Abstract
Aim: The objective of this study was to examine the clinical and biological significance of CD44 expression in conversion hepatectomy for initially unresectable colorectal liver metastases. Methods: Fifty-four patients who received chemotherapy followed by hepatectomy (conversion hepatectomy) for initially unresectable liver metastases were enrolled. CD44 expression and its clinical significance were examined in 52 resected specimens; two specimens revealed no residual cancer cells. The biological significance of CD44 expression in the chemoresistance response to fluorouracil, oxaliplatin or irinotecan, three major anti-cancer agents for colon cancer in the clinical setting, was examined using colon cancer cell lines. Results: Membrane CD44 expression in the residual cancer cells after chemotherapy for colorectal liver metastases was detectable in 19 patients (37%), and was significantly associated with high proliferative activity represented by Ki-67 expression (P = 0.003). CD44 expression was also significantly associated with shorter disease-free survival and worse overall survival after hepatectomy (hazard ratio and P-values were 2.570, 0.007 and 3.457, 0.026, respectively). In SW480 and HT29 colon cancer cells, siRNA-mediated CD44 knockdown attenuated cell growth. Additionally, CD44 knockdown overcame chemoresistance in response to fluorouracil and oxaliplatin with enhanced apoptosis and p27 upregulation, respectively. For irinotecan, CD44 knockdown showed no additional effect in chemoresistance. Conclusions: CD44 enhances chemoresistance in response to anti-cancer drugs (fluorouracil and oxaliplatin) in colon cancer cells. CD44 expression in liver metastases after chemotherapy implies the presence of occult micrometastases and is a worse prognostic factor in patients with conversion hepatectomy for initially unresectable colorectal liver metastases.
Keywords: CD44, cancer stem cell marker, chemoresistance, colorectal liver metastasis, conversion therapy
Introduction
Colorectal cancer (CRC) is the second most common malignant neoplasia worldwide, and approximately 50% of colon cancer patients will be diagnosed with hepatic metastases either at the time of initial presentation or as a result of disease recurrence [1]. The development of oxaliplatin- or irinotecan-based systemic chemotherapies with or without molecularly targeted agents has dramatically improved the tumor response rates and prognostic outcomes in patients with liver metastases from CRC [2-6]. Highly effective chemotherapy regimens enable subsequent complete hepatic resection even for initially unresectable liver metastasis from CRC, which is termed conversion hepatectomy (or conversion therapy). Conversion hepatectomy after oxaliplatin- or irinotecan-based systemic chemotherapy with molecular targets for initially unresectable liver metastasis has been achieved in approximately 12-49% of patients [5,7-9]. Conversion hepatectomy can produce favorable prognostic outcomes relative to patients with initially unresectable liver metastases from CRC [10]. Although conversion hepatectomy is successful, disease relapse is high and occurs in approximately two-thirds of patients [1,10]. These findings emphasize that most patients still harbor biologically active micrometastases with chemoresistance (so-called occult micrometastasis) even after effective chemotherapy and conversion hepatectomy. Identifying patients with a high risk of disease recurrence after conversion hepatectomy and understanding the biological mechanism of chemoresistance in their residual cancer cells may lead to the development of novel therapeutic strategies.
CD44, a major adhesion molecule of the extracellular matrix, has been implicated in a wide variety of physiological processes, including leukocyte homing and activation, wound healing, and cell migration [9,10]. Cells produce CD44 protein isoforms through the process of alternative mRNA splicing. The CD44 standard isoform (CD44s) is expressed predominantly in hematopoietic cells and normal epithelial cell subsets, whereas the variant isoform (CD44v) is expressed by some epithelial cells during embryonic development, during lymphocyte maturation and activation, and by several types of carcinoma cells. Recently, cancer stem cells in many tumors have been identified by their positive expression of CD44, either individually or in combination with other markers, and these cells have been shown to be involved in tumor progression, metastasis, and chemoresistance [10-16]. The present study set out to investigate the clinical impact and biological significance of CD44 expression in patients undergoing conversion hepatectomy for initially unresectable colorectal liver metastases.
Materials and methods
The present study was designed to assess the prognostic value of CD44 expression levels in patients receiving oxaliplatin- or irinotecan-based systemic chemotherapy followed by hepatectomy for liver-confined metastases from CRC. Patients were retrospectively enrolled in this study based on the following inclusion criteria: (1) initially unresectable or marginally unresectable colorectal metastases confined to the liver by radiological imaging assessment; and (2) oxaliplatin- or irinotecan-based systemic chemotherapy followed by hepatectomy. Patients who received systemic chemotherapy in other institutes were excluded because it was unclear whether their liver metastases were initially judged to be resectable or unresectable. In a previous study [10], we examined Ki-67 expression and its prognostic impact in patients who underwent oxaliplatin- or irinotecan-based systemic chemotherapy followed by hepatectomy for colorectal liver-confined metastases in the same setting, and we used a similar group in the present study. The details of patient selection were as follows. Between January 2005 and March 2013, among 261 consecutive patients who had been treated for colorectal liver metastases in the Department of Gastroenterological Surgery, Kumamoto University, 145 patients were treated for liver-confined metastases. Forty-two patients were eligible for straight hepatectomy without chemotherapy (initially resectable cases) (mean follow-up of 43 months after surgery). Two patients underwent local ablation therapy. Treatment with best supportive care was chosen for six patients. The remaining 95 patients received systemic chemotherapy because of initially unresectable colorectal liver-confined metastases. Eighty-six of these 95 patients were treated with oxaliplatin- or irinotecan-based chemotherapy with or without molecular targets. Among the remaining nine patients, seven were treated with other regimens (hepatic arterial infusion in four patients, oral fluoropyrimidine [S-1] in one patient, and S-1/leucovorin in two patients) and the two others were excluded from this study because they received systemic chemotherapy in other institutes. Of the 86 patients with oxaliplatin- or irinotecan-based chemotherapy, 54 who had been treated with chemotherapy followed by hepatectomy were enrolled in the present study. In 12 of these 54 patients, elective local ablation therapy was simultaneously used for unresectable liver metastases because of the small size and deep location of the tumor during hepatectomy [11]. The remaining 32 patients could not achieve hepatectomy because of ineffective chemotherapy. Most patients received adjuvant chemotherapy after hepatectomy, except for those who died postoperatively. After a mean follow-up of 33 months after surgery, 38 of 54 patients were alive (16 with no evidence of disease; 22 alive with disease recurrence), and 14 had died of disease recurrence. Two patients died of other disease within one year. The characteristics of these patients are listed in Table 1. Two of the 54 patients displayed no detectable residual cancer cells by hematoxylin and eosin staining in the resected liver specimens and were therefore not eligible for CD44 staining. This study was approved by the Human Ethics Review Committee of the Graduate School of Medicine, Kumamoto University (Kumamoto, Japan). Written informed consent regarding the use of biological specimens for investigational purposes was obtained from all patients.
Table 1.
Baseline characteristics of 54 patients who received conversion hepatectomy for initially unresectable colorectal liver metastases
| Age median (range), years | 65 (35-83) |
| Male, n (%) | 31 (58%) |
| Platelet count, median (range), × 104/μL | 19.1 (6.5-37.5) |
| Total bilirubin, median (range) mg/dL | 0.7 (0.3-1.3) |
| Serum albumin, median (range), g/L | 3.9 (3.0-4.4) |
| Prothrombin activity (%) | 108 (54-137) |
| Serum CEA level, ng/mL | 6.1 (1.0-172.6) |
| Size of largest tumor, median (range), mm | 25.5 (5-160) |
| Solitary tumor, n (%) | 15 (28%) |
| Tumor number, median (range) | 2.5 (1-19) |
| Synchronous/Metachronous | 34/19 |
| Molecular targets (present), n (%) | 27 (50%) |
| Chemotherapy cycles | 6 (2-38) |
| RECIST CR/PR/SD/PD | 2/32/15/5 |
CEA, carcinoembryonic antigen; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Immunohistochemical staining
Sample processing and immunohistochemical procedures were performed as described in previous reports [12,13]. Formalin-fixed, paraffin-embedded blocks of colorectal liver metastases were cut into 3-µm sections, which were autoclave-pretreated in Histofine antigen retrieval solution (pH 9) (Nichirei). Endogenous tissue peroxidase activity was blocked using 3% hydrogen peroxide, and the sections were then incubated with diluted primary antibodies. A subsequent reaction was performed with a biotin-free horseradish peroxidase enzyme-labeled polymer from the EnVision+ detection system (Dako). A positive reaction was visualized with the addition of diaminobenzidine solution, which was followed by counterstaining with Mayer’s hematoxylin. Primary mouse monoclonal antibodies (mAbs) against CD44 (1:300 dilution; Bender MedSystems) and Ki-67 (1:100 dilution; Dako) were used for this study. Negative controls for immunostaining were prepared by omitting the primary antibody. Two investigators (Y. H. and T. H.) independently scored all of the immunohistochemical staining results. The presence of membrane CD44 staining was defined as a positive result. In the assessment for Ki-67 expression level, five random visual high-power fields per lesion were evaluated to determine the highest number of Ki-67-positive nuclei as previously reported [10]. For example, in cases with multiple tumors, at least two or three tumors were chosen in order of size to evaluate and determine Ki-67 expression level. The samples were divided into Ki-67-positive expression (> 30%) and Ki-67-negative expression (≤ 30%) groups.
Cell lines, culture conditions, and reagents
The human colon cancer lines SW620, SW480, Lovo, HCT15, COLO201, COLO320, DLD1, COLO205, and HT29 were purchased from the Japanese Collection of Research Bioresources. The cells were routinely maintained in Dulbecco’s Modified Eagle’s Medium (Invitrogen) supplemented with 10% FBS (Invitrogen). The cells were incubated at 37°C in a 5% CO2 air-humidified atmosphere.
Real-time PCR and siRNA transfection
Total RNA extraction, cDNA synthesis, and quantitative reverse transcription PCR (qRT-PCR) were carried out as previously described [12-14]. Total cellular RNA was extracted using the RNeasy Mini Kit (Qiagen), and cDNA was synthesized with the SuperScript III Transcriptor First Strand cDNA Synthesis System for RT-PCR (Invitrogen) according to the manufacturers’ instructions. qRT-PCR was carried out using a LightCycler 480 II instrument (Roche), with primers designed using the Universal Probe Library (Roche) following the manufacturer’s recommendations. Relative mRNA levels were normalized to the level of β-Actin. The following primers were used: CD44 forward, 5’-GACACCATGGACAAGTTTTGG-3’; CD44 reverse, 5’-CGGCAGGTTATATTCAAATCG-3’; β-Actin forward, 5’-ATTGGCAATGAGCGGTTC-3’; β-Actin reverse, 5’-CGTGGATGCCACAGGACT-3’. Colon cancer cells (SW480 with standard CD44 expression and HT29 with variant CD44 expression) were used. Expression of both standard and variant CD44 was transiently downregulated using a predesigned siRNA duplex directed against CD44, and a nontargeting siRNA was used as a negative control. The sequences of the siRNA (chimeric RNA-DNA) duplexes (Japan Bioservice) were as follows [14]: CD44 siRNA, 5’-AAAUGGUCGCUACAGCAUCTT-3’ and 5’-GAUGCUGUAGCGACCAUUUTT-3’, and control siRNA, 5’-CGUACGCGGAAUACUUCGATT-3’ and 5’-UCGAAGUAUUCCGCGUACGTT-3’. Colon cancer cells were transfected with the annealed siRNA for 24 h using Lipofectamine 2000.
Protein extraction and western blot analysis
Protein extraction from cultivated cells and western blot analyses were carried out as previously described [13,14]. Briefly, the cells were lysed in cell lysis buffer containing 25 mM Tris (pH 7.4), 100 mmol/L NaCl, and 1% Tween 20. Equal amounts of proteins were loaded onto 10% gels and separated using SDS-PAGE. The resolved proteins were electrophoretically transferred to polyvinylidene fluoride membranes (Bio-Rad). The membranes were blocked with 5% low-fat dry milk in TBS-T [25 mmol/L Tris (pH 7.4), 125 mmol/L, NaCl, 0.4% Tween 20] for 1 h at room temperature, followed by incubation with a primary antibody at 4°C overnight. The blots were extensively washed with TBS-T and incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody diluted 1:2,000 in TBS-T for 1 h at room temperature. The membranes were washed and visualized using a Chemiluminescent Detection Reagent Kit (ECL; GE Healthcare). Primary antibodies for CD44s (1:1,000 dilution; Bender MedSystems), cleaved-caspase 3 (1:500 dilution; Cell Signaling Technology), cleaved PARP (1:1,000 dilution; Cell Signaling Technology), DPD (1:1,000 dilution; IBL), ERCC1 (1:500 dilution; Santa Cruz Biotechnology), p21 (1:200 dilution; Santa Cruz Biotechnology), p27 (1:1000 dilution; BD Bioscience), and β-actin (1:1,000 dilution; Cell Signaling Technology) were used for this study.
Statistical analyses
Statistical analyses were performed using a commercial statistical software package (SPSS for Windows, version 26.0; SPSS). Continuous values were evaluated using the Mann-Whitney U-test. Categorical variables were compared using the χ2 test. Overall survival and disease-free survival were calculated using the Kaplan-Meier method and were compared using a log-rank test. A P-value of less than 0.05 (two-tailed t-test) was considered statistically significant.
Results
Clinical significance of CD44 expression in patients who received conversion hepatectomy for initially unresectable liver metastases
CD44 is known as a cancer stem cell marker and plays a crucial role in cancer aggressiveness and chemoresistance. We performed immunohistochemical staining for CD44 in the resected liver metastases after systemic chemotherapy to assess the clinical significance of CD44 expression in patients who underwent chemotherapy followed by hepatectomy. Of 52 patients, membrane CD44 expression in the residual cancer cells after chemotherapy for liver metastases was detectable in 19 patients (37%) (Figure 1A), whereas the other 33 patients (63%) were CD44-negative (Figure 1B; Table 2). CD44 expression did not show significant association with the residual tumor size prior to conversion hepatectomy, or with RECIST PR (Table 2). Interestingly, CD44 expression was significantly associated with Ki-67 expression in the residual cancer cells (Table 2). This finding suggests that CD44-positive cancer cells are highly proliferative even after chemotherapy (Figure 1C). Furthermore, we investigated the prognostic implications of the biological activity represented by CD44-positive cancer cells. In survival curve analyses, CD44-positive expression in liver metastasis was significantly associated with a shorter disease-free survival compared to CD44-negative expression (P < 0.01) (Figure 1D). More patients with CD44-positive expression displayed early recurrence within one year after hepatectomy (25 of 33 patients) than in the CD44-negative expression group (three of 19 patients) (76% vs. 16%). Additionally, patients with CD44-positive expression in liver metastasis had significantly worse overall survival than those with CD44-negative expression (P = 0.02) (Figure 1E). In Cox regression analyses, moreover, CD44-positive expression in the residual cancer cells was significantly associated with shorter disease-free survival [hazard ratio [HR] 2.570, 95% confidence interval (CI) 1.293-5.111] and worse overall survival [HR 3.457, 95% CI 1.159-10.316] (Table 3). On the other hand, as a control, we examined CD44 expression of resected liver metastases without systemic chemotherapy (straight hepatectomy group) in 42 patients. In this group, positive membrane CD44 expression was detectable in 24 patients (57%), whereas membrane CD44 expression was negative in 18 patients (43%). Interestingly, in contrast to patients undergoing conversion hepatectomy, there was no significant difference in disease-free survival and overall survival between positive and negative CD44 expression groups in patients with straight hepatectomy (P = 0.39 and 0.12, respectively) (Figure 1F and 1G). Thus, CD44 expression in the residual cancer cells after chemotherapy showed an association with high proliferative activity in the residual cancer cells rather than clinical effects in the disappeared cancer cells such as RECIST PR. These findings suggest that CD44 plays an important role in tumor aggressiveness and chemoresistance in the residual cancer cells after chemotherapy for liver metastases. CD44-positive expression after systemic chemotherapy implied occult micrometastases, and was a worse prognostic factor in patients with conversion hepatectomy for liver metastases, but not in patients with straight hepatectomy.
Figure 1.
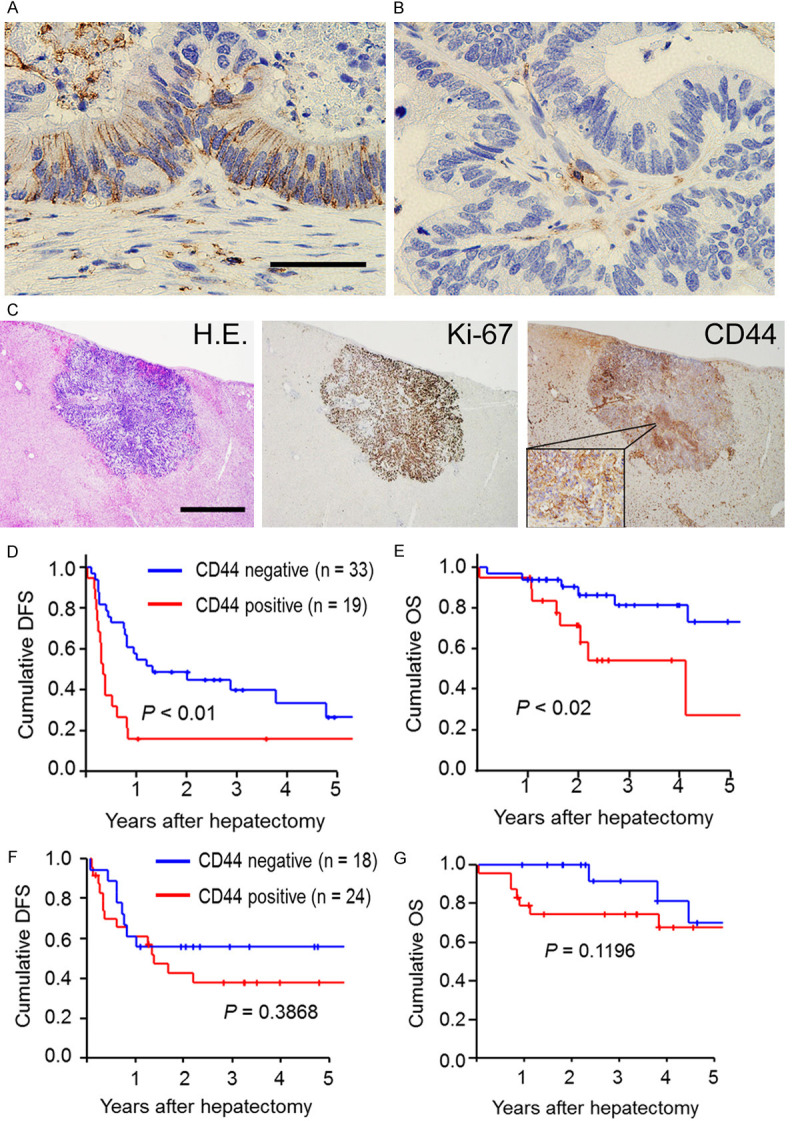
CD44 expression in residual cancer cells after chemotherapy for colorectal liver metastases and its clinical impacts on survival outcomes. (A) A representative picture of membranous CD44-positive expression (Scale bars, 50 µm). (B) A representative picture of membranous CD44-negative expression. (C) Pictures of CD44 and Ki-67 expression in the a residual micrometastasis after chemotherapy. Left) Hematoxylin-eosin staining (Scale bar, 1 mm). Middle) Ki-67 expression. Right) CD44 expression. (D and E) A Kaplan-Meier survival analysis of disease-free survival (D) and overall survival (E) in comparisons between positive and negative CD44 expression in patients who underwent conversion hepatectomy (chemotherapy and then hepatectomy) for initially unresectable colorectal liver metastases. The log-rank test was used. (F and G) A Kaplan-Meier survival analysis of disease-free survival (F) and overall survival (G) in comparisons between positive and negative CD44 expression in patients who underwent straight hepatectomy (without chemotherapy) for initially resectable colorectal liver metastases. The log-rank test was used.
Table 2.
Comparison of clinico-pathological factors between negative and positive CD44 expression in patients with conversion hepatectomy (n = 52)
| Variable | CD44- (n = 33) | CD44+ (n = 19) | P-value |
|---|---|---|---|
| Age | 67 (45-82) | 61 (35-83) | 0.203 |
| Sex (male/female) | 20/13 | 11/8 | 0.848 |
| Synchronous/Metachronous | 23/10 | 11/8 | 0.389 |
| Molecular targets (present/absent) | 13/20 | 13/6 | 0.044 |
| Tumor size prior to hepatectomy (mm) | 24 (5-160) | 35 (11-60) | 0.549 |
| Tumor number prior to hepatectomy | 2 (1-13) | 3 (1-19) | 0.250 |
| CEA prior to hepatectomy (ng/mL) | 6.3 (1.3-173) | 6.2 (1-161) | 0.768 |
| RECIST PR/SD or PD | 22/11 | 10/9 | 0.316 |
| Ki-67 in the residual cancer cells (negative/positive) | 17/16 | 2/17 | 0.003 |
CEA, carcinoembryonic antigen; PR, partial response; SD, stable disease; PD, progressive disease.
Table 3.
Prognostic analyses of clinico-pathological parameters in patients with conversion hepatectomy for initially unresectable colorectal liver metastases (n = 52)
| Parameter | HR | Exp (B) | P-value |
|---|---|---|---|
| Disease-free survival | |||
| Tumor size prior to hepatectomy > 3 cm | 1.882 | 0.975-3.632 | 0.060 |
| Tumor number prior to hepatectomy ≥ 3 | 1.525 | 0.789-2.945 | 0.209 |
| CEA prior to hepatectomy > 7 ng/mL | 2.795 | 1.388-5.631 | 0.004 |
| RECIST SD or PD | 1.674 | 0.849-3.302 | 0.137 |
| CD44-positive in the residual cancer cells | 2.570 | 1.293-5.111 | 0.007 |
| Overall survival | |||
| Tumor size prior to hepatectomy > 3 cm | 1.825 | 0.656-5.081 | 0.249 |
| Tumor number prior to hepatectomy ≥ 3 | 1.471 | 0.546-3.964 | 0.446 |
| CEA prior to hepatectomy > 7 ng/mL | 2.521 | 0.874-7.275 | 0.087 |
| RECIST SD or PD | 4.355 | 1.569-12.087 | 0.005 |
| CD44-positive in the residual cancer cells | 3.457 | 1.159-10.316 | 0.026 |
Cox regression analyses were used. CEA, carcinoembryonic antigen; SD, stable disease; PD, progressive disease.
Biological roles of CD44 expression in chemoresistance
We examined the biological impact of CD44 expression on chemoresistance using colon cancer cell lines. In nine colon cancer cell lines, CD44 expression levels were examined by western blotting (Figure 2A) (Supplementary Figure 1A). We found that SW480 and Lovo strongly expressed CD44s protein, whereas HT29 expressed CD44v protein. To assess the functional role of CD44s or CD44v expression, we downregulated CD44 expression by siRNA, which suppresses both standard and variant CD44 mRNA expression (Figure 2B). Downregulation of CD44 protein expression suppressed the growth activity both in SW480 cells (with CD44s expression) and in HT29 cells (with CD44v expression) (Figure 2C-F) (Supplementary Figure 1B and 1C). Next, we assessed the biological significance of CD44 expression in chemoresistance using SW480 cells (with CD44s expression) and HT29 cells (with CD44v expression). Downregulation of CD44s expression in SW480 cells and CD44v expression in HT29 cells attenuated cell survival under 5-FU treatment compared to controls (Figure 3A and 3B), and enhanced cleaved-PARP and -caspase 3 expression by western blot analysis (Figure 3C and 3D) (Supplementary Figure 2A). On the other hand, the expression level of DPD, an enzyme involved in the metabolism of 80-90% of 5-FU, was unaffected by the downregulation of CD44s or CD44v expression (Figure 3C and 3D) (Supplementary Figure 2A). These results indicated that standard and variant CD44 participated in chemoresistance against 5-FU in colon cancer cells, and that inhibition of standard or variant CD44 expression accelerated cancer cell death induced by 5-FU.
Figure 2.
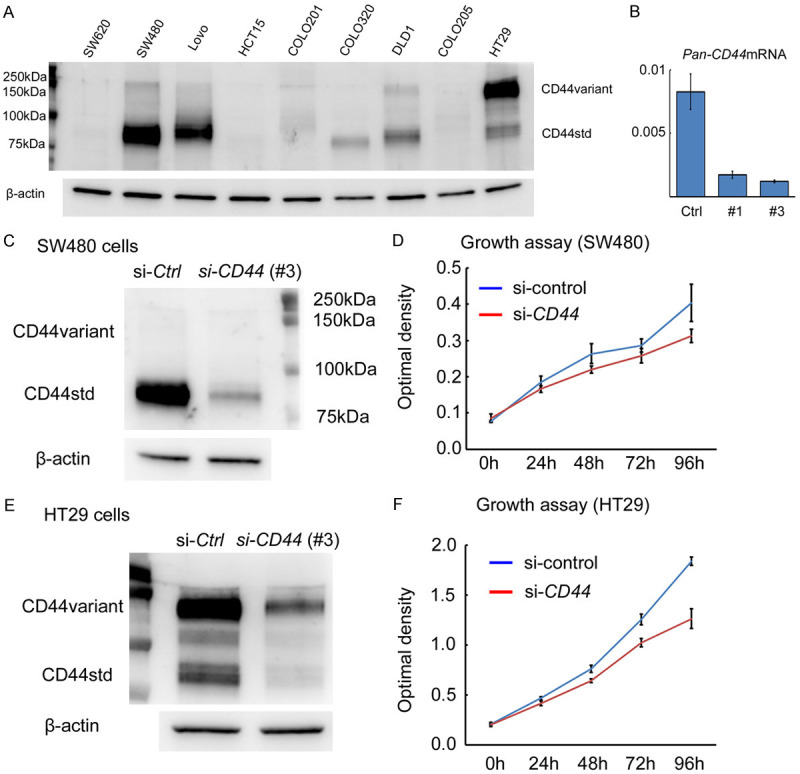
CD44 protein is expressed in colon cancer cell lines and its downregulation attenuates cell growth. A. CD44 protein expression in nine colon cancer cell lines by western blotting. SW480 and Lovo strongly expressed CD44s protein, whereas HT29 expressed CD44v protein. β-actin is used as an internal control. B. Downregulation of Pan-CD44 mRNA using siRNA in SW480 cells at 24 h. C. Downregulation of CD44 protein expression in SW480 cells (with CD44s protein) by siRNA at 48 h. D. Downregulation of CD44 expression inhibits cell growth in SW480 cells compared to control. E. Downregulation of CD44 protein expression in HT29 cells (with CD44v protein) by siRNA at 48 h. F. Downregulation of CD44 expression inhibits cell growth in HT29 cells compared to control.
Figure 3.
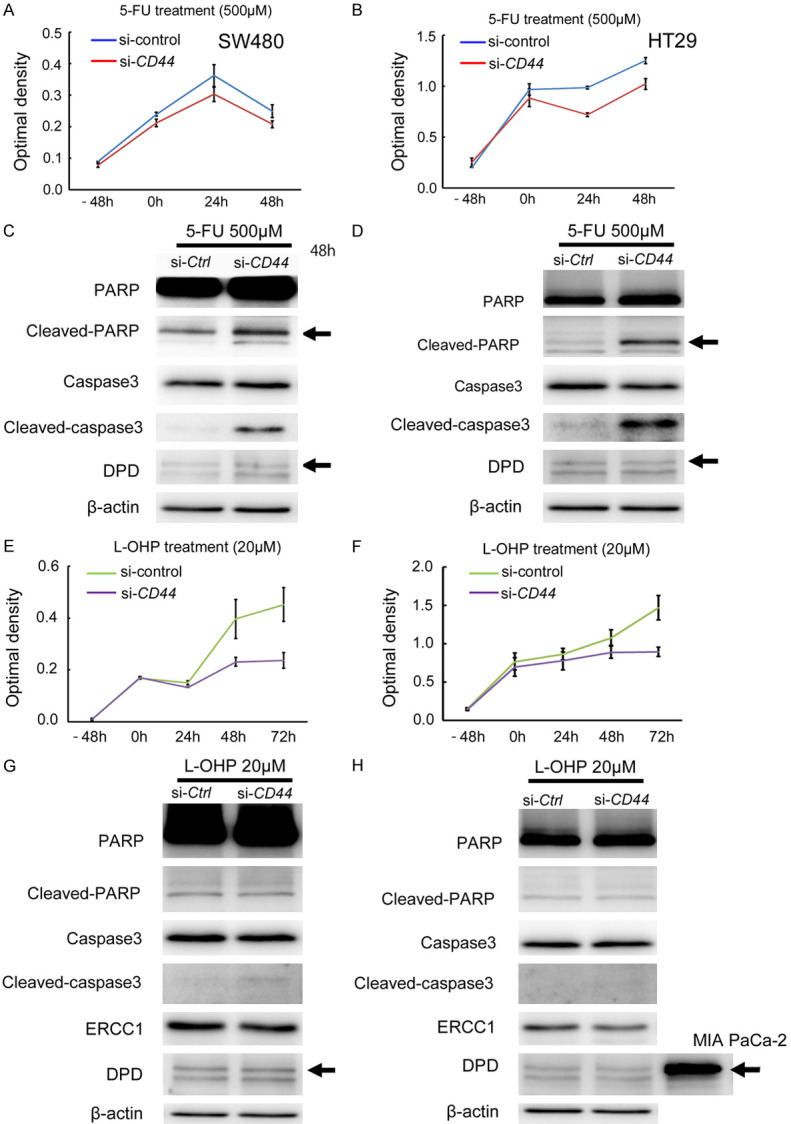
Functional role of CD44 expression in chemoresistance in colon cancer cells. A. Downregulation of CD44 expression inhibits cell growth under 5-FU treatment (500 μM) in SW480 cells (with CD44s protein). B. Downregulation of CD44 expression inhibits cell growth under 5-FU treatment (500 μM) in HT29 cells (with CD44v protein). C. Downregulation of CD44 expression enhances cleaved-PARP and cleaved-caspase 3 expression in SW480 cells under 5-FU treatment (500 μM) by western blot analysis at 48 h. β-actin is used as an internal control. D. Downregulation of CD44 expression enhances cleaved-PARP and cleaved-caspase 3 expression in HT29 cells under 5-FU treatment (500 μM) by western blot analysis at 48 h. β-actin is used as an internal control. E. Downregulation of CD44 expression inhibits cell growth under L-OHP treatment (20 μM) in SW480 cells (with CD44s protein). F. Downregulation of CD44 expression inhibits cell growth under L-OHP treatment (20 μM) in HT29 cells (with CD44v protein). G. Downregulation of CD44 expression does not affect protein expression levels of cleaved-PARP, cleaved-caspase 3, DPD, or ERCC1 in SW480 cells under L-OHP treatment (20 μM) by western blot analysis at 48 h. H. Downregulation of CD44 expression does not affect protein expression levels of cleaved-PARP, cleaved-caspase 3, DPD, or ERCC1 in HT29 cells under L-OHP treatment (20 μM) by western blot analysis at 48 h. MIA Paca-2 cells were used as a positive control for DPD expression.
Oxaliplatin (L-OHP) is another anti-cancer agent that is often used in colon cancer patients. In SW480 cells (with CD44s expression) and HT29 cells (with CD44v expression), downregulation of CD44s or CD44v expression suppressed cell growth under L-OHP treatment compared to controls (Figure 3E and 3F), but did not enhance cleaved-PARP and -caspase 3 expression in either cell line (Figure 3G and 3H) (Supplementary Figure 2A). The expression level of ERCC1, which is involved in the repair of oxaliplatin-induced DNA damage and is associated with L-OHP resistance, did not show any change following the downregulation of CD44s or CD44v expression (Figure 3G and 3H) (Supplementary Figure 2A). Although the downregulation of CD44 expression attenuated cell growth compared to controls under L-OHP, cell death represented by cleaved-PARP and -caspase 3 expression was not affected, unlike the treatment with 5-FU. To further assess the inhibitory effect on cell growth under L-OHP treatment by downregulation of CD44 expression, we examined cell cycle-related protein expression. In SW480 cells (with CD44s expression) and HT29 cells (with CD44v expression) treated with L-OHP, downregulation of CD44 expression upregulated p27 protein expression but not p21 expression, in both colon cancer cell lines (Figure 4) (Supplementary Figure 2B). The increased p27 protein expression upon downregulation of CD44 expression was undetectable under treatment with 5-FU. Additionally, we examined the biological impact of CD44 expression on chemoresistance under treatment with irinotecan, another anti-cancer agent often used in colon cancer patients (Figure 5). Interestingly, downregulation of CD44 expression did not affect chemoresistance under irinotecan treatment either in SW480 (with CD44s expression) or in HT29 cells (with CD44v expression), in contract to 5-FU and L-OHP.
Figure 4.
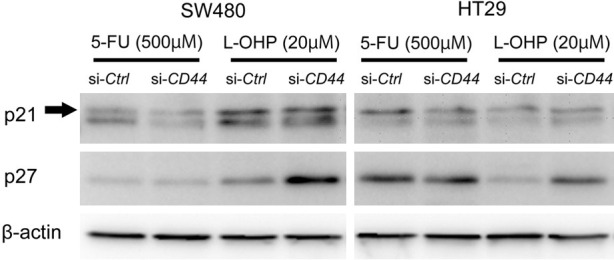
Downregulation of CD44 upregulates p27 protein, but not p21 protein, in colon cancer cells under oxaliplatin (L-OHP) treatment. β-actin is used as an internal control.
Figure 5.
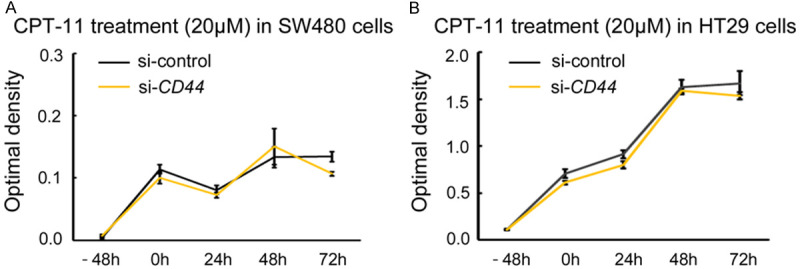
Downregulation of CD44 expression does not affect chemoresistance under irinotecan (CPT-11) treatment in colon cancer cell lines. A. Downregulation of CD44 expression does not affect cell growth under CPT-11 treatment (20 μM) in SW480 cells (with CD44s protein). B. Downregulation of CD44 expression does not affect cell growth under CPT-11 treatment (20 μM) in HT29 cells (with CD44v protein). β-actin is used as an internal control.
Collectively, these findings suggest that standard and variant CD44 expression enhance chemoresistance (anti-apoptotic activity under 5-FU treatment and pro-proliferative activity under L-OHP treatment), and CD44 may therefore be a therapeutic target in strategies to overcome chemoresistance to 5-FU and L-OHP in colon cancer cells.
Discussion
Conversion hepatectomy (chemotherapy followed by hepatectomy) provided significantly improved prognostic outcomes compared to patients with initially unresectable liver metastases in previous studies [15,16]. One remaining major concern is the high recurrence rate after conversion therapy. In the present study, there was no significant association between CD44 expression and tumor size or number, whereas CD44 expression displayed a significant association with biologically active cancer cells even after chemotherapy, as represented by their high Ki-67 expression. Furthermore, CD44 expression was a worse prognostic factor of recurrence and overall survival in patients with conversion hepatectomy for initially unresectable colorectal liver metastases.
CD44 has recently been proposed as a cancer stem cell marker in several cancers, including colon cancer, and its overexpression leads to high cancer aggressiveness and chemoresistance [14,17,18]. Some reports have indicated the mechanism underlying CD44-related chemoresistance. Cancer cells are often exposed to high levels of reactive oxygen species (ROS) during tumor progression and under treatment with anti-cancer drugs. The ability to avoid such exposure is required for cancer cell survival. The redox balance is maintained in cancer cells due to their marked antioxidant capacity using the glutathione antioxidant system. Expression of CD44 contributes to ROS defense through upregulation of glutathione [18,19]. CD44 ablation by siRNA increases metabolic flux to mitochondrial respiration and concomitantly inhibits entry into glycolysis and the pentose phosphate pathway in cancer cells [20]. Such metabolic changes reduce cellular glutathione and increase the intracellular level of ROS in glycolytic cancer cells, and thus enhance the effect of chemotherapeutic drugs.
Expression of the cancer stem cell marker CD44 in the residual liver tumor may be associated with aggressive properties even after chemotherapy for liver metastases. Although conversion hepatectomy improves the prognostic outcomes compared to patients with initially unresectable liver metastases, the high rate of relapse, which frequently occurs within one year after conversion therapy, is a major concern in the clinical setting. To develop a novel strategy for patients with a high risk of cancer relapse and death, molecular characterization is the logical step. In this study, CD44 expression in the residual cancer cells after chemotherapy was associated with highly proliferative cancer cells represented by high Ki-67 expression. These findings support the notion that radical hepatectomy can be a gold standard treatment option for colorectal liver metastases in the current clinical setting. Additionally, our findings suggest that CD44 expression in the resected liver metastasis is a biomarker of occult micrometastases after conversion hepatectomy. On the other hand, in contrast to conversion hepatectomy, CD44 expression did not show any significant association with the prognostic outcomes in patients with straight hepatectomy (without chemotherapy). These results suggest that CD44 plays a crucial role in chemoresistance in patients with colorectal liver metastases. CD44 could be a therapeutic target to enhance the chemosensitivity in patients with colorectal liver metastases. The use of a perioperative treatment targeting CD44 might therefore be able to abolish occult micrometastases in the body.
In conclusion, CD44 enhances chemoresistance in response to anti-cancer drugs (fluorouracil and oxaliplatin) in colon cancer cells. CD44 expression in the residual cancer cells after chemotherapy implies the presence of occult micrometastases and is a useful biomarker of high risk for early recurrence and death after conversion hepatectomy (chemotherapy and subsequent hepatectomy) for initially unresectable colorectal liver metastases.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.H.); and the Takeda Science Foundation, Japan (to H.H.).
Disclosure of conflict of interest
None.
Abbreviations
- CRC
colorectal cancer
- S-1
an oral fluoropyrimidine
- CEA
carcinoembryonic antigen
- L-OHP
oxaliplatin
- CPT-11
irinotecan
- ROS
reactive oxygen species
Supporting Information
References
- 1.Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer. 2002;38:1023–1033. doi: 10.1016/s0959-8049(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 2.Pozzo C, Basso M, Cassano A, Quirino M, Schinzari G, Trigila N, Vellone M, Giuliante F, Nuzzo G, Barone C. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–939. doi: 10.1093/annonc/mdh217. [DOI] [PubMed] [Google Scholar]
- 3.Kohne CH, van Cutsem E, Wils J, Bokemeyer C, El-Serafi M, Lutz MP, Lorenz M, Reichardt P, Ruckle-Lanz H, Frickhofen N, Fuchs R, Mergenthaler HG, Langenbuch T, Vanhoefer U, Rougier P, Voigtmann R, Muller L, Genicot B, Anak O, Nordlinger B. Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J. Clin. Oncol. 2005;23:4856–4865. doi: 10.1200/JCO.2005.05.546. [DOI] [PubMed] [Google Scholar]
- 4.Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, Levitt R, Rowland K, Nair S, Sargent DJ, Donohue JH. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a north central cancer treatment group phase II study. J. Clin. Oncol. 2005;23:9243–9249. doi: 10.1200/JCO.2005.07.740. [DOI] [PubMed] [Google Scholar]
- 5.Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, Konopke R, Stroszczynski C, Liersch T, Ockert D, Herrmann T, Goekkurt E, Parisi F, Kohne CH. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 6.Beppu T, Miyamoto Y, Sakamoto Y, Imai K, Nitta H, Hayashi H, Chikamoto A, Watanabe M, Ishiko T, Baba H. Chemotherapy and targeted therapy for patients with initially unresectable colorectal liver metastases, focusing on conversion hepatectomy and long-term survival. Ann Surg Oncol. 2014;21(Suppl 3):S405–13. doi: 10.1245/s10434-014-3577-x. [DOI] [PubMed] [Google Scholar]
- 7.Okines A, Puerto OD, Cunningham D, Chau I, Van Cutsem E, Saltz L, Cassidy J. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br J Cancer. 2009;101:1033–1038. doi: 10.1038/sj.bjc.6605259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohne CH, Lenz HJ. Chemotherapy with targeted agents for the treatment of metastatic colorectal cancer. Oncologist. 2009;14:478–488. doi: 10.1634/theoncologist.2008-0202. [DOI] [PubMed] [Google Scholar]
- 9.Masi G, Loupakis F, Pollina L, Vasile E, Cupini S, Ricci S, Brunetti IM, Ferraldeschi R, Naso G, Filipponi F, Pietrabissa A, Goletti O, Baldi G, Fornaro L, Andreuccetti M, Falcone A. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg. 2009;249:420–425. doi: 10.1097/SLA.0b013e31819a0486. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi H, Beppu T, Sakamoto Y, Miyamoto Y, Yokoyama N, Higashi T, Nitta H, Hashimoto D, Chikamoto A, Baba H. Prognostic value of Ki-67 expression in conversion therapy for colorectal liver-limited metastases. Am J Cancer Res. 2015;5:1225–1233. [PMC free article] [PubMed] [Google Scholar]
- 11.Mima K, Beppu T, Chikamoto A, Miyamoto Y, Nakagawa S, Kuroki H, Okabe H, Hayashi H, Sakamoto Y, Watanabe M, Kikuchi K, Baba H. Hepatic resection combined with radiofrequency ablation for initially unresectable colorectal liver metastases after effective chemotherapy is a safe procedure with a low incidence of local recurrence. Int J Clin Oncol. 2013;18:847–855. doi: 10.1007/s10147-012-0471-z. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi H, Sakai K, Baba H, Sakai T. Thrombospondin-1 is a novel negative regulator of liver regeneration after partial hepatectomy through transforming growth factor-beta1 activation in mice. Hepatology. 2012;55:1562–1573. doi: 10.1002/hep.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi H, Higashi T, Yokoyama N, Kaida T, Sakamoto K, Fukushima Y, Ishimoto T, Kuroki H, Nitta H, Hashimoto D, Chikamoto A, Oki E, Beppu T, Baba H. An imbalance in TAZ and YAP expression in hepatocellular carcinoma confers cancer stem cell-like behaviors contributing to disease progression. Cancer Res. 2015;75:4985–4997. doi: 10.1158/0008-5472.CAN-15-0291. [DOI] [PubMed] [Google Scholar]
- 14.Mima K, Okabe H, Ishimoto T, Hayashi H, Nakagawa S, Kuroki H, Watanabe M, Beppu T, Tamada M, Nagano O, Saya H, Baba H. CD44s regulates the TGF-beta-mediated mesenchymal phenotype and is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res. 2012;72:3414–3423. doi: 10.1158/0008-5472.CAN-12-0299. [DOI] [PubMed] [Google Scholar]
- 15.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghemard O, Levi F, Bismuth H. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. discussion 657-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adam R, Wicherts DA, de Haas RJ, Ciacio O, Levi F, Paule B, Ducreux M, Azoulay D, Bismuth H, Castaing D. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J. Clin. Oncol. 2009;27:1829–1835. doi: 10.1200/JCO.2008.19.9273. [DOI] [PubMed] [Google Scholar]
- 17.Bates RC, Edwards NS, Burns GF, Fisher DE. A CD44 survival pathway triggers chemoresistance via lyn kinase and phosphoinositide 3-kinase/Akt in colon carcinoma cells. Cancer Res. 2001;61:5275–5283. [PubMed] [Google Scholar]
- 18.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Nagano O, Okazaki S, Saya H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene. 2013;32:5191–5198. doi: 10.1038/onc.2012.638. [DOI] [PubMed] [Google Scholar]
- 20.Tamada M, Nagano O, Tateyama S, Ohmura M, Yae T, Ishimoto T, Sugihara E, Onishi N, Yamamoto T, Yanagawa H, Suematsu M, Saya H. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res. 2012;72:1438–1448. doi: 10.1158/0008-5472.CAN-11-3024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


