Abstract
In the absence of disease, ageing in the human brain is accompanied by mild cognitive dysfunction, gradual volumetric atrophy, a lack of significant cell loss, moderate neuroinflammation, and an increase in the amyloid beta (Aβ) and tau proteins. Conversely, pathologic age-related conditions, particularly Alzheimer's disease (AD), result in extensive neocortical and hippocampal atrophy, neuron death, substantial Aβ plaque and tau-associated neurofibrillary tangle pathologies, glial activation and severe cognitive decline. Humans are considered uniquely susceptible to neurodegenerative disorders, although recent studies have revealed Aβ and tau pathology in non-human primate brains. Here, we investigate the effect of age and AD-like pathology on cell density in a large sample of postmortem chimpanzee brains (n = 28, ages 12–62 years). Using a stereologic, unbiased design, we quantified neuron density, glia density and glia:neuron ratio in the dorsolateral prefrontal cortex, middle temporal gyrus, and CA1 and CA3 hippocampal subfields. Ageing was associated with decreased CA1 and CA3 neuron densities, while AD pathologies were not correlated with changes in neuron or glia densities. Differing from cerebral ageing and AD in humans, these data indicate that chimpanzees exhibit regional neuron loss with ageing but appear protected from the severe cell death found in AD.
This article is part of the theme issue ‘Evolution of the primate ageing process’.
Keywords: neuron, glia, brain, chimpanzee, ageing, Alzheimer's disease
1. Introduction
As human longevity increases, distinguishing the neurological basis for age-related cognitive decline is imperative. Common cognitive deficits in elderly people include difficulties with complex tasks, word recall, episodic memory and processing speed [1]. Based on magnetic resonance imaging (MRI) studies, these cognitive alterations coincide with decreased brain weight, increased white matter hyperintensity, enlarged lateral ventricles and mild regional volumetric atrophy [2]. Accompanying these gross changes are modest modifications in neurons, dendritic spines, synapses and neurotransmitters along with increased glial activation, reduced cerebral blood flow and a weakening of the blood-brain barrier [3–5]. Ageing also contributes to an individual's risk for developing Alzheimer's disease (AD), the most prevalent form of dementia. AD is a progressive, irreversible brain disorder that results in extensive neocortical and hippocampal neuronal loss and atrophy, amyloid beta (Aβ) protein-containing plaques and vascular deposition, tau-associated neurofibrillary tangles (NFT), neuroinflammation and severe cognitive impairment [6,7].
Distinguishing the earliest stages of AD from healthy ageing remains an area of great interest and a difficult diagnostic problem, although certain metrics, such as regional neuron loss and glial activation, have been established [1]. Age-related decline in neuron numbers is modest in the dentate gyrus and subiculum, while the CA1-CA3, entorhinal cortex, as well as the neocortex are preserved [8–12]. In striking contrast, AD brains exhibit profound neuronal death in the prefrontal and temporal cortex, entorhinal cortex, CA1 of the hippocampus, dentate gyrus and subiculum [8,9,13–17]. Another discriminating factor between ageing and AD is the severity of neuroinflammation, which is assessed, in part, by changes in glial density, activation and morphology [18]. Total glial density does not change in the human neocortex during the normal course of ageing or AD [19–21]. However, glial subtypes, such as microglia and astrocytes, are altered in both conditions [22,23]. As the brain's primary immune cell, microglia activate and proliferate with age in the neocortex, including the hippocampus and entorhinal cortex, of healthy humans [23,24]. AD brains display greater microglial activation and proliferation concomitant with Aβ plaques, particularly in the hippocampus [25–27]. Astrocytes provide metabolic and structural support to neurons, regulate neurogenesis, and modulate synaptic activity and neurotransmitter homeostasis [28]. Astrogliosis, as indicated by the upregulation of glial fibrillary acidic protein (GFAP), hypertrophy of the soma and cellular processes, and loss of domain organization, has been associated with normal ageing, and to a larger extent in AD [28–32].
Humans are considered uniquely susceptible to neurodegenerative disease, such as AD, but several recent studies have revealed AD-like pathology in the brains of non-human primates [33–37]. Aged lemurs, prosimians, monkeys and great apes exhibit diffuse and neuritic amyloid plaques as well as vascular amyloid, although cognitive changes based on plaque burden were not observed in aged macaques [34,35,38–46]. Furthermore, the presence of hyperphosphorylated tau has been reported in lemurs, squirrel and rhesus monkeys, baboons, and gorillas adjacent to Aβ deposition, and African green monkeys and aged chimpanzees exhibited NFT [34,35,46–49]. Closest in phylogeny to humans, non-human primates also exhibit senescence-related changes [33,50,51]. Mouse lemurs, marmosets, rhesus monkeys and apes show mild cognitive variations with age in spatial memory and executive function [52–57]. Some MRI studies in ageing mouse lemurs and rhesus macaques show progressive volumetric atrophy in the prefrontal cortex (PFC) and decreased brain weight in chimpanzees [55,58,59]. However, a more recent, comprehensive MRI analysis found no overt atrophy of volume in the neocortex and white matter in chimpanzees [51,59]. Postmortem analyses in primates have detected mild age-related dendritic atrophy, synapse loss, white matter damage, gliosis (i.e. activation of astrocytes and microglia), neurotransmitter alterations, and increases of Aβ in brain parenchyma and in cerebral vasculature [34,60–63].
Previous studies of ageing in non-human primates reported a lack of neuron loss in the neocortex, including CA1, entorhinal cortex and subiculum, with age [64–69]. Additionally, microglial activation has been observed in the frontal cortex, cingulum bundle and primary visual cortex, although numbers of activated microglia did not increase significantly with age in the visual cortex, substantia nigra and ventral tegmental area of aged rhesus monkeys [50,70,71]. Heightened expression of major histocompatibility complex class II (MHC-II) antigen, a marker of activated microglia, also was identified in the cerebral cortex and white matter of pig-tailed macaques [72]. Astrocyte activation in the form of higher GFAP expression was detected in the aged rhesus monkey hippocampus and PFC, although astrocyte density did not vary [73].
Formerly, we analysed the brains of 20 aged chimpanzees for evidence of Aβ and tau lesions as well as microglia and astrocyte activation [34,74,75]. Aβ was observed in plaques and, most predominantly, in blood vessels, which correlated with increased tau. Tau lesions were found in the form of AT8-immunoreactive (ir) pretangles, NFT and neuritic clusters (NC) of aggregated dystrophic neurites, and NFT were observed in apes that exhibited plaques and moderate or severe cerebral amyloid angiopathy (CAA), a condition in which amyloid accumulates in the brain's vasculature. Age correlated with greater volumes of Aβ plaques and vessels, but not tau or activated microglia and astrocyte densities. Like AD, Aβ42 deposition was positively associated with higher hippocampal microglial activation in chimpanzees, while astrogliosis occurred in both the hippocampus and PFC layer I in conjunction with Aβ and tau proteins. Contrary to AD, activated microglia density was not significantly correlated with tau lesions and astrogliosis was not identified in other cortical layers in chimpanzees. Despite certain comparable age-related and AD-like pathologies identified in non-human primate brains, only humans exhibit major neuronal loss and severe cognitive decline as observed in clinical AD. Thus, building on our prior investigations, we quantified regional neuron density, glia density, and glia:neuron ratios in the dorsolateral PFC, middle temporal gyrus (MTG), and CA1 and CA3 hippocampal subfields of chimpanzees to determine if ageing or AD pathology correlates with regional neuron loss or glial activation.
2. Material and methods
(a). Specimens and sample processing
Postmortem brain samples from 12 male (ages 17–62 years) and 16 female (ages 12–58 years) chimpanzees (Pan troglodytes, electronic supplementary material, table S1) were acquired from Association of Zoos and Aquariums-accredited zoos and American Association for Accreditation of Laboratory Animal Care-accredited research institutions through the National Chimpanzee Brain Resource (NIH grant: NS092988). The chimpanzees included in this study did not participate in formal behavioural or cognitive testing and were maintained in accordance with each institution's animal care and use guidelines. Available health information for each animal has been included in electronic supplementary material, table S2. Depending on availability, samples were taken from the right or left hemispheres. Brains were collected postmortem after death from natural causes (approximately less than or equal to 20 h PMI), immersion-fixed in 10% buffered formalin for a minimum of 10 days, and transferred to a 0.1 M buffered saline solution containing 0.1% sodium azide at 4°C for storage. Samples were cryoprotected in a graded series of sucrose solutions (10, 20 and 30%) and cut frozen into 40 µm-thick sections in a coronal plane using a Leica SM2000R freezing sliding microtome (Buffalo Grove, IL). Sections then were placed into individual centrifuge tubes containing a cryo-protection solution (30% dH2O, 30% ethylene glycol, 30% glycerol, 10% 0.244 M phosphate-buffered saline (PBS)), numbered sequentially, and stored at −20°C until histological or immunohistochemical processing. Every 10th section in each region was stained for Nissl substance with a 0.5% cresyl violet solution to reveal cell somata, define cytoarchitectural boundaries, and quantify neuron density (Nv), glia density (Gv) and glia to neuron (G : N) ratio (figure 1). Previously, immunohistochemistry was performed for markers of hyperphosphorylated tau (AT8), Aβ42, microglia (Iba1, ionized calcium-binding adaptor molecule 1), and astrocytes (GFAP, glial fibrillary acidic protein) in the same regions using the avidin-biotin-peroxidase method and 3,3′-diaminobenzidine (DAB) with nickel enhancement or NovaRed (figure 2) [34].
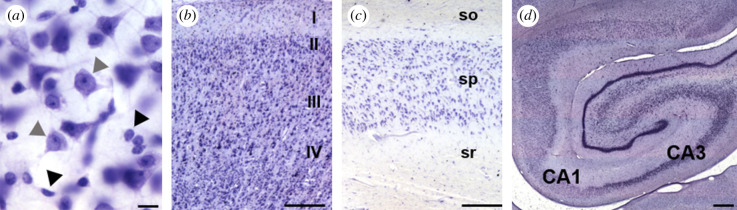
Figure 1.
Photomicrographs of Nissl staining in the chimpanzee brain: (a) classification of neurons (grey arrowhead) and glia (black arrowhead), (b) layer III in the MTG, (c) stratum pyramidale layer of the CA1 and (d) a photo montage delineating the CA1 and CA3 hippocampal subfields. Abbreviations: so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum. Scale bar = 25 µm (a), 250 µm (b–d). (Online version in colour.)
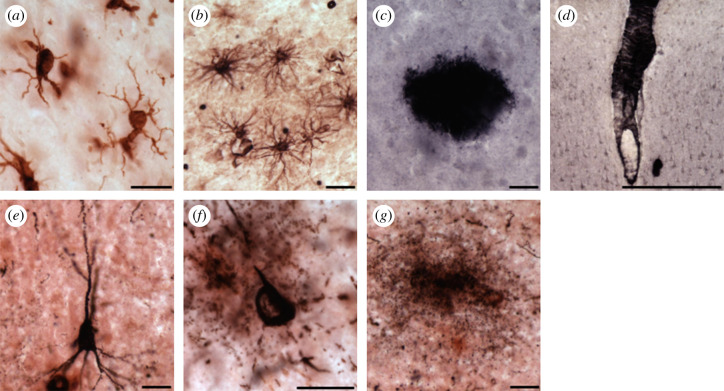
Figure 2.
Photomicrographs of Iba1-ir microglia (a), GFAP-ir astrocytes (b), Aβ42-ir plaque (c), leptomeningeal vessel (d), AT8-ir pretangle (e), NFT (f) and tau neuritic cluster (g) in the chimpanzee PFC (a–d,f) and hippocampus (e,g). Scale bars = 25 µm (a–c,e–g), 250 µm (d). (Online version in colour.)
(b). Regions of interest
We analysed layer III in Brodmann's areas 9 and 10 of the dorsolateral PFC and Brodmann's area 21 of the MTG, as well as the stratum pyramidale in the hippocampal subfields CA1 and CA3 (figure 1). Sampled areas were selected based on prior reports that demonstrated involvement of these regions in both ageing and AD pathology [76–78]. Humans with AD display extensive neuron and synapse loss in layers III and V of the neocortex and stratum pyramidale in the CA1 field, and neuritic Aβ plaques and NFT are most prevalent in these cortical layers [5,16,17,79,80]. Chimpanzees also display Aβ and tau pathologies in the neocortex and hippocampus [34,40].
(c). Stereologic data acquisition
Quantitative analyses were performed using computer-assisted stereology with an Olympus BX-51 photomicroscope equipped with a digital camera and StereoInvestigator software v. 11 (MBF Bioscience, Williston, VT) by two observers blinded to age, sex and pathology. Initial subsampling techniques were performed for each probe to determine appropriate sampling parameters [81]. Regional Nv and Gv were obtained using the optical fractionator probe at 40× (N.A. 0.75) under Köhler illumination. Counting frames were set at 100 × 100 µm with a grid size of 250 × 250 µm, a disector height of 7 µm, and a guard zone of 2%. Beginning at a random starting point, three equidistant sections (every 10th section) per region of interest and animal were selected for analysis. A marker was placed on the nucleus of neurons and on glia when encountered within the optical disector frame, and mounted section thickness was measured at every fifth sampling site. Neurons were identified by the presence of a nucleus, nucleolus and axonal hillock, while glia were distinguished by their lack of nuclei and granules of heterochromatin, giving a speckled appearance. For each region, Nv and Gv (per mm3) were calculated as the population estimate divided by sum volume of the examined disectors, and the G : N ratio was calculated as Gv/Nv [82]. The percentage (%) of gain or loss between old and young animals was determined with the following equation: (aged density or ratio/young density or ratio × 100) − 100. To correct for tissue shrinkage in the z-axis, the height of the disector was multiplied by the ratio of section thickness to the actual weighted mean thickness after mounting and dehydration. No correction was necessary for the x and y dimensions, because shrinkage in section surface area is minimal [83]. The mean number of sampling sites per individual was 32 ± 6 (mean ± s.d.) for PFC and MTG and 65 ± 7 for the CA1 and CA3. The mean number of markers per individual for neurons was 375 ± 97 and for glia was 610 ± 227 in the PFC and MTG. In the CA1 and CA3, the mean number of markers per individual for neurons was 397 ± 33 and for glia was 960 ± 72. The average CE for all regional neuron densities was 0.06 and for glia densities was 0.05.
(d). Statistical analyses
Data were previously collected for Aβ42 plaque and vessel volume (%), AT8-immunoreactive (ir) pretangle, NFT and tau NC densities, Iba1-ir microglia densities, and GFAP-ir astrocyte densities from aged apes (i.e. greater than or equal to 37 years old; figure 2) [34,74,75]. Young chimpanzees (i.e. greater than or equal to 35 years) were assessed for AD pathology, which was absent with the exception of a few AT8-ir pretangles in the PFC of one 12-year-old female that died from peritonitis, and Iba1-ir microglia densities and GFAP-ir astrocyte densities [34,74,75]. All pathology densities and volumes were checked for linearity, and because of skewness of means close to zero, densities and volumes were transformed using the formula: arcsin (sqrt (density/1000)). To evaluate neuropathologic changes for each individual, a brain age value from 0 to 60 was computed using a pathology scoring system adapted from staging guidelines for Aβ and NFT deposition in AD and CAA [34]. Principal component analysis (PCA) was performed to reduce the number of pathological variables to the most relevant factors for brain age in chimpanzees. Four factors—AT8-ir NC densities, NFT density, and AB plaque and vessel volumes—explained 57% of the variance, and all variables had primary loadings between 0.67 and 0.87. Regression factors (PCA-generated pathology factor) created from this prior analysis were employed for further regression analyses with Nv and Gv in this study. Data were tested for a normal distribution and for outliers using the ROUT method (Q = 1), and outliers were excluded. Linear regression analyses were used to determine relationships between the dependent variables of regional Nv, Gv and G : N, and independent variables of chronological age, PCA-generated pathology factor, Aβ42 plaque and vessel volumes (%), and pretangle, NFT, and tau NC densities (per mm3). Sex and brain region variations were examined using two-way (sex) or mixed model (region) ANOVAs with Bonferroni post hoc tests. Statistical analyses were conducted using GraphPad Prism 8.3.0 (San Diego, CA), and the level of significance (α) was set at 0.05.
3. Results
Regional Nv, Gv and G : N were quantified for layer III of the PFC and MTG as well as the pyramidal layer of the CA1 and CA3 hippocampal subfields (figure 1). Average densities and ratios for young (n = 8, 12–35 years), aged (n = 20, 37–62 years), and all chimpanzees in addition to the per cent gain or loss between old and young animals for each region and variable are shown in electronic supplementary material, table S3. Using previously collected microglia and astrocyte densities from tissue in the same animals, along with the total glia density from the current study, the breakdown of glial subtypes was calculated for each region examined with an average across all areas of 81% oligodendrocytes, 8% astrocytes and 11% microglia (electronic supplementary material, table S4) [74,75].
(a). Region and sex
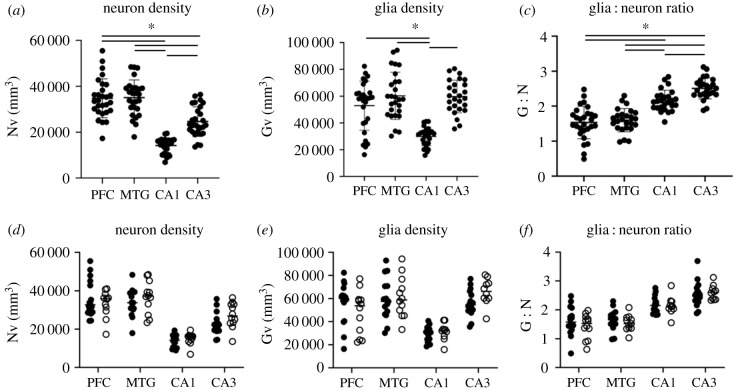
Mixed model ANOVA with Bonferroni multiple comparison tests revealed that PFC and MTG Nv are significantly higher than CA1 (PFC: t27 = 12.01, MTG: t26 = 13.54, ps ≤ 0.01) and CA3 (PFC: t27 = 4.97, MTG: t26 = 5.97, ps ≤ 0.01) but do not differ from each other (t26 = 0.17, p = 0.99; figure 3a). CA3 Nv was higher than CA1 (t27 = 12.44, p ≤ 0.01). Glia density was significantly higher in the PFC and MTG than CA1 (PFC: t25 = 5.77, MTG: t24 = 8.69, ps ≤ 0.01), but not CA3 (PFC: t25 = 1.43, MTG: t24 = 0.11, ps ≥ 0.99; figure 3b). PFC Gv did not vary from MTG (t26 = 2.14, p = 0.25). As with Nv, CA3 Gv was greater than CA1 (t25 = 16.26, p ≤ 0.01). Both PFC and MTG G : N ratios were significantly lower than CA1 (PFC: t25 = 5.14, MTG: t22 = 5.94, ps ≤ 0.01) and CA3 (PFC24: t = 8.18, MTG: t21 = 9.48, ps ≤ 0.01), while CA3 was significantly greater than CA1 (t24 = 5.76, p ≤ 0.01; figure 3c). G : N in the PFC did not differ from MTG (t24 = 1.01, p = 0.99). Two-way ANOVA revealed no sex differences in Nv (F1,103 = 1.61, p = 0.21), Gv (F1,99 = 0.24, p = 0.63) or G : N (F1,97 = 0.61, p = 0.44; figure 3d–f) for any region examined.
Figure 3.
Scatter plots of regional neuron density (a), glia density (b) and glia:neuron ratios (c) in the chimpanzee brain. Although variations were found by region (a–c), sex differences were not observed (d–f; solid circle, females; open circle, males). *p ≤ 0.05.
(b). Age
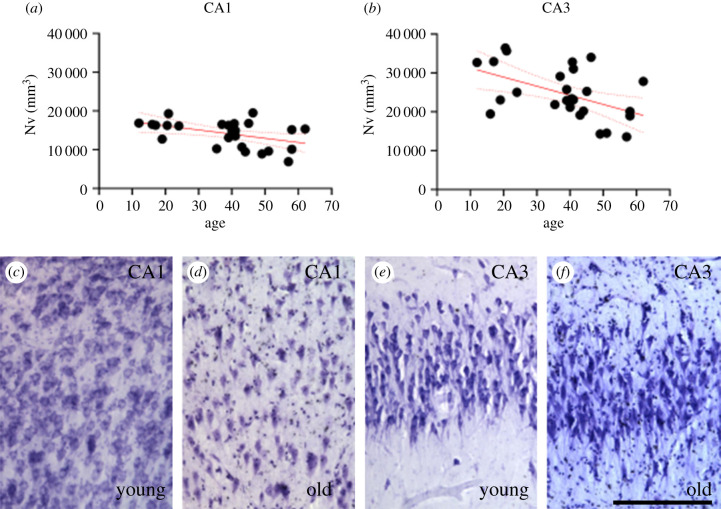
Age was associated with significantly decreased Nv in both CA1 and CA3 (figure 4, ps ≤ 0.02; electronic supplementary material, table S5). Age-related changes were not observed in PFC or MTG Nv (electronic supplementary material, figure S1A-B, ps ≥ 0.29). There were no age-related changes for Gv (electronic supplementary material, figure S1C-F, ps ≥ 0.07), or G : N in any of the regions examined (electronic supplementary material, figure S1G-J, ps ≥ 0.10; electronic supplementary material, table S5).
Figure 4.
A decrease in CA1 and CA3 neuron densities (Nv, mm3; a,b) was correlated with older age in the chimpanzee brain (ps ≤ 0.02). Photomicrographs of Nissl staining in hippocampal subfields CA1 (c,d) and CA3 (e,f) in a young chimpanzee (c,e) and an old (d,f) chimpanzee brain. Scale bar = 250 µm. (Online version in colour.)
(c). Alzheimer’s disease pathology
To determine an overall Aβ and tau score for each chimpanzee, a PCA-generated pathology factor was calculated as previously described [34]. Linear regression analyses were used to investigate the association of regional Nv, Gv or GN with PCA-generated pathology factors and regional AD pathology measurements previously collected in the 20 oldest apes (i.e. greater than or equal to 37 years [34]). Pathologic markers included Aβ42-ir plaque and vessel volumes (%) and AT8-ir pretangle, NFT and NC densities (per mm3) collected in the PFC, MTG, CA1 and CA3. Briefly, plaques were defined as extracellular accumulations of insoluble Aβ42, while vascular amyloid was quantified when Aβ42 deposition was present in the vessel walls. Pretangles were characterized as having an intact cell soma, the presence of diffuse punctate hyperphosphorylated tau (AT8) immunostaining in the cytoplasm, well-preserved dendrites and a nucleus. NFT were identified based on intraneuronal aggregates of hyperphosphorylated tau, and distorted, shortened or absent dendrites and axons. Tau NC contained clusters of dystrophic neurites, consisting of AT8-ir swollen axons and dendrites, or diffuse, punctate staining.
Regional Nv, Gv and G : N were not correlated with PCA-based pathology factors or AD pathologies with two exceptions (electronic supplementary material, table S6, ps ≥ 0.07). In the MTG only, Aβ42 vessel volume was associated with decreased Nv (R2 = 0.23, p = 0.05; electronic supplementary material, figure S2A), even after excluding two outliers with significantly high levels of amyloid deposition (R2 = 0.28, p = 0.04; electronic supplementary material, figure S2B,E-F). However, to correct for multiple comparison testing error, we performed a Bonferroni correction (p = 0.002), and the correlation of MTG Aβ42 vessel volume and Nv was no longer significant. Additionally, pretangle density was negatively correlated with Nv in the CA1 (R2 = 0.19, p = 0.05; electronic supplementary material, figure S2C), but after removal of two outliers with high pretangle numbers, the association was non-significant (R2 = 0.00, p = 0.88; electronic supplementary material, figure S2D,G-H).
4. Discussion
Several studies identified senescence-related changes and AD-like pathology in the brains of non-human primates [33–35,37,38]. Yet, none established whether Aβ or tau pathologies were associated with the profound neuron loss and neuroinflammation observed in the human AD brain. To address this knowledge gap, we quantified Nv, Gv and G : N in postmortem tissue obtained from a large cohort of chimpanzee brains with markers of AD pathology.
Neuron density decreased moderately with age in the CA1 (12%) and CA3 (19%) hippocampal subfields in this sample of chimpanzees. These data diverge from prior investigations in older non-human primates and humans [5,12,65,84]. Previously, no changes in neuron number with age were found in CA1 and CA3 for chimpanzees and rhesus macaques [65,84]. However, both studies were limited in the number of animals (chimpanzees = 6; macaques = 8) compared to the current study of 28 apes. Neuron numbers were reported rather than densities, and both the polymorphic and pyramidal layers were measured in macaques. In elderly humans, the majority of studies of CA1 and CA3 neurons demonstrated preservation during physiological ageing [5,9,10,12,85]. Although the human and chimpanzee hippocampus appears to be differentially affected by ageing, this region is vulnerable to neuronal death in both species with age and neurodegeneration.
A non-significant trend of neuron loss (20-24%) was associated with Aβ42 vascular deposition in the temporal cortex of chimpanzees. Previously, we found that Aβ42 was threefold higher in neocortical vessels compared to the hippocampus [34]. In addition, Aβ plaque and vessel volumes were significantly greater in older apes, suggesting that age-related increases contribute to neuronal toxicity in MTG of aged chimpanzees. This concept is further supported by the lack of neuron loss with age in this region. Notably, pretangle densities were highest in the MTG compared to the hippocampus, although pretangles did not correlate with neuron loss [34,40]. AD pathologic markers in great apes demonstrate similar regional staging progression as humans with Aβ deposits occurring first in the neocortex, whereas NFT initiate in the medial temporal lobe and brainstem [86,87]. Thus, MTG may represent an area in which Aβ and tau pathologies converge in chimpanzees. Moreover, MTG exhibits the largest, albeit nonsignificant, decrease in Gv (18%) and G : N (34%) with age and the lowest density of microglia in these apes (electronic supplementary material, table S3). In AD, microglia play an important role in the removal of Aβ peptides through phagocytosis, and physiological senescence in microglia is accompanied by the release of inflammatory cytokines, which have detrimental effects on neurons [88]. Consequently, in chimpanzees, the low number of microglia in MTG may result in decreased Aβ42 phagocytosis, exacerbated by an age-associated increase in Aβ42-ir vasculature and high numbers of pretangles, leading to neuronal death.
Humans with AD differ from chimpanzees in that significant neuronal loss occurs in the PFC and CA1 as well as the temporal cortex [5,14,16,89,90]. The absence of cell death in the chimpanzee hippocampus could be due to the increase in severity of NFT pathology in AD relative to milder NFT densities in aged chimpanzees. In AD, NFT correlates strongly with neuronal loss and cognitive decline [91,92]. Therefore, we compared CA1 Nv in apes with pretangles (13 581 mm−3) or NFT (115 959 mm−3) to those without (15 025 mm−3), and observed a trend of mild decline with tau pathologies. Moreover, a 57-year-old male chimpanzee with the highest CA1 pretangle and NFT densities exhibited the lowest Nv (6902 mm−3) of all the apes and was an outlier in our original analysis, which showed a negative correlation of CA1 pretangle density and Nv (electronic supplementary material, figure S2C). Once this animal was removed, the association no longer remained (electronic supplementary material, figure S2D). Furthermore, like humans, Aβ plaque and vascular levels were higher in the neocortical regions compared to the hippocampal subfields in these chimpanzees.
Neocortical and hippocampal glia densities were not associated with age or AD pathology in chimpanzees. Our previous findings support the lack of age and pathology-related variation in microglia and astrocyte densities in the same apes and brain regions [74,75]. Human studies also found that astrocyte numbers did not change with age or AD pathology, and microglia density did not differ in the temporal cortex of control and AD brains [19,20,93]. Instead, expression of GFAP and MHC-II, markers of astrocyte and microglia activation, increased in AD patients, implicating a phenotypic change instead of a marked proliferation [93]. Greater microglial activation was also detected in the hippocampus in elderly non-demented subjects [23]. Non-human primates demonstrate a similar pattern. Rhesus monkeys exhibited both senescence-associated expression of MHC-II and activated microglia concomitant with fibrillar Aβ plaques with clusters of phosphorylated tau-containing swollen neurites [72,94,95]. Likewise, Aβ oligomers trigger astrocyte and microglial activation in long-tailed macaques, and microglial activation was observed in the brains of aged common marmosets in conjunction with Aβ and hyperphosphorylated tau deposition [46,96]. In chimpanzees, greater volumes of Aβ42 plaque and vessel deposition were correlated with higher hippocampal microglial activation, while tau deposition was significantly increased in activated, intermediate microglia [34]. This evidence suggests that overall glia densities are not impacted by age or AD pathology in great apes, although glial activation increases in proximity to Aβ and tau deposition.
Unexpectedly, the breakdown of glial subtypes in chimpanzees (81% oligodendrocytes, 8% astrocytes and 11% microglia) diverges from estimates in humans (males: 75% oligodendrocytes, 20% astrocytes, 5% microglia), who have a higher percentage of astrocytes and lower percentage of microglia [20]. Rhesus monkey brains have a distribution of 35% oligodendrocytes, 57% astrocytes and 7% microglial cells in the cortex [97]. Although potentially a result of different quantification methods, the species-related variation in glial makeup may be an important difference in humans and could play a role in the reduced neuroinflammation observed in ape brains during ageing, although further analyses are required.
Glia : neuron ratios were not associated with age or pathology in chimpanzees. Average G : N were higher in the hippocampus (2.36) compared to the neocortex (1.57). These results are in accordance with previous studies in other non-human primates and humans. A neocortical G : N of 1.70 was reported in rhesus monkeys and 1.98 in chimpanzees, while human cortical grey matter ranges from 0.6 to 4.0 with an average of 1.79 [72,98,99]. In addition, a G : N of 1.37 was found in the neocortex of AD patients and controls, suggesting the ratio does not change with disease [21].
Comparing differences in the brains of humans and great apes can enhance our understanding of the selective vulnerability of humans to neurodegenerative diseases, such as AD (table 1). In the current study, we found that chimpanzees experience limited decreases in Nv in association with physiologic and pathologic ageing, although not to the same severity as AD. A caveat of this investigation, though, is the lack of antemortem cognitive testing, which is necessary to determine whether neuronal loss is associated with cognitive decline in aged chimpanzees. Moreover, the contribution of life-history factors, such as social environment, diet, body metabolism and physical activity, that may affect neurological variation were not examined in these animals. These data highlight and further support the role for non-human primates as models of ageing and neurodegenerative diseases due to their long lifespans, genomic similarities, and complex physiology and cognition.
Table 1.
Summary of neuropathologic changes in ageing and Alzheimer's disease in chimpanzees and humans.
| pathology | Alzheimer's disease |
ageing |
||
|---|---|---|---|---|
| chimpanzee | human | chimpanzee | human | |
| Aβ | Aβ is primarily in blood vessels and occurs prior to plaque formation | Aβ is primarily in plaques, although CAA occurs in 80% of AD patients | chimpanzees demonstrate increased intravascular Aβ deposition with age | humans exhibit increased Aβ deposition in plaques and vessels with age |
| Aβ pathology is associated with increased tau pathology | ||||
| tau | neuritic clusters contain dystrophic tau neurites but lack an Aβ core | neuritic plaques contain an Aβ core | pretangles in the neocortex increase with age | NFT increase with age in the hippocampus |
| neuro-inflammation | Aβ42 is correlated with increased microglial activation (i.e. plaques in humans, vessels in chimpanzees) | age is not associated with increased microglial or astrocyte density or activation in chimpanzees | humans display both increased microglial activation and density with age | |
| microglial activation is correlated with Aβ but not NFT lesions | microglial activation is associated with Aβ and NFT pathology | |||
| neuron loss | neuron loss occurs in the temporal cortex in association with Aβ deposition in the brain's blood vessels | selective neuronal loss occurs in the prefrontal cortex and hippocampus | neuron loss was observed in the hippocampus of chimpanzees | selective neuronal loss occurs in the subiculum and dentate gyrus but not CA1-CA3 subfields |
| cognitive impairment | antemortem cognitive testing in chimpanzees with AD pathology has not been performed yet | severe memory, cognitive and behavioural deficits are observed | cognitive testing is rare in aged apes; mild cognitive deficits in spatial memory, attention, executive function, and cognitive flexibility have been noted | processing speed, attention, memory and cognitive flexibility gradually decline during ageing |
Supplementary Material
Acknowledgements
We thank Cheryl Stimpson and Bridget Wicinski for their technical assistance.
Ethics
All samples were acquired postmortem from American Zoo and Aquarium-accredited zoos and American Association for Accreditation of Laboratory Animal Care-accredited research institutions through the National Chimpanzee Brain Resource.
Data accessibility
Data will be available on the National Chimpanzee Brain Resource website: https://www.chimpanzeebrain.org.
Authors' contributions
All authors have approved this manuscript. M.K.E., P.R.H. and M.A.R. designed research; C.C.S., W.D.H., J.J.E., J.M.E. and P.R.H. provided samples; M.K.E. and E.L.M. collected data; M.K.E., R.S.M. and M.A.R. analysed data; and M.K.E., C.C.S., R.S.M., W.D.H., J.J.E., J.M.E., E.J.M., P.R.H. and M.A.R. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Funding
This work was supported and funded by the NSF (BCS-1316829, M.A.R.), NIH (NS042867, NS073134, NS092988, W.D.H., C.C.S.; AG017802, J.J.E.; AG014308, J.M.E.; AG005138, P.R.H.; AG014449, AG043375, E.J.M.), James S. McDonnell Foundation (220020293, C.C.S.), Sigma Xi (M.K.E.), KSU Research Council (M.A.R.) and KSU Graduate Student Senate (M.K.E.).
References
- 1.Toepper M. 2017. Dissociating normal aging from Alzheimer's disease: a view from cognitive neuroscience. J. Alzheimers Dis. 57, 331–352. ( 10.3233/JAD-161099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedman AM, van Haren NEM, Schnack HG, Kahn RS, Hulshoff Pol HE. 2012. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum. Brain Mapp. 33, 1987–2002. ( 10.1002/hbm.21334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan DG. 1987. The dopamine and serotonin systems during aging in human and rodent brain. A brief review. Prog. Neuro-Psychopharmacol. Biol. Psychiat. 11, 153–157. ( 10.1016/0278-5846(87)90053-4) [DOI] [PubMed] [Google Scholar]
- 4.Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. 1993. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology 43, 192–197. ( 10.1212/WNL.43.1_Part_1.192) [DOI] [PubMed] [Google Scholar]
- 5.West MJ, Coleman PD, Flood DG, Troncoso JC. 1994. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet 344, 769–772. ( 10.1016/S0140-6736(94)92338-8) [DOI] [PubMed] [Google Scholar]
- 6.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. 2011. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 1, a006189 ( 10.1101/cshperspect.a006189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heppner FL, Ransohoff RM, Becher B. 2015. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16, 358–372. ( 10.1038/nrn3880) [DOI] [PubMed] [Google Scholar]
- 8.Gómez-Isla T, Price JL, McKeel DW Jr, Morris JC, Growdon JH, Hyman BT. 1996. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J. Neurosci. 16, 4491–4500. ( 10.1523/JNEUROSCI.16-14-04491.1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simić G, Kostović I, Winblad B, Bogdanović N. 1997. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer's disease. J. Comp. Neurol. 379, 482–494. () [DOI] [PubMed] [Google Scholar]
- 10.Terry RD, Deteresa R, Hansen LA. 1987. Neocortical cell counts in normal human adult aging. Ann. Neurol. 21, 530–539. ( 10.1002/ana.410210603) [DOI] [PubMed] [Google Scholar]
- 11.Pakkenberg B, Gundersen HJG. 1997. Neocortical neuron number in humans: effect of sex and age. J. Comp. Neurol. 384, 312–320. () [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Pinilla E, Ordóñez C, del Valle E, Navarro A, Tolivia J. 2016. Regional and gender study of neuronal density in brain during aging and in Alzheimer's disease. Front. Aging Neurosci. 8, 1–12. ( 10.3389/fnagi.2016.00213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison JH, Hof PR. 1997. Life and death of neurons in the aging brain. Science 278, 412–419. ( 10.1126/science.278.5337.412) [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. 1997. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann. Neurol. 41, 17–24. ( 10.1002/ana.410410106) [DOI] [PubMed] [Google Scholar]
- 15.West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. 2004. Hippocampal neurons in pre-clinical Alzheimer's disease. Neurobiol. Aging 25, 1205–1212. ( 10.1016/j.neurobiolaging.2003.12.005) [DOI] [PubMed] [Google Scholar]
- 16.Giannakopoulos P, Hof PR, Kovari E, Vallet PG, Herrmann FR, Bouras C. 1996. Distinct patterns of neuronal loss and Alzheimer's disease lesion distribution in elderly individuals older than 90 years. J. Neuropathol. Exp. Neurol. 55, 1210–1220. ( 10.1097/00005072-199612000-00004) [DOI] [PubMed] [Google Scholar]
- 17.Bussière T, Gold G, Kövari E, Giannakopoulos P, Bouras C, Perl DP, Morrison JH, Hof PR. 2003. Stereologic analysis of neurofibrillary tangle formation in prefrontal cortex area 9 in aging and Alzheimer's disease. Neuroscience 117, 577–592. ( 10.1016/S0306-4522(02)00942-9) [DOI] [PubMed] [Google Scholar]
- 18.Meraz Rios MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernández J, Campos-Peña V. 2013. Inflammatory process in Alzheimer's disease. Front. Integr. Neurosci. 7, 59 ( 10.3389/fnint.2013.00059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJG, Nyengaard JR, Regeur L. 2003. Aging and the human neocortex. Exp. Gerontol. 38, 95–99. ( 10.1016/S0531-5565(02)00151-1) [DOI] [PubMed] [Google Scholar]
- 20.Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. 2008. Neocortical glial cell numbers in human brains. Neurobiol. Aging 29, 1754–1762. ( 10.1016/j.neurobiolaging.2007.04.013) [DOI] [PubMed] [Google Scholar]
- 21.Pelvig DP, Pakkenberg H, Regeur L, Oster S, Pakkenberg B. 2003. Neocortical glial cell numbers in Alzheimer's disease. A stereological study. Dement. Geriatr. Cogn. Disord. 16, 212–219. ( 10.1159/000072805) [DOI] [PubMed] [Google Scholar]
- 22.Von Bernhardi R, Tichauer JE, Eugenin J. 2010. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J. Neurochem. 112, 1099–1114. ( 10.1111/j.1471-4159.2009.06537.x) [DOI] [PubMed] [Google Scholar]
- 23.DiPatre PL, Gelman BB. 1997. Microglial cell activation in aging and Alzheimer disease: partial linkage with neurofibrillary tangle burden in the hippocampus. J. Neuropathol. Exp. Neurol. 56, 143–149. ( 10.1097/00005072-199702000-00004) [DOI] [PubMed] [Google Scholar]
- 24.Schuitemaker A, et al. 2012. Microglial activation in healthy aging. Neurobiol. Aging 33, 1067–1072. ( 10.1016/j.neurobiolaging.2010.09.016) [DOI] [PubMed] [Google Scholar]
- 25.Ekonomou A, et al. 2015. Stage-specific changes in neurogenic and glial markers in Alzheimer's disease. Biol. Psychiatry 77, 711–719. ( 10.1016/j.biopsych.2014.05.021) [DOI] [PubMed] [Google Scholar]
- 26.Meda L, Cassatella MA, Szendrei GI, Otvos LJ, Baron P, Villalba M, Ferrari D, Rossi F. 1995. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 374, 647–650. ( 10.1038/374647a0) [DOI] [PubMed] [Google Scholar]
- 27.Rozemuller AJM, van Gool WA, Eikelenboom P. 2005. The neuroinflammatory response in plaques and amyloid angiopathy in Alzheimer's disease: therapeutic implications. Curr. Drug Targets CNS Neurol. Disord. 4, 223–233. ( 10.2174/1568007054038229) [DOI] [PubMed] [Google Scholar]
- 28.Dossi E, Vasile F, Rouach N. 2018. Human astrocytes in the diseased brain. Brain Res. Bull. 136, 139–156. ( 10.1016/j.brainresbull.2017.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozovsky I, Finch CE, Morgan TE. 1998. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol. Aging 19, 97–103. ( 10.1016/S0197-4580(97)00169-3) [DOI] [PubMed] [Google Scholar]
- 30.Sofroniew MV, Vinters HV. 2010. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35. ( 10.1007/s00401-009-0619-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beach TG, Walker R, McGeer EG. 1989. Patterns of gliosis in Alzheimer's disease and aging cerebrum. Glia 2, 420–436. ( 10.1002/glia.440020605) [DOI] [PubMed] [Google Scholar]
- 32.Cotrina ML, Nedergaard M. 2002. Astrocytes in the aging brain. J. Neurosci. Res. 67, 1–10. ( 10.1002/jnr.10121) [DOI] [PubMed] [Google Scholar]
- 33.Hof PR, Gilissen EP, Sherwood CC. 2002. Comparative neuropathology of brain aging in primates. In Aging in nonhuman primates (eds Erwin JM, Hof PR), pp. 130–154. Basel, Switzerland: Karger. [Google Scholar]
- 34.Edler MK, Sherwood CC, Meindl RS, Hopkins WD, Ely JJ, Erwin JM, Mufson EJ, Hof PR, Raghanti MA. 2017. Aged chimpanzees exhibit pathologic hallmarks of Alzheimer's disease. Neurobiol. Aging 59, 107–120. ( 10.1016/j.neurobiolaging.2017.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cramer PE, et al. 2018. Aging African green monkeys manifest transcriptional, pathological, and cognitive hallmarks of human Alzheimer's disease. Neurobiol. Aging 64, 92–106. ( 10.1016/j.neurobiolaging.2017.12.011) [DOI] [PubMed] [Google Scholar]
- 36.Mufson EJ, Benzing WC, Cole GM, Wang H, Emerich DF, Sladek JR, Morrison JH, Kordower JH. 1994. Apolipoprotein E-immunoreactivity in aged rhesus monkey cortex: colocalization with amyloid plaques. Neurobiol. Aging 15, 621–627. ( 10.1016/0197-4580(94)00064-6) [DOI] [PubMed] [Google Scholar]
- 37.Perez SE, Sherwood CC, Cranfield MR, Erwin JM, Mudakikwa A, Hof PR, Mufson EJ. 2016. Early Alzheimer's disease-type pathology in the frontal cortex of wild mountain gorillas (Gorilla beringei beringei). Neurobiol. Aging. 39, 195–201. ( 10.1016/j.neurobiolaging.2015.12.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gearing M, Tigges J, Mori H, Mirra SS. 1996. Aβ40 is a major form of β-amyloid in nonhuman primates. Neurobiol. Aging 17, 903–908. ( 10.1016/S0197-4580(96)00164-9) [DOI] [PubMed] [Google Scholar]
- 39.Gearing M, Rebeck GW, Hyman BT, Tigges J, Mirra SS. 1994. Neuropathology and apolipoprotein E profile of aged chimpanzees: implications for Alzheimer disease. Proc. Natl Acad. Sci. USA 91, 9382–9386. ( 10.1073/pnas.91.20.9382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosen RF, et al. 2008. Tauopathy with paired helical filaments in an aged chimpanzee. J. Comp. Neurol. 509, 259–270. ( 10.1002/cne.21744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemere CA, Oh J, Stanish HA, Peng Y, Pepivani I, Fagan AM, Yamaguchi H, Westmoreland SV, Mansfield KG. 2008. Cerebral amyloid-beta protein accumulation with aging in cotton-top tamarins: a model of early Alzheimer's disease? Rejuvenation Res. 11, 321–332. ( 10.1089/rej.2008.0677) [DOI] [PubMed] [Google Scholar]
- 42.Mestre-Francés N, Keller E, Calenda A, Barelli H, Checler F, Bons N. 2000. Immunohistochemical analysis of cerebral cortical and vascular lesions in the primate Microcebus murinus reveal distinct amyloid β1–42 and β1–40 immunoreactivity profiles. Neurobiol. Dis. 7, 1–8. ( 10.1006/nbdi.1999.0270) [DOI] [PubMed] [Google Scholar]
- 43.Kraska A, et al. 2011. Age-associated cerebral atrophy in mouse lemur primates. Neurobiol. Aging 32, 894–906. ( 10.1016/j.neurobiolaging.2009.05.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sloane JA, Pietropaolo MF, Rosene DL, Moss MB, Peters A, Kemper T, Abraham CR. 1997. Lack of correlation between plaque burden and cognition in the aged monkey. Acta Neuropathol. 94, 471–478. ( 10.1007/s004010050735) [DOI] [PubMed] [Google Scholar]
- 45.Latimer CS, et al. 2019. A nonhuman primate model of early Alzheimer's disease pathologic change: implications for disease pathogenesis. Alzheimer's Dementia 15, 93–105. ( 10.1016/j.jalz.2018.06.3057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-Callejas JD, Fuchs E, Perez-Cruz C, Perez-Cruz C. 2016. Evidence of tau hyperphosphorylation and dystrophic microglia in the common marmoset. Front. Aging Neurosci. 8, 1–15. ( 10.3389/fnagi.2016.00315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delacourte A, Sautiere PE, Wattez A, Mourton-Gilles C, Petter A, Bons N. 1995. Biochemical characterization of Tau proteins during cerebral aging of the lemurian primate Microcebus murinus. C. R. Acad. Sci. III 318, 85–89. [PubMed] [Google Scholar]
- 48.Elfenbein HA, Rosen RF, Stephens SL, Switzer RC, Smith Y, Pare J, Mehta PD, Warzok R, Walker LC. 2007. Cerebral beta-amyloid angiopathy in aged squirrel monkeys. Histol. Histopathol. 22, 155–167. ( 10.14670/HH-22.155) [DOI] [PubMed] [Google Scholar]
- 49.Schultz C, Hubbard GB, Rüb U, Braak E, Braak H. 2000. Age-related progression of tau pathology in brains of baboons. Neurobiol. Aging 21, 905–912. ( 10.1016/S0197-4580(00)00176-7) [DOI] [PubMed] [Google Scholar]
- 50.Peters A, Verderosa A, Sethaers C, Sethares C. 2008. The neuroglial population in the primary visual cortex of the aging rhesus monkey. Glia 56, 1151–1161. ( 10.1002/glia.20686) [DOI] [PubMed] [Google Scholar]
- 51.Sherwood CC, Gordon AD, Allen JS, Phillips KA, Erwin JM, Hof PR, Hopkins WD. 2011. Aging of the cerebral cortex differs between humans and chimpanzees. Proc. Natl Acad. Sci. USA 108, 13 029–13 034. ( 10.1073/pnas.1016709108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lacreuse A, Herndon JG. 2009. Nonhuman primate models of cognitive aging. In Animal models of human cognitive aging (eds Bizon JL, Woods AG), pp. 29–58. Totowa, NJ: Humana Press. [Google Scholar]
- 53.Parr L, Lacreuse A, Parr L, Chennareddi L, Herndon JG. 2018. Age-related decline in cognitive flexibility in female chimpanzees. Neurobiol. Aging 72, 83–88. ( 10.1016/j.neurobiolaging.2018.08.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herndon JG, Moss MB, Rosene DL, Killiany RJ. 1997. Patterns of cognitive decline in aged rhesus monkeys. Behav. Brain Res. 87, 25–34. ( 10.1016/S0166-4328(96)02256-5) [DOI] [PubMed] [Google Scholar]
- 55.Picq J-L, Aujard F, Volk A, Dhenain M. 2012. Age-related cerebral atrophy in nonhuman primates predicts cognitive impairments. Neurobiol. Aging 33, 1096–1109. ( 10.1016/j.neurobiolaging.2010.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rapp PR, Amaral DG. 1991. Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol. Aging 12, 481–486. ( 10.1016/0197-4580(91)90077-W) [DOI] [PubMed] [Google Scholar]
- 57.Munger EL, Takemoto A, Raghanti MA, Nakamura K. 2017. Visual discrimination and reversal learning in aged common marmosets (Callithrix jacchus). Neurosci. Res. 124, 57–62. ( 10.1016/j.neures.2017.06.002) [DOI] [PubMed] [Google Scholar]
- 58.Shamy JL, Habeck C, Hof PR, Amaral DG, Fong SG, Buonocore MH, Stern Y, Barnes CA, Rapp PR. 2011. Volumetric correlates of spatiotemporal working and recognition memory impairment in aged rhesus monkeys. Cereb. Cortex 21, 1559–1573. ( 10.1093/cercor/bhq210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herndon JG, Tigges J, Anderson DC, Klumpp SA, McClure HM. 1999. Brain weight throughout the life span of the chimpanzee. J. Comp. Neurol. 409, 567–572. () [DOI] [PubMed] [Google Scholar]
- 60.Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. 2003. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb. Cortex 13, 950–961. ( 10.1093/cercor/13.9.950) [DOI] [PubMed] [Google Scholar]
- 61.Cargill R, Kohama SG, Struve J, Su W, Banine F, Witkowski E, Back SA, Sherman LS. 2012. Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan. Neurobiol. Aging 33, 830.e13–830.e24. ( 10.1016/j.neurobiolaging.2011.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harada N, Nishiyama S, Satoh K, Fukumoto D, Kakiuchi T, Tsukada H. 2002. Age-related changes in the striatal dopaminergic system in the living brain: a multiparametric PET study in conscious monkeys. Synapse 45, 38–45. ( 10.1002/syn.10082) [DOI] [PubMed] [Google Scholar]
- 63.Coskren PJ, Luebke JI, Kabaso D, Wearne SL, Yadav A, Rumbell T, Hof PR, Weaver CM. 2015. Functional consequences of age-related morphologic changes to pyramidal neurons of the rhesus monkey prefrontal cortex. J. Comp. Neurosci. 38, 263–283. ( 10.1007/s10827-014-0541-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erwin JM, Nimchinsky EA, Gannon PJ, Perl DP, Hof PR. 2001. The study of brain aging in great apes. In Functional neurobiology of aging, pp. 447–455. San Diego, CA: Academic Press; See https://www.sciencedirect.com/science/article/pii/B9780123518309500317. [Google Scholar]
- 65.Perl DP, Good PF, Bussière T, Morrison JH, Erwin JM, Hof PR. 2000. Practical approaches to stereology in the setting of aging- and disease-related brain banks. J. Chem. Neuroanat. 20, 7–19. ( 10.1016/S0891-0618(00)00077-6) [DOI] [PubMed] [Google Scholar]
- 66.Peters A, Sethares C, Moss MB. 1998. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb. Cortex 8, 671–678. ( 10.1093/cercor/8.8.671) [DOI] [PubMed] [Google Scholar]
- 67.Rosene DL. 1993. Comparing age-related changes in the basal forebrain and hippocampus of the rhesus monkey. Neurobiol. Aging 14, 669–670. ( 10.1016/0197-4580(93)90065-J) [DOI] [PubMed] [Google Scholar]
- 68.West MJ, Amaral DJ, Rapp PR. 1993. Preserved hippocampal cell number in aged monkeys with recognition memory deficits. Soc. Neurosci. Abstr. 19, 599. [Google Scholar]
- 69.Gazzaley AH, Thakker MM, Hof PR, Morrison JH. 1997. Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol. Aging 18, 549–553. ( 10.1016/S0197-4580(97)00112-7) [DOI] [PubMed] [Google Scholar]
- 70.Shobin E, et al. 2017. Microglia activation and phagocytosis: relationship with aging and cognitive impairment in the rhesus monkey. GeroScience 39, 199–220. ( 10.1007/s11357-017-9965-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sloane JA, Hollander W, Moss MB, Rosene DL, Abraham CR. 1999. Increased microglial activation and protein nitration in white matter of the aging monkey. Neurobiol. Aging 20, 395–405. ( 10.1016/S0197-4580(99)00066-4) [DOI] [PubMed] [Google Scholar]
- 72.Sheffield LG, Berman NE. 1998. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol. Aging 19, 47–55. ( 10.1016/S0197-4580(97)00168-1) [DOI] [PubMed] [Google Scholar]
- 73.Haley GE, Kohama SG, Urbanski HF, Raber J. 2010. Age-related decreases in SYN levels associated with increases in MAP-2, ApoE, and GFAP levels in the rhesus macaque prefrontal cortex and hippocampus. Age (Omaha) 32, 283–296. ( 10.1007/s11357-010-9137-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edler MK, et al. 2018. Microglia changes associated to Alzheimer's disease pathology in aged chimpanzees. J. Comp. Neurol. 526, 1–16. ( 10.1002/cne.24484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Munger EL, et al. 2019. Astrocytic changes with aging and Alzheimer's disease-type pathology in chimpanzees. J. Comp. Neurol. 527, 1179–1195. ( 10.1002/cne.24610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderton BH. 2002. Ageing of the brain. Mech. Ageing Dev. 123, 811–817. ( 10.1016/S0047-6374(01)00426-2) [DOI] [PubMed] [Google Scholar]
- 77.Bakkour A, Morris JC, Wolk DA, Dickerson BC. 2013. The effects of aging and Alzheimer's disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage 76, 332–344. ( 10.1016/j.neuroimage.2013.02.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Montine TJ, et al. 2012. National Institute on Aging-Alzheimer's association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 123, 1–11. ( 10.1007/s00401-011-0910-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hof PR, Morrison JH. 1994. The cellular basis of cortical disconnection in Alzheimer's disease and related dementing conditions. In Alzheimer's disease (eds Terry RD, Katzman R, Bick KL), pp. 197–229. New York, NY: Raven. [Google Scholar]
- 80.Hof PR, Morrison JH. 2004. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 27, 607–613. ( 10.1016/j.tins.2004.07.013) [DOI] [PubMed] [Google Scholar]
- 81.Slomianka L, West MJ. 2005. Estimators of the precision of stereological estimates: an example based on the CA1 pyramidal cell layer of rats. Neuroscience 136, 757–767. ( 10.1016/j.neuroscience.2005.06.086) [DOI] [PubMed] [Google Scholar]
- 82.Sherwood CC, Raghanti MA, Wenstrup JJ. 2005. Is humanlike cytoarchitectural asymmetry present in another species with complex social vocalization? A stereologic analysis of mustached bat auditory cortex. Brain Res. 1045, 164–174. ( 10.1016/j.brainres.2005.03.023) [DOI] [PubMed] [Google Scholar]
- 83.Dorph-Petersen KA, Nyengaard JR, Gundersen HJ. 2001. Tissue shrinkage and unbiased stereological estimation of particle number and size. J. Microsc. 204, 232–246. ( 10.1046/j.1365-2818.2001.00958.x) [DOI] [PubMed] [Google Scholar]
- 84.Keuker JIH, Luiten PGM, Fuchs E. 2003. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol. Aging 24, 157–165. ( 10.1016/S0197-4580(02)00062-3) [DOI] [PubMed] [Google Scholar]
- 85.West MJ. 1993. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol. Aging 14, 287–293. ( 10.1016/0197-4580(93)90113-P) [DOI] [PubMed] [Google Scholar]
- 86.Braak H, Braak E. 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259. ( 10.1007/BF00308809) [DOI] [PubMed] [Google Scholar]
- 87.Thal DR, Rüb U, Orantes M, Braak H. 2002. Phases of a beta-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800. ( 10.1212/WNL.58.12.1791) [DOI] [PubMed] [Google Scholar]
- 88.Nirzhor SSR, Khan RI, Neelotpol S. 2018. The biology of glial cells and their complex roles in Alzheimer's disease: new opportunities in therapy. Biomolecules 8, 93 ( 10.3390/biom8030093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bussière T, et al. 2004. Morphological characterization of thioflavin-S-positive amyloid plaques in transgenic Alzheimer mice and effect of passive Aβ immunotherapy on their clearance. Am. J. Pathol. 165, 987–995. ( 10.1016/S0002-9440(10)63360-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Von Gunten A, Kövari E, Rivara CB, Bouras C, Hof PR, Giannakopoulos P. 2005. Stereologic analysis of hippocampal Alzheimer's disease pathology in the oldest-old: evidence for sparing of the entorhinal cortex and CA1 field. Exp. Neurol. 193, 198–206. ( 10.1016/j.expneurol.2004.12.005) [DOI] [PubMed] [Google Scholar]
- 91.Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR. 2003. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology 60, 1495–1500. ( 10.1212/01.WNL.0000063311.58879.01) [DOI] [PubMed] [Google Scholar]
- 92.Giannakopoulos P, Kövari E, Herrmann FR, Hof PR, Bouras C. 2009. Interhemispheric distribution of Alzheimer disease and vascular pathology in brain aging. Stroke 40, 983–986. ( 10.1161/STROKEAHA.108.530337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Serrano-Pozo A, Gomez-Isla T, Growdon JH, Frosch MP, Hyman BT. 2013. A phenotypic change but not proliferation underlies glial responses in Alzheimer disease. Am. J. Pathol. 182, 2332–2344. ( 10.1016/j.ajpath.2013.02.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shah P, Lal N, Leung E, Traul DE, Gonzalo-Ruiz A, Geula C. 2010. Neuronal and axonal loss are selectively linked to fibrillar amyloid-β within plaques of the aged primate cerebral cortex. Am. J. Pathol. 177, 325–333. ( 10.2353/ajpath.2010.090937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geula C, Wu CK, Saroff D, Lorenzo A, Yuan M, Yankner BA. 1998. Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. Nat. Med. 4, 827–831. ( 10.1038/nm0798-827) [DOI] [PubMed] [Google Scholar]
- 96.Forny-Germano L, et al. 2014. Alzheimer's disease-like pathology induced by Aβ oligomers in non-human primates. J. Neurosci. 34, 13 629–13 643. ( 10.1523/JNEUROSCI.1353-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peters A, Josephson K, Vincent SL. 1991. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat. Rec. 229, 384–398. ( 10.1002/ar.1092290311) [DOI] [PubMed] [Google Scholar]
- 98.Bahney J, Biology C, von Bartheld CS, Bahney J, Herculano-Houzel S. 2017. The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J. Comp. Neurol. 524, 3865–3895. ( 10.1002/cne.24040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sherwood CC, et al. 2006. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc. Natl Acad. Sci. USA 103, 13 606–13 611. ( 10.1073/pnas.0605843103) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available on the National Chimpanzee Brain Resource website: https://www.chimpanzeebrain.org.