Abstract
On 22 August, a common whitethroat in the Netherlands tested positive for West Nile virus lineage 2. The same bird had tested negative in spring. Subsequent testing of Culex mosquitoes collected in August and early September in the same location generated two of 44 positive mosquito pools, providing first evidence for enzootic transmission in the Netherlands. Sequences generated from the positive mosquito pools clustered with sequences that originate from Germany, Austria and the Czech Republic.
Keywords: West Nile Virus; Europe; the Netherlands; zoonotic infections; viral infections; West Nile fever; outbreaks; surveillance; epidemiology; molecular methods, sequencing
Extensive surveillance of mosquitoes, dead and live birds was set up in 2016 to monitor the introduction and spread of a selection of high-risk arboviruses in the Netherlands. The presence of Usutu virus (USUV) infections was confirmed first in 2016, and genomic sequencing provided evidence for continued enzootic presence of USUV in subsequent years [1,2]. West Nile virus (WNV), however, had so far not been detected. Here we report the first locally acquired WNV detection in birds in the Netherlands.
Surveillance in mosquitoes, dead birds and live birds
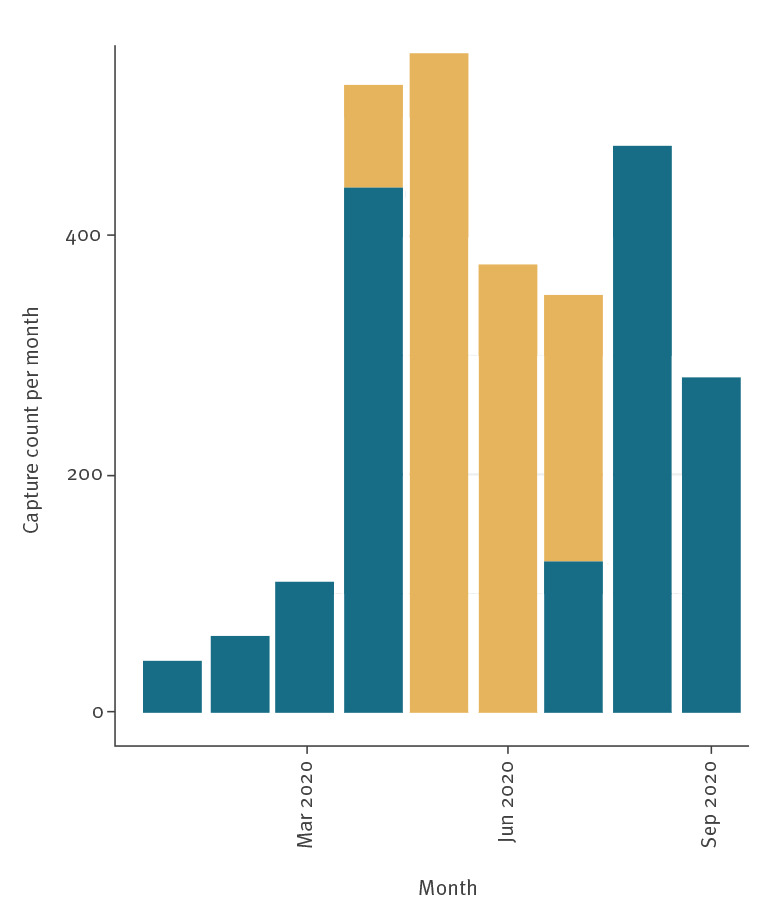
Since January 2020, 2,783 live birds have been caught and sampled in the Netherlands to detect possible introduction and spread of zoonotic viruses. Wild birds were randomly captured using mist nets and other trapping methods and sampled as part of a wider study of the presence of zoonotic viruses in birds in the Netherlands [1,2]. Captured birds were ringed, weighed and measured, sex and age were determined, and throat (and for larger species cloacal) swabs were collected and stored in virus transport medium at −80 °C until use. Recapturing and resampling of ringed birds occurs frequently during the breeding season. Because of COVID-19, it was not possible to test samples received between 29 April and 24 July. To date 1,477 of 2,783 birds have been tested for WNV (Figures 1 and 2).
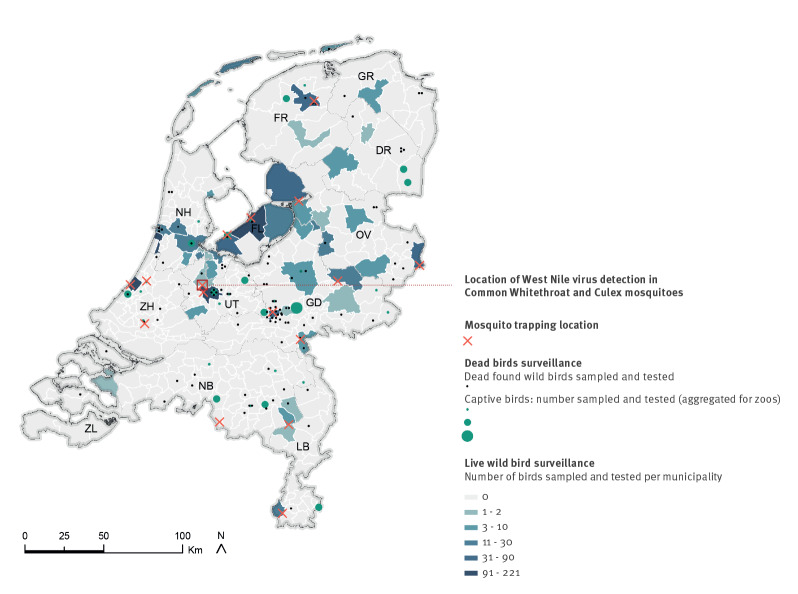
Figure 1.
Surveillance network for zoonotic viruses in birds and mosquitoes and location of West Nile Virus detection, the Netherlands, 2020
DR: Drenthe; FL: Flevoland; FR: Friesland; GD: Gelderland; GR: Groningen; LB: Limburg; NB: Noord Brabant; NH: Noord Holland; OV: Overijssel; UT: Utrecht; ZH: Zuid Holland; ZL: Zeeland.
Crosses indicate mosquito trapping locations. Dots indicate locations of dead birds collected in 2020. Captive dead birds are aggregated by zoo. The number of live birds sampled and tested in 2020 is indicated at the municipality level. Source administrative boundaries: Statistics the Netherlands (CBS). Created using ArcGIS 10.6 software by ESRI.
Figure 2.
Number of live birds caught and sampled, the Netherlands, 2020 (n = 2,783)
Yellow: samples that have been sampled, but not yet tested owing to COVID-19. Blue: samples that have been tested for West Nile virus.
Mortality in captive and wild birds is reported through a citizen science-based alerting system. In 2020, a total of 221 birds were reported and collected for further research (Figure 1). For all birds, an autopsy was performed and brain samples were taken.
In parallel to the sampling of live and dead birds, a network of 16 mosquito trapping sites was set up in July 2020. At each site, mosquitoes were collected on a weekly basis using molasses fermentation-baited BG-pro traps (Biogents GmbH, Regensburg, Germany) without additional lures, following a slightly modified molasses fermentation protocol [3]. The mosquitoes were morphologically identified. Individuals belonging to the Culex pipiens complex were pooled, based on trap location and time point of sampling, with a maximum of 10 individuals per pool. Mosquito pools were homogenised in medium (DMEM, NaHCO3, Hepes-buffered saline, penicillin/streptomycin, amphotericin).
All samples (brain samples of dead birds, throat and cloaca (pooled, per bird), swabs of live birds and mosquito pools) were screened for the presence of WNV and USUV using a real-time PCR with primers and probes as described previously, with phocine distemper virus (PDV) used as an internal control [4]. Positive results were confirmed with a second PCR targeting another region of the genome (Table). In addition, all RT-PCR-positive samples were subjected to sequencing.
Table. Primer sequences for screening and confirmation RT-PCR for West Nile virus, the Netherlands, 2020.
| Virus | Forward primer '5 -> 3' | Reverse primer '5 -> 3' | Probe '5 -> 3' | Goal |
|---|---|---|---|---|
| WNV | CCACCGGAAGTTGAGTAGACG | TTTGGTCACCCAGTCCTCCT | TGCTGCTGCCTGCGGCTCAACCC | Screening |
| WNV lineage 2 |
CCATCTGYTCCGCWGTGCC | ATCCATTCTCCTTTTGCGTGRAT | TGGGTTCCCACRGGGCGYACCACYTG | Confirmation |
WNV: West Nile virus.
Results of the West Nile virus screening
One bird, a common whitethroat (Curruca communis) was caught on 22 August in the municipality of Utrecht, the Netherlands and tested positive for WNV lineage 2 (cycle threshold (Ct) value: 31.8 and 32.5 in the screening and confirmation PCR, respectively). The bird appeared healthy at the time of capture. The USUV RT-PCR was negative. The bird was caught multiple times at the same location between 2018 and 2020. On 21 July 2018 the bird was in first year plumage. In May 2020, the bird was identified as a sexually active adult male based on the shape of its cloaca and tested negative for WNV. A total of 173 birds caught at the same location in 2020 tested negative for WNV.
At the same location, two of 44 pools of C. pipiens mosquitoes tested positive for WNV RNA. Positive pools were collected in August and September 2020, and came from two traps ca 250 m apart. The first pool (Ct value: 18.6) was collected in the same week as the positive bird (17–23 August). The second positive pool (Ct value: 26.1) was collected in the week from 31 August to 6 September. Mosquitoes from other locations have not yet been screened for WNV.
Sequencing
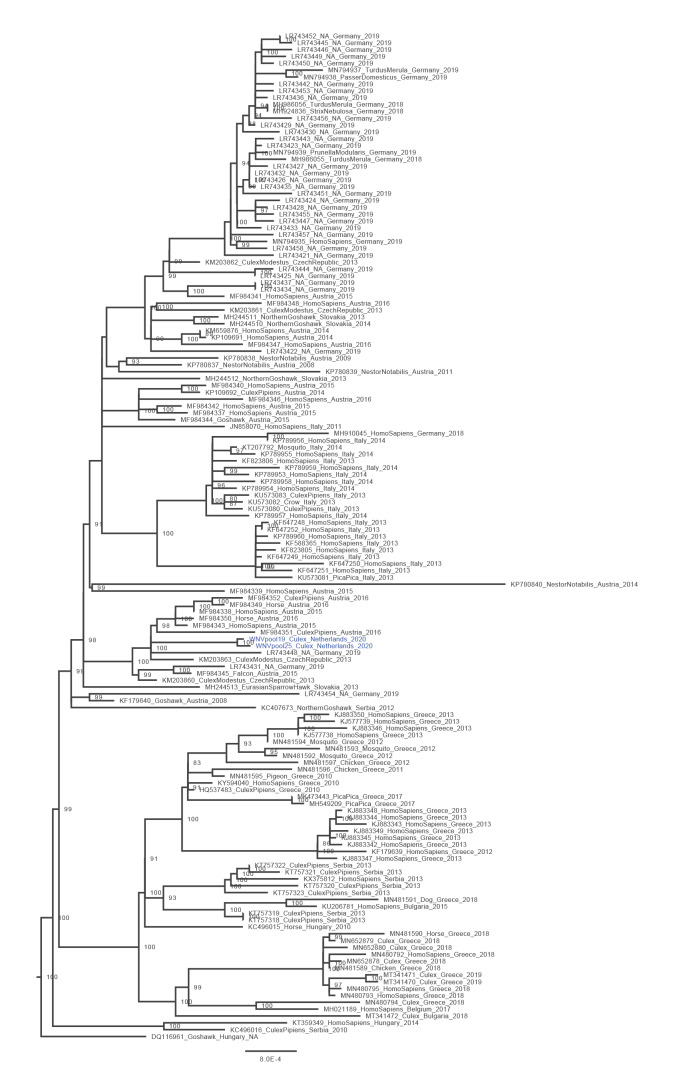
We developed a WNV-specific amplicon-based whole genome sequencing PCR similar to what has previously been described for USUV and SARS-CoV-2, using Nanopore sequencing [2,5]. Sequencing was performed as previously described [2,5]. Primer sequences are available in Supplementary Table S1. We downloaded all full-length WNV sequences available in GenBank on 4 September 2020 [6] and aligned them using MUSCLE software [7]. We downsampled the number of reference sequences to include all lineage 2 WNV sequences, but only a small selection of WNV lineage 1. Phylogenetic analysis using IQ-TREE [8] under the GTR + F + I + G4 model, as determined by the best model prediction option, confirmed the presence of WNV lineage 2 in the two mosquito pools for which we had nearly complete genome coverage (Figure 3). The viral load in the bird sample was too low for full genome sequencing but yielded a 500 bp sequence matching with lineage 2 WNV. The genomes from the two mosquito pools (Genbank accession numbers MW036633; MW036634) differed by 3 nt from each other and clustered with sequences that originate from Germany (in 2019) and older sequences found in Austria and the Czech Republic (Figure 3).
Figure 3.
Phylogenetic tree of all full genomes of West Nile virus lineage 2 available in GenBank on 4 September 2020 (n = 147)
The figure includes two sequences acquired from two mosquito pools in this study, in blue. The scale bar represents units of substitutions per site.
Discussion
Most human and animal cases of WNV in Europe are reported from countries in the southern and south-eastern parts but the geographic distribution has expanded considerably over the past 10 years, from six countries reporting human or animal cases in 2010 in the European Union (EU) to 12 in 2019 [9,10]. In 2020, a total of 209 human cases have been reported so far in the EU, in Greece, Italy, Romania, Hungary and Spain, as well as multiple asymptomatic cases found in blood donor screening and nine clinical cases in humans in Germany [11]. Considering that most people do not develop symptoms and only human cases with known place of infection on NUTS3 level are included in the overviews from the European Centre for Disease Prevention and Control (ECDC), these numbers are likely to be an underestimation.
In contrast to the United States, the main amplification host species for WNV in Europe have not been defined with certainty [12]. However, a wide range of birds is susceptible, including a variety of migrating bird species, which can play an important role in the national and international spread of the virus [13]. Mosquitoes can acquire the virus by feeding on a viraemic bird and can transmit it to other birds, animals or humans following an intrinsic incubation period [14]. The main vectors in Europe belong to the C. pipiens complex, which are present throughout Europe [15]. Moreover, northern and north-western Culex mosquitoes have been shown to be highly competent for both WNV lineages, and wild-caught Dutch jackdaws and carrion crows are susceptible experimentally [15-18]. Humans and equids are considered dead-end hosts but in highly enzootic areas, a much broader range of species can be infected [19]. During the 2020 transmission season, WNV infections in equids were observed in France, Spain, Italy, Germany and Portugal but only in Germany, WNV circulation in birds was officially reported. In contrast to humans and equids, reporting of WNV in birds is not notifiable [11].
The simultaneous detection of WNV in a local common whitethroat and Culex mosquitoes provides definitive evidence for enzootic transmission in the Netherlands. In 2016, five of 265 screened bird serum samples collected in the Netherlands were found WNV antibody-positive [20], but no virus was found. The two nearly complete whole genome sequences differed from each other by 3 nt, which could indicate local evolution, although separate introductions are also possible. The most recent sequences that clustered with the Dutch WNV sequences originated from Germany, which may be the origin of the virus we found.
Common whitethroats are long-distance migrants that arrive in the Netherlands in the second half of April, while passage of more northern populations occurs until the end of May. Post-breeding dispersal is notable from the beginning of July and southward migration starts in August. Extensive ringing data indicate that nearly all whitethroats captured in the Netherlands between April and July have a local origin [21]. Of the six previous local captures of the positive bird, two occurred in the breeding season of a sexually active male, indicating that this bird was a local breeder. In addition to the negative WNV result in May 2020 and the finding of the WNV-positive mosquitoes at the same site, this strongly suggests that the bird has contracted the virus locally.
The detection in the bird and two positive mosquito pools followed a heatwave, lasting 13 days, including 8 days with temperatures above 30 °C, and average minimum temperatures just below 20 °C [22]. Elevated temperatures are known to shorten the viral extrinsic incubation period in mosquitoes, thereby increasing transmission efficiency. Moreover, virus replication increases, further facilitating arbovirus circulation.
This finding was the result of an extensive surveillance programme in live birds, dead birds and mosquitoes. To date, no locally acquired human or equine cases have been reported in the Netherlands, but it has been shown that detection of WNV circulation in domestic birds or mosquitoes can precede the first human detections by several weeks [23]. Therefore, we will increase our surveillance effort in the area where WNV was first detected as well as areas that are most at risk of WNV establishment according to a previous risk assessment in the Netherlands [24]. Increased monitoring will encompass surveillance in live and dead birds as well as humans, mosquitoes, livestock, equids and wildlife using a multidisciplinary One Health approach.
Acknowledgements
We thank all bird ringers that were involved in catching and sampling live birds, as well as reporting bird mortality and trapping mosquitoes. We also thank the general public for reporting mortality and submitting specimens of dead birds, and the staff of the DWHC for performing the necropsies and collecting the samples.
This work is part of the research programme One Health PACT with project number 109986, which is (partly) financed by the Dutch Research Council (NWO). Part of the surveillance activities that are reported in this manuscript were set up under the Eco-alert project (ZonMW project number 522001004) between 2014 and 2018.
Supplementary Data
Conflict of interest: None declared.
Authors’ contributions: RS: writing the manuscript. RS, MK, HJ, CK, MS: coordination and design of surveillance activities. EM, IC, AL, MB, RM, BOM, MK: sequencing and sequence analysis. JB: coordination, pathology and sampling of dead birds. MS, LK, RB, CK, JvB: mosquito sampling and species determination. JM, TB, TM, HJ: coordination and sampling of live birds. RS, BOM, HJ, MK: overall data analysis and interpretation. All authors were involved in critical assessment of the final manuscript.
References
- 1. Rijks JM, Kik ML, Slaterus R, Foppen R, Stroo A, IJzer J, et al. Widespread Usutu virus outbreak in birds in the Netherlands, 2016. Euro Surveill. 2016;21(45):30391. 10.2807/1560-7917.ES.2016.21.45.30391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oude Munnink BB, Münger E, Nieuwenhuijse DF, Kohl R, van der Linden A, Schapendonk CME, et al. Genomic monitoring to understand the emergence and spread of Usutu virus in the Netherlands, 2016-2018. Sci Rep. 2020;10(1):2798. 10.1038/s41598-020-59692-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mweresa CK, Omusula P, Otieno B, van Loon JJA, Takken W, Mukabana WR. Molasses as a source of carbon dioxide for attracting the malaria mosquitoes Anopheles gambiae and Anopheles funestus. Malar J. 2014;13(1):160. 10.1186/1475-2875-13-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim SM, Koraka P, Osterhaus ADME, Martina BEE. Development of a strand-specific real-time qRT-PCR for the accurate detection and quantitation of West Nile virus RNA. J Virol Methods. 2013;194(1-2):146-53. 10.1016/j.jviromet.2013.07.050 [DOI] [PubMed] [Google Scholar]
- 5. Oude Munnink BB, Nieuwenhuijse DF, Stein M, O’Toole Á, Haverkate M, Mollers M, et al. Dutch-Covid-19 response team Rapid SARS-CoV-2 whole-genome sequencing and analysis for informed public health decision-making in the Netherlands. Nat Med. 2020;26(9):1405-10. 10.1038/s41591-020-0997-y [DOI] [PubMed] [Google Scholar]
- 6. Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2010;38(Database issue) suppl_1;D46-51. 10.1093/nar/gkp1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37(5):1530-4. 10.1093/molbev/msaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792-7. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control (ECDC). West Nile fever cases by affected areas in Europe and the Mediterranean, 2010 transmission season. Stockholm: ECDC. [Accessed: 24 Sep 2020]. Available from: https://www.ecdc.europa.eu/en/publications-data/west-nile-fever-cases-affected-areas-europe-and-mediterranean-2010-transmission
- 10.European Centre for Disease Prevention and Control (ECDC). Epidemiological update: West Nile virus transmission season in Europe, 2019. Stockholm: ECDC; 2019. Available from: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2019
- 11.European Centre for Disease Prevention and Control (ECDC). Weekly updates: 2020 West Nile virus transmission season. Stockholm: ECDC. [Accessed: 21 Sep 2020]. Available from: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc
- 12. Esser HJ, Mögling R, Cleton NB, van der Jeugd H, Sprong H, Stroo A, et al. Risk factors associated with sustained circulation of six zoonotic arboviruses: a systematic review for selection of surveillance sites in non-endemic areas. Parasit Vectors. 2019;12(1):265. 10.1186/s13071-019-3515-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Owen J, Moore F, Panella N, Edwards E, Bru R, Hughes M, et al. Migrating birds as dispersal vehicles for West Nile virus. EcoHealth. 2006;3(2):79-85. 10.1007/s10393-006-0025-9 [DOI] [Google Scholar]
- 14. Colpitts TM, Conway MJ, Montgomery RR, Fikrig E. West Nile Virus: biology, transmission, and human infection. Clin Microbiol Rev. 2012;25(4):635-48. 10.1128/CMR.00045-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vogels CB, Göertz GP, Pijlman GP, Koenraadt CJ. Vector competence of European mosquitoes for West Nile virus. Emerg Microbes Infect. 2017;6(11):e96. 10.1038/emi.2017.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fros JJ, Geertsema C, Vogels CB, Roosjen PP, Failloux A-B, Vlak JM, et al. West Nile virus: high transmission rate in north-western European mosquitoes indicates its epidemic potential and warrants increased surveillance. PLoS Negl Trop Dis. 2015;9(7):e0003956. 10.1371/journal.pntd.0003956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lim SM, Brault AC, van Amerongen G, Sewbalaksing VD, Osterhaus ADME, Martina BEE, et al. Susceptibility of European jackdaws (Corvus monedula) to experimental infection with lineage 1 and 2 West Nile viruses. J Gen Virol. 2014;95(Pt 6):1320-9. 10.1099/vir.0.063651-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim SM, Brault AC, van Amerongen G, Bosco-Lauth AM, Romo H, Sewbalaksing VD, et al. Susceptibility of carrion crows to experimental infection with lineage 1 and 2 West Nile viruses. Emerg Infect Dis. 2015;21(8):1357-65. 10.3201/eid2108.140714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Root JJ, Bosco-Lauth AM. West Nile virus associations in wild mammals: an update. Viruses. 2019;11(5):E459. 10.3390/v11050459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lim SM, Geervliet M, Verhagen JH, Müskens GJDM, Majoor FA, Osterhaus ADME, et al. Serologic evidence of West Nile virus and Usutu virus infections in Eurasian coots in the Netherlands. Zoonoses Public Health. 2018;65(1):96-102. 10.1111/zph.12375 [DOI] [PubMed] [Google Scholar]
- 21.Vogeltrekstation. [Dutch Centre for Avian Migration and Demography]. Grasmus Sylvia communis. [Warbler Sylvia communis]. Wageningen; Vogeltrekstation. [Accessed: 29 Sep 2020]. Dutch. Available from: http://www.vogeltrekatlas.nl/soortzoek2.html?-0-Grasmus-Totaal
- 22.Royal Netherlands Meteorological Institute (KNMI). Augustus vol warmterecords. [English title]. De Bilt: KNMI; 2020. Dutch. Available from: https://www.knmi.nl/over-het-knmi/nieuws/augustus-vol-warmterecords
- 23. Gossner CM, Marrama L, Carson M, Allerberger F, Calistri P, Dilaveris D, et al. West Nile virus surveillance in Europe: moving towards an integrated animal-human-vector approach. Euro Surveill. 2017;22(18):30526. 10.2807/1560-7917.ES.2017.22.18.30526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Esser HJ, Liefting Y, Ibáñez-Justicia A, van der Jeugd H, van Turnhout CAM, Stroo A, et al. Spatial risk analysis for the introduction and circulation of six arboviruses in the Netherlands. Parasit Vectors. 2020;13(1):464. 10.1186/s13071-020-04339-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.