Abstract
Many studies have shown that ozone (O3) can inhibit inflammation in osteoarthritis (OA) and regulate the metabolic balance of articular cartilage, but the mechanisms of this process are not well understood. Our study investigated the therapeutic mechanism of O3 in OA. OA models were established, and the MWT and PWL were measured. HE staining and safranin O-fast green staining were used to observe cartilage degeneration. The levels of MMP-13, collagen-2, LC3II and P62 were measured by immunohistochemistry, and the levels of TNF-α and IL-6 were measured by ELISA. The results showed that intra-articular injection of O3 can effectively alleviate pain and inhibit cartilage degeneration in OA rats. O3 can also reduce the concentrations of TNF-α and IL-6, inhibit the expression of MMP-13 and the degradation of collagen-2, upregulate the autophagy-related protein LC3II and inhibit P62. This effect is associated with the upregulation of chondrocyte autophagy in OA.
Keywords: Medical ozone (O3), osteoarthritis (OA), cartilage degeneration, autophagy, MMP-13, collagen-2
Introduction
Osteoarthritis (OA) is the most common type of arthritis, affecting more than half of people over the age of 65. Additionally, OA is considered one of the most common causes of disability, affecting the knee, spine, hip and hand joints. The signs and symptoms of OA usually include pain, joint stiffness, muscle weakness and swelling of the knee [1,2]. In general, OA is strongly associated with overweight or obesity, gender and previous injury. The common features of knee OA include loss of cartilage, narrowing of joint spaces, hypertrophic bone changes and formation of osteophytes [3]. Many studies have identified OA as a complex disease featuring complicated changes in the homeostasis of articular cartilage and subchondral bone, but the molecular and cellular mechanisms of this process are not well understood.
The main pathological changes in osteoarthritis include a reduction in the number of chondrocytes and the degradation of cartilage matrix. Recent studies have shown that autophagy is a critical part of the role of chondrocytes in OA. The process by which eukaryotic cells degrade cytoplasmic proteins and organelles through the lysosomal pathway to maintain survival is called autophagy. Autophagy removes damaged organelles and long-acting macromolecules and is an indispensable mechanism for maintaining cell homeostasis [4]. Several studies, including one by Cheng NT [5], have found that maintaining autophagy in chondrocytes can prevent cartilage degeneration in OA animal models. The progression of OA is associated with a decrease in the level of autophagy in chondrocytes. Therefore, increasing autophagy in chondrocytes may be a potential mechanism for OA treatment.
Intra-articular O3 injection therapy has been widely used in clinical treatment. In an OA model, intra-articular injection of O3 promotes the repair of the articular cartilage matrix collagen network. Our previous work demonstrated that O3 could remediate the decreased autophagy of chondrocytes stimulated with IL-1β in vitro [6]. Accordingly, we hypothesized that O3 might inhibit OA inflammation by upregulating cartilage autophagy and regulating the metabolic balance of articular cartilage.
Materials and methods
OA model and ozone treatment
Female Wistar rats (200-220 g) were randomly divided into 3 groups (n=8 per group), as follows: in the Sham group 50 μL saline was injected into the right knee joint; in the MIA group, an OA model was established by injection of monoiodoacetate (MIA) 3 mg (50 μL) in the right knee joint; in the MIA+O3 group, MIA was injected in the right knee joint, and 0.5 mL O3 (30 μg/mL) was injected in the right knee joint 7, 14, and 21 days later. All rats were purchased from the Experimental Animal Center of Shandong University. All procedures involving the use of animals were conducted in compliance with the guidelines of the National Institute of Health and were approved by the Animal Care and Use Committee of the Shandong Provincial Hospital affiliated with Shandong University. All possible efforts were made to minimize animal suffering and the number of animals used.
Behavior observation
The mechanical withdrawal threshold (MWT) and paw withdrawal latency (PWL) were measured 1 day before and 1, 3, 7, 14, 21, and 28 days after MIA injection. The effect of O3 on pain in the OA rat model was observed.
MWT detection
Behavioral experiments were conducted from 8:00 to 12:00 in the morning, and the rats were placed in the observation box for 30 min every day for the first 3 days of the experiment so that they could adapt to the environment of the observation box as soon as possible.
A transparent plexiglass box with a length, width and height of 30 cm was placed on a 30 cm high metal shelf; the upper part of the shelf was made of wire mesh with small holes. After the rats adapted to the surrounding environment, the experimenter held the Von Frey filaments through the wire mesh and vertically stimulated the hind paws of the rats (avoiding the insensitive parts of the pads). The Von Frey filaments were advanced against the paw until they slightly bent into an S shape, and they were held there for approximately 6~8 s. A positive response (“X”) was noted if the paw was sharply withdrawn during the stimulation time. Flinching immediately upon withdrawal of the hair was also considered a positive response. Ambulation was considered an ambiguous response and was not recorded as a positive reaction.
After a positive reaction, the rats were stimulated with a smaller scale of Von Frey filaments; if a negative response (“O”) was recorded, a larger filament was applied. Descending stimuli were tested until the minimum stimulus was reached. The intertrial interval was at least 10 s. The results of 5 consecutive experiments and the velocity values of the filaments were recorded. The 50% withdrawal threshold refers to the mechanical strength at which 50% of mechanical stimuli cause contraction reactions. The formula is 10log(x)+kδ, where X is the velocity value of the filament used for the last stimulation, k is the coefficient of the specific stimulation mode, and δ=0.224.
PWL detection
The PWL was determined according to the method of Hargreaves K [7]. A heat source was focused on part of the hind paw, and radiant heat stimulation was applied. When the hind paw of the rat moved rapidly or was lifted up, the time from the start of irradiation to the withdrawal of the paw was recorded as PWL. If the stimulation time extended beyond 20 s and the paw was still not lifted, the stimulation was stopped to prevent local tissue damage. The intertrial interval was 10 min; the measurement was performed 3 times in succession and averaged.
HE staining and safranin O-fast green staining
Twenty-eight days after MIA injection, rats were sacrificed and perfused with 4% paraformaldehyde for fixation. Then the right knee joint was removed, fixed, decalcified, embedded in paraffin, and sliced. HE staining and safranin O-fast green staining were performed, and the Mankin’s score was measured to observe the effect of O3 on the degeneration of cartilage in OA.
The expression of MMP-13, collagen0142 2, LC3II and P62 in cartilage was measured by immunohistochemistry
Twenty-eight days after MIA injection, rats were sacrificed and perfused with 4% paraformaldehyde for fixation. Then, the right knee joint was removed, fixed, decalcified, embedded in paraffin, and sliced. After dewaxing, hydration, antigen retrieval, blocking, primary and secondary antibody incubation, DAB color development, hematoxylin counterstaining and sealing were performed, the expression of MMP-13, collagen 2, LC3II and P62 was observed to explore the effect of O3 on cartilage degeneration and cartilage autophagy.
The concentrations of TNF-α and IL-6 in peripheral blood were determined by ELISA
Twenty-eight days after MIA injection, 5 mL of peripheral blood was collected under anesthesia and centrifuged at 3000 rpm for 10 min to obtain serum for testing. The standard samples, experimental samples and antibody were added to the wells of the test plate. After 3 washes, the TMB colorimetric reagent and the stop solution were added in succession. Finally, the concentrations of TNF-α and IL-6 were measured with a microplate reader to explore the effect of O3 on inflammation in OA.
Results
At 14 days, 21 days and 28 days after MIA injection, the MWT and PWL of the MIA group were significantly lower than those of the Sham group (P<0.01), and the MWT and PWL of the MIA+O3 group were significantly increased compared with those of the MIA group (P<0.01) (Figure 1). The results of HE staining and safranin O-fast green cartilage staining confirmed that injection of O3 delayed cartilage degeneration in OA. The Mankin’s score of the MIA group was significantly higher than that of the Sham group (P<0.01), and the Mankin’s score of the MIA+O3 group was significantly lower than that of the MIA group (P<0.01) (Figure 2). Compared with the MIA group, the expression of MMP-13 in cartilage was decreased, while the expression of collagen-2 was increased, in the MIA+O3 group (P<0.05) (Figure 3). The expression of LC3II was significantly higher in the MIA+O3 group than in the MIA group (P<0.01), while the expression of P62 was lower than that of the MIA group (P<0.05) (Figure 4). Compared with the Sham group, the MIA group had a significantly increased concentration of IL-6 (P<0.01) and an increased concentration of TNF-α (P<0.05). Compared with the MIA group, the MIA+O3 group had decreased concentrations of IL-6 (P<0.01) and TNF-α (P<0.05) (Figure 5). The results showed that O3 had an inhibitory effect on OA inflammation.
Figure 1.
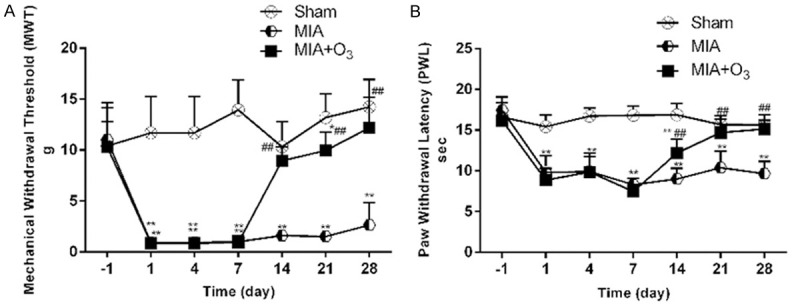
Effects of O3 on the mechanical withdrawal threshold (MWT) and paw withdrawal latency (PWL) in osteoarthritic rats (n=8). A. O3 increases the MWT in osteoarthritic rats. B. O3 increases the PWL in osteoarthritic rats. *P<0.05, **P<0.01 vs. Sham; #P<0.05, ##P<0.01 vs. MIA.
Figure 2.
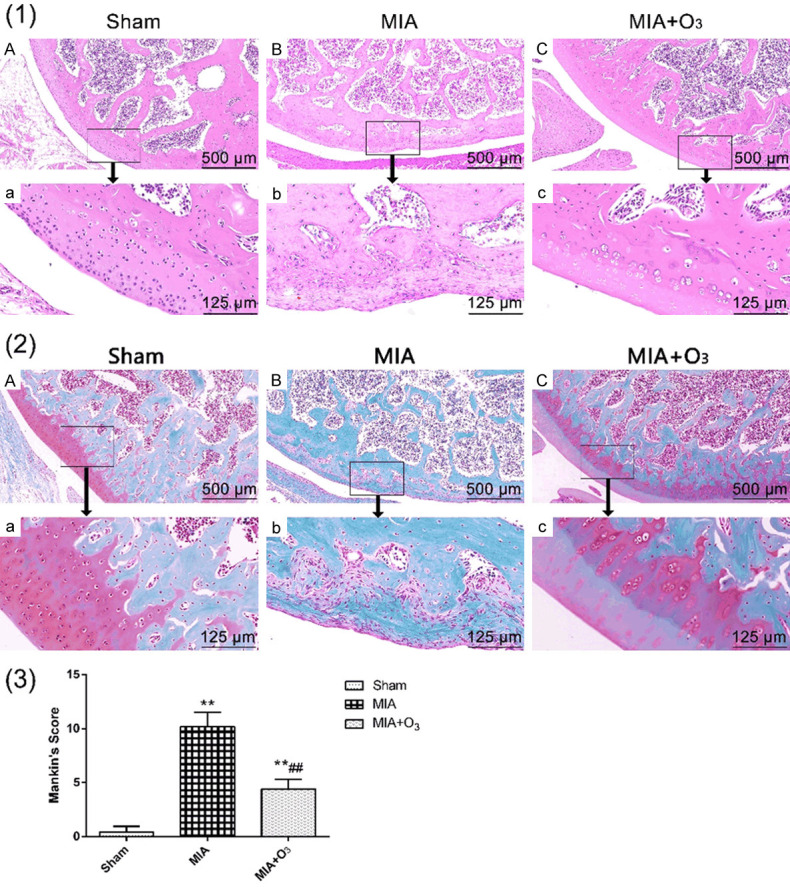
Effects of O3 on HE staining and safranin O-fast green staining of knee joints of OA rats (n=8). (1) The cartilage surface in the MIA group was rough, with a degenerate shape and a significantly decreased number of chondrocytes. Compared with the MIA group, the MIA+O3 group had increased cartilage thickness and a smooth cartilage surface. The MIA+O3 group had significantly deeper matrix staining and more uniform coloring than the MIA group. (2) The structure was disordered in the MIA group, and the tidal line was fuzzy and interrupted. Safranin O staining markedly decreased or even disappeared in the MIA group. The articular cartilage structure in the MIA+O3 group was still clear, and the tidal line was continuous. Safranin O staining slightly diminished. (3) The Mankin’s score of the MIA group was significantly higher than that of the Sham group, while the Mankin’s score of the MIA+O3 group was significantly lower than that of the MIA group. *P<0.05, **P<0.01 vs. Sham; #P<0.05, ##P<0.01, vs. MIA.
Figure 3.
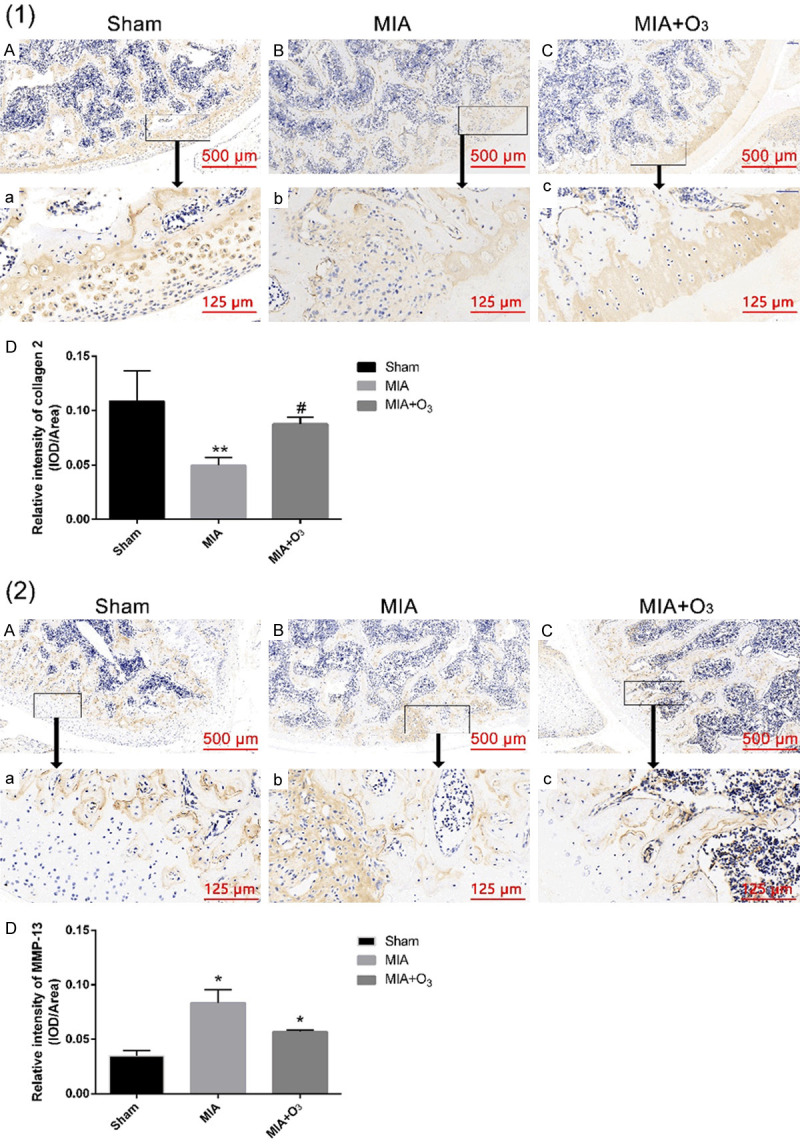
The expression of collagen 2 and MMP-13 in rat knee cartilage by immunohistochemistry. (1) The expression of collagen 2 in the MIA+O3 group was higher than that in the MIA group. (2) The expression of MMP-13 in the MIA group and MIA+O3 group was higher than that in the Sham group, while the expression of MMP-13 in the MIA+O3 group was lower than that in the MIA group. *P<0.05, **P<0.01 vs. Sham; #P<0.05, ##P<0.01 vs. MIA.
Figure 4.
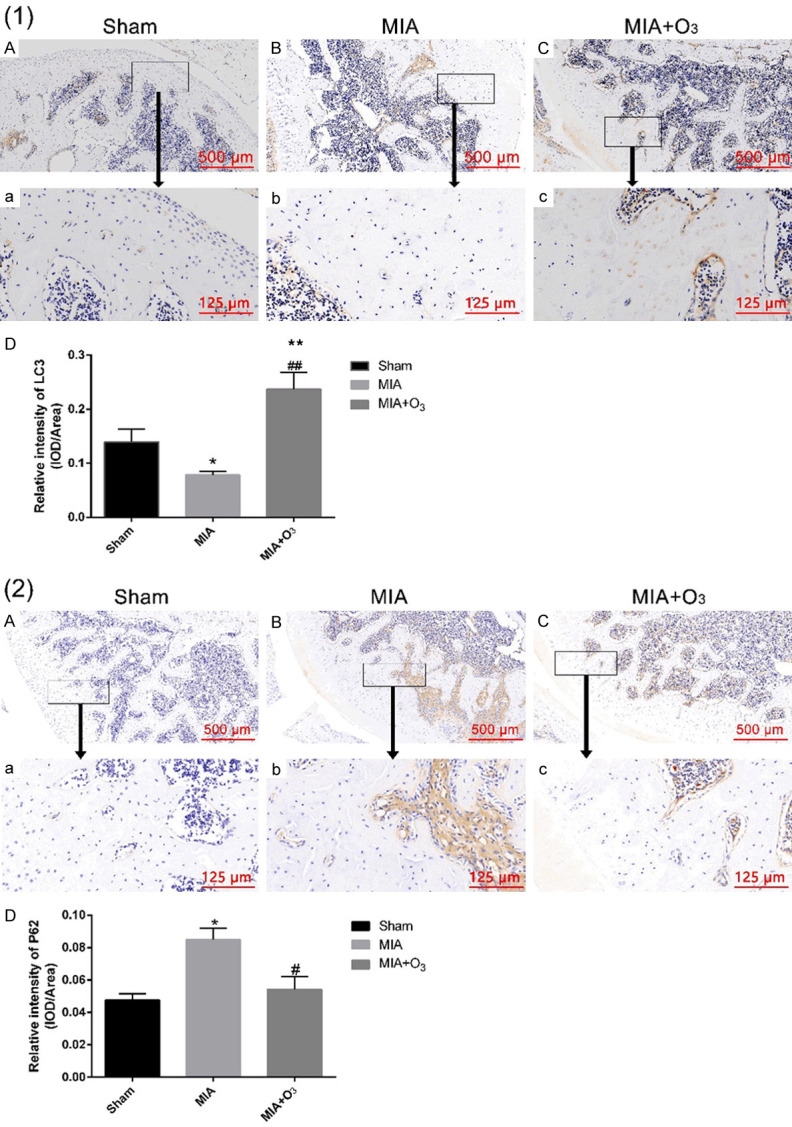
The expression of LC3II and P62 in rat knee cartilage by immunohistochemistry. (1) The expression of LC3II in the MIA+O3 group was significantly higher than that in the Sham and MIA groups. (2) The expression of P62 in the MIA+O3 group was lower than that in the MIA group. *P<0.05, **P<0.01 vs. Sham; #P<0.05, ##P<0.01 vs. MIA.
Figure 5.
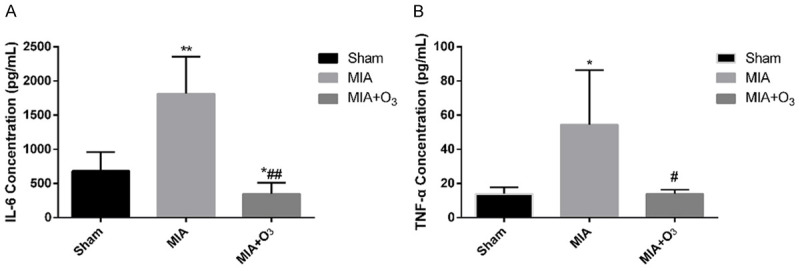
Concentrations of IL-6 and TNF-α in the peripheral blood of rats by ELISA. A. Compared with the MIA group, the concentration of IL-6 in the MIA+O3 group decreased significantly. B. The concentration of TNF-α in the MIA+O3 group decreased significantly compared with that in the MIA group. *P<0.05, **P<0.01 vs. Sham; #P<0.05, ##P<0.01 vs. MIA.
Discussion
OA is the most common joint disease worldwide. According to epidemiological surveys, 9.6% of males and 18% of females over the age of 60 have OA. As the population ages, it is expected that by 2020, OA may become the fourth leading cause of disability [8], which will bring a heavy social burden. Current research suggests that pathological changes in OA joints include destruction and gradual loss of articular cartilage, hyperplasia of subchondral bone, formation of calluses, synovial inflammation, degeneration of the ligaments and meniscus, and hypertrophy of the joint capsule [3]. Drugs are effective in treating OA. In addition, pain is one of the typical symptoms of OA, which seriously affects the quality of life of patients. The study found that the pain intensity of OA was not associated with joint deterioration, mainly related to the mechanism of peripheral pain and the mechanism of central pain [9]. Dimitroulas T [10] concluded that approximately 30% of OA patients have neuropathic pain, which also provides an idea for effective relief of OA pain.
O3 is a mixture of ozone and oxygen, and its application in clinical treatment has been nearly a hundred years old. O3 in a certain concentration range has the effects of killing multiple pathogenic bacteria, reconstructing redox balance, regulating immunity and analgesia and has been widely used in clinical treatment. At present, intra-articular injection of O3 therapy for OA has been widely used in domestic clinical practice and has achieved good results. In recent years, the mechanism of intra-articular injection of O3 to treat OA has attracted attention and has become a research hotspot. Studies have found that O3 can reduce the release of MMPs, such as collagenase and gelatinase, and reduce the destruction of articular cartilage. On the other hand, O3 can increase antioxidant enzymes and oxidative shock proteins, such as heme oxygenase and heme oxigenase-1 (HO-1), and inhibit the cytokines IL-4 and IL-10 and transforming growth factor-β (TGF-β), which ultimately stimulates chondrocytes to facilitate the synthesis of proteoglycans, glycosaminoglycans and collagen, which is beneficial for joint repair [11,12]. However, the mechanism of action of O3 on the therapeutic molecules of OA is not clear.
To explore the specific mechanism of action of O3 on OA, this study used one of the most typical rat OA pain models, intra-articular injection of metabolic inhibitor MIA, inhibited chondrocyte activity, resulting in destruction of chondrocyte glycolysis, eventually leading to cell death [13,14]. The progressive loss of chondrocytes leads to histomorphological changes in articular cartilage, which is similar to the joints of OA patients. However, the study of pain behavior in the MIA-induced OA model has only recently begun. Clinical observation found that intra-articular injection of O3 can alleviate OA pain. Therefore, 7 days after the establishment of the MIA injection-induced OA model in this study, intra-articular injection of O3 was given 1 day before MIA injection and 1 day after injection. The general behavior of the rats was observed on days 4, 7, 14, 21 and 28, and the pain-related behaviors MWT and PWL were determined. The results showed that the rats in each group had a normal diet and flexible activities without sputum and leg movements. There was no significant difference in MWT and PWL between the groups. At 1, 4, and 7 days after MIA injection, the MWT and PWL were significantly lower in the MIA group and MIA+O3 group. Compared with the Sham group, the difference was statistically significant (P<0.01), and the activities of the two groups were decreased. The time of landing on the right hind limb is shortened, and there is a performance of lameness and an increased frequency of lameness. This demonstrates the successful establishment of an MIA-induced OA pain model. At 14 days after MIA injection (7 days after O3 injection), the MWT of the MIA+O3 group was significantly increased compared with that of the MIA group (P<0.01) but slightly decreased compared with that of the Sham group, and the difference was not statistically significant. The PWL of the MIA+O3 group was significantly higher than that of the MIA group (P<0.01) and significantly decreased compared with that of the Sham group (P<0.01), indicating that O3 can improve the MWA induced by MIA and improve the PWL. Fernihough J [15] reported that gabapentin can effectively improve the mechanical hyperalgesia induced by MIA, but the effect on the improvement of the thermal pain threshold is not consistent. At 21 days and 28 days after MIA injection (14 days and 21 days after O3 injection), the MWT and PWL of the MIA+O3 group were significantly increased compared with those of the MIA group (P<0.01), indicating that O3 injection can effectively alleviate OA. However, the specific mechanism of O3 treatment remains to be further studied.
In the development of OA, cartilage degeneration is one of the main pathological changes. To investigate whether O3 can inhibit the degeneration of OA cartilage, this study observed the general appearance of the rat knee joint, HE staining of the joint and red-solid green staining, and clarified the effect of O3 on OA cartilage. Through the appearance observation, the Sham group had a smooth articular surface and no obvious damage. The MIA group had a rough joint surface and scattered bleeding points, which were grayish white, while the joints after O3 injection were slightly rough, indicating that the injection of O3 had no obvious damage to the OA joint. In this study, HE staining showed that the apoptosis and necrosis of chondrocytes in the articular cartilage of the MIA group disappeared, the cartilage layer was destroyed, and the subchondral bone was hardened. In the MIA+O3 group, the chondrocytes were arranged neatly, and local chondrocytes appeared hyperplastic. Through safranin O-fast green staining observation, it was found that the Sham group had a clear tidal line, safranin O staining did not decrease, and the MIA group had obvious fibroblasts on the surface of the articular cartilage. The cartilage layer showed a large number of clustered cell clusters, the tidal line was interrupted, and safranin O staining was severely decreased or even disappeared, demonstrating that the MIA-induced OA model was successful in this study. The structure of articular cartilage in the MIA+O3 group was clear, and the tidal line was continuous, but there were multiple tidal lines, and safranin O staining was mildly decreased, indicating that OA has an inhibitory effect on cartilage degeneration. To further investigate the effect of O3 on OA cartilage degeneration, Mankin’s score of joints based on HE staining and safranin O-fast green staining showed that the Mankin’s score of the MIA group was significantly higher than that of the Sham group, while the Mankin’s score of the MIA+O3 group was higher than that of the MIA+O3 group. The Sham group, which was significantly lower than the MIA group, also indicated that the MIA-induced OA model was successful, and O3 injection inhibited cartilage degeneration of OA.
The pathological changes in OA-affected joints are mainly stimulated by the cytokines IL-1β, IL-6, IL-8 and TNFα to stimulate nuclear factor κB (NF-κB) and MAPK signaling pathways, promoting catabolism and thereby making articular cartilage, subchondral bone and slip change. Membrane metabolism changes [16]. In the development of OA, matrix metalloproteinases (MMPs) play an important role in the decomposition process, and MMP-13 plays the most important role in the pathology of OA. MMP-13 cleavage of Collagen 2 is required for chondrocyte and matrix mineralization [17]. Studies have found that the expression of collagen-2 in chondrocytes was significantly lower in OA patients than in normal subjects. In this study, the effects of O3 on the expression of MMP-13 and collagen-2 in OA cartilage were observed. The results showed that the expression of MMP-13 in the MIA group and MIA+O3 group was higher than that in the Sham group, while the expression of MMP-13 in the MIA+O3 group was lower than that in the MIA group and collagen-2 was significantly reduced in the MIA group. The expression of collagen-2 in the MIA+O3 group was upregulated compared with that in the MIA group, indicating that O3 inhibited the expression of MMP-13 and the degradation of collagen-2 in OA.
With the in-depth study of the pathogenesis of OA, the mechanism of the occurrence and progression of OA associated with the decline of autophagy in articular chondrocytes was discovered [18]. Therefore, increasing autophagy in chondrocytes is one of the targets for the treatment of OA. Autophagy is a cellular process in which organelles, proteins and different macromolecules are transported to lysosomes for degradation. It is an important means of maintaining energy balance in cells and is involved in cell differentiation, apoptosis, and the immune response [19,20]. In mammalian cells, different signal transduction pathways may affect autophagy, but the two major regulators of autophagy are mTOR and AMPK kinase. Takayama K [21] reported that intra-articular injection of rapamycin (an mTOR inhibitor and autophagy activator) can activate the autophagy-associated protein LC3II in articular cartilage, inhibit the expression of MMP-13, and delay the development of articular cartilage degeneration in OA. The results showed that compared with the Sham group, the expression of LC3II was decreased in the MIA group and increased in the MIA+O3 group. In contrast, compared with the Sham group, the expression of P62 was increased in the MIA group and decreased in the MIA+O3 group. O3 can increase the autophagy of chondrocytes in OA and inhibit cartilage degeneration. Studies have confirmed that O3 has an anti-inflammatory function. For this reason, the effects of O3 on IL-6 and TNF-α in OA were examined. The results showed that O3 reduced IL-6 and TNF-α in peripheral blood in OA.
Acknowledgements
This research was funded by grants from the National Natural Science Foundation of China (81771199, 81271346).
Disclosure of conflict of interest
None.
References
- 1.Reginster JY. The prevalence and burden of arthritis. Rheumatology (Oxford) 2002;41(Supp 1):3–6. [PubMed] [Google Scholar]
- 2.Mobasheri A. The future of osteoarthritis therapeutics: emerging biological therapy. Curr Rheumatol Rep. 2013;15:385. doi: 10.1007/s11926-013-0385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Lam GY, Brumell JH. Autophagy signaling through reactive oxygen species. Antioxid Redox Signal. 2011;14:2215–2231. doi: 10.1089/ars.2010.3554. [DOI] [PubMed] [Google Scholar]
- 5.Cheng NT, Meng H, Ma LF, Zhang L, Yu HM, Wang ZZ, Guo A. Role of autophagy in the progression of osteoarthritis: the autophagy inhibitor, 3-methyladenine, aggravates the severity of experimental osteoarthritis. Int J Mol Med. 2017;39:1224–1232. doi: 10.3892/ijmm.2017.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X, Li Y, Lin X, Wang J, Zhao X, Xie J, Sun T, Fu Z. Ozone induces autophagy in rat chondrocytes stimulated with IL-1β through the AMPK/mTOR signaling pathway. J Pain Res. 2018;11:3003–3017. doi: 10.2147/JPR.S183594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 8.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 9.Perrot S. Osteoarthritis pain. Best Pract Res Clin Rheumatol. 2015;29:90–97. doi: 10.1016/j.berh.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Dimitroulas T, Duarte RV, Behura A, Kitas GD, Raphael JH. Neuropathic pain in osteoarthritis: a review of pathophysiological mechanisms and implications for treatment. Semin Arthritis Rheum. 2014;44:145–154. doi: 10.1016/j.semarthrit.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Vendruscolo CDP, Moreira JJ, Seidel SRT, Fülber J, Neuenschwander HM, Bonagura G, Agreste FR, Baccarin RYA. Effects of medical ozone upon healthy equine joints: clinical and laboratorial aspects. PLoS One. 2018;13:e0197736. doi: 10.1371/journal.pone.0197736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bocci V. How a calculated oxidative stress can yield multiple therapeutic effects. Free Radic Res. 2012;46:1068–1075. doi: 10.3109/10715762.2012.693609. [DOI] [PubMed] [Google Scholar]
- 13.Guzman RE, Evans MG, Bove S, Morenko B, Kilgore K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol. 2003;31:619–624. doi: 10.1080/01926230390241800. [DOI] [PubMed] [Google Scholar]
- 14.Kalbhen DA. Chemical model of osteoarthritis--a pharmacological evaluation. J Rheumatol. 1987;14:130–1. [PubMed] [Google Scholar]
- 15.Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112:83–93. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13:302–311. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 17.Wu CW, Tchetina EV, Mwale F, Hasty K, Pidoux I, Reiner A, Chen J, Van Wart HE, Poole AR. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J Bone Miner Res. 2002;17:639–51. doi: 10.1359/jbmr.2002.17.4.639. [DOI] [PubMed] [Google Scholar]
- 18.López de Figueroa P, Lotz MK, Blanco FJ, Caramés B. Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheumatol. 2015;67:966–76. doi: 10.1002/art.39025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariño G, López-Otín C. Autophagy: molecular mechanisms, physiological functions and relevance in human pathology. Cell Mol Life Sci. 2004;61:1439–54. doi: 10.1007/s00018-004-4012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takayama K, Kawakami Y, Kobayashi M, Greco N, Cummins JH, Matsushita T, Kuroda R, Kurosaka M, Fu FH, Huard J. Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res Ther. 2014;16:482. doi: 10.1186/s13075-014-0482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


