Abstract
Poor diet quality is associated with poor cognition and increased neurodegeneration, including Alzheimer’s disease (AD). We are interested in the role of diet quality on cognitive functioning (by sex and increasing genetic risk for AD), in a sample of African American (AA) middle-aged adults. We analyzed a sub-group of participants (~55% women; mean follow-up time~4.7y) from the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study with a genetic risk score for AD (hAlzScore). The Healthy Eating Index (HEI-2010), Dietary Approaches to Stop Hypertension (DASH), and the Mean Adequacy Ratio (MAR) computed at baseline (2004–2009) and follow-up visits (2009–2013) were used to assess initial diet quality (DQ) and change over time. Linear mixed-effects regression models were utilized, adjusting for select covariates, selection bias and multiple testing. DQ change (ΔDQ) was associated with California Verbal Learning Test- List A (CVLT- List A) - overall (0.15±0.06, p=0.008) and in women (0.21±0.08, p=0.006), at highest AD risk, indicating protective effects over time. Greater AD risk was longitudinally associated with poorer Clock Command Test scores in men. Poor diet quality was positively and cross-sectionally associated with Trails B scores, but in women only. Better-quality diet was associated with a slower decline in verbal memory among AA women, with greater AD risk. Insufficient clinical evidence and/or mixed findings dictate the need for more studies are needed to investigate brain morphology and volume changes in relation to diet quality in an at-risk population for AD, over time.
Keywords: Alzheimer’s disease, genetic risk, diet quality, cognitive decline, health disparities, adults
INTRODUCTION
Diet quality has profound and long-term consequences on cognitive function1–3. An emerging literature is reporting protective benefits of some dietary factors (such as vitamins D and E, polyunsaturated fatty acids (PUFAs) etc.) against cognitive decline as well as delayed onset and progression of Alzheimer’s disease (AD)4–6. Vitamin D has been implicated in cognitive decline due to possible neuronal loss with reduced number of vitamin D receptors (VDR) in brain regions like the hippocampus and AD risk because of lower hippocampal VDR mRNA7. PUFAs (and their precursors) have numerous beneficial effects for improved brain health and cognition via optimal neurotransmission, better cell survival and reducing neuroinflammation- in addition to influencing fluid intelligence, memory, gray and white matter volume and related microstructures8. Epidemiological evidence demonstrates a role for dietary intervention in the primary prevention of chronic diseases, even in old age9. Increasing evidence implicates certain dietary patterns as beneficial to brain health1,5,10. For instance, the Mediterranean diet, typically characterized by higher intakes of fruit, vegetables, whole grains, fish, unsaturated fatty acids and moderate alcohol consumption, is important for its role in preserving cognitive health11. A systematic review from 2016 found memory (i.e., delayed recognition, long-term, and working memory), executive function, and visual constructs benefited from Mediterranean diets2. However, the study population was predominantly white across the board, with a couple of exceptions that included Hispanic participants. Another recent review looking into the Mediterranean, DASH and MIND diet suggested that that higher adherence to these diets is associated with less cognitive decline and a lower risk of AD12. Diet that is very similar to a Mediterranean diet in composition, also widely used and recommended in the US, is the DASH13 (Dietary Approaches to Stop Hypertension) diet. While most primary studies on diet quality and cognition focused on one or possibly two diet quality measures, this study aimed to expand the current literature on three validated scoring systems to measure diet quality- establishing a comprehensive approach.
There is a paucity of research on association studies investigating diet and cognitive performance/ decline among different racial groups in the progression of AD14–18. In fact, most research on the relation of race to cognitive function in AD has been cross-sectional19. Longitudinal studies assess rates of cognitive decline, but few have examined the association between cognitive decline20 and diet quality with genetic risk for AD. African Americans (AA) in particular suffer from higher incidence rates of AD, perhaps due to undiscovered genetic factors, disproportionately higher rates of risk factor diseases21 (such as diabetes and stroke), biological or environmental exposures that erode ‘cognitive reserve’ which may protect against or accelerate disease expression, or detection bias of existing testing methods22. They also struggle to adhere to a healthy diet more than their White counterparts23–25.
In the present study, we examined the cross-sectional and longitudinal relationships of diet quality and cognition in a socio-economically diverse sample of African American middle-aged adults. We hypothesized that initial better diet quality would be associated with higher baseline cognitive functioning. We also examined whether those relationships differ across sex and by increasing genetic risk for AD.
MATERIALS AND METHODS
Database
HANDLS is a prospective cohort study initiated in 2004 to investigate health disparities in medical, metabolic, and cognitive outcomes in a socioeconomically diverse sample of Whites and African Americans (30–64 years old at baseline) recruited from select neighborhoods in Baltimore, Maryland. Initial data were collected in two phases (Visit 1: 2004–2009). Phase 1 consisted of screening, recruitment, first 24-hr dietary recall, and household interviews in participants’ homes. Phase 2 consisted of the second 24-hr dietary recall and physical examinations in mobile Medical Research Vehicles (MRVs). The first follow-up examinations were performed approximately five years later (Visit 2: 2009–2013; mean follow-up time±SE of 4.62y±0.95; range: 0.42–8.20) at which two 24-hr dietary recalls were also collected. Neuropsychological tests were administered at both visits on the MRVs. The numbers of participants with at least one of 11 cognitive test scores at visits 1 or 2, dietary and covariate data at baseline ranged from 123 to 228 (k=1.70–1.95 observations per participant), which yielded 5–15% (k=1.0–1.7) missing observations.
Written informed consent was obtained from all participants at each visit during which they were provided with a protocol booklet in layman’s terms and a video that described all procedures and future re-contacts. HANDLS study was ethically approved by the National Institutes of Health, National Institute of Environmental Health Sciences (NIEHS/NIH) Institutional Review Board (IRB).
Study sample
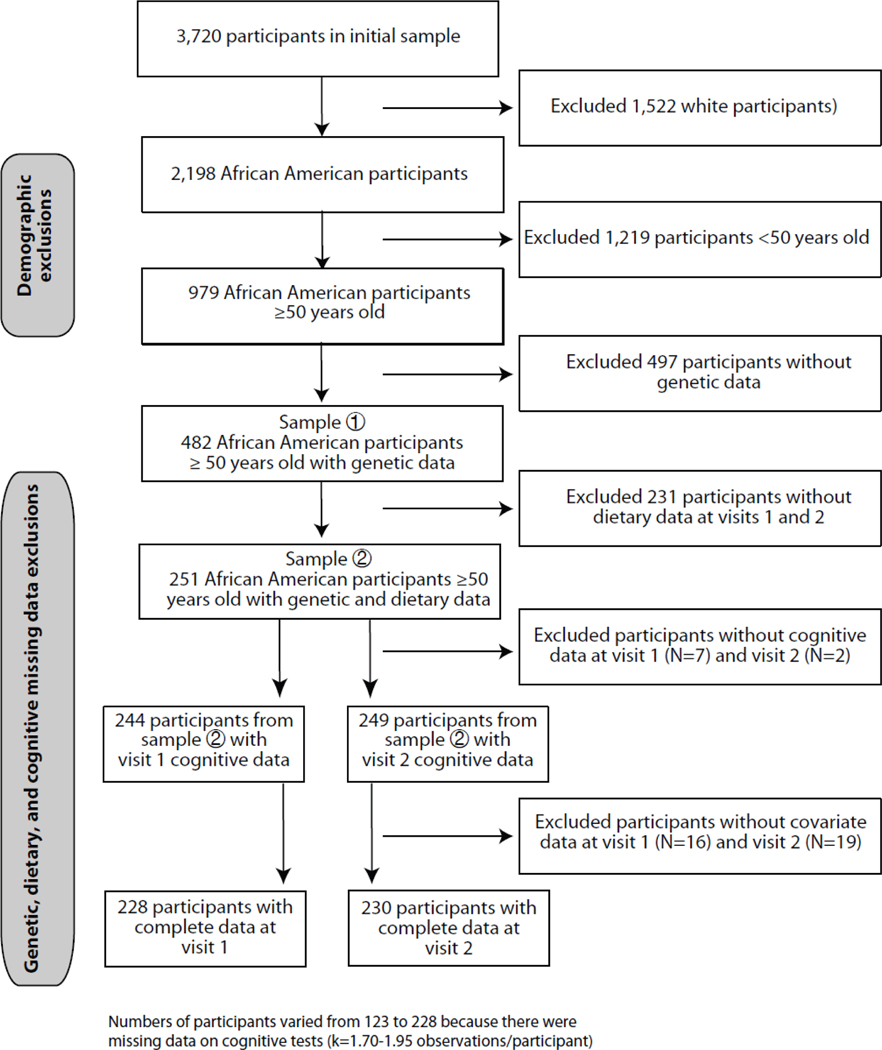
The initial HANDLS sample recruited at visit 1 (N=3,720) with complete data on demographics. In this study, we excluded participants from European ancestry (N=1,522) due to non-availability of genetic data in this group and examined only African American participants (N=2,198). Restricting our sample to participants over 50 years (N=979) and then excluding participants with missing data on valid cognitive tests, dietary assessment and genetic polymorphisms, yielded a sample of N=342 at visit 1 and N=268 at visit 2. We restricted our sample to “over 50 years” for a greater variability in cognitive decline measures across both racial groups compared. We calculated change in diet quality over time for visit 1 (N=244) and visit 2 (N=249). After excluding participants with incomplete covariate data, our final sample for analyses was N=228 for visit 1 and N=230 for visit 2 (Fig. 1). This sample selectivity was adjusted for using a 2-stage Heckman selection model26.
Figure 1.
Flowchart of participant selection
Cognitive Measures
A cognitive battery of tests was administered to participants consisting of: Mini-Mental State Examination (MMSE); California Verbal Learning Test–List A (CVLT-List-A); California Verbal Learning Test–Free Recall Long Delay (FRLD); Benton Visual Retention Test (BVRT); Brief Test of Attention; Trailmaking Test A (Trails A); Trailmaking Test B (Trails B); Digits Span Forward Test; Digits Span Backward Test; Clock Command Test; Identical Pictures Test; Card Rotation Test; and Animal Fluency Test. Details of these tests are available in Appendix 1. Except for the BVRT and the Trailmaking Tests, higher scores reflect better cognition. For BVRT and Trailmaking Tests parts A and B, better performance on BVRT was measured by fewer errors; the Trailmaking Tests was measured by faster performance. Cognitive performance test scores at baseline (Visit 1), follow-up (Visit 2), and change between visits, by sex, for HANDLS participants >50y is presented in Table S1.
Genetic Data
In total, 1,024 HANDLS participants were successfully genotyped to 907,763 single nucleotide polymorphisms (SNPs) at the equivalent of Illumina 1M array coverage. Sample exclusion criteria were (1) call rate <95%, discordance between self-reported sex and sex estimated from X-chromosome heterogeneity, cryptic relatedness, discordance between self-reported African ancestry and ancestry confirmed by genetic data. SNP exclusion criteria were (1) Hardy-Weinberg equilibrium p-value <10–7, minor allele frequency <0.01, and call rate <95%. Genotype quality control and data management was conducted using PLINKv1.06 (PMID: 17701901). Cryptic relatedness was estimated via pairwise identity by descent analyses in PLINK and confirmed using RELPAIR (PMID: 11032786). HANDLS participant genotypes were imputed using MACH/minimac version 2.0 (https://genome.sph.umich.edu/wiki/Minimac) based on combined haplotype data for the 1000 Genomes Populations project phase 3 version 5 multi-ethnic reference panel. Our final sample includes subjects with complete genetic data as they are further narrowed down by the availability of complete dietary, cognitive and covariate information.
Genetic Risk Score Calculation
Previously reported genetic variants at specific genetic loci implicated with phenotypes of (AD) were used for genetic risk score calculation (Table S2). Of the one hundred-thirty reported genetic variants, seventy-seven had valid SNP identifier. Seventy out of seventy-seven SNPs had imputed genotype data in the HANDLS study. After excluding two SNPs with poor imputation quality score (R-squared <0.30), there were 68 SNPs for the final analysis. These SNPs were then screened for significant associations with the MMSE from the published literature. This was primarily because few studies used more than two tests (including MMSE) to measure cognitive performance. Only 12 of the 68 showed a significant association with baseline cognitive performance, across sex, age, race and geographical location27–34. The genotype dosages of the risk alleles of these 12 SNPs were used for the calculation of the HANDLS AD genetic risk score (hAlzScore). The online Supplementary Table S2 describes those SNPs. Table 5 presents with individual SNP and hAlzScore correlation. The SNPs were located on the following genes: Transferrin, TF (n=1); Cystatin 3, CST3 (n=1); Presenilin 1, PSEN1 (n=1); Prion Protein, PRNP (n=1); Insulin Degrading Enzyme, IDE (n=1); Transcription Factor A, Mitochondrial, TFAM (n=1); Apolipoprotein E, APOE (n=2); Angiotensin I Converting Enzyme, ACE (n=2); Glyceraldehyde-3-Phosphate Dehydrogenase, GAPDH (n=1) and Cholinergic Receptor Nicotinic Beta 2 Subunit, CHRNB2 (n=1).
Diet Quality Assessment
Method
All 24-hr dietary recalls were collected using the United States Department of Agriculture (USDA) computerized Automated Multiple-Pass Method (AMPM)35. The AMPM was designed to provide prompts throughout all 5-steps of the recall to capture all the foods and drinks consumed throughout the previous day. The steps are described in detail elsewhere13. Trained interviewers provided an illustrated food model booklet, rulers, and measuring cups and spoons to participants to help them estimate accurate quantities of foods and beverages consumed. The approximate time between recalls was 4 to 10 days. Each recall was coded using the USDA Survey Net data processing system, matching foods consumed with codes in the Food and Nutrient Database for Dietary Studies36. Of the 3,720 participants examined at visit 1, 2,177 individuals and at visit 2, 2,140 persons completed two 24-hr dietary recalls.
Healthy Eating Index 2010 (HEI2010)
Food-based diet quality was also evaluated with the HEI-2010. The National Cancer Institute’s Applied Research website provided the basic steps for calculating the HEI-2010 component and total scores and statistical codes for 24-hr dietary recalls37. A detailed description of the procedure used for this study is available on the HANDLS website 38. The HEI-2010 includes 12 components, 9 of which assess adequacy of the diet and the remaining 3 should be consumed in moderation. Among the 9 components are: 1) total fruit; 2) total vegetables; 3) whole fruit; 4) greens and beans; 5) whole grains; 6) dairy; 7) total protein foods; 8) seafood and plant proteins; and 9) fatty acids. Refined grains, sodium, and empty calories reflect components that should be consumed in moderation39. Component and total HEI-2010 scores were calculated for each recall day and were averaged to obtain the mean for both days combined.
Dietary Approaches to Stop Hypertension (DASH)
The score for DASH diet adherence, based on 8 nutrients, was determined for each participant using the formula reported by Mellen et al.40. The nine target nutrients were total fat, saturated fat, protein, fiber, cholesterol, calcium, magnesium, sodium and potassium. Micronutrient goals were expressed per 1000 kcal. The total DASH score was generated by the sum of all nutrient targets met. If the participant achieved the DASH target for a nutrient, a value of 1 was assigned, and if the intermediate target for a nutrient was achieved, a value of 0.5 was assigned. A value of zero was assigned if neither target was met. The maximum DASH score was 8; individuals meeting approximately half of the DASH targets (DASH score=4.5) were considered DASH adherent 40.
Mean Adequacy Ratio (MAR)
Diet quality was also assessed using Nutrient Adequacy Ratio (NAR) for 17 micronutrients and Mean Adequacy Ratio (MAR) scores41,42. The NAR score was determined by dividing each participant’s daily intake of a micronutrient divided by the Recommended Dietary Allowance (RDA) for that micronutrient. The micronutrients were vitamins A, C, D, E, B6, B12, folate, iron, thiamin, riboflavin, niacin, copper, zinc, calcium, magnesium, phosphorus, and selenium. The RDA was adjusted for participants’ ages and sexes and vitamin C was adjusted for smokers43. The NAR score was converted into a percent with values exceeding 100 truncated to 100. The formula used to calculate the MAR score was: MAR= (∑NAR scores)/1744. NAR and MAR were calculated separately for each daily-intake and then averaged. MAR scores represented nutrient-based diet quality since they were based on intakes of foods and beverages and no supplements.
Diet Quality (DQ) score
Two principal components analyses45 were conducted whereby baseline diet quality indices (HEI-2010, DASH and MAR) as well as their annual rates of change were reduced into two measures, namely DQ and ΔDQ, respectively, using the Kaiser rule for component extraction (Eigen value>1) and examining the scree plot. In both cases, 46–54% of the total variance was explained by the single component.45 Those measures were used in the main analysis, for data reduction purposes.
Covariates
Sociodemographic, lifestyle, and health-related potential confounders
All regression models adjusted for sociodemographic factors, namely age, sex, race, educational levels (less than High School [HS] coded as ‘0’; HS coded as ‘1’; and more than HS coded as ‘2’) and poverty status (below vs. above 125% the federal poverty line). Additional adjustment factors include body mass index (kg/m2), current drug use (“opiates, marijuana or cocaine”=1 vs. not=0) and current smoking status ( “never or former smoker”=0 vs. “current smoker”=1). These models were further adjusted for self-reported history of type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease (stroke, congestive heart failure, non-fatal myocardial infarction or atrial fibrillation), inflammatory disease (multiple sclerosis, systemic lupus, gout, rheumatoid arthritis, psoriasis, thyroid disorder and Crohn’s disease) and use of NSAIDs (prescription and over-the- counter) during visit 1.
Statistical analysis
Stata 15.0 (StataCorp, College Station, TX) was used to conduct all analyses. First, participants’ characteristics, including covariates and exposures, were compared by sex using t- tests for continuous variables and χ2 tests for categorical variables. Second, several mixed-effects regression models on continuous initial Diet Quality (DQ) and Diet Quality Change (ΔDQ) scores calculated from total scores of HEI-2010, MAR and individual components, were conducted to test associations with cognitive performance measures, while adjusting for potential confounders. We used linear mixed-effects models to characterize the overall pattern of change in cognitive function, and to examine the relation of a specific predictor (e.g. diet quality or hAlzScore) to initial level of cognitive function and annual rate of change. In this approach both initial level of cognition and individual rate of change are explicitly modeled as sources of random variability and possible correlates of how rapidly cognition changes. Everyone is assumed to follow the mean path of the group except for random effects which cause the initial level of cognition to be higher or lower and the rate of change to be faster or slower. Thus, we added a random effect for the intercept and another for the slope. Specifically, each model included years elapsed between visits (TIME), exposures/covariate main effects and 2-way interaction terms between TIME and exposures/covariates. We assumed the unavailability of outcomes to be missing at random.46 Sex-specific associations were examined through stratified analyses separately among men and women. Effect modification by sex was formally tested for effects of hAlzScore/DQ/ΔDQ on baseline cognitive performance (2-way interaction terms) and on cognitive change over time (3-way interaction terms). These models were adjusted for covariates (See Covariates section) that include socio-demographics, lifestyle and health-related factors. Scores for Trails A and B were log-transformed before modeling due to the extreme distribution of both. All other cognitive tests were not skewed.
Three sets of models were tested: (1) Models with hAlzScore as the main predictor, for cross-sectional and longitudinal cognitive performance (2) Models with DQ and ΔDQ as the main predictors for cross-sectional and longitudinal cognitive performance, and (3) Models with DQ and ΔDQ interacting with hAlzScore to determine cross-sectional and longitudinal cognitive performance. In addition, to test for clinical significance, the exposures and outcomes were transformed into z-scores. They were then run in the same mixed models in lie of the unstandardized variables and the effect sizes were noted. An effect size >0.2 was considered strong, while >0.1 was moderate.
To account for multiple testing, given that there were 2 exposures, type I error was reduced to 0.05/2=0.025 for main effects and for interaction terms for the mixed-effects regression models. 3-way interaction terms were deemed statistically significant at an α-error level of 0.05.
RESULTS
Descriptive findings are outlined in Table 1. Women had higher HEI-2010 and DASH scores than men represented by means across visits (48.0 and 2.3 vs. 43.6 and 1.4, p=0.03 and p=0.004) respectively. Other notable differences include current smoking status, current use of illicit drugs and Body Mass Index (BMI). Table 2 displays findings from the linear mixed-effects regression models for AlzScore on cognitive test performance over time. After adjustment for multiple testing, none of the tests were associated with hAlzScore longitudinally, except Clock Command in men (0.04± 0.01, p=0.01), showing a protective effect. However, hAlzScore was significantly associated with a decline in CVLT-DFR (−0.41± 0.14, p= 0.004) in men and BVRT (0.69± 0.26, p=0.009) in women. Other longitudinal effects were inconsistent overall and within sex. Table 3 presents the associations between diet quality and cognitive change by time. None of the tests survived multiple testing, except Trails B in women: longitudinal association with diet quality change reflecting a worsening of performance (−0.04±0.01, p=0.01). We also conducted a sensitivity analysis with total energy intake (data not shown), that did not affect our current findings.
Table 1.
Characteristics of HANDLS study participants (>50y) by sex (mean across waves)a
| By sex (% or Mean±SEM) | Pb | ||
|---|---|---|---|
| Men (n=102) | Women (n=126) | Men vs. women | |
| Main Exposures | |||
| Healthy Eating Index 2010 (HEI-2010) | 43.6±1.4 | 48.0±1.6 | 0.03 |
| Dietary Approaches to Stop Hypertension (DASH) | 1.4±0.1 | 2.3±0.2 | 0.004 |
| Mean Adequacy Ratio (MAR) | 80.5±1.7 | 76.4±2.4 | 0.17 |
| hAlzScore | 12.7±0.2 | 12.4±0.2 | 0.36 |
| Changes in Diet Quality | |||
| Delta HEI2010 | 1.0±0.4 | 0.5±0.3 | 0.29 |
| Delta DASH | −0.0±0.02 | −0.9±0.04 | 0.10 |
| Delta MAR | −0.5±0.4 | −0.4±0.4 | 0.86 |
| Diet PCA Score | −0.01±0.2 | −0.2±0.2 | 0.17 |
| Sociodemographic characteristics | |||
| Age (y), Mean ±SEM | 57.1±0.5 | 56.0±0.8 | 0.22 |
| African-American, % | 44.7 | 55.3 | 0.80 |
| Education, % | |||
| <HS | 8.8 | 6.4 | 0.59 |
| HS | 51.0 | 57.1 | |
| >HS | 40.2 | 36.5 | |
| PIR≥125%, % | 60.0 | 73.0 | 0.89 |
| Lifestyle and health-related factors | |||
| Current smoking status, % | 47.1 | 32.5 | 0.03 |
| Current use of illicit drugs, % | 16.7 | 7.1 | 0.02 |
| Body mass index, kg/m2; Mean±SEM | 29.3±1.0 | 33.3±1.1 | 0.01 |
| Co-morbid conditions and NSAIDs | |||
| Diabetes, % | 22.0 | 30.0 | 0.17 |
| Hypertension, % | 55.0 | 88.0 | 0.08 |
| Dyslipidemia, % | 32.0 | 52.0 | 0.07 |
| Cardiovascular diseasec, % | 22.0 | 33.0 | 0.45 |
| Inflammatory conditionsd, % | 9.0 | 31.0 | 0.08 |
| NSAIDSe, % | 34.0 | 35.0 | 0.09 |
Abbreviations: HANDLS=Healthy Aging in Neighborhoods of Diversity Across the Lifespan; hAlzScore= HANDLS Alzheimer’s Risk Score; HS=High School; PIR=Poverty Income Ratio; SEM=standard error of the mean; PCA= Principal Component Analysis
Values are percent or Mean±SEM or %±SE.
P-value was based on independent samples t-test when row variable is continuous and χ2 test when row variable is categorical.
Cardiovascular disease include self-reported stroke, congestive heart failure, non-fatal myocardial infarction or atrial fibrillation.
Inflammatory conditions include multiple sclerosis, systemic lupus, gout, rheumatoid arthritis, psoraiasis, thyroid disorder and Crohn’s disease.
Non-steroidal anti-inflammatory drugs (NSAIDS) include over the counter and prescription drugs in that category.
Table 2:
Coefficient estimates (β±SE) for associations between cognitive test performance and hAlzScore by time, for HANDLS participants >50y of age with complete and reliable cognitive test scores derived from mixed-effects linear regression models
| All | Women | Men | ||||
|---|---|---|---|---|---|---|
| Mini-Mental State Exam, MMSE | N=225; k=1.82 | p-value | N=123; k=1.85 | p-value | N=102; k=1.77 | p-value |
| Time | 0.02±0.17 | 0.90 | −0.54±0.55 | 0.33 | −0.13±0.26 | 0.62 |
| hAlzScore | −0.11± −0.07* | 0.09 | −0.04± 0.09 | 0.62 | −0.13± 0.11 | 0.20 |
| hAlzScore × Time | 0.02± −0.02 | 0.31 | 0.01± 0.02 | 0.74 | 0.02± 0.03 | 0.39 |
| California Verbal Learning Test CVLT, List A | N=223; k=1.76 | N=122; k=1.8 | N=101; k=1.71 | |||
| Time | −0.68±0.44 | 0.12 | −2.32±1.58 | 0.14 | −0.56±0.54 | 0.30 |
| hAlzScore | −0.32± −0.2 | 0.10 | −0.39± 0.25 | 0.11 | −0.15± 0.32 | 0.63 |
| hAlzScore × Time | 0.05± −0.05 | 0.33 | 0.13*± 0.07 | 0.07 | −0.06± 0.07 | 0.42 |
| California Verbal Learning Test CVLT, DFR | N=219; k=1.74 | N=121; k=1.78 | N=98; k=1.7 | |||
| Time | −0.38±0.19 | 0.05 | −0.94±0.63 | 0.14 | −0.84±0.27 | 0.002 |
| hAlzScore a | −0.19± −0.1* | 0.18 | 0.04± 0.14 | 0.75 | −0.41± 0.14*** | 0.004 |
| hAlzScore × Time | 0.03± −0.02 | 0.18 | 0.01± 0.03 | 0.62 | 0.03± 0.03 | 0.37 |
| Benton Visual Retention Test, BVRT | N=227; k=1.89 | N=123; k=1.92 | N=104; k=1.85 | |||
| Time | 0.77±0.42 | 0.08 | −2.97±1.48 | 0.05 | 0.96±0.48 | 0.05 |
| hAlzScore a | 0.25± −0.19 | 0.05 | 0.69± 0.26*** | 0.009 | −0.14± 0.26 | 0.59 |
| hAlzScore × Time | −0.06± −0.05 | 0.24 | −0.10± 0.07 | 0.12 | 0.02± 0.06 | 0.68 |
| Brief Test of Attention | N=220; k=1.78 | N=121; k=1.81 | N=99; k=1.75 | |||
| Time | 0.21±0.15 | 0.17 | 0.18±0.54 | 0.73 | 0.27±0.25 | 0.28 |
| hAlzScore | −0.07± −0.08 | 0.40 | −0.13± 0.11 | 0.27 | −0.05± 0.13 | 0.69 |
| hAlzScore × Time | 0.01± −0.02 | 0.71 | 0.00± 0.02 | 0.85 | 0.02± 0.03 | 0.40 |
| Animal Fluency | N=228; k=1.95 | N=124; k=1.96 | N=104; k=1.93 | |||
| Time | 0.32±0.27 | 0.23 | 0.60±0.78 | 0.45 | 0.67±0.39 | 0.08 |
| hAlzScore | 0.02± −0.18 | 0.90 | 0.08± 0.22 | 0.71 | 0.12± 0.28 | 0.67 |
| hAlzScore × Time | −0.03± −0.03 | 0.31 | −0.02± 0.03 | 0.61 | −0.08± 0.04* | 0.07 |
| Digits Span, Forward | N=226; k=1.85 | N=123; k=1.83 | N=103; k=1.86 | |||
| Time | 0.13±0.11 | 0.25 | 0.27±0.35 | 0.45 | 0.27±0.16 | 0.09 |
| hAlzScore | 0.02± −0.07 | 0.74 | 0.00± 0.09 | 0.10 | 0.12± 0.10 | 0.24 |
| hAlzScore × Time | 0± −0.01 | 0.96 | 0.00± 0.02 | 0.94 | −0.00± 0.02 | 0.10 |
| Digits Span, Backward | N=226; k=1.84 | N=123; k=1.82 | N=103; k=1.86 | |||
| Time | −0.23±0.15 | 0.12 | −0.30±0.51 | 0.55 | −0.15±0.21 | 0.46 |
| hAlzScore b | −0.04± −0.07 | 0.51 | −0.16± 0.09* | 0.08 | 0.11± 0.09 | 0.23 |
| hAlzScore × Time | 0.01± −0.02 | 0.73 | 0.03± 0.02 | 0.23 | −0.01± 0.02 | 0.64 |
| Clock, Command | N=228; k=1.93 | N=125; k=1.95 | N=103; k=1.89 | |||
| Time | 0.07±0.09 | 0.46 | 0.15±0.32 | 0.64 | 0.04±0.12 | 0.73 |
| hAlzScore | 0± −0.04 | 0.93 | −0.01± 0.06 | 0.86 | −0.03± 0.07 | 0.64 |
| hAlzScore × Time | 0.01± −0.01 | 0.37 | −0.01± 0.01 | 0.40 | 0.04± 0.01** | 0.01 |
| Trailmaking Test, Part A | N=224; k=1.84 | N=123; k=1.87 | N=101; k=1.81 | |||
| Time | −0.00±0.04 | 0.84 | 0.09±0.12 | 0.46 | 0.03±0.05 | 0.59 |
| hAlzScore | 0.00±0.02 | 0.94 | 0.03±0.02 | 0.27 | 0.00±0.02 | 0.93 |
| hAlzScore × Time | −0.00± 0.00 | 0.24 | −0.00± 0.01 | 0.42 | −0.01± 0.01 | 0.17 |
| Trailmaking Test, Part B | N=222; k=1.77 | N=123; k=1.76 | N=99; k=1.78 | |||
| Time | −0.07±0.05 | 0.16 | −0.32±0.15 | 0.04 | −0.06±0.06 | 0.26 |
| hAlzScore | −0.00±0.02 | 0.98 | 0.00± 0.04 | 0.86 | −0.02± 0.03 | 0.54 |
| hAlzScore × Time | 0.00±0.01 | 0.93 | −0.00± −0.01 | 0.87 | 0.00± 0.00 | 0.56 |
Abbreviations: hAlzScore= Alzheimer’s Risk Score; MMSE= Mini-Mental State Examination; CVLT-List A= California Verbal Learning test- List A; CVLT- DFR= California Verbal Learning Test-Long-Delayed Free Recall; BVRT= Benton Visual Retention Test; Attention= Brief Test of Attention; Trails A= Trailmaking Test A; Trails B= Trailmaking Test B; Digit Span Forward= Digits Span Forward Test; Digit Span Backward= Digits Span Backward Test; Clock Command= Clock Command Test; Identical Pictures= Identical Pictures Test; Card Rotation= Card rotation Test; Animal Fluency= Animal Fluency Test.
a× Continuous covariates were mean-centered.
*** p<0.01,
** p<0.05,
* p<0.10;
a indicates significant interaction with se× at the p<0.5 level;
b indicates significant interaction with se× at the p<0.10 level k= the total number of observations/total number of groups per test
Table 3:
Coefficient estimates (β±SE) for associations between diet quality and cognitive change by time, for HANDLS participants >50y of age with complete and reliable cognitive test scores derived from mixed-effects linear regression models
| All | Women | Men | |
|---|---|---|---|
| Mini-Mental State E×am, MMSE | N=225 | N=123 | N=181 |
| Time | 0.02±0.17 | 0.11±0.21 | −0.10±0.26 |
| Diet Change# | 0.02±0.14 | −0.09±0.16 | 0.07±0.25 |
| Diet Change × Time | −0.01±0.04 | 0.04±0.04 | 0.02±0.07 |
| Initial Diet^ | −0.06±0.11 | −0.17±0.13 | −0.00±0.21 |
| Initial Diet × Time | 0.01±0.03 | 0.03±0.03 | 0.05±0.05 |
| California Verbal Learning Test CVLT, List Aa1 | N=223 | N=122 | N=173 |
| Time | −0.64±0.43 | −1.31**±0.66 | −0.56±0.52 |
| Diet Change | 0.23±0.36 | 0.16±0.42 | 0.75±0.63 |
| Diet Change × Time | −0.01±0.10 | −0.15±0.13 | 0.22±0.14 |
| Initial Diet | −0.06±0.31 | −0.05±0.37 | −0.12±0.55 |
| Initial Diet × Time | −0.03±0.08 | −0.08±0.11 | 0.06±0.12 |
| California Verbal Learning Test CVLT, Free Recall Long Delay FRLDa1,a2 | N=219 | N=121 | N=167 |
| Time | −0.34*±0.19 | −0.36±0.28 | −0.79***±0.26 |
| Diet Change | −0.06±0.18 | −0.25±0.23 | 0.38±0.27 |
| Diet Change × Time | 0.03±0.04 | 0.07±0.06 | −0.03±0.07 |
| Initial Diet | −0.02±0.16 | −0.16±0.21 | 0.29±0.24 |
| Initial Diet × Time | −0.02±0.04 | 0.01±0.05 | −0.09±0.06 |
| Benton Visual Retention Test, BVRT a1 | N=227 | N=123 | N=192 |
| Time | 0.64±0.42 | 0.14±0.68 | 1.03**±0.48 |
| Diet Change | −0.30±0.37 | −0.16±0.47 | −0.51±0.61 |
| Diet Change × Time | −0.07±0.10 | −0.05±0.14 | −0.07±0.15 |
| Initial Diet | −0.20±0.31 | −0.20±0.38 | 0.06±0.52 |
| Initial Diet × Time | 0.01±0.08 | 0.08±0.10 | −0.15±0.12 |
| Brief Test of Attentionb1,b2,c2,d2 | N=220 | N=121 | N=173 |
| Time | 0.24±0.24 | 0.19±0.20 | 0.26±0.24 |
| Diet Change | −0.25*±0.17 | −0.24±0.20 | −0.22±0.27 |
| Diet Change × Time | 0.04±0.05 | 0.02±0.04 | 0.13*±0.07 |
| Initial Diet | −0.35**±0.15 | −0.45***±0.17 | 0.01±0.24 |
| Initial Diet × Time | 0.03±0.02 | 0.04±0.03 | 0.01±0.06 |
| Animal Fluencya2,d2 | N=228 | N=124 | N=201 |
| Time | 0.31±0.27 | 0.10±0.66 | 0.67*±0.39 |
| Diet Change | −0.08±0.35 | −0.10±0.43 | 0.28±0.65 |
| Diet Change × Time | 0.11*±0.06 | 0.16±0.14 | −0.01±0.11 |
| Initial Diet | 0.06±0.29 | 0.19±0.36 | −0.23±0.55 |
| Initial Diet × Time | 0.02±0.05 | 0.03±0.11 | −0.03±0.09 |
| Digits Span, Forwarda2,c1 | N=226 | N=123 | N=192 |
| Time | 0.15±0.11 | −0.05±0.15 | 0.29*±0.16 |
| Diet Change | 0.25**±0.11 | 0.39**±0.16 | 0.19±0.24 |
| Diet Change × Time | 0.00±0.03 | 0.01±0.03 | −0.02±0.05 |
| Initial Diet | 0.14±0.11 | 0.19±0.14 | 0.13±0.20 |
| Initial Diet × Time | −0.01±0.02 | −0.01±0.03 | −0.01±0.04 |
| Digits Span, Backwarda1,e2 | N=226 | N=123 | N=192 |
| Time | −0.23±0.15 | −0.43**±0.21 | −0.12±0.21 |
| Diet Change | 0.06±0.13 | 0.11±0.17 | 0.00±0.22 |
| Diet Change × Time | 0.02±0.04 | 0.06±0.05 | −0.01±0.06 |
| Initial Diet | −0.13±0.11 | −0.20±0.14 | 0.06±0.19 |
| Initial Diet × Time | 0.05*±0.03 | 0.06*±0.04 | 0.01±0.05 |
| Clock, Commandb1 | N=228 | N=125 | N=195 |
| Time | 0.08±0.09 | 0.02±0.14 | 0.05±0.13 |
| Diet Change | 0.12±0.08 | 0.10±0.10 | 0.15±0.15 |
| Diet Change × Time | −0.02±0.02 | −0.02±0.03 | −0.02±0.04 |
| Initial Diet | −0.00±0.07 | −0.10±0.09 | 0.10±0.13 |
| Initial Diet × Time | 0.02±0.02 | 0.03±0.02 | 0.02±0.03 |
| Trailmaking Test, Part Ac1,d1 | N=224 | N=123 | N=101 |
| Time | −0.01±0.04 | −0.04±0.05 | 0.02±0.05 |
| Diet Change | 0.03±0.04 | −1.81±5.50 | 0.03±0.06 |
| Diet Change × Time | −0.01±0.01 | 1.02±1.87 | −0.02±0.01 |
| Initial Diet | −0.01±0.03 | 0.37±4.52 | 0.00±0.05 |
| Initial Diet × Time | −0.01±0.00 | 2.74***±1.39 | 0.00±0.01 |
| Trailmaking Test, Part Ba1,c2,d1,d2,e2 | N=222 | N=123 | N=99 |
| Time | −0.07±0.05 | −0.14**±0.06 | −0.06±0.05 |
| Diet Change | 0.07±0.05 | 0.13**±0.06 | −0.06±0.07 |
| Diet Change × Time | −0.02±0.01 | −0.04***±0.01 | 0.02±0.02 |
| Initial Diet | −0.02±0.04 | −0.01±0.01 | −0.03±0.06 |
| Initial Diet × Time | −0.00±0.01 | −0.01±0.01 | −0.03**±0.06 |
Abbreviations: HANDLS= Healthy Aging in Neighborhoods of Diversity Across the Lifespan; hAlzScore= HANDLS Alzheimer’s Risk Score; MMSE= Mini-Mental State Examination; CVLT-List A= California Verbal Learning test- List A; CVLT-DFR= California Verbal Learning Test-Delayed Free Recall; BVRT= Benton Visual Retention Test; Attention= Brief Test of Attention; Trails A= Trailmaking Test A; Trails B= Trailmaking Test B; Digit Span Forward= Digits Span Forward Test; Digit Span Backward= Digits Span Backward Test; Clock Command= Clock Command Test; Identical Pictures= Identical Pictures Test; Card Rotation= Card rotation Test; Animal Fluency= Animal Fluency Test.
Represents change in diet quality over time (~5 years from baseline)
Represents diet quality at baseline (Time 0)
Continuous covariates were mean-centered.
p<0.01,
p<0.05,
p<0.10;
indicates significant interaction between time and se× at the p<0.05 level;
indicates significant interaction between time and se× at the p<0.10 level;
indicates significant interaction between se× and diet at the p<0.05 level;
indicates significant interaction between se× and diet at the p<0.10 level;
indicates significant interaction between se× and diet (change) at the p<0.05 level;
indicates significant interaction between se× and diet (change) at the p<0.10 level;
indicates significant interaction between se× and diet (change) and time at the p<0.05 level;
indicates significant interaction between se× and diet (change) and time at the p<0.10 level;
indicates significant interaction between se× and diet and time at the p<0.05 level;
indicates significant interaction between se× and diet and time at the p<0.10 level;
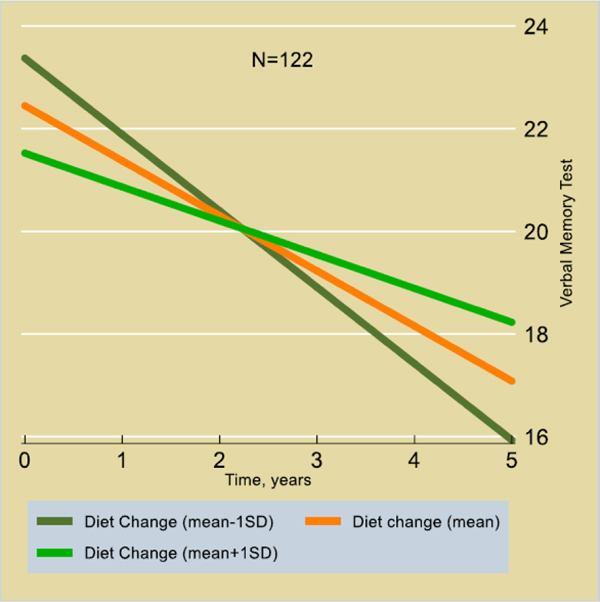
Table S3 shows cross-sectional (baseline vs. baseline) and longitudinal (baseline vs. change, change vs. change) associations between cognitive test scores and hAlzScore, and diet quality (DQ and ΔDQ). Annual rate of change in the California Verbal Learning Test- List A (CVLT- List A) was associated with an interaction between diet quality change and hAlzScore in the total population (Time×ΔDQ×hAlzScore: 0.15±0.06, p=0.008) as well as in women (Fig. 2) (0.21±0.08, p=0.006), indicating protective effects of diet quality at higher AD risk levels. No other associations were statistically significant after correcting for multiple testing.
Figure 2.
Predictive margins for CVLT – List A by Time, across levels of diet change, among women (N=122) with highest genetic risk for AD; Mixed effects linear regression models
Finally, to tease apart the dietary index/indices driving the findings, we conducted two additional sensitivity analyses with just main findings from our principal component analyses. The results for diet quality in cognition is presented in Table S4, while the results of gene X diet interactions are presented in Table S5. We found that, all three indices had significant contributions to Trails B test scores over time. However, only HEI-2010 and DASH scores influenced the gene X diet interactions for CVLT- List A and delayed free recall.
DISCUSSION
This study prospectively examined the relationship between change in diet quality and a genetic risk for AD on cognition in urban-dwelling African American adults. Our findings indicated that improvements in diet quality over time were associated with a slower rate of decline on a test of verbal memory particularly among African American women with higher genetic risk for AD (Fig. 2). The association was not present in men but persisted overall in mixed-sex analyses. No cross-sectional associations (initial diet and related findings) were detected in our present analyses, except for Trails A and B in women only.
AD is a progressive cognitive decline that diminishes social and occupational functioning. AD is typically characterized by memory deficits, cognitive deterioration, functional impairment in activities of daily living, and neuropsychiatric symptoms47. It has been poorly identified and assessed in African Americans,48,49 resulting in an escalating public health crisis as reflected by an increased prevalence of the disease in African Americans.
Examining gene variations may be one pioneering method to explain the pathophysiologic and clinical symptoms observed in persons with AD, a multifactorial disorder. The pathogenesis of AD in African American elders may be related to the amyloid-β cascade and pathogenesis of neuropsychiatric symptoms. Several neuroanatomical structures and neurotransmitters are shared in the pathogenesis of AD and neuropsychiatric symptoms such as schizophrenia, major depression, and personality alterations. These derive from abnormalities in the limbic system and frontal and temporoparietal regions with altered function of the serotonergic, noradrenergic, and cholinergic systems in the brain. Collectively, these neurochemical and neuroanatomical changes can result in the clinical symptoms manifested in African American elders with AD. This theory of the pathogenesis of AD in African American elders with AD also may support the temporal nature of the clinical symptoms given the increased abnormalities in neurotransmitters and neuroanatomy specific to the limbic system. However, further analysis is warranted about this theory since it is based on the limited number of clinical symptoms reported and examined in African American elders with AD as well as indications of mixed-pathologies50.
In terms of the genetics of AD, Apolipoprotein E (ApoE) ε4 increases the risk of both age-related cognitive decline and the transition from mild to severe cognitive impairment51. Moreover, there is evidence that AD patients who are ε4 carriers have a faster rate of cognitive decline52,53 although the data are equivocal. A few studies have investigated this issue reporting that ε4 carriers exhibit a phenotype characterized by greater memory impairment54. In other words, AD patients who have memory complaints are significantly more likely to be ε4 carriers. In addition, greater memory deficits on formal neuropsychological testing have been observed in AD patients who are ε4 carriers. Studies on ApoE ε4 status and episodic memory have involved predominantly White samples except for Fillenbaum et al. who compared the effects of ε4 status on baseline cognitive functioning in African American versus White AD patients55. Our risk score in HANDLS (hAlzScore) contained two ApoE SNPs which could elucidate the observed, long-term memory association in women. Although we have ApoE information on all 1,024 genotyped HANDLS participants, the current analyses did not specifically focus on the overlapping subjects (those included in the final sample who also had complete ApoE data) as we continued with the risk score calculation. The lack of more current literature on racial difference in AD further justifies the need for studying ApoE in a unique study population such as ours.
Interestingly, and in contrast to our current finding, there were racial differences in cognitive abilities such that the ε4 allele was related to faster decline in semantic memory and working memory for Whites but not for African Americans.
Dietary modification may have the potential to reduce the risk of developing AD. A recent meta-analysis (N=34,168) showed that the highest Mediterranean diet score was associated with reduced risk of developing cognitive disorders (RR=0·79, 95%CI 0·70, 0·90)56 while supplementation of the with olive oil or nuts was associated with improved cognitive function57. A study that investigated a relationship between Southern diet (high in added fats, fried food, eggs, processed meats and sugar-sweetened beverages) and Prudent diet (rich in vegetables, fruit, cereals and legumes, whole grains, rice/pasta, fish, low-fat dairy, poultry, and water) in individuals at risk for AD found an association between Southern diet and reduced cognitive performance among African Americans58. In both Whites and African American adults, greater adherence to a Prudent dietary pattern was associated with better cognitive outcomes suggesting differential effects of diet on cognitive function in middle-aged individuals at high risk for AD. This suggests that diet could be a non-pharmaceutical tool to reduce cognitive decline in racially diverse populations59. Mediterranean, Dietary Approaches to Stop Hypertension (DASH)60–62, and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND)63,64 have all been linked to reduced risk of AD and lower cognitive decline in a recent publication12. Suggested mechanisms include: Olive serves as one of the building block components of MedDi and MIND diets and the exerted potential health beneficial might be suggested due to the presence of its bioactive constituents such as oleic acids and phenolic compounds in olives, for example, as well as the combined neuroprotective functions of the antioxidants, mono and polyunsaturated fatty acids MUFAs and PUFAs.
Confidence in our findings is strengthened by several factors. First, we used a longitudinal design to ascertain temporality of these relationships while stratifying by sex that is important in cognitive decline. Second, we used a composite measure of 11 cognitive tests that assessed a range of cognitive abilities, reducing the opportunity for floor and ceiling effects and other sources of measurement error to affect results. Finally, the availability of a mean of repeated measurements of cognition per individual allowed us to simultaneously but separately model initial level of cognition and rate of change, thereby allowing us to more effectively adjust for the former while testing for sex differences in the latter.
Nevertheless, some study limitations should be noted. First, our final sample size after using multiple selection variables was rather small. We were also unable to determine the statistical power of our selected samples since the process in mixed models is more complex than in linear models and requires more assumptions65. It is also often estimated using simulations which are not always reliable. Second, although our models were adjusted for a wealth of potentially confounding covariates, causality cannot be inferred given the observational nature study and the possible role played by residual confounding. Third, outcome measures were only repeated up to twice over an average follow-up of 5y, leaving room for improvement in studies with 3 or more timepoints. Fourth, although we performed our risk score calculation based on over one-hundred AD-related genes and reported SNPs, hundreds of more SNPs have been discovered since the Nature publication66, and we are unable to claim our list as comprehensive. Fifth, we excluded those <50y to have greater variability in cognitive decline measures at the expense of statistical power with a larger sample size. Finally, no additional analyses were performed with complete apolipoprotein E allele status to further explore the associations.
This study aimed to investigate longitudinal associations of genetic risk for AD and diet quality with cognitive outcomes, in a sample of less than 500 people. While we were well powered to do the study, we might have missed significant gene variations while creating our genetic risk score. It might be equally important to study who are <50years in hopes of detecting some early changes, that was outside the scope of this study. In addition, because of the projected growth of minority populations in the coming decades, larger multi-racial/ethnic studies of cognitive function in older people are needed.
Conclusions and implications
We conclude that Among African American women with increased genetic risk for AD, a better-quality diet was associated with a slower rate of decline in verbal memory. It is evident that diet quality and its change over time can impact memory in the long run, especially in people with higher risk for AD. Mechanistically speaking, the changes observed begin long before the detected impairments are manifest. While we cannot change the genetic risk for a disease, shifting to a better-quality diet offers possible long-term health benefits, as it has been well-established in the literature. More studies are needed to investigate brain morphology and volume changes in relation to diet quality, in an at-risk population for AD, over time.
Supplementary Material
Table 4:
Coefficient estimates (β±SE) for associations between cognitive test performance and hAlzScore by time and diet, for HANDLS participants >50y of age with complete and reliable cognitive test scores derived from mixed-effects linear regression models
| All | Women | Men | |
|---|---|---|---|
| Mini-Mental State E×am, MMSEe2,f1,h1 | N=225; k=1.82 | N=123; k=1.85 | N=181; k=1.77 |
| Time | −0.0±0.17 | −0.64±0.55 | −0.10±0.26 |
| hAlzScore | −0.12*±0.07 | −0.05±0.09 | −0.15±0.11 |
| hAlzScore × Time | 0.02±0.02 | 0.01±0.02 | 0.02±0.03 |
| Diet Change# | 0.06±0.14 | −0.09±0.16 | 0.02±0.26 |
| Diet Change × Time | −0.02±0.04 | 0.05±0.04 | 0.02±0.07 |
| hAlzScore × Diet Change | −0.00±0.08 | −0.06±0.09 | 0.19±0.14 |
| hAlzScore × Diet Change × Time | 0.03±0.02 | 0.04**±0.02 | −0.04±0.04 |
| Initial Diet^ | −0.05±0.11 | −0.18±0.13 | −0.10±0.22 |
| Initial Diet × Time | 0.01±0.03 | 0.04±0.03 | 0.06±0.06 |
| hAlzScore × Initial Diet | 0.05±0.06 | −0.02±0.07 | 0.25**±0.12 |
| hAlzScore × Initial Diet ×Time | 0.02±0.01 | 0.02±0.01 | −0.03±0.03 |
| California Verbal Learning Test CVLT, List Ab1,d2 | N=223; k=1.76 | N=122; k=1.8 | N=173; k=1.71 |
| Time | −0.80*±0.43 | −2.22±1.52 | −0.57±0.53 |
| hAlzScore | −0.36*±0.20 | −0.44*±0.24 | −0.24±0.33 |
| hAlzScore × Time | 0.05±0.05 | 0.16**±0.07 | −0.08±0.07 |
| Diet Change | 0.36±0.36 | 0.06±0.42 | 0.99±0.70 |
| Diet Change × Time | −0.04±0.10 | −0.16±0.13 | 0.24±0.15 |
| hAlzScore × Diet Change | −0.22±0.22 | −0.17±0.26 | −0.25±0.38 |
| hAlzScore × Diet Change × Time | 0.15***±0.06 | 0.21***±0.08 | 0.02±0.08 |
| Initial Diet | −0.07±0.31 | −0.13±0.36 | −0.07±0.57 |
| Initial Diet × Time | −0.04±0.08 | −0.06±0.10 | 0.06±0.13 |
| hAlzScore × Initial Diet | −0.10±0.18 | −0.30±0.21 | −0.05±0.32 |
| hAlzScore × Initial Diet ×Time | 0.12***±0.04 | 0.18***±0.06 | 0.03±0.07 |
| California Verbal Learning Test CVLT, Free Recall Long Delay FRLD | N=219; k=1.74 | N=121; k=1.78 | N=167; k=1.7 |
| Time | −0.41**±0.19 | −1.15*±0.63 | −0.83**±0.27 |
| hAlzScore | −0.21**±0.10 | 0.02±0.13 | −0.50***±0.14 |
| hAlzScore × Time | 0.02±0.02 | 0.02±0.03 | 0.04±0.04 |
| Diet Change | −0.05±0.18 | −0.29±0.23 | 0.71**±0.29 |
| Diet Change × Time | 0.03±0.04 | 0.08±0.06 | −0.05±0.07 |
| hAlzScore × Diet Change | 0.11±0.11 | 0.19±0.14 | −0.09±0.16 |
| hAlzScore × Diet Change × Time | 0.03±0.02 | 0.04±0.03 | 0.03±0.04 |
| Initial Diet | −0.03±0.16 | −0.15±0.21 | 0.42*±0.24 |
| Initial Diet × Time | −0.02±0.03 | 0.02±0.04 | −0.10*±0.06 |
| hAlzScore × Initial Diet | 0.03±0.09 | 0.02±0.12 | −0.14±0.14 |
| hAlzScore × Initial Diet ×Time | 0.04**±0.02 | 0.05**±0.03 | 0.05±0.04 |
| Benton Visual Retention Test, BVRT a2 | N=227; k=1.89 | N=123; k=1.92 | N=192; k=1.85 |
| Time | 0.80*±0.41 | −2.84*±1.46 | 1.04**±0.48 |
| hAlzScore | 0.29±0.19 | 0.71***±0.26 | −0.09±0.27 |
| hAlzScore × Time | −0.07±0.04 | −0.10±0.06 | 0.02±0.06 |
| Diet Change | −0.31±0.37 | −0.35±0.46 | −0.48±0.67 |
| Diet Change × Time | −0.07±0.10 | 0.00±0.13 | −0.08±0.16 |
| hAlzScore × Diet Change | −0.20±0.23 | −0.29±0.30 | 0.27±0.35 |
| hAlzScore × Diet Change × Time | 0.05±0.06 | 0.06±0.08 | −0.02±0.08 |
| Initial Diet | −0.16±0.31 | −0.16±0.37 | −0.07±0.55 |
| Initial Diet × Time | 0.00±0.07 | 0.07±0.10 | −0.11±0.12 |
| hAlzScore × Initial Diet | 0.07±0.17 | 0.05±0.20 | 0.36±0.30 |
| hAlzScore × Initial Diet ×Time | −0.10**±0.04 | −0.10**±0.05 | −0.07±0.07 |
| Brief Test of Attention | N=220; k=1.78 | N=121; k=1.81 | N=173; k=1.75 |
| Time | 0.27±0.24 | 0.11±0.55 | 0.21±0.24 |
| hAlzScore | −0.04±0.09 | −0.14±0.11 | −0.01±0.13 |
| hAlzScore × Time | −0.01±0.03 | 0.00±0.02 | 0.01±0.03 |
| Diet Change | −0.29*±0.18 | −0.32±0.20 | −0.19±0.30 |
| Diet Change × Time | 0.04±0.06 | 0.02±0.04 | 0.10±0.07 |
| hAlzScore × Diet Change | 0.08±0.10 | 0.23*±0.12 | −0.10±0.16 |
| hAlzScore × Diet Change × Time | −0.00±0.03 | −0.01±0.02 | 0.04±0.04 |
| Initial Diet | −0.37**±0.15 | −0.50***±0.17 | 0.03±0.26 |
| Initial Diet × Time | 0.04±0.04 | 0.04±0.03 | −0.03±0.06 |
| hAlzScore × Initial Diet | 0.09±0.09 | −0.00±0.09 | −0.01±0.15 |
| hAlzScore × Initial Diet ×Time | −0.01±0.02 | −0.01±0.02 | 0.05±0.04 |
| Animal Fluency | N=228; k=1.95 | N=124; k=1.95 | N=201; k=1.93 |
| Time | 0.33±0.26 | 0.48±1.65 | 0.63±0.39 |
| hAlzScore | 0.05±0.18 | 0.13±0.25 | 0.15±0.29 |
| hAlzScore × Time | −0.04±0.03 | −0.05±0.07 | −0.09*±0.05 |
| Diet Change | −0.14±0.35 | −0.05±0.44 | −0.20±0.71 |
| Diet Change × Time | 0.11*±0.06 | 0.16±0.14 | 0.03±0.12 |
| hAlzScore × Diet Change | 0.00±0.21 | −0.18±0.26 | 0.12±0.37 |
| hAlzScore × Diet Change × Time | 0.02±0.04 | 0.01±0.08 | 0.06±0.06 |
| Initial Diet | 0.04±0.29 | 0.30±0.36 | −0.59±0.59 |
| Initial Diet × Time | 0.02±0.05 | 0.02±0.11 | −0.00±0.10 |
| hAlzScore × Initial Diet | 0.32**±0.16 | 0.30±0.20 | 0.32±0.32 |
| hAlzScore × Initial Diet ×Time | −0.03±0.03 | −0.05±0.06 | 0.04±0.05 |
| Digits Span, Forwardh2 | N=226; k=1.85 | N=123; k=1.83 | N=192; k=1.86 |
| Time | 0.14±0.11 | 0.25±0.36 | 0.31*±0.16 |
| hAlzScore | 0.01±0.07 | −0.02±0.09 | 0.13±0.11 |
| hAlzScore × Time | −0.00±0.01 | −0.00±0.02 | −0.00±0.02 |
| Diet Change | 0.24*±0.14 | 0.44***±0.17 | 0.06±0.26 |
| Diet Change × Time | −0.00±0.03 | 0.01±0.03 | −0.01±0.05 |
| hAlzScore × Diet Change | −0.01±0.08 | 0.03±0.10 | −0.00±0.14 |
| hAlzScore × Diet Change × Time | 0.00±0.02 | 0.00±0.02 | −0.02±0.03 |
| Initial Diet | 0.14±0.11 | 0.20±0.14 | 0.03±0.21 |
| Initial Diet × Time | −0.01±0.02 | −0.02±0.03 | 0.00±0.04 |
| hAlzScore × Initial Diet | −0.03±0.06 | −0.08±0.07 | 0.10±0.12 |
| hAlzScore × Initial Diet ×Time | −0.00±0.01 | 0.01±0.01 | −0.03±0.02 |
| Digits Span, Backwarda1 | N=226; k=1.84 | N=123; k=1.82 | N=192; k=1.86 |
| Time | −0.26*±0.15 | −0.40±0.51 | −0.17±0.21 |
| hAlzScore | −0.06±0.07 | −0.19**±0.09 | 0.13±0.10 |
| hAlzScore × Time | 0.01±0.02 | 0.03±0.02 | −0.00±0.03 |
| Diet Change | 0.07±0.13 | 0.14±0.16 | −0.04±0.24 |
| Diet Change × Time | 0.02±0.04 | 0.05±0.05 | −0.00±0.06 |
| hAlzScore × Diet Change | 0.00±0.08 | 0.05±0.10 | −0.08±0.13 |
| hAlzScore × Diet Change × Time | 0.02±0.02 | 0.05*±0.03 | −0.03±0.03 |
| Initial Diet | −0.13±0.11 | −0.21±0.14 | 0.08±0.19 |
| Initial Diet × Time | 0.05±0.03 | 0.06*±0.04 | 0.00±0.05 |
| hAlzScore × Initial Diet | −0.05±0.06 | −0.06±0.07 | −0.11±0.11 |
| hAlzScore × Initial Diet ×Time | 0.02±0.02 | 0.01±0.02 | 0.01±0.03 |
| Clock, Commandb1 | N=228; k=1.93 | N=125; k=1.95 | N=195; k=1.89 |
| Time | 0.06±0.09 | 0.18±0.32 | 0.03±0.12 |
| hAlzScore | −0.01±0.04 | −0.02±0.06 | −0.04±0.07 |
| hAlzScore × Time | 0.01±0.01 | −0.01±0.01 | 0.04***±0.02 |
| Diet Change | 0.14*±0.08 | 0.03±0.10 | 0.26*±0.16 |
| Diet Change × Time | −0.02±0.02 | −0.02±0.03 | −0.04±0.04 |
| hAlzScore × Diet Change | −0.02±0.05 | 0.01±0.06 | −0.12±0.08 |
| hAlzScore × Diet Change × Time | −0.00±0.01 | 0.00±0.02 | −0.01±0.02 |
| Initial Diet | 0.01±0.07 | −0.12±0.08 | 0.13±0.13 |
| Initial Diet × Time | 0.02±0.02 | 0.02±0.02 | −0.00±0.03 |
| hAlzScore × Initial Diet | 0.05±0.04 | 0.05±0.05 | 0.01±0.07 |
| hAlzScore × Initial Diet ×Time | 0.01±0.01 | 0.01±0.01 | 0.01±0.02 |
| Trailmaking Test, Part A | N=224; k=1.84 | N=123; k=1.87 | N=101; k=1.81 |
| Time | −0.01±0.04 | 0.13±0.11 | 0.02±0.05 |
| hAlzScore | −0.00±0.02 | 0.02±0.02 | 0.00±0.02 |
| hAlzScore × Time | 0.03±0.03 | −0.01±0.01 | −0.00±0.00 |
| Diet Change | −0.01±0.01 | −0.00±0.04 | 0.03±0.06 |
| Diet Change × Time | 0.01±0.02 | −0.01±0.01 | −0.03±0.02 |
| hAlzScore × Diet Change | 1.07±0.75 | 0.02±0.03 | −0.01±0.03 |
| hAlzScore × Diet Change × Time | −0.00±0.00 | −0.01±0.01 | 0.00±0.00 |
| Initial Diet | −0.01±0.03 | −0.05±0.03 | −0.00±0.05 |
| Initial Diet × Time | −0.01±0.01 | −0.02***±0.01 | 0.00±0.01 |
| hAlzScore × Initial Diet | −0.00±0.02 | −0.02±0.02 | 0.01±0.03 |
| hAlzScore × Initial Diet ×Time | 0.00±0.00 | 0.00±0.00 | 0.00±0.01 |
| Trailmaking Test, Part Bc1,d1,g1 | N=222; k=1.77 | N=123; k=1.76 | N=99; k=1.78 |
| Time | −0.07±0.05 | −0.25*±0.15 | −0.06±0.06 |
| hAlzScore | −0.06±0.02 | −0.00±0.04 | −0.08±0.03 |
| hAlzScore × Time | 0.00±0.01 | −0.00±0.01 | 0.00±0.07 |
| Diet Change | 0.06±0.05 | 0.12*±0.07 | −0.09±0.08 |
| Diet Change × Time | −0.02±0.01 | −0.03***±0.01 | 0.01±0.01 |
| hAlzScore × Diet Change | 0.02±0.03 | 0.04±0.04 | 0.02±0.04 |
| hAlzScore × Diet Change × Time | −0.00±0.01 | −0.01±0.01 | 0.06±0.01 |
| Initial Diet | −0.02±0.04 | −0.01±0.05 | −0.06±0.06 |
| Initial Diet × Time | −0.00±0.01 | −0.01±0.01 | 0.03±0.01 |
| hAlzScore × Initial Diet | 0.05±0.02 | 0.03±0.03 | 0.06±0.03 |
| hAlzScore × Initial Diet ×Time | −0.00±0.00 | −0.00±0.01 | −0.00±0.01 |
Abbreviations: HANDLS= Healthy Aging in Neighborhoods of Diversity Across the Lifespan; hAlzScore= HANDLS Alzheimer’s Risk Score; MMSE= Mini-Mental State Examination; CVLT-List A= California Verbal Learning test- List A; CVLT-DFR= California Verbal Learning Test-Long-Delayed Free Recall; BVRT= Benton Visual Retention Test; Attention= Brief Test of Attention; Trails A= Trailmaking Test A; Trails B= Trailmaking Test B; Digit Span Forward= Digits Span Forward Test; Digit Span Backward= Digits Span Backward Test; Clock Command= Clock Command Test; Identical Pictures= Identical Pictures Test; Card Rotation= Card rotation Test; Animal Fluency= Animal Fluency Test.
2Represents change in diet quality over time (~5 years from baseline)
22Represents diet quality at baseline (Time 0)
2Continuous covariates were mean-centered.
2k= the total number of observations/total number of groups per test
p<0.01,
p<0.05,
p<0.10;
indicates significant interaction between se× and hAlzScore at the p<0.05 level;
22indicates significant interaction between se× and hAlzScore at the p<0.10 level;
indicates significant interaction between se× and hAlzScore and time at the p<0.05 level;
indicates significant interaction between se× and hAlzScore and time at the p<0.10 level;
2indicates significant interaction between se× and diet (change) at the p<0.05 level;
2indicates significant interaction between se× and diet (change) at the p<0.10 level;
2indicates significant interaction between se× and diet (change) and time at the p<0.05 level;
22indicates significant interaction between se× and diet (change) and time at the p<0.10 level;
indicates significant interaction between se× and diet (change) and hAlzScore at the p<0.05 level;
indicates significant interaction between se× and diet (change) and hAlzScore at the p<0.10 level;
indicates significant interaction between se× and diet (change) and hAlzScore and time at the p<0.05 level;
indicates significant interaction between se× and diet (change) and hAlzScore and time at the p<0.10 level;
indicates significant interaction between se× and diet and time at the p<0.05 level;
indicates significant interaction between se× and diet and time at the p<0.10 level;
indicates significant interaction between se× and diet and hAlzScore at the p<0.05 level;
indicates significant interaction between se× and diet and hAlzScore at the p<0.10 level;
ACKNOWLEDGEMENT
This work was fully supported by the Intramural Research Program (Grant#ZIA-AG000195) of the National Institutes of Health, National Institute on Aging, NIA/NIH/IRP.
Sources of funding: This research was supported entirely by the Intramural Research Program of the NIH (Grant number: ZIA-AG000195), National Institute on Aging.
ABBREVIATIONS
- (AA)
African Americans
- (AD)
Alzheimer’s disease
- (BMI)
Body mass index
- (HS)
High School
- (HANDLS)
Healthy Aging in Neighborhoods of Diversity across the Life Span
- (HEI-2010)
study Healthy Eating Index
- (DASH)
total score Dietary Approaches to Stop Hypertension
- (MAR)
Mean Adequacy Ratio
- (hAlzScore)
HANDLS Alzheimer’s risk score
Footnotes
Disclaimer: The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of the Army/Navy/Air Force, Department of Defense, or the U.S. Government.
This peer-reviewed article has been accepted for publication but not yet copyedited or typeset, and so may be subject to change during the production process. The article is considered published and may be cited using its DOI 10.1017/S0007114520001269
CONFLICT OF INTEREST: None declared.
REFERENCES
- 1.Alosco ML, Spitznagel MB, Raz N, et al. Dietary habits moderate the association between heart failure and cognitive impairment. Journal of nutrition in gerontology and geriatrics. 2013;32(2):106–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A. Adherence to a Mediterranean- Style Diet and Effects on Cognition in Adults: A Qualitative Evaluation and Systematic Review of Longitudinal and Prospective Trials. Frontiers in nutrition. 2016;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersson SD, Philippou E. Mediterranean Diet, Cognitive Function, and Dementia: A Systematic Review of the Evidence. Advances in nutrition (Bethesda, Md). 2016;7(5):889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu Y, Scarmeas N. Dietary patterns in Alzheimer’s disease and cognitive aging. Current Alzheimer research. 2011;8(5):510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solfrizzi V, Panza F, Frisardi V, et al. Diet and Alzheimer’s disease risk factors or prevention: the current evidence. Expert review of neurotherapeutics. 2011;11(5):677–708. [DOI] [PubMed] [Google Scholar]
- 6.Yannakoulia M, Kontogianni M, Scarmeas N. Cognitive health and Mediterranean diet: just diet or lifestyle pattern? Ageing research reviews. 2015;20:74–78. [DOI] [PubMed] [Google Scholar]
- 7.Wilson VK, Houston DK, Kilpatrick L, et al. Relationship between 25-hydroxyvitamin D and cognitive function in older adults: the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2014;62(4):636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richard EL, Laughlin GA, Kritz-Silverstein D, Reas ET, Barrett-Connor E, McEvoy LK. Dietary Patterns and Cognitive Function among Older Community-Dwelling Adults. Nutrients. 2018;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlton KE. Eating well: ageing gracefully! Asia Pacific journal of clinical nutrition. 2002;11 Suppl 3:S607–617. [DOI] [PubMed] [Google Scholar]
- 10.Dacks PA, Shineman DW, Fillit HM. Current evidence for the clinical use of long-chain polyunsaturated n-3 fatty acids to prevent age-related cognitive decline and Alzheimer’s disease. The journal of nutrition, health & aging. 2013;17(3):240–251. [DOI] [PubMed] [Google Scholar]
- 11.Moore K, Hughes CF, Ward M, Hoey L, McNulty H. Diet, nutrition and the ageing brain: current evidence and new directions. The Proceedings of the Nutrition Society. 2018;77(2):152–163. [DOI] [PubMed] [Google Scholar]
- 12.van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Advances in nutrition (Bethesda, Md). 2019;10(6):1040–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. The New England journal of medicine. 1997;336(16):1117–1124. [DOI] [PubMed] [Google Scholar]
- 14.Graff-Radford NR, Besser LM, Crook JE, Kukull WA, Dickson DW. Neuropathologic differences by race from the National Alzheimer’s Coordinating Center. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2016;12(6):669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris JC, Schindler SE, McCue LM, et al. Assessment of Racial Disparities in Biomarkers for Alzheimer Disease. JAMA neurology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavett BE, Fletcher E, Harvey D, et al. Ethnoracial differences in brain structure change and cognitive change. Neuropsychology. 2018;32(5):529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajan KB, Barnes LL, Wilson RS, Weuve J, McAninch EA, Evans DA. Apolipoprotein E Genotypes, Age, Race, and Cognitive Decline in a Population Sample. Journal of the American Geriatrics Society. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weuve J, Barnes LL, Mendes de Leon CF, et al. Cognitive Aging in Black and White Americans: Cognition, Cognitive Decline, and Incidence of Alzheimer Disease Dementia. Epidemiology (Cambridge, Mass). 2018;29(1):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes LL, Wilson RS, Li Y, Gilley DW, Bennett DA, Evans DA. Change in cognitive function in Alzheimer’s disease in African-American and white persons. Neuroepidemiology. 2006;26(1):16–22. [DOI] [PubMed] [Google Scholar]
- 20.Wilson RS, Li Y, Aggarwal NT, et al. Cognitive decline and survival in Alzheimer’s disease. International journal of geriatric psychiatry. 2006;21(4):356–362. [DOI] [PubMed] [Google Scholar]
- 21.Hajjar I, Wharton W, Mack WJ, Levey AI, Goldstein FC. Racial Disparity in Cognitive and Functional Disability in Hypertension and All-Cause Mortality. American journal of hypertension. 2016;29(2):185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachman DL, Stuckey M, Ebeling M, et al. Establishment of a predominantly African-American cohort for the study of Alzheimer’s disease: the South Carolina Alzheimer’s disease clinical core. Dementia and geriatric cognitive disorders. 2009;27(4):329–336. [DOI] [PubMed] [Google Scholar]
- 23.Hossain S, Beydoun MA, Kuczmarski MF, Tajuddin S, Evans MK, Zonderman AB. The Interplay of Diet Quality and Alzheimer’s Disease Genetic Risk Score in Relation to Cognitive Performance Among Urban African Americans. Nutrients. 2019;11(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savoca MR, Arcury TA, Leng X, et al. The diet quality of rural older adults in the South as measured by healthy eating index-2005 varies by ethnicity. Journal of the American Dietetic Association. 2009;109(12):2063–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards Adams IK, Figueroa W, Hatsu I, et al. An Examination of Demographic and Psychosocial Factors, Barriers to Healthy Eating, and Diet Quality Among African American Adults. Nutrients. 2019;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yosef Hochberg ACT. Multiple comparison procedures. JOHN WILEY & SONS, New York: 1987. [Google Scholar]
- 27.Antonell A, Balasa M, Oliva R, et al. A novel PSEN1 gene mutation (L235R) associated with familial early-onset Alzheimer’s disease. Neuroscience letters. 2011;496(1):40–42. [DOI] [PubMed] [Google Scholar]
- 28.Berr C, Richard F, Dufouil C, Amant C, Alperovitch A, Amouyel P. Polymorphism of the prion protein is associated with cognitive impairment in the elderly: the EVA study. Neurology. 1998;51(3):734–737. [DOI] [PubMed] [Google Scholar]
- 29.de Oliveira FF, Bertolucci PH, Chen ES, Smith MC. Brain-penetrating angiotensin-converting enzyme inhibitors and cognitive change in patients with dementia due to Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2014;42 Suppl 3:S321–324. [DOI] [PubMed] [Google Scholar]
- 30.Finckh U, von der Kammer H, Velden J, et al. Genetic association of a cystatin C gene polymorphism with late-onset Alzheimer disease. Archives of neurology. 2000;57(11):1579–1583. [DOI] [PubMed] [Google Scholar]
- 31.Lillenes MS, Stoen M, Gunther CC, et al. Mitochondrial transcription factor A (TFAM) rs1937 and AP endonuclease 1 (APE1) rs1130409 alleles are associated with reduced cognitive performance. Neuroscience letters. 2017;645:46–52. [DOI] [PubMed] [Google Scholar]
- 32.Ma C, Zhang Y, Li X, et al. The TT allele of rs405509 synergizes with APOE epsilon4 in the impairment of cognition and its underlying default mode network in non-demented elderly. Current Alzheimer research. 2016;13(6):708–717. [DOI] [PubMed] [Google Scholar]
- 33.Ozturk A, DeKosky ST, Kamboh MI. Lack of association of 5 SNPs in the vicinity of the insulin- degrading enzyme (IDE) gene with late-onset Alzheimer’s disease. Neuroscience letters. 2006;406(3):265–269. [DOI] [PubMed] [Google Scholar]
- 34.Tisato V, Zuliani G, Vigliano M, et al. Gene-gene interactions among coding genes of iron- homeostasis proteins and APOE-alleles in cognitive impairment diseases. PloS one. 2018;13(3):e0193867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple- Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–332. [DOI] [PubMed] [Google Scholar]
- 36.FNDDS. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds/Accessed.
- 37.HEI Tools for Researchers. https://epi.grants.cancer.gov/hei/tools.html. Accessed.
- 38.Healthy Eating Index 2010. https://handls.nih.gov/06Coll-w01HEI.htm Accessed. [Google Scholar]
- 39.Guenther PM, Kirkpatrick Si Fau - Reedy J, Reedy J Fau - Krebs-Smith SM, et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. (1541–6100 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mellen PB, Gao Sk Fau - Vitolins MZ, Vitolins Mz Fau - Goff DC Jr., Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. (0003–9926 (Print)). [DOI] [PubMed] [Google Scholar]
- 41.Murphy SP, Foote JA, Wilkens LR, et al. Simple measures of dietary variety are associated with improved dietary quality. Journal of the American Dietetic Association. 2006;106(3):425–429. [DOI] [PubMed] [Google Scholar]
- 42.Fanelli Kuczmarski M, Mason MA, Beydoun MA, Allegro D, Zonderman AB, Evans MK. Dietary patterns and sarcopenia in an urban African American and White population in the United States. Journal of nutrition in gerontology and geriatrics. 2013;32(4):291–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitamin C Fact Sheet. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/Accessed. [Google Scholar]
- 44.Fanelli Kuczmarski M, Bodt BA, Stave Shupe E, Zonderman AB, Evans MK. Dietary Patterns Associated with Lower 10-Year Atherosclerotic Cardiovascular Disease Risk among Urban African-American and White Adults Consuming Western Diets. Nutrients. 2018;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jake Lever MKNA. Principal component analysis. Nature Methods. 2017;14: 641–642. [Google Scholar]
- 46.Ibrahim JG, Molenberghs G. Missing data methods in longitudinal studies: a review. Test. 2009;18(1):1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holston EC. Stigmatization in Alzheimer’s disease research on African American elders. Issues in mental health nursing. 2005;26(10):1103–1127. [DOI] [PubMed] [Google Scholar]
- 48.Wilson RS, Capuano AW, Sytsma J, Bennett DA, Barnes LL. Cognitive aging in older Black and White persons. Psychology and aging. 2015;30(2):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corsi M, Di Raimo T, Di Lorenzo C, et al. Cognitive disability in alzheimer’s disease and its management. La Clinica terapeutica. 2016;167(5):e123–e126. [DOI] [PubMed] [Google Scholar]
- 50.Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85(6):528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mount DL, Ashley AV, Lah JJ, Levey AI, Goldstein FC. Is ApoE epsilon4 associated with cognitive functioning in African Americans diagnosed with Alzheimer Disease? An exploratory study. Southern medical journal. 2009;102(9):890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hendrie HC, Murrell J, Baiyewu O, et al. APOE epsilon4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. International psychogeriatrics. 2014;26(6):977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart R, Russ C, Richards M, Brayne C, Lovestone S, Mann A. Apolipoprotein E genotype, vascular risk and early cognitive impairment in an African Caribbean population. Dementia and geriatric cognitive disorders. 2001;12(4):251–256. [DOI] [PubMed] [Google Scholar]
- 54.Potter GG, Plassman BL, Burke JR, et al. Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2009;5(6):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barnes LL, Arvanitakis Z, Yu L, Kelly J, De Jager PL, Bennett DA. Apolipoprotein E and change in episodic memory in blacks and whites. Neuroepidemiology. 2013;40(3):211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu L, Sun D. Adherence to Mediterranean diet and risk of developing cognitive disorders: An updated systematic review and meta-analysis of prospective cohort studies. Scientific reports. 2017;7:41317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajaram S, Valls-Pedret C, Cofán M, et al. The Walnuts and Healthy Aging Study (WAHA): Protocol for a Nutritional Intervention Trial with Walnuts on Brain Aging. Frontiers in aging neuroscience. 2016;8:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nutaitis AC, Tharwani SD, Serra MC, et al. Diet as a Risk Factor for Cognitive Decline in African Americans and Caucasians with a Parental History of Alzheimer’s Disease: A Cross-Sectional Pilot Study Dietary Patterns. The journal of prevention of Alzheimer’s disease. 2019;6(1):50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gorelick PB, Furie KL, Iadecola C, et al. Defining Optimal Brain Health in Adults: A Presidential Advisory From the American Heart Association/American Stroke Association. Stroke. 2017;48(10):e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu J, Song X, Chen GC, et al. Dietary pattern in midlife and cognitive impairment in late life: a prospective study in Chinese adults. The American journal of clinical nutrition. 2019;110(4):912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abbatecola AM, Russo M, Barbieri M. Dietary patterns and cognition in older persons. Current opinion in clinical nutrition and metabolic care. 2018;21(1):10–13. [DOI] [PubMed] [Google Scholar]
- 62.Berendsen AAM, Kang JH, van de Rest O, Feskens EJM, de Groot L, Grodstein F. The Dietary Approaches to Stop Hypertension Diet, Cognitive Function, and Cognitive Decline in American Older Women. Journal of the American Medical Directors Association. 2017;18(5):427–432. [DOI] [PubMed] [Google Scholar]
- 63.Omar SH. Mediterranean and MIND Diets Containing Olive Biophenols Reduces the Prevalence of Alzheimer’s Disease. International journal of molecular sciences. 2019;20(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cherian L, Wang Y, Fakuda K, Leurgans S, Aggarwal N, Morris M. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) Diet Slows Cognitive Decline After Stroke. The journal of prevention of Alzheimer’s disease. 2019;6(4):267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evangelos Kontopantelis DAS, Rosa Parisi, David Reeves. Simulation-Based Power Calculations for Mixed Effects Modeling: idpower in Stata. Journal of Statistical Software. 2016;74(12):25. [Google Scholar]
- 66.Bertram L, McQueen Mb Fau - Mullin K, Mullin K Fau - Blacker D, Blacker D Fau - Tanzi RE, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. (1061–4036 (Print)). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.