Abstract
About 20 years after the identification of immunoglobulin E (IgE) and its key role in allergic hypersensitivity reactions against normally harmless substances, scientists have started inventing strategies to block its pathophysiological activity in 1986. The initial concept of specific IgE targeting through the use of anti-IgE antibodies has gained a lot of momentum and within a few years independent research groups have reported successful generation of first murine monoclonal anti-IgE antibodies. Subsequent generation of optimized chimeric and humanized versions of these antibodies has paved the way for the development of therapeutic anti-IgE biologicals as we know them today. With omalizumab, there is currently still only one therapeutic anti-IgE antibody approved for the treatment of allergic conditions. Since its application is limited to the treatment of moderate-to-severe persistent asthma and chronic spontaneous urticaria, major efforts have been undertaken to develop alternative anti-IgE biologicals that could potentially be used in a broader spectrum of allergic diseases. Several new drug candidates have been generated and are currently assessed in pre-clinical studies or clinical trials. In this review, we highlight the molecular properties of past and present anti-IgE biologicals and suggest concepts that might improve treatment efficacy of future drug candidates.
1. Introduction
The discovery of immunoglobulin E (IgE) in 19678,9 together with the identification of its central role in the pathogenesis of allergic inflammation (reviewed elsewhere13,14) has set the stage for the development of therapeutic anti-IgE strategies. The generation of detailed knowledge about the molecular and structural characteristics of IgE has further accelerated this process. IgE consists of two identical heavy and light chains. Each heavy chain includes four constant epsilon domains (Cε1–4)15. Overall, the IgE antibody features high flexibility. Particularly the IgE-Fc part has been described to undergo important conformational changes depending on its interaction partner. While IgE binding to the high affinity receptor FcεRI on allergic effector cells induces an open Fc conformation, low affinity receptor FcεRII/CD23 binding on antigen presenting cells (APC), airway epithelial cells (AEC) or B-cells forces IgE in a closed Fc conformation and thereby ensures mutually exclusive interaction with the two receptors16–18. In addition to the free form of IgE, B-cells express membrane IgE (mIgE) that is part of the B-cell receptor (BCR) and contains an extracellular membrane proximal domain (EMPD)19–21. Identification of these features have been key for the development of efficient targeting strategies. Over the last decades, the field of anti-IgE biologicals has advanced into a competitive and active field of investigation. Different companies and research institutions are currently assessing approaches to specifically target IgE in pre-clinical studies or clinical trials (Table 1)22,23. Here, we provide a chronological overview on past achievements, present investigations and potential future approaches of anti-IgE strategies.
Table 1.
Past, present and future anti-IgE biologicals.
| Anti-IgE name | Format | Target | Indication | Clinical status | Company | Published |
|---|---|---|---|---|---|---|
| Omalizumab | Antibody | IgE, mIgE | Asthma, CSU | approved | Novartis, Genentech | 199330 |
| Ligelizumab | Antibody | IgE, mIgE, CD23-IgE | CSU | phase III | Novartis | 1999118 |
| Quilizumab | Antibody | mIgE | CSU | phase II | Genentech | 201066 |
| MEDI4212 | Antibody | IgE, mIgE | n.a. | phase I | MedImmune | 201478 |
| XmAb7195 | Antibody | IgE, mIgE | n.a. | phase I | Xencor | 201281 |
| 8D6 | Antibody | IgE, CD23-IgE | n.a. | pre-clinical | - | 201293 |
| GE2 | Fc-fusion protein | FcεRI/ CD23, FcγRII, | n.a. | pre-clinical | - | 2002101 |
| DE53-Fc | DARPin | IgE, FcεRI-IgE, FcγRIIb | n.a. | pre-clinical | - | 2011107 |
| bi53_79 | DARPin | IgE, FcεRI-IgE | n.a. | pre-clinical | - | 201491 |
| 026 sdab | Nanobody | IgE, FcεRI-IgE | n.a. | pre-clinical | Ablynx | 2018111 |
2. The Past: Approved anti-IgE biologicals
In 1986, Dr. Christoph Heusser and his colleagues at the former Swiss pharmaceutical company Ciba-Geigy proposed the development of neutralizing non-anaphylactogenic anti-IgE antibodies24. Around the same time, the research team of Dr. Tse Wen Chang at the US biopharmaceutical company TANOX came up with the idea to generate a monoclonal antibody against IgE that specifically targets and eliminates IgE-secreting B-cells10. While the group at Ciba-Geigy demonstrated proof-of-concept in different mouse models11,25, TANOX generated the first monoclonal murine anti-human IgE antibody called TES-C2126 (Fig. 1). In 1990, the two companies entered a partnership with the goal to join their anti-IgE programs and to generate a therapeutic anti-IgE antibody that would block the biologic activity of IgE based on three criteria: i) neutralization of free serum IgE, ii) targeting of IgE-producing B-cells and iii) non-reactivity with other IgE bearing cells. Therefore, they further engineered TES-C21 and developed the chimeric mouse/human monoclonal antibody TESC-2, later renamed to CGP5190126. In clinical phase I and II trials with allergic rhinitis patients CGP51901 showed dose-dependent reduction of free serum IgE levels, inhibition of rhinitis symptoms and high tolerability27,28. The cooperation between TANOX and Ciba-Geigy ultimately gave rise to the fully humanized monoclonal anti-IgE antibody CGP56901, also known as TNX-901 or Talizumab29. Later in 1996, Ciba-Geigy fused with the pharmaceutical company Sandoz to form Novartis. During that time, Genentech has also been generating a murine anti-human IgE antibody called MaE11, which has been further developed into the humanized monoclonal antibody rhuMAb-E2530. To reduce competition and maximize synergies, Genentech, TANOX and Novartis formed a tripartite partnership. Despite the fact that both antibody candidates expressed similar key properties31, the three partners decided to select rhuMAb-E25 from Genentech over CGP56901 for further development due to already established manufacturing processes. This collaboration ultimately led to the approval of rhuMAb-E25 - today best known as omalizumab (Xolair®) – as the first therapeutic anti-IgE antibody in 200312.
Fig. 1. Chronological appearance of anti-IgE compounds.
Following the discovery of IgE various anti-IgE compounds have been reported. The years of their appearance (blue) are marked on the timeline (grey arrow). The inter-relationship between different anti-IgE compounds is highlighted with arrows (dotted black). TES-C21: monoclonal murine anti-IgE antibody; TESC-2/ CGP51901: monoclonal chimeric mouse/human anti-IgE antibody; TNX-901: humanized monoclonal anti-IgE antibody; rhuMAb-E25/ omalizumab: humanized monoclonal anti-IgE antibody; GE2: fusion protein of IgG1-Fc3–4 and IgE-Fc2–4; DE53-Fc: fusion protein of anti-IgE DARPin and IgG1-Fc3–4; XmAb7195: humanized affinity maturated version of parental omalizumab antibody; CMAB007: omalizumab biosimilar; ligelizumab: high-affinity version of TNX-901; quilizumab: afucosylated humanized monoclonal anti-IgE (EMPD) antibody; MEDI4212: afucosylated monoclonal anti-IgE antibody; bi53_79: bispecific disruptive anti-IgE DARPin; 39D11: anti-IgE nanobody; 026 sdab: humanized affinity maturated anti-IgE nanobody.
Until now, omalizumab is still the only approved therapeutic anti-IgE antibody. Despite its restricted indication for the treatment of moderate-to-severe persistent asthma and chronic spontaneous urticaria (CSU), omalizumab is frequently used off-label in allergic rhinitis, food allergies and allergen-specific immunotherapy32–34. Several meta-analyses have assessed the efficacy of omalizumab treatment in allergic asthma and CSU. These studies came to the conclusion that omalizumab efficiently reduces asthma exacerbations, leads to overall improvement in quality of life and allows the reduction or withdrawal of inhaled corticosteroids35–39. However, the use of omalizumab in severe eosinophilic asthma in corticosteroid-resistant patients is controversially discussed35,40,41. Further studies on this subject are needed, especially since there are other approved biologics that might be superior to omalizumab in this context42. The primary mode-of-action of this humanized monoclonal anti-IgE antibody of IgG1 subclass is the neutralization of free IgE. It binds with nanomolar affinity (KD ∼ 7 × 10−9 M)43 to the Cε3 domain of IgE. Recent analysis of crystal structures composed of omalizumab Fab fragments and IgE-Fc3–4 variants revealed that omalizumab-mediated inhibition of IgE binding to FcεRI does not occur via direct competition for binding sites on IgE as stated in many previous reports44. Omalizumab shares only minor overlap with FcεRIα binding epitopes on IgE and mainly inhibits IgE receptor binding via steric inhibition44,45 (Fig. 2a). On the other hand, the binding footprints of omalizumab and CD23 on IgE show a high degree of overlap44,45. Therefore, the inhibition of IgE:CD23 interactions is primarily dependent on direct binding competition. Importantly, omalizumab inhibits IgE receptor interactions with both, FcεRI and CD23 (Fig. 2b) and does not aggregate receptor-bound IgE ensuring its non-anaphylactogenic profile44. In rare cases (~0.1%), however, treatment with omalizumab has induced adverse reactions including skin inflammation and anaphylaxis in the absence of anti-drug antibodies46–48. Recent studies, have provided strong evidence that omalizumab can bind and cross-link IgE-Fc3–4:FcεRIα complexes due to the missing Cε2 domain, which in the intact IgE molecule masks the omalizumab binding epitope on IgE44. Whether IgE-Fc3–4:FcεRIα complexes exist in vivo and whether such binding events might play a role in the limited cases of omalizumab-induced anaphylaxis is currently not known. Another study revealed that omalizumab-IgE immune complexes activate human neutrophils that may induce skin inflammation and anaphylaxis in humanized mice by engaging activating Fc-gamma receptors and binding of complement factors49. Although this is an interesting finding, it does not explain the rarity of such omalizumab-induced adverse reactions in humans.
Fig. 2. Binding characteristics and modes-of-action of omalizumab.
a) Schematic overview of omalizumab (blue), FcεRIα (left panel) and FcεRII/CD23 (right panel) binding sites as well as their relative overlap on IgE-Fc3–4 (purple). b) Omalizumab mediated inhibition of free IgE binding to FcεRI or CD23. c) Omalizumab mediated accelerated dissociation of pre-formed IgE:FcεRI and IgE:CD23 complexes. d) Potential mechanisms leading to suppression of IgE production by omalizumab.
As first discovered by Dr. Donald MacGlashan in 199750,51 and confirmed by several studies with omalizumab, anti-IgE treatment reduces FcεRI levels on allergic effector cells such as mast cells and basophils52. This beneficial side effect is a consequence of free IgE clearance, which leads to the lack of IgE-mediated FcεRI stabilization on the surface of these cells53. Additionally, omalizumab has been described to accelerate the dissociation of IgE from FcεRI54 and CD2345 (Fig. 2c). Recently an alternative omalizumab Fab fragment has been generated by introducing three mutations in the variable and constant light chain. This so called FabXol3 antibody fragment showed superior activity in dissociating IgE from FcεRIα than the original full length antibody55. Whether the increased disruptive efficacy of omalizumab would result in an additional treatment benefit still requires careful evaluation.
There is emerging evidence that omalizumab in addition to its primary mode-of-action has the ability to decrease IgE production by targeting mIgE positive B-cells56,57. Several studies have shown reduced B-cell numbers and IgE secretion upon long-term omalizumab treatment58–60 (Fig. 2d). While these observations are highly interesting, the exact mechanisms underlying omalizumab-dependent regulation of IgE production are not fully understood yet.
3. The present: Monoclonal anti-IgE antibodies in clinical trials
So far, the field of anti-IgE biologicals has largely been dominated by monoclonal antibody approaches and different companies have successfully reached the stage of clinical trials with their anti-IgE candidates. However, none of these have been approved for therapeutic application in the last two decades. One of the highly anticipated antibodies that is still under active investigation in clinical trials is ligelizumab (QGE031) from Novartis. This is a high affinity version of TNX-901 that was originally “put on ice” in the collaboration between Genentech, TANOX and Novartis. To our current knowledge, all other anti-IgE programs discussed in this section have been discontinued due to strategic decisions or failure to achieve primary endpoints in clinical trials.
3.1. Monoclonal anti-IgE antibody ligelizumab
While omalizumab was successfully developed in the tripartite collaboration with Novartis and Genentech, TANOX still proceeded to test the original anti-IgE antibody TNX-901 in a phase II clinical trial with peanut allergic subjects61,62. However, this program had to be stopped after an extensive legal debate with Genentech and Novartis. Recently, a high-affinity version of TNX-901, generated by TANOX, has been developed by Novartis under the name ligelizumab (QGE 031) and is currently in a phase III clinical investigation for the treatment of CSU (NCT03580356). Ligelizumab is a humanized monoclonal IgG1 antibody that binds IgE with an affinity of ∼ 1.4 × 10−10 M but does not interact with FcεRIα-bound IgE22. Unlike omalizumab it does not accelerate dissociation of pre-formed FcεRIα:IgE complexes and recent structural analysis revealed that ligelizumab recognizes a distinct IgE epitope, only partially overlapping with that of omalizumab45. Ligelizumab binds to both Cε3 domains across the IgE-Fc dimer and favours the recognition of IgE in an open Fc conformation. It shares significantly more overlap with the binding epitope of FcεRI on IgE than omalizumab and is superior in inhibiting this interaction. On the other hand, ligelizumab shows almost no overlap with the CD23 binding site and is therefore less efficient than omalizumab in blocking IgE:CD23 interactions45 (Fig. 3a). This distinct receptor inhibition profile might play an important role for its treatment efficacy in certain diseases. Based on these findings, ligelizumab is predicted to have a therapeutic advantage over omalizumab in FcεRI-driven allergic disorders. Indeed, ligelizumab showed significantly better symptom control in comparison to omalizumab in a phase IIb dose-finding trial for CSU last year63. It was also superior to omalizumab in the inhibition of primary human basophil activation and a passive systemic anaphylaxis mouse model45. On the other hand, it might be less suitable for the treatment of allergic disorders that heavily rely on CD23-dependent processes including antigen presentation or transepithelial transport (e.g. eosinophilic lung inflammation)64. Further, the distinct receptor inhibition profile provides a plausible explanation as to why ligelizumab lacked superior efficacy over omalizumab treatment in a phase II clinical trial with allergic asthma patients (NCT02075008). In line with a recent clinical trial that revealed a surprisingly long suppression of free serum IgE levels in atopic patients upon single dose injection43, it has been demonstrated that ligelizumab can suppress IgE production most likely by interacting with CD23-bound IgE on human B-cells45. However, this secondary mode-of-action that might increase treatment efficacy of ligelizumab over omalizumab in various atopic disorders needs to be further investigated.
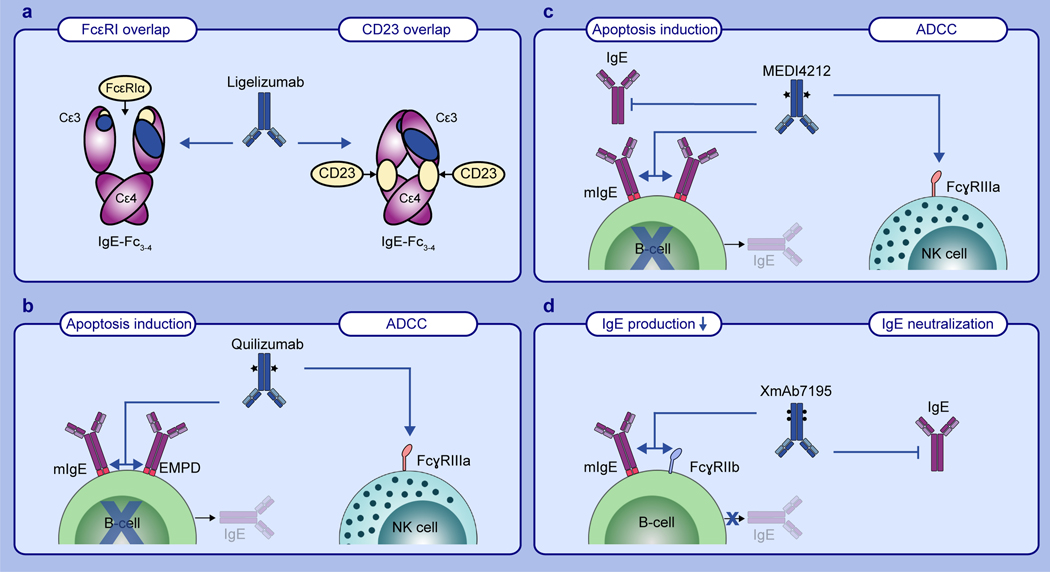
Fig. 3. Binding characteristics and modes-of-action of different anti-IgE antibodies.
a) Schematic overview of ligelizumab (blue), FcεRIα (left panel) and FcεRII/CD23 (right panel) binding sites as well as their relative overlap on IgE-Fc3–4 (purple). b) Two mechanisms of quilizumab mediated suppression of IgE production through induction of B-cell apoptosis or antibody dependent cellular cytotoxicity (ADCC). Afucosylation of quilizumab is indicated with stars. c) Mechanisms of MEDI4212 mediated neutralization of free IgE and suppression of IgE production through induction of B-cell apoptosis or antibody dependent cellular cytotoxicity (ADCC). Afucosylation of MEDI4212 is indicated with stars. d) XmAb7195 mediated neutralization of free IgE and suppression of IgE production through co-aggregation of mIgE and FcγRIIb on B-cells. Mutations in the antibody Fc-part are indicated with dots.
3.2. Monoclonal anti-IgE antibody quilizumab
Another extensively investigated approach for the treatment of allergic disorders is the specific targeting of IgE producing B-cells displaying mIgE on their surface65. Genentech has developed the monoclonal mouse anti-human IgE antibody 47H4, which recognizes the EMPD of IgE66. 47H4 has been shown to reduce serum IgE levels and the number of IgE-producing plasma cells in vivo in genetically modified mice that carry the human EMPD in the mouse IgE locus. The authors have concluded that 47H4 most likely induces apoptosis in mIgE positive B-cells66. Following these studies, an afucosylated humanized version of the antibody, known as quilizumab, has been generated. The removal of fucose from the IgG antibody increases the binding affinity to FcγRIIIa67. This modification enhances the mechanism of antibody-dependent cellular cytotoxicity (ADCC) by NK cells and in the case of quilizumab increased its efficacy to kill mIgE positive B-cells67 (Fig. 3b). In a phase I clinical trial for allergic rhinitis (NCT01160861) and a phase II clinical trial for allergen-induced asthma (NCT01196039) quilizumab treatment reduced both total and allergen-specific IgE levels by 35% and 40%, respectively, and resulted in a slight amelioration of allergen induced asthmatic airway responses68. Surprisingly, these results did not translate into phase II clinical trials with allergic asthma patients (NCT01582503) or CSU patients (NCT01987947), as quilizumab treatment only partially reduced serum IgE levels and did not significantly improve the clinical outcome69,70. The disappointing results of these trials might be due to the low frequency and transient abundance of mIgE positive B-cells71 as well as the growing evidence that most of the secreted IgE derives from IgG producing B-cells undergoing sequential isotype switching to IgE72–77.
3.3. Monoclonal anti-IgE antibody MEDI4212
A monoclonal anti-IgE antibody with the aim of combining two modes-of-action, namely the neutralization of free IgE and the enhanced killing of mIgE positive B-cells via ADCC has been developed by MedImmune. MEDI4212 has been selected using phage display technology and targeted mutagenesis of the VH and VL chains for affinity maturation78. It binds IgE with an affinity of KD ~2 pM and does not interact with FcεRI-bound IgE78. To maximize MEDI4212 mediated induction of ADCC in mIgE positive B-cells, an afucosylated version of the antibody has been produced79 (Fig. 3c). Crystal structure analysis of the MEDI4212 Fab with IgE-Fc3–4 revealed a binding epitope within the Cε3 and Cε4 domains of IgE. It shows a substantial overlap with the FcεRI binding sites on IgE and thus blocks IgE-binding to FcεRI through direct competition. In contrast, MEDI4212 has no obvious overlap with CD23 epitopes on IgE, but still inhibits binding to CD23. The authors concluded that steric or even allosteric inhibition might be involved78. A phase I study with atopic patients (NCT01544348) has been conducted with MEDI4212 in 2013. The results looked promising as MEDI4212 treatment led to more pronounced reduction of free IgE than omalizumab. However, IgE levels returned to baseline faster in the subjects treated with MEDI4212 than with omalizumab. This has most likely been due to the short serum half-life of the afucosylated antibody80. So far, no clinical follow up study has been conducted.
3.4. Monoclonal anti-IgE antibody XmAb7195
The anti-IgE antibody XmAb7195 is a humanized, affinity maturated version of the murine parental antibody of omalizumab (MaE11) with a mutated Fc-part for increased binding to the inhibitory receptor FcγRIIb81,82. It has been developed by Xencor with the aim of neutralizing free serum IgE and suppressing IgE production through aggregation of FcγRIIb and mIgE on B-cells (Fig. 3d). Indeed, XmAb7195 has been shown to reduce free IgE levels and to inhibit the formation of IgE-secreting plasma cells in a severe combined immunodeficiency (SCID) mouse model engrafted with human PBMCs81. The benefit of this dual targeting approach has also been evaluated in a phase Ia clinical trial with healthy volunteers and adults with elevated IgE levels (NCT02148744). A second phase Ib clinical trial (NCT02881853) has been completed in 2017. However, the results of these studies have not been published.
3.5. Omalizumab biosimilars
The patents for omalizumab have recently expired in the US and Europe. Therefore, various companies have started developing biosimilars. Even though the generation of an omalizumab biosimilar might be faster and less cost intense than generating a novel anti-IgE antibody from scratch, this process requires laborious optimization and thorough testing83. To get approval by official authorities biosimilars are expected to feature a similar safety and efficacy profile as the original biological. Typically, at least one phase I and one phase III clinical trial will be required to gather this information. One of the most advanced biosimilars of omalizumab is the monoclonal anti-IgE antibody CMAB00784, which is developed by the National Engineering Research Center of Antibody Medicine of China. Besides minor changes it shares the same amino acid sequence as omalizumab. CMAB007 has completed a phase I clinical trial in China with promising results similar to omalizumab84 and is currently undergoing a multi-center, randomized, double-blind, placebo-controlled phase III clinical trial for patients with allergic asthma in China (NCT03468790). Since Omalizumab has only recently been approved for the treatment of moderate-to-severe asthma in China in 2017, it will be interesting to see what will happen to CMAB007. There are several additional omalizumab biosimilars, which are currently evaluated at different stages of clinical trials (Table 2).
Table 2.
Omalizumab biosimilars in development.
| Anti-IgE name | Format | Indication | Clinical status | Company | Year |
|---|---|---|---|---|---|
| CMAB007 | Antibody | Asthma | phase III | Mabpharm | 2012 |
| STI-004 | Antibody | asthma | phase III | Sorrento Therapeutics | 2015 |
| FB317 | Antibody | Asthma, CIU | phase I | Fountain Biopharma | 2016 |
| GBR 310 | Antibody | Asthma, CSU | phase II | Glenmark | 2018 |
| CTP-39 | Antibody | n.a. | phase I | Celltrion | 2019 |
| BP001 | Antibody | Asthma, CU | phase I | Biosana | 2019 |
4. The future: Anti-IgE biologicals in pre-clinical evaluation
Based on the lessons learned from past anti-IgE approaches various novel strategies are currently evaluated in pre-clinical studies. Some of these next generation anti-IgE variants deviate from the classical monoclonal antibody framework and aim to be either more specific than the classical anti-IgE antibody strategies or to feature multi-functionality. Whether any of the alternative anti-IgE variants will make it into clinics and if they will bring superior patient benefit remains to be seen.
4.1. Anti-IgE designed ankyrin repeat proteins (DARPins)
In 2010, a set of new anti-IgE binders based on designed ankyrin repeat protein (DARPin) scaffolds has been published85. DARPins are genetically engineered alternative scaffold proteins consisting of a repetitive consensus sequence derived from naturally occurring ankyrin repeat proteins86. DARPin libraries with either two or three internal repeats have been generated by randomizing variable positions in the scaffold. For the selection of specific binders, these libraries can be screened using ribosome display87. Due to their simple structure, small size and high solubility, DARPins can be expressed at high quantities in E. coli88. Selected DARPins can be genetically joined by suitable linkers to create multivalent or even multispecific binders89. Multiple binders against human IgE were selected and some of them have been reported to bind to the constant heavy chain of human IgE with high specificity and affinity. DARPin clone E2_79 showed the highest affinity and prominently inhibited IgE-binding to FcεRI. Moreover, it suppressed allergen-mediated degranulation of human FcεRI expressing rat basophilic leukemia cells to the same degree as omalizumab85. In a follow up study, E2_79 has been found to feature a novel mode-of-action, which led to the definition of a new class of anti-IgE molecules, termed “disruptive anti-IgE inhibitors”. Namely, in addition to neutralizing free IgE, E2_79 actively removes IgE that is pre-bound to FcεRI by a facilitated dissociation mechanism90. The disruptive efficacy could be further enhanced by fusing E2_79 to a non-disruptive anti-IgE DARPin E3_53 that recognizes FcεRI-bound IgE and thus serves as an anchoring moiety in the resulting bispecific DARPin bi53_7991 (Fig. 4a). In the meantime, several pre-clinical studies have highlighted the therapeutic potential of DARPin bi53_79. It has been demonstrated to efficiently desensitize human basophils ex vivo, to prevent passive cutaneous anaphylaxis in human FcεRIα transgenic mice in vivo91 and to inhibit mast cell-dependent early airway responses in viable human atopic lung tissue ex vivo92. The possibility to desensitize allergic effector cells by actively removing IgE from the cell surface opens new therapeutic approaches that might impact the development strategies of future anti-IgE drug candidates. Particularly, such an approach might allow acute treatment, which is different to “classical” anti-IgE antibodies that have a rather slow onset of action. Overall, DARPins feature many favorable characteristics including ease of production, high stability and solubility, small molecular size and the possibility to generate multi-specific binders88. On the other hand, they need to be carefully optimized to warrant low immunogenicity and long serum half-life. Phase III trials with other DARPin-based drug candidates are currently ongoing (NCT02462486) and it will be interesting to see when the first DARPin-based biological will be approved for clinical use.
Fig. 4. Modes-of-action of potential future anti-IgE drug candidates.
a) Mechanisms of DARPin bi53_79 mediated inhibition of IgE-binding to FcεRI and CD23, suppression of IgE production and active disruption of pre-formed IgE:FcεRI complexes. b) 8D6 mediated inhibition of IgE-binding to FcεRI, binding of IgE complexes to CD23 and suppression of IgE production. c) GE2 mediated suppression of allergic effector cell activation through co-aggregation of FcεRI with FcγRIIb and suppression of IgE production by co-aggregation of CD23 with FcγRIIb. d) 026 sdab mediated inhibition of IgE-binding to FcεRI and CD23 and active disruption of pre-formed IgE:FcεRI complexes.
4.2. Monoclonal anti-IgE antibody 8D6
In 2012, another monoclonal anti-IgE antibody, called 8D6, which binds to the Cε3 domain of IgE with high affinity (KD ∼ 20 pM) was generated93,94. 8D6 features a special binding characteristic that has not been described before. It inhibits the interaction of IgE with FcεRI but does not interfere with IgE-binding to CD23. Similar to ligelizumab, 8D6 recognizes CD23-IgE complexes on the cell surface. However, unlike ligelizumab pre-formed 8D6:IgE immune complexes still bind to CD2393. Because aggregation of CD23 on B-cells has been claimed to reduce IgE production95,96, it has been speculated that 8D6 could potentially mimic this activity by cross-linking CD23-bound IgE in addition to block sensitization of allergic effector cells93,97. However, this has not been experimentally confirmed so far.
4.3. IgG-Fc-fusion proteins
An alternative strategy to suppress allergic reactions besides interfering with IgE-binding to FcεRI is to leverage the inhibitory function of FcɣRIIb on allergic effector cells98–100. A fusion protein consisting of the human IgG1-Fc3–4 and the human IgE-Fc2–4 parts, known as GE2 (or epsigam), has been generated to aggregate FcεRI with FcγRIIb on allergic effector cells and thereby exploit this mechanism of inhibition101 (Fig. 4c). GE2 has been shown to inhibit IgE-mediated degranulation of sensitized primary human basophils101 and cord blood derived human mast cells99. Further, it suppresses passive cutaneous anaphylaxis in human FcεRIα transgenic mice101, reduces IL-16 production in Langerhans-like dendritic cells (LLDCs)102, decreases isotype switching of peripheral human B-cells to IgE103, inhibits dust mite allergen-induced allergic skin reaction in rhesus monkeys dose-dependently104 and showed positive results in a non-human primate model of allergic asthma with cynomolgus monkeys105. Unfortunately, repeated injections of GE2 led to the production of anti-drug antibodies resulting in acute anaphylaxis-like reactions in monkeys105. Multiple modified versions of GE2 failed to improve efficacy to inhibit allergic effector cell activation or its safety profile106. Nevertheless, GE2 has been patented by Tunitas Therapeutics in 2015. It will be interesting to see, whether GE2 will be further developed.
A fusion protein (DE53-Fc) consisting of an IgG1-Fc and the anti-IgE anchor DARPin E3_53 has also been reported to inhibit allergic effector cells by co-engagement of FcεRI and FcγRIIb on allergic effector cells107. Compared to GE2, DE53-Fc recognizes FcεRI-bound IgE in a non-competitive manner. Moreover, a mutated version of DE53-Fc (DE53-Fc mut+) with increased affinity for FcγRIIb showed even higher efficacy to inhibit basophil activation108.
4.4. Anti-IgE nanobodies
Another alternative scaffold, known as nanobody or single domain antibody (sdab), has been used to generate specific binders against IgE. These antibody fragments are derived from camelids (e.g. camels, dromedaries and llamas) or cartilaginous fishes (e.g. nurse shark and wobbegong), which produce functional antibodies devoid of light chains109. Using phage display, nanobody libraries have been screened on cynomolgus IgE and selected clones tested for cross-reactivity to human IgE as well as for their efficacy to inhibit IgE-binding to FcεRI. 39D11 is one of the identified anti-IgE clones, which has undergone additional rounds of affinity maturation and sequence optimization (humanization)110. The resulting anti-IgE nanobody IgE026 or 026 sdab is patented by Ablynx. An independent study has shown that 026 sdab binds across the Cε3 and Cε4 domain of human IgE overlapping with the CD23 binding epitope and thus inhibiting IgE-CD23 interactions by steric hindrance111. On the other hand, there is virtually no overlap with the FcεRI binding epitope on IgE. Nevertheless, 026 sdab still inhibits the interaction of IgE with FcεRI, which has been explained by conformational hindrance111, as the IgE-Fc adopts a closed conformation in complex with 026 sdab, which is incompatible with FcεRI binding. Consequently, 026 sdab also inhibits mediator release of human FcεRI expressing RBL-SX38 cells111. 026 sdab belongs to the novel class of disruptive IgE inhibitors, as it has been shown to displace IgE from FcεRI111 (Fig. 4d). Nanobodies share many common features with DARPins such as small size, high stability and solubility as well as the possibility to generate multi-specific binders112. However, due to the lack of the Fc-part they also require optimization of pharmacokinetics109.
5. Conclusions
The classical anti-IgE approach using intact humanized monoclonal antibodies for the neutralization of IgE has led to the generation of potent IgE inhibitors such as omalizumab. Despite the successful use of the current anti-IgE therapy for the treatment of severe allergic asthma and CSU there is certainly still a lot of room for improvement. It seems that an important aspect in the development of monoclonal anti-IgE biologicals has been underestimated so far. Namely, that anti-IgE antibodies often feature fundamentally distinct receptor inhibition profiles depending on their particular binding epitopes, steric effects and the ability to induce conformational changes in IgE upon binding. Obviously, their modes-of-action will heavily depend on these features. It seems that too much emphasis has been assigned to affinity optimization of anti-IgE biologicals in the past. Careful assessment of the efficacy to inhibit IgE-binding to CD23 or FcεRI and the generation of structural information is key to identify their potential modes-of-action. This knowledge will facilitate determination of suitable therapeutic indications and ultimately increase chances of success in clinical trials. IgE-driven conditions, in which CD23-mediated processes are known to play an important role (e.g. allergic asthma113,114) should therefore be primarily treated with anti-IgE biologicals that provide strong inhibition of CD23:IgE complex formation, as shown in a model of pulmonary inflammation quite some time ago64. On the other hand, IgE-dependent conditions like CSU115,116 or food allergies117 that heavily rely on FcεRI-mediated processes require treatments with compounds that feature the corresponding inhibition profile. As already mentioned earlier in this review, the specific receptor inhibition profile could be an important reason why ligelizumab treatment did not show superior efficacy compared to omalizumab treatment in patients with allergic asthma (NCT02075008), however was well superior for the treatment of CSU patients63. While the line between FcεRI- and CD23-mediated pathogenesis cannot always be drawn strictly, this concept should still be kept in mind for the development of future anti-IgE drug candidates.
Another aspect that is likely to impact the generation of new anti-IgE biologicals is the discovery of disruptive IgE inhibitors91,111. The possibility to actively desensitize allergic effector cells might potentially shorten the onset of action and the degree of treatment benefit. Such disruptive anti-IgE inhibitors might also be of great value for rapid desensitization prior to or during allergen-specific immunotherapy (AIT). Combinatorial treatment with a disruptive IgE inhibitor could potentially enhance the outcome, reduce adverse reactions and increase the overall safety of AIT.
Taken together, we suggest that future anti-IgE biologicals should aim at targeting the involved pathomechanisms at multiple levels to get maximal treatment efficacy22. An ideal IgE inhibitor should therefore in addition to neutralizing free IgE, actively disrupt IgE:FcεRI complexes on allergic effector cells and suppress IgE production in B-cells through engagement of either mIgE, CD23-bound IgE or inhibitory co-receptors. First steps into multi-level targeting have been taken with the development of XmAb719581 or MEDI141278. However, as clinical trials have demonstrated, there is still much room for improvement of such approaches. While recent studies clearly suggest that more tailored anti-IgE approaches for different indications are required, the question whether a universal anti-IgE biological that shows high treatment efficacy in a multitude of allergic disorders could be developed by broadening the activity profile and following a multi-level IgE targeting strategy remains to be investigated.
Box 1. Major milestone discoveries.
Discovery of mast cells in 1878 and basophils in 18791,2
Discovery of anaphylaxis in 19023
Introduction of the allergy concept in 19064
Discovery of the “atopic reagin” in serum transfer experiments in 19235
Introduction of the atopy theory in 19236
Classification of hypersensitivity reactions in 19637
First anti-IgE concept 1986/87 with development of antibody prototype in 199010,11
Market approval of first anti-IgE antibody omalizumab in 200312
Box 2. Future research perspectives.
Alternative binding scaffolds instead of monoclonal anti-IgE antibodies
Disruptive IgE inhibitors for active desensitization
Active suppression of allergic effector cells by targeting inhibitory receptors
Multi-level targeting approaches to optimize treatment efficacy
Acknowledgments:
We would like to thank Dr. Daniel Brigger, Mr. Pascal Gasser and Dr. Christoph Heusser for vivid scientific discussions and proofreading of the manuscript. Dr. Guntern has nothing to disclose. Dr. Eggel reports grants and personal fees from Novartis Pharma AG, outside the submitted work.
Funding information: Research Fund of the Swiss Lung Association, Bern and the Uniscientia foundation; NIH grant HL141493.
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
References
- 1.Ehrlich P Beiträge für Theorie und Praxis der histologischen Färbung. (1878). [Google Scholar]
- 2.EHRLICH & P. Beitrage zur Kenntnis der granulierten. Bindegewebszellen und der eosinophilen Leukozyten. Arch Anat Physiol 3, 166–169 (1879). [Google Scholar]
- 3.PORTIER & P. De l’action anaphylactique de certains venins. C R Soc Biol 54, 170 (1902). [Google Scholar]
- 4.WAGNER R CLEMENS VON PIRQUET, DISCOVERER OF THE CONCEPT OF ALLERGY. Bull. N. Y. Acad. Med. 40, 229–235 (1964). [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen SG & Zelaya-Quesada M Prausnitz and Küstner phenomenon: the P-K reaction. J. Allergy Clin. Immunol. 114, 705–10 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Coca AF & Cooke RA On the Classification of the Phenomena of Hypersensitiveness. J. Immunol. 8, 163 LP – 182 (1923). [Google Scholar]
- 7.Gell PGH & Coombs R Clinical aspects of immunology,. (Blackwell Scientific, 1963). [Google Scholar]
- 8.Johansson SG, Bennich H & Wide L A new class of immunoglobulin in human serum. Immunology 14, 265–72 (1968). [PMC free article] [PubMed] [Google Scholar]
- 9.Ishizaka K & Ishizaka T Identification of gamma-E-antibodies as a carrier of reaginic activity. J. Immunol. 99, 1187–98 (1967). [PubMed] [Google Scholar]
- 10.Chang TW et al. Monoclonal Antibodies Specific for Human IgE-Producing B Cells: A Potential Therapeutic for IgE-Mediated Allergic Diseases. Nat. Biotechnol. 8, 122–126 (1990). [DOI] [PubMed] [Google Scholar]
- 11.Heusser CH et al. New Concepts of IgE Regulation. Int. Arch. Allergy Immunol. 94, 87–90 (1991). [DOI] [PubMed] [Google Scholar]
- 12.G L & Drug L,W Approval Highlights for 2003. Nurse Pract. 29, (2004). [DOI] [PubMed] [Google Scholar]
- 13.Gould HJ & Sutton BJ IgE in allergy and asthma today. 8, (2008). [DOI] [PubMed] [Google Scholar]
- 14.Galli SJ, Tsai M & Piliponsky AM The development of allergic inflammation. Nature 454, 445 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutton BJ, Davies AM, Bax HJ & Karagiannis SN IgE Antibodies: From Structure to Function and Clinical Translation. Antibodies (Basel, Switzerland) 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wurzburg BA, Garman SC & Jardetzky TS Structure of the Human IgE-Fc Cε3-Cε4 Reveals Conformational Flexibility in the Antibody Effector Domains. Immunity 13, 375–385 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Dhaliwal B et al. IgE binds asymmetrically to its B cell receptor CD23. Sci. Rep. 7, 1–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhaliwal B et al. Crystal structure of IgE bound to its B-cell receptor CD23 reveals a mechanism of reciprocal allosteric inhibition with high affinity receptor FcεRI. Proc. Natl. Acad. Sci. U. S. A. 109, 12686–91 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bestagno M et al. Membrane immunoglobulins are stabilized by interchain disulfide bonds occurring within the extracellular membrane-proximal domain. Biochemistry 40, 10686–92 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Venkitaraman AR, Williams GT, Dariavach P & Neuberger MS The B-cell antigen receptor of the five immunoglobulin classes. Nature 352, 777–81 (1991). [DOI] [PubMed] [Google Scholar]
- 21.Batista FD, Anand S, Presani G, Efremov DG & Burrone OR The two membrane isoforms of human IgE assemble into functionally distinct B cell antigen receptors. J. Exp. Med. 184, 2197–205 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasser P & Eggel A Targeting IgE in allergic disease. Curr. Opin. Immunol. 54, 86–92 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Balbino B, Conde E, Marichal T, Starkl P & Reber LL Approaches to target IgE antibodies in allergic diseases. Pharmacol. Ther. 191, 50–64 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Heusser C Immunoregulation in allergy: the potential of anti-IgE antibodies of IL-4 antagonists for the treatment of allergic diseases. Arb. Paul. Ehrlich. Inst. Bundesamt. Sera Impfstoffe. Frankf. A. M. 283–9; discussion 289–91 (1994). [PubMed] [Google Scholar]
- 25.Heusser CH et al. Demonstration of the therapeutic potential of non-anaphylactogenic anti-ige antibodies in murine models of skin reaction, lung function and inflammation. Int. Arch. Allergy Immunol. 113, 231–235 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Davis FM et al. Can anti-IgE be used to treat allergy? Springer Semin. Immunopathol. 15, 51–73 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Corne J et al. The effect of intravenous administration of a chimeric anti-IgE antibody on serum IgE levels in atopic subjects: efficacy, safety, and pharmacokinetics. J. Clin. Invest. 99, 879–87 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Racine-Poon A et al. Efficacy, pharmacodynamics, and pharmacokinetics of CGP 51901, an anti-immunoglobulin E chimeric monoclonal antibody, in patients with seasonal allergic rhinitis. Clin. Pharmacol. Ther. 62, 675–90 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Kolbinger F, Saldanha J, Hardman N & Bendig MM Humanization of a mouse anti-human IgE antibody: a potential therapeutic for IgE-mediated allergies. “Protein Eng. Des. Sel 6, 971–980 (1993). [DOI] [PubMed] [Google Scholar]
- 30.Presta LG et al. Humanization of an antibody directed against IgE. J. Immunol. 151, 2623–32 (1993). [PubMed] [Google Scholar]
- 31.Heusser C & Jardieu P Therapeutic potential of anti-IgE antibodies. Curr. Opin. Immunol. 9, 805–813 (1997). [DOI] [PubMed] [Google Scholar]
- 32.MacGinnitie AJ et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J. Allergy Clin. Immunol. 139, 873–881.e8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dantzer JA & Wood RA The use of omalizumab in allergen immunotherapy. Clin. Exp. Allergy 48, 232–240 (2018). [DOI] [PubMed] [Google Scholar]
- 34.El-Qutob D Off-Label Uses of Omalizumab. Clin. Rev. Allergy Immunol. 50, 84–96 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Normansell R, Walker S, Milan SJ, Walters EH & Nair P Omalizumab for asthma in adults and children. Cochrane Database Syst. Rev. (2014). doi: 10.1002/14651858.CD003559.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alhossan A, Lee CS, MacDonald K & Abraham I “Real-life” Effectiveness Studies of Omalizumab in Adult Patients with Severe Allergic Asthma: Meta-analysis. J. Allergy Clin. Immunol. Pract. 5, 1362–1370.e2 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Lai T et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci. Rep. 5, 8191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubini NPM, Ensina LFC, Silva EMK, Sano F & Solé D Effectiveness and safety of Omalizumab in the treatment of chronic spontaneous urticaria: Systematic review and meta-analysis. Allergol. Immunopathol. (Madr). 47, 515–522 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Zhao Z-T et al. Omalizumab for the treatment of chronic spontaneous urticaria: A meta-analysis of randomized clinical trials. J. Allergy Clin. Immunol. 137, 1742–1750.e4 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Bestagno M et al. Membrane immunoglobulins are stabilized by interchain disulfide bonds occurring within the extracellular membrane-proximal domain. Biochemistry 40, 10686–92 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Humbert M et al. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur. Respir. J. 51, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chapman KR et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy 74, 1716–1726 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arm JP et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin. Exp. Allergy 44, 1371–85 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pennington LF et al. Structural basis of omalizumab therapy and omalizumab-mediated IgE exchange. Nat. Commun. 7, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gasser P et al. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat. Commun. 2020 111 11, 1–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Limb SL, Starke PR, Lee CE & Chowdhury BA Delayed onset and protracted progression of anaphylaxis after omalizumab administration in patients with asthma. J. Allergy Clin. Immunol. 120, 1378–1381 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Lieberman PL, Jones I, Rajwanshi R, Rosén K & Umetsu DT Anaphylaxis associated with omalizumab administration: Risk factors and patient characteristics. J. Allergy Clin. Immunol. 140, 1734–1736.e4 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Lieberman PL, Umetsu DT, Carrigan GJ & Rahmaoui A Anaphylactic reactions associated with omalizumab administration: Analysis of a case-control study. J. Allergy Clin. Immunol. 138, 913–915.e2 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Balbino B et al. The anti-IgE mAb Omalizumab induces adverse reactions by engaging Fcγ receptors. J. Clin. Invest. (2019). doi: 10.1172/JCI129697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacGlashan DW et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J. Immunol. 158, 1438–45 (1997). [PubMed] [Google Scholar]
- 51.MacGlashan DW et al. Serum ige level drives basophil and mast cell ige receptor display. Int. Arch. Allergy Immunol. 113, 45–47 (1997). [DOI] [PubMed] [Google Scholar]
- 52.Holgate S et al. The use of omalizumab in the treatment of severe allergic asthma: A clinical experience update. Respir. Med. 103, 1098–1113 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Lin H et al. Omalizumab rapidly decreases nasal allergic response and FcεRI on basophils☆. J. Allergy Clin. Immunol. 113, 297–302 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Hobi G, Kim B & Buschor P Accelerated dissociation of IgE-Fc ε RI complexes by disruptive inhibitors actively desensitizes allergic effector cells. 19–21 doi: 10.1016/j.jaci.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies AM et al. Allosteric mechanism of action of the therapeutic anti-IgE antibody omalizumab. J. Biol. Chem. 292, 9975–9987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan MA, Gigliotti NM, Dotson AL & Rosenwasser LJ Omalizumab may decrease IgE synthesis by targeting membrane IgE+ human B cells. Clin. Transl. Allergy 3, 29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lowe PJ & Renard D Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br. J. Clin. Pharmacol. 72, 306–20 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanf G et al. Omalizumab decreased IgE-release and induced changes in cellular immunity in patients with allergic asthma. Allergy 61, 1141–4 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Djukanović R et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am. J. Respir. Crit. Care Med. 170, 583–93 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Plewako H et al. The effect of omalizumab on nasal allergic inflammation. J. Allergy Clin. Immunol. 110, 68–71 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Leung DYM et al. Effect of Anti-IgE Therapy in Patients with Peanut Allergy. N. Engl. J. Med. 348, 986–993 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Leung DYM, Shanahan WR, Li X-M & Sampson HA New approaches for the treatment of anaphylaxis. Novartis Found. Symp. 257, 248–60; discussion 260–4, 276–85 (2004). [PubMed] [Google Scholar]
- 63.Maurer M et al. Ligelizumab for Chronic Spontaneous Urticaria. N. Engl. J. Med. 381, 1321–1332 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Coyle AJ et al. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production: inhibition by a non-anaphylactogenic anti-IgE antibody. J. Exp. Med. 183, 1303–10 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J-B et al. Unique epitopes on C epsilon mX in IgE-B cell receptors are potentially applicable for targeting IgE-committed B cells. J. Immunol. 184, 1748–56 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Brightbill HD et al. Antibodies specific for a segment of human membrane IgE deplete IgE-producing B cells in humanized mice. J. Clin. Invest. 120, 2218–29 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brightbill H et al. Quilizumab is an Afucosylated Humanized Anti-M1 Prime Therapeutic Antibody. Clin. Anti-inflamm. Anti-Allergy Drugs 1, 24–31 (2014). [Google Scholar]
- 68.Gauvreau GM et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. 6, 1–11 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Harris JM et al. A randomized trial of the efficacy and safety of quilizumab in adults with inadequately controlled allergic asthma. Respir. Res. 17, 29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harris JM et al. A randomized trial of quilizumab in adults with refractory chronic spontaneous urticaria. J. Allergy Clin. Immunol. 138, 1730–1732 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Laffleur B et al. Self-Restrained B Cells Arise following Membrane IgE Expression. Cell Rep. 10, 900–909 (2015). [DOI] [PubMed] [Google Scholar]
- 72.Calkhoven PG et al. Relationship between IgG1 and IgG4 antibodies to foods and the development of IgE antibodies to inhalant allergens. II. Increased levels of IgG antibodies to foods in children who subsequently develop IgE antibodies to inhalant allergens. Clin. Exp. Allergy 21, 99–107 (1991). [DOI] [PubMed] [Google Scholar]
- 73.Jenmalm MC & Björkstén B Development of immunoglobulin G subclass antibodies to ovalbumin, birch and cat during the first eight years of life in atopic and non-atopic children. Pediatr. Allergy Immunol. 10, 112–21 (1999). [DOI] [PubMed] [Google Scholar]
- 74.Schwarz A et al. IgG and IgG4 to 91 allergenic molecules in early childhood by route of exposure and current and future IgE sensitization: Results from the Multicentre Allergy Study birth cohort. J. Allergy Clin. Immunol. 138, 1426–1433.e12 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Aalberse RC et al. sIgE and sIgG to airborne atopic allergens: Coupled rather than inversely related responses. Allergy 73, 2239–2242 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Saunders SP, Ma EGM, Aranda CJ & Curotto de Lafaille MA Non-classical B Cell Memory of Allergic IgE Responses. Front. Immunol. 10, 715 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiménez-Saiz R et al. Human BCR analysis of single-sorted, putative IgE+ memory B cells in food allergy. J. Allergy Clin. Immunol. 144, 336–339.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen ES et al. A novel IgE-neutralizing antibody for the treatment of severe uncontrolled asthma. MAbs 6, 755–763 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nyborg AC et al. Development of an antibody that neutralizes soluble IgE and eliminates IgE expressing B cells. Cell. Mol. Immunol. 13, 391–400 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheldon E et al. Pharmacokinetics, Pharmacodynamics, and Safety of MEDI4212, an Anti-IgE Monoclonal Antibody, in Subjects with Atopy: A Phase I Study. Adv. Ther. 33, 225–51 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Chu SY et al. Reduction of total IgE by targeted coengagement of IgE B-cell receptor and FcγRIIb with Fc-engineered antibody. J. Allergy Clin. Immunol. 129, 1102–1115 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Chu SY et al. Inhibition of B cell receptor-mediated activation of primary human B cells by coengagement of CD19 and FcγRIIb with Fc-engineered antibodies. Mol. Immunol. 45, 3926–3933 (2008). [DOI] [PubMed] [Google Scholar]
- 83.Ferrando M, Bagnasco D, Braido F, Varricchi G & Canonica GW Biosimilars in allergic diseases. Curr. Opin. Allergy Clin. Immunol. 16, 68–73 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Zhou B et al. Tolerability, pharmacokinetics and pharmacodynamics of CMAB007, a humanized anti-immunoglobulin E monoclonal antibody, in healthy Chinese subjects. MAbs 4, 110–119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baumann MJ, Eggel A, Amstutz P, Stadler BM & Vogel M DARPins against a functional IgE epitope. Immunol. Lett. 133, 78–84 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Forrer P, Stumpp MT, Binz HK & Plückthun A A novel strategy to design binding molecules harnessing the modular nature of repeat proteins. FEBS Lett. 539, 2–6 (2003). [DOI] [PubMed] [Google Scholar]
- 87.Binz HK et al. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat. Biotechnol. 22, 575–582 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Binz HK, Stumpp MT, Forrer P, Amstutz P & Plückthun A Designing repeat proteins: well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J. Mol. Biol. 332, 489–503 (2003). [DOI] [PubMed] [Google Scholar]
- 89.Eggel A, Baumann MJ, Amstutz P, Stadler BM & Vogel M DARPins as Bispecific Receptor Antagonists Analyzed for Immunoglobulin E Receptor Blockage. J. Mol. Biol. 393, 598–607 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Kim B et al. Accelerated disassembly of IgE–receptor complexes by a disruptive macromolecular inhibitor. Nature 491, 613–617 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eggel A et al. Accelerated dissociation of IgE-FcεRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. J. Allergy Clin. Immunol. 133, 1709–19.e8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Delgado SJ et al. Disruptive anti-IgE inhibitors prevent mast cell-dependent early airway response in viable atopic lung tissue. J. Allergy Clin. Immunol. (2019). doi: 10.1016/j.jaci.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 93.Shiung Y-Y et al. An anti-IgE monoclonal antibody that binds to IgE on CD23 but not on high-affinity IgE.Fc receptors. Immunobiology 217, 676–683 (2012). [DOI] [PubMed] [Google Scholar]
- 94.Chen JB et al. Structural basis for selective inhibition of immunoglobulin E-receptor interactions by an anti-IgE antibody. Sci. Rep. 8, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakamura T et al. In vitro IgE inhibition in B cells by anti-CD23 monoclonal antibodies is functionally dependent on the immunoglobulin Fc domain. Int. J. Immunopharmacol. 22, 131–41 (2000). [DOI] [PubMed] [Google Scholar]
- 96.Fellmann M, Buschor P, Röthlisberger S, Zellweger F & Vogel M High affinity targeting of CD23 inhibits IgE synthesis in human B cells. Immunity, Inflamm. Dis 3, 339–49 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sutton BJ & Davies AM Structure and dynamics of IgE-receptor interactions: FcεRI and CD23/FcεRII. Immunol. Rev. 268, 222–235 (2015). [DOI] [PubMed] [Google Scholar]
- 98.Ono M, Bolland S, Tempst P & Ravetch JV Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor FeγRIIB. Nature 383, 263–266 (1996). [DOI] [PubMed] [Google Scholar]
- 99.Kepley CL et al. Co-aggregation of FcgammaRII with FcepsilonRI on human mast cells inhibits antigen-induced secretion and involves SHIP-Grb2-Dok complexes. J. Biol. Chem. 279, 35139–49 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Cassard L, Jönsson F, Arnaud S & Daëron M Fcγ receptors inhibit mouse and human basophil activation. J. Immunol. 189, 2995–3006 (2012). [DOI] [PubMed] [Google Scholar]
- 101.Zhu D, Kepley CL, Zhang M, Zhang K & Saxon A A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat. Med. 8, 518–21 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kepley CL, Zhang K, Zhu D & Saxon A FcepsilonRI-FcgammaRII coaggregation inhibits IL-16 production from human Langerhans-like dendritic cells. Clin. Immunol. 108, 89–94 (2003). [DOI] [PubMed] [Google Scholar]
- 103.Yamada T, Zhu D, Zhang K & Saxon A Inhibition of interleukin-4-induced class switch recombination by a human immunoglobulin Fc gamma-Fc epsilon chimeric protein. J. Biol. Chem. 278, 32818–24 (2003). [DOI] [PubMed] [Google Scholar]
- 104.Zhang K et al. Inhibition of allergen-specific IgE reactivity by a human Ig Fcγ-Fcε bifunctional fusion protein. J. Allergy Clin. Immunol. 114, 321–327 (2004). [DOI] [PubMed] [Google Scholar]
- 105.Van Scott MR et al. Systemic administration of an Fcγ–Fcε-fusion protein in house dust mite sensitive nonhuman primates. Clin. Immunol. 128, 340–348 (2008). [DOI] [PubMed] [Google Scholar]
- 106.Allen LC, Kepley CL, Saxon A & Zhang K Modifications to an Fcγ-Fcɛ fusion protein alter its effectiveness in the inhibition of FcɛRI-mediated functions. J. Allergy Clin. Immunol. 120, 462–468 (2007). [DOI] [PubMed] [Google Scholar]
- 107.Eggel A et al. Inhibition of ongoing allergic reactions using a novel anti-IgE DARPin-Fc fusion protein. Allergy 66, 961–968 (2011). [DOI] [PubMed] [Google Scholar]
- 108.Buschor P, Eggel A, Zellweger F, Stadler BM & Vogel M Improved FcγRIIb Targeting Functionally Translates into Enhanced Inhibition of Basophil Activation. Int. Arch. Allergy Immunol. 163, 206–214 (2014). [DOI] [PubMed] [Google Scholar]
- 109.Harmsen MM & De Haard HJ Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 77, 13–22 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.ABLYNX NV Wo 2012/175740 A1 Immunoglobulin Single Variable Domains Directed Against IgE. (2012). [Google Scholar]
- 111.Jabs F et al. Trapping IgE in a closed conformation by mimicking CD23 binding prevents and disrupts FcϵRI interaction. Nat. Commun. 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van der Linden RH et al. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim. Biophys. Acta 1431, 37–46 (1999). [DOI] [PubMed] [Google Scholar]
- 113.Palaniyandi S, Tomei E, Li Z, Conrad DH & Zhu X CD23-Dependent Transcytosis of IgE and Immune Complex across the Polarized Human Respiratory Epithelial Cells. J. Immunol. 186, 3484–3496 (2011). [DOI] [PubMed] [Google Scholar]
- 114.Palaniyandi S et al. Inhibition of CD23-mediated IgE transcytosis suppresses the initiation and development of allergic airway inflammation. Mucosal Immunol. 8, 1262–1274 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaplan AP, Giménez-Arnau AM & Saini SS Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy 72, 519–533 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chang TW et al. The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. J. Allergy Clin. Immunol. 135, 337–42 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Yu W, Freeland DMH & Nadeau KC Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 16, 751–765 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wan M, Shiau FY, Gordon W & Wang GY Variant antibody identification by peptide mapping. Biotechnol. Bioeng. 62, 485–8 (1999). [DOI] [PubMed] [Google Scholar]