Abstract
Background
Although short-term outcomes for liver transplantation have improved, patient and graft survivals are limited by infection, cancer and other complications of immunosuppression. Rapid induction of tolerance after liver transplantation would decrease these complications, improving survival and quality of life. Tolerance to kidneys, but not thoracic organs or islets, has been achieved in nonhuman primates and humans through the induction of transient donor chimerism. Since the liver is considered to be tolerogenic, we tested the hypothesis that the renal transplant transient chimerism protocol would induce liver tolerance.
Methods
Seven cynomolgus macaques received immune conditioning followed by simultaneous donor bone marrow and liver transplantation. The more extensive liver surgery required minor adaptations of the kidney protocol to decrease complications. All immunosuppression was discontinued on POD 28. Peripheral blood chimerism, recipient immune reconstitution, liver function tests and graft survival were determined.
Results
The level and duration of chimerism in liver recipients was comparable to that previously reported in renal transplant recipients. However, unlike in the kidney model, the liver was rejected soon after immunosuppression withdrawal. Rejection was associated with proliferation of recipient CD8 T effector cells in the periphery and liver, increased serum IL-6 and IL-2, but peripheral Treg numbers did not increase. Anti-donor antibody was also detected.
Conclusions
These data show the transient chimerism protocol does not induce tolerance to livers, likely due to greater CD8 T cell responses than in the kidney model. Successful tolerance induction may depend on greater control or deletion of CD8 T cells in this model.
Introduction
Advances in surgical technique and immunosuppression have led to great improvements in short-term liver allograft survival.1,2 However, long-term sequelae of immunosuppression –including infection, graft failure and malignancy – are still the leading causes of patient death.2–5 Side effects of immunosuppression such as diabetes, hypertension, hypercholesterolemia and renal failure also decrease quality of life.3–6 The induction of tolerance early after liver transplantation (LT) would therefore be expected to improve outcomes.
Induction of mixed hematopoietic chimerism (MC) at the time of solid organ transplant consistently induces tolerance in rodents, nonhuman primates (NHP) and humans.7 In cynomolgus monkeys, simultaneous kidney and bone marrow transplantation (SKBMT) with a nonmyeloablative conditioning regimen resulted in transient MC and tolerance across MHC barriers in up to 70% of recipients.8–10 This strategy was translated to humans, where immunosuppression was discontinued successfully in 70% of SKBMT recipients.11,12 Studies in primates and humans have shown that tolerance is mediated by regulatory T cells (Tregs) in the early post-transplant period, with deletion of donor-reactive T cell clones observed later after immunosuppression withdrawal.13–15 On the other hand, rejection was associated with high numbers of pre-transplant donor-reactive memory T cells in the NHP model.16 This suggests a complex interaction between Tregs, clonal deletion and pre-existing alloreactivity determines whether tolerance is induced after transient donor chimerism.
In NHPs, the nonmyeloablative conditioning regimen does not induce tolerance to organs considered to be less tolerogenic than the kidney (e.g. islets and heart).17,18 Thus, the organ plays a critical role in the induction of tolerance with this protocol. In some animal models the liver is the most tolerogenic organ. For example, in pigs and rodents, a liver can be accepted in the absence of immunosuppression19,20 and can reverse donor-specific sensitization.21 Therefore, we hypothesized that the liver could facilitate the induction of tolerance with a nonmyeloablative transient MC protocol. Here, we report the results this strategy with LT in a preclinical cynomolgus monkey model.22 We demonstrate that despite developing donor chimerism at similar levels and duration as in the SKBMT model, liver recipients rapidly rejected their graft following withdrawal of immunosuppression. This rejection was characterized by proliferation of CD8 effector T cells in the periphery and the graft, suggesting that, with the transient MC protocol, these cells prevent tolerance induction early after LT.
Materials and Methods
Animals
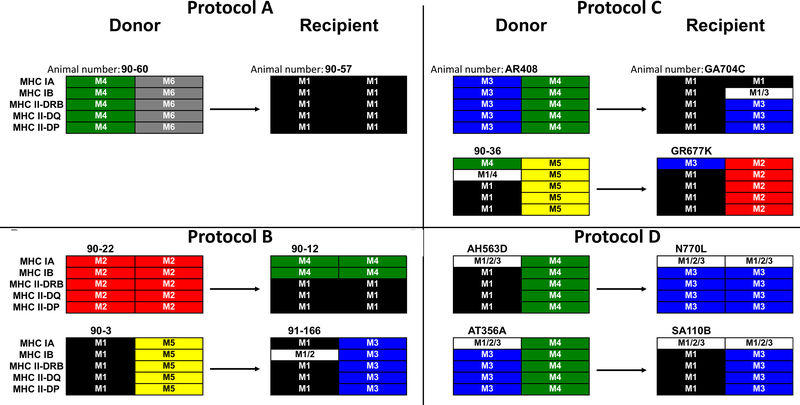
Fourteen cynomolgus macaques (7 donor-recipient pairs) weighing 6.1–10.2 kg were used (Charles River Primates, Wilmington, MA). Recipients and donors were selected for ABO compatibility and mismatching of MHC (Figure 1) as determined by the Genetic Services Unit of the Wisconsin National Primate Research Center at the University of Wisconsin-Madison (see supplemental details).23 Donors and recipients had differential binding of anti-Bw6 antibody (H38, One Lambda Canoga Park, CA) to peripheral blood mononuclear cells to allow tracking of chimerism. All macaques were housed at the Institute of Comparative Medicine (Columbia University Medical Center, New York, NY). This facility holds a current OLAW Assurance, USDA registration and is AAALAC accredited. All surgical and experimental procedures were performed in accordance with NIH guidelines for the care and use of primates and approved by the Columbia University IACUC.
Figure 1:
MHC of Animal Pairs by Regimen. Shown are MHC class I-A, I-B, II-DRB, II-DQ and II-DP for each haplotype of the donors (left side) and recipients (right side). All donor-recipient pairs were at least a full haplotype mismatch in both the host-to-graft and graft-to-host directions.
Recipient Conditioning
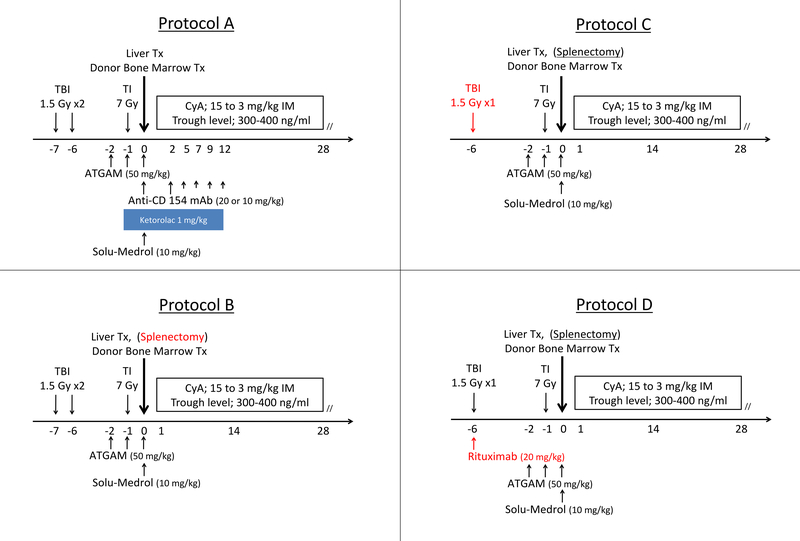
Recipients were conditioned with a nonmyeloablative regimen based on the SKBMT protocols that induced tolerance to renal transplants (Table 1, Figure 2).8,9 All animals received total body irradiation (TBI, 300–150 cGy), thymic irradiation (700 cGy), T-cell depletion with ATGAM (50 mg/kg on Day −2, −1, and 0) and a 28-day course of IM Cyclosporine (CyA, Novartis, East Hanover, NJ). In Group A, we administered anti-CD154 mAb to block T-cell co-stimulation. In Groups B-D, we performed splenectomy instead of co-stimulatory blockade as either treatment can induce tolerance to kidneys.8,9 In Groups C and D, we decreased the dose of TBI to accelerate recovery from the more extensive liver transplant procedure. In Group D, we added rituximab in an attempt at preventing the development of anti-donor antibodies.
Table 1:
Immune Conditioning Regimens. All animals received 7 Gy of Thymic Irradiation on Day −1; 50 mg/kg of ATGAM on Days −2, −1 and 0; and 28-days of Cyclosporine A with a target trough level of 300–400 ng/mL. Total Body Irradiation of 1.5 Gy per dose was given on POD −7 and −6 if x 2, or only on POD −6 if x 1
| Protocol Group | Recipient Animals | Total Body Irradiation (Gy) | Thymic Irradiation (Gy) | T-Cell Depletion (ATGAM) | Cyclosporine A | Anti-CD154 mAh or Splenectomy | Rituximab |
|---|---|---|---|---|---|---|---|
| A | 90–57 | 1.5 × 2 | 7 | 50 mg/kg Day −2, −1, 0 |
Days 0–28 | Anti-CD154 mAb | No |
| B | 90–12 90–166 |
1.5 × 2 | 7 | 50 mg/kg Day −2, −1, 0 |
Days 0–28 | Splenectomy | No |
| C | GA704C GR677K |
1.5 × 1 | 7 | 50 mg/kg Day −2, −1, 0 |
Days 0–28 | Splenectomy | No |
| D | N770L SA110B |
1.5 × 1 | 7 | 50 mg/kg Day −2, −1, 0 |
Days 0–28 | Splenectomy | 20 mg/kg Day −6 |
Figure 2:
Schema for the induction protocols for Groups A – D. CyA: Cyclosporine A. Red text indicates changes from the previous protocol.
Donor Hepatectomy and Bone Marrow Procurement
The donor liver was perfused with 500ml of CoStorSol (Preservation Solutions Inc, Elkhorn, WI) via aortic and portal cannulas. After hepatectomy and euthanasia, bone marrow was harvested from the vertebral bodies, minced to 3 mm fragments and incubated in RPMI (Life Technologies Carlsbad, CA) with 1% DNAse (Sigma-Aldrich, St. Louis, MO) and 100 U/mL Pen/Strep (Life Technologies Carlsbad, CA) for one hour at room temperature on a rotary shaker. Fragments were removed through a 70-μm filter. Marrow was washed and red cells lysed with Ammonium-Chloride-Potassium solution. The cells were counted and their number adjusted so that 3×108 nucleated cells/kg were resuspended in normal saline with 10% CP2D (Haemoneitcs, Braintree, MA).24,25
Combined Liver-Bone Marrow Transplantation (CLBMT) and Splenectomy
Details of the LT procedure were reported previously.22 In Groups B-D, splenectomy was performed before hepatic dissection. 3×108 nucleated cells/kg of donor bone marrow were infused intravenously through a blood transfusion filter set after completion of surgery.
Flow Cytometric Analysis and Detection of Chimerism
Peripheral blood cells were stained with the following mAbs two times a week after transplantation: CD56 PE (B159), CD95 PE (DX2), HLA-I PE (DX17), CD3 PerCP (SP34–2), CD4 BV510 (L200), CD4 APC (L200) (BD Biosciences, San Jose, CA); CD8 APC (BW135.80), CD20 APC-Vio770 (LT20), CD25 Pacific Blue (BC96) (BioLegend), CD45RA APC-Vio770 (T6D11), CD11b Viogreen (M1/70.15.11.5), FOXP3 (236A/E7) (Invitrogen), HLA-I PE-Vio770 (REA230) (Miltenyi Biotec Inc. San Diego, CA); CD28 BV421 (CD28.2) and CD8 BV421 (RPA-T8), (BioLegend Inc. San Diego, CA). Anti-Bw6 FITC (H38, One Lambda, Canoga Park, CA) was used to assess donor chimerism. Tregs were defined as CD3+/CD4+/CD25high/FoxP3+. The same analysis was performed on liver tissue and lymph nodes on sacrifice. Data was acquired on a FACS Canto II Flow Cytometer (BD Bioscience) and analyzed using FloJo Software (TreeStar Inc. Ashland, OR).
Mixed Lymphocyte Reaction (MLR)
Thymidine uptake MLR were performed as previously described.8
Anti-Donor Antibody
Anti-donor antibodies were detected using flow cytometry as previously described.26 Data was acquired using a FACS Canto and analyzed with FloJo Software.
Histopathology Studies
Liver biopsies and necropsy specimens were fixed in formalin and stained with H&E and trichrome. Deparaffinized sections were stained for C4d and IgG, using polyclonal rabbit predilute mAb (Cell Marque Rocklin, California) on a Leica Bond 3 platform. All histopathology slides were reviewed by a single LT pathologist. Rejection was scored using BANFF criteria.27
Cytokine Analysis
Serum samples were analyzed in duplicate using the Ivitrogen Cytokine 29-Plex Monkey Panel (ThermoFisher Waltham, MA) to measure levels of IL-2, IL-6, IL-10, IL-17, IFNγ, and TNFα. Data was acquired on a LX200 analyzer (ThermoFisher Waltham, MA) and analyzed using Exponent 3.1 software.
Statistics
Paired, single-tailed student T-tests were performed to examine MLR results and percentages of graft-infiltrating cell populations using Excel 2016 (Microsoft Redmond, WA). Statistical significance was defined as p<0.05.
Results
Standard Conditioning Regimen
Seven combined liver and bone marrow transplants (CLBMT) were performed between MHC-mismatched animals (Figure 1). Groups A and B underwent conditioning regimens that induced tolerance in SKBMT recipients (Figure 2).8,9 Animal 90–57 received anti-CD154 mAb, requiring anti-platelet prophylaxis to prevent thrombotic complications and developed bleeding refractory to transfusion.28 To avoid this complication, splenectomy was performed in lieu of anti-CD154 therapy, as either reduce alloantibody formation in the monkey SKBMT protocol.8 Group B animals also manifested early complications. Animal 90–12 suffered an intraoperative anesthetic complication and was humanely euthanized prior to anesthetic weaning. Animal 90–166 a pleural effusion requiring thoracentesis; on post-transplant day (PTD) 4 it was humanely euthanized due to respiratory complications. Given the post-transplant course of these initial animals, subsequent animals received only 1.5 Gy of TBI to decrease toxicity and CMV-related complications observed in animals receiving similar regimens at our Center, and to accelerate hematopoietic recovery from conditioning (Groups C and D).
Modified Regimen: Immune Depletion and Induction of Mixed Chimerism
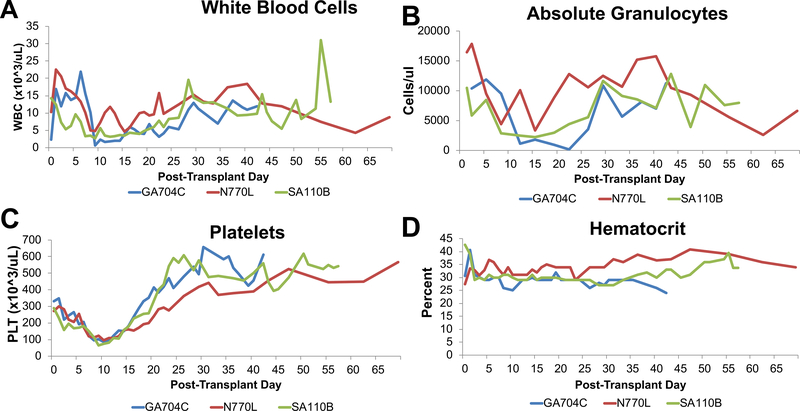
Four recipients underwent immune conditioning that included 1.5 Gy of TBI (Figure 2). One animal, GR677K, suffered hemorrhage due to vascular line migration, and was sacrificed on PTD 10. The 3 remaining animals had successful immune conditioning, with leukocyte counts reaching a nadir by PTD 10 (Figure 3A), but recovering soon after. Animal GA704C had transient neutropenia on PTD 22. Both N770L and SA110B maintained granulocyte levels >2000 cells/μL (Figure 3B) and none required neutropenic prophylaxis. Although platelets (Figure 3C) reached a nadir by day 10, levels never fell below 65 k/ul and none of the animals experienced bleeding complications. Hematocrit remained stable (Figure 3D) and none of the three animals required a post-operative blood transfusion.
Figure 3: Hematologic responses of long-term survivors.
(A) WBC values reached a nadir near POD 10 and recovered thereafter. (B) Absolute granulocyte counts. Granulocytes decreased after conditioning and GA704C had absolute counts less than 400 cells/ul but recovered quickly. (C) Platelet (Plt) counts reached a nadir near POD 10 and recovered thereafter. (D) Hematocrit. All animals maintained stable hematocrits after transplant and none required transfusions. Blue lines show results for GA704C, Red lines show results for N770L, and Green lines show results for SA110B.
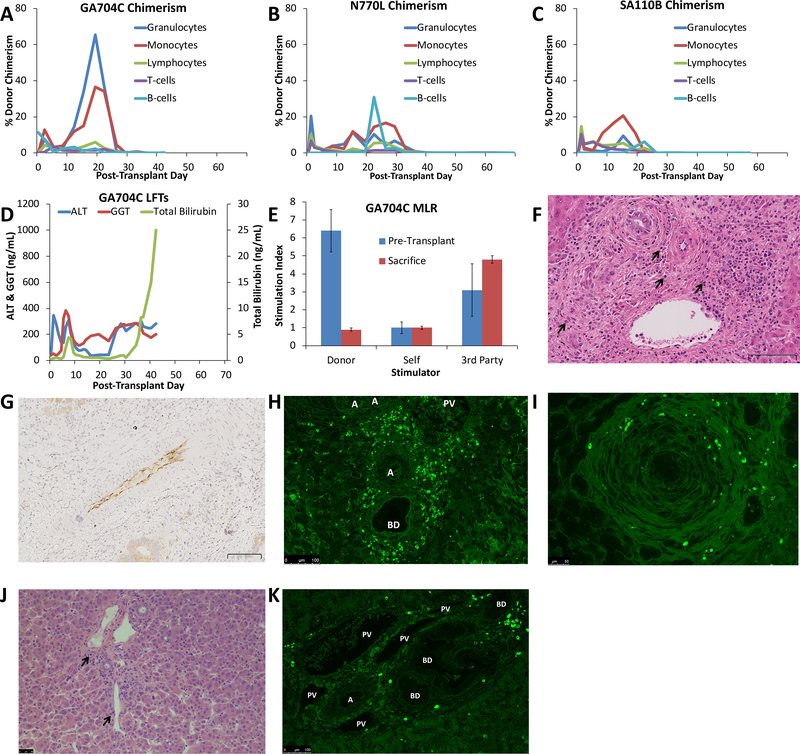
Transient (4–5 weeks) multi-lineage MC was achieved in all three animals that survived long-term (Table 1, Figure 4). Maximum donor MC was generally achieved in the myeloid lineage 2–4 weeks after CLBMT (Figure 4A–C). In contrast, lymphoid chimerism reached maximum levels immediately after transplant, with a second peak occurring at the same time as peak myeloid chimerism (Figure 4A–C). B-cell chimerism reached a peak in the 3rd week after transplant (Figure 4A–C). Donor chimerism was undetectable by day 26 in GA704C, day 40 in N770L, and day 26 in SA110B.
Figure 4: Results of Liver Transplants Without Rituximab.
(A-C) Percent Donor Chimerism. Animal numbers are indicated above graphs. Blue lines show the %Donor Granulocytes, Red lines show %Donor Monocytes, Green lines show %Donor Lymphocytes, Purple lines show %Donor CD3+ T cells; Light Blue lines show %Donor CD20+ B cells. Lymphocyte chimerism peaked early after transplant while the myeloid lineages peaked in the 3rd week after transplant. All animals lost chimerism after the 5th week post-transplant; (D) Liver function tests of GA704C. Blue lines show AST values, Red lines show ALT values, green lines show Total Bilirubin values. There was a rapid rise of LFTs after immunosuppression withdrawal; (E) Mixed Lymphocyte Reaction for Animal GA704C. MLR were performed pre-transplant (blue bars) and at the time of sacrifice (red bars). Responses are shown to donor stimulators, self-stimulation and 3rd-party stimulators and express as stimulation index normalized to negative controls without stimulators. GA704C demonstrated donor-specific unresponsiveness at the time of sacrifice. Error bars represent Std Dev; (F) GA704C liver POD 42 showing diffuse cellular infiltration with many plasma cells (black arrowheads) H&E 25x. Scale bar indicates 100 μm; G) Immunohistochemistry of GA704C showed diffuse C4d deposition in hepatic arteries at POD 42. 18x, scale bar indicates 100 μm; (H) Immunofluorescence for IgG of GA704C liver showed diffuse plasma cell infiltration on POD 42. Scale bar indicates 100μm. A-artery, BD- bile duct; (I) Immunofluorescence for IgG of GA704C autologous pancreas on POD 42 also showed plasma cell infiltration indicating a non-specific plasma cell response. Scale bar indicated 50 μm; (J) Liver histology of GR677K on POD 10 had minimal cellular infiltration while still on immunosuppression with scattered plasma cells (black arrowheads). Scale bar indicates 50 μm; (K) Immunofluorescence for IgG of GR677K liver biopsy shows scattered IgG-positive plasma cells. Scale bar indicates 100 μm. A-artery, BD-bile duct; PV-portal vein.
Plasma Cell Infiltration and Humoral Responses (Regimen C)
GA704C liver function tests recovered well from the initial rise due to ischemia-reperfusion injury. However, an early rise in bilirubin and GGT occurred on PTD 5 and resolved with antibiotic therapy, possibly representing cholangitis. This animal had a later rise in bilirubin and GGT (Figure 4D) and ultrasound examination on day 35 revealed biliary dilatation supporting the diagnosis of anastomotic biliary stricture. During revision of the biliary anastomosis on PTD 42, the animal suffered an anesthetic complication and was humanely euthanized. MLR demonstrated hyporesponsiveness to the donor with preservation of 3rd-party response (Figure 4E). Histopathology revealed a diffuse plasma cell infiltration of the graft (Figure 4F) with C4d deposition in the graft arterial vasculature (Figure 4G). Despite the absence of circulating anti-donor antibodies (not shown), immunofluorescence confirmed the presence of IgG-producing cells not only in the liver allograft (Figure 4H), but also in autologous tissues such as the pancreas (Figure 4I). GR677K also showed the presence of IgG-producing cells in the liver allograft by PTD 10 when it was sacrificed at Day 10 due to bleeding (Figure 4J, K)).
Additional B-Cell Depletion Was Associated With Severe Acute Cellular Rejection (Regimen D)
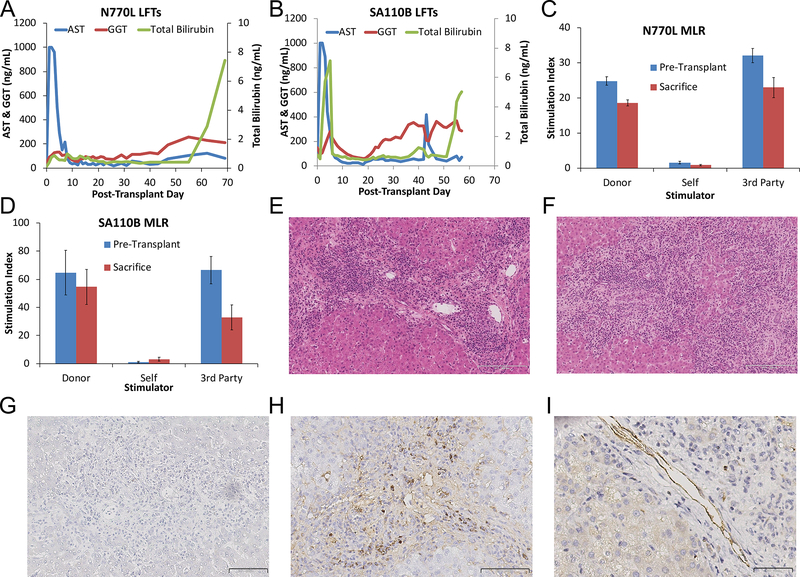
Given the plasma cell infiltration seen in the previous animals, rituximab was added for animals N770L and SA110B. N770L’s liver functioned well until laboratory values suggested acute cellular rejection (ACR) and it was humanely euthanized on PTD 69 (Figure 5A). In contrast, SA110B’s clinical course was complicated by early biliary obstruction that resolved spontaneously by PTD 8. However, signs of biliary obstruction recurred and the animal required biliary anastomosis revision on PTD 42. After initial improvement, bilirubin rose again due to rejection of the allograft and the animal was humanely euthanized on PTD 57 (Figure 5B). Although no anti-donor antibodies were detected (not shown), MLR revealed donor-specific reactivity in both N770 and SA110B (Figure 5C, D). Histology revealed severe ACR in both animals with N770L graded as Banff 8/9 and SA110B as 9/9. (Figure 5E, F). Although SA110B had minimal plasma cell infiltrates on immunohistochemistry (Figure 5G), N770L had scattered plasma cell infiltration and C4d deposition (Figure 5H, I).
Figure 5: Results of Liver Transplants with Rituximab:
(A, B) Liver function tests of animals treated with rituximab. Animal numbers are indicated above graphs. Blue lines show AST values, Red lines show ALT values, green lines show Total Bilirubin values. All animals had rapid rise of LFTs after immunosuppression withdrawal; (C, D) Mixed Lymphocyte Reactions. Animal numbers are indicated above graphs. MLR were performed pre-transplant (blue bars) and at the time of sacrifice (red bars). Responses are shown to donor stimulators, self-stimulation and 3rd-party stimulators and express as stimulation index normalized to negative controls without stimulators. N770L and SA110B maintained anti-donor responses after immunosuppression withdrawal. Error bars represent Std Dev. (E) Liver histology on POD 69 for N770L demonstrated Banff 8/9 acute cellular rejection. H&E, 14x, Scale bar indicates 200 μm; (F) Liver histology for SA110B on POD 57 revealed Banff 9/9 acute cellular rejection. H&E, 13x scale bar indicates 200 μm; (G) Immunohistochemistry for IgG on SA110B POD 57 liver tissue showed no plasma cell infiltration. 22x, scale bar indicates 100 μm; (H) Immunohistochemistry for IgG on N770L POD 69 liver tissue showed scattered plasma cell infiltration. 27x, scale bar indicates 200 μm; (I) Immunohistochemistry for C4d showed deposition on endothelium. 40x, scale bar indicates 50 μm.
Lymphocyte Recovery, Expansion of CD8+ Effector Memory T cells and Cytokine Concentrations
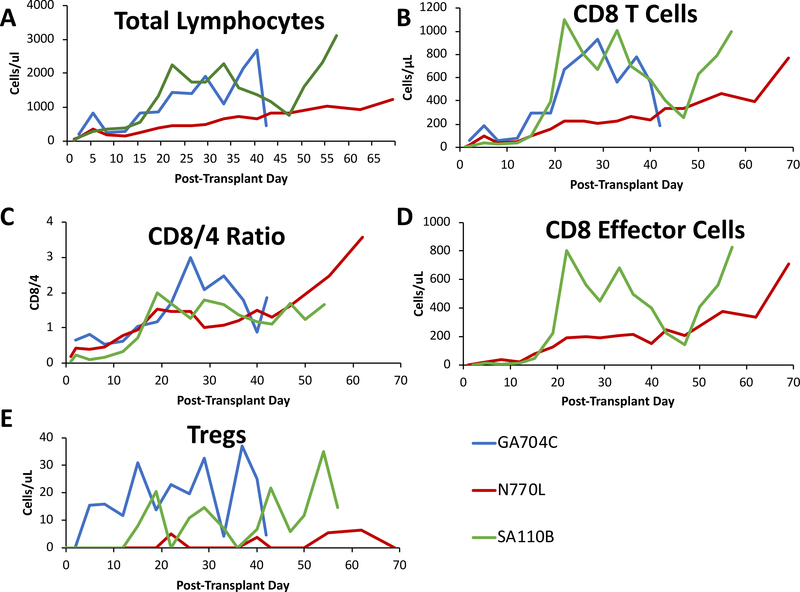
After an initial spike in total lymphocyte count on PTD 5, all three Group C and D animals reached a nadir on PTD 10 and had variable rates of recovery thereafter. GA704C and SA110B both reached >500 lymphocytes/μL by PTD 15, while N770L did not cross the same threshold until PTD 30 (Figure 6A). Reconstitution of lymphocytes in all three animals was dominated by CD8+ T cells that became more abundant than CD4+ T cells by the 3rd week after transplant, (Figure 6B, C). The greater the CD8+ predominance among T cells, the earlier the loss of MC occurred. Both GA704C and SA110B lost chimerism by PTD 26, by which time there were 2–3 times as many CD8+ T cells as there were CD4+ T cells. N770L also had up to 1.7 times as many CD8+ T cells at PTD 40, when chimerism was lost.
Figure 6: Lymphocyte Reconstitution:
(A) Total lymphocytes counts. Peripheral lymphocyte numbers reached a nadir by day 10, but recovered soon after. (B) Absolute numbers of peripheral CD8 T cells. CD8 T cell numbers increased rapidly after the transplant and accounted for the majority of circulating lymphocytes. (C) Ratio of circulating CD8 to CD4 T cells. CD8/4 ratios of the three surviving animals revealed a shift in the normal ratio where CD4 T cells are more abundant to where CD8 cells predominate. (D) Absolute numbers of circulating CD8 T-effector memory cells. The vast majority of CD8 cells were effector memory T cells defined as CD95+/CD28- CD8 T cells. (E) Absolute numbers of circulating CD4/CD25hi/FoxP3+ Tregs/microliter in peripheral blood. Blue lines show results for GA704C, Red lines show results for N770L, and Green lines show results for SA110B.
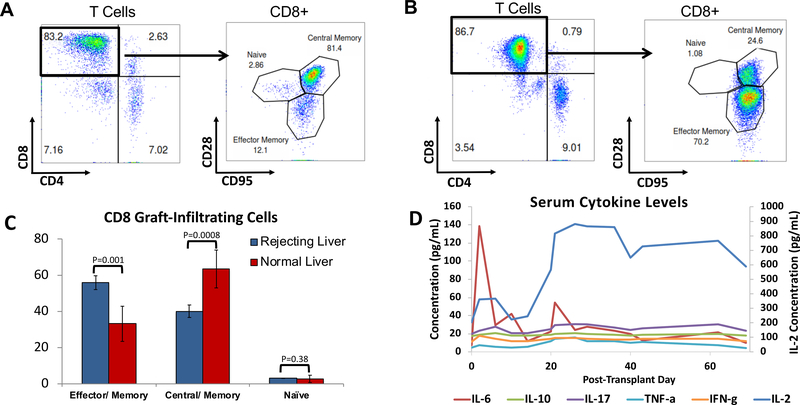
Further characterization of CD8+ T cells was performed using flow cytometry by co-staining for CD95 and CD28 to define naïve (Tn), central memory (Tcm) and effector memory (Tem) phenotypes in Group D animals29,30. In contrast to normal animals,31 peripheral CD8+ cells in Group D animals consisted of very few Tn cells and a marked predominance of Tem (Figure 6D). The absolute number of Tregs in the peripheral blood remained fairly constant after transplantation (Figure 6E). By the time MC was no longer detectable, Tem comprised up to 80% of CD8+ cells. Graft-infiltrating cells were also examined in Group D animals at the time of liver biopsies and sacrifice. Although the normal ratio of CD8:CD4 cells was maintained in rejecting livers, the CD8 cells had a significant shift from a predominance of Tcm in naïve livers to predominantly Tem and almost no naïve CD8 cells were detected (Figure 7A–C).
Figure 7: Resident and Graft-Infiltrating Lymphocytes of liver biopsies and cytokine levels.
(A) Resident T cells in normal livers are predominately CD8, among which Tcm dominate. (B) Graft infiltrating T cells are also predominately CD8 cells but are comprised of more Tem. (C) Resident CD8 T cells in the liver shifts from predominately Tcm to Tem in rejecting livers, with almost no Tn cells Error bars represent Std Dev. (D) Average levels of serum cytokines in peripheral blood. All cytokine values are indicated on the left Y axis except for IL-2, with values indicated on the right Y axis.
We measured the concentration of serum cytokines in peripheral blood post-transplantation to quantify the levels of pro-inflammatory or suppressive cytokines (Figure 7D). There was an early elevation in IL-6 post-operatively and a second rise near the time of chimerism loss. IL-2 was suppressed early after the transplant while the animals were being treated with CyA, but rose after its discontinuation. There were no large changes in the peripheral concentrations of the other cytokines we measured, including IL-10.
Discussion
Tolerance remains the “Holy Grail” of transplant immunology. In contrast to other solid organs, the liver has shown a propensity for operational tolerance. In a European multicenter trial with adult deceased donor recipients, 41 of 98 patients successfully had immunosuppression withdrawn. Operational tolerance in this study was only observed in patients that had been maintained on immunosuppression at least 3 years, with greatest rates of success achieved in patients who had been weaned 10.6 years after transplantation.32 However, weaning trials include only highly-selected patients with stable graft function for a number of years after transplantation. This limits their application to a small fraction of LT recipients. Additionally, immunosuppression is weaned after years of stable graft function, during which time patients are exposed to risks of infections and drug toxicities. Thus, rapid and reliable induction of tolerance after LT remains an elusive but desirable goal.
There have been few reports of prospective tolerance induction in LT. Todo et al. recently reported a pilot series of 10 patients that received an infusion of ex vivo expanded Tregs in living donor transplantation. Immunosuppression was withdrawn within 18 months and, at the time of report, 7 patients had been drug free for 16–33 months.33 The other 3 patients had autoimmune diseases and experienced acute cellular rejection, leading to suspension of the trial pending long-term follow-up. Others have infused donor bone marrow at the time of LT without myelosuppressive conditioning.34–37 In these studies, persistent donor chimerism was not detected in recipients and the rate of tolerance induction did not improve on the rate of tolerance achieved in weaning trials.
In this study, we chose to examine the ability of non-myeloablative conditioning protocols that result in transient MC to induce tolerance to LT in NHPs. These protocols were based on similar studies conducted on kidney transplants in NHP8,9,12 that were successfully translated to humans.11,12 The mechanism of renal tolerance that is induced with transient MC is not completely defined. Studies in NHP and humans suggest that early allograft acceptance is due to regulatory mechanisms,13–15,38 with deletion of alloreactive T-cell clones occurring in the late phases of graft acceptance.39 Additionally, the kidney appears to play a pivotal role in tolerance induction with this protocol.40 When this strategy was applied to NHP models of lung or islet transplantation, tolerance did not develop.41,42 The importance of the kidney is underscored in studies of cardiac tolerance. With a simultaneous heart and bone marrow transplant, allograft survival is prolonged but tolerance is not induced.18 However, if a donor kidney is transplanted along with the heart, tolerance to both grafts is induced as long as the kidney is present.43 These results suggest a critical intrinsic or soluble factor in the kidney that actively influences the immune response, possibly through Th3-mediated Treg development or by renal tubular epithelial cell expression of PD-L1.40,43
Since the liver is considered tolerogenic and in rodent studies has been reported to promote the development of Tregs44 and modulate the immune response through PD-L1 expression45 as has been reported for the kidney, we decided to test the hypothesis that a nonmyeloablative conditioning regimen that induces transient MC would induce tolerance to LT. In developing the liver model, small modifications of the SKBMT protocol were made due to technical and clinical differences between the renal and liver models. Although renal tolerance in the NHPs occurs at similar rates whether splenectomy or anti-CD154 mAb are used8,9, we initially chose to use anti-CD154 mAb as part of our conditioning regimen (Group A). However, the thrombogenic side effects of anti-CD154 required the administration of ketorolac to prevent platelet activation28 and led to clinically significant bleeding in one animal. Therefore, in Groups B-D we performed a splenectomy instead of giving anti-CD154 mAb. To accelerate recovery after conditioning and reduce the risk of CMV reactivation, we lowered the dose of TBI in regimens C and D. In the NHP SKBMT model, none of the animals that received only 1.5 Gy of TBI developed donor MC or tolerance to the kidney. In our liver model, despite the lower radiation dose, multilineage MC was achieved at comparable levels and duration to that achieved in the SKBMT models using 3 Gy TBI.8,9 Importantly, T-cell chimerism was seen in all recipients of CLBMT. This is significant given that lymphocyte chimerism is highly correlated with the development of tolerance in the NHP renal transplant model.9 The greater ease at achieving MC with liver compared to kidney transplants in combination with BMT suggests that passenger lymphocytes, especially T cells, coming from the liver graft may contribute to chimerism. This is further supported by the observation that lymphoid and T cell chimerism is seen at high levels on POD 1 in the liver but not the renal model.
In contrast to the results achieved in the NHP kidney and heart models, despite developing MC, liver allografts were rejected soon after immunosuppression withdrawal. Both grafts in Group C showed plasma cell infiltration as early as 10 days after transplantation but had undetectable levels of anti-donor antibodies. The lack of donor-specific antibodies detected in the serum could indicate absorption by the liver, or, given the evidence of autoimmunity in autologous tissues in one animal, the antibodies may be directed against autoantigens. In Group D, the addition of B-cell depletion appeared to decrease plasma cell infiltration. Thereafter, the major pathologic finding was T-cell infiltration of the rejected allografts. In naive monkeys, peripheral T cells are predominantly CD4+ and the distribution of peripheral CD8+ cells is approximately 60% Tem, 20% Tcm, and 20% Tn.29 Shortly before the loss of chimerism in each animal, peripheral CD8 cells became more abundant than CD4 cells and they continued to expand until liver grafts were rejected, by which time >80% of CD8 T cells were Tem. Graft-infiltrating cells isolated from liver biopsies or at the time of sacrifice were also predominantly CD8 Tem, whereas naïve liver T cells are predominantly CD8 Tcm. There were no major changes in the number of peripheral Tregs in the animals around the time of graft loss. The cytokine profile was dominated by IL-6 early and IL-2 levels rose soon after CyA was stopped. Either or both cytokines may have contributed to the expansion of the CD8 T cells and IL-6 may have been driving the expansion of CD8 T effectors.46 Thus, in our model, the CD8 Tem responses seem to be the primary determinant of chimerism loss and ACR of the liver allograft.
The failure of this protocol to induce tolerance to liver grafts, as opposed to kidneys, may reflect several factors. LT is associated with a profound inflammatory response associated with ischemia-reperfusion injury.47 Intestinal ischemia produced by temporary portal clamping during the LT is associated with the upregulation of inflammatory cytokines, including IL-6 and IL-17, by the intestine.48 The liver is then exposed to the portal blood after reperfusion. Although the liver is normally considered tolerogenic, the inflammatory environment induced by the transplant surgery may alter the phenotype of antigen-presenting cells (APC) in the liver from tolerogenic to immune activating,49 or act directly on T cells during their activation. Thus, a prolonged period of immunosuppression may be required before withdrawal to allow inflammation to clear before APC-T cell interactions become “tolerogenic”. As mentioned above, the liver is a reservoir of T cell memory and a site of memory T cell homing. Memory T cells are a barrier to clinical tolerance induction, and may be responsible for the failure of many regimens that induce tolerance in rodents to translate to NHP or humans.16,50–53 The liver itself is an immune organ that contains large numbers of lymphocytes and memory T cells.54–56 In a clinical trial of immunosuppression withdrawal, the inability to wean immunosuppression after LT was associated with expansion of memory CD8 T cells.57 A similar CD8 T cell expansion was reported after stopping standard immunosuppression in rhesus macaques.58 Additionally, inflammatory conditions present after LT favor generation, activation and differentiation of memory T cells.59 Our study is the first report to suggest that CD8 T cell memory is a barrier to tolerance induction to livers in a translational NHP model. We therefore hypothesize that tolerance in this model will require more efficient depletion of CD8 memory T cells, especially during the inflammatory phase post-transplantation. However, we cannot rule out that the kidney is a unique organ with respect to its ability to induce tolerance with this protocol, and that the liver lacks an important cell or factor that is present only in the kidney.
The results of this study are limited by the small number of animals that survived until rejection and the small group sizes of animals that received identical induction therapy. The small group size is due to the difficulty of performing liver transplants in a macaque model that required modifications of early protocols to achieve better clinical outcomes.58,60 Despite these modifications, transient mixed chimerism was observed at levels and durations that would be expected to facilitate tolerance induction in kidney transplant recipients.61 Another shortcoming is that the histology of rejected grafts showed different patterns of rejection depending on whether rituximab was given or not. However, the proliferation of effector CD8 T cells in the periphery and the graft, coupled with supporting data from other NHP and human studies,16,57,58 strongly suggests these cells represent a major barrier to tolerance induction in this model. Studies designed to confirm this hypothesis by targeting effector CD8 T cells are ongoing.
In conclusion, transient MC induced by nonmyeloablative conditioning does not promote tolerance to liver allografts in NHP. Despite modifying the regimen to reduce the overall toxicity and prevent humoral alloresponses while still achieving chimerism, severe ACR occurred soon after withdrawal of immunosuppression. Rejection was associated with expansion of memory CD8 T cells. The increased response of effector CD8 T cells to liver versus kidney grafts may be associated with either the liver being a reservoir of memory T cells, increased levels of inflammatory cytokines, or the lack of a factor specific to the kidney in the transient MC protocol.
Acknowledgments
Funding: ATGAM used in this study was generously provided by Pfizer, Inc. SC, JW and DW were supported by the NIH 5T32HL007854-19 grant. AG was supported by the Louis J. Gerstner, Jr. Foundation Award and the American Association for the Study of Liver Diseases Career Development Award in the Memory of the University of Michigan Transplant Team. The study was supported by the NIH-NIAID R56 AI122332 and by the National Center for Advancing Translational Sciences, National Institutes of Health through Grant Number UL1TR001873. Data reported in this manuscript was acquired in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health under awards S10RR027050 and S10OD020056. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors acknowledge Mr. Michael and Mrs. Susan Kerr and the Glickman Family for generous financial support of this work.
Abbreviations
- ACR
Acute Cellular Rejection
- APC
Antigen-Presenting Cell
- CLBMT
Combined Liver and Bone Marrow Transplantation
- CyA
Cyclosporine A
- LT
Liver Transplantation
- MC
Mixed Hematopoietic Chimerism
- MLR
Mixed Lymphocyte Reaction
- NHP
Nonhuman Primate
- PTD
Post-Transplant Day
- SKBMT
Simultaneous Kidney and Bone Marrow Transplantation
- TBI
Total Body Irradiation
- Tcm
Central Memory T cell
- Tem
Effector Memory T cell
- Tn
Naïve T cell
- Treg
Regulatory T cell
Footnotes
Disclosure: The authors declare no conflicts of interest.
Supplemental Methods
Genomic DNAs isolated from whole blood served as templates for PCR with a panel of primers that flank the highly polymorphic peptide binding domains encoded by exon 2 of MHC class I (Mafa-A, -B, -I, -E) and class II (Mafa-DRB, -DQA, -DQB, -DPA and -DPB) loci. These PCR products were generated with Fluidigm Access Arrays. After cleanup and pooling, these amplicons were sequenced on an Illumina MiSeq and the sequence reads were mapped against a custom database of Mauritian cynomolgus macaque class I and class II.62
References
- 1.Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. Am J Transplant. 2011;11(6): 1226–1235. [DOI] [PubMed] [Google Scholar]
- 2.Rana A, Ackah RL, Webb GJ, et al. No Gains in Long-Term Survival After Liver Transplantation Over the Past Three Decades. Ann Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 3.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25): 2601–2614. [DOI] [PubMed] [Google Scholar]
- 4.Mynarek M, Schober T, Behrends U, Maecker-Kolhoff B. Posttransplant lymphoproliferative disease after pediatric solid organ transplantation. Clin Dev Immunol. 2013;2013: 814973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel KE, Eickhoff J, Lucey MR. Why do patients die after a liver transplantation? Clin Transplant. 2017;31(3). [DOI] [PubMed] [Google Scholar]
- 6.Issa N, Kukla A, Ibrahim HN. Calcineurin inhibitor nephrotoxicity: a review and perspective of the evidence. Am J Nephrol. 2013;37(6): 602–612. [DOI] [PubMed] [Google Scholar]
- 7.Sachs DH. Tolerance: of mice and men. J Clin Invest. 2003;111(12): 1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59(2): 256–262. [PubMed] [Google Scholar]
- 9.Kawai T, Sogawa H, Boskovic S, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4(9): 1391–1398. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Cosimi AB, Sachs DH. Preclinical and clinical studies on the induction of renal allograft tolerance through transient mixed chimerism. Curr Opin Organ Transplant. 2011;16(4): 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4): 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai T, Sachs DH, Sykes M, Cosimi AB, Immune Tolerance N. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2013;368(19): 1850–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotta K, Aoyama A, Oura T, et al. Induced regulatory T cells in allograft tolerance via transient mixed chimerism. JCI Insight. 2016;1(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreola G, Chittenden M, Shaffer J, et al. Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation. Am J Transplant. 2011;11(6): 1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprangers B, DeWolf S, Savage TM, et al. Origin of Enriched Regulatory T Cells in Patients Receiving Combined Kidney-Bone Marrow Transplantation to Induce Transplantation Tolerance. Am J Transplant. 2017;17(8): 2020–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadazdin O, Boskovic S, Murakami T, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011;3(86): 86ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai T, Sogawa H, Koulmanda M, et al. Long-term islet allograft function in the absence of chronic immunosuppression: a case report of a nonhuman primate previously made tolerant to a renal allograft from the same donor. Transplantation. 2001;72(2): 351–354. [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Cosimi AB, Wee SL, et al. Effect of mixed hematopoietic chimerism on cardiac allograft survival in cynomolgus monkeys. Transplantation. 2002;73(11): 1757–1764. [DOI] [PubMed] [Google Scholar]
- 19.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223(5205): 472–476. [DOI] [PubMed] [Google Scholar]
- 20.Kamada N, Wight DG. Antigen-specific immunosuppression induced by liver transplantation in the rat. Transplantation. 1984;38(3): 217–221. [DOI] [PubMed] [Google Scholar]
- 21.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19(4): 916–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato Y, Griesemer AD, Wu A, et al. Novel H-shunt Venovenous Bypass for Liver Transplantation in Cynomolgus Macaques. Comp Med. 2017;67(5): 436–441. [PMC free article] [PubMed] [Google Scholar]
- 23.Budde ML, Wiseman RW, Karl JA, Hanczaruk B, Simen BB, O’Connor DH. Characterization of Mauritian cynomolgus macaque major histocompatibility complex class I haplotypes by high-resolution pyrosequencing. Immunogenetics. 2010;62(11–12): 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennington LR, Sakamoto K, Popitz-Bergez FA, et al. Bone marrow transplantation in miniature swine. I. Development of the model. Transplantation. 1988;45(1): 21–26. [DOI] [PubMed] [Google Scholar]
- 25.Griesemer A, Liang F, Hirakata A, et al. Occurrence of specific humoral non-responsiveness to swine antigens following administration of GalT-KO bone marrow to baboons. Xenotransplantation. 2010;17(4): 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boskovic S, Kawai T, Smith RN, et al. Monitoring antidonor alloantibodies as a predictive assay for renal allograft tolerance/long-term observations in nonhuman primates. Transplantation. 2006;82(6): 819–825. [DOI] [PubMed] [Google Scholar]
- 27.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25(3): 658–663. [DOI] [PubMed] [Google Scholar]
- 28.Koyama I, Kawai T, Andrews D, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77(3): 460–462. [DOI] [PubMed] [Google Scholar]
- 29.Nadazdin O, Boskovic S, Murakami T, et al. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. Am J Transplant. 2010;10(6): 1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo DJ, Weaver TA, Stempora L, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am J Transplant. 2011;11(1): 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zitsman JS, Alonso-Guallart P, Ovanez C, et al. Distinctive Leukocyte Subpopulations According to Organ Type in Cynomolgus Macaques. Comp Med. 2016;66(4): 308–323. [PMC free article] [PubMed] [Google Scholar]
- 32.Benitez C, Londono MC, Miquel R, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58(5): 1824–1835. [DOI] [PubMed] [Google Scholar]
- 33.Todo S, Yamashita K, Goto R, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64(2): 632–643. [DOI] [PubMed] [Google Scholar]
- 34.Rolles K, Burroughs AK, Davidson BR, Karatapanis S, Prentice HG, Hamon MD. Donor-specific bone marrow infusion after orthotopic liver transplantation. Lancet. 1994;343(8892): 263–265. [DOI] [PubMed] [Google Scholar]
- 35.Ricordi C, Karatzas T, Nery J, et al. High-dose donor bone marrow infusions to enhance allograft survival: the effect of timing. Transplantation. 1997;63(1): 7–11. [DOI] [PubMed] [Google Scholar]
- 36.Tryphonopoulos P, Tzakis AG, Weppler D, et al. The role of donor bone marrow infusions in withdrawal of immunosuppression in adult liver allotransplantation. Am J Transplant. 2005;5(3): 608–613. [DOI] [PubMed] [Google Scholar]
- 37.Donckier V, Troisi R, Le Moine A, et al. Early immunosuppression withdrawal after living donor liver transplantation and donor stem cell infusion. Liver Transpl. 2006;12(10): 1523–1528. [DOI] [PubMed] [Google Scholar]
- 38.LoCascio SA, Morokata T, Chittenden M, et al. Mixed chimerism, lymphocyte recovery, and evidence for early donor-specific unresponsiveness in patients receiving combined kidney and bone marrow transplantation to induce tolerance. Transplantation. 2010;90(12): 1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris H, DeWolf S, Robins H, et al. Tracking donor-reactive T cells: Evidence for clonal deletion in tolerant kidney transplant patients. Sci Transl Med. 2015;7(272): 272ra210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hull TD, Benichou G, Madsen JC. Why some organ allografts are tolerated better than others: new insights for an old question. Curr Opin Organ Transplant. 2019;24(1): 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aoyama A, Ng CY, Millington TM, et al. Comparison of lung and kidney allografts in induction of tolerance by a mixed-chimerism approach in cynomolgus monkeys. Transplant Proc. 2009;41(1): 429–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oura T, Ko DS, Boskovic S, et al. Kidney versus Islet Allograft Survival after Induction of Mixed Chimerism with Combined Donor Bone Marrow Transplantation. Cell Transplant. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasaki H, Oura T, Spitzer TR, et al. Preclinical and clinical studies for transplant tolerance via the mixed chimerism approach. Hum Immunol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Kuhr CS, Zheng XX, et al. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am J Transplant. 2008;8(8): 1639–1651. [DOI] [PubMed] [Google Scholar]
- 45.Morita M, Fujino M, Jiang G, et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am J Transplant. 2010;10(1): 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okada M, Kitahara M, Kishimoto S, Matsuda T, Hirano T, Kishimoto T. IL-6/BSF-2 functions as a killer helper factor in the in vitro induction of cytotoxic T cells. J Immunol. 1988;141(5): 1543–1549. [PubMed] [Google Scholar]
- 47.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10(2): 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee HT, Kim M, Kim JY, et al. Critical role of interleukin-17A in murine intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2013;304(1): G12–25. [DOI] [PubMed] [Google Scholar]
- 49.Bowen DG, McCaughan GW, Bertolino P. Intrahepatic immunity: a tale of two sites? Trends Immunol. 2005;26(10): 512–517. [DOI] [PubMed] [Google Scholar]
- 50.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12): 1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada Y, Boskovic S, Aoyama A, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012;12(2): 330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koyama I, Nadazdin O, Boskovic S, et al. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7(5): 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tonsho M, Lee S, Aoyama A, et al. Tolerance of Lung Allografts Achieved in Nonhuman Primates via Mixed Hematopoietic Chimerism. Am J Transplant. 2015;15(8): 2231–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su YC, Lee CC, Kung JT. Effector function-deficient memory CD8+ T cells clonally expand in the liver and give rise to peripheral memory CD8+ T cells. J Immunol. 2010;185(12): 7498–7506. [DOI] [PubMed] [Google Scholar]
- 55.Obhrai JS, Oberbarnscheidt MH, Hand TW, Diggs L, Chalasani G, Lakkis FG. Effector T cell differentiation and memory T cell maintenance outside secondary lymphoid organs. J Immunol. 2006;176(7): 4051–4058. [DOI] [PubMed] [Google Scholar]
- 56.Bogdanos DP, Gao B, Gershwin ME. Liver immunology. Compr Physiol. 2013;3(2): 567–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donckier V, Craciun L, Miqueu P, et al. Expansion of memory-type CD8+ T cells correlates with the failure of early immunosuppression withdrawal after cadaver liver transplantation using high-dose ATG induction and rapamycin. Transplantation. 2013;96(3): 306–315. [DOI] [PubMed] [Google Scholar]
- 58.Kim H, Kim H, Lee SK, et al. Memory T cells are significantly increased in rejected liver allografts of rhesus monkeys. Liver Transpl. 2018;24(2): 256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jubin V, Ventre E, Leverrier Y, et al. T inflammatory memory CD8 T cells participate to antiviral response and generate secondary memory cells with an advantage in XCL1 production. Immunol Res. 2012;52(3): 284–293. [DOI] [PubMed] [Google Scholar]
- 60.Oura T, Yamashita K, Suzuki T, et al. A technique for orthotopic liver transplantation in cynomolgus monkeys. Transplantation. 2014;98(6): e58–60. [DOI] [PubMed] [Google Scholar]
- 61.Thaiss CC, Oura T, Sasaki H, et al. Importance of Hematopoietic Mixed Chimerism for Induction of Renal Allograft Tolerance in Nonhuman Primates. Transplantation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karl JA, Bohn PS, Wiseman RW, et al. Major histocompatibility complex class I haplotype diversity in Chinese rhesus macaques. G3 (Bethesda). 2013;3(7): 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]