Abstract
Periodontitis is a chronic inflammatory disease caused by complex interactions between the host immune system and pathogens that affect the integrity of periodontium. To prevent disease progression and thus preserve alveolar bone structure, simultaneous anti-inflammatory and osteogenic intervention are essential. Hence, a glycogen synthase kinase 3 beta inhibitor (BIO) was selected as a potent inflammation modulator and osteogenic agent to achieve this treatment objective. BIO’s lack of osteotropicity, poor water solubility, and potential long-term systemic side effects, however, have hampered its clinical applications. To address these limitations, pyrophosphorylated Pluronic F127 (F127-PPi) was synthesized and mixed with regular F127 to prepare an injectable and thermoresponsive hydrogel formulation (PF127) of BIO, which could adhere to hard tissue and gradually release BIO to exert its therapeutic effects locally. Comparing to F127 hydrogel, PF127 hydrogels exhibited stronger binding to hydroxyapatite (HA). Additionally, BIO’s solubility in PF127 solution was dramatically improved over F127 solution and the improvement was proportional to the polymer concentration. When evaluated on a rat model of periodontitis, PF127-BIO hydrogel treatment was found to be very effective in preserving alveolar bone and ligament, and preventing periodontal inflammation, as shown by the micro-CT and histological data, respectively. Altogether, these findings suggested that the thermoresponsive PF127 hydrogel is an effective local drug delivery system for better clinical management of periodontitis and associated pathologies.
Keywords: Periodontitis, GSK3 inhibitor, Pluronic F127, Pyrophosphate, Thermoresponsive hydrogel, Osteotropic
Graphical Abstract
INTRODUCTION
Periodontal disease is a prevalent and chronic inflammatory condition that affects the surrounding tissues of the teeth including gingiva, periodontal ligament, and alveolar bone resulting in pocket formation, mobility, bone loss, and eventually lead to the loss of tooth. In addition to the pathogenic bacterial population, the host immune response, which aims at protecting host tissues from bacterial aggression, also acts as a mediator of the periodontal damage[1]. Current treatment strategies aimed at reducing bacterial load by mechanical therapy and administration of antimicrobial agents[2,3]. Additionally, host modulation agents have been utilized to ameliorate inflammation and prevent disease progression[4,5]. Their efficacy on halting the alveolar bone loss, which is the major destructive and non-reversible hallmark of periodontal disease, is limited. Therefore, there is an unmet clinical need to develop novel therapies that would prevent bone erosion and regenerate the lost alveolar bone.
Glycogen synthase kinase 3 beta (GSK3β) is a multi-tasking serine/threonine kinase with crucial roles in several physiological processes including inflammation and bone homeostasis. It has been shown to play a critical role in the host inflammatory response[6–8] and bone homeostasis[9] as a negative regulator, suggesting that inhibitors of GSK3β may provide therapeutic effects for inflammatory and bone metabolic diseases[10]. Particularly, a GSK3 inhibitor (SB216763) has been studied in periodontal disease and data confirmed its therapeutic benefits in preventing alveolar bone loss associated with periodontal disease[11]. During the last decade, several selective GSK3 inhibitors have been synthesized and tested in clinical trials at various phases[12,13]. In particular, 6-bromoindirubin-3’-oxime (BIO), a potent GSK3 inhibitor with an enzymatic IC50 of 5 nM, has exhibited anti-inflammatory [7,8] and strong bone and teeth anabolic effects [14–20]. Due to the involvement of GSK3 in multiple physiological processes, however, systemic administration may cause serious adverse side effects (e.g., diarrhea, hypoglycemia, tumorigenesis)[21–23]. Hence, it is necessary to limit and restrict its biological action primarily at the intended site of action. Local delivery of BIO into the periodontal pocket will permit direct targeting of periodontal tissue, achieving high local concentrations along with minimizing systemic toxicities. However, the poor aqueous solubility and rapid clearance of BIO from the periodontal pockets post major challenges to its effective local delivery.
Thermoresponsive hydrogel formulations injected into the periodontal pocket would be a promising option for the local delivery of BIO to prevent the bony defects associated with periodontitis. Poloxamer 407 (Pluronic F127), a frequently used formulation excipient that has been approved by U.S. FDA for pharmaceutical applications, is a triblock amphiphilic copolymer consisting of a center block of polypropylene oxide flanked by two polyethylene oxide blocks (PEO101 PPO56 PEO101)[24–27]. It has been used extensively in controlled drug and cell delivery[28]. Its unique thermoresponsive gelation property in aqueous solutions (20–35% w/v) makes it an appealing carrier material for periodontal drug delivery and other similar applications[29,30]. The amphiphilic F127 polymer enhances the solubility of hydrophobic drugs at room temperature by forming micelles. When exposed to physiological temperature, the polymer solution forms a hydrogel, holding encapsulated drugs in its collapsed micellar structure to provide sustained release kinetics. F127 is also known to be non-toxic and biocompatible[30]. The constant flow of crevicular fluid, the poor bone-adhesion and mechanical properties of F127 hydrogel, however, would significantly limit the bioavailability of the payload drug in the periodontal pocket.
Therefore, to improve binding of F127 hydrogel to the hard tissues in the periodontal pocket, we here report the synthesis of a novel thermoresponsive pyrophosphorylated F127 (F127-PPi), which was used to formulate an osteotropic (bone-binding) thermoresponsive hydrogel system (PF127, mixtures of F127-PPi and regular F127) for the delivery of BIO. After a detailed characterization of its physicochemical properties, the therapeutic potential of the BIO-loaded PF127 delivery system was evaluated in a rat model of experimental periodontitis.
METHODS AND MATERIALS
Materials and Reagents
Pluronic F127 and pyrophosphate were purchased from Sigma-Aldrich (Saint Louis in MO, USA). BIO was synthesized according to literature[31]. Dense Ceramic Hydroxyapatite discs (0.5” diameter and .08” Thick) were obtained from Clarkson Chromatography Products, Inc. (South Williamsport, PA USA). Mouse osteoblast MC3T3-E1 cells were acquired from ATCC (Manassas, VA, USA). Fetal bovine serum (FBS, BenchMark™) was acquired from Gemini BenchMark (West Sacramento, CA). Minimum Essential Media (alpha-MEM), and trypsinEDTA were purchased from GIBCO (Grand Island, NY, USA). Cell Counting Kit-8 (CCK-8) was bought from Dojindo Molecular Technologies, Inc. (Rockville, MD USA). All other solvents and reagents, if not specified, were acquired from either Acros Organics (Morris Plains, NJ, USA) or Fisher Scientific (Pittsburgh, PA, USA).
Synthesis of Pyrophosphorylated Pluronic F127 (F127-PPi)
Synthesis of Tosylated Pluronic F127
Pluronic F127 (1.0 g, 0.079 mmol) and 4-toluenesulfonyl chloride (151 mg, 0.79 mmol) were dissolved in dry dichloromethane (DCM). Pyridine (62 μL, 0.79 mmol) was then added and the resulting solution was stirred for 24 hr at 21 °C. After adding DCM (80 mL), the resulting solution was washed with HCl (1 M, 20 mL) and brine (80 mL × 2). After washing, the organic phase was separated and dried over MgSO4. The solution was filtered, concentrated and the residue was purified on a LH-20 column to produce 922 mg of the final product, yield: 90%. 1H NMR (CDCl3, 500 MHz) δ ppm 7.78 (d, J = 3.0 Hz, 4H), 7.33 (d, J = 3.0 Hz 4H), 4.14 (t, J = 5.0 Hz, 4H), 3.77 (t, J = 4.5 Hz, 8H), 3.59–3.55 (m, 872 H), 3.50–3.44 (m, 142 H), 3.38 (m, 65H), 2.44 (s, 6H), 1.12 (t, J = 5.0 Hz, 195H); 13C NMR (CDCl3, 125 MHz) δ ppm 144.7, 133.0, 129.8, 127.9 , 75.5, 75.4, 75.1, 73.4, 73.0, 72.9, 72.7, 69.2, 68.7, 21.6, 17.5, 17.3.
Synthesis of Pyrophosphorylated Pluronic F127
The tosylated Pluronic F127 (1.0 g, 0.077 mmol) and tris(tetra-n-butylammonium) hydrogen diphosphate [(n-Bu4N)3(HO)P2O6] (0.31 mmol, 280 mg) were dissolved in dry acetonitrile. The solution was stirred at 21 °C until the starting materials completely disappeared (~ 3 hr, monitored by TLC). After removal of the solvents, the residue was dissolved in water (20 mL) and dialyzed (MWCO = 12–14 kDa) against NaCl solution (0.1 mol/L) overnight to exchange tetrabutyl ammonium to sodium. The resulting solution was then dialyzed against distilled water to remove the excess of NaCl. The resulting solution was then lyophilized to obtain 859 mg of the final pyrophosphorylated Pluronic F127 (F127-PPi) product, yield: 85%.
1H NMR (CDCl3, 500 MHz) δ ppm 4.16 (t, J = 5.0 Hz, 4H), 3.78 (t, J = 4.5 Hz, 8H), 3.59–3.54 (m, 872H), 3.50–3.44 (m, 142 H), 3.39–3.36 (m, 65H), 1.13 (t, J = 5.0 Hz, 195H); 13C NMR (CDCl3, 125 MHz) δ ppm 75.5, 75.4, 75.1, 73.4, 73.0, 72.9, 72.8, 72.7, 70.6, 17.5, 17.3; 31P NMR (202.5MHz, CDCl3): δ (ppm) = −7.70 (d, J = 20.2Hz), −7.91 (d, J = 20.2Hz).
To determine the PPi content, F127-PPi was hydrolyzed in 1 M HCl for 2 hr at 100 °C to release the phosphate. After removal of F127 with chloroform extraction, an equal volume of 1 M HCl solution containing 0.5% (w/v) ammonium molybdate and 2% (w/v) ascorbic acid was added. The samples were incubated at 37 °C for 2 hr then their absorbance at 820 nm was measured using a UV spectrometer[49]. Eighty percent of terminal hydroxyl groups of F127 was found to has been pyrophosphorylated.
Preparation of BIO-loaded Thermoresponsive Hydrogel
PF127 hydrogel formulations with predetermined polymer concentrations (20, 25 and 30% w/v) were prepared by mixing F127-PPi and F127 at different ratio (0:100, 25:75, 50:50, 75:25, 100:0% w/w). Briefly, the desired amount of F127-PPi and F127 was dissolved in PBS (pH 7.4) with stirring in an ice-water bath (~ 4 °C, to prevent gelation) until a clear solution (PF127) was obtained and then stored at 4 °C overnight. BIO was then dissolved in the polymer solutions by continuous stirring at 4 °C, the obtained solutions were filtered through 0.8 μm filter syringes.
Binding Potential to Model Bone (Hydroxyapatite, or HA)
To assess the binding potential of the formulated hydrogels to hydroxyapatite (HA), which constitute the main inorganic component of bone and teeth, an in vitro binding study was done using HA discs. Briefly, polymer solutions (25% w/v) were prepared, containing 100 μM of BIO, with different ratios of F127-PPi and F127 (0:100, 25:75, 50:50, 75:25, and 100:0% w/w) in order to optimize binding affinity. Hydrogels (1 mL) were formed on HA disc placed in plastic wells at 37 °C, and the hydrogels were allowed to stabilize for 15 min. After that, HA discs, on which hydrogels were formed, were inverted with the temperature maintained at 37 °C by keeping the inverted hydrogels inside the water bath (37 °C). The time for the hydrogel to remain attached to the HA disk was measured as the hydrogel’s binding time to HA disc. The binding experiment was performed in triplicate. Based on this study, an optimal formulation was selected for all subsequent experiments.
Evaluation of BIO’s Solubility in PF127 and F127 Solutions
The aqueous solubility of BIO in PF127 and F127 solutions was assessed in this experiment. The solubility values of BIO were measured at dissolution equilibrium after adding the BIO to the PF127 and F127-containing media for a predetermined period of time, as reported previously[32]. Specifically, an excessive amount of BIO was added to different concentrations of PF127 or F127 solutions in microcentrifuge tubes. The suspensions were mixed on a rotor at 4 °C for 48 hr to achieve dissolution equilibrium. At 4 °C, the suspensions were centrifuged at 2,000 rpm for 5 min to settle the undissolved drug to the bottom of the microcentrifuge tube. The supernatants were then filtered through 0.8 μm syringe filters to obtain the saturated BIO solutions. A UV SpectraMax® M2 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) was used to measure BIO concentrations at 260 nm.
In vitro Release of BIO from Hydrogel
The release rate of the physically entrapped BIO from PF127 hydrogels (20, 25, and 30% w/v), was studied by a membrane-less experiment, as reported previously[33–35]. Briefly, samples of 1 mL of polymer solutions containing 0.5 mg (1.5 mM) or 35 μg (100μM) of BIO were transferred into screw-capped Pyrex® glass tubes and incubated in a water bath at 37 °C until the gels were formed. After gelation, phosphate-buffered saline (PBS, pH 7.4, 1 mL) containing 0.5 % Tween 80 pre-equilibrated at 37 °C were gently laid over the surface of the hydrogels and incubated in a water bath at 37 °C with continuous gentle shaking. To measure the release of BIO, the supernatant was withdrawn at regular time intervals and replaced with pre-equilibrated fresh releasing buffer (1 mL). The concentration of BIO was determined at 260 nm on a SpectraMax® M2 microplate reader (Molecular Devices, Sunnyvale, CA, USA) and a NanoDrop™ One Microvolume UV-Vis Spectrophotometer (Thermo Scientific™, Waltham, MA, USA). The release study was performed in triplicate.
In vitro Hydrogel Erosion
The erosion time of PF127-BIO hydrogel formulations (20, 25, and 30% w/v) was determined by performing the weight remaining (%) experiment, as reported by others[33–35]. Briefly, samples of 1 mL of the formulations were transferred into screw-capped Pyrex® glass tubes and incubated in a water bath at 37 °C until the gels were formed. After gelation, the original weight of the hydrogel samples was measured as (W0). Subsequently, 2 mL of PBS (pH 7.4) pre-equilibrated at 37 °C were gently laid over the surface of the hydrogels and incubated in a water bath at 37 °C with continuous gentle shaking. The weight of remaining hydrogels (Wt) was measured at regular time intervals after completely blotting off the buffer. Erosion study was performed in triplicate. Weight remaining (%) was calculated as:
Biocompatibility of PF127 Hydrogel
The impact of selected PF127 hydrogel formulation (25% w/v of mixed F127-PPi and F127, 50:50% w/w) and F127 hydrogel (25% w/v) (with or without BIO) on cell viability was assessed using CCK-8 assay. The BIO concentration in all BIO-containing formulations was 100 nM. Briefly, mouse preosteoblast MC3T3-E1 cell line was cultured in cell culture medium (alpha-MEM) with 10% (v/v) FBS and 1% (v/v) Penicillin/Streptomycin. The cells were incubated to 90% confluence under standard conditions at 37 °C in humidified atmosphere with 5% CO2. The hydrogels were extracted with alpha-MEM at 37 °C for 24 hr according to the ISO Standard 10993–12[34,35]. The ratio between the surface area of the hydrogels and the volume of medium was 1.25 cm2/mL. Undiluted extracts were used for the assay. Cells were grown in 96 well plates (1 × 104 cells/well) and incubated for 24 hr at 37 °C. The cells were then treated with either media only, PF127 extract, PF127-BIO extract, F127 extract, F127-BIO extract, or free BIO, and the plates were incubated at 37 °C for 24 and 48 hr. At each follow-up time point, CCK-8 reagent (10 μL) was added to each well and further incubated for 4 hr at 37 °C. The absorbance was measured at 450 nm using a SpectraMax® M2 microplate reader (Molecular Device, Sunnyvale, CA, USA).
Gelation and Viscosity Studies
Gelation temperature was determined by measuring the storage modulus (G’) and loss modulus (G”) of samples in the temperature sweep mode[36,37]. Solutions with w/v concentrations ranging between 20 and 40% were prepared as described above. Each sample was uniformly loaded between the Peltier plate of the AR1500ex rheometer (TA Instruments, New Castle, DE, USA) and a 60 mm-diameter, 1° cone geometry at 3 °C. The linear viscoelastic region of each sample was pre-determined at 45 °C by using the oscillation amplitude function, and the following condition was chosen: strain of 0.1%, and angular frequency of 1 rad/s. Then, the G’ and G” of each sample were measured for the temperature range from 3 °C to 45 °C by using the oscillation temperature sweep function of the rheometer (heating step: 1 °C, soak time: 2 min for each temperature increase). The gelation temperature of each sample was determined as the temperature when G’ and G” became equal.
The viscosity of the gel phase was also investigated at constant temperature by flow sweep[30] (37 °C, shear rate from 1 to 100 s−1) using the rheometer and a 25 mm-diameter parallel plate. Samples were dispensed on the Peltier plate of the rheometer, heated to 37 °C and maintained at 37 °C for 15 min to reach thermal stability before isothermally tested.
The Therapeutic Efficacy of BIO-containing Hydrogels on a Rat Model of Experimental Periodontitis
Ten-month-old female Sprague Dawley rats (retired breeders, Envigo) were acclimated for one week prior to experiment. The animals (n = 48) were randomly assigned into the following groups (Table 1): healthy control, experimental periodontitis (EP) treated with saline, EP treated with 25% w/v of mixed F127-PPi and F127 hydrogel (50:50% w/w, containing 100 μM of BIO) (PF127-BIO), EP treated with 25% w/v of F127 hydrogel (containing 100 μM of BIO) (F127-BIO), EP treated with 25% w/v of PF127 hydrogel, and EP treated with free BIO (100 μM). Using silk ligatures, the experimental periodontitis was induced as described previously[32,38]. Briefly, rats were first anesthetized in an isoflurane chamber with a 1% to 4% isoflurane in 100% O2. After taking the body weight, the rats were positioned with a nose cone supplied with 0.5% to 2% isoflurane and 100% O2 to maintain anesthesia during the entire procedure. To induce experimental periodontitis, a 4–0 silk ligature was gently tightened subgingivally around the maxillary 2nd molars (M2). Following ligature placement, different treatments (10 μL) were locally delivered between the maxillary 1st molar (M1) and 2nd molar (M2) once each week for 3 weeks. After one week, the ligatures were removed. All animals were euthanized at week 4. The entire palate including all three molars was dissected and fixed in 10% formalin prior to micro-CT and histological analyses. The subgingival placement of silk ligature is a standard method to enhance bacterial collection on the ligature placed next to the periodontal tissue attachment to the tooth, inducing inflammation and bone loss. This model is pertinent due to its resemblance to man with respect to periodontal anatomy, development and composition of dental (bacterial) plaque, histopathology of periodontal lesions, and basic immunobiology[39]. The role of bacterial infection in this model is further supported by the fact that topical antimicrobial dose of minocycline reduced inflammation and bone loss better than a systemic subantimicrobial dose[40]. All animals-related experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska Medical Center (UNMC).
Table 1.
In vivo experiment group arrangement and the schedule of experimental procedures.
| Group | # of animals | Week 1 | Week 2 | Week 3 | Week 4 |
|---|---|---|---|---|---|
| Untreated healthy control (Con+) | 8 | - | - | - | Euthanized |
| EP/Saline (Con−) | 8 | Ligatures, Saline | Remove Ligatures, Saline | Saline | Euthanized |
| EP/PF127 | 8 | Ligatures, PF127 | Remove Ligatures, PF127 | PF127 | Euthanized |
| EP/BIO | 8 | Ligatures, BIO | Remove Ligatures, BIO | BIO | Euthanized |
| EP/F127-BIO | 8 | Ligatures, F127-BIO | Remove, Ligatures, F127-BIO | F127-BIO | Euthanized |
| EP/PF127-BIO | 8 | Ligatures, PF127-BIO | Remove Ligatures, PF127-BIO | PF127-BIO | Euthanized |
Micro-computed Tomography (μ-CT) Analysis of Periodontal Bone
All palate samples (including all three molars) were evaluated for alveolar bone quality using a high-resolution X-ray microtomography system (Skyscan 1172, Bruker, Kontich, Belgium), references to previous studies[32,38]. The X-ray source was set as follows: 70 kV and 141μA, resolution 12.9 μm, exposure time 1880 ms, and aluminum filter 0.5 mm-thick. To generate 3D images, scanning raw data were reconstructed using NRecon software. Sagittal sections were generated using CT-Analyzer software. To evaluate bone erosion, the linear distance from cementoenamel junction (CEJ) to alveolar bone crest (ABC) was determined (CEJ-ABC) using Skyscan Data viewer software. The distance for each sample was measured in the M1-M2 interproximal and from two points: distopalatal of M1 and mesiopalatal of M2. Sagittal sections as shown in Figure 4a, where roots in an even plane with their respective canal spaces widest in the mesiodistal direction, were used for (CEJ-ABC) measurement. A longer distance from CEJ to ABC suggests more bone loss. For the analysis of histomorphometric parameters, such as bone volume (BV) and trabecular thickness (Tb.Th), coronal sections were obtained using the Skyscan DataViewer and a polygonal region of interest (ROI, excluding the roots) between M1 and M2 was identified. The ROI was determined from the distopalatal of M1 to the mesiopalatal of M2 (length), 130 slices below the CEJ of M1 and M2 (height), and from the palatal side to the buccal side of M1 and M2 (width).
Figure 4.
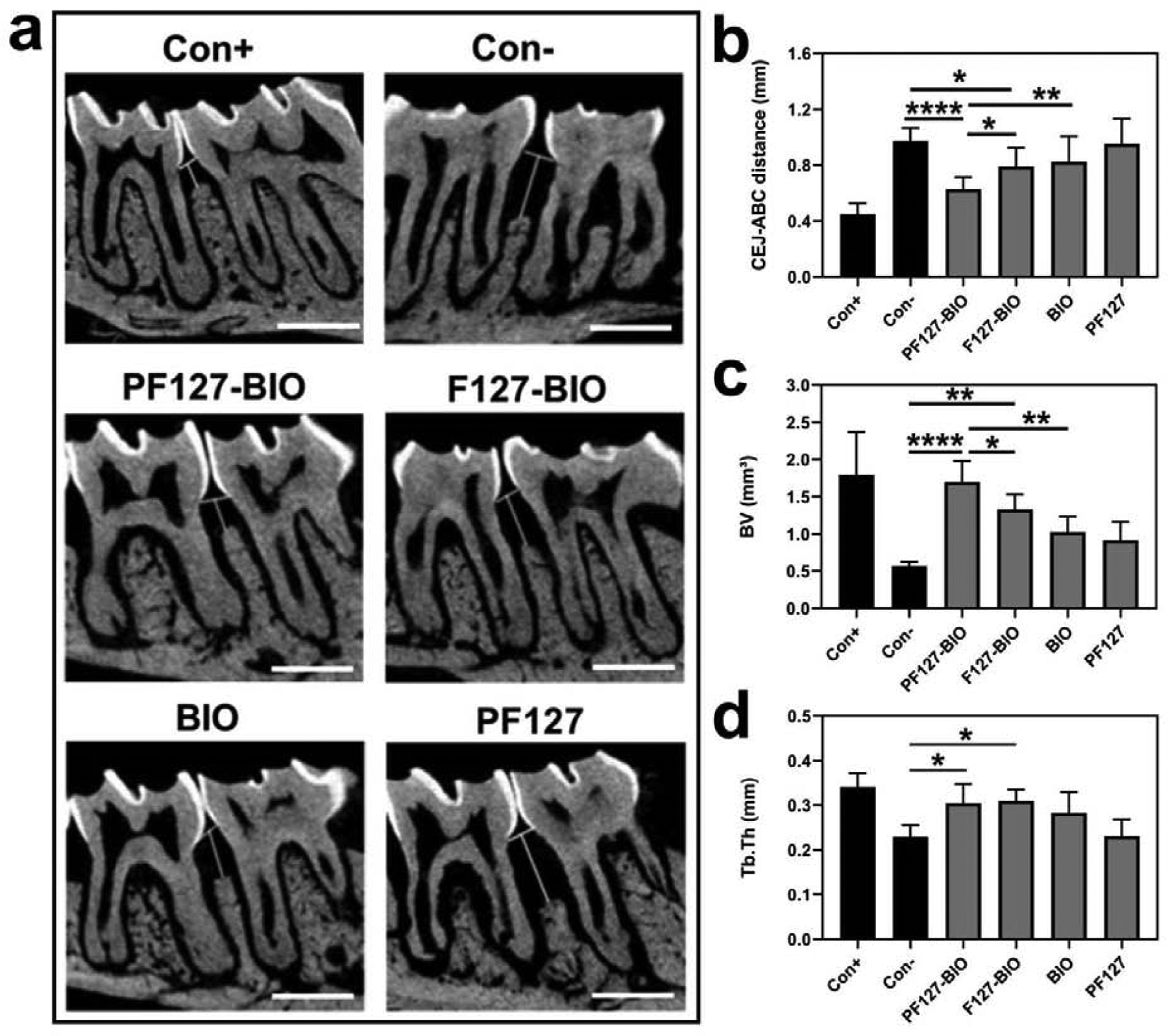
The in vivo evaluation of PF127-BIO hydrogel’s therapeutic efficacy in an experimental periodontitis rat model. (a) Micro-CT sagittal images showing the effect of different treatments on maxillary molar cementoenamel junction (CEJ) to alveolar bone crest (ABC). White vertical lines indicate ABC-CEJ distance. Scale bar = 1 mm. (b) Measurement of the linear distance between CEJ and ABC. (c-d) Quantitative analysis of alveolar bone quality after different treatments. Bone volume (BV), trabecular thickness (Tb.Th). Values are presented as the mean ± SD. *P < 0.05, **P < 0.01, and ****P < 0.0001 (one-way ANOVA with Tukey’s multiple comparisons).
As described previously[41], femurs of these tested rats were also collected and scanned to assess the potential systemic anabolic effect of BIO. The X-ray source was set as the follows: voltage was 70 kV, current was 141 μA, exposure time was 700 ms, resolution was 8.6 μm, and aluminum filter was 0.5 mm thick. The 3-D imagine were generated using the Skyscan NRecon and Skyscan DataViewer software. For bone quality analysis, a consistent polygonal ROI of trabecular bone at the distal femur was selected and the ROI was determined from 20 slices to 100 slices proximal to the growth plate. The bone histomorphometric parameters, including mean bone volume (BV), bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th) and bone mineral density (BMD) were determined using CT-Analyzer software.
Histological Analysis
At the completion of the μ-CT scanning, palates were decalcified for two weeks using 14 % EDTA solution. Following decalcification, tissues were embedded in paraffin to obtain 4 μm thick sagittal sections. For each specimen, serial slices were obtained in a mesiodistal direction, where roots of M1 and M2 aligned in one plane with their respective canal spaces widest. ROI was determined as the M1-M2 interproximal. The slides were hematoxylin and eosin (H&E) stained for microscopic observation. To probe the presence of inflammatory cells between M1 and M2 and osteoclasts in the alveolar crest, a pathologist (SML) blinded to experimental group assignment, evaluated four slides per specimen semi-quantitatively using an Olympus BX53 microscope at the area of interest (M1-M2 interproximal), specifically at the ligature site in the connective tissue of the papilla adjacent to the M2 sulcular epithelium. A semi-quantitative scoring system [32,42,43] was used to evaluate inflammatory cells, where 0 is negative, 1 is less than 30 % of the affected tissues, 2 is some inflammatory cells (30 – 60 %), and 3 is many inflammatory cells (>60 %). Similarly, osteoclasts on the alveolar bone edges were evaluated using a semi-quantitative scoring system[32,44] where 0 is negative, 1 is a few osteoclasts lining less than 5% of alveolar bone surface, 2 is some osteoclasts (5 – 25%), and 3 is many osteoclasts (25 – 50%). Immunohistochemical (IHC) staining of β-catenin was performed using primary antibody (rabbit monoclonal anti-β-catenin antibody, Abcam, ab32572; 1:400 dilution). After deparaffinization and rehydration, sections were incubated in citrate buffer (pH = 6.0, 0.1 M) for antigen retrieval, washed, and then incubated in hydrogen peroxide. Sections were then blocked and incubated with the primary antibody, followed by incubation with the secondary antibody. The antibody complexes were visualized using the DAB chromogen. Hematoxylin was used for counterstaining. The staining intensity (represented as dark brown-stained tissues) for three slides per specimen was independently evaluated at the area of interest (M1-M2 interproximal) by the pathologist using a scale of from 0 to 3, where 0 is negative, 1 is weak staining, 2 is moderate staining, and 3 is strong staining[19,45]. The pathologist’s scoring was validated by another examiner (YA) who evaluated a random set of slides independently and then compared to the pathologist’s scores.
Statistical Analysis
All the generated data were expressed as the mean ± SD (standard deviation). Statistical analyses were carried out using Prism 8.0 software (GraphPad, San Diego, CA). The Analysis of Variance (ANOVA) was used to analyze continuous outcomes among more than three groups. Tukey’s pairwise post-hoc testing was performed for multiple comparisons. P-value < 0.05 was considered statistically significant.
RESULTS
Hydrogel Binding to Hydroxyapatite (HA)
The in vitro binding of the PF127 hydrogel (25% w/v) to HA was analyzed to predict their affinity to bone in vivo. As shown in Figure 1a, the binding time increased as the F127-PPi content was increased in the hydrogel. Among different ratios of F127-PPi, 50, 75, and 100 (w/w %) showed the longest binding time to HA disc (24.6, 25.6, and 28.2 min), respectively. Their binding time was statistically significant when compared to F127 hydrogel (P < 0.0001). The ratio of 25 (w/w %) also exhibited considerable binding time (18.2 min) which was significantly higher than F127 hydrogel (P < 0.01). F127 hydrogel had the lowest binding time (8.5 min) among all the formulations. Based on this observation, the formulation of 50:50% w/w ratio of F127-PPi and F127 was used in the subsequent experiments as it showed strong binding affinity with relatively low PPi content.
Figure 1.
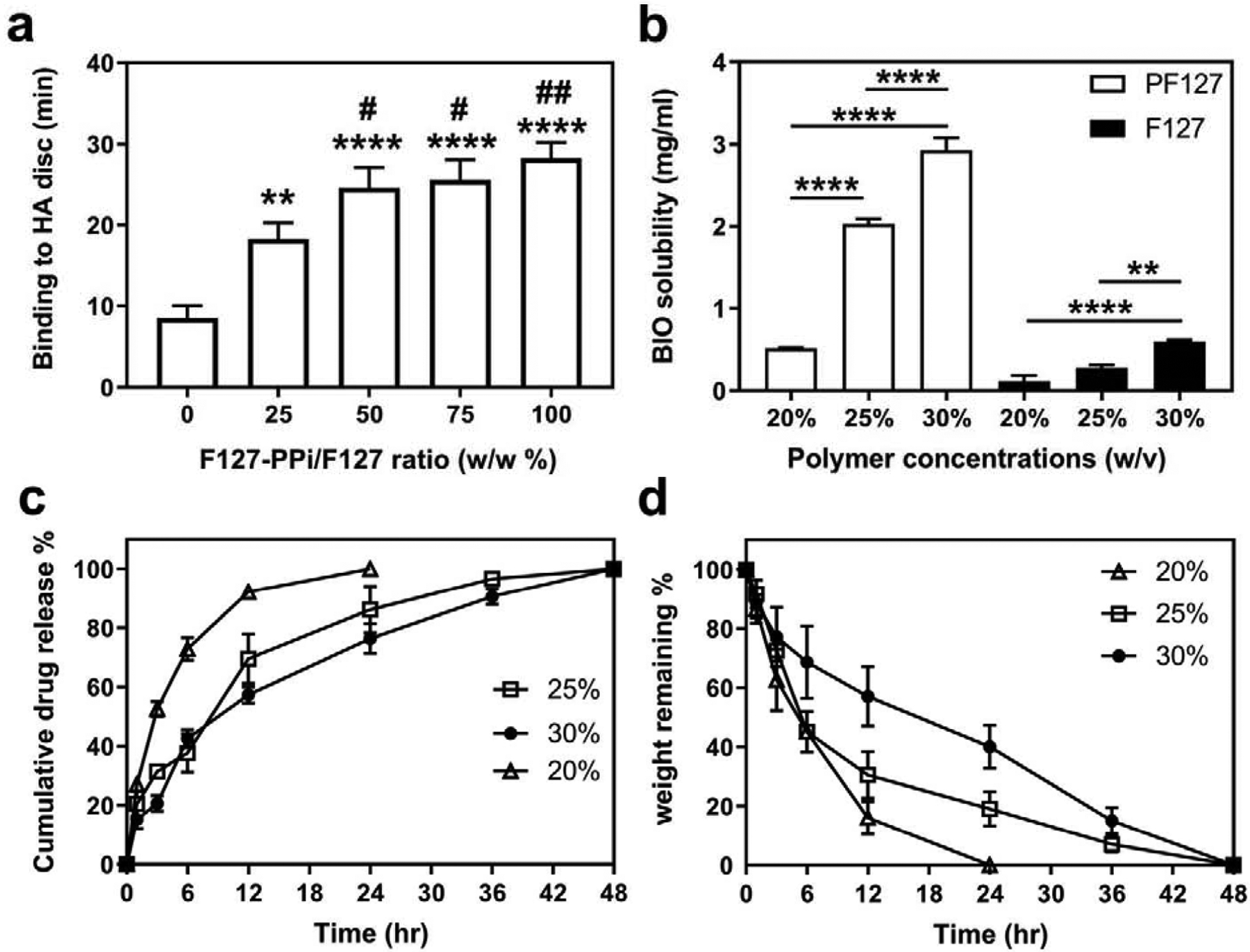
In vitro characterization of PF127 hydrogels. (a) Assessment of hydroxyapatite (HA) binding of 25% w/v PF127 hydrogels prepared with different ratio of F127-PPi and F127. **P < 0.01, and ****P < 0.0001 when compared to F127 hydrogel (ratio of 0); #P < 0.05 and ##P < 0.01 when compared to ratio of 25 (one-way ANOVA with Tukey’s multiple comparisons). (b) Solubility of BIO in PF127 vs F127 solutions at 4 °C. **P < 0.01 and ****P < 0.0001 (one-way ANOVA with Tukey’s multiple comparisons). (c) Cumulative BIO (1.5 mM) release from PF127 hydrogels at 37 °C. (d) Erosion time of PF127 hydrogels incubated at 37 °C measured by weight remaining (%). Values are presented as the mean ± SD, n = 3.
Solubility of BIO in PF127 vs F127
The solubility of BIO in PF127 and F127-containing solutions of different concentrations was analyzed and compared at 4 °C with solution pH = 7. As shown in Figure 1b, the solubility of BIO appears to be proportionally dependent upon polymer concentration. The solubility of BIO in 20% PF127 was determined to be 0.5 mg/mL (1.4 mM) when compared to the 0.1 mg/mL (281 μM) in 20% F127. Furthermore, when polymer concentration was increased to 25%, BIO’s solubility in PF127 was greatly improved to 2 mg/mL (5.6 mM), while the value was only improved to 0.3 mg/mL (842 μM) in F127 solution. When concentration further increased to 30%, solubility in PF127 continued to increase to 3 mg/mL (8.4 mM), while in F127 improved to 0.6 mg/mL (1.7 mM). Clearly, PF127 can significantly improve BIO’s aqueous solubility.
In vitro Release of BIO from PF127 Hydrogel
The releasing kinetics of BIO (1.5 mM) from PF127 hydrogels (20, 25, and 30% w/v) was studied at 37 °C. As shown in Figure 1c, the releasing kinetics of BIO from the hydrogels were found to be dependent upon the concentration of polymers: the higher the polymer concentration, the slower the release rate. The total release (~ 99 %) of BIO from 20% w/v hydrogel occurred in 24 hr with burst release (~ 50%) in the first 3 hr. BIO release from 25% w/v hydrogel was shown with a burst release in the first 12 hr followed by a sustained release in the next 48 hr. For 30% w/v hydrogel, the release of BIO was sustained for over 48 hr. This releasing kinetics of BIO seemed to be not dependent upon the concentration of BIO in the PF127-BIO hydrogel. As shown in Figure S1 (see Supporting Information), the releasing kinetics of BIO from PF127 hydrogel (25% w/v, [BIO] = 100 μM) followed the same trend as that from PF127 hydrogel (25% w/v, [BIO] = 1.5 mM).
In vitro Hydrogel Erosion
The PF127 hydrogels erosion behavior was characterized by measuring weight remaining (%) at regular incubation time intervals. The results correlate with the release study and shown to be a function of the concentration of hydrogel. As shown in Figure 1d, the hydrogel with 20% w/v concentration was completely eroded within 24 hr, ~50% was eroded in the first 3 hr. While 25% w/v hydrogel was completed eroded in 48 hr, ~60% weight loss was observed in the first 6 hr (burst erosion) followed by sustained erosion. Hydrogel with 30% w/v concentration was completely eroded in 48 hr in a more sustained erosion manner.
Biocompatibility of PF127-based Hydrogel
To assess the safety of PF127 hydrogel comparing to F127 which was known to be biocompatible, mouse osteoblast MC3T3-E1 cells were treated with PF127, PF127-BIO, F127 and F127-BIO culture media extracts, or free BIO (100 nM) for 24 hr and 48 hr, and cell viability was defined using the CCK-8 assay (Figure 2). The viability percentage of MC3T3-E1 cells treated with PF127 25% w/v, when compared to media-treated control, was slightly reduced to 84.5% after 24 hr and further decreased to 79.5% after 48 hr. The viability of MC3T3-E1 cells treated with F127 extract was 87.5% and 83.5% after 24 and 48 hr, respectively. When BIO was added to hydrogels, cell viability was not changed significantly. However, when cells treated with 100 nM of free BIO, the viability was determined to be 102% and 104.5% comparing to media-treated control.
Figure 2.
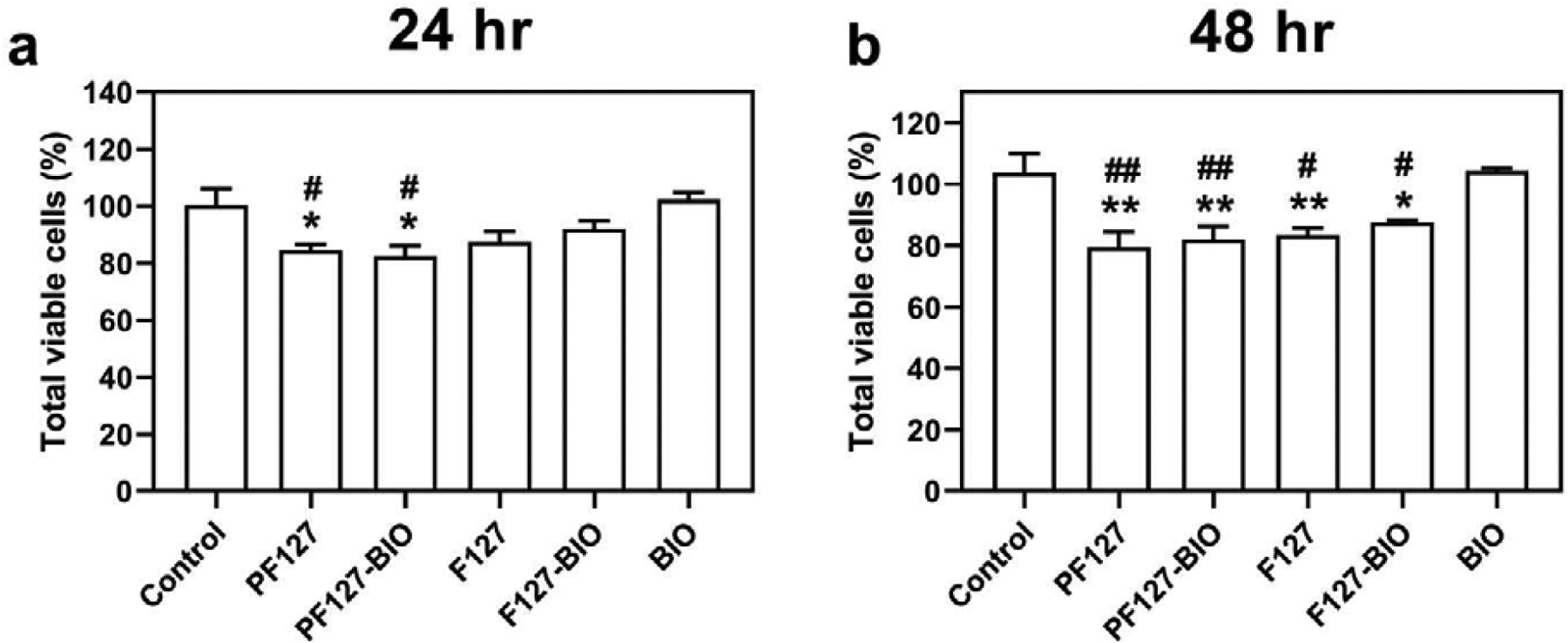
Effect of different treatments on growth of MC3T3-E1 cells measured by CCK-8 assay following (a) 24 hr and (b) 48 hr exposure. Data are shown as the mean ± SD. *P < 0.05 when compared to control group. #P < 0.05 when compared to BIO group. **P < 0.01 when compared to control group. ##P < 0.01 when compared to BIO group.
Gelation and Viscosity Analysis of the PF127 Hydrogel
Gelation temperature (Tgel) was determined based on the temperature sweep measurements of storage and loss modulus, G’ and G” (Figure 3) (for additional data, see Supporting Information Figure S2 & S3,). Samples showed fluid-like behaviors at low temperature (T < Tgel) because G’ was much smaller than G”. As samples were heated, both G’ and G” increased sharply near Tgel, and G’ became larger than G” at high temperature (T > Tgel), which shows that samples underwent gelation and demonstrated solid-like behaviors. Therefore, Tgel was determined according to the cross-point between the G’ and G” curves, and Table 2 summarizes the Tgel values obtained.
Figure 3.
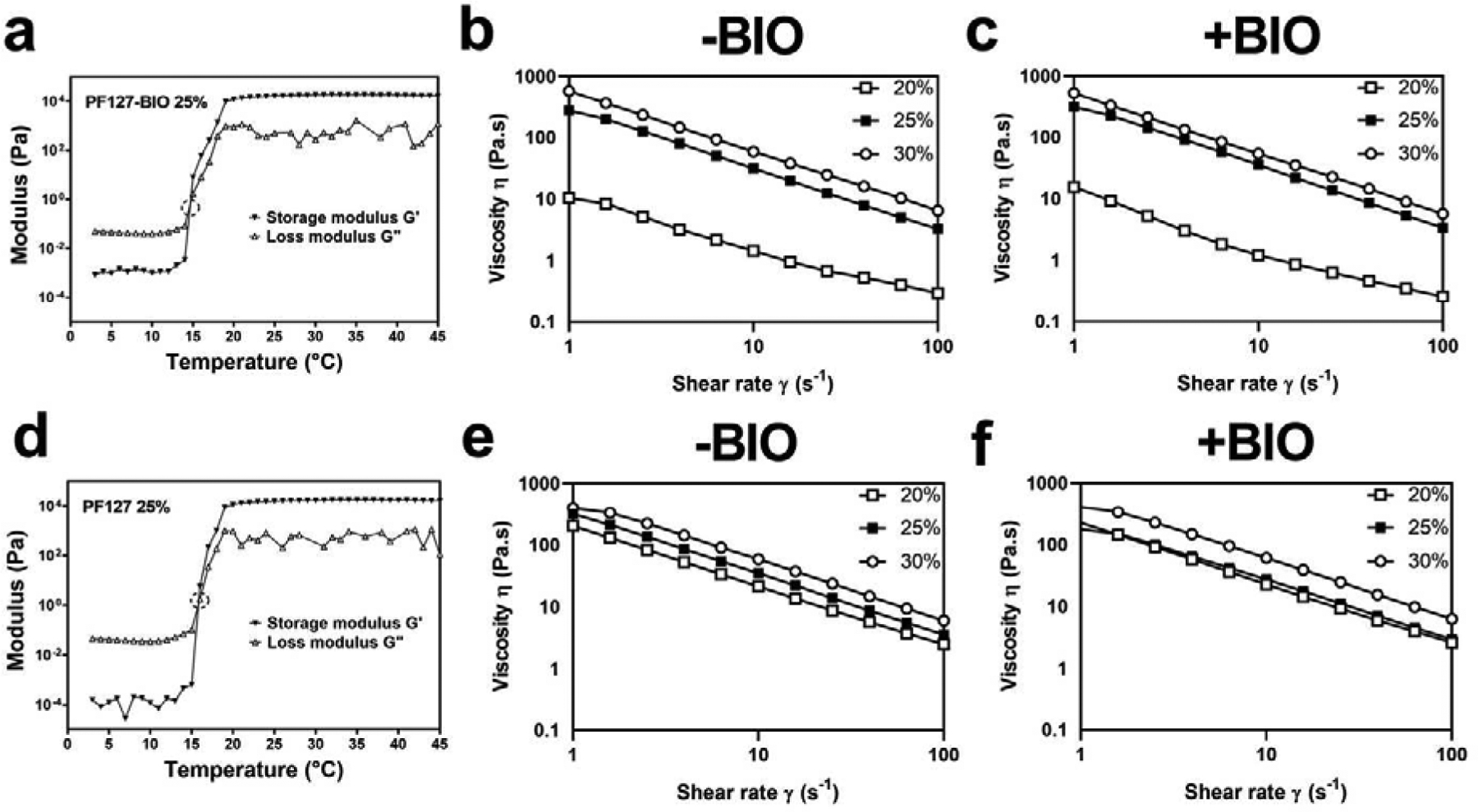
Physical properties of hydrogels. (a&d) Storage and loss modulus (G’ and G”) that determined gelation temperature (Tgel) values of PF127-BIO and PF127 hydrogels. The viscosity of (b) PF127 and (c) PF127-BIO hydrogels at different shear rates. The viscosity of (e) F127 and (f) F127-BIO hydrogels at different shear rates.
Table 2.
The gelation temperature Tgel (°C) for different thermoresponsive hydrogel formulations.
| Concentration (w/v) | F127 | PF127 | F127-BIO | PF127-BIO |
|---|---|---|---|---|
| 20% | 18.0 | 19.0 | 19.0 | 19.0 |
| 25% | 16.0 | 15.0 | 14.0 | 14.0 |
| 30% | 13.0 | 12.1 | 11.1 | 11.0 |
Viscosity measurements were also performed on PF127 and F127 hydrogels with and without BIO at 37 °C to study the effect of the pyrophosphorolation and drug content on their viscous property (Figure 3). All hydrogels showed a typical shear-thinning behavior (nonNewtonian) and the viscosity decreased as a function of shear rate. No notable differences in viscosity were observed between PF127 and F127 hydrogels within the same concentration, except 20% w/v PF127 which showed low viscosity and forms a very weak hydrogel. BIO addition seemed to have no significant impact on hydrogels’ viscous property.
Micro-computed Tomography (μ-CT) Analysis of the Periodontal Bone
As presented in Figure 4a, it was evident that the PF127-BIO hydrogel prevented alveolar bone loss compared to the other treatments. The linear measurement (CEJ to ABC) indicated that the PF127-BIO hydrogel-treated group had the shortest distance among all other treated groups. While F127-BIO treated group exhibited statistically significant difference only when compared to the Saline group (Con−), free BIO-treated group did not show a statistically significant difference comparing to the Saline group (Con−) (Figure 4b). Bone volume (BV, Figure 4c) and trabecular thickness (Tb.Th, Figure 4d) were also quantified to further validate bone quality. The value of BV for the PF127-BIO treated group was significantly higher when compared to all the other treated groups. In contrast, F127-BIO treated group exhibited a statistically significant difference only when compared to the Saline group (Con−). The values of Tb.Th for the PF127-BIO and F127-BIO treated groups were significantly higher when compared to the saline group. No statistically significant difference between Free BIO treated group and the saline group was observed in the above μ-CT parameters. 3-D movies showing the periodontal bone quality after different treatments can be found in Supporting Information. Femur analyses data did not show any significant difference among all treatment groups (Figure S4, Supporting Information).
Histological Evaluation
To study the impacts of different treatment on inflammatory cells, osteoclasts, and βcatenin, images of stained tissue sections (Figures 5a, 6a) were assessed semi-quantitatively. Histology scores of inflammatory cells were shown in Figure 5b. As expected, Healthy (Con+) group had a score of 0 for both inflammatory cells and osteoclasts, which was significantly lower than all the treatment groups except PF127-BIO group. There was no statistically significant difference between Healthy (Con+) and PF127-BIO group. PF127-BIO hydrogel-treated group had the lowest inflammatory cell score when compared to all the other groups (P < 0.0001 compared to Saline group (Con−), P < 0.01 compared to F127-BIO and free BIO treated groups), while F127-BIO and free BIO treated groups exhibited a statistically significant difference (P < 0.01) only when compared to the Saline group (Con−). PF127-BIO hydrogel treated group had the lowest osteoclast score comparing to all treatment groups except Healthy (Con+) group and was significantly (P < 0.001) lower than that of the Saline group (Con−) (Figure 5c). Scores of F127-BIO and free BIO treated groups were significantly lower than the saline Con− group. βCatenin positive cell score of the PF127-BIO hydrogel-treated group was the highest among all treatment groups (Figure 6b, P < 0.0001 compared to Saline group (Con−), P < 0.05 compared to F127-BIO and free BIO treated groups, P < 0.001 when compared to Healthy group (Con+)). Scores of F127-BIO and free BIO-treated groups were not significantly different (P > 0.05) from the Saline (Con−), PF127, and Healthy (Con+) groups.
Figure 5.
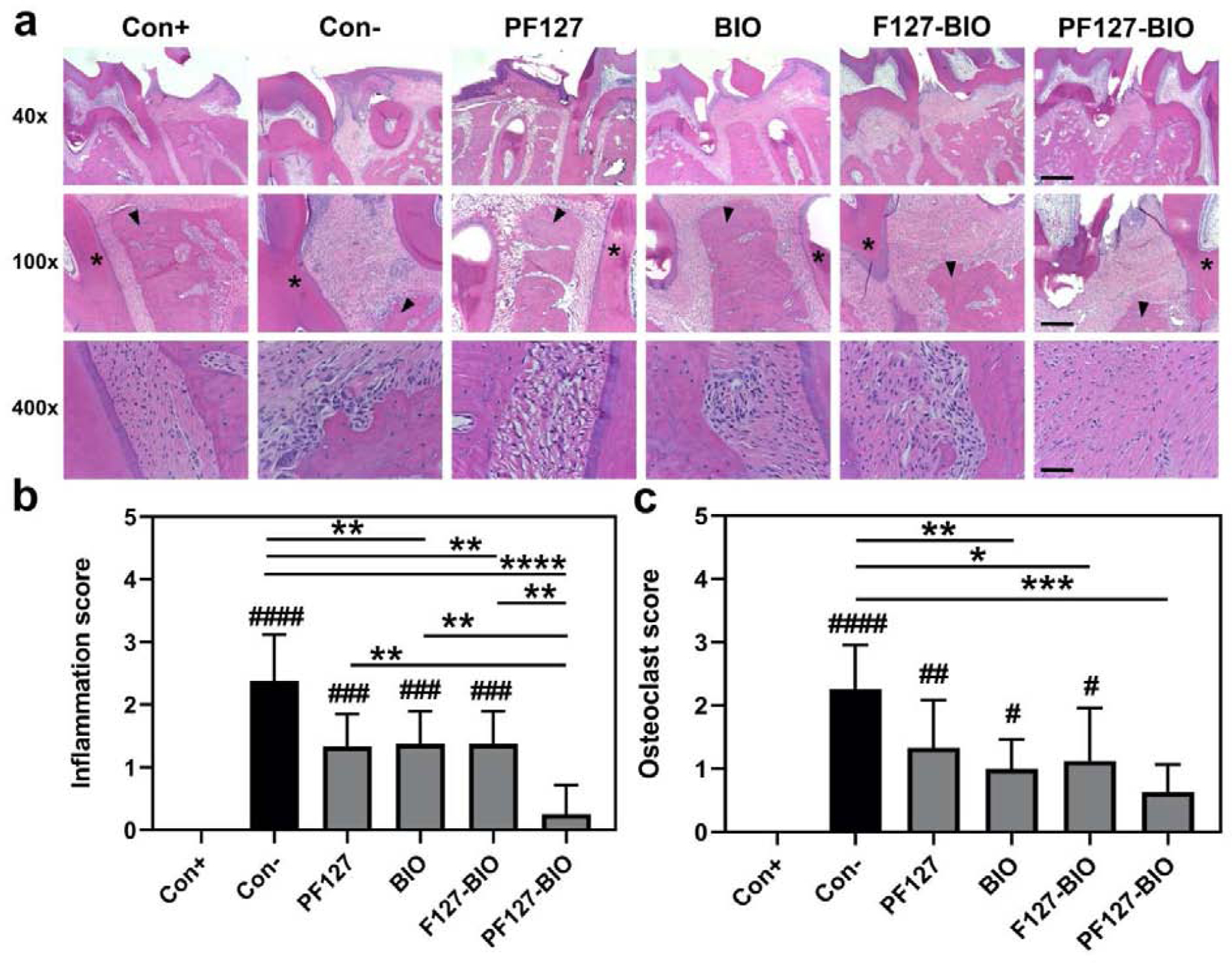
Histological analysis of papillary connective tissue and alveolar bone between first molar (M1) and second molar (M2) after three weeks of different treatments. (a) Representative images of H&E stained tissue sections from different treatment groups. Semi-quantitative assessment of (b) inflammatory cells and (c) osteoclasts. Values are presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001 when compared to Con+ group. (one-way ANOVA). Scale bar = 500 μm, 200 μm and 50 μm for 40x, 100× and 400×, respectively. Black arrows indicate alveolar bone and asterisks indicate first molar (M1).
Figure 6.
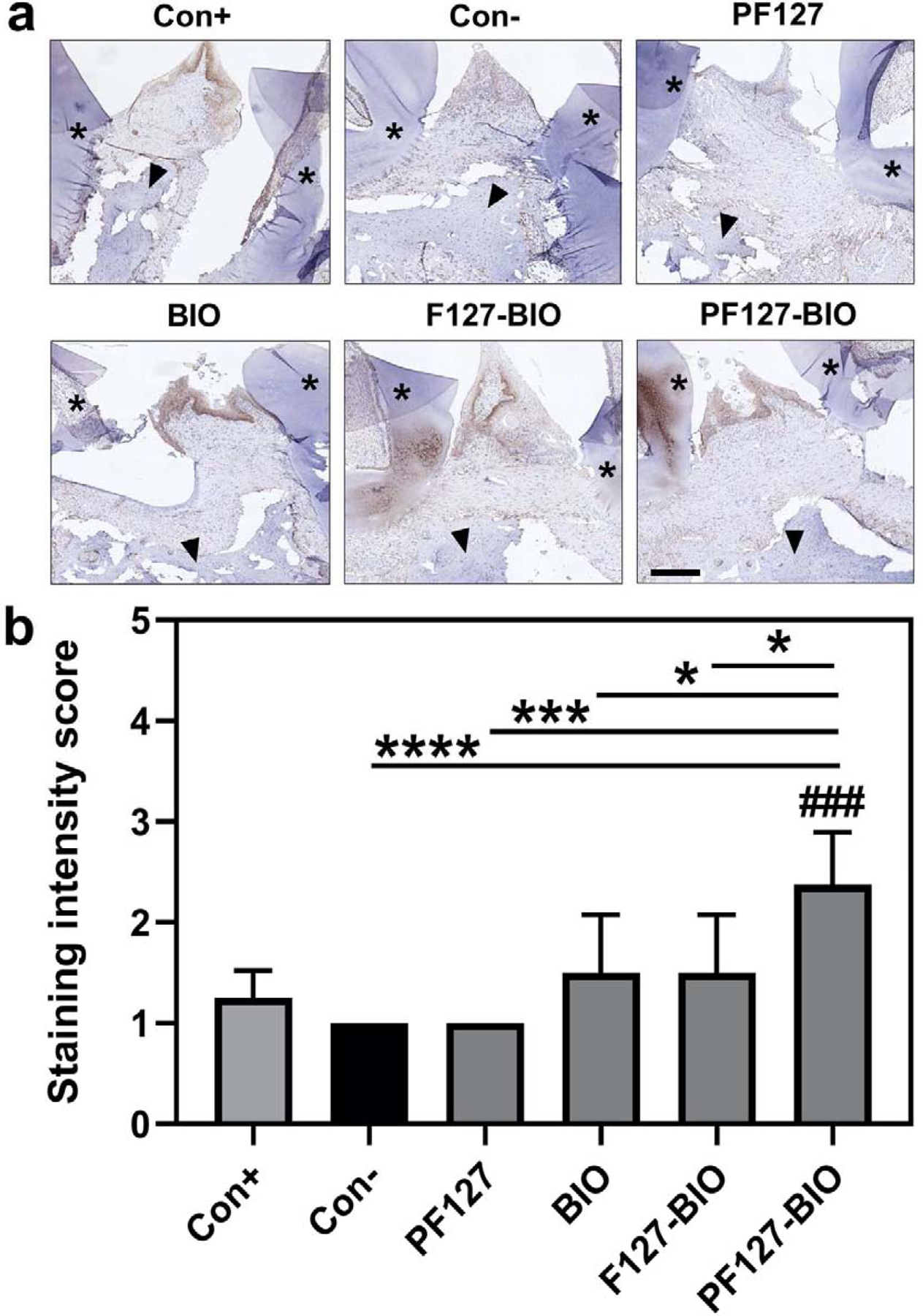
Immunohistochemistry (IHC) analysis of β-catenin at the treatment area. (a) Representative images from different treatment groups of IHC staining of β-catenin of connective tissue above the alveolar bone crest (ABC) and between first molar (M1) and second molar (M2). (b) Semi-quantitative assessment of β-catenin positive cells interproximally between first molar (M1) and second molar (M2). Values are presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. ###P < 0.001 when compared to Con+ group. (one-way ANOVA). Scale bar = 200 μm. Brown color indicates β-catenin staining, black arrows indicate alveolar bone, and asterisks indicate first molar (M1) and second molar (M2).
DISCUSSION
Inflammation-induced alveolar bone loss in response to periodontal infection has long been the focus in clinical management of periodontal disease. To prevent disease progression, simultaneous anti-inflammatory and osteogenic interventions are considered to be essential. Glycogen synthase kinase 3 (GSK3) plays a substantial role in regulating the production of pro- and anti-inflammatory cytokines in innate and adaptive immune cells[6]. It acts as a downstream effector molecule in the PI3K pathway that stimulates the production of proinflammatory cytokines including TNF-α, IL-6, IL-12, and IL-1β, known to promote osteoclastogenesis, leading to alveolar bone loss[11]. GSK3 is also a cytosolic Wnt signaling inhibitor that induces β-catenin degradation and thus suppresses osteoblast differentiation[9,14,17,46]. Hence, there is a strong rationale to consider GSK3 as an attractive therapeutic target for periodontal disease. Pharmacological inhibition of GSK3 using 6-bromoindirubin-3’-oxime (BIO) has been found to alleviate different inflammatory diseases by positively regulating inflammatory cytokines[11,18,19,47–49]. Additionally, BIO has also been found to promote robust osteoblast differentiation, potent bone anabolism[14,46], natural tooth repair[20] and to prevent mandibular cartilage pathological changes[19]. Hence, BIO was proposed in this study to prevent periodontal disease progression because of its potency as an anti-inflammatory and an osteogenic agent. However, in addition to its poor water solubility and lack of osteotropicity, oncogenic concerns[22] have limited BIO’s clinical applications.
To overcome these limitations, we have developed a novel osteotropic hydrogel for local delivery of BIO with better water solubility and direct access to periodontal tissues and thus minimize potential long-term side effects associated with Wnt/β-catenin signaling agonists[22,23]. In this study, the osteointegration or ionic bonding of Pluronic F127 hydrogel was improved through a straightforward two-step reaction, optimizing this delivery system for bone regeneration[50]. The chain termini of Pluronic F127 were modified with pyrophosphate (PPi) without compromising gelation and viscous properties of F127. In addition to the unique thermoresponsive characteristics of F127 hydrogel, this design was also a step forward from our previous studies in which Pluronic copolymers were modified with biomineral-binding moieties (i.e., PPi, phosphorylated serine) to develop dentotropic micelle formulations as an effective and safe delivery tool for antimicrobials to improve dental plaque prevention and treatment[51]. Bisphosphonates (BP) are first-line antiresorptive agents used clinically for the treatment of osteoporosis. They are known to have strong bone affinity and have been used extensively as bone-targeting ligands[52]. While the utility of BP in these particular applications is of great value, its dental application has been frowned upon due to the association of BP with osteonecrosis of the jaw (ONJ)[53,54]. To circumvent the limitations of BP, we ventured to utilize PPi to replace BP as bone-targeting ligand for the thermosresponsive PF127 delivery platform. Similar to BP, PPi has strong affinity to hydroxyapatite. Due to its biodegradability via phosphatase into phosphate[55], PPi does not have the safety concerns associated with BP, especially at the low concentration as a targeting moiety. It has also been used extensively and safely in toothpaste (e.g., Crest®) as an anti-tartar agent. The shelf-life of PPi-containing formulations is not a concern because over the years, the oral hygiene industry has proven that under optimal pH values and buffering conditions, PPi can be used as a toothpaste ingredient with long shelf-life.
The concept of osteotropic hydrogel has been reported previously [56–59]. The use of BP in these studies as the bone-targeting ligands prevents them from being used as delivery vehicle for oral care. Since none of the hydrogel system described was thermoresponsive, they cannot be administered as injections either. Different from these previously reported systems, we envisioned that the thermoresponsive PF127 formulation can be easily adapted into the current clinical management periodontitis. This novel formulation can be conveniently injected into the periodontal pocket after routine scaling and root planing (see Figure S5 and a video illustrating PF127-BIO’s injectability in Supporting Information). The osteotropic hydrogel formed will be capable of interacting with the periodontal hard tissues and achieving the ultimate goal of sustained local drug delivery to assist the clinical management of periodontal disease. The results from the present study support our initial design objective and validate the periodontal bone preservation and anti-inflammatory capacity of the BIO loaded-PF127 hydrogel.
The binding experiment (Figure 1a) showed that the PPi-containing hydrogel formulations (PF127, mixtures of regular F127 and F127-PPi) had stronger binding to HA discs than F127 hydrogel, confirming that PF127 hydrogel was a viable carrier system for local drug delivery to hard tissues. Interestingly, BIO’s solubility in PF127 solution compared to F127 was greatly improved and was proportional to the increase of the polymer concentration. This observation could be explained partially by the improved aqueous solubility of F127-PPi attributes to the conjugation of the highly water-soluble and negatively charged PPi to the chain termini of F127. The cell viability data indicated that the PF127 design retains the good biocompatibility of Pluronic F127[30] even after loading BIO. The observed slight decrease in cell viability associated with F127 and PF127 formulations (Figure 2), which is similar to what has been reported previously[60], may be attributed to the cell membrane disruption caused by Pluronic F127 as a surfactant. This potential biocompatibility concern, however, was not supported by the in vivo observation. As shown in Figure 5, the periodontal tissue exposed to PF127 gel was observed with lower inflammation and osteoclast scores when compared to the Saline group (Con−), supporting the in vivo safety of PF127 as a drug carrier system. We speculate that different from their continuous exposure to cells in culture, the access of PF127 or F127 to the periodontal epithelium after their local deposition in the periodontal pocket may be limited due to the presence desmosomes and occasionally gap junctions [61].
It was shown that PF127 hydrogels with or without BIO loading preserved the thermoresponsive and viscous properties of F127 hydrogels (Figure 3; see also Supporting Information, Figures S2, S3). As the temperature increased, solubility decreased and thus micelle formation was promoted, leading to the tangling of micelles’ coronas and hence gel formation [62]. It was noted that the 20% w/v PF127 exhibited low viscosity and formed weak hydrogel which might be due to the higher aqueous solubility of F127-PPi, leading to increased critical micellar concentration (CMC). In vitro release of BIO through the PF127 hydrogels and erosion profile was affected by the amount of polymer in the hydrogel. The viscosity of the hydrogel increases as a function of polymer concentration which ultimately reduces the drug release rate and surface erosion process, indicating that BIO release was mainly driven by a combination of hydrogel surface erosion and potentially diffusion. It is important to note that the in vitro experiments performed and illustrated in Figure 1 were intended to demonstrate the impact of different factors that would affect the PF127-BIO system’s affinity to hydroxyapatite, the releasing profile of BIO and the erosion of PF127-BIO. These in vitro experimental conditions did not recapitulate the unique pathophysiology of the periodontal pocket, where the PF127-BIO was deposited. For example, contrary to the large volume of the releasing buffer, the flow of gingival crevicular fluid (GCF) in the periodontal pocket is rather slow. No GCF data is available for rats. For human with periodontitis, the GCF is ~ 1 uL/30 s pool. For healthy periodontal tissue, the value is about 0.5 uL/30 s pool[63]. Given such a low volume of releasing media, the strong affinity of PF127-BIO to periodontal hard tissue and the semi-enclosed deposition site (periodontal pocket), we expect that the PF127-BIO hydrogel’s resident time post administration would be much longer than 48 hr. Since there was no visual observation of the PF127-BIO gel in the periodontal pocket at the time of necropsy and tissue processing, it is also clear that the hydrogel did not survive the entire course of the study (4 weeks). Future nearinfrared optical imaging with special probes that may access the rodent oral cavity would be necessary to fully appreciate the kinetics of the PF127-BIO hydrogel’s presence in the periodontal pocket of the rat ligature model.
When tested in vivo on the rat periodontitis model, micro-CT analysis of alveolar bone demonstrated that PF127-BIO hydrogel could most effectively preserve the alveolar bone among all the treatment groups (Figure 4, see also videos showing periodontal bone quality after different treatment in Supporting Information). It mitigated alveolar crest loss and retained bone volume (BV) when compared to all other groups. In confirmation of the μ-CT findings, the histological assessment suggests that comparing to other groups, PF127-BIO hydrogel showed superior anti-inflammatory effects and inhibition of osteoclastic bone resorption. It significantly increased β-catenin positive cell score and reduced inflammatory cell score when compared to all other groups. It also has a lower osteoclast score when compared to saline control (Figure 5), suggesting reduced erosion of the mineralized tissue within the periodontium. These observations could be attributed to the osteogenic [14,46,64] and anti-inflammatory activities of BIO [7,18,46,47], respectively. PF127 hydrogel alone did not produce any osteoprotective effects against the periodontal bone loss, suggesting that the carrier system itself did not contribute to alveolar bone preservation. Moreover, the tissue preservation/regeneration associated with PF127-BIO treatment was not limited to the periodontal bone alone. As can be seen from Figure 4, the higher bone levels seen particularly with PF127-BIO have periodontal ligament (PDL) spaces at the same level as the bone crest. This was corroborated in Figure 5, especially at 100×, showing fibrous PDL between the bone crest area (black arrow) and the tooth root (*). Therefore, the action of the PF127-BIO formulation seemed to have affected both bone and PDL.
As an important safety consideration for Wnt/β-catenin agonists, they may be drained into the circulation after the local release, leading to systemic off-target adverse side effects (e.g., diarrhea, hypoglycemia, tumorigenesis)[21–23]. Therefore, as a surrogate for the potential side effects of systemic GSK3 inhibition, the femoral bone quality was analyzed to gauge the potential systemic osteogenic effects of BIO. No treatment groups showed any significant changes in the femoral bone quality when compared to control (Figure S4, Supporting Information), suggesting that the effect of BIO was restricted locally to periodontal tissues. We believe that the small localized doses of Wnt/β-catenin agonists used in animal studies were significantly lower than those used in clinical studies, posting a lower risk to produce any systemic response[20]. The finding of the absence of systemic anabolism after local PF127-BIO administration in the experimental periodontitis model is significant, as it may have provided a path forward for the clinical translation of Wnt/β-catenin agonists (including GSK3 inhibitors), which have been hindered by their systemic off-target adverse effects.
Comparing to F127-BIO hydrogel and free BIO treatments, the weekly periodontal administration of PF127-BIO hydrogel was more effective in mitigating periodontitis-associated alveolar bone loss and PDL damage. We believe that after local administration, PF127-BIO hydrogel’s strong adhesion to periodontal hard tissues enhanced its local retention, thus ensuring the sustained release of BIO at the desired site, leading to significantly improved preservation/regeneration of the periodontal bone and ligament. This working mechanism explains the superior therapeutic effect of PF127-BIO hydrogel when compared to F127-BIO and dose equivalent free BIO treatment in prevention of alveolar bone loss associated with periodontitis. Clinically, periodontal bone erosion should be treated when diagnosis is made. Hence, the treatments were given at the time of ligature placement to make it clinically relevant. In the human, repeated placement of PF127-BIO hydrogel in periodontitis lesions during periodontal maintenance appointments (for scaling and root planing) may prove to be sufficient to control the progression of bone loss in aggressive cases.
CONCLUSION
In the present study, we have designed, synthesized and formulated a novel osteotropic thermoresponsive hydrogel delivery system by conjugating pyrophosphates (PPi) to the chain termini of Pluronic F127 and mixing it with regular F127. This osteotropic hydrogel system not only showed increased drug loading capacity, but also exhibited strong binding to hydroxyapatite (HA), without disruption of the unique thermoresponsive properties of Pluronic F127. When tested on a rat model of experimental periodontitis, PF127-BIO hydrogel effectively preserved periodontal bone/ligament and mitigated further progression of the pathology. As evidenced in these results, we envision that upon further optimization, this novel and safe thermoresponsive hydrogel-based drug carrier system may have the potential to be translated as an effective therapy for clinical management of aggressive periodontitis.
Supplementary Material
Scheme 1.
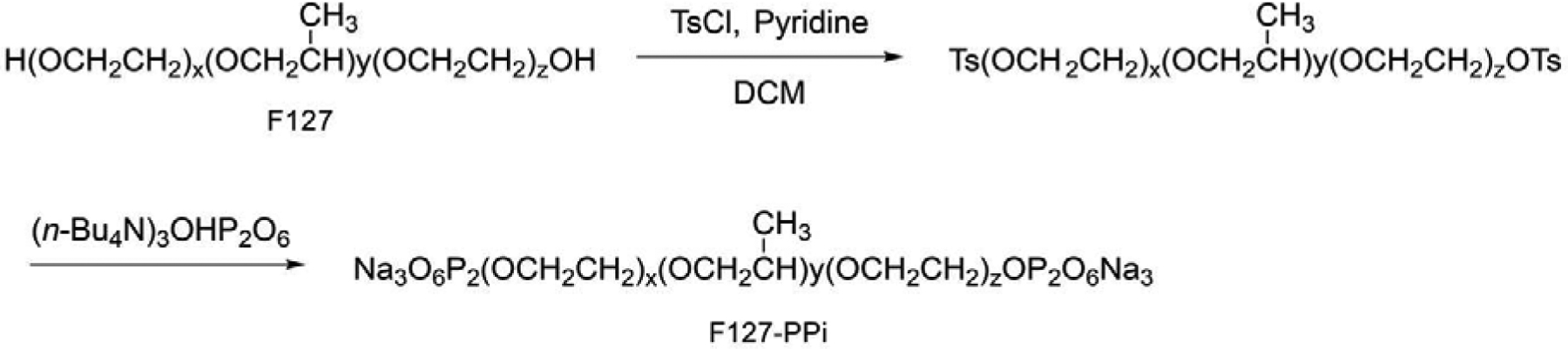
The synthesis of pyrophosphorylated Pluronic F127 (F127-PPi).
ACKNOWLEDGMENT
This work was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, United States (R01 AI119090) and the College of Pharmacy of the University of Nebraska Medical Center, United States. Y.A. was supported by the Jazan University Scholarship, Jazan University, Saudi Arabia. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
- Figure S1. Cumulative BIO (100 μM) release from 25% w/v PF127 hydrogel at 37 °C.
- Figure S2. Storage and loss modulus (G’ and G”) that determined gelation temperature (Tgel) values of PF127-BIO and PF127 hydrogels with different concentrations.
- Figure S3. Storage and loss modulus (G’ and G”) that determined gelation temperature (Tgel) values of F127-BIO and F127 hydrogels with different concentrations.
- Figure S4. Quantitative analysis of rat femurs quality after different treatments.
- Figure S5. Pictures of the 25% w/v PF127-BIO hydrogel at 4 °C and at 37 °C.
- Video, illustrating of the PF127-BIO’s injectability.
- Videos, illustrating of the periodontal bone quality after different treatments.
Declaration of interests
DW, YA, and RGR are co-inventors of a PCT patent application covering the PF127 technology. DW claims an equity position in Bohe Biotechnology, a start-up company that has licensed the technology for further preclinical and translational development. The rest of the coauthors declare no competing interest.
REFERENCES
- [1].Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. The Lancet. 2005. November;366(9499):1809–1820. [DOI] [PubMed] [Google Scholar]
- [2].Herrera D, Sanz M, Jepsen S, Needleman I, Roldán S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. Journal of Clinical Periodontology. 2002. December;29:136–159. [DOI] [PubMed] [Google Scholar]
- [3].Greenstein G. Local drug delivery in the treatment of periodontal diseases: assessing the clinical significance of the results. Journal of Periodontology. 2006. April;77(4):565–578. [DOI] [PubMed] [Google Scholar]
- [4].Williams RC, Jeffcoat MK, Howell TH, Reddy MS, Johnson HG, Hall CM, Goldhaber P. Ibuprofen: an inhibitor of alveolar bone resorption in beagles. Journal of Periodontal Research. 1988. July;23(4):225–229. [DOI] [PubMed] [Google Scholar]
- [5].Raja S, Byakod G, Pudakalkatti P. Growth factors in periodontal regeneration. International Journal of Dental Hygiene. 2009. May;7(2):82–89. [DOI] [PubMed] [Google Scholar]
- [6].Wang H, Brown J, Martin M. Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine. 2011. February;53(2):130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huang WC, Lin YS, Wang CY, Tsai CC, Tseng HC, Chen CL, Lu PJ, Chen PS, Qian L, Hong JS, Lin CF. Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology. 2009. September;128(1 Pt 2):e275–e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Whittle BJ, Varga C, Pósa A, Molnár A, Collin M, Thiemermann C. Reduction of experimental colitis in the rat by inhibitors of glycogen synthase kinase-3β. British Journal of Pharmacology. 2006. March;147(5):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Arioka M, Takahashi-Yanaga F, Sasaki M, Yoshihara T, Morimoto S, Hirata M, Mori Y, Sasaguri T. Acceleration of bone regeneration by local application of lithium: Wnt signal-mediated osteoblastogenesis and Wnt signal-independent suppression of osteoclastogenesis. Biochemical Pharmacology. 2014. August;90(4):397–405. [DOI] [PubMed] [Google Scholar]
- [10].Martinez A, Castro A, Dorronsoro I, Alonso M. Glycogen synthase kinase 3 (GSK-3) inhibitors as new promising drugs for diabetes, neurodegeneration, cancer, and inflammation. Medicinal Research Reviews. 2002. July;22(4):373–384. [DOI] [PubMed] [Google Scholar]
- [11].Adamowicz K, Wang H, Jotwani R, Zeller I, Potempa J, Scott DA. Inhibition of GSK3 abolishes bacterial-induced periodontal bone loss in mice. Molecular Medicine. 2012. August;18(8):1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xie J, Méndez JD, Méndez-Valenzuela V, Aguilar-Hernández MM. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cellular Signaling. 2013. November;25(11):2185–2197. [DOI] [PubMed] [Google Scholar]
- [13].Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P. Pentosidine effects on human osteoblasts in vitro. Annals of the New York Academy of Sciences. 2008. April;1126(1):166–172. [DOI] [PubMed] [Google Scholar]
- [14].Wang FS, Ko JY, Weng LH, Yeh DW, Ke HJ, Wu SL. Inhibition of glycogen synthase kinase-3β attenuates glucocorticoid-induced bone loss. Life Sciences. 2009. November;85(1920):685–692. [DOI] [PubMed] [Google Scholar]
- [15].Krause U, Harris S, Green A, Ylostalo J, Zeitouni S, Lee N, Gregory CA. Pharmaceutical modulation of canonical Wnt signaling in multipotent stromal cells for improved osteoinductive therapy. Proceedings of the National Academy of Sciences. 2010. March;107(9):4147–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fukuda T, Kokabu S, Ohte S, Sasanuma H, Kanomata K, Yoneyama K, Kato H, Akita M, Oda H, Katagiri T. Canonical Wnts and BMPs cooperatively induce osteoblastic differentiation through a GSK3β-dependent and β-catenin-independent mechanism. Differentiation. 2010. July;80(1):46–52. [DOI] [PubMed] [Google Scholar]
- [17].Piters E, Boudin E, Van Hul W. Wnt signaling: a win for bone. Archives of Biochemistry and Biophysics. 2008. May;473(2):112–116. [DOI] [PubMed] [Google Scholar]
- [18].Kwon YJ, Yoon CH, Lee SW, Park YB, Lee SK, Park MC. Inhibition of glycogen synthase kinase-3β suppresses inflammatory responses in rheumatoid arthritis fibroblastlike synoviocytes and collagen-induced arthritis. Joint Bone Spine. 2014. May;81(3):240246. [DOI] [PubMed] [Google Scholar]
- [19].Jiang YY, Wen J, Gong C, Lin S, Zhang CX, Chen S, Cheng W, Li H. BIO alleviated compressive mechanical force-mediated mandibular cartilage pathological changes through Wnt/β-catenin signaling activation. Journal of Orthopaedic Research. 2018. April;36(4):1228–1237. [DOI] [PubMed] [Google Scholar]
- [20].Neves VC, Babb R, Chandrasekaran D, Sharpe PT. Promotion of natural tooth repair by small molecule GSK3 antagonists. Scientific Reports. 2017. January;7:39654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Takahashi-Yanaga F. Activator or inhibitor? GSK-3 as a new drug target. Biochemical Pharmacology. 2013. July;86(2):191–199. [DOI] [PubMed] [Google Scholar]
- [22].Kahn M. Can we safely target the WNT pathway? Nature Reviews Drug Discovery. 2014. July;13(7):513–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang P, Yan R, Zhang X, Wang L, Ke X, Qu Y. Activating Wnt/β-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacology & Therapeutics. 2019. April;196:79–90. [DOI] [PubMed] [Google Scholar]
- [24].Giuliano E, Paolino D, Fresta M, Cosco D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics. 2018. September;10(3):E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharmaceutical Research. 2006. December;23(12):2709–2728. [DOI] [PubMed] [Google Scholar]
- [26].Fakhar-ud-Din Khan GM. Development and characterisation of levosulpiride-loaded suppositories with improved bioavailability in vivo. Pharmaceutical Development and Technology. 2019. January;24(1):63–69. [DOI] [PubMed] [Google Scholar]
- [27].Monti D, Burgalassi S, Rossato MS, Albertini B, Passerini N, Rodriguez L, Chetoni P. Poloxamer 407 microspheres for orotransmucosal drug delivery. Part II: In vitro/in vivo evaluation. International Journal of Pharmaceutics. 2010. November;400(1–2):32–36. [DOI] [PubMed] [Google Scholar]
- [28].Rangabhatla AS, Tantishaiyakul V, Boonrat O, Hirun N, Ouiyangkul P. Novel in situ mucoadhesive gels based on Pluronic F127 and xyloglucan containing metronidazole for treatment of periodontal disease. Iranian Polymer Journal. 2017. November;26(11):851–859. [Google Scholar]
- [29].Akash MS, Rehman K, Li N, Gao JQ, Sun H, Chen S. Sustained delivery of IL-1Ra from pluronic F127-based thermosensitive gel prolongs its therapeutic potentials. Pharmaceutical Research. 2012. December 1;29(12):3475–3485. [DOI] [PubMed] [Google Scholar]
- [30].Diniz IM, Chen C, Xu X, Ansari S, Zadeh HH, Marques MM, Shi S, Moshaverinia A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. Journal of Materials Science: Materials in Medicine. 2015. March;26(3):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Polychronopoulos P, Magiatis P, Skaltsounis AL, Myrianthopoulos V, Mikros E, Tarricone A, Musacchio A, Roe SM, Pearl L, Leost M, Greengard P. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. Journal of Medicinal Chemistry. 2004. February;47(4):935–946. [DOI] [PubMed] [Google Scholar]
- [32].Wang X, Jia Z, Almoshari Y, Lele SM, Reinhardt RA, Wang D. Local application of pyrophosphorylated simvastatin prevents experimental periodontitis. Pharmaceutical Research. 2018. August;35(8):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Deshmukh M, Singh Y, Gunaseelan S, Gao D, Stein S, Sinko PJ. Biodegradable poly (ethylene glycol) hydrogels based on a self-elimination degradation mechanism. Biomaterials. 2010. September;31(26):6675–6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li Y, Cao J, Han S, Liang Y, Zhang T, Zhao H, Wang L, Sun Y. ECM based injectable thermo-sensitive hydrogel on the recovery of injured cartilage induced by osteoarthritis. Artificial Cells, Nanomedicine, and Biotechnology. 2018. November;46(sup2):152–160. [DOI] [PubMed] [Google Scholar]
- [35].Ma X, He Z, Han F, Zhong Z, Chen L, Li B. Preparation of collagen/hydroxyapatite/alendronate hybrid hydrogels as potential scaffolds for bone regeneration. Colloids and Surfaces B: Biointerfaces. 2016. July;143:81–87. [DOI] [PubMed] [Google Scholar]
- [36].Winter HH. Can the gel point of a cross-linking polymer be detected by the G′ – G″ crossover? Polymer Engineering and Science. 1987. December;27(22):1698–1702. [Google Scholar]
- [37].Bercea M, Darie RN, Nita LE, Morariu S. Temperature Responsive Gels Based on Pluronic F127 and Poly(vinyl alcohol). Industrial & Engineering Chemistry Research. 2011. March;50(7):4199–4206. [Google Scholar]
- [38].Bradley AD, Zhang Y, Jia Z, Zhao G, Wang X, Pranke L, Schmid MJ, Wang D, Reinhardt RA. Effect of simvastatin prodrug on experimental periodontitis. Journal of Periodontology. 2016. May;87(5):577–582. [DOI] [PubMed] [Google Scholar]
- [39].Klausen. Microbiological and immunological aspects of experimental periodontal disease in rats: a review article. Journal of periodontology. 1991. January;62(1):59–73. [DOI] [PubMed] [Google Scholar]
- [40].Xu Y, Wei W. A comparative study of systemic subantimicrobial and topical treatment of minocycline in experimental periodontitis of rats. Archives of oral biology. 2006. September 1;51(9):794–803. [DOI] [PubMed] [Google Scholar]
- [41].Jia Z, Wang X, Wei X, Zhao G, Foster KW, Qiu F, Gao Y, Yuan F, Yu F, Thiele GM, Bronich TK, O’Dell JR, Wang D. Micelle-Forming Dexamethasone Prodrug Attenuates Nephritis in Lupus-Prone Mice without Apparent Glucocorticoid Side Effects. ACS Nano. 2018. July;12(8):7663–7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gibson-Corley KN, Olivier AK, Meyerholz DK. Principles for valid histopathologic scoring in research. Veterinary Pathology. 2013. November;50(6):1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schett G, Stolina M, Bolon B, Middleton S, Adlam M, Brown H, Zhu L, Feige U, Zack DJ. Analysis of the kinetics of osteoclastogenesis in arthritic rats. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 2005. October;52(10):3192–3201. [DOI] [PubMed] [Google Scholar]
- [44].Bolon B, Morony S, Cheng Y, Hu YL, Feige U. Osteoclast numbers in Lewis rats with adjuvant-induced arthritis: identification of preferred sites and parameters for rapid quantitative analysis. Veterinary Pathology. 2004. January;41(1):30–36. [DOI] [PubMed] [Google Scholar]
- [45].Song S, Ajani JA, Honjo S, Maru DM, Chen Q, Scott AW, Heallen TR, Xiao L, Hofstetter WL, Weston B, Lee JH. Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Research. 2014. August;74(15):4170–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Georgiou KR, King TJ, Scherer MA, Zhou H, Foster BK, Xian CJ. Attenuated Wnt/β-catenin signalling mediates methotrexate chemotherapy-induced bone loss and marrow adiposity in rats. Bone. 2012. June;50(6):1223–1233. [DOI] [PubMed] [Google Scholar]
- [47].Park DW, Jiang S, Liu Y, Siegal GP, Inoki K, Abraham E, Zmijewski JW. GSK3β-dependent inhibition of AMPK potentiates activation of neutrophils and macrophages and enhances severity of acute lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2014. September;307(10):L735–L745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang Y, Huang WC, Wang CY, Tsai CC, Chen CL, Chang YT, Kai JI, Lin CF. Inhibiting glycogen synthase kinase-3 reduces endotoxaemic acute renal failure by downregulating inflammation and renal cell apoptosis. British Journal of Pharmacology. 2009. July;157(6):1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Klamer G, Shen S, Song E, Rice AM, Knight R, Lindeman R, O’Brien TA, Dolnikov A. GSK3 inhibition prevents lethal GVHD in mice. Experimental Hematology. 2013. January;41(1):39–55. [DOI] [PubMed] [Google Scholar]
- [50].Bai X, Gao M, Syed S, Zhuang J, Xu X, Zhang XQ. Bioactive hydrogels for bone regeneration. Bioactive Materials. 2018. December;3(4):401–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chen F, Jia Z, Rice KC, Reinhardt RA, Bayles KW, Wang D. The development of dentotropic micelles with biodegradable tooth-binding moieties. Pharmaceutical Research. 2013. November;30(11):2808–2817. [DOI] [PubMed] [Google Scholar]
- [52].Wang D, Miller SC, Kopečková P, Kopeček J. Bone-targeting macromolecular therapeutics. Advanced Drug Delivery Reviews. 2005. May;57(7):1049–1076. [DOI] [PubMed] [Google Scholar]
- [53].Purcell PM, Boyd IW. Bisphosphonates and osteonecrosis of the jaw. Medical Journal of Australia. 2005. April;182(8):417–418. [DOI] [PubMed] [Google Scholar]
- [54].Kennel KA, Drake MT. Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clinic Proceedings 2009. July; 84(7):632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shellis RP, Addy M, Rees GD. In vitro studies on the effect of sodium tripolyphosphate on the interactions of stain and salivary protein with hydroxyapatite. Journal of Dentistry. 2005. April 1;33(4):313–24. [DOI] [PubMed] [Google Scholar]
- [56].Ossipov DA. Bisphosphonate-modified Biomaterials for Drug Delivery and Bone Tissue Engineering. Expert Opinion on Drug Delivery. 2015. March;12(9):1443–58. [DOI] [PubMed] [Google Scholar]
- [57].Varghese OP, Sun W. Hilborn J, Ossipov DA. In Situ Cross-Linkable High Molecular Weight Hyaluronan-Bisphosphonate Conjugate for Localized Delivery and Cell-Specific Targeting: A Hydrogel Linked Prodrug Approach. Journal of American Chemical Society. 2009. July 1; 131(25):8781–3 [DOI] [PubMed] [Google Scholar]
- [58].Rayment EA, Dargaville TR, Shooter GK, George GA, Upton Z. Attenuation of Protease Activity in Chronic Wound Fluid with Bisphosphonate-Functionalised Hydrogels. Biomaterials. 2008. April; 29(12):1785–95 [DOI] [PubMed] [Google Scholar]
- [59].Nejadnik MR, Yang X, Mimura T, Birgani ZT, Habibovic P, Itatani K, Jansen JA, Hilborn J, Ossipov D, Mikos AG, Leeuwenburgh SC. Calcium-mediated Secondary Cross-Linking of Bisphosphonated Oligo(poly(ethylene Glycol) Fumarate) Hydrogels. Macromolecular Bioscience. 2013. October;13(10):1308–13. [DOI] [PubMed] [Google Scholar]
- [60].Wang B, Shao J, Jansen JA, Walboomers XF, Yang F. A Novel Thermoresponsive Gel as a Potential Delivery System for Lipoxin. Journal of Dental Research. 2019. March;98(3):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fujita T, Yoshimoto T, Kajiya M, Ouhara K, Matsuda S, Takemura T, Akutagawa K, Takeda K, Mizuno N, Kurihara H. Regulation of Defensive Function on Gingival Epithelial Cells Can Prevent Periodontal Disease. Japanese Dental Science Review. 2018. May; 54(2):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sharma PK, Bhatia SR. Effect of anti-inflammatories on Pluronic® F127: micellar assembly, gelation and partitioning. International Journal of Pharmaceutics. 2004. July;278(2):361–377. [DOI] [PubMed] [Google Scholar]
- [63].Ho W, Eubank T, Leblebicioglu B, Marsh C, Walters J. Azithromycin decreases crevicular fluid volume and mediator content. Journal of dental research. 2010. August;89(8):831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Low SA, Galliford CV, Yang J, Low PS, Kopeček J. Biodistribution of fracture-targeted gsk3β inhibitor-loaded micelles for improved fracture healing. Biomacromolecules. 2015. September;16(10):3145–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


