Abstract
Recreational substance use (SU) can emerge or worsen in the aftermath of psychological trauma. Anhedonia is one reason for this problematic SU. Symptoms of posttraumatic stress disorder (PTSD) that represent anhedonia (post-trauma anhedonia; PTA) have been consistently linked to SU disorders. However, no prospective studies have examined whether changes in PTA over time are associated with problematic SU in recently-traumatized people, which was the goal of this study. 165 men and women were recruited as part of a prospective PTSD study in the emergency department of a Level 1 trauma center. Clinical assessments of PTSD and SU were administered at three and six months post-trauma. Compared to participants with minimal SU at six months post-trauma, high substance users at six months post-trauma showed significant increases in PTA during the three to six month time period. This relationship was significant even after accounting for variance associated with other factors, including PTSD symptoms such as re-experiencing and hyperarousal. Participants who demonstrated increases in SU during this time also showed significant increases in PTA, unlike those who demonstrated consistently minimal/no SU during this time. These findings indicate that PTA may be a mechanism through which SU problems emerge in recently-traumatized individuals.
Keywords: Anhedonia, Substance use, Trauma, PTSD, emergency department
1. Introduction
Substance use is one way through which traumatized people cope with their symptoms. Prevalence rates of Substance Use Disorders (SUD) in posttraumatic stress disorder (PTSD) are high. Some national estimates indicate ~46% of people with PTSD are addicted to substances(Pietrzak et al., 2011). Similarly, among people with SUD seeking treatment, rates of lifetime PTSD are remarkably high (~40%) (McCauley et al., 2012). Self-medication hypotheses(Khantzian, 1997) suggest that PTSD symptoms may be a causal mechanism through which SUDs develop(Chilcoat and Breslau, 1998). Substances may be used to ameliorate the presence and severity of symptoms—to avoid painful memories, to be able to fall asleep, to reduce anxiety, or to elevate mood and enhance pleasure in activities. A number of substances are abused in traumatized people(Cougle et al., 2011; Kevorkian et al., 2015), with alcohol, nicotine, and cannabis use disorders among the highest in terms of PTSD/SUD co-morbidity(Breslau et al., 2003; Pietrzak et al., 2011). Evidence indicates that PTSD, and not simply trauma exposure, is a risk factor for SUD (Kevorkian et al., 2015), given that PTSD symptoms sometimes precede the onset of SUDs(Bremner et al., 1996).
A subset of PTSD symptoms that reflect anhedonia, or an impaired ability to experience pleasure, appear to be the most frequently associated with substance use problems(Mathew et al., 2015; McDevitt-Murphy et al., 2010). Feelings of detachment from others, numbing, and loss of interest in activities reflect an anhedonic dimension of PTSD, which has been identified in earlier factor analytic studies (Liu et al., 2014; Yang et al., 2017). These post-trauma anhedonia symptoms are also highly correlated with anhedonic symptoms of depression (Kashdan et al., 2006). Anhedonia has been frequently reported in SUD populations(Garfield et al., 2014), and is thought to be a source of susceptibility for substance use and relapse (Guillot et al., 2016; Leventhal and Zvolensky, 2015). Lower hedonic tone predicts susceptibility to drug cravings over time(Cook et al., 2004), suggesting that people may be likely to turn to drugs to increase their feelings of happiness and interest. Anhedonia also predicts persistence of use (Leventhal et al., 2009) increased withdrawal symptoms(Janiri et al., 2005) and problems with cessation(Cook et al., 2010; Doran et al., 2006; Hatzigiakoumis et al., 2011). People at high risk for mental health disorders have reported using substances to enhance positive mood and decrease anhedonia(Gill et al., 2015). Dysregulated hedonic homeostasis has been theorized to contribute to SUD maintenance(Koob, 2008; Koob and Le Moal, 1997), and increasingly, abnormalities in appetitive processes have been implicated in the development of these problems.
Longitudinal studies of adolescents have shown that trajectories of escalating drug use over time correspond with significant increases in anhedonia(Leventhal et al., 2017; Lichenstein et al., 2017). Results of a National Epidemiologic Survey (N~43,000) of adults revealed that anhedonia was associated with lifetime substance (and specifically, amphetamine and cocaine) dependence (Leventhal et al., 2010). Among those who never developed substance dependence, people who exhibited anhedonia were more likely to initiate drug use than those without anhedonia, and the effects of anhedonia on lifetime substance use were far greater than those found with depressive symptoms in general(Leventhal et al., 2010). This supports the idea that anhedonia itself may motivate substance use, and influence the transition from use to misuse to dependence.
Further, inflammation is thought to be a potential contributor to the emergence of anhedonic symptoms, which, in turn, may increase risk for SUDs. Increased inflammatory activity has been consistently associated with changes in brain regions associated with appetitive processing, and in turn, anhedonic symptoms(Dantzer et al., 2008; Swardfager et al., 2016). Inflammatory mediators such as C-reactive Protein (CRP) have been identified as predictors of substance use in prospective studies (Costello et al., 2013). Other recent studies show links between proinflammatory markers, substance use and disturbances in mood and behavior (Chang et al., 2019; Goldstein et al., 2015). Inflammation is also thought to influence the development of substance use and alterations in reward-related response in the aftermath of traumatic brain injury (Cannella et al., 2019; Merkel et al., 2017). Trauma may be a trigger for immune activation, which may mediate outcomes such as anhedonia and substance use (Andersen, 2019). As such, immune activation is an important factor to consider in the development of problematic substance use and mood disturbance after trauma.
Post-trauma anhedonia may be a mechanism through which people develop substance use disorders after trauma. However, this has not been examined in studies with a prospective design, which permits investigation of the temporality of the relationship between substance use and post-trauma symptoms. Thus, our goal was to use a prospective design to assess relationships between substance use and post-trauma anhedonia in people that were followed immediately after trauma exposure (recruited from the emergency department; ED). We assessed for the presence and severity of substance use at six months following trauma exposure, and measured changes in post-trauma anhedonia over time.
Specifically, we examined whether changes in post-trauma anhedonia from three to six months post-trauma corresponded with risky or high levels of substance use at six months post-trauma. Additionally, we assessed whether changes in substance use corresponded with changes in post-trauma anhedonia. We hypothesized that increases in post-trauma anhedonia over time would be associated with significant substance use at six months post-trauma, and we expected that no such increases in post-trauma anhedonia would be observed in those who showed consistently minimal or no substance use during this time period. We examined changes in post-trauma anhedonia from three to six months since this period has been frequently highlighted as a time frame during which PTSD symptom resolution or escalation occurs(Blanchard et al., 1995; Perez Benitez et al., 2013; Schock et al., 2016; Warren et al., 2014). Given that inflammatory mediators such as CRP have been associated with substance use and anhedonia (Costello et al., 2013) as well as mood disruptions (Chang et al., 2019; Goldstein et al., 2015) and PTSD symptoms (Costello et al., 2013; Eraly et al., 2014; Michopoulos et al., 2015), we included this as a covariate in our statistical models and also accounted for variance associated with non-anhedonic PTSD symptoms. We conducted secondary analyses to examine whether changes in depressive symptoms were similarly associated with these substance use patterns.
2. Methods
2.1. Participants
A total of 165 men and women aged 18–63 years (Mean=36.5, SD=12.5) were recruited as part of a larger prospective study of PTSD conducted in the ED of a Level 1 trauma center (MH094757), as described in earlier publications(Fani et al., 2019; Michopoulos et al., 2019; Stevens et al., 2013; van Rooij et al., 2017). Eligible patients were approached in the ED after initial medical evaluation, appropriate laboratory testing, and medical clearance had occurred. Once a patient signed informed consent, trained assessors collected demographic information and assessments that included information on prior trauma, substance abuse, current and past depression and PTSD symptoms, and details concerning the presenting trauma. Patients were queried about past and current medical conditions and medications. Participants who had experienced a DSM-IV criterion A trauma in the past 24 hours were eligible for the study but were not included if they had current suicidal ideation or attempt in last three months, current intoxication, loss of consciousness as a result of the trauma. Participants were also assessed for traumatic brain injury by ED physicians using the Glasgow Coma Scale, and people with scores of less than 15 were excluded from the study. Participants received the MINI Neuropsychiatric Interview (Sheehan et al., 1998) within 1 month of trauma exposure to assess for the lifetime and current presence of mood, psychotic, and substance use disorders. Participants who endorsed symptoms consistent with a current psychotic disorder were excluded from the current analyses. Table 1 describes demographic and clinical characteristics of this sample.
Table 1.
Demographic and Clinical Characteristics
| No Drug Use at 6 Months Post- trauma (n=118) | Risky/High Drug Use at 6 Months Post-trauma (n=47) | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | F | |
| Age (years) | 38.9 (12.5) | 30.7 (10.6) | 15.7** |
| % | % | Chi square | |
| Males | 49.2 | 56.3 | .69 |
| Fisher’s Exact | |||
| Race | 9.1* | ||
| Black | 74.6 | 70.8 | |
| White/Caucasian | 19.5 | 12.5 | |
| Asian | 1.7 | 0 | |
| American Indian or Alaska Native | 0 | 0 | |
| Native Hawaiian or Pacific Islander | 0 | 0 | |
| Mixed | 1.7 | 12.5 | |
| Other | 2.5 | 4.2 | |
| Fisher’s Exact | |||
| Household Monthly Income | 7.5* | ||
| 0–249 | 5.3 | 8.3 | |
| 250–499 | 6.2 | 6.3 | |
| 500–999 | 14.2 | 25 | |
| 1000–1999 | 16.8 | 25 | |
| 2000+ | 58 | 35.4 | |
| Mean (SD) | F | ||
| hsCRP | 3.6 | 3.7 | .05 |
| BDI total score | 9.6 (9.8) | 13.1 (11) | 4.4* |
| BDI anhedonia score | 1.6 (2) | 1.8 (1.9) | .9 |
| PSS total score | 10.2 (11) | 13 (10.6) | .1 |
| PSS anhedonia score | 1.3 (2.3) | 2 (2.5) | 3.6 |
=p<.05
= p<.01
hsCRP= high-sensitivity C-reactive Protein
BDI= Beck Depression Inventory II
PSS= PTSD Symptom Scale
2.2. Clinical Assessments
The PTSD Symptom Scale (PSS) The PTSD Symptom Scale (PSS; (Foa et al., 1993) was administered to assess PTSD symptoms in accordance with DSMIV criteria (American Psychiatric Association, 1994) at baseline screening (in the ED), three and six months post-trauma. A post-trauma anhedonia subscale score was created from three PSS items; data from factor analytic studies have repeatedly demonstrated that these three items cluster together, forming an anhedonia dimension of PTSD, and are highly correlated with depressive anhedonia (Kashdan et al., 2006; Liu et al., 2014; Yang et al., 2017). We examined changes in post-trauma anhedonia from three to six months post-trauma, given that this period has been frequently highlighted as a time frame during which PTSD symptom resolution or escalation occurs(Blanchard et al., 1995; Perez Benitez et al., 2013; Schock et al., 2016; Warren et al., 2014). At the 3 month assessment, Cronbach’s alpha for this measure at =.9; at the 6 month assessment, Cronbach’s alpha=.91.
The Beck Depression Inventory version 2 (BDI-II(Beck et al., 1996)), a self-report measure of depression symptoms in the past two weeks, was given to assess current depression symptoms at all timepoints. Both the total score and anhedonia subscale were used in secondary statistical analyses.
The Drug Abuse Screening Test (DAST (Staley and el-Guebaly, 1990)) was used to assess for the presence and severity of substance use at baseline screening, three and six months post-trauma. Other institutions (e.g., Oregon Health and Science University, Dept of Family Medicine, Screening, Brief Intervention, Referral to Treatment clinic; http://www.sbirtoregon.org) designated DAST short form (DAST 10) scores of 1–2 as being high enough to warrant brief intervention, which is currently the cutoff being used in their web-based screening application. p<.05).
C-reactive protein (CRP) is a marker of systemic inflammation that was assayed in serum samples collected in the ED after trauma exposure. Serum samples were stored at −80C until the time of CRP assay. Serum concentrations of CRP were measured using an immunoturbidometric assay from Sekisui Diagnostics (www.sekisuidiagnostics.com) on the Beckman AU480 chemistry analyzer, with an inter-assay CV of 5.2% and an intra-assay CV of 3.1%. Individuals with CRP levels >20 mg/L were excluded from the current analysis.
2.3. Statistical Analyses.
Two repeated measures ANCOVAs were conducted. In the first, we compared changes in post-trauma anhedonia from three to six months post-trauma between people who endorsed problematic substance use (assessed by the DAST) at six months post-trauma and participants who showed no/minimal substance use at that timepoint. In the second, we examined differences in post-trauma anhedonia change over time (three to six months) between two groups of participants; those who had increased their drug use from three to six months (n=22) as compared to those who maintained minimal/no drug use or decreased their drug use over time (n=141). Covariates included: CRP; age, which was significantly different between the two groups at six months post-trauma; and change in non-anhedonic PTSD symptoms from three to six months post-trauma. Secondarily, to determine specificity of effects, we repeated our first model and substituted other PTSD symptom clusters (re-experiencing and hyperarousal) with post-trauma anhedonia to examine potential interactions with drug use at six months post-trauma.
3. Results
3.1. Participant characteristics.
In this sample we found that, at three months post-trauma, mean post-trauma anhedonia=1.2 (SD=2.2), and mean post-trauma anhedonia at six months post-trauma=1.5 (SD=2.4). The anhedonia subscales of the BDI and PSS were correlated at the three month (r=.58, p<.0001) and six month (r=.56, p<.0001) timepoints.
DAST scores ranged from 0–7. At all timepoints, approximately half of the participants in the sample endorsed no drug use. At baseline, mean DAST score=1.1 (SD=1.6), at three months, mean DAST score = .8 (SD=1.4), at six months, mean DAST=.8 (SD=1.3). We used the 6 month DAST to assign participants to one of two groups: the high substance use group (DAST>=2; n=47) or the no/minimal drug use group (DAST<2; n=118). In this sample, 86% of participants maintained minimal/no drug use status from three to six months post-trauma, whereas 14% increased their drug use within this time frame.
Serum CRP concentrations in the current sample averaged 3.36 ± 4.57 mg/L and ranged from 0.01 to 18.9 mg/L. CRP data were natural log-transformed due to non-normal distribution. CRP was negatively correlated with six month DAST score within the no/minimal drug use group (r=.3, p=.001).
3.2. Post-trauma anhedonia and changes in substance use between three to six months post-trauma.
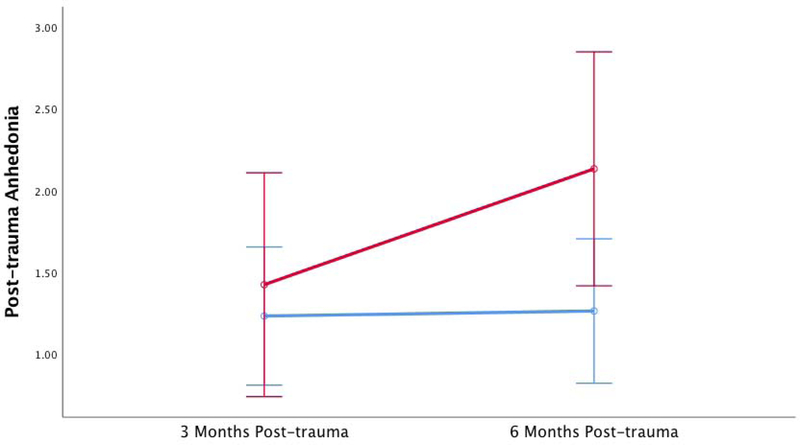
When comparing change in post-trauma anhedonia over time, no main effects of post-trauma anhedonia were observed (F1,160=.14, p=.71). However, we found a significant time by group interaction with substance use and post-trauma anhedonia change over time, (F1,160=4.3, p=.04) indicating that, compared to those who had minimal substance use at six months post-trauma, high substance users at six months post-trauma showed significant increases in post-trauma anhedonia from three to six month time period (Figure 1), after accounting for age, change in non-anhedonic PTSD symptoms, and CRP concentrations. Age, CRP concentrations and non-post-trauma anhedonia symptom change between three to six months did not contribute significantly to this model (all ps>05). A PSS symptom change by post-trauma anhedonia interaction was observed with substance use (F1,160=37.05, p<.0001). When sex was added to the model, no interactive effects were observed (p=.58). When the analysis was repeated with depressive anhedonic symptoms in place of post-trauma anhedonia (BDI anhedonia subscale), the model was not significant (F1,160=.65, p=.42). In addition, total depressive symptoms (BDI total score) at three and six months also did not yield a significant overall model (F1,160=.68, p=.41).
Figure 1. Increased Post-trauma Anhedonia is Associated with Greater Substance Use at Six Months Post-trauma.
Blue line represents participants with low/no substance use at six months post-trauma, red line represents participants with high levels of substance use at six months post-trauma. Error bars represent 95% confidence interval.
3.3. PTSD re-experiencing and hyperarousal symptoms and changes in substance use between three to six months post-trauma.
The statistical model was repeated to examine whether change in re-experiencing or hyperarousal from three to six months significantly contributed to drug use status at six months post-trauma, after accounting for effects of age and CRP level. With both re-experiencing (F1,160=2.1, p=.15) and hyperarousal (F1,160=.36, p=.55), the models were not statistically significant, with no main or interactive effects observed.
3.4. Participants with increased substance use over time vs no/minimal changes in substance use over time.
We compared participants who demonstrated increases in substance use during this three to six month post-trauma time period (n=21) to those who demonstrated a consistently minimal (or absent) level of substance use (n=139). We again accounted for age, change in non-anhedonic PTSD symptoms and CRP concentrations. No main effects of post-trauma anhedonia were observed (F1,155=1.54, p=.21). However a significant time by group interaction with substance use and post-trauma anhedonia was observed; participants who demonstrated increases in substance use from three to six months post-trauma had significant increases in post-trauma anhedonia, unlike those who did not show increases in substance use over time; (F1,155=6.59, p=.01). Age, CRP concentrations and non-post-trauma anhedonia symptom change between three to six months did not contribute significantly to this model (all ps>05). A PSS symptom change by post-trauma anhedonia interaction was observed with substance use (F1,155=35.31, p<.0001).
4. Discussion
In the current study, we examined associations between changes in anhedonic PTSD symptoms over time and substance use at six months post-trauma in a sample of recently traumatized patients recruited from the ED. Our findings indicated that participants with relatively high levels of substance use at six months post-trauma demonstrated significant increases in post-trauma anhedonia, or post-trauma anhedonia, between three to six months post-trauma, whereas those with minimal/no drug use at the six month timepoint demonstrated no increases in post-trauma anhedonia over time. These associations remained significant even after controlling for age, change in other PTSD symptoms and inflammation at the time of trauma (CRP level). We did not find that changes in other types of PTSD symptoms or depression were associated with patterns of high substance use at this six month time point. Similarly, we found that escalations in substance use over time were associated with changes in post-trauma anhedonia and not other types of PTSD symptoms during this time period.
To our knowledge, this is the first prospective study to investigate associations between changes in anhedonic symptoms of PTSD and drug use in the aftermath of trauma. Collectively, our findings indicate that post-trauma anhedonia may contribute significantly to the development of substance use problems in recently-traumatized individuals. Anhedonia is being increasingly recognized as not just a feature of withdrawal or craving in those with substance use problems (Garfield et al., 2014), but as an independent causative agent (Destoop et al., 2019; Sussman and Leventhal, 2014). Data from prospective studies provide support for this notion. In adolescents, depressive anhedonia at the initial point of assessment has been associated with escalating usage of marijuana over time (Leventhal et al., 2017), and escalating patterns of use have been linked to similar escalations in anhedonia over time (Lichtenstein 2017). In some traumatized people, changing patterns of substance or alcohol use are likely to emerge when they experience difficulties with positive affect. The diminished responsiveness to rewarding stimuli that characterizes post-trauma anhedonia may lead some traumatized people to turn to substances to self-medicate their symptoms. They may use substances to enhance muted feelings of pleasure, as well as diminish negative affect and related physiological arousal. Over time, this can lead to patterns of increased and otherwise problematic use, fueling substance tolerance and interfering with various aspects of functioning.
It is possible that these manifestations of anhedonia exist as a trait-like vulnerability for some individuals, and the experience of trauma may interact with this vulnerability to enhance risk for substance use or relapse. Anhedonia has been associated with motivation for use, and it has predicted the initiation of drug use. Trait anhedonia has been associated with shorter latency to initiate use and increased usage overall during an experimental task (Leventhal et al., 2014b). Similarly, low hedonic capacity, which refers to the ability to experience pleasure in response to a rewarding stimulus, has been associated with an increased risk of smoking overall and escalating use over time (18 months) in adolescents (Audrain-McGovern et al., 2012). Although depression has a significant impact on substance use disorder risk, some studies have found that anhedonia is uniquely associated with the likelihood of developing and maintaining substance use problems. For example, Leventhal and colleagues (2014) found that, even after adjusting for depressive symptoms overall, a lifetime history of anhedonia symptoms specifically predicted relapse in the year following smoking cessation treatment (Leventhal et al., 2014a). Anhedonia has also been found to mediate the relationship between familial risk for substance use and substance use trajectories in adolescents (Cho et al., 2019).
Anhedonia is thought to be related to dysfunction in dopamine transmission (Gorwood, 2008; Stein, 2008), which also characterizes substance use disorders. As such, vulnerability for developing anhedonia may also be evident at the level of the brain, and it may be linked to disrupted structure and function in neural networks that are involved with reward processing and emotion regulation. A recent review highlights the role of stress/trauma in producing changes in critical reward-processing brain pathways, which in turn influence the development of anhedonia, increasing risk for problems such as depression and SUD (Sheth et al., 2017). Volumetric changes in nodes of brain reward processing networks, specifically, the orbitofrontal cortex (OFC), have corresponded with increased anhedonia, and similarly, increased alcohol and marijuana use over time (Luby et al., 2018). Smaller OFC volumes have predicted earlier onset and greater severity of drug use in earlier studies of adolescents (Cheetham et al., 2012). The OFC communicates with limbic regions, particularly, the amygdala to engage in reward valuation and outcome expectancies (Schoenbaum et al., 2006). Notably, earlier age of substance use onset has been associated with larger amygdala volumes in youths (Yucel et al., 2006). We recently observed that decrements in a white matter pathway involved with emotion regulation, the uncinate fasciculus, at around the time of trauma predicted later development of post-trauma anhedonia in recently-traumatized individuals(Fani et al., 2019). The present findings build on this body of work, indicating that post-trauma anhedonia development in recently-traumatized individuals may influence the course and extent of substance use.
Among the different reward functions—motivation for, learning and “liking” of a reward stimulus-- post-trauma anhedonia appears to be most aligned with deficits in reward liking, also termed consummatory anhedonia. As a treatment target, post-trauma anhedonia may be malleable and responsive to psychotherapeutic and pharmaceutic interventions designed to enhance this aspect of appetitive functioning (Craske et al., 2016). Among the various interventions that have been proposed, behavioral activation, mindfulness meditation, and positive psychology interventions may be the most promising, given that they are designed to enhance hedonic capacity. Medications that target dopamine transmission, such as buproprion, may prove to be best-suited for people with post-trauma anhedonia. In addition, oxytocin has been suggested as a way to normalize reward functioning in PTSD and improve the ability to “appreciate” socially rewarding stimuli by enhancing feelings of safety and trust in social interactions (Olff et al., 2010). In the context of recent trauma, assessments for anhedonia can be used to detect people who are at greatest risk for developing both post-trauma anhedonia and SUD; once assessed, prevention strategies aimed at improving positive affect may be best suited for this subset of traumatized people.
The present findings must be interpreted in light of certain limitations. Despite the large sample size of this study, only a minority of individuals in this sample endorsed severe substance use disorder problems, which may impact the generalizability of these findings to traumatized people with severe substance use disorder. The data from these participants represents a relatively acute period of recovery from trauma (within one year post-trauma), and severe substance use problems may emerge outside of this window of time. As such, the high substance use group in our traumatized population may reflect a group that is at significantly greater risk for developing substance use disorders in the future. Longer-term longitudinal studies are needed to ascertain whether these trajectories persist and worsen in some recently-traumatized individuals. Given our findings with post-trauma anhedonia and its correlations with depressive anhedonia, we were surprised to find that changes in depressive anhedonia were not associated with substance use at six months post-trauma. This may indicate that anhedonic symptoms of PTSD, which are more related to social anhedonia, are more specifically involved in influencing substance use patterns following trauma exposure.
In conclusion, in this sample of recently-traumatized individuals, we found that increases in anhedonic symptoms, but not other symptoms of PTSD or depression, were associated with increased substance use at six months post-trauma. Our findings suggest that post-trauma anhedonia may be a mechanism through which substance use problems emerge in the aftermath of trauma. Additionally, the results indicate the potential utility of targeting these particular manifestations of PTSD in the prevention and treatment of disordered substance use in traumatized people.
Highlights.
Anhedonic symptoms of PTSD may play a role in problematic substance use after trauma
We examined changes in post-trauma anhedonia (PTA) and substance use in recently-traumatized people
Changes in PTA were associated with problematic substance use at 6 months post-trauma
PTA may be a mechanism through which substance use emerges or worsens in the aftermath of trauma
Funding and Acknowledgments:
This work was primarily supported by National Institutes of Mental Health R01 MH094757 (to KJR, BOR), U01 MH110925 (to KJR), K12 HD085850 (to VM), and MH101380 (to NF). We would like to thank Debra Houry, M.D. and Abigail Hankin-Wei, M.D., for their collaborative efforts on this study. We also thank Erin Renner, Alex O. Rothbaum, Thomas Crow, Heather Grinstead, Rebecca C. Hinrichs, Jessica Maples-Keller, Lydia Odenat, Loren M. Post, Liza C. Zwiebach, Devika Fiorillo, Kathryn Breazeale, Jessica Morgan, Natasha Mehta, Elicia D. Skelton, Taleesha S. Booker, Jonathan Zebrowski, Zachary Clifford, and Sterling Winters for their work in the emergency department recruiting and assessing participants.
Footnotes
Disclosures: The authors have no financial conflicts of interest to disclose.
Negar Fani: Conceptualization, data analysis, manuscript writing and editing, supervision; Jahnvi Jain: Data curation, editing; Lauren Hudak: Writing, reviewing and editing, Barbara O. Rothbaum: resources, editing; Kerry J. Ressler, MD, PhD: Conceptualization, resources, editing; Vasiliki Michopoulos: Data curation, editing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association, 1994. Diagnostic and Statistical Manual of Mental Disorders, 4th Ed. American Psychiatric Association, Washington, DC. [Google Scholar]
- Andersen SL, 2019. Stress, sensitive periods, and substance abuse. Neurobiol Stress 10, 100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Leventhal AM, Cuevas J, Rodgers K, Sass J, 2012. Where is the pleasure in that? Low hedonic capacity predicts smoking onset and escalation. Nicotine Tob Res 14 (10), 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown G, 1996. Manual for Beck Depression Inventory II (BDI-II). Psychology Corporation, San Antonio, Texas. [Google Scholar]
- Blanchard EB, Hickling EJ, Vollmer AJ, Loos WR, Buckley TC, Jaccard J, 1995. Short-term follow-up of post-traumatic stress symptoms in motor vehicle accident victims. Behav Res Ther 33 (4), 369–377. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Darnell A, Charney DS, 1996. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry 153 (3), 369–375. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR, 2003. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry 60 (3), 289–294. [DOI] [PubMed] [Google Scholar]
- Cannella LA, McGary H, Ramirez SH, 2019. Brain interrupted: Early life traumatic brain injury and addiction vulnerability. Exp Neurol 317, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HB, Munroe S, Gray K, Porta G, Douaihy A, Marsland A, Brent D, Melhem NM, 2019. The role of substance use, smoking, and inflammation in risk for suicidal behavior. J Affect Disord 243, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yucel M, Lubman DI, 2012. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol Psychiatry 71 (8), 684–692. [DOI] [PubMed] [Google Scholar]
- Chilcoat HD, Breslau N, 1998. Investigations of causal pathways between PTSD and drug use disorders. Addict Behav 23 (6), 827–840. [DOI] [PubMed] [Google Scholar]
- Cho J, Stone MD, Leventhal AM, 2019. Anhedonia as a Phenotypic Marker of Familial Transmission of Polysubstance Use Trajectories Across Midadolescence. Psychology of Addictive Behaviors 33 (1), 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J, Spring B, McChargue D, Doran N, 2010. Effects of anhedonia on days to relapse among smokers with a history of depression: a brief report. Nicotine Tob Res 12 (9), 978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D, 2004. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine Tob Res 6 (1), 39–47. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Copeland WE, Shanahan L, Worthman CM, Angold A, 2013. Creactive protein and substance use disorders in adolescence and early adulthood: a prospective analysis. Drug Alcohol Depend 133 (2), 712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougle JR, Bonn-Miller MO, Vujanovic AA, Zvolensky MJ, Hawkins KA, 2011. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav 25 (3), 554–558. [DOI] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour HJ, 2016. Treatment for Anhedonia: A Neuroscience Driven Approach. Depress Anxiety 33 (10), 927–938. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9 (1), 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destoop M, Morrens M, Coppens V, Dom G, 2019. Addiction, Anhedonia, and Comorbid Mood Disorder. A Narrative Review. Front Psychiatry 10, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Spring B, Borrelli B, McChargue D, Hitsman B, Niaura R, Hedeker D, 2006. Elevated positive mood: a mixed blessing for abstinence. Psychol Addict Behav 20 (1), 36–43. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O’Connor DT, Baker DG, Team MRS, 2014. Assessment of Plasma C-Reactive Protein as a Biomarker of Posttraumatic Stress Disorder Risk. JAMA Psychiatry 71 (4), 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Michopoulos V, van Rooij SJH, Clendinen C, Hardy RA, Jovanovic T, Rothbaum BO, Ressler KJ, Stevens JS, 2019. Structural connectivity and risk for anhedonia after trauma: A prospective study and replication. J Psychiatr Res 116, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO, 1993. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress 6, 459–473. [Google Scholar]
- Garfield JB, Lubman DI, Yucel M, 2014. Anhedonia in substance use disorders: a systematic review of its nature, course and clinical correlates. Aust N Z J Psychiatry 48 (1), 36–51. [DOI] [PubMed] [Google Scholar]
- Gill KE, Poe L, Azimov N, Ben-David S, Vadhan NP, Girgis R, Moore H, Cressman V, Corcoran CM, 2015. Reasons for cannabis use among youths at ultra high risk for psychosis. Early Interv Psychiatry 9 (3), 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Lotrich F, Axelson DA, Gill MK, Hower H, Goldstein TR, Fan J, Yen S, Diler R, Dickstein D, Strober MA, Iyengar S, Ryan ND, Keller MB, Birmaher B, 2015. Inflammatory markers among adolescents and young adults with bipolar spectrum disorders. J Clin Psychiatry 76 (11), 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorwood P, 2008. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci 10 (3), 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot CR, Bello MS, Tsai JY, Huh J, Leventhal AM, Sussman S, 2016. Longitudinal Associations between Anhedonia and Internet-Related Addictive Behaviors in Emerging Adults. Comput Human Behav 62, 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L, 2011. Anhedonia and substance dependence: clinical correlates and treatment options. Front Psychiatry 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri L, Martinotti G, Dario T, Reina D, Paparello F, Pozzi G, Addolorato G, Di Giannantonio M, De Risio S, 2005. Anhedonia and substance-related symptoms in detoxified substance-dependent subjects: a correlation study. Neuropsychobiology 52 (1), 37–44. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Elhai JD, Frueh BC, 2006. Anhedonia and emotional numbing in combat veterans with PTSD. Behav Res Ther 44 (3), 457–467. [DOI] [PubMed] [Google Scholar]
- Kevorkian S, Bonn-Miller MO, Belendiuk K, Carney DM, Roberson-Nay R, Berenz EC, 2015. Associations among trauma, posttraumatic stress disorder, cannabis use, and cannabis use disorder in a nationally representative epidemiologic sample. Psychol Addict Behav 29 (3), 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ, 1997. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry 4 (5), 231–244. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2008. Hedonic Homeostatic Dysregulation as a Driver of Drug-Seeking Behavior. Drug Discov Today Dis Models 5 (4), 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 1997. Drug abuse: hedonic homeostatic dysregulation. Science 278 (5335), 52–58. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Brightman M, Ameringer KJ, Greenberg J, Mickens L, Ray LA, Sun P, Sussman S, 2010. Anhedonia associated with stimulant use and dependence in a population-based sample of American adults. Exp Clin Psychopharmacol 18 (6), 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Cho J, Stone MD, Barrington-Trimis JL, Chou CP, Sussman SY, Riggs NR, Unger JB, Audrain-McGovern J, Strong DR, 2017. Associations between anhedonia and marijuana use escalation across mid-adolescence. Addiction 112 (12), 2182–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Zimmerman M, 2009. Refining the depression-nicotine dependence link: patterns of depressive symptoms in psychiatric outpatients with current, past, and no history of nicotine dependence. Addict Behav 34 (3), 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Piper ME, Japuntich SJ, Baker TB, Cook JW, 2014a. Anhedonia, depressed mood, and smoking cessation outcome. J Consult Clin Psychol 82 (1), 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Trujillo M, Ameringer KJ, Tidey JW, Sussman S, Kahler CW, 2014b. Anhedonia and the relative reward value of drug and nondrug reinforcers in cigarette smokers. J Abnorm Psychol 123 (2), 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ, 2015. Anxiety, depression, and cigarette smoking: a transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychol Bull 141 (1), 176–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichenstein SD, Musselman S, Shaw DS, Sitnick S, Forbes EE, 2017. Nucleus accumbens functional connectivity at age 20 is associated with trajectory of adolescent cannabis use and predicts psychosocial functioning in young adulthood. Addiction 112 (11), 1961–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wang L, Cao C, Wang R, Zhang J, Zhang B, Wu Q, Zhang H, Zhao Z, Fan G, Elhai JD, 2014. The underlying dimensions of DSM-5 posttraumatic stress disorder symptoms in an epidemiological sample of Chinese earthquake survivors. J Anxiety Disord 28 (4), 345–351. [DOI] [PubMed] [Google Scholar]
- Luby JL, Agrawal A, Belden A, Whalen D, Tillman R, Barch DM, 2018. Developmental Trajectories of the Orbitofrontal Cortex and Anhedonia in Middle Childhood and Risk for Substance Use in Adolescence in a Longitudinal Sample of Depressed and Healthy Preschoolers. Am J Psychiatry, appiajp201817070777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew AR, Cook JW, Japuntich SJ, Leventhal AM, 2015. Post-traumatic stress disorder symptoms, underlying affective vulnerabilities, and smoking for affect regulation. Am J Addict 24 (1), 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley JL, Killeen T, Gros DF, Brady KT, Back SE, 2012. Posttraumatic Stress Disorder and Co-Occurring Substance Use Disorders: Advances in Assessment and Treatment. Clin Psychol (New York) 19 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt-Murphy ME, Murphy JG, Monahan CM, Flood AM, Weathers FW, 2010. Unique Patterns of Substance Misuse associated with PTSD, Depression, and Social Phobia. J Dual Diagn 6 (2), 94–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel SF, Cannella LA, Razmpour R, Lutton E, Raghupathi R, Rawls SM, Ramirez SH, 2017. Factors affecting increased risk for substance use disorders following traumatic brain injury: What we can learn from animal models. Neurosci Biobehav Rev 77, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Maples-Keller J, Roger EI, Beaudoin FL, Sumner JA, Rothbaum BO, Hudak L, Gillespie CF, Kronish IM, McLean SA, Ressler KJ, 2019. Nausea in the peri-traumatic period is associated with prospective risk for PTSD symptom development. Neuropsychopharmacology 44 (4), 668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, Gillespie CF, Ressler KJ, 2015. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry 172 (4), 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Langeland W, Witteveen A, Denys D, 2010. A psychobiological rationale for oxytocin in the treatment of posttraumatic stress disorder. CNS Spectr 15 (8), 522–530. [DOI] [PubMed] [Google Scholar]
- Perez Benitez CI, Zlotnick C, Dyck I, Stout R, Angert E, Weisberg R, Keller M, 2013. Predictors of the long-term course of comorbid PTSD: a naturalistic prospective study. Int J Psychiatry Clin Pract 17 (3), 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, Grant BF, 2011. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord 25 (3), 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock K, Bottche M, Rosner R, Wenk-Ansohn M, Knaevelsrud C, 2016. Impact of new traumatic or stressful life events on pre-existing PTSD in traumatized refugees: results of a longitudinal study. Eur J Psychotraumatol 7, 32106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, 2006. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci 29 (2), 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Sheth C, McGlade E, Yurgelun-Todd D, 2017. Chronic Stress in Adolescents and Its Neurobiological and Psychopathological Consequences: An RDoC Perspective. Chronic Stress (Thousand Oaks) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley D, el-Guebaly N, 1990. Psychometric properties of the Drug Abuse Screening Test in a psychiatric patient population. Addict Behav 15 (3), 257–264. [DOI] [PubMed] [Google Scholar]
- Stein DJ, 2008. Depression, anhedonia, and psychomotor symptoms: the role of dopaminergic neurocircuitry. CNS Spectr 13 (7), 561–565. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ, 2013. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman S, Leventhal A, 2014. Substance misuse prevention: addressing anhedonia. New Dir Youth Dev 2014 (141), 45–56, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager W, Rosenblat JD, Benlamri M, McIntyre RS, 2016. Mapping inflammation onto mood; inflammatory mediators of anhedonia. Neurosci Biobehav Rev. [DOI] [PubMed] [Google Scholar]
- van Rooij SJH, Stevens JS, Ely TD, Hinrichs R, Michopoulos V, Winters SJ,Ogbonmwan YE, Shin J, Nugent NR, Hudak LA, Rothbaum BO, Ressler KJ, Jovanovic T, 2017. The Role of the Hippocampus in Predicting Future Posttraumatic Stress Disorder Symptoms in Recently Traumatized Civilians. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren AM, Foreman ML, Bennett MM, Petrey LB, Reynolds M, Patel S, Roden-Foreman K, 2014. Posttraumatic stress disorder following traumatic injury at 6 months: associations with alcohol use and depression. J Trauma Acute Care Surg 76 (2), 517–522. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang L, Cao C, Cao X, Fang R, Zhang J, Elhai JD, 2017. The underlying dimensions of DSM-5 PTSD symptoms and their relations with anxiety and depression in a sample of adolescents exposed to an explosion accident. Eur J Psychotraumatol 8 (1), 1272789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Lubman DI, Velakoulis D, Wong MT, Wood SJ, Condello A, Brewer WJ, Pantelis C, 2006. Structural brain correlates of alcohol and cannabis use in recreational users. Acta Neuropsychiatr 18 (5), 226–229. [DOI] [PubMed] [Google Scholar]