Abstract
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract infections among infants with most infections occurring in the first year of life. Multiple RSV exposures are required for children to mount adult-like immune responses. Although adult RSV immunity is associated with less severe disease, the protection induced through natural infection is short-lived. Therefore, vaccination of RSV-experienced young children may accelerate immunity and provide long-term protection from RSV reinfection. However, the extent to which different Th-biased vaccine regimens influence pre-existing humoral and cellular immunity in RSV-experienced young children is unknown. To address this question, infant BALB/c mice were RSV-infected and subsequently immunized with the prefusion RSV F (PreF) antigen formulated with either a Th2-skewing (Alum) or Th1/Th2-balanced (Advax-SM) adjuvant. These studies show that both adjuvants boosted neutralizing antibody and protected from RSV reinfection, but Advax-SM adjuvant prevented the Th2-skewed immunity observed in RSV-experienced young mice immunized with PreF/Alum.
Keywords: Young, Th1/Th2-balanced, vaccination, mice, RSV
1. Introduction
In the first year of life, approximately 70% of infants are infected with RSV and by two years of age, 50% of children have been infected multiple times [1]. Humoral immunity is largely dependent on neutralizing antibody directed against RSV F protein and 3–6 seasons of RSV exposure are required for children’s serum neutralizing antibody titers to reach levels comparable to those seen in adulthood [2]. Furthermore, infant RSV memory T cell responses are insufficient to prevent reinfection [3] and IFNγ-producing T cells are reduced and delayed compared to adults [2]. Thus, a RSV vaccine that accelerates humoral and cellular immunity in RSV-experienced children may confer protection from RSV reinfections. However, the ability of such a vaccine to safely and effectively alter pre-existing infant RSV immunity has not yet been evaluated.
To determine the extent to which RSV F protein subunit immunization affects pre-existing humoral and cellular immunity as well as safety and efficacy, infant BALB/c mice were RSV infected and immunized 3 weeks later with the prefusion conformation of RSV F protein (PreF) formulated with Alum (Th2-polarizing) or Advax-SM (Th1/Th2-balanced) adjuvants. Neutralizing and PreF-specific antibody titers were equivalent among both groups of immunized mice with complete viral protection following RSV challenge. PreF/Alum immunization elicited robust Th2 immunity and increased mucus production, whereas PreF/Advax-SM immunization increased cytolytic CD8+ T cells. Together, these data demonstrate that despite pre-existing immunity generated during infant RSV infection, adjuvants with different Th profiles boost antibody responses and produce discrete cellular immunity when used in PreF immunization of RSV-experienced young mice.
2. Materials and methods
2.1. Mice, Vaccine Administration, and Viral Quantification
Infant mice born to Balb/cJ dams (The Jackson Laboratory, Bar Harbor, ME) were infected with 5x105 pfu/gm RSV L19 at post-natal day 5–6, as previously described [4]. Three weeks later, mice were primed via intramuscular (i.m.) injection (0.37” needle) with 50µl of vehicle (PBS), RSV PreF (DS-Cav1) (10μg/mouse; Jason McLellan, University of Texas at Austin) formulated with Advax-SM™ (Lot#:Vax-SPL-1910–11; 1 mg/mouse; Vaxine Pty Ltd, Bedford Park, Australia) or alum (Lot#:5531; PreF/Alum; 10 mg/mL, Alhydrogel, InvivoGen) and boosted with their respective vaccine formulation 3 weeks later. At 1-week post-boost, mice were intranasally (i.n.) challenged with 5x105 pfu/gm RSV L19 and culled at 4- or 8-days post-infection (dpi). RSV L19 was propagated and viral titers quantified as previously described [5].
2.2. Cell preparation, stimulation, and flow cytometry
Bronchoalveolar lavage (BAL) and lower right lung lobes were collected, processed, and enumerated, as previously described [6]. Cells were stimulated and processed for flow cytometry, as described in Supplemental Methods. Samples were run on a BD LSRFortessa managed by the United Flow Core, University of Pittsburgh. Data was analyzed using FlowJo V10 software (FLOWJO, LLC, OR).
2.3. Histology
Left lungs were gravity-filled with 10% formalin at 4- and 8dpi, as previously described [7]. Lungs were processed and stained with hematoxylin and eosin or Periodic Acid-Schiff (PAS) at the McGowan Institute for Regenerative Medicine (University of Pittsburgh, PA). Lung inflammation and mucus hypersecretion were quantified, as previously described [4, 8].
2.4. Neutralizing and RSV-specific IgG subtype
Serum was collected via submandibular bleed 2–3 days prior to secondary RSV challenge and separated using Gel-Z Serum Separator Tubes (Sarstedt, Germany). Serum was stored at −80°C until heat inactivation (56°C for 30 minutes). Neutralizing antibody titers were determined using a Renilla Luciferase RSV reporter assay; RSV PreF-specific IgG subtypes were determined via ELISA, as detailed in Supplemental Methods.
2.6. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8 software (GraphPad Software, La Jolla, CA). Results are displayed as the mean ± SEM and p values <0.05 were considered significant.
3. Results
3.1. RSV PreF-immunization of RSV-experienced young mice increases neutralizing antibody titers.
To determine the extent to which antibody responses were increased, RSV-experienced young mice were immunized with RSV-PreF adjuvanted with alum (Th2-skewing) or Advax-SM (Th1/Th2-balanced) and serum was collected immediately prior to secondary challenge (Fig. 1A). PreF/Advax-SM and PreF/Alum immunization increased RSV neutralizing antibodies relative to the PBS group and had undetectable virus in the lungs at 4dpi (Fig. 1B–C). PreF/Advax-SM and PreF/Alum groups had elevated levels of IgG2a compared to PBS mice, whereas only PreF/Alum-vaccinated animals had increased IgG1 (Fig. 1D–E). Both PreF/Advax-SM and PreF/Alum groups had ratios < 1 (Fig. 1F), suggesting a Th2-skewed response.
Figure 1. Vaccination of RSV-experienced young mice elicits high RSV neutralizing antibody titers and protects against secondary infection.
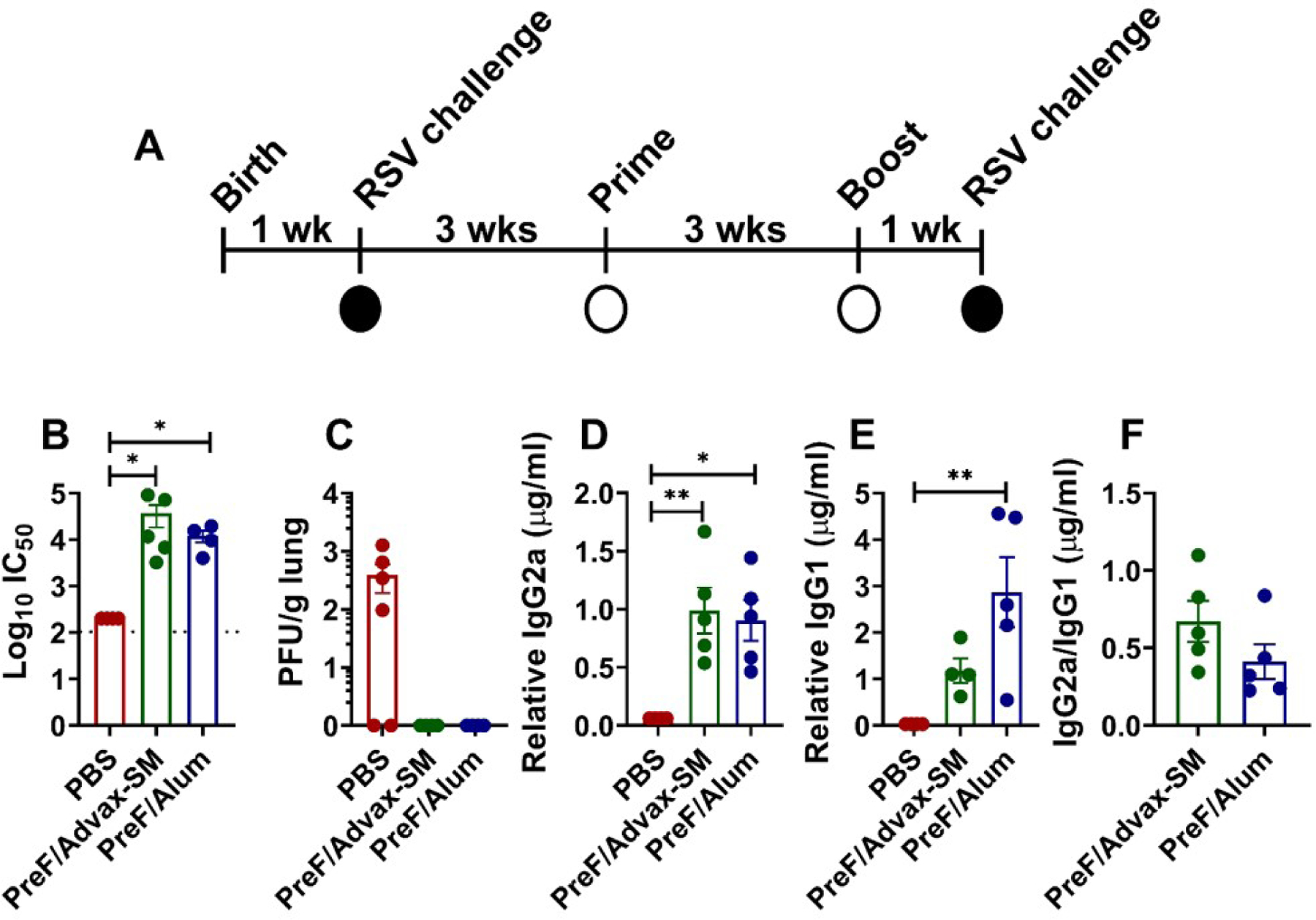
At 5 to 6 days of age, infant BALB/cJ mice were infected with 5x105 PFU/gram of RSV L19. Three weeks later, female mice were immunized with Prefusion RSV F protein (PreF)/Advax-SM, PreF/Alum, or mock-vaccinated with PBS, then boosted 3 weeks after initial immunization. One-week post-boost, mice were bled and subsequently challenged with 5x105 PFU/g of RSV L19. Mice were culled for sample collection at 4- or 8-days post-infection (dpi) (A). RSV neutralizing antibody levels (B), PreF-specific IgG2a (D), PreF-specific IgG1 (E), and IgG2a/IgG1 ratio (F) were obtained from pre-challenge serum. IgG2a/IgG1 ratio was determined by dividing PreF-specific relative IgG2a (µg/mL) by PreF-specific relative IgG1(µg/mL). Left lungs or upper right lungs were harvested to assess viral titers at 4dpi with quantification using H&E plaque assays (C). Viral titers were performed in triplicate and data within each group represent the mean titer for each animal. Data are represented as mean ± SEM. Statistical significance between vaccination groups was calculated using one-way ANOVA with Dunn’s multiple comparison post-test (B and C), one-way ANOVA with Tukey’s multiple comparison post-test (D and E), or unpaired t-test (F) between all groups. *p<0.05 and **p<0.01.
3.2. PreF/Alum elicits Th2-associated innate immunity in RSV-experienced young mice.
To elucidate differential cellular responses in PreF/Advax-SM- and PreF/Alum-immunized mice, innate immune cells were quantified in bronchoalveolar lavage (BAL) and lung at 4dpi. In the BAL, eosinophils were dramatically increased in PreF/Alum-immunized animals, whereas neutrophil and monocyte populations did not differ significantly across groups (Fig. 2A–C). In lung, type 2 innate lymphoid cells (ILC2) and ILC2s producing IL-5 and IL-13 increased in PreF/Alum animals as compared to PBS and PreF/Advax-SM (Fig. 2D–F). Collectively, increased eosinophils and activated ILC2s suggest that PreF/Alum, but not PreF/Advax-SM immunization of RSV-experienced young mice induced a Th2-associated innate cellular profile.
Figure 2. Alum-adjuvanted RSV PreF vaccination elicits a type-2 innate immune response.
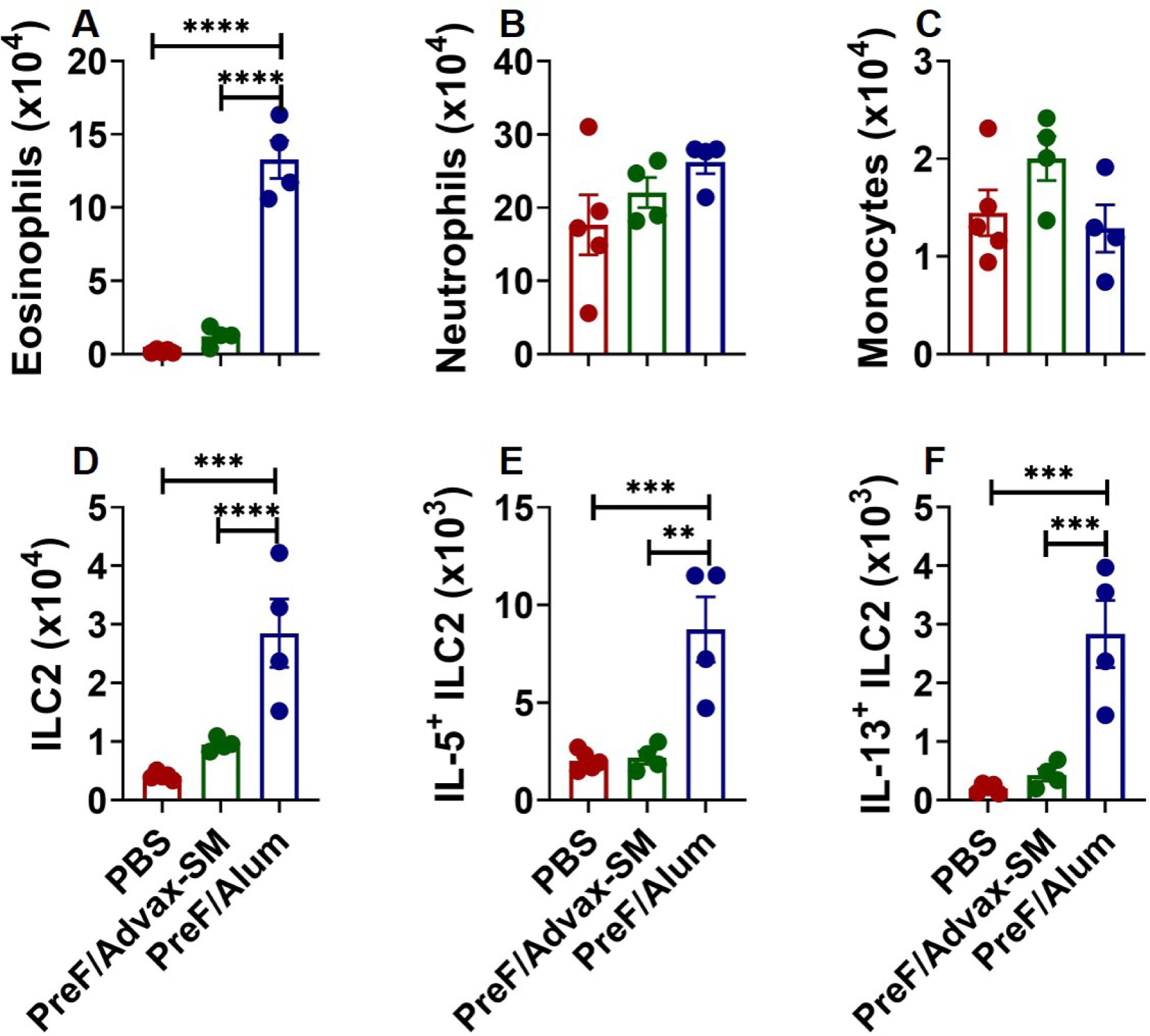
Mice were challenged, immunized, and re-challenged with RSV as described in Figure 1. At 4dpi, BAL was harvested for quantification of eosinophils (A), neutrophils (B), and monocytes (C). Lungs were harvested and homogenized for quantification of ILC2s (D) and ILC2 intracellular cytokine staining of IL-5 (E) and IL-13 (F). Data are represented as mean ± SEM. Statistical significance between vaccination groups was calculated using one-way ANOVA with Tukey’s multiple comparison post-test between all groups. **p<0.01, ***p<0.001, and ****p<0.0001.
3.3. PreF/Alum generates a CD4+ Th2 response, while PreF/Advax-SM promotes cytotoxic CD8+ T cells.
To determine if T cell responses correlate with the Th2-associated innate immunity observed in PreF/Alum immunized mice, T-helper subtypes were analyzed from the BAL. More CD4+ T cells were recovered from PreF/Advax-SM and PreF/Alum-vaccinated mice at 4dpi and remained elevated in PreF/Alum mice at 8dpi compared to PBS and PreF/Advax-SM groups (Fig. 3A). PreF/Advax-SM immunization generated a trend toward greater IFNγ+ CD4+ T cells at 4dpi (Fig. 3B). By 8dpi, similar increases in IFNγ+ CD4+ T cells were observed in all immunization groups. Validating the Th2-associated innate response, PreF/Alum immunization induced an increase in IL-4+ CD4+ T cells at 8dpi (Fig. 3C) coupled with increases in IL-5+ and IL-13+ CD4+ T cells at 4dpi that remained elevated through 8dpi (Fig. 3D–E). Alternatively, PreF/Advax-SM immunization generated greater numbers of CD8+ T cells at 4 and 8dpi, though significance was lost by 8dpi (Fig. 3F). Moreover, CD8+ T cells exhibited a cytotoxic phenotype in PreF/Advax-SM-vaccinated mice, with increased Granzyme B expression at both time points and increased IFNγ+ CD8+ T cells at 8dpi (Fig. 3G–H). Together, these data demonstrate that immunization of RSV-experienced young mice with PreF/Alum skews towards Th2 immunity as compared to PreF/Advax-SM, which favors a cytolytic CD8+ T cell response.
Figure 3. Alum-adjuvanted RSV PreF vaccination elicits a type-2 T cell response.
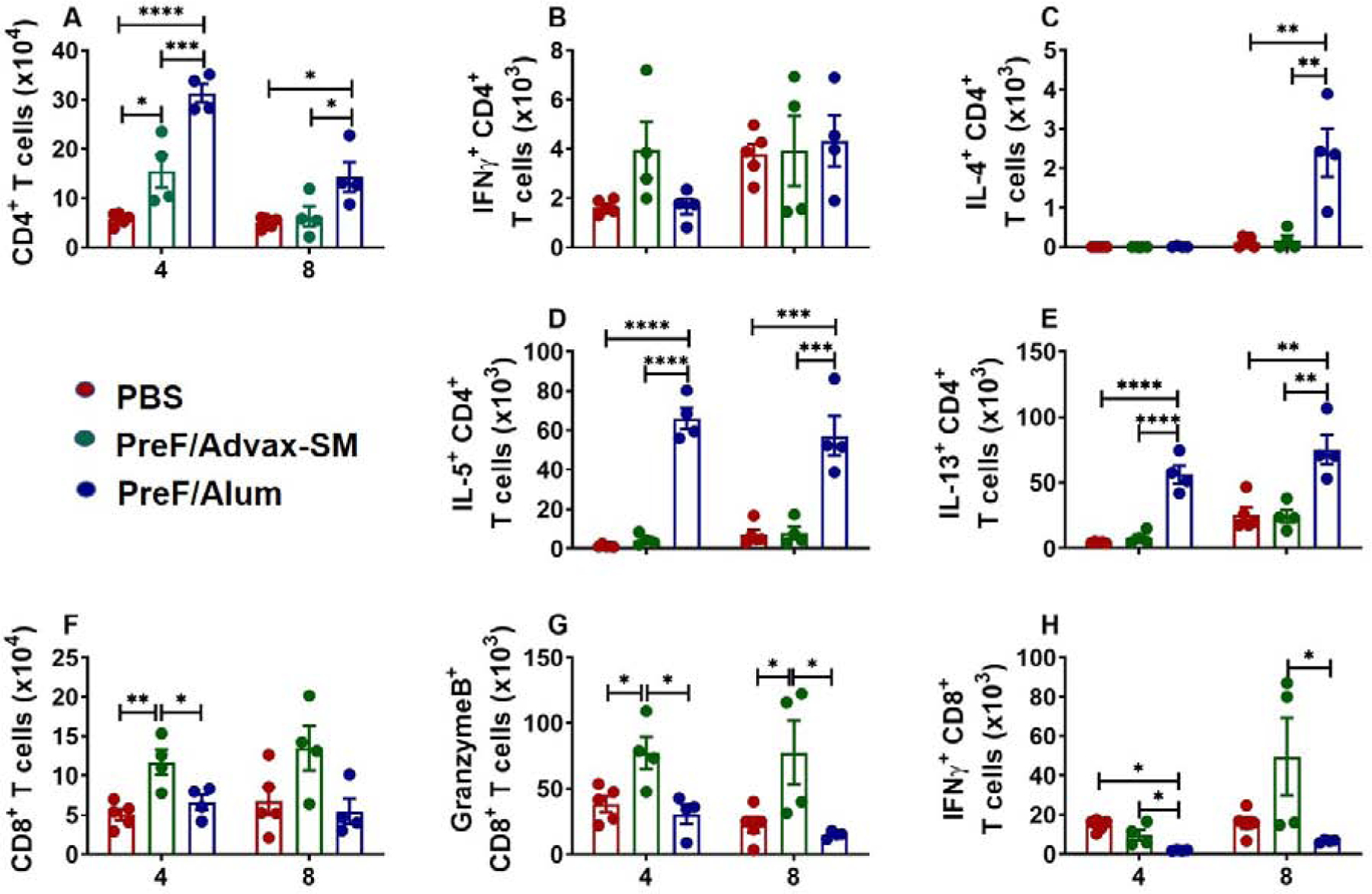
Mice were challenged, immunized, and re-challenged with RSV as described in Figure 1. At 4 or 8dpi, BAL was harvested for quantification of CD4+ T cells (A) and T cell intracellular cytokine staining of IFNγ (B), IL-4 (C), IL-5 (D), and IL-13 (E). CD8+ T cells were also quantified from the BAL at 4 and 8dpi (F) with intracellular cytokine staining of granzyme B (G) and IFNγ (H). Data are represented as mean ± SEM. Statistical significance between vaccination groups at each time point was calculated using one-way ANOVA with Tukey’s multiple comparison post-test between all groups. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
3.4. PreF/Alum-immunized young mice have increased airway mucus production following secondary RSV challenge.
To evaluate whether the differential immune responses in PreF/Advax-SM-versus PreF/Alum-vaccinated animals corresponded with differences in pathology, lung sections were examined for inflammation and mucus production. At 4dpi, all mice had peribronchial and perivascular inflammation but only PBS- and PreF/Alum-vaccinated animals had severity scores of 4 (Fig. 4Aa–f, Supplemental Fig. 1). Overall, inflammation declined by 8dpi (Fig. 4B–C) but PreF/Advax-SM-vaccinated mice had the greatest reduction in inflammation (Fig. 4C).
Figure 4. Airway mucus production is elevated in RSV-experienced young mice vaccinated with alum-adjuvanted RSV PreF.
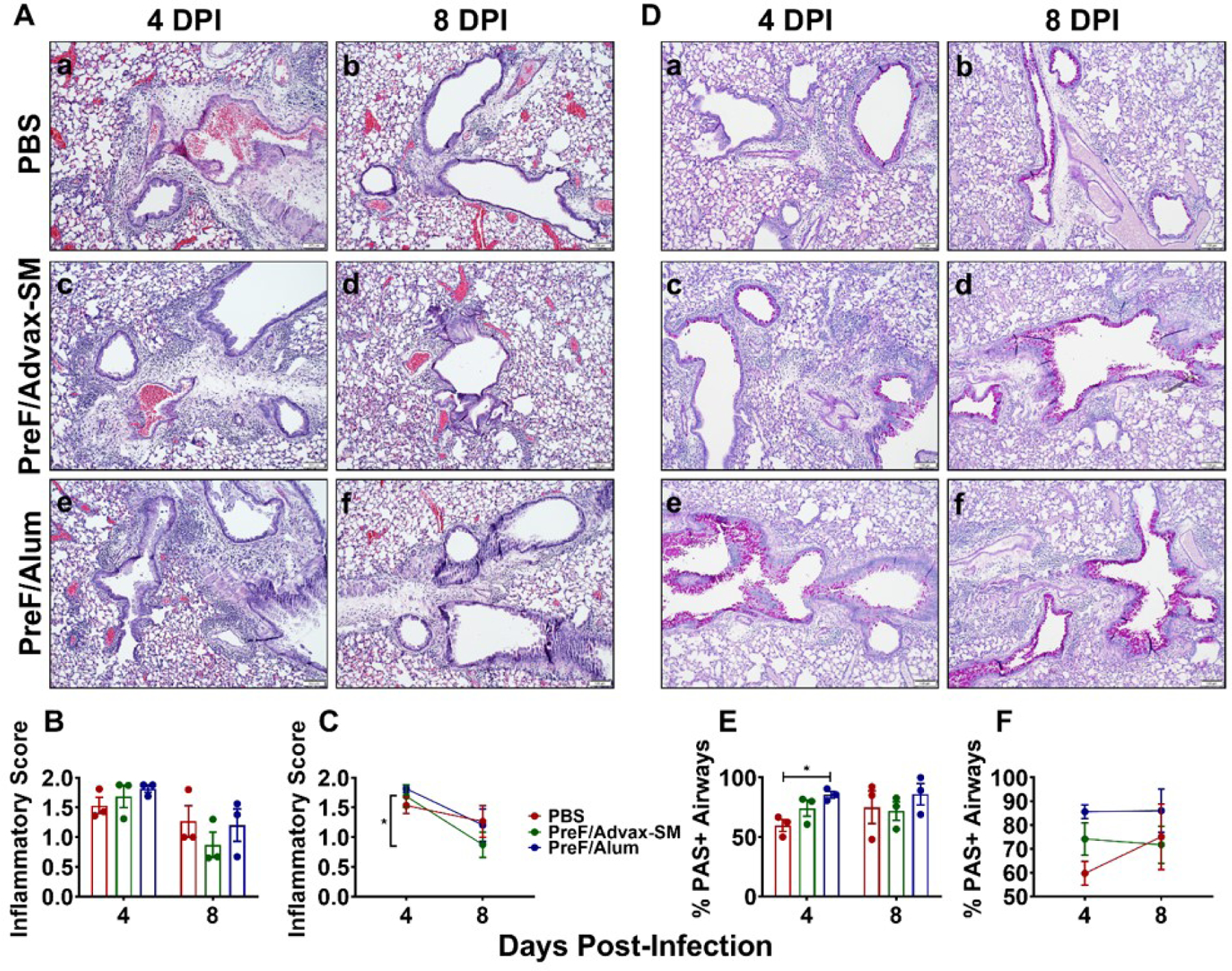
Mice were challenged, immunized, and re-challenged with RSV as described in Figure 1. At 4 or 8dpi, lungs were formalin-filled, paraffin-embedded and sectioned for staining with H&E (Aa-f). Lungs were scored, averaged, and inflammatory score graphed. Data is represented as mean ± SEM (n=3) (B). Inflammatory score trends from 4 to 8dpi are shown in (C). PAS staining was also performed at 4dpi (De-f). To quantify the extent of PAS staining, lungs were scored as described in the methods. Scores were averaged and the total percentage of PAS+ airways were graphed (E). PAS staining quantification trends from 4 to 8dpi are shown in (F). Statistical significance between vaccination groups was calculated in using one-way ANOVA with Tukey’s multiple comparison post-test (B and E) or 2-way ANOVA with Sidak’s multiple comparison post-test (C and F) between all groups. *p<0.05.
Airway mucus production, another hallmark of RSV-mediated lung pathology, was lower in airways of PBS- versus PreF/Alum-immunized mice at 4dpi (Fig. 4E), as determined by Periodic Acid-Schiff (PAS+) staining (Fig. 4Da–f). However, the proportion of PAS+ airways trended up in the PBS group between 4 and 8dpi, with > 1/3 of airways receiving severity scores of 4 (Fig. 4F & Supplemental Fig. 1). Although the proportion of PAS+ airways in the PreF/Advax-SM mice trended lower than PreF/Alum at 4dpi, the difference was not statistically significant. When evaluated over time, PAS+ airways increased in PBS mice, but remained low in the PreF/Advax-SM group (Fig. 4E–F, Supplemental Fig. 1). Alternatively, PreF/Alum-immunized mice maintained the highest proportion of PAS+ airways through 8dpi (Fig. 4E–F, Supplemental Fig. 1). Overall, these data show similar levels of inflammation and mucus production across immunization groups, with notable early and persistently elevated levels of PAS+ airways with PreF/Alum immunization and faster resolution of inflammation in PreF/Advax-SM immunization mice.
4. Discussion
Our results demonstrate that despite prior infant RSV infection, PreF immunization of young mice can boost neutralizing antibody responses and produce discrete cellular immunity that is largely dependent on the vaccine adjuvant. Furthermore, our results suggest that high serum titers of vaccine-induced neutralizing antibodies and undetectable viral replication in the lungs do not guarantee protection from lung pathology in RSV-experienced immunized young mice. Future studies involving RSV PreF vaccination of RSV-experienced young mice should consider evaluating additional indicators of vaccine response, such as T cell immunity and physiological measures of lung function, in determining the overall safety profile of potential RSV vaccines.
RSV exposure occurs early in life, with nearly 70% of infants infected by the age of 1 [1]. These early-life responses to RSV are inefficient, characterized by an inability to produce neutralizing antibody and requiring multiple reinfections to develop short-term protective immunity [2, 9]. The recent stabilization of the prefusion conformation of RSV F protein (PreF) and its ability to generate potent neutralizing antibody when combined with both Th2-polarizing and Th1/Th2-balanced adjuvants has reinvigorated RSV vaccine development [10, 11]. Our results demonstrated that PreF-vaccination with either alum or Advax-SM adjuvants boosted RSV neutralizing antibody production in RSV-experienced young mice compared to PBS-immunized controls and conferred protection from reinfection.
RSV-infected human and murine neonates display an inability to mount strong IFNγ+ T cell responses [2, 4, 12], a characteristic that is associated with more severe acute disease [12] and exaggerated Th2-mediated pathology upon reinfection in mouse models [13, 14]. Therefore, in the context of pre-existing Th2-biased infant RSV immunity, vaccine formulations that promote IFNγ-producing T cell responses may offer an improved safety and efficacy profile. Our results show that PreF immunization, when paired with the Th1/Th2-balanced adjuvant, Advax-SM, boosted neutralizing antibody production and induced an IFNγ+ cytotoxic CD8+ T cell response in RSV-experienced young mice. In contrast, PreF/Alum immunization generated robust Th2 immunity, characterized by increased airway eosinophils, IL-5+ and IL-13+ ILC2s, and Th2 CD4+ T cells, despite complete protection against viral replication. Contrary to previously published reports [13, 14], PBS controls in our study did not mount overt conventional Th2 immunity upon reinfection but was the only group to demonstrate an upward trajectory in the percentage of PAS+ airways between 4 and 8dpi. This worsening over time suggests a possible delay in Th2 kinetics or unconventional sources of the mucus-inducing cytokine, IL-13.
While the elevated and sustained mucus hypersecretion in PreF/Alum-vaccinated mice was unsurprising given overwhelming Th2 immunity, mucus production in PreF/Advax-SM-vaccinated mice was unexpected. Although the evaluation of cellular immunity was limited to the airspace, PreF/Advax-SM-vaccinated mice had no appreciable type 2 response and no classic source of IL-13. Further T cell analysis demonstrated an increase in IL-13+ CD8+ T cells across all groups by 8dpi with a notable increase in PreF/Advax-SM-vaccinated mice as compared to PreF/Alum-immunized mice (Supplemental Fig. 2). This is consistent with the CD8+ Tc2 cell subset, which can express IL-13 and has been shown to play important roles during viral infection and allergic lung inflammation [15, 16]. Moreover, PBS controls generated a 61-fold increase in IL-13+ CD8+ T cells by 8dpi (38.9- and 5.5-fold increases for PreF/Advax-SM and PreF/Alum, respectively), suggesting that delayed, unconventional sources of Th2 cytokines may contribute to lung pathology following repeated RSV infections. However, the increase in IFNγ+ CD8 T cells in PreF/Advax-SM-vaccinated animals at 8dpi may have counteracted the increase in IL-13+ CD8 T cells, mitigating the increase in mucus production observed in PBS controls [17]. This is the first known report of CD8+ Tc2 in a young mouse model of RSV immunization and their role in RSV-mediated histopathology requires further study.
Most infants are exposed to RSV within their first year of life [1] but numerous exposures over multiple RSV seasons are required to achieve adult-like immunity [2]. Although adult RSV immune responses are associated with less severe disease than infants and children, the protection afforded through natural RSV infection in adulthood is short-lived [9]. Therefore, the goal of immunizing RSV-experienced young children is to generate Th1/Th2-balanced RSV immunity that provides long-lasting protection. Using a novel, clinically relevant murine model, we show for the first time that RSV Pre F immunization of RSV-experienced young mice can protect from reinfection. These data further demonstrate that adjuvants with greater Th1-skewing potential may ameliorate aspects of histopathology resulting from RSV memory generated during primary infant RSV infection.
Supplementary Material
Highlights:
PreF+Th1/Th2 adjuvant boosts neutralizing antibodies in RSV exposed young mice
Th2-skewed immunity in PreF/alum-immunized young mice despite RSV protection
PreF+Th1/Th2 adjuvant increases cytotoxic CD8 T cell responses in young mice
Acknowledgements
We would like to especially thank Dr. Nikolai Petrovsky for providing Advax-SM and Dr. Jason McLellan for providing DS-Cav1 (the RSV preF immunogen used in this study).
Funding
Financial support was provided by: 1R43AI140941-01 (PI: Yondola/Empey), David and Betty Brenneman Fund (PI: Empey), 5T32AI089443-07 (K. Eichinger/PI: Shlomchik), 1TL1TR001858 (K. Eichinger/PI: Kapoor), R03-RHD080874A (PI: Empey), American Heart Association (AHA) Individual postdoctoral fellowship:19POST34370078 (Perkins) and the Center for Clinical Pharmaceutical Sciences, University of Pittsburgh School of Pharmacy. Development of Advax-SM adjuvant was supported by National Institutes of Health contracts HHSN272201400053C, HHSN272200800039C and AI061142. This work benefitted from SPECIAL BD LSRFORTESSATM funded by NIH 1S10OD011925-01 (PI: Borghesi). This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of any funding agency.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–6. [DOI] [PubMed] [Google Scholar]
- [2].Green CA, Sande CJ, de Lara C, Thompson AJ, Silva-Reyes L, Napolitano F, et al. Humoral and cellular immunity to RSV in infants, children and adults. Vaccine. 2018;36:6183–90. [DOI] [PubMed] [Google Scholar]
- [3].Bont L, Versteegh J, Swelsen WT, Heijnen CJ, Kavelaars A, Brus F, et al. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatr Res 2002;52:363–7. [DOI] [PubMed] [Google Scholar]
- [4].Empey KM, Orend JG, Peebles RS Jr., Egana L, Norris KA, Oury TD, et al. Stimulation of immature lung macrophages with intranasal interferon gamma in a novel neonatal mouse model of respiratory syncytial virus infection. PLoS One. 2012;7:e40499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol 1988;26:153–62. [DOI] [PubMed] [Google Scholar]
- [6].Eichinger KM, Kosanovich JL, Empey KM. Localization of the T-cell response to RSV infection is altered in infant mice. Pediatr Pulmonol 2018;53:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eichinger KM, Egana L, Orend JG, Resetar E, Anderson KB, Patel R, et al. Alveolar macrophages support interferon gamma-mediated viral clearance in RSV-infected neonatal mice. Respir Res 2015;16:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Perkins TN, Oczypok EA, Dutz RE, Donnell ML, Myerburg MM, Oury TD. The receptor for advanced glycation end products is a critical mediator of type 2 cytokine signaling in the lungs. J Allergy Clin Immunol 2019;144:796–808 e12. [DOI] [PubMed] [Google Scholar]
- [9].Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 1991;163:693–8. [DOI] [PubMed] [Google Scholar]
- [10].McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sastry M, Zhang B, Chen M, Joyce MG, Kong WP, Chuang GY, et al. Adjuvants and the vaccine response to the DS-Cav1-stabilized fusion glycoprotein of respiratory syncytial virus. PLoS One. 2017;12:e0186854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 2003;168:633–9. [DOI] [PubMed] [Google Scholar]
- [13].Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med 2002;196:1381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dakhama A, Park JW, Taube C, Joetham A, Balhorn A, Miyahara N, et al. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J Immunol 2005;175:1876–83. [DOI] [PubMed] [Google Scholar]
- [15].Cerwenka A, Morgan TM, Harmsen AG, Dutton RW. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J Exp Med 1999;189:423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Miyahara N, Takeda K, Kodama T, Joetham A, Taube C, Park JW, et al. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J Immunol 2004;172:2549–58. [DOI] [PubMed] [Google Scholar]
- [17].Cohn L, Homer RJ, Niu N, Bottomly K. T helper 1 cells and interferon gamma regulate allergic airway inflammation and mucus production. J Exp Med 1999;190:1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


