannabinoid turnover. Adv Pharmacol 2017;80:31–66. [DOI] [PubMed] [Google Scholar]
- 59. Trostchansky A, Bonilla L, Gonzalez-Perilli L, Rubbo H.. Nitro-fatty acids: formation, redox signaling, and therapeutic potential. Antioxid Redox Signal 2013;19:1257–1265. [DOI] [PubMed] [Google Scholar]
- 60. Caro AA, Cederbaum AI.. Role of cytochrome P450 in phospholipase A2- and arachidonic acid-mediated cytotoxicity. Free Radic Biol Med 2006;40:364–375. [DOI] [PubMed] [Google Scholar]
- 61. Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res 2009;50:S52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Spector AA, Fang X, Snyder GD, Weintraub NL.. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 2004;43:55–90. [DOI] [PubMed] [Google Scholar]
- 63. Spector AA, Kim HY.. Cytochrome P epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim Biophys Acta 2015;1851:356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM.. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv Nutr 2015;6:513–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Takenaga M, Hirai A, Terano T, Tamura Y, Kitagawa H, Yoshida S.. Comparison of the in vitro effect of eicosapentaenoic acid (EPA)-derived lipoxygenase metabolites on human platelet function with those of arachidonic acid. Thromb Res 1986;41:373–384. [DOI] [PubMed] [Google Scholar]
- 66. Goetzl EJ, Brash AR, Tauber AI, Oates JA, Hubbard WC.. Modulation of human neutrophil function by monohydroxy-eicosatetraenoic acids. Immunology 1980;39:491–501. [PMC free article] [PubMed] [Google Scholar]
- 67. Heidel JR, Taylor SM, Laegreid WW, Silflow RM, Liggitt HD, Leid RW.. In vivo chemotaxis of bovine neutrophils induced by 5-lipoxygenase metabolites of arachidonic and eicosapentaenoic acid. Am J Pathol 1989;134:671–676. [PMC free article] [PubMed] [Google Scholar]
- 68. Valone FH, Franklin M, Sun FF, Goetzl EJ.. Alveolar macrophage lipoxygenase products of arachidonic acid: isolation and recognition as the predominant constituents of the neutrophil chemotactic activity elaborated by alveolar macrophages. Cell Immunol 1980;54:390–401. [DOI] [PubMed] [Google Scholar]
- 69. Kogure R, Toyama K, Hiyamuta S, Kojima I, Takeda S.. 5-Hydroxy-eicosapentaenoic acid is an endogenous GPR119 agonist and enhances glucose-dependent insulin secretion. Biochem Biophys Res Commun 2011;416:58–63. [DOI] [PubMed] [Google Scholar]
- 70. Powell WS, Gravel S, Gravelle F.. Formation of a 5-oxo metabolite of 5,8,11,14,17-eicosapentaenoic acid and its effects on human neutrophils and eosinophils. J Lipid Res 1995;36:2590–2598. [PubMed] [Google Scholar]
- 71. Terano T, Salmon JA, Moncada S.. Biosynthesis and biological activity of leukotriene B5. Prostaglandins 1984;27:217–232. [DOI] [PubMed] [Google Scholar]
- 72. Hafstrom I, Palmblad J, Malmsten CL, Radmark O, Samuelsson B.. Leukotriene B4—a stereospecific stimulator for release of lysosomal enzymes from neutrophils. FEBS Lett 1981;130:146–148. [DOI] [PubMed] [Google Scholar]
- 73. Juan H, Peskar BA, Simmet T.. Effect of exogenous 5,8,11,14,17-eicosapentaenoic acid on cardiac anaphylaxis. Br J Pharmacol 1987;90:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Maddox JF, Serhan CN.. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med 1996;183:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Patricia MK, Kim JA, Harper CM, Shih PT, Berliner JA, Natarajan R, Nadler JL, Hedrick CC.. Lipoxygenase products increase monocyte adhesion to human aortic endothelial cells. Arterioscler Thromb Vasc Biol 1999;19:2615–2622. [DOI] [PubMed] [Google Scholar]
- 76. Reilly KB, Srinivasan S, Hatley ME, Patricia MK, Lannigan J, Bolick DT, Vandenhoff G, Pei H, Natarajan R, Nadler JL, Hedrick CC.. 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J Biol Chem 2004;279:9440–9450. [DOI] [PubMed] [Google Scholar]
- 77. Nazarewicz RR, Zenebe WJ, Parihar A, Parihar MS, Vaccaro M, Rink C, Sen CK, Ghafourifar P.. 12(S)-hydroperoxyeicosatetraenoic acid (12-HETE) increases mitochondrial nitric oxide by increasing intramitochondrial calcium. Arch Biochem Biophys 2007;468:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thollon C, Iliou JP, Cambarrat C, Robin F, Vilaine JP.. Nature of the cardiomyocyte injury induced by lipid hydroperoxides. Cardiovasc Res 1995;30:648–655. [PubMed] [Google Scholar]
- 79. Matsuda H, Miyatake K, Dahlen SE.. Pharmacodynamics of 15(S)-hydroperoxyeicosatetraenoic (15-HPETE) and 15(S)-hydroxyeicosatetraenoic acid (15-HETE) in isolated arteries from guinea pig, rabbit, rat and human. J Pharmacol Exp Ther 1995;273:1182–1189. [PubMed] [Google Scholar]
- 80. Hersberger M. Potential role of the lipoxygenase derived lipid mediators in atherosclerosis: leukotrienes, lipoxins and resolvins. Clin Chem Lab Med 2010;48:1063–1073. [DOI] [PubMed] [Google Scholar]
- 81. Weylandt KH, Krause LF, Gomolka B, Chiu CY, Bilal S, Nadolny A, Waechter SF, Fischer A, Rothe M, Kang JX.. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-alpha. Carcinogenesis 2011;32:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Endo J, Sano M, Isobe Y, Fukuda K, Kang JX, Arai H, Arita M.. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J Exp Med 2014;211:1673–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, da Costa Goncalves A, Huang Y, Luft FC, Gollasch M.. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol 2009;29:54–60. [DOI] [PubMed] [Google Scholar]
- 84. Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC.. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res 1998;83:932–939. [DOI] [PubMed] [Google Scholar]
- 85. Proctor KG, Falck JR, Capdevila J.. Intestinal vasodilation by epoxyeicosatrienoic acids: arachidonic acid metabolites produced by a cytochrome P450 monooxygenase. Circ Res 1987;60:50–59. [DOI] [PubMed] [Google Scholar]
- 86. Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, Patel PK, Jacobs ER, Medhora M.. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol 2008;294:H724–H735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. VanRollins M. Epoxygenase metabolites of docosahexaenoic and eicosapentaenoic acids inhibit platelet aggregation at concentrations below those affecting thromboxane synthesis. J Pharmacol Exp Ther 1995;274:798–804. [PubMed] [Google Scholar]
- 88. Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M.. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am J Physiol Heart Circul Physiol 2001;280:H2430–H2440. [DOI] [PubMed] [Google Scholar]
- 89. Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK.. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999;285:1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Campbell WB, Deeter C, Gauthier KM, Ingraham RH, Falck JR, Li P1.. 15-Dihydroxyeicosatrienoic acid relaxes bovine coronary arteries by activation of K(Ca) channels. Am J Physiol Heart Circ Physiol 2002;282:H1656–H1664. [DOI] [PubMed] [Google Scholar]
- 91. López-Vicario C, Alcaraz-Quiles J, García-Alonso V, Rius B, Hwang SH, Titos E, Lopategi A, Hammock BD, Arroyo V, Clària J.. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides. Proc Natl Acad Sci U S A 2015;112:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hercule HC, Salanova B, Essin K, Honeck H, Falck JR, Sausbier M, Ruth P, Schunck WH, Luft FC, Gollasch M.. The vasodilator 17,18-epoxyeicosatetraenoic acid targets the pore-forming BK alpha channel subunit in rodents. Exp Physiol 2007;92:1067–1076. [DOI] [PubMed] [Google Scholar]
- 93. Lu T, Katakam PV, VanRollins M, Weintraub NL, Spector AA, Lee HC.. Dihydroxyeicosatrienoic acids are potent activators of Ca(2+)-activated K(+) channels in isolated rat coronary arterial myocytes. J Physiol 2001;534:651–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ballantyne CM, Manku MS, Bays HE, Philip S, Granowitz C, Doyle RT Jr, Juliano RA.. Icosapent ethyl effects on fatty acid profiles in statin-treated patients with high triglycerides: the randomized, placebo-controlled ANCHOR study. Cardiol Ther 2019;8:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shearer GC, Borkowski K, Puumala SL, Harris WS, Pedersen TL, Newman JW.. Abnormal lipoprotein oxylipins in metabolic syndrome and partial correction by omega-3 fatty acids. Prostagland Leukot Ess 2018;128:1–10. [DOI] [PubMed] [Google Scholar]
- 96. Shearer GC, Harris WS, Pedersen TL, Newman JW.. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res 2010;51:2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Burr GO, Burr MM.. On the nature and role of the fatty acids essential in nutrition. J Biol Chem 1930;86:587. [Google Scholar]
- 98. Burr GO, Burr MM, Miller ES.. On the fatty acids essential in nutrition. III. J Biol Chem 1932;97:1–9. [Google Scholar]
- 99. Spector AA, Kim HY.. Discovery of essential fatty acids. J Lipid Res 2015;56:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Moon SH, Liu X, Cedars AM, Yang K, Kiebish MA, Joseph SM, Kelley J, Jenkins CM, Gross RW.. Heart failure-induced activation of phospholipase iPLA2gamma generates hydroxyeicosatetraenoic acids opening the mitochondrial permeability transition pore. J Biol Chem 2018;293:115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Laneuville O, Breuer DK, Xu N, Huang ZH, Gage DA, Watson JT, Lagarde M, DeWitt DL, Smith WL.. Fatty acid substrate specificities of human prostaglandin-endoperoxide H synthase-1 and -2. Formation of 12-hydroxy-(9Z, 13E/Z, 15Z)- octadecatrienoic acids from alpha-linolenic acid. J Biol Chem 1995;270:19330–19336. [DOI] [PubMed] [Google Scholar]
- 102. Milligan G, Shimpukade B, Ulven T, Hudson BD.. Complex pharmacology of free fatty acid receptors. Chem Rev 2017;117:67–110. [DOI] [PubMed] [Google Scholar]
- 103. Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI.. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 2003;278:11303–11311. [DOI] [PubMed] [Google Scholar]
- 104. Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M.. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 2003;422:173–176. [DOI] [PubMed] [Google Scholar]
- 105. Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B.. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun 2003;301:406–410. [DOI] [PubMed] [Google Scholar]
- 106. Edfalk S, Steneberg P, Edlund H.. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008;57:2280–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE, Wank S.. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 2011;140:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F.. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 2009;52:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tan JK, McKenzie C, Marino E, Macia L, Mackay CR.. Metabolite-sensing G protein-coupled receptors-facilitators of diet-related immune regulation. Annu Rev Immunol 2017;35:371–402. [DOI] [PubMed] [Google Scholar]
- 110. Khan MZ, He L.. The role of polyunsaturated fatty acids and GPR40 receptor in brain. Neuropharmacology 2017;113:639–651. [DOI] [PubMed] [Google Scholar]
- 111. Eclov JA, Qian Q, Redetzke R, Chen Q, Wu SC, Healy CL, Ortmeier SB, Harmon E, Shearer GC, O’Connell TD.. EPA, not DHA, prevents fibrosis in pressure overload induced heart failure; potential role of free fatty acid receptor 4. J Lipid Res 2015;56:2297–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G.. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 2005;11:90–94. [DOI] [PubMed] [Google Scholar]
- 113. Burns RN, Moniri NH.. Agonism with the omega-3 fatty acids alpha-linolenic acid and docosahexaenoic acid mediates phosphorylation of both the short and long isoforms of the human GPR120 receptor. Biochem Biophys Res Commun 2010;396:1030–1035. [DOI] [PubMed] [Google Scholar]
- 114. Watson SJ, Brown AJ, Holliday ND.. Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol Pharmacol 2012;81:631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tanaka T, Yano T, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G.. Cloning and characterization of the rat free fatty acid receptor GPR120: in vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch Pharmacol 2008;377:515–522. [DOI] [PubMed] [Google Scholar]
- 116. Cornall LM, Mathai ML, Hryciw DH, McAinch AJ.. Diet-induced obesity up-regulates the abundance of GPR43 and GPR120 in a tissue specific manner. Cell Physiol Biochem 2011;28:949–958. [DOI] [PubMed] [Google Scholar]
- 117. Xiong Y, Swaminath G, Cao Q, Yang L, Guo Q, Salomonis H, Lu J, Houze JB, Dransfield PJ, Wang Y, Liu JJ, Wong S, Schwandner R, Steger F, Baribault H, Liu L, Coberly S, Miao L, Zhang J, Lin DC, Schwarz M.. Activation of FFA1 mediates GLP-1 secretion in mice. Evidence for allosterism at FFA1. Mol Cell Endocrinol 2013;369:119–129. [DOI] [PubMed] [Google Scholar]
- 118. Suckow AT, Polidori D, Yan W, Chon S, Ma JY, Leonard J, Briscoe CP.. Alteration of the glucagon axis in GPR120 (FFAR4) knockout mice: a role for GPR120 in glucagon secretion. J Biol Chem 2014;289:15751–15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stone VM, Dhayal S, Brocklehurst KJ, Lenaghan CS, Winzell M, Hammar M, Xu X, Smith DM, Morgan NG.. GPR120 (FFAR4) is preferentially expressed in pancreatic delta cells and regulates somatostatin secretion from murine islets of Langerhans. Diabetologia 2014;57:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Moran BM, Abdel-Wahab YH, Flatt PR, McKillop AM.. Evaluation of the insulin-releasing and glucose-lowering effects of GPR120 activation in pancreatic beta-cells. Diabetes Obes Metab 2014;16:1128–1139. [DOI] [PubMed] [Google Scholar]
- 121. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM.. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Christiansen E, Watterson KR, Stocker CJ, Sokol E, Jenkins L, Simon K, Grundmann M, Petersen RK, Wargent ET, Hudson BD, Kostenis E, Ejsing CS, Cawthorne MA, Milligan G, Ulven T.. Activity of dietary fatty acids on FFA1 and FFA4 and characterisation of pinolenic acid as a dual FFA1/FFA4 agonist with potential effect against metabolic diseases. Br J Nutr 2015;113:1677–1688. [DOI] [PubMed] [Google Scholar]
- 123. Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, Patel RT, Lee J, Chen S, Peroni OD, Dhaneshwar AS, Hammarstedt A, Smith U, McGraw TE, Saghatelian A, Kahn BB.. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014;159:318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hudson BD, Shimpukade B, Mackenzie AE, Butcher AJ, Pediani JD, Christiansen E, Heathcote H, Tobin AB, Ulven T, Milligan G.. The pharmacology of TUG-891, a potent and selective agonist of the free fatty acid receptor 4 (FFA4/GPR120), demonstrates both potential opportunity and possible challenges to therapeutic agonism. Mol Pharmacol 2013;84:710–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Liu Y, Chen LY, Sokolowska M, Eberlein M, Alsaaty S, Martinez-Anton A, Logun C, Qi HY, Shelhamer JH.. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A(2) via GPR120 receptor to produce prostaglandin E(2) and plays an anti-inflammatory role in macrophages. Immunology 2014;143:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nohr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW.. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab 2013;2:376–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Galindo MM, Voigt N, Stein J, van Lengerich J, Raguse JD, Hofmann T, Meyerhof W, Behrens M.. G protein-coupled receptors in human fat taste perception. Chem Senses 2012;37:123–139. [DOI] [PubMed] [Google Scholar]
- 128. Milligan G, Alvarez-Curto E, Watterson KR, Ulven T, Hudson BD.. Characterizing pharmacological ligands to study the long-chain fatty acid receptors GPR40/FFA1 and GPR120/FFA4. Br J Pharmacol 2015;172:3254–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen J, Shearer GC, Chen Q, Healy CL, Beyer AJ, Nareddy VB, Gerdes AM, Harris WS, O'Connell TD, Wang D.. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation 2011;123:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV.. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol 2015;84:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Nagai T, Anzai T, Mano Y, Kaneko H, Anzai A, Sugano Y, Maekawa Y, Takahashi T, Yoshikawa T, Fukuda K.. Eicosapentaenoic acid suppresses adverse effects of C-reactive protein overexpression on pressure overload-induced cardiac remodeling. Heart Vessels 2013;28:404–411. [DOI] [PubMed] [Google Scholar]
- 132. Ghule AE, Kandhare AD, Jadhav SS, Zanwar AA, Bodhankar SL.. Omega-3-fatty acid adds to the protective effect of flax lignan concentrate in pressure overload-induced myocardial hypertrophy in rats via modulation of oxidative stress and apoptosis. Int Immunopharmacol 2015;28:751–763. [DOI] [PubMed] [Google Scholar]
- 133. Mayyas F, Jaradat R, Alzoubi KH.. Cardiac effects of fish oil in a rat model of streptozotocin-induced diabetes. Nutr Metab Cardiovasc Dis 2018;28:592–599. [DOI] [PubMed] [Google Scholar]
- 134. Poudyal H, Panchal SK, Ward LC, Brown L.. Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J Nutr Biochem 2013;24:1041–1052. [DOI] [PubMed] [Google Scholar]
- 135. Ramadeen A, Connelly KA, Leong-Poi H, Hu X, Fujii H, Laurent G, Domenichiello AF, Bazinet RP, Dorian P.. Docosahexaenoic acid, but not eicosapentaenoic acid, supplementation reduces vulnerability to atrial fibrillation. Circ Arrhythm Electrophysiol 2012;5:978–983. [DOI] [PubMed] [Google Scholar]
- 136. Ramadeen A, Laurent G, dos Santos CC, Hu X, Connelly KA, Holub BJ, Mangat I, Dorian P. N- 3 polyunsaturated fatty acids alter expression of fibrotic and hypertrophic genes in a dog model of atrial cardiomyopathy. Heart Rhythm 2010;7:520–528. [DOI] [PubMed] [Google Scholar]
- 137. Kitamura K, Shibata R, Tsuji Y, Shimano M, Inden Y, Murohara T.. Eicosapentaenoic acid prevents atrial fibrillation associated with heart failure in a rabbit model. Am J Physiol Heart Circ Physiol 2011;300:H1814–H1821. [DOI] [PubMed] [Google Scholar]
- 138. Li X, Ballantyne LL, Mewburn JD,, Kang JX,, Barkley RM,, Murphy RC,, Yu Y,, Funk CD.. Endogenously generated omega-3 fatty acids attenuate vascular inflammation and neointimal hyperplasia by interaction with free fatty acid receptor 4 in mice. J Am Heart Assoc 2015;4:e001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Han L, Shen WJ, Bittner S, Kraemer FB, Azhar S.. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-beta/delta and PPAR-gamma. Future Cardiol 2017;13:279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O'Rahilly S, Palmer CN, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W.. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev 2006;58:726–741. [DOI] [PubMed] [Google Scholar]
- 141. Glass CK, Ogawa S.. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol 2006;6:44–55. [DOI] [PubMed] [Google Scholar]
- 142. Bervejillo L, Ferreira AM.. Understanding peroxisome proliferator-activated receptors: from the structure to the regulatory actions on metabolism. Adv Exp Med Biol 2019;1127:39–57. [DOI] [PubMed] [Google Scholar]
- 143. Brown JD, Plutzky J.. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation 2007;115:518–533. [DOI] [PubMed] [Google Scholar]
- 144. Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications—a review. Nutr J 2014;13: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Berger JP, Akiyama TE, Meinke PT.. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci 2005;26:244–251. [DOI] [PubMed] [Google Scholar]
- 146. Evans RM, Barish GD, Wang YX.. PPARs and the complex journey to obesity. Nat Med 2004;10:355–361. [DOI] [PubMed] [Google Scholar]
- 147. Cheang WS, Tian XY, Wong WT, Huang Y.. The peroxisome proliferator-activated receptors in cardiovascular diseases: experimental benefits and clinical challenges. Br J Pharmacol 2015;172:5512–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV.. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 1999;3:397–403. [DOI] [PubMed] [Google Scholar]
- 149. Nelson JR, Wani O, May HT, Budoff M.. Potential benefits of eicosapentaenoic acid on atherosclerotic plaques. Vasc Pharmacol 2017;91:1–9. [DOI] [PubMed] [Google Scholar]
- 150. Wong ND, Toth PP, Amsterdam EA; American Society for Preventive Cardiology. Most important advances in preventive cardiology during this past decade: viewpoint from the American Society for Preventive Cardiology. Trends Cardiovasc Med 2019;doi: 10.1016/j.tcm.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 151. Jackson SM, Parhami F, Xi XP, Berliner JA, Hsueh WA, Law RE, Demer LL.. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler Thromb Vasc Biol 1999;19:2094–2104. [DOI] [PubMed] [Google Scholar]
- 152. Marx N, Sukhova GK, Collins T, Libby P, Plutzky J.. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation 1999;99:3125–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sethi S, Ziouzenkova O, Ni H, Wagner DD, Plutzky J, Mayadas TN.. Oxidized omega-3 fatty acids in fish oil inhibit leukocyte-endothelial interactions through activation of PPAR alpha. Blood 2002;100:1340–1346. [DOI] [PubMed] [Google Scholar]
- 154. Huang PH, Sata M, Nishimatsu H, Sumi M, Hirata Y, Nagai R.. Pioglitazone ameliorates endothelial dysfunction and restores ischemia-induced angiogenesis in diabetic mice. Biomed Pharmacother 2008;62:46–52. [DOI] [PubMed] [Google Scholar]
- 155. Quintela AM, Jiménez R, Gómez-Guzmán M, Zarzuelo MJ, Galindo P, Sánchez M, Vargas F, Cogolludo Á, Tamargo J, Pérez-Vizcaíno F, Duarte J.. Activation of peroxisome proliferator-activated receptor-beta/-delta (PPARbeta/delta) prevents endothelial dysfunction in type 1 diabetic rats. Free Radic Biol Med 2012;53:730–741. [DOI] [PubMed] [Google Scholar]
- 156. Tian XY, Wong WT, Wang N, Lu Y, Cheang WS, Liu J, Liu L, Liu Y, Lee SS, Chen ZY, Cooke JP, Yao X, Huang Y.. PPARdelta activation protects endothelial function in diabetic mice. Diabetes 2012;61:3285–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wong WT, Tian XY, Xu A, Yu J, Lau CW, Hoo RL, Wang Y, Lee VW, Lam KS, Vanhoutte PM, Huang Y.. Adiponectin is required for PPARgamma-mediated improvement of endothelial function in diabetic mice. Cell Metab 2011;14:104–115. [DOI] [PubMed] [Google Scholar]
- 158. Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf J, Tedgui A.. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature 1998;393:790–793. [DOI] [PubMed] [Google Scholar]
- 159. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK.. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998;391:79–82. [DOI] [PubMed] [Google Scholar]
- 160. Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM.. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med 2001;7:48–52. [DOI] [PubMed] [Google Scholar]
- 161. Tordjman KM, Semenkovich CF, Coleman T, Yudovich R, Bak S, Osher E, Vechoropoulos M, Stern N.. Absence of peroxisome proliferator-activated receptor-alpha abolishes hypertension and attenuates atherosclerosis in the Tsukuba hypertensive mouse. Hypertension 2007;50:945–951. [DOI] [PubMed] [Google Scholar]
- 162. Babaev VR, Ishiguro H, Ding L, Yancey PG, Dove DE, Kovacs WJ, Semenkovich CF, Fazio S, Linton MF.. Macrophage expression of peroxisome proliferator-activated receptor-alpha reduces atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2007;116:1404–1412. [DOI] [PubMed] [Google Scholar]
- 163. Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J, Michel T, Plutzky J.. PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. J Clin Invest 2009;119:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Jung UJ, Torrejon C, Chang CL, Hamai H, Worgall TS, Deckelbaum RJ.. Fatty acids regulate endothelial lipase and inflammatory markers in macrophages and in mouse aorta: a role for PPARgamma. Arterioscler Thromb Vasc Biol 2012;32:2929–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Chang HY, Lee HN, Kim W, Surh YJ.. Docosahexaenoic acid induces M2 macrophage polarization through peroxisome proliferator-activated receptor gamma activation. Life Sci 2015;120:39–47. [DOI] [PubMed] [Google Scholar]
- 166. Jung TW, Park HS, Choi GH, Kim D, Ahn SH, Kim DS, Lee T, Jeong JH.. Maresin 1 attenuates pro-inflammatory reactions and ER stress in HUVECs via PPARalpha-mediated pathway. Mol Cell Biochem 2018;448:335–347. [DOI] [PubMed] [Google Scholar]
- 167.AIM-HIGHInvestigators, Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W.. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 168. Davidson MH, Rosenson RS, Maki KC, Nicholls SJ, Ballantyne CM, Mazzone T, Carlson DM, Williams LA, Kelly MT, Camp HS, Lele A, Stolzenbach JC.. Effects of fenofibric acid on carotid intima-media thickness in patients with mixed dyslipidemia on atorvastatin therapy: randomized, placebo-controlled study (FIRST). Arterioscler Thromb Vasc Biol 2014;34:1298–1306. [DOI] [PubMed] [Google Scholar]
- 169.ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC Jr, Cushman WC, Simons-Morton DG, Byington RP.. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J.. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–212. [DOI] [PubMed] [Google Scholar]
- 171. Karwi QG, Uddin GM, Ho KL, Lopaschuk GD.. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med 2018;5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kanda H, Nohara R, Hasegawa K, Kishimoto C, Sasayama S.. A nuclear complex containing PPARalpha/RXRalpha is markedly downregulated in the hypertrophied rat left ventricular myocardium with normal systolic function. Heart Vessels 2000;15:191–196. [DOI] [PubMed] [Google Scholar]
- 173. Oka S, Alcendor R, Zhai P, Park JY, Shao D, Cho J, Yamamoto T, Tian B, Sadoshima J.. PPARalpha-Sirt1 complex mediates cardiac hypertrophy and failure through suppression of the ERR transcriptional pathway. Cell Metab 2011;14:598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Guellich A, Damy T, Lecarpentier Y, Conti M, Claes V, Samuel JL, Quillard J, Hebert JL, Pineau T, Coirault C.. Role of oxidative stress in cardiac dysfunction of PPARalpha-/- mice. Am J Physiol Heart Circ Physiol 2007;293:H93–H102. [DOI] [PubMed] [Google Scholar]
- 175. Smeets PJ, Teunissen BE, Willemsen PH, van Nieuwenhoven FA, Brouns AE, Janssen BJ, Cleutjens JP, Staels B, van der Vusse GJ, van Bilsen M.. Cardiac hypertrophy is enhanced in PPAR alpha-/- mice in response to chronic pressure overload. Cardiovasc Res 2008;78:79–89. [DOI] [PubMed] [Google Scholar]
- 176. Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP.. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest 2002;109:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ding G, Fu M, Qin Q, Lewis W, Kim HW, Fukai T, Bacanamwo M, Chen YE, Schneider MD, Mangelsdorf DJ, Evans RM, Yang Q.. Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc Res 2007;76:269–279. [DOI] [PubMed] [Google Scholar]
- 178. Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM.. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res 2005;97:372–379. [DOI] [PubMed] [Google Scholar]
- 179. Smeets PJ, Teunissen BE, Planavila A, de Vogel-van den Bosch H, Willemsen PH, van der Vusse GJ, van Bilsen M.. Inflammatory pathways are activated during cardiomyocyte hypertrophy and attenuated by peroxisome proliferator-activated receptors PPARalpha and PPARdelta. J Biol Chem 2008;283:29109–29118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q.. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med 2004;10:1245–1250. [DOI] [PubMed] [Google Scholar]
- 181. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G; Gissi-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1223–1230. [DOI] [PubMed] [Google Scholar]
- 182. Shimojo N, Jesmin S, Sakai S, Maeda S, Miyauchi T, Mizutani T, Aonuma K, Kawano S.. Fish oil constituent eicosapentaenoic acid inhibits endothelin-induced cardiomyocyte hypertrophy via PPAR-alpha. Life Sci 2014;118:173–178. [DOI] [PubMed] [Google Scholar]
- 183. Baranowski M, Blachnio-Zabielska A, Gorski J.. Peroxisome proliferator-activated receptor alpha activation induces unfavourable changes in fatty acid composition of myocardial phospholipids. J Physiol Pharmacol 2009;60:13–20. [PubMed] [Google Scholar]
- 184. Iwami D, Zhang Q, Aramaki O, Nonomura K, Shirasugi N, Niimi M.. Purified eicosapentaenoic acid induces prolonged survival of cardiac allografts and generates regulatory T cells. Am J Transplant 2009;9:1294–1307. [DOI] [PubMed] [Google Scholar]
- 185. Yin R, Huang H, Zhang J, Zhu J, Jing H, Li Z.. Dietary n-3 fatty acids attenuate cardiac allograft vasculopathy via activating peroxisome proliferator-activated receptor-gamma. Pediatr Transplant 2008;12:550–556. [DOI] [PubMed] [Google Scholar]
- 186. Anderson EJ, Thayne KA, Harris M, Shaikh SR, Darden TM, Lark DS, Williams JM, Chitwood WR, Kypson AP, Rodriguez E.. Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium via PPARgamma activation? Antioxid Redox Signal 2014;21:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]