Abstract
Background:
Air pollution is a carcinogen and causes pulmonary and cardiac complications. We examined the association of fine particulate matter pollution (PM2.5) and mortality from cancer and all-causes among pediatric, adolescent, and young adult (AYA) cancer patients in Utah, a state with considerable variation in PM2.5.
Methods:
We followed 2,444 pediatric (diagnosed ages 0–14) and 13,459 AYA (diagnosed ages 15–39) patients diagnosed 1986–2015 from diagnosis to five and ten years post-diagnosis, death, or emigration. We measured average monthly PM2.5 by ZIP code during follow-up. Separate pediatric and AYA multivariable Cox models estimated the association of PM2.5 and mortality. Among AYAs we examined effect modification of PM2.5 and mortality by stage while controlling for cancer type.
Results:
Increases in PM2.5 per 5 µg/m3 were associated with cancer mortality in pediatric lymphomas and CNS tumors at both time points, and all-cause mortality in lymphoid leukemias (HR5-years=1.32 [1.02–1.71]). Among AYAs, PM2.5 per 5 µg/m3 was associated with cancer mortality in CNS tumors and carcinomas at both time points, and all-cause mortality for all AYA cancer types (HR5-year =1.06 [1.01–1.13]). PM2.5≥12µg/m3 was associated with cancer mortality among breast (HR5-year =.50 [1.29–1.74]; HR10-year =1.30 [1.13–1.50]) and colorectal cancers (HR5-year =1.74 [1.29–2.35]; HR10-year =1.67 [1.20–2.31]) at both time points. Effect modification by stage was significant, with local tumors at highest risk.
Conclusion:
PM2.5 was associated with mortality in pediatric and AYA patients with specific cancers.
Impact:
Limiting PM2.5 exposure may be important for young cancer patients with certain cancers.
Keywords: Air pollution, pediatric cancer, adolescent and young adult cancer, cancer mortality, all-cause mortality
INTRODUCTION
Air pollution is classified as a carcinogen and is associated with mortality from cancer, pulmonary, and cardiac causes (1–4). Fine particulate matter air pollution (PM2.5) is a risk factor for cancer incidence and mortality among the general adult population (3,5–7), but its effect among cancer patients after diagnosis and treatment is largely understudied. Continued exposure to PM2.5 after diagnosis may accelerate cancer progression and increase risk for cancer mortality. Increased PM2.5 exposure is associated with cancer mortality among adult breast, liver, and lung cancer patients (8–12). PM2.5 may have a similar association with mortality from cancer or additional causes in young cancer patients. A study of childhood cancer survivors provides evidence that PM2.5 may be a significant contributor to pulmonary morbidity (13), which is a leading cause of death in childhood cancer survivors (14).
To our best knowledge, no studies have investigated how PM2.5 exposure affects mortality in pediatric, adolescent, and young adult (AYA) cancer patients (13). Studies examining disparities in pediatric cancer mortality primarily focus on genetics, cancer biology, treatment-related factors, or race and health behaviors (15,16). Similarly, studies of AYA cancer mortality include investigations of delays in diagnosis, lack of access to specialists, and histologic differences between cancers in AYAs and older adults (17–19). Since low-income and minority populations who have worse cancer outcomes are more likely to live in communities with higher levels of air pollution (20,21), pollution may be unaccounted for in these studies. As cancers in young patients are unique in the types of cancers that occur and their underlying biology (17,19,22), studies of the association of PM2.5 and mortality in older adult patient populations cannot be easily extrapolated to younger cancer patients.
PM2.5 is a major public health problem in the state of Utah (23–26). Population density is growing rapidly with a minimum of 80% of Utah’s population living on 20% of its landmass (27,28). Heavy reliance on cars for transportation and close residential proximity to major roadways exposes the population to air pollution (29,30). This same majority population lives in county-sized valley basins surrounded by mountains. During the winter, cold temperatures create a layer of air that traps pollutants over the most populated counties, resulting in periods of hazardous PM2.5 concentrations (31). The effects of chronic and acute PM2.5 exposure on the morbidity and mortality of the Utah population has been studied extensively (23–25,32,33), but the effect of PM2.5 on mortality of Utah’s cancer patient population is unknown.
We examined the association between PM2.5 and mortality from cancer and all-causes among pediatric and AYA cancer patients in Utah. Previous studies quantified PM2.5 exposure by residence at diagnosis but could not account for residential history post-diagnosis (8–10). Our cohort was derived from a statewide database that allowed us to document patients’ residential ZIP codes and the dates associated with those locations after diagnosis. Since PM2.5 is postulated to accelerate cancer progression (8–11), we examined effect modification of the association of PM2.5 and mortality by stage at diagnosis among AYA patients.
MATERIALS AND METHODS
Data Sources and Cohort
We identified pediatric (0 to 14 years) and AYA cancer patients (15 to 39 years) diagnosed while residing in Utah using the Utah Cancer Registry (UCR) from 1986–2015. UCR provided month and year of diagnosis, race/ethnicity, age at diagnosis, cancer diagnosis, histology, and stage for AYA cancers using the SEER summary stage (local, regional, distant, and unstaged). Patients were classified by year of diagnosis (1986 to 1995, 1996 to 2005, and 2006 to 2015). Exact day of diagnosis was not available from UCR, so the 1st was used as a substitute.
UCR records were linked to statewide inpatient hospitalization records from the Utah Department of Health, and administrative records (marriage and divorce, driver license, vital records) from the Utah Population Database (UPDB), which provided multiple records on each patient. The UPDB was also linked to electronic emergency department (ED) data from two healthcare systems serving >85% of Utah’s population and to outpatient records from one of the same healthcare systems. All healthcare records started from a patient’s first appearance in the system prior to cancer diagnosis and ended at death or last known record. Healthcare records contained race and the ICD-9/10 codes associated with the visits. We were provided every record with residential history for our cohort from a person’s first appearance in the database to their last known date of residence in Utah or death. UPDB provided sex, race, and date and cause of death.
We included patients diagnosed with malignant tumors (n=26,492). We excluded patients who survived <1 month from diagnosis (n=537), were missing month of diagnosis (n=157), or were missing stage (n=1), and who died from injury, accidents, poisonings, pregnancy, and congenital conditions.
We followed patients from diagnosis to the clinically-relevant time points of five years and ten years after diagnosis (34,35). If a patient’s last known date in Utah occurred before the end of either follow-up period, the date of their last record in Utah was used as their end of follow-up. All-cause and cancer-specific mortality were defined by ICD-9 and ICD-10 codes in UPDB records. In models examining effect modification by stage, we also examined one-year mortality estimates to determine if mortality shortly following a diagnosis was also associated with PM2.5.
Residential histories and PM2.5 exposure
We constructed residential histories using ZIP codes and counties found in all records from first cancer diagnosis to the end of follow-up. Each month during follow-up was assigned a ZIP code. For subjects aged <18 years, parental administrative records and other UPDB records tracked their residential ZIP codes.
Stationary monitors in four Utah counties that contain 80% of Utah’s population and major cities, including Salt Lake City (28), measured PM10 from 1986–1998. For those years we imputed daily county-level PM2.5 using no intercept regression models correlating PM10 and PM2.5 while accounting for stagnation, an approach used in other studies in Utah (36). Data from stationary PM2.5 monitors across the state were used to generate ZIP code estimates from 1999–2015. We first estimated daily PM2.5 from 1999 to 2015 for the 2010 population-weighted centroid of each residential ZIP code using data from the U.S. Environmental Protection Agency (EPA) Datamart (37). Using topographic features, we delineated 20 air basins across the state. Air basins were defined as areas where lateral air movement was reduced due to mountain ranges. Six air basins were in the four counties containing 80% of Utah’s population. We assigned each monitor and ZIP code centroid to the air basin where it was located and estimated daily PM2.5 using inverse distance weighting, with estimates limited to each air basin because we assumed each basin had a distinct pollution profile.
We calculated each patient’s cumulative average PM2.5 exposure for the entirety of follow-up, starting at diagnosis. If a patient was followed between 1986 and 1998, we calculated each patient’s cumulative average county-level PM2.5 exposure using the imputed PM2.5 values. If a patient was followed from 1999 onwards we calculated their cumulative average PM2.5 at every patient’s ZIP code. If PM2.5 was missing, we substituted the county-level PM2.5.
We excluded patients who were missing PM2.5 exposure information at the time of diagnosis (n=79) and stopped follow-up when PM2.5 exposure information became missing (n=273).
Cancer variables
Pediatric patient diagnoses were classified using the International Classification for Childhood Cancer (ICCC) Chapters which each have a unique staging system rarely captured by cancer registries (38,39). The schema may include patient age, stage, lymph node involvement, tumor location, tissue histology, or a combination of these criteria. Guided by the Children’s Oncology Group criteria for pediatric cancer staging and input from an oncologist, we determined which cancers had staging criteria that could be approximated using the adult staging criteria (lymphomas, central nervous system (CNS) tumors, malignant bone tumors, germ cell, other malignant, and other/unspecified neoplasms), cancers requiring both stage and histology (soft tissue sarcomas, neuroblastoma, hepatic tumors), cancers requiring histology alone (renal tumors), and cancers for which staging or risk group criteria were not available (leukemias, retinoblastomas)(40–49).
AYA patients were classified using AYA SEER groupings (50). AYA carcinomas were combined with SEER site codes to identify breast, cervical, colorectal, kidney and renal pelvis, lung, testicular, thyroid, and other carcinomas. AYA cancer stage was defined by the adult cancer stage. Staging does not apply to leukemias which are all categorized as distant. The final AYA stage variable consisted of the categories local, regional, distant, unstaged, and NA (leukemias only).
Other variables
Race/ethnicity (White, non-Hispanic or non-White) was ascertained from all records. If any record mentioned that a participant was not white, they were indicated as such. If race/ethnicity was missing, race/ethnicity was obtained from UPDB birth records containing the self-reported race of the participant’s parents.
Census tract socioeconomic status (SES) at diagnosis was computed by UCR using the Yost index (51). If census tract at diagnosis was unavailable, the Yost index was calculated by county. Patients were categorized into one of four quartiles (Highest, high, low, and lowest SES).
Smoking among AYAs prior to diagnosis was ascertained using ICD-9 (305.1, 649.0–649.04, 989.84, V15.82) and ICD-10 codes (F17.21, 099.330-O99.335, P04.2, P96.81, T65.22, Z57.31, Z71.6, Z72.0, Z77.22, and Z87.891) in the healthcare records that were linked to our cohort. Healthcare records with smoking ICD codes were only available from 1996 onwards.
Statistical models
Multi-level discrete time survival analysis modeled the association between cumulative PM2.5 exposure and mortality from cancer and all-causes. Follow-up, measured in months, started one month after diagnosis and ended at the month of death, emigration from Utah, missing PM2.5, or end of follow-up. In the cause-specific cancer models, individuals were censored if the cause of death was not cancer. Cumulative average PM2.5 was measured using a time-varying lag covariate that averaged exposure from the month of diagnosis (t0) to the month prior to observation (t-1). We modeled PM2.5 in continuous (per 5ug/m3) and categorical fashions (EPA 3-year standard of <12µg/m3 or ≥12µg/m3).
Pediatric models were stratified by ICCC Chapters with sufficient numbers to produce reliable estimates (leukemias, lymphoid leukemias, lymphomas, CNS tumors, neuroblastomas, bone tumors, soft tissue sarcomas, and hepatic tumors) and for all pediatric cancers together. Pediatric models for specific ICCC Chapters controlled for sex, diagnosis age, race/ethnicity, census tract SES clustered by county, and stage and/or histology when applicable. The leukemia-specific model did not include risk groups. The model containing all pediatric cancers included a separate baseline hazard for each ICCC Chapter. Due to the diverse methods of categorizing pediatric cancer stage, this model did not control for stage or histology.
We also ran models that were stratified by specific AYA Group (leukemias, lymphomas, CNS tumors, bone tumors, melanomas, carcinomas, sarcomas) and a model that included all AYA cancers together. We also ran models that stratified the AYA carcinomas by SEER site. All AYA models controlled for sex, diagnosis age, race/ethnicity, census tract SES at diagnosis clustered by county, and included a separate baseline hazard for stage except for the leukemia-specific AYA model. Models for AYA cancer of all types included a separate baseline hazard for each cancer and stage (leukemias staged as “NA”).
Effect modification by stage, smoking, and SES
We examined effect modification of PM2.5 by stage using an interaction term among all AYA cancers for which stage applies (lymphomas, CNS tumors, bone tumors, melanomas, carcinomas, soft tissue sarcomas, miscellaneous specified, unspecified malignant). AYA leukemias were excluded from this analysis. Models for the effect modification by stage controlled for sex, diagnosis age, race/ethnicity, census tract SES at diagnosis clustered by county, and included a separate baseline hazard for each AYA cancer group. We did not examine effect modification by stage for all pediatric cancers due to the unique classification of stage for each ICCC Chapter.
We examined effect modification by smoking among AYAs diagnosed from 1996 onwards using an interaction term between smoking (Yes/No) and PM2.5 per 5µg/m3. We conducted a sensitivity analysis to assess the impact of smoking in models of PM2.5 and mortality among AYA cancers. We also examined effect modification of the association of PM2.5 and cancer mortality by census tract SES among pediatric and AYA cancers of all types at 5 and 10 years after diagnosis. In a post-hoc analyses we stratified models for cervical cancer by stage.
We display model results for cancers with stable effect estimates defined by event count ≥10 and stability of the confidence interval. We indicate imprecise confidence intervals defined by an upper-to-lower 95% confidence interval ratio (CIR) ≥3 (52,53). Results are considered significant if the confidence interval does not include the null value. Effect modification is significant if the p≥0.05 for the test of trend.
RESULTS
We included 2,444 pediatric and 13,459 AYA cancer patients diagnosed from 1986 to 2015 who were largely White-Caucasian (Table 1). Roughly 14% of AYA patients diagnosed from 1996 to 2015 had a record of smoking. The most common pediatric cancers were leukemias, CNS tumors, and lymphomas. The most common AYA cancers were carcinomas, lymphomas, and melanomas. Breast, testicular, and thyroid cancers were the most predominant carcinomas.
Table 1.
Characteristics of pediatric, and adolescent and young adult (AYA) cancer patients
| Pediatric patientsa (N=2,444) | AYA patientsa (N=13,459) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Female | 1,150 | 47.1 | 7,938 | 59.0 |
| White, non-Hispanic | 1,950 | 79.8 | 11,113 | 82.6 |
| Smoking prior to diagnosis (diagnosed 1996–2015)b | - | - | 1,434 | 10.7 |
| Diagnosis year | ||||
| 1986 to 1995 | 603 | 24.7 | 2,955 | 22.0 |
| 1996 to 2005 | 795 | 32.5 | 4,296 | 31.9 |
| 2006 to 2015 | 1,046 | 42.8 | 6,208 | 46.1 |
| Census tract SES quartilesc | ||||
| Highest SES | 762 | 31.2 | 3,867 | 28.7 |
| High SES | 660 | 27.0 | 3,897 | 29.0 |
| Low SES | 591 | 24.2 | 3,113 | 23.1 |
| Lowest SES | 431 | 17.6 | 2,582 | 19.2 |
| Cancer diagnosis | ||||
| Leukemiasd | 722 | 29.5 | 586 | 4.4 |
| Lymphoid Leukemia | 581 | 23.8 | ||
| Lymphomasd | 256 | 10.5 | 1,420 | 10.6 |
| Hodgkin lymphomas | 84 | 3.4 | 729 | 5.9 |
| Non-Hodgkin lymphomas | 96 | 3.9 | 628 | 4.7 |
| Central nervous system, cranial, and spinal neoplasmsd | 569 | 23.3 | 714 | 5.3 |
| Malignant bone cancersd | 120 | 4.9 | 286 | 2.1 |
| Sarcomas, all sitesd | 356 | 2.7 | ||
| Soft tissue and heart | 160 | 6.6 | 283 | 2.1 |
| Germ cell, trophoblastic, and gonad tumorsd | 93 | 3.8 | 184 | 1.4 |
| Neuroblastoma and peripheral nervous cell tumorse | 177 | 7.2 | - | - |
| Retinoblastomae | 55 | 2.3 | - | - |
| Renal tumorse | 124 | 5.1 | - | - |
| Hepatic tumorse | 48 | 2.0 | - | - |
| Other malignant epithelial neoplasm and melanomase | 108 | 4.4 | - | - |
| Other and unspecified malignant neoplasmse | 12 | 0.5 | - | - |
| Melanoma and skin carcinomasf | - | - | 2,140 | 15.9 |
| Carcinomasf | - | - | ||
| Breast | - | - | 1,365 | 10.1 |
| Cervical | - | - | 714 | 5.3 |
| Colorectal | - | - | 555 | 4.1 |
| Kidney and renal pelvis | - | - | 182 | 1.4 |
| Lung | - | - | 123 | 0.9 |
| Testicular | - | - | 1,323 | 9.8 |
| Thyroid | - | - | 2,292 | 17.0 |
| Other | - | - | 807 | 6.0 |
| Miscellaneous specified neoplasms, NOSf | - | - | ||
| Unspecified Malignant Neoplasmsf | 12 | 0.5 | 16 | 0.1 |
| Stage at diagnosis | ||||
| Distant | 373 | 15.3 | 1,369 | 10.2 |
| Excluding Leukemias | ||||
| Localized | 917 | 37.5 | 7,922 | 58.9 |
| Regional | 381 | 15.6 | 3,241 | 23.9 |
| Unstaged | 51 | 2.1 | 319 | 2.4 |
| Deaths within 10 year | ||||
| All-cause deaths | 428 | 17.5 | 2,156 | 16.0 |
| Cancer-related deaths | 380 | 15.6 | 1,753 | 13.0 |
Pediatric patients diagnosed with their first primary cancer 0–14 years; AYA patients diagnosed with their first primary cancer 15–39 years;
Denominator is AYA 10,501 patients diagnosed from 1996 to 2015;
Computed using the Yost index;
Common cancers between the International Classification of Childhood Cancer (ICCC) and AYA ICD-O-3/WHO 200 classification (AYA/WHO) systems;
Cancers specific to the ICCC;
Cancers specific to the AYA/WHO; Italics indicate values for a subset.
After 10 years, approximately 15% of both cohorts were deceased with 88.8% of pediatric and 81.3% of AYA deaths attributed to cancer. Most deaths occurred within five years of diagnosis (Pediatric: 89.7%, AYA: 83.1%). On average, pediatric patients had 1.8 residential ZIP codes (range: 1–9) and AYA patients had 1.8 ZIP codes (range: 1–16). After 10 years the mean cumulative average PM2.5 was 10.5µg/m3 (4.96–15.41) among pediatric patients and 10.4µg/m3 (4.6–15.5) among AYA patients. AYA and pediatric patients with cumulative average PM2.5≥12µg/m3 were in the upper 90% of PM2.5 exposure.
We found significant positive associations between PM2.5 per 5µg/m3 and cancer mortality among pediatric lymphomas and CNS tumors at 5 and 10 years post-diagnosis (Table 2). We found significant associations between PM2.5 and all-cause mortality among patients diagnosed with lymphomas and CNS tumors at both time points, lymphoid leukemias at 5 years post-diagnosis, and hepatic tumors at 10 years post-diagnosis. Among pediatric cancers of all types, the associations between PM2.5 and mortality from cancer or all-causes at both time points are marginally nonsignificant, but positive and precise with a CIR of 1. In the categorical analysis representing pediatric patients in the upper 90% of those exposed, PM2.5 ≥12µg/m3 and all-cause mortality was significant among lymphoid leukemias at 5 years post-diagnosis.
Table 2.
Fine particulate matter (PM2.5) and mortality among pediatric cancer patients
| PM2.5 per 5 ug/m3 | PM2.5 ≥12 ug/m3 | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 years post-diagnosis | 10 years post-diagnosis | 5 years post-diagnosis | 10 years post-diagnosis | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Cancer mortality | ||||||||
| All cancer typesd | 1.12 | 0.98–1.28 | 1.09 | 0.98–1.22 | 1.09 | 0.75–1.58 | 1.07 | 0.79–1.45 |
| Leukemias, all typesd | 1.10 | 0.87–1.39 | 1.04 | 0.86–1.27 | 0.96 | 0.61–1.50 | 0.88 | 0.59–1.31 |
| Lymphoid leukemiad | 1.22 | 0.87–1.71 | 1.06 | 0.72–1.58 | 1.33 | 0.78–2.26b | 1.05 | 0.64–1.71b |
| Lymphomase | 1.34a | 1.06–1.68 | 1.34a,c | 1.06–1.68 | -- | -- | ||
| Central nervous system and intracranial/spinal neoplasmse | 1.30a | 1.08–1.56 | 1.27a | 1.05–1.52 | 1.41 | 0.83–2.38b | 1.41 | 0.91–2.16 |
| Malignant bone tumorse | 1.04 | 0.37–2.90b | 0.99 | 0.42–2.30b | 0.42 | 0.07–2.66b | 0.39 | 0.06–2.60b |
| Neuroblastoma and other peripheral nervous tumorsf | 1.12 | 0.85–1.48 | 1.20 | 0.95–1.51 | 1.30 | 0.64–2.62b | 1.41 | 0.68–2.95b |
| Soft tissue sarcomasf | 0.81 | 0.44–1.47b | 0.75 | 0.42–1.36b | 0.73 | 0.31–1.72 | 0.67 | 0.29–1.54b |
| Hepatic tumorsf | -- | 2.10 | 0.73–6.06b | -- | 2.14 | 0.84–5.44b | ||
| All-cause mortality | ||||||||
| All cancer typesd | 1.09 | 0.98–1.20 | 1.05 | 0.96–1.15 | 1.15 | 0.87–1.51 | 1.10 | 0.87–1.39 |
| Leukemias, all typesd | 1.15 | 0.94–1.39 | 1.08 | 0.91–1.28 | 1.23 | 0.91–1.66 | 1.11 | 0.84–1.48 |
| Lymphoid leukemiad | 1.32a | 1.02–1.71 | 1.15 | 0.82–1.61 | 1.69a | 1.13–2.52 | 1.33 | 0.90–1.97 |
| Lymphomase | 1.29a | 1.03–1.62 | 1.33a | 1.11–1.60 | -- | -- | ||
| Central nervous system and intracranial/spinal neoplasmse | 1.25a | 1.09–1.44 | 1.22a | 1.04–1.42 | 1.41 | 0.91–2.19 | 1.38 | 0.96–1.99 |
| Malignant bone tumorse | 0.90 | 0.37–2.19b | 0.89 | 0.43–1.85b | 0.34 | 0.06–1.98b | 0.31 | 0.05–1.89b |
| Neuroblastoma and other peripheral nervous tumorsf | 1.00 | 0.70–1.41 | 1.10 | 0.81–1.50 | 1.30 | 0.63–2.68b | 1.40 | 0.67–2.92b |
| Soft tissue sarcomasf | 0.79 | 0.43–1.47b | 0.67 | 0.41–1.09b | 0.70 | 0.29–1.70b | 0.56 | 0.27–1.17b |
| Hepatic tumorsf | 2.40 | 0.71–8.11b | 1.20a | 1.07–1.35 | -- | -- | ||
HR=hazard ratio; 95% CI=95% confidence interval; Cumulative average monthly PM2.5 over all residential ZIP codes over 5 and 10 years post-diagnosis; Models for all cancer types are adjusted for cancer diagnosis, and all models are adjusted for sex, race/ethnicity, age at diagnosis, and census tract SES clustered by county; Estimates rounded to the nearest hundredth;
Hazard ratios significant if null value not included in the 95% CI;
Ratio of upper-to-lower 95% confidence interval is ≥3;
No additional deaths;
Stage or risk group not included in model;
Stage included in model;
Stage and histology in model;
Event count <10.
Among AYA patients (Table 3), we found significant associations between PM2.5 per 5µg/m3 and cancer mortality among AYA CNS tumors and carcinomas at 5 and 10 years post-diagnosis. The association of PM2.5 and cancer mortality among all AYA cancers is marginally nonsignificant, but positive and precise. The association for PM2.5 and cancer mortality among sarcomas at 10 year post-diagnosis is inverse and marginally nonsignificant, likely driven by sarcomas of other sites. PM2.5 had a significant positive association per 5µg/m3 with all-cause mortality among all AYA cancer patients at 5 years post-diagnosis and AYA CNS tumor and carcinoma patients at both time points.
Table 3.
Fine particulate matter (PM2.5) and mortality among adolescent and young adult (AYA) cancer patients
| PM2.5 per 5 ug/m3 | PM2.5 ≥12 ug/m3 | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 years post-diagnosis | 10 years post-diagnosis | 5 years post-diagnosis | 10 years post-diagnosis | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Cancer mortality | ||||||||
| All cancer types | 1.06 | 0.99–1.12 | 1.04 | 0.96–1.12 | 1.21a | 1.16–1.26 | 1.21a | 1.14–1.28 |
| Leukemias | 0.98 | 0.78–1.22 | 0.93 | 0.77–1.13 | 1.26 | 0.91–1.74 | 1.27 | 0.96–1.67 |
| Lymphomas | 0.89 | 0.73–1.08 | 0.91 | 0.76–1.09 | 1.19 | 0.85–1.66 | 1.33 | 0.94–1.87 |
| Central nervous system and intracranial/spinal neoplasms | 1.20a | 1.06–1.36 | 1.20a | 1.04–1.38 | 1.49a | 1.24–1.79 | 1.63a | 1.45–1.83 |
| Bone tumors | 1.00 | 0.81–1.23 | 0.96 | 0.75–1.24 | 0.94 | 0.55–1.59b | 0.97 | 0.63–1.49 |
| Melanoma and skin carcinomas | 1.17 | 0.90–1.52 | 1.13 | 0.83–1.52 | 1.53a | 1.17–2.01 | 1.33 | 0.97–1.82 |
| Carcinomas | 1.14a | 1.06–1.22 | 1.10a | 1.02–1.18 | 1.24a | 1.18–1.31 | 1.18a | 1.11–1.25 |
| Sarcomas, all sites | 0.89 | 0.79–1.01 | 0.87 | 0.76–1.00 | 0.85 | 0.61–1.17 | 0.82 | 0.59–1.14 |
| Soft tissue and heart sarcomas | 0.93 | 0.74–1.17 | 0.93 | 0.74–1.17 | 0.96 | 0.67–1.37 | 0.88 | 0.62–1.24 |
| Sarcomas, other sites | 0.77 | 0.30–1.94b | 0.77 | 0.30–1.94b | 0.53 | 0.11–2.67b | 0.66 | 0.21–2.04b |
| All-cause mortality | ||||||||
| All cancer types | 1.06a | 1.01–1.13 | 1.04 | 0.96–1.12 | 1.25a | 1.22–1.29 | 1.23a | 1.17–1.28 |
| Leukemias | 0.98 | 0.77–1.25 | 0.94 | 0.76–1.16 | 1.18 | 0.89–1.56 | 1.18 | 0.92–1.50 |
| Lymphomas | 0.96 | 0.75–1.23 | 0.95 | 0.75–1.18 | 1.27a | 1.02–1.58 | 1.26 | 0.98–1.61 |
| Central nervous system and intracranial/spinal neoplasms | 1.22a | 1.12–1.33 | 1.19a | 1.05–1.36 | 1.47a | 1.26–1.71 | 1.56a | 1.33–1.83 |
| Bone tumors | 1.00 | 0.79–1.26 | 0.97 | 0.73–1.28 | 1.13 | 0.91–1.39 | 1.18 | 0.93–1.49 |
| Melanoma and skin carcinomas | 1.13 | 0.91–1.41 | 1.08 | 0.86–1.37 | 1.76a | 1.43–2.16 | 1.56a | 1.30–1.88 |
| Carcinomas | 1.14a | 1.08–1.20 | 1.09a | 1.03–1.16 | 1.27a | 1.18–1.37 | 1.19a | 1.13–1.26 |
| Sarcomas, all sites | 0.85 | 0.71–1.01 | 0.84 | 0.69–1.01 | 0.81 | 0.56–1.18 | 0.79 | 0.55–1.13 |
| Soft tissue and heart sarcomas | 0.87 | 0.66–1.16 | 0.84 | 0.61–1.15 | 0.91 | 0.66–1.26 | 0.84 | 0.60–1.17 |
| Sarcomas, other sites | 0.77 | 0.30–1.94b | 0.88 | 0.48–1.59b | 0.53 | 0.11–2.67b | 0.66 | 0.21–2.04b |
HR=hazard ratio; 95% CI=95% confidence interval; Cumulative average monthly PM2.5 over all residential ZIP codes over 5 and 10 years; Models for all cancer types are adjusted for cancer diagnosis, and all models are adjusted for sex, race/ethnicity, age at diagnosis, and census tract SES at diagnosis clustered by county; Separate baseline hazard included for stage of diagnosis; Estimates rounded to the nearest hundredth;
Hazard ratios significant if null value not included in the 95% CI;
Ratio of upper-to-lower 95% confidence interval is ≥3.
We report significant associations between PM2.5≥12µg/m3 and cancer mortality among patients of all AYA cancer types, CNS tumors, and carcinomas both time points, and melanoma patients at 5 years post-diagnosis. The association between PM2.5 ≥12µg/m3 and all-cause mortality was positive and significant among AYA patients of all cancer types, CNS tumors, melanomas, and carcinomas at 5 and 10 years post-diagnosis, and among AYAs diagnosed with lymphomas at 5 years post-diagnosis.
Among AYA carcinoma patients (Table 4), we found positive significant associations between PM2.5 per 5µg/m3 and cancer mortality among AYA colorectal cancers and kidney cancers at 5 and 10 years post-diagnosis (Table 4). The point estimate for kidney cancer is large, but the CIR suggests that these estimates are not precise or stable. The association of PM2.5 and mortality from cancer or all-causes among breast cancers is marginally nonsignificant but positive with a precise CIR. Among cervical cancers, PM2.5 had a significant inverse association with all-cause mortality at 10 years post-diagnosis. This inverse association is driven by later stage cervical cancers (Supplemental Table 1). Local stage cervical cancers have a positive but nonsignificant association between PM2.5 and any type of mortality. Results for PM2.5 ≥12µg/m3 are similar with the addition of a significant association between PM2.5 and mortality from all-causes or cancer among AYA breast cancer patients.
Table 4.
Fine particulate matter (PM2.5) and mortality among adolescent and young adult cancer carcinoma patients
| PM2.5 per 5 ug/m3 | PM2.5 ≥12 ug/m3 | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 years post-diagnosis | 10 years post-diagnosis | 5 years post-diagnosis | 10 years post-diagnosis | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Cancer mortality | ||||||||
| Breast | 1.18 | 0.91–1.54 | 1.16 | 0.97–1.39 | 1.50a | 1.29–1.74 | 1.30a | 1.13–1.50 |
| Cervical | 0.84 | 0.62–1.13 | 0.83 | 0.67–1.02 | 0.83 | 0.61–1.13 | 0.78 | 0.57–1.06 |
| Colorectal | 1.36a | 1.06–1.75 | 1.23a | 1.00–1.52 | 1.74a | 1.29–2.35 | 1.67a | 1.20–2.31 |
| Kidney and renal pelvis | 4.06a | 2.06–7.99b | 6.95a | 3.10–15.59b | -- | -- | ||
| Lung | 0.94 | 0.68–1.29 | 0.86 | 0.59–1.26 | -- | -- | ||
| Testicular | 0.92 | 0.54–1.58b | 0.88 | 0.54–1.42b | -- | -- | ||
| Thyroid | -- | 1.16 | 0.61–2.19b | -- | -- | |||
| Other | 1.09 | 0.98–1.22 | 1.05 | 0.94–1.19 | 1.03 | 0.88–1.20 | 1.00 | 0.84–1.18 |
| All-cause mortality | ||||||||
| Breast | 1.26 | 0.98–1.62 | 1.20 | 0.99–1.45 | 1.62a | 1.42–1.84 | 1.36a | 1.19–1.55 |
| Cervical | 0.86 | 0.74–1.01 | 0.83a | 0.76–0.90 | 0.85 | 0.62–1.18 | 0.73a | 0.54–0.99 |
| Colorectal | 1.31a | 1.04–1.66 | 1.21 | 0.99–1.49 | 1.65a | 1.35–2.03 | 1.64a | 1.31–2.04 |
| Kidney and renal pelvis | 2.86a | 2.09–3.91 | 2.09a | 1.16–3.79b | -- | -- | ||
| Lung | 1.02 | 0.77–1.34 | 0.93 | 0.67–1.29 | -- | -- | ||
| Testicular | 0.99 | 0.58–1.70b | 0.95 | 0.62–1.46 | -- | -- | ||
| Thyroid | -- | 1.28 | 0.74–2.23b | -- | -- | |||
| Other | 1.07 | 0.98–1.18 | 1.04 | 0.94–1.15 | 1.01 | 0.84–1.21 | 0.99 | 0.82–1.19 |
HR=hazard ratio; 95% CI=95% confidence interval; Cumulative average monthly PM2.5 over all residential ZIP codes over 5 and 10 years; Models adjusted for sex, race/ethnicity, age at diagnosis, and census tract SES at diagnosis clustered by county; Separate baseline hazard included for stage of diagnosis; Estimates rounded to the nearest hundredth;
Hazard ratios significant if null value not included in the 95% CI;
Ratio of upper-to-lower 95% confidence interval is ≥3;
Event count <10.
We examined effect modification by stage of the association of PM2.5 ≥12µg/m3 and mortality among all AYA cancers. There was significant effect modification of the association of PM2.5 and mortality at one, five, and ten years post-diagnosis (Table 5). Local tumors had the highest effect estimates and the estimates declined in a dose-response fashion in the order of local, regional, distant, and unstaged tumors. We also found evidence of significant effect modification by smoking for the association of PM2.5 and all-cause mortality among all AYA cancer patients at 5 years after diagnosis (No smoke HR=1.06, CI=0.94–1.19; Smoke HR=0.82, CI=0.68–0.99; p-value for interaction=0.02). We also found significant effect modification of the association of PM2.5 and mortality from cancer and all-causes by census tract SES among AYA cancer patients (Figure 1) but not among pediatric patients.
Table 5.
Effect modification of the association of cumulative average fine particulate matter (PM2.5) ≥ 12µg/m3 and mortality among adolescent and young adult cancer patients by stage at diagnosis
| 1 year post-diagnosis | 5 years post-diagnosis | 10 years post-diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | Joint test p-value | HR | 95% CI c | Joint test p-value | HR | 95% CI | Joint test p-value | |
| Cancer mortality | |||||||||
| Local | 1.15 | 0.82–1.60 | <0.001c | 1.44b | 1.29–1.61 | <0.001c | 1.44b | 1.24–1.66 | <0.0001c |
| Regional | 1.46 | 0.95–2.25 | 1.25b | 1.04–1.49 | 1.07 | 0.96–1.19 | |||
| Distant | 0.95 | 0.78–1.16 | 1.09 | 0.93–1.26 | 1.16b | 1.03–1.30 | |||
| Unstaged | 0.62b | 0.45–0.87 | 0.82 | 0.57–1.18 | 0.88 | 0.67–1.17 | |||
| All-cause mortality | |||||||||
| Local | 1.30 | 0.92–1.83 | <0.001c | 1.54b | 1.37–1.73 | <0.001c | 1.43b | 1.22–1.67 | <0.0001c |
| Regional | 1.63b | 1.05–2.53 | 1.32b | 1.09–1.60 | 1.17b | 1.03–1.32 | |||
| Distant | 0.89 | 0.75–1.05 | 1.05 | 0.85–1.29 | 1.11 | 0.94–1.32 | |||
| Unstaged | 0.78 | 0.55–1.10 | 1.05 | 0.75–1.48 | 1.06 | 0.80–1.41 | |||
HR=hazard ratio; 95% CI=95% confidence interval; Models used 5- and 10-year average PM2.5 for all residential ZIP codes categorized by EPA yearly standard of 12 µg/m3 PM2.5; 95% confidence interval ratio is ≤2 for all analyses; Models adjusted for sex, age at diagnosis, race/ethnicity, census tract SES at diagnosis clustered by county; Separate baseline hazard for cancer type included, leukemias excluded from this analysis; Estimates rounded to the nearest hundredth;
Hazard ratios significant if null value not included in the 95% CI;
Ratio of upper-to-lower 95% confidence interval is ≥3;
p-value for effect modification significant at p<0.05.
Figure 1. Effect modification by census-tract socioeconomic status of the association of fine particulate matter (PM2.5) and cancer mortality among AYA cancer patients.
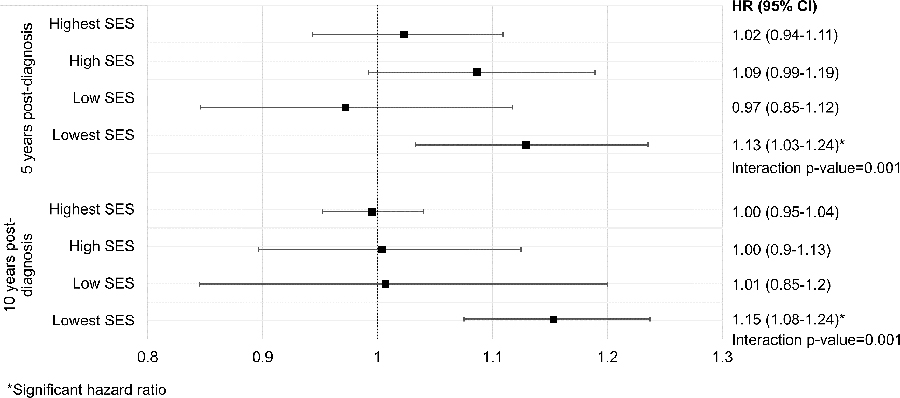
A forest plot for the effect modification by census tract socioeconomic status (SES) of the association of PM2.5 and cancer mortality among AYA patients is shown. Cancer mortality was measured at 5- and 10-years after diagnosis. The black squares denote the SES quartile-specific hazard ratios and the capped horizontal bars indicate the bounds of the 95% confidence intervals. The p-value for the interaction term of SES and PM2.5 in the main model is written underneath the hazard ratio for the lowest SES quartile. The p-values for the interaction term in the 5-and 10-year models are shown.
We did not see significant differences in the association of PM2.5 and mortality between the smoking-adjusted or smoking-unadjusted models (Supplemental Table 2). The sensitivity analysis only included cancer patients diagnosed from 1996 onwards which excluded 22% of our sample. Thus results for the sensitivity analysis are different than the main tables.
DISCUSSION
PM2.5 is associated with short- and longer-term mortality for young patients diagnosed with specific cancers in this statewide cohort. Pediatric patients with lymphoma and CNS tumors had a minimum of a 25% increase in risk for cancer mortality per 5 µg/m3 increase in PM2.5 after five and ten years from diagnosis. We found significant positive associations between PM2.5 and mortality for AYA patients with CNS tumors, carcinomas, melanomas, breast, and colorectal cancers, which also aligns with studies of older adult cancer patients (3,9,10).
A longitudinal study of the Medicare population found a significant association between PM2.5 and an increase in all-cause mortality of 7.3% (CL7.1–7.5) per 10µg/m3 of PM2.5 (54). Although our study is not directly comparable with the Medicare study, our results for suggests that PM2.5 may have a greater association with mortality among certain groups of pediatric and AYA cancer patients than the Medicare population. Pediatric cancer survivors diagnosed before the age of 21 have rates of frailty similar to older adults, suggestive of early aging attributed to cancer, its therapies, and morbidities common to the aging process (55). Although not directly comparable, early aging could explain why our pediatric and AYA cohort has risk estimates similar to or greater than the elderly.
AYAs with cancer may face different risks from PM2.5 than older cancer patients. We found a significant positive association between PM2.5 and mortality among AYA colorectal cancer patients that was not present in a study of older adults with cancer (3). Although our kidney cancer sample is small with an imprecise CIR, the hazard ratio for the association of PM2.5 and cancer mortality 10 years after diagnosis among AYA kidney cancer patients is greater than the adult kidney cancer hazard ratio of 1.14 (CI=1.03–1.27) for PM2.5 per 4.4 µg/m3 (3). Although nonsignificant the estimate for PM2.5 and all-cause mortality among AYA kidney cancer patients is more precise and shows a hazard ratio greater than seen in adult kidney cancer patients. The association of PM2.5 and all-cause mortality in our AYA breast cancer patients is similar to adult studies reporting a hazard ratio of 1.12 (95% CI: 0.96–1.30) per 10µg/m3. Breast cancer patients in the upper 90% of those exposed may have the greatest risk for PM2.5-related mortality from all-causes or cancer (10,56). Further investigation is required to confirm these results.
Stage of diagnosis may play a role in PM2.5 and cancer mortality. We report significant effect modification by stage at diagnosis among AYA cancers while controlling for cancer type. Similar to studies of adult lung cancer patients (8), the association between PM2.5 and mortality was highest among patients with localized tumors, suggesting that PM2.5 may be driving cancer progression in susceptible tissues. Regional and distant stage tumors may be so developed that PM2.5 does not affect further progression of the cancer, or patients diagnosed at later stages may not survive long enough for the adverse effects of chronic PM2.5 exposure to be observed. At the same time, patients with localized stage disease may remain active or spend more time outdoors, potentially increasing their exposure to PM2.5 more than patients with more advanced disease.
Low SES and residence in low-income neighborhoods are associated with elevated residential exposure to air pollutants, advanced cancer diagnosis, and mortality from cancer among individuals aged <65 years (21,57,58). We found significant effect modification of the association of PM2.5 and mortality by census tract SES for AYA cancers of all types, with the greatest effects among the lowest SES patients. Since PM2.5 is correlated with residence in a low-income neighborhood (21), PM2.5 could operate along a separate etiologic pathway to increase risk for mortality among residents of low-income neighborhoods and is an important area for future research.
PM2.5 levels in the United States have increased over the past two years with changes in regulatory policy (59). Our results suggest that chronic PM2.5 exposure higher than the current EPA standard of PM2.5≥12µg/m3 may be particularly deleterious for young patients diagnosed with certain cancers. For example, the risk for cancer mortality among AYA colorectal cancer patients chronically exposed to PM2.5 ≥12µg/m3 is approximately 20–30% higher than patients with less exposure. Also, the pediatric and AYA cancers with positive associations between PM2.5 and mortality in this study had prior evidence, to varying degrees, of an association with PM2.5 and incidence of those same cancers. PM2.5 is associated with incident pediatric leukemia, lymphomas, brain astrocytomas, and adult breast and colorectal cancers (60–65). Further research is needed to confirm our findings and elucidate the underlying mechanisms of these associations.
Air pollution’s relationship to mortality among cancer patients could be induced through the mechanisms that initially caused the cancer. Air pollution is a mixture of compounds with genotoxic, cytotoxic, and inflammatory properties (8,66). In addition to PM2.5, air pollution also includes benzene, heavy metals, and polycyclic aromatic hydrocarbons that may promote cancer progression through the aforementioned mechanisms (67–70). PM2.5 particles can also promote the proliferation of ER-positive breast cancer cells and inhibit E2-induced cell proliferation (71). Thus PM2.5 exerts both estrogenic and anti-estrogenic abilities in vitro, qualifying PM2.5 as a xenoestrogen (9).
Furthermore, we found that at PM2.5 exposure ≥12µg/m3, AYA colorectal cancers and breast cancer patients had the largest risk estimates for the association between PM2.5 and mortality. These cancers have estrogenic components to their etiology (72–78). Although estrogen is thought to protect against colorectal cancer (78,79), the effects of endogenous and exogenous estrogen on the risk of colorectal cancer is still unclear (75). Xenoestrogens may also have different effects on colorectal cancers than endogenous hormones (80). Our results support the theory that PM2.5 could induce cancer mortality by acting as a xenoestrogen on hormone-sensitive tissue.
Smoking is associated with mortality among melanoma patients and immunosuppression is strongly proposed as the underlying mechanism for this association (81–84). The immunosuppressant effect of smoking may increase risk for all-cause mortality by suppressing the immune system’s ability to fight off the cancer and non-cancer infections. PM2.5 may also act as an immunosuppressant in cancer patients by reducing function of T cells and macrophages (32,85–88). Our significant results for PM2.5 ≥12 µg/m3 and mortality among AYA melanoma patients implies that PM2.5 may have a similar immunosuppressant effect in melanoma cancer patients.
A nationwide study found a significant positive association between PM2.5 and mortality among cervical cancer patients (89). We report an inverse association for PM2.5 and mortality in cervical cancer patients but our sample size is limited. This inverse association appears to be driven by stage at diagnosis, but warrents additional research.
Certain limitations exist with our study. Although our AYA cohort was robust in size, our pediatric cohort was smaller with less precise confidence intervals to provide adequate conclusions for certain sites. Loss-to-follow-up may also have occurred as 14% of pediatric and 18% of AYA cancer patients had dates of last residence in Utah that occurred before the end of follow-up.
Modeling PM2.5 values for the years 1986 to 1999 could be a source of measurement error. This potential measurement error could have also produced an over or underestimate of effect, particularly in lower-populated counties where air pollution monitoring data were not available. This measurement error may be responsible for the inverse associations seen in the cervical cancer patients in counties with smaller populations.
Although we used the adult staging and/or pediatric histology to approximate the pediatric staging, these approximations are not direct substitutes for the actual pediatric risk classifications. We were not able to control for risk group in the leukemias. An additional limitation was a lack of molecular sub-type data. For example, as PM2.5 may exert xenogeneic effects, future studies should examine the association between PM2.5 and mortality in breast cancer patients by ER+, PR+, HER2+, and triple negative tumor status. We did not control for smoking in the main models. However, Utah has the nation’s lowest percent of historic and current smokers so smoking in patients diagnosed from 1986 to 1995 is likely to be similarly low (90).
While Utah’s low smoking limits generalizability, this low smoking rate and low potential exposure to secondhand smoke may increase our ability to the detect effects of PM2.5 in this population. Our majority White-Caucasian patient population limits our ability to apply our results to states with a different demographic profile or on a national level. In addition, our cohort is relatively small compared to cancer patient populations in larger states. Further investigation in a larger patient population is needed to confirm our findings.
Strengths of this study include the inclusion of residential histories which reduces exposure misclassification from using ZIP code at diagnosis as the only measure of patient residence. We also implemented a novel model that reduces bias from the high correlation between short survival and high PM2.5 exposure. Despite our small sample size the majority of our reported estimates are precise with upper-to-lower CIRs ≤2.
This study is the first to identify PM2.5 air pollution as a significant risk factor for cancer mortality in young patients diagnosed with specific cancers and for all AYA cancers. We also provide support for studies that theorize how air pollution can influence the progression of cancer after diagnosis, thereby building upon the theoretical foundation that supports this work.
Cancer is a leading cause of death in the United States and worldwide (91). While improvements in detection and treatment are of great importance to reducing cancer mortality, understanding how continued exposure to pollutants with known carcinogenic effects such as PM2.5 is also important but largely unknown. The majority of cancer patients and survivors live in the same places in which they resided before their diagnosis (92). Their unchanged environmental context contains pollutants and other extrinsic factors that likely contributed to their cancer and may further their risk for mortality after diagnosis. Studies such as this can lead to patient recommendations to reduce their personal exposure to air pollution through home-based or behavioral interventions. One means is through expanding air pollution alerts to target cancer patients. More importantly, current changes in policies and protocols have reduced the ability of regulatory bodies to enforce standards for PM2.5 and other pollutants (93,94). Studies are needed to support existing policies and to advocate for further protections of vulnerable populations who may be at great risk for illness and death due to this preventable exposure.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Douglas Fair, MD, MS, for his input on the clinical variables in our analyses. We acknowledge the contributions of the Cancer Biostatistics Shared Resource at Huntsman Cancer Institute. Funding for this study originated from the National Cancer Institute’s Geographical Management of Cancer Health Disparities (GMaP) program at the Huntsman Cancer Institute (J Ou), St. Baldrick’s Foundation Grant (J Ou, A Kirchhoff), and the NIH/NCI Cancer Center Support Grant.
Financial support: National Cancer Institute’s Geographic Management of Cancer Health Disparities (GMaP) program at the Huntsman Cancer Institute, St. Baldrick’s Foundation Grant, and the NIH/NCI Cancer Center Support Grant.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Pope CA 3rd, Lefler, Ezzati M, Higbee JD, Marshall JD, Kim SY, et al. Mortality Risk and Fine Particulate Air Pollution in a Large, Representative Cohort of U.S. Adults. Environ Health Perspect 2019;127(7):77007 doi 10.1289/ehp4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah ASV, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, et al. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ 2015;350 doi 10.1136/bmj.h1295. [DOI] [PMC free article] [PubMed]
- 3.Turner MC, Krewski D, Diver WR, Pope CA 3rd, Burnett RT, Jerrett M, et al. Ambient Air Pollution and Cancer Mortality in the Cancer Prevention Study II. Environ Health Perspect 2017;125(8):087013 doi 10.1289/ehp1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Outdoor Air Pollution. IARC Monogr Eval Carcinog Risks Hum 2016;109:9–444. [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison RM, Smith DJT, Kibble AJ. What is responsible for the carcinogenicity of PM2.5? Occup Environ Med 2004;61(10):799–805 doi 10.1136/oem.2003.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Li M, Wan X, Sun Y, Cheng K, Zhao X, et al. Spatiotemporal analysis of PM2.5 and pancreatic cancer mortality in China. Environ Res 2018;164:132–9 doi 10.1016/j.envres.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Moore JX, Akinyemiju T, Wang HE. Pollution and regional variations of lung cancer mortality in the United States. Cancer Epidemiol 2017;49:118–27 doi 10.1016/j.canep.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckel SP, Cockburn M, Shu YH, Deng H, Lurmann FW, Liu L, et al. Air pollution affects lung cancer survival. Thorax 2016;71(10):891–8 doi 10.1136/thoraxjnl-2015-207927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huo Q, Zhang N, Wang X, Jiang L, Ma T, Yang Q. Effects of Ambient Particulate Matter on Human Breast Cancer: Is Xenogenesis Responsible? PLoS One 2013;8(10):e76609 doi 10.1371/journal.pone.0076609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tagliabue G, Borgini A, Tittarelli A, van Donkelaar A, Martin RV, Bertoldi M, et al. Atmospheric fine particulate matter and breast cancer mortality: a population-based cohort study. BMJ Open 2016;6(11) doi 10.1136/bmjopen-2016-012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Ha S, Kan H, Hu H, Curbow BA, Lissaker CT. Health effects of air pollution on length of respiratory cancer survival. BMC Public Health 2013;13:800 doi 10.1186/1471-2458-13-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng H, Eckel SP, Liu L, Lurmann FW, Cockburn MG, Gilliland FD. Particulate matter air pollution and liver cancer survival. Int J Cancer 2017;141(4):744–9 doi 10.1002/ijc.30779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou JY, Hanson HA, Ramsay JM, Leiser CL, Zhang Y, VanDerslice JA, et al. Fine Particulate Matter and Respiratory Healthcare Encounters among Survivors of Childhood Cancers. Int J Environ Res Public Health 2019;16(6) doi 10.3390/ijerph16061081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang TT, Hudson MM, Stokes DC, Krasin MJ, Spunt SL, Ness KK. Pulmonary outcomes in survivors of childhood cancer: a systematic review. Chest 2011;140(4):881–901 doi 10.1378/chest.10-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia S Disparities in cancer outcomes: Lessons learned from children with cancer. Pediatr Blood Cancer 2011;56(6):994–1002 doi 10.1002/pbc.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, Leisenring WM, Ness KK, Robison LL, Armstrong GT, Yasui Y, et al. Racial/Ethnic Differences in Adverse Outcomes Among Childhood Cancer Survivors: The Childhood Cancer Survivor Study. J Clin Oncol 2016. doi 10.1200/jco.2015.66.3567. [DOI] [PMC free article] [PubMed]
- 17.Smith AW, Seibel NL, Lewis DR, Albritton KH, Blair DF, Blanke CD, et al. Next steps for adolescent and young adult oncology workshop: an update on progress and recommendations for the future. Cancer 2016;122(7):988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bleyer A Young adult oncology: the patients and their survival challenges. CA Cancer J Clin 2007;57(4):242–55. [DOI] [PubMed] [Google Scholar]
- 19.Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B. The distinctive biology of cancer in adolescents and young adults. Nature Reviews Cancer 2008;8(4):288. [DOI] [PubMed] [Google Scholar]
- 20.Scheurer ME, Lupo PJ, Schüz J, Spector LG, Wiemels JL, Aplenc R, et al. An overview of disparities in childhood cancer: Report on the Inaugural Symposium on Childhood Cancer Health Disparities, Houston, Texas, 2016. Pediatr Hematol Oncol 2018;35(2):95–110 doi 10.1080/08880018.2018.1464088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajat A, Hsia C, O’Neill MS. Socioeconomic Disparities and Air Pollution Exposure: A Global Review. Current environmental health reports 2015;2(4):440–50 doi 10.1007/s40572-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coccia PF. Overview of Adolescent and Young Adult Oncology. J Oncol Pract 2019;15(5):235–7 doi 10.1200/jop.19.00075. [DOI] [PubMed] [Google Scholar]
- 23.Bennion K Utah cities atop EPA’s worst-air-quality list. The Salt Lake Tribune 2013. 1/21/2013.
- 24.Penrod E As Utah areas exceed ozone pollution standards, gov recommends feds step in. The Salt Lake Tribune 2016.
- 25.Pope CA 3rd, Hill RW, Villegas GM. Particulate air pollution and daily mortality on Utah’s Wasatch Front. Environ Health Perspect 1999;107(7):567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Lung Association. 2017. July 20 Most Polluted Cities. American Lung Association, <http://www.lung.org/our-initiatives/healthy-air/sota/city-rankings/most-polluted-cities.html>. Accessed 2017 July 20. [Google Scholar]
- 27.Pirozzi CS, Jones BE, VanDerslice JA, Zhang Y, Paine R 3rd, Dean NC. Short-Term Air Pollution and Incident Pneumonia: A Case-Crossover Study. Ann Am Thorac Soc 2017. doi 10.1513/AnnalsATS.201706-495OC. [DOI] [PubMed]
- 28.Davidson L Urbanites: Nine of 10 Utahns live on 1 percent of state’s land. Salt Lake Tribune 2012.
- 29.Martin R, Coulombe R, Brain R. Utah Air Quality: PM 2.5. 2016.
- 30.Utah Department of Environmental Quality, Division of Air Quality. 2019. January 28 Understanding Utah’s Air Quality. Utah Department of Environmental Quality; <https://deq.utah.gov/communication/news/featured/understanding-utahs-air-quality>. Accessed 2019 January 28. [Google Scholar]
- 31.Silcox GD, Kelly KE, Crosman ET, Whiteman CD, Allen BL. Wintertime PM2.5 concentrations during persistent, multi-day cold-air pools in a mountain valley. Atmos Environ 2012;46(Supplement C):17–24 doi 10.1016/j.atmosenv.2011.10.041. [DOI] [Google Scholar]
- 32.Horne BD, Joy EA, Hofmann MG, Gesteland PH, Cannon JB, Lefler JS, et al. Short-term Elevation of Fine Particulate Matter Air Pollution and Acute Lower Respiratory Infection. Am J Respir Crit Care Med 2018. doi 10.1164/rccm.201709-1883OC. [DOI] [PubMed]
- 33.Beard JD, Beck C, Graham R, Packham SC, Traphagan M, Giles RT, et al. Winter Temperature Inversions and Emergency Department Visits for Asthma in Salt Lake County, Utah, 2003–2008. Environ Health Perspect 2012;120(10):1385–90 doi 10.1289/ehp.1104349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenner H Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. The Lancet 2002;360(9340):1131–5 doi 10.1016/S0140-6736(02)11199-8. [DOI] [PubMed] [Google Scholar]
- 35.Ries LG, Young J, Keel G, Eisner M, Lin Y, Horner M. SEER survival monograph: cancer survival among adults: US SEER program, 1988–2001, patient and tumor characteristics. National Cancer Institute, SEER Program, NIH Pub 2007(07–6215):193–202. [Google Scholar]
- 36.Pope CA, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation 2006;114(23):2443–8. [DOI] [PubMed] [Google Scholar]
- 37.US EPA Air Quality System. 11 April 2016. Air Quality Stytem Data Mart. <https://aqs.epa.gov/aqsweb/documents/data_mart_welcome.html>. Accessed 2016 11 April 2016.
- 38.Gupta S, Aitken JF, Bartels U, Brierley J, Dolendo M, Friedrich P, et al. Paediatric cancer stage in population-based cancer registries: the Toronto consensus principles and guidelines. The Lancet Oncology 2016;17(4):e163–e72. [DOI] [PubMed] [Google Scholar]
- 39.National Cancer Institute S, Epidemiology, and End Results (SEER) Program,. 2019. June 10 International Classification of Childhood Cancer (ICCC). U.S. Department of Health and Human Services, National Institutes of Health, <https://seer.cancer.gov/iccc/>. Accessed 2019 June 10. [Google Scholar]
- 40.Rodriguez‐Galindo C, Krailo M, Frazier L, Chintagumpala M, Amatruda J, Katzenstein H, et al. Children’s Oncology Group’s 2013 blueprint for research: rare tumors. Pediatr Blood Cancer 2013;60(6):1016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bollard CM, Lim MS, Gross TG, Committee CNHL. Children’s Oncology Group’s 2013 blueprint for research: Non‐Hodgkin lymphoma. Pediatr Blood Cancer 2013;60(6):979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freyer DR, Felgenhauer J, Perentesis J, Adolescent C, Committee YAOD. Children’s Oncology Group’s 2013 blueprint for research: adolescent and young adult oncology. Pediatr Blood Cancer 2013;60(6):1055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorlick R, Janeway K, Lessnick S, Randall RL, Marina N, Committee CBT. Children’s Oncology Group’s 2013 blueprint for research: bone tumors. Pediatr Blood Cancer 2013;60(6):1009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly KM, Hodgson D, Appel B, Chen L, Cole PD, Horton T, et al. Children’s Oncology Group’s 2013 blueprint for research: Hodgkin lymphoma. Pediatr Blood Cancer 2013;60(6):972–8. [DOI] [PubMed] [Google Scholar]
- 45.Hawkins DS, Spunt SL, Skapek SX, Committee CSTS. Children’s Oncology Group’s 2013 blueprint for research: Soft tissue sarcomas. Pediatr Blood Cancer 2013;60(6):1001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunger SP, Loh ML, Whitlock JA, Winick NJ, Carroll WL, Devidas M, et al. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer 2013;60(6):957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gajjar A, Packer RJ, Foreman N, Cohen K, Haas‐Kogan D, Merchant TE, et al. Children’s Oncology Group’s 2013 blueprint for research: central nervous system tumors. Pediatr Blood Cancer 2013;60(6):1022–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dome JS, Fernandez CV, Mullen EA, Kalapurakal JA, Geller JI, Huff V, et al. Children’s Oncology Group’s 2013 blueprint for research: renal tumors. Pediatr Blood Cancer 2013;60(6):994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park JR, Bagatell R, London WB, Maris JM, Cohn SL, Mattay KM, et al. Children’s Oncology Group’s 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer 2013;60(6):985–93. [DOI] [PubMed] [Google Scholar]
- 50.National Cancer Institute S, Epidemiology, and End Results (SEER) Program,. 2019. June 12 AYA Site Recode. <https://seer.cancer.gov/ayarecode/>. Accessed 2019 June 12.
- 51.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 2001;12(8):703–11. [DOI] [PubMed] [Google Scholar]
- 52.Baker M Statisticians issue warning over misuse of P values. Nature News 2016;531(7593):151. [DOI] [PubMed] [Google Scholar]
- 53.Poole C Low P-values or narrow confidence intervals: which are more durable? Epidemiology 2001;12(3):291–4. [DOI] [PubMed] [Google Scholar]
- 54.Di Q, Dai L, Wang Y, et al. Association of short-term exposure to air pollution with mortality in older adults. JAMA 2017;318(24):2446–56 doi 10.1001/jama.2017.17923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henderson TO, Ness KK, Cohen HJ. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am Soc Clin Oncol Educ Book 2014:e423–30 doi 10.14694/EdBook_AM.2014.34.e423. [DOI] [PubMed] [Google Scholar]
- 56.DuPre NC, Hart JE, Holmes MD, Poole EM, James P, Kraft P, et al. Particulate Matter and Traffic-Related Exposures in Relation to Breast Cancer Survival. Cancer Epidemiol Biomarkers Prev 2019;28(4):751–9 doi 10.1158/1055-9965.Epi-18-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL, et al. The impact of socioeconomic status on survival after cancer in the United States : findings from the National Program of Cancer Registries Patterns of Care Study. Cancer 2008;113(3):582–91 doi 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 58.Hastert TA, Beresford SA, Sheppard L, White E. Disparities in cancer incidence and mortality by area-level socioeconomic status: a multilevel analysis. J Epidemiol Community Health 2015;69(2):168–76 doi 10.1136/jech-2014-204417. [DOI] [PubMed] [Google Scholar]
- 59.Clay K, Muller NZ. Recent increasesin air pollution: Evidence and implications for mortality National Bureau of Economic Research 2019. doi 10.3386/w26381. [DOI]
- 60.Lavigne É, Bélair M-A, Do MT, Stieb DM, Hystad P, van Donkelaar A, et al. Maternal exposure to ambient air pollution and risk of early childhood cancers: A population-based study in Ontario, Canada. Environ Int 2017;100:139–47 doi 10.1016/j.envint.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Raaschou-Nielsen O, Hertel O, Thomsen BL, Olsen JH. Air pollution from traffic at the residence of children with cancer. Am J Epidemiol 2001;153(5):433–43 doi 10.1093/aje/153.5.433. [DOI] [PubMed] [Google Scholar]
- 62.Raaschou-Nielsen O, Reynolds P. Air pollution and childhood cancer: a review of the epidemiological literature. Int J Cancer 2006;118(12):2920–9 doi 10.1002/ijc.21787. [DOI] [PubMed] [Google Scholar]
- 63.White AJ, Keller JP, Zhao S, Carroll R, Kaufman JD, Sandler DP. Air Pollution, Clustering of Particulate Matter Components, and Breast Cancer in the Sister Study: A U.S.-Wide Cohort Environ Health Perspect 2019;127(10) doi 10.1289/EHP5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogala D, Marchwińska-Wyrwał E, Spychała A, Hajok l. Incidence of Colorectal Cancer in Urban Population Exposed to Cadmium. Polish Journal of Environmenal Studies 2019;28(5):3395–400 doi 10.15244/pjoes/92707 [DOI] [Google Scholar]
- 65.Filippini T, Heck JE, Malagoli C, Del Giovane C, Vinceti M. A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2015;33(1):36–66 doi 10.1080/10590501.2015.1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol 2011;2011:487074 doi 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Darbre PD. Overview of air pollution and endocrine disorders. Int J Gen Med 2018;11:191–207 doi 10.2147/IJGM.S102230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crouse DL, Goldberg MS, Ross NA, Chen H, Labrèche F. Postmenopausal Breast Cancer Is Associated with Exposure to Traffic-Related Air Pollution in Montreal, Canada: A Case–Control Study. Enviromental Health Perspectives 2010;118(1):1578–83 doi 10.1289/ehp.1002221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.International Agency for Research on Cancer. Diesel and Gasoline Engine Exhausts and Some Nitroarenes IARC Monogr Eval Carcinog Risks Hum. Volume 105 Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 70.International Agency for Research on Cancer. Outdoor Air Pollution. IARC Monogr Eval Carcinog Risks Hum 2016. p 454. [PMC free article] [PubMed]
- 71.Chen ST, Lin CC, Liu YS, Lin C, Hung PT, Jao CW, et al. Airborne particulate collected from central Taiwan induces DNA strand breaks, Poly(ADP-ribose) polymerase-1 activation, and estrogen-disrupting activity in human breast carcinoma cell lines. J Environ Sci Health A Tox Hazard Subst Environ Eng 2013;48(2):173–81 doi 10.1080/10934529.2012.717809. [DOI] [PubMed] [Google Scholar]
- 72.Yu X, Zhou S, Wang J, Zhang Q, Hou J, Zhu L, et al. Hormone replacement therapy and breast cancer survival: a systematic review and meta-analysis of observational studies. Breast Cancer 2017;24(5):643–57 doi 10.1007/s12282-017-0789-5. [DOI] [PubMed] [Google Scholar]
- 73.Sufrin G, Mirand EA, Moore RH, Chu TM, Murphy GP. Hormones in renal cancer. Trans Am Assoc Genitourin Surg 1976;68:115–20. [PubMed] [Google Scholar]
- 74.Ding J, Yeh C-R, Sun Y, Lin C, Chou J, Ou Z, et al. Estrogen receptor β promotes renal cell carcinoma progression via regulating LncRNA HOTAIR-miR-138/200c/204/217 associated CeRNA network. Oncogene 2018;37(37):5037–53 doi 10.1038/s41388-018-0175-6. [DOI] [PubMed] [Google Scholar]
- 75.Rennert G Reproductive factors, hormones and colorectal cancer-still unresolved. Br J Cancer 2017;116(1):1–3 doi 10.1038/bjc.2016.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu CP, Ho JY, Huang YT, Cha TL, Sun GH, Yu DS, et al. Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-beta activation. PLoS One 2013;8(2):e56667 doi 10.1371/journal.pone.0056667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bennett NC, Rajandram R, Ng KL, Gobe GC. Evaluation of steroid hormones and their receptors in development and progression of renal cell carcinoma. J Kidney Cancer VHL 2014;1(2):17–25 doi 10.15586/jkcvhl.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Botteri E, Støer NC, Sakshaug S, Graff-Iversen S, Vangen S, Hofvind S, et al. Menopausal hormone therapy and colorectal cancer: a linkage between nationwide registries in Norway. BMJ Open 2017;7(11):e017639-e doi 10.1136/bmjopen-2017-017639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barzi A, Lenz AM, Labonte MJ, Lenz H-J. Molecular pathways: Estrogen pathway in colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2013;19(21):5842–8 doi 10.1158/1078-0432.CCR-13-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marino M Xenoestrogens challenge 17β-estradiol protective effects in colon cancer. World J Gastrointest Oncol 2014;6(3):67–73 doi 10.4251/wjgo.v6.i3.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer 2013;132(2):401–10 doi 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- 82.Pozniak J, Nsengimana J, Laye JP, O’Shea SJ, Diaz JMS, Droop AP, et al. Genetic and Environmental Determinants of Immune Response to Cutaneous Melanoma. Cancer Res 2019;79(10):2684–96 doi 10.1158/0008-5472.Can-18-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones MS, Jones PC, Stern SL, Elashoff D, Hoon DSB, Thompson J, et al. The Impact of Smoking on Sentinel Node Metastasis of Primary Cutaneous Melanoma. Ann Surg Oncol 2017;24(8):2089–94 doi 10.1245/s10434-017-5775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qiu F, Liang C-L, Liu H, Zeng Y-Q, Hou S, Huang S, et al. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017;8(1):268–84 doi 10.18632/oncotarget.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pirozzi CJB, VanDerslice J, Jephson A, Dean N Short-term effects of particulate air pollution exposure on incidence and severity of pneumonia. American Thoracic Society International Conference Seattle, WA2015. [Google Scholar]
- 86.Jaligama S, Saravia J, You D, Yadav N, Lee GI, Shrestha B, et al. Regulatory T cells and IL10 suppress pulmonary host defense during early-life exposure to radical containing combustion derived ultrafine particulate matter. Respir Res 2017;18(1):15- doi 10.1186/s12931-016-0487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ciencewicki J, Jaspers I. Air pollution and respiratory viral infection. Inhal Toxicol 2007;19(14):1135–46 doi 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- 88.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol 2010;126(4):845–52.e10 doi 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 89.Turner MC, Krewski D, Chen Y, Pope CA 3rd, Gapstur SM, Thun MJ. Radon and nonrespiratory mortality in the American Cancer Society cohort. Am J Epidemiol 2012;176(9):808–14 doi 10.1093/aje/kws198. [DOI] [PubMed] [Google Scholar]
- 90.Public Health Indicator Based Information System (IBIS). 2016. February 1 Complete Health Indicator Report of Lung Cancer Deaths. Utah Department of Health; <https://ibis.health.utah.gov/indicator/complete_profile/LungCADth.html>. Accessed 2017 February 1. [Google Scholar]
- 91.National Cancer Institute. 2018. February 25 Cancer Statistics. National Institutes of Health; <https://www.cancer.gov/about-cancer/understanding/statistics>. Accessed 2019 February 25. [Google Scholar]
- 92.Muralidhar V, Nguyen PL, Tucker-Seeley RD. Recent relocation and decreased survival following a cancer diagnosis. Prev Med 2016;89:245–50 doi 10.1016/j.ypmed.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goldman GT, Dominici F. Don’t abandon evidence and process on air pollution policy. Science 2019;363(6434):1398–400. [DOI] [PubMed] [Google Scholar]
- 94.Davenport C EPA to eliminate office that advises agency chief on science. The New York Times 2018;27.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


