Abstract
Non‐Uremic Calciphylaxis (NUC) is a rare condition that often manifests as intractable and painful integumentary wounds, afflicting patients with a high burden of co‐morbidity. The Endocannabinoid System (ECS) is a ubiquitous signalling system that is theorised to be dysregulated within wound beds and associated peri‐wound tissues. Preclinical research has shown that the dominant chemical classes derived from the cannabis plant, cannabinoids, terpenes, and flavonoids, interact with the integumentary ECS to promote wound closure and analgesia. This is a prospective open label cohort study involving two elderly Caucasian females with recalcitrant NUC leg ulcers of greater than 6 months duration. Topical Cannabis‐Based Medicines (TCBM) composed of cannabinoids, terpenes, and flavonoids were applied daily to both the wound bed and peri‐wound tissues until complete wound closure was achieved. Wounds were photographed regularly, and the digital images were subjected to planimetric analysis to objectively quantify the degree of granulation and epithelization. Analgesic utilisation, as a surrogate/proxy for pain scores, was also tracked. The cohort had a mean M3 multimorbidity index score of 3.31. Complete wound closure was achieved in a mean of 76.3 days. Additionally, no analgesics were required after a mean of 63 days. The treatments were well tolerated with no adverse reactions. The positive results demonstrated in very challenging wounds such as NUC, among highly complex patients, suggest that TCBM may have an even broader role within integumentary and wound management. This treatment paradigm warrants being trialled in other wound types and classes, and ultimately should be subjected to randomised controlled trials.
Keywords: topical cannabis‐based medicines, non‐uremic Calciphylaxis, endocannabinoid system, wound closure, wound‐related pain
1. INTRODUCTION
Calciphylaxis is a rare enigmatic integumentary condition associated with high levels of morbidity and mortality. 1 It typically manifests as painful necrotic lesions with high propensity to become infected. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 Calciphylaxis is predominately observed in patients with end‐stage kidney disease (ESKD), termed uremic calciphylaxis (UC), or calcific uremic arteriolopathy (CUA), but may also present in patients without ESKD, termed non‐uremic calciphylaxis (NUC). 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 The 1‐year mortality rate in patients with calciphylaxis associated with UC/CUA has been reported at 45% to 80%, while those with NUC have a 1‐year mortality of 25% to 45%. 3 , 4 , 5 Clinically, the integumentary lesions in UC/CUA and NUC are morphologically similar with intensely painful necrotic ulcers, eschars, indurated nodules, and peri‐wound livedo, with lesions most commonly located on the lower extremities. 5 , 8
A recent systematic review and meta‐analysis in UC/CUA found no significant clinical benefit from the five most frequently used treatment modalities that included sodium thiosulfate (50.3% of patients), surgical parathyroidectomy (28.7% of patients), cinacalcet (25.3% of patients), hyperbaric oxygen (15.3% of patients), and bisphosphates (5.9% of patients). 9 It is important to note that all of the aforementioned treatments involve therapies that are systemic, invasive (intravenous and/or intralesional injections), associated with significant side effect burden, and financial costs. 10 Therefore, calciphylaxis is truly an “orphaned” disease state that merits investigation into novel treatments that are non‐invasive, safe, and may be self‐administered.
The endocannabinoid system (ECS) is a chemical signalling system that holds a ubiquitous presence in all organ systems among mammalian species. 9 Moreover, the ECS is embodied throughout all levels, components, and appendages of the integumentary system, both cutaneous and mucous membranes. 9 , 11 , 12 , 13 , 14 The evolving study of the ESC has recognised that ECS signalling goes beyond the classic cannabinoid receptors, CB1 and CB2, by involving other extracellular receptors such as TRPV, GPR, and 5‐HT, as well as acting on nuclear receptors such as PPAR. 11 It has been theorised that dysregulated ECS signalling is central to the pathophysiology of integumentary and wound conditions. 9 , 11 , 12 , 13 , 14 Dubbed the “entourage effect”, 15 it has been postulated that synergistic and potentiated positive healthcare outcomes, including healing of integumentary wounds, may be achieved through the activity of the main molecular families derived from legalised medical grade cannabis. 15 , 16 This study was conducted in Toronto, Canada, where medical cannabis was federally legalised in 2001. There is a global trend towards its legalisation. As of early 2020, medical cannabis has been legalised in 40 countries and 33 American states. 17
The conceptual framework that has guided global wound management for over two decades is the “Wound Bed Preparation” (WBP) paradigm. 18 Its main limitation is that it does not fully address the state of peri‐wound tissues. New scientific insights reflect that both wound beds and peri‐wound tissues harbour pathophysiologic features, such as inflammation, hypoxia, acidosis, etc., that predispose to wound chronicity and deterioration. 19 , 20 , 21 This study involves the topical application of Topical Cannabis‐Based Medicines (TCBM) (Table 1) to the wound bed (VS‐12) and to the peri‐wound tissues (VS‐14).
TABLE 1.
Specifications of VS‐12 and VS‐14
| Components | VS‐12 | VS‐14 |
|---|---|---|
| Applied to Wound Bed | Applied to Peri‐Wound | |
| Base carrier | Hyaluronic acid + aloe vera gel 1/1 v/v | Liposomal base a |
| CBD b | 3.75 mg/mL | 3.75 mg/mL |
| THC c | <1 mg/mL | <1 mg/mL |
| Quercetin | 31.25 mg/mL | 31.25 mg/mL |
| Disomin | 25.31 mg/mL | 25.31 mg/mL |
| Hersperidin | 2.5 mg/mL | 2.5 mg/mL |
| Beta carophyllene | 152.69 mg/mL | 152.69 mg/mL |
Promotes penetration of cannabinoids, terpenes, and flavonoids through stratum corneum and into peri‐wound tissues.
Cannabidiol.
Delta‐9 tetrahydrocannabinol.
2. METHODS
The two patients reported in this paper were among 35 patients with intractable integumentary wounds, 33 involving cutaneous membrane and 2 involving mucous membranes, that were recruited for a prospective open label clinical trial (Study ID #ISRCTN16488940) using Topical Cannabis‐Based Medicines (TCBM) to assess their effect on wound healing and wound‐related pain. Two female patients with painful and non‐healing leg ulcers, of greater than 6 months duration, were referred to a regional consultative wound management clinic in Toronto, Canada. Both patients failed all available best practices in accordance with WBP. 18 Daily topical applications of Cannabis‐Based Medicines, VS‐12 and VS‐14 (Table 1), composed of mixtures of cannabinoids, terpenes, and flavonoids, were applied topically to the wound beds and peri‐wound tissues. VS‐12 and VS‐14 are chemically equivalent but compounded in separate vehicles that promote absorption through a wound bed and intact integument, respectively. The daily treatments were continued until complete wound closure, defined as the wound bed being 100% epithelialized. The overall clinical trial was approved by the Research Ethics Board at the William Osler Health System in Brampton, Ontario, Canada (Study 18‐0038).
On their initial visits, their degrees of global medical complexity were calculated using the M3 multimorbidity index tool. 22 , 23 Additionally, both patients consented to 4 mm punch biopsies of their wounds for histopathologic and immunofluorescent evaluation that confirmed NUC. Following gentle cleansing with sterile normal saline, each patient underwent daily application of evenly applied thin layers of VS‐12 to the wound beds, and VS‐14 to a 4 to 6 cm radial cuff of peri‐wound integument. Tissues were then covered with one layer each of Jelonet and Mesorb, followed by spiral bandaging of the lower limb, sequentially, using gauze kling roll, Comprilan, and Easifix, between the level of the metatarsal phalangeal joints and the infra‐popliteal space.
3. STATISTICS
To track the treatment outcomes and perform statistical analyses, images of the wound were taken with a smartphone camera (iPhone 6 and XS, Apple Inc.). Using a simple planimetric wound image analysis technique, 24 the wound area on each image was manually contoured, and the relative wound area change and relative wound composition change, in terms of granulation and reepithelization, was calculated. Data were also fitted to a linear regression model to report the general trend and the estimated time to complete wound closure. The daily utilisation of opioid analgesics was prospectively documented as a proxy/surrogate to monitor and gauge pain severity.
4. RESULTS
4.1. Patient A
An 85‐year‐old Caucasian woman presented with a 6‐month history of painful ulcerations involving her right leg. Her medical history included chronic congestive heart failure, valvular heart disease, pulmonary hypertension, moderate dementia, Type 2 diabetes mellitus, atrial fibrillation (Xarelto 2.5 mg bid), systemic hypertension, osteoarthritis, surgically fused right ankle, and hyperlipidemia. Her M3 comorbidity index was 3.59. Her laboratory parameters are summarised in Table 2. Patient A could not express her pain level in terms of numeric rating scores. However, her caregiver indicated that at the onset of this study, the patient had previously never been in a similar level of distress. Her caregiver shared a questionable history of “allergy” to strong opioids and thus elected to only use TYLENOL with Codeine No. 3 tablets, USP (300 mg/30 mg) for pain relief.
TABLE 2.
Laboratory data
| Lab Tests | Normal Ranges | Patient A | Patient B |
|---|---|---|---|
| Hb (g/L) | 115 to 160 | 123 | 113 |
| Albumin (g/L) | 35 to 50 | 30 | 30 |
| eGFR (mL/min) | >60 | 73 | 89 |
| Creatinine (μmol/L) | 49 to 90 | 66 | 57 |
| Calcium (mmol/L) | 2.02 to 2.62 | 2.32 | 2.43 |
| Phosphate (mmol/L) | 0.70 to 1.50 | 0.75 | 1.27 |
| Rheumatoid factor (IU/L) | <20 | <20 | 92.3 |
| Cardiolipin IGM (CU) | <20 | 23.0 | <2.6 |
| Arterial toe‐brachial iIndex | >0.65 | R leg 0.7 |
R leg 0.63 L leg 0.52 |
| Venous reflux | – | R leg + |
R leg ++ L leg ++ |
On clinical examination, Patient A had five necrotic ulcerations involving the antero‐lateral aspect of her right leg. Mild veno‐lymphedema was noted.
27 good‐quality images of this wound region, captured over 74 days, were available for analysis, with representative images at different stages of treatment shown in Figure 1A. The wound area was 50% closed on day 37 and completely closed on day 74 (2.5 months). According to the linear regression model, the wound was expected to close in 77.0 days (fitted slope = −1.4%/day), illustrated in Figure 1B.
FIGURE 1.
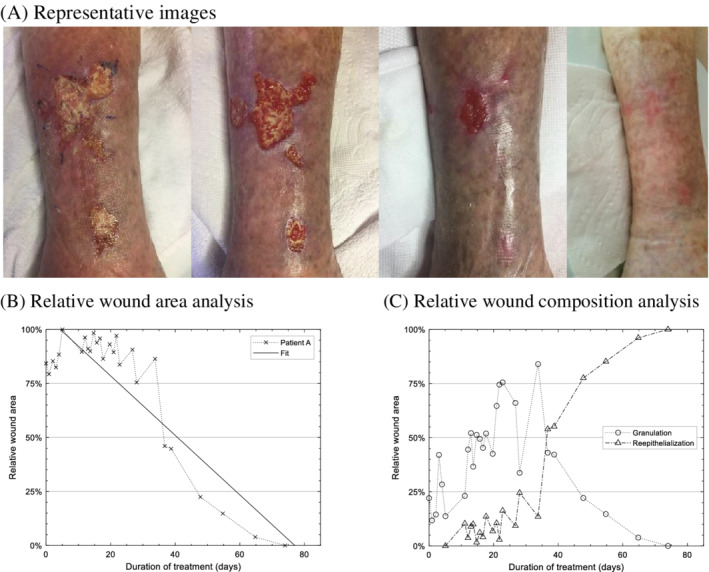
Patient A: A, Representative images of the wound region of Patient A on day 0, 27, 54, and 74. B, Result of tracking of wound area through duration of treatment. The wound was completely closed on day 74. When fitted to a linear regression model, the expected wound closure date is 77.0 days. C, Result of wound composition analysis showing the relative area of granulated tissue vs reepithelialized tissue
Planimetric wound image analysis 24 assessed the gross phenotypic changes in the wound bed during the treatment phase, with results illustrated in Figure 1C. A characteristic two‐phase wound closure process is observed: during the first half of the treatment, granulation dominates the wound healing landscape with relatively small decrease in the total wound area (slope of granulation for first 34 days = +1.8%/day). During the second half of the treatment, the reepithelization process quickly caught up by replacing the granulated tissue and rapidly contracting the wound area, achieving ultimate wound closure (slope of reepithelization from day 34 till closure = +1.8%/day).
Regarding pain management, Patient A initially required 10 tablets of TYLENOL with Codeine No. 3 tablets per day. A 33% reduction in analgesic requirements, analogous to a clinically significant degree of pain relief 25 occurred on day 18. On day 57 of treatment, TYLENOL with Codeine No. 3 tablets were no longer required by the patient for analgesia.
4.2. Patient B
A 69‐year‐old Caucasian woman presented with an 8‐month history of painful ulcerations involving her right leg, and a 4‐month history of ulcerations involving her left leg. Her medical history reflected Type 2 diabetes mellitus, rheumatoid arthritis, systemic hypertension, osteoarthritis, and hyperlipidemia. Her M3 comorbidity index was 3.02. Her laboratory parameters are summarised in Table 2. During the 3 weeks prior to the start of the trial, Patient B had been rendered completely bed‐bound and dependent on others for personal care owing to her extreme pain. On clinical examination, Patient B had numerous necrotic ulcerations involving both legs that were circumferential. Mild veno‐lymphedema was noted.
About 44 good‐quality images of the posterior aspects of both legs, captured over 81 days, were included in the analysis for each of the two legs, shown in Figure 2A,B, respectively. As illustrated in Figure 2C, the left leg wound area was 50% closed around day 36 to 41 and was seen completely closed on day 79 (2.6 months) (fitted slope = −0.8%/day). The right leg wound healed slightly faster overall (Figure 2D). It was 50% closed at approximately day 32 to 36 and was completely closed on day 74 (2.4 months) (fitted slope = −1.2%/day).
FIGURE 2.
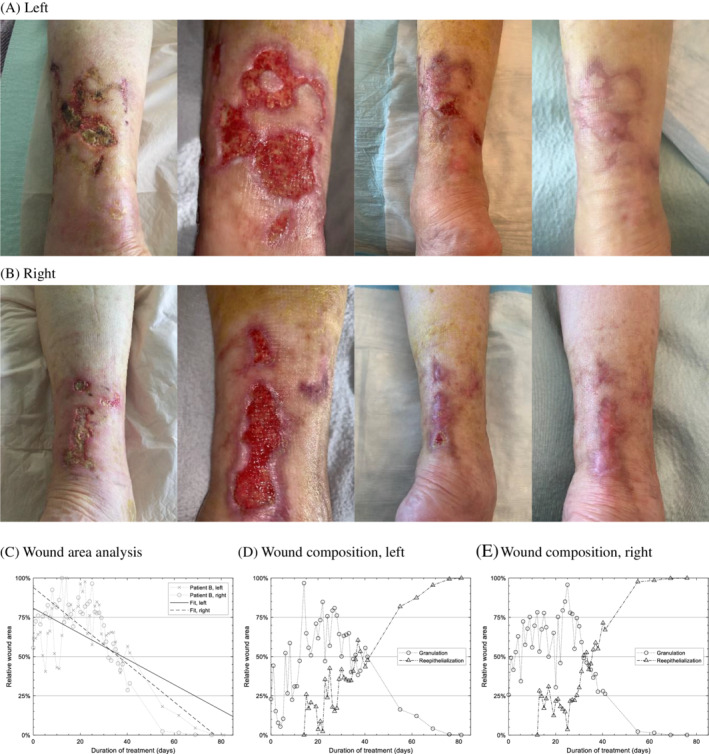
Patient B: A,B, Representative images of the wound region of (A) Patient A's left leg on day 0, 27, 55, and 81 (2 days after closure), and (B) Patient A's right leg on day 0, 27, 55, and 76. C, Result of tracking of wound area through duration of treatment for both legs. The wound was seen completely closed on day 79 and 76, respectively. When fitted to a linear regression model, the expected wound closure dates are 100 and 77 days, respectively. D,E, Result of wound composition analysis showing the relative area of granulated tissue vs reepithelialized tissue for the two legs
Planimetric wound image analysis, as shown in Figure 2E, demonstrated a very similar two‐phase wound healing response to Patient A described above. The first half of the treatment was characterised by increase in granulation tissue (slope of granulation for first 25 days: left leg = +2.3%/day, right leg = +1.1%/day), and the second half was characterised by rapid reepithelization and closure of wound (slope of reepithelization from day 25 till closure: left leg = +1.5%/day, right left = +1.8%/day).
Patient B initially required 188 mg of oral morphine sulfate equivalents per day. A 33% reduction in analgesic requirements, analogous to a clinically significant degree of pain relief 25 occurred on day 19. She began to ambulate with assistance on day 21 and became fully mobile and independent on day 54. On day 68 of treatment, she no longer required any form of analgesia.
Throughout the entire course of this trial, there were no significant side effects, systemically, regionally, or locally, experienced by either of the patients.
5. DISCUSSION
This is the first study to report the use of TCBM to promote complete integumentary wound closure in human subjects, specifically, two Caucasian females with a mean age of 77 years, with 3 lower limbs afflicted with intractable and biopsy proven NUC. The mean M3 index for this cohort was 3.31, reflective of a high level of medical comorbidity when one considers that almost two thirds of typical populations score zero on the M3 index. 22 , 23 This study reports the most rapid complete closure of NUC wounds in the existing peer‐reviewed literature with a mean of 2.5 months. According to the surrogate/proxy measures employed, the patients achieved clinically significant analgesia 25 after a mean of 0.6 months, while requiring zero analgesics after a mean of 2.1 months of treatment. In addition to being non‐invasive and non‐systemic, it was not associated with any additional discomfort to the patients, significant side effects, or toxicities.
Table 3 summarises the existing published case reports of NUC treated with the IV medications pamidronate, zoledronate, and sodium thiosulfate (STS), as well as intralesional STS. Two studies using IV pamidronate reported complete closure after 6 months of treatment. 26 , 28 One study using IV pamidronate did not report the outcome of complete closure. 28 The report of zoledronate treatment did not report the outcome of complete closure. 27 Three studies using IV STS treatment reported complete closure after 4 months, 6 months, and 42 weeks (9.8 months). 29 , 33 , 34 Three other studies using IV STS did not the outcome of complete closure. 30 , 31 , 32 The report of intralesional STS described four cases, three with NUC and one with UC, of which 75% of cases were “healed completely or almost completely” healed after 6 to 11 months of treatment. 35 All of the studies in Table 3 involved treatments that are invasive and/or systemic and were associated significant side effects, and in the case of intralesional STS, all patients reported pain with the injections. It is important to note that the studies in Table 3 neither consistently reported clinically significant analgesia nor consistency in their objective measures of pain.
TABLE 3.
Comparison of existing treatments reported in literature
| Publication | Treatment | Failure of Other Medical Wound Therapy? | Total Wound Closure Achieved? | Time to Wound Closure |
|---|---|---|---|---|
| Truong et al (2019) 26 | IV pamidronate | No | Yes | 6 months |
| Fergie et al (2017) 27 | IV zoledronate | No | No. Healing slowly at 6 months | N/A |
| Lorriaux et al (2015) (Patient 1) 28 | IV pamidronate | Yes (IV STS) | Response after 8 infusions | N/A |
| Lorriaux et al (2015) (Patient 2) 28 | IV Pamidronate | Yes (IV STS) | Yes | 6 months |
| Ning et al (2013) 29 | IV STS | No | Yes | 6 months |
| Smith et al (2012) 30 | IV STS | No | Unknown. Response at 3 months | N/A |
| Ong et al (2011) 31 | IV STS | Yes (IV pamidronate) | No | N/A |
| Stanciu et al (2011) 32 | IV STS | No | No | N/A |
| Kalajian et al (2009) 33 | IV STS | No a | Yes | 4 months |
| Hackett et al (2009) 34 | IV STS | Yes (IV pamidronate) | Yes | 42 weeks |
| Isoherranen et al (2017) 35 , b | IL STS | N/A | Variable | 6 to 11 months |
Oral cinacalcet hydrochloride (30 mg daily), sevelamer hydrochloride (1600 mg 3 times daily), and ergocalciferol (50 000 U twice weekly) used as concurrent therapies.
Isoherranen et al report four cases treated with IL STS. Three of four patients had normal renal function (NUC). Time to wound closure was not specified for each patient individually, so the data is displayed as pooled. Three patients demonstrated complete healing, and one of these patients experienced wound relapse.
Clinically significant relief of wound‐related pain, using medical cannabis oils containing both THC and CBD, has been reported in small case series involving malignant wounds and pyoderma gangrenosum. 36 , 37 A case series in which medical cannabis oil containing only CBD was applied to children with epidermolysis bullosa demonstrated both analgesia and a trend towards wound healing. 36 Opioid sparing was observed in all three reports. 36 , 37 , 38
The chemical composition of the TCBM used is this study was based on a meta‐synthesis of all of the available preclinical and human evidence related to integumentary wound healing using the various molecular classes expressed by the cannabis plant. Furthermore, the TCBM was created to be compliant with guidelines for Cannabis‐Based Medicinal products published by the UK National Institute for Health and Clinical Excellence (NICE). 39 However, the precise mechanism of action of the TCBM used in this study remains theoretical and under investigation. We postulate that the positive outcomes reported are the result of a potentiation and synergy between cannabinoids, terpenes, and flavonoids, acting on both wound bed and peri‐wound tissues. Chronic non‐healing wounds are known to be stalled in a state of extreme inflammation that arrests the normal wound healing cascade. 40 Based upon published preclinical data, it is theorised that VS‐12/VS‐14 components such as the cannabinoids, THC, CBD, through their intrinsic anti‐inflammatory properties, 41 , 42 may be able to reduce inflammation to a more physiologic and homeostatic level, thereby allowing wounds to progress towards the subsequent stages of wound healing that include granulation tissue formation, angiogenesis, re‐epithelialization, and tissue remodelling. The anti‐inflammatory properties of cannabinoids may operate through their ability to reduce levels of TNFα, 42 reactive oxygen species, 43 and lipoxygenases. 44 , 45 Furthermore, cannabinoids also have demonstrated ability to improve tissue perfusion and oxygenation via direct vasodilation 46 and nitric oxide‐related mechanisms. 47 Cannabinoids influence various physiologic processes through numerous cellular signalling pathways. 9 , 11 , 12 , 13 , 14 These include, but are not limited to, signalling through classical cannabinoid receptors (CB1 and CB2), novel cannabinoid receptors (GPR), ionotropic receptors (TRPV, TRPA, TRPM), nuclear receptors (PPARγ, PPARα, PPARδ, NF‐κB), and non‐cannabinoid targets (5‐HT, GlyR, A2A, α2R). 9 , 11 , 12 , 13 , 14 Through their capacity to interact with intracellular receptors such as the PPAR family of nuclear receptors, cannabinoids may also potentially promote wound healing through epigenetic mechanisms. 48 , 49 , 50
In addition to cannabinoids, VS‐12 and VS‐14 contain the Terpene, β‐Carophyllene, and the Flavonoids, Quercetin, Disomin, and Hesperidin. β‐Carophyllene is a strong CB2 agonist and thus is associated with analgesic and anti‐inflammatory properties. 51 A recently published mouse model in which β‐Carophyllene led to enhanced re‐epithelialization that was demonstrated to be mediated through epigenetic mechanisms. 52 Flavonoids have long been the key components of numerous nutraceuticals and polyherbal integumentary and wound treatments. Flavonoids, as a class, possess anti‐inflammatory and antioxidant properties. 53 In a preclinical model, Quercetin accelerated cutaneous wound healing by increasing levels of Vascular endothelial growth factor (VEGF) and Transforming growth factor (TGF‐β1). 54 Both VS‐12 and VS‐14 contain Diosmin and Hesperidin in the same proportions as found in the oral tablet, Daflon 500 mg. A meta‐analysis of humans with venous leg ulcers treated with Daflon 500 mg as an adjuvant therapy, demonstrated accelerated healing. 54 The combination of Diosmin and Hesperidin has been demonstrated to be phlebotonic and venoactive through their inhibition of the expression of vascular cell adhesion molecule (VCAM), endothelial intercellular adhesion molecule 1 (ICAM‐1), and other leucocyte adhesion molecules. 55
The obvious limitations of this study include small cohort size and lack of control measures. Although it is theorised that the positive results observed were due to local absorption and associated activities within the tissues of the wound bed and peri‐wound, it is not known if systemic absorption of the various components of TCBM played a significant role.
6. CONCLUSION
Topical Cannabis‐Based Medicines, applied to both wound beds and peri‐wound tissues, represent a promising novel, non‐invasive, and safe treatment option for NUC leg ulcers. The ease and simplicity of its application also allows for potential self‐application and self‐titration by patients. Given that TCBM demonstrated both rapid wound closure and relief of wound‐related pain, in very challenging wounds such as NUC, among highly complex patients, they may be poised for an even broader role within overall integumentary and wound management. Therefore, this novel treatment paradigm warrants being trialled in other wound types and classes, and ultimately should be subjected to randomised controlled trials. Moreover, future studies should also include methodologies to distinguish between local and systemic effects.
ACKNOWLEDMENTS
We wish to thank, Anna Mann, our hospital librarian for her assistance and guidance.
CONFLICT OF INTEREST
The primary author is the President and CEO of the company which holds intellectual property and patents related to the formulae and methodology used in this study. The other authors declare no conflicts of interest.
Maida V, Shi RB, Fazzari FGT, Zomparelli L. Topical cannabis‐based medicines – A novel paradigm and treatment for non‐uremic calciphylaxis leg ulcers: An open label trial. Int Wound J. 2020;17:1508–1516. 10.1111/iwj.13484
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704‐1714. [DOI] [PubMed] [Google Scholar]
- 2. Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27(11):3421‐3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCarthy JT, el‐Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91(10):1384‐1394. [DOI] [PubMed] [Google Scholar]
- 4. Fine A, Zacharias J. Calciphylaxis is usually non‐ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61(6):2210‐2217. [DOI] [PubMed] [Google Scholar]
- 5. Ghosh T, Winchester DS, Davis MDP, El‐Azhary R, Comfere NI. Early clinical presentations and progression of calciphylaxis. Int J Dermatol. 2017;56(8):856‐861. [DOI] [PubMed] [Google Scholar]
- 6. Chen TY, Lehman JS, Gibson LE, Lohse CM, El‐Azhary RA. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol [Internet]. 2017;39(11):795‐802. Available from: www.amjdermatopathology.com. [DOI] [PubMed] [Google Scholar]
- 7. Weenig RH, Sewell LD, Davis MDP, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56(4):569‐579. [DOI] [PubMed] [Google Scholar]
- 8. Nigwekar SU, Wolf M, Sterns RH, Hix JK. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3(4):1139‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maccarrone M, Bab I, Bíró T, et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci. 2015;36:277‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Udomkarnjananun S, Kongnatthasate K, Praditpornsilpa K, Eiam‐Ong S, Jaber BL, Susantitaphong P. Treatment of calciphylaxis in CKD: a systematic review and meta‐analysis. Kidney Int Reports. 2019;4(2):231‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tóth KF, Ádám D, Bíró T, Oláh A. Cannabinoid signaling in the skin: therapeutic potential of the “c(ut)annabinoid” system. Molecules. 2019;24:918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bíró T, Tóth BI, Haskó G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oddi S, Maccarrone M. Endocannabinoids and skin barrier function: molecular pathways and therapeutic opportunities. In: Wondrak GT, ed. Skin Stress Response Pathways: Environmental Factors and Molecular Opportunities [Internet]. Cham: Springer International Publishing; 2016:301‐323. Available from: 10.1007/978-3-319-43157-4_15. [DOI] [Google Scholar]
- 14. del RC, Millán E, García V, Appendino G, DeMesa J, Muñoz E. The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem Pharmacol. 2018;157:122‐133. [DOI] [PubMed] [Google Scholar]
- 15. Russo EB. The case for the entourage effect and conventional breeding of clinical cannabis: no “strain,” no gain. Front Plant Sci. 2019;9:1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mansouri K, Norooznezhad AH. Cannabinoids: a possible treatment for chronic cutaneous wounds. J Dermatol Treat. 2019;1‐2. 10.1080/09546634.2019.1690620. [DOI] [PubMed] [Google Scholar]
- 17. PwC Canada . PwC Canada's cannabis series: Chapter 9 ‐ Cannabis in the pharmaceutical industry [Internet]; 2019. Available from: https://www.pwc.com/ca/en/industries/cannabis/pwc-cannabis-series-chapter-9-cannabis-in-the-pharmaceutical-industry.html.
- 18. Harries RL, Bosanquet DC, Harding KG. Wound bed preparation: TIME for an update. Int Wound J. 2016;13:8‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polak A, Kucio C, Kloth LC, et al. A randomized, controlled clinical study to assess the effect of anodal and cathodal electrical stimulation on Periwound skin blood flow and pressure ulcer size reduction in persons with neurological injuries. Ostomy Wound Manag [Internet]. 2018;64(2):10‐29. Available from: www.o-wm.comFEATURE. [PubMed] [Google Scholar]
- 20. Dowsett C, Gronemann MN, Harding K. Others. Taking wound assessment beyond the edge. Wounds Int. 2015;6(1):19‐23. [Google Scholar]
- 21. Onesti MG, Fioramonti P, Carella S, Maruccia M. The importance of periwound skin in the treatment of “difficult wound”. G Chir [Internet]. 2011;32(1–2):83‐88. Available from: http://europepmc.org/abstract/MED/21352717. [PubMed] [Google Scholar]
- 22. Stanley J, Sarfati D. The new measuring multimorbidity index predicted mortality better than Charlson and Elixhauser indices among the general population. J Clin Epidemiol. 2017;92:99‐110. [DOI] [PubMed] [Google Scholar]
- 23. Gurney JK, Stanley J, Sarfati D. The M3 multimorbidity index outperformed both Charlson and Elixhauser indices when predicting adverse outcomes in people with diabetes. J Clin Epidemiol. 2018;99:144‐152. [DOI] [PubMed] [Google Scholar]
- 24. Shi RB, Qiu J, Maida V. Towards algorithm‐enabled home wound monitoring with smartphone photography: a hue‐saturation‐value colour space thresholding technique for wound content tracking. Int Wound J. 2019;16(1):211‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farrar JT, Berlin JA, Strom BL. Clinically important changes in acute pain outcome measures. A validation study. J Pain Symptom Manage. 2003;25(5):406‐411. [DOI] [PubMed] [Google Scholar]
- 26. Truong DH, Riedhammer MM, Zinszer K. Non‐uraemic calciphylaxis successfully treated with pamidronate infusion. Int Wound J. 2019;16(1):250‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fergie B, Valecha N, Miller A. A case of nonuremic calciphylaxis in a Caucasian woman. Case Rep Dermatol Med. 2017;2017:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lorriaux A, Chaby G, Dhaille F, et al. Nonuraemic calciphylaxis: response to treatment with pamidronate and negative pressure therapy. Clin Exp Dermatol. 2015;40(1):52‐65. [DOI] [PubMed] [Google Scholar]
- 29. Ning MS, Dahir KM, Castellanos EH, McGirt LY. Sodium thiosulfate in the treatment of non‐uremic calciphylaxis. J Dermatol. 2013;40(8):649‐652. [DOI] [PubMed] [Google Scholar]
- 30. Smith VM, Oliphant T, Shareef M, Merchant W, Wilkinson SM. Calciphylaxis with normal renal function: treated with intravenous sodium thiosulfate. Clin Exp Dermatol. 2012;37(8):874‐878. [DOI] [PubMed] [Google Scholar]
- 31. Ong S, Coulson IH. Normo‐renal calciphylaxis: response to sodium thiosulfate. J Am Acad Dermatol [Internet]. 2011;64(5):e82‐e84. Available from: http://resolver.scholarsportal.info/resolve/01909622/v64i0005/e82_ncrtst. [DOI] [PubMed] [Google Scholar]
- 32. Stanciu M, Gagné‐Henley A, Thérien G. Unusual case of proximal calciphylaxis without renal failure. J Cutan Med Surg. 2011;15(5):290‐292. [DOI] [PubMed] [Google Scholar]
- 33. Kalajian AH, Malhotra PS, Callen JP, Parker LP. Calciphylaxis with normal renal and parathyroid function: not as rare as previously believed. Arch Dermatol. 2009;145(4):451‐458. [DOI] [PubMed] [Google Scholar]
- 34. Hackett BC, McAleer MA, Sheehan G, Powell FC, O'Donnell BF. Calciphylaxis in a patient with normal renal function: response to treatment with sodium thiosulfate. Clin Exp Dermatol. 2009;34(1):39‐42. [DOI] [PubMed] [Google Scholar]
- 35. Isoherranen K, Bouchard L, Kluger N. Benefits of intralesional injections of sodium thiosulfate in the treatment of calciphylaxis. Int Wound J. 2017;14(6):955‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chelliah MP, Zinn Z, Khuu P, Teng JMC. Self‐initiated use of topical cannabidiol oil for epidermolysis bullosa. Pediatr Dermatol. 2018;35(4):e224‐e227. [DOI] [PubMed] [Google Scholar]
- 37. Maida V. Medical cannabis in the palliation of malignant wounds—a case report. J Pain Symptom Manage. 2017;53:e4‐e6. [DOI] [PubMed] [Google Scholar]
- 38. Maida V, Corban J. Topical medical cannabis: a new treatment for wound pain—three cases of pyoderma gangrenosum. J Pain Symptom Manage. 2017;54(5):732‐736. [DOI] [PubMed] [Google Scholar]
- 39. Chang‐Douglass S, Mulvihill C, Pilling S. Cannabis‐based medicinal products: summary of NICE guidance. BMJ [Internet]. 2020;369:m1108. Available from: http://www.bmj.com/lookup/doi/10.1136/bmj.m1108. [DOI] [PubMed] [Google Scholar]
- 40. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17:2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zurier RB, Burstein SH. Cannabinoids, inflammation, and fibrosis. FASEB J. 2016;30:3682‐3689. [DOI] [PubMed] [Google Scholar]
- 42. Sangiovanni E, Fumagalli M, Pacchetti B, et al. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phyther Res. 2019;33(8):2083‐2093. [DOI] [PubMed] [Google Scholar]
- 43. Han KH, Lim S, Ryu J, et al. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res. 2009;84(3):378‐386. [DOI] [PubMed] [Google Scholar]
- 44. Takeda S, Jiang R, Aramaki H, et al. Δ9‐tetrahydrocannabinol and its major metabolite Δ9‐tetrahydrocannabinol‐11‐oic acid as 15‐lipoxygenase inhibitors. J Pharm Sci. 2011;100(3):1206‐1211. [DOI] [PubMed] [Google Scholar]
- 45. Takeda S, Usami N, Yamamoto I, Watanabe K. Cannabidiol‐2′,6′‐dimethyl ether, a cannabidiol derivative, is a highly potent and selective 15‐lipoxygenase inhibitor. Drug Metab Dispos. 2009;37(8):1733‐1737. [DOI] [PubMed] [Google Scholar]
- 46. Richter JS, Quenardelle V, Rouyer O, et al. A systematic review of the complex effects of cannabinoids on cerebral and peripheral circulation in animal models. Front Physiol. 2018;9:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stefano GB, Quinn E, Kream RM. Endocannabinoid Stimulated Release of Nitric Oxide and its Mitochondrial Influence Triggering Vascular Pathology. Pharm Anal Acta. 2015;6(6):378. 10.4172/21532435.1000378. [DOI] [Google Scholar]
- 48. Pucci M, Rapino C, Di Francesco A, Dainese E, D'Addario C, Maccarrone M. Epigenetic control of skin differentiation genes by phytocannabinoids. Br J Pharmacol. 2013;170(3):581‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. D'Addario C, Di Francesco A, Pucci M, Finazzi Agrò A, MacCarrone M. Epigenetic mechanisms and endocannabinoid signalling. FEBS J. 2013;280:1905‐1917. [DOI] [PubMed] [Google Scholar]
- 50. Lewis CJ, Mardaryev AN, Sharov AA, Fessing MY, Botchkarev VA. The epigenetic regulation of wound healing. Adv Wound Care. 2014;3(7):468‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guimarães AG, Serafini MR, Quintans‐Júnior LJ. Terpenes and derivatives as a new perspective for pain treatment: a patent review. Expert Opin Ther Pat. 2014;24:243‐265. [DOI] [PubMed] [Google Scholar]
- 52. Koyama S, Purk A, Kaur M, et al. Beta‐caryophyllene enhances wound healing through multiple routes. PLoS One. 2019;14(12):e0216104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Serra R, Grande R, Butrico L, et al. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Int Wound J. 2016;13(1):88‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gopalakrishnan A, Ram M, Kumawat S, Tandan SK, Kumar D. Quercetin accelerated cutaneous wound healing in rats by increasing levels of VEGF and TGF‐β1. Indian J Exp Biol. 2016;54:187‐195. [PubMed] [Google Scholar]
- 55. Coleridge‐Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta‐analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005;30(2):198‐208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


