Abstract
The disease tuberculosis is fatal if untreated. It is caused by the acid‐fast bacilli Mycobacterium tuberculosis. Mycobacterium resides and replicates within the alveolar macrophages, causing inflammation and granuloma, wherein macrophage‐T cell interactions enhance the inflammation‐causing pulmonary caseous lesions. The first interactions between Mycobacterium and the receptors on macrophages decide the fate of Mycobacterium because of phagolysosomal impairments and the expression of several miRNAs, which may regulate CD40 expression on macrophages. While the altered phagolysosomal functions impede antigen presentation to the T cell‐expressed antigen receptor, the interactions between the macrophage‐expressed CD40 and the T cell‐expressed CD40‐ligand (CD40L or CD154) provide signals to T cells and Mycobacterium‐infected macrophages. These two functions significantly influence the resolution or persistence of Mycobacterium infection. CD40 controls T‐cell polarisation and host‐protective immunity by eliciting interleukin‐12p40, nitric oxide, reactive oxygen species and IFN‐γ production. Indeed, CD40‐deficient mice succumb to low‐dose aerosol infection with Mycobacterium because of deficient interleukin (IL)‐12 production leading to impaired IFN‐γ‐secreting T‐cell response. In contrast, despite generating fewer granulomas, the CD40L‐deficient mice developed anti‐mycobacterial T‐cell responses to the levels observed in the wild‐type mice. These host‐protective responses are significantly subdued by the Mycobacterium‐infected macrophage produced TGF‐β and IL‐10, which promote pro‐mycobacterial T‐cell responses. The CD40‐CD40L‐induced counteractive immune responses against Mycobacterium thus present a conundrum that we explain here with a reconciliatory hypothesis. Experimental validation of the hypothesis will provide a rationale for designing anti‐tubercular immunotherapy.
Keywords: anti‐mycobacterial T‐cell response, CD40‐CD40L interaction, mannose receptor, microRNA, Mycobacterium tuberculosis, Mycobacterium–macrophage interactions
In the review, we discuss the regulation of macrophage functions by CD40‐CD40L interactions in the context of positive and negative regulators, and the role of miRNAs in reorienting the outcome of the process of mycobacterial infection.
Introduction
Mycobacterium tuberculosis (Mtb) is an acid‐fast bacillus that resides and replicates within monocytes and macrophages, in particular, alveolar macrophages (AMs), which play key roles in developing robust innate and adaptive immune responses against the bacterium. 1 , 2 While the bacterium is eliminated or pushed to dormancy in a resistant host, the pathogen inflicts the disease tuberculosis in a susceptible host. Thus, M. tuberculosis infection presents two paradoxes: one, functional duality of the macrophages as a supporting host or as an eliminator, and two, alternate fates of the pathogen in resistant versus susceptible hosts. In principle, the macrophages from the resistant and the susceptible hosts have intrinsic or genetic differences that result in either elimination or growth of Mycobacteria. 3 , 4 Complementary to the intrinsic ability or inability of the host cells to control the infection, the pathogen can suppress the anti‐mycobacterial killing mechanisms in the susceptible macrophages. It fails in the resistant macrophages, linking the alternate outcomes of the infection to the virulence of the pathogen. 5 The macrophages are well known to also act as the antigen‐presenting cells to the antigen‐specific T cells. 6 This innate control of Mycobacterium may be linked to the T‐cell responses that may further accentuate the initial control of the pathogen. Intracellular signalling regulates three major integrated processes: (1) macrophage–Mycobacterium interaction, (2) macrophage–T cell interaction and (3) T‐cell regulation of macrophage functions. Here, we analyse these dualities of macrophage functions and alternate outcomes of infection with special reference to CD40‐CD40L interaction.
Macrophage–Mycobacterium interaction
Once internalised following multiple ligand–receptor interactions, Mycobacterium lives within phagosomal vesicles, which are formed during the phagocytosis of the pathogen. The phagosomes fuse with the lysosomes, which are rich in hydrolytic enzymes, proteases and lipases. The phagosome–lysosome fusion eventuates in the death of the intra‐phagolysosomal Mycobacteria. The internally degraded antigens are complexed with MHC class I or with MHC class II, and these two classes of antigens are presented to CD8+ or CD4+ T cells, respectively. Once these T cells are activated, the cytotoxic activities of the CD8+ T cells may directly destroy the mycobacterial antigen‐expressing macrophages, 7 , 8 or the cytokines from the activated CD4+ T cells may activate the macrophages to kill the intracellular Mycobacteria through reactive nitric oxide. 9 , 10 , 11
Conventionally, it was believed that cytotoxic activities of CD8+ T cells and cytokine secretion by CD4+ T cells are suppressed in susceptible host exemplifying a scenario, which may be more complex in reality. In many diseases/infections, it has been found that the polyfunctionality and antigenic responses against pathogens are controlled by the metabolic pathways operating in immune cells. 12 T‐cell metabolic machinery is regulated in anergy and exhaustion. 13 , 14 Such mechanisms may be the underlying causes of the lack of optimal anti‐bacterial responses as observed in a susceptible host. A resistant host may mount a strong TH1/TH17 response because of the relatively active intracellular metabolic process including glycolysis and upregulation of amino acid transporters SLC1A/EEAT2/GLT‐1. Furthermore, the upregulation of CD98 and transferrin receptor can facilitate the cellular energetics positively via activation of the Akt‐mTOR axis and control of protein translation of crucial anti‐bacterial cytokines and molecules. 15 Indeed, Mtb‐specific‐CD4+ TH1 response moderates protective immunity by producing cytokines such as IFN‐γ or TNF‐α. 16 CD40L depression strongly correlates with IFN‐γ levels in TB patients. In fact, a soluble agonist of CD40L was enough to restore IFN‐γ production from PBMCs isolated from TB patients, but not from healthy tuberculin reactor controls, which in turn conjures that defects in CD40L expression in TB patients contribute to diminished levels of IFN‐γ. 17 Both interleukin (IL)‐12 and IFN‐γ productions from human peripheral blood T cells are regulated by mTOR and STAT3. 18 Moreover, the IFN‐γ‐driven control of M. tuberculosis inside infected macrophages requires both iNOS and HIF‐1α. Nitric oxide may regulate aerobic glycolysis along with HIF‐1α to control intracellular Mtb replication. 19 Similarly, in chronic Mtb infection, circulating T cells may exhibit an exhausted phenotype characterised by gradual loss of secretion of IL‐2 and effectors IFN‐γ and TNF‐α. 20 The blockade of markers of T‐cell exhaustion TIM‐3 and PD‐1 may restore the functions of TB‐specific CD4+CXCR5+ T cells. 21 One study describes the presence of such exhausted T cells overexpressing checkpoint marker PD‐1 on TH1 cytokine‐producing Mtb‐specific CD4+ T cells in peripheral blood of TB patients. These cells are associated with poor prognosis, and blockade of PD1/PD‐L1 checkpoint (using anti‐PD‐L1 antibodies) can augment the IFN‐γ secretion but not the proliferation of CD4+ T cells. 22 By contrast, these processes are suppressed in a susceptible host that results in full‐blown disease tuberculosis. The strategies for survival are therefore lined up as soon as Mycobacteria attach to the macrophages. One of these strategies is to intercept CD40 expression and function that influences Mtb survival or elimination.
Mycobacterium attachment and internalisation
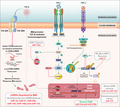
Mycobacterium infection starts with its attachment to the receptors on the macrophage surface and its subsequent internalisation by phagocytosis or receptor‐mediated endocytosis aided by opsonisation with serum complements 23 or natural antibodies. 24 Besides, the AM‐expressed mannose receptor and surfactant protein A (Sp‐A) receptor facilitate endocytosis through recognition of lipoarabinomannan (LAM) and Sp‐A on Mycobacterium, respectively. 25 , 26 The scavenger receptors bind the mycobacterial cell wall lipoteichoic acid to enhance the phagocytosis of the bacteria. 27 Besides these receptors, Toll‐like receptors (TLRs) are also implicated in the internalisation of Mycobacteria. The mycobacterial surface lipoglycoprotein MPT83 and LAM are recognised by TLR2 and TLR4, respectively, to enhance Mtb internalisation. 28 , 29 Dectin‐1 ligands that are expressed by Mtb await their purification and structural characterisation, as Mycobacteria do not express β‐1,3‐glucans, the known Dectin‐1 ligands. Different receptors on macrophages or dendritic cells thus enhance Mtb internalisation (Table 1; Figure 1) but exactly how and to what extent these receptors modulate its subsequent intracellular survival remains elusive.
Table 1.
Receptors mediating the internalisation of Mycobacterium tuberculosis
| No. | Receptors expressed by host cells | Ligand binding mechanism | Implication |
|---|---|---|---|
| 1. | CD14 receptors | The entry of nonopsonised tubercle bacilli into brain microglia | Promoting TNF‐α production |
| 2. | CR1 (CR1, CD35) | Binding to complement fragments C3b/C4b deposited on mycobacteria | Licensing entry inside macrophages |
| 3. | CR3 (CD11b, CD18) | Opsonised Mycobacterium tuberculosis binds CR3 at its iC3b binding domain; Nonopsonised Mycobacterium tuberculosis uses its endogenous capsular polysaccharides to interact with the β‐glucan binding site near the C terminus of CD11b | Uptake of complement opsonised bacterium and activating the alternative complement pathway |
| 4. | CR4 (CD11c, CD18) | Mycobacterium tuberculosis macrophage binding in the absence of serum | Tyrosine phosphorylation of a major 60‐kDa protein in host cells (p60src) |
| 5. | DC‐SIGN | Binding with LAM | Potentiate TLR‐4‐mediated IL‐10 secretion by LPS‐stimulated MoDCs |
| 6. | Dectin‐1 | Binding with an unknown ligand on Mtb | Promoting mycobacterial‐induced IL‐12p40 production by DC |
| 7. | Fcγ receptors | Immunoglobulin G (IgG)‐mediated opsonisation of Mtb bacilli | Redirecting intracellular trafficking of Mtb containing vesicles with ferritin‐loaded lysosomes |
| 8. | Fibronectin (Fn) | The interaction may occur through the binding of bacterial fibronectin‐binding proteins (FnBPs) with fibronectin | Dispensable for Mtb attachment and internalisation |
| 9. | Mannose Receptor (CD206) |
LAM mediated binding to MR1 ManLAM inhibits phagosome maturation |
Synthesis of IL‐10, IL‐1R; inhibiting IL‐12 production; blocking of phagosome maturation |
| 10. | Mincle | Recognition of Mtb ligand glycolate trehalose dimycolate | Mincle‐mediated secretion of inflammatory cytokines/chemokines and promotion of granuloma |
| 11. | Scavenger receptor class A (MARCO) | Interaction with bacterial cell wall components and LDL | Mtb ‘tether’ cell wall glycolipid, trehalose 6,6′‐dimycolate TDM/Cord factor to the macrophage and to activate the TLR2 signalling pathway |
| 12. | Scavenger receptor class B (SR‐B1/ CD36) | ManLAM and LM; diglycerides lipoteichoic acid (LTA) | Facilitating the availability of lipoproteins to TLR2 heterodimers |
| 13. | SIGNR3 | LM and ManLAM; lipoprotein LpqH | SIGNR3 can ‘collaborate’ with TLR2 for inducing pro‐inflammatory cytokine secretion |
| 14. |
Surfactant protein A (Sp‐A) |
Mtb binding to SP‐A is dependent on calcium and glycosylation of Sp‐A | It enhances binding and phagocytosis of Mtb |
| 15. |
Surfactant protein D (Sp‐D) |
SP‐D through its carbohydrate recognition domain binds to the terminal mannose caps of LAM | Agglutination; Reducing phagocytic uptake; increasing PL fusion |
| 16. | TLR2 | Reported Mtb ligands for TLR2: LAM, LM, PIM, and lipoglycan binding; lipoproteins LpqH and LprG; Rv0577 and hsp70; PE_PGRS33 | ERK1/2 phosphorylation and TNFα production; macrophage apoptosis, consequently promoting containment of Mtb |
| 17. | TLR4 | Mtb 50S ribosomal protein Rv0652; H37Rv | Inducing IRF3 to encourage IFN‐β secretion |
| 18. | TLR9 (Intracellular) | Undermethylated CG motifs (CpG) within bacterial DNA | Inducing IL‐12p40 and TNF‐α production |
Figure 1.
Receptors implicated in the internalisation of Mycobacterium sp. and intracellular sensors. (1) Pulmonary Mycobacterium infection begins with the bacilli entering into the airway where airway epithelial cells (AEC) respond by synthesising antimicrobial peptides (AMPs) and proteins, for example, Collectins Surfactant protein A (SP‐A) and SP‐D proteins. (2) These proteins opsonise the bacteria and facilitate phagocytic uptake by alveolar macrophages through SP‐A receptors. (3) Mycolic acid and lipoteichoic acid in the Mtb cell membrane play distinct roles in receptor‐mediated internalisation. Lipoteichoic acid binds to the MARCO (Class‐A scavenger receptor) and affects cytokine production in a TLR2‐dependent manner. (4) The entry of Mycobacterium is also supported by an array of pattern recognition receptors (PRRs) including, Toll‐Like receptors (TLRs) TLR4‐CD14, TLR2, Mannose receptors, immunoglobulin G (IgG)‐coated Mycobacteria via FcγRs and Scavenger receptors that bind lipopolysaccharides of gram‐negative bacteria and lipoteichoic acid of gram‐positive bacteria. (5) The abundance of C3 and C3bi proteins in broncho‐alveolar lavage fluid marks the Mycobacterium for complement‐mediated lysis through the alternative pathway. This binding also enhances its phagocytic uptake via complement receptors expressed by alveolar APCs. (6) Serum‐derived ligands facilitate pathogen uptake via receptors CR1, CR3 and CR4 and translocated to membrane‐bound phagosomes. CR3 through its interaction with Mannosyl‐phosphatidyl‐Myo‐inositol‐based glycolipids (PIM) can also facilitate Mycobacterium uptake. (7) A battery of major virulence proteins from mycobacterial species are reported. Lipoarabinomannan (LAM) and mannosylated LAM (ManLAM) are two major representatives. Others include HSP60/65, 38kDa protein/Ag38, Mtb resuscitation‐promoting factor (RpfB), ESAT‐6, CFP‐10, MPT83, PE‐PGRS33, and these factors have different mechanisms of binding to the host cells. (8) The C‐type lectin and DC‐Sign receptors (CD209) mediate Mtb entry via binding to PIM similarly. Other CLRs are Mincle and Dectin‐1 that may also mediate the internalisation process through unknown mechanisms. Intracellular Sensors: (9) During Mtb infection, the lysosomal release of cathepsin B (CTS‐B) plays an important role in NLRP3‐inflammasome activation and subsequent rise in IL‐1β production. This pathway may also control pyroptosis. (10) TLR3 and TLR8 are activated by mycobacterial RNA while undermethylated CpG motifs from the Mtb genome may activate TLR9 to elicit the cytokine biosynthesis. (11) Many other intracellular sensors of mycobacterial moieties have been reported. These include RIG‐1, MDA‐5 and PKR that may contribute to the upregulation of Type‐I IFNs. NOD2 activation may occur through GMDP which is a metabolite of the Mtb cell wall. (12) Cytosolic DNA sensor AIM‐2 mediates IL‐1β and IL‐18 production in response to Mtb. IFI16 is an innate immune sensor for intracellular DNA that may lead to the activation of the cytosolic surveillance pathway (CSP).
Mycobacterial alteration of host microRNA
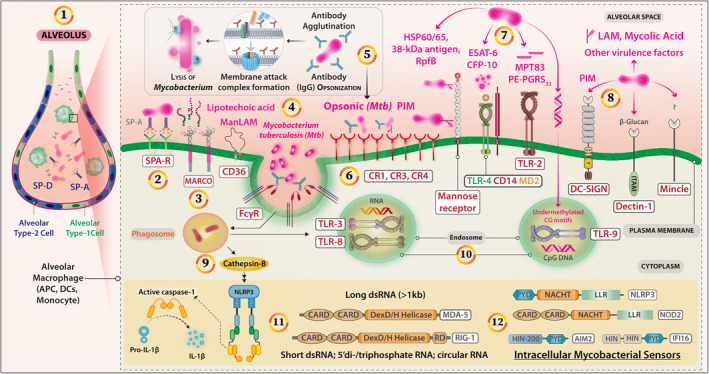
Although the mechanism of early Mycobacterium–macrophage interaction influencing the subsequent macrophage response is not worked out, the pathogen internalisation is followed by alterations in a huge number of microRNAs (Table 2). In Mtb‐infected macrophages, miR‐23a, miR‐125a, miR‐146a, miR‐579, miR‐708, miR‐27a, miR‐30a, miR‐129, miR‐1178 and miR‐1958 expressions were enhanced, whereas miR‐20b and miR‐26a expressions were downregulated. 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 miR23a modulates TLR2/MyD88/NF‐κB signalling to result in enhanced intracellular Mtb survival and prevention of macrophage autophagy, as miR‐23a inhibitors attenuated Mtb survival but enhanced autophagy. 30 Mycobacterial surface sulfoglycolipids act as competitive antagonists of TLR2 and inhibit NF‐κB activation to impair cytokine production or costimulatory molecule expression. 31 Similarly, Mtb lipoproteins LprG, the glycolipid phosphatidylinositol mannoside‐6, and the lipoglycan lipomannan bind TLR2 to induce ERK‐1/2‐dependent TNF‐α production in macrophages. 32 In Mtb‐infected macrophages, TLR4‐enhanced miR‐125a directly targets TRAF6 negatively regulating NF‐κB to suppress cytokines, attenuate immune response, and promote mycobacterial survival. 33 , 34 Apparently, miR‐708 supported Mycobacterium survival and inflammatory response. 35 miR‐579 downregulated its mRNA targets – SIRT1 and PDK1 – to enhance macrophage apoptosis and death 36 in human macrophages. miR‐1178 overexpression enhanced the intracellular growth of Mycobacteria but attenuated the accumulation of IFN‐γ, IL‐6, IL‐1β and TNF‐α, while miR‐1178 knockdown suppressed the Mycobacteria survival and enhanced the expression of these pro‐inflammatory cytokines in human macrophages. 42 The TLR2/MyD88/NF‐κB signalling‐induced miR‐27b expression suppressed the NF‐κB‐mediated induction of pro‐inflammatory factors but increased p53‐dependent production of reactive oxygen species and bactericidal functions of macrophages. 43 miR‐26b negatively regulated the NF‐κB pathway by directly targeting TGF‐β‐activated kinase‐1 (TAK1), resulting in inhibition of immune response, and promotion of Mtb replication and gene expression. 44 miR‐106b targeted the 3′‐UTR of Cathepsin S resulting in its silencing and impaired antigen processing by the Mtb‐infected macrophages. 45 Similar regulations were observed with miR‐20b in tuberculosis patients and M. tuberculosis‐infected mice. 46 During Mtb infection, miR‐26a facilitated arginase activity but reduced iNOS activity, 47 and iNOS expression was also reduced by miR‐146a by the inhibition of TRAF6, p38MAPK and NF‐κB. 48 miR‐26a directly targeted the transcription factor KLF4 to prevent lysosomal trafficking of Mtb and to regulate Mtb survival in macrophages. 47 Enhanced miR‐155 expression in Mtb‐infected macrophages suppressed the lipidation and autophagosome formation in dendritic cells enhancing mycobacterial survival. 49 While studying a network of 77 putative miRNAs in early Mtb‐infected macrophages, miR‐155 was found to exhibit dual roles in the survival of the Mtb‐infected macrophages and the Mtb‐specific T cells through SHIP‐1/protein kinase B (Akt) pathway. 50 On the one hand, miR‐155 generated a favorable niche for the pathogen, and on the other hand, it enabled an effective adaptive immune response. 49 , 50 , 51 Similarly, Mtb‐induced miR‐33 is reported to modulate an array of responses in the host ranging from autophagy, lysosomal function and fatty acid oxidation to support Mtb replication. 52 These observations suggest that during the early interaction with TLRs and other receptors on host macrophages, Mtb triggers intricate intracellular signalling pathways that selectively regulate the miRNAs that counteractively control Mtb fates in macrophage (Figure 2). The miRNA manipulated phagolysosomal compartment affects antigen processing and presentation influencing the T‐cell responses. It remains to be investigated whether the miRNAs show kinetic regulation of their expression to match the requirements of the immune system to mount a host‐protective immune response. Dissection of the miRNAs specificity for intracellular signalling, accompanied by kinetic regulation of each of those miRNAs, will lead to the scientific rationale for a plausible miRNA based anti‐mycobacterial therapy.
Table 2.
Mycobacterium infection results in alterations in a large number of microRNAs within the host cells these up‐ and down‐regulations are highly dependent upon the infecting Mycobacterial species and responding host cell types to produce context‐dependent cellular effects
| miRNA | Status a | Targets | Cell types | Mycobacterium species | Comment/mechanism(s) of action |
|---|---|---|---|---|---|
| Undetermined | |||||
| 1. miR‐125b | – | κB‐Ras2 3′UTR | Primary human mϕ | – | Estradiol represses NF‐κB activation through induction of κB‐Ras2 |
| 2. miR‐129 | – | SP3 | Predicted | Mycobacterium tuberculosis | SP3 maintains M1/M2 plasticity |
|
3. miR‐150↓; miR‐485‐3p↑ |
– | – | THP‐1 | Mtb Beijing/W, non‐Beijing/W clinical strains | Alterations in the Wnt pathway, insulin pathway, TGF‐β pathway, and glycosaminoglycan biosynthesis |
| 4. miR‐33 | – | NOD2 | Predicted | Mycobacterium tuberculosis | Downregulation of NOD2 dampens the inflammatory response |
| 5. miR‐365 | – | IL‐6 | HEK293 | – | Post‐transcriptional level regulation of IL‐6 by miRNA‐365 |
| 6. miR‐455‐5p | – | SOCS3 | Predicted | Mycobacterium tuberculosis | The expression of miR‐455‐3p downregulate SOCS3 expression which promotes M2 phenotype |
| 7. Sp110 | – | miR‐125a; miR‐146a; miR‐155; miR‐21a; miR‐99b | RAW264.7 | Mycobacterium tuberculosis H37Ra | Sp110‐mediated macrophage resistance to Mtb underlines the inhibiting of multiple miRNAs and modulating host immune response |
| Upregulated | |||||
| 8. hsa‐let‐7b‐5p | ↑ | APO‐1/FAS/CD95 | THP‐1 | Mycobacterium tuberculosis | hsa‐let‐7b‐5p helps intracellular survival of Mtb in THP‐1 cells by downregulating Fas protein level |
| 9. hsa‐miR‐144‐5p | ↑ | DRAM2 | TB patients; PBCs; Tissues | Mycobacterium tuberculosis | Inhibiting anti‐bacterial autophagy |
| 10. miR let‐7e | ↑ | CASP3 | Human MDMs | Mycobacterium avium | Interfering with the regulation of apoptosis |
| 11. miR‐106b‐5p | ↑ | Cathepsin S | Human mϕ | Mycobacterium tuberculosis | Mtb avoids exposure to degradative enzymes in the endocytic pathway |
| 12. miR‐1178 | ↑ | TLR4 | Human mϕ; HTP‐1; U937 cells | Mycobacterium tuberculosis | Reduction of pro‐inflammatory cytokines‐ IFN‐γ, IL‐6, IL‐1β, and TNF‐α |
| 13. miR‐124 | ↑ |
MyD88; TRAF6; TLR6 |
TB patient Leucocytes; RAW264.7 AM |
Mycobacterium tuberculosis; Mycobacterium bovis (BCG) |
Negative regulatory role of miR‐124 in the fine‐tuning inflammatory response in alveolar macrophages |
| 14. miR‐125a | ↑ | UVRAG | RAW264.7; J774A.1 | Mycobacterium tuberculosis | Inhibiting autophagosome formation thereby promoting intracellular growth of Mycobacterium tuberculosis |
| 15. miR‐129‐3p | ↑ | Atg4b | RAW264.7 | Mycobacterium tuberculosis | Inhibiting autophagy favors Mtb survival |
|
16. miR‐132; miR‐26a |
↑ | p300 | Primary human mϕ | Mycobacterium tuberculosis | Limiting macrophage responses to IFN‐γ |
| 17. miR‐140 | ↑ | TRAF6 | TB patient PBMCs; THP‐1 and U937 | Mycobacterium tuberculosis | miR‐140 promotes Mtb survival by suppressing pro‐inflammatory cytokines production |
| 18. miR‐142‐3p | ↑ | N‐Wasp | J774A.1; Primary Human mϕ | Mycobacterium smegmatis | Alterations of actin filament assembly affecting other early events of phagolysosome biogenesis |
|
19. miR‐143; miR‐365 |
↑ | c‐Maf, Bach‐1, and Elmo‐1 | BMDMs | Mtb clinical Beijing strain HN878 | miRNA‐mediated regulation of c‐Maf, Bach‐1, and Elmo‐1 in Mtb‐infected (IL‐4/IL‐13) macrophages |
| 20. miR‐144 | ↑ | ‐ | TB patients PBMCs | Mycobacterium tuberculosis | Inhibiting TNF‐α and IFN‐γ production and T‐cell proliferation |
| 21. miR‐144‐3p | ↑ | ATG4a | RAW264.7 |
Mycobacterium bovis (BCG) Mycobacterium tuberculosis |
Inhibiting the formation of autophagosomes. |
| 22. miR‐145 | ↑ | TIRAP | MDMs | Virulent H37Rv | Elicited only by virulent H37Rv infection |
| 23. miR‐146 | ↑ | IRAK1; TGFBR2 | Bovine mϕ cell line (Bomac) | Mycobacterium bovis | Post‐transcriptional regulation of IL‐1, TLR signalling via IRAK1 |
| 24. miR‐146a | ↑ | TRAF6 | RAW264.7; BMDMs | Mycobacterium tuberculosis | Suppressing nitric oxide production via iNOS |
| 25. miR‐146a | ↑ | – | Human PBMCs | Mycobacterium abscessus | Mycobacterium abscessus may promote a neutrophil‐dependent growth niche |
| 26. miR‐155 | ↑ | ATG3 | Human DC | Mycobacterium tuberculosis | Subverting autophagy |
| 27. miR‐155 | ↑ | SHIP1 | Mϕ |
Mycobacterium tuberculosis Mycobacterium bovis (BCG) |
miR‐155 regulating macrophage survival and T‐cell expansion through SHIP1; miR‐155 repressing the expression of SHIP1 and modulating ROS production |
| 28. miR‐155 | ↑ | Rheb | BMDMs; RAW264.7 | Mycobacterium tuberculosis | Induction of miR‐155, in turn, activates autophagy by targeting Rheb |
| 29. miR‐155 | ↑ | FOXO3 | THP‑1; TB Patients PBMCs | Mycobacterium tuberculosis | Apoptosis inhibition through regulating FOXO3 target genes |
|
30. miR‐155; miR‐ 31 |
↑ | PP2A (Ppp2r5a) | RAW264.7; BMDMs | Mycobacterium bovis (BCG) | Mycobacterium bovis BCG‐induced miR‐155 and miR‐ 31 are required for activating the WNT‐SHH pathway and autophagy regulation |
| 31. miR‐1958 | ↑ | Atg5 | RAW264.7 | Mycobacterium tuberculosis | Inhibiting autophagy by interacting with Atg5 and supporting intracellular Mtb survival |
| 32. miR‐199a | ↑ | TBK1 | J774A.1; BMDM | Mycobacterium bovis | Suppressing maturation of autophagosomes and interferon‐β (IFN‐β) production |
| 33. miR‐206 | ↑ | TIMP‐3 | THP‐1 | Mycobacterium tuberculosis | miR‐206 is a regulator of inflammation and MMP‐9 by targeting TIMP3 |
| 34. miR‐21 | ↑ | PFK‐M | BMDM; Human MDM; RAW267.4 |
Mycobacterium tuberculosis H37Ra, H37Rv |
Mtb limits glycolysis in host macrophages through sustained induction of anti‐inflammatory miR‐21 |
| 35. miR‐21 | ↑ | IL‐12p35; Bcl‐2 | BMDMs; BMDCs | Mycobacterium bovis (BCG) | Modulating anti‐mycobacterial TH1 response inefficacy of BCG vaccination |
| 36. miR‐223 | ↑ | CXCL2; CCL3; IL‐6 | TB patients; Murine myeloid cells | Mycobacterium tuberculosis | miR‐223 regulating leucocyte chemotaxis via chemoattractants |
| 37. miR‐22‐3p | ↑ | Unknown | TB patient | Mycobacterium tuberculosis | Plasma biomarker |
| 38. miR‐23a‐5p | ↑ | TLR2 | RAW264.7 | Mycobacterium tuberculosis | Modulation of TLR2/MyD88/NF‐κB signalling |
| 39. miR‐27a | ↑ | CACNA2D3 | Human PBMCs | Mycobacterium tuberculosis | Inhibiting autophagosome formation and promoting the intracellular survival of Mtb |
| 40. miR‐27b | ↑ | Bag2 | RAW264.7; HEK293T | Mycobacterium tuberculosis | miR‐27b positively regulates apoptosis by directly targeting Bag2 and increasing the activity of the p53–ROS signalling pathway |
| 41. miR‐29a | ↑ | CASP7 | Human MDMs | Mycobacterium avium | Interfering with the regulation of apoptosis |
| 42. miR‐30a | ↑ | MyD88 | THP‐1 cells | Mycobacterium tuberculosis H37Rv | Inhibiting TLR/MyD88 activation and cytokine (TNF‐α, IL‐6, IL‐8) expression |
|
43. miR‐31; miR‐150 |
↑ | MyD88 | TB patients PBMCs; BMDMs | Mycobacterium bovis (BCG) | Sonic hedgehog signalling‐responsive miR‐31 and miR‐150 target MyD88 suppressing TLR2 signalling |
| 44. miR‐3178 | ↑ | TRAF‐3 | THP‐1 | Mycobacterium tuberculosis H37Rv | More research is required for inferring definitive roles of this miRNA in the context of Mtb infection |
| 45. miR‐32‐5p | ↑ | FSTL1 | THP‐1 and U937 | Mycobacterium tuberculosis | TLR‐4/miRNA‐32‐5p/FSTL1 axis modulating host defence against mycobacterial infection |
| 46. miR‐33 | ↑ | ABCA1; ATG5; LAMP1 | THP‐1 | Mycobacterium tuberculosis | Target's autophagy suppression, and compromisation of lysosomal function, and lipid homeostasis |
| 47. miR‐3619‐5p | ↑ | Cathepsin S (CTSS) | THP‐1 | Mycobacterium bovis (BCG) | CTSS targeting by miR‐3619‐5p impairs the degradation of autophagic substrates thus blocking autophagosome‐lysosome processing |
| 48. miR‐381‐3p | ↑ | CD1c | TB patient DCs | Bacillus calmette‐Guérin (BCG) | Suppression of lipid antigen presentation and induction of IL‐10 |
| 49. miR‐579 | ↑ | SIRT1; PDK1 | Human mϕ | Mycobacterium tuberculosis | Macrophage cell death and apoptosis |
| 50. miR‐‐5p | ↑ | Bcl‐2; TLR4 | RAW264.7 and THP‐1 | Mycobacterium tuberculosis | Enhances Mtb survival and apoptosis, by attenuating the secretion of inflammatory cytokines (IL‐1β, IL‐6, and TNF‐α) |
| 51. miR‐708‐5p | ↑ | TLR4 | Human mϕ | Mycobacterium tuberculosis | Reduction of pro‐inflammatory cytokines‐ IFN‐γ, IL‐6, IL‐1β, and TNF‐α |
| 52. miR‐889 | ↑ | TWEAK | Latent TB patients | Mycobacterium tuberculosis | miR‐889 inhibits autophagy via suppression of TWEAK expression |
| 53. miR‐99b | ↑ |
TNFRSF4/ OX40; TNF‐α |
DC and mϕ | Mycobacterium tuberculosis H37Rv | The knockdown of miR‐99b in DCs reduces Mtb growth owing to increasing levels of IL‐1β, TNF‐α |
| Downregulated | |||||
| 54. miR let‐7f | ↓ | A20/TNFAIP3 | RAW264.7 | Mycobacterium tuberculosis | Mycobacterium tuberculosis macrophage infection leads to ESAT‐6‐dependent miRNA let‐7f downregulation |
| 55. miR‐125b | ↓ | TNF mRNA | Human mϕ | Mycobacterium smegmatis | TLR2‐dependent MAPK p38 and the PI3K/Akt pathway with the production of steady‐state TNF mRNA |
| 56. miR‐144 | ↓ | Tpl2/MAP3K8 | MDMs | Mycobacterium tuberculosis | Suppression of TNF‐α, IL‐1β, and IL‐6 via the ERK1/2 pathway |
| 57. miR‐17 | ↓ | ULK1; Beclin 1; ATG7; MCL‐1 ATG16L1; p62; STAT3 | RAW264.7 | Mycobacterium tuberculosis | miR‐17/PKC δ/STAT3 axis regulates autophagy during Mtb infection |
| 58. miR‐20b | ↓ | NLRP3 | TB patient mϕ | Mycobacterium tuberculosis | Deactivating the NLRP3/caspase‐1/IL‐1β pathway in TB mice; Mitigating the inflammation and pyroptosis |
| 59. miR‐20b‐5p | ↓ | Mcl‐1 | RAW264.7 | Mycobacterium tuberculosis | Enhancing Mtb survival via attenuating the cell apoptosis by Mcl‐1 upregulation |
| 60. miR‐26a | ↓ | KLF4 | RAW264.7 | Mycobacterium tuberculosis | Facilitates upregulation of KLF4 consequently increases arginase and decreases iNOS activity; affecting the trafficking of Mtb to lysosomes |
| 61. miR‐26b | ↓ | TAK‐1 | THP‐1 | Mycobacterium tuberculosis | miR‐26b suppresses the TNFα‐induced NF‐κB signalling in THP‐1 cells |
| 62. miR‐27a | ↓ | TAB 2/3 | RAW264.7; BMDMs | Mycobacterium avium subspecies paratuberculosis | Inhibiting the activation of the MAPK‐p38 signalling |
| 63. miR‐27a | ↓ | IRAK4 | THP‐1 | Mycobacterium tuberculosis | miR‐27a inhibiting the release of inflammatory factors and promoting mycobacterial survival |
| 64. miR‐29 | ↓ | IFN‐γ mRNA | NK cell; CD4+; CD8+ T cells | Mycobacterium bovis (BCG) | miR‐29 suppresses IFN‐γ production by directly targeting IFN‐γ mRNA |
| 65. miR‐3178 | ↓ | TRAF‐3 | GES‐1 cells | Helicobacter pylori | Contrasting to Mtb infection, miR‐3178 is downregulated, controls inflammation, and gastric carcinogenesis in this model |
Status of miRNAs indicating Downregulation (↓) and Upregulation (↑)
Figure 2.
Regulatory miRNAs network that modulates the process of autophagy and promotes intracellular survival of Mycobacterium sp. (1) The process of autophagy in Mtb‐infected macrophages/APCs is shown. Mycobacterium modulates the miRNAs by either up‐ or downregulating the expression of certain miRNAs that have an impact on autophagy and hence its intracellular survival. Many of these miRNAs do influence CD40 expression, CD40 signalling, and subsequent survival or elimination of Mycobacterium. However, the use of miRNAs as pathogenic biomarkers for tuberculosis requires consideration of the Mycobacterium species and host cell type and genetics of the host. miRNA targeting using antagomiRs–oligonucleotides for devising an anti‐mycobacterial strategy seems feasible.
miRNA regulation of CD40 expression in M. tuberculosis‐infected macrophages and dendritic cells
An effective anti‐mycobacterial therapy would require appropriate T‐cell response, which is dependent on CD40‐CD40L interactions. While CD40 signals through many signalling pathways in macrophages, 53 signalling through CD40L potentiates the T‐cell antigen‐specific receptor‐activated T‐cell functions. 54 It is reported that several microRNAs regulate CD40 expression in various cell types. For example, miR‐145 down‐regulates CD40 expression specifically in vascular smooth muscle cells 55 and in human monocyte‐derived macrophages. 56 TNF‐α increases CD40 expression in a model of atherosclerosis but reduce miR‐145 expression. 57 miR‐146a targets TRAF6 and IRAK1 to repress CD40 expression in PBMCs obtained from patients with myasthenia gravis 58 and perhaps also in other cell types.
IFN‐γ and TNF‐α – the cytokines that activate macrophages to kill Mtb – are shown to enhance CD40 expression. In Mtb‐infected macrophages, IFN‐γ that inhibits miR‐21 enhances CD40 expression and anti‐mycobacterial functions. 59 , 60 Opposing IFN‐γ and TNF‐α, transforming growth factor‐β (TGF‐β) deactivates macrophages to impair anti‐mycobacterial functions and reduces CD40 expression in macrophages. 60 miR‐21 thus inhibits TNF‐α‐induced CD40 expression via the SIRT1‐NF‐κB signalling pathway. 61 IFN‐γ activates STAT‐1 homodimerisation to execute its effects. Mtb upregulates expression of miR146a that targets STAT1 to reduce CD40 expression. 62 miR‐29a augments CD40 expression in bone marrow‐derived DCs. 63 While miR‐29a targeted IFN‐γ mRNA reduces its expression, IFN‐γ reciprocally inhibited miR‐29a expression in T cells. 64 In TB patients, miR‐16 is significantly elevated but miR‐155 is reduced. 65 While TLR4 stimulation reduces the level of miR‐16 that negatively regulates the CD40 expression, 66 Helicobacter pylori infection enhances the expression of miR‐155 that promotes CD40 and TNF‐α expression. 67 Thus, Mtb infection modulates the expression of miR‐16, miR‐21, miR‐29a, miR‐145, miR‐146a and miR‐155, which in turn regulate CD40 expression (Figure 3).
Figure 3.
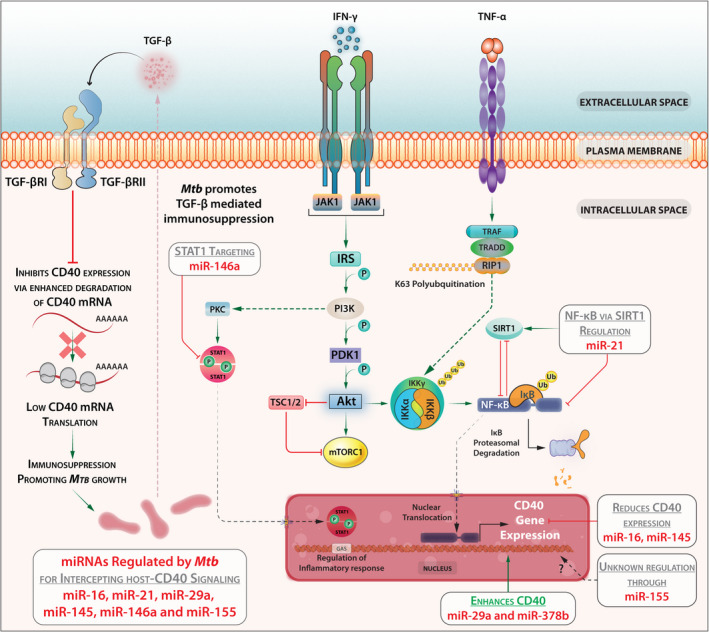
Mtb infection modulates the expression of miR‐16, miR‐21, miR‐29a, miR‐145, miR‐146a and miR‐155 which in turn regulate CD40 expression.
Transcription factors regulate CD40 expression
Besides microRNAs, other factors regulate CD40 expression in macrophages. NF‐κB may function as a central regulator of CD40 expression, 68 , 69 perhaps through TLR4‐CD40 and TLR9‐CD40 feed‐forward motifs as shown in the case of another intra‐macrophage pathogen, Leishmania major. 70 The mitogen‐activated protein kinases (MAPKs) – JNK and p38MAPK but not ERK – may activate NF‐κB to augment CD40 expression in both mouse and human macrophages. 71 LPS/TLR4‐induced CD40 expression involves the endogenous production of the cytokine IFN‐β. IFN‐β induces not only STAT‐1α‐dependent CD40 expression but also SOCS‐1 that inhibits cytokine signalling affecting CD40 expression in macrophages and microglia. IFN‐β‐induced CD40 gene expression is thus self‐limited by IFN‐β‐induced SOCS‐1 expression. 72 Besides NF‐κB, IRF8 is another key transcription factor that regulates CpG‐promoted CD40 expression. TRAF6 and IRAK1 may also be targeted by miRNA‐146a to reduce CD40 expression in DCs. 73 It is known that the virulent Mtb strain H37Rv invades macrophages quicker than the avirulent H37Ra but the avirulent strain induces significantly higher nitric oxide and hydrogen peroxide, IL‐12, TNF‐α and IFN‐γ productions from the infected macrophages. It remains to be investigated whether CD40‐CD40L interaction is a key factor in Mtb virulence 74 , 75 and vice versa.
CD40 expression in various circulating and alveolar cells of TB patients
Most of the studies mentioning the roles of miRNAs concerning the modulation of CD40 levels are either performed in vitro or using mouse models. Through an exhaustive analysis, Fu et al. 76 has found a huge number of microRNAs in the serum of active pulmonary tuberculosis patients, but it is yet to be determined whether these circulating miRNAs have targets that are involved in CD40 pathways that may produce specific changes in the effector/memory T cells or APCs. However, this proposition also requires experimental validation. We have summarised the roles of such miRNAs (Table 2) in the modulation of CD40‐signalling during Mtb infection. In general, AMs rely less on glycolysis but more on OXPHOS for meeting their energy requirements under steady‐state conditions. 77 AMs exhibit low‐efficiency antigen presentation and very low‐level expression of costimulatory molecules 78 including CD40. However, infection or other stimulation could enhance the CD40 level among this lung residential APC population. 79 Patients suffering from hyper‐IgM syndrome, caused by the mutations in CD40L and thereby defects in CD40 signalling, may have increased susceptibility to intracellular pathogens 80 including Mycobacterium. 81
Although the AMs were traditionally believed to be the only host cell for Mtb proliferation, recent findings support that the pathogen could thrive in many different phagocytes within the lung microenvironment. Kinetic studies further defend the concept that the initial distribution of the pathogen remains associated with AMs, but during the chronic phase of infection, the disseminating bacilli and plausibly latent bacteria may spread among other phagocytes including interstitial macrophages perpetuating the infection. This observation supports that diverse macrophage populations in the lungs rather serve as the Mtb growth permissive environment in a temporal manner. 82
Macrophage CD40 expression can be enhanced by IFN‐γ through activation of the transcription factors STAT‐1 and NF‐κB via an autocrine positive feedback loop including IFN‐γ‐induced TNF‐α. IFN‐γ‐induced CD40 expression is suppressed by antilipidaemic agent simvastatin that inhibits 3‐hydroxy‐3‐methylglutaryl (HMG)‐CoA reductase – an enzyme required for the synthesis of isoprenoids and STAT‐1 expression. The inhibition of the prenylation of Rho family proteins a family of small GTPases inhibits CD40 and STAT‐1 expression. As a consequence, STAT‐1α and RNA Polymerase II recruitment to the CD40 promoter are diminished and H3 and H4 histone acetylation is reduced. 83 Functional analysis of CD40 promoter in microglial cells indicates that STAT‐1 binds to two IFN‐γ‐activated sequence elements. The transcription factors PU.1 and/or Spi‐B bind to the Ets elements. 84 , 85 IL‐4‐activated transcription factor STAT6 binds to these two proximal and distal IFN‐γ‐activated sequences and represses CD40 expression. 86 Thus, several transcription factors act in tandem to regulate CD40 gene expression in cells of the macrophage lineage (Figure 4).
Figure 4.
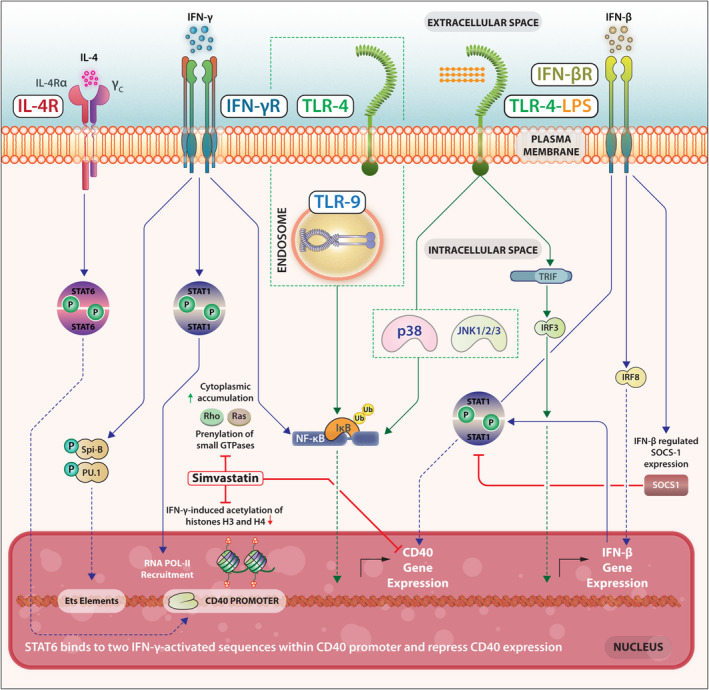
Several transcription factors act in tandem to regulate CD40 gene expression in cells of the macrophage lineage.
The induction of CD40‐CD40L expression in B cells, DCs and endothelial cells can also be of therapeutic importance. As CD40 engagement on the DCs membrane directly augments the cytokine production, cross‐antigen presentation and maturation, CD40 regulates DCs activation and differentiation. Similarly, in the case of B cells, CD40 signalling promotes cell survival, germinal centre formation, Ig class switching and somatic hypermutation of the Ig to enhance Ag affinity and formation of memory and plasma B cells. 87 The involvement of the CD40‐CD40L pathway in Mtb infection is paradoxical, although targeting this pathway provides long‐term clinical benefits in many diseases including organ transplantation 88 and autoimmunity. 89 Similar beneficial effects of CD40‐CD40L expression/signalling may constitute a futuristic anti‐TB therapy.
Altered antigen processing in Mtb‐infected macrophages or dendritic cells
Mtb antigen processing is preceded by its uptake into the phagosomal vesicles. One way to survive within the host cells is to stall further maturation of the phagosomes and thereby antigen processing, too. 90 Phagosomal maturation involves fusion with lysosomes (the vesicular organelle rich in hydrolases, proteases, lipases and other enzymes that are required for degradation of the pathogen and the pathogen‐derived antigens) so that the resulting peptides can be complexed with MHC class‐I or MHC class‐II molecules for presentation to T cells as the phagolysosomal vesicles are acidified. Mtb inhibits this phagosomal maturation to ensure persistence in the immature phagosomes (Figure 5).
Figure 5.
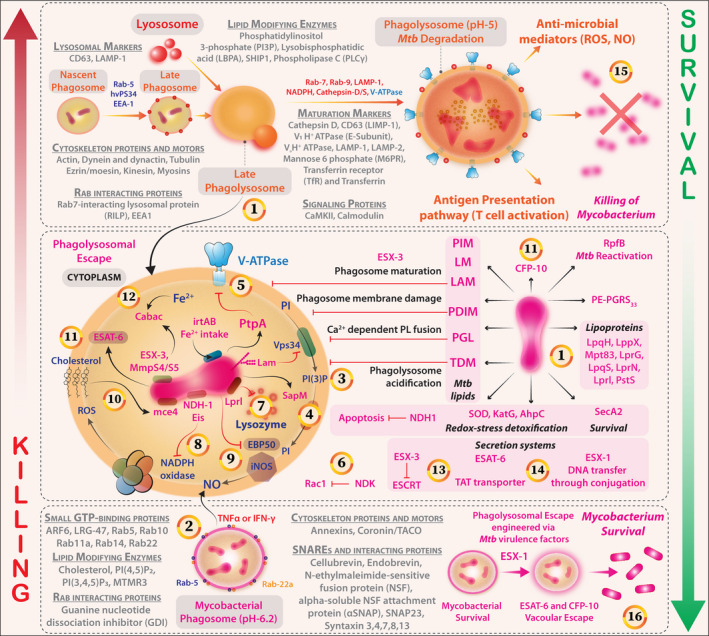
Pathway of phagosome biogenesis, maturation, and phagolysosome fusion for efficient clearance of Mtb. Ploys of immunoevasion via Mtb virulence factors are also shown. (1) Mycobacterium deploys several factors that subvert the phagosome biogenesis, maturation and acidification steps that follow its internalisation. Pathogenic Mycobacteria reside within compartments devoid of lysosomal contents because of blocking of Ca2+ fluxes and receive nutrients through modulation of Rab‐dependent vesicular trafficking. LAM and PIM drive these processes. (2) Mycobacterial phagosomes (Bottom) through various proteins counter the independent stress factors such as reactive oxygen species (ROS) and reactive nitrogen species (RNS); however, immunological activation with TNFα or IFN‐γ results in the maturation of phagosomes by the maturation marker expression and lysosomal fusion (Their distinct markers and associated proteins are represented with Grey Font). (3) LAM inhibits Ca2+ influx and PI3P‐dependent delivery of lysosomal components (V‐ATPase and Cathepsin) from the Trans‐Golgi network (TGN) to the phagosome. (4) Mycobacterium, perhaps through secretory acid phosphatase (SapM), targets small GTPases – Rabs, Rhos or ARFs – to affect Coronin‐1/TACO‐dependent actin cytoskeleton rearrangements and phagosome maturation. (5) The mycobacterial protein tyrosine phosphatase (PtpA) inhibits V‐ATPase and phagosomal acidification. (6) The nucleotide diphosphate kinase (NDK‐1) of mycobacterium may inactivate small GTPase Rac‐1 and attenuate NADPH oxidase‐mediated host protection. (7) Lprl, a mycobacterial Lipoprotein, inactivates the lysozyme. (8) The Type‐I NADH dehydrogenase and Eis protein inhibit the NADPH oxidase activity limiting the ROS availability. (9) Mycobacterium effectively attenuates NO production by interfering with EBP50 and iNOS recruitment. (10) The mammalian cell entry protein‐Mce4 scavenges cholesterol from host membranes and potentiates lipid body accumulation and mycobacterial survival. (11) Early secretory antigenic target‐6 (ESAT‐6), a major virulence factor that controls NF‐κB and interferon‐regulatory factors, and CFP‐10 engineer vacuolar escape and intracellular survival of Mycobacterium. (12) Mtb hitchhikes intracellular Fe2+ stores a major siderophore mediating this process is Carboxymycobactin. (13) ESX‐3 secretion system (composed of EsxG and EsxH) leads to impairment of ESCRT‐mediated endomembrane repair. (14) ESX‐1 mediates the process of phagosomal to cytosolic translocation. (15) A potent phagosomal maturation and intracellular degradation of Mtb by the acquisition of indicated markers (Grey fonts). Results in potentiation of APC‐T‐cell antigenic presentation pathway and confers T cell‐based protection against the bacterium. (16) In contrast, the association between Mtb virulence factors (Factors that are associated with Mtb are shown in pink colour) and potent immunosuppression, steps of phagosomal, maturation, acidification, neutralisation/detoxification of redox stress and inhibition of autophagic processes together induce permissive niches for Mtb replication and dissemination.
Mtb‐secreted EspB [Early Secretory Antigenic Target 6 (ESAT‐6) system 1 (ESX‐1) secretion‐associated protein B)] and EspA suppress antigen‐processing functions of the Mtb‐infected macrophages 91 reduce IFN‐γRI expression and inhibit IFN‐γ‐activated STAT1 phosphorylation. 92 , 93 Avirulent Mtb is perhaps deficient in this system and may, therefore, be unable to survive in macrophages. Molecular analyses show that LRRK2 (leucine‐rich repeat kinase 2) negatively regulates phagosome maturation via the recruitment of phosphatidylinositol‐3 kinase (PI3K) complex and Rubicon to the phagosome in macrophages, 94 as LRRK2 inhibition and LRRK2‐deficiency enhance phagosome maturation and significantly reduce Mtb burden in macrophages 94 but lysophosphatidylcholine promotes phagosome maturation via cAMP‐induced activation of the PKA‐PI3K‐p38MAPK pathway and controls Mtb infection through Ca2+ and ROS‐dependent pathways. 95 As CD40 also induces the host‐protective pathway of PI3K and p38MAPK in macrophages, CD40 stimulation in Mtb‐infected macrophages would also reduce bacterial burden. CD40 appears to be a likely target of the bacteria, as CD40 expression is reduced in Mtb‐infected macrophages.
Virulent Mtb causes marked disorganisation of actin filaments and F‐actin fragmentation in the cytoplasm of infected macrophages, which contributes to delayed phagolysosomal fusion. 96 Mycobacterial polyunsaturated lipids bind ATP and its receptor P2X7 regulating actin polymerisation. 97 cAMP‐dependent inhibition of actin polymerisation in phagosomes containing virulent Mtb prevents phagolysosomal fusion supporting bacterial growth 98 (Figure 5). Hence, the ability of the lipid/ATP/P2X7 axis to destabilise actin polymerisation and consequently delay phagosome maturation deserves further investigation.
The intravesicular pH in the Mtb‐inhabited phagosomes is between 6.3 and 6.0, whereas the lysosomal lytic enzymes require a pH lower than 3.0 (Figure 5). Even if these LAMP‐1‐positive phagosomes fuse with lysosomes, the vacuolar‐ATPase that is required for pumping protons into the vesicular lumen is extruded. 99 The impaired acidification associated with vacuolar‐ATPase exclusion has negative effects on antigen processing and presentation, as vacuolar‐ATPase‐dependent phagosomal acidification is necessary for generating processed Mtb antigens. 100 The initial Mtb–macrophage interaction dictates the state of phagosomal maturation, as TLR2 blockade, but not CR3 blockade, promotes phagosomal acidification and bacterial death 101 (Figure 5).
CD40 at the interface of macrophage and T cells
The characteristic caseous lesions in the lung are the sequel of a strong granulomatous response mediated by activated T cells (Figure 6). The T cells are activated by at least two signals: (1) T‐cell receptor signal triggered by the recognition of Mtb antigens presented by the AMs or dendritic cells in the context of MHC‐II or MHC‐I molecules and (2) the costimulatory signal from CD28 that interacts with the CD80 and CD86 expressed on the antigen‐presenting AMs or dendritic cells. During the macrophage–T cell interaction, the T cell‐expressed CD40‐ligand (CD40L) binds to the macrophage‐expressed CD40 and triggers CD40 signals in the macrophage. CD40 is known to signal through a cascade of kinases to induce NF‐κB‐dependent IL‐12 expression that leads to TH1 cell differentiation and host protection. Additionally, the same CD40 can also signal through a different pathway to generate IL‐10 and TGF‐β that aggravate the disease by deactivation of macrophages and differentiation of T‐reg cells (Figure 6). The antigen‐presenting cell‐secreted IL‐12 works on the T cells through IL‐12R to trigger the STAT4‐dependent induction of IFN‐γ. IFN‐γ activates the Mycobacterium‐infected macrophages to elicit STAT‐1‐dependent iNOS‐catalysed nitric oxide‐mediated mycobactericidal functions of macrophages. IL‐4, IL‐10 and TGF‐β antagonise these host‐protective functions. Therefore, it is possible that these two counteractive effector functions of CD40‐CD40L interaction determine the outcome of Mtb infection.
Figure 6.
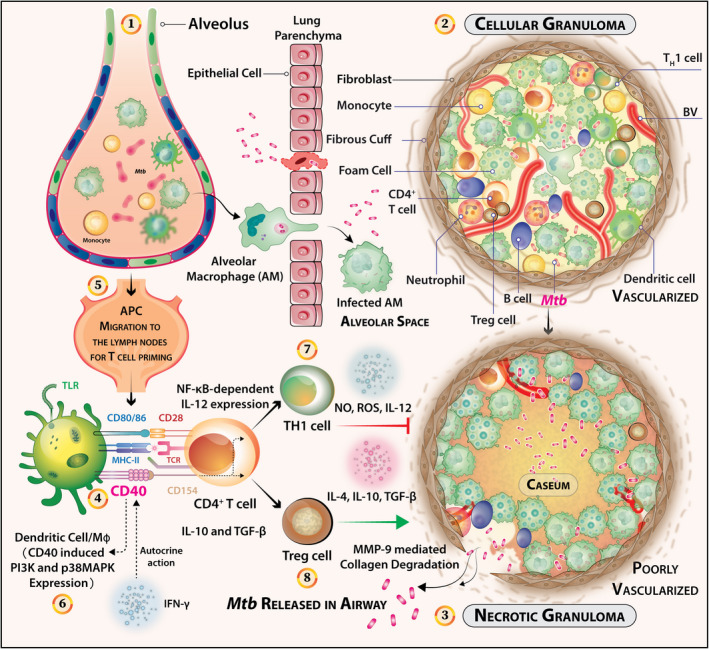
CD40 signals regulate the effector T‐cell responses that in turn control the growth of Mtb, balancing granuloma pathogenesis and subsequent dissemination of the bacilli in the airway. (1) The advent of the tuberculosis disease occurs via confrontation of Mtb bacilli and the alveolar macrophages. The infection initiates with receptor‐mediated internalisation and triggers a cascade of events that govern the subsequent fates of the pathogen both intracellular and extracellular. (2) Infected macrophages may recruit other cell types such as CD4+ T cells, monocytes, neutrophils, B cells and DCs. The granulomatous niche can occur as safe houses for reinitiating latent TB infection. (3) However, incapacitated immune responses can lead to the formation of necrotic granulomas indicative of chronic or latent TB infection. These type of granuloma are poorly vascularised and calcified to the core with the characteristic caseous centre. An abundance of foam cells with peripheral fibrotic cuffs abstaining T and B cells can also be marked histologically. Altogether, caseous granulomas can harbour drug‐tolerant Mtb. (4) Nonetheless, the Mtb containment strategy of the host can turn on radically upon itself when necrotic granulomas are formed. A strong TH1 cell response may circumvent this critical transition into which receptors like CD40 may have previously unexplored roles. (5) Within the draining/thoracic lymph nodes, the T cells are primed slowly at about 12–20 Days post‐Mtb infection, as indicated in animal models. (6) CD40‐CD40L crosstalk between T cells, B cells and DCs may promote signals to DCs to induce IL‐12 secretion resulting in TH1 cell differentiation and IFN‐γ‐mediated anti‐mycobacterial effects. (7) CD40 is known to signal through a cascade of kinases to generate NF‐κB‐dependent IL‐12 expression that leads to host protection by TH1 cells. (8) On the contrary, the same CD40 can also signal through a different pathway to generate IL‐10 and TGF‐β that aggravate the disease by deactivation of macrophages and differentiation of T‐reg cells. Therefore, more information is required to dissect the underlying roles of CD40 in mediating the pathogenesis of Mtb granulomatous response.
Vaccine‐based protection to Mtb heavily relies on the induction of IFN‐γ‐producing CD4+ T cells. IL‐17A and IFN‐γ are two important cornerstones for vaccine‐induced protection against experimental tuberculosis. Through the adoptive transfer of exogenously primed activated DCs into the lungs of vaccinated mice at the time of Mtb infection may overcome the lag required for the generation of vaccine‐induced memory CD4+ T cells. This effect can be accelerated by the induction of endogenous CD103+ DC and activation of the CD40 pathway through the TLR ligand amph‐CpG, coupled with CD40 agonist FGK4.5. 102 Additionally, out of numerous receptor–ligand interactions occurring at the APC‐T cell synapses, the CD40‐CD154 interaction is vital for the optimal activation of CD4+ T cells. In the case of Mtb‐infected DCs, their interaction with T cells is required for inducing protective IL‐17 response. Blocking the CD40‐CD40L interaction with the anti‐CD40L antibody MR1 attenuates the IL‐17 response to Mtb‐infected DCs despite stimulation with CD40LT. 103 This effect is also independent of the low Mtb‐antigenic concentration during the initial phase of infection as observed by others. 104 Therefore, CD40‐mediated costimulation may polarise TH17 cells independent of the antigenic loads in airway tissue, which may perhaps be a crucial event in restricting early replication of Mtb. 103 These protective effects can also be augmented by signals that are dependent on PRRs as another study advocates that a latency associated protein resuscitation‐promoting factor (Rpf) E can induce TLR4‐dependent DC maturation and promotes TH1/TH17 type immunity in vivo. 105 Our group showed that TLR4 and CD40 can modulate each other's expression in the experimental model of cutaneous leishmaniasis. 70 Possibly, TLR4‐CD40 cross‐regulation may be controlling the protective immunity against Mtb infection.
Presentation of the processed Mycobacterial antigens to T cells
The antigen presentation to T cells involves presenting an antigenic peptide in a complex with either MHC class I or MHC class II for recognition by the antigen‐specific T‐cell receptor (Figure 7). Many of the mycobacterial ligands are elicitors of the cytosolic surveillance pathway (CSP). These pathways are activated by mycobacterial ESX‐1 secretion system‐mediated extrusion of DNA/RNA allowing activation of host mobile intracellular pathogen sensors including RIG‐1, MDA‐5, c‐GAS/STING/TBK‐1, PKR, NLRP3, AIM‐2 and others (Figures 1 and 7). The activation of CSP‐pathway relates to robust Type‐I IFN signatures in response to this pathogen. 106 Although Type‐I IFNs may defend against viruses, their induction by bacteria is detrimental to the host. 107
Figure 7.
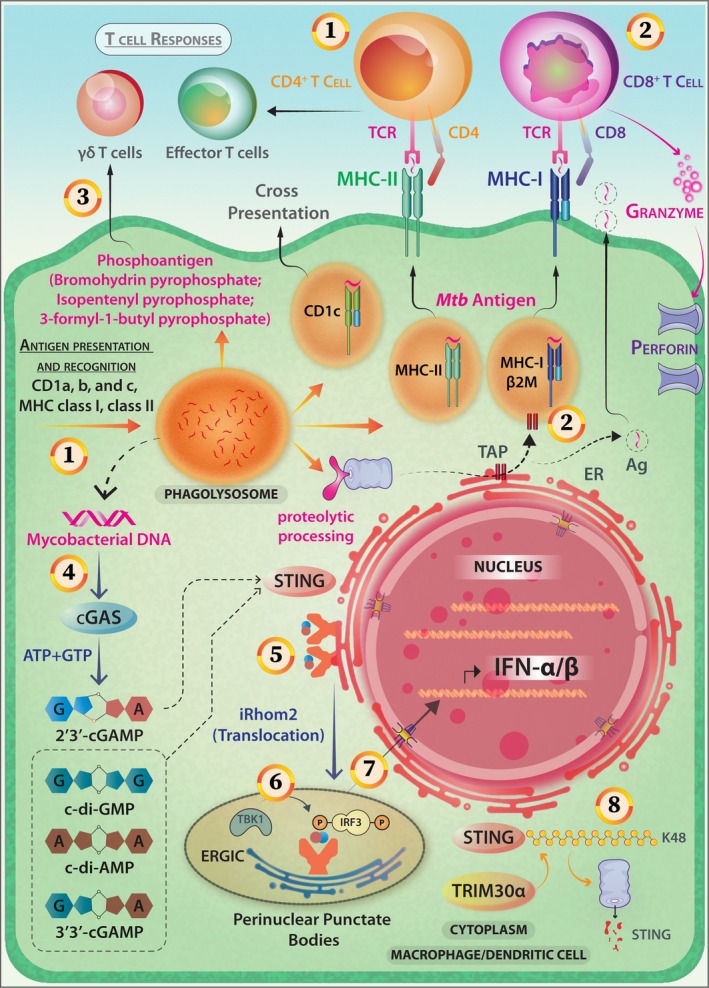
Antigen presentation, T‐cell responses and activation of the cytosolic surveillance pathway (CSP) in the case of Mycobacterial infection of APCs. (1) Mycobacterial antigens can access both cytosolic and vacuolar antigen‐processing pathways and are presented by the class‐II MHC pathway inducing a potent CD4 response. (2) Presentation in the context of MHC‐I (whereby CD8+ T cell is activated) and CD1 (lipidic antigen) is also reported. (3) Novel phospholigands like bromohydrin pyrophosphate (BrHPP), Mycobacterial antigens (Isopentenyl Pyrophosphate, IPP and non‐prenyl phosphoantigen 3‐formyl‐1‐butyl‐pyrophosphate) are potent elicitors of Vγ9vδ2+ T cells. (4) Mycobacterium activates cytosolic sensor c‐GAS, the STING/TBK1/IRF3 pathway through c‐GAMP and induces Type I IFN‐mediated innate immune responses. (5) Cyclic dinucleotides binding on STING induces its migration from the endoplasmic reticulum (ER) to form perinuclear punctate structures. This intracellular trafficking is mediated by iRhom2. (6) TBK‐1 phosphorylates CTD‐of STING and that results in IRF‐3 recruitment and phosphorylation. (7) The IRF‐3 homodimers translocate to the nucleus to activate the gene transcription of type‐I IFNs. (8) TRIM30α, which is a negative‐feedback regulator of STING via K48‐linked polyubiquitination, marks it for proteasomal degradation. (9) Additionally, Mycobacterium actively employs SecA2 and ESX‐1 secretion systems for releasing RNA into host cells and elicits IFN‐β production through STING and IRF3 activation. Mycobacterial RNA activates the RIG‐1‐MAVS‐TBK1‐IRF‐7 pathway (not shown).
The number of antigen‐loaded MHC molecules and the accessibility of the T‐cell receptor to the presented peptide antigen decide the efficacy of this antigen presentation. Mtb‐infected macrophages express significantly fewer MHC‐I and MHC‐II molecules on the surface, 108 , 109 the T‐cell receptors' accessibility to the peptide–MHC complex remains to be investigated. TLR2‐Mtb lipoprotein interaction inhibits IFN‐γ‐induced MHC‐II expression and processing of soluble antigens in a Class II transactivator (CIITA) IV‐dependent and MAPK‐dependent manner. 110 Repressed MHC‐II expression and enhanced TLR2‐driven macrophage apoptosis decrease antigen recognition by CD4+ T cells. IL‐10 plays a significant role in this process. 111
Expression of costimulatory molecules on Mycobacterium‐infected macrophages
The Mtb‐infected BALB/c‐derived macrophages have reduced CD80, but enhanced ICAM‐1, expression 112 perhaps mediated by a 10kDa antigen from Mtb. 113 Consistent with the enhanced IL‐10 production by the Mtb‐infected macrophages, IL‐10 is shown to downregulate the expression of costimulatory molecules on macrophages. 114 As T‐cell activation through T‐cell antigen receptor in the absence of the costimulatory signal results in T‐cell anergy, the antigen presentation by significantly low CD80‐expressing Mtb‐infected macrophages leads to T‐cell anergy 115 that has been attributed to IL‐10 from the antigen‐presenting macrophages. 116 Besides anergy, T‐cell response is further reduced by higher levels of PD‐L1 expression on Mtb‐infected macrophages and PD‐1 on T cells. 117 CD80‐mediated T‐cell costimulation is thus balanced by the negative effects of PD1‐PD‐L1 interaction. However, CD40‐CD40L interaction can significantly influence this balance in T‐cell response.
CD40‐CD40L as a crucial costimulatory receptor–ligand pair in tuberculosis
CD40 signalling, albeit uncharacterised in Mtb‐infected macrophages, appears to play important roles in eliciting T‐cell responses. CD40‐CD40L interaction is shown to enhance the IL‐12‐ and IL‐18‐dependent, CREB‐ and c‐Jun‐promoted IFN‐γ production by Mtb‐responsive CD8+ T cells that also execute perforin‐ and granulysin‐mediated cytotoxicity on Mtb‐infected macrophages. 7 CD40‐deficient mice show aggravated Mtb infection because of inadequate IL‐12 and IFN‐γ responses as compared to the wild‐type control. 118 An agonistic anti‐CD40 antibody elicited strong CD40 signalling in both uninfected and BCG‐infected DCs resulting in increased expression of MHC‐II and costimulatory molecules, mRNA production related to pro‐inflammatory cytokines and IL‐12. 119 CD40‐deficient Mtb‐infected, but not the uninfected, DCs failed to elicit antigen‐specific TH17 cells. 120 CD40L treatment of human monocytes resulted in anti‐mycobacterial activities. 119 By contrast, compared with the wild‐type mice, CD40L‐deficient mice remain resistant to Mtb infection, although these mice had fewer granulomas and fewer CD4+ T cells in granulomas. 121 While these observations indicate that CD40 plays a significant role in anti‐tubercular T cell‐mediated host protection, some observations suggest otherwise leading to a paradox.
The paradox stems from the following findings. Firstly, the direct CD40 engagement on chronically Mtb‐infected macrophages failed to elicit mycobactericidal activities 120 possibly because of complete subversion or switching to probacterial CD40 signalling. In fact, such observations were reported with L. major infection of BALB/c‐derived macrophages. 122 Yet, whether similar possibilities exist in Mtb infection remains unexplored. Secondly, CD40‐deficient mice succumbed to aerosolic low‐dose Mtb infection because of deficient IL‐12 production leading to impaired priming of IFN‐γ‐secreting T‐cell responses but the CD40L‐deficient mice remained resistant to the same infection. 121 These paradoxical results in CD40‐deficient and CD40L‐deficient mice implied the presence of an alternative ligand for CD40. Indeed, mycobacterial Hsp70 has been proposed to be an alternative ligand for CD40, as Hsp70 was coimmunoprecipitated with CD40 from Mtb‐infected monocytic cell lines. 123 However, as Hsp70 is conserved from bacteria through humans, it remains to be seen whether mouse or human mono‐mac cells expressed Hsp70 evokes intracellular signalling similar to that triggered by CD40L and elicits protection against the Mtb infection. Thirdly, CD40L‐deficient mice developed anti‐mycobacterial T‐cell responses to the levels observed in the wild‐type mice.
The data generated using the CD40‐deficient or CD40L‐deficient mice, or the mono‐mac cells thereof, thus present a conundrum about the role of CD40 in Mtb infection. Recent mass‐spectrometry based studies have identified nitric oxide‐induced alterations in the expression of 1713 proteins in Mtb‐infected macrophage‐like cell line. 124 Nitric oxide can be generated in situ by the inducible nitric oxide synthetase, which can be induced by CD40 signalling. 125 It has also been shown that in response to such oxidative stresses, Mtb alters the phosphorylation of serine, threonine and tyrosine kinases. 126 It is possible that CD40‐induced IL‐10 exerts pro‐mycobacterial effects, as reported for Leishmania infection in macrophages. 125 This would fit the conundrum, as CD40 was shown to induce IL‐12 and IL‐12‐induced IFN‐γ was shown to activate macrophages to trigger anti‐mycobacterial effects such as by NO and reactive oxygen species productions. 127 Therefore, the same receptor CD40 signals in a contrasting manner when macrophages are chronically infected, or not, with M. tuberculosis and trigger counteractive effector functions.
Concluding remarks
It is clear from the above account that Mycobacterium redirects or suppresses the immune response by intercepting the following processes: (1) the processing of the mycobacterial antigens by the antigen‐presenting cells such as macrophages and DCs, (2) presentation of the processed Mycobacteria‐derived antigens, (3) responsiveness of the T cells to the antigen‐derived first signal and the ancillary signals from the costimulatory molecules and cytokines and (4) response of the Mycobacterium‐infected macrophages to different cytokines. The conclusions from the analyses are expected to reveal the regulation of macrophage functions by CD40‐CD40L interactions, negative regulators and dynamicity in the infection process. Such understanding will brace up novel aspects of macrophage–Mycobacterium interactions including the mechanisms of pathogenesis and possible immunotherapeutic targets.
Author Contributions
Prashant chauhan: Resources; Software; Visualization; Writing‐review & editing. Jagneshwar Dandapat: Supervision. Arup Sarkar: Supervision. Bhaskar Saha: Conceptualization; Formal analysis; Resources; Supervision; Writing‐original draft; Writing‐review & editing.
Conflict of interest
The authors declares no conflict of interest.
Acknowledgment
The work had financial assistance from JC Bose National Fellowship (India) to BS [JCB/2018/000027].
References
- 1. Stanley SA, Cox JS. Host‐pathogen interactions during Mycobacterium tuberculosis infections. Curr Top Microbiol Immunol 2013; 374: 211–241. [DOI] [PubMed] [Google Scholar]
- 2. Urdahl KB. Understanding and overcoming the barriers to T cell‐mediated immunity against tuberculosis. Semin Immunol 2014; 26: 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kramnik I. Genetic dissection of host resistance to Mycobacterium tuberculosis: the sst1 locus and the Ipr1 gene. Curr Top Microbiol Immunol 2008; 321: 123–148. [DOI] [PubMed] [Google Scholar]
- 4. Skamene E. Genetic control of resistance to mycobacterial infection. Curr Top Microbiol Immunol 1986; 124: 49–66. [DOI] [PubMed] [Google Scholar]
- 5. Li AH, Waddell SJ, Hinds J et al Contrasting transcriptional responses of a virulent and an attenuated strain of Mycobacterium tuberculosis‐infected macrophages. PLoS One 2010; 5: e11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mcdonough KA, Kress Y, Bloom BR. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun 1993; 61: 2763–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samten B, Wizel B, Shams H et al CD40 ligand trimer enhances the response of CD8+ T cells to Mycobacterium tuberculosis . J Immunol 2003; 170: 3180–3186. [DOI] [PubMed] [Google Scholar]
- 8. Lin PL, Flynn JL. CD8+ T cells and Mycobacterium tuberculosis infection. Semin Immunopathol 2015; 37: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med 1992; 175: 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denis M. Interferon‐γ‐treated murine macrophages inhibit the growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cellular Immunol 1991; 132: 150–157. [DOI] [PubMed] [Google Scholar]
- 11. Liu W, Peng Y, Yin Y, Zhou Z, Zhou W, Dai Y. The involvement of NADPH oxidase‐mediated ROS in cytokine secretion from macrophages induced by Mycobacterium tuberculosis ESAT‐6. Inflammation 2014; 37: 880–892. [DOI] [PubMed] [Google Scholar]
- 12. Chauhan P, Sarkar A, Saha B. Interplay between metabolic sensors and immune cell signaling In Torrado E, Silvestre R. (eds), Metabolic Interactions in Infection. New York, NY: Springer International Publishing; 2018; 115–196. [DOI] [PubMed] [Google Scholar]
- 13. Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. J Immunol 2009; 183: 6095–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bengsch B, Johnson AL, Kurachi M et al Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD‐1 are an early driver of CD8+ T cell exhaustion. Immunity 2016; 45: 358–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhat KH, Yaseen I. Mycobacterium tuberculosis: macrophage takeover and modulation of innate effector responses In Ribón W. (Ed.), Mycobacterium ‐ Research and Development. London, UK: IntechOpen; 2018; 12–39. [Google Scholar]
- 16. Prezzemolo T, Guggino G, La Manna MP, Di Liberto D, Dieli F, Caccamo N. Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis . Front Immunol 2014; 5: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samten B, Thomas EK, Gong J, Barnes PF. Depressed CD40 ligand expression contributes to reduced γ‐interferon production in human tuberculosis. Infect Immun 2000; 68: 3002–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kusaba H, Ghosh P, Derin R et al Interleukin‐12‐induced interferon‐γ production by human peripheral blood T cells is regulated by the mammalian target of rapamycin (mTOR). J Biol Chem 2005; 280: 1037–1043. [DOI] [PubMed] [Google Scholar]
- 19. Braverman J, Stanley SA. Nitric oxide modulates macrophage responses to Mycobacterium tuberculosis infection through activation of HIF‐1α and repression of NF‐κB. J Immunol 2017; 199: 1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jayaraman P, Jacques MK, Zhu C et al TIM3 mediates T cell exhaustion during Mycobacterium tuberculosis infection. PLoS Patho 2016; 12: e1005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bosco MJ, Wei M, Hou H et al The exhausted CD4+ CXCR5+ T cells involve the pathogenesis of human tuberculosis disease. Int J Infect Dis 2018; 74: 1–9. [DOI] [PubMed] [Google Scholar]
- 22. Day CL, Abrahams DA, Bunjun R et al PD‐1 expression on Mycobacterium tuberculosis‐specific CD4 T cells is associated with bacterial load in human tuberculosis. Front Immunol 2018; 9: 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferguson JS, Weis JJ, Martin JL, Schlesinger LS. Complement protein C3 binding to Mycobacterium tuberculosis is initiated by the classical pathway in human bronchoalveolar lavage fluid. Infect Immun 2004; 72: 2564–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar SK, Singh P, Sinha S. Naturally produced opsonizing antibodies restrict the survival of Mycobacterium tuberculosis in human macrophages by augmenting phagosome maturation. Open Biol 2015; 5: 150–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kang PB, Azad AK, Torrelles JB et al The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan‐mediated phagosome biogenesis. J Exp Med 2005; 202: 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pasula R, Downing JF, Wright JR, Kachel DL, Davis TE Jr, Martin WJ. Surfactant protein A (SP‐A) mediates attachment of Mycobacterium tuberculosis to murine alveolar macrophages. Am J Res Cell Mol Biol 1997; 17: 209–217. [DOI] [PubMed] [Google Scholar]
- 27. Dunne DW, Resnick D, Greenberg J, Krieger M, Joiner KA. The type I macrophage scavenger receptor binds to gram‐positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci USA 1994; 91: 1863–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shams H, Wizel B, Lakey DL et al The CD14 receptor does not mediate entry of Mycobacterium tuberculosis into human mononuclear phagocytes. FEMS Immunol Med Microbiol 2003; 36: 63–69. [DOI] [PubMed] [Google Scholar]
- 29. Yadav M, Schorey JS. The β‐glucan receptor dectin‐1 functions together with TLR2 to mediate macrophage activation by Mycobacteria . Blood 2006; 108: 3168–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gu X, Gao Y, Mu DG, Fu EQ. MiR‐23a‐5p modulates mycobacterial survival and autophagy during Mycobacterium tuberculosis infection through TLR2/MyD88/NF‐κB pathway by targeting TLR2. Exp Cell Res 2017; 354: 71–77. [DOI] [PubMed] [Google Scholar]
- 31. Blanc L, Gilleron M, Prandi J et al Mycobacterium tuberculosis inhibits human innate immune responses via the production of TLR2 antagonist glycolipids. Proc Natl Acad Sci USA 2017; 114: 11205–11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shukla S, Richardson ET, Drage MG, Boom WH, Harding CV. Mycobacterium tuberculosis lipoprotein and lipoglycan binding to toll‐like receptor 2 correlates with agonist activity and functional outcomes. Infect Immun 2018; 86: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niu W, Sun B, Li M, Cui J, Huang J, Zhang L. TLR‐4/microRNA‐125a/NF‐κB signaling modulates the immune response to Mycobacterium tuberculosis infection. Cell Cycle 2018; 17: 1931–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim JK, Yuk JM, Kim SY et al MicroRNA‐125a inhibits autophagy activation and antimicrobial responses during mycobacterial infection. J Immunol 2015; 194: 5355–5365. [DOI] [PubMed] [Google Scholar]
- 35. Li WT, Zhang Q. MicroRNA‐708‐5p regulates mycobacterial vitality and the secretion of inflammatory factors in Mycobacterium tuberculosis‐infected macrophages by targeting TLR4. Eur Rev Med Pharmacol Sci 2019; 23: 8028–8038. [DOI] [PubMed] [Google Scholar]
- 36. Ma J, Chen XL, Sun Q. microRNA‐579 upregulation mediates death of human macrophages with Mycobacterium tuberculosis infection. Biochem Biophys Res Comm 2019; 518: 219–226. [DOI] [PubMed] [Google Scholar]
- 37. Liu F, Chen J, Wang P et al MicroRNA‐27a controls the intracellular survival of Mycobacterium tuberculosis by regulating calcium‐associated autophagy. Nat Comm 2018; 9: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Behura A, Mishra A, Chugh S et al ESAT‐6 modulates Calcimycin‐induced autophagy through microRNA‐30a in Mycobacteria infected macrophages. J Infect 2019; 79: 139–152. [DOI] [PubMed] [Google Scholar]
- 39. Qu Y, Ding S, Ma Z et al MiR‐129‐3p favors intracellular BCG survival in RAW264. 7 cells by inhibiting autophagy via Atg4b. Cellular Immunol 2019; 337: 22–32. [DOI] [PubMed] [Google Scholar]
- 40. Ding S, Qu Y, Yang S, Zhao YE, Xu G. Novel miR‐1958 promotes Mycobacterium tuberculosis survival in RAW264. 7 cells by inhibiting autophagy via Atg5. J Microbiol Biotechnol 2019; 29: 989–998. [DOI] [PubMed] [Google Scholar]
- 41. Zhang D, Yi Z, Fu Y. Downregulation of miR‐20b‐5p facilitates Mycobacterium tuberculosis survival in RAW 264.7 macrophages via attenuating the cell apoptosis by Mcl‐1 upregulation. J Cellular Biochem 2019; 120: 5889–5896. [DOI] [PubMed] [Google Scholar]
- 42. Shi G, Mao G, Xie K, Wu D, Wang W. MiR‐1178 regulates mycobacterial survival and inflammatory responses in Mycobacterium tuberculosis‐infected macrophages partly via TLR4. J Cellular Biochem 2018; 119: 7449–7457. [DOI] [PubMed] [Google Scholar]
- 43. Liang S, Song Z, Wu Y et al MicroRNA‐27b modulates inflammatory response and apoptosis during Mycobacterium tuberculosis infection. J Immunol 2018; 200: 3506–3518. [DOI] [PubMed] [Google Scholar]
- 44. Li H, Wang Y, Song Y. MicroRNA‐26b inhibits the immune response to Mycobacterium tuberculosis (Mtb) infection in THP‐1 cells via targeting TGFβ‐activated kinase‐1 (TAK1), a promoter of the NF‐κB pathway. Int J Clin Exp Pathol 2018; 11: 1218–1227. [PMC free article] [PubMed] [Google Scholar]
- 45. Pires D, Bernard EM, Pombo JP et al Mycobacterium tuberculosis modulates miR‐106b‐5p to control cathepsin S expression resulting in higher pathogen survival and poor T‐cell activation. Front Immunol 2017; 8: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lou J, Wang Y, Zhang Z, Qiu W. MiR‐20b inhibits Mycobacterium tuberculosis induced inflammation in the lung of mice through targeting NLRP3. Exp Cell Res 2017; 358: 120–128. [DOI] [PubMed] [Google Scholar]
- 47. Sahu SK, Kumar M, Chakraborty S et al MicroRNA 26a (miR‐26a)/KLF4 and CREB‐C/EBPβ regulate innate immune signaling, the polarization of macrophages and the trafficking of Mycobacterium tuberculosis to lysosomes during infection. PLoS Pathol 2017; 13: e1006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li M, Wang J, Fang Y et al microRNA‐146a promotes mycobacterial survival in macrophages through suppressing nitric oxide production. Sci Rep 2016; 6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Etna MP, Sinigaglia A, Grassi A et al Mycobacterium tuberculosis‐induced miR‐155 subverts autophagy by targeting ATG3 in human dendritic cells. PLoS Pathol 2018; 14: e1006790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang J, Jiao J, Xu W et al MiR‐155 is upregulated in patients with active tuberculosis and inhibits apoptosis of monocytes by targeting FOXO3. Mol Med Rep 2015; 12: 7102–7108. [DOI] [PubMed] [Google Scholar]
- 51. Iwai H, Funatogawa K, Matsumura K et al MicroRNA‐155 knockout mice are susceptible to Mycobacterium tuberculosis infection. Tuberculosis 2015; 95: 246–250. [DOI] [PubMed] [Google Scholar]
- 52. Ouimet M, Koster S, Sakowski E et al Mycobacterium tuberculosis induces the miR‐33 locus to reprogram autophagy and host lipid metabolism. Nat Immunol 2016; 17: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T‐cell activation. Immunol Rev 1996; 153: 85–106. [DOI] [PubMed] [Google Scholar]
- 54. Rub A, Dey R, Jadhav M et al Cholesterol depletion associated with Leishmania major infection alters macrophage CD40 signalosome composition and effector function. Nat Immunol 2009; 10: 273–280. [DOI] [PubMed] [Google Scholar]
- 55. Li Y, Huang J, Jiang Z et al MicroRNA‐145 regulates platelet‐derived growth factor‐induced human aortic vascular smooth muscle cell proliferation and migration by targeting CD40. Am J Trans Res 2016; 8: 1813–1825. [PMC free article] [PubMed] [Google Scholar]
- 56. Furci L, Schena E, Miotto P, Cirillo DM. Alteration of human macrophages microRNA expression profile upon infection with Mycobacterium tuberculosis . Int J Mycobacteriol 2013; 2: 128–134. [DOI] [PubMed] [Google Scholar]
- 57. Guo X, Yu L, Chen M et al miR‐145 mediated the role of aspirin in resisting VSMCs proliferation and anti‐inflammation through CD40. J Translat Med 2016; 14: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lu J, Yan M, Wang Y et al Altered expression of miR‐146a in myasthenia gravis. Neurosci Lett 2013; 555: 85–90. [DOI] [PubMed] [Google Scholar]
- 59. Hackett EE, Charles‐Messance H, O'Leary SM et al Mycobacterium tuberculosis limits host glycolysis and IL‐1β by restriction of PFK‐M via MicroRNA‐21. Cell Rep 2020; 30: 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nguyen VT, Walker WS, Benveniste EN. Post‐transcriptional inhibition of CD40 gene expression in microglia by transforming growth factor‐β. Eur J Immunol 1998; 28: 2537–2548. [DOI] [PubMed] [Google Scholar]
- 61. Lin Q, Geng Y, Zhao M, Lin S, Zhu Q, Tian Z. MiR‐21 regulates TNF‐α‐induced CD40 expression via the SIRT1‐NF‐κB pathway in renal inner medullary collecting duct cells. Cell Physiol Biochem 2017; 41: 124–136. [DOI] [PubMed] [Google Scholar]
- 62. Cho S, Lee HM, Yu IS et al Differential cell‐intrinsic regulations of germinal center B and T cells by miR‐146a and miR‐146b. Nat Comm 2018; 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shah AU, Cao Y, Siddique N, Lin J, Yang Q. miR29a and miR378b Influence CpG‐Stimulated Dendritic Cells and Regulate cGAS/STING Pathway. Vaccines 2019; 7: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ma F, Xu S, Liu X et al The microRNA miR‐29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon‐γ. Nat Immunol 2011; 12: 861–869. [DOI] [PubMed] [Google Scholar]
- 65. Wagh V, Urhekar A, Modi D. Levels of microRNA miR‐16 and miR‐155 are altered in serum of patients with tuberculosis and associate with responses to therapy. Tuberculosis 2017; 102: 24–30. [DOI] [PubMed] [Google Scholar]
- 66. Li QQ, Xi J, Li BQ, Li N. MiR‐16, as a potential NF‐κB‐related miRNA, exerts anti‐inflammatory effects on LPS‐induced myocarditis via mediating CD40 expression: A preliminary study. J Biochem Mol Toxicol 2020; 34: e22426. [DOI] [PubMed] [Google Scholar]
- 67. Wang J, Deng Z, Wang Z et al MicroRNA‐155 in exosomes secreted from Helicobacter pylori infection macrophages immunomodulates inflammatory response. Am J Transl Res 2016; 8: 3700–3709. [PMC free article] [PubMed] [Google Scholar]
- 68. Nguyen VT, Benveniste EN. Critical role of tumor necrosis factor‐α and NF‐κB in interferon‐γ‐induced CD40 expression in microglia/macrophages. J Biol Chem 2002; 277: 13796–13803. [DOI] [PubMed] [Google Scholar]
- 69. Benveniste EN, Nguyen VT, Wesemann DR. Molecular regulation of CD40 gene expression in macrophages and microglia. Brain Behav Immun 2004; 18: 7–12. [DOI] [PubMed] [Google Scholar]
- 70. Chandel HS, Pandey SP, Shukla D et al Toll‐like receptors and CD40 modulate each other's expression affecting Leishmania major infection. Clin Exp Immunol 2014; 176: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu W, Alexis NE, Chen X, Bromberg PA, Peden DB. Involvement of mitogen‐activated protein kinases and NFκB in LPS‐induced CD40 expression on human monocytic cells. Toxicol App Pharmacol 2008; 228: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Qin H, Wilson CA, Lee SJ et al IFN‐β‐induced SOCS‐1 negatively regulates CD40 gene expression in macrophages and microglia. FASEB J 2006; 20: 985–987. [DOI] [PubMed] [Google Scholar]
- 73. Hua C, Sun L, Yang Y, Tan R, Hou Y. Mechanisms of CpG‐induced CD40 expression on murine bone marrow‐derived dendritic cells. Autoimmunity 2013; 46: 177–187. [DOI] [PubMed] [Google Scholar]
- 74. He ZL, Du FW, Du XZ. The viable Mycobacterium tuberculosis H37Ra strain induces a stronger mouse macrophage response compared to the heat‐inactivated H37Rv strain. Mol Med Rep 2013; 7: 1597–1602. [DOI] [PubMed] [Google Scholar]
- 75. Mogga SJ, Mustafa T, Sviland L, Nilsen R. In Situ Expression of CD40, CD40L (CD154), IL‐12, TNF‐α, IFN‐γ and TGF‐β1 in murine lungs during slowly progressive primary tuberculosis. Scand J Immunol 2003; 58: 327–334. [DOI] [PubMed] [Google Scholar]
- 76. Fu Y, Yi Z, Wu X, Li J, Xu F. Circulating microRNAs in patients with active pulmonary tuberculosis. J Clin Microbiol 2011; 49: 4246–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Woods PS, Kimmig LM, Meliton AY et al Tissue‐Resident alveolar macrophages do not rely on glycolysis for LPS‐induced inflammation. Am J Res Cell and Mol Biol 2020; 62: 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chelen CJ, Fang Y, Freeman GJ et al Human alveolar macrophages present antigen ineffectively due to defective expression of B7 costimulatory cell surface molecules. J Clin Inv 1995; 95: 1415–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ordway D, Henao‐Tamayo M, Orme IM, Gonzalez‐Juarrero M. Foamy macrophages within lung granulomas of mice infected with Mycobacterium tuberculosis express molecules characteristic of dendritic cells and antiapoptotic markers of the TNF receptor‐associated factor family. J Immunol 2005; 175: 3873–3881. [DOI] [PubMed] [Google Scholar]
- 80. Callard RE, Armitage RJ, Fanslow WC, Spriggs MK. CD40 ligand and its role in X‐linked hyper‐IgM syndrome. Immunol Today 1993; 14: 559–564. [DOI] [PubMed] [Google Scholar]
- 81. Krishnan VP, Taur P, Pandrowala A, Madkaikar M, Desai M. X‐Linked Hyper IgM Syndrome presenting with recurrent tuberculosis—a Case Report. J Clin Immunol 2020; 22: 1–3. [DOI] [PubMed] [Google Scholar]
- 82. Srivastava S, Ernst JD, Desvignes L. Beyond macrophages: the diversity of mononuclear cells in tuberculosis. Immunol Rev 2014; 262: 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lee SJ, Qin H, Benveniste EN. Simvastatin inhibits IFN‐γ‐induced CD40 gene expression by suppressing STAT‐1α. J Leukoc Biol 2007; 82: 436–447. [DOI] [PubMed] [Google Scholar]
- 84. Nguyen VT, Benveniste EN. Involvement of STAT‐1 and ets family members in interferon‐γ induction of CD40 transcription in microglia/macrophages. J Biol Chem 2000; 275: 23674–23684. [DOI] [PubMed] [Google Scholar]
- 85. Wesemann DR, Dong Y, O'Keefe GM, Nguyen VT, Benveniste EN. Suppressor of cytokine signaling 1 inhibits cytokine induction of CD40 expression in macrophages. J Immunol 2002; 169: 2354–2360. [DOI] [PubMed] [Google Scholar]
- 86. Nguyen VT, Benveniste EN. IL‐4‐activated STAT‐6 inhibits IFN‐γ‐induced CD40 gene expression in macrophages/microglia. J Immunol 2000; 165: 6235–6243. [DOI] [PubMed] [Google Scholar]
- 87. Elgueta R, Benson MJ, De Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009; 229: 152–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Quezada SA, Fuller B, Jarvinen LZ et al Mechanisms of donor‐specific transfusion tolerance: preemptive induction of clonal T‐cell exhaustion via indirect presentation. Blood 2003; 102: 1920–1926. [DOI] [PubMed] [Google Scholar]
- 89. Visvanathan S, Daniluk S, Ptaszyński R et al Effects of BI 655064, an antagonistic anti‐CD40 antibody, on clinical and biomarker variables in patients with active rheumatoid arthritis: a randomised, double‐blind, placebo‐controlled, phase IIa study. Ann Rheum Dis 2019; 78: 754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Clemens DL, Horwitz MA. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med 1995; 181: 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xu J, Laine O, Masciocchi M et al A unique Mycobacterium ESX‐1 protein co‐secretes with CFP‐10/ESAT‐6 and is necessary for inhibiting phagosome maturation. Mol Microbiol 2007; 66: 787–800. [DOI] [PubMed] [Google Scholar]
- 92. Huang D, Bao L. Mycobacterium tuberculosis EspB protein suppresses interferon‐γ‐induced autophagy in murine macrophages. J Microbiol Immunol Infect 2016; 49: 859–865. [DOI] [PubMed] [Google Scholar]
- 93. MacGurn JA, Cox JS. A genetic screen for Mycobacterium tuberculosis mutants defective for phagosome maturation arrest identifies components of the ESX‐1 secretion system. Infect Immun 2007; 75: 2668–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Härtlova A, Herbst S, Peltier J et al LRRK2 is a negative regulator of Mycobacterium tuberculosis phagosome maturation in macrophages. EMBO J 2018; 37: e98694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lee HJ, Ko HJ, Song DK, Jung YJ. Lysophosphatidylcholine promotes phagosome maturation and regulates inflammatory mediator production through the Protein Kinase A‐Phosphatidylinositol 3 Kinase–p38 Mitogen‐Activated Protein Kinase signaling pathway during Mycobacterium tuberculosis infection in mouse macrophages. Front Immunol 2018; 9: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Guérin I, de Chastellier C. Pathogenic Mycobacteria disrupt the macrophage actin filament network. Infect Immun 2000; 68: 2655–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kuehnel MP, Rybin V, Anand PK, Anes E, Griffiths G. Lipids regulate P2X7‐receptor‐dependent actin assembly by phagosomes via ADP translocation and ATP synthesis in the phagosome lumen. J Cell Sci 2009; 122: 499–504. [DOI] [PubMed] [Google Scholar]
- 98. Kalamidas SA, Kuehnel MP, Peyron P et al cAMP synthesis and degradation by phagosomes regulate actin assembly and fusion events: consequences for Mycobacteria . J Cell Sci 2006; 119: 3686–3694. [DOI] [PubMed] [Google Scholar]
- 99. Sturgill‐Koszycki S, Schlesinger PH, Chakraborty P et al Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton‐ATPase. Science 1994; 263: 678–681. [DOI] [PubMed] [Google Scholar]
- 100. Singh CR, Moulton RA, Armitige LY et al Processing and presentation of a mycobacterial antigen 85B epitope by murine macrophages is dependent on the phagosomal acquisition of vacuolar proton ATPase and in situ activation of cathepsin D. J Immunol 2006; 177: 3250–3259. [DOI] [PubMed] [Google Scholar]
- 101. Souza CD, Evanson OA, Weiss DJ. Role of cell membrane receptors in the suppression of monocyte anti‐microbial activity against Mycobacterium avium subsp. paratuberculosis . Microb Pathog 2008; 44: 215–223. [DOI] [PubMed] [Google Scholar]
- 102. Griffiths KL, Ahmed M, Das S et al Targeting dendritic cells to accelerate T‐cell activation overcomes a bottleneck in tuberculosis vaccine efficacy. Nat Commun 2016; 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sia JK, Bizzell E, Madan‐Lala R, Rengarajan J. Engaging the CD40‐CD40L pathway augments T‐helper cell responses and improves control of Mycobacterium tuberculosis infection. PLoS Pathog 2017; 13: e1006530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40‐CD40L cross‐talk integrates strong antigenic signals and microbial stimuli to induce development of IL‐17‐producing CD4+ T cells. Proc Natl Acad Sci USA 2009; 106: 876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Choi HG, Kim WS, Back YW et al Mycobacterium tuberculosis RpfE promotes simultaneous TH1‐and TH17‐type T‐cell immunity via TLR4‐dependent maturation of dendritic cells. Eur J Immunol 2015; 45: 1957–1971. [DOI] [PubMed] [Google Scholar]
- 106. Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. Mycobacterium tuberculosis activates the DNA‐dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 2012; 11: 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Watson RO, Bell SL, MacDuff DA et al The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 2015; 17: 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chang ST, Linderman JJ, Kirschner DE. Multiple mechanisms allow Mycobacterium tuberculosis to continuously inhibit MHC class II‐mediated antigen presentation by macrophages. Proc Natl Acad Sci USA 2005; 102: 4530–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Noss EH, Pai RK, Sellati TJ et al Toll‐like receptor 2‐dependent inhibition of macrophage class II MHC expression and antigen processing by 19‐kDa lipoprotein of Mycobacterium tuberculosis . J Immunol 2001; 167: 910–918. [DOI] [PubMed] [Google Scholar]
- 110. Su H, Zhu S, Zhu L et al Recombinant lipoprotein Rv1016c derived from Mycobacterium tuberculosis is a TLR‐2 ligand that induces macrophages apoptosis and inhibits MHC‐II antigen processing. Front Cell Infect Microbiol 2016; 47: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Simmons DP, Canaday DH, Liu Y et al Mycobacterium tuberculosis and TLR2 agonists inhibit induction of type I IFN and class I MHC antigen cross processing by TLR9. J Immunol 2010; 185: 2405–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Saha B, Das G, Vohra H, Ganguly NK, Mishra GC. Macrophage–T cell interaction in experimental mycobacterial infection. Selective regulation of co‐stimulatory molecules on Mycobacterium‐infected macrophages and its implication in the suppression of cell‐mediated immune response. Eur J Immunol 1994; 24: 2618–2624. [DOI] [PubMed] [Google Scholar]
- 113. Singh B, Singh G, Trajkovic V, Sharma P. Intracellular expression of Mycobacterium tuberculosis‐specific 10‐kDa antigen down‐regulates macrophage B7.1 expression and nitric oxide release. Clin Exp Immunol 2003; 134: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. De la Barrera S, Alemán M, Musella R et al IL‐10 down‐regulates costimulatory molecules on Mycobacterium tuberculosis‐pulsed macrophages and impairs the lytic activity of CD4 and CD8 CTL in tuberculosis patients. Clin Exp Immunol 2004; 138: 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Vordermeier HM, Harris DP, Friscia G et al T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38‐kDa antigen in patients with active disease. Eur J Immunol 1992; 22: 2631–2637. [DOI] [PubMed] [Google Scholar]
- 116. Boussiotis VA, Tsai EY, Yunis EJ et al IL‐10‐producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Inv 2000; 105: 1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sakhno LV, Tikhonova MA, Tyrinova TV et al Cytotoxic activity of dendritic cells as a possible mechanism of negative regulation of T lymphocytes in pulmonary tuberculosis. Clin Dev Immunol 2012; 628635: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hogan LH, Markofski W, Bock A, Barger B, Morrissey JD, Sandor M. Mycobacterium bovis BCG‐induced granuloma formation depends on γ interferon and CD40 ligand but does not require CD28. Infect Immun 2001; 69: 2596–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Demangel C, Palendira U, Feng CG, Heath AW, Bean AG, Britton WJ. Stimulation of Dendritic Cells via CD40 enhances immune responses to Mycobacterium tuberculosis infection. Infect Immun 2001; 69: 2456–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Flórido M, Gonçalves AS, Gomes MS, Appelberg R. CD40 is required for the optimal induction of protective immunity to Mycobacterium avium . Immunology 2004; 111: 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lazarevic V, Myers AJ, Scanga CA, Flynn JL. CD40, but not CD40L, is required for the optimal priming of T cells and control of aerosol M. tuberculosis infection. Immunity 2003; 19: 823–835. [DOI] [PubMed] [Google Scholar]
- 122. Awasthi A, Mathur R, Khan A et al CD40 signaling is impaired in L. major–infected macrophages and is rescued by a p38MAPK activator establishing a host‐protective memory T cell response. J Exp Med 2003; 197: 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang Y, Kelly CG, Karttunen JT et al CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC‐chemokines. Immunity 2001; 15: 971–983. [DOI] [PubMed] [Google Scholar]
- 124. Ganief N, Sjouerman J, Albeldas C et al Associating H2O2‐and NO‐related changes in the proteome of Mycobacterium smegmatis with enhanced survival in macrophage. Emerg Microbes Infect 2018; 7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mathur RK, Awasthi A, Wadhone P, Ramanamurthy B, Saha B. Reciprocal CD40 signals through p38MAPK and ERK‐1/2 induce counteracting immune responses. Nat Med 2004; 10: 540–544. [DOI] [PubMed] [Google Scholar]
- 126. Calder B, Albeldas C, Blackburn JM, Soares NC. Mass spectrometry offers insight into the role of Ser/Thr/Tyr phosphorylation in the Mycobacteria . Front Microbiol 2016; 7: 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mendez‐Samperio P, Ayala‐Verdin HE, Trejo‐Echeverria A. Interleukin‐12 regulates the production of Bacille Calmette‐Guerin‐induced interferon‐γ from human cells in a CD40‐dependent manner. Scand J Immunol 1999; 50: 61–67. [PubMed] [Google Scholar]


