Abstract
Background
Antiangiogenic therapy with bevacizumab has failed to provide substantial gains in overall survival. Epithelial membrane protein 2 (EMP2) is a cell surface protein that has been previously shown to be expressed in glioblastoma, correlate with poor survival, and regulate neoangiogenesis in cell lines. Thus, the relationship between bevacizumab and EMP2 was investigated.
Methods
Tumor samples were obtained from 12 patients with newly diagnosed glioblastoma at 2 time points: (1) during the initial surgery and (2) during a subsequent surgery following disease recurrence post-bevacizumab treatment. Clinical characteristics and survival data from these patients were collected, and tumor samples were stained for EMP2 expression. The IVY Glioblastoma Atlas Project database was used to evaluate EMP2 expression levels in 270 samples by differing histological areas of the tumor.
Results
Patients with high EMP2 staining at initial diagnosis had decreased progression-free and overall survival after bevacizumab (median progression-free survival 4.6 months vs 5.9 months; log-rank P = .076 and overall survival 7.7 months vs 14.4 months; log-rank P = .011). There was increased EMP2 staining in samples obtained after bevacizumab treatment in both unpaired (mean H-score 2.31 vs 1.76; P = .006) and paired analyses (mean difference 0.571; P = .019). This expression increase correlated with length of bevacizumab therapy (R2 = 0.449; Pearson P = .024).
Conclusions
Bevacizumab treatment increased EMP2 protein expression. This increase in EMP2 correlated with reduced mean survival time post-bevacizumab therapy. We hypothesize a role of EMP2 in clinical bevacizumab resistance and as a potential antiangiogenic therapeutic target in glioblastoma.
Keywords: angiogenesis, bevacizumab, EMP2, glioblastoma
Key Points.
EMP2 expression was associated with survival after bevacizumab therapy.
EMP2 expression increased after bevacizumab treatment in glioblastoma.
Importance of the Study.
We have previously established EMP2 as a proangiogenic protein in glioblastoma. In this study, we further characterize the biology of EMP2 in glioblastoma by evaluating protein expression in matched clinical glioblastoma samples prior to and after bevacizumab treatment. In addition, we investigate localization of the EMP2 in glioblastoma and its effect on survival in regard to bevacizumab. Overall, we supply data EMP2 to potentially implicate a prognostic and antiangiogenic therapeutic target for glioblastoma.
A pathologic hallmark and diagnostic criterion of glioblastoma is the recruitment and proliferation of blood vessels to facilitate further tumor growth.1,2 Given this association, antiangiogenics have been investigated as a potential therapeutic strategy for glioblastoma. The first and best characterized antiangiogenic strategy has been the use of bevacizumab,3,4 an antibody that binds and inactivates vascular endothelial growth factor A (VEGF-A).5 In addition, the following alternative strategies for modulating angiogenesis through the VEGF pathway have been investigated: (1) aflibercept (VEGF trap), a decoy receptor for VEGF-A and B,6 (2) anti-VEGF receptor antibodies,7,8 and (3) intracellular tyrosine kinase inhibitors (cediranib and vandetanib) blocking downstream signaling.9–12 There are several randomized controlled trials studying these anti-VEGF agents in glioblastoma.13–18 In robust meta-analyses of these data, the overall data suggest a progression-free survival but not overall survival benefit of antiangiogenic therapy as adjuvant treatment in new or recurrent glioblastoma, with or without concurrent chemotherapy.19 Given these data, it is clear that targeting angiogenesis in glioblastoma with a monotherapy targeting the VEGF pathway is insufficient, and thus, there is an urgent need to identify novel targets that can predict therapeutic response or be used in concert to improve results.
We have previously identified epithelial membrane protein 2 (EMP2) as a cell surface protein present in a number of tumors including glioblastoma but not in nonpathologic brain tissue.20,21 In in vitro and in vivo models of glioblastoma, EMP2 expression was associated with increased tumor size, vascularity, and VEGF-A expression.22 Furthermore, humanized monoclonal anti-EMP2 antibody leads to significant decreases in tumor load and angiogenesis.22 These experimental data suggest a potential antiangiogenic target in EMP2. In this study, we evaluate the relationship between EMP2 and bevacizumab in a series of clinical glioblastoma specimens, and we identify increased EMP2 expression in tumor samples after bevacizumab treatment, proportional to the length of bevacizumab treatment. These clinical data supplement our existing data suggesting EMP2 as a potential therapeutic target in glioblastoma.
Methods and Materials
Patient Selection
This study was approved by the institutional review board and informed research consent was acquired in all cases. Tumor tissue was prospectively collected for patients undergoing surgery for suspected glioblastoma. Inclusion criteria for this study included (1) adult patients, (2) patients with new tumors, (3) pathologically proven WHO IV glioblastoma, (4) acquired tumor tissue both prior to and after bevacizumab treatment, and (5) clinical and radiologic follow-up of at least 3 months. After the implementation of the above inclusion criteria, 12 patients were included in this study.
The Cancer Genome Atlas and IVY Glioblastoma Atlas Project Data
Using the The Cancer Genome Atlas (TCGA) Data Portal (http://tcga-data.nci.nih.giv/tcga/), robust multichip average normalized mRNA expression data from the Affymetrix HG-U133A Gene Chip was downloaded along with associated survival data and genomic subtype data.23,24 The IVY Glioblastoma Atlas Project (IVY GAP) Data Portal (http://glioblastoma.alleninstitute.org/)25 was used to collect RSEM normalized RNA sequencing data along with histological annotation of the sample source.
Immunohistochemistry
Paraffin-embedded clinical tumor samples and control tissue were sliced into 5-µm-thick samples to be used for immunohistochemistry (IHC). First, sections were deparaffinized in 3 washes of xylene and then rehydrated in serial dilutions of ethanol. Antigen retrieval was performed by placing the slides in a container of 0.1 mol/L citrate, pH 6.0, at 95°C for 25 min. Sections were incubated with polyclonal rabbit antisera against human EMP2,26 and staining visualized using DAKO HRP Anti-Mouse followed by DAB (3,3′-diaminobenzidine) as the substrate. Counterstaining was performed with hematoxylin. Slides were then dehydrated and mounted. An isotype control (rabbit sera) was used as a negative control.
IHC staining was evaluated by a neuropathologist (S.T.), blinded to patient outcomes, based on both the staining intensity (0 = no detection; 1 = weak; 2 = moderate; 3 = strong) and the positive staining percentage (0–100%). The product of the 2 values was used to generate a histologic score (H-score).
Statistical Analysis
Statistical analysis was carried out on GraphPad Prism v6.0h (GraphPad Software, Inc.). Kaplan–Meier survival analysis was carried out using the dependent variables progression-free survival, surgery-free survival, and overall survival stratified by high and low EMP2 H-score from both a clinical cohort and TCGA cohort. Linear regression analysis was implemented to evaluate the relationship between the length of bevacizumab treatment and EMP2 H-score. Unpaired and paired t-tests with Welch’s correction for unequal variance were used to evaluate differences in EMP2 H-score before and after bevacizumab treatment. Errors are presented as the standard error of the mean (SEM) or standard deviation (SD).
Results
EMP2 Is Localized to Areas of Vascular Proliferation in Glioblastoma
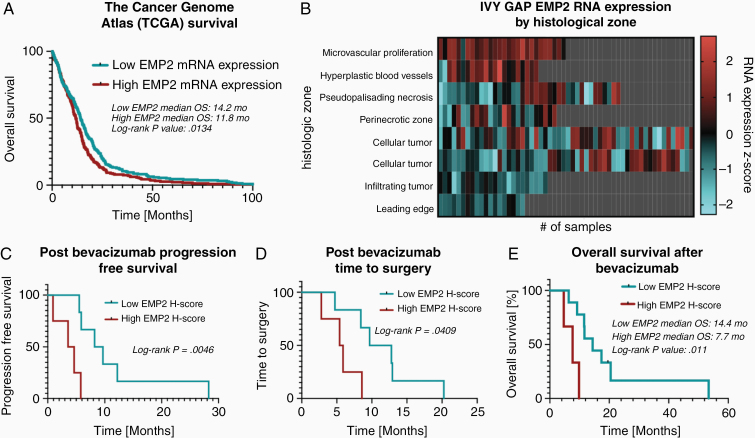
We have previously published the expression pattern of EMP2 using a tissue microarray containing 110 patients diagnosed with glioblastoma and shown that high levels correlate with poor survival.20 To expand on this initial observation, the TCGA dataset was queried for RNA expression data and overall survival for patients with glioblastoma. In 525 patients stratified by median EMP2 mRNA expression, there was decreased overall survival in patients with high EMP2 mRNA expression (median survival 11.8 months vs 14.2 months; log-rank P = .0134; Figure 1A).
Figure 1.
Increased EMP2 is associated with decreased survival after bevacizumab therapy. The Cancer Genome Atlas (TCGA) database was queried for EMP2 RNA expression data and specimens were stratified by high or low EMP2 expression. Kaplan–Meier analysis using the TCGA dataset (A). The IVY Glioblastoma Atlas Project (IVY GAP) database was used to identify RNA expression through normalized z-score at different areas of the tumor as defined by tumor histology (B). Kaplan–Meier analysis using clinical data for progression-free survival (C), time to repeat surgery (D), and overall survival (E), after bevacizumab therapy was initiated.
Our previous studies suggested a role for EMP2 in regulating neoangiogenesis in glioblastoma cell lines.20,22 In order to extend this observation to patient tumors, the IVY GAP Database was queried for RNA sequencing data on EMP2 in clinical glioblastoma specimens. Z-score-normalized RNA expression values were obtained from a total of 270 samples from 37 tumors. Each sample had an associated histological identifier, corresponding to the pathological description of the region from which the sample was obtained. These included areas of microvascular proliferation, hyperplastic blood vessels, pseudopalisading necrosis, perinecrotic zone, cellular tumor, infiltrating tumor, and the leading edge of the tumor. A heat map was created from RNA expression values by histological identifier and there was increased EMP2 RNA expression in areas of microvascular proliferation and hyperplastic blood vessels relative to other areas of the tumor (Figure 1B).
Patient Characteristics
The data above collectively suggested that EMP2 may help regulate neoangiogenesis within the tumor parenchyma as well as tumor cell invasion along the leading edge. Given the widespread usage of antiangiogenics in glioblastoma treatment, we examined the effects of bevacizumab on EMP2 levels. We hypothesized that bevacizumab treatment, a monoclonal antibody against soluble VEGF, may correlate with increased EMP2 levels given the similarities observed with EMP2 upregulation and bevacizumab resistance.22,27
A total of 12 patients with tissue samples from surgical resection at our institution between 2003 and 2013 at the following time points (1) upon initial presentation and (2) after bevacizumab treatment were included in this study. Clinical and tumor characteristics are included in Table 1. There were 8 (67%) males and 4 (33%) females. The average age was 55 years (range 46–73 years). Tumor locations included were temporal for 5 (42%), frontal for 4 (33%), and parietal for 3 (25%). Gross total resection was achieved in 10 (83%) patients. Two tumors (17%) had EGFR amplifications and 1 tumor (8%) had an IDH1 R132H mutation. All patients had newly diagnosed tumors initially treated with surgical resection and adjuvant temozolomide and radiation therapy. Upon subsequent recurrence, all patients had bevacizumab with or without additional chemotherapy. Additional therapy included 1 patient with aflibercept (VEGF trap) prior to bevacizumab treatment, 1 patient with ofranergene obadenovec (VB-111) after bevacizumab treatment, and 2 patients with dendritic cell vaccine (DCvax-L) after bevacizumab treatment. Average length of bevacizumab treatment was 9.5 weeks (range 4–24 weeks). Average progression-free survival after bevacizumab treatment was 6.4 months (range 1–13 months). Average time to surgery after bevacizumab treatment was 7.7 months (range 3–13 months). Overall survival after bevacizumab treatment was 15.5 months (range 5–53 months) and overall survival from initial diagnosis was 39 months (range 10–147 months).
Table 1.
Patient Characteristics
| Characteristic | N = 12 |
|---|---|
| Age (years) | 55 (range 46–73 years) |
| Gender | |
| Male | 8 (67%) |
| Female | 4 (33%) |
| Tumor location | |
| Temporal | 5 (42%) |
| Frontal | 4 (33%) |
| Parietal | 3 (25%) |
| Gross total resection | 10 (83%) |
| EGFR amplifications | 2 (17%) |
| IDH1 R132H mutation | 1 (8%) |
| Additional chemotherapy | |
| Aflibercept (VEGF trap) | 1 (8%) |
| Ofranergene obadenovec (VB-111) | 1 (8%) |
| Dendritic cell vaccine (DCvax-L) | 2 (17%) |
| Bevacizumab treatment | |
| Treatment length | 9.5 (range 4–24 weeks) |
| Progression-free survival after treatment | 6.4 (range 1–13 months) |
| Time to surgery after treatment | 7.7 (range 3–13 months) |
| Survival after treatment | 15.5 (range 5–53 months) |
| Survival from the initial diagnosis | 39 (range 10–147 months) |
EMP2 Expression Is Associated With Decreased Survival With Bevacizumab Treatment
Twelve patients had tumor samples from newly diagnosed glioblastoma available for IHC analysis. The average staining intensity (scored from 0 to 3) was 2.1 (SD = 0.43; range 1–3), the average percent staining was 85% (SD = 12.1; range 60–100), and the average H-score was 183 (SD = 55; range 112.5–300). A cutoff of H-score at least 200 was used to stratify high initial EMP2 from low initial EMP2 levels for further analyses. There was no difference in clinical characteristics between these 2 groups including age (57.6 vs 51.8; P = .29), gender (29% female vs 40% female; P = .71), or gross total resection rate (86% vs 80%; P = .82). To investigate the interaction between EMP2 levels and response to bevacizumab treatment, we evaluated survival after bevacizumab treatment stratified by EMP2 H-score. In patients with a high EMP2 H-score, there was decreased progression-free survival after bevacizumab (median survival 4.6 months vs 5.9 months; log-rank P = .0046; Figure 1C), decreased time to repeat surgery (median length 5.9 months vs 8.4 months; log-rank P = .0409; Figure 1D), and decreased overall survival (median survival 7.7 months vs 14.4 months; log-rank P = .011; Figure 1E).
EMP2 Expression Increases After Bevacizumab Treatment in Clinical Glioblastoma Specimens
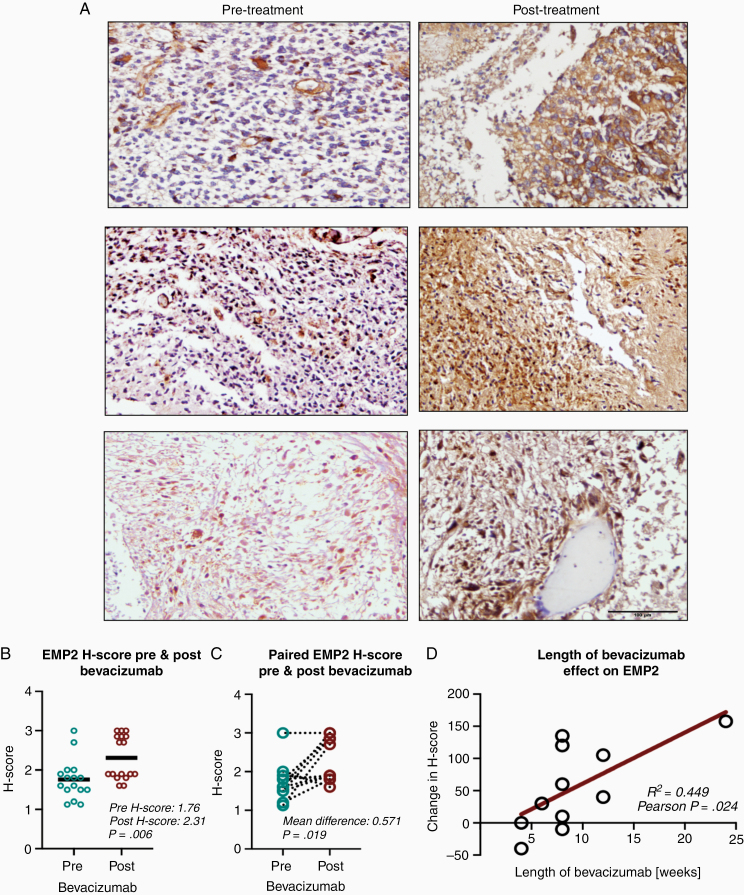
EMP2 H-scores from 16 tumor samples obtained from patients undergoing initial resection for newly diagnosed glioblastoma were compared to EMP H-scores obtained from 17 patients undergoing subsequent resection after bevacizumab treatment for recurrent glioblastoma (Figure 2A). EMP2 H-scores were significantly higher in recurrent tumor samples after bevacizumab treatment (mean H-score 2.31 vs 1.76; P = .006; Figure 2B). A cohort of 12 patients had paired tumor tissue obtained from initial resection for newly diagnosed glioblastoma as well as from recurrent tumor resection after bevacizumab treatment. EMP2 H-scores were significantly higher in recurrent tumor samples after bevacizumab treatment relative to paired initial tumor samples (mean difference 0.571; P = .019; Figure 2C). We evaluated whether the length of bevacizumab treatment affected changes in EMP2 H-score before and after bevacizumab treatment. There was a positive linear correlation between length of bevacizumab treatment and change in EMP2 H-score (R2 = 0.449; Pearson P = .024; Figure 2D). Two patients were treated with medications that target angiogenesis in addition to bevacizumab prior to recurrent surgery: 1 patient with aflibercept (VEGF trap) and 1 patient with ofranergene obadenovec (VB-111). While not statistically powered, these patients interestingly showed increased H-scores relative to those without dual treatment (mean H-score 300 vs 220; P = .063).
Figure 2.
Bevacizumab therapy increases EMP2 levels. (A) Protein expression of EMP2 was visualized using standard IHC in clinical samples before and following bevacizumab treatment and eventual resistance. The expression pattern from 2 patients is shown. Magnification: 200×. Scale bar = 100 µm. (B and C) Protein expression of EMP2 in clinical samples increased after bevacizumab therapy in both unpaired (B) and paired (C) analyses. (D) Increase in protein expression was positively correlated with length of bevacizumab therapy.
Discussion
Following accelerated approval from the US Food and Drug Administration in 2009, bevacizumab showed improved response rates and 6-month progression-free survival compared to historical controls, but since then, it has been shown that most patients’ tumors progress after a median time of 3–5 months.4,28 Given these results, novel methods are needed to quickly detect bevacizumab resistance as well as perhaps serve as a novel target for combination therapy. We have previously identified EMP2 as a protein associated with angiogenesis in glioblastoma22 and shown in glioblastoma cell lines that overexpression of this protein leads to increased tumor vascularity. In this study, we attempt to build upon our laboratory work with an investigation of EMP2 in clinical glioblastoma samples, and we specifically examine the effects of EMP2 following antiangiogenic treatment.
In this study, increased levels of EMP2 were observed in clinical tumor samples after bevacizumab treatment in both unpaired and paired analyses. We find that this increase in EMP2 levels correlated with the length of bevacizumab treatment and the presence of additional VEGF targeting therapeutics. We have previously shown that EMP2 may at least partially serve to increase tumor vascularity through upregulation of VEGF-A within the tumor parenchyma.22 Resistance to bevacizumab is a well-studied topic, with hypoxia-dependent and hypoxia-independent recruitment of alternative kinase signaling pathways,29 changes in autophagy,30,31 and upregulation of alternative promotors of angiogenesis32–38 as potential mechanisms for resistance. Interestingly, in previous gene expression studies in tumors before and after bevacizumab, EMP2 has not been identified as a highly upregulated gene.38–41 In this study, we focus on protein expression of this cell surface molecule and see consistent increases in expression after bevacizumab treatment.
Furthermore, differences in progression-free and overall survival in patients correlated with altered levels of EMP2 from tumor tissue after resection of newly diagnosed glioblastoma. Specifically, higher levels of EMP2 were associated with decreased survival following bevacizumab treatment. While the numbers tested are relatively small, we hypothesize that EMP2 levels may correlate with the efficacy of bevacizumab treatment. This will need to be confirmed by comparing survival to EMP2 levels in patients without bevacizumab treatment.
Lastly, we find an association between areas of vascular proliferation and EMP2 expression, suggesting increased utilization of EMP2 in glioma cells surrounding areas of angiogenesis. This has consequences for potential therapeutic delivery of anti-EMP2 agents as the target protein resides in areas around abnormal blood vessels without an intact blood–brain barrier. Overall, we attempt to build upon our previous work investigating EMP2 as a potential antiangiogenic therapy. We hypothesize that the EMP2 correlation to bevacizumab treatment may warrant future study as a potential mechanism for bevacizumab resistance and antiangiogenic therapy.
Finally, we note several limitations to the study. This study is susceptible to confounding bias given (1) small sample size and (2) heterogeneity in treatment including a few patients with therapy beyond the standard of care chemotherapy and radiation therapy (ie, immunotherapy). In addition, given all patients received temozolomide and radiation therapy, it is unclear whether these have an effect on EMP2 expression and is not specifically tested in our study. In addition, EMP2 may increase in recurrent tumors independent of treatment modalities. Lastly, given the intratumoral heterogeneity with EMP2 expression, EMP2 expression levels may be biased depending on the areas of sample selection. Despite these limitations, we believe this clinical data translate previous work in cell lines to highlight the potential of EMP2 as a biomarker for antiangiogenic therapy in glioblastoma.
Conclusions
EMP2 is a cell surface protein found in glioblastoma that serves to increase angiogenesis in cell line models. High levels of EMP2 are associated with decreased survival after bevacizumab therapy. After bevacizumab therapy, EMP2 protein expression levels increase in a manner proportional to the length of therapy, and specifically, protein expression was enriched in areas of angiogenesis in glioblastoma. Overall, we hypothesize that these data suggest further study on EMP2 in the treatment response and resistance to bevacizumab as well as further evaluation as a tool to assess antiangiogenic therapies in glioblastoma.
Funding
This work was generously supported by Eli & Edythe Broad Stem Cell Institute at UCLA Fellowship (K.P.) NIH/NCI P50-CA211015 (M.W. and W.Y.), and NCI R01 CA163971 (M.W.).
Conflict of interest statement. M.W and L.K.G. are inventors on the University of California patents related to anti-EMP2 monoclonal antibodies. M.W. was a consultant for OncoResponse, Inc., Seattle, WA.
Authorship statement: Conception of study: K.P., L.G., T.C., L.L., W.Y., I.Y., and M.W; acquisition of data: K.P., S.K., S.T., C.D., A.M., L.L., W.Y., I.Y., and M.W; analysis of data: K.P., S.K., S.T., C.D., A.M., L.L., W.Y., I.Y., and M.W; drafting of manuscript: K.P., S.K., C.D., I.Y., and M.W; critical review of manuscript: all authors; study supervision: L.G., T.C., L.L., W.Y., I.Y., and M.W.
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Gupta A, Dwivedi T. A simplified overview of World Health Organization classification update of central nervous system tumors 2016. J Neurosci Rural Pract. 2017;8(4):629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 4. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sivakumar B, Harry LE, Paleolog EM. Modulating angiogenesis: more vs less. JAMA. 2004;292(8):972–977. [DOI] [PubMed] [Google Scholar]
- 6. Nayak L, de Groot J, Wefel JS, et al. Phase I trial of aflibercept (VEGF trap) with radiation therapy and concomitant and adjuvant temozolomide in patients with high-grade gliomas. J Neurooncol. 2017;132(1):181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atzori MG, Tentori L, Ruffini F, et al. The anti-vascular endothelial growth factor receptor-1 monoclonal antibody D16F7 inhibits invasiveness of human glioblastoma and glioblastoma stem cells. J Exp Clin Cancer Res. 2017;36(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peterson TE, Kirkpatrick ND, Huang Y, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci U S A. 2016;113(16):4470–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown N, McBain C, Nash S, et al. Multi-center randomized phase II study comparing cediranib plus gefitinib with cediranib plus placebo in subjects with recurrent/progressive glioblastoma. PLoS One. 2016;11(5):e0156369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dietrich J, Wang D, Batchelor TT. Cediranib: profile of a novel anti-angiogenic agent in patients with glioblastoma. Expert Opin Investig Drugs. 2009;18(10):1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee EQ, Kaley TJ, Duda DG, et al. A multicenter, phase II, randomized, noncomparative clinical trial of radiation and temozolomide with or without vandetanib in newly diagnosed glioblastoma patients. Clin Cancer Res. 2015;21(16):3610–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 14. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrlinger U, Schäfer N, Steinbach JP, et al. Bevacizumab plus irinotecan versus temozolomide in newly diagnosed O6-methylguanine-DNA methyltransferase nonmethylated glioblastoma: the randomized GLARIUS trial. J Clin Oncol. 2016;34(14):1611–1619. [DOI] [PubMed] [Google Scholar]
- 16. Chauffert B, Feuvret L, Bonnetain F, et al. Randomized phase II trial of irinotecan and bevacizumab as neo-adjuvant and adjuvant to temozolomide-based chemoradiation compared with temozolomide-chemoradiation for unresectable glioblastoma: final results of the TEMAVIR study from ANOCEF†. Ann Oncol. 2014;25(7):1442–1447. [DOI] [PubMed] [Google Scholar]
- 17. Balana C, De Las Penas R, Sepúlveda JM, et al. Bevacizumab and temozolomide versus temozolomide alone as neoadjuvant treatment in unresected glioblastoma: the GENOM 009 randomized phase II trial. J Neurooncol. 2016;127(3):569–579. [DOI] [PubMed] [Google Scholar]
- 18. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 19. Ameratunga M, Pavlakis N, Wheeler H, Grant R, Simes J, Khasraw M. Anti-angiogenic therapy for high-grade glioma. Cochrane Database Syst Rev. 2018;11:CD008218. doi: 10.1002/14651858.CD008218.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qin Y, Fu M, Takahashi M, et al. Epithelial membrane protein-2 (EMP2) activates Src protein and is a novel therapeutic target for glioblastoma. J Biol Chem. 2014;289(20):13974–13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel KS, Kejriwal S, Sun MM, et al. Identification of epithelial membrane protein 2 (EMP2) as a molecular marker and correlate for angiogenesis in meningioma. J Neurooncol. 2020;147(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qin Y, Takahashi M, Sheets K, et al. Epithelial membrane protein-2 (EMP2) promotes angiogenesis in glioblastoma multiforme. J Neurooncol. 2017;134(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network . The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verhaak RG, Hoadley KA, Purdom E, et al. ; Cancer Genome Atlas Research Network . Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Puchalski RB, Shah N, Miller J, et al. An anatomic transcriptional atlas of human glioblastoma. Science. 2018;360(6389):660–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wadehra M, Natarajan S, Seligson DB, et al. Expression of epithelial membrane protein-2 is associated with endometrial adenocarcinoma of unfavorable outcome. Cancer. 2006;107(1):90–98. [DOI] [PubMed] [Google Scholar]
- 27. Li Y, Ali S, Clarke J, Cha S. Bevacizumab in recurrent glioma: patterns of treatment failure and implications. Brain Tumor Res Treat. 2017;5(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pope WB, Xia Q, Paton VE, et al. Patterns of progression in patients with recurrent glioblastoma treated with bevacizumab. Neurology. 2011;76(5):432–437. [DOI] [PubMed] [Google Scholar]
- 29. Ramezani S, Vousooghi N, Joghataei MT, Chabok SY. The role of kinase signaling in resistance to bevacizumab therapy for glioblastoma multiforme. Cancer Biother Radiopharm. 2019;34(6):345–354. [DOI] [PubMed] [Google Scholar]
- 30. Abdul Rahim SA, Dirkse A, Oudin A, et al. Regulation of hypoxia-induced autophagy in glioblastoma involves ATG9A. Br J Cancer. 2017;117(6):813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang H, Song J, Liu Z, Pan L, Xu G. Autophagy activation promotes bevacizumab resistance in glioblastoma by suppressing Akt/mTOR signaling pathway. Oncol Lett. 2018;15(2):1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scholz A, Harter PN, Cremer S, et al. Endothelial cell-derived angiopoietin-2 is a therapeutic target in treatment-naive and bevacizumab-resistant glioblastoma. EMBO Mol Med. 2016;8(1):39–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Labussière M, Cheneau C, Prahst C, et al. Angiopoietin-2 may be involved in the resistance to bevacizumab in recurrent glioblastoma. Cancer Invest. 2016;34(1):39–44. [DOI] [PubMed] [Google Scholar]
- 34. Michaelsen SR, Staberg M, Pedersen H, et al. VEGF-C sustains VEGFR2 activation under bevacizumab therapy and promotes glioblastoma maintenance. Neuro Oncol. 2018;20(11):1462–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cho HR, Hong B, Kim H, et al. Assessment of bevacizumab resistance increased by expression of BCAT1 in IDH1 wild-type glioblastoma: application of DSC perfusion MR imaging. Oncotarget. 2016;7(43):69606–69615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mao XG, Wang C, Liu DY, et al. Hypoxia upregulates HIG2 expression and contributes to bevacizumab resistance in glioblastoma. Oncotarget. 2016;7(30):47808–47820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mastrella G, Hou M, Li M, et al. Targeting APLN/APLNR improves antiangiogenic efficiency and blunts proinvasive side effects of VEGFA/VEGFR2 blockade in glioblastoma. Cancer Res. 2019;79(9):2298–2313. [DOI] [PubMed] [Google Scholar]
- 38. Urup T, Staunstrup LM, Michaelsen SR, et al. Transcriptional changes induced by bevacizumab combination therapy in responding and non-responding recurrent glioblastoma patients. BMC Cancer. 2017;17(1):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Piao Y, Liang J, Holmes L, Henry V, Sulman E, de Groot JF. Acquired resistance to anti-VEGF therapy in glioblastoma is associated with a mesenchymal transition. Clin Cancer Res. 2013;19(16):4392–4403. [DOI] [PubMed] [Google Scholar]
- 40. Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15(14):4589–4599. [DOI] [PubMed] [Google Scholar]
- 41. DeLay M, Jahangiri A, Carbonell WS, et al. Microarray analysis verifies two distinct phenotypes of glioblastomas resistant to antiangiogenic therapy. Clin Cancer Res. 2012;18(10):2930–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]