Abstract
Background
Brain metastases (BM) are a frequent complication of advanced cancer and are characterized by a variety of neurological symptoms. Although the presence of neurological symptoms is included in the response assessment in patients with primary brain tumors, to the authors' knowledge little is known regarding the prognostic impact of neurological symptoms in patients with BM.
Methods
Patients with newly diagnosed BM from non–small cell lung cancer were identified from the Vienna Brain Metastasis Registry and were evaluated according to the incidence, distribution, and prognostic impact of neurological symptoms at the time of diagnosis of BM.
Results
A total of 1608 patients (57.3% male and 42.7% female; median age, 62 years) were available for further analyses. Neurological symptoms including focal deficits (985 patients; 61.3%), signs of increased intracranial pressure (483 patients; 30.0%), epileptic seizures (224 patients; 13.9%), and neuropsychological symptoms (233 patients; 14.5%) were documented in 1186 of the 1608 patients (73.8%). Patients with asymptomatic BM presented with a longer median overall survival after the diagnosis of BM compared with patients with symptomatic BM (11 months vs 7 months; P < .001). In multivariate analysis with a diagnosis‐specific graded prognostic assessment (hazard ratio, 1.41; 95% CI, 1.33‐1.50 [P < .001]), the presence of neurological symptoms (hazard ratio, 1.39; 95% CI, 1.23‐1.57 [P < .001]) was found to be independently associated with survival prognosis from the time of diagnosis of BM.
Conclusions
Neurological symptoms at the time of BM diagnosis demonstrated a strong and independent association with survival prognosis. The results of the current study have highlighted the need for the integration of the presence of neurological symptoms into the prognostic assessment of patients with BM from non–small cell lung cancer.
Lay Summary
Neurological symptom evaluation is included regularly in the assessment of patients with primary brain tumors. However, to the authors' knowledge, little is known regarding the prognostic impact in patients with newly diagnosed brain metastases (BM).
The current study has provided a detailed clinical characterization of the incidence, distribution, and prognostic impact of neurological symptoms in a large, real‐life cohort of patients with BM from non–small cell lung cancer.
In this cohort, neurological symptoms at the time of diagnosis of BM demonstrated a strong, independent prognostic impact on the survival prognosis.
The results of the current study have highlighted the need for the integration of neurological symptom burden into the prognostic assessment of patients with BM from non–small cell lung cancer.
Keywords: neurological assessment, neurological symptoms, prognostic factors in brain metastases, survival prognosis in symptomatic brain metastases, symptomatic burden
Short abstract
Neurological symptoms at the time of diagnosis of brain metastases demonstrate a strong, independent prognostic impact on prognosis. The current study highlights the integration of neurological symptom burden into the prognostic assessment of patients with brain metastases from non–small cell lung cancer.
Introduction
Brain metastases (BM) are associated with a wide range of neurological symptoms, including signs of increased intracranial pressure as well as focal deficits such as vertigo, neurocognitive impairment, seizures, dysphasia, motor weakness, and ataxia. 1 , 2 , 3 Historical series have indicated that up to 80% of patients present with considerable symptoms, even in the presence of only a single BM. In patients with primary brain tumors, the symptomatic burden is measured using the Neurologic Assessment in Neuro‐Oncology (NANO) scale and regularly is included in the Response Assessment in Neuro‐Oncology (RANO) guidelines for primary brain tumors. 4
However, in patients with BM, the prognostic assessment and the resulting treatment decisions rely mainly on clinical factors, including the histology of the primary tumor, the number and size of BM, the presence of the primary tumor, and the age of the patient. 5 Several recent clinical trials specifically included patients with asymptomatic BM from non–small cell lung cancer (NSCLC) and demonstrated the promising clinical activity of targeted therapies also within the context of BM. 6 , 7 , 8 , 9 , 10 Indeed, a risk‐stratified treatment approach is of particular importance in the population of patients with BM because survival ranges from a few weeks to several months and even years. 11 Consequently, not only efficacy but also short‐term as well as long‐term toxicities need to be considered in treatment planning. 12 , 13 Therefore, further insight into the incidence and clinical impact of symptomatic burden in patients with BM is needed to guide the further development of clinical trials in this population of particular high clinical need. 12 To our knowledge, no systematic evaluation of neurological symptoms in newly diagnosed patients with BM from NSCLC with regard to the prognostic impact has been performed to date. In the current study, we took advantage of the Vienna Brain Metastasis Registry, allowing for the evaluation of neurological symptoms in a real‐life cohort of patients with NSCLC BM who were recruited over a period of over 30 years. 11
Materials and Methods
Patients
The medical files of patients who were treated for newly diagnosed NSCLC BM between 1986 and 2019 at the Medical University of Vienna were retrieved from the Vienna Brain Metastasis Registry. 11 All clinical characteristics were evaluated retrospectively by chart review. Written results from computed tomography (CT) or magnetic resonance imaging were used for the initial diagnosis as well restaging of BM. If both imaging procedures were available, magnetic resonance imaging results were preferred for intracranial staging. If no written reports were available, the localizations and sizes of the BM were considered as not precisely given and therefore recorded as “missing.” The diagnosis‐specific graded prognostic assessment (DS‐GPA) for lung cancer was calculated based on clinical characteristics as published previously, including age, number of BM, status of extracranial disease, and Karnofsky performance status. 14 Synchronous diagnosis was defined as the diagnosis of the primary tumor and BM within 30 days. Extracranial and intracranial restaging was performed routinely every 2 to 4 months from the time of diagnosis of the primary tumor until death or time of last follow‐up. Disease status at the end of life was evaluated based on the last available complete restaging including extracranial as well as intracranial imaging within a time period of 60 days before death. Death due to intracranial disease progression was indicated in the absence of extracranial disease progression but an increase in the intracranial tumor burden. In contrast, death due to extracranial disease progression was defined in the absence of intracranial disease progression but an increase in extracranial tumor burden at the time of the last restaging. Patient data were collected in a password‐secured database and handled anonymously. The study and the data collection were approved by the ethics committee of the Medical University of Vienna (vote 078/2004).
Evaluation of Neurological Symptoms
A clinical examination and history with regard to the neurological status of patients were performed routinely at the time of diagnosis of BM and documented in the patients' charts. Patients were categorized as symptomatic if any neurological symptom in relation to the diagnosed BM was documented in the patient file at the time of diagnosis of BM. Patients with neurological symptoms due to any other disease present before the diagnosis of BM were excluded from the analysis. Neurological symptoms were defined as the presence of either focal deficits, signs of increased intracranial pressure, epileptic seizures, or neuropsychological symptoms. Signs of increased cranial pressure were defined as the presence of ≥1 of the following: headache, nausea, or emesis as published previously. 15 , 16 , 17 Neuropsychological symptoms were defined as memory disorder, cognitive impairment, or organic brain disorder. In the event of memory problems or episodes of forgetfulness at the time of diagnosis of BM, symptoms were summarized as memory disorders. Cognitive impairment was defined in the event of slow thinking and processing of information at the time of BM diagnosis. In the event of hallucinations, delusions, personality changes, or delirium, symptoms were summarized as an organic brain disorder. Table 1 lists all evaluated neurological symptoms in detail. The patient was defined as asymptomatic in the event of absent neurological symptoms at the time of diagnosis of BM. BM diagnosis through screening and/or staging was defined as BM diagnosis in an asymptomatic patient.
TABLE 1.
Neurological Symptoms Evaluated at the Time of BM Diagnosis
| Focal deficits |
| Ataxia |
| Vertigo |
| Motor disorders |
| Paresis of one extremity |
| Hemiparesis |
| Facial nerve palsy |
| Cranial nerve palsy |
| Hypesthesia |
| Aphasia |
| Signs of increased intracranial pressure |
| Headache |
| Nausea and emesis |
| Epileptic seizures |
| Generalized seizures |
| Focal seizures |
| Neuropsychological symptoms |
| Memory disorder |
| Cognitive impairment |
| Organic brain disorder |
Abbreviation: BM, brain metastases.
Statistical Analysis
Overall survival (OS) from the time of diagnosis of the primary tumor was defined as time from the initial histological diagnosis of the primary tumor to death or last follow‐up. OS from the time of BM diagnosis was defined as the time from the initial radiological diagnosis of BM to death or last follow‐up. Brain metastatic‐free survival was defined as the time from the diagnosis of the primary tumor to the radiological diagnosis of BM. Patients with a synchronous diagnosis (within 30 days) of primary tumor and BM were excluded from analysis investigating the brain metastatic‐free survival period. The Kaplan‐Meier product‐limit method was used to estimate OS. To estimate OS differences between groups, the log‐rank test was used. Parameters according to neurological symptoms with a statistically significant association with survival prognosis in the univariate analysis were included separately in a multivariate analysis with the DS‐GPA using the Cox proportional hazards model. A 2‐tailed P value ≤.05 was considered to indicate statistical significance. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 23.0 software.
Results
Patient Characteristics
A total of 1608 patients (922 males [57.3%] and 686 females [42.7%]) with newly diagnosed BM from NSCLC were available for further analysis. The median age of the patients at the time of diagnosis of BM was 62 years (range, 27‐88 years). Of the 1608 patients, 875 (54.4%) presented with a synchronous diagnosis of BM and primary tumor, whereas 733 patients (45.6%) subsequently were diagnosed with BM. Of 529 patients (32.9%) with adenocarcinoma, information regarding ALK or EGFR mutational status was available. Of these 529 patients, 94 (17.8%) presented with EGFR‐mutated NSCLC and 23 (4.3%) presented with ALK‐mutated NSCLC. The median BM‐free survival from the time of diagnosis of the primary tumor to the diagnosis of BM was 13 months (range, 1‐293 months). Table 2 and Supporting Table 1 list additional patient characteristics.
TABLE 2.
Patient and Clinical Characteristics of the Entire Study Population (N = 1608)
| Characteristics at the Time of BM Diagnosis | Entire Population | |
|---|---|---|
| No. | % | |
| Sex | ||
| Male | 922 | 57.3 |
| Female | 686 | 42.7 |
| Y of BM diagnosis | ||
| 1986‐1999 | 146 | 9.1 |
| CT imaging at BM diagnosis | 80 | 5.0 |
| MRT imaging at BM diagnosis | 66 | 4.1 |
| 2000‐2009 | 636 | 40.0 |
| 2010‐2019 | 826 | 50.9 |
| Symptoms at BM diagnosis | ||
| Present | 1186 | 73.8 |
| Absent (diagnosis during screening) | 422 | 26.2 |
| Focal deficits | ||
| Present | 985 | 61.3 |
| Motor disorders | 486 | 30.2 |
| Motor disfunction of 1 extremity | 205 | 12.7 |
| Hemiparesis | 220 | 13.7 |
| Facial nerve palsy | 61 | 3.8 |
| Ataxia | 95 | 5.9 |
| Cranial nerve palsy | 204 | 12.7 |
| Hypesthesia | 124 | 7.7 |
| Aphasia | 164 | 10.2 |
| Vertigo | 481 | 29.9 |
| Absent | 623 | 38.7 |
| Signs of increased intracranial pressure | ||
| Present | 483 | |
| Headache | 397 | 24.7 |
| Nausea and emesis | 231 | 14.3 |
| Absent | 1125 | 75.3 |
| Seizures | ||
| Present | 224 | 13.9 |
| Generalized seizures | 123 | 7.6 |
| Focal seizures | 91 | 3.1 |
| Generalized and focal seizures | 10 | 0.6 |
| Absent | 1384 | 86.1 |
| Neuropsychological symptoms | ||
| Present | 233 | 14.5 |
| Organic brain disorder | 125 | 7.8 |
| Cognitive impairment | 87 | 5.4 |
| Memory disorders | 87 | 5.4 |
| Absent | 1375 | 85.5 |
Abbreviations: BM, brain metastases; CT, computed tomography; MRI, Magnetic resonance imaging .
Neurological Symptoms at the Time of Diagnosis of BM
Of the 1608 patients, 1186 patients (73.8%) presented with neurological symptoms at the time of diagnosis of BM, whereas 422 patients (26.2%) presented with asymptomatic brain metastatic disease that was diagnosed during screening and/or staging procedures in the absence of any BM‐specific symptoms. In more detail, approximately 61.3% of the 1608 patients (985 patients) presented with focal deficits, 30.0% (483 patients) presented with signs of increased intracranial pressure, 14.5% (233 patients) presented with neuropsychological symptoms, and 13.9% (224 patients) presented with epileptic seizures. None of the included patients presented with a loss of consciousness except during epileptic seizures.
Focal deficits
The most commonly observed neurological deficit was motor disorders in 486 of 1608 patients (30.2%). Hemiparesis was evident in 220 patients (13.7%) followed by paresis of 1 extremity (205 patients; 12.7%) and facial nerve palsy (61 patients; 3.8%). A total of 164 of 1608 patients (10.2%) presented with aphasia, 124 (7.7%) presented with hypesthesia, and ataxia was present in 95 of 1608 patients (5.9%).
Signs of increased intracranial pressure
A total of 454 of 1608 patients (28.2%) presented with at least 1 sign of increased intracranial pressure. Headache was present in 400 patients (24.9%), and nausea and/or emesis was reported in 231 patients (14.4%).
Epileptic seizures
A total of 224 of 1608 patients (13.9%) presented with epileptic seizures, including 123 patients (7.6%) with generalized seizures, 91 patients (5.6%) with focal seizures, and 10 patients (0.6%) with focal and generalized seizures.
Neuropsychological symptoms
A total of 233 of 1608 patients (14.5%) presented with neuropsychological symptoms, including organic brain syndrome in 125 patients (7.8%) as well as memory disorders and cognitive impairment in 87 patients (5.4%).
Additional patient and clinical characteristics presented are listed in Table 2, Supporting Table 1, and Figure 1A.
FIGURE 1.
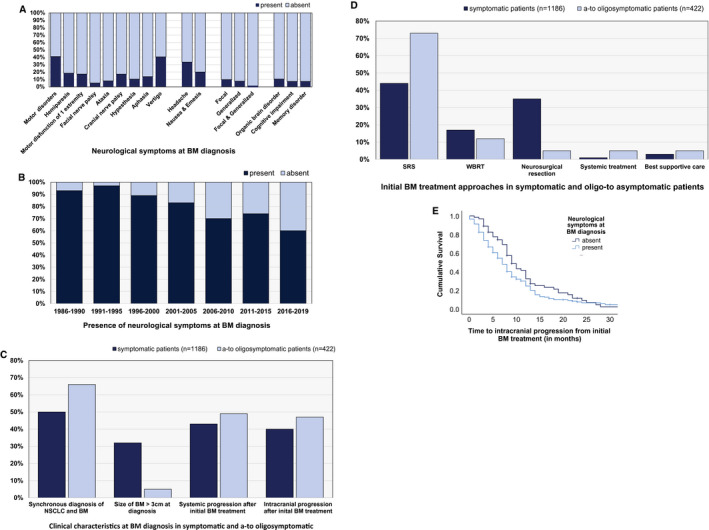
(A) Presence of neurological symptoms at the time of diagnosis of brain metastases (BM) according to (B) year of BM diagnosis, (C) clinical characteristics at the time of BM diagnosis, (D) initial BM‐directed treatment approaches, and (E) time to intracranial disease progression after BM‐directed therapy. NSCLC indicates non–small cell lung cancer; SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy.
Characteristics Associated With Neurological Symptoms at the Time of BM Diagnosis
The percentage of patients diagnosed with asymptomatic disease through screening and/or staging imaging constantly increased throughout the inclusion period from 1986 to 2019. Of the 146 of 1608 patients (9.1%) diagnosed between 1986 and 1999, 4 patients (2.7%) presented with asymptomatic BM disease. In comparison, 152 of 636 patients (23.9%) who were diagnosed between 2000 and 2009 and 266 of 826 patients (32.2%) who were diagnosed between 2010 and 2019 were diagnosed with an asymptomatic BM status (P < .001, chi‐square test) (Fig. 1B) (Table 3).
TABLE 3.
Patient and Clinical Characteristics in Dependence of Neurological Symptoms at Time of BM Diagnosis
| Characteristics | Symptomatic Patients N = 1186 | Asymptomatic Patients N = 422 | P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Characteristics at time of BM diagnosis | |||||
| Y of diagnosis | <.001 | ||||
| 1986‐1999 | 142 | 12.0 | 4 | 1.0 | |
| 2000‐2009 | 484 | 40.8 | 152 | 36.0 | |
| 2010‐2019 | 560 | 47.2 | 266 | 63.0 | |
| Histology of the primary tumor | .590 | ||||
| Adenocarcinoma | 877 | 73.9 | 314 | 74.4 | |
| Squamous cell carcinoma | 140 | 11.8 | 55 | 13.0 | |
| Synchronous diagnosis of NSCLC and BM | <.001 | ||||
| Yes | 597 | 50.3 | 278 | 65.9 | |
| No | 589 | 49.7 | 144 | 34.1 | |
| No. of BM at the time of diagnosis | .741 | ||||
| 1 | 577 | 48.7 | 199 | 47.2 | |
| 2‐3 | 350 | 29.5 | 133 | 31.5 | |
| ≥4 | 259 | 21.8 | 90 | 21.3 | |
| Localization of BM | .634 | ||||
| Supratentorial | 747 | 63.0 | 272 | 64.5 | |
| Infratentorial | 160 | 13.5 | 49 | 11.6 | |
| Both | 279 | 23.5 | 101 | 23.9 | |
| Localization side of BM | .152 | ||||
| Right | 366 | 30.9 | 122 | 28.9 | |
| Left | 396 | 33.4 | 126 | 29.9 | |
| Both | 410 | 34.6 | 171 | 40.5 | |
| Not available | 14 | 1.2 | 3 | 0.7 | |
| Size of BM at the time of diagnosis | .001 | ||||
| ≤3 cm | 740 | 68.3 | 367 | 87.0 | |
| >3 cm | 343 | 31.7 | 21 | 5.0 | |
| Missing | 34 | 8.0 | |||
| Leptomeningeal carcinomatosis | .013 | ||||
| Yes | 38 | 3.2 | 4 | 0.9 | |
| No | 1148 | 96.8 | 418 | 99.1 | |
| Characteristics after BM diagnosis | |||||
| First‐line treatment of BM | <.001 | ||||
| SRS | 521 | 43.9 | 309 | 73.2 | |
| WBRT | 202 | 17.0 | 51 | 12.1 | |
| Neurosurgical surgical resection | 416 | 35.1 | 21 | 5.0 | |
| Systemic treatment | 11 | 0.9 | 21 | 5.0 | |
| Best supportive care | 36 | 3.0 | 20 | 4.7 | |
| Systemic disease progression after first‐line BM treatment | .027 | ||||
| Yes | 508 | 42.8 | 207 | 49.1 | |
| No | 678 | 57.2 | 215 | 50.9 | |
| Median time from first BM treatment to systemic disease progression (range), mo | 4 (0‐207) | 5 (0‐44) | .721 | ||
| Intracranial progression after first‐line BM treatment | .014 | ||||
| Yes | 472 | 39.8 | 197 | 46.7 | |
| No | 714 | 60.2 | 225 | 53.3 | |
| Median time from first BM treatment to intracranial disease progression (range), mo | 7 (0‐71) | 9 (1‐42) | .072 | ||
| Status at last follow‐up | <.001 | ||||
| Deceased | 1069 | 90.1 | 332 | 78.7 | |
| Alive | 117 | 9.9 | 90 | 21.3 | |
| Median OS from the time of diagnosis of BM (range), mo | 7 (6‐8) | 11 (10‐12) | <.001 | ||
| Cause of death | .003 | ||||
| Intracranial progression | 42 | 6.8 | 14 | 7.7 | |
| Extracranial progression | 221 | 35.7 | 91 | 50.3 | |
| Intracranial and extracranial disease progression | 306 | 49.4 | 66 | 36.5 | |
| Other reasons | 50 | 8.1 | 10 | 5.5 | |
Abbreviations: BM, brain metastases; NSCLC, non–small cell lung cancer; OS, overall survival; SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy.
Although the presence of neurological symptoms was found to be irrespective of the number of BM as well as the localization of the BM (P > .05, chi‐square test) (Table 3), the size of the BM at the time of diagnosis demonstrated a significant association with neurological symptoms. Of 364 of 1608 patients (22.6%) with at least 1 BM measuring ≥3 cm, 94.2% (343 of 364 patients) presented with neurological symptoms at the time of BM diagnosis. In comparison, among the 1107 of 1608 patients (68.8%) presenting only with BM measuring <3 cm, only 66.8% (740 of 1107 patients) were found to demonstrate symptomatic BM disease (P < .001, chi‐square test) (Fig. 1C) (Table 3). Furthermore, the presence of leptomeningeal carcinomatosis at the time of diagnosis of BM was found to be significantly associated with neurological symptoms. Approximately 90.5% of the patients with leptomeningeal spread (38 of 42 patients) presented with neurological symptoms compared with 4 of 42 patients with asymptomatic disease (10%) despite the radiological presence of leptomeningeal carcinomatosis (P = .013) (Table 3).
Impact of Neurological Symptoms on Clinical Course
The applied initial treatment approach differed between patients with symptomatic and patients with asymptomatic BM. Stereotactic radiosurgery (SRS) was performed statistically significantly more frequently as the initial treatment approach in patients with asymptomatic BM compared with patients with symptomatic BM (73.2% vs 43.9%; P < .001, chi‐square test) (Fig. 1D) (Table 3). Neurosurgical resection was statistically significantly more frequently performed in patients with symptomatic BM (35.1% vs 5.0%; P < .001, chi‐square test) (Fig. 1D) (Table 3). Moreover, in approximately 54.4% of patients with signs of increased intracranial pressure, neurosurgical resection was performed as the initial treatment approach for BM.
Furthermore, treatment approaches changed over the decades according to the presence of asymptomatic and symptomatic BM (see Supporting Table 2) (see Supporting Fig. 1A). Between 1986 and 1999, approximately 82.4% of symptomatic patients (117 of 142 patients) were treated with neurosurgical resection, whereas 9.9% of patients (14 of 142 patients) underwent SRS as their initial treatment approach (P = .008, chi‐square test) (see Supporting Table 2) (see Supporting Fig. 1A). In comparison, between 2010 and 2019, approximately 20.9% of symptomatic patients (117 of 560 patients) underwent a neurosurgical resection, whereas 47.0% (263 of 560 patients) were treated with SRS (P < .001, chi‐square test) (see Supporting Table 2) (see Supporting Figs. 1A and 1B). In asymptomatic patients, the application of whole brain radiotherapy and systemic treatment as the initial treatment approach at the time of diagnosis of BM increased over the decades. Approximately 3.3% of patients (5 of 152 patients) were treated with systemic therapy as the initial treatment approach between 2000 and 2009, and 6% (16 of 266 patients) were treated with systemic therapy as the initial treatment approach between 2010 and 2019. Nine of 152 patients (5.9%) were treated with whole brain radiotherapy between 2000 and 2009 at the time of the initial BM diagnosis, compared with 42 of 266 patients (15.8%) between 2010 and 2019 (P < .001, chi‐square test) (see Supporting Table 2) (see Supporting Figs. 1A and 1B).
Intracranial (70.6% vs 29.4%; P = .014, chi‐square test) (Fig. 1C) (Table 3) as well as extracranial (71.0% vs 29.0%; P = .027, chi‐square test) (Fig 1C) (Table 3) disease progression was observed more frequently in patients with symptomatic BM compared with patients with asymptomatic BM. The median time to intracranial disease progression from first‐line BM‐directed treatment was 7 months (range, 0‐71 months) in symptomatic patients and 9 months (range, 1‐42 months) in patients with asymptomatic BM (P = .072, log‐rank test) (Fig. 1E) (Table 3). The median time to extracranial disease progression was 4 months (range, 0‐207 months) in patients with symptomatic BM compared with 5 months (range, 0‐44 months) in patients with asymptomatic BM (P = .721, log‐rank test) (Table 3).
Cause of death based on a complete restaging within 60 days before death could be evaluated in 800 of 1608 patients (49.8%). Patients with neurological symptoms at the time of BM diagnosis presented more frequently with combined intracranial and extracranial progressing disease as their cause of death, whereas patients with asymptomatic BM presented more frequently with death due to extracranial disease progression (P = .003, chi‐square test) (Fig. 2A) (Table 3).
FIGURE 2.
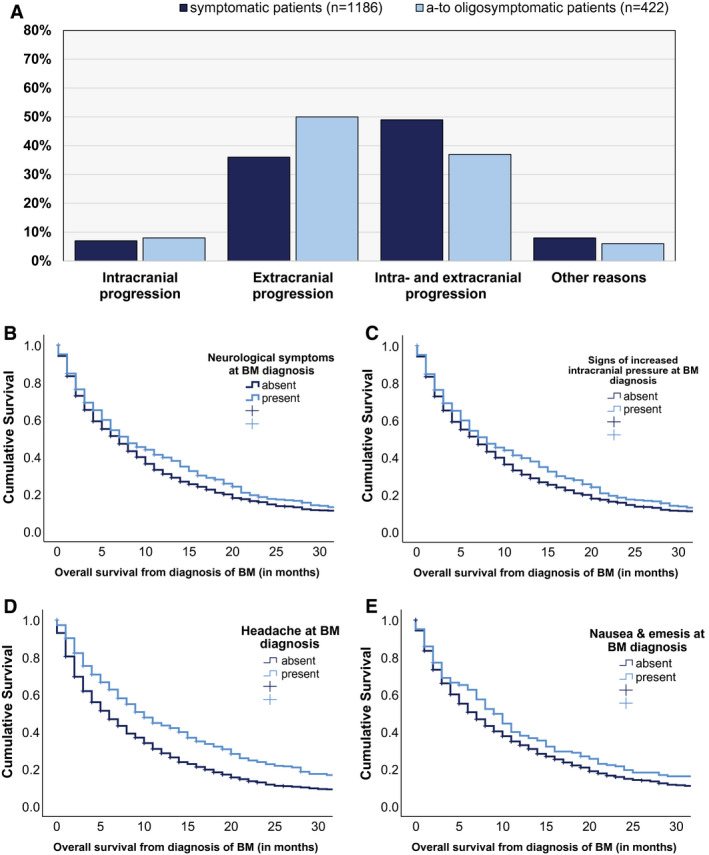
Association between neurological symptoms and (A) cause of death and median overall survival prognosis according to (B) the presence of neurological symptoms at the time of diagnosis of brain metastases (BM) and (C) increased intracranial pressure at the time of BM diagnosis, including (D) headache and (E) nausea and emesis (vomiting).
Impact of Neurological Symptoms on Survival Prognosis
The median OS from the time of diagnosis of BM was 7 months in the entire cohort (range, 0‐267 months) (see Supporting Table 1). Patients with asymptomatic BM presented with a longer median OS after the diagnosis of BM compared with patients with symptomatic BM (11 months vs 7 months; P < .001) (Fig. 2B) (Table 3). The impact of neurological symptoms on survival prognosis differed between the DS‐GPA classes. In the good prognostic class (3.5‐4 points; class I) (see Supporting Table 3), only a numerical association was present because symptomatic patients in the DS‐GPA class I category presented with a median OS of 18 months (range, 14‐22 months) compared with 14 months in asymptomatic patients (range, 8‐20 months; P = .291, log‐rank test) (see Supporting Table 3). In the lower prognostic classes of II to IV (3 to 0 points), patients with neurological symptoms presented with a statistically significantly impaired prognosis compared with patients with asymptomatic BM (class II: 14 vs 11 [P = .011, log‐rank test]; class III: 10 vs 5 [P < .001, log‐rank test]; and class IV: 6 vs 3 [P = .017, log‐rank test]) (see Supporting Table 3).
The Graded Prognostic Assessment for Lung Cancer using Molecular Markers (Lung‐molGPA) could be validated in a subgroup analyses of 529 of 1608 patients (32.9%) for whom information regarding mutational status was available (P < .001, log‐rank test) (see Supporting Table 3). The impact of neurological symptoms on survival prognosis also was found to differ by Lung‐molGPA classes. In the good prognostic class (3.5‐4 points; class I) as well as in the lower prognostic classes (0.0‐2.5 points), only a numeric association was present in favor of asymptomatic patients (class I: 24 months vs 17 months; class III: 9 months vs 8 months; and class IV: 4 months vs 3 months; P > .05, log‐rank test) (see Supporting Table 3). In the prognostic class II (2.5‐3.5 points), patients with neurological symptoms presented with a statistically significantly impaired prognosis compared with patients with asymptomatic BM (class II: 24 vs 13; P < .001, log‐rank test) (see Supporting Table 3).
We further investigated the prognostic impact of the presenting neurological symptoms in the patients with symptomatic BM. In these patients, signs of increased intracranial pressure were found to be associated with an improved survival prognosis compared with other neurological symptoms (8 months vs 6 months; P = .032, log‐rank test) (Fig. 2C) (see Supporting Table 4). In more detail, patients who experienced headache (10 months vs 6 months; P < .001) (Fig. 2D) (see Supporting Table 4) or nausea and/or emesis (9 months vs 7 months; P = 0.040, log‐rank test) (Fig. 2E) (see Supporting Table 4) presented with longer survival times compared with patients with other neurological symptoms. We further investigated whether the observed association between OS and increased intracranial pressure also was present in the subgroup of patients with leptomeningeal disease and large (>3 cm) BM. Patients with leptomeningeal metastases at the time of diagnosis of BM demonstrated a numerically impaired survival prognosis when presenting with signs of increased intracranial pressure; however, this finding was not statistically significant (3 months vs 2 months; P = .530, log‐rank test) (see Supporting Table 3). Patients with BM measuring >3 cm and signs of increased intracranial pressure at the time of diagnosis presented with a significantly improved median OS (9 months vs 7 months; P = .032, log‐rank test) (see Supporting Table 3).
All other presenting neurological symptoms presented only with a numerical association with survival prognosis (Figs. 3A‐3C) (see Supporting Table 4).
FIGURE 3.
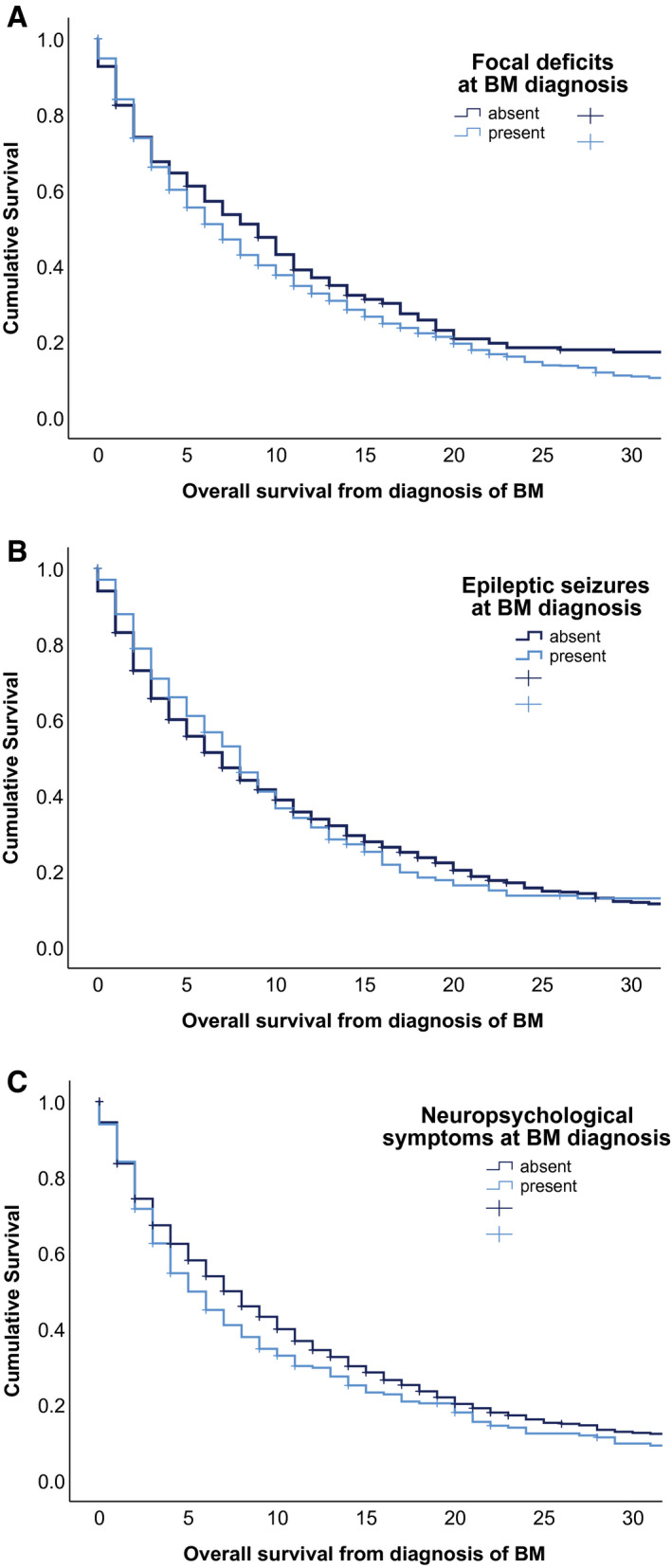
Association between median overall survival and (A) the presence of focal deficits at the time of diagnosis of brain metastases (BM), (B) the presence of epileptic seizures at the time of BM diagnosis, and (C) the presence of neuropsychological symptoms at the time of BM diagnosis.
Multivariate Analysis Including Neurological Symptoms and DS‐GPA Score
In multivariate analysis including DS‐GPA and neurological symptoms overall (including focal deficits, signs of increased intracranial pressure, epileptic seizures, and neuropsychological symptoms), both neurological symptoms (hazard ratio [HR], 1.39; 95% CI, 1.23‐1.57 [P < .001, Cox regression model]) (see Supporting Table 5) and DS‐GPA (HR, 1.41; 95% CI, 1.33‐1.50 [P < .001, Cox regression model]) (see Supporting Table 5) remained statistically significant. In multivariate analysis including DS‐GPA and signs of increased intracranial pressure (including headache, nausea, and emesis) (HR, 1.0; 95% CI, 0.85‐1.10 [P = .613, Cox regression model]) (see Supporting Table 5), only DS‐GPA (HR, 1.43; 95% CI, 1.34‐1.53 [P < .001, Cox regression model]) (see Supporting Table 5) remained statistically significant.
In the multivariate analysis of the subgroup of patients for whom information regarding mutational status was available, including the Lung‐molGPA and neurological symptoms, both neurological symptoms (HR, 1.40; 95% CI, 1.13‐1.75 [P = .002, Cox regression model]) (see Supporting Table 5) and Lung‐molGPA (HR, 1.54; 95% CI, 1.35‐1.76 [P < .001, Cox regression model]) (see Supporting Table 5) remained statistically significant. In multivariate analysis of signs of increased intracranial pressure (HR, 0.97; 95% CI, 0.78‐1.20 [P = .767, Cox regression model]) (see Supporting Table 5) and Lung‐molGPA, only Lung‐molGPA remained statistically significant (HR, 1.57; 95% CI, 1.38‐1.79 [P < .001, Cox regression model]) (see Supporting Table 5).
Discussion
In the current study, patients with BM from NSCLC demonstrated a wide range of neurological symptoms at the time of diagnosis. The percentage of patients with asymptomatic disease has significantly increased over the last 20 years, underscoring the need to adapt diagnostic and treatment algorithms previously designed for symptomatic BM populations before the broad application of BM screening. 18 Indeed, the neurological symptom burden is included in the response assessment of patients with primary brain tumors, whereas the prospective evaluation of neurological symptoms is not regularly included in the prognostic assessment of patients with BM. 4 , 5 In the real‐life cohort in the current study that included 1608 patients with newly diagnosed BM from NSCLC, neurological symptoms at the time of BM diagnosis were found to have a strong, independent prognostic impact on the survival prognosis, thereby supporting the inclusion of neurological symptoms in the prognostic assessment as well as in the clinical decision making among patients with BM.
The presence of neurological symptoms was found to be an independent prognostic factor for OS from the time of diagnosis of BM in the large real‐life cohort of 1608 patients with newly diagnosed NSCLC BM in the current study. In approximately 32.9% of the patients, the recently introduced Lung‐molGPA, which incorporates molecular information in the prognostic estimation of patients with BM, could be validated. Moreover, in multivariate analysis, both Lung‐molGPA and the presence of neurological symptoms demonstrated a statistically significant association with survival prognosis. Therefore, neurological symptoms appear to be independently associated with the survival prognosis and should be evaluated prospectively for integration into the prognostic assessment of patients with NSCLC BM.
To our knowledge, previous studies regarding neurological symptoms have focused on patients with primary brain tumors; to the best of our knowledge, the current study is the largest study to date performed among patients with newly diagnosed BM. The focal deficits before surgery and cognitive function, as well as seizure reduction, were shown to correlate with survival prognosis in patients with primary brain tumors because worsening of seizures during the disease course may indicate disease progression in patients with lower grade gliomas. 19 , 20 , 21 , 22 , 23 It is interesting to note that signs of increased intracranial pressure were found to be associated with a prolonged survival prognosis compared with other BM‐specific neurological symptoms. Peritumoral edema, frequently causing signs of increased intracranial pressure, also previously was shown to be associated with improved survival prognosis because patients with large peritumoral edema were reported to present with an improved survival prognosis compared with patients with small edema. 24 Herein, an expanding growth pattern rather than a glioma‐like infiltrative growth pattern was found to be correlated with large edema and consequently improved survival. 24 Furthermore, symptomatic patients, especially patients with signs of increased intracranial pressure, were more frequently treated with neurosurgical resection, potentially also resulting in a longer survival time. In keeping with this finding, previous studies in patients with BM have argued that neurosurgical resection can successfully reduce the neurological symptom burden and especially the symptoms of increased neurological pressure. 25
The NANO scale, which assesses the neurological symptom burden using a standardized approach, is included in the response assessment in patients with primary brain tumors. 26 Currently, the prognostic assessment in patients with BM is based on few clinical factors, including the histology of the primary tumor and the age and Karnofsky performance score of the patient as well as the number of BM and the status of the extracranial disease. 5 The strong and independent association between neurological symptoms and survival prognosis in patients with BM noted in the current study argues for the inclusion and necessity of a symptom‐adapted treatment approach for prognostic assessment in these patients. Indeed, the presence of neurological symptoms is increasingly reflected in the therapeutic considerations of BM treatment because several recent clinical trials have specifically included patients with asymptomatic BM. 6 , 7 , 8 , 27 Consequently, the European Association of Neuro‐Oncology guidelines for the treatment of BM recommend immediate local treatment approaches in patients with a high symptomatic burden, whereas primarily systemic treatment should be evaluated in patients with asymptomatic disease. 18 Several clinical trials currently are recruiting to investigate the possibility of postponing local treatments in patients with asymptomatic NSCLC BM and applying systemic treatments with high intracranial activity such as a tyrosine kinase inhibitors in patients with NSCLC with driver mutations (ClinicalTrial.gov identifier NCT03769103).
Although in the current study we were able to investigate the presence of neurological symptoms in a large, real‐life cohort, some limitations have to be considered in the interpretation of the results. The findings of the current study certainly were limited by the disadvantages of its retrospective design, although to the best of our knowledge the current analysis was one of the first to address not only the frequency of neurological symptoms but also their impact on survival prognosis. In the current study, approximately 14.5% of patients presented with neuropsychological symptoms at the time of BM diagnosis. In contrast, a review published in 2003 listed cognitive or mental status changes as the most frequent presenting symptom at the time of BM diagnosis in approximately 34% of patients. 28 This discrepancy may be the result of the retrospective design of the current study because neuropsychological symptoms were not assessed regularly using validated testing batteries and therefore might be underreported. Furthermore, individual grading for every neurological symptom described in the study and the duration between first symptoms and the diagnosis of BM could not be investigated. A further potential limitation of the current study was that the volume of BM was not included in analyses. Some patients had a diagnosis made based only on CT. It is important to note that CT is no longer considered the gold standard for BM diagnosis because small BM and leptomeningeal metastases are underreported. However, the number and localization of BM at the time of diagnosis were not found to be significantly associated with the presence of neurological symptoms in the current study cohort. We were able to analyze a unique cohort of 1608 patients with newly diagnosed NSCLC BM and provide detailed information regarding the presence of neurological symptoms at the time of diagnosis of BM. The long inclusion period from 1986 to 2019 potentially biased the current study findings because imaging modalities changed, but this also allowed for the analysis of changes occurring over the last 30 years. Therefore, the results of the current study have provided the unique opportunity to gain deeper insight into the incidence, distribution, and prognostic impact of the neurological symptom burden in patients with NSCLC BM.
The results of the current study presented a detailed characterization of the incidence, distribution, and prognostic impact of neurological symptoms at the time of diagnosis of BM in a unique, large, real‐life cohort of patients with NSCLC BM. The study highlighted the integration of neurological symptoms into the prognostic assessment of patients with NSCLC BM. Future clinical trials should investigate an adapted treatment approach according to neurological symptoms in patients with NSCLC BM.
Funding Support
The costs for this project were covered by the research budget of the Clinical Division of Oncology of the Department of Medicine I at the Medical University of Vienna, and an unrestricted research grant from Daiichi Sankyo (UE71101084) was used for the establishment of the Vienna Brain Metastasis Registry.
Conflict of Interest Disclosures
Matthias Preusser has received honoraria for lectures, consultation, or advisory board participation (all <€5000) from Bayer, Bristol‐Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, AstraZeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dohme, and Tocagen and has received research support from Bohringer‐Ingelheim, Bristol‐Myers Squibb, Roche, Daiichi Sankyo, Merck Sharp & Dohme, NovoCure, GlaxoSmithKline, and AbbVie for work performed outside of the current study. Anna Sophie Berghoff has received grants and personal fees from Daiichi Sankyo; honoraria for lectures, consultation, or advisory board participation from Roche, Bristol‐Myers Squibb, and Merck; and nonfinancial support from Roche, Amgen, and AbbVie for work performed as part of the current study and grants from Daiichi Sankyo and personal fees from Roche and Bristol‐Myers Squibb for work performed outside of the current study. The other authors made no disclosures.
Author Contributions
Ariane Steindl and Anna Sophie Berghoff designed the study. Ariane Steindl, Sarah Yadavalli, Katharina‐Anna Gruber, and Maria Seiwald collected the information from the Vienna Brain Metastasis Registry. Ariane Steindl performed the statistical analyses. Ariane Steindl and Anna Sophie Berghoff wrote the article. Brigitte Gatterbauer, Karin Dieckmann, Josa M. Frischer, Thomas Klikovits, Sabine Zöchbauer‐Müller, Mir Ali Reza Hoda, Christine Marosi, Georg Widhalm, and Matthias Preusser collaborated in drafting the article and revising it critically for important intellectual interdisciplinary content. All authors read and approved the final article.
Supporting information
Fig S1
Supplementary Material
Supplementary Material
Steindl A, Yadavalli S, Gruber K‐A, Seiwald M, Gatterbauer B, Dieckmann K, Frischer JM, Klikovits T, Zöchbauer‐Müller S, Grisold A, Hoda MAR, Marosi C, Widhalm G, Preusser M, Berghoff AS. Neurological symptom burden impacts survival prognosis in patients with newly diagnosed non–small cell lung cancer brain metastases. Cancer. 2020:126:4341–4352. 10.1002/cncr.33085
Presented as a poster discussion at the European Society for Medical Oncology 2019 Annual Congress; September 27‐October 1, 2019; Barcelona, Spain.
This study was performed within the PhD thesis of Ariane Steindl with the title “Immunological and Genetic Drivers of Brain Metastasis Progression” in the Clinical Neuroscience program (CLINS) at the Medical University of Vienna.
References
- 1. Jena A, Taneja S, Talwar V, Sharma JB. Magnetic resonance (MR) patterns of brain metastasis in lung cancer patients: correlation of imaging findings with symptom. J Thorac Oncol. 2008;3:140‐144. doi:10.1097/JTO.0b013e318161d775 [DOI] [PubMed] [Google Scholar]
- 2. Brennum J, Kosteljanetz M, Roed HM. Brain metastases [in Danish]. Ugeskr Laeger. 2002;164:3522‐3526. [PubMed] [Google Scholar]
- 3. Rodin D, Banihashemi B, Wang L, et al. The Brain Metastases Symptom Checklist as a novel tool for symptom measurement in patients with brain metastases undergoing whole‐brain radiotherapy. Curr Oncol. 2016;23:e239‐e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mason WP. NANO, a practical scale for neurologic assessments in patients with brain tumors? Neuro Oncol. 2017;19:603‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis‐specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaw AT, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment‐naive advanced ALK‐positive non–small cell lung cancer (NSCLC): primary results of the global phase III ALEX study. J Clin Oncol. 2017;35:LBA9008. [Google Scholar]
- 7. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non–small‐cell lung cancer and untreated brain metastases: early analysis of a non‐randomised, open‐label, phase 2 trial. Lancet Oncol. 2016;17:976‐983. doi:10.1016/S1470‐2045(16)30053‐5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open‐label, phase 2 trial. Lancet Oncol. 2012;13:459‐465. [DOI] [PubMed] [Google Scholar]
- 9. Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAFV600E‐mutant metastatic non–small cell lung cancer: an open‐label, multicentre phase 2 trial. Lancet Oncol. 2016;17:984‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR‐mutated advanced non–small‐cell lung cancer. J Clin Oncol. 2018;36:3290‐3297. [DOI] [PubMed] [Google Scholar]
- 11. Berghoff AS, Schur S, Fureder LM, et al. Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open. 2016;1:e000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Preusser M, Winkler F, Collette L, et al. Trial design on prophylaxis and treatment of brain metastases: lessons learned from the EORTC Brain Metastases Strategic Meeting 2012. Eur J Cancer. 2012;48:3439‐3447. doi:10.1016/j.ejca.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 13. Preusser M, Winkler F, Valiente M, et al. Recent advances in the biology and treatment of brain metastases of non–small cell lung cancer: summary of a multidisciplinary roundtable discussion. ESMO Open. 2018;3:e000262. doi:10.1136/esmoopen‐2017‐000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis‐specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi‐institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655‐661. [DOI] [PubMed] [Google Scholar]
- 15. Esquenazi Y, Lo VP, Lee K. Critical care management of cerebral edema in brain tumors. J Intensive Care Med. 2017;32:15‐24. doi:10.1177/0885066615619618 [DOI] [PubMed] [Google Scholar]
- 16. Forsyth PA, Posner JB. Headaches in patients with brain tumors: a study of 111 patients. Neurology. 1993;43:1678‐1683. [DOI] [PubMed] [Google Scholar]
- 17. Cadena R, Shoykhet M, Ratcliff JJ. Emergency neurological life support: intracranial hypertension and herniation. Neurocrit Care. 2017;27(suppl 1):82‐88. doi:10.1007/s12028‐017‐0454‐z [DOI] [PubMed] [Google Scholar]
- 18. Soffietti R, Abacioglu U, Baumert B, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro‐Oncology (EANO). Neuro Oncol. 2017;19:162‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low‐grade glioma. J Clin Oncol. 2002;20:2076‐2084. doi:10.1200/JCO.2002.08.121 [DOI] [PubMed] [Google Scholar]
- 20. Koekkoek JA, Dirven L, Heimans JJ, et al. Seizure reduction is a prognostic marker in low‐grade glioma patients treated with temozolomide. J Neurooncol. 2016;126:347‐354. doi:10.1007/s11060‐015‐1975‐y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3:159‐168. doi:10.1016/S1474‐4422(04)00680‐5 [DOI] [PubMed] [Google Scholar]
- 22. Gorlia MT, Wu W, Wang M, et al. New validated prognostic models and prognostic calculators in patients with low‐grade gliomas diagnosed by central pathology review: a pooled analysis of EORTC/RTOG/NCCTG phase III clinical trials. Neuro Oncol. 2013;15:1568‐1579. doi:10.1093/neuonc/not117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vecht CJ, Kerkhof M, Duran‐Pena A. Seizure prognosis in brain tumors: new insights and evidence‐based management. Oncologist. 2014;19:751‐759. doi:10.1634/theoncologist.2014‐0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spanberger T, Berghoff AS, Dinhof C, et al. Extent of peritumoral brain edema correlates with prognosis, tumoral growth pattern, HIF1a expression and angiogenic activity in patients with single brain metastases. Clin Exp Metastasis. 2013;30:357‐368. [DOI] [PubMed] [Google Scholar]
- 25. Schodel P, Schebesch KM, Brawanski A, Proescholdt MA. Surgical resection of brain metastases–impact on neurological outcome. Int J Mol Sci. 2013;14:8708‐8718. doi:10.3390/ijms14058708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nayak L, Deangelis LM, Brandes AA, et al. The Neurologic Assessment in Neuro‐Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro‐Oncology (RANO) criteria. Neuro Oncol. 2017;19:625‐635. doi:10.1093/neuonc/nox029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600‐mutant melanoma brain metastases (COMBI‐MB): a multicentre, multicohort, open‐label, phase 2 trial. Lancet Oncol. 2017;18:863‐873. doi:10.1016/S1470‐2045(17)30429‐1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lassmann A, Deangelis LM. Brain metastases. Curr Ther Neurol Dis. 2003;21:265‐267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Supplementary Material
Supplementary Material


