Summary
The symbiotic relationship between legumes and rhizobium bacteria in root nodules has a high demand for iron, and questions remain regarding which transporters are involved. Here, we characterize two nodule‐specific Vacuolar iron Transporter‐Like (VTL) proteins in Medicago truncatula.
Localization of fluorescent fusion proteins and mutant studies were carried out to correlate with existing RNA‐seq data showing differential expression of VTL4 and VTL8 during early and late infection, respectively.
The vtl4 insertion lines showed decreased nitrogen fixation capacity associated with more immature nodules and less elongated bacteroids. A mutant line lacking the tandemly‐arranged VTL4–VTL8 genes, named 13U, was unable to develop functional nodules and failed to fix nitrogen, which was almost fully restored by expression of VTL8 alone. Using a newly developed lux reporter to monitor iron status of the bacteroids, a moderate decrease in luminescence signal was observed in vtl4 mutant nodules and a strong decrease in 13U nodules. Iron transport capability of VTL4 and VTL8 was shown by yeast complementation.
These data indicate that VTL8, the closest homologue of SEN1 in Lotus japonicus, is the main route for delivering iron to symbiotic rhizobia. We propose that a failure in iron protein maturation leads to early senescence of the bacteroids.
Keywords: iron, Medicago, micronutrient, nitrogen fixation, nodulin‐21, symbiosis, vacuole
Introduction
Legumes and a small number of other plant species (Parasponia sp.) are able to form a symbiosis with rhizobium bacteria which enables the host plant to access N2 as a source of nitrogen. The host plant provides carbohydrates derived from photosynthesis for the energy‐demanding reduction of N2 to ammonium, carried out by the bacterial nitrogenase enzyme. Successful establishment of the symbiosis in specialized structures called root nodules depends on signalling and recognition between the rhizobia and the host plant, as well as later checkpoints during nodule development (reviewed in Oldroyd et al., 2011; Suzaki et al., 2015).
Root nodules have a high requirement for iron owing to abundant proteins that use iron as a cofactor. Infected plant cells produce large amounts of haem for leghaemoglobins, which maintain a microaerobic environment for the oxygen‐sensitive nitrogenase enzyme (Downie, 2005; Ott et al., 2005; Garrocho‐Villegas et al., 2007). The bacterial nitrogenase enzyme (NifH + NifDK) binds 12 iron in the form of iron–sulphur clusters and another seven in the iron–molybdenum cofactor (Dean et al., 1993; Howard & Rees, 2006). The nitrogen‐fixing bacteria also contain numerous cytochromes and other iron proteins. When the plant is starved of iron, nodule initiation and further development is strongly impaired (O’Hara et al., 1988; Tang et al., 1990; Brear et al., 2013).
Nodule development is initiated by a signalling cascade between plant roots and the bacteria, entrapment of the bacteria in curled root hairs and formation of infection threads along which the bacteria travel into the root cortex. Division of root cortical cells leads to formation of the nodule, considered a specialized root outgrowth. The nodules formed by Medicago truncatula in symbiosis with Sinorhizobium meliloti or S. medicae are of the indeterminate type. The meristem persists over time and generates a gradient of cells at progressing developmental stages (Vasse et al., 1990; Roux et al., 2014), commonly divided into four histological zones, with the meristem as Zone I. Zone II corresponds to the infection zone where bacteria are released from the infection threads, divide and form symbiosomes – intracellular bacteria surrounded by a plant‐derived membrane. In older cells of Zone II, bacterial cell division stops while genome replication continues, resulting in elongated polyploid bacteroids (Mergaert et al., 2006). Synchrotron X‐ray fluorescence (XRF) studies on M. truncatula indicated that iron is delivered to Zone II by the vascular bundles in the nodule (Rodríguez‐Haas et al., 2013). In the latter study ‘iron‐rich bodies’ were observed in and near the vascular bundles which may correspond to the iron storage protein ferritin, the expression of which is induced in Zone II (Strozycki et al., 2007; Roux et al., 2014). In the Interzone (transition of Zone II to III), infected plant cells and the bacteroids complete their differentiation with the last rounds of endoreduplication and enlargement, resulting in a striking cell morphology of tightly packed elongated rhizobia surrounding a central vacuole. Zone III comprises the major part of a functional nodule and this is where nitrogen‐fixation takes place. In older nodules, a zone of senescent cells, Zone IV, is formed from which nutrients such as iron are recycled from degraded plant cells and symbionts (Van de Velde et al., 2006; Rodríguez‐Haas et al., 2013).
Forward and reverse genetic studies have identified several transporters involved in iron delivery to the nodules and symbiosomes. The closely related genes Lotus japonicus MATE1 and M. truncatula MATE67 are highly induced during nodule development, specifically in the infection zone (Takanashi et al., 2013; Wang et al., 2017; Kryvoruchko et al., 2018). Studies in Xenopus oocytes showed that the LjMATE1 and MtMATE67 proteins can transport citrate, an organic chelator of Fe3+ in the xylem (Takanashi et al., 2013; Kryvoruchko et al., 2018). The MtMATE67 protein is localized to vascular bundles but also to the symbiosome membrane (Kryvoruchko et al., 2018). An RNAi mutant of LjMATE1 accumulated high levels of iron in the vascular bundle of roots and nodules, whereas iron staining was fainter in the infection zone of mutant nodules, particularly in the central region, compared to wild‐type (Takanashi et al., 2013). These two studies indicate that the host plant delivers iron to the nodules via the xylem.
Uptake of iron into the infected cells may involve MtNRAMP1, one of seven members of this gene family in M. truncatula with the highest relative expression in roots and nodules (Tejada‐Jiménez et al., 2015). Yeast complementation studies showed that MtNRAMP1 can transport iron and manganese, similar to its closest Arabidopsis homologue which is the primary route for manganese into the cell (Cailliatte et al., 2010). MtNRAMP1 is localized to plasma membranes, including the plasma membrane of infected cells. A transposon insertion mutant of MtNRAMP1 has impaired nodule development, but still exhibited 60% nitrogenase activity compared to wild‐type (Tejada‐Jiménez et al., 2015).
For iron to reach the bacteroid, it needs to be exported by the infected plant cell and imported across the bacteroid membrane. Five different allelic mutants in the LjSEN1 gene, encoding a Vacuolar iron Transporter‐Like (VTL) protein, were identified in a screen for ineffective symbiotic (Fix‐) mutants in L. japonicus (Hakoyama et al., 2011). Promoter‐GUS studies showed that LjSEN1 is expressed exclusively in rhizobia‐infected cells, but its subcellular localization and function in iron transport has not been documented to date. The VTL proteins differ from the Vacuolar Iron Transporter (VIT) proteins in that the former lack a cytosolic loop thought to mediate Fe2+/H+ antiport in VIT, see diagrams in Fig. 1a (Labarbuta et al., 2017; Kato et al., 2019). VIT proteins are well characterized in yeast, plants and Plasmodium, mediating iron transport into the vacuole for detoxifying excess iron, or for building up iron stores in seeds (Kim et al., 2006; Kato et al., 2019). In contrast, the physiological function of VTLs is less clear, although expression patterns and functional studies in Arabidopsis suggest that VTLs also play a role in iron homeostasis (Gollhofer et al., 2011).
Fig. 1.
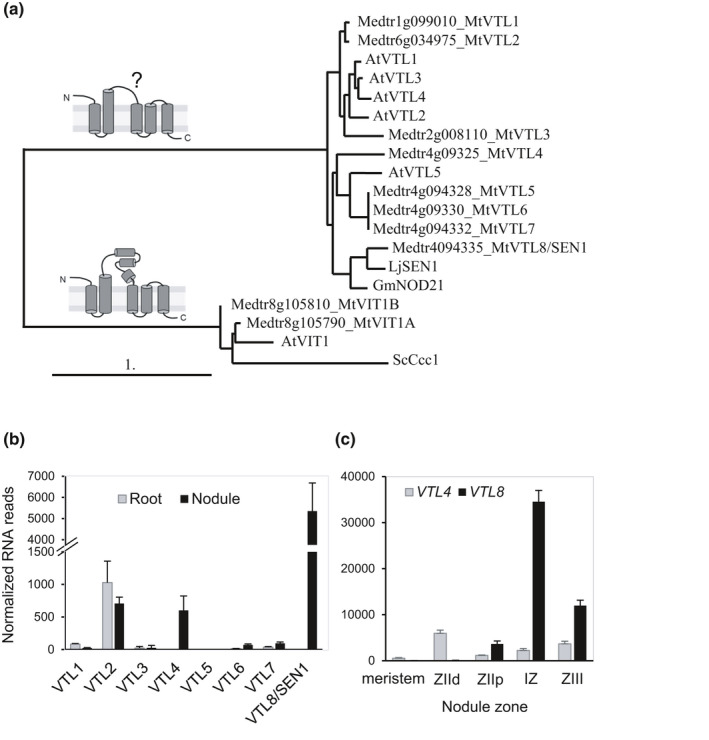
Vacuolar iron Transporter‐Like (VTL) proteins in Medicago truncatula. (a) Phylogenetic relationship and predicted domain structure of VTL protein sequences identified in the M. truncatula genome and those previously reported in Arabidopsis thaliana (AtVTL1, AT1G21140; AtVTL2, AT1G76800; AtVTL3, AT3G43630; AtVTL4, At3G43660; AtVTL5, AT3G25190). The ‘true’ Vacuolar Iron Transporter (VIT) sequences including Ccc1 from Saccharomyces cerevisiae served as an outgroup. The domain structure of VIT proteins is adapted from Kato et al. (2019). (b, c) Expression of M. truncatula VTL genes in roots and nodules (b) and in different nodule zones (c). Data were obtained from the Symbimics website (https://iant.toulouse.inra.fr/symbimics), and are Ribominus‐like values for (b) and DESeq‐normalized reads from laser‐capture microdissection fractions for (c). ZIId and ZIIp, distal and proximal fractions of Zone II, respectively. IZ, Interzone. ZIII, nitrogen‐fixing Zone III. Error bars represent SE.
To better understand the role of VTLs in nodule development and biological nitrogen fixation, we characterized two VTL genes in M. truncatula, VTL4 and VTL8, which are specifically expressed in nodules. Mutant studies showed they play a role at early and late stages, respectively, of nodule development. In particular VTL8 is critical for delivering iron to the bacteroids and for establishment of the nitrogen‐fixing bacteria. While this article was undergoing review, two studies describing a specific VTL in soybean nodule development were also accepted for publication (Brear et al., 2020; Liu et al., 2020).
Materials and Methods
Plant lines and growth
Medicago truncatula Jemalong and M. truncatula subsp. tricycla R108 genotypes were used as wild‐type controls for the 13U and vtl4 mutant lines, respectively. The Tnt1‐insertion mutants vtl4‐1 (NF17463) and vtl4‐2 (NF21016) were identified by reverse screening using PCR and purchased from the Samuel Roberts Noble Foundation (Ardmore, OK, USA). These lines have approximately 25 insertions each, and a list of the positions of all known insertions is given in Supporting Information Table S1. While most insertions are outside of annotated genes, the 3–5 insertions that could potentially affect other genes (if homozygous) are not specifically expressed in nodules, except for VTL4 (Limpens et al., 2013; Roux et al., 2014). Primer sequences for genotyping vtl4 mutants are listed in Table S2.
The 13U mutant line was isolated from a collection of fast neutron radiation mutants (Domonkos et al., 2013) and the deletion was identified using a combined approach of genetic mapping and microarray‐based Affymetrix GeneChip hybridization (Murray et al., 2011). The boundaries of the deletion were determined by PCR, see Table S2 for primer sequences.
Seeds were scarified, surface sterilized with 10% (w/v) sodium hypochlorite, stratified at 4°C for 3 to 7 d and then germinated at 20°C. The next day, seedlings were plated out on modified Fahraeus medium (0.7 mM KH2PO4, 0.8 mM Na2HPO4, 0.50 mM MgSO4, 0.7 mM CaCl2, 20 µM Fe‐citrate, pH 6.0), and micronutrients (5 µM H3BO3, 9 µM MnSO4, 0.8 µM ZnSO4, 0.3 µM CuSO4 and 0.5 µM H2MoO4), or planted out on a 1 : 1 mixture of Terragreen–sand, or on zeolite (Geoproduct Kft., Mád, Hungary), as indicated in figure legends. Plants were grown in long‐day conditions (16 h : 8 h, light : dark) at 22°C, light intensity 180–200 μmol photons m−2 s−1.
Bacterial strains and growth
Two different bacterial strains were used for nodulating M. truncatula, as indicated in the figure legends. Sinorhizobium (Ensifer) medicae WSM419 (pXLGD4 PhemA:lacZ, tetR) was generally used for nodulating M. truncatula Jemalong and the 13U mutant; S. meliloti 1021 (PnifA:lacZ, tetR) was used for M. truncatula subsp. tricycla R108 and the vtl4 mutants. Bacteria were grown in TY medium (1% (w/v) tryptone, 0.3% (w/v) yeast extract, 6 mM CaCl2), either in liquid medium for inoculation or on solid medium with 1% (w/v) agar for maintaining the strains. For testing iron‐dependent regulation of PmbfA:lux, Rhizobium leguminosarum bv. viciae strain J251 was used as wild‐type and its derivative J386 carrying a Tn5::lacZ insertion in irr (Wexler et al., 2003).
Agrobacterium rhizogenes‐mediated complementation of M. truncatula mutants
DNA fragments containing the VTL4 and VTL8 genes including the 1.5 and 1.6 kb promoter sequences and 0.9 and 1.2 kb 3′‐untranslated regions, respectively, were amplified with oligonucleotides (see Table S2) from BAC (bacterial artificial chromosome) clone mth2‐28d20. The destination vector pKGW‐R (www.gateway.psb.ugent.be/vector/show/pKGW_RedRoot/) was linearized with restriction enzymes AatII and SpeI. The gene fragments and the linearized vector were fused using the In‐Fusion HD Cloning Kit (Clontech Laboratories/Takara Bio, Mountain View, CA, USA) according to the manufacturer's protocol. Constructs were introduced into the ARqua1 strain of Agrobacterium rhizogenes and used for hairy root transformation as described in The Medicago truncatula Handbook (Chabaud et al., 2006). Transformed roots were identified by fluorescence of the DsRed marker protein.
Protein localization and fluorescence microscopy
The VTL4 promoter (2.5 kb), VTL8 promoter (2.1 kb) and coding sequences were domesticated for Golden Gate assembly. A glycine‐rich linker sequence (Table S2) was added to the C‐terminus followed by the coding sequence of mCherry. As a marker for membranes, the Arabidopsis thaliana PIP2A (AT3G53420) sequence was fused to eGFP and placed behind the promoter of L. japonicus UBIQUITIN (GenBank DQ249171.1) The two sequences were assembled together into vector pL1‐R1 and transformed into Agrobacterium rhizogenes ARqua1 for hairy root transformation of M. truncatula R108. Nodules were harvested after 21 d and imaged using a Leica TCS SP5 confocal microscope with standard settings for eGFP, mCherry and DAPI (4′,6‐diamidino‐2‐phenylindole). To analyse nodule occupancy by rhizobia, nodule sections were stained with the DNA‐binding fluorescent dye SYTO13 (Invitrogen, Eugene, OR, USA) as previously described (Domonkos et al., 2013).
PmbfA:lux reporter and activity assays
Nucleotides −487 to +104, containing the promoter sequence and part of the coding sequence of the mbfA gene (SMc00359) were PCR‐amplified from S. meliloti 1021 (see Table S2 for primer sequences) and cloned using the BamHI and KpnI restriction sites into pIJ11268 upstream of the lux operon (Frederix et al., 2014). A de‐repressed version of the reporter was made by mutating the Iron Control Element (ICE) from TTCTAA to AGCTTC (−19 to −14) by site‐directed mutagenesis. The pIJ11282 plasmid expressing lux constitutively has been described previously (Frederix et al., 2014). The plasmids were transferred from Escherichia coli to S. meliloti or R. leguminosarum by conjugation using an E. coli helper strain carrying plasmid pRK2013. For luminescence assays, overnight bacterial cultures were diluted in UMS medium (Wheatley et al., 2017) without or with 40 µM iron sulphate (FeSO4) as indicated. Luminescence and OD600 of triplicate cultures were measured in a ClarioStar microplate reader (BMG Labtech, Ortenberg, Germany).
Luminescence imaging and quantification
Plants growing on Terragreen–sand mixture were inoculated with S. meliloti 1021 carrying the PmbfA:lux reporter plasmid or the mutated PmbfAICE:lux reporter at 7 d post‐germination, and grown for a further 21 d. Plants were dug up, roots rinsed with distilled water, blotted dry and imaged using the NightOWL II LB 983 in vivo imaging system with IndiGO software (Berthold Technologies, Bad Wildbad, Germany). Luminescent nodules were identified using the automated peak picking tool. Luminescence values in photons per square millimetre were calculated from the output. At least five plants per line were analysed with each inoculum.
Miscellaneous methods
For details on gene expression analysis and yeast complementation assays, see Methods S1. Acetylene reduction assays were performed as previously described (Domonkos et al., 2013). Purified bacteroids were stained with propidium iodide to determine their length using confocal microscopy and imagej software. Protein blot analysis with antibodies against Pisum sativum ferritin and Arabidopsis hemoglobin 2 (Agrisera, Vännas, Sweden) was carried out following supplier's protocols. The iron concentration of nodules was determined photospectrometrically using the colorimetric chelator ferene (3‐(2‐pyridyl)‐5,6(5‐sulfo‐2‐furyl)‐1,2,4‐triazine) on mineralized tissue samples. Iron staining of nodules was performed using the Perls’ method (Meguro et al., 2007).
Results
Medicago truncatula has eight VTL genes of which two are specifically expressed in nodules
The M. truncatula genome (Mt4.0v1) was searched for homologues of Arabidopsis thaliana VIT and VTL genes. We identified two homologues of VIT and eight homologues of VTL as compared to one and five genes, respectively, in Arabidopsis thaliana. Amino acid alignment of the eight MtVTLs with the Arabidopsis thaliana homologues showed only a limited phylogenetic relationship between different VTLs of the two species (Fig. 1a). Based on this phylogeny, the M. truncatula genes were assigned as VTL1–VTL8. MtVTL1, MtVTL2 and MtVTL3 are most similar to Arabidopsis thaliana VTL1–VTL4. MtVTL4, MtVTL5, MtVTL6 and MtVTL7 are closely related paralogues on chromosome 4 which are more similar to Arabidopsis thaliana VTL5 than to other AtVTL genes. In contrast, MtVTL8 has no direct homologue in Arabidopsis thaliana, but is closely related to SEN1 in L. japonicus (Hakoyama et al., 2011) and NODULIN‐21 of soybean (GmNod21, Delauney et al., 1990; renamed GmVTL1 in Brear et al., 2020 and Liu et al., 2020). The amino acid sequences of MtVTL8 and LjSEN1 share 75.7% similarity (63.0% identity). Between MtVTL8 and GmNod21 the similarity is 58.7%, and between LjSEN1 and GmNod21 similarity is 63.2%.
Insight into the expression of M. truncatula VTLs was primarily obtained from publicly available resources. Three independent studies found that MtVTL4 transcripts are highly enriched in nodules, compared to low levels in roots and virtually no expression in aerial parts (Benedito et al., 2008; Limpens et al., 2013; Roux et al., 2014). RNA‐seq data (Roux et al., 2014; https://iant.toulouse.inra.fr/symbimics) showed that VTL8 is exclusively expressed in nodules (there is no probe for VTL8 in the Affymetrix chip used in the other two studies), at levels that are > seven‐fold greater than those of VTL4 (Fig. 1b). Of the other MtVTLs, only VTL2 is abundantly expressed in nodules, but the levels are similar in roots. Low expression of VTL1 and VTL6/7 in nodules are in agreement with Northern blot data for the closest L. japonicus homologues, represented by ESTs MWM137c08 and MWM075h10, respectively (Hakoyama et al., 2011).
During nodule development, the expression of MtVTL4 is highest in the infection zone, Zone IId (Roux et al., 2014; Fig. 1c) and > four‐fold enriched in infected cells relative to noninfected cells (Limpens et al., 2013). MtVTL8 expression peaks in the Interzone (Zones II–III). This expression pattern is similar to that of LjSEN1 which increased as the nodule matured (Hakoyama et al., 2011). Our own data using the MtVTL8 promoter for expression of VTL8‐mCherry showed fluorescence signal in infected cells only (see later).
In summary, of the eight VTL genes encoded in the M. truncatula genome, VTL8 is the closest homologue of the nodule‐specific LjSEN1 and GmNod21 and has a similar expression pattern. In addition, VTL4 is also specifically induced in nodules, but earlier in the infection process.
MtVTL4 and MtVTL8 localize to membranes in infected cells
To investigate the expression pattern and localization of the VTL4 and VTL8 proteins in nodules, the coding sequences were fused in frame to mCherry using a short C‐terminal linker peptide and expressed under the control of their own promoters in roots of R108 wild‐type seedlings. The presence of a glycine‐rich linker peptide has previously been shown to improve membrane insertion and stability of Arabidopsis thaliana VTLs expressed in Baker's yeast (Gollhofer et al., 2014). The same vector also contained a constitutively expressed GFP fusion of aquaporin AtPIP2 to visualize membranes (Cutler et al., 2002). AtPIP2A has previously been used as a plasma membrane marker in M. truncatula (Ivanov & Harrison, 2014) and was shown to internalize to membranes surrounding the infection thread during rhizobium infection (Fournier et al., 2008), and to perihyphal membranes and the arbuscule trunk domain of the periarbuscular membrane in mycorrhizal symbiosis (Pumplin & Harrison, 2009). From several membrane markers that we tested, LjUBIprom:AtPIP2A‐eGFP was expressed in all nodule cell types and GFP was found in the plasma membrane as well as symbiosomes (Fig. 2), in agreement with reports that the symbiosome membrane contains proteins of plasma membrane origin as well as late endosome markers (Limpens et al., 2009).
Fig. 2.
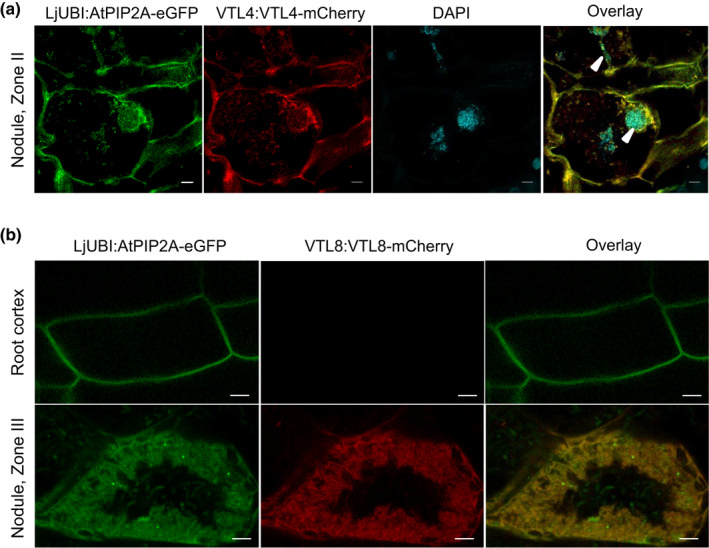
Localization of VTL4 and VTL8 in Medicago truncatula. Translational fusions of VTL4 (a) and VTL8 (b) with mCherry fluorescent protein were expressed in roots of wild‐type seedlings, which were nodulated with Sinorhizobium meliloti 1021. The VTL genes were placed under the control of their own promoters, and co‐expressed with the membrane marker AtPIP2A‐eGFP using the constitutive Lotus japonicus UBIQUITIN (UBI) promoter. (a) Localization of VTL4‐mCherry. The image is of cells in Zone II (infection zone). DAPI staining visualizes DNA including the bacteria in infection threads (arrow heads). Bars, 5 µm. (b) Localization of VTL8‐mCherry. Images of fluorescence signals in the root cortex and Zone III (nitrogen‐fixing zone) are shown. Bars, 5 µm.
In roots expressing VTL4prom:VTL4‐mCherry, a specific fluorescence signal was found associated with membranes in cells of the infection zone, such as the cell with an infection thread shown in Fig. 2(a). VTL4‐mCherry co‐localized with AtPIP2A‐eGFP to the plasma membrane and was also found in membranes surrounding the infection thread (Fig. 2a, arrow head in merged image).
VTL8‐mCherry was only detected in the characteristically shaped infected cells of the Interzone and not in noninfected neighbouring cells or in root cells (Fig. 2b). This expression pattern is in agreement with RNA‐seq data (Fig. 1b,c), and protein localization studies of GmNod21/GmVTL1 in soybean (Brear et al., 2020; Liu et al., 2020). While the fluorescence signal was weak, resulting in low‐resolution images, it was clearly confined to the mass of bacteroids surrounding the central vacuole. Co‐localization with AtPIP2A‐eGFP and assuming that both proteins are membrane bound, suggest that VTL8 is localized to the symbiosome membrane. The soybean homologue was indeed found in the enriched symbiosome membrane fraction by proteomics (Brear et al., 2020). The weak fluorescence signal of VTL8‐mCherry is surprising given the relatively high transcript levels of endogenous VTL8. This may be due to lower transcriptional activity of the domesticated VTL8 promoter sequence; lack of the native 3′UTR in this construct; or less efficient membrane insertion of the fusion protein leading to protein instability. Although further studies would be beneficial, these results are a first indication that VTL4 and VTL8 localize to intracellular host plant membranes associated with rhizobia.
VTL8 is required for nodule development and nitrogen fixation
To study the role of VTL4 and VTL8 in nodule development, mutants were obtained from different sources. Two lines with a Tnt1 insertion in the coding sequence of VTL4 were identified in the Noble Foundation collection (Fig. 3a), but none were found for VTL8 despite extensive screening by PCR. However, a mutant line lacking several VTL genes was isolated from a collection of Fix‐mutants generated by fast neutron radiation. The rough map position of the deletion in the 13U mutant had been identified previously (Domonkos et al., 2013). Microarray hybridization using the genomic DNA of 13U identified a probe set corresponding to the gene Medtr4g094325 (VTL4). Further analysis by PCR amplifications identified a 30‐kb deletion in the 13U genome spanning VTL4 to VTL8 (Fig. 3a). Gene expression analysis confirmed the absence of VTL4 transcript in the vtl4 mutants and the absence of both VTL4 and VTL8 expression in the 13U line (Fig. 3b,c).
Fig. 3.
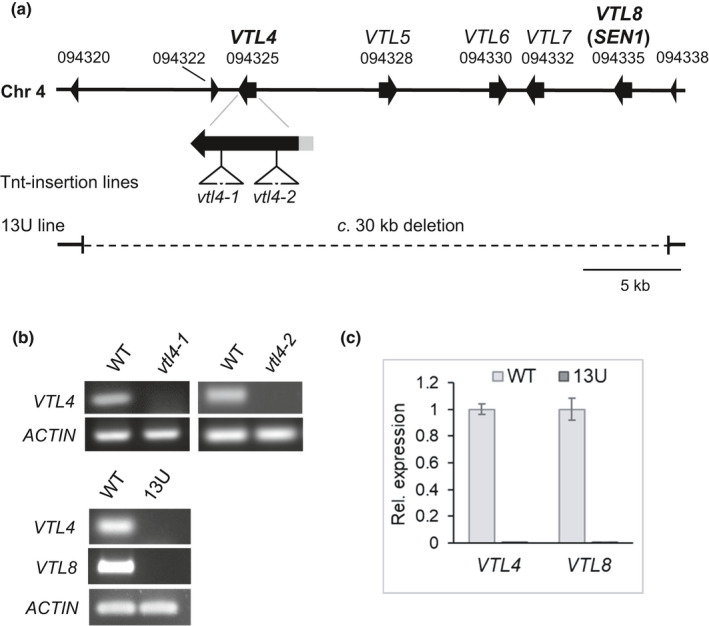
Medicago truncatula mutant lines affected in VTL gene expression. (a) Arrangement of VTL paralogues on chromosome 4. The position of two different Tnt1 insertions in VTL4 are indicated by triangles. The deleted segment in the 13U line is indicated by a dashed line. Medtr4g094320, Medtr4g094322 and Medtr4g094338 encode hypothetical proteins of less than 100 amino acids. (b) Upper panel, RT‐PCR of VTL4 transcript levels in nodules (28 d post inoculation, dpi) of the parental wild‐type (WT, cultivar R108) and the vtl4‐1 and vtl4‐2 Tnt1 insertion lines, NF17463 and NF21016, respectively. Lower pane, RT‐PCR of VTL4 and VTL8 transcript levels in nodules of the parental wild‐type (WT, cultivar Jemalong) and the 13U line. (c) Quantitative RT‐PCR results for the expression of VTL4 and VTL8 in nodules (28 dpi) of the 13U line relative to the parental wild‐type. The expression was normalized to that of the UPL7 gene. Error bars represent the SE of the mean from three technical replicates, using cDNA from two different plants for each line.
The 13U plants grown in low‐nitrogen substrate were chlorotic and smaller than the parental wild‐type (Fig. 4a), but grew normally upon nitrogen fertilization (Domonkos et al., 2013; Fig. S1). The development of 13U nodules was arrested at an early stage and they lacked the typical pink colour of leghaemoglobin (Fig. 4b). Previous studies on 13U nodules showed bacterial colonization of the infection zone and Interzone but only sporadic infection within the nitrogen fixation zone, and no detectable nitrogenase activity (Domonkos et al., 2013). To further investigate the defective development of the rhizobia into bacteroids, nodule sections were stained with the nucleic acid binding dye SYTO13. The overall morphology of bacteria in 13U plants appeared similar to wild‐type, but in the interzone they were disorganized rather than orientated toward the vacuoles of infected cells (Fig. S2). Size classification of isolated rhizobia showed that 13U nodules contained mostly poorly elongated bacterial cells, contrasting with a large population of elongated cells between 5 and 9 µm in wild‐type nodules (Fig. S3). The shift to smaller‐sized rhizobial cells is reminiscent of the dnf7‐2 ineffective mutant identified previously (Horvath et al., 2015), suggesting a similar defect in differentiation of the bacteroids in the 13U line.
Fig. 4.
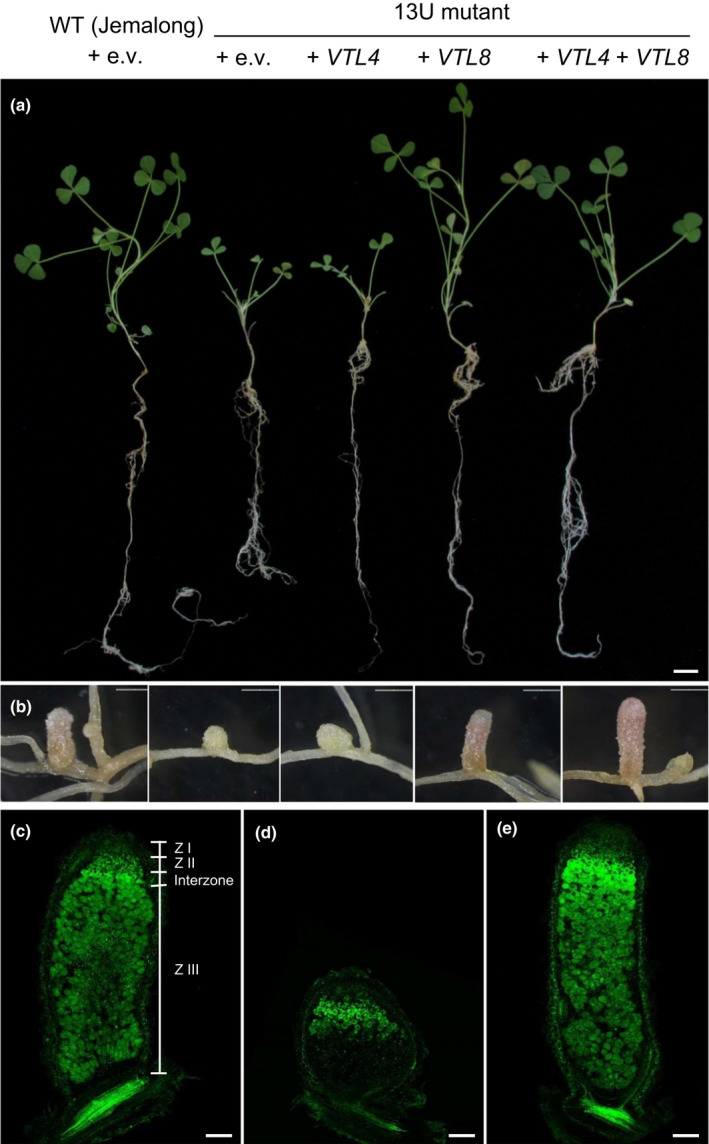
VTL8 is required for development of functional nodules. (a) Wild‐type Medicago truncatula plants (WT, Jemalong) and the 13U mutant transformed with VTL4, VTL8, VTL4 + VTL8 or empty vector (e.v.), as indicated. Roots were inoculated with Sinorhizobium medicae and images taken 31 d post inoculation (dpi). Bar, 1 cm. (b) Root nodules from plants in (a). Transformed roots were confirmed by DsRed fluorescent protein marker (not shown). Bars, 1 mm. (c) Wild‐type; (d), 13U + e.v. and (e), 13U + VTL8, longitudinal sections of nodules stained with SYTO13. Bars, (c–e), 200 μm.
In order to demonstrate which gene or genes caused the symbiotic phenotype of 13U, genetic complementation experiments were carried out. Transformation with constructs expressing VTL4 and VTL8 individually or together showed that expression of VTL8 alone in the 13U mutant reverted the mutant phenotype to wild‐type. Specifically, vegetative growth of 13U plants + VTL8 was similar to wild‐type (Fig. 4a), nodules developed fully with the typical pink colour of leghaemoglobin (Fig. 4b), a large Zone III (Fig. 4e) and normal fresh weight per nodule (Fig. S4), indicating that nitrogen fixation was restored. Expression of VTL4 alone in the 13U mutant had little effect (Fig. 4a,b), although co‐expression with VTL8 increased nodule fresh weight to above wild‐type values (Fig. S4). Thus, VTL8 is essential for nodule maturation, whereas VTL4 has a minor function and VTL5, VTL6 and VTL7 do not appear to have a function in nodules or in another aspect of plant development.
MtVTL4 is required for early bacteroid development
To further investigate whether VTL4 has a role in nodule development, the two vtl4 mutant lines and wild‐type (R108) were grown on low‐nitrogen substrate and inoculated with rhizobia. While there were no major defects in vegetative development (Fig. 5a), fresh weight measurements showed that vtl4‐1 and vtl4‐2 plants had significantly less biomass than wild‐type under both low‐ and high‐nitrogen growth conditions (Fig. S1). The nitrogen fixation capacity, measured as acetylene reduction activity in excised roots, showed a c. 50% decrease in the vtl4 mutant lines compared to wild‐type at 28 d post inoculation (dpi) (Fig. 5b). These data indicate that the impaired growth of the two vtl4 mutant lines may only be partially related to lower nitrogen fixation rates, and it should be noted at this point that the mutant lines were not backcrossed to remove other Tnt1 insertions (Table S1).
Fig. 5.
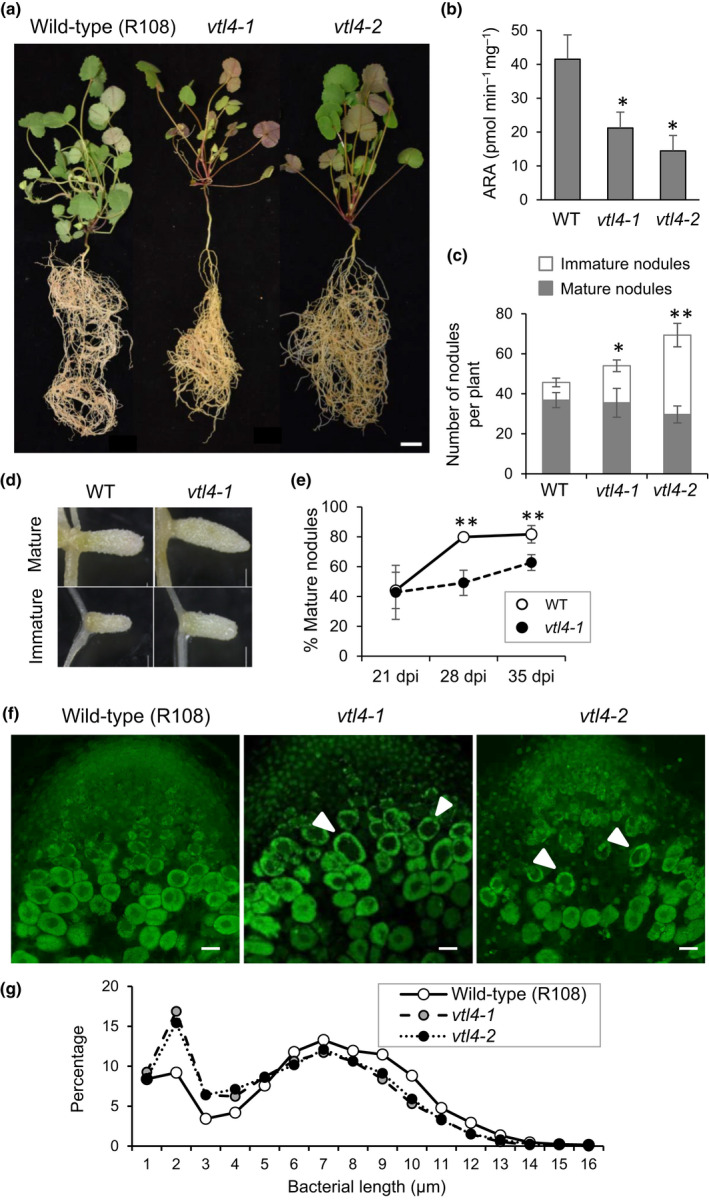
Disruption of VTL4 delays infection. (a) Wild‐type Medicago truncatula (R108) and vtl4 mutant plants grown under nodulating conditions. The representative images are of 40‐d‐old plants, 35 d after inoculation with Sinorhizobium meliloti 1021. Bar, 1 cm. (b) Acetylene reduction activity (ARA), per milligram nodule fresh weight, of the indicated plant lines at 28 d post inoculation (dpi). Values represent the mean ± SE of three measurements, each on two pooled root systems per vial. *, P < 0.1, Student's t‐test. (c) Number of mature and immature nodules in vtl4 mutants relative to wild‐type, at 35 dpi. Values represent the mean ± SE of six plants: *, P < 0.1; **, P < 0.01 (number of white nodules), Student’s t‐test. (d) Representative images of mature and immature nodules in vtl4‐1 and wild‐type. Bars, 1 mm. (e) Percentage mature nodules per plant at 21, 28 and 35 dpi in wild‐type (open circles) and the vtl4‐1 mutant (closed circles). Error bars represent SE, *, P < 0.1; **, P < 0.01 (n = 5–15 plants), Student's t‐test. (f) Longitudinal sections of nodules stained with SYTO13, showing part of the infection zone (Zone II). Arrow heads point to infected cells with a larger vacuole and smaller bacteroids. Bars, 100 µm. (g) The distribution of bacteroid cell sizes in wild‐type nodules (WT, R108 represented by open circles) and vtl4 nodules (grey and black closed circles). The lengths of at least 2500 bacterial cells were measured and their relative distribution in size classes is presented.
The roots of vtl4 plants were nodulated, but they had a significantly higher number of immature nodules (lacking leghemoglobin) (Fig. 5c). The total number of nodules per plant also tended to be higher in the mutants, but only significantly so for the vtl4‐2 data set. There were no macroscopic differences in nodules of vtl4 mutants and wild‐type (Fig. 5d), suggesting that the higher proportion of immature nodules is due to a delay in development and/or increased initiation of nodule primordia. We followed the percentage of mature nodules over time in the vtl4‐1 mutant, harvesting plants at weekly intervals. At 21 dpi, the percentage of mature nodules was close to 40% in both wild‐type and vtl4‐1 plants. At 28 dpi, wild‐type plants had 80% mature nodules, whereas the vtl4‐1 mutant had only c. 50%, increasing to 63% mature nodules at 35 dpi (Fig. 5e).
Cross‐sections stained with SYTO13 showed that the infection of plant cells and bacteroid development proceeded normally in the vtl4 mutants, except for a subtly different morphology of the infected cells in Zone II (Fig. 5f) where VTL4 expression reaches its highest levels (Fig. 1c). The early‐stage infected cells appeared ‘hollow’, with a larger vacuole and smaller bacteroids (Fig. 5f, white arrow heads). Measurements of isolated rhizobia showed that vtl4 mutants have a higher proportion of shorter bacterial cells than wild‐type (Fig. 5g). While this could simply be due to the higher ratio of immature nodules, the decreased nitrogenase activity supports the idea that bacterial development is impaired in the two vtl4 mutants.
VTL4 and VTL8 mediate iron transport in yeast
LjSEN1 and its homologues have been suggested to transport iron out of the infected plant cell into the peribacteroid space, based on sequence homology between VTLs and VIT proteins (Hakoyama et al., 2011; Brear et al., 2013; González‐Guerrero et al., 2016). To confirm that the VTL4 and VTL8 proteins are able to transport iron, the genes were expressed in yeast for functional complementation assays. The VTL genes were cloned into the pYES2 plasmid under the control of a galactose‐inducible promoter. The plasmids were transformed into a Δccc1 yeast mutant which lacks the vacuolar iron transporter Ccc1 (Li et al., 2001). In this mutant, the inability to store iron in the vacuole leads to a severe growth defect in the presence of excess iron in the medium (Fig. 6). Growth can be restored by expressing another vacuolar iron transporter such as Arabidopsis VIT1 (Kim et al., 2006), used here as a positive control alongside the wild‐type strain (Fig. 6). VTL4 as well as VTL8 were able to rescue growth of the Δccc1 strain on medium with 5 mM FeSO4. Functional complementation was only seen using Δccc1 in the DY150 (W303 derivative) genetic background, but not in the BY4741 strain. This may be due to differences in salt tolerance between the two strains causing an indirect effect on iron homeostasis (Petrezselyova et al., 2010). Nevertheless, the results show that MtVTL4 and MtVTL8 are able to transport iron out of the cytosol, either across the vacuolar membrane or plasma membrane.
Fig. 6.
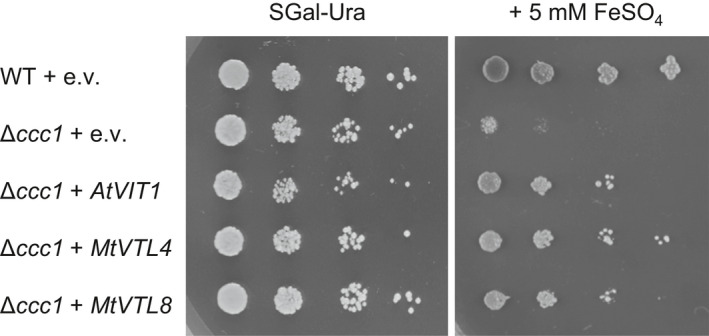
Expression of VTL4 or VTL8 restores vacuolar iron transport in Δccc1 yeast. Yeast transformed with pYES2 empty vector (e.v.) or with the indicated Medicago truncatula VTL genes were grown on minimal synthetic medium with galactose (SGal) lacking uracil (‐Ura) in a five‐fold serial dilution. Addition of 5 mM iron sulphate to the same medium selects for strains able to transport iron across the vacuolar membrane.
Development of a transcriptionally regulated iron reporter in bacteroids
To provide evidence for iron transport in planta, we sought to develop an iron‐sensing reporter in symbiotic rhizobium bacteria. Recently, Pini et al. (2017) demonstrated the use of transcriptional lux reporters for spatio‐temporal mapping of organic molecules in the pea‐rhizobium interaction. The same approach was followed here, but using an iron‐inducible promoter. While iron regulatory mechanisms in free‐living rhizobia are well characterized (Rudolph et al., 2006; Todd et al., 2006; O’Brian, 2015), much less is known in symbiotic rhizobia. Expression data indicate that most genes involved in iron homeostasis in free‐living rhizobia, for example those for biosynthesis of the siderophore rhizobactin (rhb) or for haem‐iron uptake (hmu), are not expressed during nodule development (Supporting Information Table S3; Roux et al., 2014). However, one gene that is strongly induced is mbfA (membrane‐bound ferritin A), with percentage‐wise the highest transcript levels in the proximal Zone II and Interzone of M. truncatula nodules, whereas expression is very low in non‐differentiated bacteria of Zone IId. MbfA has been characterized as an iron exporter in Bradyrhizobium japonicum and Agrobacterium tumefaciens (Bhubhanil et al., 2014; Sankari & O’Brian, 2014) and its expression was shown to be regulated by iron in B. japonicum (Rudolph et al., 2006).
We cloned the promoter region of S. meliloti mbfA, including part of an upstream gene and c. 100 nucleotides into the coding sequence, and placed it upstream of the lux operon on a plasmid, called PmbfA:lux (Fig. 7a). To confirm that S. meliloti mbfA, and thus PmbfA:lux, are regulated by iron, bacteria were grown in iron‐limited and iron‐replete medium to mid‐log phase. Transcript levels of the endogenous mbfA gene were analysed by quantitative reverse transcription polymerase chain reaction (RT‐qPCR), and found to be three‐fold induced in the presence of iron (Fig. 7b). Luminescence was measured in bacteria grown under the same conditions but transferred to a plate reader with luminescence detection. Sinorhizobium meliloti carrying a promotor‐less lux plasmid was used to establish the background signal (Fig. 7c). In the absence of iron, S. meliloti carrying the PmbfA:lux plasmid gave a luminescence reading that was only slightly above background, showing that mbfA promoter activity was strongly repressed. In iron‐replete medium, luminescence was more than seven‐fold increased. Similar results were obtained for early log‐phase and late log‐phase cell cultures (not shown).
Fig. 7.
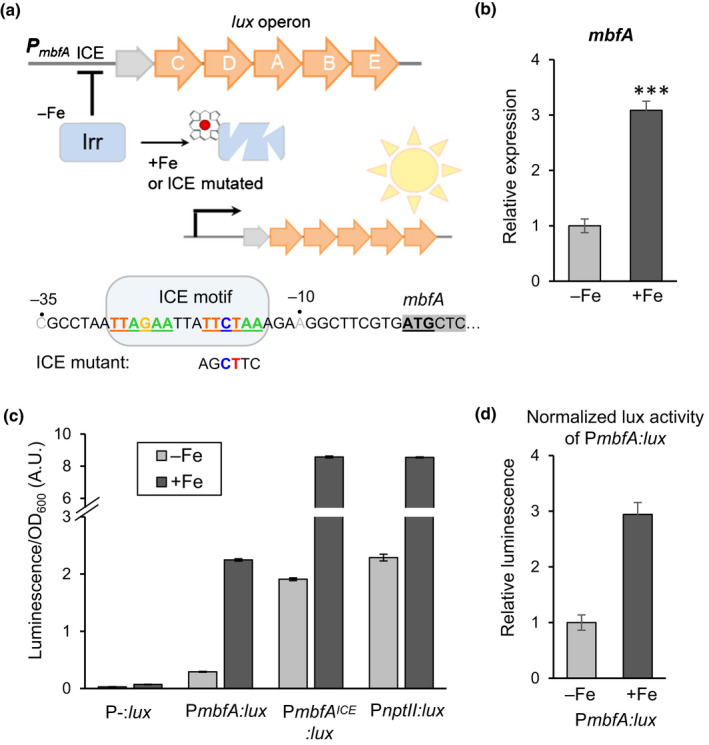
Development of an iron reporter for symbiotic rhizobium bacteria. (a) Diagram of the bacterial PmbfA:lux reporter and regulation of its expression by the iron response regulator (Irr) which binds the upstream Iron Control Element (ICE). In iron‐replete conditions, Irr binds iron in the form of haem and is degraded, leading to de‐repression (activation) of target gene expression. (b) Expression of the endogenous mbfA gene in free‐living Sinorhizobium meliloti 1021 in response to iron. Bacteria were grown in UMS medium without (−Fe) or with 40 µM iron sulphate (+Fe) until mid‐log phase, then harvested for RNA extraction. mbfA transcript levels were assayed by RT‐qPCR. Values were normalized to the housekeeping gene gapA, and are the mean ± SE of three biological replicates (***, P = 0.0001, Bio‐Rad CFX Maestro software). (c) Sinorhizobium meliloti 1021 carrying the lux plasmid without promoter (P‐:lux); the PmbfA:lux reporter; PmbfA:lux with a mutated ICE motif; or a constitutive lux reporter with the nptII promoter were grown as in (b). Luminescence was measured in a plate reader and corrected for cell density (OD600). Values are the mean ± SE of three biological replicates (cell cultures grown in parallel). A.U., arbitrary units. (d) PmbfA:lux activity normalized for constitutive lux activity of the PnptII:lux reporter in response to iron. Error bars represent SE.
Scrutiny of the S. meliloti mbfA promoter sequence revealed an ICE motif immediately upstream of the ATG start codon, overlapping with the −35 and −10 elements of the core promoter (Fig. 7a). The ICE motif is the cis‐acting element for the iron response regulator Irr in B. japonicum (Rudolph et al., 2006) and R. leguminosarum (Todd et al., 2006). The stability of Irr is controlled by haem binding and its regulatory activity is thus highly specific for iron (Yang et al., 2005). To show that the iron‐induced PmbfA:lux activity is regulated by the ICE motif and Irr, we mutated four nucleotides of the ICE motif with the aim of preventing Irr binding (Fig. 7a). When bacteria were grown in iron‐deficient medium, the ICE mutant of PmbfA:lux gave a luminescence signal similar to that of wild‐type PmbfA:lux plus iron. Thus, binding of the Irr repressor is abolished in this mutant, without affecting maximal transcriptional activity of the promoter. To further confirm that PmbfA:lux activity was regulated by Irr, the Pmbfa:lux plasmid was transferred into irrA ‐ mutant and wild‐type strains of R. leguminosarum (Wexler et al., 2003). In the irrA ‐ mutant, PmbfA:lux was not repressed in low‐iron medium, with a luminescence output similar to a constitutive lux reporter construct (Fig. S5a,b).
We noted that the ICE mutant of Pmbfa:lux has c. four‐fold more luminescence in medium with iron, and thus seems to be induced by iron despite the mutation. However, the same effect was observed for a construct with constitutive lux expression, PnptII:lux, containing the neomycin phosphotransferase II promoter (Frederix et al., 2014). Luciferase activity is highly dependent on ATP and FAD, which are more available in healthy cells grown in iron‐replete medium. Lack of iron in the medium strongly limits bacterial growth (Fig. S5c), because iron is an essential element for respiration and many other metabolic processes, affecting the energy status of the cell. When the change in luminescence signal resulting from PmbfA:lux expression in response to iron is normalized for luminescence in PnptII:lux, the calculated three‐fold increase matches the three‐fold increase in mbfA transcript abundance (Fig. 7d).
In summary, tests in free‐living rhizobia show that the PmbfA:lux reporter is almost fully repressed in the absence of iron and that, in the presence of iron, the luminescence signal is induced to levels that are comparable to constitutive lux expression. Moreover, expression of PmbfA:lux is regulated by the cis‐acting ICE motif and trans‐acting Irr transcription factor, which respond specifically to iron.
Host plant VTLs are required for iron delivery to the bacteroids
To assess the iron status of bacteroids in root nodules, M. truncatula seedlings were inoculated with bacteria expressing the PmbfA:lux reporter, and luminescence was detected with a NightOWL imaging system. Inoculated seedlings grown on vertical agar plates showed no luminescence until the initiation of nodules, similar to the transcriptional lux reporters responding to sucrose and gamma‐aminobutyric acid in pea (Pini et al., 2017). Luminescence was associated with the central part of the nodule (Fig. 8a), correlating with published RNA‐seq data for mbfA expression (Table S3).
Fig. 8.
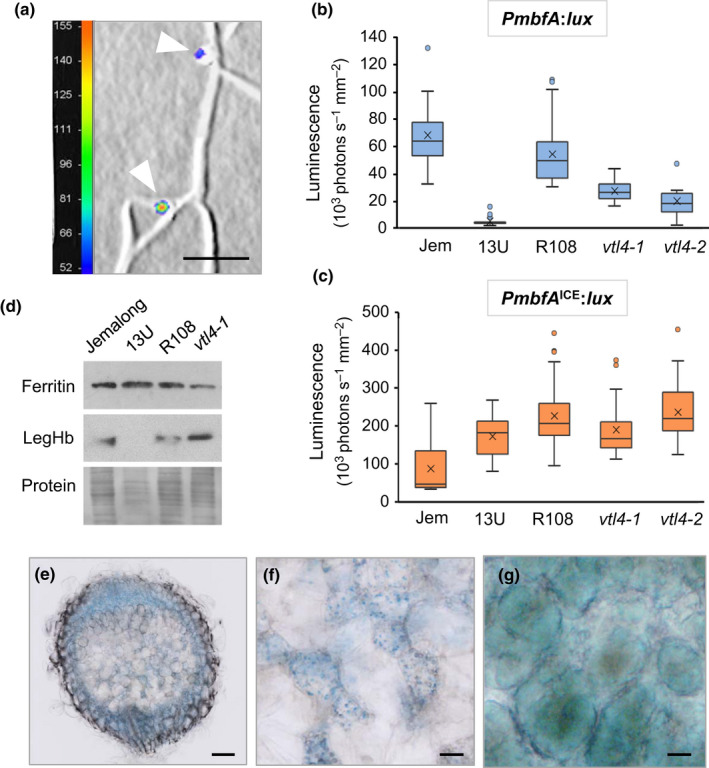
Symbiotic bacteria are iron deficient in nodules lacking VTL4 and VTL8 despite normal iron levels in plant tissues. (a) Detail of an Medicago truncatula R108 root inoculated with Sinorhizobium meliloti 1021 expressing PmbfA:lux at 21 d post inoculation (dpi). Luminescence is represented by a colour scale in photons per second, superimposed on a grey‐scale image. The white arrow heads point to nodules. Bars, 5 mm. (b, c) Expression of the bacterial iron reporter PmbfA:lux (b) and the deregulated Iron Control Element (ICE) mutant (c) in root nodules at 21 dpi, measured as luminescence with a NightOWL camera. Medicago truncatula Jemalong (Jem) was used as wild‐type for the 13U mutant, and M. truncatula R108 as wild‐type for the vtl4 mutants. The data are presented in a box plot, with the box marking the upper and lower quartiles, the middle line represents the median and the symbol × indicates the mean. The whiskers show the spread of the data within the 1.5‐fold interquartile range (n = 9 plants for vtl4‐2, and n ≥ 13 for other lines). (d) Protein blot analysis of ferritin and leghaemoglobin (LegHb) in nodules (21 dpi) of the indicated genotypes. Ponceau S staining of the blot was used as protein loading control (lower panel). (e–g) Histological iron staining of 28‐dpi nodules from (e) 13U mutant plant, (f) enlarged image of the senesced Zone III in 13U, and (g) infected cells in Zone III of wild‐type Jemalong, see Supporting Information Fig. S6 for an image of a whole nodule. Haem‐iron in (g) has a different hue from non‐haem iron. Bars, 0.1 mm (e) and 20 µm (f, g).
To investigate whether mutations in VTL4 and VTL8 affect the amount of iron perceived by endosymbiotic bacteria, the mutant lines (13U, vtl4‐1 and vtl4‐2) and their relevant wild‐types (R108 and A17) were grown on Terragreen–sand to obtain a well‐developed root system. Sinorhizobium meliloti 1021 was used to inoculate all plant genotypes. While less effective in nodulating M. truncatula Jemalong, the parent line of the 13U mutant, the number of nodules were sufficient for quantitative analysis. At 21 dpi, plants were dug up, washed and the roots were imaged for luminescence. The values (in photons per second, normalized to area) are presented as a box plot, a statistical graph method that summarizes the whole data set. The 13U mutant nodules showed 90% decrease in luminescence, and vtl4 mutant nodules had a reduction in luminescence signal of 50%, compared to their respective wild‐types (Fig. 8b). Because the lower PmbfA:lux signal could be due to fewer or ATP‐depleted bacteria in the mutant nodules, we also analysed luminescence in roots inoculated with the constitutive ICE mutant reporter, PmbfAICE:lux. As with the wild‐type reporter, luminescence signal was restricted to the nodules, but the area‐normalized values were similar in the vtl4 mutants and R108 parental line. In the 13U line, luminescence from the PmbfAICE:lux reporter was slightly increased compared to the control line (Fig. 8c). Thus, the number or total mass of energy‐replete bacteria in wild‐type and mutant nodules are comparable, regardless of differences in development, but the lower PmbfA:lux signal indicates that the bacteria in vtl4 and 13U mutants are iron deficient.
To investigate to what extent iron homeostasis in the plant is altered in the 13U and vtl4 mutants, which could indirectly affect the iron status of the bacteroids, protein extracts of nodules were analysed for ferritin. The expression of this iron storage protein is strongly correlated with intracellular iron levels (Briat et al., 2010). The ferritin levels in 13U and vtl4 nodules were similar in their respective wild‐type controls, despite a complete lack of leghaemoglobin in 13U nodules (Fig. 8d). In agreement with these findings, the total iron content was 1.05 ± 0.05 mg g−1 dry weight for wild‐type nodules and 1.14 ± 0.14 mg g−1 for 13U nodules. Interestingly, staining of nonhaem iron in 13U nodules using Perls’ reagent showed a mosaic of cells with and without iron in the senescing region which corresponds to the nitrogen fixation zone, Zone III (Fig. 8e). At higher magnification, the iron appears associated with small organelles (Fig. 8f). In wild‐type nodules, the infected cells in Zone III contain a high concentration of haem‐iron which stains a greenish‐blue, whereas uninfected cells have little iron staining (Figs 8g, S6).
In summary, these data indicate that VTL4 and VTL8 are specifically required for iron delivery to the bacteroids, but not for transport of iron into host plant cells.
Discussion
Because of a high demand for metals, nodules provide an interesting system to study the function of transporters and other metal homeostasis genes in a compartmentalized plant cell. After iron is imported across the plasma membrane into an infected cell, it is distributed between the mitochondria, plastids and the differentiating bacteroids. Our functional characterization of two VTL genes that are specifically expressed in root nodules of M. truncatula strongly indicates they are required for iron delivery to the bacteroids. Evidence for iron as the substrate of VTL4 and VTL8 is based on (1) homology with the vacuolar iron transporter VIT; (2) yeast complementation studies, and (3) the use of a bacterial iron reporter. VIT proteins function as proton‐dependent antiporters of Fe2+ and other divalent ions including Mn2+ and Co2+, although the transport of iron is clearly the main physiological function (e.g. Kim et al., 2006). VTLs lack the cytosolic loop proposed to confer substrate specificity (Kato et al., 2019), however, the ability of VTLs to partially restore growth of yeast defective in vacuolar iron transport, indicate that iron is indeed a substrate. Nevertheless, transport of other metals such as Mn2+ should be investigated, for example using yeast complementation (pmr1 mutant), Xenopus oocytes studies, or in vitro transport studies with reconstituted membrane vesicles.
The third piece of evidence for iron transport is that signal output of a bacterial iron reporter was decreased in the plant vtl mutants. Because the transcriptional lux reporter was newly developed for this study, we will discuss the pros and cons here.
Specificity and sensitivity are key parameters for any reporter or biosensor. Building on published knowledge regarding iron regulation in rhizobia and other alpha‐proteobacteria (O’Brian, 2015), we showed that the S. meliloti mbfA promoter used for the transcriptional reporter is repressed by the Irr transcription factor binding to the ICE motif. Haem‐dependent control of Irr stability makes this regulatory mechanism highly specific for iron. Irr responds to micromolar iron concentrations in the medium (Yang et al., 2005), however in our assays we used 40 µM iron, to make sure the bacterial culture would remain iron‐replete during growth of the culture and sequestration of iron into biomass. The iron concentration of the medium is likely to be directly correlated with the intracellular ‘free’ iron concentration, by means of specific iron transporters, which in turn is correlated with haem biosynthesis and regulation by Irr. However, it is technically challenging to obtain a precise figure for intracellular ‘free’ iron or haem, which are kept within a narrow range by homeostatic control. Thus, the reporter cannot be used to measure the extracellular or intracellular iron concentration, but merely shows whether the cells are iron‐limited or iron‐replete (the ‘iron status’ of the cell).
Luciferase activity was chosen as a reporter because it can be used in vivo with light‐sensitive CCD cameras (Pini et al., 2017), has a greater dynamic range and better signal‐to‐noise ratio than GFP. In particular the virtually zero background signal in plant tissue and in the repressed state of the mbfA promoter, are critical. The resolution of CCD cameras is unfortunately low (in millimetres), although systems with 0.1 mm resolution are now available. A drawback of luciferase is that it is dependent on ATP and thus the energy status of the cell.
We tested the reporter in free‐living bacteria, with the caveat that the results are not necessarily transferrable to bacteroids. The latter are much larger in size and do no longer divide. However, expression of mbfA and irr in bacteroids (Table S3) suggest that Irr operates in both free‐living and symbiotic bacteria. Another potential issue with the reporter system was that the 13U mutant does not have fully developed bacteroids, and the nodules may not have many live bacteria at all. This was refuted using the ICE mutant construct, which showed sufficient luminescence signal in 13U nodules (and thus bacteria that were not ATP‐limited), compared to low luminescence of the wild‐type reporter. This allowed us to conclude that the strongly decreased luminescence signal is due to repression of mbfA:lux transcription and thus to iron deficiency.
Expression patterns and our mutant studies showed that the two nodule‐specific VTL genes in M. truncatula are involved in iron transport at different times of the infection process. This may reflect the findings of older studies in peanut and lupin, that iron is required for both nodule initiation and further development (O’Hara et al., 1988; Tang et al., 1990). Phenotypic observations of the vtl4 and 13U mutants are in agreement with the differential expression patterns of VTL4 and VTL8 in nodules. VTL4 is thought to provide iron to the bacteria when they are dividing in the infection thread. Lack of VTL4 results in less elongated bacteroids and more immature nodules, either because nodule development is delayed or because more nodules are initiated. These phenotypes could be due to sub‐optimal iron delivery, analogous to the compromised growth of iron‐limited free‐living bacteria (Fig. S3c). The minor effects of knocking out VTL4 on nodule and bacteroid development suggest that VTL4 is not the only source of iron. At this stage, the bacteria may still have some iron stored from before infection, or simply need little iron. In contrast, VTL8 is critical for full differentiation of the bacteroids into nitrogen‐fixing symbionts. In line with this, VTL8 expression peaks just before the induction of the bacterial nif genes for nitrogenase when large amounts of iron are needed for incorporation in the iron–sulphur cofactors of the abundant nitrogenase protein (Brear et al., 2013). It was previously shown that bacteria unable to express nifD or nifK, two main structural proteins of nitrogenase, differentiate into late‐stage bacteroids, but then undergo early senescence (Hirsch et al., 1983).
Interestingly, iron homeostasis in the nodules is hardly affected in 13U mutants. For instance, no accumulation of iron was observed in the vascular bundles or other parts, unlike the LjMATE mutants (Takanashi et al., 2013; Kryvoruchko et al., 2018). Iron‐rich bodies were observed in the equivalent of Zone III, possibly proplastids unable to use iron for haem biosynthesis, or degenerated symbiosomes before iron is recycled. Thus, the cells in Zone III of the 13U mutant receive sufficient iron, and providing more iron to the plant is unlikely to bypass the block in iron delivery to the bacteroids. In Arabidopsis, iron accumulation is suppressed by BRUTUS, an iron‐regulated E3 ligase (Rodriguez‐Celma et al. 2019; Selote et al., 2015). The L. japonicus homologue of BRUTUS was shown to play an important role in nodule development (Shimomura et al., 2006), although iron homeostasis in the Lj brutus mutant remains to be investigated.
Phylogenetic analysis shows that VTL8, LjSEN1 and GmNOD21 form a separate clade of VTL genes. It is therefore likely that the gene was recruited specifically for symbiosis in the ancestral legume species. Why was a VIT‐like gene, but not VIT, co‐opted for this function? Perhaps VIT could not easily be recruited to the symbiosome membrane during bacteroid development as it is specifically localized to the vacuole membrane, and symbiosomes do not acquire vacuole marker proteins until shortly before senescence (Limpens et al., 2009). Alternatively, it is possible that VTLs transport both Fe2+ and Fe3+, or Fe3+ exclusively, because the cytosolic loop is missing in VTLs. Interestingly, VTLs have a high degree of sequence similarity to the transmembrane domain of MbfA, which also lacks the cytosolic loop but has an additional N‐terminal ferritin‐like domain (Bhubhanil et al., 2014; Sankari & O’Brian, 2014). It has been suggested that this domain oxidizes Fe2+ to Fe3+, which is then transported by the membrane domain. It would be interesting to see if plant ferritin, which accumulates transiently in Zone II, is able to associate with VTL8 to deliver iron to the peribacteroid space.
It is still not clear how iron is taken up by the bacteroids. As noted earlier, iron uptake genes that are active in free‐living rhizobia (Johnston et al., 2001) are generally not induced in the symbiont stage (Table S3, Roux et al., 2014, and reviewed in Abreu et al. (2019)). The Fe2+ transporter FeoAB plays a role in iron transport in Bradyrhizobium and feoA or feoB deletion strains produced ineffective nodules on soybean (Sankari & O’Brian, 2016). However, there are no feoAB genes in the S. meliloti genome. Alternatively, iron may be taken up as Fe3+‐citrate or Fe3+‐malate via tricarboxylic acid transporters, which are highly induced in expression. In any case, solubility of iron in the peribacteroid space should not be an issue for efficient iron uptake, because of acidification of the peribacteroid space towards the onset of nitrogen fixation (Pierre et al., 2013). The iron‐regulated lux reporter developed for our study should help to characterize the bacterial iron transporters in nodules. It is important to note that the expression of MbfA indicates that bacteroids are saturated with iron, and need to export it back to the peribacteroid space to protect themselves from oxidative stress. Thus, low‐affinity uptake systems may be sufficient for the iron requirements of bacteroids.
In summary, this study provides confirmation that VTL8/SEN1 is the main iron transporter across the symbiosome membrane, but it also opens up new questions regarding iron homeostasis in nodules. In addition to identifying the iron species that is exported by the infected plant cell (and by the bacteria through mbfA), key questions are how iron is delivered to VTL8/SEN1 and how it is partitioned between haem biosynthesis for leghaemoglobin and export to the bacteroids. Additionally, the role of VTLs in iron homeostasis in plants in general needs to be further addressed.
Author contributions
JHW, RTG, GK‐K, AD, BH, EMB and MF performed the research and helped with designing experiments and data analysis; PK and JB analysed and interpreted the data and wrote the manuscript. JHW and GK‐K contributed equally to this work.
Supporting information
Fig. S1 Growth of wild‐type, 13U and vtl4 Medicago truncatula with and without nitrogen.
Fig. S2 Cytology of infected cells in the 13U mutant.
Fig. S3 Distribution of bacteroid cell sizes in nodules of wild‐type and the 13U mutant.
Fig. S4 Nodule fresh weight of complemented 13U lines.
Fig. S5 Regulation of mbfA expression by IrrA and iron.
Fig. S6 Iron staining of nodules using Perls’ reagent.
Methods S1 Additional information on Materials and Methods.
Table S1 Tnt1 insertions in Medicago truncatula lines NF17463 (vtl4‐1) and NF21016 (vtl4‐2).
Table S2 Primers and other oligonucleotides.
Table S3 Expression of Sinorhizobium meliloti genes involved in iron homeostasis during nodule development.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
The authors thank Jeremy Murray, Andy Breakspear and Giles Oldroyd for their generous advice to JHW’s PhD research project. The authors would also like to thank Giles Oldroyd for providing gene sequences for Golden Gate cloning; Jeremy Murray for help with identification of the deleted genome region in the 13U line; Allan Downie (John Innes Centre) for plasmid pIJ11268; Penelope Smith (La Trobe University, Melbourne, Australia) for sending AtVIT1 and MtVTL4 in pYES2; Thomas Buckhout (Humboldt University Berlin, Germany) for the Δccc1 and wild‐type yeast strains; Jon Todd (University of East Anglia) for the R. leguminosarum strains; Eva Wegel (John Innes Centre) and Zoltán Tóth (Agricultural Biotechnology Institute) for confocal microscopy; and Krisztián Laczi (Department of Biotechnology, University of Szeged, Hungary) for conducting acetylene reduction assays, Hannah Justice and Heather Bland (John Innes Centre/University of East Anglia) for phenotype analysis. The authors thank Krisztina Miró (ABC, Gödöllő, Hungary) for skillful technical assistance. This study was supported by Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Grants BB/J004553/1 and BB/P012574/1 (RTG and JB), the Gatsby Charitable Foundation (JHW), the Hungarian National Research Fund and National Research, Development and Innovation Office grants OTKA 67576, 119652, 129547 and the Hungarian Ministry for National Economy GINOP‐2.3.2‐15‐2016‐00001 (PK).
References
- Abreu I, Mihelj P, Raimunda D. 2019. Transition metal transporters in rhizobia: tuning the inorganic micronutrient requirements to different living styles. Metallomics 11: 735–755. [DOI] [PubMed] [Google Scholar]
- Benedito VA, Torres‐Jerez I, Murray JD, Andriankaja A, Allen S, Kakar K, Wandrey M, Verdier J, Zuber H, Ott T et al 2008. A gene expression atlas of the model legume Medicago truncatula . The Plant Journal 55: 504–513. [DOI] [PubMed] [Google Scholar]
- Bhubhanil S, Chamsing J, Sittipo P, Chaoprasid P, Sukchawalit R, Mongkolsuk S. 2014. Roles of Agrobacterium tumefaciens membrane‐bound ferritin (MbfA) in iron transport and resistance to iron under acidic conditions. Microbiology 160: 863–871. [DOI] [PubMed] [Google Scholar]
- Brear EM, Bedon F, Gavrin A, Kryvoruchko IS, Torres‐Jerez I, Udvardi MK, Day DA, Smith PMC. 2020. GmVTL1a is an iron transporter on the symbiosome membrane of soybean with an important role in nitrogen fixation. New Phytologist 228: 667–681. [DOI] [PubMed] [Google Scholar]
- Brear EM, Day DA, Smith PMC. 2013. Iron: an essential micronutrient for the legume‐rhizobium symbiosis. Frontiers in Plant Science 4: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat JF, Duc C, Ravet K, Gaymard F. 2010. Ferritins and iron storage in plants. Biochimica et Biophysica Acta 1800: 806–814. [DOI] [PubMed] [Google Scholar]
- Cailliatte R, Schikora A, Briat JF, Mari S, Curie C. 2010. High‐affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell 22: 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud M, Boisson‐Dernier A, Zhang J, Taylor CG, Yu O, Barker DG. 2006. Agrobacterium rhizogenes‐mediated root transformation In: Mathesius U, Journet EP, Sumner LW, eds. The Medicago truncatula handbook. Manchester, UK: Noble. [Google Scholar]
- Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR. 2002. Random GFP:cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proceedings of the National Academy of Sciences, USA 97: 3718–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DR, Bolin JT, Zheng L. 1993. Nitrogenase metalloclusters: structures, organization, and synthesis. Journal of Bacteriology 175: 6737–6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delauney AJ, Cheon C‐I, Snyder PJ, Verma DPS. 1990. A nodule‐specific sequence encoding a methionine‐rich polypeptide. Plant Molecular Biology 14: 449–451. [DOI] [PubMed] [Google Scholar]
- Domonkos A, Horvath B, Marsh JF, Halasz G, Ayaydin F, Oldroyd GED, Kalo P. 2013. The identification of novel loci required for appropriate nodule development in Medicago truncatula . BMC Plant Biology 13: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie JA. 2005. Legume haemoglobins: symbiotic nitrogen fixation needs bloody nodules. Current Biology 15: R196–R198. [DOI] [PubMed] [Google Scholar]
- Fournier J, Timmers ACJ, Sieberer J, Jauneau A, Chabaud M, Barker DG. 2008. Mechanism of infection thread elongation in root hairs of Medicago truncatula and dynamic interplay with associated rhizobial colonization. Plant Physiology 148: 1985–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederix M, Edwards A, Swiderska A, Stanger A, Karunakaran R, Williams A, Abbruscato P, Sanchez‐Contreras M, Poole PS, Downie JA. 2014. Mutation of praR in Rhizobium leguminosarum enhances root biofilms, improving nodulation competitiveness by increased expression of attachment proteins. Molecular Microbiology 93: 464–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrocho‐Villegas V, Gopalasubramaniam SK, Arredondo‐Peter R. 2007. Plant hemoglobins: what we know six decades after their discovery. Gene 398: 78–85. [DOI] [PubMed] [Google Scholar]
- Gollhofer J, Schläwicke C, Jungnick N, Schmidt W, Buckhout TJ. 2011. Members of a small family of nodulin‐like genes are regulated under iron deficiency in roots of Arabidopsis thaliana . Plant Physiology and Biochemistry 49: 557–564. [DOI] [PubMed] [Google Scholar]
- Gollhofer J, Timofeev R, Lan P, Schmidt W, Buckhout TJ. 2014. Vacuolar‐Iron‐Transporter1‐like proteins mediate iron homeostasis in Arabidopsis. PLoS ONE 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Guerrero M, Escudero V, Saéz Á, Tejada‐Jiménez M. 2016. Transition metal transport in plants and associated endosymbionts: arbuscular mycorrhizal fungi and Rhizobia. Frontiers in Plant Science 7: 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakoyama T, Sandal N, Suganuma N, Niimi K, Umehara Y, Kouchi H, Nakamura Y, Tamaoki M, Isobe S, Tabata S et al 2011. The integral membrane protein SEN1 is required for symbiotic nitrogen fixation in Lotus japonicus nodules. Plant and Cell Physiology 53: 225–236. [DOI] [PubMed] [Google Scholar]
- Hirsch AM, Bang M, Ausubel FM. 1983. Ultrastructural analysis of ineffective alfalfa nodules formed by nif:Tn5 mutants of Rhizobium meliloti . Journal of Bacteriology 155: 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B, Domonkos A, Kereszt A, Szucs A, Abraham E, Ayaydin F, Boka K, Chen Y, Chen R, Murray JD et al 2015. Loss of the nodule‐specific cysteine rich peptide, NCR169, abolishes symbiotic nitrogen fixation in the Medicago truncatula dnf7 mutant. Proceedings of the National Academy of Sciences, USA 112: 15232–15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JB, Rees DC. 2006. How many metals does it take to fix N2? A mechanistic overview of biological nitrogen fixation. Proceedings of the National Academy of Sciences, USA 103: 17088–17093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov S, Harrison MJ. 2014. A set of fluorescent protein‐based markers expressed from constitutive and arbuscular mycorrhiza‐inducible promoters to label organelles, membranes and cytoskeletal elements in Medicago truncatula . The Plant Journal 80: 1151–1163. [DOI] [PubMed] [Google Scholar]
- Johnston AW, Yeoman KH, Wexler M. 2001. Metals and the rhizobial–legume symbiosis – uptake, utilization and signalling. Advances in Microbial Physiology 45: 113–156. [DOI] [PubMed] [Google Scholar]
- Kato T, Kumazaki K, Wada M, Taniguchi R, Nakane T, Yamashita K, Hirata K, Ishitani R, Ito K, Nishizawa T et al 2019. Crystal structure of plant vacuolar iron transporter VIT1. Nature Plants 5: 308–315. [DOI] [PubMed] [Google Scholar]
- Kim S, Punshon T, Lanzirotti A, Li LT, Alonso JM, Ecker JR, Kaplan J, Guerinot ML. 2006. Localization of iron in Arabidopsis seeds requires the vacuolar membrane transporter VIT1. Science 314: 1295–1298. [DOI] [PubMed] [Google Scholar]
- Kryvoruchko IS, Benedito VA, Roberts DM, Finney LA, Tejada‐Jiménez M, Nakashima J, Udvardi MK, Torres‐Jerez I, Pislariu CI, Routray P et al 2018. An iron‐activated citrate transporter, MtMATE67, is required for symbiotic nitrogen fixation. Plant Physiology 176: 2315–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarbuta P, Duckett K, Botting CH, Chahrour O, Malone J, Dalton JP, Law CJ. 2017. Recombinant vacuolar iron transporter family homologue PfVIT from human malaria‐causing Plasmodium falciparum is a Fe2+/H+ exchanger. Scientific Reports 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen O, McVey Ward D, Kaplan J. 2001. CCC1 is a transporter that mediates vacuolar iron storage in yeast. Journal of Biological Chemistry 276: 29515–29519. [DOI] [PubMed] [Google Scholar]
- Limpens E, Lvanov S, Van Esse W, Voets G, Fedorova E, Bisseling T. 2009. Medicago N2‐fixing symbiosomes acquire the endocytic identity marker Rab7 but delay the acquisition of vacuolar identity. Plant Cell 21: 2811–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpens E, Moling S, Hooiveld G, Pereira PA, Bisseling T, Becker JD, Küster H. 2013. Cell‐ and tissue‐specific transcriptome analyses of Medicago truncatula root nodules. PLoS ONE 8: e64377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Liao LL, Nie MM, Peng WT, Zhang MS, Lei JN, Zhong YJ, Liao H, Chen ZC. 2020. A VIT‐like transporter facilitates iron transport into nodule symbiosomes for nitrogen fixation in soybean. New Phytologist 226: 1413–1428. [DOI] [PubMed] [Google Scholar]
- Meguro R, Asoano Y, Odagiri S, Li C, Iwatsuki H, Shoumura K. 2007. Nonheme‐iron histochemistry for light and electron microscopy: a historical, theoretical and technical review. Archives of Histology and Cytology 70: 1–19. [DOI] [PubMed] [Google Scholar]
- Mergaert P, Uchiumi T, Alunni B, Evanno G, Cheron A, Catrice O, Mausset A‐E, Barloy‐Hubler F, Galibert F, Kondorosi A et al 2006. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium‐legume symbiosis. Proceedings of the National Academy of Sciences, USA 103: 5230–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD, Muni RRD, Torres‐Jerez I, Tang Y, Allen S, Andriankaja M, Li G, Laxmi A, Cheng X, Wen J et al 2011. Vapyrin, a gene essential for intracellular progression of arbuscular mycorrhizal symbiosis, is also essential for infection by rhizobia in the nodule symbiosis of Medicago truncatula . The Plant Journal 65: 244–252. [DOI] [PubMed] [Google Scholar]
- O’Brian MR. 2015. Perception and homeostatic control of iron in the rhizobia and related bacteria. Annual Review of Microbiology 69: 229–245. [DOI] [PubMed] [Google Scholar]
- O’Hara GW, Dilworth MJ, Boonkerd N, Parkpian P. 1988. Iron‐deficiency specifically limits nodule development in peanut inoculated with Bradyrhizobium sp. New Phytologist 108: 51–57. [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Murray JD, Poole PS, Downie JA. 2011. The rules of engagement in the legume‐Rhizobial symbiosis. Annual Review of Genetics 45: 119–144. [DOI] [PubMed] [Google Scholar]
- Ott T, Van Dongen JT, Günther C, Krusell L, Desbrosses G, Vigeolas H, Bock V, Czechowski T, Geigenberger P, Udvardi MK. 2005. Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Current Biology 15: 531–535. [DOI] [PubMed] [Google Scholar]
- Petrezselyova S, Zahradka J, Sychrova H. 2010. Saccharomyces cerevisiae BY4741 and W303–1A laboratory strains differ in salt tolerance. Fungal Biology 114: 144–150. [DOI] [PubMed] [Google Scholar]
- Pierre O, Engler G, Hopkins J, Brau F, Boncompagni E, Hérouart D. 2013. Peribacteroid space acidification: a marker of mature bacteroid functioning in Medicago truncatula nodules. Plant, Cell & Environment 36: 2059–2070. [DOI] [PubMed] [Google Scholar]
- Pini F, East AK, Appia‐Ayme C, Tomek J, Karunakaran R, Mendoza‐Suárez M, Edwards A, Terpolilli JJ, Roworth J, Downie JA et al 2017. Bacterial biosensors for in vivo spatiotemporal mapping of root secretion. Plant Physiology 174: 1289–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin N, Harrison MJ. 2009. Live‐cell imaging reveals periarbuscular membrane domains and organelle location in Medicago truncatula roots during arbuscular mycorrhizal symbiosis. Plant Physiology 151: 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Celma J, Chou H, Kobayashi T, Long TA, Balk J. 2019. Hemerythrin E3 ubiquitin ligases as negative regulators of iron homeostasis in plants. Frontiers in Plant Science 10: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Haas B, Finney L, Vogt S, González‐Guerrero M, Rodríguez‐Haas B, Imperial J, González‐Melendi P. 2013. Iron distribution through the developmental stages of Medicago truncatula nodules. Metallomics 5: 1247. [DOI] [PubMed] [Google Scholar]
- Roux B, Rodde N, Jardinaud MF, Timmers T, Sauviac L, Cottret L, Carrère S, Sallet E, Courcelle E, Moreau S et al 2014. An integrated analysis of plant and bacterial gene expression in symbiotic root nodules using laser‐capture microdissection coupled to RNA sequencing. The Plant Journal 77: 817–837. [DOI] [PubMed] [Google Scholar]
- Rudolph G, Semini G, Hauser F, Lindemann A, Friberg M, Hennecke H, Fischer HM. 2006. The Iron Control Element, acting in positive and negative control is a target for the Irr protein. Journal of Bacteriology 188: 9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankari S, O’Brian MR. 2014. A bacterial iron exporter for maintenance of iron homeostasis. Journal of Biological Chemistry 289: 16498–16507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankari S, O’Brian MR. 2016. The Bradyrhizobium japonicum ferrous iron transporter FeoAB is required for ferric iron utilization in free living aerobic cells and for symbiosis. Journal of Biological Chemistry 291: 15653–15662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selote D, Samira R, Matthiadis A, Gillikin JW, Long TA. 2015. Iron‐binding E3 ligase mediates iron response in plants by targeting basic Helix‐Loop‐Helix transcription factors. Plant Physiology 167: 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura K, Nomura M, Tajima S, Kouchi H. 2006. LjnsRING, a novel RING finger protein, is required for symbiotic interactions between Mesorhizobium loti and Lotus japonicus . Plant and Cell Physiology 47: 1572–1581. [DOI] [PubMed] [Google Scholar]
- Strozycki PM, Szczurek A, Lotocka B, Figlerowicz M, Legocki AB. 2007. Ferritins and nodulation in Lupinus luteus: iron management in indeterminate type nodules. Journal of Experimental Botany 58: 3145–3153. [DOI] [PubMed] [Google Scholar]
- Suzaki T, Yoro E, Kawaguchi M. 2015. Leguminous plants: inventors of root nodules to accommodate symbiotic bacteria In: Jeon KW, ed. International review of cell and molecular biology. Amsterdam, the Netherlands: Elsevier, 111–158. [DOI] [PubMed] [Google Scholar]
- Takanashi K, Yokosho K, Saeki K, Sugiyama A, Sato S, Tabata S, Ma JF, Yazaki K. 2013. LjMATE1: a citrate transporter responsible for iron supply to the nodule infection zone of Lotus japonicus . Plant and Cell Physiology 54: 585–594. [DOI] [PubMed] [Google Scholar]
- Tang C, Robson AD, Dilworth MJ. 1990. A split‐root experiment shows that iron is required for nodule initiation in Lupinus angustifolius L. New Phytologist 115: 61–67. [DOI] [PubMed] [Google Scholar]
- Tejada‐Jiménez M, González‐Guerrero M, Imperial J, Kryvoruchko I, Lucas MM, Udvardi M, Castro‐Rodríguez R, Tejada‐Jiménez M. 2015. Medicago truncatula natural resistance‐associated macrophage Protein1 is required for iron uptake by Rhizobia‐infected nodule cells. Plant Physiology 168: 258–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JD, Sawers G, Rodionov DA, Johnston AWB. 2006. The Rhizobium leguminosarum regulator IrrA affects the transcription of a wide range of genes in response to Fe availability. Molecular Genetics and Genomics 275: 564–577. [DOI] [PubMed] [Google Scholar]
- Van de Velde W, Pérez Guerra JC, De Keyser A, De Rycke R, Rombauts S, Maunoury N, Mergaert P, Kondorosi E, Holsters M, Goormachtich S. 2006. Aging in legume symbiosis. A molecular view on nodule senescence in Medicago truncatula . Plant Physiology 141: 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasse J, De Billy F, Camut S, Truchet G. 1990. Correlation between ultrastructural differentiation of bacteriods and nitrogen fixation in alfalfa nodules. Journal of Bacteriology 172: 4295–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hou Q, Li P, Yang L, Sun X, Benedito VA, Wen J, Chen B, Mysore KS, Zhao J. 2017. Diverse functions of multidrug and toxin extrusion (MATE) transporters in citric acid efflux and metal homeostasis in Medicago truncatula . The Plant Journal 90: 79–95. [DOI] [PubMed] [Google Scholar]
- Wexler M, Todd JD, Kolade O, Bellini D, Hemmings AM, Sawers G, Johnston AWB. 2003. Fur is not the global regulator of iron uptake genes in Rhizobium leguminosarum . Microbiology 149: 1357–1365. [DOI] [PubMed] [Google Scholar]
- Wheatley RM, Ramachandran VK, Geddes BA, Perry BJ, Yost CK, Poole P. 2017. Role of O2 in the growth of Rhizobium leguminosarum bv. viciae 3841 on glucose and succinate. Journal of Bacteriology 199: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ishimori K, O’Brian MR. 2005. Two heme binding sites are involved in the regulated degradation of the bacterial iron response regulator (Irr) protein. Journal of Biological Chemistry 280: 7671–7676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Growth of wild‐type, 13U and vtl4 Medicago truncatula with and without nitrogen.
Fig. S2 Cytology of infected cells in the 13U mutant.
Fig. S3 Distribution of bacteroid cell sizes in nodules of wild‐type and the 13U mutant.
Fig. S4 Nodule fresh weight of complemented 13U lines.
Fig. S5 Regulation of mbfA expression by IrrA and iron.
Fig. S6 Iron staining of nodules using Perls’ reagent.
Methods S1 Additional information on Materials and Methods.
Table S1 Tnt1 insertions in Medicago truncatula lines NF17463 (vtl4‐1) and NF21016 (vtl4‐2).
Table S2 Primers and other oligonucleotides.
Table S3 Expression of Sinorhizobium meliloti genes involved in iron homeostasis during nodule development.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.


