Abstract
Purpose
Animal models have demonstrated a link between decreases in retinal dopamine levels and the development of form-deprivation myopia (FDM). However, the consistency of dopamine's role in the other major form of experimental myopia, that of lens-induced myopia (LIM), is less clear, raising the question as to what extent dopamine plays a role in human myopia. Therefore, to better define the role of dopamine in both forms of experimental myopia, we examined how consistent the protection afforded by dopamine and the dopamine agonist 6-amino-5,6,7,8-tetrahydronaphthalene-2,3-diol hydrobromide (ADTN) is between FDM and LIM.
Methods
Intravitreal injections of dopamine (0.002, 0.015, 0.150, 1.500 µmol) or ADTN (0.001, 0.010, 0.100, 1.000 µmol) were administered daily to chicks developing FDM or LIM. Axial length and refraction were measured following 4 days of treatment. To determine the receptor subtype by which dopamine and ADTN inhibit FDM and LIM, both compounds were coadministered with either the dopamine D2-like antagonist spiperone (0.005 µmol) or the D1-like antagonist SCH-23390 (0.005 µmol).
Results
Intravitreal administration of dopamine or ADTN inhibited the development of FDM (ED50 = 0.003 µmol and ED50 = 0.011 µmol, respectively) and LIM (ED50 = 0.002 µmol and ED50 = 0.010 µmol, respectively) in a dose-dependent manner, with a similar degree of protection observed in both paradigms (P = 0.471 and P = 0.969, respectively). Coadministration with spiperone, but not SCH-23390, inhibited the protective effects of dopamine and ADTN against the development of both FDM (P = 0.214 and P = 0.138, respectively) and LIM (P = 0.116 and P = 0.100, respectively).
Conclusions
pharmacological targeting of the retinal dopamine system inhibits FDM and LIM in a similar dose-dependent manner through a D2-like mechanism.
Keywords: myopia, dopamine, ADTN, refractive error, ocular growth, animal models
Myopia (short-sightedness) is a refractive disorder arising from a mismatch between the axial length and optical power of the eye that generally results from excessive elongation of the eye during development and into early adulthood1.1 Work in animal models has implicated changes in retinal dopaminergic functions as being critical to the development of myopia.2–4 Specifically, in chicks,5 guinea pigs,6 tree shrews,7 primates,8 and mice9–11 (although with some variability in mice12), ocular levels of dopamine and its primary metabolite, 3,4-dihydroxyphenylacetic acid (DOPAC), are diminished during the development of form-deprivation myopia (FDM). Furthermore, the administration of dopaminergic agonists (chicks,5,13–17 guinea pigs,18 mice,19 tree shrews,20 primates21), exogenous dopamine (rabbits22), or its precursor levodopa (chicks,23,24 mice,25 guinea pigs26,27) significantly inhibits the development of FDM. In contrast, blocking dopaminergic functions in chicks, through administration of dopaminergic antagonists, prevents the growth suppression associated with brief periods of normal vision (diffuser13,28 and, to an extent, negative lens removal),16 as well as the protective effects of bright-light exposure against FDM.29,30 Similarly, retina-specific tyrosine hydroxylase knockout mice and mice treated with 6-hydroxydopamine, which depletes the retina of dopaminergic neurons, show a myopic shift in refraction.10,31 However, such a myopic shift is not seen in otherwise untreated chicks that were administered 6-hydroxydopamine.32
What role dopamine plays in the other major form of experimental myopia, that of lens-induced myopia (LIM), is less clear with conflicting results observed.18,28,33,34 While an earlier study by Bartmann et al.33 observed no change in dopamine levels during the development of LIM in chicks, a finding also seen in guinea pigs,18 a later study in chicks by Guo et al.34 did observe a significant decrease in dopamine levels in response to hyperopic defocus. At a pharmacological level, although both FDM and LIM can be inhibited by the administration of the dopaminergic agonist quinpirole13,16 or the dopamine precursor levodopa23,24 in chicks, another dopaminergic agonist, apomorphine, has been reported to inhibit FDM5,17 but demonstrates inconsistent effects in LIM in chicks and guinea pigs.16–18 Furthermore, administration of 6-hydroxydopamine, which depletes dopaminergic neurons, has been reported to unexpectedly inhibit rather than enhance the development of FDM32,35 but appears to have no effect on the development of LIM32 in chicks. Finally, in chicks, the D2-like dopamine antagonist spiperone has been reported to abolish the protective effects of brief periods of normal vision against FDM13,28 but not consistently in LIM.16,28 Together, these results suggest that, unlike what is seen in FDM,5,13,15,16,20,29,36 the dopaminergic system may not play the same critical role in LIM and that any effect may not be generated through the dopamine D2-like receptor family.
In addition to potential differences in the role of the dopaminergic system between the two major forms of experimental myopia, several other subtle mechanistic differences have been observed between FDM and LIM (for review, see Morgan et al.37). Such dissimilarities between the two experimental models may be driven by how the retina responds to these different optical approaches for inducing myopic growth. Specifically, FDM involves an open-loop system in which the loss of form vision leads to increased ocular growth rates for as long as the diffuser remains attached and developmental plasticity remains.38,39 In contrast, LIM is a closed-loop system in which the eye elongates until compensation is achieved for the imposed defocus.40,41
Therefore, to address whether dopamine does play a role in LIM, this study investigates whether pharmacological manipulation of the dopaminergic system has similar effects on the development of FDM and LIM. To this end, this study first compares the dose-dependent responses of FDM and LIM to two dopaminergic interventions previously shown to be effective against the development of FDM, that of intravitreal administration of dopamine itself22 and intravitreal administration of the dopamine receptor agonist 6-amino-5,6,7,8-tetrahydronaphthalene-2,3-diol hydrobromide (ADTN).13 Second, this study investigates whether any inhibition of LIM by the abovementioned dopaminergic compounds occurs through modulation of the dopamine D2 receptor family, as seen for FDM in chicks.5,13,15,16,29,36 To test this, dopamine and ADTN were coadministered with the D1-like dopaminergic antagonist SCH-23390 and the D2-like antagonist spiperone for both FDM and LIM.
Methods
Animals and Housing
Day-old male White-Leghorn chickens were obtained from Barter & Sons Hatchery (Horsley Park, NSW, Australia). Chicks were kept in temperature-controlled rooms and under normal laboratory lighting (500 lux, fluorescent lights) on a 12:12-hour light/dark cycle with lights on at 9 am and off at 9 pm. Chicks were given access to unlimited amounts of food and water and 5 days to adjust to their surroundings before experiments commenced.
Authorization to conduct experiments using animals was approved by the University of Canberra Animal Ethics Committee under the ACT Animal Welfare Act 1992 (project number: CEAE 20-98) and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Myopia Induction and Measurement of Ocular Parameters
Myopia was induced by placing either a translucent diffuser (FDM) or negative lens (–10 D, LIM) over the treated (left) eye as previously described.23,42,43 In short, on the day prior to treatment, Velcro mounts were fitted around the left eye with Loctite super glue (Henkel, Kilsyth, VIC, Australia). On the following day, immediately following the first drug treatment, translucent diffusers or –10-D lenses fitted to matching Velcro rings were placed onto the mounts, with the right eye remaining untreated to serve as a contralateral control.
Axial length and refractive measurements were carried out prior to the start of treatment and on the day after the completion of the experimental period using A-scan ultrasonography (Biometer AL-100, resolution: 0.01 mm; Tomey Corporation, Nagoya, Japan) and automated infrared photoretinoscopy (system provided courtesy of Professor Frank Schaeffel, University of Tuebingen, Germany) as previously described.23 In short, refraction was measured for treated (left) and contralateral control (right) eyes using automated infrared photoretinoscopy with refractive values representing the mean spherical equivalent of 10 measurements per eye. For axis alignment, the Purkinje image was centered within the pupil to obtain the correct refractive axis. Illumination levels within the room were held at less than 5 lux to avoid light reflections in the pupil arising from aberrant sources. Axial length was measured using A-scan ultrasonography, with each scan representing the mean of 10 measurements and the average of three scans taken for each eye. For axial length measures, animals were anaesthetized under light isoflurane (5% in 1 L of medical-grade oxygen per minute; Veterinary Companies of Australia, Kings Park, NSW, Australia) using a vaporizer gas system (Stinger Research Anaesthetic Gas Machine [2848]; Advanced Anaesthesia Specialists, Payson, AZ, USA).
Drug Preparation and Administration
Dopamine hydrochloride (H8502; Sigma-Aldrich, St. Louis, MO, USA), ADTN hydrobromide (ab120150; Abcam, Cambridge, MA, USA), SCH-23390 (D054; Sigma-Aldrich), or spiperone (S7395; Sigma-Aldrich) was dissolved fresh in a solution containing 0.1% w/v ascorbic acid in 1× PBS, pH 6.0 (Table 1). Chicks were administered a 10-µL intravitreal injection once daily (9 am), using a 30-gauge needle (Terumo, Macquarie Park, NSW, Australia) fitted to a Hamilton syringe (100-µL capacity) to their treated eye on 4 consecutive days. For intravitreal administration, chicks were anaesthetized under light isoflurane (as described above). For coadministration experiments, a dopaminergic antagonist, spiperone or SCH-23390, was added to the above dopamine or ADTN solutions and administered as a single 10-µL intravitreal injection each day.
Table 1.
Drug Preparations
| Drug | Drug Action | Volume Given Daily (µL) | Amount Administered (µmol/d) | Concentration: Drug Solution (mM) | Estimated Vitreal Concentration (µM) |
|---|---|---|---|---|---|
| Dopamine | Natural ligand | 10 | 1.500 | 150.00 | 7500 |
| 0.150 | 15.00 | 750 | |||
| 0.015 | 1.50 | 75 | |||
| 0.002 | 0.15 | 10 | |||
| ADTN | Nonspecific agonist | 10 | 1.000 | 100.00 | 5000 |
| 0.100 | 10.00 | 500 | |||
| 0.010 | 1.00 | 50 | |||
| 0.001 | 0.10 | 5 | |||
| Dopamine/SCH-23390 | Natural ligand/D1-like antagonist | 10 | 0.150/0.005 | 15.00/0.50 | 750/25 |
| Dopamine/spiperone | Natural ligand/D2-like antagonist | 10 | 0.150/0.005 | 15.00/0.50 | 750/25 |
| ADTN/SCH-23390 | Nonspecific agonist/D1-like antagonist | 10 | 0.100/0.005 | 10.00/0.50 | 500/25 |
| ADTN/spiperone | Nonspecific agonist/D2-like antagonist | 10 | 0.100/0.005 | 10.00/0.50 | 500/25 |
Each drug preparation was administered to both FDM- and LIM-treated eyes; 0.150 µmol dopamine and 0.100 µmol ADTN were also administered to eyes receiving no other ocular treatment to examine their effects on normal ocular development. Vitreal concentration was estimated from an average vitreous volume of 200 µL at this age. Molar mass (g/mol)—dopamine (189.64), ADTN (260.13), SCH-23390 (324.24), and spiperone (395.47).
Control Paradigms
Each of the following three experimental paradigms (outlined below) were undertaken in separate weeks alongside their own control groups, which included FDM only (n = 10 per experiment), LIM only (–10 D, n = 10 per experiment), and age-matched untreated control animals (n = 10 per experiment).
Experiment 1: Dopamine Dose-Response Curves for FDM and LIM
To establish whether intravitreal administration of dopamine showed a similar dose-dependent protection against the development of FDM and LIM, chicks were allocated to treatment groups as outlined in Table 2. In short, chicks undergoing FDM or LIM were given a daily intravitreal injection of one of four doses of dopamine (0.002, 0.015, 0.150, 1.500 µmol) for a period of 4 days. At the end of the experimental period, the axial length and refractive measurements from these chicks were compared to those from the left eyes of FDM only, LIM only, and age-matched untreated control animals. This experiment also examined the effects of intravitreal injections of the vehicle solution (0.1% ascorbic acid in 1× PBS) on the development of FDM and LIM. As vehicle treatment had no effect on the development of FDM or LIM, vehicle-treated groups were not included in subsequent experiments. Finally, this experiment examined the effects of dopamine treatment on normal ocular development by administering dopamine, at its highest dose (1.500 µmol), to chicks receiving no visual treatment.
Table 2.
Allocation of Animals Across the Three Experimental Paradigms Investigated
| Drug Solution | Dose Administered (µmol) | Numbers Fitted With Translucent Diffuser (FDM) | Numbers Fitted With Negative Lens (−10 D, LIM) | Numbers With No Optical Treatment |
|---|---|---|---|---|
| Experiment 1 | ||||
| None | — | 10 | 10 | 10 |
| Vehicle | — | 10 | 10 | — |
| Dopamine | 0.002 | 6 | 8 | — |
| Dopamine | 0.015 | 8 | 8 | — |
| Dopamine | 0.150 | 9 | 9 | — |
| Dopamine | 1.500 | 9 | 9 | 6 |
| Experiment 2 | ||||
| None | — | 10 | 10 | 10 |
| ADTN | 0.001 | 8 | 7 | — |
| ADTN | 0.010 | 6 | 10 | — |
| ADTN | 0.100 | 8 | 8 | — |
| ADTN | 1.000 | 8 | 7 | 8 |
| Experiment 3 | ||||
| None | — | 10 | 10 | 10 |
| Dopamine | 0.150 | 9 | 9 | — |
| Dopamine/SCH-23390 | 0.150/0.005 | 8 | 8 | — |
| Dopamine/spiperone | 0.150/0.005 | 8 | 8 | — |
| ADTN | 0.100 | 8 | 8 | — |
| ADTN/SCH-23390 | 0.100/0.005 | 8 | 8 | — |
| ADTN/spiperone | 0.100/0.005 | 8 | 8 | — |
| SCH-23390 | 0.005 | — | — | 6 |
| Spiperone | 0.005 | — | — | 6 |
Each of the three tested paradigms was investigated in separate weeks and therefore contained their own control groups (FDM only, LIM only, and age-matched untreated controls), which received no drug solution. In addition to examining the effect of each drug on FDM and LIM, 1.500 µmol dopamine, 1.000 µmol ADTN, 0.005 µmol SCH-23390, and 0.005 µmol spiperone were also administered to eyes receiving no other ocular treatment to examine their effects on normal ocular development. Vehicle solution represents 0.1% w/v ascorbic acid in 1× PBS (pH 6.0).
Experiment 2: ADTN Dose-Response Curves for FDM and LIM
Like the work undertaken for dopamine, to establish whether intravitreal administration of the dopamine agonist ADTN showed a similar dose-dependent protection against the development of FDM and LIM, chicks were allocated to treatment groups as outlined in Table 2. Like experiment 1, chicks undergoing FDM or LIM were given a daily intravitreal injection of one of four doses of ADTN (0.001, 0.010, 0.100, 1.000 µmol) for a period of 4 days. At the end of the experimental period, the axial length and refractive measurements from these chicks were compared to those from the left eyes of FDM only, LIM only, and age-matched untreated control animals. This experiment also examined the effects of ADTN treatment on normal ocular development by administering ADTN, at its highest dose (1.000 µmol), to chicks receiving no visual treatment.
Experiment 3: Effects of Dopaminergic Antagonism
To establish whether the protective effects of dopamine and ADTN against the development of FDM and LIM can be similarly disrupted by dopaminergic antagonism, and whether the same receptor family is responsible in both forms of experimental myopia, chicks were allocated to treatment groups as outlined in Table 2. Dopamine (0.150 µmol) or ADTN (0.100 µmol) were coadministered as a single 10-µL injection with either the D1-like receptor antagonist SCH-23390 (0.005 µmol) or the D2-like receptor antagonist spiperone (0.005 µmol) (based on doses used previously)13,24 to examine whether their protective effects against FDM and LIM can be pharmacologically disrupted by either family of antagonists.
Statistical Analysis
A power calculation was undertaken to determine the group sizes required to achieve 80% power in observing a 0.8-D change in refraction when the standard deviation is approximately 0.5 D:
To account for fluctuations in standard deviation, as well as potential dropouts due to diffuser or lens removal (at which point chicks were removed from the experiment and not reported), group sizes were set at n = 10.
All values reported represent the means ± the standard error of the means. Percent protection against the development of FDM or LIM was calculated by comparing the change in axial length or refraction in drug-treated groups to the change in axial length or refraction in FDM- or LIM-only animals:
Prior to statistical analysis, all data were first tested for normality and homogeneity of variance (Shapiro-Wilk test). Before analyzing the effects of drug treatment, an analysis of the control paradigms across all experiments was undertaken. First, ocular development in age-matched untreated control or contralateral control eyes was compared across all three experiments using a one-way univariate ANOVA. To compare ocular development between age-matched untreated control and contralateral control eyes across all three experiments, a multivariate ANOVA (MANOVA) was undertaken.
To evaluate the development of myopia across all three experiments, form-deprived or lens-treated eyes were compared to age-matched untreated control eyes using a MANOVA. The degree of myopia development between experiments was then compared using an ANOVA.
The effect of pharmacological treatment was analyzed in each experiment using an ANOVA, followed by a Student's unpaired t-test with Bonferroni correction for multiple testing for analysis of specific between-group effects. To compare FDM and LIM dose-response curves, or to compare dopamine and ADTN dose-response curves, a MANOVA was undertaken. All analyses were reviewed by a statistician and undertaken using the program IBM SPSS Statistics package 25 (SPSS, Inc., Armonk, NY, USA) with a statistical cutoff of 0.05.
Results
Analysis of Control Paradigms
No differences in refraction or axial length were observed between groups or between eyes prior to the commencement of treatment. As summarized in Table 3 (ANOVA), there was no difference in age-matched untreated control or contralateral control values across the three experiments. Nor were there any differences between age-matched and contralateral control eyes (Table 3, MANOVA). Therefore, for ease of analysis, all statistical comparisons of treatment effect against normal ocular development in the following sections were made against age-matched untreated control animals only.
Table 3.
Statistical Analysis of Untreated Control Paradigms Across Experiments 1, 2, and 3
| Ocular Parameter | Test | Age Matched | Contralateral | Age Matched vs Contralateral |
|---|---|---|---|---|
| Axial length | ANOVA | F(2, 28) = 0.663, P = 0.524 | F(2, 88) = 0.146, P = 0.932 | — |
| MANOVA | — | — | Wilks’ λ = 0.463, F(1, 59) = 1.221, P = 0.359 | |
| Refraction | ANOVA | F(2, 28) = 1.353, P = 0.277 | F(2, 88) = 2.155, P = 0.121 | — |
| MANOVA | — | — | Wilks’ λ = 0.532, F(1, 59) = 1.763, P = 0.284 |
ANOVAs were undertaken to compare whether similar ocular biometry was seen in age-matched untreated control eyes across all three experimental groups. The same analysis was undertaken for contralateral control values across experiments. To expand on this, MANOVAs were undertaken to compare whether the ocular biometry observed between the two control groups (age-matched untreated controls and contralateral controls) were similar across all three experiments.
Form deprivation and negative lens wear led to chicks developing significantly longer axial lengths and more myopic refractions relative to age-matched untreated control animals, with no observed differences in the control groups across experiments (Table 4). Therefore, all comparisons of the effect of drug treatment on the development of experimental myopia were made to each experiment's respective control groups (Table 5). In experiment 1, daily intravitreal treatment with the vehicle solution (0.1% ascorbic acid in 1× PBS), over a period of 4 days, did not alter the development of FDM or LIM (Fig. 1, raw data—Table 6). As there was no effect, a vehicle-treated group was not included in experiments 2 and 3.
Table 4.
Statistical Analysis of Myopia Control Paradigms Across Experiments 1, 2, and 3
| Ocular Parameter | Test | FDM | LIM |
|---|---|---|---|
| Axial length | MANOVA | Wilks’ λ = 0.165, F(1, 59) = 42.915, P < 0.001 | Wilks’ λ = 0.212, F(1, 59) = 31.540, P < 0.001 |
| ANOVA | F(2,28) = 0.407, P = 0.670 | F(2,28) = 0.199, P = 0.821 | |
| Refraction | MANOVA | Wilks’ λ = 0.015, F(1, 59) = 570.141, P < 0.001 | Wilks’ λ = 0.046, F(1, 59) = 175.450, P < 0.001 |
| ANOVA | F(2,28) = 0.022, P = 0.978 | F(2,28) = 2.042, P = 0.151 |
MANOVAs were undertaken to determine whether form deprivation and negative lens wear induced ocular changes across all three experimental groups relative to age-matched untreated control values. ANOVAs were undertaken to compare whether the degree of myopia development was different across experiments. Statistically significant outcomes (P < 0.05) are presented in bold.
Table 5.
Statistical Analysis of the Ability of Dopamine and ADTN, Across Their Full Dose Range, to Inhibit the Development of Experimental Myopia
| Dopamine | ADTN | |||
|---|---|---|---|---|
| FDM | LIM | LIM | FDM | |
| Axial length (M) | F( 4, 38) = 4.740, P < 0.01 | F( 4, 40) = 8.783, P < 0.001 | F( 4, 36) = 6.020, P < 0.01 | F( 4, 38) = 7.014, P < 0.001 |
| Axial length (U) | F(4, 38) = 1.668, P = 0.176 | F(4, 40) = 1.281, P = 0.296 | F( 4, 36) = 5.728, P < 0.01 | F(4, 38) = 1.332, P = 0.276 |
| ACD (M) | F(4, 38) = 0.789, P = 0.539 | F(4, 40) = 1.094, P = 0.375 | F(4, 36) = 0.553, P = 0.699 | F(4, 38) = 2.160, P = 0.092 |
| Lens thickness (M) | F(4, 38) = 1.454, P = 0.234 | F(4, 40) = 1.669, P = 0.179 | F(4, 36) = 1.016, P = 0.414 | F(4, 38) = 1.564, P = 0.204 |
| VCD (M) | F( 4 , 38) = 3.871, P < 0.01 | F( 4 , 40) = 8.302, P < 0.001 | F( 4 , 36) = 4.391, P < 0.01 | F( 4 , 38) = 6.800, P < 0.001 |
| Refraction (M) | F( 4 , 38) = 12.309, P < 0.001 | F( 4 , 40) = 7.523, P < 0.01 | F( 4 , 36) = 14.977, P < 0.001 | F( 4 , 38) = 5.864, P < 0.01 |
| Refraction (U) | F( 4 , 38) = 18.170, P < 0.001 | F( 4 , 40) = 10.343, P < 0.001 | F( 4 , 36) = 20.784, P < 0.001 | F( 4 , 38) = 23.148, P < 0.001 |
ANOVAs were undertaken to compare the effect of dopamine and ADTN treatment on ocular biometry and refraction relative to FDM- or LIM-only values (M) or age-matched untreated control values (U). ACD, lens thickness, and VCD were only compared to myopia treatment (M) to demonstrate that protection against axial elongation was driven by changes in VCD rather than ACD and lens thickness. Statistically significant outcomes (P < 0.05) are presented in bold. ACD, anterior chamber depth; VCD, vitreal chamber depth.
Figure 1.
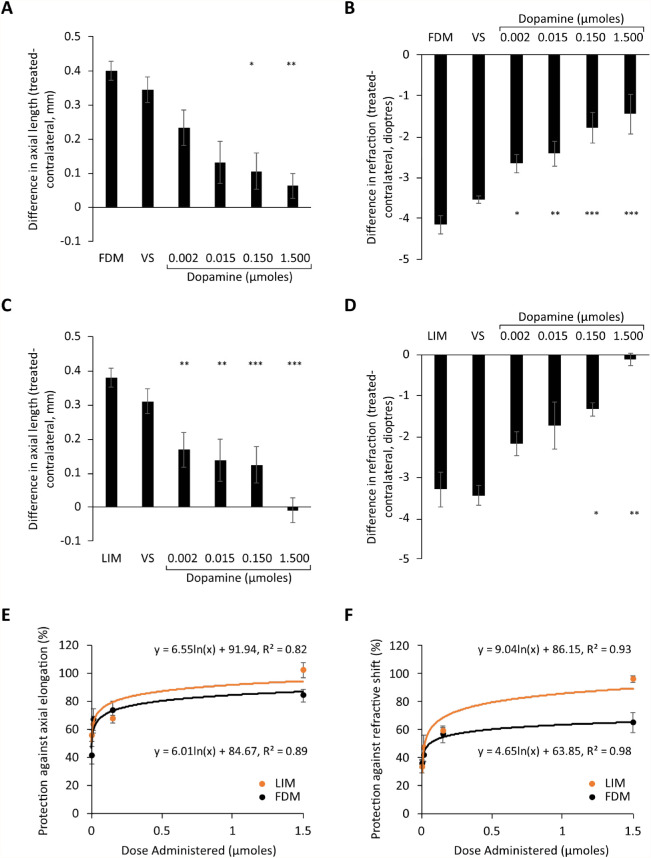
Dopamine dose-response curves for FDM and LIM following 4 days of treatment. (A) Axial length and (B) refraction measurements from FDM chicks, (C) axial length and (D) refraction measurements from LIM chicks, and percent protection against the (E) axial elongation and (F) shift in refraction associated with experimental myopia development. Data represent the means ± standard error of the means. VS, treatment with vehicle solution in FDM or LIM eyes. Sample sizes (min n = 6 per group) can be found in Table 2. Statistics denote difference of treated eyes relative to FDM or LIM only. *P < 0.05. **P < 0.01. ***P < 0.001.
Table 6.
Axial Length and Refractive Measurements—Intravitreal Dopamine Treatment
| Axial Length | Refraction | |||||||
|---|---|---|---|---|---|---|---|---|
| Condition | Left Eye | Right Eye | Compared to Myopia | Compared to Untreated | Left Eye | Right Eye | Compared to Myopia | Compared to Untreated |
| Untreated | 8.66 ± 0.05 | 8.67 ± 0.04 | P < 0.001 | — | 2.66 ± 0.03 | 2.62 ± 0.12 | P < 0.001 | — |
| FDM only | 9.08 ± 0.04 | 8.68 ± 0.04 | — | P < 0.001 | −1.48 ± 0.24 | 2.68 ± 0.04 | — | P < 0.001 |
| LIM only | 8.95 ± 0.05 | 8.61 ± 0.02 | — | P < 0.001 | −0.70 ± 0.25 | 2.60 ± 0.28 | — | P < 0.001 |
| FDM vehicle | 9.13 ± 0.04 | 8.78 ± 0.05 | P = 0.729 | P < 0.001 | −0.80 ± 0.11 | 2.73 ± 0.06 | P = 0.881 | P < 0.001 |
| LIM vehicle | 8.99 ± 0.04 | 8.69 ± 0.02 | P = 0.670 | P < 0.01 | −1.09 ± 0.25 | 2.36 ± 0.12 | P = 0.650 | P < 0.001 |
| 1.500 µmol alone | 8.70 ± 0.06 | 8.77 ± 0.03 | P < 0.05 | P = 0.987 | 2.18 ± 0.22 | 2.18 ± 0.16 | P < 0.001 | P = 0.453 |
| FDM + dopamine | ||||||||
| 0.002 µmol | 8.88 ± 0.08 | 8.65 ± 0.04 | P = 0.624 | P = 0.486 | 0.03 ± 0.24 | 2.68 ± 0.07 | P < 0.05 | P < 0.001 |
| 0.015 µmol | 8.85 ± 0.06 | 8.72 ± 0.03 | P = 0.130 | P = 0.473 | 0.08 ± 0.28 | 2.45 ± 0.20 | P < 0.01 | P < 0.001 |
| 0.150 µmol | 8.76 ± 0.07 | 8.65 ± 0.03 | P < 0.05 | P = 0.999 | 0.79 ± 0.31 | 2.58 ± 0.08 | P < 0.001 | P < 0.001 |
| 1.500 µmol | 8.76 ± 0.06 | 8.70 ± 0.04 | P < 0.01 | P = 0.999 | 0.96 ± 0.38 | 2.40 ± 0.18 | P < 0.001 | P < 0.001 |
| LIM + dopamine | ||||||||
| 0.002 µmol | 8.62 ± 0.07 | 8.44 ± 0.07 | P < 0.01 | P = 0.999 | −0.14 ± 0.21 | 2.04 ± 0.15 | P = 0.999 | P < 0.001 |
| 0.015 µmol | 8.64 ± 0.06 | 8.49 ± 0.04 | P < 0.01 | P = 0.999 | 0.54 ± 0.27 | 2.28 ± 0.15 | P = 0.219 | P < 0.01 |
| 0.150 µmol | 8.57 ± 0.05 | 8.46 ± 0.06 | P < 0.001 | P = 0.999 | 0.96 ± 0.11 | 2.30 ± 0.15 | P < 0.05 | P < 0.05 |
| 1.500 µmol | 8.48 ± 0.05 | 8.50 ± 0.02 | P < 0.001 | P = 0.999 | 1.77 ± 0.27 | 1.89 ± 0.21 | P < 0.01 | P = 0.355 |
Data are presented as the means ± standard error of the means, and statistics are presented as pairwise comparisons with Bonferroni correction of treated (left) eyes to myopia only or age-matched untreated groups, with significant comparisons (P < 0.05) presented in bold. Untreated: age-matched untreated controls.
Finally, administration of dopamine (raw data—Table 6), ADTN (raw data—Table 7), SCH-23390 (raw data—Table 8), or spiperone (raw data—Table 8) into otherwise untreated eyes did not lead to any changes in axial length or refraction following 4 days of treatment relative to those values seen in age-matched untreated control animals.
Table 7.
Axial Length and Refractive Measurements—Intravitreal ADTN Treatment
| Axial Length | Refraction | |||||||
|---|---|---|---|---|---|---|---|---|
| Condition | Left Eye | Right Eye | Compared to Myopia | Compared to Untreated | Left Eye | Right Eye | Compared to Myopia | Compared to Untreated |
| Untreated | 8.71 ± 0.04 | 8.66 ± 0.05 | P < 0.001 | — | 2.50 ± 0.11 | 2.52 ± 0.15 | P < 0.001 | — |
| FDM only | 9.03 ± 0.04 | 8.66 ± 0.05 | — | P < 0.001 | −1.81 ± 0.21 | 2.40 ± 0.11 | — | P < 0.001 |
| LIM only | 9.08 ± 0.03 | 8.72 ± 0.03 | — | P < 0.001 | −1.39 ± 0.37 | 2.63 ± 0.17 | — | P < 0.001 |
| 1.000 µmol alone | 8.68 ± 0.05 | 8.66 ± 0.04 | P < 0.05 | P = 0.816 | 2.10 ± 0.13 | 2.08 ± 0.11 | P < 0.001 | P = 0.274 |
| FDM + ADTN | ||||||||
| 0.001 µmol | 9.00 ± 0.04 | 8.73 ± 0.03 | P = 0.999 | P < 0.01 | −0.71 ± 0.20 | 2.59 ± 0.07 | P = 0.082 | P < 0.001 |
| 0.010 µmol | 8.87 ± 0.06 | 8.69 ± 0.04 | P = 0.954 | P = 0.388 | 0.20 ± 0.35 | 2.50 ± 0.18 | P < 0.01 | P < 0.001 |
| 0.100 µmol | 8.84 ± 0.05 | 8.72 ± 0.06 | P < 0.05 | P = 0.222 | 0.09 ± 0.28 | 2.10 ± 0.10 | P < 0.001 | P < 0.001 |
| 1.000 µmol | 8.67 ± 0.07 | 8.66 ± 0.05 | P < 0.01 | P = 0.999 | 1.03 ± 0.32 | 2.26 ± 0.16 | P < 0.001 | P < 0.01 |
| LIM + ADTN | ||||||||
| 0.001 µmol | 8.89 ± 0.05 | 8.62 ± 0.04 | P = 0.412 | P = 0.811 | −0.88 ± 0.34 | 2.39 ± 0.19 | P = 0.999 | P < 0.001 |
| 0.010 µmol | 8.77 ± 0.05 | 8.58 ± 0.03 | P < 0.01 | P = 0.999 | −0.26 ± 0.21 | 2.14 ± 0.10 | P = 0.136 | P < 0.001 |
| 0.100 µmol | 8.73 ± 0.07 | 8.65 ± 0.05 | P < 0.01 | P = 0.999 | 0.41 ± 0.31 | 2.34 ± 0.17 | P < 0.01 | P < 0.001 |
| 1.000 µmol | 8.68 ± 0.08 | 8.62 ± 0.08 | P < 0.01 | P = 0.999 | 0.46 ± 0.24 | 2.32 ± 0.13 | P < 0.01 | P < 0.001 |
Data are presented as the means ± standard error of the means, and statistics are presented as pairwise comparisons with Bonferroni correction of treated (left) eyes to myopia only or age-matched untreated groups, with significant comparisons (P < 0.05) presented in bold.
Table 8.
Axial Length and Refractive Measurements—Coadministration of Dopamine and ADTN With Dopaminergic Antagonists
| Axial Length | Refraction | |||||||
|---|---|---|---|---|---|---|---|---|
| Condition | Left Eye | Right Eye | Compared to Myopia | Compared to Untreated | Left Eye | Right Eye | Compared to Myopia | Compared to Untreated |
| Untreated | 8.69 ± 0.03 | 8.67 ± 0.04 | P < 0.001 | — | 2.49 ± 0.11 | 2.67 ± 0.04 | P < 0.001 | — |
| FDM only | 9.03 ± 0.04 | 8.69 ± 0.04 | — | P < 0.001 | −1.54 ± 0.29 | 2.54 ± 0.09 | — | P < 0.001 |
| LIM only | 9.05 ± 0.04 | 8.68 ± 0.03 | — | P < 0.001 | −1.44 ± 0.23 | 2.33 ± 0.19 | — | P < 0.001 |
| FDM + dopamine or dopamine/antagonist | ||||||||
| 0.150 µmol | 8.75 ± 0.07 | 8.65 ± 0.03 | P < 0.001 | P = 0.379 | 0.79 ± 0.30 | 2.58 ± 0.08 | P < 0.001 | P < 0.001 |
| 0.150 µmol/D1 | 8.80 ± 0.08 | 8.66 ± 0.04 | P < 0.05 | P = 0.186 | 0.68 ± 0.24 | 2.50 ± 0.08 | P < 0.001 | P < 0.001 |
| 0.150 µmol/D2 | 8.94 ± 0.06 | 8.68 ± 0.03 | P = 0.214 | P < 0.01 | −0.75 ± 0.10 | 2.40 ± 0.11 | P = 0.108 | P < 0.001 |
| LIM + dopamine or dopamine/antagonist | ||||||||
| 0.150 µmol | 8.57 ± 0.04 | 8.45 ± 0.04 | P < 0.001 | P = 0.108 | 1.00 ± 0.12 | 2.23 ± 0.15 | P < 0.001 | P < 0.001 |
| 0.150 µmol/D1 | 8.61 ± 0.06 | 8.46 ± 0.05 | P < 0.001 | P = 0.298 | 0.90 ± 0.11 | 2.23 ± 0.13 | P < 0.001 | P < 0.01 |
| 0.150 µmol/D2 | 8.94 ± 0.03 | 8.65 ± 0.07 | P = 0.116 | P < 0.001 | −1.33 ± 0.33 | 2.28 ± 0.05 | P = 0.814 | P < 0.001 |
| FDM + ADTN or ADTN/antagonist | ||||||||
| 0.100 µmol | 8.87 ± 0.05 | 8.74 ± 0.05 | P < 0.01 | P = 0.129 | 0.09 ± 0.28 | 2.10 ± 0.10 | P < 0.001 | P < 0.001 |
| 0.100 µmol/D1 | 8.75 ± 0.03 | 8.63 ± 0.02 | P < 0.01 | P = 0.384 | −0.05 ± 0.12 | 2.15 ± 0.04 | P < 0.01 | P < 0.001 |
| 0.100 µmol/D2 | 8.94 ± 0.03 | 8.66 ± 0.04 | P = 0.138 | P < 0.001 | −0.82 ± 0.07 | 2.58 ± 0.08 | P = 0.100 | P < 0.001 |
| LIM + ADTN or ADTN/antagonist | ||||||||
| 0.100 µmol | 8.73 ± 0.07 | 8.65 ± 0.05 | P < 0.001 | P = 0.517 | 0.73 ± 0.30 | 2.30 ± 0.17 | P < 0.001 | P < 0.01 |
| 0.100 µmol/D1 | 8.77 ± 0.05 | 8.65 ± 0.07 | P < 0.001 | P = 0.141 | 0.35 ± 0.15 | 2.23 ± 0.08 | P < 0.001 | P < 0.001 |
| 0.100 µmol/D2 | 8.89 ± 0.06 | 8.63 ± 0.02 | P = 0.056 | P < 0.01 | −0.57 ± 0.18 | 2.10 ± 0.05 | P = 0.089 | P < 0.001 |
| No diffuser or lens | ||||||||
| 0.005 µmol D1 | 8.58 ± 0.06 | 8.66 ± 0.04 | — | P = 0.290 | 2.28 ± 0.25 | 2.30 ± 0.14 | — | P = 0.947 |
| 0.005 µmol D2 | 8.59 ± 0.04 | 8.65 ± 0.03 | — | P = 0.253 | 2.28 ± 0.14 | 2.38 ± 0.11 | — | P = 0.584 |
Data are presented as the means ± standard error of the means, and statistics are presented as pairwise comparisons with Bonferroni correction, with significant comparisons (P < 0.05) presented in bold. D1, SCH-23390 (D1-like antagonist); D2: spiperone (D2-like antagonist).
Dopamine Dose-Response Curves for FDM and LIM
Daily intravitreal administration of dopamine significantly inhibited the excessive axial elongation and myopic shift in refraction associated with FDM and LIM (Fig. 1, ANOVA analysis—Table 5, raw data—Table 6). This occurred in a dose-dependent manner that was best described by a logarithmic function (Figs. 1E, 1F). As expected, there was a strong correlation between the changes seen in refraction and axial length in response to administration of dopamine into form-deprived (R2 = 0.61, Supplementary Fig. S1A) or negative lens-treated eyes (R2 = 0.70, Supplementary Fig. S1B).
Based on the axial length data, dopamine showed a similar ED50 for both FDM (ED50 = 0.003 µmol) and LIM (ED50 = 0.002 µmol). In accordance, there was no statistically significant difference in the level of protection afforded against axial elongation (Wilks’ λ = 0.775, F(1, 65) = 0.941, P = 0.471; Fig. 1E) and the myopic shift in refraction (Wilks’ λ = 0.795, F(1, 65) = 0.579, P = 0.686; Fig. 1F) between FDM/dopamine- and LIM/dopamine-treated chicks when compared across all doses.
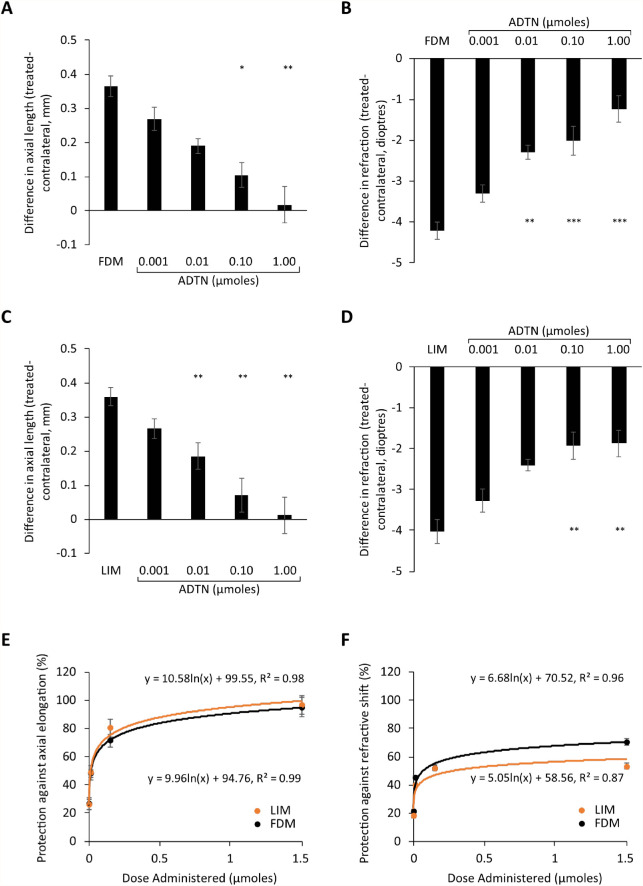
ADTN Dose-Response Curves for FDM and LIM
Like that seen for dopamine, daily intravitreal administration of ADTN significantly inhibited the excessive axial elongation and myopic shift in refraction associated with FDM and LIM (Fig. 2; ANOVA analysis—Table 5, raw data—Table 7). This occurred in a dose-dependent manner that was best described by a logarithmic function (Figs. 2E, 2F). As with dopamine, there was a strong correlation between the changes seen in refraction and axial length in response to the administration of ADTN into form-deprived (R2 = 0.70, Supplementary Fig. S1C) or negative lens-treated eyes (R2 = 0.65, Supplementary Fig. S1D).
Figure 2.
ADTN dose-response curves for FDM and LIM following 4 days of treatment. (A) Axial length and (B) refraction measurements from FDM chicks, (C) axial length and (D) refraction measurements from LIM chicks, and percent protection against the (E) axial elongation and (F) shift in refraction associated with experimental myopia development. Data represent the means ± standard error of the means. Sample sizes (min n = 6 per group) can be found in Table 2. Statistics denote difference of treated eyes relative to FDM- or LIM-only animals. *P < 0.05. **P < 0.01. ***P < 0.001.
Like dopamine, ADTN showed similar ED50 values for both FDM (ED50 = 0.011 µmol) and LIM (ED50 = 0.010 µmol). As such, there was no statistically significant difference in the level of protection afforded against axial elongation (Wilks’ λ = 0.962, F(1, 61) = 0.128, P = 0.969; Fig. 2E) and the myopic shift in refraction (Wilks’ λ = 0.805, F(1, 61) = 0.787, P = 0.554; Fig. 2F) between FDM/ADTN- and LIM/ADTN-treated chicks when compared across all doses tested.
Comparison of the Protection Provided by Dopamine Versus ADTN
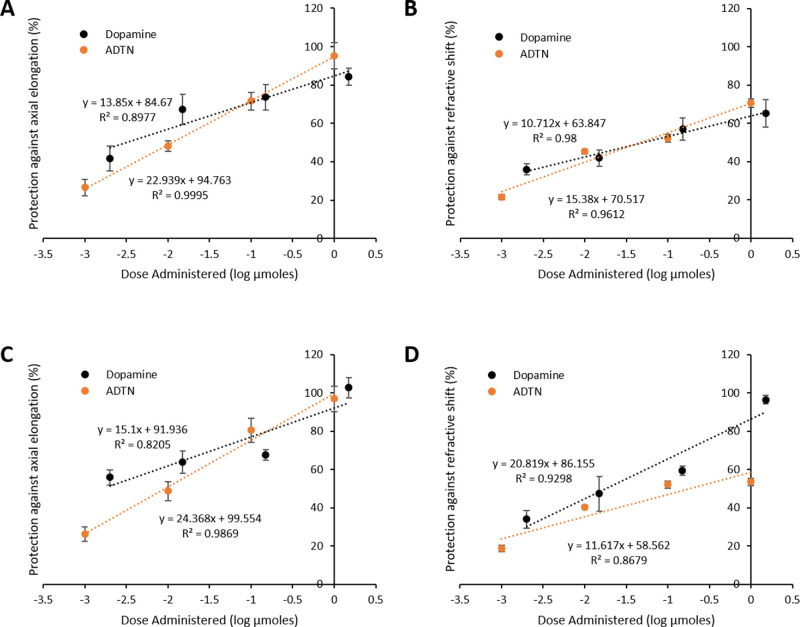
As the amount of dopamine and ADTN administered was similar across the dose range investigated (Table 1), their effectiveness relative to each other was compared via MANOVA analysis. When administered to form-deprived eyes, there was no statistically significant difference in the protection afforded against axial elongation (Wilks’ λ = 0.869, F(1, 61) = 0.451, P = 0.770; Fig. 3A) or the myopic shift in refraction (Wilks’ λ = 0.606, F(1, 61) = 1.949, P = 0.167; Fig. 3B) between dopamine- and ADTN-treated chicks. The same was seen for LIM, with both compounds eliciting similar protection across all doses tested (axial length: Wilks’ λ = 0.646, F(1, 65) = 1.921, P = 0.163, Fig. 3C; refraction: Wilks’ λ = 0.683, F(1, 65) = 1.511, P = 0.256, Fig. 3D).
Figure 3.
Comparisons of dose-dependent protection elicited by dopamine and ADTN. Percent protection against the (A) axial elongation and (B) shift in refraction associated with FDM; percent protection against the (C) axial elongation and (D) shift in refraction associated with LIM. Data are plotted as percent protection with respect to the log of the dose administered per day (µmol/d). Data represent the means ± standard error of the means.
Effects of Dopaminergic Antagonism
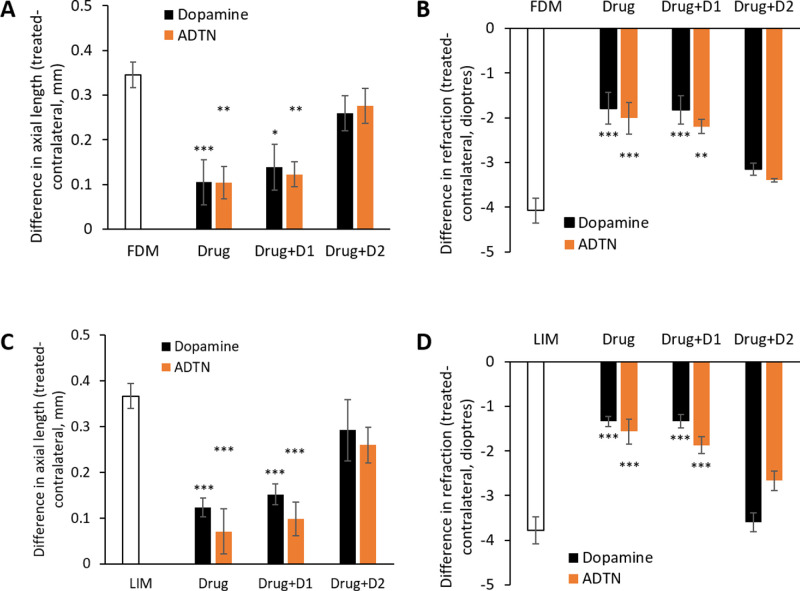
The protection elicited by dopamine (0.150 µmol) against the development of experimental myopia persisted when coadministered with the D1-like dopamine antagonist SCH-23390 (0.005 µmol) with respect to the changes seen in both axial length and refraction (Fig. 4, raw data—Table 8). In contrast, when dopamine was coadministered with the D2-like dopamine antagonist spiperone (0.005 µmol), the protective effects of dopamine were significantly inhibited (Fig. 4, raw data—Table 8), with cotreated eyes no different from their FDM- or LIM-only counterparts in axial length and refraction.
Figure 4.
Coadministration with dopaminergic antagonists. (A) Axial length and (B) refraction measurements from chicks developing FDM and (C) axial length and (D) refraction measurements from chicks developing LIM. Data represent the means ± standard error of the means. Drug: the administration of dopamine or ADTN into FDM- or LIM-treated eyes; Drug+D1: dopamine or ADTN coadministered with the D1-like antagonist SCH-23390 in FDM- or LIM-treated eyes; Drug+D2: dopamine or ADTN coadministered with the D2-like antagonist spiperone in FDM- or LIM-treated eyes. Sample sizes (min n = 6 per group) can be found in Table 2. Statistics denote difference of treated eyes relative to FDM or LIM only. *P < 0.05. **P < 0.01. ***P < 0.001.
Similarly, the protective effects of 0.100 µmol ADTN against the development of FDM and LIM remained when coadministered with SCH-23390 (Fig. 4, raw data—Table 8). However, as with dopamine, when ADTN was coadministered with spiperone, these protective effects were significantly inhibited (Fig. 4, raw data—Table 8), with cotreated eyes no different from their FDM- or LIM-only counterparts in axial length and refraction.
Discussion
Several subtle differences have been reported in the biochemical changes observed between the two major forms of experimental myopia (for review, see Morgan et al.37), including suggested differences in the role of dopamine.18,28,33 However, the findings of this current study indicate that FDM and LIM appear to be similarly influenced by pharmacological manipulation of the dopaminergic system. Specifically, intravitreal application of dopamine or the dopaminergic receptor agonist ADTN inhibited the development of both FDM and LIM. Importantly, each of these dopaminergic compounds inhibited both forms of experimental myopia in a similar dose-dependent manner that was best described by a logarithmic relationship. The current findings indicate that the protection afforded by both compounds was driven by stimulation of the dopamine D2-like receptor family in both FDM and LIM, demonstrating mechanistic similarities.
The Level of Protection Elicited by Dopamine and ADTN is Similar Between FDM and LIM
To be able to appropriately characterize the action of both dopaminergic compounds, their effect on the development of experimental myopia was investigated over multiple doses rather than a single dose (for review, see Waud44 and Saha and Brannath45). From this, we can see that the development of FDM and LIM was inhibited, in a dose-dependent manner, to the same degree by the daily intravitreal injection of dopamine or the dopaminergic agonist ADTN. This strongly suggests that the dopaminergic system is similarly important to both experimental paradigms. These dose-based analyses also demonstrated that the degree of protection offered by both dopaminergic compounds was very similar.
Similarities in the Role of Dopamine in FDM and LIM
Although a large range of dopaminergic compounds have been tested against the development of FDM, only a handful have also been tested against LIM. Of these, only the nonspecific agonist apomorphine has been concurrently tested against both paradigms. In the first of these studies, Schmid and Wildsoet17 reported that apomorphine inhibited the development of both FDM and LIM in chicks, with the effect against LIM seen over a greater dose range. Similar findings have been reported by studies investigating the effect of apomorphine on either FDM (chicks,5,15 primates,21 mice19) or LIM (chicks16) alone. In contrast, the only other concurrent study of both forms of experimental myopia reported that despite inhibiting FDM, apomorphine did not affect the development of LIM across five doses in guinea pigs.18 This may suggest differences between birds and members of the Rodentia family with respect to the role of dopamine in LIM or the effect of dopaminergic agents in LIM. This may not be surprising, as unlike that seen in chicks5,13,15,16,29,36 and tree shrews,20 in which dopamine appears to inhibit experimental myopia through a D2-like receptor mechanism, in rodents (guinea pigs46 and mice47), this protection appears to be driven through a D1-like mechanism, with D2 receptor activation reported to enhance myopia. Thus, apomorphine may have different effects in rodents with respect to eye growth.
Other than apomorphine, the only other agonist tested against both forms of experimental myopia, although in separate studies, is that of the D2-like receptor agonist quinpirole. In both FDM (chicks13 and tree shrews)20 and LIM (chicks),16 daily administration of this agonist was shown to inhibit the development of both forms of experimental myopia. More recently, we have also shown that administration of the dopaminergic precursor levodopa inhibits FDM23 and LIM24 in a similar dose-dependent manner when given either topically or intravitreally over a period of 4 days. Taken together with the current findings, the pharmacological data would suggest that stimulation of the dopaminergic system can similarly inhibit FDM and LIM, although with potential receptor subfamily differences in rodents.
Based on the suggested role of dopamine in inhibiting ocular growth, a small number of studies have also examined whether blocking dopaminergic signaling, through administration of antagonists, can prevent the protective effects of brief periods of normal vision against the development of FDM and LIM. Initial studies reported that administration of the dopamine D2-like receptor antagonist spiperone inhibited the protective effects of brief periods of normal vision against the development of both FDM13 and LIM,28 although to a lesser extent in LIM. However, a later study by one of the groups was unable to replicate the initial report of spiperone's ability to block the protective effects of normal vision against LIM.16 Our current findings, although not looking at the paradigm of normal vision, indicate that dopaminergic protection against the development of FDM and LIM can be similarly blocked by the administration of spiperone but not the D1-like antagonist SCH-23390, correlating with the expected D2-like receptor mechanism.5,13,15,16,20,29,36 Similarly, we have previously reported that the ability of the dopamine precursor levodopa to inhibit both forms of experimental myopia can also be blocked by the coadministration of spiperone.24 Finally, the ability of bright-light exposure, which increases retinal dopamine levels,48–51 to inhibit the development of both forms of experimental myopia can similarly be blocked by the administration of spiperone in chicks29 (Karouta C, et al. IOVS 2018;59:ARVO E-Abstract 676). Together, the majority of evidence would suggest, at least in chicks, that stimulation of the D2-like receptors underlies the ability of dopamine to inhibit the development of both forms of experimental myopia.
The pharmacological evidence noted above would suggest that the dopaminergic system plays a role in both forms of experimental myopia; therefore, one would expect that retinal dopamine levels are similarly affected in both FDM and LIM. Although dopamine levels have consistently been shown to be reduced during the development of FDM in all species studied (chicks,5,23,52 guinea pigs,53 tree shrews,7 primates8), this is not consistently seen in LIM. Specifically, initial studies reported no change in dopamine levels during the development of LIM in chicks33 and guinea pigs.18 However, more recent work in chicks has suggested that dopamine levels may well be reduced in LIM34 (Sarfare S, et al. IOVS 2019;60:ARVO E-Abstract 5883), which, if confirmed, would further indicate that the two myopia paradigms are similar with respect to the role of dopamine.
Overall, the current literature ultimately supports a similar role for dopamine in the development of both forms of experimental myopia. However, as discussed above, members of the Rodentia family appear to show differences in receptor mechanism that must be taken into account when characterizing the role of dopamine in eye growth.
Conclusion
Here we show that intravitreal injection of either dopamine or the dopaminergic agonist ADTN inhibits the development of FDM and LIM in a dose-dependent manner. Importantly, we show that the degree of protection elicited by each compound is similar between the two experimental paradigms and between drugs. We also report that the protection afforded by dopamine and ADTN is elicited through a D2-like receptor mechanism in both FDM and LIM. Together, these findings indicate that dopamine may well play a role in both forms of experimental myopia.
Acknowledgments
Partially funded by ANU Connect Ventures through a Discovery Translation Fund grant (Project ID: DTF311).
Disclosure: K. Thomson, None; C. Karouta, None; R. Ashby, PCT application currently under review (P)
References
- 1. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012; 379: 1739–1748. [DOI] [PubMed] [Google Scholar]
- 2. Feldkaemper M, Schaeffel F.. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013; 114: 106–119. [DOI] [PubMed] [Google Scholar]
- 3. Troilo D, Smith EL III, Nickla DL, et al.. IMI—report on experimental models of emmetropization and myopia. Invest Ophthalmol Vis Sci. 2019; 60: M31–M88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou X, Pardue MT, Iuvone PM, Qu J. Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res. 2017; 61: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA. 1989; 86: 704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mao JF, Liu SZ, Qin WJ, Xiang Q. Modulation of TGFbeta(2) and dopamine by PKC in retinal Muller cells of guinea pig myopic eye. Int J Ophthalmol. 2011; 4: 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McBrien NA, Cottriall CL, Annies R. Retinal acetylcholine content in normal and myopic eyes: a role in ocular growth control? Vis Neurosci. 2001; 18: 571–580. [DOI] [PubMed] [Google Scholar]
- 8. Iuvone PM, Tigges M, Fernandes A, Tigges J. Dopamine synthesis and metabolism in rhesus monkey retina: development, aging, and the effects of monocular visual deprivation. Vis Neurosci. 1989; 2: 465–471. [DOI] [PubMed] [Google Scholar]
- 9. Park H, Jabbar SB, Tan CC, et al.. Visually-driven ocular growth in mice requires functional rod photoreceptors. Invest Ophthalmol Vis Sci. 2014; 55: 6272–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu XH, Qian KW, Xu GZ, et al.. The role of retinal dopamine in C57BL/6 mouse refractive development as revealed by intravitreal administration of 6-hydroxydopamine. Invest Ophthalmol Vis Sci. 2016; 57: 5393–5404. [DOI] [PubMed] [Google Scholar]
- 11. Park H, Tan CC, Faulkner A, et al.. Retinal degeneration increases susceptibility to myopia in mice. Mol Vis. 2013; 19: 2068–2079. [PMC free article] [PubMed] [Google Scholar]
- 12. Markand S, Baskin NL, Chakraborty R, et al.. IRBP deficiency permits precocious ocular development and myopia. Mol Vis. 2016; 22: 1291–1308. [PMC free article] [PubMed] [Google Scholar]
- 13. McCarthy CS, Megaw P, Devadas M, Morgan IG. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp Eye Res. 2007; 84: 100–107. [DOI] [PubMed] [Google Scholar]
- 14. Ashby R, McCarthy CS, Maleszka R, Megaw P, Morgan IG. A muscarinic cholinergic antagonist and a dopamine agonist rapidly increase ZENK mRNA expression in the form-deprived chicken retina. Exp Eye Res. 2007; 85: 15–22. [DOI] [PubMed] [Google Scholar]
- 15. Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci. 1993; 10: 447–453. [DOI] [PubMed] [Google Scholar]
- 16. Nickla DL, Totonelly K, Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res. 2010; 91: 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmid KL, Wildsoet CF.. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci. 2004; 81: 137–147. [DOI] [PubMed] [Google Scholar]
- 18. Dong F, Zhi ZN, Pan MZ, et al.. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Molecular Vision. 2011; 17: 2824–2834. [PMC free article] [PubMed] [Google Scholar]
- 19. Yan T, Xiong W, Huang F, et al.. Daily injection but not continuous infusion of apomorphine inhibits form-deprivation myopia in mice. Invest Ophthalmol Vis Sci. 2015; 56: 2475–2485. [DOI] [PubMed] [Google Scholar]
- 20. Ward AH, Siegwart JT, Frost MR, Norton TT. Intravitreally-administered dopamine D2-like (and D4), but not D1-like, receptor agonists reduce form-deprivation myopia in tree shrews. Vis Neurosci. 2017; 34: E003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci. 1991; 32: 1674–1677. [PubMed] [Google Scholar]
- 22. Gao Q, Liu Q, Ma P, Zhong X, Wu J, Ge J. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch Clin Exp Ophthalmol. 2006; 244: 1329–1335. [DOI] [PubMed] [Google Scholar]
- 23. Thomson K, Karouta C, Morgan I, Kelly T, Ashby R. Effectiveness and safety of topical levodopa in a chick model of myopia. Sci Rep. 2019; 9: 18345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomson K, Morgan I, Karouta C, Ashby R. Levodopa inhibits the development of lens-induced myopia in chicks. Sci Rep. 2020; 10: 13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Landis EG, Chrenek MA, Chakraborty R, et al.. Increased endogenous dopamine prevents myopia in mice. Exp Eye Res. 2020; 193: 107956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mao J, Liu S, Qin W, Li F, Wu X, Tan Q. Levodopa inhibits the development of form-deprivation myopia in guinea pigs. Optom Vis Sci. 2010; 87: 53–60. [DOI] [PubMed] [Google Scholar]
- 27. Mao J, Liu S.. Different roles of retinal dopamine in albino guinea pig myopia. Neurosci Lett. 2017; 639: 94–97. [DOI] [PubMed] [Google Scholar]
- 28. Nickla DL, Totonelly K. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res. 2011; 93: 782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashby RS, Schaeffel F.. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010; 51: 5247–5253. [DOI] [PubMed] [Google Scholar]
- 30. Chen S, Zhi Z, Ruan Q, et al.. Bright light suppresses form-deprivation myopia development with activation of dopamine D1 receptor signaling in the ON pathway in retina. Invest Ophthalmol Vis Sci. 2017; 58: 2306–2316. [DOI] [PubMed] [Google Scholar]
- 31. Bergen MA, Park HN, Chakraborty R, et al.. Altered refractive development in mice with reduced levels of retinal dopamine. Invest Ophthalmol Vis Sci. 2016; 57: 4412–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schaeffel F, Hagel G, Bartmann M, Kohler K, Zrenner E. 6-Hydroxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Res. 1994; 34: 143–149. [DOI] [PubMed] [Google Scholar]
- 33. Bartmann M, Schaeffel F, Hagel G, Zrenner E. Constant light affects retinal dopamine levels and blocks deprivation myopia but not lens-induced refractive errors in chickens. Vis Neurosci. 1994; 11: 199–208. [DOI] [PubMed] [Google Scholar]
- 34. Guo SS, Sivak JG, Callender MG, Diehl-Jones B. Retinal dopamine and lens-induced refractive errors in chicks. Curr Eye Res. 1995; 14: 385–389. [DOI] [PubMed] [Google Scholar]
- 35. Li XX, Schaeffel F, Kohler K, Zrenner E. Dose-dependent effects of 6-hydroxy dopamine on deprivation myopia, electroretinograms, and dopaminergic amacrine cells in chickens. Vis Neurosci. 1992; 9: 483–492. [DOI] [PubMed] [Google Scholar]
- 36. Schaeffel F, Bartmann M, Hagel G, Zrenner E. Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res. 1995; 35: 1247–1264. [DOI] [PubMed] [Google Scholar]
- 37. Morgan IG, Ashby RS, Nickla DL. Form deprivation and lens-induced myopia: are they different? Ophthalmic Physiol Opt. 2013; 33: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wiesel TN, Raviola E.. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977; 266: 66–68. [DOI] [PubMed] [Google Scholar]
- 39. Raviola E, Wiesel TN.. An animal model of myopia. N Engl J Med. 1985; 312: 1609–1615. [DOI] [PubMed] [Google Scholar]
- 40. Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988; 28: 639–657. [DOI] [PubMed] [Google Scholar]
- 41. Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995; 1: 761–765. [DOI] [PubMed] [Google Scholar]
- 42. Karouta C, Ashby RS.. Correlation between light levels and the development of deprivation myopia. Invest Ophthalmol Vis Sci. 2014; 56: 299–309. [DOI] [PubMed] [Google Scholar]
- 43. Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009; 50: 5348–5354. [DOI] [PubMed] [Google Scholar]
- 44. Waud DR. Analysis of dose—response curves. In Smooth Muscle. Boston, MA: Springer; 1975: 471–506. [Google Scholar]
- 45. Saha S, Brannath W.. Comparison of different approaches for dose response analysis. Biom J. 2019; 61: 83–100. [DOI] [PubMed] [Google Scholar]
- 46. Jiang LQ, Long K, Zhou X, Qu J. Reciprocal activities of two type dopamine receptors determine the myopia development. Invest Ophthalmol Vis Sci. 2012; 53: 3437–3437. [Google Scholar]
- 47. Huang F, Yan T, Shi F, et al.. Activation of dopamine D2 receptor is critical for the development of form-deprivation myopia in the C57BL/6 mouse. Invest Ophthalmol Vis Sci. 2014; 55: 5537–5544. [DOI] [PubMed] [Google Scholar]
- 48. Megaw P, Morgan I, Boelen M. Vitreal dihydroxyphenylacetic acid (DOPAC) as an index of retinal dopamine release. J Neurochem. 2001; 76: 1636–1644. [DOI] [PubMed] [Google Scholar]
- 49. Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012; 103: 33–40. [DOI] [PubMed] [Google Scholar]
- 50. Proll MA, Kamp CW, Morgan WW. Use of liquid chromatography with electrochemistry to measure effects of varying intensities of white light on DOPA accumulation in rat retinas. Life Sci. 1982; 30: 11–19. [DOI] [PubMed] [Google Scholar]
- 51. Mathis U, Feldkaemper M, Wang M, Schaeffel F. Studies on retinal mechanisms possibly related to myopia inhibition by atropine in the chicken. Graefes Arch Clin Exp Ophthalmol. 2020; 258: 319–333. [DOI] [PubMed] [Google Scholar]
- 52. Ohngemach S, Hagel G, Schaeffel F. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Vis Neurosci. 1997; 14: 493–505. [DOI] [PubMed] [Google Scholar]
- 53. Mao J, Liu S, Qin W, Xiang Q, Wu X. Exogenous levodopa increases the neuro retinal dopamine of guinea pig myopic eyes in vitro. Eye Sci. 2011; 26: 211–216. [DOI] [PubMed] [Google Scholar]