Abstract
Molecular phenotype discordance between primary and metastatic tumors exists in a small proportion of breast cancer (BC) patients with accessible synchronous metastases. Reduced therapeutic effect and delays in treatment can occur when decisions on systemic therapy are determined by ignoring the differences in tumor type. Here we report a 54-year-old post-menopausal locally advanced BC patient, who showed no tumor response following routine treatment which included targeting anti-HER2, based on the phenotype of primary tumor (Luminal B, HER2-positive), during neoadjuvant therapy. However, following a secondary biopsy of the metastatic subclavian lymph node, a distinct pathological feature (Triple-negative) was revealed; chemotherapy was adjusted accordingly and resulted in a positive tumor response. Various subclones within primary and metastatic lesions were identified which might be attributed to tumor heterogeneity and in turn resulting in the phenotypic discordance in the receptor status. The patient died due to tumor progression related to triple-negative-featured lung metastasis, with overall survival time of 26.4 months. This study strengthens the value of concurrent biopsies of both primary and synchronous metastatic lesions in BC patients, and provides a reference for treating this kind of tumor when discordance in the molecular phenotype is observed.
Keywords: Breast cancer, cancer stem cells, concurrent biopsies, phenotypic discordance, synchronous metastasis
Introduction
Breast cancer (BC) is the most frequently diagnosed malignancy and the leading cause of cancer death among women worldwide; China’s proportional contribution to global rates is increasing, accounting for 12.2% of all newly diagnosed BCs and 9.6% of all deaths from the disease [1,2]. The current therapeutic model for treating BC involves individualized treatment, which is mainly based on the molecular classification of the tumor [3]. Due to the heterogeneity of BC, the pathological molecular phenotypes between the primary and metastatic lesions could be discordant, which could significantly impact the treatment regimen of BC [4]. Thus, several guidelines [5,6] for the diagnosis and treatment of BC recommended reviewing the status of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) upon tumor recurrence or progression. However, synchronous metastasis can be discovered in 5%-10% BC patients in clinical practice during the first diagnosis [7]. For these patients, clinical treatment is usually established by the pathological characteristics of the primary lesion where the possibilities of differences between the primary and metastatic lesions are invariably ignored [8]. Disregarding the variation between lesions may lead to poor therapeutic effect and delays in treatment time. This article reports the diagnosis and treatment of a BC patient whose receptor status of the primary lesion (Luminal B, HER2-positive) and metastatic lesions, including subclavian lymph node and left lung (Triple-negative), were totally different. The report focuses on the significance of concurrent biopsies of primary and synchronous metastatic lesions in BC patients, mechanisms underlying the molecular phenotype differences between primary and metastatic lesions, and also the current knowledge for treating these types of tumors.
Case presentation
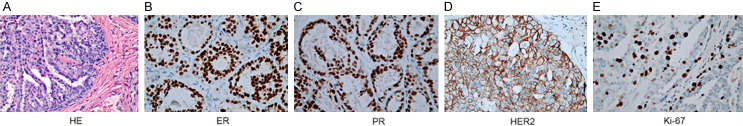
A 54-year-old post-menopausal woman was admitted to the local hospital for evaluation due to a newly discovered right breast mass in early March 2017. Physical examination revealed a right breast mass (6.0*5.0 cm) as well as enlarged right axillary and right subclavian lymph nodes (largest: 2.0*2.0 cm). Without baseline imaging assessment, a simple excision of the right breast mass was conducted revealing a malignant tumor. Histopathological analysis showed infiltrating ductal carcinoma, Nottingham grade II out of III, and a 7.0 cm diameter tumor (Figure 1A). The tumor was found to be ER 2+, PR 2+, HER2 2+ and Ki-67 20% positive by immunohistochemistry (IHC) (Figure 1B-E). Without fluorescence in situ hybridization (FISH) analysis to confirm the status of HER2 gene amplification, she received systemic chemotherapy with cyclophosphoramide/epirubicin/5-fluorouracil (CEF) after surgery.
Figure 1.
Histopathologic characterization identified the right breast mass of the patient as a Luminal B and HER2-positive breast cancer. A. HE staining showed infiltrating ductal carcinoma, Nottingham grade II out of III (200×). B-E. Immunohistochemical staining showed the tumor was positive for ER (2+), PR (2+), HER2 (2+), and Ki-67 (20%) (400×). Abbreviations: BC, breast cancer; ER, estrogen receptor; HE, hematoxylin & eosin; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Two weeks following chemotherapy, she was referred to our institution for further medical help. Physical examination revealed enlargement of both right axillary and subclavian lymph nodes, the largest one increased to 5.0*5.0 cm; no mass was discovered in the right or contralateral breast. Positron emission computed tomography (PET/CT) scan revealed right axillary and right subclavian lymph node metastases with no evidence of distant metastasis (Figure 2A, 2B). The patient had an Eastern Cooperative Oncology Group (ECOG) score of 1. Blood, liver and renal function tests, urine test and ECG were normal. Serum CEA and CA153 were both within normal range. HER2 gene amplification status in the pathologic specimen of excised breast mass was confirmed to be positive by FISH analysis (Figure 2C). Based on the patient’s molecular findings, prior therapeutic drug usage, health condition and treatment willingness, paclitaxel and carboplatin combined with trastuzumab (TCH) were administrated for 2 cycles as a neoadjuvant therapy, but the targeted metastatic lymph node lesions showed no significant shrinkage.
Figure 2.
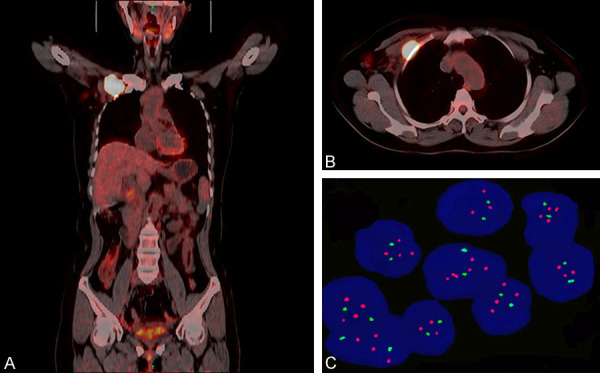
Imaging and molecular characteristics following the patient’s first visit at our institution. A and B. PET-CT scans showed heterogeneous fluorodeoxyglucose uptake in both right axillary and right subclavian lymph nodes, but not in distant organs, indicating the patient had locally advanced BC. C. FISH analysis confirmed HER2 gene amplification within the primary lesion. Abbreviations: FISH, Fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; PET-CT, Positron emission tomography/computed tomography.
The patient stopped treatment, due to personal reasons, for 3 months. The right subclavian lesion was increased to 12.0*8.0 cm following readmission. Biopsy of the right subclavian lesion revealed a poorly differentiated carcinoma originated from the mammary gland (Figure 3A). The lesion was found to be ER negative, PR negative, HER2 negative and Ki-67 90% positive by IHC analysis (Figure 3B-E). When reviewing the evolution of the patient’s molecular phenotype, we further labeled tumors of both primary and metastatic lesions with cancer stem cells (CSCs) molecular markers such as CD44, CD24, and ALDH1. The primary antibodies used are listed in Supplementary Table 1. The IHC results of the primary lesion showed CD44lowCD24+ALDH1low, while metastatic lesions showed CD44highCD24+ALDH1high (Figure 4A-F). Moreover, a comprehensive assessment of the tumor burden in the patient was performed and a highly radioactive area in the right clavicle by the emission computed tomography (ECT) was observed. Considering the patient’s disease progression, chemotherapeutic regimens were switched to gemcitabine combined with cisplatin (GP). The patient was also given zoledronic acid to inhibit bone damage. This treatment regimen significantly ameliorated her clinical symptoms. The right metastatic axillary lymph node almost disappeared, and the mass on the right subclavian area was reduced to 6.0*5.5 cm after 6 treatment cycles (Supplementary Figure 1). During chemotherapy, the patient experienced bone marrow suppression (CTCAE grade 3) and recovered after application of granulocyte stimulating factors. Considering both efficacy bottleneck and side effects associated with chemotherapy, regional radiotherapy of the right chest wall was conducted. However, the disease subsequently progressed by metastasizing to the left lung as evidenced by imaging following radiotherapy (Figure 5A). Following patient confirmation, biopsy of the left lung lesion was performed and metastatic infiltrating carcinoma was revealed (Figure 5B). In line with the results of right subclavian lesion, the left lung lesion was also identified as triple-negative (Figure 5C-E). However, an unsatisfactory staining of Ki-67 was observed by IHC analysis (Data not shown). The patient then underwent a variety of treatments including vinorelbine, capecitabine and albumin paclitaxel; however, due to intolerable repeated marrow suppression (CTCAE grade 1-3) and hand-foot syndrome (CTCAE grade 3), the patient eventually abandoned treatment and died of advanced tumor progression on May 5, 2019. Her overall survival (OS) time was 26.4 months from the first diagnosis.
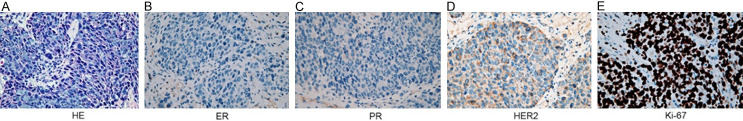
Figure 3.
Histopathologic characterization identified the subclavian lymph node of the BC patient as a triple-negative metastatic lesion. A. HE staining revealed a poorly differentiated carcinoma originated from the mammary gland (400×). B-E. Immunohistochemical staining showed the tumor was negative for ER, PR, and HER2, but positive for Ki-67 (90%) (400×). Abbreviations: BC, breast cancer; ER, estrogen receptor; HE, hematoxylin & eosin; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Figure 4.
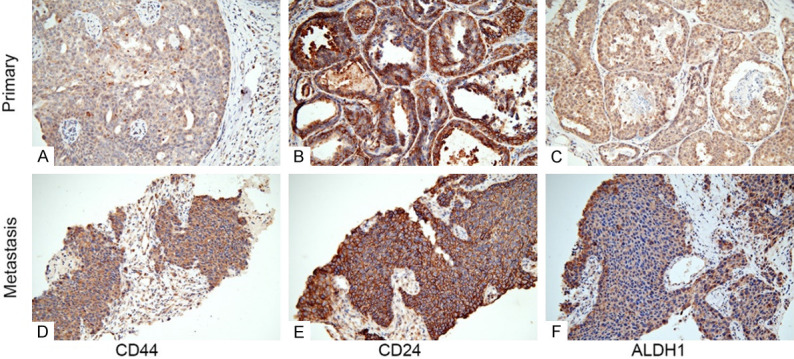
Immunohistochemical staining of cancer stem cell markers revealed a significant difference between the primary lesion and metastatic subclavian lymph node. A and D. CD44 showed low expression in the primary lesion but high expression in the metastatic lesion, localized in both the membrane and cytoplasm of tumor cells (200×); B and E. CD24 showed high membranous expression in both the primary and metastatic lesions (200×); C and F. ALDH1 showed moderate expression in both nucleus and cytoplasm of tumor cells localized in the primary lesion; however, it showed high expression mainly localized to the cytoplasm of the metastatic lesion (200×). Abbreviations: ALDH1, aldehyde dehydrogenase; CD, cluster of differentiation.
Figure 5.
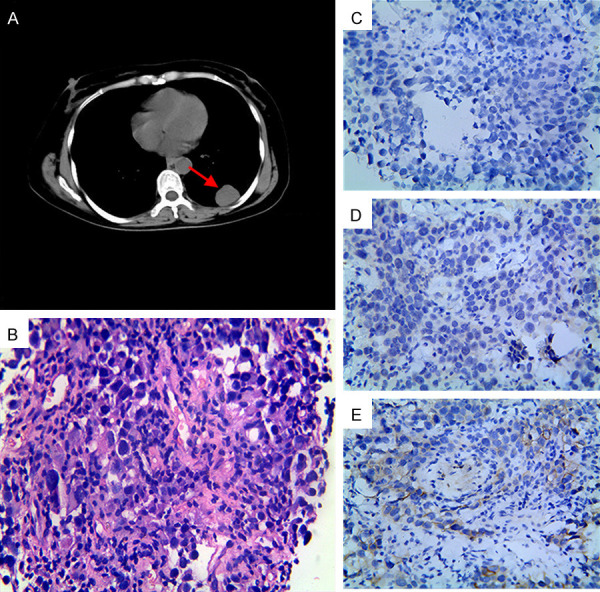
Imaging and histopathologic characterization revealed a left lung metastasis with a triple-negative pathological feature. A. CT scan showed a lesion in the left lower lung, as indicated by the arrowhead; B. HE staining indicated the lesion as metastatic infiltrating carcinoma (400×); C-E. Immunohistochemical staining showed the metastatic lesion was negative for ER, PR, and HER2 (400×). Abbreviations: BC, breast cancer; CT, computed tomography; ER, estrogen receptor; HE, hematoxylin & eosin; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Discussion
BC is the most common cancer among women in China as in most other countries [2]. Compared with developed countries and taking into consideration the unevenness in the distribution of medical resources in China, the level of diagnosis and treatment of BC in some areas is relatively backward; many patients cannot obtain the newly designed standard of care in early stages of disease development [2]. Therefore, the treatment experience of this patient is not uncommon. She was diagnosed with stage IIIc (cT3N3aM0, AJCC cancer staging manual, 7th edition) BC at her first visit. Without baseline examination or biopsy of the metastatic regional lymph node, she underwent nonstandard surgery and chemotherapy. Following surgery and chemotherapy, the patient was referred to our department; however, the right subclavian mass was still enlarged after neoadjuvant treatment with TCH regimen according to the molecular phenotype of the primary tumor (Luminal B, HER2-positive). Then, according to the receptor status of the right subclavian metastasis (Triple-negative), treatment was adjusted to the commonly used GP regimen for the treatment of triple-negative metastatic BC and the lesion rapidly shrank. Therefore, it is of vital significance not only for diagnosis, but also for treatment, to conduct concurrent biopsies of both primary and synchronous metastatic tumors in BC patients.
Neoadjuvant chemotherapy often leads to changes in the status of receptors in BC patients [9,10], but patient data from individuals receiving less than 4-6 cycles of chemotherapy are lacking. The patient in this study received initial treatment of CEF regimen for only 1 cycle and was insensitive to TCH regimen from the beginning. Therefore, the possibility of changes in the status of receptors induced by chemotherapy, while not able to be completely ruled out, remains low. Molecular phenotype discordance between the primary and metastatic lesions can be found in BC patients with local metastasis [8]; this may be related to the heterogeneity of BC. BC is a highly heterogeneous malignant tumor [11], and the discovery of BC stem cells (BCSCs) provides an important evidence for its heterogeneity [12]. As reported, CD44 and ALDH1 are mature markers used for the identification of BCSCs [13,14]. These two markers were positively expressed in both primary and metastatic lesions in this case, so we considered the tumor from this patient may have originated from BCSCs. It has been shown that CD24 may promote invasion and metastasis of BC, and has been related to poor patient prognosis [15]. The patient in this case showed high expression of CD24 in both primary and metastatic lesions, and experienced local metastasis in the first diagnosis which rapidly progressed during the treatment stage. In view of the production of subclones by BCSCs with different phenotypes [16], we considered that the primary tumor may be composed of a mixture of subclones with Lumina B, HER2-overexpressed tumor cells and triple-negative tumor cells. Subclones with Lumina B and HER2-overexpressed tumor cells were superior in number in the primary tumor and were easily detectable by a single biopsy; however, the subclones of the triple-negative subtype were relatively small, difficult to detect, but more likely to metastasize due to high malignancy. The patient’s subsequent tumor evolution, including lung metastasis with a pathological triple-negative feature, supports this notion. In reviewing the expression pattern of the BCSC markers, it was discovered that both CD44 and ALDH1 showed high expression in the metastatic subclavian lesion than primary lesion. Consistent with the patient’s results, both biomarkers have been shown to enhance invasiveness and promote metastasis of BC cells [17-19]. Thus, the tumor’s biological characteristics and the molecular phenotype discordance suggested that the early metastasis likely originated from the small proportion of triple-negative subclones in the primary lesion, and that metastasis may be associated with the increased expression of CD44 and ALDH1. Interestingly, we found that there were differences in the subcellular localization of ALDH1 in the primary and metastatic lesions. ALDH1 was expressed in both nucleus and cytoplasm of primary tumor cells, but predominantly expressed in the cytoplasm of metastatic tumor cells. The mechanism and significance behind this phenomenon remain to be elucidated.
During the care of this BC patient, we consecutively evaluated the primary and metastatic lesions and then switched her to an effective treatment regimen, which prolonged her life. We believe that had biopsies of both the primary and synchronous metastatic lesions been performed concurrently at the beginning of the diagnosis the treatment strategies would have been drastically changed. The patient would have had less exposure to ineffective treatments, experienced a better clinical outcome and ultimately, her tumor progression to lung metastasis may have been delayed or avoided altogether. Coincidentally, a Korean BC patient was also reported to have benefited from the use of concurrent biopsies [20]. Therefore, this case report emphasizes that concurrent biopsies of the primary and metastatic lesions in BC patients with synchronous metastasis are vital for the overall treatment at the first diagnosis. For patients with discordance in the tumor biomarkers, we recommend making treatment decisions based on the receptor status of the more aggressive metastatic lesion. We have shown that determining anti-cancer treatment on the basis of receptor status change can affect the disease outcome.
In summary, regarding the concept of individualized therapy based on molecular phenotypes for BC patients, a single biopsy of the primary lesion may not fully reflect the whole tumor biology and cannot meet the patient’s needs for diagnosis and treatment. The approach of obtaining concurrent biopsies of both primary and synchronous metastatic lesions remains beneficial for revealing the molecular pathological features of BC, which is crucial to decision making and predicting prognosis based on the molecular heterogeneity in BC. When significant discordance exists in the tumor biomarkers between primary and synchronous metastatic lesions, we suggest that treatment be preferentially focused on the basis of receptor status of the aggressive metastatic lesion. This may affect the clinical decisions and provide a better outcome for the patient.
Acknowledgements
This work was supported by the grant from Bengbu Medical College Key Project of Translational Medicine (No. BYTM2019009), and the internal grants from both Leading New Technology Project (No. 2018126) and Science Fund for Distinguished Young Scholars (No. 2019byyfyjq02) of the First Affiliated Hospital of Bengbu Medical College. Written informed consent was obtained from the family member of the patient for publication of this manuscript and accompanying images.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM, Goss PE. Breast cancer in China. Lancet Oncol. 2014;15:e279–289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 3.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Li N, Li X, Lei L, Wang X. The prognostic impact of hormonal receptor and HER-2 expression discordance in metastatic breast cancer patients. Onco Targets Ther. 2020;13:853–863. doi: 10.2147/OTT.S231493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCCN. Clinical Practice Guidelines in Oncology. Breast Cancer, Version 1. 2020. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed February 2, 2020.
- 6.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, Harbeck N, Aguilar Lopez B, Barrios CH, Bergh J, Biganzoli L, Boers-Doets CB, Cardoso MJ, Carey LA, Cortés J, Curigliano G, Diéras V, El Saghir NS, Eniu A, Fallowfield L, Francis PA, Gelmon K, Johnston SRD, Kaufman B, Koppikar S, Krop IE, Mayer M, Nakigudde G, Offersen BV, Ohno S, Pagani O, Paluch-Shimon S, Penault-Llorca F, Prat A, Rugo HS, Sledge GW, Spence D, Thomssen C, Vorobiof DA, Xu B, Norton L, Winer EP. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E ESMO Guidelines Working Group. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii11–19. doi: 10.1093/annonc/mds232. [DOI] [PubMed] [Google Scholar]
- 8.Rossi S, Basso M, Strippoli A, Dadduzio V, Cerchiaro E, Barile R, D’Argento E, Cassano A, Schinzari G, Barone C. Hormone receptor status and HER2 expression in primary breast cancer compared with synchronous axillary metastases or recurrent metastatic disease. Clin Breast Cancer. 2015;15:307–312. doi: 10.1016/j.clbc.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Gahlaut R, Bennett A, Fatayer H, Dall BJ, Sharma N, Velikova G, Perren T, Dodwell D, Lansdown M, Shaaban AM. Effect of neoadjuvant chemotherapy on breast cancer phenotype, ER/PR and HER2 expression-implications for the practising oncologist. Eur J Cancer. 2016;60:40–48. doi: 10.1016/j.ejca.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Niikura N, Tomotaki A, Miyata H, Iwamoto T, Kawai M, Anan K, Hayashi N, Aogi K, Ishida T, Masuoka H, Iijima K, Masuda S, Tsugawa K, Kinoshita T, Nakamura S, Tokuda Y. Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann Oncol. 2016;27:480–487. doi: 10.1093/annonc/mdv611. [DOI] [PubMed] [Google Scholar]
- 11.Ellsworth RE, Blackburn HL, Shriver CD, Soon-Shiong P, Ellsworth DL. Molecular heterogeneity in breast cancer: state of the science and implications for patient care. Semin Cell Dev Biol. 2017;64:65–72. doi: 10.1016/j.semcdb.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Dick JE. Breast cancer stem cells revealed. Proc Natl Acad Sci U S A. 2003;100:3547–3549. doi: 10.1073/pnas.0830967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali HR, Dawson SJ, Blows FM, Provenzano E, Pharoah PD, Caldas C. Cancer stem cell markers in breast cancer: pathological, clinical and prognostic significance. Breast Cancer Res. 2011;13:R118. doi: 10.1186/bcr3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulenburg A, Blatt K, Cernyreiterer S, Sadovnik I, Herrmann H, Marian B, Grunt TW, Zielinski CC, Valent P. Cancer stem cells in basic science and in translational oncology: can we translate into clinical application? J Hematol Oncol. 2015;8:16. doi: 10.1186/s13045-015-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon MJ, Han J, Seo JH, Song K, Jeong HM, Choi JS, Kim YJ, Lee SH, Choi YL, Shin YK. CD24 overexpression is associated with poor prognosis in luminal A and triple-negative breast cancer. PLoS One. 2015;10:e0139112. doi: 10.1371/journal.pone.0139112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22:457–472. doi: 10.1038/cr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery N, Hill A, Mcfarlane S, Neisen J, O’Grady A, Conlon S, Jirstrom K, Kay EW, Waugh DJ. CD44 enhances invasion of basal-like breast cancer cells by upregulating serine protease and collagen-degrading enzymatic expression and activity. Breast Cancer Res. 2012;14:R84. doi: 10.1186/bcr3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alamgeer M, Ganju V, Kumar B, Fox J, Hart S, White M, Harris M, Stuckey J, Prodanovic Z, Schneider-Kolsky ME, Watkins DN. Changes in aldehyde dehydrogenase-1 expression during neoadjuvant chemotherapy predict outcome in locally advanced breast cancer. Breast Cancer Res. 2014;16:R44. doi: 10.1186/bcr3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Zuo X, Xie K, Wei D. The role of CD44 and cancer stem cells. Methods Mol Biol. 2018;1692:31–42. doi: 10.1007/978-1-4939-7401-6_3. [DOI] [PubMed] [Google Scholar]
- 20.Hahm MH, Kim HJ, Shin KM, Cho SH, Park JY, Jung JH, Jeong JY, Bae JH. Concurrent invasive ductal carcinoma of the breast and malignant follicular lymphoma, initially suspected to be metastatic breast cancer: a case report. J Breast Cancer. 2014;17:91–97. doi: 10.4048/jbc.2014.17.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.