SUMMARY
Cell division requires the assembly and organization of a microtubule spindle for the proper separation of chromosomes in mitosis and meiosis. Phase separation is an emerging paradigm for understanding spatial and temporal regulation of a variety of cellular processes, including cell division. Phase-separated condensates have been recently discovered at many structures during cell division as a possible mechanism for properly localizing, organizing, and activating proteins involved in cell division. Here, we review how these condensates play roles in regulating microtubule density and organization, spindle assembly and function, and activating some of the key players in cell division. We conclude with perspectives on areas of future research for this exciting and rapidly advancing field.
Keywords: Phase separation, phase-separated condensates, membraneless compartments, cell division, microtubule-based spindle
eTOC Blurb
The process of cell division requires spatial and temporal control over microtubule-associated proteins and their regulatory factors to promote the assembly and function of the microtubule pindle. In this review, Ong and Torres describe the role of phase separation in cell division and in coordinating and organizing the assembly of cell division factors and the microtubule spindle.
INTRODUCTION
In biology, phase separation is the phenomenon in which biomolecules form phase-separated condensates (also called membraneless compartments or coacervates) that have different physical and biological functions than the free, soluble macromolecule (Banani et al., 2017; Boeynaems et al., 2018). This process has recently emerged as being important for cellular processes like RNA function (Bouchard et al., 2018), transcription (Han et al., 2020; Sabari et al., 2018), DNA repair (Duan et al., 2019), ubiquitylation (Bouchard et al., 2018; Dao et al., 2018; Sun et al., 2018; Yasuda et al., 2020), nuclear pore complex trafficking (Celetti et al., 2020; Milles et al., 2013), and cell division (Ding et al., 2019; Jiang et al., 2015; So et al., 2019; Trivedi et al., 2019). Underscoring the importance of phase-separated macromolecules to human physiology, their dysregulation has been linked to multiple disease states, including neurodegenerative diseases and cancer (Spannl et al., 2019), and possibly developmental disorders (Desai and Pethe, 2020; Hubstenberger et al., 2013; Plys et al., 2019; Schoenfelder et al., 2015).
In particular, the cell division field has seen an emergence of advancements in understanding the molecular function of phase separated macromolecules. Cell division is a highly dynamic process that relies on the proper formation of a bipolar microtubule-based spindle that functions as the primary mechanical structure for driving chromosome congression to the metaphase plate and for the equal segregation of DNA into two daughter cells (Lacroix et al., 2018). Three key features essential to microtubule spindle function are the spindle poles, the microtubule spindle itself, and kinetochores. The spindle poles anchor the minus ends of microtubules and serve as the nucleation sites for spindle microtubules. In most animal cells, the centrosome serves as the primary microtubule organizing center (MTOC) and spindle pole for the dividing cell (Azimzadeh, 2014). The spindle microtubules provide the structural framework for the spindle (Prosser and Pelletier, 2017) and include astral microtubules, which radiate toward the cell periphery; k-fibers (or kinetochore microtubules), which are captured at the kinetochore; and polar (or interpolar) microtubules, which extend toward the chromosomes but usually form an attachment to an opposing polar microtubule. The kinetochore, a large protein structure that bridges the interaction between a k-fiber and the centromeric DNA of chromosomes (Musacchio and Salmon, 2007), is important for the capture and movement of chromosomes.
Although the repertoire of proteins necessary for cell division has been well-documented and characterized, what roles phase separation play in the fidelity of cell division is an exciting area of ongoing research. Phase separation serves as a novel and attractive mechanism for orchestrating the cell division machinery and for coordinating and organizing complex spatially and temporally sensitive processes. Phase separation has been documented at key cell division structures, namely: the spindle pole (centrosomal and acentrosomal), spindle body, kinetochore, inner centromere, chromosomes, and elsewhere (Figure 1, Table 1). In this Review, we focus on the function, properties, and key components of phase separation at these cell division structures and provide perspectives on future avenues for understanding the role of phase separation within the context of mitosis and meiosis.
Figure 1. Potential Roles for Phase Separation in Cell Division.

Many aspects of mitosis and meiosis are regulated by phase separation. Phase separation may play important roles in the organization of cellular structures and in spatial and temporal regulation of factors involved in cell division.
Table 1.
Summary of Phase-separated Condensates in Cell Division
| Structure | Key components | Properties | Biological Function | System |
|---|---|---|---|---|
| Spindle poles |
Centrosome SPD-5 (C. elegans), Plk4 (X. laevis extracts and cultured human cells) |
|
|
C. elegans embryos (SPD-5) (Woodruff et al., 2017) |
|
|
Recombinant Plk4 and X. laevis extracts (Gouveia et al., 2019), cultured human cells (Park et al., 2019) | ||
|
Acentrosomal Centrosome and centrosome-associated proteins |
|
|
Recombinant proteins and mammalian oocytes (So et al., 2019) | |
| Spindle body | Tubulin, microtubule motors and crosslinkers |
|
|
X. laevis extracts (Brugues and Needleman, 2014; Gatlin et al., 2009), purified tubulin and recombinant proteins (Edozie et al., 2019) |
| Tau |
|
|
Recombinant proteins (Hernandez-Vega et al., 2017) | |
| TPX2 |
|
|
Recombinant proteins, X. laevis extracts (King and Petry, 2020) | |
| BuGZ |
|
|
X. laevis extracts, recombinant proteins, and cultured human cells (Huang et al., 2018; Jiang et al., 2015) | |
| Centromere | Chromosomal passenger complex (INCENP, Borealin, Survivin) |
|
|
Cultured human cells (Trivedi et al., 2019) |
| Chromosomes |
Mitotic Ki-67 |
|
|
Cultured human cells (Cuylen et al., 2016) |
|
Meiotic RNA binding proteins and lncRNA |
|
|
S. pombe (Ding et al., 2019) | |
| Other | Mostly stress granules, RNA splicing speckles, centrosome/pericentri olar matrix |
|
|
Cultured human cells (Rai et al., 2018) |
Abbreviations: MT, microtubule; MTOC, microtubule organizing center; NEBD, nuclear envelope breakdown
Properties of phase separation
Broadly, phase separated components share a number of properties (Figure 2) (Banani et al., 2017; Boeynaems et al., 2018). Free, soluble macromolecules, usually RNA, proteins, or RNA-protein complexes, can undergo phase separation to form membraneless condensates (Figure 2A). These condensates are usually spherical in appearance due to surface tension (Banani et al., 2017; Boeynaems et al., 2018). Moreover, their contents are selective (Banani et al., 2017; Boeynaems et al., 2018): some macromolecules are incorporated within the condensates (dark blue macromolecules), some are excluded from the condensates (red macromolecules), and some are neither included nor excluded and have an equal concentration within and outside the condensate. Macromolecules incorporated into the condensate tend to have higher concentrations than macromolecules in the bulk solution. In the case of enzymes and their substrates, this may lead to faster rates of reaction (Banani et al., 2017).
Figure 2. Phase-separated Condensates Have Unique Chemical and Physical Properties.
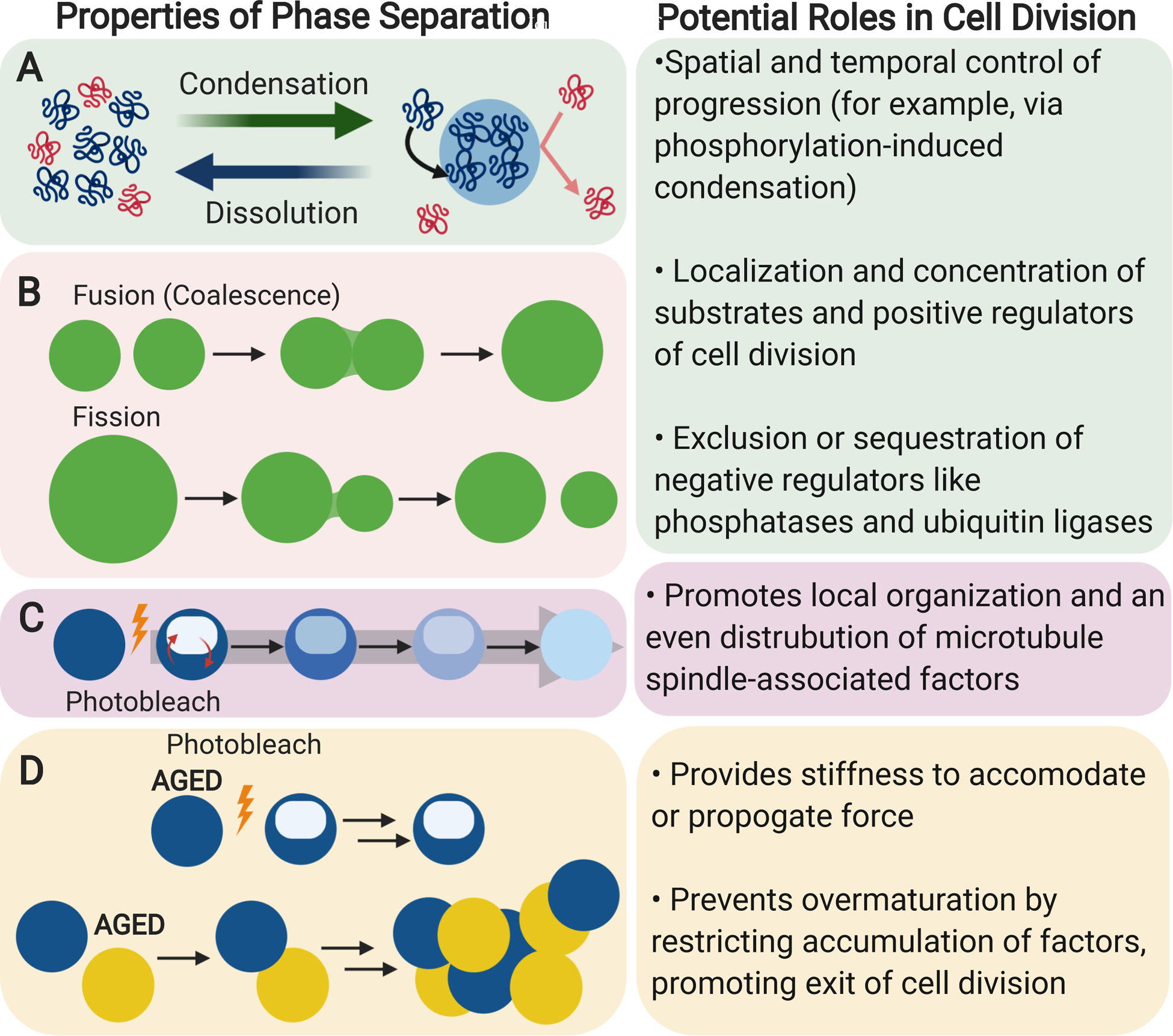
(A) Soluble macromolecules can condense to form phase-separated compartments. These compartments are selective in which macromolecules are incorporated and are distinct from the bulk solution. Condensates may dissolve back into free macromolecules. The factors that affect condensation or dissolution vary.
(B) Phase-separated condensates may exhibit liquid-like droplet behavior. Two or more condensates may fuse, increasing their size and mixing their contents. A condensate may also undergo fission to form two or more smaller condensates, usually in response to sheer force.
(C) Within phase-separated condensates, macromolecules usually have faster rates of diffusion and internal rearrangement relative to macromolecules in solid or solid-like structures. Macromolecules in phase-separated condensates also tend to have fast rates of exchange with macromolecules in free solution.
(D) Phase-separated condensates can undergo “aging” wherein they harden over time. Macromolecules within aged condensates do not diffuse easily and aged condensates tend to aggregate instead of fusing.
The formation of phase-separated condensates depends on a variety of factors. In general, macromolecules with intrinsically disordered, low complexity, or repetitive regions tend to form phase-separated condensates (Banani et al., 2017; Boeynaems et al., 2018). Condensation is usually mediated by post-translational modifications such as phosphorylation or poly(ADP-ribosylation) (PARylation) (Duan et al., 2019) and by multivalent interactions (Banani et al., 2017), particularly via hydrophobic or charged amino acid residue-mediated protein-protein interactions, RNA-RNA interactions, or protein-RNA interactions (Mittag and Parker, 2018). Condensation is generally promoted by molecular crowding (either induced by high concentrations of proteins or by the addition of a crowding agent such as PEG (Milles et al., 2013)) and lower ionic strength solutions (Lin et al., 2015). Caution must be used when interpreting in vitro experiments using purified protein, however, as the concentrations of purified protein used to observe phase separation by microscopy are often much higher than physiological conditions. Moreover, experiments using crowding agents such as PEG must similarly be interpreted carefully, as use of reagents like PEG may result in artifacts that are not physiologically relevant. While high concentrations of proteins and the use of crowding agents are used as aids to form and study phase-separated condensates in vitro, further experiments are necessary to confirm the existence and understand the role of phase-separated condensates within a cell. Phase-separated condensates may dissolve when diluted, in high salt solutions, or in the presence of small molecules that disrupts hydrophobic interactions such as 1,6-hexanediol (Ding et al., 2019). In yeast, autophagy-related phase separated condensates were promoted by an acidic pH of 6, the approximate pH of the yeast cytoplasm during starvation conditions (Fujioka et al., 2020). Temperature may also play a role in phase separation, though how much of a role this plays within a mammalian system is unclear (Jiang et al., 2015).
Many phase-separated condensates have a liquid-like character, sometimes earning them the name liquid-liquid phase-separated (LLPS) condensates, as the liquid-like condensates are separated (another common term is “de-mixed”) from the liquid of the cytoplasm (Hyman et al., 2014). These condensates are distinct from the bulk solution. When in close proximity, these condensates can fuse together, mixing their components and growing in size (Figure 2B) (Banani et al., 2017). They may also undergo fission in the presence of an external force such as a sheer force (Figure 2B) (Banani et al., 2017). Within liquid-like condensates, macromolecules diffuse quickly and the contents of the condensate undergo internal rearrangements at fast timescales, usually demonstrated by photobleaching experiments (Figure 2C) (Banani et al., 2017). Macromolecules can also exchange in and out of the liquid-like condensate. For example, nuclear transport receptors enriched in nuclear pore complex-based gels recover quickly after photobleaching, suggesting that the nuclear transport receptors within the condensate exchange quickly and freely with their soluble counterpart (Schmidt and Görlich, 2015). In contrast, the nuclear pore complex-based gels, which are in a solid-like condensate due to aging over time (Celetti et al., 2020; Milles et al., 2013), do not recover after photobleaching, suggesting that the nuclear pore complex-based gels are more static and do not freely diffuse within the condensate or exchange with solution. In biological systems, this dynamic aspect of condensates may allow for the equal distribution of protein complexes over a large cellular substructure.
Some condensates have been observed to “age” over time. Aged condensates can “harden” into solid-like or gel-like states (Celetti et al., 2020; Milles et al., 2013) wherein the dynamics of their internal rearrangements become slower, as demonstrated via photobleaching experiments (Figure 2D, top) (Gouveia et al., 2019; Lin et al., 2015; Park et al., 2019), and their ability to fuse decreases (Figure 2D, bottom) (Patel et al., 2015). Instead of fusing, aged condensates tend to clump, sometimes forming networks and amyloid-like aggregates (Lin et al., 2015). For proteins involved in amyloid diseases, the formation of these amyloids can be affected by patient-derived mutations and may play a role in disease progression (Conicella et al., 2016; Mackenzie et al., 2017; Molliex et al., 2015; Patel et al., 2015; Wegmann et al., 2018). In the nuclear pore complex, the formation of gel-like states may play a role in nuclear trafficking (Celetti et al., 2020; Milles et al., 2013; Schmidt and Görlich, 2015). The physiological function, if any, of the aggregation of aged condensates in other biological systems is largely unknown.
Phase separation at the centrosome
The centrosome is the main microtubule organizing center (MTOC) of most eukaryotic cells and usually serves as the spindle pole in mitosis (Nigg and Holland, 2018; Prosser and Pelletier, 2017). Centrosomes are composed of two centrioles surrounded by pericentriolar matrix (PCM) that serves to scaffold proteins necessary for microtubule nucleation and anchoring (Azimzadeh, 2014). During interphase, the centrioles undergo a complex round of duplication and maturation into a centrosome before it can serve as a proper MTOC for cell division (Nigg and Holland, 2018). Many microtubule-associated proteins localize at the centrosome to provide structural support and cohesion and to promote microtubule nucleation, polymerization, and organization (Nigg and Holland, 2018). Of interest, recent examples have surfaced where the phase separation of key centrosomal proteins is essential for centrosome maturation and homeostasis.
In the C. elegans embryo, SPD-5 is a protein with roles in centrosome maturation and loss of SPD-5 leads to defects in spindle assembly (Laos et al., 2015). In a study to reconstitute centrosome morphology and microtubule organization in vitro, SPD-5 was found to form phase-separated condensates as a function of molecular crowding (Woodruff et al., 2017). Whereas newly-formed, “young” SPD-5 condensates incorporated other SPD-5 into their condensates, older, “aged” SPD-5 condensates did not exhibit dynamic exchange with the solution or internal rearrangement after photobleaching in vitro (Woodruff et al., 2017). Within the embryo, the dynamics of SPD-5 at the centrosome also resembled that of the aged SPD-5 condensates (Woodruff et al., 2017). These data suggest that, in vivo, SPD-5 undergoes a liquid-like to a solid-like rearrangement as a function of time or droplet size. Droplet formation was enhanced by Plk1 kinase activity and via incorporation of SPD-2 (mammalian homolog: Cep192), and in cells, key centrosomal proteins Plk1, SPD-2, TPXL-1 (mammalian homolog: TPX2), and Zyg-9 (mammalian homolog: CKAP5, also known as ch-TOG or the mammalian homolog of XMAP215) incorporated into these condensates at the centrosomes (Woodruff et al., 2017). The microtubule-associated protein EB1 was not incorporated into SPD-5 condensates in vitro, suggesting that these condensates are selective (Woodruff et al., 2017). In the presence of TPXL-1 and Zyg-9, SPD-5 condensates incorporated and concentrated tubulin both in vitro and in vivo (Woodruff et al., 2017). These SPD-5, Zyg-9, and TPXL-1 condensates also formed microtubule asters in vitro (Woodruff et al., 2017). These data indicate that phase separation at the centrosome is critical for its function as a MTOC.
In Xenopus egg extracts, the ability of Plk4 to form condensates was dependent on its kinase activity (Gouveia et al., 2019); in particular, in cultured human cells, Plk4 autophosphorylation on key residues in its cryptic polo box (CPB) domain promoted phase-separated condensates (Park et al., 2019). These Plk4 condensates concentrated tubulin and acted as the MTOC that recruited and ordered the centrosomal protein STIL and gamma-tubulin (Gouveia et al., 2019). During centrosome biogenesis and maturation, Plk4-Cep152 binding in a ring-like structure is first necessary for Plk4 localization to the centrioles (Park et al., 2014), then Plk4-STIL binding in a dot-like structure is necessary for the initiation of procentriole formation (Moyer and Holland, 2019). When Plk4 formed phase separated condensates, the binding between Plk4 and Cep152 became weaker whereas the binding between Plk4 and STIL became stronger (Park et al., 2019), suggesting a role for phase separation in procentriole formation. When in condensates, Plk4 also resisted ubiquitin-mediated degradation by excluding the ubiquitin ligase β-TrCP (Park et al., 2019), suggesting that phase separation may stabilize proteins by excluding the factors that degrade them. Unlike many other condensates, condensates made from recombinant Plk4 in vitro did not recover after photobleaching experiments, suggesting that Plk4 condensates resemble a more solid gel-like state (Gouveia et al., 2019; Park et al., 2019). These data suggest that, during centrosome maturation, Plk4 may transition from a liquid-like to a solid gel-like state (in a manner similar to SPD-5 in the C. elegans embryo), perhaps to prevent degradation factors or further maturation factors from accumulating at the centrosome. Alternatively, the hardening of the centrosome may serve to “cement” microtubules within the centrosome, to maintain an ordered array of microtubules, and to resist forces generated by microtubule motors. Phase separation then may serve as a means by which different protein interactions are controlled through time to spatially and temporally regulate centrosome maturation.
Phase separation at the acentrosomal meiotic spindle pole and spindle body
Whereas all eukaryotic cells form a microtubule spindle to divide, not all cells use centrosomes to nucleate microtubule spindles. For example, plants, fungi, and some algae all divide without centrosomes, instead using other organelles or protein complexes to organize their microtubules (Yi and Goshima, 2018). Among mammalian cells, the mammalian oocyte is unique in that while it expresses many centrosomal proteins, the oocyte lacks a centrosome and undergoes an acentrosomal cell division (Dumont and Desai, 2012). How these proteins are localized and organized to form a microtubule spindle within the oocyte, a large cell relative to other mammalian cells, is an active area of investigation.
Immunofluorescence microscopy of centrosome- and spindle-related proteins in mouse metaphase I oocytes identified a number of proteins that localized at the acentrosomal MTOC at the spindle poles (So et al., 2019). However, a number of other proteins localized in a structure that encompassed the MTOC as well as the spindle itself. This structure exhibited droplet-like behavior and high rates of diffusion, suggestive of phase separation (So et al., 2019). This structure, which the authors termed the liquid-like meiotic spindle domain (LISD), was disrupted by depletion of microtubule-associated protein TACC3, by depletion of CHC17 (a binding partner of TACC3), and by inhibition of Aurora A kinase activity (So et al., 2019).
In contrast to phase separation at the centrosome, the LISD did not appear to concentrate tubulin (So et al., 2019). However, disruption of the LISD resulted in reduced density of k-fibers and interpolar microtubules, spindle volume, and resulted in delays in meiotic progression (So et al., 2019). Consequently, the authors speculate the LISD may serve at least two purposes: first, as the oocyte is a large cell, the formation of the LISD may sequester and concentrate important meiotic spindle proteins; second, the LISD may promote an even distribution of spindle proteins across the entire spindle, preventing the accumulation of a particular protein in a confined area (So et al., 2019).
Phase separation at the microtubule spindle
The microtubule spindle is a large and dynamic cellular structure that needs to be assembled and disassembled every cell division. Moreover, it is a complicated machine: during cell division, microtubules undergo dynamic instability, wherein their lengths change rapidly due to fluctuating periods of growth and depolymerization, and the spindle itself is permeated with a number of microtubule motors and microtubule-associated proteins that also affect microtubule polymerization dynamics, force generation, and mechanical stress propagation (Prosser and Pelletier, 2017). How the spindle is organized across many length scales and how spindle architecture gives rise to the mechanical properties of the microtubule spindle is still under study (Lacroix et al., 2018). Phase separation has begun to describe how the microtubule spindle is organized at small and large length scales.
The foundation of the microtubule spindle is the tubulin dimer. Purified tubulin was observed not only to polymerize into filaments but also to form spindle-shaped, highly oriented domains (Edozie et al., 2019). These micron-scale almond shaped domains, which the authors termed tactoids, formed condense bundles in the presence of the Arabidopsis thaliana microtubule crosslinker MAP65 (human ortholog: PRC1) (Edozie et al., 2019). Tactoids that elongated and approached a neighboring tactoid fused together into a longer tactoid, but only if the two tactoids were parallel along the long axis (end-to-end) (Edozie et al., 2019). Interestingly, via photobleaching experiments of the tactoids, MAP65 is able to quickly diffuse within the tactoid but the polymerized tubulin of the microtubule itself cannot (Edozie et al., 2019). The ability of MAP65 to diffuse within the tactoid is reminiscent of the diffusion of proteins in the meiotic LISD and may suggest that phase separation-based diffusion may serve to distribute microtubule-associated proteins evenly within the meiotic and mitotic spindles. As these results come from an in vitro system employing the use of methylcellulose as a crowding agent and GMPCPP to stabilize the microtubule filaments, this system fails to account for many important aspects of microtubule dynamics, including the actions of molecular motors in shaping the microtubule spindle as well as the property of dynamic instability at microtubule ends. Nonetheless, these experiments suggest that, at least under some conditions, microtubules and microtubule crosslinkers such as MAP65 have the capacity to self-organize microtubules into local domains.
Mitotic spindles isolated from frog extracts display similar properties (Gatlin et al., 2009). Using microneedle manipulation, two metaphase mitotic spindles brought close together would fuse into one mitotic spindle (Gatlin et al., 2009). Unlike tubulin tactoids, mitotic spindles could fuse under a variety of orientations: both parallel and perpendicular mitotic spindles fused (Gatlin et al., 2009). The ability of these spindles to fuse was dependent on the activity of the motor protein dynein (Gatlin et al., 2009). Later experiments that used quantitative polarized light microscopy and computational modeling to study the X. laevis metaphase mitotic spindle demonstrated that the spindle is self-organized via local domains governed by microtubule polymerization dynamics, cross-linkers, and motors (Brugués and Needleman, 2014). Given that recent experiments using microneedle manipulation to probe biomechanical properties of the spindle demonstrated that different regions of the spindle have different degrees of stiffness and viscosities (Suresh et al., 2020; Takagi et al., 2019), local phase-separated domains may serve to give rise to the morphology of the mitotic spindle and accounts for the generation and propagation of mechanical stress.
Phase separation may thus play a role in providing a physical means for microtubules to find and attach to kinetochores in the otherwise crowded microtubule spindle body. Which microtubule-associated proteins grant different biomechanical properties, how they are localized within the entire microtubule spindle, and how they are affected by phase separation within sub-regions or sub-condensates of the microtubule spindle, however, is not entirely clear. Moreover, tubulin itself is a highly modified protein (Janke and Magiera, 2020), and these modifications play important roles in cell division (Barisic and Maiato, 2016). Because post-translational modifications regulate the formation of phase-separated condensates, tubulin modifications that affect the phase separation properties of the microtubule spindle may also affect the organization, morphology, and mechanical properties of the microtubule spindle. For example, the velocity and the time bound to microtubules of molecular motor dynein, which is necessary for the fusion of Xenopus mitotic spindles (Gatlin et al., 2009), increases when dynein binds to acetylated tubulin (Alper et al., 2014). While it is unknown whether or not tubulin post-translational modifications have any effect on the ability of dynein or other microtubule-associated proteins to induce phase separation of tubulin, these modifications nonetheless may regulate the phase separation properties of the microtubule spindle either directly through the ability of tubulin to assemble and associate with itself or indirectly, via the ability of microtubule-associated proteins to bind to microtubules.
Key proteins involved in assembling the microtubule spindle also exhibit phase separation properties. We highlight the roles of microtubule binding proteins tau, TPX2, and BugZ. Tau is a microtubule stabilizing protein which has been highly studied because aggregates of tau have been implicated in a number of neurodegenerative diseases (Wang and Mandelkow, 2016). While a clear role for tau in mitosis or spindle assembly has not yet been identified, tau is differentially phosphorylated in mitosis (Pope et al., 1994; Tatebayashi et al., 2006) and decorates the microtubule spindle (Connolly et al., 1977; Preuss and Mandelkow, 1998). Tau regulates the activity of molecular motors like kinesins and severing enzymes like the katanins (Siahaan et al., 2019), two families of enzymes that play roles in shaping the mitotic spindle (Cheung et al., 2016; Mayr et al., 2007; Splinter et al., 2010; Stumpff et al., 2008), and overexpression of tau led to the formation of monopolar spindles and mitotic arrest in the Drosophila melanogaster wing disc (Bouge and Parmentier, 2016). In experiments with purified protein, tau formed liquid-like condensates (Hernández-Vega et al., 2017; Wegmann et al., 2018) that incorporated and concentrated tubulin dimers. These tau-tubulin drops served as sites for microtubule nucleation and the tau droplets spread out along the emerging microtubule (Hernández-Vega et al., 2017). Tau-coated microtubules could fuse or bundle with each other, forming a network of parallel filaments (Hernández-Vega et al., 2017). Similar to MAP65 on tubulin tactoids, photobleaching experiments demonstrated that the fluorescence of tau on microtubule bundles, but not the tubulin itself, quickly recovered after photobleaching, suggesting that tau was able to diffuse along the microtubule length and with free tau in solution (Hernández-Vega et al., 2017). Moreover, tau condensates were observed to age and harden, promoting the formation of tau aggregates (Wegmann et al., 2018), though whether this aspect of tau condensates has any function in normal spindle assembly or is part of the progression of neurodegenerative diseases is unknown.
Similarly, purified TPX2, a microtubule nucleator that promotes branched microtubules from an existing microtubule filament (Petry et al., 2013), formed phase-separated condensates that incorporated and concentrated tubulin (King and Petry, 2020). In solution, these TPX2-tubulin condensates formed microtubule asters, and in Xenopus egg extracts, these TPX2-tubulin condensates served as microtubule nucleation points (King and Petry, 2020). Purified TPX2 preferentially formed droplets on existing microtubules and enhanced branched microtubule nucleation (King and Petry, 2020). Interestingly, importin-α/β, inhibitors of TPX2 nucleation (Schatz et al., 2003), dissolved TPX2-tubulin condensates (King and Petry, 2020). Given that the concentration of importin that can inhibit TPX2 is low near the chromosomes by nature of a Ran-GTP gradient (Kalab et al., 2002), importin-mediated dissolution of TPX2 condensates may partly explain why TPX2 microtubule nucleation is high near the chromosomes and how TPX2 promotes correct spindle size and function (Bird and Hyman, 2008). Moreover, as TPX2 protects Aurora A from proteasome-mediated degradation (Giubettini et al., 2011), TPX2-based phase separation may play a role in stabilizing Aurora A by excluding ubiquitin ligases that target Aurora A for destruction.
BuGZ is a mitotic spindle (Jiang et al., 2014; Toledo et al., 2014) and kinetochore-associated (Jiang et al., 2014) protein that binds to and stabilizes Bub3, a protein that scaffolds and recruits proteins that monitor the proper attachment between microtubules and chromosomes via a mechanism known as the spindle assembly checkpoint to prevent improper cell division (Musacchio and Salmon, 2007). During mitosis, BuGZ facilitates the loading of Bub3 onto the kinetochore and loss of BuGZ leads to a weakening of the spindle assembly checkpoint (Jiang et al., 2014; Toledo et al., 2014). Recombinant BuGZ was found to phase separate in a temperature-dependent manner, as BuGZ condensates were disrupted at colder temperatures (Jiang et al., 2015). In Xenopus egg extracts, which allow for the study of kinetochore-independent roles of BuGZ, disruption of BuGZ phase separation resulted in fewer microtubule-associated proteins associated with MTOCs generated from Aurora A kinase (Jiang et al., 2015). The microtubule aster-like MTOCs formed in the presence of phase-separation deficient BuGZ were also smaller and contained fewer microtubules than phase-separation capable BuGZ (Jiang et al., 2015). Moreover, in vitro, Aurora A could incorporate into BuGZ droplets, promoting Aurora A kinase activity (Huang et al., 2018).
The incorporation of Aurora A into BuGZ phase-separated condensates is particularly interesting given the kinetochore localization and function of BuGZ. While Aurora A predominately functions in centrosome maturation and spindle assembly (Joukov and De Nicolo, 2018), Aurora A has also been demonstrated to phosphorylate a component of the kinetochore, Hec1 (DeLuca et al., 2018). BuGZ-based phase-separation may thus function to enhance Aurora A phosphorylation both at the centrosome and at the kinetochores. However, the experiments demonstrating the phase separation properties of BuGZ were performed in Xenopus egg extracts. Xenopus egg extracts allow for the study of phase separation of BuGZ with Aurora A (Huang et al., 2018) and microtubule-associated proteins (Jiang et al., 2015), but because these extracts lack chromosomes and kinetochores, the role of BuGZ phase separation at the kinetochores is unclear. For example, expression of mutant forms of BuGZ that lacked the ability to phase separate resulted in abnormal mitotic spindles in HeLa cells (Jiang et al., 2015). However, these mutant BuGZ constructs had a weaker association with tubulin (Jiang et al., 2015). Since BuGZ promotes Bub3 loading on the kinetochores in a microtubule-dependent manner (Jiang et al., 2014; Toledo et al., 2014), it is possible that the observed spindle assembly defects may be attributed to errors in kinetochore-microtubule attachment from a lack of Bub3 and not necessarily to the phase separation properties of BuGZ. Further experiments are necessary to determine if BuGZ phase separation, the BuGZ-Bub3 interaction, or both are necessary for proper microtubule spindle formation. Interestingly, Aurora A binding partners TACC3 (So et al., 2019), TPX2 (King and Petry, 2020), and BuGZ (Huang et al., 2018) are all involved in phase-separated microtubule assembly processes, potentially suggesting an important role of Aurora A in mediating spindle assembly via phase separation.
Phase separation at the centromere
The centromere is a cellular substructure assembled around CENP-A nucleosomes located at centromeric DNA (McKinley and Cheeseman, 2016). One of the major regulators of cell division located within the centromere is the Aurora B kinase, whose kinase activity generally destabilizes incorrect kinetochore-microtubule attachments, delaying anaphase onset until proper kinetochore-microtubule attachments are formed (Hindriksen et al., 2017). Aurora B, together with Survivin, Borealin, and INCENP, form the chromosomal passenger complex (CPC) (Carmena et al., 2012).
Purified Survivin, Borealin, and INCENP formed phase-separated droplets in vitro and in cells at the inner centromere (Trivedi et al., 2019). In vitro, components of the inner centromere, including α-satellite RNA and DNA, histone H3 phosphorylated at Thr3, SGO1, phosphorylated HP1α, microtubules, and Aurora B, either promoted the formation of these phase-separated droplets, were enriched in these phase-separated droplets, or both (Trivedi et al., 2019). In contrast, Mad2, an outer kinetochore protein, was excluded from CPC-based droplets (Trivedi et al., 2019). In vitro, the ability of Borealin to form phase-separated droplets was enhanced by Cdk1/Cyclin B phosphorylation. Disruption of phase separation of the CPC via expression of a mutant form of Borealin resulted in reduced localization of the CPC and Aurora B at the inner centromere and spindle midbody by about half (Trivedi et al., 2019). This result suggests that phase separation mediated by Borealin contributes to or enhances CPC localization and assembly but is not the only factor that contributes to CPC localization or assembly (Carmena et al., 2012; Hindriksen et al., 2017). Loss of CPC phase separation also resulted in a delay in mitotic progression, a weakening of the spindle assembly checkpoint, and an increase in the number of lagging chromosomes, though whether this is simply due to less Aurora B activity from reduced Aurora B localization or some other aspect of phase separation is unclear (Trivedi et al., 2019).
Given that Aurora B phosphorylates components of the kinetochore in early mitosis (Broad et al., 2020), phase separation of the centromere may serve to spatially separate Aurora B and the inner centromere from kinetochore proteins. This hypothesis is particularly intriguing given that Mad2 was excluded from the CPC-based droplets. In this way, the formation of phase-separated condensates at the centromere serves as a physical means of separating the kinetochore and centromere. Phase separation, then, along with other physical methods (Akiyoshi et al., 2010), may serve to decrease Aurora B activity at the kinetochore and promote the formation of stable kinetochore-microtubule attachments.
Aurora C is a homolog of Aurora B that is expressed predominantly in gametes and has important functions in meiosis (Quartuccio and Schindler, 2015). Similar to Aurora B, Aurora C binds INCENP and performs similar functions during meiotic divisions (Abdul Azeez et al., 2019). Whether or not Aurora C also undergoes phase-separation during meiosis is unknown. However, because the phase separation described here was reconstituted with Survivin, Borealin, and INCENP, all of which are also present and active in meiosis, phase separation may also play a role in the progression of meiosis and gamete formation. Perhaps Aurora C can change the localization and function of CPC-based condensates in meiosis to fine-tune the CPC for function in meiosis.
Phase separation at the chromosomes
Whereas chromatin and transcription factors form phase-separated compartments that aid in transcription (Han et al., 2020; Sabari et al., 2018), chromosomes themselves play roles in phase separation-mediated processes in cell division. Via an siRNA screen for proteins involved in chromosome separation, loss of Ki-67 in HeLa cells was observed to cause chromosomes to clump together (Cuylen et al., 2016). Microtubules were not able to access the kinetochores of clumped chromosomes, leading to mitotic failure (Cuylen et al., 2016). No one domain of Ki-67 was responsible for the proper separation of chromosomes; rather, Ki-67 acted as a molecular surfactant, coating the surface of the chromosome and using electrostatic and/or steric interactions to separate chromosomes from each other and to promote a functional mitosis (Cuylen et al., 2016).
In contrast, in meiosis, long noncoding RNA (lncRNA) may serve to join homologous chromosomes together. In S. pombe meiosis, sme2 RNA is a lncRNA that accumulates at its gene locus and plays a role in facilitating the pairing of homologous recombination (Ding et al., 2019). Via microscopy of proteins that localized near the sme2 lncRNA foci, two other genetic loci were identified (Ding et al., 2019). These two loci also coded for meiosis-specific lncRNA, and, similar to sme2 lncRNA, these two novel lncRNAs also accumulated and formed foci at their respective genomic locations (Ding et al., 2019). Each of the three chromosomes of S. pombe had one gene locus that exhibited fusion under physiological conditions (Ding et al., 2019). In particular, two sme2 lncRNA foci (one on each homologous chromosome) would fuse, bringing the two chromosomes together for homologous recombination; the other two lncRNA behaved likewise (Ding et al., 2019). Interestingly, the different lncRNAs were phase separated from each other and would only fuse with the compartment that shared the same RNA species (Ding et al., 2019). In meiosis, then, phase separation of protein-lncRNA foci served to exclude nonhomologous chromosomes and to physically join homologous chromosomes.
Other factors in phase separation during cell division
Cell division is highly regulated by kinases such as the cyclin-dependent kinases, Polo kinases, and Aurora kinases (Malumbres and Barbacid, 2009; Mistry et al., 2008; van Vugt et al., 2010). Evidence for the role of kinases in promoting the formation of phase-separated condensates is growing. Many of the discussed condensates are promoted by phosphorylation (centrosomal SPD-5 by Plk1, centrosomal Plk4 by Plk4; the meiotic LISD by Aurora A; centromeric CPC by Cdk1) and, at least in one case, kinase activity is enhanced by the formation of a phase-separated condensate (BuGZ enhancing Aurora A). A prime example of a kinase regulating condensate formation is DYRK3 (Wippich et al., 2013), which is a cell-cycled regulated kinase that increases in concentration from G1 to M phase and is degraded in late mitosis by the APC/C (Rai et al., 2018). DYRK3 localizes to and associates with proteins of multiple membraneless compartments such as the centrosome, stress granules, and splicing speckles (Rai et al., 2018). Overexpression of DYRK3 led to dissolution of these membraneless compartments, and chemical inhibition of DYRK3 stabilized them (Rai et al., 2018). These membraneless compartments dissolve during mitosis and reform at the end of mitosis dependent on DYRK3 kinase activity and the ratio of DYRK3 to substrate, which increases at nuclear envelope break down (NEBD) due to the mixing of the nucleoplasm and cytoplasm (Rai et al., 2018). Inhibition of DYRK3 kinase activity led to the persistence of membraneless compartments during mitosis and the sequestration of mitotic regulator BuGZ (Rai et al., 2018). The inhibition of DYRK3 consequently resulted in multipolar spindle formation and an increase in mitotic timing (Rai et al., 2018). Therefore, DYRK3 kinase activity may serve as a means of solubilizing or freeing mitotic factors from membraneless compartments in early mitosis for proper spindle assembly and cell division. However, these experiments were performed with only one chemical inhibitor of DYRK3, GSK-626616, and at the relatively high concentration of 1 μM. Given that GSK-626616 has known off-target effects at 1 μM, including other DYRK kinases and important signaling and cell-cycle related kinases like ERK8, PIM1, JAK kinases, and NEK kinases (Wippich et al., 2013), further study of the role of DYRK3 and other kinases is necessary to understand how condensate formation and dissolution may serve as regulators of mitosis.
CONCLUSION AND PERSPECTIVES
Phase separation is an increasingly studied phenomenon with emerging roles in regulating the fidelity of cell division. At the molecular level, phase separated condensates concentrate and localize proteins of interest: thus, microtubules are more easily nucleated at the centromere, microtubule-associated proteins and motors are diffuse and spread throughout the microtubule spindle, and meiotic factors are sequestered and spread within a particular domain of the otherwise large oocyte. Phase separated condensates also sequester other factors and provide a means of spatial regulation, as Plk4-condensates exclude ubiquitin ligases and the CPC-based centromeric condensate excluded the kinetochore protein Mad2. Finally, as the centrosome and centromere were both observed to harden over time and as lncRNA complexes in meiosis keep homologous chromosomes together, phase separated condensates may serve a structural role, allowing for the formation of an ordered microtubule array at the centrosome and for the accommodation of stresses and forces at the centromere and within the microtubule spindle.
To what extend does phase separation serve to organize cellular substructures? Understanding whether or not certain organelles or substructures exhibit properties of phase separation and how, if at all, those properties affect their function is an active area of research. For example, here, we highlighted the phase separation properties of centrosomal protein SPD-5 and Plk4 in centrosome maturation. However, other proteins involved in centrosome maturation do not undergo phase separation. Purified D. melanogaster centrosomal protein Centrosomin (human ortholog CDK5RAP2; probable Drosophila ortholog for C. elegans SPD-5), for example, was found to form structures that resembled phase-separated aggregates by microscopy. In contrast to most phase-separated systems, however, Centrosomin did not dynamically rearrange after photobleaching, suggesting that these Centrosomin structures are not liquid-like phase-separated aggregates but a micron-scale ordered structure or a solid-like phase-separated condensate (Feng et al., 2017).
Do these data suggest that the centrosome is not regulated by phase separation? It is possible that the dynamic liquid-like state of Centrosomin was unable to be observed due to technical reasons. Certain nuclear pore complex proteins have long been known to form aggregate-like gels (Milles et al., 2013), but it was only recently discovered, via innovative microfluidics and imaging systems, that nuclear pore complex proteins first transition through a liquid-like state before maturing into gel-like states (Celetti et al., 2020). There is a possibility that solid-like structures, such as Centrosomin-based structures, are simply “aged” liquid-like phase-separated structures, and that technical details prevent the capture of the liquid-like states. Alternatively, cellular structures such as the centrosome may simply be composed of both ordered structures and phase separated structures.
Much work has focused on how assembly of mitotic and meiotic structures are assembled by phase separation, but less work has focused on the relationship between phase separation and disassembly. As cells progress through cell division, the activity of kinases like Plk1 or Aurora kinases generally decreases as they are degraded by the APC/C (Lindon and Pines, 2004; Stewart and Fang, 2005). Given the importance of kinase activity in forming phase-separated condensates (So et al., 2019; Woodruff et al., 2017), it is reasonable to hypothesize that condensates may dissolve as kinases and other mitotic and meiotic factors are degraded. Is it possible that force by the microtubule spindle has a role in physically breaking apart phase-separated condensates or in reducing the concentration of factors such that they no longer undergo phase separation? For example, CPC phase-separated condensates, which localize at the centromeres of two homologous chromosomes, likely experience both a physical force during the metaphase to anaphase transition and a change in the local concentration of CPC components, both factors which may cause its dissolution.
How a cell commits to enter and complete cell division has been an active area of study. In particular, the rapid events in cell division need to be executed in a non-reversible, temporally regulated, and sequential manner. Characteristics of kinases have been shown to assist in decisively executing events in cell division. For example, in mitosis, Cdk1 exhibits hysteresis and bistability, meaning that, once Cdk1 is active, a small decrease in the concentration of its activator Cyclin B does not significantly inhibit Cdk1 activity (Pomerening et al., 2003; Sha et al., 2003). This mechanism prevents regression from mitosis due to a small change in Cyclin B levels. Moreover, Cdk1 is regulated by positive feedback loops at the entry to mitosis, allowing for a rapid and irreversible entry into mitosis (Santos et al., 2012). Can phase separation play a similar role in promoting rapid and irreversible temporal regulation of cell division? As cell division-promoting factors accumulate, they may spontaneously phase separate as a function of concentration or other cellular events, such as nuclear envelope breakdown (Rai et al., 2018). These phase-separated condensates may activate kinases by concentrating kinases and their substrates, excluding phosphatases or ubiquitin ligases, or other means (Fujioka et al., 2020; Huang et al., 2018). Thus, the formation of phase separated condensates may also serve to enhance rapid and irreversible progression through the steps of cell division.
Post-translational modifications, particularly via kinases and ubiquitin ligases, play crucial roles at the heart of cell cycle progression and in the establishment of phase separation (Bouchard et al., 2018; Dao et al., 2018; Heinkel et al., 2019; Park et al., 2019; Su et al., 2016; Sun et al., 2018; Yasuda et al., 2020). While phosphatases have been studied in regulating phase separation in other systems, they are largely unstudied in cell division. For example, phosphatases have been shown to abrogate phase separation in T-cell receptors (Su et al., 2016) and in M. tuberculosis membrane proteins (Heinkel et al., 2019). Interestingly, while the M. tuberculosis phosphatase was incorporated into the phase-separated condensate, the phosphatase was also localized to distinct foci within the condensate, perhaps representing selective enrichment or phase-within-phase separation. Whether these findings apply to phosphatases in cell division remains to be uncovered. When kinase activity is essential, are phosphatases sequestered within their own condensates, and when phosphatase activity is required, do these condensates then fuse with condensates containing phosphorylated substrates? Such a mechanism would allow for spatial regulation of phosphatases and their substrates.
Similarly, while phase separation may also be driven by ubiquitylation, the main ubiquitin ligase in cell division, the APC/C (Pines, 2011), has not yet been shown to undergo phase separation. However, the APC/C is active at and localizes to many structures during cell division (Acquaviva et al., 2004; Huang and Raff, 2002; Melloy and Holloway, 2004; Torres et al., 2010), many of which have been demonstrated to be sites of phase separation, so phase separation involving the APC/C or its substrates may be possible. While the presence of poly-ubiquitin chains have been shown to induce phase separation (Sun et al., 2018), no deubiquitylases have yet been identified as agents of ubiquitin-mediated phase-separated condensate dissolution as phosphatases have for kinase-mediated condensates.
ACKNOWLEDGMENTS
We apologize to colleagues whose work could not be cited to keep this review to a reasonable length. This material is based upon work supported by the National Institutes of Health NIGMS grant number R01GM117475 and National Science Foundation grant number MCB1912837 to J.Z.T. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Institutes of Health or the National Science Foundation. This work was also supported by an NIH-NIGMS Ruth L. Kirschstein National Research Service Award GM007185 and a National Science Foundation Graduate Research Fellowship DGE-1650604 to J.Y.O. Both Figures 1 and 2 were created with BioRender.com
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATIONS OF INTEREST
The authors declare no competing interests.
REFERENCES
- Abdul Azeez KR, Chatterjee S, Yu C, Golub TR, Sobott F, and Elkins JM (2019). Structural mechanism of synergistic activation of Aurora kinase B/C by phosphorylated INCENP. Nat. Commun. 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acquaviva C, Herzog F, Kraft C, and Pines J (2004). The anaphase promoting complex/cyclosome is recruited to centromeres by the spindle assembly checkpoint. Nat. Cell Biol 6, 892–898. [DOI] [PubMed] [Google Scholar]
- Akiyoshi B, Sarangapani KK, Powers AF, Nelson CR, Reichow SL, Arellano-Santoyo H, Gonen T, Ranish JA, Asbury CL, and Biggins S (2010). Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 468, 576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper JD, Decker F, Agana B, and Howard J (2014). The Motility of Axonemal Dynein Is Regulated by the Tubulin Code. Biophys. J 107, 2872–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh J (2014). Exploring the evolutionary history of centrosomes. Philos. Trans. R. Soc. B Biol. Sci 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani SF, Lee HO, Hyman AA, and Rosen MK (2017). Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol 18, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisic M, and Maiato H (2016). The Tubulin Code: A Navigation System for Chromosomes during Mitosis. Trends Cell Biol. 26, 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird AW, and Hyman AA (2008). Building a spindle of the correct length in human cells requires the interaction between TPX2 and Aurora A. J. Cell Biol 182, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, et al. (2018). Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol. 28, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard JJ, Otero JH, Scott DC, Szulc E, Martin EW, Sabri N, Granata D, Marzahn MR, Lindorff-Larsen K, Salvatella X, et al. (2018). Cancer Mutations of the Tumor Suppressor SPOP Disrupt the Formation of Active, Phase-Separated Compartments. Mol. Cell 72, 19–36.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouge AL, and Parmentier ML (2016). Tau excess impairs mitosis and kinesin-5 function, leading to aneuploidy and cell death. DMM Dis. Model. Mech 9, 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad AJ, DeLuca KF, and DeLuca JG (2020). Aurora B kinase is recruited to multiple discrete kinetochore and centromere regions in human cells. J. Cell Biol 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugués J, and Needleman D (2014). Physical basis of spindle self-organization. 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena M, Wheelock M, Funabiki H, and Earnshaw WC (2012). The chromosomal passenger complex (CPC): From easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol 13, 789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celetti G, Paci G, Caria J, VanDelinder V, Bachand G, and Lemke EA (2020). The liquid state of FG-nucleoporins mimics permeability barrier properties of nuclear pore complexes. J. Cell Biol 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K, Senese S, Kuang J, Bui N, Ongpipattanakul C, Gholkar A, Cohn W, Capri J, Whitelegge JP, and Torres JZ (2016). Proteomic Analysis of the Mammalian Katanin Family of Microtubule-severing Enzymes Defines Katanin p80 subunit B-like 1 (KATNBL1) as a Regulator of Mammalian Katanin Microtubule-severing. Mol. Cell. Proteomics 15, 1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conicella AE, Zerze GH, Mittal J, and Fawzi NL (2016). ALS Mutations Disrupt Phase Separation Mediated by α-Helical Structure in the TDP-43 Low-Complexity C-Terminal Domain. Structure 24, 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JA, Kalnins VI, Cleveland DW, and Kirschner MW (1977). Immunofluorescent staining of cytoplasmic and spindle microtubules in mouse fibroblasts with antibody to tau protein. Proc. Natl. Acad. Sci. U. S. A 74, 2437–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuylen S, Blaukopf C, Politi AZ, Müller-Reichert T, Neumann B, Poser I, Ellenberg J, Hyman AA, and Gerlich DW (2016). Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao TP, Kolaitis RM, Kim HJ, O’Donovan K, Martyniak B, Colicino E, Hehnly H, Taylor JP, and Castañeda CA (2018). Ubiquitin Modulates Liquid-Liquid Phase Separation of UBQLN2 via Disruption of Multivalent Interactions. Mol. Cell 69, 965–978.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca KF, Meppelink A, Broad AJ, Mick JE, Peersen OB, Pektas S, Lens SMA, and DeLuca JG (2018). Aurora A kinase phosphorylates Hec1 to regulate metaphase kinetochore-microtubule dynamics. J. Cell Biol 217, 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai D, and Pethe P (2020). Polycomb repressive complex 1: Regulators of neurogenesis from embryonic to adult stage. J. Cell. Physiol 235, 4031–4045. [DOI] [PubMed] [Google Scholar]
- Ding DQ, Okamasa K, Katou Y, Oya E, Nakayama J ichi, Chikashige Y, Shirahige K, Haraguchi T, and Hiraoka Y (2019). Chromosome-associated RNA–protein complexes promote pairing of homologous chromosomes during meiosis in Schizosaccharomyces pombe. Nat. Commun 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Du A, Gu J, Duan G, Wang C, Gui X, Ma Z, Qian B, Deng X, Zhang K, et al. (2019). PARylation regulates stress granule dynamics, phase separation, and neurotoxicity of disease-related RNA-binding proteins. Cell Res. 29, 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J, and Desai A (2012). Acentrosomal spindle assembly and chromosome segregation during oocyte meiosis. Trends Cell Biol. 22, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edozie B, Sahu S, Pitta M, Englert A, Do Rosario CF, and Ross JL (2019). Self-organization of spindle-like microtubule structures. Soft Matter 15, 4797–4807. [DOI] [PubMed] [Google Scholar]
- Feng Z, Caballe A, Wainman A, Johnson S, Haensele AFM, Cottee MA, Conduit PT, Lea SM, and Raff JW (2017). Structural Basis for Mitotic Centrosome Assembly in Flies. Cell 169, 1078–1089.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y, Alam JM, Noshiro D, Mouri K, Ando T, Okada Y, May AI, Knorr RL, Suzuki K, Ohsumi Y, et al. (2020). Phase separation organizes the site of autophagosome formation. Nature 578, 301–305. [DOI] [PubMed] [Google Scholar]
- Gatlin JC, Matov A, Groen AC, Needleman DJ, Maresca TJ, Danuser G, Mitchison TJ, and Salmon ED (2009). Spindle Fusion Requires Dynein-Mediated Sliding of Oppositely Oriented Microtubules. Curr. Biol 19, 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giubettini M, Asteriti IA, Scrofani J, De Luca M, Lindon C, Lavia P, and Guarguaglini G (2011). Control of Aurora-A stability through interaction with TPX2. J. Cell Sci 124, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia SM, Zitouni S, Kong D, Duarte P, Gomes BF, Sousa AL, Tranfield EM, Hyman A, Loncarek J, and Bettencourt-Dias M (2019). PLK4 is a microtubule-associated protein that self-assembles promoting de novo MTOC formation. J. Cell Sci 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Yu D, Gu R, Jia Y, Wang Q, Jaganathan A, Yang X, Yu M, Babault N, Zhao C, et al. (2020). Roles of the BRD4 short isoform in phase separation and active gene transcription. Nat. Struct. Mol. Biol 27, 333–341. [DOI] [PubMed] [Google Scholar]
- Heinkel F, Abraham L, Ko M, Chao J, Bach H, Hui LT, Li H, Zhu M, Ling YM, Rogalski JC, et al. (2019). Phase separation and clustering of an ABC transporter in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A 116, 16326–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Vega A, Braun M, Scharrel L, Jahnel M, Wegmann S, Hyman BT, Alberti S, Diez S, and Hyman AA (2017). Local Nucleation of Microtubule Bundles through Tubulin Concentration into a Condensed Tau Phase. Cell Rep. 20, 2304–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindriksen S, Lens SMA, and Hadders MA (2017). The Ins and Outs of Aurora B Inner Centromere Localization. Front. Cell Dev. Biol 5, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, and Raff JW (2002). The dynamic localisation of the Drosophila APC/C: evidence for the existence of multiple complexes that perform distinct functions and are differentially localised. J. Cell Sci 115, 2847–2856. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li T, Ems-McClung SC, Walczak CE, Prigent C, Zhu X, Zhang X, and Zheng Y (2018). Aurora A activation in mitosis promoted by BuGZ. J. Cell Biol 217, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubstenberger A, Noble SL, Cameron C, and Evans TC (2013). Translation repressors, an RNA helicase, and developmental cues control RNP phase transitions during early development. Dev. Cell 27, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, Weber CA, and Jülicher F (2014). Liquid-Liquid Phase Separation in Biology. Annu. Rev. Cell Dev. Biol 30, 39–58. [DOI] [PubMed] [Google Scholar]
- Janke C, and Magiera MM (2020). The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol [DOI] [PubMed] [Google Scholar]
- Jiang H, He X, Wang S, Jia J, Wan Y, Wang Y, Zeng R, Yates J, Zhu X, and Zheng Y (2014). A Microtubule-Associated Zinc Finger Protein, BuGZ, Regulates Mitotic Chromosome Alignment by Ensuring Bub3 Stability and Kinetochore Targeting. Dev. Cell 28, 268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang S, Huang Y, He X, Cui H, Zhu X, and Zheng Y (2015). Phase Transition of Spindle-Associated Protein Regulate Spindle Apparatus Assembly. Cell 163, 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V, and De Nicolo A (2018). Aurora-PLK1 cascades as key signaling modules in the regulation of mitosis. Sci. Signal 11. [DOI] [PubMed] [Google Scholar]
- Kalab P, Weis K, and Heald R (2002). Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science (80-.) 295, 2452–2456. [DOI] [PubMed] [Google Scholar]
- King MR, and Petry S (2020). Phase separation of TPX2 enhances and spatially coordinates microtubule nucleation. Nat. Commun 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix B, Letort G, Pitayu L, Sallé J, Stefanutti M, Maton G, Ladouceur A-M, Canman JC, Maddox PS, Maddox AS, et al. (2018). Microtubule Dynamics Scale with Cell Size to Set Spindle Length and Assembly Timing. Dev. Cell 45, 496–511.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laos T, Cabral G, and Dammermann A (2015). Isotropic incorporation of SPD-5 underlies centrosome assembly in C. Elegans. Curr. Biol 25, R648–R649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Protter DSW, Rosen MK, and Parker R (2015). Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol. Cell 60, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindon C, and Pines J (2004). Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J. Cell Biol 164, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Nicholson AM, Sarkar M, Messing J, Purice MD, Pottier C, Annu K, Baker M, Perkerson RB, Kurti A, et al. (2017). TIA1 Mutations in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia Promote Phase Separation and Alter Stress Granule Dynamics. Neuron 95, 808–816.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, and Barbacid M (2009). Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9, 153–166. [DOI] [PubMed] [Google Scholar]
- Mayr MI, Hümmer S, Bormann J, Grüner T, Adio S, Woehlke G, and Mayer TU (2007). The Human Kinesin Kif18A Is a Motile Microtubule Depolymerase Essential for Chromosome Congression. Curr. Biol 17, 488–498. [DOI] [PubMed] [Google Scholar]
- McKinley KL, and Cheeseman IM (2016). The molecular basis for centromere identity and function. Nat. Rev. Mol. Cell Biol 17, 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloy PG, and Holloway SL (2004). Changes in the localization of the Saccharomyces cerevisiae anaphase-promoting complex upon microtubule depolymerization and spindle checkpoint activation. Genetics 167, 1079–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milles S, Huy Bui K, Koehler C, Eltsov M, Beck M, and Lemke EA (2013). Facilitated aggregation of FG nucleoporins under molecular crowding conditions. EMBO Rep. 14, 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry HB, MacCallum DE, Jackson RC, Chaplain MAJ, and Davidson FA (2008). Modeling the temporal evolution of the spindle assembly checkpoint and role of Aurora B kinase. Proc. Natl. Acad. Sci. U. S. A 105, 20215–20220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittag T, and Parker R (2018). Multiple Modes of Protein–Protein Interactions Promote RNP Granule Assembly. J. Mol. Biol 430, 4636–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, and Taylor JP (2015). Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization. Cell 163, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer TC, and Holland AJ (2019). Plk4 promotes centriole duplication by phosphorylating stil to link the procentriole cartwheel to the microtubule wall. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, and Salmon ED (2007). The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol 8, 379–393. [DOI] [PubMed] [Google Scholar]
- Nigg EA, and Holland AJ (2018). Once and only once: Mechanisms of centriole duplication and their deregulation in diseases. Nat. Rev. Mol. Cell Biol 19, 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-E, Zhang L, Bang JK, Andresson T, DiMaio F, and Lee KS (2019). Phase separation of Polo-like kinase 4 by autoactivation and clustering drives centriole biogenesis. Nat. Commun 10, 4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Park JE, Kim TS, Kim JH, Kwak MJ, Ku B, Tian L, Murugan RN, Ahn M, Komiya S, et al. (2014). Molecular basis for unidirectional scaffold switching of human Plk4 in centriole biogenesis. Nat. Struct. Mol. Biol 21, 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, et al. (2015). A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 162, 1066–1077. [DOI] [PubMed] [Google Scholar]
- Petry S, Groen AC, Ishihara K, Mitchison TJ, and Vale RD (2013). Branching microtubule nucleation in xenopus egg extracts mediated by augmin and TPX2. Cell 152, 768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J (2011). Cubism and the cell cycle: the many faces of the APC/C. Nat. Rev. Mol. Cell Biol 12, 427–438. [DOI] [PubMed] [Google Scholar]
- Plys AJ, Davis CP, Kim J, Rizki G, Keenen MM, Marr SK, and Kingston RE (2019). Phase separation of polycomb-repressive complex 1 is governed by a charged disordered region of CBX2. Genes Dev. 33, 799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerening JR, Sontag ED, and Ferrell JE (2003). Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat. Cell Biol 5, 346–351. [DOI] [PubMed] [Google Scholar]
- Pope WB, Lambert MP, Leypold B, Seupaul R, Sletten L, Krafft G, and Klein WL (1994). Microtubule-associated protein tau is hyperphosphorylated during mitosis in the human neuroblastoma cell line SH-SY5Y. Exp. Neurol 126, 185–194. [DOI] [PubMed] [Google Scholar]
- Preuss U, and Mandelkow EM (1998). Mitotic phosphorylation of tau protein in neuronal cell lines resembles phosphorylation in Alzheimer’s disease. Eur. J. Cell Biol 76, 176–184. [DOI] [PubMed] [Google Scholar]
- Prosser SL, and Pelletier L (2017). Mitotic spindle assembly in animal cells: A fine balancing act. Nat. Rev. Mol. Cell Biol 18, 187–201. [DOI] [PubMed] [Google Scholar]
- Quartuccio SM, and Schindler K (2015). Functions of Aurora kinase C in meiosis and cancer. Front. Cell Dev. Biol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai AK, Chen J-X, Selbach M, and Pelkmans L (2018). Kinase-controlled phase transition of membraneless organelles in mitosis. Nature 559, 211–216. [DOI] [PubMed] [Google Scholar]
- Sabari BR, Dall’Agnese A, Boija A, Klein IA, Coffey EL, Shrinivas K, Abraham BJ, Hannett NM, Zamudio AV, Manteiga JC, et al. (2018). Coactivator condensation at super-enhancers links phase separation and gene control. Science (80-.) 361, eaar3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SDM, Wollman R, Meyer T, and Ferrell JE (2012). Spatial positive feedback at the onset of mitosis. Cell 149, 1500–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz CA, Santarella R, Hoenger A, Karsenti E, Mattaj IW, Gruss OJ, and Carazo-Salas RE (2003). Importin alpha-regulated nucleation of microtubules by TPX2. EMBO J. 22, 2060–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HB rode, and Görlich D (2015). Nup98 FG domains from diverse species spontaneously phase-separate into particles with nuclear pore-like permselectivity. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfelder S, Sugar R, Dimond A, Javierre BM, Armstrong H, Mifsud B, Dimitrova E, Matheson L, Tavares-Cadete F, Furlan-Magaril M, et al. (2015). Polycomb repressive complex PRC1 spatially constrains the mouse embryonic stem cell genome. Nat. Genet 47, 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha W, Moore J, Chen K, Lassaletta AD, Yi C-S, Tyson JJ, and Sible JC (2003). Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proc. Natl. Acad. Sci 100, 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siahaan V, Krattenmacher J, Hyman AA, Diez S, Hernández-Vega A, Lansky Z, and Braun M (2019). Kinetically distinct phases of tau on microtubules regulate kinesin motors and severing enzymes. Nat. Cell Biol 21, 1086–1092. [DOI] [PubMed] [Google Scholar]
- So C, Seres KB, Steyer AM, Mönnich E, Clift D, Pejkovska A, Möbius W, and Schuh M (2019). A liquid-like spindle domain promotes acentrosomal spindle assembly in mammalian oocytes. Science (80-.) 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spannl S, Tereshchenko M, Mastromarco GJ, Ihn SJ, and Lee HO (2019). Biomolecular condensates in neurodegeneration and cancer. Traffic 20, 890–911. [DOI] [PubMed] [Google Scholar]
- Splinter D, Tanenbaum ME, Lindqvist A, Jaarsma D, Flotho A, Yu K Lou, Grigoriev I, Engelsma D, Haasdijk ED, Keijzer N, et al. (2010). Bicaudal D2, dynein, and kinesin-1 associate with nuclear pore complexes and regulate centrosome and nuclear positioning during mitotic entry. PLoS Biol. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S, and Fang G (2005). Destruction box-dependent degradation of Aurora B is mediated by the anaphase-promoting complex/cyclosome and Cdh1. Cancer Res. 65, 8730–8735. [DOI] [PubMed] [Google Scholar]
- Stumpff J, von Dassow G, Wagenbach M, Asbury C, and Wordeman L (2008). The Kinesin-8 Motor Kif18A Suppresses Kinetochore Movements to Control Mitotic Chromosome Alignment. Dev. Cell 14, 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Ditlev JA, Hui E, Xing W, Banjade S, Okrut J, King DS, Taunton J, Rosen MK, and Vale RD (2016). Phase separation of signaling molecules promotes T cell receptor signal transduction. Science (80-.) 352, 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Wu R, Zheng J, Li P, and Yu L (2018). Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 28, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh P, Long AF, and Dumont S (2020). Microneedle manipulation of the mammalian spindle reveals specialized, short-lived reinforcement near chromosomes. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi J, Sakamoto R, Shiratsuchi G, Maeda YT, and Shimamoto Y (2019). Mechanically Distinct Microtubule Arrays Determine the Length and Force Response of the Meiotic Spindle. Dev. Cell 49, 267–278.e5. [DOI] [PubMed] [Google Scholar]
- Tatebayashi Y, Planel E, Chui D, Sato S, Miyasaka T, Sahara N, Murayama M, Kikuchi N, Yoshioka K, Rivka R, et al. (2006). c‐jun N‐terminal kinase hyperphosphorylates R406W tau at the PHF‐1 site during mitosis. FASEB J. 20, 762–764. [DOI] [PubMed] [Google Scholar]
- Toledo CM, Herman JA, Olsen JB, Ding Y, Corrin P, Girard EJ, Olson JM, Emili A, DeLuca JG, and Paddison PJ (2014). BuGZ Is Required for Bub3 Stability, Bub1 Kinetochore Function, and Chromosome Alignment. Dev. Cell 28, 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JZ, Ban KH, and Jackson PK (2010). A Specific Form of Phospho Protein Phosphatase 2 Regulates Anaphase-promoting Complex/Cyclosome Association with Spindle Poles. Mol. Biol. Cell 21, 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Palomba F, Niedzialkowska E, Digman MA, Gratton E, and Stukenberg PT (2019). The inner centromere is a biomolecular condensate scaffolded by the chromosomal passenger complex. Nat. Cell Biol 21, 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt MATM, Gardino AK, Linding R, Ostheimer GJ, Reinhardt HC, Ong S-E, Tan CS, Miao H, Keezer SM, Li J, et al. (2010). A mitotic phosphorylation feedback network connects Cdk1, Plk1, 53BP1, and Chk2 to inactivate the G(2)/M DNA damage checkpoint. PLoS Biol. 8, e1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, and Mandelkow E (2016). Tau in physiology and pathology. Nat. Rev. Neurosci 17, 5–21. [DOI] [PubMed] [Google Scholar]
- Wegmann S, Eftekharzadeh B, Tepper K, Zoltowska KM, Bennett RE, Dujardin S, Laskowski PR, MacKenzie D, Kamath T, Commins C, et al. (2018). Tau protein liquid–liquid phase separation can initiate tau aggregation. EMBO J. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wippich F, Bodenmiller B, Trajkovska MG, Wanka S, Aebersold R, and Pelkmans L (2013). Dual specificity kinase DYRK3 couples stress granule condensation/ dissolution to mTORC1 signaling. Cell 152, 791–805. [DOI] [PubMed] [Google Scholar]
- Woodruff JB, Ferreira Gomes B, Widlund O, Mahamid J, Honigmann A, and Hyman AA (2017). The Centrosome Is a Selective Condensate that Nucleates Microtubules by Concentrating Tubulin. Cell 169. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Tsuchiya H, Kaiho A, Guo Q, Ikeuchi K, Endo A, Arai N, Ohtake F, Murata S, Inada T, et al. (2020). Stress- and ubiquitylation-dependent phase separation of the proteasome. Nature 578, 296–300. [DOI] [PubMed] [Google Scholar]
- Yi P, and Goshima G (2018). Microtubule nucleation and organization without centrosomes. Curr. Opin. Plant Biol 46, 1–7. [DOI] [PubMed] [Google Scholar]


