Abstract
The nociceptin/orphanin FQ peptide (NOP) receptor-related ligands have been demonstrated in preclinical studies for several therapeutic applications. This article highlights (1) how nonhuman primates (NHP) were used to facilitate the development and application of positron emission tomography tracers in humans; (2) effects of an endogenous NOP ligand, nociceptin/orphanin FQ, and its interaction with mu opioid peptide (MOP) receptor agonists; and (3) promising functional profiles of NOP-related agonists in NHP as analgesics and treatment for substance use disorders. NHP models offer the most phylogenetically appropriate evaluation of opioid and non-opioid receptor functions and drug effects. Based on preclinical and clinical data of ligands with mixed NOP/MOP receptor agonist activity, several factors including their intrinsic efficacies for activating NOP versus MOP receptors and different study endpoints in NHP could contribute to different pharmacological profiles. Ample evidence from NHP studies indicates that bifunctional NOP/MOP receptor agonists have opened an exciting avenue for developing safe, effective medications with fewer side effects for treating pain and drug addiction. In particular, bifunctional NOP/MOP partial agonists hold a great potential as (1) effective spinal analgesics without itch side effects; (2) safe, nonaddictive analgesics without opioid side effects such as respiratory depression; and (3) effective medications for substance use disorders.
Keywords: Analgesics, Bifunctional ligands, Chronic pain, Drug abuse, Inflammatory pain, MOP receptor, NOP receptor, Opioids, Parkinson’s disease, Primate, Spinal cord
1. The N/OoFQ-NOP Receptor System
In 1994, several groups of scientists discovered a G protein-coupled receptor with high homology to classical opioid receptors, and this receptor was initially named opioid receptor-like 1 (ORL1) (Bunzow et al. 1994; Fukuda et al. 1994; Mollereau et al. 1994; Wang et al. 1994). A year later, two groups of scientists isolated an endogenous 17-amino acid peptide (FGGFTGARKSARKLANQ) which is selective for the ORL1 receptor. This peptide was named “nociceptin,” because following intracerebroventricular injection, it produced hyperalgesia in mice (Meunier et al. 1995). The same peptide was named “orphanin FQ” based on the recognition of the ORL1 receptor and its first and last amino acid residues (Reinscheid et al. 1995). According to the nomenclature guidelines recommended by the International Union of Basic and Clinical Pharmacology, the peptide was named “nociceptin/orphanin FQ” (N/OFQ), and the ORL1 receptor was renamed “N/OFQ peptide” (NOP) receptor (Cox et al. 2015). This ligand-receptor system has been extensively studied in the past 25 years. Several articles have provided comprehensive overview about the biological actions, medicinal chemistry, pharmacology, and therapeutic applications of the N/OFQ-NOP receptor system (Calo’ and Guerrini 2013; Kiguchi et al. 2016; Lambert 2008; Toll et al. 2016; Witkin et al. 2014; Zaveri 2016). This review particularly highlights the functional profiles of NOP-related ligands in nonhuman primates (NHP) and discusses the therapeutic potential of NOP receptor-targeted ligands.
1.1. Cloning of the Rhesus Monkey NOP Receptor
Similar to classical opioid receptors, NOP receptor is coupled to pertussis toxin-sensitive Gi/o proteins which inhibit adenylate cyclase and voltage-gated calcium channels and activate inward potassium channels (Hawes et al. 2000; Margas et al. 2008; Vaughan and Christie 1996). NOP receptor activation reduces synaptic transmission by either inhibiting neuronal excitability via postsynaptically located NOP receptors or reducing neurotransmitter release via presynaptically located NOP receptors (Calo’ and Guerrini 2013; Moran et al. 2000; Schlicker and Morari 2000). The NOP receptor has been implicated in numerous therapeutic applications based on burgeoning preclinical animal studies (Lambert 2008; Witkin et al. 2014). Given the species differences in receptor activation and signaling cascades between rodents and primates (Chen et al. 2013; Li et al. 2002; Schattauer et al. 2012), it is important to know if the NOP receptor functions differently between NHP and humans.
Scientists have succeeded to clone the rhesus monkey NOP receptor and found that the nucleotide sequence and amino acid sequence of the rhesus monkey NOP receptor were 95.9% and 97.8% identical to those of the human NOP receptor, respectively (Koga et al. 2009). The identified seven amino acid differences between the monkey and the human NOP receptor did not affect the potency of (+)J-113397, a NOP receptor antagonist, in the inhibition of N/OFQ-stimulated [35S]GTPγS binding. There was no significant difference between the monkey and the human NOP receptor in terms of the binding affinity of 125I[Tyr14]N/OFQ, the [35S]GTPγS binding stimulated by N/OFQ, and the antagonist activity of (+)J-113397 (Koga et al. 2009). N/OFQ seems to activate both monkey and the human NOP receptors without significant species differences.
1.2. Imaging Studies of the NOP Receptor
The distribution of 125I[Tyr14]N/OFQ binding sites has been optimized and determined in the brain and spinal cord of cynomolgus macaques (Bridge et al. 2003). The binding sites of 125I[Tyr14]N/OFQ were widespread in the NHP central nervous system and largely consistent with the mRNA expression pattern of the NOP receptor in the human central nervous system (Peluso et al. 1998). The highest levels of 125I[Tyr14]N/OFQ binding were detected in NHP neocortical areas (e.g., frontal cortex and cingulate cortex), hippocampus, amygdala, thalamus, and caudate putamen. There are some differences in several regions regarding low- versus high-binding levels, including the hippocampus, spinal cord, caudate putamen, ventral tegmental area, and dorsal raphe nucleus between NHP (Bridge et al. 2003) and rodents (Anton et al. 1996; Letchworth et al. 2000; Neal et al. 1999). The extensive distribution of 125I[Tyr14]N/OFQ binding sites in NHP not only supports the multiple functional roles of the N/OFQ-NOP receptor system (Lambert 2008; Witkin et al. 2014) but also indicates that some NOP receptor functions may be species-selective (Bridge et al. 2003).
Positron emission tomography (PET) is a powerful noninvasive in vivo imaging technique to measure the receptor occupancy and target expression and for visualization of metabolic processes (Giovacchini et al. 2011). The development of selective PET radiotracers for the NOP receptor has been successful (Hostetler et al. 2013; Pedregal et al. 2012; Pike et al. 2011). Among reported NOP PET tracers, 11C-NOP-1A was initially demonstrated as a useful radioligand to quantify NOP receptor in the rhesus monkey brain (Kimura et al. 2011). 11C-NOP-1A showed good, stable brain uptake, and a selective NOP antagonist, SB-612111, decreased its distribution volume (VT; a measure of receptor density) by approximately 50–70% in all brain regions, indicating that most brain uptake was specifically bound to NOP receptors (Kimura et al. 2011). Subsequently, 11C-NOP-1A was further demonstrated as a promising PET ligand to reliably quantify NOP receptors in the human brain (Lohith et al. 2012, 2014). Whole-body scans showed radioactivity of 11C-NOP-1A in the brain and peripheral organs expressing NOP receptors, such as heart, lungs, and pancreas; and its effective dose is similar to that of other 11C-labeled radioligands in humans (Lohith et al. 2012). Recently, 11C-NOP-1A was used to measure the in vivo binding to NOP receptors in alcohol-dependent individuals, and regional distribution volume of 11C-NOP-1A was not significantly different from that of healthy individuals in the control group (Narendran et al. 2018). These findings may indicate that central NOP receptor density remains unchanged in alcohol-dependent individuals.
Another promising NOP PET tracer is [18F]MK-0911 (Hostetler et al. 2013). The pattern of [18F]MK-0911 binding density in the rhesus monkey brain, such as cortex, caudate putamen, hippocampus, and cerebellum, is consistent with the localization of 125I[Tyr14]N/OFQ binding sites in the macaque brain (Bridge et al. 2003). [18F] MK-0911 displayed reversible NOP receptor-specific binding in the rhesus monkey brain, as its binding was blocked dose-dependently by selective NOP antagonists in different structures; and baseline PET scans with [18F]MK-0911 in healthy humans showed similar tracer distribution and kinetics as compared to those in rhesus monkeys (Hostetler et al. 2013). Importantly, increasing doses of MK-5757, a selective NOP antagonist (Satoh et al. 2009), prior to [18F]MK-0911 were associated with higher levels of the NOP receptor occupancy (Hostetler et al. 2013). Such receptor occupancy studies with selective NOP PET tracers will provide essential dose-selection guidance for future clinical development of NOP receptor antagonists. Collectively, NOP PET tracers are valuable tools to investigate the functional roles of NOP receptors and endogenous N/OFQ in humans under different disease states, such as mental disorders and substance abuse disorders, and facilitate the development of NOP-targeted ligands for different therapeutic applications.
2. Effects of N/OFQ in Nonhuman Primates
N/OFQ has been administered through different delivery routes to determine its role for modulating pain and itch in NHP. Originally, N/OFQ was co-administered with capsaicin into the monkey’s tail to illustrate its peripheral antiallodynic effects, which could be blocked by a NOP receptor antagonist (Ko et al. 2002a). This early study provides the first functional evidence that activation of peripheral NOP receptors in primates could be a viable therapeutic target for alleviating peripherally elicited pain. Indeed, NOP receptors were present in most of small- and large-diameter human dorsal root ganglion (DRG) neurons, and activation of NOP receptors inhibited capsaicin-induced calcium flux in human DRG neurons (Anand et al. 2016). NOP receptors were also found on epidermal keratinocytes and small unmyelinated and large myelinated nerve fibers in humans. The expression of NOP receptors in plantar skin affected by pachyonychia congenital was relatively lower than that of unaffected skin (Pan et al. 2018). These findings together support the notion that peripheral NOP receptor activation may be a treatment option for managing neuropathic pain.
Intrathecal delivery of mu opioid peptide (MOP) receptor agonists has become part of a routine regimen for perioperative analgesia (e.g., during caesarean section) and been used successfully in different clinical settings in the past four decades (Brill et al. 2003; Schug et al. 2006). However, itch (pruritus) is a common side effect derived from intrathecal morphine and compromises the use of spinal opioids in pain management (Ganesh and Maxwell 2007; Waxler et al. 2005). Interestingly, similar to human responses, intrathecal morphine produced long-lasting itch sensation and pain relief simultaneously in NHP (Ko and Naughton 2000). Intrathecal N/OFQ dose-dependently produced antinociception without eliciting itch scratching responses in NHP, and this effect was reversed by a NOP receptor antagonist (Ko et al. 2006). Along with the mass spectrometry, N/OFQ(2–17) was identified as the major fragment of N/OFQ in the NHP cerebrospinal fluid, and N/OFQ(2–17) did not interfere with intrathecal N/OFQ-induced antinociception (Ko et al. 2006). Given that rodents did not display robust scratching responses following intrathecal morphine (Lee et al. 2003; Sukhtankar and Ko 2013), NHP could serve as a surrogate species to build up a translational bridge for identifying novel spinal analgesics without itch side effects.
Intrathecal N/OFQ in ultralow doses (i.e., in femto moles) in mice produced pain-like behaviors manifested by biting, scratching, and licking behaviors (Sakurada et al. 1999). Unlike dual actions (i.e., pronociception in low doses and antinociception in high doses) of spinal N/OFQ in rodents (Hao et al. 1998; Inoue et al. 1999), intrathecal N/OFQ over a wide dose range, from 1 fmol to 1 μmol, only produced antinociception in NHP (Ko and Naughton 2009). More importantly, intrathecal N/OFQ did not exert anti-morphine action as N/OFQ dose-dependently enhanced intrathecal morphine-induced antinociception without attenuating morphine-induced scratching (Ko and Naughton 2009). In a NHP model of inflammatory pain, intrathecal N/OFQ was found to be the most potent peptide among all endogenous opioid-related peptides for exerting antihyperalgesia (Lee and Ko 2015). Taken together, these findings suggest that spinal N/OFQ-NOP receptor system plays a pivotal role in pain inhibition and the NOP receptor represents an attractive target as spinal analgesics (Kiguchi et al. 2016).
Supraspinal N/OFQ-NOP receptor system plays a pronociceptive role in rodents, as several studies have shown that intracerebroventricular administration of N/OFQ and NOP receptor agonists produced hyperalgesia and attenuated morphine-induced antinociception (Calo et al. 1998; Meunier et al. 1995; Reinscheid et al. 1995). With the advance of surgical techniques, an intrathecal catheter was implanted, and the catheter tip was placed in the cisterna magna of NHP for supraspinal drug delivery (Ding et al. 2015). The intracisternal administration of neuropeptides mimics the “volume transmission” of endogenous peptides transported to multiple sites in the brain (Veening et al. 2012). Unlike substance P eliciting allodynia-/hyperalgesia-like responses, intracisternal administration of N/OFQ produced NOP antagonist-reversible antinociceptive effects, and intracisternal N/OFQ did not attenuate morphine antinociception in NHP (Ding et al. 2015). These findings provide distinct functional profiles of supraspinal N/OFQ-NOP receptor system between NHP and rodents. NHP with the intracisternal catheter could further improve our understanding of diverse neuropeptides involved in top-down, descending pain modulation in primates.
To our knowledge, NOP-related ligands have been studied in NHP in three therapeutic areas, i.e., treatment potential for (1) pain, (2) substance use disorders, and (3) Parkinson’s disease. As Morari’s research team has recently reviewed effects of NOP-related ligands in the NHP model of Parkinson’s disease (Mercatelli et al. 2019), below we specifically discuss the effects of NOP-related ligands as analgesics and a treatment option for substance use disorders.
3. NOP-Related Agonists as Analgesics
Ample evidence indicates that NOP-related agonists exerted antinociceptive and antihypersensitive effects in rodents under a variety of pain modalities (Kiguchi et al. 2016; Schroder et al. 2014). As intrathecal and systemic administration are common drug delivery routes in the clinic, this section reviews the antinociceptive and antihypersensitive effects of NOP-related agonists following intrathecal and systemic administration in NHP.
3.1. Effects of Intrathecal Administration of NOP-Related Agonists
3.1.1. Selective NOP Receptor Agonists
The spinal dorsal horn is the major locus not only for the integration of peripheral sensory input and descending supraspinal modulation but also for regulating peripherally and centrally mediated pain (Peirs and Seal 2016). In particular, intrathecal drug delivery can provide effective, long-lasting pain relief as an alternative delivery route (Caraway et al. 2015; Smyth et al. 2015). Through chemical modification of N/OFQ by increasing its potency and decreasing its degradation, a selective NOP agonist UFP-112 exerted antinociceptive effects with higher potency and longer duration of action than N/OFQ in mice (Calo et al. 2011; Rizzi et al. 2007). Such findings can be translated to NHP as intrathecal UFP-112 was approximately ten times more potent than morphine with similar duration of action for attenuating acute pain and capsaicin-induced thermal allodynia in NHP (Hu et al. 2010).
Using an innovative chemical strategy, peptide welding technology (PWT) (Calo et al. 2018), scientists have generated different tetrabranched derivatives of N/OFQ. PWT2-N/OFQ was demonstrated to be a high-affinity, potent, and selective NOP agonist. In particular, PWT2-N/OFQ was about 40-fold more potent than N/OFQ and produced 5 h duration of antinociception in mice (Rizzi et al. 2015). More importantly, these promising findings (e.g., largely increased potency and improved duration of action of PWT2-N/OFQ) can be translated from rodents to primates. Intrathecal PWT2-N/OFQ potently exerted full antinociceptive effects lasted for more than 24 h without eliciting scratching in NHP (Rizzi et al. 2015). For a side-by-side comparison, PWT2-N/OFQ (i.e., 3 nmol) is approximately 30-fold more potent than N/OFQ (100 nmol), and the duration of antinociceptive action of PWT2-N/OFQ (~24 h) is tenfold longer than that of N/OFQ (~2.5 h) in NHP (Ko et al. 2006; Rizzi et al. 2015). These findings indicate that PWT derivatives of N/OFQ-related peptides are viable candidates for future spinal analgesics with improved therapeutic profiles.
3.1.2. Ligands with Mixed NOP/MOP Receptor Agonist Activity
In rat neuropathic pain models, intrathecal N/OFQ not only exerted antihyperalgesia but also synergistically enhanced antihyperalgesic effects of intrathecal morphine (Courteix et al. 2004). This antinociceptive synergism by coadministration of NOP and MOP receptor agonists intrathecally has also been found in NHP (Hu et al. 2010; Ko and Naughton 2009). In order to investigate the pharmacological profile of a single molecule with mixed NOP/MOP agonist activity, scientists have identified several mixed NOP/MOP receptor agonists. [Dmt1]N/OFQ(1–13)-NH2 displayed similar potency and efficacy like N/OFQ in vitro, but intrathecal [Dmt1]N/OFQ (1–13)-NH2 was approximately 30-fold more potent than N/OFQ in producing antinociception in NHP (Molinari et al. 2013). Moreover, intrathecal PWT2-[Dmt1]N/OFQ(1–13) exerted full antinociceptive effects with higher potency and much longer duration of action in NHP (Cerlesi et al. 2017).
Intrathecal administration of small molecules with mixed NOP/MOP partial agonist activity, such as BU08028 and SR16435, also potently and effectively attenuated hypersensitivity in mouse models of neuropathic and inflammatory pain (Sukhtankar et al. 2013). More importantly, repeated administration of intrathecal SR16435 showed slower development of tolerance to its antiallodynic effects as compared to a partial MOP agonist buprenorphine (Sukhtankar et al. 2013). Recently, scientists have identified a naltrexone-derived analog with mixed NOP/MOP partial agonist activity, BU10038, and found that intrathecal administration of BU10038 potently produced antinociception and antihypersensitivity without scratching, and intrathecal BU10038 did not cause tolerance, as compared to morphine, after chronic 4-week administration in NHP (Kiguchi et al. 2019). Collectively, these findings together strongly support the notion that mixed NOP/MOP receptor agonists display higher potency, wider therapeutic window, and slower tolerance development and such ligands should be developed as a new generation of spinal analgesics.
3.2. Effects of Systemic Administration of NOP-Related Agonists
3.2.1. Selective NOP Receptor Agonists
Behavioral effects of systemic administration of NOP-related agonists are integrated from peripheral, spinal, and supraspinal actions of NOP receptor activation. Following subcutaneous, intramuscular, or intravenous administration, selective NOP receptor agonists, such as Ro 64–6198 and SCH 221510, dose-dependently produced antinociceptive effects against different noxious stimuli in NHP (Kangas and Bergman 2014; Ko et al. 2009; Podlesnik et al. 2011; Sukhtankar et al. 2014b). In particular, systemic NOP receptor agonists effectively increased thermal nociceptive thresholds (Cremeans et al. 2012; Kangas and Bergman 2014) and attenuated capsaicin-induced allodynia (Ko et al. 2009) and carrageenan-induced hyperalgesia (Sukhtankar et al. 2014b). Compared to clinically used MOP receptor agonists, selective NOP receptor agonists did not cause adverse effects typically associated with MOP agonists, such as respiratory depression, itch, abuse liability, constipation, and physical dependence (Ding et al. 2016; Ko et al. 2009; Wladischkin et al. 2012). However, Ro 64–6198 caused sedation at a dose which was 30-fold higher than its full antinociceptive dose in NHP (Podlesnik et al. 2011). This functional profile of selective NOP agonists is still considered promising because antinociceptive doses of MOP agonists produced respiratory depression and reinforcing effects (Butelman et al. 1993; Ko et al. 2002b), kappa opioid peptide (KOP) receptor agonists produced sedation and dysphoria (Butelman et al. 2001; Ko et al. 1999), and delta opioid peptide (DOP) receptor agonists produced convulsions (Negus et al. 1994; Sukhtankar et al. 2014b) in NHP.
Selective NOP agonists did not consistently increase thermal nociceptive thresholds across different groups of NHP (Cornelissen et al. 2019; Saccone et al. 2016). It should be noted that the tail-withdrawal latency from an acute noxious stimulus, 50°C water, is not relevant to the clinical setting in which patients experience spontaneous pain and mechanical hypersensitivity (Brix Finnerup et al. 2013). This procedure has been used commonly by NHP investigators to study opioid-related ligands (Butelman et al. 2001; Ko et al. 1999; Negus et al. 1994); however, it is not useful for non-opioid analgesics with different mechanisms (Hawkinson et al. 2007; Sukhtankar et al. 2014b). Moreover, antinociceptive doses of MOP agonists measured by the NHP warm water tail-withdrawal assay impaired NHP’s food-maintained operant behavior (Withey et al. 2018). These results indicate that antinociceptive doses of clinically used MOP agonists detected in NHP might be too high, i.e., no behavioral selectivity as the same antinociceptive dose has suppressed other behavioral responses. In particular, the antinociceptive dose 10 mg/kg of morphine in NHP (Cornelissen et al. 2019) was much higher than the analgesic doses of morphine (i.e., 0.1–0.2 mg/kg) used in humans (Aubrun et al. 2012), indicating that these NHP needed much higher doses of morphine to suppress their tail-withdrawal responses. As behaviorally disruptive effects of (–)Ro 64–6198 peaked at 100 min after intramuscular administration, using a 15-min inter-injection interval to assess behavioral effects of (–)Ro 64–6198 (Cornelissen et al. 2019) was a significant experimental design flaw. Without recognizing promising clinical data of cebranopadol, a mixed NOP/opioid receptor agonist (Calo and Lambert 2018; Raffa et al. 2017; see Sect. 3.2.2), Cornelissen et al. (2019) made an inappropriate conclusion about the opioid-sparing potential of NOP agonists. Nevertheless, it is worth noting that SCH 221510 significantly produced morphine-like antinociceptive effects in a NHP “operant” nociceptive assay with behavioral selectivity (Kangas and Bergman 2014). Such findings agree with those from reflex-based assays (Podlesnik et al. 2011; Sukhtankar et al. 2014b) and support the analgesic potential of selective NOP agonists. As the functional plasticity of NOP receptors and the efficacy of NOP agonists may change along with different pain modalities (Kiguchi et al. 2016; Schroder et al. 2014), NHP studies with different outcome measures including operant behavior and hypersensitivity will advance our understanding of the analgesic potential of NOP-related ligands.
3.2.2. Ligands with Mixed NOP/MOP Receptor Agonist Activity
In addition to MOP agonist-induced antinociception enhanced by NOP agonists at the spinal level (Hu et al. 2010; Ko and Naughton 2009), the isobologram analysis demonstrates that systemic NOP receptor agonists, Ro 64–6198 and SCH 221510, synergistically enhanced buprenorphine-induced antinociception without causing respiratory depression in NHP (Cremeans et al. 2012). Buprenorphine has much lower binding affinity at NOP receptors, i.e., its Ki values range from 77 to 285 nM, and good binding selectivity for MOP over NOP receptors (i.e., from 50- to 930-fold) (Ding et al. 2018c; Khroyan et al. 2009; Spagnolo et al. 2008). In the functional assay of NOP agonist-stimulated [35S]GTPγS binding, buprenorphine displayed no stimulation (Spagnolo et al. 2008) or mild stimulation (i.e., 10–15% as compared to N/OFQ) at much higher concentration (i.e., >250 nM) (Ding et al. 2018c; Khroyan et al. 2009). The receptor binding and efficacy profile of buprenorphine fits very well with its MOP partial agonist profile in NHP as the NOP receptor antagonist could not shift the dose-response curve of buprenorphine-induced antinociception (Cremeans et al. 2012).
Buprenorphine has been widely used in both humans and veterinary medicine to effectively alleviate a variety of pain conditions including neuropathic pain (Hans 2013; Raffa et al. 2014). However, buprenorphine is not completely devoid of abuse potential (Lavonas et al. 2014). Given the inhibition of dopamine neurotransmission by the NOP receptor (Flau et al. 2002; Murphy et al. 1996) and synergistic antinociception between NOP agonists and buprenorphine (Cremeans et al. 2012), we initially formed a hypothesis that coactivation of NOP and MOP receptors may potently produce analgesia with fewer side effects (Lin and Ko 2013). Despite that NOP receptor activation attenuated MOP receptor-mediated antinociception in rodents (Khroyan et al. 2009), our hypothesis that bifunctional NOP/MOP agonists may have a wider therapeutic window as compared to selective MOP or NOP agonists in primates (Lin and Ko 2013) is supported by the functional profiles of three ligands with mixed NOP/MOP agonist activity discussed below (Fig. 1).
Fig. 1.
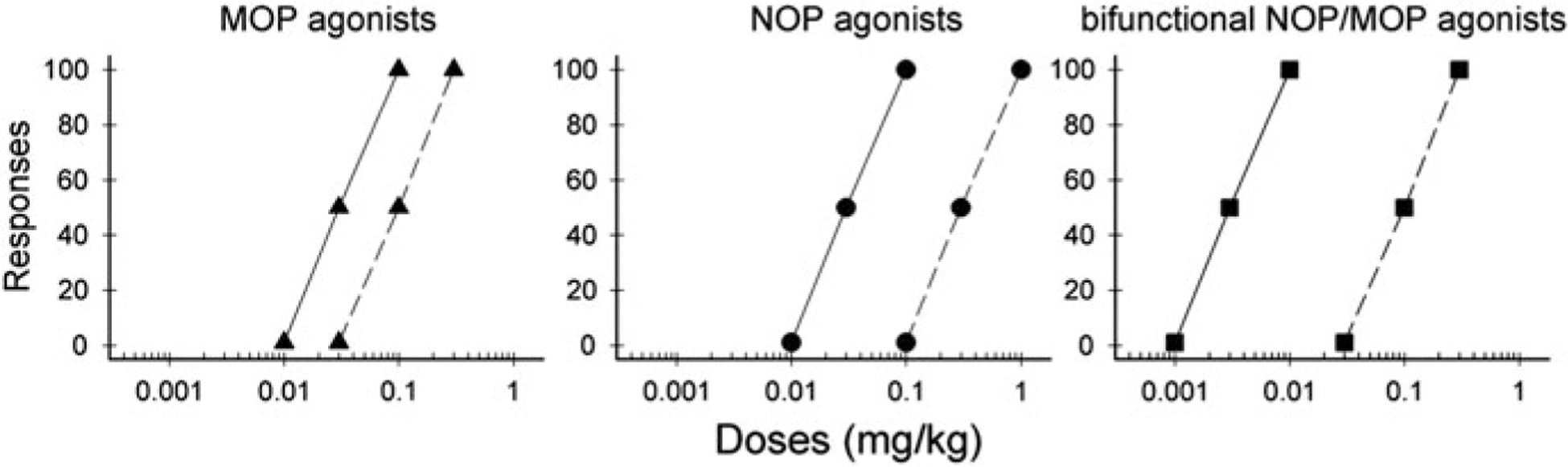
A general hypothetical framework of comparison of the therapeutic windows of MOP, NOP, and bifunctional NOP/MOP agonists based on current literature. Solid lines indicate the doses at which antinociception/analgesia occurs. Dashed lines indicate the doses at which side effects, especially respiratory depression and sedation, emerge. Reprinted with permission from Lin and Ko (2013)
BU08028, a recently developed buprenorphine analog, strikingly displays a similar receptor binding profile like buprenorphine (i.e., Ki: 1–10 nM for MOP, KOP, and DOP receptors) with improved binding affinity (Ki: 8 nM) and efficacy (~48% stimulation of [35S]GTPγS binding) on NOP receptors (Khroyan et al. 2011). BU08028 exerted an extra-long duration of antinociceptive and antihypersensitive effects, up to 30 h, in NHP (Ding et al. 2016). Unlike rodent studies in which a NOP antagonist potentiated BU08028-induced antinociception (Khroyan et al. 2011), both NOP and MOP antagonists produced the same degree of the rightward shift of the dose-response curve for BU08028-induced antinociception in NHP (Ding et al. 2016). Under the progressive-ratio schedule of drug self-administration, BU08028 did not produce reinforcing effects (i.e., abuse potential) as compared to other drugs of abuse, including cocaine and buprenorphine. More importantly, unlike fentanyl which quickly caused respiratory depression, BU08028 at ~30 times higher than its antinociceptive dose did not change NHP’s respiratory and cardiovascular activities. These findings provide the first functional evidence that BU08028 with mixed NOP/MOP agonist activity is a safe, nonaddictive analgesic in NHP (Ding et al. 2016).
In order to test our hypothesis by a non-morphinan chemical structure, AT-121 was identified as a bifunctional NOP/MOP partial agonist, which showed high potency (EC50, 20–35 nM) and partial agonist efficacy (NOP, 41%; MOP, 14% stimulation of [35S]GTPγS binding) at both NOP and MOP receptors (Ding et al. 2018c). Through a series of NHP assays, AT-121 exerted morphine-like antinociceptive and antihypersensitive effects and did not compromise respiratory and cardiovascular activities. Unlike morphine, AT-121 did not produce opioid-induced hyperalgesia and physical dependence and has a much slower development of analgesic tolerance than morphine. Slower development of tolerance to AT-121’s antinociception supports the notion that coactivation of NOP and MOP receptors reserves most functional receptor reservoirs and repeated administration of a bifunctional NOP/MOP agonist may cause a smaller degree of receptor desensitization (Dumas and Pollack 2008; Lin and Ko 2013). More importantly, daily pretreatment with AT-121 attenuated reinforcing effects of oxycodone without disrupting food-maintained operant behavior, indicative of selective inhibition of opioid-reinforced operant behavior (Ding et al. 2018c). These findings together not only support our hypothesis that bifunctional NOP/MOP agonists are safe, nonaddictive analgesics with a wider therapeutic window (Lin and Ko 2013) but also provide functional evidence that such agonists could have a dual therapeutic action for treatment of pain and opioid addiction (Ding et al. 2018c). It is worth noting that opioid and non-opioid “partial” agonists generally have proven therapeutic efficacy with favorable safety and tolerability (Kane et al. 2016; Kantola et al. 2017; van Niel et al. 2016). Similar to buprenorphine’s intrinsic efficacy (e.g., ~17% stimulation of [35S]GTPγS binding at MOP receptors) (Spagnolo et al. 2008), BU08028 and AT-121 are expected to exert analgesic efficacy equal to or more than buprenorphine, but with little or no abuse liability.
Cebranopadol binds to NOP, MOP, and KOP receptors with Ki values of 1–3 nM, and it has nearly full agonist activity at human NOP, MOP, and DOP receptors and partial agonist activity at KOP receptors, based on the [35S]GTPγS binding assay (Linz et al. 2014). As Calo and Lambert (2018) has recently provided a comprehensive review of cebranopadol, we only briefly discuss this drug herein. Through a series of preclinical pain models in rodents, cebranopadol is highly potent (e.g., ED50 values, 0.5–5 μg/kg in rats with chronic pain) and fully effective in producing antinociceptive and antihypersensitive effects (Calo and Lambert 2018; Linz et al. 2014; Raffa et al. 2017). In rat models of spinal nerve ligation-induced neuropathy and arthritic pain, both NOP and MOP receptors mainly contributed to antihypersensitive effects of cebranopadol (Linz et al. 2014; Schiene et al. 2018). Cebranopadol also potently (1–5.6 μg/kg, subcutaneous) produced antinociceptive and antihypersensitive effects in NHP. After intrathecal administration, 1 μg of cebranopadol produced antinociception without eliciting scratching responses (Trapella et al. 2018). More importantly, recent clinical studies have reported promising results of cebranopadol’s efficacy and tolerability. For example, an analgesic dose of cebranopadol produced respiratory depression with an estimate for minimum ventilation greater than zero l/min, which is different from full MOP agonists such as fentanyl and indicative of potential ceiling, in healthy individuals (Dahan et al. 2017). In the first clinical trial in patients with chronic low back pain, cebranopadol was effective, safe, and displayed beneficial effects, such as improved sleep and functionality, with an acceptable tolerability profile (Christoph et al. 2017). In patients experiencing moderate to severe pain following bunionectomy, cebranopadol was better tolerated and received a better overall rating than morphine controlled release (Scholz et al. 2018). In patients with moderate-to-severe cancer pain, cebranopadol was effective, safe, and well-tolerated than morphine prolonged release (Eerdekens et al. 2018). Overall, these clinical data of cebranopadol support the hypothesis that ligands with mixed NOP/MOP agonists have the improved analgesic potency and wider therapeutic window (Kiguchi et al. 2016; Lin and Ko 2013).
4. NOP-Related Ligands for Treatment of Substance Use Disorders
4.1. Effects of Selective NOP Receptor Agonists
Given that activation of NOP receptors inhibited dopamine release in the nucleus accumbens (Di Giannuario and Pieretti 2000; Murphy et al. 1996), NOP receptor agonists may not produce reward-related behaviors and may inhibit MOP receptor-mediated reward. Unlike MOP agonists, NOP agonists did not produce conditioned place preference (CPP) (Devine et al. 1996) and reinforcing effects measured by drug self-administration (Sukhtankar et al. 2014a), and they blocked MOP agonist-induced CPP in rodents (Toll et al. 2016). In NHP, the discriminative stimulus effects of Ro 64–6198 partially generalized to diazepam (Saccone et al. 2016), but Ro 64–6198 did not produce reinforcing effects as compared to alfentanil, cocaine, and methohexital (Ko et al. 2009).
Although Ro 64–6198 attenuated reinforcing effects of remifentanil, its attenuation only occurred in NHP showing sedation (Podlesnik et al. 2011). It is known that Ro 64–6198 has a limited bioavailability (Heinig et al. 2010). However, when another NOP agonist, SCH221510, was administered intracisternally, it attenuated reinforcing effects of both remifentanil and sucrose pellets, indicative of no behavioral selectivity in rodents (Sukhtankar et al. 2014a). It is not clear to what degree central NOP receptor activation can “selectively” attenuate reinforcing effects of MOP agonists or other classes of drugs of abuse without sedation in NHP. It should be noted that reinforcing effects determined by drug self-administration (operant behavior) procedures, not CPP, is considered a gold standard to assess drug’s abuse liability and effective medications for substance abuse disorders (Ator and Griffiths 2003; Mello and Negus 1996). It is also important to note that MOP agonists can produce reward and reinforcing effects through mechanisms that do not require dopamine neurotransmission (Fields and Margolis 2015; Hiranita et al. 2013; Ide et al. 2017). Such evidence may explain the limited efficacy of NOP agonists for attenuating reinforcing effects of MOP agonists (Podlesnik et al. 2011; Sukhtankar et al. 2014a).
4.2. Effects of Ligands with Mixed NOP/MOP Receptor Agonist Activity
Compared to remifentanil, buprenorphine, and oxycodone, bifunctional NOP/MOP partial agonists, such as AT-121 and BU08028, did not produce reinforcing effects in NHP (Ding et al. 2016, 2018c). However, cebranopadol with full NOP and MOP agonist activity produced reinforcing effects in the fixed-ratio schedule (FR30) of reinforcement, and the reinforcing strength of cebranopadol was lower than that of fentanyl under the progressive-ratio schedule in NHP (Trapella et al. 2018). These findings are similar to a recent human study, reporting that cebranopadol produced some drug-liking effects, but cebranopadol has lower abuse potential than a MOP agonist, hydromorphone (Gohler et al. 2019). Comparing the reinforcing effects of AT-121, BU08028, and cebranopadol under the same NHP drug self-administration procedure, NOP receptor activation seems able to attenuate reinforcing effects mediated by partial, but not full, MOP receptor agonism. Future studies using more ligands with different intrinsic efficacies at NOP versus MOP receptors will advance our understanding of the functional role of NOP receptors in modulating reinforcing effects of MOP agonists.
In a session of daily pretreatment for 5 days, AT-121 acutely attenuated and continued to attenuate reinforcing effects of oxycodone without disrupting food-maintained operant behavior; and the degree of attenuation was similar to the inhibitory effects of buprenorphine (Ding et al. 2018c). Such attenuation could be due to partial MOP agonism and/or NOP agonism. Nonetheless, AT-121 is the first ligand to demonstrate the functional efficacy of a bifunctional NOP/MOP agonist in blocking reinforcing effects of a prescription opioid oxycodone with behavioral selectivity (Ding et al. 2018c). Furthermore, BU08028 was recently found to selectively decrease alcohol drinking without altering food-maintained operant behavior following acute and chronic dosing regimens in NHP (Czoty et al. 2017). As AT-121 and BU08028 alone did not produce reinforcing effects (Ding et al. 2016, 2018c), bifunctional NOP/MOP partial agonists have opened a new avenue for developing safe, effective medications with few side effects for treating substance use disorders.
5. Conclusions
Taken together, functional profiles of NOP-related agonists in NHP have shown promising therapeutic potential for treating pain and drug abuse. NHP models offer the most phylogenetically appropriate evaluation of opioid and non-opioid receptor functions and drug effects (Chen et al. 2013; Lin and Ko 2013; Phillips et al. 2014). Often exciting findings from rodents cannot be translated to primates. For example, a recently discovered G protein signaling-biased MOP agonist, PZM21, lacked opioid rewarding effects in mice (Manglik et al. 2016). However, like oxycodone, PZM21 produced reinforcing effects in the NHP drug self-administration assay (Ding et al. 2018b). As pain and/or drug addiction is embedded in chronic diseases which cause dysregulation of multiple ligand-receptor systems in NHP and humans (Ding et al. 2018a; Ferguson et al. 2018; Kiguchi et al. 2017; Wang et al. 2011), ligands with dual or multiple targets or combined pharmacotherapy may be more effective with favorable side effect profiles. Depending upon the intrinsic efficacies for activating NOP and MOP receptors and therapeutic applications, bifunctional NOP/MOP agonists certainly provide a viable treatment option for pain and substance use disorders.
Acknowledgments
The US National Institutes of Health, National Institute on Drug Abuse (DA032568, DA035359, DA040104, and DA044775), National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR059193 and AR064456), and the US Department of Defense (W81XWH-13-2-0045) supported part of findings described in this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US federal agencies.
Footnotes
Conflict of Interest N.K. and M.C.K. declare that there is no conflict of interest.
Contributor Information
Norikazu Kiguchi, Department of Pharmacology, Wakayama Medical University, Wakayama, Japan.
Mei-Chuan Ko, Department of Physiology and Pharmacology, Wake Forest School of Medicine, Winston-Salem, NC, USA.
References
- Anand P, Yiangou Y, Anand U, Mukerji G, Sinisi M, Fox M, McQuillan A, Quick T, Korchev YE, Hein P (2016) Nociceptin/orphanin FQ receptor expression in clinical pain disorders and functional effects in cultured neurons. Pain 157:1960–1969 [DOI] [PubMed] [Google Scholar]
- Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ (1996) Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J Comp Neurol 368:229–251 [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR (2003) Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend 70:S55–S72 [DOI] [PubMed] [Google Scholar]
- Aubrun F, Mazoit JX, Riou B (2012) Postoperative intravenous morphine titration. Br J Anaesth 108:193–201 [DOI] [PubMed] [Google Scholar]
- Bridge KE, Wainwright A, Reilly K, Oliver KR (2003) Autoradiographic localization of (125)i[Tyr (14)] nociceptin/orphanin FQ binding sites in macaque primate CNS. Neuroscience 118:513–523 [DOI] [PubMed] [Google Scholar]
- Brill S, Gurman GM, Fisher A (2003) A history of neuraxial administration of local analgesics and opioids. Eur J Anaesthesiol 20:682–689 [DOI] [PubMed] [Google Scholar]
- Brix Finnerup N, Hein Sindrup S, Staehelin Jensen T (2013) Management of painful neuropathies. Handb Clin Neurol 115:279–290 [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK (1994) Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett 347:284–288 [DOI] [PubMed] [Google Scholar]
- Butelman ER, France CP, Woods JH (1993) Apparent pA2 analysis on the respiratory depressant effects of alfentanil, etonitazene, ethylketocyclazocine (EKC) and Mr2033 in rhesus monkeys. J Pharmacol Exp Ther 264:145–151 [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Traynor JR, Vivian JA, Kreek MJ, Woods JH (2001) GR89,696: a potent kappa-opioid agonist with subtype selectivity in rhesus monkeys. J Pharmacol Exp Ther 298:1049–1059 [PubMed] [Google Scholar]
- Calo’ G, Guerrini R (2013) Medicinal chemistry, pharmacology, and biological actions of peptide ligands selective for the nociceptin/orphanin FQ receptor In: Ko MC, Husbands SM (eds) Research and development of opioid-related ligands. American Chemical Society, Washington, pp 275–325. 10.1021/bk-2013-1131.ch1015 [DOI] [Google Scholar]
- Calo G, Lambert DG (2018) Nociceptin/orphanin FQ receptor ligands and translational challenges: focus on cebranopadol as an innovative analgesic. Br J Anaesth 121:1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo G, Rizzi A, Cifani C, Micioni Di Bonaventura MV, Regoli D, Massi M, Salvadori S, Lambert DG, Guerrini R (2011) UFP-112 a potent and long-lasting agonist selective for the nociceptin/orphanin FQ receptor. CNS Neurosci Ther 17:178–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo G, Rizzi A, Marzola G, Guerrini R, Salvadori S, Beani L, Regoli D, Bianchi C (1998) Pharmacological characterization of the nociceptin receptor mediating hyperalgesia in the mouse tail withdrawal assay. Br J Pharmacol 125:373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo G, Rizzi A, Ruzza C, Ferrari F, Pacifico S, Gavioli EC, Salvadori S, Guerrini R (2018) Peptide welding technology – a simple strategy for generating innovative ligands for G protein coupled receptors. Peptides 99:195–204 [DOI] [PubMed] [Google Scholar]
- Caraway D, Walker V, Becker L, Hinnenthal J (2015) Successful discontinuation of systemic opioids after implantation of an intrathecal drug delivery system. Neuromodulation 18:508–515. Discussion 515–506 [DOI] [PubMed] [Google Scholar]
- Cerlesi MC, Ding H, Bird MF, Kiguchi N, Ferrari F, Malfacini D, Rizzi A, Ruzza C, Lambert DG, Ko MC, Calo G, Guerrini R (2017) Pharmacological studies on the NOP and opioid receptor agonist PWT2-[Dmt(1)]N/OFQ(1–13). Eur J Pharmacol 794:115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B, Kim D (2013) Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun 4:2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph A, Eerdekens MH, Kok M, Volkers G, Freynhagen R (2017) Cebranopadol, a novel firstin-class analgesic drug candidate: first experience in patients with chronic low back pain in a randomized clinical trial. Pain 158:1813–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen JC, Steele FF, Tenney RD, Obeng S, Rice KC, Zhang Y, Banks ML (2019) Role of mu-opioid agonist efficacy on antinociceptive interactions between mu agonists and the nociceptin opioid peptide agonist Ro 64–6198 in rhesus monkeys. Eur J Pharmacol 844:175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courteix C, Coudore-Civiale MA, Privat AM, Pelissier T, Eschalier A, Fialip J (2004) Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain. Pain 110:236–245 [DOI] [PubMed] [Google Scholar]
- Cox BM, Christie MJ, Devi L, Toll L, Traynor JR (2015) Challenges for opioid receptor nomenclature: IUPHAR review 9. Br J Pharmacol 172:317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremeans CM, Gruley E, Kyle DJ, Ko MC (2012) Roles of mu-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther 343:72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Epperly P, Davenport A, Ko MC, Husbands SM, Flynn S (2017) Effects of BU08028, a mixed mu opioid receptor and nociceptin/orphanin FQ peptide (NOP) receptor agonist, on alcohol drinking in rhesus monkeys. Neuropsychopharmacology 43:S630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Boom M, Sarton E, Hay J, Groeneveld GJ, Neukirchen M, Bothmer J, Aarts L, Olofsen E (2017) Respiratory effects of the nociceptin/orphanin FQ peptide and opioid receptor agonist, cebranopadol, in healthy human volunteers. Anesthesiology 126:697–707 [DOI] [PubMed] [Google Scholar]
- Devine DP, Reinscheid RK, Monsma FJ Jr, Civelli O, Akil H (1996) The novel neuropeptide orphanin FQ fails to produce conditioned place preference or aversion. Brain Res 727:225–229 [DOI] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S (2000) Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides 21:1125–1130 [DOI] [PubMed] [Google Scholar]
- Ding H, Czoty PW, Kiguchi N, Cami-Kobeci G, Sukhtankar DD, Nader MA, Husbands SM, Ko MC (2016) A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci U S A 113:E5511–E5518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Hayashida K, Suto T, Sukhtankar DD, Kimura M, Mendenhall V, Ko MC (2015) Supraspinal actions of nociceptin/orphanin FQ, morphine and substance P in regulating pain and itch in non-human primates. Br J Pharmacol 172:3302–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Kishioka S, Ma T, Peters CM, Ko MC (2018a) Differential mRNA expression of neuroinflammatory modulators in the spinal cord and thalamus of type 2 diabetic monkeys. J Diabetes 10:886–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Perrey DA, Nguyen T, Czoty PW, Zhang Y, Ko MC (2018b) Reinforcing, antinociceptive, and pruritic effects of a G protein-biased mu opioid receptor agonist, PZM21, in primates. FASEB J 32(1_Suppl):683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Yasuda D, Daga PR, Polgar WE, Lu JJ, Czoty PW, Kishioka S, Zaveri NT, Ko MC (2018c) A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci Transl Med 10:eaar3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas EO, Pollack GM (2008) Opioid tolerance development: a pharmacokinetic/pharmacodynamic perspective. AAPS J 10:537–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerdekens MH, Kapanadze S, Koch ED, Kralidis G, Volkers G, Ahmedzai SH, Meissner W (2018) Cancer-related chronic pain: investigation of the novel analgesic drug candidate cebranopadol in a randomized, double-blind, noninferiority trial. Eur J Pain 10.1002/ejp.1331 [DOI] [PubMed] [Google Scholar]
- Ferguson LB, Harris RA, Mayfield RD (2018) From gene networks to drugs: systems pharmacology approaches for AUD. Psychopharmacology (Berl) 235:1635–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Margolis EB (2015) Understanding opioid reward. Trends Neurosci 38:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flau K, Redmer A, Liedtke S, Kathmann M, Schlicker E (2002) Inhibition of striatal and retinal dopamine release via nociceptin/orphanin FQ receptors. Br J Pharmacol 137:1355–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Sugimoto T (1994) cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett 343:42–46 [DOI] [PubMed] [Google Scholar]
- Ganesh A, Maxwell LG (2007) Pathophysiology and management of opioid-induced pruritus. Drugs 67:2323–2333 [DOI] [PubMed] [Google Scholar]
- Giovacchini G, Squitieri F, Esmaeilzadeh M, Milano A, Mansi L, Ciarmiello A (2011) PET translates neurophysiology into images: a review to stimulate a network between neuroimaging and basic research. J Cell Physiol 226:948–961 [DOI] [PubMed] [Google Scholar]
- Gohler K, Sokolowska M, Schoedel KA, Nemeth R, Kleideiter E, Szeto I, Eerdekens MH (2019) Assessment of the abuse potential of cebranopadol in nondependent recreational opioid users: a phase 1 randomized controlled study. J Clin Psychopharmacol 39:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans GH (2013) Buprenorphine in the treatment of neuropathic pain In: Ko MC, Husbands SM (eds) Research and development of opioid-related ligands. American Chemical Society, Washington, pp 103–123. 10.1021/bk-2013-1131.ch1006 [DOI] [Google Scholar]
- Hao JX, Xu IS, Wiesenfeld-Hallin Z, Xu XJ (1998) Anti-hyperalgesic and anti-allodynic effects of intrathecal nociceptin/orphanin FQ in rats after spinal cord injury, peripheral nerve injury and inflammation. Pain 76:385–393 [DOI] [PubMed] [Google Scholar]
- Hawes BE, Graziano MP, Lambert DG (2000) Cellular actions of nociceptin: transduction mechanisms. Peptides 21:961–967 [DOI] [PubMed] [Google Scholar]
- Hawkinson JE, Szoke BG, Garofalo AW, Hom DS, Zhang H, Dreyer M, Fukuda JY, Chen L, Samant B, Simmonds S, Zeitz KP, Wadsworth A, Liao A, Chavez RA, Zmolek W, Ruslim L, Bova MP, Holcomb R, Butelman ER, Ko MC, Malmberg AB (2007) Pharmacological, pharmacokinetic, and primate analgesic efficacy profile of the novel bradykinin B1 receptor antagonist ELN441958. J Pharmacol Exp Ther 322:619–630 [DOI] [PubMed] [Google Scholar]
- Heinig K, Kratochwil N, Bucheli F, Thomae A (2010) Bioanalytics and pharmacokinetics of the nociceptin/orphanin FQ peptide receptor agonist RO0646198 in Wistar rats and Cynomolgus monkeys. J Chromatogr B Anal Technol Biomed Life Sci 878:2101–2105 [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Kopajtic TA, Katz JL (2013) Stimulants as specific inducers of dopamine-independent sigma agonist self-administration in rats. J Pharmacol Exp Ther 347:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler ED, Sanabria-Bohorquez S, Eng W, Joshi AD, Patel S, Gibson RE, O’Malley S, Krause SM, Ryan C, Riffel K, Bi S, Okamoto O, Kawamoto H, Ozaki S, Ohta H, de Groot T, Bormans G, Depre M, de Hoon J, De Lepeleire I, Reynders T, Cook JJ, Burns HD, Egan M, Cho W, van Laere K, Hargreaves RJ (2013) Evaluation of [(1)(8)F]MK-0911, a positron emission tomography (PET) tracer for opioid receptor-like 1 (ORL1), in rhesus monkey and human. NeuroImage 68:1–10 [DOI] [PubMed] [Google Scholar]
- Hu E, Calo G, Guerrini R, Ko MC (2010) Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain 148:107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide S, Takahashi T, Takamatsu Y, Uhl GR, Niki H, Sora I, Ikeda K (2017) Distinct roles of opioid and dopamine systems in lateral hypothalamic intracranial self-stimulation. Int J Neuropsychopharmacol 20:403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Shimohira I, Yoshida A, Zimmer A, Takeshima H, Sakurada T, Ueda H (1999) Dose-related opposite modulation by nociceptin/orphanin FQ of substance P nociception in the nociceptors and spinal cord. J Pharmacol Exp Ther 291:308–313 [PubMed] [Google Scholar]
- Kane JM, Skuban A, Hobart M, Ouyang J, Weiller E, Weiss C, Correll CU (2016) Overview of short- and long-term tolerability and safety of brexpiprazole in patients with schizophrenia. Schizophr Res 174:93–98 [DOI] [PubMed] [Google Scholar]
- Kangas BD, Bergman J (2014) Operant nociception in nonhuman primates. Pain 155:1821–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantola I, Scheinin M, Gulbrandsen T, Meland N, Smerud KT (2017) Safety, tolerability, and antihypertensive effect of SER100, an opiate receptor-like 1 (ORL-1) partial agonist, in patients with isolated systolic hypertension. Clin Pharmacol Drug Dev 6:584–591 [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Cami-Kobeci G, Husbands SM, Zaveri NT, Toll L (2011) The first universal opioid ligand, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-meth oxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028): characterization of the in vitro profile and in vivo behavioral effects in mouse models of acute pain and cocaine-induced reward. J Pharmacol Exp Ther 336:952–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Jiang F, Zaveri NT, Toll L (2009) Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists. J Pharmacol Exp Ther 331:946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Cami-Kobeci G, Sukhtankar DD, Czoty PW, DeLoid HB, Hsu FC, Toll L, Husbands SM, Ko MC (2019) BU10038 as a safe opioid analgesic with fewer side effects after systemic and intrathecal administration in primates. Br J Anaesth. 10.1016/j.bja.2018.1010.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Ko MC (2016) Central N/OFQ-NOP receptor system in pain modulation. Adv Pharmacol 75:217–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Peters CM, Kock ND, Kishioka S, Cline JM, Wagner JD, Ko MC (2017) Altered expression of glial markers, chemokines, and opioid receptors in the spinal cord of type 2 diabetic monkeys. Biochim Biophys Acta 1863:274–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Fujita M, Hong J, Lohith TG, Gladding RL, Zoghbi SS, Tauscher JA, Goebl N, Rash KS, Chen Z, Pedregal C, Barth VN, Pike VW, Innis RB (2011) Brain and whole-body imaging in rhesus monkeys of 11C-NOP-1A, a promising PET radioligand for nociceptin/orphanin FQ peptide receptors. J Nucl Med 52:1638–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI, Woods JH (1999) Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J Pharmacol Exp Ther 291:1113–1120 [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN (2000) An experimental itch model in monkeys: characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology 92:795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN (2009) Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys. J Pain 10:509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN, Traynor JR, Song MS, Woods JH, Rice KC, McKnight AT (2002a) Orphanin FQ inhibits capsaicin-induced thermal nociception in monkeys by activation of peripheral ORL1 receptors. Br J Pharmacol 135:943–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Terner J, Hursh S, Woods JH, Winger G (2002b) Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther 301:698–704 [DOI] [PubMed] [Google Scholar]
- Ko MC, Wei H, Woods JH, Kennedy RT (2006) Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies. J Pharmacol Exp Ther 318:1257–1264 [DOI] [PubMed] [Google Scholar]
- Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP (2009) Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology 34:2088–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Ichikawa D, Nambu H, Azuma-Kanoh T, Sakai N, Takaki-Kawagoe H, Ozaki S, Ohta H (2009) Cloning and characterization of the rhesus monkey nociceptin/orphanin FQ receptor. Genes Genet Syst 84:319–325 [DOI] [PubMed] [Google Scholar]
- Lambert DG (2008) The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov 7:694–710 [DOI] [PubMed] [Google Scholar]
- Lavonas EJ, Severtson SG, Martinez EM, Bucher-Bartelson B, Le Lait MC, Green JL, Murrelle LE, Cicero TJ, Kurtz SP, Rosenblum A, Surratt HL, Dart RC (2014) Abuse and diversion of buprenorphine sublingual tablets and film. J Subst Abus Treat 47:27–34 [DOI] [PubMed] [Google Scholar]
- Lee H, Ko MC (2015) Distinct functions of opioid-related peptides and gastrin-releasing peptide in regulating itch and pain in the spinal cord of primates. Sci Rep 5:11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Naughton NN, Woods JH, Ko MC (2003) Characterization of scratching responses in rats following centrally administered morphine or bombesin. Behav Pharmacol 14:501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth SR, Mathis JP, Rossi GC, Bodnar RJ, Pasternak GW (2000) Autoradiographic localization of (125)I[Tyr(14)]orphanin FQ/nociceptin and (125)I[Tyr(10)]orphanin FQ/nociceptin (1–11) binding sites in rat brain. J Comp Neurol 423:319–329 [PubMed] [Google Scholar]
- Li J, Li JG, Chen C, Zhang F, Liu-Chen LY (2002) Molecular basis of differences in (–)(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidiny)-cyclohexyl]benzeneacetamide-induced desensitization and phosphorylation between human and rat kappa-opioid receptors expressed in Chinese hamster ovary cells. Mol Pharmacol 61:73–84 [DOI] [PubMed] [Google Scholar]
- Lin AP, Ko MC (2013) The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci 4:214–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz K, Christoph T, Tzschentke TM, Koch T, Schiene K, Gautrois M, Schroder W, Kogel BY, Beier H, Englberger W, Schunk S, De Vry J, Jahnel U, Frosch S (2014) Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther 349:535–548 [DOI] [PubMed] [Google Scholar]
- Lohith TG, Zoghbi SS, Morse CL, Araneta MD, Barth VN, Goebl NA, Tauscher JT, Pike VW, Innis RB, Fujita M (2014) Retest imaging of [11C]NOP-1A binding to nociceptin/orphanin FQ peptide (NOP) receptors in the brain of healthy humans. NeuroImage 87:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohith TG, Zoghbi SS, Morse CL, Araneta MF, Barth VN, Goebl NA, Tauscher JT, Pike VW, Innis RB, Fujita M (2012) Brain and whole-body imaging of nociceptin/orphanin FQ peptide receptor in humans using the PET ligand 11C-NOP-1A. J Nucl Med 53:385–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hubner H, Huang XP, Sassano MF, Giguere PM, Lober S, Da D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK (2016) Structure-based discovery of opioid analgesics with reduced side effects. Nature 537:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margas W, Sedeek K, Ruiz-Velasco V (2008) Coupling specificity of NOP opioid receptors to pertussis-toxin-sensitive Galpha proteins in adult rat stellate ganglion neurons using small interference RNA. J Neurophysiol 100:1420–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14:375–424 [DOI] [PubMed] [Google Scholar]
- Mercatelli D, Pisano CA, Novello S, Morari M (2019) NOP receptor ligands and Parkinson’s disease. Handb Exp Pharmacol. 10.1007/164_2018_199 [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B et al. (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377:532–535 [DOI] [PubMed] [Google Scholar]
- Molinari S, Camarda V, Rizzi A, Marzola G, Salvadori S, Marzola E, Molinari P, McDonald J, Ko MC, Lambert DG, Calo G, Guerrini R (2013) [Dmt1]N/OFQ(1–13)-NH2: a potent nociceptin/orphanin FQ and opioid receptor universal agonist. Br J Pharmacol 168:151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC (1994) ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett 341:33–38 [DOI] [PubMed] [Google Scholar]
- Moran TD, Abdulla FA, Smith PA (2000) Cellular neurophysiological actions of nociceptin/orphanin FQ. Peptides 21:969–976 [DOI] [PubMed] [Google Scholar]
- Murphy NP, Ly HT, Maidment NT (1996) Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience 75:1–4 [DOI] [PubMed] [Google Scholar]
- Narendran R, Ciccocioppo R, Lopresti B, Paris J, Himes ML, Mason NS (2018) Nociceptin receptors in alcohol use disorders: a positron emission tomography study using [(11)C]NOP-1A. Biol Psychiatry 84:708–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal CR Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ Jr (1999) Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol 412:563–605 [PubMed] [Google Scholar]
- Negus SS, Butelman ER, Chang KJ, DeCosta B, Winger G, Woods JH (1994) Behavioral effects of the systemically active delta opioid agonist BW373U86 in rhesus monkeys. J Pharmacol Exp Ther 270:1025–1034 [PubMed] [Google Scholar]
- Pan B, Schroder W, Jostock R, Schwartz M, Rosson G, Polydefkis M (2018) Nociceptin/orphanin FQ opioid peptide-receptor expression in pachyonychia congenita. J Peripher Nerv Syst 23:241–248 [DOI] [PubMed] [Google Scholar]
- Pedregal C, Joshi EM, Toledo MA, Lafuente C, Diaz N, Martinez-Grau MA, Jimenez A, Benito A, Navarro A, Chen Z, Mudra DR, Kahl SD, Rash KS, Statnick MA, Barth VN (2012) Development of LC-MS/MS-based receptor occupancy tracers and positron emission tomography radioligands for the nociceptin/orphanin FQ (NOP) receptor. J Med Chem 55:4955–4967 [DOI] [PubMed] [Google Scholar]
- Peirs C, Seal RP (2016) Neural circuits for pain: recent advances and current views. Science 354:578–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso J, LaForge KS, Matthes HW, Kreek MJ, Kieffer BL, Gaveriaux-Ruff C (1998) Distribution of nociceptin/orphanin FQ receptor transcript in human central nervous system and immune cells. J Neuroimmunol 81:184–192 [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t’Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML (2014) Why primate models matter. Am J Primatol 76:801–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike VW, Rash KS, Chen Z, Pedregal C, Statnick MA, Kimura Y, Hong J, Zoghbi SS, Fujita M, Toledo MA, Diaz N, Gackenheimer SL, Tauscher JT, Barth VN, Innis RB (2011) Synthesis and evaluation of radioligands for imaging brain nociceptin/orphanin FQ peptide (NOP) receptors with positron emission tomography. J Med Chem 54:2687–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlesnik CA, Ko MC, Winger G, Wichmann J, Prinssen EP, Woods JH (2011) The effects of nociceptin/orphanin FQ receptor agonist Ro 64–6198 and diazepam on antinociception and remifentanil self-administration in rhesus monkeys. Psychopharmacology (Berl) 213:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa RB, Burdge G, Gambrah J, Kinecki HE, Lin F, Lu B, Nguyen JT, Phan V, Ruan A, Sesay MA, Watkins TN (2017) Cebranopadol: novel dual opioid/NOP receptor agonist analgesic. J Clin Pharm Ther 42:8–17 [DOI] [PubMed] [Google Scholar]
- Raffa RB, Haidery M, Huang HM, Kalladeen K, Lockstein DE, Ono H, Shope MJ, Sowunmi OA, Tran JK, Pergolizzi JV Jr (2014) The clinical analgesic efficacy of buprenorphine. J Clin Pharm Ther 39:577–583 [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ Jr, Civelli O (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270:792–794 [DOI] [PubMed] [Google Scholar]
- Rizzi A, Spagnolo B, Wainford RD, Fischetti C, Guerrini R, Marzola G, Baldisserotto A, Salvadori S, Regoli D, Kapusta DR, Calo G (2007) In vitro and in vivo studies on UFP-112, a novel potent and long lasting agonist selective for the nociceptin/orphanin FQ receptor. Peptides 28:1240–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi A, Sukhtankar DD, Ding H, Hayashida K, Ruzza C, Guerrini R, Calo G, Ko MC (2015) Spinal antinociceptive effects of the novel NOP receptor agonist PWT2-nociceptin/orphanin FQ in mice and monkeys. Br J Pharmacol 172:3661–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone PA, Zelenock KA, Lindsey AM, Sulima A, Rice KC, Prinssen EP, Wichmann J, Woods JH (2016) Characterization of the discriminative stimulus effects of a NOP receptor agonist Ro 64–6198 in rhesus monkeys. J Pharmacol Exp Ther 357:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurada T, Katsuyama S, Sakurada S, Inoue M, Tan-No K, Kisara K, Sakurada C, Ueda H, Sasaki J (1999) Nociceptin-induced scratching, biting and licking in mice: involvement of spinal NK1 receptors. Br J Pharmacol 127:1712–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Sagara T, Sakoh H, Hashimoto M, Nakashima H, Kato T, Goto Y, Mizutani S, Azuma-Kanoh T, Tani T, Okuda S, Okamoto O, Ozaki S, Iwasawa Y, Ohta H, Kawamoto H (2009) Identification of an orally active opioid receptor-like 1 (ORL1) receptor antagonist 4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-1H-benzimidazol-1-yl}−1-[(1S,3S,4R)-spiro[bicycle 2.2.1]heptane-2,1′-cyclopropan]-3-ylmethyl]piperidine as clinical candidate. J Med Chem 52:4091–4094 [DOI] [PubMed] [Google Scholar]
- Schattauer SS, Miyatake M, Shankar H, Zietz C, Levin JR, Liu-Chen LY, Gurevich VV, Rieder MJ, Chavkin C (2012) Ligand directed signaling differences between rodent and human kappa-opioid receptors. J Biol Chem 287:41595–41607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiene K, Schroder W, Linz K, Frosch S, Tzschentke TM, Jansen U, Christoph T (2018) Nociceptin/orphanin FQ opioid peptide (NOP) receptor and micro-opioid peptide (MOP) receptors both contribute to the anti-hypersensitive effect of cebranopadol in a rat model of arthritic pain. Eur J Pharmacol 832:90–95 [DOI] [PubMed] [Google Scholar]
- Schlicker E, Morari M (2000) Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides 21:1023–1029 [DOI] [PubMed] [Google Scholar]
- Scholz A, Bothmer J, Kok M, Hoschen K, Daniels S (2018) Cebranopadol: a novel, first-in-class, strong analgesic: results from a randomized phase IIa clinical trial in postoperative acute pain. Pain Physician 21:E193–E206 [PubMed] [Google Scholar]
- Schroder W, Lambert DG, Ko MC, Koch T (2014) Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists. Br J Pharmacol 171:3777–3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug SA, Saunders D, Kurowski I, Paech MJ (2006) Neuraxial drug administration: a review of treatment options for anaesthesia and analgesia. CNS Drugs 20:917–933 [DOI] [PubMed] [Google Scholar]
- Smyth C, Ahmadzai N, Wentzell J, Pardoe A, Tse A, Nguyen T, Goddard Y, Nair S, Poulin PA, Skidmore B, Ansari MT (2015) Intrathecal analgesia for chronic refractory pain: current and future prospects. Drugs 75:1957–1980 [DOI] [PubMed] [Google Scholar]
- Spagnolo B, Calo G, Polgar WE, Jiang F, Olsen CM, Berzetei-Gurske I, Khroyan TV, Husbands SM, Lewis JW, Toll L, Zaveri NT (2008) Activities of mixed NOP and mu-opioid receptor ligands. Br J Pharmacol 153:609–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Ko MC (2013) Physiological function of gastrin-releasing peptide and neuromedin B receptors in regulating itch scratching behavior in the spinal cord of mice. PLoS One 8:e67422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Lagorio CH, Ko MC (2014a) Effects of the NOP agonist SCH221510 on producing and attenuating reinforcing effects as measured by drug self-administration in rats. Eur J Pharmacol 745:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Lee H, Rice KC, Ko MC (2014b) Differential effects of opioid-related ligands and NSAIDs in nonhuman primate models of acute and inflammatory pain. Psychopharmacology 231:1377–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Zaveri NT, Husbands SM, Ko MC (2013) Effects of spinally administered bifunctional nociceptin/orphanin FQ peptide receptor/mu-opioid receptor ligands in mouse models of neuropathic and inflammatory pain. J Pharmacol Exp Ther 346:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT (2016) Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol Rev 68:419–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapella C, Ding H, Kiguchi N, Calo G, Ko MC (2018) Reinforcing and antinociceptive effects of a mixed opioid and NOP receptor agonist, cebranopadol, in non-human primates. In: The 17th world congress on pain (IASP): meeting abstract, PST538 [Google Scholar]
- van Niel JC, Schneider J, Tzschentke TM (2016) Efficacy of full micro-opioid receptor agonists is not impaired by concomitant buprenorphine or mixed opioid agonists/antagonists – preclinical and clinical evidence. Drug Res 66:562–570 [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ (1996) Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. Br J Pharmacol 117:1609–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Gerrits PO, Barendregt HP (2012) Volume transmission of beta-endorphin via the cerebrospinal fluid; a review. Fluids Barriers CNS 9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yuan W, Li MD (2011) Genes and pathways co-associated with the exposure to multiple drugs of abuse, including alcohol, amphetamine/methamphetamine, cocaine, marijuana, morphine, and/or nicotine: a review of proteomics analyses. Mol Neurobiol 44:269–286 [DOI] [PubMed] [Google Scholar]
- Wang JB, Johnson PS, Imai Y, Persico AM, Ozenberger BA, Eppler CM, Uhl GR (1994) cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett 348:75–79 [DOI] [PubMed] [Google Scholar]
- Waxler B, Dadabhoy ZP, Stojiljkovic L, Rabito SF (2005) Primer of postoperative pruritus for anesthesiologists. Anesthesiology 103:168–178 [DOI] [PubMed] [Google Scholar]
- Withey SL, Paronis CA, Bergman J (2018) Concurrent assessment of the antinociceptive and behaviorally disruptive effects of opioids in squirrel monkeys. J Pain 19:728–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, Tucker RC, Ciccocioppo R (2014) The biology of nociceptin/orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol Ther 141:283–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wladischkin KA, Dysko RC, Collins GT, Ko YA, Winger G, Ko MC (2012) Pharmacological characterization of NOP receptor agonists as abuse-free and constipation-free analgesics in monkeys. FASEB J 26(Suppl):1123 [Google Scholar]
- Zaveri NT (2016) Nociceptin opioid receptor (NOP) as a therapeutic target: progress in translation from preclinical research to clinical utility. J Med Chem 59:7011–7028 [DOI] [PMC free article] [PubMed] [Google Scholar]


