Abstract
Dental clinicians have relied for centuries on traditional dental materials (polymers, ceramics, metals, and composites) to restore oral health and function to patients. Clinical outcomes for many crucial dental therapies remain poor despite many decades of intense research on these materials. Recent attention has been paid to biomolecules as a chassis for engineered preventive, restorative, and regenerative approaches in dentistry. Indeed, biomolecules represent a uniquely versatile and precise tool to enable the design and development of bioinspired multifunctional dental materials to spur advancements in dentistry. In this review, we survey the range of biomolecules that have been used across dental biomaterials. Our particular focus is on the key biological activity imparted by each biomolecule toward prevention of dental and oral diseases as well as restoration of oral health. Additional emphasis is placed on the structure–function relationships between biomolecules and their biological activity, the unique challenges of each clinical condition, limitations of conventional therapies, and the advantages of each class of biomolecule for said challenge. Biomaterials for bone regeneration are not reviewed as numerous existing reviews on the topic have been recently published. We conclude our narrative review with an outlook on the future of biomolecules in dental biomaterials and potential avenues of innovation for biomaterial-based patient oral care.
1. Introduction
The clinical need for dental biomaterial therapies is unrelenting. There are 3.5 billion cases of untreated oral conditions and, in particular, 267 million individuals with complete tooth loss globally.1 An estimated 800 million resin composite, 100 million amalgam, and millions of glass ionomer cement restorations are placed each year and are one of the most prevalent medical interventions in the human body,2 not to mention the over five million implants placed in the United States each year.3 The cost for these therapies is immense; the global dental implant market alone is 3500 million USD.4 Indeed, 141 clinical trials (October 2019; Clinicaltrials.gov) are recruiting or active for dental implants combined with another 201 for dental caries and 81 for endodontic diseases. The combined complexity and prevalence of dental diseases requires well engineered materials to optimize patient outcomes.
Advanced biomaterials are needed to provide the unique functionality required by new devices, scaffolds, and drug delivery systems to keep pace with rapid progress in dental medicine. The range of biomaterial modalities for dental therapies is wide; from load-bearing, nanoparticle-filled, photopolymerized resin composites to soft, degradable collagen membranes for guided bone regeneration. Dental biomaterials, while seemingly “limited” to the small (relative to the rest of the body) oral cavity, are required to interface not only with a diverse set of tissues, from soft oral gingiva to hard, mineralized enamel and bone; but also function under demanding environmental conditions, such as sudden changes in temperature, a wide range of salivary and biofilm-induced pH, antagonistic forces from chewing and wear from hard food debris, and an extraordinarily diverse microflora.5
Advances in the general fields of biomaterials and tissue engineering in recent decades have pushed bioengineering principles past cytocompatibility into tailoring specific biological responses; for example, fast and intimate osseointegration of dental implants or the overall commercial success of autologous cell-based therapies (such as for articular cartilage repair or wound healing).6 Indeed, many traditional dental materials only serve a space-filling role – not a biologically-instructive role – and as a result have little ability to regenerate native tissue.7 A potent toolkit to unlock specific biological responses is the diverse array of biomolecules nature provides. Biomolecules include a large series of biomacromolecules (for example, proteins, polynucleic acids, lipids, and polysaccharides) and small molecules (for example, amino acids, oligopeptides, monosaccharides, deoxyribonucleotide, and metabolic products) which are essential for physiological processes, such as cell proliferation, migration, differentiation, and overall homeostasis.8,9 Harnessing the biomolecule toolkit for biomaterial design is a bioinspired and biomimetic approach that offers different molecules with precise biological functions;10,11 the human proteome contains up to several billion protein species.12 Advances in biomolecule synthesis over the past decade, such as the now ubiquitous solid phase peptide synthesis,13 rapid expansion of metaomic technologies,14 and recombinant technologies,15 have further driven the ability of tissue engineers and biomaterial scientists to derivatize materials with biomolecules. In any case, harnessing and exploiting the full potential of the biomolecules toolkit to develop more effective, off-the-shelf, preventive and therapeutic materials to address oral diseases requires synergistic collaborations between basic, clinical, and industrial teams (Fig. 1).
Fig. 1.
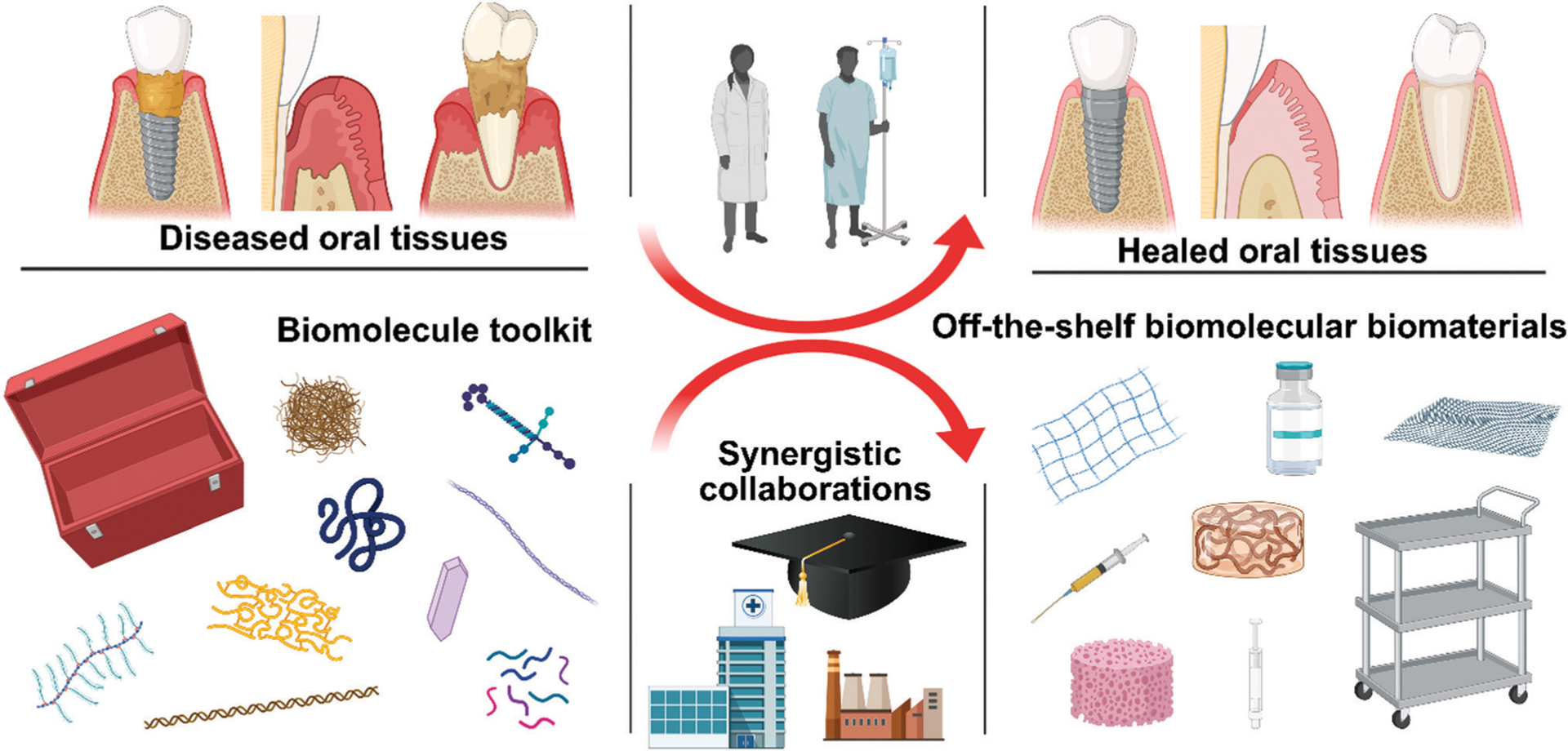
Harnessing biomolecules for bioinspired dental biomaterials. Promising and proven biomolecules include hyaluronic acid, DNA, elastin, peptides, proteins, intrinsically disordered proteins, laminin, minerals, and collagen. Dental biomaterials potentially benefitting from biomolecule incorporation include tissue grafts and membranes, adhesives, and regenerative endodontic obturation materials.
In this review, we survey the range of biomolecules used across dental biomaterials with a particular focus on the biological activity of these biomolecules toward prevention of oral disease and/or restoration of oral health. This review is organized by clinical condition to emphasize the design principles needed for each specific disease and biomaterial and the resulting biomolecules used to enhance device function. We conclude our narrative review with an outlook on the future of biomolecules in dental biomaterials and consider potential avenues of innovation using these materials for patient care.
2. Biomolecule-based dental biomaterials
2.1. Antimicrobial dental biomaterials
Infection of medical devices, dental included, is a grand challenge. Indeed, a range of medical devices (from fixation devices to catheters to dental implants) all become infected at unacceptably high rates.16–19 Infection is particularly difficult to control acknowledging that antimicrobial resistance is rapidly proliferating: the US Centers for Disease Control and Prevention estimates there are more than two million infections in the US each year from antibiotic resistant bacteria resulting in at least 23 000 deaths.20 The numbers of deaths per year and expense due to device infections is expected to dramatically rise over the coming years.21 The annual healthcare cost in the US alone for infections due to antibiotic resistant strains is already around $20 billion.22,23 Challenging regulatory environments, lack of understanding of resistance mechanisms, and reduced financial incentives, among others, have led to reduced development of new antibiotics.24 This alarm has driven the development of other, non-antibiotic based biomaterials16,25 for devices outside of dentistry.17,26,27 Here, we survey a range of biomolecules that have been harnessed to derivatize dental biomaterials with antimicrobial activity toward preventing infection.
2.1.1. Antimicrobial dental implants.
Approximately 178 million Americans are missing at least one tooth thereby causing lost self-confidence and lower self-image.1,28,29 Conservatively estimated current estimates of an approximately 10%30–32 dental implant failure rate lead to over one million implants failing worldwide per year.33 This high failure rates results in functional lifespans of dental implants of around 5 to 11 years34 yet as much as 23% of the entire adult U.S. population may possess a dental implant by 2026.35 All the evidence combined strongly suggests that dental implant infection and failure are critical healthcare concerns. Dental implant infection, or periimplantitis, is an inflammatory condition related to infection, biofilm formation, and eventual supporting tissue loss.36,37 As a result, antimicrobial dental implants are highly desirable.
2.1.1.1. Antimicrobial peptides for dental implants.
Special attention has been recently given to antimicrobial peptides (AMPs) due to their excellent antibacterial, antibiofilm properties, and generally low bacterial strain resistance.38,39 The latter is an important advantage over commercially-used antibiotics, diminishing the potential risks involved in the use of synthetic drugs (i.e., cytotoxicity, strain resistance, etc.).40 AMPs are typically small (under 50 amino acids) naturally occurring molecules that are generally cationic and amphipathic, though exceptions certainly exist.41–43 This general structure allows them to act as antimicrobial agents with broad activity spectrum, low cytotoxicity, selectivity towards microbial membranes, host immunity modulation, and the ability to bind bacterial endotoxins and neutralize their biological effects.44,45 Despite the existence of thousands of distinct AMPs in nature, which vary in size, structure, sequence, and polarity, only a handful have been applied toward dental implant applications.46 AMP immobilization onto surfaces like dental implants enhances their stability and increases the local concentration and therefore biological availability for microbe killing.47–49 Moreover, rationally designed peptides offer the ability to recapitulate the function of proteins and bypass protein’s structural complexities and expensive synthesis or isolation.50 A broad overview of AMP coatings for medical devices in general can be found elsewhere.51,52 We survey here select AMPs used on dental implants to reduce peri-implantitis.
One well-characterized AMP used to coat dental implants is GL13K, which is a self-assembling, cationic, amphipathic designer AMP derived (and later altered) from the salivary protein BPIFA2.53,54 Early work with GL13K established it could be anchored on titanium and reduce the load of Porphyromonas gingivalis.55 Subsequent work showed similar antimicrobial activity against Streptococcus gordonii56 without affecting osseointegration in a rabbit model.57 More recent work58–60 has shown GL13K’s antimicrobial behavior is dependent on the formation of twisted nanoribbon structures that is triggered by neutralization of cationic side groups before surface anchoring. It should be emphasized that AMP mechanisms are not well-established.61 GL13K has also been anchored on microgrooved substrates to promote soft issue formation with simultaneous antibiofilm activity.62
Another AMP used to coat implants is hLF1–11, which is composed of the first 11 N-terminal residues of human lactoferrin (a glycoprotein found in most human fluids63).64,65 hLF1–11 has been covalently anchored and adsorbed to titanium and shown to reduce Streptococcus sanguinis and Lacto-bacillus salivarius activity.66 Important work using hFl1–11 has shown that the resultant antimicrobial activity is sensitive to the specific covalent (such as silanization or surface initiated polymerization) anchoring method employed.67,68 Others have also shown that immobilization methods affect AMP activity.69 In response, some groups have adopted anchoring chemistry that are chemoselective to tightly regulate AMP orientation.70,71 A related concept of spacers, or the domain (such as amino acids in the case of AMPs) sometimes placed between the bioactive moiety and the residue(s) used to anchor it, is also important for optimal AMP activity,72 and has been exploited in different peptides coating configurations, including chimeric peptides.
Chimeric peptides, which are further explained and explored in Section 5, have also been harnessed as AMP coatings for dental implants. These peptides simultaneously present an implant binding peptide, identified using combinatorial phage or cell surface display technologies, and an antimicrobial domain. A common concern of peptide coatings is their durability and the ease, or difficulty, of their clinical application. Chimeric peptides provide a high affinity, material specific binding at the implant interface based on their self-assembly ability while also displaying AMPs on the site. One example showed antimicrobial activity against Streptococcus mutans, Staphylococcus epidermidis, and E. coli using different combinations of AMPs on titanium surfaces.73 Chimeric peptides have been further applied to titanium in a water-based coating and exert antimicrobial activity against S. mutans.74
Recently, titanium binding peptides (TiBP) have been combined in chimeric peptides with different AMPs using spacer domains (Fig. 2A–D).72 Spacer domains75 are introduced to provide the secondary structural features common to AMPs to enhance the antmicrobial activity of the peptide film on the implant. In this study, chimeric peptides were demonstrated to thoroughly coat titanium sufaces even in the presence of proteins and maintain antimicrobial function following toothbrushing. Correlating the structure–function relationship of the chimeric peptide film resulted in predicting the antimicrobial peptide film properties under competition as well as challenged implant surfaces.
Fig. 2.
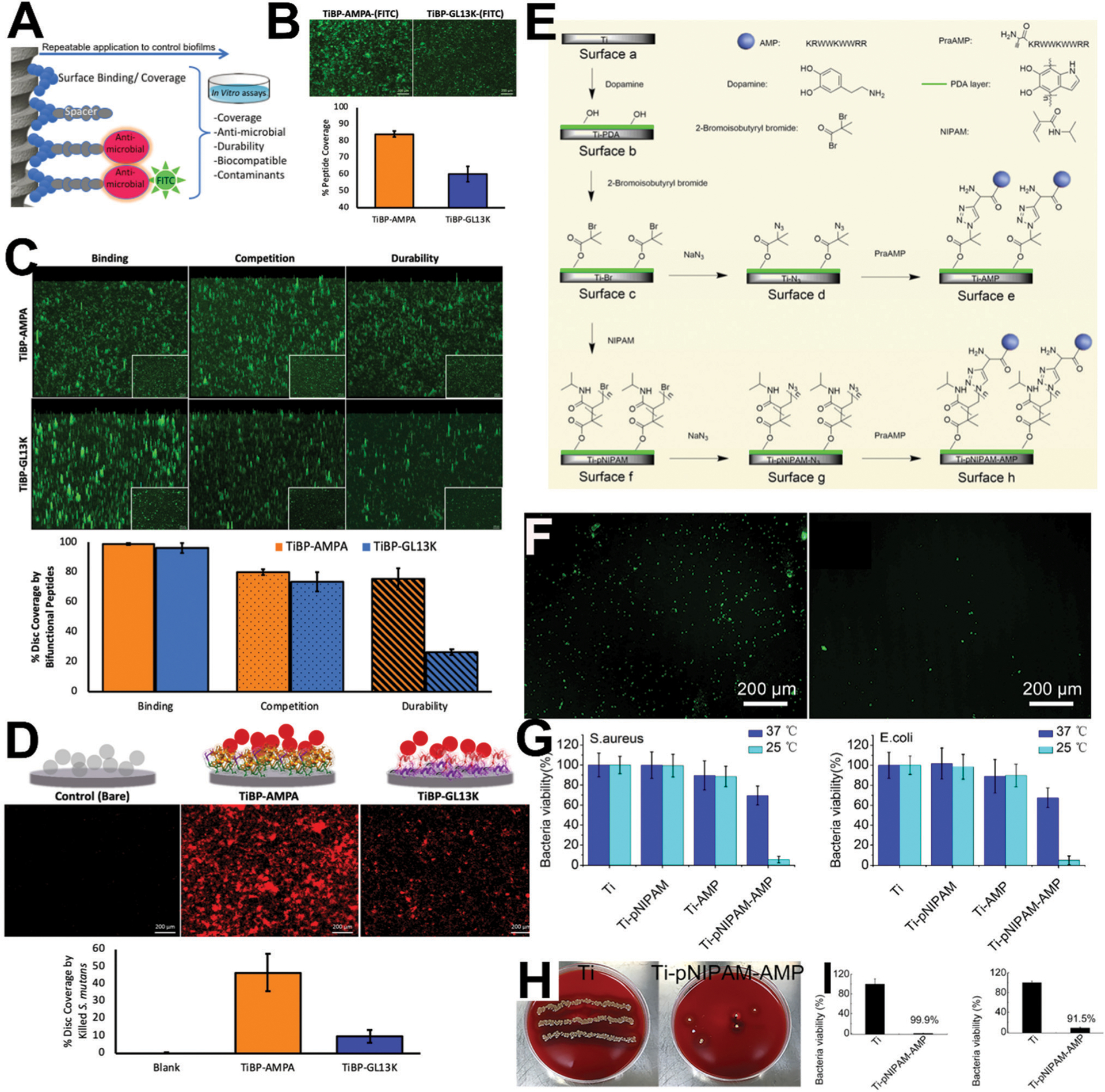
Chimeric antimicrobial peptides and temperature-sensitive immobilized antimicrobial peptides with in vivo potency. (A) Schematic representation of AMP designed with an implant/titanium binding domain (TiBP) connected to an AMP domain separated by a spacer. Two peptides designs were used in this study: TiBP-AMPA and TiBP-GL13K, which differed in their respective AMPs (AMPA vs. GL13K). (B) Visualization of FITC-labeled peptides using fluorescence microscopy after challenge by S. mutans for 24 hours. The percentage of peptide (TiBP-AMPA vs. TiBP-GL13K) coverage was determined. (C) Fluorescent microscopy images of peptides (TiBP-AMPA and TiBP-GL13K) binding to titanium implant discs, binding with competition from bovine serum albumin, and durability following 1 minute of brushing with an electric toothbrush. (D) Fluorescence microscopy images and quantification of propidium iodide (PI) staining of dead S. mutans bacteria on implant discs after challenge for 24 hours. (E) Scheme of preparation of temperature-sensitive surfaces on Ti; Ti was treated with dopamine to form surface b (Ti-PDA); then, surface b was treated with 2-bromoisobutyryl bromide to form surface c (Ti-Br); by click chemistry, surface c was first converted into surface d by adding NaN3, and then into surface e (Ti-AMP); surface e (Ti-AMP) contained AMP but lacked pNIPAM; by atom transfer radical polymerization, pNIPAM was formed on surface c to generate surface f (Ti-pNIPAM); by click chemistry, surface f was converted first into surface g (Ti-pNIPAM-N3) by adding NaN3 and then into surface h (Ti-pNIPAM-AMP) by adding HHC36. Surface f contained AMP conjugated to pNIPAM. (F) Exposure and hiding of HHC36 (fluorescently labelled in green) at lower (left; 25 °C) and higher temperature (right; 37 °C). (G) Quantitative antibacterial activity of different surfaces after incubation against S. aureus and E. coli for 2 h at 25 °C (exposed peptide) and 37 °C (hidden peptide). (H) In vivo characterization of antimicrobial activity and biocompatibility of samples after implantation in infected rabbit tibiae for 7 days; images of the Petri dishes showing the presence of bacteria (yellow spots) on samples after retrieval (left; plain Ti and right; temperature-sensitive with HHC36). (I) Antimicrobial activity of the surfaces of different samples (left) and the tissues surrounding the corresponding samples (right) after in vivo retrieval. Reprinted with permission from ref. 72 (2019) and ref. 89 (2018) American Chemical Society.
LL-37 is another AMP that has been amply used to coat implants (including its derivatives such as OP-145, P60.4ac, SAAP-148, SAAP-145, and SAAP-276).76–78 LL-37 is naturally generated through the degradation of the larger human cationic antimicrobial protein (hCAP18).79 Early work using LL-37 demonstrated contact killing of Escherichia coli.69 Exemplary work showed that LL-37 and closely related derivatives could be immobilized on substrates and retain antimicrobial activity against clinical and multidrug-resistant Staphylococcus in vivo.80 Others have also shown similar in vivo activity in rabbit intramedullary nail infection and mouse subcutaneous implant-associated infection models.81
A final family of AMPs including Tet-213 (also known as HHC3682), Tet-26, Tet-21, and Tet-2083,84 has been applied toward anti-biofilm implant coatings as well. For example, Tet-213, which was generated computationally, has been incorporated into layer-by-layer assembled structures (LBL; reviewed elsewhere85) to reduce biofilm formation of Streptococcus aureus and P. gingivalis.86 Earlier work proved the possibility of coating Tet213 on implants with retained antimicrobial activity.87,88 Fig. 2E–H shows an example of immobilized HHC36 produced with in vivo antimicrobial activity,89 which relatively few studies comprehensively evaluate for antimicrobial surfaces.90 Indeed, evaluation of antimicrobial surfaces in vivo remains an area of active debate. This particular system also features a temperature-sensitive display of AMP (hidden at 37 1C and exposed at 25 1C) to reduce potential cytotoxicity. Melamine (produced by combining portions of the antimicrobial cationic peptides mellitin and protamine91) is another surface-immobilized AMP for dental implants that has been tested in vivo and shown effective against P. aeruginosa and S. aureus.92
Multifunctional, AMP-based biomaterials have been synthesized as well. Examples include recombinant spider silk proteins (silk generally consists of β-sheet protein structures93) fused with a cell-binding domain derived from fibronectin (fibronectin structure detailed later) and anti-biofilm dispersin,94 bone-regenerating and antimicrobial surfaces,95–97 and AMP delivery from mineral coated nanotubes for antibiofilm dental implants.98,99
2.1.1.2. Antimicrobial elastin-like recombinamers.
Recombinant materials, in general, are an attractive biomolecule synthesis route because of the control in the specific molecular sequence, monodispersity, and ability to scale to large quantities.100 One example is elastin-like recombinamers (ELRs), which are defined recombinant protein-based polymers (rPBPs) derived from amino acid sequences found in the hydrophobic domains of tropoelastin, the precursor to elastin which is the structural biomolecule responsible for tissue elasticity.101 These hydrophobic amino acid domains from tropoelastin are most frequently repeats of the pentamer (VPGXG)n, where X is any amino acid except proline.102 With greater size than AMPs comes greater functional possibilities and structures. Indeed, ELRs are commonly expressed in heterologous hosts, mainly E. coli, due to their large molecular weight.103 Other domains, such as antimicrobial domains, can be added to ELRs and still retain their fundamental properties, such as reversible temperature-dependent phase-transitional behavior, biocompatibility104,105 and amenability to a variety of methods for surface functionalization, such as LBL deposition.106
Foundational work showed that the antimicrobial peptide ABP-CM4 from the Chinese silkworm could be added to an ELR sequence and show antimicrobial activity.107 Other work with similar molecules has combined both an antimicrobial peptide and RGD for further multifunctionality.108 Recent work109 synthesized an ELR with a typical polycationic backbone, a cysteine-based C-terminal grafting domain for covalent immobilization onto surfaces, and the AMP GL13K on the N-terminus. These ELR-coated surfaces showed anti-biofilm activity against S. epidermidis and S. aureus (Fig. 3). In a similar fashion, other work from the same group developed antimicrobial ELRs and showed their activity against microcosm biofilms from stocks of oral plaque samples in a drop flow bioreactor to simulate relevant conditions for biofilm formation like that found in the oral cavity.110
Fig. 3.
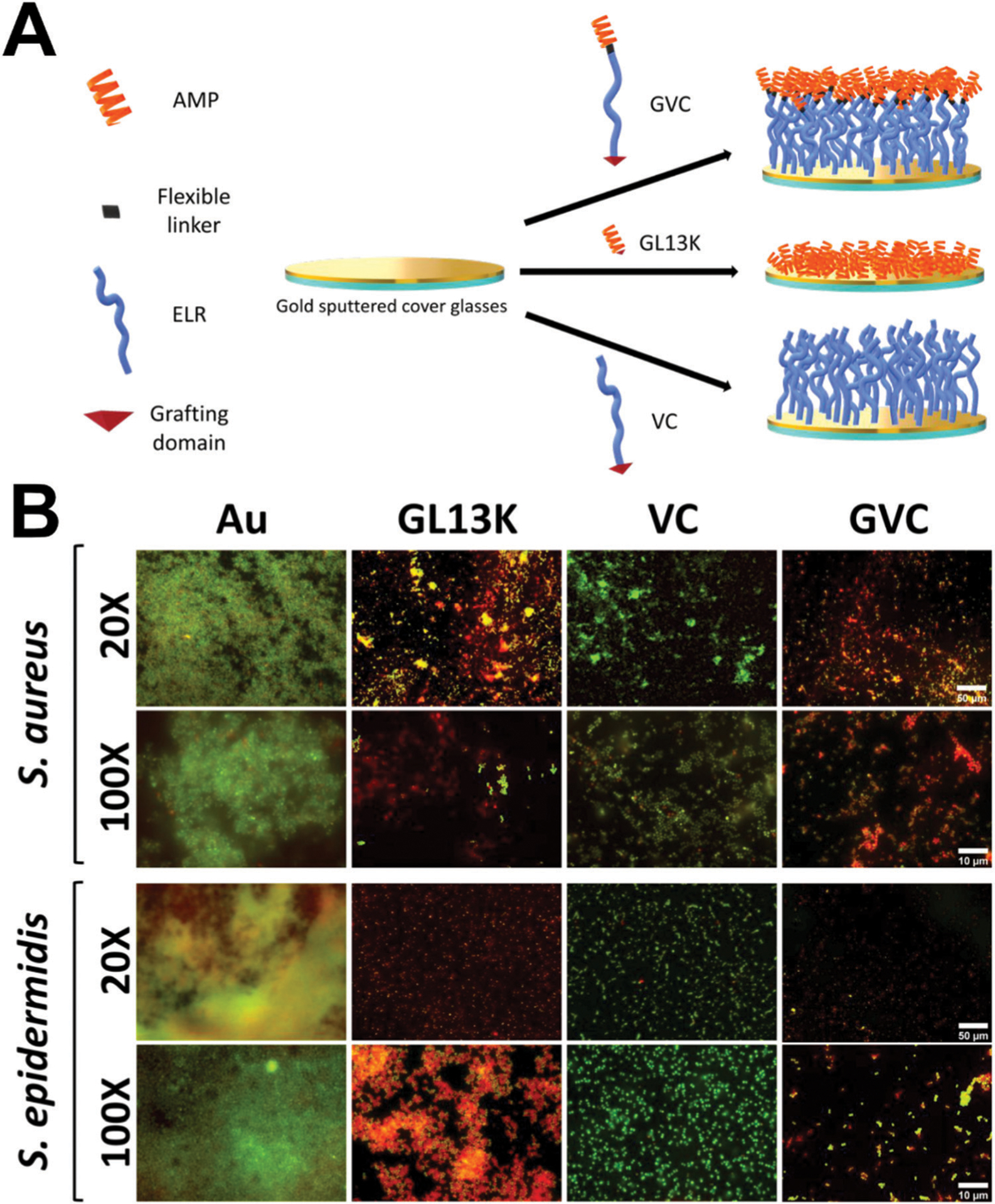
Elastin-like recombinamer coatings on dental implants for anti-biofilm potency. (A) Schematic representation of the modular composition of the antimicrobial-ELR (AM-ELR) and production of self-assembled monolayers (SAMs) on gold surfaces. (B) Live/dead staining biofilms (where green is alive and red is dead) for both (S. aureus and S. epidermidis) after 24 h of culture on negative control gold (Au) surfaces, positive control GL13K peptide surfaces (GL13K), the ELR without an AMP incorporated (VC) and then the antimicrobial AM-ELR (GVC). Reprinted with permission from ref. 109 (2019) American Chemical Society.
2.1.2. Antimicrobial biomolecules for dental restorative materials.
Dental restorations, or more colloquially “tooth fillings,” are used for the restoration of carious lesions. Caries occur in almost all adults and the majority of school children.111,112 Resin composite restoration have particularly short lifespans (around 5 years).113,114 Constant restoration replacement results in loss of irreplaceable tooth tissue with time.115 In fact, replacement of failed restorations constitutes about 50% of all operative dentistry work performed by dentists.116 Restoration failures relate to hydrophilic methacrylate-based adhesive resins infiltrating demineralized, water-rich dentin and acting as semi-permeable membranes.117 This enables penetration of gingival crevicular fluid and saliva, enzymes, bacteria, and bacterial acidic byproducts into the space between dentin and the restorative material to cause degradation and ultimately recurrent decay and premature failure.118 One preventative approach in the literature has been the modification of dental restorations using biomolecules to enhance their longevity, for instance, using antimicrobial biomolecules such as AMPs. Section 6 presents alternative approaches for expanding the lifespan of dental restorative materials based on direct modification of teeth tissues using biomolecules with different functionalities.
2.1.2.1. Dental restorative material modification with AMPs.
One AMP used to biofunctionalize dental restoration materials with antimicrobial has been nisin. Nisin is a cationic peptide from a group of AMPs named lantibiotics.119 The first variation of nisin was composed of 34 amino acids and derived from Lactococcus lactis bacteria.120 Nisin has since been applied to many industries and produced at industrial scales121 given its low cytotoxicity.122 For example, nisin has been incorporated into a dental adhesive for antimicrobial activity against S. mutans while not reducing mechanical bonding or photo-polymerization of the adhesive.123 Additional work showed this material was also antimicrobial against a saliva-derived microcosm.124 An alternative approach is the conjugation of AMPs with methacrylates to render them photopolymerizable for incorporation into dental resins. This approach125,126 has been performed with GH12 (designed de novo127) and shown to imbue the resins with antimicrobial activity while not affecting bulk mechanical properties. Another group has also incorporated an AMP derived from b defensin-3, a commonly used AMP detailed later, into an adhesive and showed disruption of S. mutans biofilms.128
2.1.3. Antimicrobial endodontic materials and treatments.
Antimicrobial agents are critical for successful endodontic treatments to combat infection in the intracanal root system and the surrounding periapical area.129 Unfortunately, around 25 million endodontic procedures are performed each year in the United States.130 The disinfection process for contaminated teeth consists of removing debris and infected pulp via mechanical instrumentation of the main root canal followed by application of irrigant and placement of an intracanal medication.131 Despite this procedure’s success (around a 90% success rate132), the root canal system is architecturally complex and secondary canals may remain untreated. Therefore, the absolute, complete elimination of microorganisms and biofilms that invade pulp is critical.133
Conventional antimicrobial agents include calcium hydro-xide, phenolic and non-phenolic compounds, biocides, iodine, antibiotics, and natural products.40,134–136 Overall, calcium hydroxide has been the intracanal dressing most used,137,138 however, calcium hydroxide may not be effective against all types of bacteria, since some studies have demonstrated that microorganisms like Enterococcus faecalis, Actinomyces radicidentis, and Candida albicans may become tolerant to increased pH produced by calcium hydroxide and result in treatment failure.139–141 Two other antimicrobials, chlorhexidine (noted for its sustained activity)142 and sodium hypochlorite dramatically reduce tooth mechanical properties. Another option is triple antibiotic paste (TAP; metronidazole, minocycline, and ciprofloxacin), but TAP is highly toxic and discolors tooth tissue.143–145 Despite these short comings, many of these existing antimicrobials have been combined with biomolecules in order to enhance the overall biological function. These hybrid materials demonstrate the benefits of combining biomolecules with conventional antimicrobial agents.
Chlorhexidine, perhaps the most ubiquitous endodontic antimicrobial, provides good examples of these hybrid materials. Chlorohexidine has been incorporated into nanotubes and used to synthesize a chlorohexidine-loaded gelatin methacryloyl (GelMA) hydrogel which shows adequate mechanical properties with sustained chlorohexidine release and in vivo cytocompatibility (Fig. 4).146 GelMA is gelatin (degraded collagen) that has been derivatized with photocrosslinkable methacrylates to combine the inherent biological activity of gelatin with the tunable physical properties of a photocrosslinking system.147 Others have loaded chlorhexidine into cellulose (a polysaccharide derived from plant cell walls148) and shown antimicrobial activity against Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, P. gingivalis, and Prevotella melaninogenica.149 However, despite the ubiquitous nature of chlorohexidine in endodontics, antimicrobial biomolecules – AMPs in particular – have been explored as alternatives to address concerns regarding antimicrobial resistance and potential cytotoxicity associated with high doses of antibiotics.150
Fig. 4.
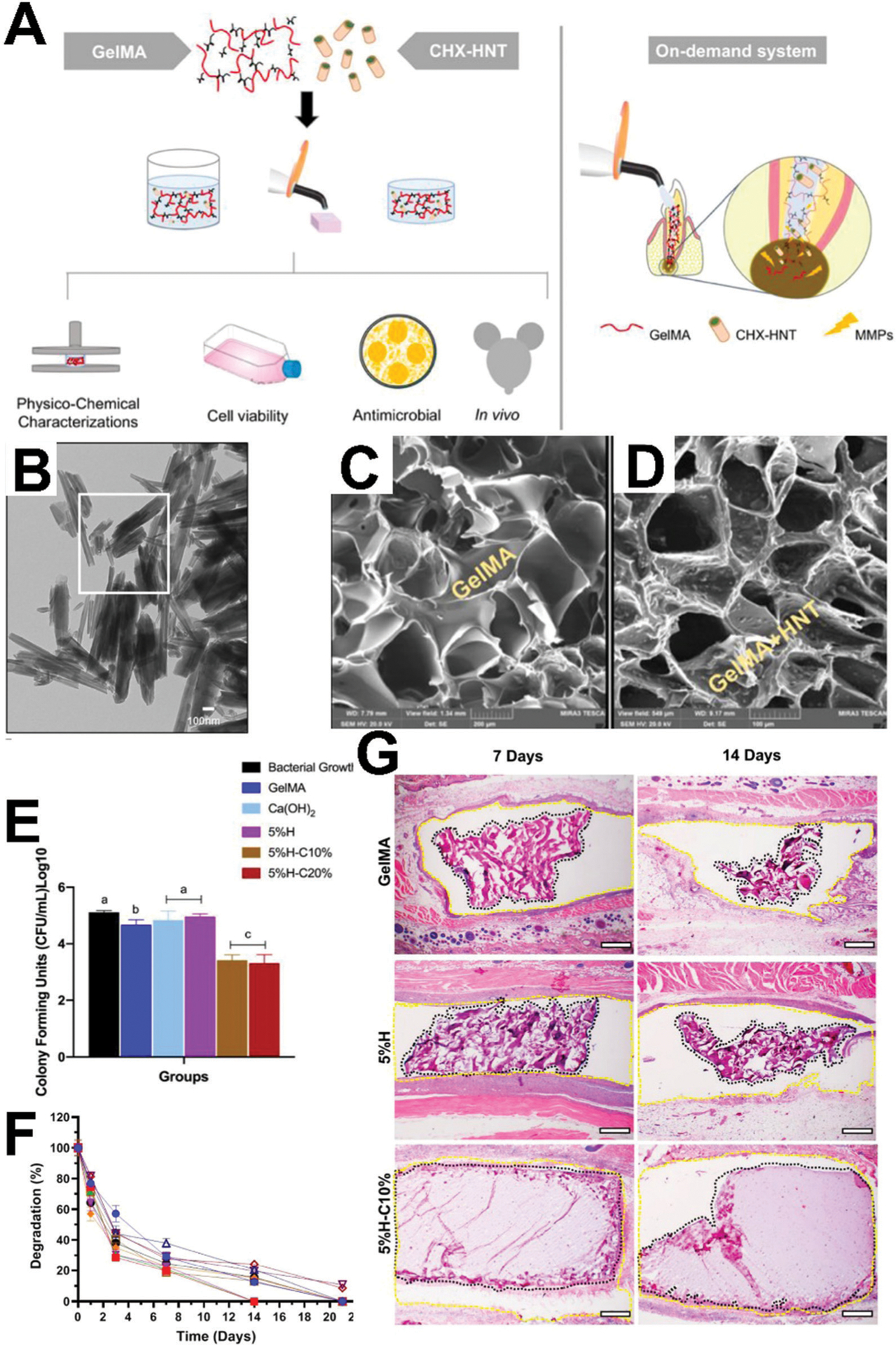
Hybrid antimicrobial biomaterial for endodontics. (A) Schematic representation of study design using a photocrosslinkable gelatin methacryloyl (GelMA) hydrogel with halloysite aluminosilicate nanotube (HNT) for release of chlorohexidine (CHX) for on-demand delivery for endodontic infection ablation. (B) Transmission electron micrographs (TEM) of HNTs. (C) Morphology (scanning electron micrographs) of GelMA hydrogel cross-section. (D) SEM cross-section of GelMA modified with CHX-loaded nanotubes. (E) Antimicrobial activity of GelMA with CHX loaded HNTs against a patient-derived oral microcosm. (F) Degradation profile of hydrogels in collagenase type I solution. (G) Histological analysis of the biopsy of the capsule surrounding indicated samples after 7 and 14 days. Reprinted with permission from ref. 146 (2020) American Chemical Society.
2.1.3.1. Antimicrobial peptides for endodontic therapy.
Conventional antimicrobials for endodontic therapies display a range of potential drawbacks, as noted. The most common repertoire of biomolecules tapped for alternative endodontic therapies is AMPs such as nisin. Nisin is more effective against Gram-positive bacteria (S. gordonii and E. faecalis, for example)151 and has been combined with low concentrations of sodium hypochlorite to reduce E. faecalis biofilm volume and thickness.152 Importantly, E. faecalis does not seem to develop resistance toward nisin.153 Another AMP with long-confirmed antimicrobial activity against oral pathogens is human b defensin-3 (HBD-3;154–156 antimicrobial activity includes S. aureus, E. coli, Fusobacterium nucleatum, Prevotella melaninogenica, Peptostreptococcus anaerobius, S. mutans, Actinomyces naeslundii, E. faecalis, and C. albicans species, for example).157 A smaller variant of HBD-3 (15 amino acids compared to; HBD3-C15158,159) is able to reduce fungal growth in an ex vivo model of C. albicans-infected root dentin with similar effects as chlorhexidine.160
Other suggested AMPs for endodontics include human neutrophil peptides 1 and 2, indolicidin, histatins 5 and 8, magainin II, cecropins B and P1, and mastoparan.150 It should be noted that not all AMPs are broad spectrum; indolicidin, magainin amide, and mastoporan are effective against Streptococcus milleri (>90% killing), whereas other listed AMPs displayed reduced antimicrobial activity (<30% killing).161 Stereochemistry of AMPs also plays an important role as past work has shown differences between l-enantiomeric and d-enantiomeric versions of DJK-5,162 DJK-2,162 and 1018163 against a root canal wall biofilm.164 d-Enantiomers versions of AMPs are usually more potent against bacteria and biofilms than their l-enantiomers counterparts, which may be associated with the higher resistance of d-enantiomers to enzymatic degradation.165–167 Finally, a continuing observation is that AMP activity is increased if the application site (usually dentin) is pre-treated with chelating agents.168 Other considerations for AMPs’ usage in endodontic therapy are reviewed elsewhere.44
2.1.4. Plant-derived antimicrobial biomolecules for periodontics.
The main entrance of pathogens into the periodontal tissue is the gingival sulcus, i.e., the area of space between a tooth and the surrounding gingiva. Untreated microbial invasion can lead to inflammation (gingivitis) and destruction of anchoring bone tissue.169 Unfortunately, around 64.7 million American have periodontitis (American Academy of Period-ontology). Nonsurgical therapies for periodontitis combine mechanical scaling and administration of antimicrobials.170 Similar antimicrobials to endodontics have historically been used. Plant extracts are an exciting source of antimicrobial biomolecules for periodontics because they are rich in secondary metabolites (such as tannins and terpenoids) that have antimicrobial activity and have been used for millennia for wound treatment.171–173 For example, plant extracts from Vitis vinifera, Pinus spp., Coffea canephora, Camellia sinensis, Vaccinium macrocarpon, Galla chinensis, Caesalpinia ferrea Martius, Psidium cattleianum have been all demonstrated enhanced anti-biofilm activity against several relevant microorganisms.174 Others have shown that extracts from Azadiracta indica are as antimicrobial as sodium hypochlorite.175
3. Soft tissue healing and attachment
3.1. Biomolecules for dental implant soft tissue integration
The oral mucosa provides protection to periodontal tissues against bacteria and other harsh stimuli in the oral cavity but is disrupted during implant placement.176 Resulting soft tissue healing and regeneration adjacent to dental implants is para-functional. The implant has a longer biologic width than natural teeth and the implant-associated mucosa is generally fragile.177 These differences in soft tissue structure and function between implants and teeth strongly contribute to peri-implantitis and implant failure.178
The effect of implant surface characteristics (such as topography or chemical composition) on bone progenitor cells and osseointegration is well understood.179–182 Several well-studied surface modification methods, such as sandblasted and acid-etched (SLA)183,184 or apatite coatings,185 offer a bounty of information on this topic.186 However, the same cannot be said for soft tissue as far fewer studies exist trying to understand surface characteristic on implant soft tissue response.187 Biomolecules offer a direct, tailored solution to enhance soft tissue healing around implants to prevent their infection and failure.
3.1.1. Peptides for enhancing dental implant soft tissue integration
3.1.1.1. RGD.
RGD188,189 is the principal integrin-binding domain present within ECM (extracellular matrix) proteins such as fibronectin, vitronectin, fibrinogen, and osteopontin. RGD surface immobilization is now a classic technique190 for the functionalization of biomaterials surface given its small size and recognition by a variety of cell types. A number of integrins show some binding affinity to RGD, such as α3β1, α5β1, α8β1, αIIbβ3, αvβ1, αvβ3, αvβ5, αvβ6, αv8β, and to some extent α2β1 and α4β1.191 The use of RGD, as compared with native ECM proteins, minimizes the risk of immune reactivity or pathogen transfer and RGD’s small size allows for a range of tunable immobilization to ocurr.192 A large body of literature exists for RGD functionalized dental implants for osseointegration but only a handful of studies exist for soft tissue.193 This may be related to the perception of RGD as “dated” and “old-fashioned” even though its simplicity makes it attractive from a manufacturing point of view.194
As a means to improve implant soft tissue healing, RGD has been conjugated to poly(l-lysine)-graft-poly(ethylene glycol) on titanium and shown to be effective in promoting epithelial and fibroblast growth.195 Others have developed multilayered coating with type I collagen and RGD-conjugated hyaluronic acid (HA, a nonsulfated glycosaminoglycan196). These coatings promoted gingival fibroblast proliferation and adhesion-related gene expression.197 Silk has been derivatized with titanium binding peptides and an RGD domain to coat titanium.198 These coatings improved fibroblast adhesion, proliferation, and strengthened mechanical cell adhesion. Some work focusing on zirconia implants immobilized RGD on typical yttria-stabilized tetragonal zirconia and a biocermet.199 Other work has immobilized linear and cyclized RGD on zirconia and showed enhanced spreading, proliferation, and focal adhesion formation from gingival fibroblasts.200 The growing demand of zirconia implants, and the prevalence of restorative abutment made of this tough and aesthetic ceramic, motivates future development of biomolecule coatings for them.201–204
3.1.1.2. Laminin-derived peptides.
Oral keratinocytes directly apposing teeth (“directly attached cells”) form a basement membrane (BM) compositionally unique to any other BM in the human body: rich in laminin332 (a heterotrimeric glycoprotein205) serving as an integrin ligand to form hemidesmosomes (HDs)206 and missing common BM proteins like collagen IV and perlecans (proteoglycans that crosslink many ECM components).207 HDs serve as the transmembrane connection between teeth and gingiva as the JE forms a protective barrier for mechanical stability of the tooth, or dental implant, and a physical barrier against biofilm colonization.176 However, HD formation on dental implants only occurs apically leaving the implant coronal surface vunerable.176 Given the difficulties in working with laminins (recombinant laminins lack post-translational modification and historical difficulties in isolation and purification from tissue culture),208 one particular peptide has been derived from the α3 globular domain 3.209 This peptide has been silanized to titanium and used to induce keratinocyte HD formation toward enhancing implant soft tissue healing.210,211 This same peptide has also been conjugated to multilayered polyelectrolyte films of poly(l-lysine)/poly(l-glutamic) acid films on titanium and shown to upregulate HD in vitro but have limited in vivo effects.212 Another laminin-derived (laminin211 derived; DLTIDDSYWYRI) peptide has been applied toward dental implant coatings as well.213–215
A number of other peptide sequences have been isolated from laminins. Laminins are critical in basement membrane assembly and the resulting supramolecular architecture. Thus, laminins are a rich repository of potential cell-signaling motifs for utilization on dental implants.216 IKVAV, from within the laminin α1 chain and traditionally associated with neurons,217 has been physisorbed to titanium and shown to increase fibroblast attachment and improve tissue integration in a subcutaneous rat model.218 YIGSR, derived from the β1 chain,219 SINNNR, derived from an α chain,220 and LRE, from laminin β2 chain,221 are all well studied laminin-derived peptides that may be advantageous for soft tissue integration with dental implants. Indeed, a systematic review convincingly supports the efficacy of laminin-derived coatings for osseointegration and new bone formation around implants.222 However, implementation for soft tissue remains unresolved. Assembled laminin-based hydrogels have become popular (such as for neural regeneration) in the literature:223 advances in understanding of laminin from such 3D systems may be useful in designing implant surfaces.
3.1.2. Whole proteins.
The most commonly used biomolecule for soft tissue attachment in the dental implant literature is collagen. Collagen, a protein consisting of a prototypical sequence of repeated G–X–Y sequences hierarchically arranged to form fibers, has numerous structural – particularly in the context of dentistry in dentition and bone – and signaling functions.224,225 The variety of collagens – there are 28 types of collagen that assemble into a variety of supramolecular structures including fibrils, network-like structures, and microfibrils – is perhaps underappreciated in the biomaterials literature, where the clear majority of work is focused on type I collagen {[α1(I)]2α2(I)}.226 A common motivation for immobilizing collagen onto implants is its native RGD and synergy domains. For example, type I collagen has been immobilized via silanization on titanium and shown to increase periodontal fibroblast proliferation.227
A typical drawback associated with immobilization of entire biomolecules on surfaces is the lack of chemoselectivity and therefore control of the active conformation, i.e. biological activity.228,229 This has been thoroughly demonstrated with type I collagen. For example, fibroblasts respond differently to collagen-laden surfaces that are manufactured with plasma-activation compared to acid etched titanium for later physisorption of collagen.230 Other work has shown differences in fibroblast behavior on type I collagen-laden titanium immobilized with silanization using either 3-chloropropyl-triethoxysilane (CPTES) or 3-glycidyloxypropyl-triethoxy-silane (GPTES) surface linkers.231
Other approaches232 avoid multi-step silanization and simply use polydopamine to immobilize type I collagen (Fig. 5) and reduce fibrous encapsulation. Polydopamine, a catecholamine, is noted to form polymeric coatings on virtually all tested substrates under mildly alkaline conditions.233 Polydopamine is reactive toward nucleophiles such as thiol, amino, and imidazole groups under mild basic conditions and derived from sea mussels.234 Indeed, in this example of type I collagen immobilization (Fig. 5), the poly-dopamine coating process yielded titanium surfaces that increased fibroblast and keratinocyte proliferation, size, focal adhesion formation, and reduced fibrous encapsulation in a subcutaneous rat model. Regardless of the immobilization method (such as simple polydopamine or multi-step silanization), there are evident benefits of presenting an entire biomolecule, with its precisely placed and plentiful binding domains perfected by evolution. However, the use of whole proteins may require strict sourcing of proteins from animal sources, protein recombination, or immunological challenges compared to other approaches.8
Fig. 5.
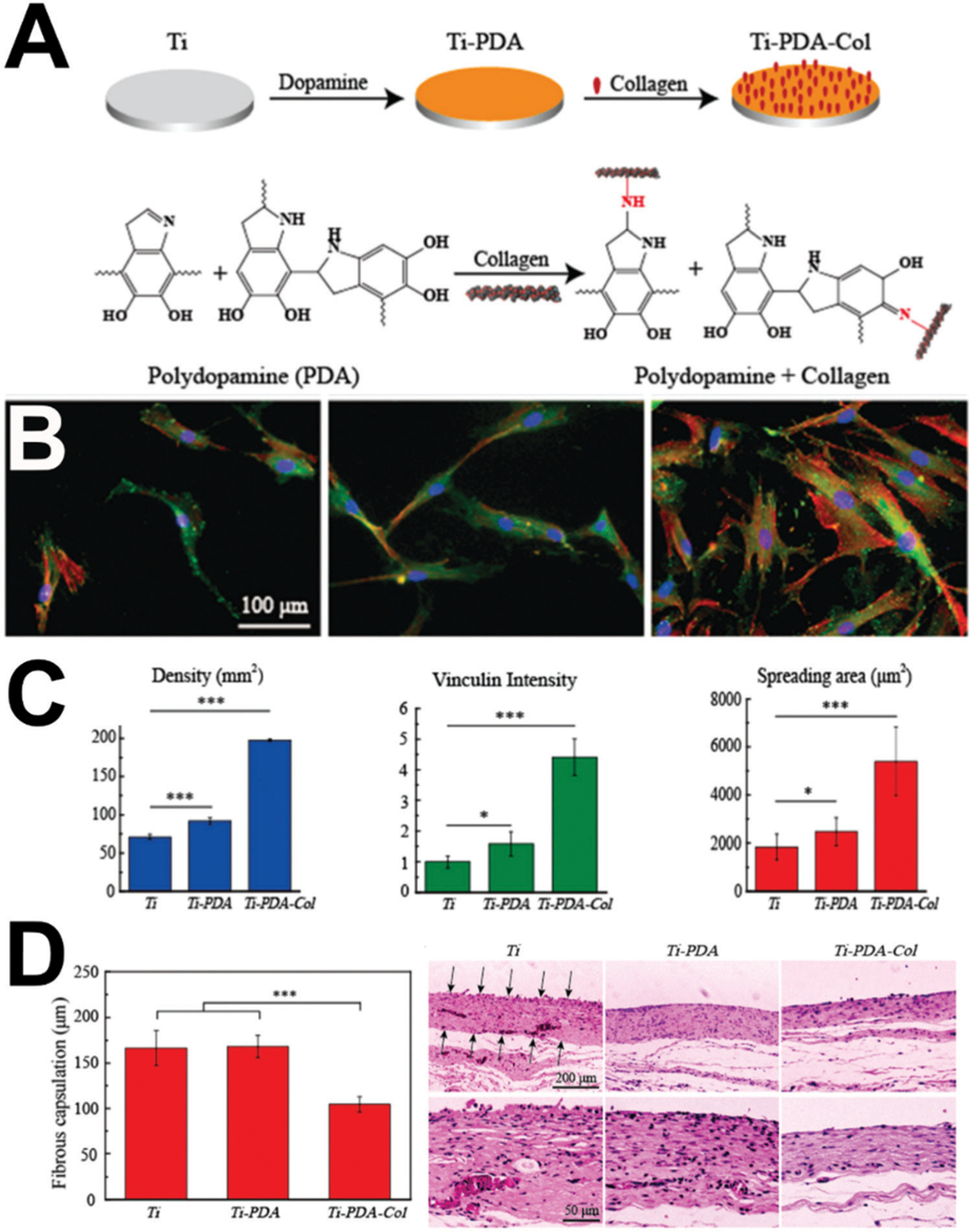
Polydopamine and whole proteins for improving soft tissue healing around dental implants. (A) Schematic of surface modification of Ti–6Al–4V (Ti). Polished titanium was first coated by a poly-dopamine (PDA) film by self-polymerization of dopamine; then, type I collagen was bonded with the PDA film via a Michael addition or Schiff base reaction. The possible structure of PDA and mechanism of the reaction between PDA and collagen is shown. (B) Adhesion of fibroblasts on (from left to right) Ti, Ti-PDA, and Ti-PDA-Col after 1 day of culture; fluorescent micrographs stained with vinculin in green, actin in red, and nuclei in blue. (C) Fibroblast surface density, vinculin intensity, and cell spreading area after 1 day of culture. (D) Histological (H&E) analysis of the biopsy of the capsule surrounding indicated samples after 30 days of implantation in rats and quantification. Reprinted with permission from ref. 231 (2019) The Royal Society of Chemistry.
3.1.2.1. Fibronectin.
Another commonly used biomolecule in biomaterials, fibronectin, has been applied to dental implants for soft tissue healing. Fibronectin is a high molecular weight dimeric glycoprotein that is organized into a fibrillar network on the cell through interactions with surface receptors, and it regulates many cell functions, such as cell adhesion, migration, growth, and differentiation.235 Fibronectin has been physisorbed to titanium implants and resulted in an increase in proliferation of epithelial and fibroblast cells.236 Fibronectin has been silanized to titanium and shown to increase fibroblast proliferation, spreading, focal adhesion formation, and soft tissue attachment in a subcutaneous sheep model.237 Fibronectin has also been used to coat hydroxyapatite-coated porous titanium and increase cell infiltration into the pores.238 Like collagen, recent work has also suggested the sensitivity the conformation of fibronectin to physiochemical properties that causes downstream signaling effects.239–243 Like with many whole biomolecules, the individual contribution of each motif from the entire biomolecule can be recapitulated using multiple individual motifs.244
3.1.2.2. Histatin-1.
Saliva presents a wealth of biomolecules that offer potential for dental implant therapies. One such molecules is histatin-1, which is a multifunctional histidine-rich peptide (57 amino acids) secreted by salivary glands, a critical molecule for oral mucosal wounds to heal faster and more efficiently than analogous skin wounds.245 Histatin-1 has been physisorbed to titanium and shown to enhance the attachment and spreading of oral epithelial cells and fibroblasts, and when presented in solution, shown to increase barrier integrity and reduce translocation of bacteria across cell monolayers.246–248 Other useful molecules may exist for increasing the success of dental implants given saliva’s wealth of biomolecules, but they remain unexplored.
3.1.2.3. Growth factors.
Growth factors are biological mediators that regulate important cellular events involved in tissue repair and wound healing.249 These biomolecules are attractive targets to stimulate soft tissue integration with implants given their role in wound healing. Some examples of this include platelet-derived growth factor (PDGF; induces epithelial proliferation250) and enamel matrix derivative (EMD; mostly composed of amelogenins251) physisorbed to implants and placed subcutaneously in rats.252 PDGF increased soft tissue penetration into the implants grooves while simultaneously reducing fibrous connective tissue thickness. Other work253 has soaked apatite-coated titanium in fibroblast growth factor 2 (FGF2; typically associated with angiogenesis253) and placed the implants in rabbit tibias. This FGF2 adsorption enhanced wound healing, reduced inflammation, and induced Sharpey’s fiber-like tissue formation.254
3.1.3. DNA.
DNA (deoxyribonucleic acid) offers a number of intriguing benefits as a biomolecule to improve dental implant soft tissue integration. DNA is highly charged which allows for sequestering of biomolecules non-covalently (such as a LBL approach).255,256 Low immunogenicity and tunable immuno-modulation are other benefits of using DNA for bioactivation of dental implant surfaces.257 Early work for enhanced soft tissue attachment used poly-d-lysine and poly(allylamine) hydro-chloride with DNA for LBL coatings on titanium.258 These surfaces promoted fibroblast proliferation but showed no effects in a subcutaneous rat model. An alternative approach is the delivery of laminin332 γ2 DNA for uptake and processing by keratinocytes to promote laminin332 production; this approach has been demonstrated effective in vitro.259 Other work has shown similar results using laminin332 α3 DNA on chitosan/collagen coated titanium with nanotube topography in vivo.260 Chitosan, as detailed later, is a natural polymer derived from the shells of shrimp and other crustaceans.261 Polyethylenimine plasmid DNA nanoplexes encoding for platelet derived growth factor-BB (PDGF-BB) have also been coated on titanium for enhanced soft tissue integration.262
3.1.4. Other attractive biomolecules.
Intrinsic to the ability for keratinocytes to form a barrier against bacteria on implant surfaces is cell–cell attachment.263 For example, in adherens junctions, the transmembrane protein E-cadherin associates with vinculin, which in turn binds catenins to link the complex to the cytoskeleton.264 Inspired by this, the extracellular domain from E-cadherin has been used physisorbed to titanium and shown to increases metabolic activity, cell area, and attachment of keratinocytes.265 A protease-activated receptor 4 (PAR4) – activating peptide conjugated to titanium, in combination with platelet rich plasma, has been shown to induce proliferation and collagen IV secretion, a key molecule for basement membranes, in keratinocytes.266 Other peptides,267 such as one derived from ameloblastin – a protein found in enamel and secreted by ameloblasts268 – has also been used to upregulate HDs when silanized to titanium simultaneously with a peptide from laminin α3 globular domain 3 (Fig. 6).210
Fig. 6.
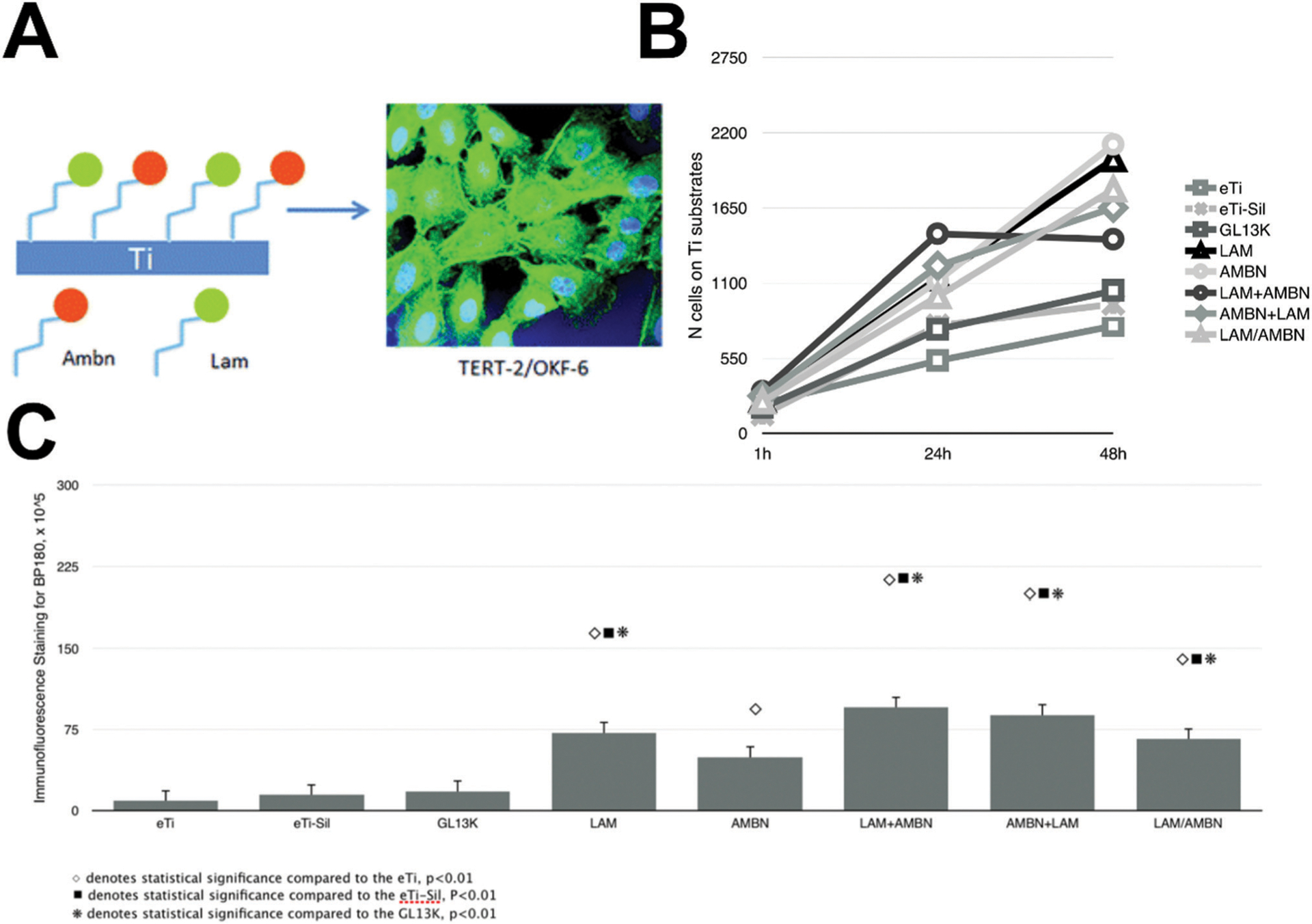
Peptides for enhancing dental implant soft tissue healing. (A) Schematic of surface modification co-immobilizing a peptide derived from ameloblastin (denoted as ABMN) and the laminin α3 globular domain 3 (denoted as LAM) to upregulate hemidesmosome formation on titanium for percutaneous devices such as dental implants. (B) Proliferation of keratinocytes through 48 hours (2 days) of culture on mono- and co-immobilized surfaces. (C) Hemidesmosome formation (immunofluorescence of collagen XVII) after 1 day of culture. Reprinted with permission from ref. 210 (2018) The Royal Society of Chemistry.
Given its role in nature, intact laminin332 is perhaps the most intuitive biomolecule to use to enhance soft tissue attachment to dental implants. Indeed, laminin332 has been used to upregulate keratinocyte HD formation after physical adsorption to titanium;269 passivation prior to adsorption seems to significantly increase the HD formation compared to nonpassivated titanium.270 Alternatively, controlled adsorption of biomolecules, such as laminin332, on tooth surfaces may be an another way to improve soft tissue interactions.271
Phenolic compounds, while typically used for immobilizing or crosslinking molecules, have been used for direct cellular effects. The most common, polydopamine, has been used to coat titanium and increase fibroblast proliferation and collagen and fibronectin synthesis.272 While simple approaches like this are attractive, some work has shown off-target effects from polydopamine on bone.273 Other phenolic compounds such as a quercitrin have been silanized to titanium and increased proliferation and ECM production by gingival fibroblasts.274 Titanium coated with polydopamine and chitosan increases proliferation and type I collagen secretion from fibroblasts.275
4. Biomolecules and mineralization for dental biomaterials
Perhaps the most prominent feature of the oral cavity is teeth. The outer covering of teeth, enamel, is the most highly mineralized tissue in the human body and withstands cyclic masticatory loading up to 770 N276 around one million cycles per year.277 The fundamental unit of enamel is the enamel prism; highly packed, hard, hydroxyapatite (carbonated calcium phosphate) mineral (approximately 95 wt% of enamel), with around 1 wt% organic matrix and 4 wt% water.278–281 Underlying enamel as a tougher mechanical support is dentin; mineralized collagen (approximately 45 vol% apatite crystals, 30 vol% collagen, and 25 vol% water).282 The basic ultrastructure of dentin – mineralized collagen – is structurally similar to bone.283 The triple-helical collagen molecules (right-handed) are packed in a quasi-hexagonal structure to form nanometer sized microfibrils which further assemble into fibrils.284 Collagen molecules align in a staggered, parallel array; this arrangement forms a characteristic 67 nm D-periodic banding pattern (“D-banding”) with an overlap zone of 32 nm and a gap zone of 35 nm.285,286 Hydroxyapatite crystals in dentin and bone are nanometric287 with their c axis preferentially aligned with the long axis of the collagen fibrils, leading to an inter-penetrating organic–inorganic nanocomposite.288
A number of major diseases afflict enamel and dentin. Approximately 2.4 billion people worldwide suffer from caries.111 Dental caries is the most common chronic childhood disease in the United States, disproportionally afflicting low income children.289 As a result, many approaches have been developed in order to remineralize and restore tooth structure using biomolecules as a biomimetic guide for regeneration. Such synthetic mineralization platforms emulate specific features of natural mineralized supramolecular matrices and may spur design of materials capable of recreating the structure and function of tissues such as enamel, dentin, or bone.290
While a number of models have been developed to mechanistically describe collagen mineralization (such as that observed in dentin),291 models based on mineralization of collagen with hydroxyapatite using non-classical pathways have been dominant in recent years.292,293 In nature, the mineralization of collagen is believed to be mediated by interactions between negatively charged complexes of ACP (amorphous calcium phosphate) precursors with the collagen fibers. The ACPs precursors are formed due to interactions between ionic components in the physiological media with soluble templates; proteins that inhibit/promote mineral deposition and phase transformation precipation.292 The ACP precursors penetrate the collagen fibrillary matrix and then they transform into hydroxyapatite. Indeed, the thorough infiltration of hydroxyapatite in the collagen matrix is considered the foundation of the excellent mechanical properties of hybrid human mineralized tissues, such as dentin and bone.287,294
In nature, non-collagenous proteins (NCPs), such as osteopontin (OPN), phosphorylated dentin phosphoprotein (DPP), fetuin and dentin matrix protein (DMP1)295 regulate the mineralization process of the insoluble collagen matrix, possibly acting as soluble templates.296 NCPs are intrinsically disordered proteins (IDPs); that is, dynamic, flexible molecules without a well-defined, kinetically stable, folded structure.297 Moreover, NCPs are highly acidic proteins with a high number of aspartic and glutamic acids and/or phosphorylated residues, such as phosphoserine.296 As NCPs are highly negatively-charged IDPs, they can sequester ions in solution to form stabilized ACPs that mediate bone mineralization.296,298–300 The small integrin binding N-glycosylated proteins, known as SIBLING proteins,301 are a family of NCPs that comprises OPN, DMP1,302 cleavage products of dentin sialophosphoprotein (DSPP),296 and bone sialoprotein296 (among others303). SIBLING proteins are known to interact with hydroxyapatite through electrostatic and hydrophobic interactions and regulate the biomineralization process of bone and dentin.296
Inspired by the role of proteins in the mineralization of dental tissues, a number of biomolecule-based dental biomaterial processes have been developed to help restore mineralization to diseased tissues and idealized as restorative therapies. One synthetic mineralization method is the polymer-induced liquid precursor process (PILP), which substitutes charged naturally-derived macromolecules (such as NCPs) with other macromolecules [most classically poly-aspartic acid (pAsp); a polyanion].304,305 Densified, crosslinked collagen hybrid matrices can be manufactured with remarkably biomimetic mechanical properties (combined strength and resilience) using PILP.306 It was discovered in the original study examining the PILP system307 that pAsp-mediated mineralization could create helical morphologies of calcium carbonate with a spherulitic twisted crystal growth, stabilized by the pAsp. Later, the same authors305 showed that pAsp triggers a liquid–liquid phase separation alongside the mineral amorphous phase precursor. Similarly, such processes can be applied to other biominerals and solid fibrillary templates, such as silicification of collagen for collagen– silica composite with unique hierarchical structures308 or cellulose–hydroxyapatite nanohybrids.309,310 Moreover, alternatives to the use of pAsp as synthetic soluble template in the PILP process have been explored, most notably poly-acrylic acid (PAA),311 so that, for instance, fibrillar mineralization can be controlled by modifications of PAA molecular weight and/or concentration.298 Recently, the synthetic soluble template has been substituted by natural NCPs, such as OPN, in vitro.312–314 The PILP process has also been widely used as a biomimetic system to discern the mechanism by which collagen is intrafibrillarly mineralized in nature.298,306,309,310,315–317 However, this is a topic under debate. Notably, the versatility of the PILP process has already spurned development of biomineralization processes for restorative dentistry and treatment of hypomineralization-based diseases.315–317
A number of biomolecules, mostly derived from NCPs and other IDPs, have been used to control biomineralization processes both as a mechanism of fundamental study and for the creation of therapies for the treatment of dental-related diseases. Below, we survey at few of these biomolecules that have resulted in the restoration of function or regeneration of dental tissues.
4.1. Elastin-like recombinamers for mineralization and biomaterials
Elastin-like recombinamers (ELRs), with their positively charged (VPGXG)n domains, have been mineralized with a PILP-based approach. One factor critical to the ability of ELRs to guide mineralization is a conformational change from disordered random coils into ordered β-sheet structures upon interaction with the developing enamel crystals318 (the same is also true for IDPs).319 In fact, the β-spiral structure and an unperturbed fibrillar structure play a critical role in ELR mineralization, more than electrostatic interactions or specific bioactive sequences.320 This process is highly tunable just based on ELR structure. For example, one can vary ELR crosslinking during manufacturing solvent evaporation to control ELR disorder–order ratios to alter structural hierarchy of the resultant mineralized structures and consequently the properties (mechanical, for example) of the functional material.318 This approach has also been applied to ELRs with a statherin-derived moiety to form layered and ordered fluorapatite, perhaps useful as an enamel therapeutic.321 A similar ELR with a statherin-derived moiety promoted bone regeneration in vivo.322 The versatility of the ELR structures also enables the biomimetic mineralization of these molecules in different micro-structures, such as hydrogels,323 membranes,318 fibers,320 and implant surfaces.324
4.2. Amelogenin for mineralization and biomaterials
Amelogenin (AMELX) is an IDP shown to play an important role in biomineralization, is the most abundant protein of forming enamel, and is capable of self-assembly to form nanospheres.325 AMELX is comprised of three domains: a 45 amino acid tyrosine-rich N-terminal domain, a large, hydrophobic central domain, and an 11 amino acid hydrophilic C-terminal domain.326 Previous work327 has reported that AMLEX undergoes a structural change from disordered, random coils to ordered β-sheet upon interaction with the developing enamel crystal. The highly conserved N-terminus contains the only post-translational modification in AMELX (phosphorylation of serine-16).328 Not surprisingly, studies329,330 have shown the role of this single phosphorylation altering conformation and protein–mineral interactions to improve its capacity to stabilize ACPs.331 The critical role of pS-16 vs. S-16 has also been elegantly shown in vivo using a knock-in animal model.332 Foundational work319 observed that AMELX self-assembles into “nanospheres” in the presence of enamel. These nanospheres prevented mineral growth in the a- and b-axis and promoted crystal formation in the c-axis, as is biomimetic. The role of C-terminus has been shown to affect the pre-nucleation clusters and assembly into nanosphere.333 Another, more applied, example by others334,335 showed that the distinctive hierarchical structure of mature enamel requires distinct conformational organization of AMELX into amyloid-like nanoribbons. A modular design for amelogenin was suggested correlating the domain of the amelogenin protein with specific mutations using protein engineering and transgenic animal studies.336,337 Using a bioinformatics scoring matrix, short peptide sequences were identified from the native amelogenin protein. These amelogenin derived peptides were demonstrated to promote formation of a cementum-like hydroxyapatite mineral layer on demineralized root dentin,338 similar to recombinant AMELX promoting pulp-like regenerative and hard tissue organization in an root apex closure model.339 A similar peptide approach regulates orientation and regrowth of aprismatic enamel on dentition.340
4.3. Statherin for mineralization and biomaterials
Statherin (STATH) is a 43 residue acidic phosphopeptide highly expressed in saliva.341 The primary sequence of statherin is: D1pSpSEEKFLRRIGRFGYGYGPYQPVPEQPLYPLQPY-QPQYQQYTF; pS are phosphorylated serines. The first five amino acids in the N terminus, and more generally the 15 terminal N terminal amino acids,342 are critical for adsorption to hydroxyapatite.343,344 The four basic residues (K and R) are likewise critical for adsorption.345,346 The C-terminus is also reported to fold into an α-helix upon adsorption.347 STATH is known to generally modulate mineralization by (1) sequestering calcium ions to suppress immediate calcium phosphate crystallization on mineralized surfaces such as dentin and (2) adsorbing onto/around nucleated crystals to inhibit their further growth.348 STATH and peptides derived from it have been applied to enamel remineralization for anti-caries applications.349–353
4.4. Osteopontin and other natural biopolymers for mineralization and biomaterials
OPN is a highly acidic, disordered protein with many negatively charged amino acids, phosphorylated serine residues, a po-lyaspartic acid cluster, and an acidic serine- and aspartate-rich (ASARM) motif, all of which are known to be critical to its biomineralization properties.354–356 OPN-mediated biomineralization has been used to direct the formation of nanoscale hydroxyapatite in the interstices of collagen around encapsulated human mesenchymal stem cells in 3D and used as a model to study prostate cancer.312 Similar worked showed effects of such OPN-mineralized materials on pericyte differentiation and vascularization.313 This is based in natural processes, for example, where OPN inhibits calcium oxalate growth and kidney stone formation; this process is dependent on OPN’s carboxylate groups and phosphorylation status.357,358 Increased OPN in vivo leads to bone hypomineralization,359 related to upstream pyrophosphate activity and osteoclastogenesis regulation.360,361 This serves as a reminder that while many of these biomolecules regulate mineralization from a structural perspective [biomolecule/crystal (or pre-cursor interactions)], biomolecules regulate mineralization together with other hormones, transcription factors, regulatory proteins, and enzymes through traditional cellular signal transduction and biochemistry.362
Other concepts from these biomineralization systems (and others reviewed in detail elsewhere290) have driven development of other advances in biomolecule-based dental biomaterials. For example, chitosan-based extrafibrillar dentin demineralization has been introduced as a bonding strategy to reduce endogenous collagen degradation, prevent water permeation into the hybrid layer, enhance antimicrobial activity, and promote longer bond stability.363,364 Other possibilities include adapting these collagen biomineralization strategies for more effective remineralization in general, such as caries-preventation.365–367 Bone-mimetic materials may also be valuable for studying cancer and bone metastases368 or pre-dentin formation.369
5. Chimeric peptides as biomolecules for dental biomaterials
An alternative and attractive approach for generating biomolecules is combining different features of multiple biomolecules into one multifunctional or multi-domain molecules. A chimeric molecule refers to an engineered construct where different functional domains in a biomolecule can be linked to form a novel biological agent.370–372 This method has historically be applied to drug delivery where one domain is designed to target the cell specific molecule and the other one carries a drug molecule.373,374 Depending on the nature of the molecules, several linker features have been applied including hydrazine, disulfide moieties, as well as click chemistries where regio-selective moieties cam be integrated in to the design.375 The concept is similar to fusion proteins where the two domains encoded by different genes can be joined to a transcript and translated as a single polypeptide.370 Extended examples includes fusion proteins having fusion partners facilitating purification of cloned genes, reporting expression levels and visualization of the proteins in a biological environment. Although this approach been commonly applied to drug delivery, it can facilitate biological activity on an implant, solid material, or tissue interface via increased activity and stability of the bioactivity by controlling molecular orientation and facilitating biomolecular interactions. In the last decades, short peptide sequences selected from combinatorial libraries, including phage and cell surface technologies, have emerged as attractive tools to bind to solid materials with high affinity.376–379 An important aspect of chimeric peptides is their properties can be improved using computational modeling and predictive tools.380–382 Peptides are particularly attractive for this purpose because of their ease of manufacture.383 A relatively common way to generate such biomolecules is to pair a bioactive domain (such as growth factor, signaling molecule, etc.) with a domain with affinity for a substrate. While we have mentioned a few chimeric peptide examples previously, we spotlight here this class of biomolecule owing to their tunability and multifunctionality.
One exciting set of chimeric peptides is those with affinity to dental hard tissue such as hydroxyapatite. Hydroxyapatite binding peptides (HABPs) selected by phage display, for example, have been conjugated to the N-terminus of a green fluorescence protein variant (GFPuv) to produce GFPuv–HABP used to induce mineralization at the adhesive/dentin interface.384 Prior work with these HABPs showed that these molecules induced calcium phosphate mineralization by exhibiting control over the mineralization kinetics and particle morphology on hydroxyapatite under specific conditions.385 In another study, another novel apatite binding peptide identified using phage display386 was shown to increase adhesion strength and adhesion specificity of various cell types, as well as control differentiation, to enhance bone regeneration in a mouse model.387–389 Others have used chimeric peptides composed of cell binding sequence combined with apatite affinity sequence to inhibit osteoblast mineralization.390
A relevant type of chimeric peptides for dental applications includes those with affinity for titanium implant materials (titanium binding peptides; TiBPs) to provide titanium with bioactivity or antimicrobial potency, such as the previously shown in Fig. 2. For example, previous examples of TiBPs have demonstrated antimicrobial activity of chimeric TiBPs-AMPs against S. mutans, S. epidermidis, and E. coli391,392 and enhanced osteoblast activity.393 Other TiBP-AMP examples showed antimicrobial potency against S. gordonii, Streptococcus oralis, and S. sanguinis.394,395 The use of chimeric peptides is also an exciting avenue of investigation for drug release systems due to their labile, non-covalent interactions with materials.396–398 Similar chimeric peptides have also been developed for polymers.399,400 Another class of chimeric peptides has been developed to bind to titanium and promote soft tissue healing around dental implants.401 In short, chimeric peptides offer an interesting avenue for multifunctionality within one short peptide sequence and opportunities for new, targeted designs that incorporate the biological activity of chimeras.
6. Oral hard tissue modification with biomolecules
An alternative approach for extending the lifespans of dental restorative materials is not the development of new restorative materials per se but rather enhance of the existing tooth structure. This is an attractive approach as decades of work have focused on novel restorative materials that show exciting laboratory results but are then never brought to market.402 An additional benefit of reinforcing enamel or dentin is the potential universal compatibility with any restorative material.
An alternative approach to protect collagen degradation at the resin/dentin adhesive interface and prevent premature failure of resin composite restorations is collagen crosslinking. Plant-derived proanthocyanidins (polyphenolic compounds that induce intraand inter collagen crosslinking)403 have been used for extending the lifespans of restorative materials. Proanthocyanidins can be “painted” onto tooth surfaces or encapsulated in dental materials.404 Application of proanthocyanidins on dentin has been shown to reduce dentin permeability,405 increase tensile (among many) mechanical properties,406 reduce degradation due to water and enzymes,407 and increase bonding.408 Other work has shown that proanthocyanidins may help protect dental pulp from restoration-associated cytotoxicity.409 Recent clinical trials have suggested limitations of proanthocyanidin application to extend restoration lifespans.410,411
Another dentin modification strategy has featured the AMP GL13K to form robust coatings that take advantage of the amphiphilicity of GL13K with strong affinity for deproteinated, negatively-charged hydroxyapatite-rich peritubular dentin. GL13K thereby forms hydrophobic, antimicrobial, highly stable coatings on dentin that reduce microleakage but do not alter mechanical adhesion between dentin and restorative materials.412,413 These simple coatings may be able to reduce recurrent caries of existing restorative materials without tedious and time-consuming restorative product development. Others have developed a peptide combining an AMP domain and a domain with high affinity for hydroxyapatite to engineer antimicrobial enamel.414–416 Other similar strategies have been developed using monomers.417,418 Additional work developed a coating process for dentin whereby lysozyme (an antimicrobial enzyme part of the immune system)419 is emulsified in a solution of PEG (polyethylene glycol) to form amyloid-like lysozyme oligomer aggregates and result in an antifouling coating against proteins and S. mutans and induce remineralization under specific conditions.420 Another well-studied, though not necessarily biomolecule-derived, hard tissue modification, is silver diamine fluoride.421,422
7. Oral tissue regeneration using biomolecules in dental biomaterials
The tooth is comprised of hydroxyapatite and soft matter (collagen fibrils, pulp-like cells, and other connective tissue). The outermost layer of teeth is of enamel, which is underlaid by less mineralized dentin and non-mineralized pulp tissue (Fig. 7).423 The supporting tissues surrounding the tooth (i.e., the periodontium), consist of alveolar bone, cementum, and periodontal ligament.424 Infections inevitably occur and inflammation leads to endodontic/periodontal diseases that require tissue substitution, repair, or regeneration.425 Tissue regeneration of dental and periodontal tissues is particularly challenging given that loss of tooth vitality frequently leads to complete removal of the pulpal tissue,426 and periodontium infection may lead to supra- or subgingival superficial scaling,427 or even complete tooth removal.428
Fig. 7.
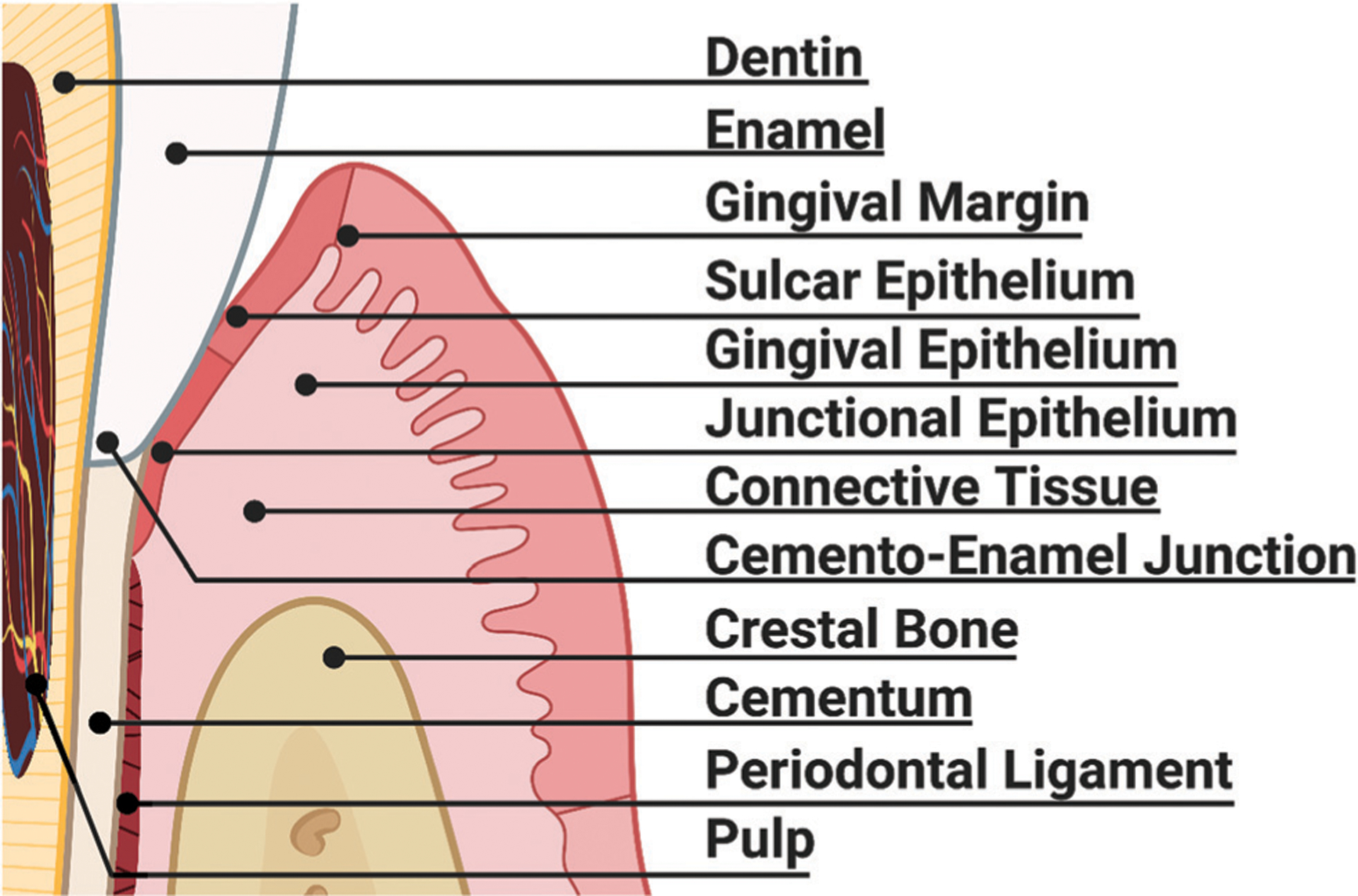
Hard and soft periodontal tissues susceptible to disease and infection necessitating bioinspired dental biomaterial therapies. The tooth, primarily composed of enamel and dentin, is filled with blood vessels and is innervated. The tooth root is covered in cementum and partially anchored into the oral cavity through periodontal ligaments as the tooth sits in a bone socket. The surrounding gingiva, composed of sulcar epithelium and connective tissue, seals the tooth from the harsh oral cavity at the junctional epithelium, near the cemento-enamel junction, and is marked by a distinctive gingival margin and epithelium in healthy individuals.
7.1. Pulp regeneration
Tooth vitality relies on a healthy pulp free of microbiology contamination. Nevertheless, dental trauma or caries may result in pulp contamination and inflammation.429,430 Teeth can lose vitality, become necrotic, and form a periapical lesion. In these cases, necrotic pulp must be removed, the intracanal system disinfected (antimicrobial options for this are discussed in section 2.1.3), and the pulp chamber filled with a restorative material.426,431 The absence of living tissue in the intracanal space prevents the possibility of pulp regeneration when using conventional endodontic therapy (root canal treatment).432 Endodontic therapies are performed frequently but the success can vary widely; some reports show long term success rates below 50%.433 As a result, a number of approaches have been developed toward regenerative endodontic therapies for pulp regeneration.
Pulp regeneration is dependent on the presence of stem cells in the desired site capable of differentiation into specialized cells (e.g., odontoblasts) and the absence of infection/contamination.429,434 Regeneration is especially relevant in the case of immature permanent teeth.435 Immature teeth possesses some anatomical characteristics (e.g., wide open apex and fragility) that do not support root canal treatment.430 Consequently, apexification and the evoked bleeding method are currently used to treat necrotic immature permanent teeth. Both approaches utilize the body’s natural biomolecule delivery responses to regenerate pulp.
Apexification induces apical closure by forming a mineralized barrier (details are reviewed elsewhere436) but does not complete root maturation.430 In contrast, the evoked bleeding method may induce root maturation.432 The evoked bleeding consists of performing a laceration of the periapical tissue to provoke bleeding into the canal system, i.e., formation of a blood clot, thus forming a natural, fibrin-based scaffold filling with apical stem cells.437 This blood clot enriches the site with growth factors FGF2, vascular endothelial growth factor (VEGF; pro-angiogenic438), nerve growth factor (NGF; anti-apoptotic439), among others.435,440 However, the regenerated tissues are heterogeneous in morphology, including cementum-, periodontal- and bone-like tissues.441 In response, treating dentin with a conditioning agent [e.g., chelators like ethylenediaminetetraacetic acid (EDTA)] beforehand can partially demineralize inorganic dentin contents to favor release of growth factors and matrix biomolecules (such as transforming growth factor β1) (TGF-β; enhances odontogensis442), bone morphogenic protein-2 (BMP-2; enhances odontogensis443), and PDGF.444–446 These released biomolecules are chemotactic toward dental pulp stem cells (DPSCs)430 and improve cell attachment to the canal walls and stem cell differentiation.447,448
One commonly used cocktail of biomolecules in pulp regeneration is platelet-rich plasma (PRP), or the fraction of a volume of plasma that possesses a greater concentration of platelets and amount of growth factors as compared to peripheral blood.449–451 One would think it would be highly regenerative considering the high concentration of growth factors (reviewed elsewhere452–454), but according to some studies,449,455 newly formed pulp within PRP-filled root canals is absent of any odontoblasts. Nevertheless, it seems that PRP-based techniques positively influence tooth survival.456 The recent results of the first randomized, controlled phase I/II clinical trial for delivery of mesenchymal stem/stromal cells (MSCs) encapsulated in platelet-poor plasma (PPP) showed that PPP/MSC treatment increased pulp response compared to a non-regenerative endodontic control.457
A wide array of manufactured, biomolecule-based scaffolds has been used for pulp and dentin–pulp complex regeneration.458 Indeed, the range of biomolecules used for pulp regeneration encompasses the range of biomolecules highlighted in this review. In a series of early, pioneering reports, DPSCs were encapsulated in alginate and placed subcutaneously into the backs of nude mice; histological analysis showed odontoblast-like cells initiated dentin-like hard tissue formation ectopically.459,460 Others have encapsulated human umbilical vein endothelial cells (HUVECs) and DPSCs in GelMA and showed native cell infiltration with establishment of well-organized neovasculature formation and pulp cells that attached to the inner dentin surface and infiltrated into the dentin tubules.461 Of note, for encapsulation of HUVECs and DPSCs in GelMA, a light-driven process was used: the hydrogel was incorporated with light-sensitive photoinitiators and then photo-polymerized using ultra-violet (UV) light. Despite being a conventional method, UV light may produce DNA damage and impair cellular function, so that the alternative light sources like the visible-light typically found in dental curing dental devices would contribute for a more biocompatible scenario for pulp regeneration, as suggested by others.462
Similar scaffolds have been encapsulated with FGF2 to drive DMP1 and nestin (odontoblast differentiation biomolecule463) expression in the dentin defect near the amputated pulp.464 HA gels fabricated by freeze-drying, when implanted in amputated pulp, showed formation of reparative dentin toward residual dental pulp under the dentin defect to a greater extent that collagen controls.465 Other HA-based injectable gel seeded with stem cells from apical papilla (SCAPs) enhance the differentiation of the cells into an odontoblastic phenotype capable of mineralization.466 Decellularized materials are also attractive materials.467 Recently, DPSCs have been encapsulated in lowand high-stiffness oligomeric collagen matrices and long-term cell survival demonstrated, as well as endothelial and odontogenic differentiation.468
Chitosan has been added to a fibrin hydrogel to promote dental pulp tissue neoformation and collagenous matrix production.469 Porous silk fibroin scaffolds fabricated using freeze-drying and physically loaded with basic fibroblast growth factor (bFGF) showed pulp-like tissue regeneration with vascularity, matrix deposition, and dentin-like tissue formation.470 Similarly, silk fibroin scaffolds loaded with RGD and DMP1 showed no hard tissue growth. This negative result suggests that processing and handling protocol of all biomolecules and biomolecule-derived biomaterials may be critical to the final biological activity.471
Heparin (a common glycosaminoglycan472) has been crosslinked with gelatin in hierarchical nanofibrous microspheres to load and sequester VEGF as an injectable, microsphere system for full-length pulp regeneration.473 Results showed successful regeneration of pulp-like tissues that filled the apical and middle third root space with notable vascular regeneration in mice. These results claim, for the first time, complete pulp tissue regeneration in a full-length root canal. An alternative strategy is the fabrication of “scaffold-free” 3D constructs composed of DPSCs in their own secreted, biomolecule-rich matrix. These constructs and DPSCs are able to differentiate into odontoblast-like mineralizing cells and form blood vessel-rich pulp-like tissues.474,475
7.2. Periodontal tissue regeneration
The periodontal tissue is comprised of cementum, periodontal ligament and the alveolar bone acting together to anchor the tooth.476 The alveolar bone lining the tooth socket shows a continuous remodeling process; a balance between bone formation and bone resorption.426,477 Periodontitis is a chronic inflammatory disease induced by bacterial infection and the host response thereto, which may lead to significant destruction of the periodontium.169 Around 796 million people worldwide have severe periodontitis.1 Periodontal regeneration was first demonstrated using guided tissue regeneration (GTR) techniques in which epithelial migration into the regenerating area is prevented.478 GTR techniques vary according to the material used to induce the regenerative process, such as bone grafts (replace the missing alveolar bone); periodontal barriers (cover the remaining alveolar bone present in the defect); and biological mediators (bioactive materials administered into the periodontal defect).479 Periodontal regeneration remains clinically challenging because of the involvement of the three distinct tissues forming the periodontium.480 The most notable challenge in periodontal regeneration is ensuring that the periodontal ligament is intercalated, integrated, and inserted into both cementum and bone (i.e., functional Sharpey’s fibers).481 A noted deficiency in the use of bone grafts and periodontal barriers is their outcomes cannot be predicted. Attempted biological mediators include biomolecules, which may induce effective migration of progenitor cells and their proliferation toward sustainable formation of a new periodontium.482
7.2.1. Biomolecules for periodontal regeneration.
Collagen is the gold standard material for periodontal regeneration and has been reviewed elsewhere,483 but is typically not stiff enough and frequently becomes exposed.484 In response, relatively advanced biomolecules release systems have been developed with materials other than collagen. For example, core/shell fibrous super-assembled frameworks have been loaded with bFGF and BMP-2 burst release for few days of bFGF followed by a slow and steady release of BMP-2 for up to four weeks. This material showed new bone formation as well as periodontal ligament and cementum regeneration when implanted in vivo.485 Others have combined stromal cell derived factor-1 (SDF-1, a chemoattractant)486 and BMP-2 with hydrogelator Nap-Phe-Phe-Tyr-OH (NapFFY) by simply dissolving and cooling the mixture to induce assembly.487 Release profiles were steady through one month and results from a maxillary critical-sized periodontal bone defect showed regeneration of periodontal tissue supporting bone.
More complex mixtures of biomolecules, multi-phasic materials, have been fabricated as well. One example includes tri-layered nanocomposites composed of chitin, bioactive glass, cementum protein 1 (CEMP1, known to induce differentiation of periodontal cells488), FGF2, and PRP that were implanted into rabbit maxillary periodontal defects.489 The results showed formation of new cementum lined with cementoblasts on the root surface, periodontal ligament formation and new alveolar bone formation. Multiphasic constructs are indeed frequently used for periodontal tissue regeneration.490 Other approaches include a photocrosslinkable HA system enriched with platelet lysate that showed a growth-factor mediated response by periodontal ligament fibroblasts and antimicrobial activity.491 Platelet lysate has also been encapsulated in HA to increase overall periodontal healing scores and restrict formation of long epithelial junctions.492 A somewhat unusual biomolecule, wool keratin (usually obtained from low quality wool processing493), has been successfully applied to promote similar periodontal tissue regeneration as collagen but while being derived from industrial waste streams.494 Other work has used polydopamine coatings on poly(ε-caprolactone) (PCL)495 or solvent cast and thermally annealed silk fibroin496 to regenerate periodontal tissue.
7.2.2. Chitosan for periodontal tissue regeneration.
A common strategy in periodontal tissue engineering, besides multi-phasic approaches, is the use of chitosan. Chitosan is generally antimicrobial (depending on processing)497 with low cytotoxicity and has been applied to many other fields including biopharmaceutics and food science.498 Chitosan is noted for its easy processability into hydrogels, fibers, beads, particles, etc., biodegradability, and ability to hydrate wounds.499 Many studies have demonstrated the beneficial effects of adding chitosan into dental materials to improve their physical, and mechanical properties.500–505 Perhaps more interestingly, chitosan has been used to enhance periodontal tissue regeneration in many forms.506
Some examples of periodontal tissue regeneration using chitosan include fabricating electrospun collagen/chitosan membranes and regenerating periodontal tissue in a rat model.506 Chitosan and gelatin has also been successfully combined to regenerate periodontal tissue.507 Multiphasic, oriented chitosan fibers have been created to form a densely mineralized matrix with the new mineral on the dentin surface in a nude mouse model.508 Chitosan seems immunomodulatory toward periodontal tissues which may be useful in promoting tissue regeneration.509 Other have combined chitosan with metallic nanoparticles for enhanced antimicrobial activity510 or bioactive glass particles for enhanced bone formation.511
7.2.3. Gene delivery for periodontal tissue regeneration.
Gene delivery is an advanced biomolecules delivery strategy to yield sustained local production and secretion of proteins to avoid immunological, half-life, and timing problems of classical biomolecule delivery.512 Delivery constructs can be divided into nonviral vectors (plasmids) and the viral-based vectors.482 Both vector options are effective but viral options may cause irreversible modifications to the host’s DNA.513 Dosing514 and proteolysis of vectors515 remain as a concern, but a few examples of gene delivery for periodontal regeneration exist.
One approach is delivery of vectors encoding growth factors like PDGF-BB or BMP-7 with proved effectiveness for periodontal tissue regeneration in a large alveolar bone defects with similar mechanical properties to native tissue and mature expression of collagen III and periostin (a matricellular periosteum protein).516,517 Another alternative approach is the reduction of the expression of a particular gene, such as using an adeno-associated virus cathepsin K (Ctsk; an important regulator of osteoclast mineral dissolution518) with small hairpin (sh)RNAs.519 Another study showed that transfecting an adenovirus containing Wnt10b (a Wnt that promotes osteogenesis via β-catenin signaling520) significantly increased osteogenesis and decreased adipogenesis, which may be useful for periodontal tissue regeneration.521 Other relevant approaches include ex vivo delivery to stem cells for later stem cell therapies for periodontal defect regeneration.522,523 Vector delivery vehicles include a range of other biomaterials and synthetic polymers.524
7.2.4. Bioprinting for periodontal regeneration.
Bioprinting is a fairly novel reconstructive process that holds potential to fabricate three-dimensional, defect-specific vascularized periodontal/bone tissues. In a recent study, bone formation was considerably increased upon the use of a 3D-printable bioink comprised of an ECM hydrogel and amorphous magnesium phosphate particles (Fig. 8).525 Two distinct concentrations of the foregoing particles were tested (0.5 and 1.0 wt%) and compared to an unfilled ECM. After encapsulation of DPSCs in the bioinks, the cell-laden constructs were tested for osteogenic differentiation potential and for in vivo bone regeneration; despite cell viability was similarly obtained as compared with the control, cell morphology features were improved, and mineralization and osteogenic gene expression were increased, leading to gradual bone healing. It is worth mentioning that these modified-bioinks are free of growth factors, and this fact did not prevent bioactivity outcomes to occur. Overall, it was demonstrated that by combining the tested bioink with bioactive compounds (amorphous magnesium phosphate), bone formation is substantially enhanced, so that this manufacturing method shows promise for effective in situ bioprinting strategies.
Fig. 8.
Novel bioink biomaterial for increasing bioactivity and bone healing. (A) Representative macrophotographs (upper images) showing the formulation process of the extracellular matrix/amorphous magnesium phosphate (ECM/AMP) bioink, and schematic of the ECM/AMP cell-laden bioink printing and the optical images (lower images) of the cell-free/cell-laden, AMP-based composites with and without cells. (B) Calcein AM (green) and PI (red) staining assay for live and dead analysis of dental pulp stem cells (DPSCs) after short period (1 day) in the cell-laden ECM and ECM/AMP-bioprinted constructs, showing more elongated morphology for DPSCs when combined with the AMP-modified constructs (red arrows denote dead cells). (C) Graphs showing similar cell viability among the tested bioink constructs, but a significant overall increased osteogenic differentiation (alkaline phosphatase activity, Alizarin Red S absorbance, and osteogenic gene expression at days 14 [lower left graph] and 21 [lower right graph]) for the ECM/AMP-modified bioinks as compared with the AMP-free control. (D) Representative macrophotographs for the application of the tested constructs in the in vivo rat model, showing the polytetrafluoroethylene (PTFE) membrane (cytoplast) used as a carrier for the printed constructs (a), its cutting into square-shaped pieces (7 × 7 mm2) (b) and combination with the constructs (c), and implantation in prepared defect (d and e) and suture (f). (E) Micro-CT results showing rat skull 3D rendering at 4 and 8 weeks for defects left empty or filled with the PTFE membrane alone, ECM and 1.0 wt% ECM/AMP-modified (ECM/1.0AMP) constructs; bone volume per total volume (BV/TV) and bone density were substantially higher for defects treated with the AMP-modified material. (F) H&E and MT staining after 8 weeks of implantation of tested groups and controls, indicating healing of the defects with new bone formation restricted to the area close to the border of the defects, with the ECM and ECM/1.0AMP groups showing thicker bone formation; inset legends – connective tissue (CT), osteoblast (OB), new bone (NB), blood vessel (BV), osteocytes (OC), woven bone (WB; blue), lamellar bone (LB; red). Reprinted with permission from ref. 514 (2020) American Chemical Society.
7.3. Biomolecule-based strategies for salivary gland regeneration
Hyposalivation, which is a characteristic of xerostomia (or dry mouth syndrome), can significantly affect the quality of life of patients and is commonly caused by Sjögren’s syndromes, various medications, and side effect of cancer-related radiation therapy.526 The prevalence of hyposalivation or xerostomia is difficult to estimate but is present in 90–100% of head and neck cancer patients.527 Treatment to regain full salivary gland function is difficult and typically temporary. As a result, a number of tissue engineering approaches have been undertaken to engineer artificial salivary tissues to mitigate effects of hyposalivation and increase salivation.528,529
Many approaches to regenerative salivary glands have used biomolecules in order to mimic the complex structure of salivary glands,530 which are generally cells from the acini (the basic secretory units of salivary glands) surrounded by ECM:, myoepithelial cells, myofibroblasts, endothelial cells, stromal cells and nerve fibers in addition to the immunological system.531 As a result of this complex structure – and in particular the necessary ductal structures – almost no examples possess all appropriate vascularization, innervation, and secretory function.530,532
Laminin111 derived peptides, which avoid cost and immunological problems associated with whole laminin proteins, have been used as one driver of salivary gland differentiation and development when conjugated to fibrin.533 Fibrin (a protein formed by proteolytic activity of thrombin on fibrinogen)534 scaffolds conjugated with these peptides have been able to partially regenerate a damaged mouse submandibular gland.535 Fibroblast growth factor 7 (FGF7) seems to be responsible for the remarkable new nodes/clusters formation within such fibrin hydrogels.536 Fibrin is an attractive biomolecule because degradation products have no adverse effects on cell function or viability.537
Matrigelt, as in a number of other regenerative medicine applications, has been successfully used to culture salivary gland constructs; particularly as a means to propagate differentiated cell types.538 However, it should be noted that Matrigelt does not have a well-defined composition539,540 as it is an assortment of ECM like laminin111, collagen IV and nidogens (crosslink laminins and collagens541).539,540 A perlecan domain IV peptide has been shown to trigger differentiation of salivary gland cells into self-assembling acini-like structures that express necessary biomarkers and secrete α-amylase to a similar extent as Matrigelt and may be a good substitute for Matrigelt.542 Other work has shown the critical role of FGF7 for branching of salivary gland ducts in 3D.536
Other biomolecules used for regeneration salivary glands includes elastin which promotes apicobasal polarization of salivary gland epithelial cells when electrospun (process reviewed elsewhere543) with poly(lactic-co-glycolic acid) (PLGA).544 Hyaluronic acid has been crosslinked with mono-2-(acryloyloxy)ethyl succinate to form hydrolytically degradable hydrogels that support multicellular spherical aggregates and stable maintenance of a stem cell phenotype.545 The addition of polydopamine to hyaluronic acid seems to further support required salivary gland differentiation.546 Other work with hyaluronic acid hydrogels has generated acini-like structures that activated the salivary fluid production molecular pathway547 and induced expression of cholinergic neurotransmitter receptors, which are necessary for salivary gland function.548 Fibronectin has been reported to help differentiation and expression of functional proteins in the acinus and adhesion-related cell markers from human salivary biopsies into acinar cells.549 Silk fibroin hydrogels aide salivary gland epithelial cells response to isoproterenol by increasing enzyme release, just as healthy salivary glands do.550 The many utilizations of chitosan for salivary gland regeneration have been previously reviewed,551 as have gene delivery approaches.552
7.4. Peptide amphiphiles for oral tissue regeneration
The self-assembly of molecules is an attractive strategy for engineering biomaterials because of the highly tunable, free energy-driven process that spontaneously organizes such molecules into finely ordered structures mimicking nature.553 One class of molecules able to do this is peptide amphiphiles (PA), which first reported to self-assemble into long nanofibers, form hydrogels, and mimic the ECM under the control of pH and presence and concentration of ions.554,555 A typical fiber-forming PA includes a peptide sequence (normally less than 10 amino acids) linked to an aliphatic tail (at least 10 carbons). This peptide sequence contains a critical domain near the aliphatic tail with a high propensity to form β-sheet secondary structures and charged residues to facilitate self-assembly; bioactive domains can be incorporated, RGD for example.556
The first families of PAs were designed to nucleate apatite crystals with specific orientation and mimic the nanostructure of dentin and bone. Since then, other structures have been achieved including spheres, filaments, 2D-sheets, networks, tubes, and helices, among others.557 A number of functionalities are can be enabled by PAs given their modularity: enzymatically cleavable domains,558 self-repair,559 biphasic release profiles,560 drug-triggered crosslinking,561 and peptide–DNA hybrids,562 among others. PAs have been used for a number of regenerative medicine applications including immunomodulation,563 angiogenesis,564 neurogenesis,565 and replication of the multi-hierarchical self-assembly of collagen,566 among others.
PAs have also been applied to dentistry. For example, PAs have been used to encapsulate DPSCs with MMP-degradability and RGD to generate mineral for toward dentin repair.567,568 Others569 have also suggested the use of PAs for dental pulp regeneration. Dentonin, a peptide shown to help repair dentin,570 has been incorporated into a PA for dentin repair.571 Similar work has also incorporated basic fibroblast growth factor (bFGF),572 TGF-β1,442 and VEGF into the PA via heparin binding for pulp regeneration.573 Others have used PAs to ectopically generate enamel-like tissue.574 Similarly, self assembling leucine zipper hydrogel systems have also been applied for regenerative dentistry.575,576
8. Future perspectives and conclusion
This review has emphasized the relevant and central role of biomolecules in designing and developing new generations of preventive and therapeutic technologies to address oral health issues both for manufacturing and modifying dental materials. In spite of the specific functional and signaling diversity and specificity of the biomolecules, which justifies the excitement and focus on them, mechanical (elasticity, toughness, etc.) and structural (porosity, roughness, etc.) characteristics of biomaterials can be overriding signaling cues for cells.577 Thus, the smart and guided combination of physical and biochemical features in dental biomaterials should be further explored. Release of biomolecules from the biomaterials structural components is another promising technology to control the biological outcome of biomaterials. However, despite decades of work focusing on biomolecule release kinetics, striking recent work has shown the importance of the temporal and spatial organization and density of biomolecules in their delivery scaffold.578–581 Patient-specific materials (cells582 or perhaps even biomolecules, such as is commonly performed with PRP) and their incorporation into therapeutic treatment strategies is another potential avenue for advancement in dental biomaterials.583 Thus, processing, sourcing, and functional display of biomolecules (release profile, encapsulation, immobilization, etc.), with or without cells, can exert strong control over the biological activity of biomolecules.584,585
One rising approach to govern biological activity of dental biomaterials is the use of immunomodulatory systems, such as for dental implants586 given the pivotal role the immune system plays in the response to materials. Strategies such as growth factor sequestration587 are well-proven routes to take advantage of immunomodulation.
Other classes of biomaterials such as polysaccharides (long chains of carbohydrates) beyond chitosan may further be explored in dentistry given their commonness in other regenerative medicine fields.588 Proteoglycans [glycosaminoglycan chains covalently attached to protein core (with the exception of hyaluronan) (or hyaluronic acid) that lack a protein core589] are widely used in cartilage tissue engineering.590 However, even though proteoglycans are found in dental pulp and important to odontogenesis, dental biomaterials composed of proteoglycans are scarce.591,592
Extracellular vesicles (EVs) are nanoparticles secreted by all cells that contain lipids, proteins, and nucleic acids and function as cell-to-cell communicators; up to 850 different proteins, over 200 mRNAs, and around 60 miRNAs.593,594 As a result, EVs are functionally active intercellular messengers that may be beneficial for delivering biomolecules towards regeneration595 and repairing tissue through immune modulation, angiogenesis, inhibition of apoptosis, reduction of fibrosis, and other pathways.595 One attractive opportunity for EVs is their delivery through biomaterials and tissue engineering constructs.596–598 EVs play a critical role in development and homeostasis of the oral cavity. For example, ~100 nm exosomes are secreted by the epithelium and mesenchyme of a developing tooth organ where epithelium exosomes induce dentin sialoprotein production and mineralization. Mesenchyme exosomes induce ameloblastin and amelogenin secretion.599,600 EVs have been used to treat bisphosphonate-related osteonecrosis of the jaw (BRONJ) through promotion of angiogenesis and bone regeneration in rats.601 Future development in dental biomaterial may exploit this potent delivery mechanism of biomolecules.602
Some key factors must be considered as materials move toward translation. The use of different cell types can, due to genetic variability and diversification even with common cell lines, result in different results between investigators.603 Disparities in biomolecule sources (naturally extracted, recombinant, de novo designed, etc.) may directly affect biological activity.194,604 High throughput screening techniques may enable rapid screening of engineering materials to accelerate this pipeline.605–608 Practicability of the materials for clinicians and potential to scale manufacturing, with environmental constraints, are also realities that promising biomaterial therapies must handle.609 Strategies to mitigate the relatively short half-lives of biomolecules and transportation and storage of biomolecule-decorated biomaterials must be faced.610 Finally, a recent trend has been toward rather complex multi-functional materials.611 While academically meritorious, financial aspects limit their potential successful clinical translation due to both required manufacturing regulation and sheer cost of all the components.612 A balance between increased complexity and practicability and cost-effectiveness is likely necessary.
Advantages in dental biomaterials must not be limited to dentistry itself but rather enriched with ideas from the vast field of tissue engineering. Indeed, there were 66 ongoing or completed clinical trials of tissue engineering-related products between 2011–2018 and over $9 billion in sales in 2017 alone.613 A further, rather concerning challenge in biomaterials is the artisanal nature of current materials, which are neither immediately accessible nor easily sent from lab to lab. This undermines biomaterials exploitation by the entire biomedical community. Filling these gaps and overcoming these knowledge barriers is essential to invigorate the biomaterial and dental communities, with input from and others in the biomedical community at large, toward shared goals and prioritization of the most essential oral and systemic health challenges.
Acknowledgements
The authors thank Jiahe He for proofreading. Figures were partially created with BioRender. This study was supported by the National Institute for Dental and Craniofacial Research of the National Institutes of Health (grant numbers R01DE026117 to C. A.; R01DE026578 and K08DE023552 to M. C. B.; R01DE025476 to C. T.; T90DE0227232 and F30DE029105 to N. G. F). N. G. F. acknowledges a 3M Science and Technology Fellowship. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies
Nicholas G. Fischer

Nicholas G. Fischer is a graduate fellow in the University of Minnesota’s Dental Scientist Training Program. His current research with Prof. Conrado Aparicio focuses on the functionalization of biomaterial surfaces at the Minnesota Dental Research Center for Biomaterials and Biomechanics. Nicholas is also interested in levering the secreted matrix to instruct cellular responses to biomaterials. Nicholas completed his undergraduate degree at Creighton University in Environmental Sciences and Biology.
Eliseu A. Münchow

Eliseu Münchow is a Professor in the School of Dentistry at the Federal University of Rio Grande do Sul (Brazil). He obtained a PhD in Dentistry from the Graduate Program in Dentistry at the Federal University of Pelotas (Brazil) in 2015, and works on the development and characterization of dental biomaterials, with emphasis in resin-based restorations, adhesive bonding agents, and nano-technology-designed materials. He was also a research scholar in the Indiana University School of Dentistry from 2014 to 2015, gaining experience in the synthesis and applicability of polymeric scaff olds/membranes with bioactive and drug-releasing properties.
Candan Tamerler

Candan Tamerler is the Wesley Cramer Professor in Mechanical Engineering Department and Bioengineering Program, and the Director of Bioenabled and Biomimetic Materials at the Institute of Bioengineering Research at the University of Kansas (KU). She received her degrees in Bogazici University and postdoctoral trainings in Westminster University. Prior to KU, she has been a faculty member in Istanbul Technical University and University of Washington. Her research interests include molecular biomimetics, peptidomimetics and bio-functional interfaces, surfaces and biomaterials design. She has published more than 150 referred journal articles and have several patents. Tamerler is a Fellow of AAAS, AIMBE and TUBA.
Marco C. Bottino

Marco C. Bottino is Associate Professor (tenured) of Dentistry at the University of Michigan in Ann Arbor. He obtained his Doctor of Dental Surgery (DDS) degree in 2001 from Universidade Paulista (São Paulo, Brazil), his master’s degree in Nuclear Technology in 2005 from the University of São Paulo (IPEN, São Paulo, Brazil), and his doctorate in Materials Science in 2010 from The University of Alabama at Birmingham (Birmingham, AL). His current research focuses on the development of drug delivery systems for oral infection ablation, injectable scaffolds for dental tissue regeneration, and bioprinting strategies for craniofacial tissue reconstruction.
Conrado Aparicio

Conrado Aparicio is Professor and Deputy Director in the Department of Restorative Sciences and the Minnesota Dental Research Center for Biomaterials and Biomechanics at the University of Minnesota. Conrado is a biomaterials scientist and obtained his PhD in Biomaterials from Barcelona-Tech in 2005. He is a leader in functionalization of biomaterial surfaces and tissue interfaces with bio-inspired molecules for boosting their bioactivity and antimicrobial properties. He also explores applications for biomimetic processes of mineralization and biomolecular self-assembly. His lab has studied bio/non-bio interactions and developed technologies for implants, constructs and scaffolds in the dental, orthopedic, and other biomedical fields.
Footnotes
Conflicts of interest
There are no conflicts of interest to declare.
References
- 1.Bernabe E, Marcenes W, Hernandez CR, Bailey J, Abreu LG, Alipour V, Amini S, Arabloo J, Arefi Z, Arora A, Ayanore MA, Bärnighausen TW, Bijani A, Cho DY, Chu DT, Crowe CS, Demoz GT, Demsie DG, Dibaji Forooshani ZS, Du M, El Tantawi M, Fischer F, Folayan MO, Futran ND, Geramo YCD, Haj-Mirzaian A, Hariyani N, Hasanzadeh A, Hassanipour S, Hay SI, Hole MK, Hostiuc S, Ilic MD, James SL, Kalhor R, Kemmer L, Keramati M, Khader YS, Kisa S, Kisa A, Koyanagi A, Lalloo R, Le Nguyen Q, London SD, Manohar ND, Massenburg BB, Mathur MR, Meles HG, Mestrovic T, Mohammadian-Hafshejani A, Mohammadpourhodki R, Mokdad AH, Morrison SD, Nazari J, Nguyen TH, Nguyen CT, Nixon MR, Olagunju TO, Pakshir K, Pathak M, Rabiee N, Rafiei A, Ramezanzadeh K, Rios-Blancas MJ, Roro EM, Sabour S, Samy AM, Sawhney M, Schwendicke F, Shaahmadi F, Shaikh MA, Stein C, Tovani-Palone MR, Tran BX, Unnikrishnan B, Vu GT, Vukovic A, Warouw TSS, Zaidi Z, Zhang ZJ and Kassebaum NJ, J. Dent. Res, 2020, 99, 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heintze SD, Ilie N, Hickel R, Reis A, Loguercio A and Rousson V, Dent. Mater, 2017, 33, e101–e114. [DOI] [PubMed] [Google Scholar]
- 3.Gaviria L, Salcido JP, Guda T and Ong JL, J. Korean Assoc. Oral Maxillofac. Surg, 2014, 40, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dental Implants: Market Estimates and Trends Analysis: Grand View Research, 2017.
- 5.Xu X, Chen X and Li J, J. Mater. Chem. B, 2020, 8, 2199–2215. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell BT, Ives CJ, Mohiuddin OA and Bunnell BA, Front. Bioeng. Biotechnol, 2019, 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohn DH, Matter, 2019, 1, 1114–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang D, Xu X, Long X, Cheng K and Li J, Biomater. Sci, 2019, 7, 3984–3999. [DOI] [PubMed] [Google Scholar]
- 9.Chen FM and Liu X, Prog. Polym. Sci, 2016, 53, 86–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma PX, Adv. Drug Delivery Rev, 2008, 60, 184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta LP, Manchineella S and Govindaraju T, Biomaterials, 2019, 230, 119633. [DOI] [PubMed] [Google Scholar]
- 12.Smith LM and Kelleher NL, Nat. Methods, 2013, 10, 186–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrendt R, White P and Offer J, J. Pept. Sci, 2016, 22, 4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ufarté L, Potocki-Veronese G and Laville E, Front. Microbiol, 2015, 6, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tripathi NK and Shrivastava A, Front. Bioeng. Biotechnol, 2019, 7, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma YX, Wang CY, Li YY, Li J, Wan QQ, Chen JH, Tay FR and Niu LN, Adv. Sci, 2019, 7(1), 1901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campoccia D, Montanaro L and Arciola CR, Biomaterials, 2013, 34, 8533–8554. [DOI] [PubMed] [Google Scholar]
- 18.Campoccia D, Montanaro L and Arciola CR, Biomaterials, 2013, 34, 8018–8029. [DOI] [PubMed] [Google Scholar]
- 19.Busscher HJ, van der Mei HC, Subbiahdoss G, Jutte PC, van den Dungen JJAM, Zaat SAJ, Schultz MJ and Grainger DW, Sci. Transl. Med, 2012, 4, 153rv10. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention; Antibiotic resistance threats in the United States, Atlanta, GA, 2013. [Google Scholar]
- 21.Shrestha P, Cooper BS, Coast J, Oppong R, Do Thi Thuy N, Phodha T, Celhay O, Guerin PJ, Wertheim H and Lubell Y, Antimicrob. Resist. Infect. Control, 2018, 7, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtz SM, Lau E, Watson H, Schmier JK and Parvizi J, J. Arthroplasty, 2012, 27, 61–65. e1. [DOI] [PubMed] [Google Scholar]
- 23.Cobb LH, McCabe EM and Priddy LB, J. Orthop. Res, 2020, DOI: 10.1002/jor.24689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bin Zaman S, Hussain MA, Nye R, Mehta V, Mamun KT and Hossain N, Cureus, 2017, 9, e1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campoccia D, Montanaro L, Speziale P and Arciola CR, Biomaterials, 2010, 31, 6363–6377. [DOI] [PubMed] [Google Scholar]
- 26.Hetrick EM and Schoenfisch MH, Chem. Soc. Rev, 2006, 35, 780–789. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed W, Zhai Z and Gao C, Mater. Today Bio, 2019, 2, 100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haugejorden O, Rise J and Klock KS, Community Dent. Oral Epidemiol, 1993, 21, 57–61. [DOI] [PubMed] [Google Scholar]
- 29.American College of Prosthodontists, Annual Report, 2017.
- 30.Koldsland OC, Scheie AA and Aass AM, J. Periodontol, 2010, 81, 231–238. [DOI] [PubMed] [Google Scholar]
- 31.Tarnow DP, J. Dent. Res, 2016, 95, 7–8. [DOI] [PubMed] [Google Scholar]
- 32.Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, Chen S, Cochran D, Derks J, Figuero E, Hämmerle CHF, Heitz-Mayfield LJA, Huynh-Ba G, Iacono V, Koo K-T, Lambert F, McCauley L, Quirynen M, Renvert S, Salvi GE, Schwarz F, Tarnow D, Tomasi C, Wang H-L and Zitzmann N, J. Clin. Periodontol, 2018, 45, S286–S291. [DOI] [PubMed] [Google Scholar]
- 33.Moy PK, Medina D, Shetty V and Aghaloo TL, Int. J. Oral Maxillofac. Implants, 2005, 20, 569–577. [PubMed] [Google Scholar]
- 34.Zitzmann NU and Berglundh T, J. Clin. Periodontol, 2008, 35, 286–291. [DOI] [PubMed] [Google Scholar]
- 35.Elani HW, Starr JR, Da Silva JD and Gallucci GO, J. Dent. Res, 2018, 97, 1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heitz-Mayfield LJA, J. Clin. Periodontol, 2008, 35, 292–304. [DOI] [PubMed] [Google Scholar]
- 37.Cochran D and Froum S, J. Periodontol, 2013, 84, 436–443.23537178 [Google Scholar]
- 38.Jenssen H, Hamill P and Hancock RE, Clin. Microbiol. Rev, 2006, 19, 491–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parachin NS, Mulder KC, Viana AA, Dias SC and Franco OL, Peptides, 2012, 38, 446–456. [DOI] [PubMed] [Google Scholar]
- 40.Segura-Egea JJ, Gould K, Sen BH, Jonasson P, Cotti E, Mazzoni A, Sunay H, Tjaderhane L and Dummer PMH, Int. Endod. J, 2018, 51, 20–25. [DOI] [PubMed] [Google Scholar]
- 41.Sitaram N, Curr. Med. Chem, 2006, 13, 679–696. [DOI] [PubMed] [Google Scholar]
- 42.Fjell CD, Hiss JA, Hancock REW and Schneider G, Nat. Rev. Drug Discovery, 2012, 11, 37–51. [DOI] [PubMed] [Google Scholar]
- 43.Brogden KA, Nat. Rev. Microbiol, 2005, 3, 238–250. [DOI] [PubMed] [Google Scholar]
- 44.Mai S, Mauger MT, Niu L, Barnes JB, Kao S, Bergeron BE, Ling J and Tay FR, Acta Biomater, 2017, 49, 16–35. [DOI] [PubMed] [Google Scholar]
- 45.Jiao Y, Tay FR, Niu L and Chen J, Int. J. Oral Sci, 2019, 11, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Shen Y and Haapasalo M, J. Oral Microbiol, 2017, 9, 1327308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steckbeck JD, Deslouches B and Montelaro RC, Expert Opin. Biol. Ther, 2014, 14, 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chouirfa H, Bouloussa H, Migonney V and Falentin-Daudré C, Acta Biomater, 2019, 83, 37–54. [DOI] [PubMed] [Google Scholar]
- 49.Wang D, Haapasalo M, Gao Y, Ma J and Shen Y, Bioact. Mater, 2018, 3, 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yucesoy DT, Khatayevich D, Tamerler C and Sarikaya M, Med. Devices Sens, 2020, 3(3), e10065. [Google Scholar]
- 51.Costa F, Carvalho IF, Montelaro RC, Gomes P and Martins MCL, Acta Biomater, 2011, 7, 1431–1440. [DOI] [PubMed] [Google Scholar]
- 52.Riool M, de Breij A, Drijfhout JW, Nibbering PH and Zaat SAJ, Front. Chem, 2017, 5, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorr S-U, Abdolhosseini M, Shelar A and Sotsky J, Biochem. Soc. Trans, 2011, 39, 1028–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirt H and Gorr SU, Antimicrob. Agents Chemother, 2013, 57, 4903–4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holmberg KV, Abdolhosseini M, Li Y, Chen X, Gorr SU and Aparicio C, Acta Biomater, 2013, 9, 8224–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X, Hirt H, Li Y, Gorr S-U and Aparicio C, PLoS One, 2014, 9, e111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X, Zhou XC, Liu S, Wu RF, Aparicio C and Wu JY, J. Mater. Sci.: Mater. Med, 2017, 28, 76. [DOI] [PubMed] [Google Scholar]
- 58.Ye Z, Zhu X, Acosta S, Kumar D, Sang T and Aparicio C, Nanoscale, 2019, 11, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye Z and Aparicio C, Nanoscale Adv, 2019, 1, 4679–4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harmouche N, Aisenbrey C, Porcelli F, Xia Y, Nelson SED, Chen X, Raya J, Vermeer L, Aparicio C, Veglia G, Gorr SU and Bechinger B, Biochemistry, 2017, 56, 4269–4278. [DOI] [PubMed] [Google Scholar]
- 61.Bechinger B and Gorr S-U, J. Dent. Res, 2017, 96, 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou L, Lai Y, Huang W, Huang S, Xu Z, Chen J and Wu D, Colloids Surf., B, 2015, 128, 552–560. [DOI] [PubMed] [Google Scholar]
- 63.Rosa L, Cutone A, Lepanto M, Paesano R and Valenti P, Int. J. Mol. Sci, 2017, 18, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gifford JL, Hunter HN and Vogel HJ, Cell. Mol. Life Sci, 2005, 62, 2588–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lupetti A, Paulusma-Annema A, Welling MM, Senesi S, van Dissel JT and Nibbering PH, Antimicrob. Agents Chemother, 2000, 44, 3257–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Godoy-Gallardo M, Mas-Moruno C, Fernández-Calderón MC, Pérez-Giraldo C, Manero JM, Albericio F, Gil FJ and Rodríguez D, Acta Biomater, 2014, 10, 3522–3534. [DOI] [PubMed] [Google Scholar]
- 67.Godoy-Gallardo M, Wang Z, Shen Y, Manero JM, Gil FJ, Rodriguez D and Haapasalo M, ACS Appl. Mater. Interfaces, 2015, 7, 5992–6001. [DOI] [PubMed] [Google Scholar]
- 68.Godoy-Gallardo M, Mas-Moruno C, Yu K, Manero JM, Gil FJ, Kizhakkedathu JN and Rodriguez D, Biomacromolecules, 2015, 16, 483–496. [DOI] [PubMed] [Google Scholar]
- 69.Gabriel M, Nazmi K, Veerman EC, Amerongen AVN and Zentner A, Bioconjugate Chem, 2006, 17, 548–550. [DOI] [PubMed] [Google Scholar]
- 70.He J, Chen J, Hu G, Wang L, Zheng J, Zhan J, Zhu Y, Zhong C, Shi X, Liu S, Wang Y and Ren L, J. Mater. Chem. B, 2018, 6, 68–74. [DOI] [PubMed] [Google Scholar]
- 71.Lin W, Junjian C, Chengzhi C, Lin S, Sa L, Li R and Yingjun W, J. Mater. Chem. B, 2015, 3, 30–33. [DOI] [PubMed] [Google Scholar]
- 72.González-Fernández E, Staderini M, Avlonitis N, Murray AF, Mount AR and Bradley M, Sens. Actuators, B, 2018, 255, 3040–3046. [Google Scholar]
- 73.Yucesoy DT, Hnilova M, Boone K, Arnold PM, Snead ML and Tamerler C, JOM, 2015, 67, 754–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wisdom C, Chen C, Yuca E, Zhou Y, Tamerler C and Snead ML, JOM, 2019, 71, 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wisdom C, VanOosten SK, Boone KW, Khvostenko D, Arnold PM, Snead ML and Tamerler C, J. Mol. Eng. Mater, 2016, 04, 1640005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jacob B, Park IS, Bang JK and Shin SY, J. Pept. Sci, 2013, 19, 700–707. [DOI] [PubMed] [Google Scholar]
- 77.Méndez-Samperio P, Peptides, 2010, 31, 1791–1798. [DOI] [PubMed] [Google Scholar]
- 78.Nell MJ, Tjabringa GS, Wafelman AR, Verrijk R, Hiemstra PS, Drijfhout JW and Grote JJ, Peptides, 2006, 27, 649–660. [DOI] [PubMed] [Google Scholar]
- 79.Molhoek EM, Van Dijk A, Veldhuizen EJA, Haagsman HP and Bikker FJ, Int. J. Antimicrob. Agents, 2011, 37, 476–479. [DOI] [PubMed] [Google Scholar]
- 80.Riool M, de Breij A, de Boer L, Kwakman PHS, Cordfunke RA, Cohen O, Malanovic N, Emanuel N, Lohner K, Drijfhout JW, Nibbering PH and Zaat SAJ, Adv. Funct. Mater, 2017, 27, 1–11. [Google Scholar]
- 81.De Breij A, Riool M, Kwakman PHS, De Boer L, Cordfunke RA, Drijfhout JW, Cohen O, Emanuel N, Zaat SAJ, Nibbering PH and Moriarty TF, J. Controlled Release, 2016, 222, 1–8. [DOI] [PubMed] [Google Scholar]
- 82.Cherkasov A, Hilpert K, Jenssen H, Fjell CD, Waldbrook M, Mullaly SC, Volkmer R and Hancock REW, ACS Chem. Biol, 2009, 4, 65–74. [DOI] [PubMed] [Google Scholar]
- 83.Fjell CD, Jenssen H, Hilpert K, Cheung WA, Panté N, Hancock REW and Cherkasov A, J. Med. Chem, 2009, 52, 2006–2015. [DOI] [PubMed] [Google Scholar]
- 84.Hilpert K, Elliott M, Jenssen H, Kindrachuk J, Fjell CD, Körner J, Winkler DFH, Weaver LL, Henklein P, Ulrich AS, Chiang SHY, Farmer SW, Pante N, Volkmer R and Hancock REW, Chem. Biol, 2009, 16, 58–69. [DOI] [PubMed] [Google Scholar]
- 85.Hammond PT, Mater. Today, 2012, 15, 196–206. [Google Scholar]
- 86.Shi J, Liu Y, Wang Y, Zhang J, Zhao S and Yang G, Sci. Rep, 2015, 5, 16336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kazemzadeh-Narbat M, Kindrachuk J, Duan K, Jenssen H, Hancock REW and Wang R, Biomaterials, 2010, 31, 9519–9526. [DOI] [PubMed] [Google Scholar]
- 88.Gao G, Lange D, Hilpert K, Kindrachuk J, Zou Y, Cheng JTJ, Kazemzadeh-Narbat M, Yu K, Wang R, Straus SK, Brooks DE, Chew BH, Hancock REW and Kizhakkedathu JN, Biomaterials, 2011, 32, 3899–3909. [DOI] [PubMed] [Google Scholar]
- 89.Zhan J, Wang L, Zhu Y, Gao H, Chen Y, Chen J, Jia Y, He J, Fang Z, Zhu Y, Mao C, Ren L and Wang Y, ACS Appl. Mater. Interfaces, 2018, 10, 35830–35837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shao J, Kolwijck E, Jansen JA, Yang F and Walboomers XF, Int. J. Antimicrob. Agents, 2017, 49, 659–667. [DOI] [PubMed] [Google Scholar]
- 91.Willcox MDP, Hume EBH, Aliwarga Y, Kumar N and Cole N, J. Appl. Microbiol, 2008, 105, 1817–1825. [DOI] [PubMed] [Google Scholar]
- 92.Chen R, Willcox MDP, Ho KKK, Smyth D and Kumar N, Biomaterials, 2016, 85, 142–151. [DOI] [PubMed] [Google Scholar]
- 93.Vepari C and Kaplan DL, Prog. Polym. Sci, 2007, 32, 991–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nilebäck L, Widhe M, Seijsing J, Bysell H, Sharma PK and Hedhammar M, ACS Appl. Mater. Interfaces, 2019, 11, 24999–25007. [DOI] [PubMed] [Google Scholar]
- 95.Hoyos-Nogués M, Buxadera-Palomero J, Ginebra MP, Manero JM, Gil FJ and Mas-Moruno C, Colloids Surf., B, 2018, 169, 30–40. [DOI] [PubMed] [Google Scholar]
- 96.Hoyos-Nogués M, Velasco F, Ginebra MP, Manero JM, Gil FJ and Mas-Moruno C, ACS Appl. Mater. Interfaces, 2017, 9, 21618–21630. [DOI] [PubMed] [Google Scholar]
- 97.Mas-Moruno C, Su B and Dalby MJ, Adv. Healthcare Mater, 2019, 8, 1801103. [DOI] [PubMed] [Google Scholar]
- 98.Yazici H, Habib G, Boone K, Urgen M, Utku FS and Tamerler C, Mater. Sci. Eng., C, 2019, 94, 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kazemzadeh-Narbat M, Lai BFL, Ding C, Kizhakkedathu JN, Hancock REW and Wang R, Biomaterials, 2013, 34, 5969–5977. [DOI] [PubMed] [Google Scholar]
- 100.Acosta S, Quintanilla-Sierra L, Mbundi L, Reboto V and Rodríguez-Cabello JC, Adv. Funct. Mater, 2020, 1909050, 1909050. [Google Scholar]
- 101.Girotti A, Fernández-Colino A, López IM, Rodríguez-Cabello JC and Arias FJ, Biotechnol. J, 2011, 6, 1174–1186. [DOI] [PubMed] [Google Scholar]
- 102.Rodríguez-Cabello JC, Martín L, Girotti A, García-Arévalo C, Arias FJ and Alonso M, Nanomedicine, 2011, 6, 111–122. [DOI] [PubMed] [Google Scholar]
- 103.Ibáñez-Fonseca A, Flora T, Acosta S and Rodríguez-Cabello JC, Matrix Biol, 2019, 84, 111–126. [DOI] [PubMed] [Google Scholar]
- 104.Castellanos MI, Zenses AS, Grau A, Rodríguez-Cabello JC, Gil FJ, Manero JM and Pegueroles M, Colloids Surf., B, 2015, 127, 22–32. [DOI] [PubMed] [Google Scholar]
- 105.García-Arévalo C, Pierna M, Girotti A, Arias FJ and Rodríguez-Cabello JC, Soft Matter, 2012, 8, 3239–3249. [Google Scholar]
- 106.Govindharajulu J, Chen X, Li Y, Rodriguez-Cabello J, Battacharya M and Aparicio C, Int. J. Mol. Sci, 2017, 18, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Da Costa A, Machado R, Ribeiro A, Collins T, Thiagarajan V, Neves-Petersen MT, Rodriguez-Cabello JC, Gomes AC and Casal M, Biomacromolecules, 2015, 16(2), 625–635. [DOI] [PubMed] [Google Scholar]
- 108.Atefyekta S, Pihl M, Lindsay C, Heilshorn SC and Andersson M, Acta Biomater, 2019, 83, 245–256. [DOI] [PubMed] [Google Scholar]
- 109.Acosta S, Quintanilla L, Alonso M, Aparicio C and Rodríguez-Cabello JC, ACS Biomater. Sci. Eng, 2019, 5, 4708–4716. [DOI] [PubMed] [Google Scholar]
- 110.Acosta S, Ibañez-fonseca A, Aparicio C and Rodriguez-Cabello JC, Biomater. Sci, 2020, 22, 154. [DOI] [PubMed] [Google Scholar]
- 111.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez M-G, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT-A, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo J-P, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Memish ZA, Mensah GA, Merriman TR, Meyer A-C, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KV, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Silberberg D, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh P-H, Zaidi AK, Zheng Z-J, Zonies D, Lopez AD and Murray CJ, Lancet, 2012, 380, 2163–2196.23245607 [Google Scholar]
- 112.Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D and Dietrich T, J. Clin. Periodontol, 2017, 44, S94–S105. [DOI] [PubMed] [Google Scholar]
- 113.Rho Y-J, Namgung C, Jin B-H, Lim B-S and Cho B-H, Oper. Dent, 2013, 38, 572–582. [DOI] [PubMed] [Google Scholar]
- 114.Burke FJT, Wilson NHF, Cheung SW and Mjör IA, J. Dent, 2001, 29, 317–324. [DOI] [PubMed] [Google Scholar]
- 115.Brantley CF, Bader JD, Shugars DA and Nesbit SP, J. Am. Dent. Assoc., JADA, 1995, 126, 1407–1413. [DOI] [PubMed] [Google Scholar]
- 116.Gordan VV, Riley JL, Geraldeli S, Rindal B, Qvist V, Fellows JL, Kellum HP and Gilbert GH, J. Am. Dent. Assoc., JADA, 2012, 143, 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spencer P, Ye Q, Misra A, Goncalves SEP and Laurence JS, J. Dent. Res, 2014, 93, 1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarda K and Katz JL, Ann. Biomed. Eng, 2010, 38, 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith L and Hillman J, Curr. Opin. Microbiol, 2008, 11, 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gross E and Morell JL, J. Am. Chem. Soc, 1971, 93, 4634–4635. [DOI] [PubMed] [Google Scholar]
- 121.Özel B,Şimşek Ö, Akçelik M and Saris PEJ, Appl. Microbiol. Biotechnol, 2018, 102, 6299–6307. [DOI] [PubMed] [Google Scholar]
- 122.Shin JM, Gwak JW, Kamarajan P, Fenno JC, Rickard AH and Kapila YL, J. Appl. Microbiol, 2016, 120, 1449–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Su M, Yao S, Gu L, Huang Z and Mai S, Peptides, 2018, 99, 189–194. [DOI] [PubMed] [Google Scholar]
- 124.Zhao M, Qu Y, Liu J, Mai S and Gu L, Odontology, 2020, 108, 376–385. [DOI] [PubMed] [Google Scholar]
- 125.Xie S, Song L, Yuca E, Boone K, Sarikaya R, VanOosten SK, Misra A, Ye Q, Spencer P and Tamerler C, ACS Appl. Polym. Mater, 2020, 2, 1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xie S-X, Boone K, VanOosten SK, Yuca E, Song L, Ge X, Ye Q, Spencer P and Tamerler C, Appl. Sci, 2019, 9, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Y, Fan Y, Zhou Z, Tu H, Ren Q, Wang X, Ding L, Zhou X and Zhang L, Arch. Oral Biol, 2017, 80, 41–50. [DOI] [PubMed] [Google Scholar]
- 128.Spolidorio D, Caiaffa K, Cilli EM, Calixto G, Kreling P, Santos-Filho N, Chorilli M, Aida K and Duque C, Int. J. Nanomed, 2018, 13, 3081–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Persoon IF and Ozok AR, Curr. Oral Health Rep, 2017, 4, 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Endodontic Facts, https://www.aae.org/specialty/about-aae/news-room/endodontic-facts/, accessed 5 May 2020.
- 131.Peralta-Mamani M, Rios D, Duarte MAH, Santiago JF Jr and Honorio HM, Am. J. Dent, 2019, 32, 311–324. [PubMed] [Google Scholar]
- 132.Elemam RF and Pretty I, ISRN Dent, 2011, 2011, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rocas IN, Lima KC and Siqueira JF Jr., Int. Endod. J, 2013, 46, 681–687. [DOI] [PubMed] [Google Scholar]
- 134.Barekatain B, Hasheminia SM, Shadmehr E and Attary Z, Indian J. Dent. Res, 2012, 23, 226–229. [DOI] [PubMed] [Google Scholar]
- 135.Athanassiadis B, Abbott PV and Walsh LJ, Aust. Dent. J, 2007, 52, S64–S82. [DOI] [PubMed] [Google Scholar]
- 136.Almadi EM and Almohaimede AA, Saudi. Med. J, 2018, 39, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jia L, Zhang X, Shi H, Li T, Lv B and Xie M, Med. Sci. Monit, 2019, 25, 2908–2916. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Pieper CM, Münchow EA and Piva E, Braz. Dent. Sci, 2015, 18, 17–21. [Google Scholar]
- 139.Ballal V, Kundabala M, Acharya S and Ballal M, Aust. Dent. J, 2007, 52, 118–121. [DOI] [PubMed] [Google Scholar]
- 140.Lakhani AA, Sekhar KS, Gupta P, Tejolatha B, Gupta A, Kashyap S, Desai V and Farista S, J. Clin. Diagn. Res, 2017, 11, ZC06–ZC09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Siqueira JF Jr. and Rocas IN, J. Dent. Res, 2009, 88, 969–981. [DOI] [PubMed] [Google Scholar]
- 142.Varoni E, Tarce M, Lodi G and Carrassi A, Minerva Stomatol, 2012, 61, 399–419. [PubMed] [Google Scholar]
- 143.Vijayaraghavan R, Mathian VM, Sundaram AM, Karunakaran R and Vinodh S, J. Pharm. BioAllied Sci, 2012, 4, S230–S233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yadlapati M, Souza LC, Dorn S, Garlet GP, Letra A and Silva RM, Int. Endod. J, 2014, 47, 769–775. [DOI] [PubMed] [Google Scholar]
- 145.Kim JH, Kim Y, Shin SJ, Park JW and Jung IY, J. Endod, 2010, 36, 1086–1091. [DOI] [PubMed] [Google Scholar]
- 146.Ribeiro JS, Bordini EAF, Ferreira JA, Mei L, Dubey N, Fenno JC, Piva E, Lund RG, Schwendeman A and Bottino MC, ACS Appl. Mater. Interfaces, 2020, 12, 16006–16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Klotz BJ, Gawlitta D, Rosenberg AJWP, Malda J and Melchels FPW, Trends Biotechnol, 2016, 34, 394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hickey RJ and Pelling AE, Front. Bioeng. Biotechnol, 2019, 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tabary N, Chai F, Blanchemain N, Neut C, Pauchet L, Bertini S, Delcourt-Debruyne E, Hildebrand HF and Martel B, Acta Biomater, 2014, 10, 318–329. [DOI] [PubMed] [Google Scholar]
- 150.de FSM. Lima, G. M. de Pádua, M. G. da C. Sousa, M. de S. Freire, O. L. Franco and T. M. B. Rezende, Biotechnol. Adv, 2015, 33, 203–213. [DOI] [PubMed] [Google Scholar]
- 151.Turner SR, Love RM and Lyons KM, Int. Endod. J, 2004, 37, 664–671. [DOI] [PubMed] [Google Scholar]
- 152.Kajwadkar R, Shin JM, Lin GH, Fenno JC, Rickard AH and Kapila YL, J. Endod, 2017, 43, 989–994. [DOI] [PubMed] [Google Scholar]
- 153.Tong Z, Huang L, Ling J, Mao X, Ning Y and Deng D, PLoS One, 2014, 9, e90235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Arora A, Majhi S and Mishra A, J. Biosci, 2018, 43, 707–715. [PubMed] [Google Scholar]
- 155.Fujii G, Selsted ME and Eisenberg D, Protein Sci, 1993, 2, 1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dhople V, Krukemeyer A and Ramamoorthy A, Biochim. Biophys. Acta, Biomembr, 2006, 1758, 1499–1512. [DOI] [PubMed] [Google Scholar]
- 157.Song W, Shi Y, Xiao M, Lu H, Qu T, Li P, Wu G and Tian Y, Int. J. Antimicrob. Agents, 2009, 33, 237–243. [DOI] [PubMed] [Google Scholar]
- 158.Hoover DM, Wu Z, Tucker K, Lu W and Lubkowski J, Antimicrob. Agents Chemother, 2003, 47, 2804–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Krishnakumari V, Rangaraj N and Nagaraj R, Antimicrob. Agents Chemother, 2009, 53, 256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yoo YJ, Kwon I, Oh SR, Perinpanayagam H, Lim SM, Ahn KB, Lee Y, Han SH, Chang SW, Baek SH, Zhu Q and Kum KY, J. Endod, 2017, 43, 1857–1861. [DOI] [PubMed] [Google Scholar]
- 161.Bartie KL, Devine DA, Wilson MJ and Lewis MA, Int. Endod. J, 2008, 41, 586–592. [DOI] [PubMed] [Google Scholar]
- 162.de la Fuente-Núñez C, Reffuveille F, Mansour SC, Reckseidler-Zenteno SL, Hernández D, Brackman G, Coenye T and Hancock REW, Chem. Biol, 2015, 22, 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.de la Fuente-Núñez C, Reffuveille F, Haney EF, Straus SK and Hancock REW, PLoS Pathog, 2014, 10, e1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.hu Ye W, Yeghiasarian L, Cutler CW, Bergeron BE, Sidow S, Xu HHK, Na Niu L, Zhi Ma J and Tay FR, J. Dent, 2019, 91, 103231. [DOI] [PubMed] [Google Scholar]
- 165.Hirt H, Hall JW, Larson E and Gorr S, PLoS ONE, 2018, 13(3), e0194900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hamamoto K, Kida Y, Zhang Y, Shimizu T and Kuwano K, Microbiol. Immunol, 2002, 46, 741–749. [DOI] [PubMed] [Google Scholar]
- 167.Di YP, Lin Q, Chen C, Montelaro RC, Doi Y and Deslouches B, Sci. Adv, 2020, 6, eaay6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stevens KA, Sheldon BW, Klapes NA and Klaenhammer TR, Appl. Environ. Microbiol, 1991, 57, 3613–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Slots J, Periodontol. 2000, 2017, 75, 7–23. [DOI] [PubMed] [Google Scholar]
- 170.Krishna R and De Stefano JA, Periodontol. 2000, 2016, 71, 113–127. [DOI] [PubMed] [Google Scholar]
- 171.Chee E and Brown AC, Biomater. Sci, 2020, 8, 1089–1100. [DOI] [PubMed] [Google Scholar]
- 172.Elisha IL, Botha FS, McGaw LJ and Eloff JN, BMC Complementary Altern. Med, 2017, 17, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cowan MM, Clin. Microbiol. Rev, 1999, 12, 564–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Karygianni L, Al-Ahmad A, Argyropoulou A, Hellwig E, Anderson AC and Skaltsounis AL, Front. Microbiol, 2015, 6, 1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vinothkumar TS, Rubin MI, Balaji L and Kandaswamy D, J. Conserv. Dent, 2013, 16, 167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Atsuta I, Ayukawa Y, Kondo R, Oshiro W, Matsuura Y, Furuhashi A, Tsukiyama Y and Koyano K, J. Prosthodont. Res, 2016, 60, 3–11. [DOI] [PubMed] [Google Scholar]
- 177.Ye D and Peramo A, Br. Med. Bull, 2014, 109, 3–18. [DOI] [PubMed] [Google Scholar]
- 178.Abdallah M-N, Badran Z, Ciobanu O, Hamdan N and Tamimi F, Adv. Healthcare Mater, 2017, 6, 1700549. [DOI] [PubMed] [Google Scholar]
- 179.Souza JCM, Sordi MB, Kanazawa M, Ravindran S, Henriques B, Silva FS, Aparicio C and Cooper LF, Acta Biomater, 2019, 94, 112–131. [DOI] [PubMed] [Google Scholar]
- 180.Schliephake H and Scharnweber D, J. Mater. Chem, 2008, 18, 2404–2414. [Google Scholar]
- 181.Boyan BD, Hummert TW, Dean DD and Schwartz Z, Biomaterials, 1996, 17, 137–146. [DOI] [PubMed] [Google Scholar]
- 182.Rasouli R, Barhoum A and Uludag H, Biomater. Sci, 2018, 6, 1312–1338. [DOI] [PubMed] [Google Scholar]
- 183.Roccuzzo M, Bunino M, Prioglio F and Bianchi SD, Clin. Oral. Implants Res, 2001, 12, 572–578. [DOI] [PubMed] [Google Scholar]
- 184.Buser D, Nydegger T, Oxland T, Cochran DL, Schenk RK, Hirt HP, Snétivy D and Nolte LP, J. Biomed. Mater. Res, 1999, 45, 75–83. [DOI] [PubMed] [Google Scholar]
- 185.Yan WQ, Nakamura T, Kawanabe K, Nishigochi S, Oka M and Kokubo T, Biomaterials, 1997, 18, 1185–1190. [DOI] [PubMed] [Google Scholar]
- 186.Curry AS, Pensa NW, Barlow AM and Bellis SL, Matrix Biol, 2016, 52.–, 397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zigterman BGR, Van den Borre C, Braem A and Mommaerts MY, Biointerphases, 2019, 14, 040802. [DOI] [PubMed] [Google Scholar]
- 188.Ruoslahti E and Pierschbacher MD, Science, 1987, 238, 491–497. [DOI] [PubMed] [Google Scholar]
- 189.Pierschbacher MD and Ruoslahti E, Nature, 1984, 309, 30–33. [DOI] [PubMed] [Google Scholar]
- 190.Xiao SJ, Textor M, Spencer ND and Sigrist H, Langmuir, 1998, 14, 5507–5516. [Google Scholar]
- 191.Hersel U, Dahmen C and Kessler H, Biomaterials, 2003, 24, 4385–4415. [DOI] [PubMed] [Google Scholar]
- 192.Bellis SL, Biomaterials, 2011, 32, 4205–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schliephake H, Scharnweber D, Dard M, Rössler S, Sewing A, Meyer J and Hoogestraat D, Clin. Oral. Implants Res, 2002, 13, 312–319. [DOI] [PubMed] [Google Scholar]
- 194.Perlin L, MacNeil S and Rimmer S, Soft Matter, 2008, 4, 2331. [Google Scholar]
- 195.Schuler M, Owen GR, Hamilton DW, de Wild M, Textor M, Brunette DM and Tosatti SGP, Biomaterials, 2006, 27, 4003–4015. [DOI] [PubMed] [Google Scholar]
- 196.Collins MN and Birkinshaw C, Carbohydr. Polym, 2013, 92, 1262–1279. [DOI] [PubMed] [Google Scholar]
- 197.Jin C, Ren L, Ding H, Shi G, Lin H and Zhang F, J. Biomed. Mater. Res., Part B, 2012, 100B, 2167–2177. [DOI] [PubMed] [Google Scholar]
- 198.Vidal G, Blanchi T, Mieszawska AJ, Calabrese R, Rossi C, Vigneron P, Duval JL, Kaplan DL and Egles C, Acta Biomater, 2013, 9, 4935–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fernandez-Garcia E, Chen X, Gutierrez-Gonzalez CF, Fernandez A, Lopez-Esteban S and Aparicio C, J. Dent, 2015, 43, 1162–1174. [DOI] [PubMed] [Google Scholar]
- 200.Yang Z, Liu M, Yang Y, Zheng M, Yang Y, Liu X and Tan J, RSC Adv, 2020, 10, 6200–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Özkurt Z and Kazazoğlu E, J. Oral Implantol, 2011, 37, 367–376. [DOI] [PubMed] [Google Scholar]
- 202.Fischer NG, Wong J, Baruth A and Cerutis DR, Materials, 2017, 10, 1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Altmann B, Karygianni L, Al-Ahmad A, Butz F, Bächle M, Adolfsson E, Fürderer T, Courtois N, Palmero P, Follo M, Chevalier J, Steinberg T and Kohal RJ, Adv. Funct. Mater, 2017, 27, 1702512. [Google Scholar]
- 204.Arena A, Prete F, Rambaldi E, Bignozzi MC, Monaco C, Di Fiore A and Chevalier J, Nanomaterials, 2019, 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hormia M, Sahlberg C, Thesleff I and Airenne T, J. Dent. Res, 1998, 77, 1479–1485. [DOI] [PubMed] [Google Scholar]
- 206.Marinkovich MP, Nat. Rev. Cancer, 2007, 7, 370–380. [DOI] [PubMed] [Google Scholar]
- 207.Farach-Carson MC, Warren CR, Harrington DA and Carson DD, Matrix Biol, 2014, 34, 64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Aumailley M, Methods Cell Biol, 2018, 143, 187–205. [DOI] [PubMed] [Google Scholar]
- 209.Kim JM, Park WH and Min BM, Exp. Cell Res, 2005, 304, 317–327. [DOI] [PubMed] [Google Scholar]
- 210.Koidou VP, Argyris PP, Skoe EP, Mota Siqueira J, Chen X, Zhang L, Hinrichs JE, Costalonga M and Aparicio C, Biomater. Sci, 2018, 6, 1936–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fischer NG, He J and Aparicio C, Coatings, 2020, 10, 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Werner S, Huck O, Frisch B, Vautier D, Elkaim R, Voegel JC, Brunel G and Tenenbaum H, Biomaterials, 2009, 30, 2291–2301. [DOI] [PubMed] [Google Scholar]
- 213.Choi J, Kim S, Bin Jo S, Kang HK, Jung SY, Kim SW, Min B and Yeo IL, J. Biomed. Mater. Res., Part A, 2020, 108(5), 1214–1222. [DOI] [PubMed] [Google Scholar]
- 214.Min S-K, Kang HK, Jang DH, Jung SY, Kim OB, Min B-M and Yeo I-S, BioMed Res. Int, 2013, 2013, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kang HK, Kim OB, Min S-K, Jung SY, Jang DH, Kwon T-K, Min B-M and Yeo I-S, Biomaterials, 2013, 34, 4027–4037. [DOI] [PubMed] [Google Scholar]
- 216.Colognato H and Yurchenco PD, Dev. Dyn, 2000, 218, 213–234. [DOI] [PubMed] [Google Scholar]
- 217.Tashiro K, Sephel GC, Weeks B, Sasaki M, Martin GR, Kleinman HK and Yamada Y, J. Biol. Chem, 1989, 264, 16174–16182. [PubMed] [Google Scholar]
- 218.Lin X, Takahashi K, Liu Y and Zamora PO, Biochim. Biophys. Acta, Gen. Subj, 2006, 1760, 1403–1410. [DOI] [PubMed] [Google Scholar]
- 219.Graf J, Iwamoto Y, Sasaki M, Martin GR, Kleinman HK, Robey FA and Yamada Y, Cell, 1987, 48, 989–996. [DOI] [PubMed] [Google Scholar]
- 220.Matter ML, Cell Biol J., 1994, 124, 1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hunter DD, Shah V, Merlie JP and Sanes JR, Nature, 1989, 338, 229–234. [DOI] [PubMed] [Google Scholar]
- 222.Javed F, Al Amri MD, Kellesarian SV, Al-Askar M, Al-Kheraif AA and Romanos GE, Arch. Oral Biol, 2016, 68, 153–161. [DOI] [PubMed] [Google Scholar]
- 223.Barros D, Amaral IF and Pêgo AP, Biomacromolecules, 2020, 21(2), 276–293. [DOI] [PubMed] [Google Scholar]
- 224.Birk DE and Brückner P, The Extracellular Matrix: an Overview, Springer Berlin Heidelberg, Berlin, Heidelberg, 2011, vol. 1, pp. 77–115. [Google Scholar]
- 225.Miranda-Nieves D and Chaikof EL, ACS Biomater. Sci. Eng, 2017, 3, 694–711. [DOI] [PubMed] [Google Scholar]
- 226.Birk DE and Brückner P, The Extracellular Matrix: an Overview, Springer Berlin Heidelberg, Berlin, Heidelberg, 2011, pp. 77–115. [Google Scholar]
- 227.Sharan J, Koul V, Dinda AK, Kharbanda OP, Lale SV, Duggal R, Mishra M, Gupta G and Singh MP, Colloids Surf., B, 2018, 161, 1–9. [DOI] [PubMed] [Google Scholar]
- 228.Stewart C, Akhavan B, Wise SG and Bilek MMM, Prog. Mater. Sci, 2019, 106, 100588. [Google Scholar]
- 229.Chouirfa H, Evans MDM, Bean P, Saleh-Mghir A, Crémieux AC, Castner DG, Falentin-Daudré C and Migonney V, ACS Appl. Mater. Interfaces, 2018, 10, 1480–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Marín-Pareja N, Salvagni E, Guillem-Marti J, Aparicio C and Ginebra MP, Colloids Surf., B, 2014, 122, 601–610. [DOI] [PubMed] [Google Scholar]
- 231.Marín-Pareja N, Cantini M, González-García C, Salvagni E, Salmerón-Sánchez M and Ginebra MP, ACS Appl. Mater. Interfaces, 2015, 7, 20667–20677. [DOI] [PubMed] [Google Scholar]
- 232.Zhu Y, Liu D, Wang X, He Y, Luan W, Qi F and Ding J, J. Mater. Chem. B, 2019, 7, 2019–2031. [DOI] [PubMed] [Google Scholar]
- 233.Lyu Q, Hsueh N and Chai CLL, ACS Biomater. Sci. Eng, 2019, 5, 2708–2724. [DOI] [PubMed] [Google Scholar]
- 234.Lee H, Dellatore SM, Miller WM and Messersmith PB, Science, 2007, 318, 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pankov R and Yamada KM, J. Cell Sci, 2002, 115, 3861–3863. [DOI] [PubMed] [Google Scholar]
- 236.Dean JW, Culbertson KC and D’Angelo AM, Int. J. Oral Maxillofac. Implants, 1995, 10, 1–17. [PubMed] [Google Scholar]
- 237.Chimutengwende-Gordon M, Pendegrass C and Blunn G, Biomed. Mater, 2011, 6, 025008. [DOI] [PubMed] [Google Scholar]
- 238.Chimutengwende-Gordon M, Pendegrass C and Blunn G, Bone Jt. J, 2017, 99B, 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sprott MR, Gallego-Ferrer G, Dalby MJ, Salmerón-Sánchez M and Cantini M, Adv. Healthcare Mater, 2019, 8, 1801469. [DOI] [PubMed] [Google Scholar]
- 240.Grigoriou E, Cantini M, Dalby MJ, Petersen A and Salmeron-Sanchez M, Biomater. Sci, 2017, 5, 1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rico P, Mnatsakanyan H, Dalby MJ and Salmerón-Sánchez M, Adv. Funct. Mater, 2016, 26, 6563–6573. [Google Scholar]
- 242.Pegueroles M, Tonda-Turo C, Planell JA, Gil FJ and Aparicio C, Biointerphases, 2012, 7, 1–13. [DOI] [PubMed] [Google Scholar]
- 243.Felgueiras HP, Evans MDM and Migonney V, Acta Biomater, 2015, 28, 225–233. [DOI] [PubMed] [Google Scholar]
- 244.Herranz-Diez C, Mas-Moruno C, Neubauer S, Kessler H, Gil FJ, Pegueroles M, Manero JM and Guillem-Marti J, ACS Appl. Mater. Interfaces, 2016, 8, 2517–2525. [DOI] [PubMed] [Google Scholar]
- 245.Torres P, Castro M, Reyes M and Torres VA, Oral Dis, 2018, 24, 1150–1160. [DOI] [PubMed] [Google Scholar]
- 246.Van Dijk IA, Nazmi K, Bolscher JGM, Veerman ECI and Stap J, FASEB J, 2015, 29, 3124–3132. [DOI] [PubMed] [Google Scholar]
- 247.van Dijk IA, Beker AF, Jellema W, Nazmi K, Wu G, Wismeijer D, Krawczyk PM, Bolscher JGM, Veerman ECI and Stap J, J. Dent. Res, 2017, 96, 430–436. [DOI] [PubMed] [Google Scholar]
- 248.Van Dijk IA, Ferrando ML, Van Der Wijk AE, Hoebe RA, Nazmi K, De Jonge WJ, Krawczyk PM, Bolscher JGM, Veerman ECI and Stap J, FASEB J, 2017, 31, 3922–3933. [DOI] [PubMed] [Google Scholar]
- 249.Kaigler D, Cirelli JA and Giannobile WV, Expert Opin. Drug Delivery, 2006, 3, 647–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chen P-H, Chen X and He X, Biochim. Biophys. Acta, 2013, 1834, 2176–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rathva V, Clin., Cosmet. Invest. Dent, 2011, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bates C, Marino V, Fazzalari NL and Bartold PM, Clin. Implant Dent. Relat. Res, 2013, 15, 53–63. [DOI] [PubMed] [Google Scholar]
- 253.Bikfalvi A, Klein S, Pintucci G and Rifkin DB, Endocr. Rev, 1997, 18, 26–45. [DOI] [PubMed] [Google Scholar]
- 254.Mutsuzaki H, Ito A, Sogo Y, Sakane M, Oyane A and Ochiai N, Arch. Orthop. Trauma Surg, 2012, 132, 113–121. [DOI] [PubMed] [Google Scholar]
- 255.Scharnweber D, Bierbaum S and Wolf-Brandstetter C, FEBS Lett, 2018, 592, 2181–2196. [DOI] [PubMed] [Google Scholar]
- 256.Atluri K, Lee J, Seabold D, Elangovan S and Salem A, Int. J. Oral Maxillofac. Implants, 2017, 32, e83–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Maeda M, Kojima T, Song Y and Takayama S, Adv. Healthcare Mater, 2019, 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Van Den Beucken JJJP, Walboomers XF, Vos MRJ, Sommerdijk NAJM, Nolte RJM and Jansen JA, J. Biomed. Mater. Res., Part A, 2006, 77, 202–211. [DOI] [PubMed] [Google Scholar]
- 259.Yang G, Zhang J, Dong W, Liu L, Shi J and Wang H, Sci. Rep, 2016, 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang J, He X-T, Xu X-Y, Yin Y, Li X, Bi C-S, Hong Y-L and Chen F-M, J. Mater. Chem. B, 2019, 7, 7415–7427. [DOI] [PubMed] [Google Scholar]
- 261.Muxika A, Etxabide A, Uranga J, Guerrero P and de la Caba K, Int. J. Biol. Macromol, 2017, 105, 1358–1368. [DOI] [PubMed] [Google Scholar]
- 262.Laird NZ, Malkawi WI, Chakka JL, Acri T, Elangovan S and Salem AK, J. Tissue Eng. Regen. Med, 2020, 14(4), 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sumigray KD and Lechler T, Curr. Top. Dev. Biol, 2015, 112, 383–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lee G, Kim HJ and Kim HM, J. Dent. Res, 2016, 95, 284–291. [DOI] [PubMed] [Google Scholar]
- 265.Pendegrass CJ, Tucker B, Patel S, Dowling R and Blunn GW, J. Biomed. Mater. Res., Part A, 2012, 100A, 3463–3471. [DOI] [PubMed] [Google Scholar]
- 266.Maeno M, Lee C, Kim DM, Da Silva J, Nagai S, Sugawara S, Nara Y, Kihara H and Nagai M, J. Dent. Res, 2017, 96, 633–639. [DOI] [PubMed] [Google Scholar]
- 267.Beyeler M, Schild C, Lutz R, Chiquet M and Trueb B, Exp. Cell Res, 2010, 316, 1202–1212. [DOI] [PubMed] [Google Scholar]
- 268.Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura T, Longenecker G, Krebsbach PH, Nanci A, Kulkarni AB and Yamada Y, J. Cell Biol, 2004, 167, 973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Tamura RN, Oda D, Quaranta V, Plopper G, Lambert R, Glaser S and Jones JC, J. Periodontal Res, 1997, 32, 287–294. [DOI] [PubMed] [Google Scholar]
- 270.El-Ghannam A, Starr L and Jones J, J. Biomed. Mater. Res, 1998, 41, 30–40. [DOI] [PubMed] [Google Scholar]
- 271.Abdallah MN, Tran SD, Abughanam G, Laurenti M, Zuanazzi D, Mezour MA, Xiao Y, Cerruti M, Siqueira WL and Tamimi F, Acta Biomater, 2017, 54, 150–163. [DOI] [PubMed] [Google Scholar]
- 272.Liu M, Zhou J, Yang Y, Zheng M, Yang J and Tan J, Colloids Surf., B, 2015, 136, 74–83. [DOI] [PubMed] [Google Scholar]
- 273.Steffi C, Shi Z, Kong CH and Wang W, J. R. Soc., Interface, 2019, 16, 20180799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gomez-Florit M, Pacha-Olivenza MA, Fernández-Calderón MC, Córdoba A, González-Martín ML, Monjo M and Ramis JM, Sci. Rep, 2016, 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yang M, Jiang P, Ge Y, Lan F, Zhou X, He J and Wu Y, J. Biomater. Appl, 2018, 32, 1071–1082. [DOI] [PubMed] [Google Scholar]
- 276.Varga S, Spalj S, Lapter Varga M, Anic Milosevic S, Mestrovic S and Slaj M, Eur. J. Orthod, 2011, 33, 427–433. [DOI] [PubMed] [Google Scholar]
- 277.Gratton DG, Aquilino SA and Stanford CM, J. Prosthet. Dent, 2001, 85, 47–52. [DOI] [PubMed] [Google Scholar]
- 278.Fincham AG, Moradian-Oldak J and Simmer JP, J. Struct. Biol, 1999, 126, 270–299. [DOI] [PubMed] [Google Scholar]
- 279.Lacruz RS, Habelitz S, Wright JT and Paine ML, Physiol. Rev, 2017, 97, 939–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Beniash E, Stifler CA, Sun CY, Jung GS, Qin Z, Buehler MJ and Gilbert PUPA, Nat. Commun, 2019, 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Koldehoff J, Swain MV and Schneider GA, Acta Biomater, 2020, 104, 17–27. [DOI] [PubMed] [Google Scholar]
- 282.Goldberg M, Kulkarni AB, Young M and Boskey A, Front. Biosci. (Elite Ed.), 2011, 3, 711–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Marshall GW, Marshall SJ, Kinney JH and Balooch M, J. Dent, 1997, 25, 441–458. [DOI] [PubMed] [Google Scholar]
- 284.Goldberga I, Li R and Duer MJ, Acc. Chem. Res, 2018, 51, 1621–1629. [DOI] [PubMed] [Google Scholar]
- 285.Shoulders MD and Raines RT, Annu. Rev. Biochem, 2009, 78, 929–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Gelse K, Pöschl E and Aigner T, Adv. Drug Delivery Rev, 2003, 55, 1531–1546. [DOI] [PubMed] [Google Scholar]
- 287.Weiner S and Wagner HD, Annu. Rev. Mater. Sci, 1998, 28, 271–298. [Google Scholar]
- 288.Cölfen H, Nat. Mater, 2010, 9, 960–961. [DOI] [PubMed] [Google Scholar]
- 289.Jackson SL, Vann WF, Kotch JB, Pahel BT and Lee JY, Am. J. Public Health, 2011, 101, 1900–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Sherif E and Alvaro M, Adv. Healthcare Mater, 2018, 1800178. [Google Scholar]
- 291.Song Q, Jiao K, Tonggu L, Wang LG, Zhang SL, Yang YD, Zhang L, Bian JH, Hao DX, Wang CY, Ma YX, Arola DD, Breschi L, Chen JH, Tay FR and Niu LN, Sci. Adv, 2019, 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Nudelman F, Pieterse K, George A, Bomans PHH, Friedrich H, Brylka LJ, Hilbers PAJ, De With G and Sommerdijk NAJM, Nat. Mater, 2010, 9, 1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Olszta MJ, Cheng X, Jee SS, Kumar R, Kim YY, Kaufman MJ, Douglas EP and Gower LB, Mater. Sci. Eng., R, 2007, 58, 77–116. [Google Scholar]
- 294.Li Y and Aparicio C, PLoS One, 2013, 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.George A and Veis A, Chem. Rev, 2008, 108, 4670–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Boskey AL and Villarreal-Ramirez E, Matrix Biol, 2016, 52–54, 43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.van der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Dunker AK, Fuxreiter M, Gough J, Gsponer J, Jones DT, Kim PM, Kriwacki RW, Oldfield CJ, Pappu RV, Tompa P, Uversky VN, Wright PE and Babu MM, Chem. Rev, 2014, 114, 6589–6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Qi Y, Ye Z, Fok A, Holmes BN, Espanol M, Ginebra M and Aparicio C, ACS Biomater. Sci. Eng, 2018, 4, 2758–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.George A, Bannon L, Sabsay B, Dillon JW, Malone J, Veis A, Jenkins NA, Gilbert DJ and Copeland NG, J. Biol. Chem, 1996, 271, 32869–32873. [DOI] [PubMed] [Google Scholar]
- 300.Hunter GK, Kyle CL and Goldberg HA, Biochem. J, 1994, 300, 723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Huang B, Sun Y, Maciejewska I, Qin D, Peng T, McIntyre B, Wygant J, Butler WT and Qin C, Eur. J. Oral Sci, 2008, 116, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.He G, Gajjeraman S, Schultz D, Cookson D, Qin C, Butler WT, Hao J and George A, Biochemistry, 2005, 44, 16140–16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Zurick KM, Qin C and Bernards MT, J. Biomed. Mater. Res., Part A, 2013, 101A, 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Niu LN, Jee SE, Jiao K, Tonggu L, Li M, Wang L, Yang YD, Bian JH, Breschi L, Jang SS, Chen JH, Pashley DH and Tay FR, Nat. Mater, 2017, 16, 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Gower LB and Odom DJ, J. Cryst. Growth, 2000, 210, 719–734. [Google Scholar]
- 306.Li Y, Thula TT, Jee S, Perkins SL, Aparicio C, Douglas EP and Gower LB, Biomacromolecules, 2012, 13, 49–59. [DOI] [PubMed] [Google Scholar]
- 307.Gower LA and Tirrell DA, J. Cryst. Growth, 1998, 191, 153–160. [Google Scholar]
- 308.Niu LN, Jiao K, Ryou H, Diogenes A, Yiu CKY, Mazzoni A, Chen JH, Arola DD, Hargreaves KM, Pashley DH and Tay FR, Biomacromolecules, 2013, 14, 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Qi Y, Cheng Z, Ye Z, Zhu H and Aparicio C, ACS Appl. Mater. Interfaces, 2019, 11(31), 27598–27604. [DOI] [PubMed] [Google Scholar]
- 310.Cheng Z, Ye Z, Natan A, Ma Y, Li H, Chen Y, Wan L, Aparicio C and Zhu H, ACS Appl. Mater. Interfaces, 2019, 11, 42486–42495. [DOI] [PubMed] [Google Scholar]
- 311.Liu Y, Li N, Qi YP, Dai L, Bryan TE, Mao J, Pashley DH and Tay FR, Adv. Mater, 2011, 23, 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Thrivikraman G, Athirasala A, Gordon R, Zhang L, Bergan R, Keene DR, Jones JM, Xie H, Chen Z, Tao J, Wingender B, Gower L, Ferracane JL and Bertassoni LE, Nat. Commun, 2019, 10, 3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.França CM, Thrivikraman G, Athirasala A, Tahayeri A, Gower LB and Bertassoni LE, J. Biomed. Mater. Res., Part B, 2018, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Rodriguez DE, Thula-Mata T, Toro EJ, Yeh Y-W, Holt C, Holliday LS and Gower LB, Acta Biomater, 2014, 10, 494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Bacino M, Girn V, Nurrohman H, Saeki K, Marshall SJ, Gower L, Saeed E, Stewart R, Le T, Marshall GW and Habelitz S, Dent. Mater, 2019, 35, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Saeki K, Chien YC, Nonomura G, Chin AF, Habelitz S, Gower LB, Marshall SJ and Marshall GW, Arch. Oral Biol, 2017, 82, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Nurrohman H, Saeki K, Carneiro KMM, Chien YC, Djomehri S, Ho SP, Qin C, Gower LB, Marshall SJ, Marshall GW and Habelitz S, J. Mater. Res, 2016, 31, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Elsharkawy S, Al-Jawad M, Pantano MF, Tejeda-Montes E, Mehta K, Jamal H, Agarwal S, Shuturminska K, Rice A, Tarakina NV, Wilson RM, Bushby AJ, Alonso M, Rodriguez-Cabello JC, Barbieri E, del Río Hernández A, Stevens MM, Pugno NM, Anderson P and Mata A, Nat. Commun, 2018, 9, 2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Fincham AG, Moradian-Oldak J, Diekwisch TGH, Lyaruu DM, Wright JT, Bringas P and Slavkin HC, J. Struct. Biol, 1995, 115, 50–59. [DOI] [PubMed] [Google Scholar]
- 320.Li Y, Rodriguez-Cabello JC and Aparicio C, ACS Appl. Mater. Interfaces, 2017, 9, 5838–5846. [DOI] [PubMed] [Google Scholar]
- 321.Shuturminska K, Tarakina NV, Azevedo HS, Bushby AJ, Mata A, Anderson P and Al-Jawad M, Front. Physiol, 2017, 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Tejeda-Montes E, Klymov A, Nejadnik MR, Alonso M, Rodriguez-Cabello JC, Walboomers XF and Mata A, Biomaterials, 2014, 35, 8339–8347. [DOI] [PubMed] [Google Scholar]
- 323.Li Y, Chen X, Fok A, Rodriguez-Cabello JC and Aparicio C, ACS Appl. Mater. Interfaces, 2015, 7, 25784–25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Li Y, Chen X, Ribeiro AJ, Jensen ED, Holmberg KV, Rodriguez-Cabello JC and Aparicio C, Adv. Healthcare Mater, 2014, 3, 1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Bansal A, Shetty D, Bindal R and Pathak A, J. Oral Maxillofac. Pathol, 2012, 16, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Margolis HC, Beniash E and Fowler CE, J. Dent. Res, 2006, 85, 775–793. [DOI] [PubMed] [Google Scholar]
- 327.Beniash E, Simmer JP and Margolis HC, J. Dent. Res, 2012, 91, 967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Kwak S-Y, Kim S, Yamakoshi Y, Simmer JP, Beniash E and Margolis HC, Connect. Tissue Res, 2014, 55, 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Masica DL, Gray JJ and Shaw WJ, J. Phys. Chem. C, 2011, 115, 13775–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Le Norcy E, Kwak S-Y, Allaire M, Fratzl P, Yamakoshi Y, Simmer JP and Margolis HC, Eur. J. Oral Sci, 2011, 119, 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Le Norcy E, Kwak S-Y, Wiedemann-Bidlack FB, Beniash E, Yamakoshi Y, Simmer JP and Margolis HC, J. Dent. Res, 2011, 90, 1091–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Shin N-Y, Yamazaki H, Beniash E, Yang X, Margolis SS, Pugach MK, Simmer JP and Margolis HC, J. Biol. Chem, 2020, 295, 1943–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Brookes SJ, Robinson C, Kirkham J and Bonass WA, Arch. Oral Biol, 1995, 40, 1–14. [DOI] [PubMed] [Google Scholar]
- 334.Engelberth SA, Bacino MS, Sandhu S, Li W, Bonde J and Habelitz S, Biomacromolecules, 2018, 19, 3917–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Carneiro KMM, Zhai H, Zhu L, Horst JA, Sitlin M, Nguyen M, Wagner M, Simpliciano C, Milder M, Chen C-L, Ashby P, Bonde J, Li W and Habelitz S, Sci. Rep, 2016, 6, 23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Snead ML, Connect. Tissue Res, 2003, 44(Suppl 1), 47–51. [PubMed] [Google Scholar]
- 337.Paine ML, Luo W, Zhu D-H, Bringas P and Snead ML, J. Bone Miner. Res, 2003, 18, 466–472. [DOI] [PubMed] [Google Scholar]
- 338.Gungormus M, Oren EE, Horst JA, Fong H, Hnilova M, Somerman MJ, Snead ML, Samudrala R, Tamerler C and Sarikaya M, Int. J. Oral Sci, 2012, 4, 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Mounir MMF, Matar MA, Lei Y and Snead ML, J. Endod, 2016, 42, 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Mukherjee K, Ruan Q, Nutt S, Tao J, De Yoreo JJ and Moradian-Oldak J, ACS Omega, 2018, 3, 2546–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Ramasubbu N, Thomas LM, Bhandary KK and Levine MJ, Crit. Rev. Oral Biol. Med, 1993, 4, 363–370. [DOI] [PubMed] [Google Scholar]
- 342.Shah S, Kosoric J, Hector MP and Anderson P, Eur. J. Oral Sci, 2011, 119, 13–18. [DOI] [PubMed] [Google Scholar]
- 343.Raj PA, Johnsson M, Levine MJ and Nancollas GH, J. Biol. Chem, 1992, 267, 5968–5976. [PubMed] [Google Scholar]
- 344.Santos O, Kosoric J, Hector MP, Anderson P and Lindh L, J. Colloid Interface Sci, 2008, 318, 175–182. [DOI] [PubMed] [Google Scholar]
- 345.Ndao M, Ash JT, Stayton PS and Drobny GP, Surf. Sci, 2010, 604, L39–L42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Long JR, Shaw WJ, Stayton PS and Drobny GP, Biochemistry, 2001, 40, 15451–15455. [DOI] [PubMed] [Google Scholar]
- 347.Goobes G, Goobes R, Schueler-Furman O, Baker D, Stayton PS and Drobny GP, Proc. Natl. Acad. Sci. U. S. A, 2006, 103, 16083–16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Masica DL and Gray JJ, Biophys. J, 2009, 96, 3082–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wang K, Wang X, Li H, Zheng S, Ren Q, Wang Y, Niu Y, Li W, Zhou X and Zhang L, RSC Adv, 2018, 8, 1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Valente MT, Moffa EB, Crosara KTB, Xiao Y, de Oliveira TM, Machado MADAM and Siqueira WL, Sci. Rep, 2018, 8, 3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Yang X, Huang F, Xu X, Liu Y, Ding C, Wang K, Guo A, Li W and Li J, Chem. Mater, 2017, 29, 5663–5670. [Google Scholar]
- 352.Grohe B, Mater. Sci. Eng., C, 2017, 77, 58–68. [DOI] [PubMed] [Google Scholar]
- 353.Kosoric J, Williams RAD, Hector MP and Anderson P, Int. J. Pept. Res. Ther, 2007, 13, 497–503. [Google Scholar]
- 354.Nelea V, Chien YC, Paquette J and McKee MD, Cryst. Growth Des, 2014, 14, 979–987. [Google Scholar]
- 355.Sodek J, Ganss B and McKee MD, Crit. Rev. Oral Biol. Med, 2000, 11, 279–303. [DOI] [PubMed] [Google Scholar]
- 356.Addadi L and Weiner S, Proc. Natl. Acad. Sci. U. S. A, 1985, 82, 4110–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Chien YC, Mansouri A, Jiang W, Khan SR, Gray JJ and McKee MD, J. Struct. Biol, 2018, 204, 131–144. [DOI] [PubMed] [Google Scholar]
- 358.Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, Xiao J, Grishin NV and Dixon JE, Science, 2012, 336, 1150–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Yuan Q, Jiang Y, Zhao X, Sato T, Densmore M, Schüler C, Erben RG, McKee MD and Lanske B, J. Bone Miner. Res, 2014, 29, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Addison WN, Azari F, Sørensen ES, Kaartinen MT and McKee MD, J. Biol. Chem, 2007, 282, 15872–15883. [DOI] [PubMed] [Google Scholar]
- 361.Gericke A, Qin C, Spevak L, Fujimoto Y, Butler WT, Sørensen ES and Boskey AL, Calcif. Tissue Int, 2005, 77, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kalmar L, Homola D, Varga G and Tompa P, Bone, 2012, 51, 528–534. [DOI] [PubMed] [Google Scholar]
- 363.Mai S, Wei C-C, Gu L, Tian F, Arola DD, Chen J, Jiao Y, Pashley DH, Niu L and Tay FR, Acta Biomater, 2017, 57, 435–448. [DOI] [PubMed] [Google Scholar]
- 364.Gu LS, Cai X, Guo JM, Pashley DH, Breschi L, Xu HHK, Wang XY, Tay FR and Niu LN, J. Dent. Res, 2019, 98, 186–193. [DOI] [PubMed] [Google Scholar]
- 365.Niu L, Zhang W, Pashley DH, Breschi L, Mao J, Chen J and Tay FR, Dent. Mater, 2014, 30, 77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Huang X, Yang H, Luo T, Huang C, Tay FR and Niu L, Acta Biomater, 2018, 67, 366–377. [DOI] [PubMed] [Google Scholar]
- 367.El-Fiqi A, Kim J-H and Kim H, Mater. Sci. Eng., C, 2020, 110660. [DOI] [PubMed] [Google Scholar]
- 368.Choi S, Friedrichs J, Song YH, Werner C, Estroff LA and Fischbach C, Biomaterials, 2019, 198, 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Coyac BR, Chicatun F, Hoac B, Nelea V, Chaussain C, Nazhat SN and McKee MD, J. Dent. Res, 2013, 92, 648–654. [DOI] [PubMed] [Google Scholar]
- 370.Baldo BA, Drug Saf, 2015, 38, 455–479. [DOI] [PubMed] [Google Scholar]
- 371.Tamerler C and Sarikaya M, Acta Biomater, 2007, 3, 289–299. [DOI] [PubMed] [Google Scholar]
- 372.Sarikaya M, Tamerler C, Jen AK-Y, Schulten K and Baneyx F, Nat. Mater, 2003, 2, 577–585. [DOI] [PubMed] [Google Scholar]
- 373.Lautenslager GT and Simpson LL, Adv. Mol. Cell Biol, 1994, 9, 233–262. [Google Scholar]
- 374.Corson TW, Aberle N and Crews CM, ACS Chem. Biol, 2008, 3, 677–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Borsari C, Trader DJ, Tait A and Costi MP, J. Med. Chem, 2020, 63, 1908–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kacar T, Zin MT, So C, Wilson B, Ma H, Gul-Karaguler N, Jen AK-Y, Sarikaya M and Tamerler C, Biotechnol. Bioeng, 2009, 103, 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Zhou Y, Snead ML and Tamerler C, Nanomedicine, 2015, 11, 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Tamerler C, Khatayevich D, Gungormus M, Kacar T, Oren EE, Hnilova M and Sarikaya M, Biopolymers, 2010, 94, 78–94. [DOI] [PubMed] [Google Scholar]
- 379.Zhang S, Karaca BT, VanOosten SK, Yuca E, Mahalingam S, Edirisinghe M and Tamerler C, Macromol. Rapid Commun, 2015, 36, 1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Boone K, Camarda K, Spencer P and Tamerler C, BMC Bioinf, 2018, 19, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Tamerler C, Khatayevich D, Gungormus M, Kacar T, Oren EE, Hnilova M and Sarikaya M, Biopolymers, 2010, 94, 78–94. [DOI] [PubMed] [Google Scholar]
- 382.Wisdom EC, Zhou Y, Chen C, Tamerler C and Snead ML, ACS Biomater. Sci. Eng, 2020, 6, 2682–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Tamerler C and Sarikaya M, ACS Nano, 2009, 3, 1606–1615. [DOI] [PubMed] [Google Scholar]
- 384.Ye Q, Spencer P, Yuca E and Tamerler C, Macromol. Mater. Eng, 2017, 302, 1600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Gungormus M, Fong H, Kim IW, Evans JS, Tamerler C and Sarikaya M, Biomacromolecules, 2008, 9, 966–973. [DOI] [PubMed] [Google Scholar]
- 386.Wu C-H, Liu I-J, Lu R-M and Wu H-C, J. Biomed. Sci, 2016, 23, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Ramaraju H, Miller SJ and Kohn DH, Biomaterials, 2017, 134, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Ramaraju H, Miller SJ and Kohn DH, Connect. Tissue Res, 2014, 55, 160–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Ramaraju H and Kohn DH, Adv. Healthcare Mater, 2019, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Ramaswamy J, Nam HK, Ramaraju H, Hatch NE and Kohn DH, Biomaterials, 2015, 73, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Yazici H, O’Neill MB, Kacar T, Wilson BR, Oren EE, Sarikaya M and Tamerler C, ACS Appl. Mater. Interfaces, 2016, 8, 5070–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Geng H, Yuan Y, Adayi A, Zhang X, Song X, Gong L, Zhang X and Gao P, Mater. Sci. Eng., C, 2018, 82, 141–154. [DOI] [PubMed] [Google Scholar]
- 393.Yazici H, Fong H, Wilson B, Oren EE, Amos FA, Zhang H, Evans JS, Snead ML, Sarikaya M and Tamerler C, Acta Biomater, 2013, 9, 5341–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Zhang X, Geng H, Gong L, Zhang Q, Li H, Zhang X, Wang Y and Gao P, Int. J. Nanomed, 2018, 13, 5361–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Liu Z, Ma S, Duan S, Xuliang D, Sun Y, Zhang X, Xu X, Guan B, Wang C, Hu M, Qi X, Zhang X and Gao P, ACS Appl. Mater. Interfaces, 2016, 8, 5124–5136. [DOI] [PubMed] [Google Scholar]
- 396.Suárez-González D, Barnhart K, Migneco F, Flanagan C, Hollister SJ and Murphy WL, Biomaterials, 2012, 33, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Yu X, Biedrzycki AH, Khalil AS, Hess D, Umhoefer JM, Markel MD and Murphy WL, Adv. Mater, 2017, 29, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Hudalla GA and Murphy WL, Adv. Funct. Mater, 2011, 21, 1754–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Serizawa T, Sawada T, Matsuno H, Matsubara T and Sato T, J. Am. Chem. Soc, 2005, 127, 13780–13781. [DOI] [PubMed] [Google Scholar]
- 400.Waku T, Imanishi Y, Yoshino Y, Kunugi S, Serizawa T and Tanaka N, Biointerphases, 2017, 12, 021002. [DOI] [PubMed] [Google Scholar]
- 401.Liu Z, Ma S, Lu X, Zhang T, Sun Y, Feng W, Zheng G, Sui L, Wu X, Zhang X and Gao P, Chem. Eng. J, 2019, 356, 117–129. [Google Scholar]
- 402.Spencer P, Ye Q, Song L, Parthasarathy R, Boone K, Misra A and Tamerler C, J. Biomed. Mater. Res., Part B, 2019, 107, 2673–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Han B, Jaurequi J, Tang BW and Nimni ME, J. Biomed. Mater. Res, 2003, 65A, 118–124. [DOI] [PubMed] [Google Scholar]
- 404.Yourdkhani M, Leme-Kraus AA, Aydin B, Bedran-Russo AK and White SR, Dent. Mater, 2017, 33, 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Leme-Kraus AA, Aydin B, Vidal CMP, Phansalkar R, Nam JW, McAlpine J, Pauli GF, Chen S and Bedran-Russo AK, J. Dent. Res, 2017, 96(4), 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Bedran-Russo AKB, Pereira PNR, Duarte WR, Drummond JL and Yamauchi M, J. Biomed. Mater. Res., Part B, 2007, 80B, 268–272. [DOI] [PubMed] [Google Scholar]
- 407.Vidal CMP, Aguiar TR, Phansalkar R, McAlpine JB, Napolitano JG, Chen S-N, Araújo LSN, Pauli GF and Bedran-Russo A, Acta Biomater, 2014, 10, 3288–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Leme-Kraus AA, Phansalkar RS, dos Reis MC, Aydin B, Sousa ABS, Alania Y, McAlpine J, Chen SN, Pauli GF and Bedran-Russo AK, J. Dent. Res, 2020, 99, 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.dos Santos AF, Pacheco JM, Silva PAO, Bedran-Russo AK, Rezende TMB, Pereira PNR and Ribeiro APD, Int. Endod. J, 2019, 52, 424–438. [DOI] [PubMed] [Google Scholar]
- 410.de Souza LC, Rodrigues NS, Cunha DA, Feitosa VP, Santiago SL, Reis A, Loguercio AD, Perdigão J and de PV. A. Saboia, J. Dent., 2020, 103325. [DOI] [PubMed] [Google Scholar]
- 411.de Souza LC, Rodrigues NS, Cunha DA, Feitosa VP, Santiago SL, Reis A, Loguercio AD, de PT. Matos V Saboia de P. A. and Perdigão J, J. Dent, 2019, 81, 7–16. [DOI] [PubMed] [Google Scholar]
- 412.Moussa DG, Kirihara JA, Ye Z, Fischer NG, Khot J, Witthuhn BA and Aparicio C, J. Dent. Res, 2019, 98, 1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Moussa DG, Fok A and Aparicio C, Acta Biomater, 2019, 88, 251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Zhang L, Fang Z, Li Q and Cao CY, J. Mater. Sci.: Mater. Med, 2019, 30, 45. [DOI] [PubMed] [Google Scholar]
- 415.Huang Z-B, Shi X, Mao J and Gong S-Q, Sci. Rep, 2016, 6, 38410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Townsend L, Williams RL, Anuforom O, Berwick MR, Halstead F, Hughes E, Stamboulis A, Oppenheim B, Gough J, Grover L, Scott RAH, Webber M, Peacock AFA, Belli A, Logan A and de Cogan F, J. R. Soc., Interface, 2017, 14, 20160657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Liu Q, Wu B, Yu Q and Wang Y, Dent. Mater. J, 2019, 38, 821–829. [DOI] [PubMed] [Google Scholar]
- 418.Mei M, Li Q-L and Chu C, Materials, 2016, 9, 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Ragland SA and Criss AK, PloS Pathog, 2017, 13, e1006512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Li C, Lu D, Deng J, Zhang X and Yang P, Adv. Mater, 2019, 31, 1903973. [DOI] [PubMed] [Google Scholar]
- 421.Mei ML, Zhao IS, Ito L, Lo ECM and Chu CH, Int. Dent. J, 2016, 66, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Chu CH and Lo ECM, Oral Health Prev. Dent, 2008, 6, 315–321. [PubMed] [Google Scholar]
- 423.Balic A, Gerontology, 2018, 64, 382–388. [DOI] [PubMed] [Google Scholar]
- 424.Nanci A and Bosshardt DD, Periodontol. 2000, 2006, 40, 11–28. [DOI] [PubMed] [Google Scholar]
- 425.Larsen T and Fiehn NE, APMIS, 2017, 125, 376–384. [DOI] [PubMed] [Google Scholar]
- 426.Chugal N, Mallya SM, Kahler B and Lin LM, Dent. Clin. North Am, 2017, 61, 59–80. [DOI] [PubMed] [Google Scholar]
- 427.Pontillo V, Miziak DB, Maller A, Nassar PO and Nassar CA, J. Int. Acad. Periodontol, 2018, 20, 123–130. [PubMed] [Google Scholar]
- 428.Ramseier CA, Anerud A, Dulac M, Lulic M, Cullinan MP, Seymour GJ, Faddy MJ, Burgin W, Schatzle M and Lang NP, J. Clin. Periodontol, 2017, 44, 1182–1191. [DOI] [PubMed] [Google Scholar]
- 429.Albuquerque MTP, Valera MC, Nakashima M, Nör JE and Bottino MC, J. Dent. Res, 2014, 93, 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Galler KM, Int. Endod. J, 2016, 49, 926–936. [DOI] [PubMed] [Google Scholar]
- 431.Vishwanath V and Rao H. m., J. Conserv. Dent, 2019, 22, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Kim SG, Malek M, Sigurdsson A, Lin LM and Kahler B, Int. Endod. J, 2018, 51, 1367–1388. [DOI] [PubMed] [Google Scholar]
- 433.Salehrabi R and Rotstein I, J. Endod, 2004, 30, 846–850. [DOI] [PubMed] [Google Scholar]
- 434.Bottino MC, Pankajakshan D and Nör JE, Dent. Clin. North Am, 2017, 61, 689–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Albuquerque MT, Valera MC, Nakashima M, Nor JE and Bottino MC, J. Dent. Res, 2014, 93, 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Kaushik SN, Kim B, Walma AMC, Choi SC, Wu H, Mao JJ, Jun H-W and Cheon K, Biomater. Res, 2016, 20, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Eramo S, Natali A, Pinna R and Milia E, Int. Endod. J, 2018, 51, 405–419. [DOI] [PubMed] [Google Scholar]
- 438.Ferrara N, Gerber H-P and LeCouter J, Nat. Med, 2003, 9, 669–676. [DOI] [PubMed] [Google Scholar]
- 439.Aloe L, Rocco M, Bianchi P and Manni L, J. Transl. Med, 2012, 10, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Bottino MC, Albuquerque MTP, Azabi A, Münchow EA, Spolnik KJ, Nör JE and Edwards PC, J. Biomed. Mater. Res., Part B, 2019, 107, 1576–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Münchow EA and Bottino MC, in Clinical approaches in Endodontic Regeneration, ed. Duncan HF and Cooper PR, Springer Nature, Switzerland, 2019. [Google Scholar]
- 442.Kwak E-A and Lee NY, Cytokine, 2019, 115, 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Poon B, Kha T, Tran S and Dass CR, J. Pharm. Pharmacol, 2016, 68, 139–147. [DOI] [PubMed] [Google Scholar]
- 444.Roberts-Clark D and Smith A, Arch. Oral Biol, 2000, 45, 1013–1016. [DOI] [PubMed] [Google Scholar]
- 445.Zhao S, Sloan AJ, Murray PE, Lumley PJ and Smith AJ, Histochem. J, 2000, 32, 489–494. [DOI] [PubMed] [Google Scholar]
- 446.Bègue-Kirn C, Smith AJ, Ruch JV, Wozney JM, Purchio A, Hartmann D and Lesot H, Int. J. Dev. Biol, 1992, 36, 491–503. [PubMed] [Google Scholar]
- 447.Galler KM, D’Souza RN, Federlin M, Cavender AC, Hartgerink JD, Hecker S and Schmalz G, J. Endod, 2011, 37, 1536–1541. [DOI] [PubMed] [Google Scholar]
- 448.Yamauchi N, Nagaoka H, Yamauchi S, Teixeira FB, Miguez P and Yamauchi M, J. Endod, 2011, 37, 1636–1641. [DOI] [PubMed] [Google Scholar]
- 449.Zhang DD, Chen X, Bao ZF, Chen M, Ding ZJ and Zhong M, J. Endod, 2014, 40, 1388–1393. [DOI] [PubMed] [Google Scholar]
- 450.Marx RE, Implant Dent, 2001, 10, 225–228. [DOI] [PubMed] [Google Scholar]
- 451.Weibrich G, Kleis WK, Hafner G and Hitzler WE, J. Craniomaxillofac. Surg, 2002, 30, 97–102. [DOI] [PubMed] [Google Scholar]
- 452.Albanese A, Licata ME, Polizzi B and Campisi G, Immun. Ageing, 2013, 10, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Lubkowska A, Dolegowska B and Banfi G, J. Biol. Regul. Homeostatic Agents, 2012, 26, 3S–22S. [PubMed] [Google Scholar]
- 454.Carlson NE and Roach RB, J. Am. Dent. Assoc., JADA, 2002, 133, 1383–1386. [DOI] [PubMed] [Google Scholar]
- 455.Zhu X, Wang Y, Liu Y, Huang GT and Zhang C, J. Endod, 2014, 40, 1573–1578. [DOI] [PubMed] [Google Scholar]
- 456.Ulusoy AT, Turedi I, Cimen M and Cehreli ZC, J. Endod, 2019, 45, 560–566. [DOI] [PubMed] [Google Scholar]
- 457.Brizuela C, Meza G, Urrejola D, Quezada MA, Concha G, Ramírez V, Angelopoulos I, Cadiz MI, Tapia-Limonchi R and Khoury M, J. Dent. Res, 2020, 99, 523–529. [DOI] [PubMed] [Google Scholar]
- 458.Moussa DG and Aparicio C, J. Tissue Eng. Regener. Med, 2019, 13, 58–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Fujiwara S, Kumabe S and Iwai Y, Okajimas Folia Anat. Jpn, 2006, 83, 15–24. [DOI] [PubMed] [Google Scholar]
- 460.Kumabe S, Nakatsuka M, Kim GS, Jue SS, Aikawa F, Shin JW and Iwai Y, Okajimas Folia Anat. Jpn, 2006, 82, 147–155. [DOI] [PubMed] [Google Scholar]
- 461.Khayat A, Monteiro N, Smith EE, Pagni S, Zhang W, Khademhosseini A and Yelick PC, J. Dent. Res, 2017, 96, 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Monteiro N, Thrivikraman G, Athirasala A, Tahayeri A, França CM, Ferracane JL and Bertassoni LE, Dent. Mater, 2018, 34, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.About I, Laurent-Maquin D, Lendahl U and Mitsiadis TA, Am. J. Pathol, 2000, 157, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Ishimatsu H, Kitamura C, Morotomi T, Tabata Y, Nishihara T, Chen KK and Terashita M, J. Endod, 2009, 35, 858–865. [DOI] [PubMed] [Google Scholar]
- 465.Inuyama Y, Kitamura C, Nishihara T, Morotomi T, Nagayoshi M, Tabata Y, Matsuo K, Chen KK and Terashita M, J. Biomed. Mater. Res., Part B, 2010, 92, 120–128. [DOI] [PubMed] [Google Scholar]
- 466.Chrepa V, Austah O and Diogenes A, J. Endod, 2017, 43, 257–262. [DOI] [PubMed] [Google Scholar]
- 467.Huang C-C, Narayanan R, Warshawsky N and Ravindran S, Front. Physiol, 2018, 9, 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Pankajakshan D, Voytik-Harbin SL, Nör JE and Bottino M, ACS Appl. Bio Mater, 2020, 3(2), 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Ducret M, Montembault A, Josse J, Pasdeloup M, Celle A, Benchrih R, Mallein-Gerin F, Alliot-Licht B, David L and Farges JC, Dent. Mater, 2019, 35, 523–533. [DOI] [PubMed] [Google Scholar]
- 470.Yang JW, Zhang YF, Sun ZY, Song GT and Chen Z, J. Biomater. Appl, 2015, 30, 221–229. [DOI] [PubMed] [Google Scholar]
- 471.Zhang W, Ahluwalia IP, Literman R, Kaplan DL and Yelick PC, J. Biomed. Mater. Res., Part A, 2011, 97A, 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Sakiyama-Elbert SE, Acta Biomater, 2014, 10, 1581–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Li X, Ma C, Xie X, Sun H and Liu X, Acta Biomater, 2016, 35, 57–67. [DOI] [PubMed] [Google Scholar]
- 474.Itoh Y, Sasaki JI, Hashimoto M, Katata C, Hayashi M and Imazato S, J. Dent. Res, 2018, 97, 1137–1143. [DOI] [PubMed] [Google Scholar]
- 475.Tatsuhiro F, Seiko T, Yusuke T, Reiko TT and Kazuhito S, Int. J. Mol. Sci, 2018, 19(7), 1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Cho MI and Garant PR, Periodontol. 2000, 2000, 24, 9–27. [DOI] [PubMed] [Google Scholar]
- 477.Zou R, Wan W, Li J, Du C, Wang Y, Qian T and Niu L, Histol. Histopathol, 2018, 33, 825–833. [DOI] [PubMed] [Google Scholar]
- 478.Bottino MC and Thomas V, Front. Oral Biol, 2015, 17, 90–100. [DOI] [PubMed] [Google Scholar]
- 479.Kao RT, Nares S and Reynolds MA, J. Periodontol, 2015, 86, S77–S104. [DOI] [PubMed] [Google Scholar]
- 480.Xu XY, Li X, Wang J, He XT, Sun HH and Chen FM, Stem Cells Transl. Med, 2019, 8, 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Staples RJ, Ivanovski S and Vaquette C, J. Periodontal Res, 2020, 55(3), 331–341. [DOI] [PubMed] [Google Scholar]
- 482.Larsson L, Decker AM, Nibali L, Pilipchuk SP, Berglundh T and Giannobile WV, J. Dent. Res, 2016, 95, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Sheikh Z, Qureshi J, Alshahrani AM, Nassar H, Ikeda Y, Glogauer M and Ganss B, Odontology, 2017, 105, 1–12. [DOI] [PubMed] [Google Scholar]
- 484.Murphy KG, Int. J. Periodont. Restor. Dent, 1995, 15, 363–375. [PubMed] [Google Scholar]
- 485.Ding T, Li J, Zhang X, Du L, Li Y, Li D, Kong B and Ge S, Biomater. Sci, 2020, 8, 2459–2471. [DOI] [PubMed] [Google Scholar]
- 486.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J and Ratajczak MZ, J. Mol. Histol, 2003, 35, 233–245. [DOI] [PubMed] [Google Scholar]
- 487.Tan J, Zhang M, Hai Z, Wu C, Lin J, Kuang W, Tang H, Huang Y, Chen X and Liang G, ACS Nano, 2019, 13, 5616–5622. [DOI] [PubMed] [Google Scholar]
- 488.Komaki M, Iwasaki K, Arzate H, Narayanan AS, Izumi Y and Morita I, J. Cell. Physiol, 2012, 227, 649–657. [DOI] [PubMed] [Google Scholar]
- 489.Sowmya S, Mony U, Jayachandran P, Reshma S, Kumar RA, Arzate H, Nair SV and Jayakumar R, Adv. Healthcare Mater, 2017, 6, 1–13. [DOI] [PubMed] [Google Scholar]
- 490.Vaquette C, Saifzadeh S, Farag A, Hutmacher DW and Ivanovski S, J. Dent. Res, 2019, 98, 673–681. [DOI] [PubMed] [Google Scholar]
- 491.Babo PS, Pires RL, Santos L, Franco A, Rodrigues F, Leonor I, Reis RL and Gomes ME, ACS Biomater. Sci. Eng, 2017, 3, 1359–1369. [DOI] [PubMed] [Google Scholar]
- 492.Babo PS, Cai X, Plachokova AS, Reis RL, Jansen J, Gomes ME and Walboomers XF, J. Tissue Eng. Regener. Med, 2018, 12, e1277–e1288. [DOI] [PubMed] [Google Scholar]
- 493.Tachibana A, Furuta Y, Takeshima H, Tanabe T and Yamauchi K, J. Biotechnol, 2002, 93, 165–170. [DOI] [PubMed] [Google Scholar]
- 494.Zhang H, Wang J, Ma H, Zhou Y, Ma X, Liu J, Huang J and Yu N, ACS Biomater. Sci. Eng, 2016, 2, 2162–2175. [DOI] [PubMed] [Google Scholar]
- 495.Hasani-Sadrabadi MM, Sarrion P, Nakatsuka N, Young TD, Taghdiri N, Ansari S, Aghaloo T, Li S, Khademhosseini A, Weiss PS and Moshaverinia A, ACS Nano, 2019, 13, 3830–3838. [DOI] [PubMed] [Google Scholar]
- 496.Geão C, Costa-Pinto AR, Cunha-Reis C, Ribeiro VP, Vieira S, Oliveira JM, Reis RL and Oliveira AL, J. Mater. Sci.: Mater. Med, 2019, 30, 27. [DOI] [PubMed] [Google Scholar]
- 497.Raafat D and Sahl HG, Microb. Biotechnol, 2009, 2, 186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Aguilar A, Zein N, Harmouch E, Hafdi B, Bornert F, Offner D, Clauss F, Foiioretti F, Huck O, Benkirane-Jessel N and Hua G, Molecules, 2019, 24, 3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Xu C, Lei C, Meng L, Wang C and Song Y, J. Biomed. Mater. Res., Part B, 2012, 100B, 1435–1443. [DOI] [PubMed] [Google Scholar]
- 500.Ali S, Sangi L, Kumar N, Kumar B, Khurshid Z and Zafar MS, Technol. Heal. Care, 2020, 28, 165–173. [DOI] [PubMed] [Google Scholar]
- 501.Dragland IS, Wellendorf H, Kopperud H, Stenhagen I and Valen H, Biomater. Investig. Dent, 2019, 6, 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Machado AHS, Garcia IM, Motta ASD, Leitune VCB and Collares FM, J. Dent, 2019, 83, 33–39. [DOI] [PubMed] [Google Scholar]
- 503.Namangkalakul W, Benjavongkulchai S, Pochana T, Promchai A, Satitviboon W, Howattanapanich S, Phuprasong R, Ungvijanpunya N, Supakanjanakanti D, Chaitrakoonthong T, Muangsawat S, Thanyasrisung P and Matangkasombut O, J. Prosthet. Dent, 2020, 123, 181.e1–181.e7. [DOI] [PubMed] [Google Scholar]
- 504.Pichaiaukrit W, Thamrongananskul N, Siralertmukul K and Swasdison S, Dent. Mater. J, 2019, 38, 1036–1042. [DOI] [PubMed] [Google Scholar]
- 505.Zhou J, Xu Q, Fan C, Ren H, Xu S, Hu F, Wang L, Yang K and Ji Q, J. Mater. Sci.: Mater. Med, 2019, 30, 39. [DOI] [PubMed] [Google Scholar]
- 506.Guo S, He L, Yang R, Chen B, Xie X, Jiang B, Weidong T and Ding Y, J. Biomater. Sci., Polym. Ed, 2020, 31, 155–168. [DOI] [PubMed] [Google Scholar]
- 507.Georgopoulou A, Papadogiannis F, Batsali A, Marakis J, Alpantaki K, Eliopoulos AG, Pontikoglou C and Chatzinikolaidou M, J. Mater. Sci.: Mater. Med, 2018, 29, 59. [DOI] [PubMed] [Google Scholar]
- 508.Varoni EM, Vijayakumar S, Canciani E, Cochis A, De Nardo L, Lodi G, Rimondini L and Cerruti M, J. Dent. Res, 2018, 97, 303–311. [DOI] [PubMed] [Google Scholar]
- 509.Arancibia R, Maturana C, Silva D, Tobar N, Tapia C, Salazar JC, Martínez J and Smith PC, J. Dent. Res, 2013, 92, 740–745. [DOI] [PubMed] [Google Scholar]
- 510.Hurt AP, Kotha AK, Trivedi V and Coleman NJ, Polymer, 2015, 25, 311–316. [Google Scholar]
- 511.Mota J, Yu N, Caridade SG, Luz GM, Gomes ME, Reis RL, Jansen JA, Frank Walboomers X and Mano JF, Acta Biomater, 2012, 8, 4173–4180. [DOI] [PubMed] [Google Scholar]
- 512.Madrigal JL, Stilhano R and Silva EA, Tissue Eng., Part B, 2017, 23, 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Goker F, Larsson L, Del Fabbro M and Asa’ad F, Int. J. Mol. Sci, 2019, 20, 3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Boden SD, Orthop. Nurs, 2005, 24, 44–49. [DOI] [PubMed] [Google Scholar]
- 515.Giannobile WV, Lee CS, Tomala MP, Tejeda KM and Zhu Z, J. Periodontol, 2001, 72, 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Pilipchuk SP, Fretwurst T, Yu N, Larsson L, Kavanagh NM, Asa’ad F, Cheng KCK, Lahann J and Giannobile WV, Adv. Healthcare Mater, 2018, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Vaquette C, Pilipchuk SP, Bartold PM, Hutmacher DW, Giannobile WV and Ivanovski S, Adv. Healthcare Mater, 2018, 7, 1–20. [DOI] [PubMed] [Google Scholar]
- 518.Costa AG, Cusano NE, Silva BC, Cremers S and Bilezikian JP, Nat. Rev. Rheumatol, 2011, 7, 447–456. [DOI] [PubMed] [Google Scholar]
- 519.Chen W, Gao B, Hao L, Zhu G, Jules J, MacDougall MJ, Wang J, Han X, Zhou X and Li YP, J. Periodontal Res, 2016, 51, 647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.MacDonald BT, Tamai K and He X, Dev. Cell, 2009, 17, 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Liu H, Zhang N, Liu Y, Liu L, Yin G and En L, Hum. Gene Ther, 2018, 29, 1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Park SY, Kim KH, Gwak EH, Rhee SH, Lee JC, Shin SY, Koo KT, Lee YM and Seol YJ, J. Biomed. Mater. Res., Part A, 2015, 103, 38–47. [DOI] [PubMed] [Google Scholar]
- 523.Park SY, Kim KH, Kim S, Rhee SH, Yeo IS, Heo SJ, Lee YM and Seol YJ, Sci. Rep, 2020, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Xie Q, Jia L, Xu H, Hu X, Wang W and Jia J, Stem Cells Int, 2016, 2016, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Dubey N, Ferreira J, Malda J, Bhaduri SB and Bottino MC, ACS Appl. Mater. Interfaces, 2020, 12(21), 23752–23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Nederfors T, Adv. Dent. Res, 2000, 14, 48–56. [DOI] [PubMed] [Google Scholar]
- 527.Pinna R, Campus G, Cumbo E, Mura I and Milia E, Ther. Clin. Risk Manag, 2015, 11, 171–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Yoo C, Vines JB, Alexander G, Murdock K, Hwang P and Jun HW, Biomater. Res, 2014, 18, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Ozdemir T, Fowler EW, Hao Y, Ravikrishnan A, Harrington DA, Witt RL, Farach-Carson MC, Pradhan-Bhatt S and Jia X, Biomater. Sci, 2016, 4, 592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Borkent D and Moharamzadeh K, Tissue engineering of salivary glands, Elsevier Ltd, 2017. [Google Scholar]
- 531.Holmberg KV and Hoffman MP, Monogr. Oral Sci, 2014, 24, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Tanaka J, Ogawa M, Hojo H, Kawashima Y, Mabuchi Y, Hata K, Nakamura S, Yasuhara R, Takamatsu K, Irié T, Fukada T, Sakai T, Inoue T, Nishimura R, Ohara O, Saito I, Ohba S, Tsuji T and Mishima K, Nat. Commun, 2018, 9, 4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Nam K, Jones JP, Lei P, Andreadis ST and Baker OJ, Biomacromolecules, 2016, 17, 2293–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Pieters M and Wolberg AS, Res. Pract. Thromb. Haemost, 2019, 3, 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Nam K, Maruyama CL, Wang CS, Trump BG, Lei P, Andreadis ST and Baker OJ, PLoS One, 2017, 12, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Samuel RZ, Lei P, Nam K, Baker OJ and Andreadis ST, Acta Biomater, 2020, 105, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Nam K, Wang CS, Maruyama CLM, Lei P, Andreadis ST and Baker OJ, J. Dent. Res, 2017, 96, 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Soscia DA, Sequeira SJ, Schramm RA, Jayarathanam K, Cantara SI, Larsen M and Castracane J, Biomaterials, 2013, 34, 6773–6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Kleinman HK and Martin GR, Semin. Cancer Biol, 2005, 15, 378–386. [DOI] [PubMed] [Google Scholar]
- 540.Hughes CS, Postovit LM and Lajoie GA, Proteomics, 2010, 10, 1886–1890. [DOI] [PubMed] [Google Scholar]
- 541.Pujuguet P, Simian M, Liaw J, Timpl R, Werb Z and Bissell MJ, J. Cell Sci, 2000, 113(Pt 5), 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Pradhan S, Zhang C, Jia X, Carson DD, Witt R and Farach-Carson MC, Tissue Eng., Part A, 2009, 15, 3309–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Chen S, John JV, McCarthy A and Xie J, J. Mater. Chem. B, 2020, 8, 3733–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Foraida ZI, Kamaldinov T, Nelson DA, Larsen M and Castracane J, Acta Biomater, 2017, 62, 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Ozdemir T, Fowler EW, Liu S, Harrington DA, Witt RL, Farach-Carson MC, Pradhan-Bhatt S and Jia X, ACS Biomater. Sci. Eng, 2016, 2, 2217–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Lee S, Ryu JH, Do M, Namkoong E, Lee H and Park K, ACS Appl. Mater. Interfaces, 2020, 12(4), 4285–4294. [DOI] [PubMed] [Google Scholar]
- 547.Pradhan-Bhatt S, Harrington DA, Duncan RL, Jia X, Witt RL and Farach-Carson MC, Tissue Eng., Part A, 2013, 19, 1610–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Ozdemir T, Srinivasan PP, Zakheim DR, Harrington DA, Witt RL, Farach-Carson MC, Jia X and Pradhan-Bhatt S, Biomaterials, 2017, 142, 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Maria OM, Liu Y, El-Hakim M, Zeitouni A and Tran SD, J. Tissue Eng. Regener. Med, 2017, 11, 2643–2657. [DOI] [PubMed] [Google Scholar]
- 550.Zhang BX, Zhang ZL, Lin AL, Wang H, Pilia M, Ong JL, Dean DD, Chen XD and Yeh CK, Tissue Eng., Part A, 2015, 21, 1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Patil SV and Nanduri LSY, Int. J. Biol. Macromol, 2017, 104, 1398–1406. [DOI] [PubMed] [Google Scholar]
- 552.Samuni Y and Baum BJ, Biochim. Biophys. Acta, Mol. Basis Dis, 2011, 1812, 1515–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Hamley IW, Soft Matter, 2011, 7, 4122–4138. [Google Scholar]
- 554.Hartgerink JD, Beniash E and Stupp SI, Science, 2001, 294, 1684–1688. [DOI] [PubMed] [Google Scholar]
- 555.Hartgerink JD, Beniash E and Stupp SI, Proc. Natl. Acad. Sci. U. S. A, 2002, 99, 5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Hendricks MP, Sato K, Palmer LC and Stupp SI, Acc. Chem. Res, 2017, 50, 2440–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Cui H, Webber MJ and Stupp SI, Biopolymers, 2010, 94, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Galler KM, Aulisa L, Regan KR, D’Souza RN and Hartgerink JD, J. Am. Chem. Soc, 2010, 132, 3217–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Chen CH, Palmer LC and Stupp SI, Nano Lett, 2018, 18, 6832–6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Kumar VA, Taylor NL, Shi S, Wickremasinghe NC, D’Souza RN and Hartgerink JD, Biomaterials, 2015, 52, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Kumar VA, Shi S, Wang BK, Li IC, Jalan AA, Sarkar B, Wickremasinghe NC and Hartgerink JD, J. Am. Chem. Soc, 2015, 137, 4823–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Freeman R, Stephanopoulos N, Álvarez Z, Lewis JA, Sur S, Serrano CM, Boekhoven J, Lee SS and Stupp SI, Nat. Commun, 2017, 8, 15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Lopez-Silva TL, Leach DG, Azares A, Li I-C, Woodside DG and Hartgerink JD, Biomaterials, 2020, 231, 119667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Kumar VA, Liu Q, Wickremasinghe NC, Shi S, Cornwright TT, Deng Y, Azares A, Moore AN, Acevedo-Jake AM, Agudo NR, Pan S, Woodside DG, Vanderslice P, Willerson JT, Dixon RA and Hartgerink JD, Biomaterials, 2016, 98, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Li M, Liu L, Xi N and Wang Y, Acta Pharmacol. Sin, 2015, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.O’Leary LER, Fallas JA, Bakota EL, Kang MK and Hartgerink JD, Nat. Chem, 2011, 3, 821–828. [DOI] [PubMed] [Google Scholar]
- 567.Kang MK, Colombo JS, D’Souza RN and Hartgerink JD, Biomacromolecules, 2014, 15, 2004–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Galler KM, Cavender A, Yuwono V, Dong H, Shi S, Schmalz G, Hartgerink JD and D’Souza RN, Tissue Eng., Part A, 2008, 14, 2051–2058. [DOI] [PubMed] [Google Scholar]
- 569.Colombo JS, Moore AN, Hartgerink JD and D’Souza RN, J. Endod, 2014, 40, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Liu H, Li W, Gao C, Kumagai Y, Blacher RW and DenBesten PK, J. Dent. Res, 2004, 83, 496–499. [DOI] [PubMed] [Google Scholar]
- 571.Nguyen PK, Gao W, Patel SD, Siddiqui Z, Weiner S, Shimizu E, Sarkar B and Kumar VA, ACS Omega, 2018, 3, 5980–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Yun Y-R, Won JE, Jeon E, Lee S, Kang W, Jo H, Jang J-H, Shin US and Kim H-W, J. Tissue Eng, 2010, 1, 218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Galler KM, Hartgerink JD, Cavender AC, Schmalz G and D’Souza RN, Tissue Eng., Part A, 2012, 18, 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Huang Z, Newcomb CJ, Bringas P, Stupp SI and Snead ML, Biomaterials, 2010, 31, 9202–9211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Huang C-C, Ravindran S, Kang M, Cooper LF and George A, ACS Biomater. Sci. Eng, 2020, 6, 2913–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Huang C-C, Ravindran S, Yin Z and George A, Biomaterials, 2014, 35, 5316–5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Zhong J, Yang Y, Liao L and Zhang C, Biomater. Sci, 2020, 8, 2734–2755. [DOI] [PubMed] [Google Scholar]
- 578.Wilson MJ, Liliensiek SJ, Murphy CJ, Murphy WL and Nealey PF, Soft Matter, 2012, 8, 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Shadish JA, Benuska GM and DeForest CA, Nat. Mater, 2019, 18, 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.C. A. De Forest and Tirrell DA, Nat. Mater, 2015, 14, 523–531. [DOI] [PubMed] [Google Scholar]
- 581.Kwon MY, Vega SL, Gramlich WM, Kim M, Mauck RL and Burdick JA, Adv. Healthcare Mater, 2018, 7, 1701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Neves LS, Rodrigues MT, Reis RL and Gomes ME, Expert Rev. Precis. Med. Drug Dev, 2016, 1, 93–108. [Google Scholar]
- 583.Raman R and Langer R, Adv. Mater, 2020, 32, 1901969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Nagamune T, Nano Converg, 2017, 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Spicer CD, Pashuck ET and Stevens MM, Chem. Rev, 2018, 118, 7702–7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Liu R, Chen S, Huang P, Liu G, Luo P, Li Z, Xiao Y, Chen Z and Chen Z, Adv. Funct. Mater, 2020, 1910672, 1910672. [Google Scholar]
- 587.Teixeira SPB, Domingues RMA, Shevchuk M, Gomes ME, Peppas NA and Reis RL, Adv. Funct. Mater, 2020, 1909011, 1909011. [Google Scholar]
- 588.Hu H and Xu F, Biomater. Sci, 2020, 8, 2084–2101. [DOI] [PubMed] [Google Scholar]
- 589.Couchman JR and Pataki CA, J. Histochem. Cytochem, 2012, 60, 885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Weyers A and Linhardt RJ, FEBS J, 2013, 280, 2511–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Listik E, Azevedo Marques Gaschler J, Matias M, Neuppmann Feres MF, Toma L and Raphaelli Nahás-Scocate AC, Carbohydr. Polym, 2019, 225, 115199. [DOI] [PubMed] [Google Scholar]
- 592.Tuin A, Zandstra J, Kluijtmans SG, Bouwstra JB, Harmsen MC and van Luyn MJA, Eur. Cells Mater, 2012, 24, 320–330. [DOI] [PubMed] [Google Scholar]
- 593.Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A and Giebel B, Int. J. Mol. Sci, 2017, 18, 1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A and Lim SK, Int. J. Proteomics, 2012, 2012, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Lamichhane TN, Sokic S, Schardt JS, Raiker RS, Lin JW and Jay SM, Tissue Eng., Part B, 2015, 21, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Armstrong JPK and Stevens MM, Adv. Drug Delivery Rev, 2018, 130, 12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Brennan MÁ, Layrolle P and Mooney DJ, Adv. Funct. Mater, 2020, 1909125, 1909125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Huang C-C, Kang M, Lu Y, Shirazi S, Diaz JI, Cooper LF, Gajendrareddy P and Ravindran S, Acta Biomater, 2020, 109, 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Jiang N, Xiang L, He L, Yang G, Zheng J, Wang C, Zhang Y, Wang S, Zhou Y, Sheu TJ, Wu J, Chen K, Coelho PG, Tovar NM, Kim SH, Chen M, Zhou YH and Mao JJ, ACS Nano, 2017, 11, 7736–7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.He L, Zhou J, Chen M, Lin CS, Kim SG, Zhou Y, Xiang L, Xie M, Bai H, Yao H, Shi C, Coelho PG, Bromage TG, Hu B, Tovar N, Witek L, Wu J, Chen K, Gu W, Zheng J, Sheu TJ, Zhong J, Wen J, Niu Y, Cheng B, Gong Q, Owens DM, Stanislauskas M, Pei J, Chotkowski G, Wang S, Yang G, Zegarelli DJ, Shi X, Finkel M, Zhang W, Li J, Cheng J, Tarnow DP, Zhou X, Wang Z, Jiang X, Romanov A, Rowe DW, Wang S, Ye L, Ling J and Mao J, Nat. Mater, 2019, 18, 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Watanabe J, Sakai K, Urata Y, Toyama N, Nakamichi E and Hibi H, J. Dent. Res, 2020, 99(5), 552–560. [DOI] [PubMed] [Google Scholar]
- 602.Cooper LF, Ravindran S, Huang C-C and Kang M, Front. Physiol, 2019, 10, 1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Ben-David U, Siranosian B, Ha G, Tang H, Oren Y, Hinohara K, Strathdee CA, Dempster J, Lyons NJ, Burns R, Nag A, Kugener G, Cimini B, Tsvetkov P, Maruvka YE, O’Rourke R, Garrity A, Tubelli AA, Bandopadhayay P, Tsherniak A, Vazquez F, Wong B, Birger C, Ghandi M, Thorner AR, Bittker JA, Meyerson M, Getz G, Beroukhim R and Golub TR, Nature, 2018, 560, 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Cai X-Y, Wake A and Gouty D, Bioanalysis, 2013, 5, 517–520. [DOI] [PubMed] [Google Scholar]
- 605.Vining KH, Scherba JC, Bever AM, Alexander MR, Celiz AD and Mooney DJ, Adv. Mater, 2018, 30, 1704486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Celiz AD, Denning C, Davies MC, Alexander MR, Tibbitt MW, Anderson DG, Patel AK and Langer R, Curr. Opin. Solid State Mater. Sci, 2016, 20, 202–211. [Google Scholar]
- 607.Barrett DA, Young LE, Patel MJ, Davies MC, Epa VC, Patel AK, Rajamohan D, Winkler DA, George VT, Celiz AD, Smith JGW, Denning C, Langer R, Hook AL, Anderson DG, Allen ND, Singh T, Flatt L, Alexander MR and Hay DC, Adv. Mater, 2015, 27, 4006–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Celiz AD, Smith JGW, Patel AK, Langer R, Anderson DG, Barrett DA, Young LE, Davies MC, Denning C and Alexander MR, Biomater. Sci, 2014, 2, 1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Tavassoli H, Alhosseini SN, Tay A, Chan PPY, Weng Oh SK and Warkiani ME, Biomaterials, 2018, 181, 333–346. [DOI] [PubMed] [Google Scholar]
- 610.Mitchell AC, Briquez PS, Hubbell JA and Cochran JR, Acta Biomater, 2016, 30, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Mertgen A, Trossmann V, Guex AG, Maniura-Weber K, Scheibel T and Rottmar M, ACS Appl. Mater. Interfaces, 2020, 12(19), 21342–21367. [DOI] [PubMed] [Google Scholar]
- 612.Place ES, Evans ND and Stevens MM, Nat. Mater, 2009, 8, 457–470. [DOI] [PubMed] [Google Scholar]
- 613.Kim YS, Smoak MM, Melchiorri AJ and Mikos AG, Tissue Eng., Part A, 2019, 25, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


