Abstract
Gut microbes play prime role in human health and have shown to exert their influence on various physiological responses including neurological functions. Growing evidences in recent years have indicated a key role of gut microbiota in contributing to mental health. The connection between gut and brain is modulated by microbes via neural, neuroendocrinal and metabolic pathways that are mediated through various neurotransmitters and their precursors, hormones, cytokines and bioactive metabolites. Impaired functioning of this connection can lead to manifestation of mental disorders. Around 1 billion of the world population is reported to suffer from emotional, psychological and neurological imbalances, substance use disorders and cognitive, psychosocial and intellectual disabilities. Thus, it becomes imperative to understand the role of gut microbes in mental disorders. Since variations occur in the conditions associated with different mental disorders and some of them have overlapping symptoms, it becomes important to have a holistic understanding of gut dysbiosis in these disorders. In this review, we consolidate the recent data on alterations in the gut microbes and its consequences in various neurological, psychological and neurodegenerative disorders. Further, considering these evidences, several studies have been undertaken to specifically target the gut microbiota through different therapeutic interventions including administration of live microbes (psychobiotics) to treat mental health disorders and/or their symptoms. We review these studies and propose that an integrative and personalized approach, where combinations of microbe-based therapeutic interventions to modulate gut microbes and in-use psychological treatment practices can be integrated and based on patient’s gut microbiome can be potentially adopted for effective treatment of the mental disorders.
Keywords: Gut microbiota, Mental health, Microbiota-gut-brain axis, Psychobiotics, Neurological disorders
Introduction
Human microbiome research has mushroomed over the last two decades [1] and many of its aspects are being explored to understand its implications in maintaining human health [2–4]. Microbes in the human body have potential to synthesize or modify variety of metabolites, including hormones, vitamins and other bioactive compounds that can modulate various biological functions [5, 6]. Though the exact number of microbes in the human body or number of genes in the collective human microbiome is still not fully settled [7], it is evident that the alterations in the human microbiota composition particularly in the human gut impacts human health [8]. This imbalance of the normal gut microbiota, referred as gut dysbiosis, can lead to development of diseases [5, 8]. In the quest of finding ways to restore health of an individual by bringing back the microbiota balance, the consideration of health and well-being in a holistic manner has got global attention [3, 6, 9]. On one hand this has led to advancements towards exploring the interconnection of human with animal via ecosystem considered under ‘one health’ approach [9]. On the other hand association between microbiota and mental health that includes conditions like emotional, psychological, neurological, substance use disorders and cognitive, psychosocial and intellectual disabilities associated with them is increasingly explored.
Existence of a communication system between the gut and the mental or behavioral state (termed as gut-brain axis), has long been recognized; for instance, when we had ‘butterflies in stomach’ during nervousness or excitement or when our stomachs were ‘tied in knots’ under stress or anger or simply, when we had that ‘gut feeling’ about something. This ‘gut-brain axis’ has been implicated to be employed by the gut microbes in maintaining homeostasis in health and disease [10, 11]. Mental disorders are foremost in conferring ill-health and, worldwide, around 970 million people (~ 13% of the global population) suffer from mental disorders and disabilities [12]. Prioritizing comprehensive health as global development agenda, mental health is included in the United Nations’ Sustainable Development Goals (SDG) and also recognized in primacy for health policies around the world. This due global realization of the importance of mental well-being and recently discovered relationships between the gut microbiome and the brain have urged the scientists to decode its role in manifestation of psychiatric disorders, including major depressive disorder [13]. While there are several reviews linking gut microbiota with human health, recent information on their role in various mental health disorders has not been consolidated to bring out a complete view of the gut-brain nexus in the manifestation of many different mental health disorders, some of them characterized by overlapping symptoms. In this review, we critically analyze the role of the gut microbiota in neuropsychiatric disorders that leads to mental illness. We also provide some of the documented evidences for restoration of mental health by modulation of gut microbiota as an effect of therapeutic interventions including change in life style and dietary habits. Additionally, we put forth the need to develop and the status of the development of psychobiotics as microbe-based therapeutic strategies for the treatment of mental disorders.
The Microbiota-Gut-Brain Axis
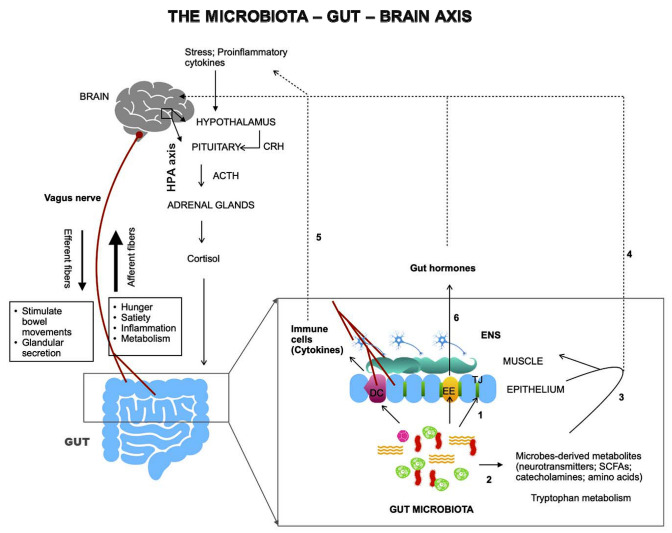
The gut-brain axis (GBA) is a bidirectional connection between a body’s central nervous system (CNS) and the enteric nervous system (ENS) [10]. Direct and indirect pathways connect emotional, memory and cognitive brain centers with the intestinal peripheral functions. A complex crosstalk exists between the endocrine (hypothalamic–pituitary–adrenal axis), immune (chemokines and cytokines) and autonomous nervous system (ANS). All these signaling stations are involved in normal physiological functions such as permeability, secretion of hormones, production of bile salts and mucus of the gastrointestinal (GI) tract [10]. Dysregulation at any point of the network affects the GI tract homeostasis. The GBA via both its neural as well as hormonal connections allow the brain to modulate activities of the functional effector cells, such as immune cells, enteric neurons, muscle cells, epithelial cells etc. of the intestine [10]. These cells are also under the effect of the gut microbiota that, therefore, impacts the GBA [10]. The significance of gut microbiota in the regulation of the gut–brain communication has led to the broadening of the concept of GBA to the Microbiota-Gut-Brain axis [11]. Figure 1 depicts the structural and functional aspects of the Microbiota-Gut-Brain axis. As depicted in the figure, the gut microbiota communicates with brain through three major pathways—the neural pathway (vagus nerve, enteric nervous system), the immune pathway (cytokines) and the endocrine pathway (HPA axis, gut hormones). (Fig. 1). Vagus nerve via efferent fibers stimulate different gut targets to modulate gut mobility, permeability, secretion of hormones, and bowel movements. Signals for hunger, satiety, inflammation or metabolism goes to brain via afferent fibers of the vagus nerve. Vagal neurons interact with the gut neuropod cells to form synapses. Gut microbiota modulates the gut-brain axis via numerous direct and indirect ways (Fig. 1, 1–6): by helping in maintaining gut permeability by modulating the integrity of tight-junctions in the gut epithelium (1), by producing a wide variety of metabolites including neurotransmitters e.g. serotonin, γ-aminobutyric acid (GABA); short chain fatty acids (SCFAs) e.g. butyric acid, propionic acid; catecholamines e.g. dopamine, norepinephrine; amino acids e.g. tryptophan; secondary bile acids, D-lactic acid and gases e.g. nitric oxide, ammonia (2). Microbes-derived metabolites can affect the CNS by acting locally on ENS and vagus nerve (3) or enter circulation and affect the brain (4). Gut microbiota also act locally on gut immune cells (e.g. dendritic cells) and enteroendocrine cells and stimulate the release of cytokines (5) and hormones (6), respectively [14].
Fig. 1.
Microbiota and the gut-brain axis. The gut-brain axis forms a bidirectional network involving three major pathways—the neural pathway (vagus nerve, enteric nervous system), the immune pathway (cytokines) and the endocrine pathway (HPA axis, gut hormones). Gut microbiota modulates this axis via numerous direct and indirect ways (1–6, as described in the text). HPA hypothalamic–pituitary–adrenal axis, EE enteroendocrine cells, DC dendritic cells, TJ tight junctions, SCFAs short chain fatty acids. Bold arrows: local interactions; dashed arrows: via circulation
Early Evidences of Association of Gut Microbiota and Mental Health
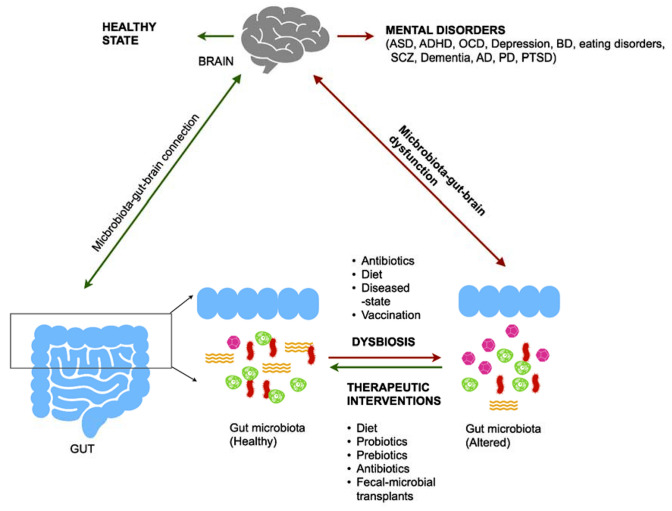
Revelation of microbiota-gut-brain connection led researchers to speculate the role of gut microbiota in mental health and behavior of individuals. An earlier study on germ-free (GF; no exposure to microorganism) rodents revealed learning deficiency and impacts on anxiety behavior and sociability [15]. The role of microbiota in the modulation of brain’s neurochemical behavior was evidenced in a preclinical study on vagotomized healthy mice administered with Lactobacillus rhamnosus [16]. L. rhamnosus (JB-1) could modulate mice’s GABAergic system therefore producing beneficial effects on mental health by reducing depression-related and anxiety-like behavior [16]. Further, alteration in the levels of gut microbial metabolites, such as SCFAs, SBAs, D-lactate, ammonia, tryptophan, histamine have shown to be associated, directly or by breaking down into neuroactive catabolites (for e.g. tryptophan breaks down along kynurenine pathway to produce many catabolites that are neuroactive) with various neurological conditions like Parkinson’s disease (PD), Anorexia Nervosa (AN), Alzheimer’s disease (AD), Autism spectrum disorder (ASD), chronic stress, depression etc.[14, 17–23]. These evidences clearly exhibited the existence of association between the microbiota-gut-brain axis and mental health and opened a way of addressing mental health disorders as a dysfunction of this axis (Fig. 2). However, whether this disruption of homeostasis in the mental health disorders is cause or effect of the alterations in gut microbiota and its functions is not emphatically clear and require more evidential studies.
Fig. 2.
Gut microbiota in mental disorders. Gut dysbiosis leads to the manifestation of several mental health disorders such as autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), depression, bipolar disorder (BD), eating disorders, schizophrenia (SCZ), dementia, Alzheimer’s disease (AD), Parkinson’s disease (PD) and post-traumatic stress disorder (PTSD). Many interventional strategies targeted at restoring healthy gut microbiota through alterations in diet, probiotics, prebiotics, antibiotics and fecal microbial transplants have been suggested as potential therapeutics for these mental disorders
Gut Dysbiosis, Mental Health Disorders and Therapeutic Interventions
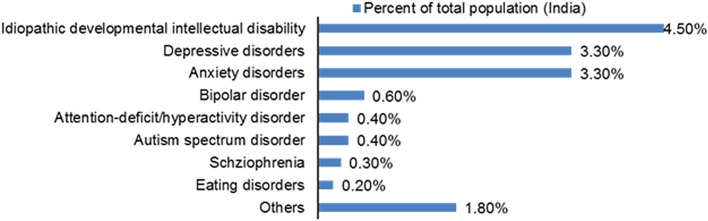
By 2017, around 197 million of people, comprising around 14% of the total population of India suffered with mental illness of one kind or another i.e. 1 in every 7 people had a mental disorder—mild to severe [24], which is almost double from the year 1990. Figure 3 depicts the burden share of various mental disorders in India.
Fig. 3.
Burden share of various mental disorders in India [24]
Considering the alarming increase in the burden of mental disorders and the evidences of association that exists between the gut microbiota and mental health (discussed in the previous section), it becomes imperative to have a comprehensive understanding of the role of the gut microbiota in various mental disorders. We consolidate these studies (Table 1) that can guide us for developing suitable therapeutic interventions to mitigate the disquieting burden of various mental disorders. Some of the employed treatment strategies and their outcomes are also included (psychobiotics are dealt later in a separate section) (Table 1).
Table 1.
Gut dysbiosis, its consequences, therapeutic interventions and their outcomes in various mental health disorders
| Mental health disorder | Change in gut microbiota | Consequences of microbial dysbiosis | Therapeutic interventions | Observations after treatment/therapy | References |
|---|---|---|---|---|---|
| Autism spectrum disorder (ASD) |
Alterations in Bacteroidetes/firmicutes ratio Increase abundance of Bacteroides, Barnesiella, Clostridium and Roseburia Decreased abundance of Bifidobacterium, Coprococcus, Dialister, Faecalibacterium, Prevotella, and Streptococcus |
Less production of butyrate and lactate; mucin degradation |
FMT Changes in diet Antibiotics |
Increased abundance of Bifidobacterium, Prevotella, and Desulfovibrio FMT associated changes in GI and ASD symptoms |
[20, 25, 26] |
| Attention-deficit/hyperactivity disorder (ADHD) |
Increase abundance of Bifdobacterium Neisseria |
Predicted enhanced biosynthesis of cyclohexadienyl dehydratase—enzyme required for dopamine synthesis | Micronutrient supplementation | Decline in abundance of Bifdobacterium | [27, 28] |
| General anxiety disorder (GAD) |
Increase abundance of Fusobacterium, Ruminococcus and Escherichia- Shigella Decrease in SCFAs-producing genera— Faecalibacterium, Eubacterium and Sutterealla |
Immune activation Degradation of mucin |
Non-probiotic (supplementary of the resistant dextrin) | Improvement in anxiety symptoms | [29, 30] |
| Depression |
Abundance of Prevotella, Klebsiella, Enterobacteriaceae and Alistipes Decrease in Faecalibacterium, Coprococcus, Dialister, Ruminococcus species Lachnospiraceae family Lactobacillus and Bifidobacterium species |
Butyrate production decline Increased intestinal inflammation |
FMT—capsule administration | Reduction in the psychological distress | [31, 32] |
| Bipolar disorder (BD) |
Increase in Bacteroidetes, Actinobacteria, class Coriobacteria, Lachnospira, Enterobacteriaceae, Flavonifractor Decrease in Firmicutes Butyrate producing genera—Roseburia, Faecalibacterium and Coprococcus, Ruminococcaceae |
Reduction in butyrate production Abnormal glucose metabolism Oxidative stress |
Special nutritional diet Pharmacotherapies including lithium, anticonvulsants, and atypical antipsychotics (AAP) |
Improvement in the symptoms of the disease | [32–36] |
| Schizophrenia (SCZ) |
Increase in Lactobacillus Streptococcus vestibularis, Lactobacillus fermentum, Enterococcus faecium, Alkaliphilus oremlandii, and Cronobacter sakazakii Cronobacter turicensis Decrease in Veillonellaceae Lachnospiraceae and Ruminococcaceae family, Proteobacteria, Firmicutes, Clostridia, Haemophilus, Sutterella |
Reduction in mucin, SCFAs Immune activation and neuroinflammation |
Fiber rich diets suggested as an effective treatment FMT- capsule administration |
Improved social-behaviors | [36–38] |
| Anorexia nervosa (AN) |
Abundance in Firmicutes, Methanobrevibacter smithii Reduction in Bacteroides Roseburia |
Reduction in butyrate Production of autoantibodies leads to cross reactivity with host neuro-peptides and hormones Decrease in appetite and food efficiency |
FMT (in GF-mice and AN patients) |
Increase in Bacteroidetes Improvement in compulsive behavior |
[39, 40] |
| Addiction (Substance abuse) |
Abundance in Bacteroidetes, Proteobacteria, Fusobacteria, Bacillaceae and Ruminococcaceae, Enterococcaceae and Staphylococcaceae Paracoccus, Thauera, and Prevotella species |
Neuroinflammation and gut inflammation | Antibiotic administration (in normal mice) | Enhanced sensitivity towards cocaine rewards | [42] |
| Post-traumatic stress disorder (PTSD) |
Abundance in Enterococcus and Escherichia-Shigella Decrease in Actinobacteria, Lentisphaerae, and Verrucomicrobia, Lachnospiraceaeae and Ruminococcaceae |
Decreased immunoregulation |
FMT Diet change |
Reduction in severity of PTSD | [43, 44] |
| Dementia |
Increase in Firmicutes/bacteroidetes ratio Decrease in Bacteroidetes |
Lower cognitive function | Probiotics | Alleviated cognitive dysfunction by modulating CNS functions | [47] |
| Alzheimer's disease (AD) |
Abundance in Escherichia and Shigella Reduction in Eubacterium rectale |
Production of proinflammatory cytokines Alteration in neurotransmitters level |
Antibiotics | Use of antibiotics Reduced inflammation but sometime with side effects | [48] |
| Parkinson's disease (PD) |
Abundance of Bifidobacteriaceae, Lactobacillaceae, Pasteurellaceae, Christensenellaceae, Lachnospiraceae and Verrucomicrobiaceae Reduction in Lachnospiraceae |
Depletion in SCFA results in inflammation in brain and colon, constipation Mucin degradation |
Drugs |
Reduction in disease symptoms with side effects Improvement in the disease symptoms |
[49, 50] |
Neurodevelopmental Disorders
In neurodevelopmental disorders the development of CNS is impaired. Brain dysfunction can lead to imbalance in motor functions, language, learning and non-verbal communications. This can lead to Intellectual Disability (ID), earlier termed as mental retardation, Autism Spectrum disorder (ASD) and Attention-Deficit Hyperactivity Disorder (ADHD). Many microbiome research studies are focused on ASD patients [20, 25]. Disturbed GI tract in ASD patients provided the sign of possible link between the gut microbiota and ASD. Alteration in the Bacteriodetes/Firmicutes ratio in ASD patients is reported, although direction of change is not consistent in studies. Increased relative abundance of bacterial genera Bacteroides, Barnesiella, Clostridium and Roseburia and reduced relative abundance of Bifidobacterium, Coprococcus, Dialister, Faecalibacterium, Prevotella, and Streptococcus have been found in different cases [25]. SCFA metabolism changes affected by changes in Faecalibacterium, Ruminococcus, and Bifidobacterium composition has also been reported [25]. Studies have focused on Fecal Microbial Transplantation (FMT)—which include transfer of fecal microbiota from healthy donors to a patient, changing the dietary habits and use of antibiotics to reverse the gut dysbiosis and few have succeeded to an extent. For instance, children with ASD showed improved behavior and 80% reduction in GI problems after a modified FMT treatment [26]. The modified FMT treatment, Microbiota Transfer Therapy (MTT), involves antibiotics administration, a bowel cleanse, a stomach-acid suppressant followed by FMT. Increased abundance of Bifidobacterium, Prevotella, and Desulfovibrio was also observed after the treatment. Improvements persisted even after two years in the gut microbiota as well as in the behavior of ASD patients [26].
Significantly reduced gut microbial diversity was observed in young patients with ADHD and elevated levels of Bacteroides and the genus Neisseria was suggested to be associated with juvenile ADHD [27]. In ADHD patients, probable role of diet in controlling the intensive behavior has been suggested [28]. Gut microbiome demonstrated the predominance of Actinobacteria, especially Bifdobacteria in ADHD patients. In a study, where micronutrients have been given to the patients, only decline in Bifdobacteria population was observed which was indicative of a positive effect [28]. However, no profound effect was observed in rest of the microbial community or symptoms. These studies reflect on the importance of determining gut microbiota in neurodevelopmental disease diagnosis so that community alteration profile can be employed as a cure.
Anxiety Disorders
Anxiety symptoms are generally observed in the patients suffering from different mental disorders. It is generally divided into Generalized Anxiety Disorder (GAD), Phobias, Panic Disorder, Social Anxiety Disorder (SAD) and Obsessive–Compulsive Disorder (OCD). Jiang et al. observed that along with decrease in richness and diversity of gut microbial community, patients suffering with GAD also showed reduction in SCFAs-producing bacteria [29]. In addition, certain genera, Escherichia, Fusobacterium, Shigella and Ruminococcus, were observed more than the normal level [29].
For the therapeutic strategies to treat anxiety disorders, among 21 studies implemented on nearly 1503 patients, 14 studies included the use of probiotic supplements to modulate intestinal microbiota while the rest of the studies focused on improving diets instead of probiotics. In these 14 studies, seven used only one type of probiotic supplement, two used two types and five used at least three types of probiotics [30]. 11 out of 21 studies showed improvement on anxiety symptoms in which five were probiotic based and six were non-probiotic based [30]. These observations indicated two points about the therapeutic strategies that can be employed for anxiety disorders; one, the non-probiotic or improvement in diet is equally effective as probiotic supplements and second, more than 50% studies (86% in case of diet regulation i.e., six out of seven), reflect that alteration in gut microbiome may be a tool for improving the anxiety symptoms.
Mood Disorders
Mood disorders can range from mild, such as, mood swings to severe, such as, depression and bipolar disorders (BD). A recent study has shown dysregulation of the HPA axis as a result of the gut dysbiosis, to be associated with the major depressive disorder and improvements in the symptoms of depression correlated with the restoration of the HPA axis [13]. In another study, population of Faecalibacterium and Coprococcus—butyrate producing organisms and of Dialister were observed declining in patients suffering from depression [31]. This study also supported a connection of dopamine production and gut microbiota as 3,4-dihydroxyphenylacetic acid, a metabolite of dopamine is correlated with mental health of the patients. Many studies have indicated that both the microbiota diversity and richness declined in patients suffering from major depressive disorder when compared to the healthy controls [32]. On the genus level, the abundance of Prevotella, Klebsiella, Enterobacteriaceae and Alistipes increased, while the abundance of family Lachnospiraceae (SCFA producers) and genus Faecalibacterium and Ruminococcus decreased [32]. The decrease in the abundance of gut-beneficial bacteria, Lactobacillus and Bifidobacterium was also observed in the depression patients [32]. The association between gut microbiota and BD has been revealed that indicated the abundance of Actinobacteria and class Coriobacteria and decline in Faecalibacterium and Ruminococcaceae in BD patients [32], Also, the family Clostridiaceae and genus Roseburia were abundant in healthier BD patients compared to depressive patients which showed more members of family Enterobacteriaceae. Change in the gut microbiota in BD patients may also relate with increased risk of metabolic changes. For instance, Flavonifractor abundance in BD patients have shown to relate with oxidative stress and inflammatory response [32]. Interestingly, both BD and major depression disorder resulted in dysbiosis in similar bacterial phyla and families such as abundant Actinobacteria, Enterobacteriaceae and low Faecalibacterium. Members of the family Lachnospiraceae were found reduced in patients suffering with depression while increased abundance of genus Lachnospira was found in the BD patients [32]. Recently, Hu et al. determined that patients with BD are likely to have more population of Bacteroidetes compared to healthy people who have more Firmicutes in their gut [33]. The study also characterized many gut microbiota-based biomarkers for diagnosis and determining outcomes for the treatment of BD.
An initial study of a 21 year old patient with both BD and ADHD showed the effect of special nutritional diet which proved remarkable in improving symptoms of the disease [34]. However, by that time, role of microbiome and gut-brain axis was not well known. Pharmacotherapies including lithium, anticonvulsants, and atypical antipsychotics (AAP) are approved for the management of BD and many studies have explored the interaction between the drugs used and the gut microbiota [35]. FMT improved by capsule administration has been shown to be likely to strongly improve the microbiota-oriented treatments in major depression and schizophrenia (SCZ) [36].
Psychotic disorders
Psychotic mental disorder patients can not differentiate between reality and imagination and undergo hallucinations, one such example is schizophrenia (SCZ). Studies investigating microbiome differences between SCZ patients and healthy individuals have given inconsistent results with Anaerococcus and Bacteroides were found increased and taxa under Proteobacteria, Clostridia, Haemophilus, Sutterella were found significantly reduced in SCZ [37]. Lactobacilli is only found to be consistently significantly elevated in SCZ and in people with increased risk of SCZ and even correlated with the severity of the SCZ symptoms. Reduced alpha-diversity was noted in SCZ patients and bacterial taxa such as Veillonellaceae and Lachnospiraceae were found to be associated with the gut of SCZ patients [37]. Metagenome-wide association study (MWAS) on 90 medication-free SCZ patients and 81 controls has delineated differences in SCFAs synthesis, tryptophan metabolism, and synthesis or degradation of neurotransmitters as functional potentials associated with SCZ [38]. Streptococcus vestibularis, Lactobacillus fermentum, Enterococcus faecium, Alkaliphilus oremlandii, and Cronobacter sakazakii/turicensis were found to be enriched in the SCZ gut [38].
Microbiome targeted treatments have not been well-studied yet in SCZ patients, however as stated above FMT improved by capsule administration has shown potential to improve the microbiota-oriented treatment [36]. Also, use of pre/probiotics have shown to be promising auxiliary treatments in SCZ [38]. For instance, transplantation of Streptococcus vestibularis into antibiotic-based microbiota depleted mice appeared to induce deficits in social behaviors, and was found to alter the neurotransmitter levels in peripheral tissues in recipient mice [38].
Eating And Addiction Disorders
Of the three major eating disorders—Anorexia Nervosa (AN), Binge Eating Disorder (BED) and Bulimia Nervosa (BN), association of AN with gut microbiota has gained attention. Roots of AN are also found associated with gut dysbiosis and low gut microbial diversity. Levels and affinities of gut microbiota-produced neuroactive compounds were found to be associated with eating disorders [17]. In the acute stages of AN, phylum Bacteroidetes were found to be reduced and Firmicutes were increased [39]. Furthermore, in AN patients the genus Methanobrevibacter and specifically, M. smithii, was found to be increased while the Roseburia species decreased [39]. Fecal transplants from the AN patients into GF mice has shown the development of AN-like behavior in the recipients [40]. This includes dysbiosis, reduced appetite, low rate of weight gain and increased compulsive behavior. Also, serotonin levels were found to be lower as compared to normal healthy mice. Administration of Bacteroides vulgatus has shown improvement in compulsive behavior but did not help in the weight gain [40].
Addiction mainly includes addiction to alcohol or substance use. The link between the gut microbiota and alcohol addiction has been established suggesting gut-inflammation, neural inflammation and change in microbiota.[41]. Lactobacillus, Bifidobacterium or Akkermansia have shown to enhance intestinal integrity and maintain the gut barrier in alcohol-use disorder patients [41]. Patients with substance abuse have also shown a sign of change in gut-microbial community. Cocaine use disorder showed increased relative abundance of Bacteroidetes. Increased relative abundance of several taxa from Proteobacteria and Fusobacteria phyla have been observed in the rats administered with the psychostimulant, methamphetamine (also known as ice). Bacillaceae and Ruminococcaceae within Firmicutes phyla were more abundant in the ice-administered mice. Reduction in Bacteroidetes/Firmicutes ratio has also been observed in the morphine-treated rodents, with expansion of potentially pathogenic bacterial families such as Enterococcaceae and Staphylococcaceae. Thauera, Paracoccus and Prevotella have also been identified as substance-related bacteria [42]. Experiments have been performed to show the relation of substance use and behavioral patterns. One such study involved reducing the gut microbiota of normal mice by administering antibiotics orally. This resulted in enhancing the sensitivity towards cocaine rewards in antibiotic-treated mice as compared to antibiotics- untreated mice [42], suggesting that the altered microbiota can enhance the response to such addictions. Although gut dysbiosis is implicated in eating and addiction disorders, more mechanistic studies utilizing antibiotics or FMT are warranted.
Stress Related Disorders
Stress affects body homeostasis and body’s response includes headache, depression, anger and anxiety which relates with change in gut microbiota, immune system function and gut motility. Stress might also be the basis of many CNS disorders discussed above. Post-traumatic stress disorder (PTSD) patients have been shown to possess similar gut-microbiota as stressed individuals but showed the decrease in Actinobacteria, Lentisphaerae, and Verrucomicrobia populations. Poor cognitive performance was observed in PTSD veteran patients with cirrhosis. This was marked with lower gut microbial diversity in PTSD with higher Enterococcus and Escherichia/Shigella and lower beneficial taxa belonging to Lachnospiraceaeae and Ruminococcaceae [43]. Recently, Xu et al. have shown alteration in the relative microbial abundance post stress conditions in rats. The stress-induced differences disappeared in adolescents, however, persisted in adults [44]. They also identified significantly altering metabolites correlated with correlated gut microbes that can be further explored for their therapeutic potential for the treatment of stress disorders [44]. FMT or dietary changes may also help in altering the severity of PTSD [43].
Another disorder linked with stress and anxiety is characterized by inability to sleep properly such as insomnia which is identified as the most prevalent sleep disorder. Gut microbiota and serotonin production are linked, as 90% of serotonin is produced by chromaffin cells in GI tract and spore forming bacteria in gut controls the release of serotonin from these cells. Also, Escherichia coli and species of Enterococcus secrete small amounts of serotonin [45]. Along with serotonin, other neurotransmitters like dopamine, GABA and melatonin, produced by gut microbes, have profound effect on sleep quality and circadian misalignment. It is also observed that the inflammatory reactions due to gut microbiome dysbiosis may lead to affect normal immune system and can influence CNS to cause insomnia-type conditions [45].
Neuro-cognitive Disorders
Neuro-cognitive disorders include delirium that involve confused thinking and less awareness about the things happening in the surroundings. Abdominal surgeries of mice model has shown that change in gut microbiota increased the occurrence of delirium condition [46]. Dementia is another neuro-cognitive disorder linked with reduced memory, language ability and problem solving and understanding abilities. It is a primary condition associated with Alzheimer’s disease (AD). Terminal-Restriction Fragment Length Polymorphism (T-RFLP) revealed that Bacteriodetes are declined in dementia patients than non-dementia patients [47]. Moreover, ratio of Firmicutes/Bacteroidetes is also increased in dementia patients [47]. AD has also been indicated to be associated with dysbiosis. An earlier study had demonstrated increased abundance of a pro-inflammatory taxa, Escherichia and Shigella, and reduction in an anti-inflammatory taxon, Eubacterium rectale in AD patients [48]. In AD, effect of antibiotics has prevalently linked changes in brain activity due to decline in gut microbial population [48]. Neuropsychiatric symptoms of anxiety, depression, apathy, sleep disorders as well as, later in the course, delirium and dementia have been associated with Parkinson’s disease (PD) which is defined by impaired motor responses characterized by slow movement, rigidity and tremor. GI symptoms (such as, constipation), colonic inflammation and appearance of neuropathological hallmarks, α-synuclein and Lewy bodies in the gut before they appear in brain clearly indicated linkage between gut and brain in the manifestation of the disease [49]. Hill-Burns et al. based on a case–control study conducted on 327 subjects (197 PD cases and 130 controls) concluded that PD is associated with gut microbiome dysbiosis [50]. This group studied the gut microbiome at taxonomic, functional and global levels and found significant alterations in the abundance of Bifidobacteriaceae, Lactobacilliaceae, Pasteurellaceae, Christensenellaceae, Lachnospiraceae, and Verrucomicrobiaceae families and revealed modulations in many metabolic pathways including metabolism of plant-derived compounds and degradation of xenobiotics [50].
Thus, different mental disorders are characterized by differential gut microbial community which is generally predominated by a bacterial genus or a family. Notably, in some of the disorders, beneficial microbial population were overpopulated. For instance, abundance of Lactobacillus and Bifidobacterium in disorders like SCZ and ADHD, respectively. The probable reason for this can be attributed to the complexity of the gut microbiota. The adult microbiome generally shows the abundance of Firmicutes and Bacteroidetes phyla over other phyla with genera such as, Clostridium and Bacteroides as most prevalent [51]. In the Indian gut, genus Prevotella is revealed to be predominant with core genera to include Prevotella, Bacteroides, Roseburia and Megasphaera [52]. Although due to variability in dietary habits, environment, health status, age, sex and season, it is impossible to set a criteria of ‘healthy’ or ‘normal’ gut microbiome but, an imbalance in gut microbiota of an individual should be considered characteristic of a disease. High abundance (more than the normal level) of beneficial genera, as found in certain mental disorders, in gut microbiome indicate imbalance and destabilized microbial community. Lower abundance of Lactobacillus and Bifidobacterium could be treated with a probiotic approach.
Role and Scope of Psychobiotics
A very important therapeutic intervention for the treatment of mental disorders is the use of live microbes to restore the integrity of gut microbiota and to provide health benefits. The possibility of utilizing modern probiotics developed using latest advanced techniques has been shown to be very important and promising in the treatment of various diseases such as in obesity-related health complications [53, 54]. The term “psychobiotics” is defined as “a live organism that, when ingested in adequate amounts, produces a health benefit in patients suffering from psychiatric illness”. Since prebiotics (indigestible food material) also have a crucial role in the growth and activities of several beneficial bacteria in the gut, the prebiotics are also included in the definition of psychobiotics [55]. The effect of psychobiotics is very broad and have been demonstrated on regulation of neuroimmune axis, neural and hormonal pathways, inflammatory reflex, sympatho-adrenal medullary axis and in treating neuropsychiatric disorders [56, 57]. Table 2 lists psychobiotic strains which have shown potential in alleviating symptoms or in treatment of various mental disorders.
Table 2.
List of psychobiotic strains (as used in combinations or individually) and outcome of their use in treatment of mental disorders
| Pyschobiotic strains | Mental disorders | Outcome of their use | References |
|---|---|---|---|
| Streptococcus vestibularis | Schizophrenia | Improved social-behaviours | [38] |
| Bifidobacterium longum | Depression and anxiety | Reduction in the psychological distress | [58] |
| Lactobacillus helveticus | |||
| Bifidobacterium longum 1714 | Psychological distress | Reduction in stress and improvement in memory | [59] |
| Bifidobacterium bifidum W23 | Mood disorders | Reduced rumination and aggressive thoughts | [60] |
| Bifidobacterium lactis W52 | |||
| Lactobacillus acidophilus W37 | |||
| Lactobacillus brevis W63 | |||
| Lactobacillus caseiW56 | |||
| Lactococcus lactis W19 | |||
| Lactococcus lactis W58 | |||
| Lactobacillus salivarius W24 | |||
| Bifidobacterium bifidum | Alzheimer’s disease | Revived cognitive and metabolic status | [61] |
| Lactobacillus acidophilus | |||
| Lactobacillus casei | |||
| Lactobacillus fermentum | |||
| Bifidobacterium bifidum | Parkinson’s disease | Downregulated cytokines and up regulated growth factor, peroxisome proliferator—activated receptor | [62] |
| Lactobacillus acidophilus | |||
| Lactobacillus fermentum | |||
| Lactobacillus reuteri | |||
| Lactobacillus plantarum | ASD children | Improved communication and behaviour | [63] |
| Bifidobacterium longum | Autism spectrum disorder (ASD) in children | Improvement in autism and gastrointestinal (GI) symptoms | [64] |
| Lactobacillus acidophilus | |||
| Lactobacillus rhamnosus | |||
| Clostridium butyricum | Vascular dementia | Alleviating cognitive dysfunction by modulating CNS functions | [65] |
Psychobiotics have been tested and implicated as a part of treatment of various mental health dis-orders. For instance, the probiotic combination of Lactobacillus helveticus and Bifidobacterium longum fed to the volunteers daily for 30 days showed significant reduction in the psychological distress which was measured on clinical depression and anxiety scale [58]. Administration of another strain of Bifidobacterium, B. longum 1714 in healthy volunteers for four weeks resulted in reduction in stress and improvement in memory [59]. Another study demarcated the effect of Bifidobacterium and Lactobacillus strains in which probiotic food supplement intervention was made for 30 days with the multispecies probiotics containing Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, and Lactococcus lactis (W19 and W58). Results showed a significantly reduced overall cognitive reactivity to sad mood, which was largely accounted for by reduced rumination and aggressive thoughts [60]. Probiotic milk containing strains of Lactobacillus, L. acidophilus, L. casei, L. fermentum and Bifidobacterium bifidum given for 12 weeks to AD patients resulted in decreased plasma and serum levels of malondialdehyde (MDA) and high-sensitivity C-reactive protein (hs-CRP), respectively. Other improvements included change in the β-cell function, insulin resistance and sensitivity in the patients indicating a positive effect on the cognitive function and some metabolic statuses in the AD patients [61]. Effect of probiotic supplements containing Bifidobacterium bifidum and Lactobacillus species including L. acidophilus, L. reuteri and L. fermentum was also observed in the Parkinson’s disease (PD) subjects for a period of twelve weeks. Movement Disorder Society-United Parkinson’s Rating Scales (MDS-UPDRS) scores were reduced in probiotic consumption group as compared to the placebo group [62]. In addition probiotic consumption also increased the insulin function in PD patients [62]. The intervention of Lactobacillus plantarum has shown to improve communication and behavior in ASD children [63] Three probiotic strains Lactobacillus acidophilus, L. rhamnosus and Bifidobacterium longum were also used in a trial on ASD children in Egypt in which Autism and gastrointestinal (GI) distress symptoms were improved in the children after 12 weeks of treatment with the probiotic strains [64]. Moreover, after probiotic supplementation, the stool samples showed increases in Bifidobacteria and Lactobacillus colonies in autistic children [64]. Another probiotic strain, Clostridium butyricum has been shown to be markedly effective in alleviating cognitive dysfunction associated with vascular dementia in mice, possibly by acting through gut-microbiome-butyrate-brain axis [65]. It modulates CNS functions and reported to have impact on behavior and brain development [65].
Psychobiotics have opened a new and novel therapeutic approach to treat patients suffering from various psychiatric illnesses. Many probiotics, however, that have shown benefits in pre-clinical trials have not shown benefits in humans. For example, administration of Lactobacillus rhamnosus (JB-1) in healthy volunteers undergoing exam-related stress showed no effect on anxiety, mood, stress, or sleep [66]. Further, probiotics intervention studies in some disorders had been inconclusive, such as in BD [67]. Psychobiotics are under expensive exploration and more and more clinical trials are being conducted to demarcate the bacterial strains as potential candidates for treatment of mental disorders. Apart from the selection of a bacteria or group of bacteria, other parameters such as ensuring their prolonged stay in the body as many people expel probiotics from their body are also looked into [68]. Further, as individual’s microbiota vary due to the specific physiology of the body, it is possible that the employment of psychobiotics and their effects may vary person to person. Thus, several factors should be considered carefully for designing the appropriate therapeutic strategies for the treatment of mental disorders.
Challenges and Future Directions
Mounting evidence over the past two decades have established the role of gut microbiome in various mental health disorders; however, before falling on the conclusive microbe-based therapeutic interventions, more robust data and clinical studies indicating the direct role of gut microbiota in restoring the mental health balance are required. There are numerous challenges that need to be addressed for better understanding of the causal or effector link between gut microbiota and wide range of mental health disorders. First, majority of the clinical studies conducted are cross-sectional and for better understanding of the impact of gut microbiota in mental health disorders or effect of any therapeutic intervention targeting gut microbiota, longitudinal studies are warranted [69]. Second, small sample size, lack of robustness and standardized sampling methodology and bioinformatics tools pose unique challenges and hamper the consistency and reproducibility of the results. Lack of comprehensive assessment of all symptoms, gender biasness and self-reporting of symptoms like in questionnaires on gastrointestinal problems, could give misleading interpretations. Though scientists have recognized these challenges and newer studies are better designed and conducted. Third, an important consideration, is that it has been extensively noted that diet plays an intricate role in the composition of an individual’s gut microbiota and even short-term changes in the diet may result in significant alterations in the diversity and richness of gut microbiota [70]. Moreover, majority of mental health disorders are known to affect the appetite and metabolism of patients thereby adding another layer of complexity. Thus, monitoring the diet and lifestyle of both patients and healthy individuals under study, may present us with better understanding for designing the treatment plans. Furthermore, information regarding other interventions like medications and co-morbidities has been lacking in certain clinical studies and must be included. Lastly, it is speculated that every individual exhibits unique gut microbiome which depends on diet, genetics and lifestyle and could display differential responsiveness to treatment methods, such as; FMT, diet therapy, psychobiotics etc. posing another major challenge. Since ‘microbiota-gut-brain’ connection is a complex system encompassing many ways to modulate the gut microbiota and many mechanisms to affect the brain functioning, an integrative approach in which various therapeutic interventions can be employed in combinations for the treatment or mitigation of the disorder or its symptoms can be adopted. Since microbiota of individuals differ from each other and under different conditions, a personalized microbial-based therapy should be considered for effective treatment. Also, studies focused on further understanding the biochemical basis of the microbiota-gut-brain interactions should be encouraged. Our understanding of the impact of gut microbiota in mental health conditions is limited by the use of rodent models and owing to the differences in humans, more clinical studies are warranted. In the coming times, advancements in new technologies and overcoming of the challenges discussed here, would allow thorough understanding of the extent gut microbiota controls the penetrance of mental health disorders and how it can be exploited for designing effective therapeutic interventions. Psychiatrists as well as microbiologists have been confronted with an important perspective of manifestation of mental health disorders; together they can work towards achieving or restoring the normal mental health balance in human population.
Acknowledgements
HV and CDR acknowledge Ramjas College, University of Delhi, Delhi, India for providing infrastructural support. SP thank Department of Biotechnology (DBT), Government of India for Junior Research Fellowship in a project. PL thank University Grants Commission (UGC), Government of India for Senior Research Fellowship. SS and RL thank The Energy and Resources Institute (TERI), New Delhi, India for providing infrastructure facilities. RL acknowledges The National Academy of Sciences, India (NASI) for support under the NASI-Senior Scientist Platinum Jubilee Fellowship Scheme.
Compliance with Ethical Standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cullen CM, Aneja KK, Beyhan S, Cho CE, Woloszynek S, Convertino M, McCoy SJ, Zhang Y, Anderson MZ, Alvarez-Ponce D, Smirnova E, Karstens L, Dorrestein PC, Li H, Sen Gupta A, Cheung K, Powers JG, Zhao Z, Rosen GL. Emerging priorities for microbiome research. Front Microbiol. 2020;11:136. doi: 10.3389/fmicb.2020.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold WM, Hill ES, Fei N, Yee AL, Garcia MS, Cralle LE, Gilbert JA. The human microbiome in health and disease. In: Netto G, Kaul K, editors. Genomic applications in pathology. Cham: Springer; 2019. pp. 607–618. [Google Scholar]
- 3.Singhvi N, Gupta V, Gaur M, Sharma V, Puri A, Singh Y, Dubey GP, Lal R. Interplay of human gut microbiome in health and wellness. Indian J Microbiol. 2020;60:26–36. doi: 10.1007/s12088-019-00825-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekanayake A, Madegedara D, Chandrasekharan V, Magana-Arachchi D. Respiratory bacterial microbiota and individual bacterial variability in lung cancer and bronchiectasis patients. Indian J Microbiol. 2020;60:196–205. doi: 10.1007/s12088-019-00850-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta V, Sood U, Kumar R, Lal R, Kalia VC. Microbiome: a new lease to microbiology. Indian J Microbiol. 2020;60:1. doi: 10.1007/s12088-019-00852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kho ZY, Lal SK. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol. 2018;9:1835. doi: 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dafale NA, Srivastava S, Purohit HJ. Zoonosis: an emerging link to antibiotic resistance under "One Health Approach". Indian J Microbiol. 2020;60:139–152. doi: 10.1007/s12088-020-00860-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 11.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am. 2017;46:77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 12.GBD Disease and Injury Incidence and Prevalence Collaborators (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet. 2017;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastiaanssen TFS, Cussotto S, Claesson MJ, Clarke G, Dinan TG, Cryan JF. Gutted! unraveling the role of the microbiome in major depressive disorder. Harv Rev Psychiatry. 2020;28:26–39. doi: 10.1097/HRP.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caspani G, Kennedy S, Foster JA, Swann J. Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microb Cell. 2019;6:454–481. doi: 10.15698/mic2019.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23(255–264):e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 16.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roubalova R, Prochazkova P, Papezova H, Smitka K, Bilej M, Tlaskalova-Hogenova H. Anorexia nervosa: gut microbiota-immune-brain interactions. Clin Nutr. 2020;39:676–684. doi: 10.1016/j.clnu.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Burmann J, Fassbender K, Schwiertz A, Schafer KH. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Wang Y, Xiayu X, Shi C, Chen W, Song N, Fu X, Zhou R, Xu YF, Huang L, Zhu H, Han Y, Qin C. Altered gut microbiota in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2017;60:1241–1257. doi: 10.3233/JAD-170020. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012;57:2096–2102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- 21.Galland L. The gut microbiome and the brain. J Med Food. 2014;17:1261–1272. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golubeva AV, Joyce SA, Moloney G, Burokas A, Sherwin E, Arboleya S, Flynn I, Khochanskiy D, Moya-Perez A, Peterson V, Rea K, Murphy K, Makarova O, Buravkov S, Hyland NP, Stanton C, Clarke G, Gahan CGM, Dinan TG, Cryan JF. Microbiota-related changes in bile acid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine. 2017;24:166–178. doi: 10.1016/j.ebiom.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YK, Shin C. The microbiota-gut-brain axis in neuropsychiatric disorders: pathophysiological mechanisms and novel treatments. Curr Neuropharmacol. 2018;16:559–573. doi: 10.2174/1570159X15666170915141036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagar R, Dandona R, Gururaj G, Dhaliwal RS, Singh A, Ferrari A, Dua T, Ganguli A, Varghese M, Chakma JK, Kumar GA, Shaji KS, Ambekar A, Rangaswamy T, Vijayakumar L, Agarwal V, Krishnankutty RP, Bhatia R, Charlson F, Chowdhary N, Erskine HE, Glenn SD, Krish V, Mantilla Herrera AM, Mutreja P, Odell CM, Pal PK, Prakash S, Santomauro D, Shukla DK, Singh R, Singh RKL, Thakur JS, ThekkePurakkal AS, Varghese CM, Reddy KS, Swaminathan S, Whiteford H, Bekedam HJ, Murray CJL, Vos T, Dandona L. The burden of mental disorders across the states of India: the global burden of disease study 1990–2017. Lancet Psychiatry. 2020;7:148–161. doi: 10.1016/S2215-0366(19)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bundgaard-Nielsen C, Knudsen J, Leutscher PDC, Lauritsen MB, Nyegaard M, Hagstrøm S, Sørensen S. Gut microbiota profiles of autism spectrum disorder and attention deficit/hyperactivity disorder: a systematic literature review. Gut Microbes. 2020 doi: 10.1080/19490976.2020.1748258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang D, Adams JB, Coleman D, Pollard EL, Maldonado J, McDonough-Means S, Caporaso JG, Krajmalnik-Brown S. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep. 2019;9:5821. doi: 10.1038/s41598-019-42183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prehn-Kristensen A, Zimmermann A, Tittmann L, Lieb W, Schreiber S, Baving L, Fischer A. Reduced microbiome alpha diversity in young patients with ADHD. PLoS ONE. 2018;13:e0200728. doi: 10.1371/journal.pone.0200728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo HD, Kim DW, Hong YS, Kim YM, Seo JH, Choe BM, Park JH, Kang JW, Yoo JH, Chueh HW, Lee JH, Kwak MJ, Kim J. Dietary patterns in children with attention deficit/hyperactivity disorder (ADHD) Nutrients. 2014;6:1539–1553. doi: 10.3390/nu6041539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang HY, Zhang X, Yu ZH, Zhang Z, Deng M, Zhao JH, Ruan B. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130–136. doi: 10.1016/j.jpsychires.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Yang B, Wei J, Ju P, Chen J. Effects of regulating intestinal microbiota on anxiety symptoms: a systematic review. Gen Psychiatr. 2019;32:e100056. doi: 10.1136/gpsych-2019-100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, Raes J. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4:623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 32.Huang TT, Lai JB, Du YL, Xu Y, Ruan LM, Hu SH. Current understanding of gut microbiota in mood disorders: an update of human studies. Front Genet. 2019;10:98. doi: 10.3389/fgene.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu S, Li A, Huang T, Lai J, Li J, Sublette ME, Lu H, Lu Q, Du Y, Hu Z, Ng CH, Zhang H, Lu J, Mou T, Lu S, Wang D, Duan J, Hu J, Huang M, Wei N, Zhou W, Ruan L, Li MD, Xu Y. Gut microbiota changes in patients with bipolar depression. Adv Sci (Weinh) 2019;6:1900752. doi: 10.1002/advs.201900752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rucklidge JJ, Harrison R. Successful treatment of bipolar disorder II and ADHD with a micronutrient formula: a case study. CNS Spectr. 2010;15:289–295. doi: 10.1017/s1092852900027516. [DOI] [PubMed] [Google Scholar]
- 35.Flowers SA, Ward KM, Clark CT. The gut microbiome in bipolar disorder and pharmacotherapy management. Neuropsychobiology. 2020;79:43–49. doi: 10.1159/000504496. [DOI] [PubMed] [Google Scholar]
- 36.Fond GB, Lagier JC, Honore S, Lancon C, Korchia T, Sunhary De Verville PL, Llorca PM, Auquier P, Guedj E, Boyer L. Microbiota-orientated treatments for major depression and schizophrenia. Nutrients. 2020;12:1024. doi: 10.3390/nu12041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szeligowski T, Yun AL, Lennox BR, Burnet PWJ. The gut microbiome and schizophrenia: the current state of the field and clinical applications. Front Psychiatry. 2020;11:156. doi: 10.3389/fpsyt.2020.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu F, Ju Y, Wang W, Wang Q, Guo R, Ma Q, Sun Q, Fan Y, Xie Y, Yang Z, Jie Z, Zhao B, Xiao L, Yang L, Zhang T, Feng J, Guo L, He X, Chen Y, Chen C, Gao C, Xu X, Yang H, Wang J, Dang Y, Madsen L, Brix S, Kristiansen K, Jia H, Ma X. Metagenome-wide association of gut microbiome features for schizophrenia. Nat Commun. 2020;11:1612. doi: 10.1038/s41467-020-15457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seitz J, Belheouane M, Schulz N, Dempfle A, Baines JF, Herpertz-Dahlmann B. The impact of starvation on the microbiome and gut-brain interaction in anorexia nervosa. Front Endocrinol (Laussanne) 2019;10:41. doi: 10.3389/fendo.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hata T, Miyata N, Takakura S, Yoshihara K, Asano Y, Kimura-Todani T, Yamashita M, Zhang XT, Watanabe N, Mikami K, Koga Y, Sudo N. The gut microbiome derived from anorexia nervosa patients impairs weight gain and behavioral performance in female mice. Endocrinology. 2019;160:2441–2452. doi: 10.1210/en.2019-00408. [DOI] [PubMed] [Google Scholar]
- 41.Leclercq S, Starkel P, Delzenne NM, de Timary P. The gut microbiota: a new target in the management of alcohol dependence? Alcohol. 2019;74:105–111. doi: 10.1016/j.alcohol.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Meckel KR, Kiraly DD. A potential role for the gut microbiome in substance use disorders. Psychopharmacology. 2019;236:1513–1530. doi: 10.1007/s00213-019-05232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bajaj JS, Sikaroodi M, Fagan A, Heuman D, Gilles H, Gavis EA, Fuchs M, Gonzalez-Maeso J, Nizam S, Gillevet PM, Wade JB. Posttraumatic stress disorder is associated with altered gut microbiota that modulates cognitive performance in veterans with cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2019;317:G661–G669. doi: 10.1152/ajpgi.00194.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu M, Wang C, Krolick KN, Shi H, Zhu J. Difference in post-stress recovery of the gut microbiome and its altered metabolism after chronic adolescent stress in rats. Sci Rep. 2020;10:3950. doi: 10.1038/s41598-020-60862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Hao Y, Fan F, Zhang B. The role of microbiome in insomnia, circadian disturbance and depression. Front Psychiatry. 2018;9:669. doi: 10.3389/fpsyt.2018.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Bi JJ, Guo GJ, Yang L, Zhu B, Zhan GF, Li S, Huang NN, Hashimoto K, Yang C, Luo AL. Abnormal composition of gut microbiota contributes to delirium-like behaviors after abdominal surgery in mice. CNS Neurosci Ther. 2019;25:685–696. doi: 10.1111/cns.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saji N, Niida S, Murotani K, Hisada T, Tsuduki T, Sugimoto T, Kimura A, Toba K, Sakurai T. Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci Rep. 2019;9:1008. doi: 10.1038/s41598-018-38218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angelucci F, Cechova K, Amlerova J, Hort J. Antibiotics, gut microbiota, and Alzheimer's disease. J Neuroinflammation. 2019;16:108. doi: 10.1186/s12974-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adler CH, Beach TG. Neuropathological basis of nonmotor manifestations of Parkinson's disease. Mov Disord. 2016;31:1114–1119. doi: 10.1002/mds.26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R, Payami H. Parkinson's disease and Parkinson's disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32:739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kulkarni AS, Kumbhare SV, Dhotre DP, Shouche YS. Mining the core gut microbiome from a sample Indian population. Indian J Microbiol. 2019;59:90–95. doi: 10.1007/s12088-018-0742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar R, Sood U, Gupta V, Singh M, Scaria J, Lal R. Recent advancements in the development of modern probiotics for restoring human gut microbiome dysbiosis. Indian J Microbiol. 2020;60:12–25. doi: 10.1007/s12088-019-00808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naumova N, Alikina T, Tupikin A, Kalmykova A, Soldatova G, Vlassov V, Kabilov M. Human gut microbiome response to short-term Bifidobacterium-based probiotic treatment. Indian J Microbiol. 2020 doi: 10.1007/s12088-020-00888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barbosa RSD, Vieira-Coelho MA. Probiotics and prebiotics: focus on psychiatric disorders—a systematic review. Nutr Rev. 2020;78:437–450. doi: 10.1093/nutrit/nuz080. [DOI] [PubMed] [Google Scholar]
- 57.Ansari F, Pourjafar H, Tabrizi A, Homayouni A. The effects of probiotics and prebiotics on mental disorders: a review on depression, anxiety, alzheimer, and autism spectrum disorders. Curr Pharm Biotechnol. 2020;21:555–565. doi: 10.2174/1389201021666200107113812. [DOI] [PubMed] [Google Scholar]
- 58.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 59.Allen AP, Hutch W, Borre YE, Kennedy PJ, Temko A, Boylan G, Murphy E, Cryan JF, Dinan TG, Clarke G. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry. 2016;6:e939. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, Hamidi GA, Salami M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, Oryan S, Mafi A, Asemi Z. Clinical and metabolic response to probiotic administration in people with Parkinson's disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38:1031–1035. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 63.Nimgampalle M, Kuna Y. Anti-Alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer's disease induced albino rats. J Clin Diagn Res. 2017;11:KC01–KC05. doi: 10.7860/JCDR/2017/26106.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaaban SY, El Gendy YG, Mehanna NS, El-Senousy WM, El-Feki HSA, Saad K, El-Asheer OM. The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr Neurosci. 2018;21:676–681. doi: 10.1080/1028415X.2017.1347746. [DOI] [PubMed] [Google Scholar]
- 65.Liu J, Sun J, Wang F, Yu X, Ling Z, Li H, Zhang H, Jin J, Chen W, Pang M, Yu J, He Y, Xu J. Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res Int. 2015;2015:412946. doi: 10.1155/2015/412946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelly JR, Allen AP, Temko A, Hutch W, Kennedy PJ, Farid N, Murphy E, Boylan G, Bienenstock J, Cryan JF, Clarke G, Dinan TG. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun. 2017;61:50–59. doi: 10.1016/j.bbi.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 67.Reininghaus EZ, Wetzlmair LC, Fellendorf FT, Platzer M, Queissner R, Birner A, Pilz R, Hamm C, Maget A, Koidl C, Riedrich K, Klampfer K, Ferk K, Dalkner N. The impact of probiotic supplements on cognitive parameters in euthymic individuals with bipolar disorder: a pilot study. Neuropsychobiology. 2020;79:63–70. doi: 10.1159/000492537. [DOI] [PubMed] [Google Scholar]
- 68.Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RB, Federici S, Cohen Y, Linevsky R, Rothschild D, Moor AE, Ben-Moshe S, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Shapiro H, Pevsner-Fischer M, Sharon I, Halpern Z, Segal E, Elinav E. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174:1388–1405.e21. doi: 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 69.Järbrink-Sehgal E, Andreasson A. The gut microbiota and mental health in adults. Curr Opin Neurobiol. 2020;62:102–114. doi: 10.1016/j.conb.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Godos J, Currenti W, Angelino D, Mena P, Castellano S, Caraci F, Galvano F, Del Rio D, Ferri R, Grosso G. Diet and mental health: review of the recent updates on molecular mechanisms. Antioxidants (Basel) 2020;9:346. doi: 10.3390/antiox9040346. [DOI] [PMC free article] [PubMed] [Google Scholar]