Abstract
Anxiety disorders in young people are frequently comorbid with other mental disorders and respond unsatisfactorily to first-line treatment in many cases. Here, we report the case of a 20-year-old man with severe social anxiety disorder, major depressive disorder, insomnia and attenuated psychotic symptoms despite ongoing treatment with cognitive behavioural therapy and mirtazapine who was treated with adjunctive cannabidiol (CBD) in doses between 200 and 800 mg/day for 6 months. During treatment with CBD, he experienced subjective benefits to his anxiety, depression and positive symptoms during treatment that were confirmed by clinicians and by standardised research instruments. Findings from this case study add to existing evidence in support of the safety of CBD and suggest that it may be useful for young people with treatment refractory anxiety and for attenuated psychotic symptoms.
Keywords: anxiety disorders (including OCD and PTSD), psychotic disorders (including schizophrenia), psychiatry (drugs and medicines)
Background
Anxiety disorders are among the most common psychiatric conditions in young people in high-income countries, affecting approximately 7% of young people at any one point in time.1 2 They typically have their onset early in life and are often associated with ongoing functional impairment and significant comorbidity. While mild in some cases, anxiety can be severe and disabling and can significantly impair a young person’s ability to maintain social and occupational functionings. The lifetime prevalence of social anxiety disorder is estimated at 4%.3
Meta-analyses and naturalistic follow-up studies suggest that treatment outcome in patients with anxiety disorders is suboptimal in many cases, with clinically meaningful treatment response lacking in up to 50% of patients.4–6 Additionally, comorbid disorders are common in young people with anxiety, and anxiety, in turn, is a frequent concern in young people with attenuated psychotic symptoms. Data from follow-up studies suggest that half of all young people who seek help for subthreshold psychotic symptoms also experience anxiety disorders.7 8 Importantly, treatment outcomes in anxiety may be worse for young people with anxiety disorders and comorbid psychiatric disorders.9 Consequently, a large number of individuals who experience anxiety disorders have ongoing symptoms and functional impairment despite current standard treatment. Effective treatment in these cases represents a clinical challenge.
Cannabidiol (CBD) can be prescribed in Australia and in several other jurisdictions and has demonstrated anxiolytic potential in several case reports.10–13 Furthermore, it has also demonstrated antipsychotic properties in at least two randomised-controlled trials (RCTs).14 15 Although CBD is receiving increasing attention, its efficacy for anxiety and early psychosis remains unclear.16 CBD is a non-psychotropic substance found in the plant Cannabis sativa, with a broad pharmacological profile, and acts primarily via cannabinoid 1 (CB1) and cannabinoid 2 (CB2) receptors, but also on the transient receptor potential cation channel subfamily V member 1 (TRPV-1) receptor and serotonin 5-HT1A receptor. It is thought to possess antipsychotic, anxiolytic and anti-inflammatory properties while possessing a generally benign side effect profile. We present a case report of a young man with severe social anxiety disorder, major depressive disorder, insomnia and attenuated psychotic symptoms who was treated with CBD for 6 months.
Case presentation
A 20-year-old man presented to a primary mental healthcare centre (headspace) in August 2018 with longstanding and severe social anxiety, low mood, chronic insomnia and subthreshold psychotic experiences. He attended in the presence of his girlfriend, who expressed concern about him reporting persecutory thoughts and not leaving the house for several consecutive days at times. At the time of presentation, he lived in a detached dwelling at the back of his parents’ house, had completed secondary education and was employed full-time as an apprentice tradesman. He had no relevant medical history.
In the initial assessment, he met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), criteria for social anxiety disorder with recurrent panic attacks that were typically triggered by social situations and severe major depressive disorder. Both diagnoses were later confirmed with the Structured Clinical Interview for DSM-5 (SCID-5). Elevated mood of up to 3 days’ duration and multiple depressive episodes in the preceding 3 years, each lasting up to 4 weeks, were noted but did not meet the diagnostic criteria for bipolar disorder. He reported long-standing insomnia with less than 4 hours of sleep per night, significant difficulties falling asleep and frequent awakenings at night. Although he did not present with any current suicidal risk at the time of the initial assessment, he reported to having two suicide attempts over the past 12 months. He reported auditory hallucinations (high-pitched ringing, commanding and non-commanding voices) about once a week, visual hallucinations (shadows) and tactile hallucinations (someone breathing on his neck) at least once a month. He fulfilled criteria for attenuated psychotic symptoms on the Comprehensive Assessment of At-Risk Mental States. He used cannabis and 3,4-methylenedioxymethamphetamine occasionally (less than once per month) prior to commencing treatment with CBD, and reported that he used these substances in effort to self-medicate from his symptoms of anxiety.
After presenting to headspace, he was initially treated with 7.5 mg mirtazapine (September 2018), which was increased to 15 mg (October 2018) and 30 mg (November 2018). Biweekly cognitive–behavioural therapy (CBT) sessions commenced in January 2019.
After 3 months of treatment with mirtazapine and 4 months of combined treatment with mirtazapine and CBT, he continued to experience severe anxiety (Overall Anxiety Severity and Impairment Scale (OASIS): 15), severe depression (Quick Inventory for Depressive Symptoms—Adolescent Version (QIDS-A17): 16, and Hamilton Anxiety Rating Scale (HAM-A): 29), attenuated psychotic symptoms, impaired social and occupational functioning (Social and Occupational Functioning Assessment Scale: 60) and was rated as markedly ill (Clinical Global Impression–Severity rating: 5) by his psychologist with no improvement in response to treatment (Clinical Global Impression–Improvement rating (CGI-I): 4).
Treatment
Treatment with CBD was initiated by the patient’s psychiatrist in March 2019 at a dose of 200 mg/day under the Australian Therapeutic Goods Administration (TGA) Clinical Trials Notification Scheme and Special Access Scheme. After 1 week, this was dose escalated to 400 mg/day and later to 600 mg after 4 weeks, and finally, to 800 mg after 8 weeks. The decision to escalate the dose was contingent on the absence of adverse events and treatment efficacy. In the absence of clear recommendation regarding the optimal dose or duration of treatment, we chose the dose based on previous research examining the safety of CBD and based on the limited evidence from clinical trials in psychiatric populations. The dose escalation protocol was chosen to identify the lowest effective dose. Biweekly CBT sessions were continued throughout the treatment, and mirtazapine was continued on a stable dose of 30 mg. Treatment with CBD was continued for 6 months, after which he was gradually titrated off over the course of 1 week. Throughout the 6 months, adverse events were continuously monitored, and blood draws to monitor liver function and routine blood investigations (full blood count, chemistry and electrolytes) took place before commencing treatment, and 1, 2 and 3 months after starting CBD.
Outcome and follow-up
During CBD treatment, the patient was initially seen every 4 weeks for the first 3 months, and then again 6 months after CBD treatment was commenced. He received concurrent CBT treatment while being treated with CBD. While minimal improvements to symptoms were noted during the first 4 weeks of treatment, the patient began reporting improvements to his anxiety and reductions of the subthreshold psychotic symptoms after a dose escalation to 600 mg/day after week 4. With regard to his subthreshold psychotic symptoms, the intensity to previously reported voices had diminished and decreased in frequency to occur about once every 2 weeks. Where these experiences were previously highly distressing, he found that he was better able to dismiss them when they did occur. He also no longer had persecutory thoughts. After a further dose escalation to 800 mg/day at week 8, the patient reported a significant reduction to his anxiety. He reported that he felt generally calmer and more in control of his anxiety. While he still endorsed having anxious thoughts, he reported that the feeling of calmness gave him an increased capacity to question the validity of any anxiety-provoking thoughts and de-escalate feelings of anxiety when faced with his usual triggers. In addition, his attenuated psychotic symptoms had mostly settled and were in remission. His overall improvement was also noted through clinical observation by his psychiatrist and psychologist. Self-reported anxiety (OASIS) and clinician-rated anxiety (HAM-A) had gradually improved from the start of treatment to 12 weeks after commencing treatment (see figure 1). Similarly, some improvements to his depressive symptoms were noted on the QIDS-A17. The overall improvement to his mental health was also reflected by a CGI-I rating of 1 (‘very much improved’) by his psychologist after 12 weeks of treatment. His symptoms continued to improve, and after 6 months of treatment with CBD, he had sustained reductions in anxiety severity and was free from psychotic experiences; however, improvements in depressive symptoms were not sustained after 6 months. Over the course of the treatment, he also reported significant improvements with his sleep, which was characterised by chronic insomnia. No side effects were noted by the patient, and no changes to blood cell counts and liver and thyroid functions were noted throughout 6 months’ treatment with CBD.
Figure 1.
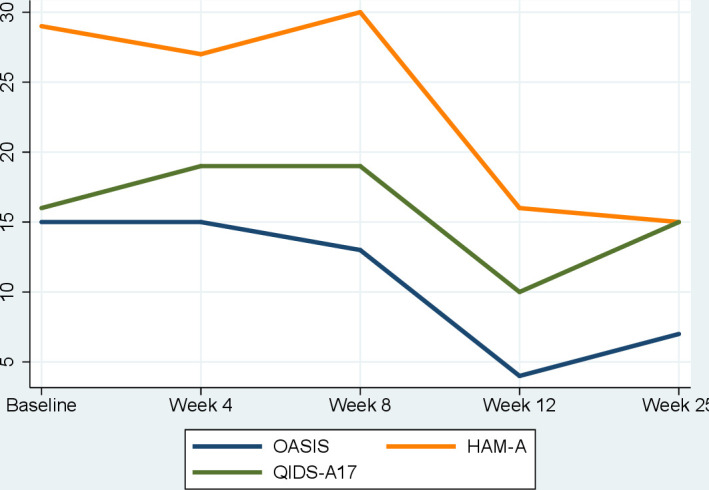
Change in symptom scores during 6 months of treatment with cannabidiol. HAM-A, Hamilton Anxiety Rating Scale; OASIS, Overall Anxiety Severity and Impairment Scale; QIDS-A17, Quick Inventory for Depressive Symptoms—Adolescent Version.
Discussion
This case report describes the successful treatment of a 20-year-old man with severe anxiety, depression and attenuated psychotic symptoms with CBD who previously failed to respond to standard treatment with CBT and antidepressant medication. The main improvements were observed in anxiety severity, sleep disturbance associated with major depressive disorder and attenuated psychotic symptoms after 8 weeks of treatment. No adverse events were noted and CBD was well tolerated by the patient. In the present case, a CBD dose of 800 mg was found to be most effective.
Current clinical guidelines for the treatment of social anxiety disorder recommend psychotherapy (eg, CBT) and antidepressant medication.17 However, incomplete remission and treatment resistance represent significant problems clinicians are regularly facing.
CBD has received increasing attention in recent years as a potential treatment for severe childhood epilepsies,18 19 psychotic disorders14 15 and, to a lesser extent, anxiety.10 12 13 20 Case reports of CBD for anxiety showed reductions in anxiety in adult patients treated with adjunctive CBD, in addition to treatment as usual12 and in one paediatric patient.13 Experimental studies of CBD for the treatment of anxiety induced by a public speaking test have similarly demonstrated that CBD is able to reduce anxiety.10 Randomised placebo-controlled clinical trials of CBD for anxiety are limited to one trial, which demonstrated significant reductions in anxiety severity in n=37 adolescent patients with social anxiety disorder following 4 weeks’ treatment with 300 mg CBD/day.21 Currently, two RCTs support antipsychotic effects of CBD in patients with first-episode psychosis and schizophrenia,14 15 and one study demonstrated that CBD normalised functional alterations in parahippocampal, striatal and midbrain areas in young individuals at ultrahigh risk of psychosis.22 While these findings appear to support the notion that CBD is potentially effective for anxiety disorders, rigorous clinical testing allowing definitive conclusions for young patients with anxiety is warranted.23
Duration and dose of treatment with CBD for anxiety and psychotic symptoms are currently unclear. In our case, we decided to commence treatment with a dose of 200 mg/day, which was increased to 800 mg/day over 8 weeks. Clinically significant improvement was observed after 8 weeks of treatment. However, it is unclear if this is due to the dose (800 mg) or duration of treatment (8 weeks). Moreover, the improvements observed in this patient may be due to the effect of CBD, continued treatment with mirtazapine and CBT, or a combination of both. Although the patient’s symptoms did not improve with treatment with mirtazapine and CBT prior to commencing treatment with CBD, it is possible that the reduction in symptoms overserved after a 12-week trial with CBD is due to treatment as usual.
The precise mechanisms by which CBD exerts antipsychotic and anxiolytic effects are currently incompletely understood. Mechanisms that are believed to be involved in anxiolytic and antipsychotic effects include allosteric modulation of the CB1 and
2 (CB 2) receptor, partial agonism at the 5-HT1A receptor, allosteric modulation of μ- and δ-opioid receptors, effects on the TRPV1 receptor.24 The endocannabinoid system plays a prominent role in the regulation of several functions relevant to fear, anxiety and psychotic pathophysiology, including the modulation of neurotransmitter systems, inflammation, sleep and cognition.25 Although currently limited, initial evidence suggests that dysregulated endocannabinoid signalling may be relevant to major psychiatric disorders.24 26
In summary, our case report suggests that CBD can be effective in treating severe and treatment refractory anxiety in combination with subthreshold psychotic symptoms. Although promising, rigorous clinical testing is required to establish whether CBD may be a potential treatment for anxiety. To further evaluate CBD and to test its safety and efficacy for young people experiencing treatment-resistant anxiety, RCTs are needed. CBD is currently listed as a Schedule four substance in Australia and can be prescribed via Special Access Schemes and Authorised Prescriber Schemes. Therefore, robust evidence regarding its safety and efficacy are urgently warranted.
Learning points.
Anxiety disorders are the most common psychiatric conditions in young people in high-income countries.
Nearly half of all young people with anxiety disorders do not remit completely with currently available treatments (eg, cognitive–behavioural therapy and selective serotonin receptor inhibitors).
Attenuated positive symptoms indicate high risk for psychotic disorders and are frequently comorbid with other psychiatric disorders.
Preliminary evidence to date demonstrates that cannabidiol (CBD) is potentially effective for anxiety and psychotic disorders.
This case shows that adjunctive treatment with CBD for 6 months led to clinically significant improvements in anxiety severity and attenuated positive symptoms.
Footnotes
Contributors: MB, EL and GPA planned the case report. MB drafted the manuscript. All authors contributed to the final version of the case report.
Funding: This case report was supported by a grant from the Lambert Initiative for Cannabinoid Therapeutics.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Copeland WE, Angold A, Shanahan L, et al. Longitudinal patterns of anxiety from childhood to adulthood: the great smoky mountains study. J Am Acad Child Adolesc Psychiatry 2014;53:21–33. 10.1016/j.jaac.2013.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodward LJ, Fergusson DM. Life course outcomes of young people with anxiety disorders in adolescence. J Am Acad Child Adolesc Psychiatry 2001;40:1086–93. 10.1097/00004583-200109000-00018 [DOI] [PubMed] [Google Scholar]
- 3.Stein DJ, Lim CCW, Roest AM, et al. The cross-national epidemiology of social anxiety disorder: data from the world mental health survey initiative. BMC Med 2017;15:143. 10.1186/s12916-017-0889-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginsburg GS, Becker EM, Keeton CP, et al. Naturalistic follow-up of youths treated for pediatric anxiety disorders. JAMA Psychiatry 2014;71:310–8. 10.1001/jamapsychiatry.2013.4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James AC, James G, Cowdrey FA, et al. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Databas Syst Rev 2015;2:Cd004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobson ET, Bloch MH, Strawn JR. Efficacy and tolerability of pharmacotherapy for pediatric anxiety disorders: a network meta-analysis. J Clin Psychiatry 2019;80:17r12064. 10.4088/JCP.17r12064 [DOI] [PubMed] [Google Scholar]
- 7.Lin A, Wood SJ, Nelson B, et al. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am J Psychiatry 2015;172:249–58. 10.1176/appi.ajp.2014.13030418 [DOI] [PubMed] [Google Scholar]
- 8.McAusland L, Buchy L, Cadenhead KS, et al. Anxiety in youth at clinical high risk for psychosis. Early Interv Psychiatry 2017;11:480–7. 10.1111/eip.12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rapee RM, Lyneham HJ, Hudson JL, et al. Effect of comorbidity on treatment of anxious children and adolescents: results from a large, combined sample. J Am Acad Child Adolesc Psychiatry 2013;52:47–56. 10.1016/j.jaac.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 10.Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology 2011;36:1219–26. 10.1038/npp.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blessing EM, Steenkamp MM, Manzanares J, et al. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 2015;12:825–36. 10.1007/s13311-015-0387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J 2019;23:18-041. 10.7812/TPP/18-041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shannon S, Opila-Lehman J. Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: a case report. Perm J 2016;20:108–11. 10.7812/TPP/16-005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2012;2:e94–e. 10.1038/tp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry 2018;175:225–31. 10.1176/appi.ajp.2017.17030325 [DOI] [PubMed] [Google Scholar]
- 16.Amminger GP, Berger M, Rice SM, et al. Novel biotherapies are needed in youth mental health. Australas Psychiatry 2017;25:117–20. 10.1177/1039856217698237 [DOI] [PubMed] [Google Scholar]
- 17.Australian Psychological Society Evidence-Based psychological interventions in the treatment of mental disorders: a literature review. fourth edition Melbourne: Australian Psychological Society, 2018. [Google Scholar]
- 18.Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 2017;376:2011–20. 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- 19.Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol 2016;15:270–8. 10.1016/S1474-4422(15)00379-8 [DOI] [PubMed] [Google Scholar]
- 20.Crippa JAS, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol 2011;25:121–30. 10.1177/0269881110379283 [DOI] [PubMed] [Google Scholar]
- 21.Masataka N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front Psychol 2019;10:2466. 10.3389/fpsyg.2019.02466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry 2018;75:1107. 10.1001/jamapsychiatry.2018.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry 2019;6:995–1010. 10.1016/S2215-0366(19)30401-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutz B, Marsicano G, Maldonado R, et al. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci 2015;16:705–18. 10.1038/nrn4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu H-C, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry 2016;79:516–25. 10.1016/j.biopsych.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minichino A, Senior M, Brondino N, et al. Measuring disturbance of the endocannabinoid system in psychosis: a systematic review and meta-analysis. JAMA Psychiatry 2019. 10.1001/jamapsychiatry.2019.0970. [Epub ahead of print: 05 Jun 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]


