Abstract
Background:
HIV-related neuroinflammation has been proposed as a catalyst for dopaminergic dysregulation in mesocortical pathways, which may contribute to the pathogenesis of depression. Abnormalities in dopaminergic neurotransmission and depression are common in people with HIV (PWH), however the link between dopamine (DA) and depression in PWH is poorly characterized. This study investigated CSF dopaminergic biomarkers, specifically DA and its metabolite, homovanillic acid (HVA), and examined their relationship with depressive symptoms and CSF neuroinflammatory markers in PWH and HIV-seronegative (HIV−) individuals.
Methods:
Participants were 102 HIV− individuals and 123 PWH (mean age=42) who underwent neuropsychiatric evaluations and lumbar puncture. Current depression severity was classified using the Beck Depression Inventory-II (BDI-II). CSF was assayed for DA and HVA using high performance liquid chromatography and neuroinflammatory markers using immunoassays. Linear regressions modelled BDI-II scores as a function of HIV, dopaminergic biomarker z-scores, and their interaction, controlling for psychosocial factors. Correlational analyses examined dopaminergic and neuroinflammatory relationships.
Results:
PWH had significantly higher BDI-II scores than HIV− participants. DA and HVA were not associated with HIV status but both significantly moderated the effect of HIV on BDI-II scores, such that PWH exhibited higher depressive symptoms than HIV− participants only at lower concentrations of HVA (z≤0.06) and DA (z≤0.11). In PWH only, lower HVA significantly correlated with higher BDI-II scores and higher neuroinflammation, including higher MCP-1 and IP-10.
Conclusions:
Results suggest that the pathophysiology of depression in PWH differs from that in HIV− individuals. Specifically, lower central dopaminergic activity was selectively associated with greater depressive symptoms and neuroinflammation in PWH. With the rise in consideration of DA agonists for the treatment of depression, these results suggest that PWH may show a greater response to these agents than their HIV− peers.
Keywords: dopamine, homovanillic acid, inflammation, depression, HIV, MCP-1, IP-10
1. Introduction
Depression, including both major depressive disorder (MDD) and subsyndromal depressive symptomatology, is a frequent and disabling co-occuring condition among people with HIV (PWH). PWH are twice as likely to develop MDD compared to HIV-seronegative (HIV−) individuals, with prevalence estimates of lifetime MDD in PWH ranging from 22–54% (Rabkin, 2008; Rooney et al., 2019; Rubin and Maki, 2019). Comorbid depression in PWH increases risk for a host of adverse biopsychosocial outcomes. Greater depressive burden is related to poorer adherence to antiretroviral therapy (ART), increased viral load, and reduced immune function (Ammassari et al., 2004; Leserman, 2003, 2008). Moreover, depression is associated with functional impairments, including dependence in activities of daily living, subjective and objective deficits in neurocognitive function, and even premature mortality (Rabkin, 2008; Rubin and Maki, 2019; Thames et al., 2011).
Dopaminergic dysfunction and neuroinflammation are distinct yet related neurobiological processes that are thought to contribute to the development of depression among PWH. Upon entry into the CNS, HIV-infected cells release neuroinflammatory cytokines and chemokines as well as neurotoxic viral proteins that disproportionately compromise dopamine-rich, fronto-striatal regions (Gaskill et al., 2017; Plessis et al., 2014). Chronic neuroinflammation is a hallmark feature of HIV-associated neurocognitive disorders (Williams et al., 2014) and neuropathological data indicate that PWH have markedly decreased concentrations of dopamine (DA) and its metabolite, homovanillic acid (HVA), in both cortical and subcortical structures (Kumar et al., 2011). Recent literature from animal and human studies indicate a robust link between chronic inflammation and dopaminergic deficiencies, including cytokine-induced reductions in the synthesis and release of DA (Felger, 2017; Felger and Miller, 2012). Importantly, inflammation-related dysregulation of dopaminergic activity dampens activity in cortico-striatal reward pathways, which may help explain depressive symptoms of anhedonia and psychomotor slowing in patients with high inflammation (Felger, 2017; Felger and Miller, 2012).
Direct and in vivo measurement of central dopaminergic function can be accomplished via assays of dopamine and its metabolites in cerebrospinal fluid (CSF). Several studies demonstrate reduced CSF levels of DA and HVA in PWH with and without neurocognitive complications in comparison to HIV− controls (Berger et al., 1994; di Rocco et al., 2000; Larsson et al., 1991). Despite the putative relevance of the dopaminergic system in the pathogenesis of depression in HIV, CSF biomarkers of dopaminergic function have not been systematically examined in relation to depressive symptoms in PWH. The present study evaluated associations between depressive symptoms and CSF DA and HVA, and whether these relationships differed by HIV serostatus. We hypothesized that: 1) consistent with the literature, PWH would report greater depressive symptoms than HIV− individuals; 2) lower CSF DA and HVA would relate to greater depressive symptoms irrespective of HIV serostatus, but that these associations would be stronger in PWH and independent of other psychosocial risk factors and antidepressant medication use; 3) in the subset of PWH with available CSF neuroinflammation markers, greater neuroinflammatory burden would relate to higher depressive symptoms and lower CSF DA and HVA.
2. Materials and Methods
2.1. Participants
Participants were 123 HIV-seropositive (HIV+) and 102 HIV-seronegative (HIV−) adults enrolled in the University of California San Diego’s (UCSD) Translational Methamphetamine AIDS Research Center (TMARC), a NIDA-funded cohort study focusing on the CNS effects of HIV and methamphetamine. Study visits took place between 2006 and 2010. All study procedures were approved by the UCSD Institutional Review Board and all participants provided written informed consent. Exclusion criteria for the current study were history of psychotic or mood disorder with psychotic features, presence of a neurological or non-HIV medical condition that may confound neurobehavioral test results, and positive urine toxicology for drugs (including methamphetamine) or positive breathalyzer test for alcohol on the day of testing. A DSM-IV diagnosis of substance use dependence within the last 5 years or abuse within the last 12 months was exclusionary for all substances except methamphetamine and cannabis, given the overarching cohort study aims. Additionally, alcohol dependence or abuse within the last 12 months was permitted.
2.2. Neuropsychiatric Assessment
Participants were evaluated for substance use disorders (dependence or abuse) and Major Depressive Disorder (MDD) diagnoses using the Composite International Diagnostic Interview (CIDI; (World Health Organization, 1998) or Structured Clinical Interview for DSM-IV (SCID-IV; (Spitzer et al., 1995), as study methodology was developed prior to the release of the DSM-5. To measure frequency and severity of current depressive symptoms, participants completed the Beck Depression Inventory-II (BDI-II; (Beck et al., 1996). The BDI-II consists of 21 items, each rated on a 4-point scale increasing in severity from 0 to 3 (possible total score range: 0 to 63). Domain-specific BDI-II scores reflecting cognitive (possible range: 0 to 27), affective (possible range: 0 to 12), and somatic (possible range: 0 to 24) symptoms of depression were computed based on a previous factor analysis of the BDI-II in 1,583 PWH (Hobkirk et al., 2015). The total BDI-II score was used as the primary outcome in analyses, while domain-specific scores were examined in secondary analyses.
A structured, clinician-administered questionnaire was used to obtain details of current antidepressant use. Participants reported use of a range of antidepressant medications, which were grouped for analysis as follows: 1) selective serotonin reuptake inhibitors (SSRIs; citalopram, escitalopram, fluoxetine, paroxetine, and sertraline); 2) serotonin and norepinephrine reuptake inhibitors (SNRIs; duloxetine and venlafaxine); 3) tricyclic antidepressants (TCAs; amitryptiline and despiramine); 4) bupropion; 5) mirtazapine; and 6) trazodone.
2.3. Neurocognitive Assessment
Participants completed a comprehensive and well-validated neuropsychological assessment that measured neurocognitive abilities across seven domains commonly impacted by HIV: verbal fluency, executive function, speed of information processing, learning, recall, working memory, and motor speed (Carey et al., 2004; Heaton et al., 2010). Raw test scores were converted to T-scores that were corrected for known demographic influences (i.e., age, sex, education, and race/ethnicity) on neurocognitive performance (Heaton et al., 2004; Heaton et al., 2003; Norman et al., 2011). T-scores were then converted to deficit scores that give differential weight to impaired over normal performance and ranged from 0 (normal) to 5 (severe) in 1-unit increments (Blackstone et al., 2012). Deficit scores were averaged within domains and across the entire battery to derive domain-specific scores and a global deficit score. Consistent with prior studies, global neurocognitive impairment was classified using a validated global deficit score cut-point of ≥0.5 (Blackstone et al., 2012; Carey et al., 2004). The deficit score variables and global neurocognitive impairment classification were examined in secondary analyses.
2.4. Neuromedical Assessment
All participants underwent a comprehensive neuromedical assessment, blood draw, and lumbar puncture. HIV disease was confirmed by MedMira Multiplo rapid test (MedMira Inc., Nova Scotia, Canada) and/or Western blot confirmation of at least two of the following HIV proteins: p24, gp41, and gp120. Clinical disease severity was categorized based on the CDC classification system. Among PWH, plasma HIV RNA was measured using reverse transcriptase-polymerase chain reaction (Amplicor, Roche Diagnostics, Indianapolis, IN) and deemed undetectable at a lower limit of quantitation (LLQ) of 50 copies/ml. Hepatitis C virus (HCV) serostatus was diagnosed by standard clinical antibody detection.
2.5. Biomarker Assays
CSF was assayed for DA and HVA using high-performance liquid chromatography (HPLC), as described in detail by Kumar et al. (Kumar et al., 2009). The reliability of HPLC for quantifying DA and HVA concentrations in CSF was reported by Kumar et al.; intra-assay coefficient of variance (% CV) was 5.4% and 10.9% for DA and HVA, respectively, and the interassay % CV was 7.1% and 11.5% for DA and HVA, respectively. DA levels were expressed as pg/ml and HVA levels were expressed as ng/ml. HVA/DA z-score ratios were calculated as a proxy for the conversion of DA into HVA, with higher ratios suggesting higher turnover of DA. A subset of participants (90 HIV+ and 66 HIV−) had additional CSF available for assays of neuroinflammation, which were conducted using commercially available immunoassays and run according to the manufacturers’ protocol. Soluble cluster of differentiation 14 (sCD14) was measured using a quantitative sandwich enzyme immunoassay (Quantikine; R&D Systems, Minneapolis, MN). Monocyte chemotactic protein (MCP-1) and interferon-inducible protein (IP-10) were measured by a multiplex bead array (EMD Millipore, Billerica, MA). Neopterin was measured by ELISA (BRAHMS Diagnostics, Hennigsdorf, Germany). CSF specimens were assayed in duplicate and measurements with coefficients of variation greater than 20% were subsequently repeated to improve precision. All biomarker values were log-transformed and converted into z-scores to reduce skewness and facilitate interpretation. A composite neuroinflammatory z-score was calculated by taking the average of the four individual neuroinflammatory z-scores.
2.6. Statistical Analysis
HIV serostatus differences on demographic, psychiatric, substance use, neuromedical, and biomarker variables were examined using analysis of variance (ANOVA), Wilcoxon/Kruskal-Wallis tests, and Chi-square statistics as appropriate. Next, Pearson’s r correlations, stratified by HIV serostatus, examined whether the composite neuroinflammation score correlated with dopaminergic biomarkers and BDI-II scores. Follow-up correlations were conducted to determine which individual neuroinflammatory biomarkers were driving composite neuroinflammation correlations with dopaminergic biomarkers and BDI-II scorees. To account for multiple comparisons, we set the FDR to 5% in the post-hoc correlational analyses involving individual neuroinflammatory biomarkers (Benjamini and Hochberg, 1995).
For the primary study aim to determine the relationship between CSF dopaminergic biomarker levels and depressive symptoms, and whether this relationship differed by HIV serostatus, multivariable linear regression modelled BDI-II scores as a function of HIV serostatus, DA z-scores, and their interaction. The same procedure was employed to examine the interaction of HIV and HVA z-scores, as well as HVA/DA z-score ratios, on BDI-II scores. The Johnson-Neyman (J-N) technique (Johnson and Neyman, 1936; Preacher et al., 2006) was conducted to probe significant interaction effects by identifying specific boundaries of dopaminergic biomarker z-scores at which HIV serostatus groups significantly differed in BDI-II scores. These boundaries are referred to as regions of significance. Region of significance analyses limited the false discovery rate (FDR) to 5% and were computed using the ‘interactions’ package in R statistical software (version 3.6.3, R Foundation for Statistical Computing, Vienna, Austria). Exploratory analyses examined correlations between dopaminergic biomarkers and individual BDI-II domains (i.e., cognitive, affective, and somatic) in order to determine the sensitivity of dopaminergic biomarkers to specific patterns of depressive symptoms.
To examine if the interactive effects of HIV and dopaminergic biomarkers on depressive symptoms were attenuated by covariates, backward model selection guided by Akaike information criteria (AIC) was applied such that final models considered background variables from Table 1 that differed by HIV serostatus at p-value < 0.10 (i.e., education, lifetime history of MDD, antidepressant use, lifetime opioid use disorder, body mass index, and hyperlipidemia). AIC models also accounted for a history of METH use disorder (none vs. lifetime only vs. current) given its high prevalence in our HIV+ and HIV− samples and potential to impact DA biomarkers and depressive symptoms. Time of lumbar puncture was also considered as a covariate given the potential influence of circadian fluctuations in DA biomarkers.
Table 1.
Study sample characteristics by HIV serostatus
| HIV− (n=102) | HIV+ (n=123) | p | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 42.1 (9.55) | 40.9 (8.10) | 0.305 |
| Sex (male), n (%) | 89 (87.3%) | 114 (92.7%) | 0.173 |
| Education (years), mean (SD) | 12.4 (2.34) | 13.1 (1.81) | 0.020 |
| Race/ethnicity | 0.562 | ||
| Non-Hispanic White, n (%) | 65 (63.7%) | 84 (68.3%) | |
| Black, n (%) | 16 (15.7%) | 14 (11.4%) | |
| Hispanic, n (%) | 16 (15.7%) | 19 (15.5%) | |
| Asian, n (%) | 2 (2.0%) | 3 (2.4%) | |
| Other, n (%) | 3 (2.9%) | 3 (2.4%) | |
| Psychiatric | |||
| Lifetime Major Depressive Disorder, n (%) | 39 (38.2%) | 67 (54.5%) | 0.022 |
| Current Major Depressive Disorder, n (%)a | 8 (7.8%) | 13 (10.6%) | 0.482 |
| Beck Depression Inventory-II, median [IQR] | 6 [2, 15] | 11 [5.5, 19.5] | 0.003 |
| Cognitive, median [IQR] | 3 [1, 7] | 2 [0, 6] | 0.115 |
| Affective, median [IQR] | 2 [0, 3] | 1 [0, 3] | 0.011 |
| Somatic, median [IQR] | 6 [3, 9] | 3 [1, 6] | <0.001 |
| On antidepressant, n (%) | 22 (21.6%) | 58 (47.2%) | <0.001 |
| SSRI, n (%) | 13 (12.7%) | 28 (22.8%) | 0.050 |
| SNRI, n (%) | 1 (1.0%) | 6 (4.9%) | 0.075 |
| TCA, n (%) | 0 (0.0%) | 10 (8.1%) | 0.002 |
| Bupropion, (n%) | 7 (6.9%) | 11 (10.8%) | 0.565 |
| Mirtazapine, (n%) | 2 (2.0%) | 6 (5.9%) | 0.226 |
| Trazodone, (n%) | 3 (2.9%) | 18 (17.6%) | 0.003 |
| Substance Use | |||
| Lifetime Use Disorder | |||
| Alcohol, n (%) | 64 (62.7%) | 85 (69.1%) | 0.388 |
| Cannabis, n (%) | 52 (51.0%) | 53 (43.1%) | 0.295 |
| Cocaine, n (%) | 34 (33.3%) | 42 (34.1%) | 1.000 |
| Methamphetamine, n (%) | 62 (60.8%) | 84 (68.3%) | 0.301 |
| Opioid, n (%) | 18 (17.6%) | 8 (6.5%) | 0.017 |
| Current Use Disordera | |||
| Alcohol, n (%) | 2 (2.0%) | 2 (1.6%) | 0.827 |
| Cannabis, n (%) | 2 (2.0%) | 2 (1.6%) | 0.827 |
| Methamphetamine, n (%) | 6 (5.9%) | 10 (8.1%) | 0.551 |
| Tobacco | |||
| Lifetime tobacco use, n (%) | 86 (84.3%) | 96 (78.0%) | 0.308 |
| Current tobacco use, n (%) | 39 (38.2%) | 53 (43.1%) | 0.548 |
| Medical Comorbidities | |||
| Hepatitis C, n (%) | 34 (33.3%) | 36 (29.3%) | 0.609 |
| Body mass index, mean (SD) | 27.8 (4.60) | 25.6 (5.79) | <0.001 |
| Diabetes, n (%) | 7 (6.9%) | 7 (5.7%) | 0.730 |
| Hypertension, n (%) | 13 (12.7%) | 23 (18.9%) | 0.212 |
| Hyperlipidemia, n (%) | 5 (4.9%) | 19 (15.6%) | 0.008 |
| HIV Disease Characteristics | |||
| AIDS diagnosis, n (%) | 60 (48.8%) | ||
| CDC Stage | |||
| A, n (%) | 53 (43.8%) | ||
| B, n (%) | 34 (28.1%) | ||
| C, n (%) | 34 (28.1%) | ||
| Estimated years of infection, median [IQR] | 7 [2, 12] | ||
| Nadir CD4 count, median [IQR] | 224 [75, 367] | ||
| Current CD4 count, median [IQR] | 471 [337, 626] | ||
| On ART, n (%) | 87 (70.7%) | ||
| Detectable plasma HIV RNA, n (%) | 59 (49.2%) |
Note. ART = antiretroviral therapy; SSRI = selective serotonin reuptake inhibitor; SNRI = serotonin-norepinephrine reuptake inhibitor; TCA = tricyclic antidepressant.
Current indicates meeting diagnostic criteria within the last 30 days.
Last, a series of secondary analyses were conducted to probe the influence of cofactors, including HIV disease characteristics, antidepressants, stimulant use, and neurocognition. This included sensitivity analyses to determine the robustness of significant associations in clinically-relevant subgroups (e.g., individuals with undetectable plasma HIV RNA, individuals not taking antidepressants) as well as correlational analyses between cofactors and dopaminergic biomarker z-scores. All statistical analyses were conducted in R.
3. Results
3.1. Participant Characteristics
The full study sample was 66% non-Hispanic White and 90% male with a mean age of 41.4 years (range: 19–65) and mean education of 12.8 years. Participant characteristics by HIV serostatus are presented in Table 1. Groups were comparable with respect to age, sex and race/ethnicity, however HIV+ participants had significantly more years of education than HIV-participants. Both groups had relatively high but comparable rates of lifetime substance use disorders, with the exception of significantly higher rates of lifetime opioid use disorder in HIV-compared to HIV+. The proportion of participants meeting diagnostic criteria for MDD in the last 30 days did not significantly differ between groups (HIV−: 8%; HIV+: 11%); however, HIV+ participants reported more extensive depressive symptomatology, as indicated by higher median BDI-II scores (HIV−: 6; HIV+: 11) and higher rates of lifetime MDD (HIV−: 38%; HIV+: 55%) and antidepressant use (HIV−: 22%; HIV+: 47%). With respect to the BDI-II, HIV+ participants reported significantly higher levels of affective (p=.011) and somatic symptoms (p<.001) than HIV− participants, but not cognitive symptoms (p=.115). The HIV+ group exhibited evidence of ART-induced immune recovery, as indicated by higher current CD4 counts (median=471 cells/mm3) compared to nadir CD4 counts (median= 224 cells/mm3) and active ART use (71%). Notably, almost half of the HIV+ sample had detectable HIV virus in plasma (49%).
3.2. HIV Serostatus Differences in Dopaminergic and Neuroinflammatory Biomarkers
CSF DA values ranged from 0.2 to 285.2 pg/mL (median=30.2, interquartile range [IQR]=6.3–59.6) and CSF HVA values ranged from 0.5 to 305.9 ng/mL (median=10.4, interquartile range [IQR]=4.4–26.9). The distributions of raw and log-transformed z-scores for DA and HVA by HIV serostatus group are presented in Figure 1A–D. DA and HVA z-scores exhibited a positive, medium-sized correlation in the full study sample (r=.41, p<.001) and within serostatus groups (HIV−: r=.39, p<.001; HIV+: r=.42, p<.001; Figure 1E).
Fig. 1.
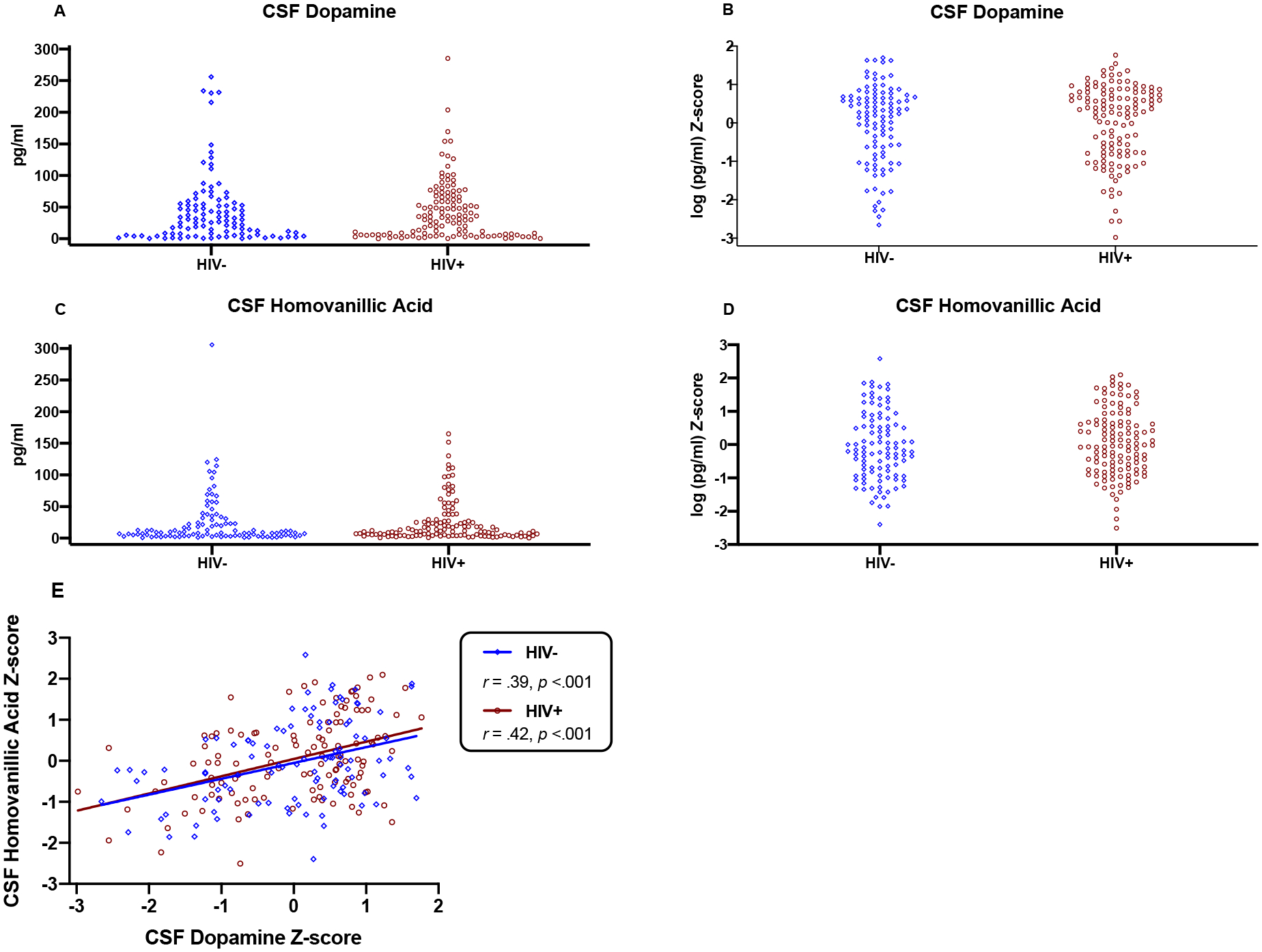
Panels A-D: Raw concentrations (pg/ml) and log-transformed z-scores for CSF dopamine and homovanillic acid by HIV serostatus. Log-transformed z-scores substantially reduced the skewness of biomarker distributions and were accordingly used for parametric analyses. Panel E: Dopamine and homovanillic acid z-scores were positively correlated in both HIV− (r=.39, p<.001) and HIV+ groups (r=.42, p<.001).
Table 2 reports serostatus group differences in CSF dopaminergic and CSF inflammatory biomarkers. Compared to HIV−, HIV+ individuals displayed comparable DA z-scores (d=0.03, p=.803), HVA z-scores (d=0.11, p=.408), and HVA/DA z-score ratios (d=0.06, p=.658). HIV+ exhibited significantly higher composite neuroinflammation z-scores than HIV− (d=1.02, p<.001). With respect to specific neuroinflammatory biomarkers, HIV+ displayed significantly higher levels of IP-10 (d=1.41, p<.001), neopterin (d=0.53, p=.001), and sCD14 (d=0.55, p<.001), and trended toward higher levels of MCP-1 (d=0.28, p=.081).
Table 2.
CSF biomarkers by HIV serostatus
| CSF Biomarker | HIV− | HIV+ | p | d |
|---|---|---|---|---|
| Dopaminergic | ||||
| Dopamine (pg/mL) | 29.2 [7.3, 55.0] | 33.6 [6.2, 66.3] | 0.803 | 0.03 |
| Homovanillic Acid (ng/mL) | 9.0 [3.6, 25.6] | 10.9 [4.4, 27.0] | 0.408 | 0.11 |
| Neuroinflammatorya | ||||
| Composite z-score | −0.4 (0.07) | 0.3 (0.06) | <.001 | 1.02 |
| IP-10 (pg/mL) | 182.6 [127.3, 291.3] | 431.8 [305.2, 683.2] | <.001 | 1.41 |
| MCP-1 (pg/mL) | 483 [345.1, 652.6] | 513.7 [413.7, 761.8] | 0.081 | 0.28 |
| Neopterin (nmol/L) | 6.1 [4.6, 8.3] | 8.9 [5.9, 13.4] | 0.001 | 0.53 |
| Soluble CD14 (pg/mL) | 68486 [47948, 103220] | 94564 [61830, 121224] | <.001 | 0.55 |
Note. Values presented as median [IQR] or mean (SD). Biomarker values were log-transformed and standardized for statistical testing and Cohen’s d estimates.
Neuroinflammatory data available for a subset of participants (90 HIV+ and 66 HIV−).
3.3. Depression and Dopaminergic Biomarker Correlations with Neuroinflammation
In the HIV+ group, lower HVA z-scores significantly correlated with higher composite neuroinflammation z-scores (r=−.26, p=.013; Figure 2A). Follow-up correlational analyses indicated significant associations between lower HVA z-scores and higher IP-10 (r=−.24, p=.022; Figure 2B) and MCP-1 z-scores (r=−.46, p<.001; Figure 2C), which remained significant after FDR correction (IP-10: p=.044; MCP-1: p<.001). HVA z-scores did not significantly correlate with composite neuroinflammation z-scores in the HIV− group (r=.01, p=.940). Similarly, correlations between DA z-scores and composite neuroinflammation z-scores did not reach statistical significance in HIV+ (r=.16, p=.133) or HIV− individuals (r=.06, p=.637).
Fig. 2.
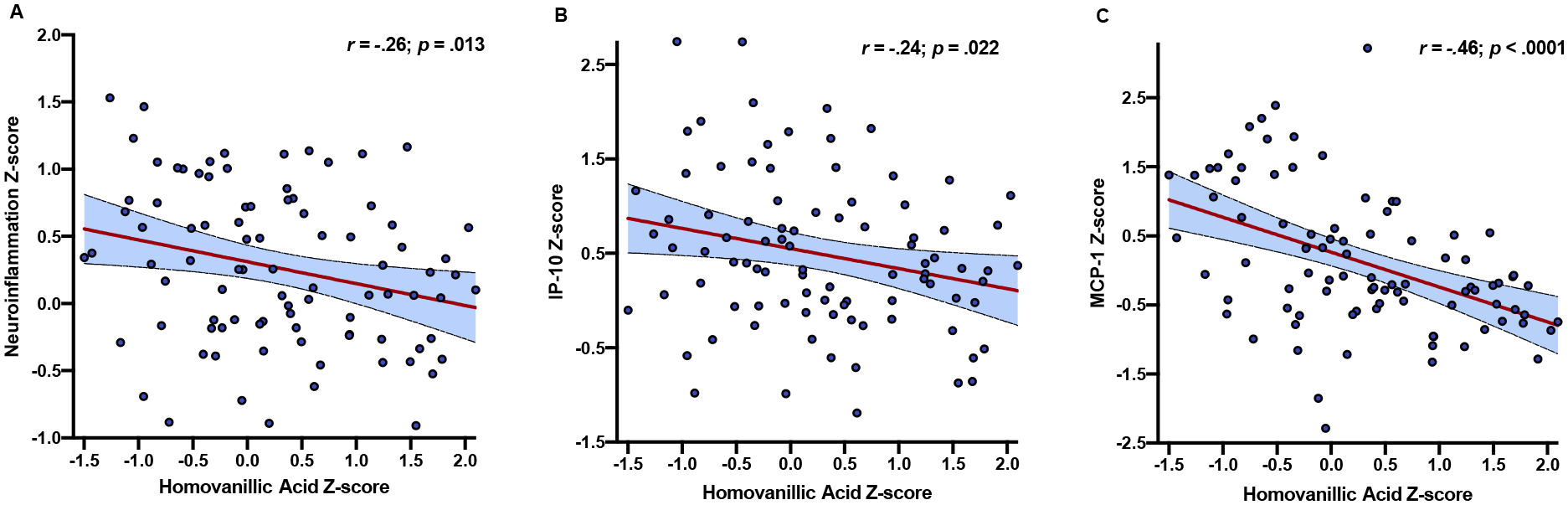
Lower CSF homovanillic acid z-scores correlate with higher composite neuroinflammation z-scores only in people with HIV (panel A). Adjusting for multiple comparisons, significant correlations were also detected between lower homovanillic acid and higher IP-10 (panel B) and MCP-1 values (panel C).
In the HIV+ group, higher BDI-II scores trended toward higher composite neuroinflammation z-scores (r=.18, p=.096). Follow-up correlational analyses indicated a significant association between higher BDI-II scores and higher IP-10 z-scores (r=.22, p=.034), however this association did not reach statistical significance after FDR correction (p=.136). BDI-II scores did not significantly correlate with composite neuroinflammation z-scores in the HIV− group (r=−.11, p=.364).
3.4. Interactive Effects of HIV and Dopaminergic Biomarkers on Depression
Table 3 presents estimates for the multivariable linear regressions examining BDI-II scores as a function of HIV serostatus, dopaminergic biomarkers, and their interaction. Results indicated a significant interaction such that the effect of HVA on BDI-II scores significantly differed between HIV+ and HIV− groups (b=−3.68 p=.012). Specifically, lower HVA z-scores significantly related to greater BDI-II scores in HIV+ (b=−2.14, r=−.19, p=.034), yet did not significantly relate to BDI-II scores in HIV− (b=1.55, r=.14, p=.146). A significant interaction effect was also detected between HIV and DA on BDI-II scores (b=−3.15, p=.033). Notably, the effect of DA on BDI-II scores was not statistically significant in either group, yet lower DA z-scores related to greater depressive symptoms in HIV+ (b=−1.53, r=−.14, p=.127) and fewer depressive symptoms in HIV− (b=1.61, r=.15, p=.134). HVA/DA ratios did not moderate the effect of HIV on BDI-II scores (p=.982) and did not relate to BDI-II scores in either group (HIV−: b=0.19, r=.03, p=.789; HIV+: b=0.16, r=.02, p=.809). In order to identify the specific range of DA and HVA z-scores at which HIV serostatus groups significantly differed on BDI-II scores, we applied the J-N technique. HIV was associated with significantly higher BDI-II scores only at the lower half of the range of DA (region of significance: z= −2.98 to 0.11; Figure 3A) and HVA z-scores (region of significance: z= −2.51 to 0.06; Figure 3B), whereas HIV serostatus did not significantly predict BDI-II scores outside the region of significance for DA (z>0.11) and HVA (z>0.06).
Table 3.
CSF DA and HVA moderate the effect of HIV on depressive symptoms, independent of psychosocial risk factors.
| DA Model | Unadjusted Model | AIC-adjusted Model | ||||
|---|---|---|---|---|---|---|
| Predictor | beta (SE) | 95% CI | p | beta (SE) | 95% CI | p |
| HIV | 3.5 (1.47) | 0.61, 6.39 | 0.018 | 1.80 (1.43) | −1.03, 4.62 | 0.212 |
| DA | 1.61 (1.07) | −0.50, 3.72 | 0.134 | 1.45 (1.00) | −0.52, 3.42 | 0.149 |
| HIV x DA | −3.15 (1.47) | −6.03, −0.26 | 0.033 | −2.79 (1.39) | −5.52, −0.05 | 0.046 |
| Lifetime MDD | - | - | - | 2.79 (1.45) | −0.06, 5.64 | 0.055 |
| Antidepressant use | - | - | - | 4.42 (1.52) | 1.41, 7.42 | 0.004 |
| Education (years) METH use disorder | - | - | - | −0.65 (0.34) | −1.31, 0.01 | 0.055 |
| Current (vs. none) | - | - | - | 11.45 (2.79) | 5.95, 16.94 | <0.001 |
| Lifetime (vs. none) | - | - | - | 4.24 (1.51) | 1.26, 7.22 | 0.006 |
| HVA Model | Unadjusted Model | AIC-adjusted Model | ||||
| Predictor | beta (SE) | 95% CI | p | beta (SE) | 95% CI | p |
| HIV | 3.52 (1.46) | 0.64, 6.4 | 0.017 | 1.8 (1.43) | −1.01, 4.62 | 0.209 |
| HVA | 1.55 (1.06) | −0.54, 3.64 | 0.146 | 1.19 (0.99) | −0.76, 3.14 | 0.232 |
| HIV x HVA | −3.68 (1.46) | −6.56, −0.81 | 0.012 | −3.20 (1.36) | −5.88, −0.53 | 0.019 |
| Lifetime MDD | - | - | - | 2.93 (1.44) | 0.09, 5.76 | 0.043 |
| Antidepressant use | - | - | - | 4.33 (1.52) | 1.33, 7.32 | 0.005 |
| Education (years) | −0.64 (0.33) | −1.30, 0.02 | 0.055 | |||
| METH use disorder | ||||||
| Current (vs. none) | - | - | - | 11.2 (2.77) | 5.74, 16.66 | <0.001 |
| Lifetime (vs. none) | - | - | - | 4.62 (1.48) | 1.71, 7.54 | 0.002 |
Note. AIC = Akaike information criteria; CI = confidence interval; DA = dopamine; HVA = homovanillic acid; MDD = Major Depressive Disorder; METH = methamphetamine; SE = standard error.
Fig. 3.
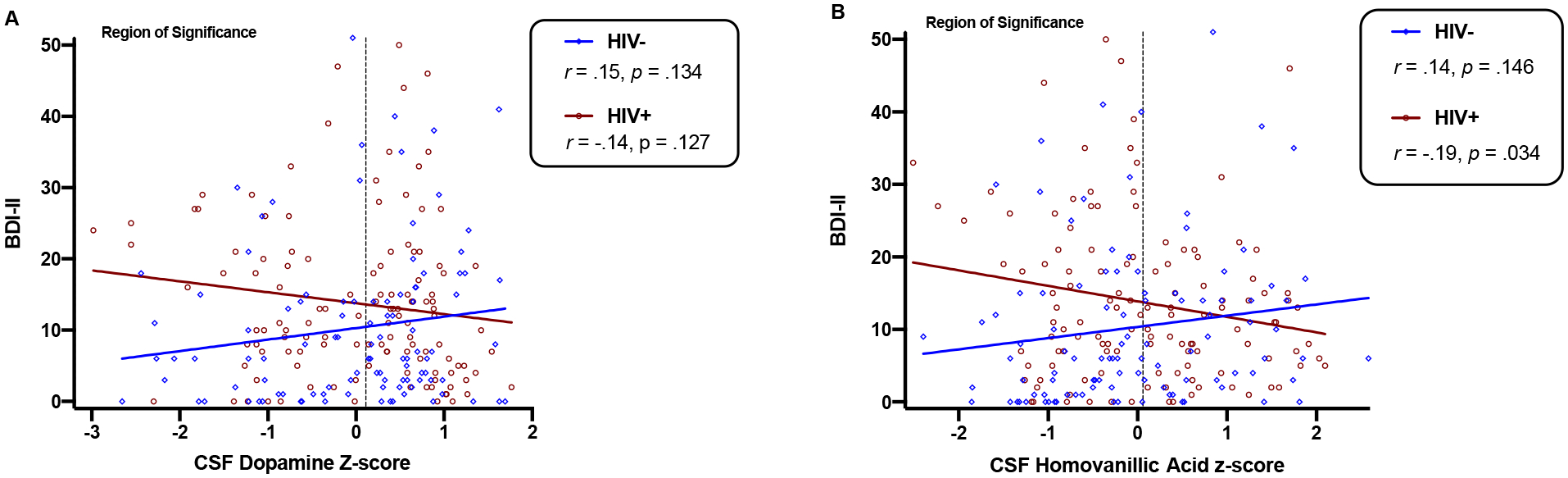
CSF dopamine (panel A) and homovanillic acid (panel B) levels significantly moderate the association between HIV and depressive symptoms. Region of significance analyses estimate that HIV+ individuals have significantly higher depressive symptoms than HIV− individuals only when concentrations of dopamine and homovanillic acid fall within the lower half of their respective distributions (dopamine z ≤ 0.11; homovanillic acid z ≤ 0.06).
3.5. Dopaminergic Biomarkers and Domains of Depression
In the HIV+ group, correlational analyses between dopaminergic biomarkers and individual BDI-II domains revealed significant associations between higher cognitive symptoms of depression and lower HVA (r=−.22, p=.016) and DA (r=−.20, p=.025) z-scores. Correlations with affective and somatic symptoms did not reach statistical significance for either HVA (affective: r=−.14, p=.118; somatic: r=−.15, p=.102) or DA z-scores (affective: r=−.07, p=.428; somatic: r=−.15, p=.101) in the HIV+ group. Dopaminergic biomarkers did not correlate with any BDI-II domains in the HIV− group (ps>.10).
3.6. AIC-guided Models with Covariates
Additional AIC-guided models that employed backward selection of covariates were conducted to determine whether the interactive effects of HIV and dopaminergic biomarkers on total BDI-II scores were attenuated by clinical and demographic factors (see Table 3). In these covariate-adjusted models, both the HIV x DA and HIV x HVA interactions remained significantly associated with BDI-II scores. With respect to covariates, a lifetime diagnosis of MDD, current antidepressant use, and a history of both current and lifetime METH use disorder significantly related to higher BDI-II scores. Fewer years of education exhibited a trend-level association with higher BDI-II (p=.055), but was retained in the models because its inclusion improved overall model fit (based on AIC criteria) compared to competing models.
3.7. Secondary Analyses
3.7.1. HIV Disease Severity
A series of secondary analyses were conducted within the HIV+ group in order to explore the influence of HIV disease characteristics (see Table 1 for a list of HIV-specific variables) on study findings. With respect to CDC staging, individuals in stage C trended toward lower DA z-scores (d=−0.42, p=.056) and exhibited significantly higher HVA/DA ratios (d=0.48, p=.030) compared to individuals in stage A. Similarly, lower nadir CD4 counts significantly related to higher HVA/DA ratios (Spearman’s rho=−.19, p=.038) and individuals with a history of AIDS trended toward higher HVA/DA ratios than those without AIDS (d=0.34, p=.060). There were no significant associations between HIV disease characteristics and HVA z-scores and the negative association between HVA and BDI-II scores remained statistically significant (b=−2.71, r=−.26, p=.040) in a sensitivity analysis restricted to HIV+ individuals with undetectable viral load (n=59).
3.7.2. Antidepressant Use
Given that antidepressant use was more prevalent in HIV+ individuals and significantly related to higher BDI-II scores, we examined whether individual classes of antidepressants impacted dopaminergic biomarker z-scores and the interactive effects of HIV and dopaminergic biomarkers on BDI-II scores. DA, HVA, and HVA/DA z-scores did not significantly differ by individuals on any antidepressant compared to non-users, nor did z-scores differ based on specific antidepressant classes compared to non-users. When treating specific antidepressant classes as individual covariates in AIC-guided regression analyses, the interactions between HIV status and dopaminergic biomarkers on BDI-II scores remained significant (DA model: p=.042; HVA model: p=.020). In these models, use of SNRIs significantly related to higher BDI-II scores (DA model: b=−14.01, p<.001; HVA model: b=−13.19, p=.001). Mirtazapine (DA model: b=−5.25, p=.160; HVA model: b=−5.22, p=.158) and trazodone (DA model: b=3.42, p=.152; HVA model: b=−3.26, p=.171 did not exhibit a significant relationship with BDI-II scores but were also retained as covariates in these models based on the AIC metric. Last, we conducted a sensitivity analysis restricted to individuals not on antidepressants (HIV−: n=80; HIV+: n=65). Although the interactions between HIV and dopaminergic biomarkers also no longer reached statistical significance in this smaller subsample, both interaction terms were still retained in models based on the AIC metric (DA model: p=.128; HVA model: p=.076).
3.7.3. Stimulant Use
Given the potential for long-term stimulant use to alter dopaminergic function and that 70% of the full sample met criteria for a lifetime use disorder of methamphetamine or cocaine (HIV−: 68.6%; HIV+: 71.3%), we examined whether dopaminergic biomarkers differed between individuals with a lifetime stimulant use disorder (STIM+) and those without (STIM-). In the full study sample, dopaminergic biomarkers did not significantly differ between STIM− and STIM+ individuals (DA: p=.895; HVA: p=.363; HVA/DA: p=.532). However, an antagonist interaction effect between HIV serostatus and STIM status was observed (p=.003) such that STIM+ individuals had significantly lower DA z-scores in the HIV+ group (d=−0.40, p=.046), yet STIM+ individuals had significantly higher DA levels in the HIV− group (d=0.48, p=.026). STIM status did not significantly relate to HVA or HVA/DA z-scores in either HIV serostatus group.
3.7.4. Neurocognition
Given that neurocognitive symptoms are common in depression and prior studies have shown low DA and HVA levels in patients with HIV-associated neurocognitive disorders, we conducted correlational analyses between dopaminergic biomarkers and global and domain-specific neurocognitive deficit scores. With respect to the dichotomous global neurocognitive impairment classification, 37.7% of the HIV+ group and 28.3% of the HIV− group exhibited global neurocognitive impairment. With respect to the continuous deficit scores, HIV+ individuals exhibited significantly higher (worse) global (d=0.28, p=.038) and verbal fluency deficit scores (d=0.39, p=.004), and trended toward higher executive function deficit scores (d=0.25, p=.071). In the HIV+ group, lower HVA z-scores significantly correlated with higher (worse) global (r=.19, p=.032), learning (r=.18, p=.042), and working memory (r=.27, p=.003) deficit scores, and also trended toward higher recall deficit scores (r=.16, p=.073). Neither DA nor HVA/DA z-scores correlated with deficit scores in HIV+. Similarly, dopaminergic biomarkers did not significantly correlate with deficit scores in the HIV− group.
4. Discussion
The clinical presentation of depression can greatly vary across individuals and there is similar heterogeneity in the pathophysiological mechanisms underlying depression (Sibille and French, 2013). Dopaminergic dysfunction is implicated in the pathogenesis of depression in HIV, yet this is understudied at the clinical level (Del Guerra et al., 2013). In the present study, depressive symptoms were expectedly greater in PWH than HIV− counterparts. This HIV-related difference in depression was moderated by central dopaminergic function, as indexed by concentrations of DA and HVA in CSF. Specifically, PWH exhibited greater depressive symptoms than HIV− individuals only at the lower half of the total range of DA and HVA concentrations. In PWH only, lower HVA significantly related to higher levels of depression as well as higher levels of neuroinflammation, a hallmark feature of HIV-associated CNS dysfunction. The high levels of depression observed in PWH with low dopaminergic tone persisted after accounting for several psychosocial risk factors for depression, as well as for antidepressant medication use, and lower HVA remained associated with higher depressive symptoms in well-treated, virally suppressed PWH. Taken together, these findings suggest that low central dopaminergic tone may be a pathophysiological signature of depression in PWH, particularly in the context of neuroinflammation.
Our interaction analyses demonstrated a significantly stronger relationship between lower CSF dopaminergic biomarkers, especially HVA, and higher depressive symptoms in PWH than HIV− individuals. Contrary to expectations, depressive symptoms did not vary as a function of dopaminergic biomarkers in HIV− individuals. In a recent meta-analysis of HIV− samples, CSF HVA was significantly decreased in patients with depression compared to controls (Hedges’s g = −0.30), and this effect appeared to be strongest in men (Ogawa et al., 2018). In contrast to the studies included in this meta-analysis, the present investigation modelled depression as a continuous range of current symptom severity and included individuals with comorbid disorders (e.g., substance use). Nevertheless, low CSF HVA is the most consistently observed CSF catecholaminergic finding in patients with depression (Hori and Kunugi, 2013) and low CSF dopamine biomarkers in other populations such as patients with or at-risk for Parkinson’s disease correlate with clinically-relevant outcomes, including greater severity of depressive symptoms, motor impairment, and future development of disease (Goldstein et al., 2018; Lian et al., 2019; Stefani et al., 2017).
Examination of individual BDI-II domain scores revealed significantly higher affective and somatic, but not cognitive, symptoms of depression in PWH than HIV−. However, CSF HVA and DA were most strongly correlated with cognitive symptoms in PWH. Although the magnitude of these associations between lower dopaminergic biomarkers and greater cognitive symptoms of depression were modest, this finding demonstrates a direct link between the dopaminergic system and depression in PWH that is unlikely to be influenced by HIV-related medical symptoms, which are more similar to the somatic and affective features of depression. In the BDI-II factor analysis reported by Hobkirk et al. (2015), from which the current study derived domains of depression, the symptom of anhedonia loaded onto the affective domain while the symptoms of fatigue and poor concentration loaded onto the somatic domain (Hobkirk et al., 2015). Given that anhedonia and psychomotor slowing are thought to emerge under conditions of dopaminergic deficiency and neuroinflammation (Belujon and Grace, 2017; Felger, 2017), future studies should aim to comprehenisvely isolate these specific symptoms independent of broader depressive domains and examine them in relation to DA biomarkers in PWH. We also did not observe a significant association between CSF dopaminergic biomarkers and neurocognitive deficits in speed of information processing and motor speed, which have previously been identified as neurocognitive domains sensitive to the transient effects of depression in PWH (Paolillo et al., 2020). However, we did observe an association in the HIV+ group between lower CSF HVA and poorer global neurocognition with specific deficits in working memory and learning, which have previously been linked to lower subcortical DA and HVA levels in PWH (Kumar et al., 2011).
We did not observe HIV group differences in CSF DA or HVA levels, however we did observe significant or trend-level associations between markers of greater HIV disease severity (i.e., CDC stage, nadir CD4, AIDS diagnosis) and lower DA and higher HVA/DA ratios. Compared to HIV− controls, some studies have reported lower CSF HVA and DA in PWH (Berger et al., 1994; Larsson et al., 1991), and within those cohorts the strongest reductions in dopaminergic levels occurred in PWH at later stages of disease progression and neurobehavioral dysfunction. In an analysis of post-mortem human brains, Kumar et al. (2009) observed significantly lower concentrations of DA in the caudate nucleus, putamen, globus pallidus, and substantia nigra of PWH compared to controls (Kumar et al., 2009). Although this neuropathological analysis importantly corroborated prior observations in CSF, the high lability of DA renders it vulnerable to decay unless brain tissue is preserved in anoxic conditions and post-mortem analyses may therefore provide an underestimation of overall DA concentrations. In contrast to advanced stages of HIV disease, increased DA and lower HVA/DA ratios have also been found in CSF of humans and macaques at earlier stages of HIV infection (Horn et al., 2013; Koutsilieri et al., 2001; Scheller et al., 2010). The mechanism underlying this hyperdopaminergic state in early disease remains unclear, although it may reflect alterations in the metabolism of DA via the MAO and COMT enzymes, the latter of which is implicated as a salient modulator of neurocognitive outcomes and CSF DA in PWH (Bousman et al., 2010; Saloner et al., 2020; Saloner et al., 2019; Sundermann et al., 2015). Regardless of mechanism, this early elevation in synaptic DA may enhance auto-oxidation of DA and, over time, contribute to neurotoxicity in dopaminergic circuitry and neurobehavioral deficits at later stages of disease (Scheller et al., 2010).
In the present study, PWH exhibited higher levels of neuroinflammation across multiple biomarkers and higher burden of neuroinflammation was in turn correlated with lower HVA concentrations. With respect to specific neuroinflammatory markers, MCP-1 and IP-10 exhibited significant associations with HVA. MCP-1 is co-localized with dopaminergic neurons in the substantia nigra in the rat brain (Banisadr et al., 2005) and higher levels of CSF MCP-1 have previously been associated with higher levels of depression in Parkinson’s disease (Lindqvist et al., 2013) and higher magnetic resonance spectroscopy estimates of choline, a marker of membrane turnover and inflammation, in the basal ganglia of PWH (Anderson et al., 2015). CSF IP-10 levels have been shown to positively correlate with depression severity in PWH (Rivera-Rivera et al., 2014) and up-regulation of IP-10 is considered a central factor in the pro-inflammatory state induced by HIV disease (Lei et al., 2019). Dopaminergic biomarkers did not correlate with neuroinflammation in the HIV− group, suggesting that the lower levels of neuroinflammation in HIV− individuals are insufficient to observe an association between dopaminergic and neuroinflammatory states.
Although some conflicting evidence has been published, the preponderance of reports in humans support that inflammation downregulates multiple aspects of dopaminergic neurotransmission, including synthesis, packaging, and release of DA (Brydon et al., 2008; Capuron et al., 2012; Felger and Miller, 2012; Felger and Treadway, 2017). For example, inflammation-induced oxidative stress decreases the bioavailability of tetrahydrobriopterin, an enzymatic cofactor critical in the synthesis of DA, which in turn has been linked to lower CSF DA and HVA (Felger et al., 2013; Neurauter et al., 2008). Inflammatory cytokines may also increase the activity of DA transporter and decrease the activity of vesicular monoamine transporter 2 (Gelman et al., 2006; Kazumori et al., 2004; Morón et al., 2003), resulting in greater reuptake of DA into presynaptic terminals and impaired sequestration of presynaptic DA into vesicles, thereby raising cytosolic DA concentrations to neurotoxic levels (Felger, 2017). Relationships between DA and inflammation also appear to be reciprocal, as DA can suppress peripheral and central immune activity. Multiple low dose DA treatments in primary human macrophages (derived from human bone marrow; (Yan et al., 2013), have been shown to control inflammation through inhibition of the NLRP3 inflammasome, potentially through D1 receptor signaling, and downregulates inflammatory responses in the periphery in response to lipopolysaccharide (Yan et al., 2015), a particularly important mechanism in HIV, as abnormal gut permeability results in translocation of microbial products including LPS (Dillon et al., 2016). DA has also been shown to confer anti-inflammatory benefits through D2 receptor signaling in microglia and astrocytes (Dominguez-Meijide et al., 2017; Shao et al., 2013; Vidal and Pacheco, 2020). In contrast, other data suggest that DA treatments in primary human macrophages, delivered acutely in concentrations that resemble CNS levels obtained during stimulant use, may prime the NLRP3 pathway through activation of NF-κB signaling (Nolan et al., 2020). Thus, the immunomodulatory effects of DA are at least partially dependent upon the concentration and duration of DA exposure and are also likely to vary based on the inflammatory disease model.
Pramipexole, a DA receptor agonist, has demonstrated efficacy in treating depressive symptoms in patients with previously treatment-resistant depression as well as in patients with Parkinson’s disease (Barone et al., 2010; Fawcett et al., 2016). Pramipexole has high affinity for D2–4 receptors and negligible affinity for D1 and D5 receptors (Kvernmo et al., 2006). Preclinical models demonstrate that pramipexole attenuates CNS inflammation in mice with experimental autoimmune encephalitis (Lieberknecht et al., 2017) and D2 and D3 receptors mediate anti-inflammatory effects in the CNS (Du et al., 2018; Escalona and Fawcett, 2017; Xia et al., 2019). In an animal model, D3 receptor expression was localized to astrocytes or microglia in the mesolimbic reward pathway, including the medial prefrontal cortex, nucleus accumbens and ventral tegmental area. (Wang et al., 2020). Moreover, the D3 receptor knockout mice displayed depressive-like behavior (Wang et al., 2020). These mechanisms are predicted to be important in HIV, since the HIV protein Tat suppresses expression of DA receptors and the innate immune HIV target cells express all the DA receptors (Basova et al., 2018; Gaskill et al., 2012). Thus, PWH with treatment-resistant depression and high inflammation may show a greater response to DA agonists than traditional antidepressants, which often have stronger effects on serotonergic and noradrenergic pathways.
The current investigation was not designed to formally evaluate the influence of antidepressant use on associations between HIV, DA and depression; however, it is noteworthy that the interactive effects of HIV and dopaminergic biomarkers on BDI-II scores remained significant after accounting for antidepressant use. The majority of antidepressants, including those reported in the present study and those retained in regression analyses, do not directly act on the dopaminergic system, which may help explain why statistical adjustment for antidepressant use did not attenuate our findings. Moreover, we did not detect any significant differences between antidepressant use, regardless of class, and non-users on DA, HVA, and HVA/DA ratios. However, given the small sample sizes for use of individual antidepressant classes in our study, our results may not generalize to studies that employ larger sample sizes with more comprehensive data on antidepressant use (e.g., duration of use, dosage). A recent study found that antidepressant use did not relate to CSF HVA levels among depressed patients, whereas CSF serotonin and norepinephrine metabolite levels did differ with antidepressant use (Yoon et al., 2017). The observation that antidepressant use was also associated with higher BDI-II scores is likely an artifact of depressed individuals being more likely to be prescribed antidepressants than those who are not depressed. The removal of these depressed individuals in the sensitivity analysis subsample of non-users also shifted the distribution of BDI-II scores toward overall less severe levels of depression, which inherently influenced our ability to detect statistically significant interactions between HIV serostatus and dopaminergic biomarkers on BDI-II scores (although these interaction terms were still retained based on the AIC metric).
Our findings and their interpretation are important to consider in the context of our sample, which consisted of individuals with a high prevalence of lifetime stimulant use disorders. TMARC, the parent study from which participants were recruited, was designed to study the combined CNS effects of HIV and methamphetamine use, given the high prevalence of comorbid substance use disorders in PWH (Soontornniyomkij et al., 2016). Thus, the HIV-group in the present study had a comparable profile of substance use to the PWH group. Depressive symptoms were substantially higher in participants who currently met criteria for methamphetamine use disorder as well as those with only a remote history of methamphetamine use disorder, yet the interaction of HIV and DA biomarkers on depressive symptoms remained significant after controlling for methamphetamine use and other psychosocial factors. In secondary analyses that compared dopaminergic biomarker levels between participants with a history of stimulant (i.e., methamphetamine or cocaine) use disorders and those without a history of stimulant use disorders, we detected opposing effects of stimulant use on DA z-scores conditional upon HIV serostatus. The observation that HIV+/STIM+ individuals had lower DA z-scores than HIV+/STIM− individuals is consistent with the known hypodopaminergic effects of chronic stimulant use (Ashok et al., 2017). Although we observed a hypodopaminergic effect of STIM use for DA z-scores in PWH, we did not observe a similar effect for HVA z-scores, which were more strongly associated with depressive symptoms than DA z-scores. With respect to the HIV− group, we unexpectedly observed higher DA levels in STIM+ individuals compared to STIM− individuals. Although it is unclear why the HIV−/STIM+ group exhibited higher DA levels, as this is conflicting with the extant literature, the opposing effects of lifetime STIM use disorders in HIV− and HIV+ individuals is consistent with the broad body of literature suggesting that the presence of HIV disease confers novel characteristics to the dopaminergic effects of STIM use (Gaskill et al., 2009; Soontornniyomkij et al., 2016). The antagonistic pattern of STIM effects on DA z-scores in the present cohort may be a result of biological effects of HIV disease as well as unmeasured psychosocial factors that differentiate the HIV− vs. HIV+ cohort.
An important limitation to our study is that the measurement of CSF DA and HVA reflects central dopaminergic function in vivo, but it is not directly reflective of brain parenchymal DA activity in any specific region. There is significant debate and lack of clarity about the precise mechanism(s) underlying depression, however our results support a role for the dopaminergic system in depression, at least in the context of HIV-associated inflammation. Of the four major dopaminergic pathways in the brain (i.e., nigrostriatal, mesolimbic, mesocortical, tuberoinfundibular), the mesolimbic and mesocortical pathways are particularly important in depression. DA projects from the ventral tegmental area to key neuroanatomical structures relevant to mood, including the nucleus accumbens (NAc), anterior cingulate cortex (ACC), and prefrontal cortex (Husain and Roiser, 2018). For example, depletion of DA in the NAc in rodents elicits an anhedonic phenotype (Salamone et al., 1994; Walton et al., 2006) and neuroimaging studies demonstrate alleviation of depressive symptoms following deep brain stimulation of the NAc or ACC (Heshmati and Russo, 2015). In the context of inflammation, higher c-reactive protein in patients with MDD was associated with a decoupling of the ventral striatum and ventromedial prefrontal cortex, which in turn related to increased anhedonia (Felger et al., 2016).
Additional limitations to the present study are worth discussing. First, our observational study design was cross-sectional and we therefore cannot rule out the potential for reverse causality (i.e., depressive symptoms in PWH contribute to dopaminergic deficits). We statistically controlled for a lifetime history of MDD to mitigate this possibility, but a longitudinal design would enhance our understanding of how changes in the dopaminergic system converge with the trajectories of depressive symptomatology in HIV. Although our data linking lower HVA levels to higher levels of neuroinflammatory markers is consistent with prior research, only a subset of the study sample had neuroinflammatory biomarker data for analysis. Thus, we are limited in our ability to model more complex associations (e.g., mediation) between DA biomarkers, neuroinflammation, and depression. Compared to more recent cohort studies, the rates of ART use and viral suppression were lower in our sample of PWH. This in part may be due to suboptimal ART adherence related to substance use as well as different treatment guidelines (ie, start ART only for CD4 < 350) and lower effectiveness of available ART during the study enrollment period. However, the relationship between CSF HVA and BDI-II scores persisted in sensitivity analyses restricted to individuals with undetectable HIV RNA, underscoring the relevance of dopaminergic dysfunction to depressive symptoms even for well-treated PWH. Consistent with the majority of neuroHIV research, our sample was predominantly comprised of men and the majority of participants were non-Hispanic White. Thus, it is critical that our study methodology be replicated in larger samples that have better representation of women and racial/ethnic minorities, who may also have distinct biological and psychosocial vulnerabilities that influence the dopaminergic system and depression.
Our data support preclinical models implicating dopaminergic dysfunction in the pathogenesis of depression in the context of elevated neuroinflammation, and are among the first to examine these relationships in PWH. Given that HIV-related neuroinflammation has been proposed as a catalyst for dopaminergic dysregulation in mesocortical pathways that contribute to depressive symptoms, enhancement of dopaminergic tone may be particularly effective in treating depression in PWH. Thus, future studies should systematically evaluate the efficacy of dopaminergic and/or anti-inflammatory treatments for PWH with depressive symptoms that are non-responsive to traditional antidepressants.
Highlights.
People with HIV (PWH) report higher levels of depression than HIV− individuals
Depression is higher in PWH only at low levels of CSF dopaminergic (DA) biomarkers
Lower CSF homovanillic acid (HVA) relates to higher depression in PWH, but not HIV−
Lower CSF HVA relates to higher CSF inflammation in PWH, but not HIV−
PWH may show a greater response to DA agonist treatments for depression than HIV−
Acknowledgments
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD), the Sanford-Burnham Medical Discovery Institute (SBMDI), and the University of California, Riverside (UCR). The TMARC comprises: Administrative Coordinating Core (ACC) – Executive Unit: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Scott L. Letendre, M.D., and Cristian L. Achim, M.D., Ph.D.; Center Manager – Mariana Cherner, Ph.D.; Associate Center Managers – Erin E. Morgan, Ph.D. and Jared Young, Ph.D.; Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Chief), Clint Cushman, B.A. (Unit Manager); ACC – Statistics Unit: Florin Vaida, Ph.D. (Unit Chief), Ian S. Abramson, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC – Participant Unit: J. Hampton Atkinson, M.D. (Unit Chief), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Behavioral Assessment and Medical (BAM) Core – Neuromedical and Laboratory Unit (NLU): Scott L. Letendre, M.D. (Core Co-Director/NLU Chief), Ronald J. Ellis, M.D., Ph.D.; BAM Core – Neuropsychiatric Unit (NPU): Robert K. Heaton, Ph.D. (Core Co-Director/NPU Chief), J. Hampton Atkinson, M.D., Thomas D. Marcotte, Ph.D., Erin E. Morgan, Ph.D., Matthew Dawson (NPU Manager); Neuroimaging (NI) Core: Gregory G. Brown, Ph.D. (Core Director), Thomas T. Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John R. Hesselink, M.D., Mary Jane Meloy, Ph.D., Craig E.L. Stark, Ph.D.; Neuroscience and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Marcus Kaul, Ph.D., Virawudh Soontornniyomkij, M.D.; Pilot and Developmental (PAD) Core: Mariana Cherner, Ph.D. (Core Director), Stuart A. Lipton, M.D., Ph.D.; Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark A. Geyer, Ph.D., Jared W. Young, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Susan F. Tapert, Ph.D., Assawin Gongvatana, Ph.D.; Project 3: Erin E. Morgan, Ph.D. (Project Director), Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director).; Project 5: Marcus Kaul, Ph.D. (Project Director).
Source funding and conflicts of interest
This research was supported by grants from the National Institute on Drug Abuse: P50DA026306 (Translational Methamphetamine AIDS Research Center [TMARC]; PI: Grant, Igor) and P01DA012065 (NeuroAIDS: Effects of Methamphetamine and HCV; PI: Grant, Igor). Stipend support to RS is funded by National Institute of Aging award F31AG064989. Stipend support to EWP is funded by National Institute of Alcohol Abuse and Alcoholism award F31AA027198. The authors declare no conflicts of interest. The funding sources had no involvement in study design, data collection, analysis, or interpretation, writing of the manuscript, or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
References
- Ammassari A, Antinori A, Aloisi MS, Trotta MP, Murri R, Bartoli L, Monforte AD, Wu AW, Starace F, 2004. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics 45, 394–402. [DOI] [PubMed] [Google Scholar]
- Anderson AM, Fennema-Notestine C, Umlauf A, Taylor MJ, Clifford DB, Marra CM, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Simpson DM, Morgello S, Grant I, Letendre SL, 2015. CSF biomarkers of monocyte activation and chemotaxis correlate with magnetic resonance spectroscopy metabolites during chronic HIV disease. Journal of neurovirology 21, 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok AH, Mizuno Y, Volkow ND, Howes OD, 2017. Association of Stimulant Use With Dopaminergic Alterations in Users of Cocaine, Amphetamine, or Methamphetamine: A Systematic Review and Meta-analysis. JAMA psychiatry 74, 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Kitabgi P, Rostene W, Parsadaniantz SM, 2005. Highly regionalized neuronal expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) in rat brain: evidence for its colocalization with neurotransmitters and neuropeptides. The Journal of comparative neurology 489, 275–292. [DOI] [PubMed] [Google Scholar]
- Barone P, Poewe W, Albrecht S, Debieuvre C, Massey D, Rascol O, Tolosa E, Weintraub D, 2010. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. The Lancet. Neurology 9, 573–580. [DOI] [PubMed] [Google Scholar]
- Basova L, Najera JA, Bortell N, Wang D, Moya R, Lindsey A, Semenova S, Ellis RJ, Marcondes MCG, 2018. Dopamine and its receptors play a role in the modulation of CCR5 expression in innate immune cells following exposure to Methamphetamine: Implications to HIV infection. PLoS One 13, e0199861–e0199861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G, 1996. Manual for Beck Depression Inventory II (BDI-II). San Antonio, TX, Psychology Corporation. [Google Scholar]
- Belujon P, Grace AA, 2017. Dopamine System Dysregulation in Major Depressive Disorders. The international journal of neuropsychopharmacology 20, 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 57, 289–300. [Google Scholar]
- Berger JR, Kumar M, Kumar A, Fernandez JB, Levin B, 1994. Cerebrospinal fluid dopamine in HIV-1 infection. AIDS (London, England) 8, 67–71. [DOI] [PubMed] [Google Scholar]
- Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, Ellis RJ, Atkinson JH, Grant I, Heaton RK, 2012. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin. Neuropsychol 26, 894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousman CA, Cherner M, Glatt SJ, Atkinson JH, Grant I, Tsuang MT, Everall IP, 2010. Impact of COMT Val158Met on executive functioning in the context of HIV and methamphetamine. Neurobehavioral HIV medicine 2010, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD, 2008. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological psychiatry 63, 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH, 2012. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 69, 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK, 2004. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J. Clin. Exp. Neuropsychol 26, 307–319. [DOI] [PubMed] [Google Scholar]
- Del Guerra FB, Fonseca JL, Figueiredo VM, Ziff EB, Konkiewitz EC, 2013. Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. Journal of neurovirology 19, 314–327. [DOI] [PubMed] [Google Scholar]
- di Rocco A, Bottiglieri T, Dorfman D, Werner P, Morrison C, Simpson D, 2000. Decreased homovanilic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clinical neuropharmacology 23, 190–194. [DOI] [PubMed] [Google Scholar]
- Dillon SM, Frank DN, Wilson CC, 2016. The gut microbiome and HIV-1 pathogenesis: a two-way street. AIDS (London, England) 30, 2737–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Meijide A, Rodriguez-Perez AI, Diaz-Ruiz C, Guerra MJ, Labandeira-Garcia JL, 2017. Dopamine modulates astroglial and microglial activity via glial renin-angiotensin system in cultures. Brain, behavior, and immunity 62, 277–290. [DOI] [PubMed] [Google Scholar]
- Du R-H, Zhou Y, Xia M-L, Lu M, Ding J-H, Hu G, 2018. α-Synuclein disrupts the anti-inflammatory role of Drd2 via interfering β-arrestin2-TAB1 interaction in astrocytes. J Neuroinflammation 15, 258–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalona R, Fawcett J, 2017. Pramipexole in Treatment Resistant-Depression, Possible Role of Inflammatory Cytokines. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 42, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Rush AJ, Vukelich J, Diaz SH, Dunklee L, Romo P, Yarns BC, Escalona R, 2016. Clinical Experience With High-Dosage Pramipexole in Patients With Treatment-Resistant Depressive Episodes in Unipolar and Bipolar Depression. The American journal of psychiatry 173, 107–111. [DOI] [PubMed] [Google Scholar]
- Felger JC, 2017. The Role of Dopamine in Inflammation-Associated Depression: Mechanisms and Therapeutic Implications. Current topics in behavioral neurosciences 31, 199–219. [DOI] [PubMed] [Google Scholar]
- Felger JC, Li L, Marvar PJ, Woolwine BJ, Harrison DG, Raison CL, Miller AH, 2013. Tyrosine metabolism during interferon-alpha administration: Association with fatigue and CSF dopamine concentrations. Brain, behavior, and immunity 31, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH, 2016. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular psychiatry 21, 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Miller AH, 2012. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol 33, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Treadway MT, 2017. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 42, 216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW, 2009. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol 175, 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Carvallo L, Eugenin EA, Berman JW, 2012. Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. J Neuroinflammation 9, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Miller DR, Gamble-George J, Yano H, Khoshbouei H, 2017. HIV, Tat and dopamine transmission. Neurobiology of disease 105, 51–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Spencer JA, Holzer CE, Soukup VM, 2006. Abnormal Striatal Dopaminergic Synapses in National NeuroAIDS Tissue Consortium Subjects with HIV Encephalitis. Journal of Neuroimmune Pharmacology 1, 410–420. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Lopez GJ, Wu T, Sharabi Y, 2018. Cerebrospinal fluid biomarkers of central dopamine deficiency predict Parkinson’s disease. Parkinsonism Relat Disord 50, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I, 2004. Revised Comprehensive Norms for an Expanded Halstead Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Psychological Assessment Resources, Inc, Lutz, FL. [Google Scholar]
- Heaton RK, Taylor MJ, Manly J, 2003. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III Clinical interpretation of the WAIS-III and WMS-III. Academic Press, San Diego, CA, US, pp. 181–210. [Google Scholar]
- Heshmati M, Russo SJ, 2015. Anhedonia and the brain reward circuitry in depression. Curr Behav Neurosci Rep 2, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobkirk AL, Starosta AJ, De Leo JA, Marra CM, Heaton RK, Earleywine M, 2015. Psychometric validation of the BDI-II among HIV-positive CHARTER study participants. Psychological assessment 27, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H, Kunugi H, 2013. Dopamine agonist-responsive depression. Psychogeriatrics : the official journal of the Japanese Psychogeriatric Society 13, 189–195. [DOI] [PubMed] [Google Scholar]
- Horn A, Scheller C, du Plessis S, Arendt G, Nolting T, Joska J, Sopper S, Maschke M, Obermann M, Husstedt IW, Hain J, Maponga T, Riederer P, Koutsilieri E, the German Competence Network, H.A., 2013. Increases in CSF dopamine in HIV patients are due to the dopamine transporter 10/10-repeat allele which is more frequent in HIV-infected individuals. Journal of Neural Transmission 120, 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, Roiser JP, 2018. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nature Reviews Neuroscience 19, 470–484. [DOI] [PubMed] [Google Scholar]
- Johnson PO, Neyman J, 1936. Tests of certain linear hypotheses and their application to some educational problems. Statistical research memoirs. [Google Scholar]
- Kazumori H, Ishihara S, Rumi MAK, Ortega-Cava CF, Kadowaki Y, Kinoshita Y, 2004. Transforming growth factor-α directly augments histidine decarboxylase and vesicular monoamine transporter 2 production in rat enterochromaffin-like cells. American Journal of Physiology-Gastrointestinal and Liver Physiology 286, G508–G514. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, ter Meulen V, Riederer P, 2001. Neurotransmission in HIV associated dementia: a short review. Journal of Neural Transmission 108, 767–775. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M, 2009. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. Journal of neurovirology 15, 257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M, 2011. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. Journal of neurovirology 17, 26–40. [DOI] [PubMed] [Google Scholar]
- Kvernmo T, Härtter S, Burger E, 2006. A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clinical Therapeutics 28, 1065–1078. [DOI] [PubMed] [Google Scholar]
- Larsson M, Hagberg L, Forsman A, Norkrans G, 1991. Cerebrospinal fluid catecholamine metabolites in HIV-infected patients. Journal of neuroscience research 28, 406–409. [DOI] [PubMed] [Google Scholar]
- Lei J, Yin X, Shang H, Jiang Y, 2019. IP-10 is highly involved in HIV infection. Cytokine 115, 97–103. [DOI] [PubMed] [Google Scholar]
- Leserman J, 2003. HIV disease progression: depression, stress, and possible mechanisms. Biological psychiatry 54, 295–306. [DOI] [PubMed] [Google Scholar]
- Leserman J, 2008. Role of depression, stress, and trauma in HIV disease progression. Psychosomatic medicine 70, 539–545. [DOI] [PubMed] [Google Scholar]
- Lian T-H, Guo P, Zuo L-J, Hu Y, Yu S-Y, Liu L, Jin Z, Yu Q-J, Wang R-D, Li L-X, Piao Y-S, Zhang W, 2019. An Investigation on the Clinical Features and Neurochemical Changes in Parkinson’s Disease With Depression. Frontiers in Psychiatry 9, 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberknecht V, Junqueira SC, Cunha MP, Barbosa TA, de Souza LF, Coelho IS, Santos ARS, Rodrigues ALS, Dafré AL, Dutra RC, 2017. Pramipexole, a Dopamine D2/D3 Receptor-Preferring Agonist, Prevents Experimental Autoimmune Encephalomyelitis Development in Mice. Molecular Neurobiology 54, 1033–1045. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, Hansson O, 2013. Cerebrospinal fluid inflammatory markers in Parkinson’s disease--associations with depression, fatigue, and cognitive impairment. Brain, behavior, and immunity 33, 183–189. [DOI] [PubMed] [Google Scholar]
- Morón JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, 2003. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. Journal of neuroscience 23, 8480–8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurauter G, Schrocksnadel K, Scholl-Burgi S, Sperner-Unterweger B, Schubert C, Ledochowski M, Fuchs D, 2008. Chronic immune stimulation correlates with reduced phenylalanine turnover. Current drug metabolism 9, 622–627. [DOI] [PubMed] [Google Scholar]
- Nolan RA, Reeb KL, Rong Y, Matt SM, Johnson HS, Runner K, Gaskill PJ, 2020. opamine activates NF-κB and primes the NLRP3 inflammasome in primary human macrophages. Brain, Behavior, & Immunity - Health 2, 100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D Jr., Cysique L, Ake C, Lazarretto D, Vaida F, Heaton RK, Group H, 2011. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol 33, 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tsuchimine S, Kunugi H, 2018. Cerebrospinal fluid monoamine metabolite concentrations in depressive disorder: A meta-analysis of historic evidence. Journal of psychiatric research 105, 137–146. [DOI] [PubMed] [Google Scholar]
- Paolillo EW, Pasipanodya EC, Moore RC, Pence BW, Atkinson JH, Grelotti DJ, Grant I, Heaton RK, Moore DJ, 2020. Cumulative Burden of Depression and Neurocognitive Decline Among Persons With HIV: A Longitudinal Study. JAIDS Journal of Acquired Immune Deficiency Syndromes 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessis SD, Vink M, Joska JA, Koutsilieri E, Stein DJ, Emsley R, 2014. HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS (London, England) 28, 803–811. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ, 2006. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat 31, 437–448. [Google Scholar]
- Rabkin JG, 2008. HIV and depression: 2008 review and update. Current HIV/AIDS Reports 5, 163–171. [DOI] [PubMed] [Google Scholar]
- Rivera-Rivera Y, García Y, Toro V, Cappas N, López P, Yamamura Y, Rivera-Amill V, 2014. Depression Correlates with Increased Plasma Levels of Inflammatory Cytokines and a Dysregulated Oxidant/Antioxidant Balance in HIV-1-Infected Subjects Undergoing Antiretroviral Therapy. Journal of clinical & cellular immunology 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney AS, Moore RC, Paolillo EW, Gouaux B, Umlauf A, Letendre SL, Jeste DV, Moore DJ, 2019. Depression and aging with HIV: Associations with health-related quality of life and positive psychological factors. Journal of affective disorders 251, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Maki PM, 2019. HIV, Depression, and Cognitive Impairment in the Era of Effective Antiretroviral Therapy. Current HIV/AIDS Reports 16, 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S, 1994. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behavioural brain research 65, 221–229. [DOI] [PubMed] [Google Scholar]
- Saloner R, Cherner M, Sundermann EE, Watson CW-M, Iudicello JE, Letendre SL, Kumar A, Ellis RJ, 2020. COMT val158met genotype alters the effects of methamphetamine dependence on dopamine and dopamine-related executive function: preliminary findings. Psychiatry Research 292, 113269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner R, Marquine MJ, Sundermann EE, Hong S, McCutchan JA, Ellis RJ, Heaton RK, Grant I, Cherner M, 2019. COMT Val158Met Polymorphism, Cardiometabolic Risk, and Nadir CD4 Synergistically Increase Risk of Neurocognitive Impairment in Men Living With HIV. Journal of acquired immune deficiency syndromes (1999) 81, e148–e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller C, Arendt G, Nolting T, Antke C, Sopper S, Maschke M, Obermann M, Angerer A, Husstedt IW, Meisner F, Neuen-Jacob E, Müller HW, Carey P, ter Meulen V, Riederer P, Koutsilieri E, 2010. Increased dopaminergic neurotransmission in therapy-naïve asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. Journal of Neural Transmission 117, 699–705. [DOI] [PubMed] [Google Scholar]
- Shao W, Zhang S. z., Tang M, Zhang X. h., Zhou Z, Yin Y. q., Zhou Q. b., Huang Y. y., Liu Y. j., Wawrousek E, Chen T, Li S. b., Xu M, Zhou J. n., Hu G, Zhou J. w., 2013. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via αB-crystallin. Nature 494, 90–94. [DOI] [PubMed] [Google Scholar]
- Sibille E, French B, 2013. Biological substrates underpinning diagnosis of major depression. The international journal of neuropsychopharmacology 16, 1893–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Kesby JP, Morgan EE, Bischoff-Grethe A, Minassian A, Brown GG, Grant I, 2016. Effects of HIV and Methamphetamine on Brain and Behavior: Evidence from Human Studies and Animal Models. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 11, 495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M, 1995. Structured Clinical Interview for DSM-IV. American Psychiatric Press, Washington, DC. [Google Scholar]
- Stefani A, Pierantozzi M, Olivola E, Galati S, Cerroni R, D’Angelo V, Hainsworth AH, Saviozzi V, Fedele E, Liguori C, 2017. Homovanillic acid in CSF of mild stage Parkinson’s disease patients correlates with motor impairment. Neurochem Int 105, 58–63. [DOI] [PubMed] [Google Scholar]
- Sundermann EE, Bishop JR, Rubin LH, Little DM, Meyer VJ, Martin E, Weber K, Cohen M, Maki PM, 2015. Genetic predictor of working memory and prefrontal function in women with HIV. Journal of neurovirology 21, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Becker BW, Marcotte TD, Hines LJ, Foley JM, Ramezani A, Singer EJ, Castellon SA, Heaton RK, Hinkin CH, 2011. Depression, cognition, and self-appraisal of functional abilities in HIV: an examination of subjective appraisal versus objective performance. The Clinical neuropsychologist 25, 224–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal PM, Pacheco R, 2020. The Cross-Talk Between the Dopaminergic and the Immune System Involved in Schizophrenia. Frontiers in Pharmacology 11, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PEM, Rushworth MFS, 2006. Weighing up the benefits of work: behavioral and neural analyses of effort-related decision making. Neural Netw 19, 1302–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lai S, Li G, Zhou T, Wang B, Cao F, Chen T, Zhang X, Chen Y, 2020. Microglial activation contributes to depressive-like behavior in dopamine D3 receptor knockout mice. Brain, behavior, and immunity 83, 226–238. [DOI] [PubMed] [Google Scholar]
- Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW, 2014. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Current HIV research 12, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 1998. Composite Diagnositic International Interview (CIDI, version 2.1). World Health Organization, Geneva, Switzerland. [Google Scholar]
- Xia Q-P, Cheng Z-Y, He L, 2019. The modulatory role of dopamine receptors in brain neuroinflammation. International Immunopharmacology 76, 105908. [DOI] [PubMed] [Google Scholar]
- Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R, 2015. Dopamine Controls Systemic Inflammation through Inhibition of NLRP3 Inflammasome. Cell 160, 62–73. [DOI] [PubMed] [Google Scholar]
- Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R, 2013. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity 38, 1154–1163. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Hattori K, Ogawa S, Sasayama D, Ota M, Teraishi T, Kunugi H, 2017. Relationships of Cerebrospinal Fluid Monoamine Metabolite Levels With Clinical Variables in Major Depressive Disorder. The Journal of clinical psychiatry 78, e947–e956. [DOI] [PubMed] [Google Scholar]


