Abstract
Post-mortem investigation in cases of fatal anaphylaxis is required to provide clarifications on the presence of macroscopic pathological changes, histological features, and immunohistochemical positivity suggestive of the diagnosis, on biochemical evidence of anaphylaxis and on the presence of serological data indicative of the allergen responsible for the anaphylactic reaction. We describe the case of a 16-year-old boy with a medical history of allergic asthma, celiac disease, and known food-induced allergy for fish, fresh milk, peanuts, hazelnuts, walnuts, apples, kiwis, and peaches. Acute onset of dyspnea followed by cyanosis of the lips and respiratory failure was described immediately after having an ice cream sandwich. Unsuccessful rescues were immediately attempted with oral administration of betamethasone, intramuscular injection of adrenaline, and cardiopulmonary resuscitation. A complete post-mortem examination was performed. Serum dosage of mast cell beta-tryptase from femoral blood detecting serum values of 41.4 mg/l. Determination of specific IgE on cadaveric blood samples confirmed the anamnestic data related to sensitization for several food allergens, including cod parvalbumin, tropomyosin, brazil nut, omega-5-gliadin of foods derived from wheat and gluten. The cause of death was identified in a cardiorespiratory failure due to anaphylactic shock in a poly-allergic subject and anaphylaxis was ascribed to the wheat contained in the ice cream sandwich eaten immediately before the onset of respiratory symptoms. The need is to implement an interdisciplinary approach capable to ascertain the sensitivity and specificity of the diagnostic tests currently in use as well as to evaluate the possibility of introducing new biomarkers in practice.
Keywords: beta tryptase, food allergens, food-induced allergy, IgE, mast-cells, Wheat
Introduction
Food-induced anaphylaxis (FIA) is defined as an adverse reaction due to a specific and reproducible immune response resulting from exposure to a particular food.1 According to international recommendations, this definition includes IgE-mediated and non-IgE mediated immune responses, as well as the combination of both mechanisms.2–3 Food allergens can cause reactions by ingestion or contact. At present, a large amount of food has been identified as a cause of IgE-mediated phenomena, although the majority of allergic reactions is attributable to a limited number of foods such as peanuts, tree nuts, egg, milk, fish, shellfish, wheat, and soy.4
Food-induced anaphylaxis is a rare cause of death and it is difficult to study as it very often represents a community event that occurs outside the hospital setting.5–6 Although the precise incidence of anaphylaxis mortality is still unknown, several retrospective post-mortem and population studies estimate the mortality rate to be 0.65%–2%. Fatal food-induced allergy usually presents acutely (from a few minutes to several hours) after exposure to a known allergen with two or more of the following symptoms: generalized urticaria, itching or redness, rhinorrhea, conjunctivitis, swelling of the lips, tongue or uvula, respiratory impairment with dyspnea, wheezing, bronchospasm and hypoxia, cardiovascular compromise with hypotension, tachycardia, and collapse. Death due to anaphylaxis is usually due to heterogeneous mechanisms, often combined, including upper airway obstruction, asphyxia from bronchospasm, cardiogenic shock and, sometimes, hemorrhagic or thromboembolic phenomena.7
Case report
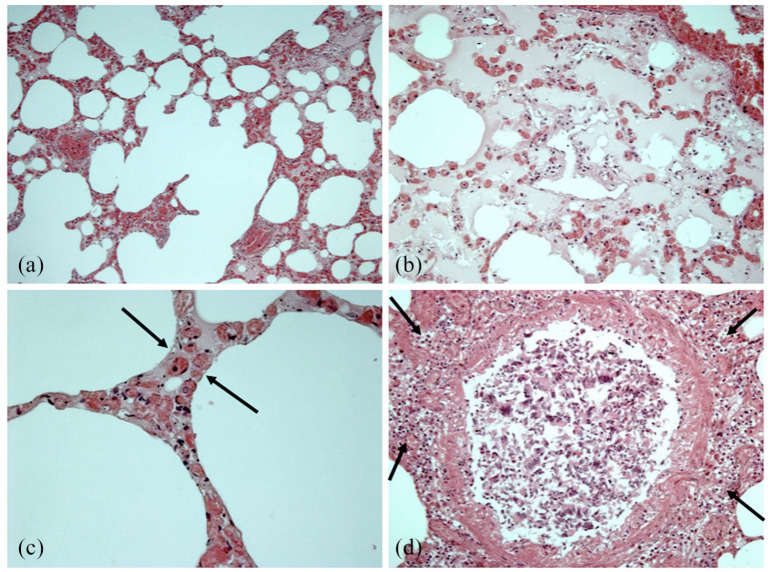
The case presented concerns a 16-year-old boy with a medical history of allergic asthma, celiac disease, and known food-induced allergy for fish, fresh milk, peanuts, hazelnuts, walnuts, apples, kiwis, and peaches. Acute onset of dyspnea followed by cyanosis of the lips and respiratory failure was described immediately after having an ice cream sandwich. Unsuccessful rescues were immediately attempted with oral administration of betamethasone, intramuscular injection of adrenaline, and cardiopulmonary resuscitation. A complete post-mortem examination was performed 2 days after death. No putrefaction phenomena were evident. External examination was unremarkable. Subpleural petechiae and heavy lungs presenting white foam on the main bronchi were observed at the autopsy investigation. Mild cerebral edema was also detected. The heart was normal, with a conical shape and slightly represented subepicardial fat. Coronary arteries were normal, and obstructions of the lumen were excluded. The laryngo-tracheo-bronchial tree did not show macroscopically detectable pathological findings. The stomach contained about 200 cc of partially digested food. All tissue specimens were fixed in formalin and embedded in paraffin, then a routine hematoxylin and eosin stain was employed. Ubiquitous acute stasis, mild cerebral edema and interstitial myocardial edema were recorded. In addition, acute pulmonary edema mixed with areas of acute pulmonary emphysema were present. Intraparenchymal hemorrhages on the spleen and adrenal glands were observed (Figure 1). An immunohistochemical technique was used to estimate mast-cell population, using the anti-tryptase antibody as a specific marker on 5 mm thick paraffin sections. Afterward, a pulmonary area of 100 mm2 was analyzed. We examined histological samples from a control group where the cause of death was clearly attributable to traumatic events. Pulmonary mast cells were identified and quantified and a great number of degranulating mast cells with tryptase-positive material outside were observed (Figure 2). Data resulting from quantitative analysis recorded a numerical increase in pulmonary mast cells in this case (average mast-cell count 12,551/100 mm2) compared with that of the traumatic control group (traumatic death) whose average mast-cell count was 3557/100 mm2. The microscopic examination of duodenal samples stained with anti-CD3 and anti-CD8 antibodies supported the clinical diagnosis of celiac disease.
Figure 1.
Lung histological findings: (a) acute emphysema; (b) focal endoalveolar edema; (c) septal edema (arrows); (d) lymphocytic, monocytic and eosinophilic peribronchial infiltration (arrows) associated with peribronchial parietal muscle thickening.
Figure 2.
Lung immunohistochemical findings: (a–d) tryptase immunoreaction (+++) (brown cell reactions) with impressive response of the mast-cells in the peribronchial area (arrows) and evident degranulation phenomena (red arrows).
Toxicological analysis on blood and urine specimens for therapeutic and non-therapeutic drugs were performed using gas chromatography-mass spectrometry and resulted negative. Tryptase was measured on a 10 cm3 sample of venous femoral blood by aspirating the vessel with a sterile single use 18-gage needle and a 10-mL syringe taken during the autopsy and stored in the freezer at –80°C using an enzyme immunoassay technique (Uni-CAP TRYPTASE Fluoroenzymeimmunoassay Pharmacia, AB Uppsala, Sweden). Serum dosage of mast cell tryptase from venous femoral blood detecting serum values of 41.4 mg/l (normal value ⩽10 mg/l).
Determination of specific IgE on cadaveric blood samples confirmed the anamnestic data related to sensitization for several food allergens, including cod parvalbumin (34.5 kUA/l), tropomyosin (40.5 kUA/l), brazil nut (5.84 kUA/l), omega-5-gliadin of foods derived from wheat (1.07 kUA/l,) and gluten (54.8 kUA/l) (Table 1).
Table 1.
Determination of specific IgE on cadaveric blood samples.
| Allergen | Specific IgE concentration (kUA/l) | Reference values (kUA/l) |
|---|---|---|
| rTri a19 (omega-5-gliadin) | 1.07 | 0.10 |
| rPru p1 (peach) | 0.02 | 0.10 |
| rPru p3 (peach) | 0.84 | 0.10 |
| rPru p4 (peach) | 0.11 | 0.10 |
| rAra h1 (peanut) | 0.05 | 0.10 |
| rAra h2 (peanut) | 0.03 | 0.10 |
| rAra h3 (peanut) | 0.08 | 0.10 |
| rCor a8 (hazelnut) | 0.26 | 0.10 |
| rGad c1 (cod parvalbumin) | 34.5 | 0.10 |
| rAra h9 (peanut) | 0.32 | 0.10 |
| rCor a1 (hazelnut) | 0.17 | 0.10 |
| nGal d2 (ovalbumin) | 0.07 | 0.10 |
| nGal d1 (ovomucoid) | 0.13 | 0.10 |
| nGal d3 (ovotransferrin) | 0.03 | 0.10 |
| rPen a1 (shrimp tropomyosin) | 40.5 | 0.10 |
| rBer e1 (Brazil nut) | 5.84 | 0.10 |
| nBos d4 (alpha-lactalbumin) | 0.17 | 0.10 |
| nBos d5 (beta-lactoglobulin) | 0.24 | 0.10 |
| nBos d8 (casein) | 0.37 | 0.10 |
| rTri a14 (gluten) | 54.8 | 0.10 |
| rPar j2 (Parietaria judaica) | 0.03 | 0.10 |
| nArt v3 (mugwort) | 0.24 | 0.10 |
The cause of death was identified in a cardiorespiratory failure due to anaphylactic shock in a poly-allergic subject and anaphylaxis was ascribed to the wheat contained in the ice cream sandwich eaten immediately before the onset of respiratory symptoms.
Discussion
In the past few decades, a definite increase in the prevalence of food allergy in infancy and childhood has been described. The actual prevalence of food allergy is approximately about 5%–8% of children and 1.2% of adults; this is easily demonstrated by the detection of IgE specific for food allergens accompanied by a convincing history of type I hypersensitivity-mediated symptoms and signs after ingestion of food. Infants and children have a predisposition to the development of food allergies but, in most cases, they acquire immunological tolerance over time. The rate of fatal food anaphylaxis is very low overall and is a rare event in pre-school children.5–6 Delay in the use of adrenaline, risky behavior (especially in adolescents), coexisting asthma and severity of prior reactions are indicated as risk factors for fatal food anaphylaxis.
Post-mortem investigation in cases of fatal anaphylaxis is required to provide clarifications on the presence of macroscopic pathological changes, histological features, and immunohistochemical positivity suggestive of the diagnosis, on biochemical evidence of anaphylaxis and on the presence of serological data indicative of the allergen responsible for the anaphylactic reaction.7 Post-mortem dosing of mast-cell tryptase is widely performed in forensic practice for the assessment of cases of suspected anaphylaxis.8–14 However, despite the extensive use of the method, the interpretation of the results is complex in relation to the presence of confounding factors including the state of conservation of the biological matrix and the increase in the levels of tryptase in other conditions different from anaphylaxis. Therefore, the combined evaluation of serum levels of tryptase and specific IgE allows to significantly improve diagnostic accuracy by eliminating the problems of differential diagnosis deriving from the finding of high tryptase values. The diagnosis of fatal anaphylaxis and the problems related to the reliability of serum tryptase values must be addressed through a precise methodology, starting from the sample collection site. Different methods (transcutaneous or directly vascular by sectioning the vessel or clamping it) and different sampling sites (arterial or venous), are described and compared in literature.15–18 Garland recommends aspirating blood samples from a clamped femoral/external iliac vein,19 as femoral vein cut down could result in a slightly elevated level in comparison to aspiration with a needle and syringe.20 Regarding the values of postmortem serum tryptase, Garland established a reference range of <23 μg/L in non-anaphylactic deaths.21 Regarding the value that can be used in the postmortem diagnosis of lethal anaphylaxis, Sun et al., performed a systematic review and meta-analysis demonstrating a cut-off level higher than 30.4 μg/L (sensitivity and specificity for anaphylaxis were 68.5% and 83.9%, respectively).22 Moreover, Tejedor-Alonso point out that the best cutoff point for postmortem tryptase in anaphylaxis is 40–60 mg/l, differentiating anaphylaxis due to drugs and Hymenoptera, comparing to anaphylaxis due to food (tryptase values were more than double of those deaths due to foods).23 Similarly, Platzgummer states that in food anaphylaxis, tryptase levels are usually lower than those observed in drug-induced anaphylaxis.24
Certainly, the argument about the validity of the current laboratory markers used for the post-mortem diagnosis of anaphylaxis is constantly evolving. Beck supports the investigation of chymase, carboxypeptidase A3, dipeptidyl peptidase I (DPPI), basogranulin, CCL-2, and platelet-activating factor (PAF) in the diagnosis of anaphylaxis.20 Further studies in the fields of proteomics, metabolomics and epigenetics could provide useful elements for post-mortem investigation of a complex immunological condition such as food-induced anaphylaxis.20,25
Conclusion
In conclusion, as demonstrated in the described case, at present it is possible to implement an interdisciplinary approach capable of documenting the existence of an anaphylactic reaction and identifying the allergen responsible for the response. However, much progress has yet to be made to ascertain the sensitivity and specificity of the diagnostic tests currently in use as well as to evaluate the possibility of introducing new biomarkers in practice.16,26–27 Furthermore, a similar methodology will enable to obtain reliable epidemiological data on a clearly underestimated phenomenon.
Footnotes
Authors’ note: Stefano D’Errico is now affiliated with Department of Medical, Surgical and Health Sciences, University of Trieste, Trieste, Italy.
Author contribution: SDE conceptualized the study and wrote the manuscript. MS, AS performed data analysis and critically reviewed the manuscript. PF, VF critically reviewed the manuscript for intellectual content.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: This case dealt with a death of judicial interest, for which ethical approval and consent were requested and obtained from the Judicial Authority.
Data processing complies with the general authorization for scientific research purposes granted by the Italian Data Protection Authority (1 March 2012 as published in Italy’s Official Journal no. 72 dated 26 March 2012) since the data do not entail any significant personalized impact on data subjects.
Approval by an institutional and/or licensing committee is not required since experimental protocols are not applied in the study.
ORCID iD: Vittorio Fineschi  https://orcid.org/0000-0002-1686-3236
https://orcid.org/0000-0002-1686-3236
References
- 1. Boyce JA, Assa’ad A, Burks AW, et al. (2010) Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. Journal of Allergy and Clinical Immunology 126: S1–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burks AW, Tang M, Sicherer S, et al. (2012) ICON: Food allergy. Journal of Allergy and Clinical Immunology 129: 906–920. [DOI] [PubMed] [Google Scholar]
- 3. Sackeyfio A, Senthinathan A, Kandaswamy P, et al. (2011) Diagnosis and assessment of food allergy in children and young people: Summary of NICE guidance. BMJ 342: d747. [DOI] [PubMed] [Google Scholar]
- 4. Brockow K, Ring J. (2009) Food anaphylaxis. Analytical and Bioanalytical Chemistry 395: 17–23. [DOI] [PubMed] [Google Scholar]
- 5. Cianferoni A, Muraro A. (2012) Food-induced anaphylaxis. Immunology and Allergy Clinics of North America 32: 165–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Umasunthar T, Leonardi-Bee J, Hodes M, et al. (2013) Incidence of fatal food anaphylaxis in people with food allergy: A systematic review and meta-analysis. Clinical & Experimental Allergy 43: 1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Byard RW. (2017) Anaphylaxis at autopsy. Forensic Science, Medicine and Pathology 13: 269–271. [DOI] [PubMed] [Google Scholar]
- 8. Wongkaewpothong P, Pacharn P, Sripramong C, et al. (2014) The utility of serum tryptase in the diagnosis of food-induced anaphylaxis. Allergy, Asthma & Immunology Research 6: 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenberger PA, Rotskoff BD, Lifschultz B. (2007) Fatal anaphylaxis: Postmortem findings and associated comorbid diseases. Annals of Allergy, Asthma & Immunology 98: 252–257. [DOI] [PubMed] [Google Scholar]
- 10. Da Broi U, Moreschi C. (2011) Post-mortem diagnosis of anaphylaxis: A difficult task in forensic medicine. Forensic Science International 204: 1–5. [DOI] [PubMed] [Google Scholar]
- 11. Pumphrey RHS, Roberts ISD. (2000) Postmortem findings after fatal anaphylactic reactions. Journal of Clinical Pathology 53: 273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fineschi V, Monasterolo G, Rosi R, et al. (1999) Fatal anaphylactic shock during a fluorescein angiography. Forensic Science International 100: 137–142. [DOI] [PubMed] [Google Scholar]
- 13. Unkrig S, Hagemeier L, Madea B. (2010) Postmortem diagnostics of assumed food anaphylaxis in an unexpected death. Forensic Science International 198: e1–e4. [DOI] [PubMed] [Google Scholar]
- 14. Riezzo I, Bello S, Neri M, et al. (2010) Ceftriaxone intradermal test-related fatal anaphylactic shock: A medico-legal nightmare. Allergy 65: 130–131. [DOI] [PubMed] [Google Scholar]
- 15. McLean-Tooke A, Goulding M, Bundell C, et al. (2014) Postmortem serum tryptase levels in anaphylactic and non-anaphylactic deaths. Journal of Clinical Pathology 67: 134–138. [DOI] [PubMed] [Google Scholar]
- 16. Edston E, van Hage-Hamsten M. (1998) Beta-tryptase measurements post-mortem in anaphylactic deaths and in controls. Forensic Science International 93: 135–142. [DOI] [PubMed] [Google Scholar]
- 17. Horn KD, Halsey JF, Zumwalt RE. (2004) Utilization of serum tryptase and immunoglobulin e assay in the postmortem diagnosis of anaphylaxis. The American Journal of Forensic Medicine and Pathology 25: 37–43. [DOI] [PubMed] [Google Scholar]
- 18. Tse R, Wong CX, Kesha K, et al. (2018) Post mortem tryptase cut-off level for anaphylactic death. Forensic Science International 284: 5–8. [DOI] [PubMed] [Google Scholar]
- 19. Garland J, Philcox W, McCarthy S, et al. (2019) The effects of different sampling techniques on peripheral post mortem tryptase levels: A recommended sampling method. International Journal of Legal Medicine 133: 1477–1483. [DOI] [PubMed] [Google Scholar]
- 20. Beck SC, Wilding T, Buka RJ, et al. (2019) Biomarkers in human anaphylaxis: A critical appraisal of current evidence and perspectives. Frontiers in Immunology 10: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garland J, Philcox W, McCarthy S, et al. (2019) Postmortem tryptase level in 120 consecutive nonanaphylactic deaths: Establishing a reference range as <23μg/L. The American Journal of Forensic Medicine and Pathology 40: 351–355. [DOI] [PubMed] [Google Scholar]
- 22. Sun KJ, He JT, Huang HY, et al. (2018) Diagnostic role of serum tryptase in anaphylactic deaths in forensic medicine: A systematic review and meta-analysis. Forensic Science, Medicine and Pathology 14: 209–215. [DOI] [PubMed] [Google Scholar]
- 23. Tejedor-Alonso MA, Vallejo-de-Torres G, Escayola EN, et al. (2020) Postmortem tryptase cutoff points and main causes of fatal anaphylaxis. The Journal of Allergy and Clinical Immunology: In Practice 8: 761–763.e6. [DOI] [PubMed] [Google Scholar]
- 24. Platzgummer S, Bizzaro N, Bilò MB, et al. (2020) Recommendations for the use of tryptase in the diagnosis of anaphylaxis and clonal mastcell disorders. European Annals of Allergy and Clinical Immunology 52: 51–61. [DOI] [PubMed] [Google Scholar]
- 25. Santurro A, Vullo AM, Borro M, et al. (2017) Personalized medicine applied to forensic sciences: New advances and perspectives for a tailored forensic approach. Current Pharmaceutical Biotechnology 18: 263–273. [DOI] [PubMed] [Google Scholar]
- 26. Nilsson C, Berthold M, Mascialino B, et al. (2020) Allergen components in diagnosing childhood hazelnut allergy: Systematic literature review and meta-analysis. Pediatric Allergy and Immunology 31: 186–196. [DOI] [PubMed] [Google Scholar]
- 27. Turillazzi E, Greco P, Neri M, et al. (2008) Anaphylactic latex reaction during anaesthesia: The silent culprit in a fatal case. Forensic Science International 179: e5–e8. [DOI] [PubMed] [Google Scholar]