Abstract
Background
Treatments for coronavirus disease 2019 (COVID-19) are limited by suboptimal efficacy.
Methods
From January 30, 2020 to March 23, 2020, we conducted a non-randomised controlled trial, in which all adult patients with laboratory-confirmed COVID-19 were assigned to three groups non-randomly and given supportive treatments: Group A, Lopinavir-Ritonavir; Group B, Huashi Baidu Formula (a Chinese medicineformula made by the China Academy of Chinese Medical Sciences to treat COVID-19, which is now in the clinical trial period) and Lopinavir-Ritonavir; and Group C, Huashi Baidu Formula. The use of antibiotics, antiviruses, and corticosteroids was permitted in Group A and B. Traditional Chinese medicine injections were permitted in Group C. The primary outcomes were clinical remission time (interval from admission to the first time the patient tested negatively for novel coronavirus or an obvious improvement was observed from chest CT) and clinical remission rate (number of patients whose clinical time was within 16 days/total number of patients).
Results
A total of 60 adult patients with COVID-19 were enrolled at sites in Wuhan, China, and the sample size of each group was 20. In Groups A, B and C, the clinical remission rates were 95.0%%(19/20), 100.0%%(20/20) and 100.0%%(20/20), respectively. Compared with Groups A and B, the clinical remission time of Group C was significantly shorter (5.9 days vs. 10.8 days, p < 0.05; 5.9 days vs. 9.7 days, p < 0.05). There was no significant difference among Groups A, B, and C in terms of the time taken to be released from quarantine. The clinical biochemical indicators and safety indexes showed no significant differences among the three groups.
Conclusions
Our findings suggest that Lopinavir-Ritonavir has some efficacy in the treatment of COVID-19, and the Huashi Baidu Formula might enhance this effect to an extent. In addition, superiority was displayed in the treatment of COVID-19 through a combination of the Huashi Baidu Formula and traditional Chinese medicine injection. In future, well-designed prospective double-blinded randomised control trials are required to confirm our findings.
Keywords: Chinese herbal medicine, Lopinavir-Ritonavir, Coronavirus disease 2019, Non-randomized controlled trial
Background
In late December 2019, a cluster of acute respiratory illnesses, now known as novel coronavirus pneumonia (NCP), occurred in Wuhan, Hubei Province, China (Hongzhou et al., 2020). The NCP rapidly spread worldwide, posing a severe threats to international public health (Hui et al., 2020, Munster et al., 2020). Subsequebtly, the World Health Organization (WHO) declared a Public Health Emergency of International Concern over the global outbreak of coronavirus disease 2019 (COVID-19) in January 31, 2020. Some COVID-19 infections can be severe, particularly in older adults with underlying medical conditions (Chen et al., 2020, Huang et al., 2020). The disease onset may result in progressive respiratory failure due to alveolar damage, and even death. China is currently directly affected by the COVID-19 pandemic. It is believed that vaccines and medicines can be developed to control and prevent this disease. However, the development of vaccines is usually hysteretic and currently there are no clinically confirmed specific antiviral treatments for COVID-19. Treatments used at present are generally supportive. Therefore, it is necessary to urgently conduct clinical trials of medicines to identify suitable treatments for COVID-19. Previous studies have indicated thatthe combination of lopinavir and ritonavir may be beneficial for patients infected with SARS-CoV and MERS-CoV (Chu et al., 2004, Arabi et al., 2018). However, corticosteroids have not been found to reduce the mortality of SARS-CoV and MERS-CoV infection based on the WHO interim guidance (Arabi et al., 2018).
Chinese herbal medicine (CHM) has been used to control infectious diseases for thousands of years, and previous studies suggest that combining Chinese with Western medicine is useful in the treatment of SARS caused by coronavirus infection (Chen and Nakamura, 2004, Jia and Gao, 2003). CHM is a potentially effective method for controlling the newly emerged NCP. From the perspective of CHM, COVID-19 belongs to the category of ‘epidemic disease’, and the total pathogenesis can be summarised as dampness, poison, blood stasis and deficiency (Qing et al., 2020). CHM treatment focuses on mobilising the body's own disease resistance, and has unique advantages in improving clinical symptoms, reducing complications, and improving quality of life. A variety of Chinese medicine treatment compounds are recommended in the Chinese 6th edition guidelines. It is highly feasible to identify and develop active components against novel coronaviruses from these compounds.
In this study, we conducted a non-randomized controlled trial (NRCT) at Jinyintan Hospital in Wuhan. We aimed to evaluate the efficacy and safety of CHM and Lopinavir-Ritonavir treatment in adult patients with COVID-19.
Methods
Study design and setting
A prospective, single-center, NRCT design was conducted from January 30 to March 23, 2020 at Jingyintan Hospital in Wuhan, Hubei province, China. All treatments were generally conducted, and baseline symptoms of patients with COVID-19 were assessed, within 24 h of enrolling. When the respiratory symptoms obviously improved and two consecutive novel coronavirus nucleic acid tests showed negative results (the interval between sampling was a minimum of one day), patients were discharged from quarantine.
The patients were non-randomly divided into three treatment groups with the patients’ informed consent: (1) Group A: Lopinavir-Ritonavir, n = 20; (2) Group B: Huashi Baidu Formula (a Chinese medicine formula made by the China Academy of Chinese Medical Sciences to treat COVID-19, which is now in the clinical trial periodformula) and Lopinavir-Ritonavir, n = 20; and (3) Group C: Huashi Baidu Formula, n = 20. The use of antibiotics, antiviruses, and corticosteroids was permitted in Groups A and B. Traditional Chinese medicine injections were permitted in Group C. The detailed treatments of the three groups were as follows: Lopinavir-Ritonavir (500 mg twice daily, orally), antibiotics (such as cefoperazone, 2 g twice daily, intravenous injection; moxifloxacin hydrochloride tablets, 0.4 g once daily, orally), corticosteroids (such as methylprednisolone, 40 mg once daily, intravenous injection; prednisone, 30 mg once daily, orally), antiviruses (such as arbidol capsule, 0.2 g three times daily, orally), Huashi Baidu Formula (137 g twice daily, orally), ShenMai injection (60 mL once daily, intravenous injection), XiYanPing injection (100 mg twice daily, intravenous injection), and XueBiJing injection (100 ml twice daily, intravenous injection). All groups received supportive therapy, including oxygen inhalation, symptomatic treatment and/or immunoglobulin intravenous injection and/or serum albumin intravenous injection, and treatment for basic diseases.
Patients
The test population was clinically diagnosed with COVID-19 in accordance with the diagnostic criteria for the diagnosis and treatment of NCP (trial edition 4) issued by the National Health Commission of the People's Republic of China and the National Administration of Traditional Chinese Medicine. Male and non-pregnant/non-lactating female patients aged 18 to 85 years were eligible if they met the diagnostic criteria for COVID-19 in the diagnosis and treatment of novel coronavirus pneumonia (trial edition 4). Exclusion criteria included patients infected with critically severe COVID-19, patients who were over 15 days of symptom onset or who had severe primary respiratory system diseases, patients who have difficulty with oral and nasal feeding, patients with serous basic diseases, including malignant tumours, mental illness, and other malignant diseases, patients who had continuously used of immunosuppressants or received organ transplants within the past six months, and those who had an allergic constitution or were allergic to Huashi Baidu Formula. The withdrawal criteria included patients who underwent severe adverse events and could not continue to take medicines as primary groups during the observation period, those whose condition worsened or resulted in death, and those who were transferred to another hospital, left the present hospital, or received a confirmed misdiagnosis. Written informed consent was obtained from all participants.
Trial oversight
The trial was designed by the China Centre for Evidence-Based Traditional Chinese Medicine and implemented in partnership with Jinyintan Hospital in Wuhan.. All details regarding trial design, conduct, and analyses can be found in the protocol (No. ChiCTR2000029400/ChiMCTR2000002940).
Outcome measures
The primary outcomes were clinical remission time (interval from admission to the first time the patient tested negatively for novel coronavirus or an obvious improvement was observed from chest CT) and clinical remission rate (number of patients whose clinical time was within 16 days/total number of patients).
The secondary outcomes were the time of release from quarantine (interval from admission to discharge), the rate of release from quarantine (number of patients discharged within 16 days/ total number of patients), and clinical biochemical indicators. Other outcomes were safety indexes, such as liver function (ALT, AST), kidney function (creatinine), and myocardial damage.
Statistical analysis
Continuous variables were described using mean ± standard deviation or median ± interquartile range (IQR). In addition, the clinical remission time and time of release from quarantine were portrayed by Kaplan-Meier plots and compared with the log-rank test. The count data were expressed as the number of cases or percentages. Differences between groups were assessed using analysis of variance or Welch's variance analysis for continuous variables and the Chi-square test or Fisher's exact test for categorical variables. Comparisons were made between baselines and outcomes of groups using paired t-tests. SPSS Ver. 21.0 (IBM Corp, Armonk, NY, USA) was used for all statistical analyses. For all comparisons, differences were tested with two-tailed tests, and p < 0.05 was considered statistically significant.
Results
General characteristics of patients with COVID-19
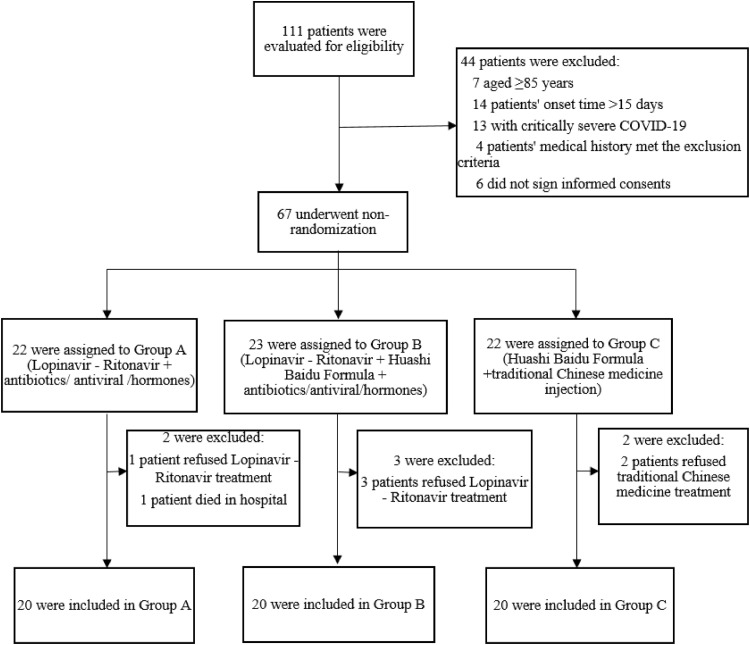
A total of 111 patients were evaluated for eligibility, and 44 patients were excluded according to the inclusion and exclusion criteria. The remaining 67 patients were assigned by non-randomized grouping, of which 22 patients were assigned to Group A, 23 patients to Group B, and 22 patients to Group C. In Group A, one patient refused Lopinavir-Ritonavir treatment, and one patient died in hospital, In Group B, three patients refused Lopinavir-Ritonavir treatment, and in Group C, two patients refused traditional Chinese medicine treatment (Fig. 1 ).
Fig. 1.
Flow program.
In the study, 66.7% (40/60) of patients were male and 33.3% (20/60) were female. The median age of the patients was 54.5 years (IQR, 45.0 to 64.8 years). The most common chronic medical illnesses were cardiovascular and cerebrovascular diseases, followed by endocrine system disease. Group B had a lower proportion of males than the other two groups. Except for lactate dehydrogenase and creatine kinase, no other major differences in age, chronic medical illness history, number of patients with a fever, and physical and chemical indexes of the three groups were observed at baseline (p > 0.05) (Table 1 , Table 2 ).
Table 1.
Demographics and baseline characteristics of 60 patients with COVID-2019 admitted to Jinyintan Hospital in Wuhan.
| Variables | Group A (n=20) | Group B (n=20) | Group C (n=20) | Total (n=60) | p-value |
|---|---|---|---|---|---|
| Age (n, %) | |||||
| Median (IQR) -year | 54.5 (42.8-67.0) | 52.0 (38.3-60.5) | 62.5 (45.8-67.5) | 54.5 (45.0-64.8) | |
| < 40 | 4 (20.0%) | 5 (25.0%) | 1 (5.0%) | 10 (16.7%) | 0.27* |
| 40-65 | 11 (55.0%) | 13 (65.0%) | 13 (65.0%) | 37 (61.7%) | |
| > 65 | 5 (25.0%) | 2 (10.0%) | 6 (30.0%) | 13 (21.7%) | |
| Sex (n, %) | |||||
| Male | 18 (90.0%) | 7 (35.0%) | 15 (75.0%) | 40 (66.7%) | 0.00069 |
| Female | 2 (10.0%) | 13 (65.0%) | 5 (25.0%) | 20 (33.3%) | |
| Chronic medical illness (n, %) | 11 (55.0%) | 9 (45.0%) | 15 (75.0%) | 35 (58.3%) | 0.15 |
| Cardiovascular and cerebrovascular diseases | 8 (40.0%) | 4 (20.0%) | 10 (50.0%) | 22 (36.7%) | 0.13 |
| Digestive system disease | 2 (10.0%) | 1 (5.0%) | 4 (20.0%) | 7 (11.7%) | 0.48* |
| Endocrine system disease | 1 (5.0%) | 3 (15.0%) | 5 (25.0%) | 9 (15.0%) | 0.27* |
| Malignant tumor | 1 (5.0%) | 3 (15.0%) | 1 (5.0%) | 5 (8.3%) | 0.60* |
| Nervous system disease | 0 (0.0%) | 0(0.0%) | 3 (15.0%) | 3 (5.0%) | 0.10* |
| Respiratory system disease | 0 (0.0%) | 1 (5.0%) | 4 (20.0%) | 5 (8.3%) | 0.12* |
| Other | 3 (15.0%) | 1 (5.0%) | 2 (10.0%) | 6 (10.0%) | 0.86* |
| Fever (n, %) | 8 (40.0%) | 7 (35.0%) | 3 (15.0%) | 18 (30.0%) | 0.19 |
COVID-2019 = Corona Virus Disease 2019; Group A = Lopinavir-Ritonavir; Group B = Huashi Baidu Formula + Lopinavir-Ritonavir; Group C = Huashi Baidu Formula; IQR ==interquartile range; * ==Fisher's exact test
Table 2.
Comparison of clinical characteristics of patients with COVID-2019 at admission and discharge.
| Variables | Group A (n=20) | Group B (n=20) | Group C (n=20) | Total (n=60) | p-value |
|---|---|---|---|---|---|
| Blood routine | |||||
| Leucocytes (× 10⁹ /l) -abnormal (n, %) | 3 (15.0%) | 6 (30.0%) | 4 (20.0%) | 13 (21.7%) | 0.63* |
| Recovery (n, %) | 3 (100.0%) | 3 (50.0%) | 3 (75.0%) | 9 (69.2%) | |
| Neutrophils (× 10⁹ /l) -abnormal (n, %) | 6 (30.0%) | 4 (20.0) | 3 (15.0%) | 13 (21.7%) | 0.63* |
| Recovery (n, %) | 3 (50.0%) | 2 (50.0%) | 2 (66.7%) | 7 (53.8%) | |
| Lymphocytes (× 10⁹ /l) -abnormal (n, %) | 13 (65.0%) | 12 (60.0%) | 6 (30.0%) | 31 (51.7%) | 0.057 |
| Recovery (n, %) | 7 (53.8%) | 5 (41.7%) | 5 (83.3%) | 17 (54.8%) | |
| Platelets (× 10⁹/ l) -abnormal (n, %) | 4 (20.0%) | 3 (15.0%) | 1 (5.0%) | 8 (13.3%) | 0.51* |
| Recovery (n, %) | 1 (25.0%) | 2 (66.7%) | 1 (100.0%) | 4 (50.0%) | |
| Haemoglobin (g/l) -abnormal (n, %) | 1 (5.0%) | 6 (30.0%) | 5 (25.0%) | 12 (20.0%) | 0.19* |
| Recovery (n, %) | 1 (100.0%) | 2 (33.3%%) | 2 (40.0%) | 5 (41.7%) | |
| Coagulation function | |||||
| Prothrombin time (s) -abnormal (n, %) | 4 (20.0%) | 3 (15.0%) | 4 (20.0%) | 11 (18.3%) | 1.0* |
| Recovery (n, %) | 1 (25.0%) | 0 (0.0%) | 1 (25.0%) | 2 (18.2%) | |
| Activated partial thromboplastin time (s) -abnormal (n, %) | 4 (20.0%) | 2 (10.0%) | 3 (15.0%) | 9 (15.0%) | 0.90* |
| Recovery (n, %) | 2 (50.0%) | 2 (100.0%) | 3 (100.0%) | 7 (77.8%) | |
| D-dimer (µg/l) -abnormal (n, %) | 8 (40.0%) | 5 (25.0%) | 8 (40.0%) | 21 (26.3%) | 0.52 |
| Recovery (n, %) | - | - | - | - | - |
| Blood biochemistry | |||||
| Albumin (g/l) -abnormal (n, %) | 19 (95.0%) | 17 (85.0%) | 17 (85.0%) | 53 (88.3%) | 0.68* |
| Recovery (n, %) | 3 (15.8%) | 3 (17.6%) | 3 (17.6%) | 9 (17.0%) | |
| Alanine aminotransferase (U/l) -abnormal (n, %) | 11 (55.0%) | 6 (30.0%) | 11 (55.0%) | 28 (46.7%) | 0.19 |
| Recovery (n, %) | 6 (54.5%) | 3 (50.0%) | 3 (27.3%) | 12 (42.9%) | |
| Glutamic-oxalacetic transaminase (U/l) -abnormal (n, %) | 9 (45.0%) | 4 (20.0%) | 10 (50.0%) | 23 (38.3%) | 0.11 |
| Recovery (n, %) | 5 (55.6%) | 2 (50.0%) | 2 (20.0%) | 9 (39.1%) | |
| Total bilirubin (μmol/l) -abnormal (n, %) | 3 (15.0%) | 1 (5.0%) | 2 (10.0%) | 6 (10.0%) | 0.86* |
| Recovery (n, %) | 2 (66.7%) | 0 (0.0%) | 0 (0.0%) | 2 (33.3%) | |
| Carbamide (mmol/l) -abnormal (n, %) | 2 (10.0%) | 2 (10.0%) | 1 (5.0%) | 5 (8.3%) | 1.0* |
| Recovery (n, %) | 2 (100.0%) | 1 (50.0%) | 1 (100.0%) | 4 (75.0%) | |
| Serum creatinin (μmol/l) -abnormal (n, %) | 7 (35.0%) | 5 (25.0%) | 8 (40.0%) | 20 (33.3%) | 0.59 |
| Recovery (n, %) | 0 (0.0%) | 1 (20.0%) | 2 (25.0%) | 3 (15.0%) | |
| Creatine kinase (U/l) -abnormal (n, %) | 9 (45.0%) | 7 (35.0%) | 6 (30.0%) | 22 (36.7%) | 0.61 |
| Recovery (n, %) | 7 (77.8%) | 4 (57.1%) | 3 (50.0%) | 14 (63.6%) | |
| Creatine phosphokinase isoenzyme (U/l) -abnormal (n, %) | 4 (20.0%) | 0 (0.0%) | 0 (0.0%) | 4 (6.7%) | 0.030* |
| Recovery (n, %) | - | - | - | - | - |
| Lactate dehydrogenase (U/l) -abnormal (n, %) | 15 (75.0%) | 7 (35.0%) | 7 (35.0%) | 29 (48.3%) | 0.014 |
| Recovery (n, %) | 9 (60.0%) | 5 (71.4%) | 5 (71.4%) | 19 (65.5%) | |
| Infection-related biomarkers | |||||
| Interleukin-6 (pg/ml) -abnormal (n, %) | 13 (65.0%) | 13 (65.0%) | 10 (50.0%) | 36 (60.0%) | 0.54 |
| Recovery (n, %) | 1 (7.7%) | 1 (7.7%) | 4 (40.0%) | 6 (16.7%) | |
| C-reactive protein (mg/l) -abnormal (n, %) | 16 (80.0%) | 15 (75.0%) | 13 (65.0%) | 44 (73.3%) | 0.55 |
| Recovery (n, %) | 6 (37.5%) | 5 (33.3%) | 6 (46.2%) | 17 (38.6%) |
Primary outcomes
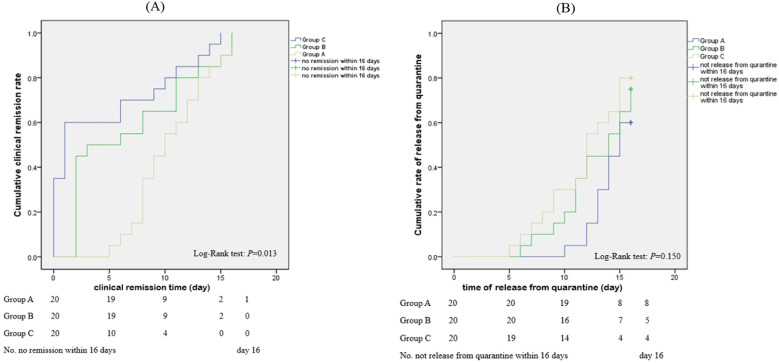
Table 3 and Fig. 2 (A) show that the clinical remission rates of Group A, B, and C were 95.0% (19/20), 100.0% (20/20), and 100.0% (20/20), respectively. The clinical remission time was 10.8 ± 4.0 days in Group A, 9.7 ± 3.7 days in Group B, and 5.9 ± 4.7 days in Group C (Table 3). According to the log-rank test, there was a significant difference among the three groups (p = 0.013). Pairwise comparisons were conducted using the LSD method, and statistical differences were identified between Group C and Group A as well as Group C and Group B (p < 0.05) (Table 3), suggesting that the curative effect of the Huashi Baidu Formula was better than that of the other two treatments.
Table 3.
Analysis of the clinical remission rate/time and rate/time of release from quarantine.
| Variables | Group A (n=20) | Group B (n=20) | Group C (n=20) | p-value |
|---|---|---|---|---|
| Clinical remission time [day, (Mean, SD)] | 10.8± 4.0a | 9.7± 3.7a | 5.9± 4.7 | 0.0010 |
| Clinical rate (n/N, %) | 19/20 (95.0%) | 20/20 (100.0%) | 20/20 (100.0%) | - |
| Time of release from quarantine [day, (Mean, SD)] | 16.7 ± 6.6 | 15.0 ± 6.6 | 14.5 ± 7.9 | 0.55 |
| Rate of being released from quarantine (n/N, %) | 12/20 (60.0%) | 15/20 (75.0%) | 16/20 (80.0%) | - |
a: there was difference between the group and Group C (p < 0.05)
Fig. 2.
(A) Cumulative clinical remission rate of the three groups–Kaplan-Meier curve; (B) Cumulative rate of release from quarantine of the three groups–Kaplan-Meier curve.
Secondary outcomes
The time and rate of release from quarantine
The time taken before release from quarantine was 16.7 ± 6.6 days in Group A, 15.0 ± 6.6 days in Group B, and 14.5 ± 7.9 days in Group C. There were no statistical differences (p > 0.05) among the three groups (Table 3). The rates of release from quarantine in Groups A, B, and C were 60.0% (12/20), 75.0% (15/20), and 80.0% (16/20), respectively (Fig. 2 (B)).
Comparative analysis of clinical characteristics of patients with COVID-19
The number and proportion of patients with improvements in biochemical indicators are described in Table 2. The recovery rate of lactate dehydrogenase in Group B and C was 71.4% (5/7), while in Group A was 60.0% (9/15).The recovery rate of leukocytes in Group A was 100.0% (3/3), while in Group B and C was 50.0% (3/6) and 75.0% (3/4) respectively. In terms of creatine kinase, the recovery rate was 77.8% (7/9) in Group A, 57.1% (4/7) in Group B and 50.0% (3/6) in Group C. In terms of lymphocytes and interleukin 6, the recovery rate in Group C (83.3%%(5/6) and 40.0% (4/10)) was significantly higher than in Group A and B.
A paired t-test was used for comparisons before and after treatments, and the results (Table 4 ) showed that there were significant differences in neutrophil, lymphocyte, albumin, glutamic-oxalacetic transaminase, serum creatinine, creatine kinase, lactate dehydrogenase, and C-reactive protein levels as well as in prothrombin, and activated partial thrombin activity times both before and after treatment in Group A (p < 0.05). Significant differences were observed in haemoglobin, albumin, total bilirubin, creatine kinase, lactate dehydrogenase, interleukin-6, and C-reactive protein level as well as prothrombin and activated partial thrombin activity times both before and after treatment in Group B (p < 0.05). In Group C, significant differences were observed in neutrophil, haemoglobin, albumin, glutamic-oxalacetic transaminase, creatine kinase, lactate dehydrogenase, interleukin-6, and C-reactive protein levels both before and after treatment (p < 0.05).
Table 4.
Comparative analysis of clinical characteristics of patients with COVID-2019.
| Variables | Normal range | Group A (n=20) | Group B (n=20) | Group C (n=20) | Total (n=60) |
|---|---|---|---|---|---|
| Blood routine | |||||
| Leucocytes (× 10⁹ /l) -baseline (n, %) | 3.5-9.5 | 5.4 (4.4-7.2) | 4.3 (3.5-5.4) | 6.1 (5.3-8.1) | 5.3 (4.1-7.0) |
| outcome (n, %) | 5.5 (4.3-6.2) | 4.6 (3.9-6.0) | 6.1 (4.9-7.1) | 5.6 (4.2-6.4) | |
| p-value | 0.20b | 0.73b | 0.22b | ||
| Neutrophils (× 10⁹ /l) -abnormal(n,%) | 1.8-6.3 | 4.2 (3.3-7.3) | 2.7 (2.1-3.4) | 4.0 (3.6-5.5) | 3.7 (2.7-5.4) |
| outcome(n,%) | 3.7 (3.1-4.9) | 2.6 (2.1-3.9) | 3.7 (2.5-4.5) | 3.5 (2.5-4.4) | |
| p-value | 0.048b | 0.47b | 0.044b | ||
| Lymphocytes (× 10⁹ /l) -abnormal (n, %) | 11.-3.2 | 0.9 (0.7-1.5) | 1.0 (0.9-1.6) | 1.4 (1.1-1.9) | 1.1 (0.9-1.7) |
| outcome (n, %) | 1.4 (0.8-1.9) | 1.4 (1.0-1.7) | 1.5 (1.4-2.2) | 1.5 (1.1-1.8) | |
| p-value | 0.00069 | 0.19 | 0.49 | ||
| Platelets (× 10⁹/ l) -abnormal (n, %) | 125-350 | 201.5 (164.5-303.5) | 173.5 (142.8-255.3) | 217.0(187.3-277.0) | 205.0 (161.0-277.0) |
| outcome (n, %) | 204.0 (143.3-285.0) | 218.0 (155.8-283.8) | 227.0 (180.3-261.5) | 217 (162.3-267.0) | |
| p-value | 0.64b | 0.23b | 0.76 | ||
| Haemoglobin (g/l) -abnormal (n, %) | 115-150 | 129.5 (119.8-143.0) | 127.0 (114.8-133.3) | 130.5(122.0-135.5) | 127.5 (119.0-136.0) |
| outcome (n, %) | 131.5 (118.0-139.0) | 125.5 (107.0-129.0) | 125.0 (115.8-131.0) | 127.0 (115.0-132.0) | |
| p-value | 0.15 | 0.040 | 0.046 | ||
| Coagulation function | |||||
| Prothrombin time (s) -abnormal (n, %) | 10.5-13.5 | 11.5 (10.8-11.7) | 11.4 (11.2-12.3) | 11.0 (10.6-11.5) | 11.3 (10.8-11.8) |
| outcome (n, %) | 10.6 (10.1-11.4) | 11.2 (10.3-11.4) | 11.0 (10.6-11.5) | 10.8 (10.3-11.4) | |
| p-value | 0.0070b | 0.0079 | 0.23 | ||
| Activated partial thromboplastin time (s) -abnormal (n, %) | 21-37 | 24.0 (22.8-27.0) | 29.5 (25.2-33.0) | 30.5 (28.0-33.1) | 28.1 (23.6-32.3) |
| outcome (n, %) | 24.9 (22.8-30.0) | 26.1 (23.8-30.4) | 30.4 (26.6-33.2) | 26.5 (24.0-31.0) | |
| p-value | 0.35 | 0.033 | 0.63b | ||
| D-dimer (µg/l) -abnormal (n, %) | 0.0-1.5 | 1.1 (0.6-2.8) | 0.4 (0.2-1.3) | 1.0 (0.4-15.0) | 0.6 (0.3-3.3) |
| outcome (n, %) | - | - | - | - | |
| Blood biochemistry | |||||
| Albumin (g/L) -abnormal (n, %) | 40-55 | 31.3 (27.1-34.2) | 36.1 (30.1-39.3) | 37.7 (32.0-56.6) | 34.1 (29.3-39.3) |
| outcome (n, %) | 37.1 (32.9-38.8) | 39.3 (35.2-40.3) | 40.7 (37.9-62.8) | 39.0 (35.3-41.8) | |
| p-value | 0.00049 | 0.040 | 0.0017 | ||
| Alanine aminotransferase (U/l) -abnormal (n, %) | 7-40 | 41.5 (26.5-52.5) | 22.0 (16.5-39.0) | 42.5 (18.3-51.5) | 33.5 (19.3-48.0) |
| outcome (n, %) | 36.0 (25.0-52.5) | 20.0 (17.3-45.5) | 38.0 (16.3-50.8) | 27.5 (18.3-49.5) | |
| p-value | 0.80b | 0.80b | 0.49b | ||
| Glutamic-oxalacetic transaminase (U/l) -abnormal (n, %) | 13-35 | 34.0 (26.3-55.5) | 26.0 (22.0-31.8) | 35.0 (23.5-42.5) | 31.5 (25.0-41.0) |
| outcome(n,%) | 20.5 (14.8-28.0) | 24.0 (17.3-32.8) | 27.0 (19.3-40.3) | 23.0 (17.3-34.5) | |
| p-value | 0.025b | 0.21b | 0.032b | ||
| Total bilirubin (μmol/l) -abnormal (n, %) | 0-21 | 12.2 (9.8-13.6) | 14.2 (10.5-16.7) | 13.1 (9.4-18.5) | 13.0 (9.8-16.4) |
| outcome (n, %) | 10.6 (7.9-14.3) | 9.2 (6.7-13.6) | 9.1 (6.4-16.8) | 9.4 (6.6-14.0) | |
| p-value | 0.079b | 0.018 | 0.068b | ||
| Carbamide (mmol/l) -abnormal (n, %) | 2.6-7.5 | 4.7 (3.6-5.5) | 3.6 (3.1-4.7) | 4.7 (4.2-6.3) | 4.5 (3.5-5.1) |
| outcome (n, %) | 5.7 (4.7-6.2) | 4.3 (3.4-5.4) | 4.8 (3.8-5.0) | 4.9 (3.8-5.7) | |
| p-value | 0.21 | 0.13 | 0.098 | ||
| Serum creatinin (μmol/l) -abnormal (n, %) | 41-73 | 70.0 (65.5-18.8) | 65.2 (54.9-75.9) | 65.4 (57.1-86.5) | 67.4 (58.7-78.4) |
| outcome (n, %) | 75.5 (72.0-82.8) | 59.5 (48.3-73.9) | 66.0 (58.0-77.2) | 70.9 (58.5-78.0) | |
| p-value | 0.018 | 0.098 | 0.19b | ||
| Creatine kinase (U/l) -abnormal (n, %) | 40-200 | 93.5 (47.0-293.8) | 55 (45.2-139.5) | 81.5 (57.3-120.5) | 68.0 (48.3-162.8) |
| outcome(n, %) | 63.0 (34.5-75.8) | 46.5 (34.5-58.3) | 58.5 (43.5-75.8) | 55.5 (39.3-72.8) | |
| p-value | 0.0020b | 0.018 | 0.022b | ||
| Creatine phosphokinase isoenzyme (U/l) -abnormal (n, %) | 0-24 | 16.5 (12.0-21.0) | 11.0 (9.0-14.0) | 11.5 (10.0-14.0) | 12.0 (10.0-17.0) |
| outcome (n, %) | - | - | - | - | |
| Lactate dehydrogenase (U/l) -abnormal (n, %) | 120-250 | 331.0 (241.5-431.5) | 225.5 (186.5-305.5) | 206.0 (181.3-270.8) | 250.0 (190.8-332.0) |
| outcome (n, %) | 222.5 (195.5-287.5) | 209.5 (183.8-220.8) | 198.5 (163.8-218.5) | 211 (183.8-234.5) | |
| p-value | 0.0017 | 0.37 | 0.034 | ||
| Infection-related biomarkers | |||||
| Interleukin-6 (pg/ml) -abnormal (n, %) | 0.0-7.0 | 8.1 (6.1-11.2) | 7.4 (6.2-8.9) | 6.8 (5.2-11.3) | 7.3 (5.9-10.4) |
| outcome (n, %) | 7.7 (5.7-10.3) | 8.4 (6.3-10.5) | 6.1 (5.0-8.9) | 7.4 (5.6-9.9) | |
| p-value | 0.75b | 0.35b | 0.013b | ||
| C-reactive protein (mg/l) -abnormal (n, %) | 0-5 | 27.5 (16.9-60.0) | 21.3 (5.1-51.8) | 7.9 (0.9-22.7) | 17.8 (4.1-46.8) |
| outcome (n, %) | 4.8 (2.3-16.1) | 5.3 (0.6-5.3) | 2.8 (1.0-7.7) | 4.3 (1.1-11.5) | |
| p-value | 0.0010b | 0.11 | 0.030b |
b = Wilcoxon Signed Rank Test
Safety indexes
In this study, the safety indexes were biochemical indicators (normal / abnormal). There was no significant difference among the three groups or within the groups in terms of the safety indexes at baseline (p > 0.05). After different treatments, the proportion of patients with abnormal creatinine in Group A increased significantly, while that in Group C increased slightly and in Group B did not change, indicating that Group B treatment was marginally safer in terms of renal function than those of the other two groups. Besides, Glutamic-pyruvic and oxalacetic transaminases could reflect liver function, and there was no statistical difference among the three groups (p > 0.05). The proportion of patients with these two abnormal indicators in Group A and C decreased, while in Group B increased. In addition, the proportion of patients with abnormal creatine kinase in the three groups decreased, which suggested that the three treatments exhibited no significant harm on cardiac function. In total, the patients in the three groups suffered no obvious adverse events (Table 5 ).
Table 5.
Clinical safety indexes of patients with COVID-19.
| Variables | Group A (n=20) | Group B (n=20) | Group C (n=20) | Total (n=60) | p-value |
|---|---|---|---|---|---|
| Alanine aminotransferase-abnormal (n/N, %) | 11/20 (55.0%) | 6/20 (30.0%) | 11/20 (55.0%) | 28/60 (46.7%) | 0.19 |
| Adverse event-(n/N, %) | 8/20 (40.0%) | 7/20 (35.0%) | 9/20 (45.0%) | 24/60 (40.0%) | 0.81 |
| p-value | 0.51 | - | 0.63 | ||
| Glutamic-oxalacetic transaminase-abnormal (n/N, %) | 9/20 (45.0%) | 4/20 (20.0%) | 10/20 (50.0%) | 23/60 (38.3%) | 0.11 |
| Adverse event-(n/N, %) | 4/20 (20.0%) | 5/20 (25.0%) | 9/20 (45.0%) | 18/60 (30.0%) | 0.19 |
| p-value | 0.063 | 1.0 | 1.0 | ||
| Serum creatinin-abnormal (n/N, %) | 7/20 (35.0%) | 5/20 (25.0%) | 8/20 (40.0%) | 20/60 (33.3%) | 0.59 |
| Adverse event-(n/N, %) | 11/20 (55.0%) | 5/20 (25.0%) | 9/20 (45.0%) | 25/60 (41.7%) | 0.15 |
| p-value | 0.13 | 1.0 | 1.0 | ||
| Creatine kinase -abnormal (n/N,%) | 9/20 (45.0%) | 7/20 (35.0%) | 6/20 (30.0%) | 22/60 (36.7%) | 0.61 |
| Adverse event-(n/N, %) | 8/20 (40.0%) | 6/20 (30.0%) | 4/20 (20.0%) | 18/60 (30.0%) | 0.39 |
| p-value | 1.0 | 1.0 | 0.63 |
Discussion
Current treatments and the potential value of TCM treatment for COVID-19
To date, no targeted vaccine or specific drugs have been developed for the prevention or treatment of NCP. Based on previous experiences relating to the treatment of SARS, MERS, or other novel influenza viruses, Lopinavir/Ritonavir, Redesivir, nucleoside analogues, neuraminidase inhibitors, and Abidor have been used in the clinical treatment of patients with COVID-19, but their clinical efficacy and safety need to be confirmed in further clinical trials (Lu, 2020). Recent studies (Wang et al., 2020) have identified Redesivir as a potential treatment for NCP. Currently, Phase 3 clinical drug trials have been launched in China, but the above studies are in the exploratory phase. For the treatment of inflammation, corticosteroids have been used frequently for severe patients with severe cases of SARS-CoV (Wong et al., 2004, He et al., 2006), MERS-CoV (Faure et al., 2014, Falzarano et al., 2013) and COVID-19. However, current evidence for SARS and MERS suggests that corticosteroids have no effect on mortality, but rather delay viral clearance (Stockman et al., 2006, Lansbury et al., 2019, Arabi et al., 2018). There is limited evidence supporting the treatment of COVID-19 with glucocorticoids (Wang et al., 2020, Russell et al., 2020). The latest Chinese guidelines state that bed rest, symptomatic support treatment, and antivirus glucocorticoid treatment are primary treatment measures for COVID-19, among which antivirus drugs mainly include alpha interferon, Lopinavir/Ritonavir, and Ribavirin. However, the effect of these treatments are limited. Both the novel coronavirus and antivirus drugs have hepatotoxicity that can cause liver injury and affect the prognosis of patients.
As traditional Chinese medicine has been used for thousands of years, it has played an important role in the prevention and treatment of infectious diseases in both ancient and modern China. Since there is no specific drug for the treatment of COVID-19 at present, the use of some proprietary Chinese medicines combined with antivirus and/or anti-inflammatory drugs have been used in the treatment of COVID-19, with some positive effects (Wang et al., 2020). Therefore, the undertaking of clinical research regarding the treatment of COVID-19 with traditional Chinese medicine is an important methodof accelerating the search for treatments to control the epidemic (Kupferschmidt and Cohen, 2020). Among CHMs, Radix Scutellariae, Forsythia, DanPi, Salvia miltiorrhiza, Ginseng, Astragalus, Radix Codonopsis, Atractylodes, and Chinese yam have all been included in the treatment plan of COVID-19. The application of these traditional Chinese medicines in the clinical treatment of COVID-19 may help to reduce the excessive inflammatory response and oxidative stress, thus curing the clinical symptoms of patients.
Evaluation of the clinical efficacy of three treatments on COVID-19
Evaluation of the clinical efficacy of Lopinavir-Ritonavir treatment on COVID-19
In the present study, the median time of release from quarantine after Lopinavir-Ritonavir treatment was 15.0 (13.0-19.0) days, which is a similar result to that in a previous study (14.0 (12.0-17.0) days) (Cao et al., 2020), indicating that the therapeutic effect of Lopinavir-Ritonavir treatment is relatively consistent. In Group A, the recovery time of leukocyte, creatine kinase, and glutamic-pyruvic transaminase levels was shorter than in the other two groups. In addition, the Lopinavir-Ritonavir treatment had some effect on the recovery rate of biochemical indicators, and the improvement in creatine kinase levels could be related to the association between the elimination of viral mRNA and Lopinavir–Ritonavir treatment (Yuan et al., 2020). A retrospective study showed Lopinavir–Ritonavir and interferon could shorten the duration of viral shedding in patients with COVID-19 (Zuo et al., 2020), thus, might have a favourable response to the course of COVID-19 infection. Results of the comparison within groups showed that Lopinavir - Ritonavir had better effects in improving lymphocyte, haemoglobin, interleukin-6, albumin, creatine kinase, and platelet levels as well as plasma prothrombin and activated partial thrombin activity time. This might be because some important proteins are encoded by novel coronaviruses, and Lopinavir can inhibit the protease activity of coronavirus. Moreover, this was an effective treatment based on the experience accumulated from the SARS and MERS outbreaks (Yao et al., 2020). Some proteins,(such as 3C-like protease, play an important role in the life cycle of these viruses, and the key drug-binding pockets are conserved among SARS-CoV, MERS-CoV, and novel coronavirus (Li and De Clercq, 2020, Zheng and Yong, 2020). The combination of Lopinavir-Ritonavir and these proteins could have inhibitory effects on novel coronaviruses and reduce the damage to human health.
Evaluation of the clinical efficacy of the combination of Lopinavir–Ritonavir and Huashi Baidu Formula treatment on COVID-19
Compared to Lopinavir–Ritonavir treatment alone, the combination of Lopinavir–Ritonavir and Huashi Baidu Formula treatment displayed advantages in terms of the clinical remission time, rate of release from quarantine, clinical remission rate, and rate of release from quarantine (9.7 days vs. 10.8 days; 15.0 days vs. 16.7 days; 100% (20/20) vs. 95% (19/20); 75.0% (15/20) vs. 60.0% (12/20)). In addition, the combination of Lopinavir–Ritonavir and Huashi Baidu Formula improved rates to a greater degree than Huashi Baidu Formula with respect to lactate dehydrogenase level (71.4% (5/7) vs. 60.0% (9/15)). Moreover, this combination also improved the treatment of abnormal lymphocytes, haemoglobin, interleukin-6, leucocyte, neutrophils, and total bilirubin. In an open-label randomised trial, using Lopinavir-Ritonavir alone showed some improvement in symptoms, and Lopinavir-Ritonavir as a component of a triple combination (interferon beta-1b, Lopinavir–Ritonavir and Ribavirin) showed some in vitro activity against coronaviruses (Hung et al., 2020). A retrospective analysis showed that the viral load was negative in 75% of patients with COVID-19 treated with Arbidol and Lopinavir–Ritonavir versus 35% of patients treated with Lopinavir-Ritonavir alone (Deng et al., 2020). In the present study, the combination of Lopinavir-Ritonavir and Huashi Baidu Formula showed better clinical efficacy than Lopinavir-Ritonavir alone. The Huashi Baidu Formula is composed of 14 CHMs, including Semen Armeniacae Amarum, Gypsum fibrosum, Glycyrrhizae Radix et Rhizoma, Poria, Pogostemonis Herba and Astragali radix, which might play a role in improving and regulating the immune system (Lu et al., 2016, Shu, 2009, Hui et al., 2006, Yuan, 2015, Wen et al., 2017, Ying, 2013) and promote the production of resistance to the novel coronavirus. The application of these Chinese medicines in the clinical treatment of COVID-19 may help to reduce the excessive inflammatory response and oxidative stress, thus reduce the clinical symptoms (such as fever, cough, and shortness of breath) (Lu et al., 2016, Shu, 2009, Hui et al., 2006, Yuan, 2015, Wen et al., 2017, Ying, 2013, Feng, 2013, Li, 2006, Min and Cheng, 2011, Jian et al., 2011, Dong, 2011, Jiao et al., 2018, Sheng et al., 2011, Hua et al., 2015) of patients.
Evaluation of the clinical efficacy of Huashi Baidu Formula treatment on COVID-19
In Group C, we adopted the method of CHM syndrome differentiation, and administered individual treatment plans according to each patient's condition, including oral Chinese medicine decoction and Chinese patent medicine, and some patients were administered traditional Chinese medicine injections. In addition, some patients used a combination of CHM decoction and injection at the early stage of admission, which was inconsistent with the use of CHM injection recommended only for severe and critically ill patients in the national program. However, this study found that the combination of the Huashi Baidu Formula and traditional Chinese medicine injection resulted in improved clinical remission times (5.9 ± 4.7 days), and the rate of release from quarantine (80.0%%(16/20)) was higher in the other two groups, with a clinical remission rate of 100.0% (20/20). On the one hand, the improvement rate of lymphocytes and interleukin-6 in Group C was higher than in the other groups, indicating the effectiveness of the Huashi Baidu Formula. On the other hand, Huashi Baidu Formula treatment also functioned well for some abnormal clinical indicators, such as haemoglobin, albumin, creatine kinase, glutamic-oxalacetic transaminase, carbamide, and lactate dehydrogenase levels as well as prothrombin and activated partial thromboplastin times. Additionally, there might be a connection between efficacy and traditional Chinese medicine injection (e.g. XueBiJing, XiYanPing, and ShenMai injections). This would be consistent with previous studies that have demonstrated that the combination of XueBiJing injection and basic antivirus treatment could improve the effectiveness of the rate of absorption of chest CT lesions in patients with COVID-19 without severe adverse events, and the XueBiJing injection could effectively improve the inflammatory markers and prognosis of patients with severe COVID-19 (Yu et al., 2020, Wen et al., 2020). The combination of XiYanPing injection and azithromycin in the treatment of mycoplasma pneumonia in children could effectively shorten the time of hospitalisation and recovery time of fevers, coughs and crackles with extremely low levels of adverse events (Qiao et al., 2019). Moreover, with the appendant of Danshen injection in the treatment of patients with severe pneumonia, the patient's clinical symptoms (fever, cough, shortness of breath, abnormal heart rate and lung rales) and recovery time could be significantly decreased, and the abnormal C-reactive protein and leukocyte levels could be reduced (Nan, 2018, Jun, 2018). Overall, these studies might explain why the combination of the Huashi Baidu Formula and traditional Chinese medicine injection be effective in the treatment of COVID-19.
Safety indexes of the three groups in the treatment of COVID-19
Several patients in this study had abnormal creatinine levels after Lopinavir-Ritonavir treatment. The number of patients with abnormal glutamic-pyruvic and oxalacetic transaminase levels after the combination of Lopinavir–Ritonavir and Huashi Baidu Formula treatment also slightly increased. However, there was no significant difference in safety indexes between and within the groups (P > 0.05). Although one patient died during hospitalization in Group A, researchers determined that it was not related to the intervention. In total, none of the patient in the study experienced severe adverse events.
Limitations and strengths
There were several limitations to this study. First, the trial was non-randomized and unblinded, thus, findings of statistical tests and P-values should be interpreted with caution, and non-significant P-values did not necessarily rule out differences between the three groups. Second, due to the small sample size and single centre site, it was difficult to assess host risk factors for disease severity and mortality using multivariable adjusted methods. A larger randomised control trial would assist in defining the clinical epidemiological characteristics and risk factors. Third, the interventions in this study were not completely in accordance with the three groups. Further studies in outpatient, primary care, or community settings are needed to follow-up the prognosis and recovery of patients.
The present study also has some strengths. First, it is one of few clinical control trials to explore the efficacy and safety of CHMs and western medicines in COVID-19 treatment. Second, we used many indicators to describe the efficacy and safety of the medicines, such as clinical remission rate, clinical remission time, and rate of release from quarantine, making the outcomes more diversified.
Conclusions
Lopinavir-Ritonavir had an effect as a treatment for COVID-19, and this effect was enhanced when combined with Huashi Baidu Formula administration. At the same time, the combined use of the Huashi Baidu Formula and traditional Chinese medicine injection in the treatment of COVID-19 produced a better effect than anticipated, and none of the three treatments had any severe adverse effects on the patients. We are cautiously optimistic about the results. Due to the limitations of this study, multi-centre, large-sample size, rigorous clinical trials are needed for further verification
Ethical approval
The trial was approved by the ethical committee of Institute of the Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences (Beijing, China) and Jinyintan hospital (Wuhan, China).
CRediT authorship contribution statement
Nannan Shi: Writing - original draft. Lanping Guo: Writing - original draft. Bin Liu: Formal analysis. Yongjun Bian: Data curation. Renbo Chen: Data curation. Suping Chen: Formal analysis. Yang Chen: Visualization. Yingying Chen: Formal analysis. Xiaodong Cong: Formal analysis. Guoju Dong: Visualization. Jing Guo: Visualization. Lijie Hu: Investigation. Jianxin Jiang: Supervision. Luxing Leng: Investigation. Bin Li: Supervision. Dongxu Li: Supervision. Hao Li: Project administration. Jing Li: Project administration. Li Li: Project administration. Jia Liu: Funding acquisition. Cheng Lu: Investigation. Wenliang Lv: Investigation. Qing Miao: Funding acquisition. Wensheng Qi: Conceptualization. Zhan Shi: Funding acquisition. Jiaheng Shi: Writing - review & editing. Huaxin Shi: Resources. Yaxin Tian: Conceptualization. Bing Wang: Writing - review & editing. Gang Wang: Resources. Jian Wang: Writing - review & editing. Wei Wang: Conceptualization. Yongyue Xian: Resources. Xiaolei Xie: Resources. Yibai Xiong: Resources. Chunyan Xu: Methodology. Ming Xu: Methodology. Bei Yan: Methodology. Jinliang Yang: Software. Li Zhang: Software. Zhenqi Zhou: Validation. Haoning Zhu: Validation. Luqi Huang: Writing - review & editing.
Declaration of Competing Interest
All authors declare that no support from any organization for the submitted work; no financial relationships with any organization that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work. The authors declare no competing interests.
Acknowledgments
The authors thank the participants and staff of the China Academy of Chinese Medical Sciences and its affiliated department for their valuable contributions. We also thank the supports from the Ministry of Science and Technology of the People's Republic of China (No. 2019YFC1712000; 2018ZX10101001-005-003; 2018ZX10101001-005-004; 2020YFC0841500). In addition, we would like to thank the Editage (www.editage.cn) for English language editing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.phymed.2020.153367.
Appendix. Supplementary materials
References
- Arabi Y.M. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- Arabi Y.M. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Nakamura T. Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phytother. Res. 2004;18(7):592–594. doi: 10.1002/ptr.1485. [DOI] [PubMed] [Google Scholar]
- Chu C.M. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J. Infect. 2020;81(1):e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.X. Pharmacological action and clinical application of magnoliae officinalis cortex. Chin. Community Doctors. 2011;13(26):151. [Google Scholar]
- Falzarano D. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat. Med. 2013;19(10):1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure E. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One. 2014;9(2):e88716. doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M.X. Study progress on chemical constituents and pharmacological effects of Descurainiae Semen Lepidii Semen. Sci. Technol. Innov. 2013;(34):71. [Google Scholar]
- He L. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006;210(3):288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongzhou L., W S.C., Yi-Wei T. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020;92(4) doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua L.X. Research progress on chemical constituents and pharmacological effects of Paeoniae Radix Rubra. Chin. Trad. Herbal Drugs. 2015;(4):595–602. [Google Scholar]
- Huang, C., et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England), 2020.395(10223): p. 497–506. [DOI] [PMC free article] [PubMed]
- Hui D.S. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis.: IJID: Off. Publ. Int. Soc. Infectious Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui Y. Overview of the pharmacology of Glycyrrhizae Radix et Rhizoma. Prog. Modern Biomed. 2006;(4):83–85. [Google Scholar]
- Hung I.F.-N. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet (London, England. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Gao W. Is traditional Chinese medicine useful in the treatment of SARS? Phytother. Res. 2003;17(7):840–841. doi: 10.1002/ptr.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian Z.Z. Reevaluation of the pharmacological effects of Atractylodis Rhizoma. Chin. J. Hospital Pharmacy. 2011;031(007):607–609. [Google Scholar]
- Jiao S.X. Research progress on chemical constituents and clinical application of ephedra plants. Chin. Pharmaceutical Affairs. 2018;32(2):201–209. [Google Scholar]
- Jun C. Clinical study of shenmai injection combined with piperacillin sodium and sulbactam sodium in the treatment of senile severe pneumonia. Shanxi Med. J. 2018;47(13):1554–1556. [Google Scholar]
- Kupferschmidt K., Cohen J. Race to find COVID-19 treatments accelerates. Science (New York, N.Y.) 2020;367(6485):1412–1413. doi: 10.1126/science.367.6485.1412. [DOI] [PubMed] [Google Scholar]
- Lansbury L. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane. Database. Syst. Rev. 2019;2 doi: 10.1002/14651858.CD010406.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nature Rev. Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Li T. Research progress on pharmacologic experiments of pinelliae rhizoma praeparatum. J. Clin. Exp. Med. 2006;005(11):1846. [Google Scholar]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- Lu L. Research progress on the pharmacological action of Armeniacae Semen Amarum. J. Jilin Med. College. 2016;037(001):63–66. [Google Scholar]
- Min D., Cheng P. Research progress on the chemical constituents and pharmacological effects of tsaoko fructus. Pharmacy Clinics Chin. Materia Med. 2011;02(4):55–59. [Google Scholar]
- Munster V.J. A Novel Coronavirus Emerging in China - Key Questions for Impact Assessment. N. Engl. J. Med. 2020;382(8):692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- Nan F.K. Clinical efficacy of shenmai injection combined with linezolid in the treatment of severe pneumonia in children and its effect on inflammatory factors. Shanxi J. Trad. Chin. Med. 2018;(9):1187–1190. [Google Scholar]
- Qiao L. Xiyanping plus azithromycin chemotherapy in pediatric patients with mycoplasma pneumoniae pneumonia: a systematic review and meta-analysis of efficacy and safety. Evidence-based Compl. Alternative Med.: eCAM. 2019;2019 doi: 10.1155/2019/2346583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing M. Understanding and thinking of novel coronavirus pneumonia in traditional Chinese medicine. J. Tradit. Chin. Med. 2020;61(04):286–288. [Google Scholar]
- Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet (London, England) 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng F.X. New research progress on the chemical constituents and pharmacological effects of Rhei Radix et Rhizoma. Chin. J. New Drugs. 2011;(16):1534–1538. [Google Scholar]
- Shu S. Study on the pharmacological action and trace elements of Gypsum Fibrosum. Chin. Med. Modern Distance Educ. China. 2009;007(5):170. [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. Clinical characteristics of 138 hospitalized patients With 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci. Trends. 2020;14(1):64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- Wen L. [Effect of Xuebijing injection on inflammatory markers and disease outcome of coronavirus disease 2019] Zhonghua wei zhong bing ji jiu yi xue. 2020;32(4):426–429. doi: 10.3760/cma.j.cn121430-20200406-00386. [DOI] [PubMed] [Google Scholar]
- Wen X. Research progress on the pharmacological action and mechanism of Pogostemonis Herba. Shanghai J. Trad. Chin. Med. 2017;(10):103–106. [Google Scholar]
- Wong C.K. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136(1) doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T.-T. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-a possible reference for coronavirus disease-19 treatment option. J. Med. Virol. 2020 doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying C.G. Research progress on pharmacological action of Astragali radix. J. North Pharmacy. 2013;000(10):53. [Google Scholar]
- Yu Z.C. Observation of the clinical efficacy of xuebijing in the treatment of COVID-19. Chin. J. Hospital Pharmacy. 2020:1–5. [Google Scholar]
- Yuan J. The correlation between viral clearance and biochemical outcomes of 94 covid-19 infected discharged patients. Inflammation Res.: Off. J. Eur. Histamine Res. Soc. 2020 doi: 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan z.Z. Research progress on pharmacology of Poria. Continuing Med. Educ. 2015;000(005):108–109. [Google Scholar]
- Zheng S., Yong F.S. A potential therapeutic agent for COVID 19 - lopinavir/ritonavir. Chin. J. Clin. Rational Drug Use. 2020;18(02):56–61. [Google Scholar]
- Zuo Y. Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID-19: A retrospective study in two designated hospitals in Anhui, China. J. Med. Virol. 2020 doi: 10.1002/jmv.26127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.