Abstract
Purpose of Review
Latin America is the scenario of great inequalities where about 32 million human beings live with diabetes. Through this review, we aimed at describing the current state of the prevalence, awareness, treatment, and control of diabetes mellitus and completion of selected guidelines of care across Latin America and identify opportunities to advance research that promotes better health outcomes.
Recent Findings
The prevalence of diabetes mellitus has been consistently increasing across the region, with some variation: higher prevalence in Mexico, Haiti, and Puerto Rico and lower in Colombia, Ecuador, Dominican Republic, Peru, and Uruguay. Prevalence assessment methods vary, and potentially underestimating the real number of persons with diabetes. Diabetes unawareness varies widely, with up to 50% of persons with diabetes who do not know they may have the disease. Glycemic, blood pressure, and LDL-C control and completion of guidelines to prevent microvascular complications are not consistently assessed across studies, and the achievement of control goals is suboptimal. On the other hand, multiple interventions, point-of-care/rapid assessment tools, and alternative models of health care delivery have been proposed and tested throughout Latin America.
Summary
The prevalence of diabetes mellitus continues to rise across Latin America, and the number of those with the disease may be underestimated. However, some local governments are embedding more comprehensive diabetes assessments in their local national surveys. Clinicians and public health advocates in the region have proposed and initiated various multi-level interventions to address this enormous challenge in the region.
Keywords: Latin America, Diabetes prevalence, Diabetes treatment, Control, Diabetes complications, Interventions, Health care
Introduction
Within the last couple of decades, non-communicable diseases (NCDs) have gained worldwide attention, especially in low- and middle-income countries (LMIC), where they have been increasingly recognized and prevalent [1, 2]. Among the NCDs, diabetes mellitus has become a global health challenge [1, 3, 4]. Type 2 diabetes mellitus—the most common form of diabetes—due to its rather silent disruption may be a current uninvited companion to over 465 million persons worldwide. In 2019, it was estimated that the number of persons with diabetes in Latin America (LatAm) was 31.6 million [5, 6] and is predicted that by 2030, the number will increase to 40.2 million, and to 49.1 million by 2045 [6].
Because of its multi-organ and multi-system impact, diabetes has been associated with both acute and long-term complications that affect not only health care needs and costs but also wellbeing and productivity [7, 8]. Within the last decade, it has also been recognized as one of the leading causes of death in some LatAm countries [9–15] and an important risk factor for cardiovascular diseases (CVD), which is the leading cause of death in LatAm [14, 16].
Far from being a monolithic group, the LatAm population is highly heterogeneous, with various populations reflecting diverse genetic ancestry, ethnicity, culture of origin, sociopolitical contexts, environmental exposures, and beliefs and practices [17, 18]. Levels of inequality in LatAm remain among the highest in the world [19–22]. All these factors—coupled with biological susceptibility, income, education, access health care, cultural influences on nutrition, health, self-image, and self-care—influence the development of diabetes in LatAm.
We conducted a review of the most current publications on the state of prevalence, awareness, treatment, and control of diabetes mellitus across LatAm. By laying out a detailed accounting of what is known, we aim to identify population, clinical, and health care needs, and opportunities for future research studies and potential interventions.
Literature Search and Review
We conducted the search using the PubMed electronic database as the primary scientific literature source. LatAm was defined as the countries in the Western hemisphere which were previously colonized by Spain, Portugal, or France. A combination of keywords was used to define the scope of the searches: diabetes prevalence, awareness, treatment, control, guidelines of care, adherence, retinopathy, nephropathy, neuropathy, foot care, fundoscopic exam, and urine albumin, and searched under LatAm and by each individual country. Hispanics/Latinos living in the USA were not included in the search.
We limited the search to publications since 2000 to reflect the most recent research on the prevalence of diabetes across LatAm countries, assessments of awareness, treatment, and control of diabetes (glycemic control), blood pressure and low-density lipoprotein cholesterol (LDL-C), and adherence to guidelines for care recommended by the American Diabetes Association (ADA) [23–25] and the Latin American Diabetes Association (ALAD) [26], and specifically hemoglobin A1c (HbA1c) measurement, fundoscopic exam, foot exam, and urine albumin excretion test. We included literature written in English, Spanish, French, and Portuguese.
In addition to PubMed, when available, we manually searched each country’s Ministry of Health and the Pan American Health Organization (PAHO) websites and accessed published and downloadable national health surveys performed during the selected timeframe. Since most available studies did not distinguish between type 1 and type 2 diabetes mellitus, our review is centered on diabetes mellitus (diabetes, henceforth) in general. Because their specific mechanisms of disease and clinical implications, gestational diabetes mellitus, and type 1 diabetes merit separate reviews.
Prevalence of Diabetes Mellitus in Latin America
The earliest contemporary reports on the prevalence of diabetes mellitus among adults throughout LatAm date from the 1950s and 1960s [27–29], when most countries were beginning to experience epidemiologic transitions [30, 31]. In 2001, Barceló reported an incidence of type 1 diabetes in LatAm in the range 0.1 cases/100,000 in Venezuela to 17.4 cases/100,000 in Puerto Rico [32]. However, the authors highlighted a handful of reports on the prevalence of type 2 diabetes and underlined the near absence of surveillance for the disease throughout the LatAm region [32].
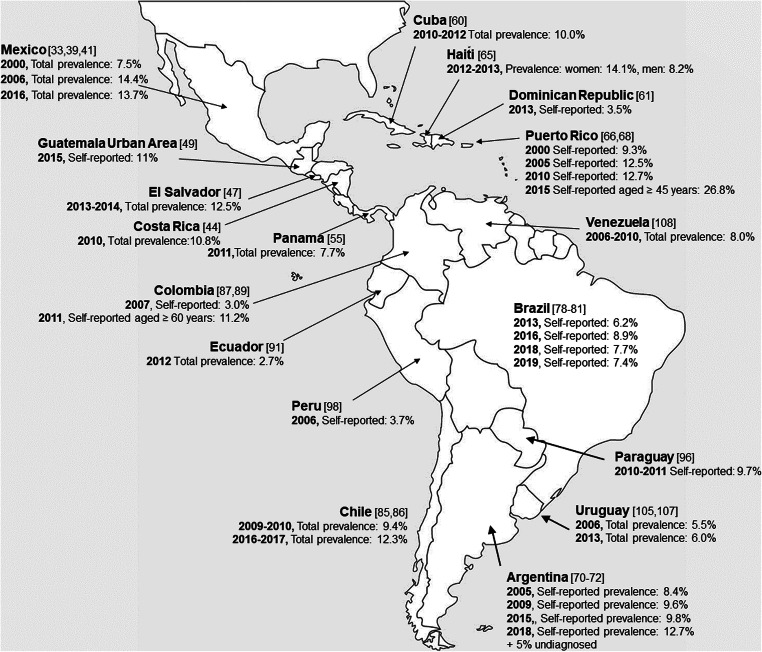
From 2005 to 2020, the prevalence of diabetes mellitus across LatAm has been assessed within individual countries and through multinational studies [33–114] and ranged between 3 and 36.3% (Fig. 1, Table 1). In our review, some national surveys assessed the prevalence of diabetes via population representative samples [33, 36, 38, 40, 42–44, 47, 49, 55, 60, 61, 65, 66, 68, 72, 85–87, 89, 91, 96, 98, 105, 107] and used similar population sampling methods (e.g., multi-stage, clustered, probabilistic sampling), whereas other studies focused on specific geographic regions or communities [34, 35, 37, 45, 46, 48–54, 56–58, 63, 67, 73–75, 77, 82–84, 92–95, 102, 110–112], recruited participants from clinical settings [51, 59, 62, 69, 95], or focused on specific age groups [37, 42, 56–58, 75, 109, 112]. Also, the age range of the population surveyed—and consequently, age-adjustment estimates—varied among surveys.
Fig. 1.
Prevalence of diabetes mellitus in Latin America Based on national surveys from 2000 to 2020. Prevalence data was extracted from national surveys, when available. Prevalence was estimated either by self-report of diabetes exclusively or in combination with glycemic tests. References next to each country’s name in [brackets]
Table 1.
Prevalence of diabetes mellitus across Latin America based on reports published from 2005 to 2020
| Author (reference) | Study period | Place (study name, if available) | Type of study | Sample characteristics | Glycemic criteria | Diabetes prevalence and other key findings |
|---|---|---|---|---|---|---|
| Mexico | ||||||
| Olaiz-Fernandez, 2007 [33] | 1999–2000 | Mexico | National Health Survey - Encuesta Nacional de Salud 2000 (ENSA 2000)) |
N = 45,294 (52% women) Age ≥ 20 years |
Self-report and capillary fasting glucose levels ≥ 126 mg/dL or random ≥ 200 mg/dL |
Total prevalence: 7.5%, of which 77.3% was self-reported Men: 7.2% (5.5% self-reported) Women: 7.8% (6.2% self-reported) Increased with age urban: 8.1%; rural: 6.5% Inverse relationship to educational attainment and income Geographic differences > in the North and lower in the South |
| Meaney, 2007 [34] | 2001–2002 | Mexico (Factores de Riesgo en México –FRIMEX) | Volunteer sample recruited via mobile clinics near public places in Monterrey, Tijuana, Guadalajara, Mexico City, Puebla, and León |
N = 140,017 (58% women) Age ≥ 18 years |
Fasting blood glucose ≥ 126 mg/dL or random ≥ 200 mg/dL Self-report and medication intake |
Total prevalence of diabetes was 10.5%.. Prevalence by sex and self-reported diabetes was not reported. |
| Stoddard, 2011 [35] | 2002 | Mexico (Mexican Family Life Survey) |
Secondary analysis Stratified multi-stage sampling |
N = 19,577 (53% women) Age ≥ 20 years |
Self-report |
Prevalence of diabetes among indigenous participants was 6%, and among non-indigenous participants was 9%. Indigenous participants were 3 times more physically active and reported half of the prevalence of smoking than non-indigenous participants but lived in settings with more fragile infrastructure. |
| Secretaría de Salud de México, Instituto Nacional de Salud Pública (INSP), 2006 [36] | 2006 | Mexico (National Health and Nutrition Survey - ENSANUT 2006) | Probabilistic, poly-stage, stratified and clustered sampling. | N = 1476 households (planned) (52.1% women) Age: all | Self-report and blood tests |
Prevalence (self-report): 7.0% Laboratory test results not available men: 6.5%, women: 7.3% Increased with age No information on urban-rural differences |
| Kumar, 2016 [37] | 2012 | Mexico (Mexican Health and Aging Study) |
Sub-analysis Cross-sectional data from the 2012 cohort. Participants recruited in four Mexican states with different urban/rural concentration, U.S.-Mexico migration patterns and diabetes prevalence |
N = 2012 (sex breakdown not reported) Age ≥ 50 years |
Self-report and HbA1c ≥ 6.5% |
Prevalence of self-reported diabetes: 21.4% S Undiagnosed diabetes: 18%. Participants living in a high US migration state had decreased odds of prediabetes and undiagnosed diabetes. |
|
Secretaría de Salud de México, Instituto Nacional de Salud Pública (INSP), 2012 [38] |
2012 | Mexico [Encuesta Nacional de Salud y Nutrición (ENSANUT 2012)] | National Health and Nutrition Survey Stratified probabilistic sampling | N = 46,303 (52.7% women) age ≥ 20 years | Self-report and blood tests |
Prevalence (self-report): 9.2% (blood test results not available) Men: 8.6% Women: 9.7% Increased with age Urban |
| Bello-Chavolla, 2016 [39] | 1993–2012 | Mexico | Review of four cycles of the National Health and Nutrition Survey “ENSANUT” | NA | Self-report and detected during the examination |
Increasing total prevalence: 1993: 6.7%, 2000: 7.5%, 2006: 14.4%; incomplete data for 2012 In 2006: 7.1% self-reported, and 7.3% newly diagnosed In 2006, higher prevalence in urban areas (15.5%) compared with rural areas (10.3%) |
| Secretaría de Salud de México, Instituto Nacional de Salud Pública (INSP), 2016 [40] | 2016 | Mexico [Encuesta Nacional de Salud y Nutrición de Medio Camino (ENSANUT-MC 2016)] |
National Health and Nutrition Survey Stratified probabilistic sampling |
N = 29,795 (51.1% women) all ages | Self-report and blood tests | Self-reported: 9.4% (blood tests not available) greater in women than in men. Regional variation. |
| Basto-Abreu, 2020 [41] | 2016 | Mexico (ENSANUT-MC 2016) | Secondary data analysis of 3700 participants with diabetes | N = 3700 (52.6% women) | Self-report and/or fasting blood glucose ≥126 mg/dL and HbA1c ≥ 6.5% |
Total prevalence = 13.7% Self-reported: 9.5%, undiagnosed: 4.1% |
| Central America | ||||||
|
Brenes-Camacho, 2007 [42] Brenes-Camacho, 2008 [43] |
2004–2006 | Costa Rica [Costa Rica: Estudio de Longevidad y Envejecimiento Saludable (CRELES)] | Nationally representative sample |
N = 3000 (sex breakdown not reported) Age ≥ 60 years |
Self-report, intake of antihyperglycemic medications and/or fasting blood glucose ≥ 126 mg/dL and HbA1c ≥ 6.5% |
Total prevalence = 23.4% (women = 27.5%; men = 18.8%) Self-reported = 21%, Undiagnosed = 2.4% among those with diabetes: 95.7% participants had health insurance 61.2% were women 54% lived in the metropolitan capital city area |
| Wong-McClure, 2015 [44] | 2010 | Costa Rica (Costa Rican National Cardiovascular Risk Factors Surveillance System) | Probabilistic sampling | N = 3653 (men = 1023; women = 2630) Age ≥ 20 years |
Previously diagnosed = self-report, use of insulin, or hypoglycemic oral treatment in past 2 weeks. Unknown diabetes = no self- report with fasting blood glucose >125 mg/dL |
Total prevalence 10.8% (women = 11.9%; men = 9.5%) Self-reported = 9.5% Undiagnosed = 1.3% prevalence increased with age. Over 75% participants had less than high school education. |
| Orantes, 2011 [45] | 2009 | El Salvador (Nefrolempa Study) |
Assessment of risk factors for chronic kidney disease (CKD) Communities represented in this study are mostly poor and primarily work in agriculture. |
N = 775 (men = 343; women = 432) Age ≥ 18 years |
Self-report or fasting blood glucose ≥ 126 mg/dL | Total prevalence = 10.3% |
| Orantes Navarro, 2015 [46] | 2009–2011 | El Salvador (Study related to the Nefrolempa Study) | Assessment of risk of CKD in women from low-income three agricultural communities |
N = 1412 all women Aged ≥18 years |
Self-report and fasting plasma glucose ≥126 mg/dL | Total prevalence = 9.3% |
| Ministerio de Salud de El Salvador, 2015 [47] | 2013–2014 | El Salvador (Encuesta Nacional de Enfermedades Crónicas No Transmisibles y Factores de Riesgo en la Población Adulta de El Salvador. ENECA-ELS 2015) |
National Survey Two-stage probabilistic sampling (STEPS) |
N = 4817 (56.4% women) Age ≥ 20 years |
Self-report and fasting plasma glucose ≥ 126 mg/dL |
Overall: 12.5% Men: 10.6%, women: 12.5% Prevalence increased with age and was higher in the metropolitan area (San Salvador). |
| Chen, 2017 [48] | 2012–2013 | Guatemala (Non-Communicable Disease Surveillance Study in Santiago de Atitlán) |
Indigenous populations Simple random sampling |
N = 350 (72.3% women) Age not specified | Self-report and fasting blood glucose |
Prevalence = 3% (1.3% previously known, 1.7% previously unknown) Despite high rates of poverty, hypertension, and dyslipidemia, there was a low rate of diabetes compared to other regions of the country. |
| Ministerio de Salud Pública y Asistencia Social de Guatemala, 2018 [49] | 2015 | Guatemala (Encuesta Nacional de Prevalencia de Enfermedades No Transmisibles y sus Factores De Riesgo Dominio I: Urbano Metropolitana) |
National Survey – Metropolitan Area only Random, stratified sampling (STEPS) |
N = 2036 (77.4% women) Age ≥ 18 years |
Self-report; fasting CBG ≥ 110 mg/dL |
Self-reported prevalence = 11.3% Women: 9.5% diagnosed within last 12 years (total 12.5%) Men: 7% diagnosed within last 12 years (9.9% total) |
| Bream, 2018 [50] | 2018 | Guatemala |
Geographic-randomized Focus on indigenous populations in the rural highland region of Atitlán. |
N = 400 (69.1% women) Age ≥ 18 years |
FBG > 7.0 mmol/L (≥ 126 mg/dL) HbA1c > 6.5% |
Total: 13.81% (women = 14.56%; men = 12.20%) Prevalence increased with age, but not BMI (kg/m2). |
| Montalván Sánchez, 2020 [51] | 2016–2017 | Honduras |
CVD burden in Copán First study on CV Risk Factors in Western Honduras Random volunteer-based, cross sectional descriptive study Attending both private and public medical institutions in the Department of Copán |
N = 384 (62% women) Age: 45–75 years |
Self-report and taking meds; fasting blood glucose >125 mg/dL |
Self-reported diabetes: women = 22.1% and men =19% (overall 21%) 6.7% with abnormal blood glucose without a previous diagnosis of diabetes. |
| Laux, 2012 [52] | 2007–2009 | Nicaragua |
Study on the prevalence of diabetes and hypertension in one urban and five rural communities in Nicaragua Five communities in the northwest (Leon and Chinandega) and one community in central Nicaragua (Matagalpa) |
N = 1355 (56.5% women) Age: 20–60 years |
Self-report or glucosuria ≥ 100 mg/dL, uncontrolled diabetes solely diagnosed as glucosuria > 100 mg/dL |
Total prevalence = 3.0% (40/1355); 33 (82.5% women). Prevalence in persons with normal blood pressure = 1.6% Prevalence in persons with hypertension = 7.7% |
| Lebov, 2015 [53] | 2010–2011 | Nicaragua (León Health and Demographic Surveillance System) | Randomly selected 50 of 208 pre-defined geographical clusters | N = 3000 (57.6% women) Age: 18–70 years | Self-report and? |
Total prevalence = 7.2%; 5% of the total (69.4% of those with diabetes) reported previous history of diabetes. Most of the participants lived in poverty. |
| Ferguson, 2020 [54] | 2012–2014 | Nicaragua |
Study of CVRF in Southwestern Nicaragua Department of Rivas agricultural communities |
N = 1227 (533 households) (56.3% women) Age range 17.4–101.8 years |
Venous blood samples obtained, but unclear if blood glucose measurements used for assessment | Overall prevalence, based on self-report = 7% |
| McDonald Posso, 2013 [55] | 2010–2011 | Panama [Primera Encuesta de Factores de Riesgo de Enfermedad Cardiovascular (PREFREC)] |
Diabetes sub-analysis Single-stage, probabilistic and randomized sampling Provinces of Panama and Colon, 5 health regions and city of Panama |
N = 1074 men and 2516 women Age ≥ 18 years |
Self-report or FBG >126 mg/dL or HbA1c ≥ 6.5% (≥ 48 mmol/mol) |
7.3% self-reported having diabetes and 2.2% were not aware of having diabetes; hence the estimated prevalence was 9.5%. The age-adjusted rate for the 2012 Panamanian population was 7.7%. Non-adjusted prevalence: 10.3% in men and 9.1% in women. Prevalence increased with age. Highest prevalence among Afro-Panamanians (11.9%), and lowest among indigenous (5.4%). |
| Caribbean | ||||||
| Da Silva Coqueiro, 2010 [56] | 1999–2000 | Cuba |
Subsample of participants from Cuba in the National Survey of Health, Wellbeing, and Aging - Encuesta Nacional de Salud, Bienestar, y Envejecimiento (SABE) study Probabilistic sampling in Havana |
N = 1905 (62.8% women) Age ≥ 60 years | Self-report |
Total prevalence based on self-report = 14.8% Diabetes was not associated with overweight. |
| De Jesús Llibre, 2011 [57] | Two phases: 2003–2006 and 2007–2010 | Cuba (10/66 Study recruited in Havana City and Matanzas) |
Sub-sample analysis First-stage sampling five municipalities in Havana City Province and the city of Matanzas |
N = 2944 (64.7% women) Age ≥ 65 years | Self-report (history and medications) and fasting glucose ≥7.0 mmol/L |
Overall prevalence = 24.8% Prevalence in women = 27.5% Prevalence in men = 19.4% |
| Herrera-Valdés, 2008 [58] | 2004–2006 | Cuba [Community-Based Epidemiological Study of Chronic Kidney Disease, Cardiovascular Disease, Diabetes Mellitus and Hypertension (ISYS)] |
“Isle of Youth Study” Focus on prevalence of obesity and its association with other conditions |
N = 14,322 (sex breakdown not reported) Age ≤ 60 years |
Self-report and laboratory tests |
Prevalence of diabetes in individuals aged ≤ 20 years: 1.3–9.5% for obese and 1.1% for non-obese Prevalence of diabetes in individuals aged ≥ 20 years: overall 4.7% in non-obese and 11.3% in obese persons. Prevalence ranged from 5.5 to 21%, by age group |
| Armas Rojas, 2008 [59] | 2006 | Cuba |
CV risk among older women in the Havana area Cross-sectional, catchment area served by the Mártires del Corynthia Polyclinic in Havana Single-stage clustering Family doctor and nurse offices selected randomly |
N = 3396 women Age ≥ 60 years |
Self-report and on treatment | Overall = 21.8% |
| Bonet-Gorbea, 2014 [60] | 2010–2011 | Cuba (III Encuesta Nacional de Factores de Riesgo y Actividades Preventivas de Enfermedades No Transmisibles. Cuba 2010–2011) |
National Health Survey Clustered, multi-stage, stratified |
N = 7928 persons, from 4150 households (50.3% women) Age ≥ 15 years |
Self-report and fasting blood glucose (≥ 7.0 mmol/L) |
Total prevalence: 10.0% (6.1% self-reported) Women: 12.9%, Men: 7.2% Urban: 11.1%, rural: 6.8%; Self-reported: 7.1% urban, and 3.0% rural Undiagnosed: 4.2% (4.3% urban, 3.9% rural) Prevalence based on skin color: “negra” (12.3%), “blanco” (10.2%) and “mulato” (8.6%) |
| Ministerio de Salud Pública de la República Dominicana, 2014 [61] | 2013 |
Dominican Republic (Encuesta Demográfica y de Salud - República Dominicana 2013) |
National Health Survey Nationally representative, probabilistic, clustered, stratified and two-stage. |
N = 39,564 (19,878 women) Age: women 15–49 years Age: men 15–59 years |
Self-report | Prevalence: 3.5% (4% women and 3% men self- reported diagnosis of diabetes)[ |
| Carrère, 2017 [62] | 2014 | Guadeloupe |
Cross-sectional multicenter study Persons undergoing a periodic health examination on invitation from the general social security fund of Guadeloupe (CGSS). |
N = 2252 (56.5% women) Age: 18–74 years | Self-report of antihyperglycemic treatment use, fasting blood glucose ≥ 7 mmol/L (≥ 126 mg/dL), HbA1c ≥ 6.5% |
Total prevalence: women 8.2%, men 5% Previously diagnosed: 6.7% in women, 3.3% in men. Higher prevalence among those with lower education. |
| Jean-Baptiste, 2006 [63] | 2002–2003 | Haiti (Prevalence of Diabetes and Hypertension in Haiti- PREDIAH) |
Population-based survey Two-stage cluster method; representative sample of Port-au-Prince, and six surrounding cities |
N = 1620 (331 men, 782 women) Age ≥ 20 years |
Casual Blood Glucose 80 mg/dL (4.4 mmol/L) Fasting blood glucose ≥ 126 mg/dL (7 mmol/L), and 2-h- post glucose load (OGTT) ≥ 200 mg/dL (11.1 mmol/dL) |
Age-standardized prevalence was 4.8% in men and 8.9% in women (77.3% men and 69.2% women known diabetes) Odds of diabetes were greater with age, abdominal obesity, hypertension, lower educational attainment, and higher income. |
| Burkhalter, 2014 [64] | 2012–2013 | Haiti | Single -enter, prospective study in Deschapelles; assessment of prevalence of CKD and associated risk factors |
N = 608 patients with full medical datasets (64.5% women) Age ≥ 18 years |
Binary data – physician answered yes/no |
Prevalence diabetes = 36.3% The authors explain that the high prevalence of diabetes may be due to selection bias. |
| Ministère de la Santé Publique et de la Population, 2018 [65] | 2016–2017 | Haiti [Enquête Mortalité, Morbidité et Utilisation des Services (EMMUS-VI)] | Random sampling, two-stage, stratified | N = 14,371 women aged 15–49; 9795 men aged 15–64, 1142 women 50–64, and 2091 men 35–64 | Hemoglobin A1c > 6.5% |
In the 35–64 age group, the prevalence of diabetes based on HbA1c > 6.5% was: 14.1% in women and 8.2% in men. Previously informed of having “hyperglycemia”: 3% of women and 2% of men. Prevalence Urban (Women 17%, men 12%) Prevalence Rural (Women 11%, men 7%) Prevalence increased with “bien-être économique du menage”. Due to the high prevalence of iron-deficiency anemia, HbA1c may have been elevated. |
| Geiss, 2012 [66] | 1995–2010 | Puerto Rico [Behavioral Risk Factors Surveillance Systems (BRFSS)] | Sub-analysis based on four cycles Random-digit-dialed telephone surveys of noninstitutionalized US civilian adults aged ≥18 years |
N = Not reported Age ≥ 18 years |
Self-reported only | Prevalence (age-adjusted for adults aged ≥18 years) 1995: 11.7%, 2000: 9.3%, 2005: 12.5%, and 2010: 12.7% |
| Pérez, 2015 [67] | 2005–2007 | Puerto Rico | Household survey in San Juan metropolitan area |
N = 857 (65.7% women) Age: 21–79 years |
Self-report and/or FPG ≥ 126 mg/dL and HbA1c ≥ 6.5% |
Age-standardized: total 25.5% (11.4% undiagnosed) 89% had health insurance; 67.2% with annual income < $20 K They compared prevalence using FPG alone or combination of FPG and HbA1c to detect undiagnosed diabetes. FPG + HbA1c yielded a higher percentage than either one alone. |
| Pickens, 2019 [68] | 2015 | Puerto Rico (2015 BRFSS) | As described above |
N = 3642 (sex breakdown not reported) Age ≥ 45 years |
Self-report only | Aged-adjusted prevalence in adults aged ≥ 45 years was 26.8% |
| Cruz, 2016 [69] | 2014 | Puerto Rico | Analysis of surgical cases from various hospitals in the San Juan metropolitan area |
N = 2603 surgical patients (56% women) Age: all |
Medical records | Prevalence = 21% but increased to 40% in patients aged ≥ 65 years. Statistically significant greater percent of complications and mortality for patients with diabetes. |
| South America | ||||||
| Ministerio de Salud de Argentina, 2011 [70] | 2009 | Argentina (2nda Encuesta Nacional de Factores de Riesgo para Enfermedades No Transmisibles) | Second National Survey on Risk Factors for Non-Communicable Diseases- 4-stage, probabilistic, clustered sampling of 24 jurisdictions |
N = 34,732 (No sex breakdown) Age ≥ 18 years |
Self-report only |
Self-report of diabetes and/or elevated blood glucose = 9.6% (an increase from 8.4% in 2005). Prevalence of diabetes in women = 10.2%, and in men = 8.9%. Prevalence of diabetes increased with age and with lower educational attainment. |
| Ministerio de Salud de Argentina, 2015 [71] | 2013 | Argentina (3ra Encuesta Nacional de Factores de Riesgo para Enfermedades No Transmisibles) | Third National Survey on Risk Factors for Non-Communicable Diseases 4-stage, probabilistic, clustered sampling | N = 32,365 (52.6% women) Age ≥ 18 years | Self-report only |
Prevalence = 9.8% with no sex differences Prevalence of diabetes increased with age. |
| Ministerio de Salud de Argentina, 2019 [72] | 2018 | Argentina (4ta Encuesta Nacional de Factores de Riesgo para Enfermedades No Transmisibles) |
Fourth National Survey on Risk Factors for Non-Communicable Diseases 4-stage, probabilistic, clustered sampling |
Three steps sampling and exam: Step 2: 16,577 and for Step 3: 5331 (No sex breakdown) Age ≥ 18 years |
Self-report and fasting blood glucose (CBG ≥ 110 mg/dL) |
Prevalence: 12.7% based on self-report (women: 13.7%, men: 11.6%) In addition, 5% who did not report having diabetes had CBG ≥ 110 mg/dL. Diabetes prevalence increased with age, and with lower educational attainment. |
| Barceló, 2001 [73] | 1998 | Bolivia | Population-based survey of households in four urban areas: La Paz, El Alto, Santa Cruz, Cochabamba |
N = 2948 adults (1036 men; 1497 women) Age ≥ 20 years |
Fasting blood glucose ≥ 126 mg/dL and OGTT |
Total prevalence = 7.2% Greater prevalence among those with more limited education. Greater prevalence among Aymara-speaking participants. |
| Kaplan, 2017 [74] | 2014–2015 | Bolivia | Assessment of CAD in the Tsimane population of Bolivia (Maniqui River) |
N = 705 (sex breakdown not specified) Age ≥ 40 years |
Fasting blood glucose > 6.9 mmol/L | Prevalence was almost zero. Other CV assessments revealed low to negligible presence of CAC, and other CV risk factors. |
| Busch Mendes, 2011 [75] | 2003 | Brazil |
São Paulo Probabilistic sampling, two-stage |
N = 842 (406 men; 436 women) Age ≥ 60 years |
Self-report |
Prevalence: 26.31% (15.54% in men, 18.89% in women) Inverse relationship with educational attainment |
| Schmidt, 2014 [76] | 2008–2010 | Brazil [Estudo Longitudinal da Saúde do Adulto (ELSA- Brasil)] | Prospective cohort study of active or retired civil servants |
N = 15,102 (6685 men, 8217 women) Age: 35–74 years |
Self-report Fasting plasma glucose ≥ 126 mg/dL, 2 h-OGTT ≥ 200 mg/dL or HbA1c ≥ 6.5% |
Prevalence (by self-report or medication use): 19.7% Percent undiagnosed: 50.4% of the total Higher prevalence of diabetes among those with less than primary education, Asian, black, and indigenous participants. |
| Dal Fabbro, 2014 [77] | 2008–2012 | Brazil | Descriptive study on health of Xavante Indians from Mato Grosso | N = 948 (463 men; 485 women) Age ≥ 20 years | Capillary sample, although venous samples were obtained for other biomarkers; HbA1c | Total age-adjusted: 28.3% (18.4% in men, 40.6% in women) |
| Ministerio do Planejamento, Orçamento e Gestão, 2014 [78] | 2013 | Brazil (Pesquisa Nacional de Saúde -PNS 2013) | Brazilian National Health Survey Random, clustered, three-stage sampling | N = 62,986 households (no sex breakdown) Age ≥ 18 years | Self-report; HbA1c was tested, but results not presented in this report |
Prevalence based on self-report = 6.2% (7.0% in women, 5.4% in men) Prevalence increased with age and with lower educational attainment. |
| de Oliveira, 2018 [79] | 2006–2016 |
Brazil [Surveillance Systems of Risk and Protection Factors for Chronic Diseases by Telephone Survey (Vigitel)] |
Secondary analysis National Telephone Survey Quantitative review |
N = 572,437 adults (sum of all years) (no sex breakdown) Age ≥ 18 years |
Self-report |
Prevalence (2016): 8.9% Increased from 5.5% in 2006 Higher prevalence among women, with lower income, and with lower education. |
| Ministério da Saúde do Brasil, 2019 [80] | 2018 | Brazil Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico (VIGITEL BRASIL 2018) |
National Telephone Survey Capital cities of each of the 26 states and the Federal District, land lines Random, stratified |
N = 52,395 19,039 men, 33,356 women Age ≥ 18 years |
Self-report only |
Prevalence self-reported: 7.7% (8.1% in women, 7.1% in men) Diabetes prevalence Increased with age. |
| Ministério da Saúde do Brasil, 2020 [81] | 2019 | Brazil Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico (VIGITEL BRASIL 2019) | National Telephone Survey |
N = 52,443 18,354 men, 34,089 women Age ≥ 18 years |
Self-report only | Prevalence self-reported: 7.4% (7.8% in women, 7.1% in men). Diabetes prevalence Increased with age. |
| Santos, 2001 [82] | 1997 | Chile | Aymara in Northern Chile living in rural areas in the highlands |
N = 196 (78 men, 118 women) Age ≥ 20 years |
Fasting blood glucose and 2 h- post glucose load (OGTT) | Prevalence of 1.3% in men and 1.7% in women |
| Carrasco, 2004 [83] | NR | Chile | Mapuche and Aymara living in four urban communities of Santiago and northern Chile; volunteers |
Mapuche (42 men, 105 women) Aymara (42 men, 118 women) Age ≥ 20 years |
Fasting blood glucose and 2-h post glucose load (OGTT) |
Assessed two indigenous groups, Aymara and Mapuche Prevalence among Aymara: 6.9% (2.4% in men, 8.5% in women) Prevalence among Mapuche: 8.2% (14.3% in men, 5.7% in women) |
| Cuevas, 2008 [84] | 1993–2001 | Chile |
Cross-sectional epidemiology study Only from urban sector La Florida in Santiago, Mid socioeconomic level |
N = 964 (336 men, 628 women) Age ≥ 18 years |
Fasting plasma glucose ≥ 126 mg/dL and/or self-reported diagnosis |
Total prevalence in 2001: 10.1% (10.7% women., 8.9% men) The total prevalence of diabetes in 1993 was 3.8%. |
| Ministerio de Salud de Chile, 2010 [85] | 2009–2010 | Chile (Encuesta Nacional de Salud, ENS Chile 2009–2010) | Random sampling of households (multi-stage and stratified), representative of the national, regional, and urban/rural zones, cross-sectional analysis | N = 5, 416 (59% women) Age ≥ 15 years | Self-report and FPG ≥ 126 mg/dL and HbA1c |
Total prevalence = 9.4% (8.4% in men, 10.4% in women) based on self-report and FPG. Diabetes prevalence increased with age and with lower educational attainment. Greatest prevalence in women of the lowest educational level |
| Ministerio de Salud de Chile, 2017 [86] | 2016–2017 | Chile (Encuesta Nacional de Salud, ENS Chile 2016–2017) | National random sampling of households (multi-stage and stratified), representative of the national, regional, and urban/rural zones, cross-sectional analysis | N = 6233 (62.9% women) Age ≥ 15 years | Self-report and FPG ≥ 126 mg/dL |
Total prevalence = 12.3% (10.6% in men, 14.0% in women) Diabetes prevalence increased with age (30.6% in persons aged ≥ 65 years) and with lower educational attainment (24.8% with < 8 years of education) |
| Rodríguez, 2009 [87] | 2007 |
Colombia [Encuesta Nacional de Salud (ENS)] |
National Health Survey Probabilistic, national representative including 41,543 households | N = 164,474 persons (52.5% women) Subsample of those in the 18–69-year age group had additional interviews and exams (glycemia) | Self-report |
Prevalence = 3.0% based on self-report, per Executive Summary |
| Camacho, 2020 [88] | 2005–2009 | Colombia [Prospective Urban Rural Epidemiology (PURE) Study] | Sub analysis of data from Colombia | N = 7485 (64.1% women) Age: 35–70 years | Self-report | Prevalence: 5.7% (6.0% in women, 5.1% in men) Greater prevalence with lower education |
| Profamilia, 2011 [89] | 2010 | Colombia [Encuesta Nacional de Demografía y Salud (ENDS 2010)] | National Health Survey Nationally-representative, in urban and rural settings, probabilistic, clustered, stratified and poly-staged. |
N = 17,574 No sex breakdown Age > 60 years |
Self-report | Prevalence only reported for adults aged ≥ 60 years Self-reported prevalence: 11.2% (12.2% urban, 8.3% rural; 12.8% in women and 9.0% in men) |
| Orces, 2018 [90] | 2010 | Ecuador [National Survey of Health, Wellbeing, and Aging - Encuesta Nacional de Salud, Bienestar, y Envejecimiento (SABE)] | Secondary data analysis Probability sampling in Andes Mountains and coastal regions, multi-stage sampling |
N = 2298 (1041 men, 1257 women) Age ≥ 60 years |
Self-report or FPG ≥ 126 mg/dL |
Prevalence = 16.7% Higher among women, blacks, urban coastal, and obese individuals. Higher in urban coastal areas. |
| Ministerio de Salud Pública de Ecuador [91] | 2012 | Ecuador [Encuesta de Salud y Nutrición del Ecuador (ENSANUT-ECU 2012)] |
National Health and Nutrition Survey Probabilistic, stratified, three-stage, and cluster sampling |
N = 15,916 (49% women) Age: 10–59 years | Self-report and FPG ≥ 126 mg/dL |
Overall prevalence = 2.7% Diabetes prevalence increased with age. No sex differences. Higher prevalence among Afro-Ecuadorian: 3.1% Higher prevalence in urban (3.2%) compared to rural (1.6%) areas. Prevalence was higher in persons from coastal than mountain regions. |
| Tufton, 2015 [92] | 2012 | Ecuador |
Santa Cruz Island, Galápagos Santa Cruz is the main island Diabetes screening program at the main local clinic |
N = 141 (59.6% women) Age ≥ 18 years | Medical history and fasting blood glucose > 126 mg/dL |
Prevalence based on self-report: 16.3% Undiagnosed: 11.3% who had fasting blood glucose > 126 mg/dL |
| Alexander, 2017 [93] | 2014 | Ecuador |
Isabela, Galápagos Secondary data analysis -source unknown |
N = 534 (67% women) Age ≥ 21 years | Fasting blood glucose, postprandial glucose |
Prevalence in persons aged ≥ 50 years: 24% Prevalence in persons aged < 50 years: 8% |
| Bonilla-Sierra, 2020 [94] | 2019 | Ecuador |
Loja, Ecuador 10th most populous town Patients attending health care centers of the Health Ministry of Ecuador or living in the geriatric center |
N = 283 (130 women) Age ≥ 60 years |
Self-report | Total prevalence = 28.27% |
| Chaves, 2015 [95] | 2006–2013 | Paraguay [Asunción, Modificación de Factores de Riesgo Cardiovascular – (AsuRiesgo)] |
Urban area of Asunción In-hospital and outpatient clinic patients, in waiting rooms invited to participate. Single-center, prospective study |
N = 18,287 (67.5% women) Ages ≥ 18 years | Self-report and fasting blood glucose | Overall Prevalence: 13.3% (14% in women, 11.8% in men) |
| Ministerio de Salud Pública y Bienestar Social de Paraguay, 2012 [96] | 2010–2011 | Paraguay (Primera Encuesta Nacional de Factores de Riesgo de Enfermedades No Transmisibles en Población General) | First National Health Survey Probabilistic, three-stage sampling |
N = 2538 (49.4% women) Ages: 15–74 years |
Self-report | Overall: 9.7% (women 11.1%, men 7.9%) Increased with age |
| Segura-Vega, 2006 [97] | 2004 | Peru (TORNASOL I) | Cross-sectional, random sampling in 26 cities across the whole country | N = 14,826 (50.5% women) Age ≥ 18 years | Self-report |
Overall = 3.3% self-reported, with no lab assessment performed. Higher prevalence in men. Lower prevalence in the highlands. Prevalence increased with SES and having health insurance. |
| Ministerio de Salud de Perú, 2006 [98] | 2005 | Peru [Encuesta Nacional de Indicadores Nutricionales, Bioquímicos, Socioeconómicos y Culturales Relacionados con las Enfermedades Crónico Degenerativas (ENINBSC-ECNT 2005)] |
National Survey Stratified and clustered sampling |
N = 4206 (50.1% women) Age ≥ 20 years | Blood glucose ≥ 100 mg/dL with self-report, random ≥ 200 mg/dL with no previous history, or taking diabetes medications |
Previous diagnosis: 3.7% Unaware: 2.8% Higher prevalence in men and with increasing age. Higher diabetes prevalence in metropolitan area Lima (6%) and lowest in the Sierra Urbana (0.9%) |
| Miranda, 2011 [99] | 2007–2008 |
Peru (PERU MIGRANT) |
Cross-sectional survey of three population-based groups: rural, rural-urban migrants, and urban Single-stage random sampling |
N = 1706 (52.8% women) Age > 30 years | Fasting glucose, HbA1c | A gradient was reported for age-standardized prevalence of diabetes: 0.8% rural, 2.8% rural-to-urban migrants, and 6.3% urban. |
| Segura-Vega, 2013 [100] | 2010–2011 | Peru (TORNASOL II) |
Comparison with first wave Similar sampling and 26 cities |
N = 14, 675 (50.8% women) Age ≥ 18 years |
Self-report |
Diabetes prevalence: 4.4% Prevalence increased with socioeconomic status and having health insurance. |
| Seclen, 2015 [101] | 2010–2012 | Peru (PERUDIAB) | Random cluster sampling of urban and suburban areas |
N = 1677 (sex breakdown not reported) Age ≥ 25 years |
Self-report and fasting plasma glucose ≥ 126 mg/dL History of pharmacological treatment |
7.0% (National), 8.4% in Lima (7. 01% in men, 7.04% in women) Diabetes prevalence was higher in coastal (8%) than in highlands (4%), and significantly higher among those without formal education. |
| Bernabé-Ortiz, 2016 [102] | 2010–2011 | Peru (CRONICAS) | Single-stage random sampling | N = 3135 (48.5% men) Age ≥ 35 years | Fasting blood glucose ≥ 126 mg/dL or self-report and taking meds | Baseline prevalence was 7.1%; 121 new cases in mean 2.4 years. |
| Krishnadath, 2016 [103] | 2013 | Suriname (Suriname Health Study) | Stratified multistage cluster sample of households |
N = 3393 (48.5% men) Age: 15–65 years |
Fasting blood glucose ≥ 7.0 mmol/L or self-reported diabetes medication use |
Prevalence: 13.0% Highest prevalence for Hindustanis (23.3%). Higher prevalence for lower income. Lower prevalence in rural areas. |
| Minderhoud, 2015 [104] | 2013–2014 | Suriname [The Rapid Assessment of Avoidable Blindness (RAAB)] |
Random clusters Survey; sub-analysis |
N = 2806 689 had diabetes (274 men, 415 women) Age > 50 years |
Previously diagnosed, receiving treatment, random blood glucose of ≥ 200 mg/dL |
Prevalence: 24.6% Highest prevalence for Hindustanis and urban dwellers |
| Ministerio de Salud Pública de Uruguay, 2007 [105] | 2006 | Uruguay (Primera Encuesta Nacional de Factores de Riesgo de Enfermedades Crónicas No Transmisibles - ENFRECNT) |
National Health Survey Multi-stage Cluster stratification Representative sampling of urban areas |
N = 2008 (1324 women) Age: 25–64 years |
Self-report; fasting blood glucose ≥ 110 mg/dL |
Total prevalence: 5.5% Men = 6.2%, Women = 4.7% No sex differences |
| Fort, 2012 [106] | 2008–2011 | Uruguay | CVRF assessment of national health insurance card applicants Cross-sectional, electronic records |
N = 74,420 patients (51% women) Age ≥ 15 years |
Self-report and/or fasting blood glucose > 125 mg/dL |
Prevalence in men: 2.4–20.2% (6.8%) Prevalence in women: 1.5–14.3% (6.1%) |
| Ministerio de Salud Pública de Uruguay, 2014 [107] | 2013 | Uruguay (Segunda Encuesta Nacional de Factores de Riesgo de Enfermedades No Transmisibles – ENFRENT) |
National Health Survey Representative sampling of urban areas Cluster stratification |
N = 3204 (1539 women) Age: 15–64 years |
Self-report and taking meds; fasting blood glucose ≥ 126 mg/dL |
Total prevalence: 6.0% (25–64, men 7.4%, women 7.8%; 55–64 = 16.8%) Undiagnosed: 50.2% Non-diagnosed and non-treated 66.3% men, 30.7% women, overall 48.9% |
| Nieto-Martínez, 2018 [108] | 2006–2010 | Venezuela [Venezuela Metabolic Syndrome, Obesity and Lifestyle Study (VEMSOLS)] | Multi-stage stratified random sampling Andes, Western and Capital District |
N = 1334 (men = 419, women = 915) Age ≥ 20 years |
Self-report and blood samples (plasma glucose) | Age-adjusted prevalence = 8.0% Higher among men |
| Multinational studies | ||||||
| Menéndez, 2005 [109] | 2000–2001 | Argentina, Cuba, Mexico, Uruguay, Chile and Brazil [National Survey of Health, Wellbeing, and Aging- Encuesta Nacional de Salud, Bienestar, y Envejecimiento (SABE)] |
Sub-analysis Multi-stage probabilistic sampling in capital cities |
N = 10,891 (58.9–65.7% women across sites) Age ≥ 60 years |
Self-report | Buenos Aires: 12.5%, São Paulo: 17.7%, Santiago: 13.3%, Mexico City: 21.9%, and Montevideo: 13.0% |
| Escobedo, 2009 [110] | 2003–2005 |
Venezuela, Colombia, Argentina, Peru, Mexico, Ecuador, and Chile [Cardiovascular Risk Factor Multiple Evaluation in Latin America (CARMELA)] |
Cross-sectional, population-based, observational study. Equiprobabilistic sampling of households; only urban sites |
N = 11,550 (38.58–49.53% men across sites) Age: 25–64 years |
Fasting blood glucose ≥ 7.0 mmol/L or self-reported diagnosis |
Prevalence of DM was 7% (range 4–9%) Weight adjusted: (Barquisimeto: 6.0%, Bogotá: 8.1%, Lima: 4.4%, Mexico City: 8.9%, Quito:5.9%, Santiago:7.2%) Generally higher in women, increasing prevalence with age. |
| Barceló, 2012 [111] | 2003–2006 |
Belize, Costa Rica El Salvador Guatemala Honduras Nicaragua [Central America Diabetes Initiative (CAMDI)] |
Cross-sectional survey of six Central American Populations Probabilistic sampling; it included the entire population of Belize and samples from urban areas in the other countries. |
N = 10,822 (50.2% women) 7234 underwent anthropometry measurement and laboratory tests |
Self-report, fasting blood glucose ≥ 126 mg/dL, 2-h OGTT ≥200 mg/dL |
Belize: 12.9% (men: 8.3%, women: 17.6%) Costa Rica: 8.8% (men: 9.6%, women: 8.0%) El Salvador: 7.6% (men: 8.7%, women: 6.8%) Guatemala: 7.3% (men: 7.8%, women: 6.8%) Honduras: 5.4% (men: 5.5%, women: 5.4%) Nicaragua: 9.8% (men: 9.1%, women: 10.5%) Total prevalence across all sites: 8.5% 40% were undiagnosed. |
| Salas, 2016 [112] | 2003–2009 | Cuba Dominican Republic Puerto Rico Venezuela Peru Mexico (10/66 Dementia Research Group) |
Sub-analysis Population-based studies in 13 catchment areas in six Latin American countries: urban areas in Cuba, Dominican Republic, Puerto Rico and Venezuela, and urban and rural areas in Peru and Mexico |
N = 17, 945 including sites in India, China and Nigeria Age ≥ 65 years |
Self-report and fasting blood glucose > 7 mmol/L BP and TG and TC were also assessed |
Self-report: Cuba: 18.3% (women > men) Dominican Republic: 14.0% (women > men) Peru Urban: 8.7% (men > women) Peru Rural: 10.3% (women> men) Venezuela: 16.2% (no difference) Mexico Urban: 24.9% (no difference) Mexico Rural: 19.2% (women > men) Puerto Rico: 32.2% (men > women) Undiagnosed: Cuba: 5.7% (men > women) Dominican Republic: 3.3% (women > men) Peru Urban: 3.3% (men > women) Venezuela: 4.9% (men > women) Mexico Urban: 2.5% (no difference) Mexico Rural: 4.8% (men > women) Puerto Rico: 11.6% (men > women) |
| Rubinstein, 2015 [113] | 2010–2011 | Argentina, Chile and Uruguay [Centro de Excelencia en Salud Cardiovascular para el Cono Sur I (CESCAS I)] |
4 small- to mid-size cities 4-stage stratified sampling |
N = 7524 men and women Age: 35–74 years |
Fasting blood glucose ≥ 110 mg/dL or taking medications for diabetes | Prevalence diabetes women 14%, men 9.4% (Marcos Paz 11.9%; Bariloche 8.4%, Temuco 14.3%; Barrios Blancos 14.2%) |
| Macincko, 2019 [114] | 2013–2014 | Brazil, Colombia El Salvador, Jamaica, Mexico, and Panama (Inter-American Development Bank’s International Primary Care Survey) |
Sub-analysis National sample adults, noninstitutionalized selected nationwide list of households and interviewed by phone (including mobile phones and landlines); 1500 interviews per country |
N = NR (sex breakdown not reported) Age ≥ 18 years |
Self-report |
19% had diabetes only. In addition to diabetes, six additional chronic conditions were assessed. 30.7% had one additional condition, 25.6% had 2 additional conditions, and 24.8% had 3 or more. |
Most of the studies (especially national surveys) reported the overall prevalence of diabetes without differentiating between type 1 and type 2 diabetes mellitus and many estimated the prevalence of the disease based on self-report (being aware of having diabetes and/or taking antihyperglycemic medications) only. Some national surveys and independent studies estimated the prevalence based on the sum of self-report and identifying individuals without history of diabetes but hyperglycemia within the diabetes range [26, 115]. The latter group was considered to have “suspected,” “undiagnosed,” or “unknown” diabetes. Hyperglycemia within the diabetes range was assessed by measuring fasting blood or plasma glucose (FBG or FPG) only, FBG/FPG and 2-h oral glucose tolerance test (OGTT), FBG/FPG and hemoglobin A1c (HbA1c), HbA1c only, or the combination of FBG/FPG, OGTT, and HbA1c, or glucose levels in urine. Some studies measured capillary blood glucose (CBG), while most studies measured venous blood or plasma glucose. While multiple studies used the ADA/ALAD-recommended glucose/HbA1c cut points for the diagnosis of diabetes [26, 115], some studies used different thresholds (e.g., fasting glucose ≥ 100 mg/dL (per CBG), random blood glucose ≥ 140 mg/dL, or random blood glucose ≥ 200 mg/dL).
Although the differences in the methodology described above limit the ability to perform cross-sectional or trend comparisons among countries, we note several commonalities. During 2005–2020, some countries reported an increase in the prevalence of diabetes [33, 36, 38–40, 66, 70–72, 79, 85, 86], consistent with previously published reviews [5, 15, 32, 116–123]. Compared with the rest of the region, and as previously reported [5, 15, 32, 124, 125], diabetes prevalence varies across the region, with higher prevalence in Mexico (13.7%), Haiti (14.1% in women and 8.2% in men), and Puerto Rico (12.5–12.7% in the population aged 18 ≥ years and 26.8% in the population aged ≥ 45 years), and lower in Colombia (3.0% in the population aged 18 ≥ years, but 11.2% in age group ≥ 60 years), Dominican Republic (3.5%), Ecuador (2.7%), Peru (3.7%), and Uruguay (5.5–6.0%) (Fig. 1, Table 1). Multiple studies reported a greater prevalence of diabetes among women [36, 38, 40, 42, 44, 47, 49–52, 57, 60, 62, 63, 65, 70, 72, 75, 78, 79, 83–86, 90, 95, 96, 110, 113], and with increasing age, especially over age 60 years [33, 36, 44, 47, 50, 55, 69–72, 78, 80, 81, 86, 91, 93, 110]. Some studies reported an inverse relationship between diabetes and socioeconomic status (SES) [33, 79, 103] or educational attainment [33, 44, 62, 63, 70, 72, 73, 75, 76, 78, 79, 86, 101]. Other studies reported a direct relationship between having health insurance and self-reported diabetes [42, 70, 97, 100], implying that persons who have health insurance—proxy of access to health care services—would be aware of their health issues and report them accordingly. This interaction also poses questions about not only the access to health care but also the timeliness and quality of the care, and health literacy (or the lack of) that persons in the lowest SES—and at the highest risk of diabetes—would experience. Some studies reported a lower prevalence of diabetes among indigenous populations [35, 48, 74], with one study proposing that exposure to urbanicity was associated with an increased prevalence of diabetes among some indigenous communities [83]. Indeed, rural to urban migration (or living in rural compared with urban areas) has been associated with increased prevalence or risk of developing diabetes in Peru [126, 127], and multiple countries reported a lower diabetes prevalence in rural compared with urban settings [33, 39, 47, 60, 65, 89–91, 99, 103].
The number of epidemiological studies published since 2005 indicates greater public health awareness about diabetes mellitus across LatAm. Multiple countries have performed at least one national survey on chronic non-communicable diseases in which self-reported diabetes mellitus and/or elevated glycemia has been included (Table 1). Some surveys have also included at least one laboratory test (i.e., fasting or random blood glucose measurement or HbA1c), which could identify individuals at risk of developing diabetes or those who may have it and are not aware of it. Because hyperglycemia may be mediated by at least two mechanisms of disease—increased hepatic glucose output manifested as fasting hyperglycemia and uncoupled postprandial insulin secretion manifested as postprandial hyperglycemia [115, 128]—a single blood test or measurement may not identify all or most of individuals affected by the disease [115]. Therefore, the actual prevalence of diabetes may still be underestimated in many countries, as highlighted in previous reviews [5, 15, 124].
The etiologies of diabetes mellitus are complex. Thus, the increasing prevalence of diabetes experienced across LatAm may reflect the convergence or interaction of multiple factors [18, 125, 129]. For instance, the increasing prevalence of overweight and obesity documented across LatAm has paralleled the increasing prevalence of diabetes in the region [84, 125, 130, 131]. In addition to increased adiposity, type 2 diabetes mellitus and insulin resistance have also been linked to malnutrition (at different life stages) in some LMICs [130, 132–134]. Stress associated with chronic poverty, intergenerational poverty, natural disasters, and other adverse events [1, 129, 132, 135] has been linked to chronic systemic inflammation and epigenetic changes, potential common denominators of multiple NCDs [136, 137]. Many LatAm major cities may be epicenters where a fragile built environment and infrastructure and changes in lifestyle and nutrition intersect increasing the cumulative risk of developing diabetes in low-income communities [30, 126, 127, 129, 135, 138, 139]. Increased life expectancy has been associated with increased diabetes prevalence [4, 16, 30, 125, 140], whereas higher educational attainment, increased access to health care, and higher health literacy level are associated with increased awareness of the disease [117]. These are all factors to consider upon designing comprehensive diabetes prevention and treatment strategies across LatAm countries.
In addition, the growing prevalence of diabetes mellitus across LatAm and the complexity of the disease suggest opportunities to create or strengthen collaborations towards its prevention and early detection [141–144]. For example, multinational and multidisciplinary research–public health–health care policy–clinical care partnerships which already exist in formal or informal platforms may be well-positioned to evaluate the impact of nutrition, health insurance, housing, and other public policies [79, 141, 143, 145–154] on health outcomes and assess their potential translation into preventive strategies at the public health and clinical care levels. At the same time, the eventual implementation of such strategies will be strengthened by local governments’ commitment to prioritize the prevention and treatment of NCDs, in this case, diabetes, as previously voiced by experts and advocates in the region [79, 141, 155–158].
Diabetes Awareness, Treatment, and Control
Diabetes Awareness
Although fewer than studies focused on prevalence, a considerable number of reports centered on diabetes awareness, treatment, and control across LatAm were published between 2005 and 2020 (Table 2) [33, 37, 40, 41, 43, 44, 49, 51, 60–63, 65, 72, 73, 87, 98, 101, 103, 104, 111, 112, 159–189]. A few of the studies evaluated diabetes awareness, treatment, and control altogether [85, 185, 189]. Most studies did not use the term “diabetes awareness,” but equated it (or more appropriately, diabetes unawareness) to “suspected,” “undiagnosed,” “unknown,” or “new” diabetes or “elevated glycemia.”
Table 2.
Diabetes awareness, treatment, and control across Latin America based on reports published from 2005 to 2020
| Author (reference) | Study period | Place | Type of Study and participant characteristics | Diabetes awareness (%) | Diabetes treatment (%) | Glycemic, blood pressure, and LDL-C goals | Attainment of three goals (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Glycemic (%) | Blood pressure < 130/80 mmHg (%) | < 100 mg/dL (%) | |||||||
| LDL-C < 100 mg/dL (%) | |||||||||
| Mexico | |||||||||
| Olaiz, 2007 [33] | 2000 | Mexico | ENSA 2000 – (study described in Table 1.) | 77.3 | NR | 55.9 on treatment had random BG > 200 | NR | NR | NR |
| Secretaría de Salud de México, Instituto Nacional de Salud Pública (INSP), 2016 [40] | 2006–2016 | Mexico |
ENSANUT 2016 MC (study described in Table 1) The report has information on 2006, 2012, and 2016 cycles. |
Only self-reported available: In 2006: 7.2 In 2012: 9.2 In 2016: 9.4 |
Only insulin: 2006: 6.8 2012: 6.5 2016: 11.1 Only oral meds 2006: 84.8 2012: 72.4 2016: 67.9 Both 2006: 2.5 2012: 6.6 2016: 8.8 None 2006: 5.9 2012: 14.5 2016 12.2 |
In 2016, 87.8 | NR | NR | NR |
| López-López, 2012 [159] | 2001–2008 | Mexico | Sub-analysis 2000 and 2006 ENSANUT data for the state of Hidalgo and comparison with local diabetes program, n = 2856 (73.1% women) | All participants had diabetes | NR | 35.6 | 99.6 | 15.5 | NR |
| Kumar, 2016 [37] | 2012 | Mexico | Based on the Mexican Health and Aging Study (Study described in Table 1.) | Self-reported: 21.4% | NR | NR | NR | NR | NR |
| Flores-Hernández, 2015 [160] | 2006, 2012 | Mexico |
Cross-sectional analysis based on ENSANUT 2006 and 2012 data from participants who self-reported diabetes N = 2965 in 2006 and N = 4483 in 2012; Age ≥ 20 years |
All participants had self-reported diabetes | NR |
HbA1c < 7% In 2006: 3.5 In 2012: 25.6 |
NR | NR | NR |
| Fanghänel Salmon, 2011 [161] | Mexico |
Secondary analysis using data for Mexico from the International Diabetes Management Practices Study) Total world-wide N = 17,232 N = 2620 from Mexico |
All participants had diabetes |
Oral meds: 66.0 Insulin only: 11.0 Both: 18.0 Diet only: 5.0 |
HbA1c < 7%: 31.1 | 25.2 | 29.7 | 0.7 | |
| Hernández- Romieu, 2011 [162] | 2005 | Mexico |
Probabilistic sampling N = 937 of self-reported diabetes (65.85% women, and mean age = 56) HbA1c was measured. The study was performed in urban and rural zones of seven Mexican states |
All participants had diabetes | 85.0 |
HbA1c < 7%: 30.0 HbA1c > 9.5%: 50.0 |
NR | NR | NR |
| Lavalle-González, 2012 [163] | 2007 | Mexico |
Secondary analysis using data for Mexico from the International Diabetes Management Practices Study) N = 2642 from Mexico (91% patients with type 1 and 89% patients with type 2 diabetes living in urban areas) |
All patients had diabetes |
Type 2 Oral only: 63.0 Insulin only: 10.9 Both: 22.3 Diet and exercise: 3.8 |
HbA1c < 7% Type 1: 20.9 Type 2: 36.8 |
Type 1: 67.3 Type 2: 41.3 |
NR | 4.0 |
| Wacher, 2016 [164] | 2000–2003 and 2006- 2009 | Mexico City |
Family Medicine Clinics under the Instituto Mexicano del Seguro Social in the Mexico City’s metropolitan area |
All participants had diabetes |
In 2003: 59.2 In 2006: 71.9 |
HbA1c < 7% In 2003: 38.9 In 2006: 21.4 |
In 2003: 78.2 In 2006: 46.9 |
In 2003: 51.9 In 2006: 12.2 |
NR |
|
Secondary data analysis of a database of 1170 patients with type 2 diabetes with disease diagnosed within 3 years N = 638 (women 68.2%), mean age = 51.8 years |
|||||||||
| Basto-Abreu, 2020 [41] | 2016 | Mexico | Encuesta Nacional de Salud y Nutrición de Medio Camino (ENSANUT-MC 2016) (Study described in Table 1.) | Total prevalence: 13.8 (4.1 undiagnosed) 70.1 aware | 10.1 were not taking medications |
HbA1c < 7%: 31.8 HbA1c 7–8%: 16.4 |
NR | NR | NR |
| Central America | |||||||||
| Gough, 2009 [165] | 2006 | Belize | CAMDI – Belize (Study described in Table 1.) | Total prevalence: 13.1 (5.4 undiagnosed) 58.8 aware | 69.1 on prescribed treatment; 95.9 taking medications | NR | NR | LDL-C ≤ 130 mg/dL: 12.0 | NR |
| Dekker, 2017 [166] | 2014–2015 | Belize |
Toledo, Belize Hillside Health Care International Clinic Diverse population, poorest district in Belize Mixed methods: medical chart review and health care provider and patient interviews Ages ≥ 18 years N = 178 charts |
Not applicable - medical chart reviews of patients with diabetes | All patients on pharmacological treatment: 9% on insulin | Glycemic control “good only for 26%” | 50.0 | NR | NR |
| Ministerio de Salud Pública de Costa Rica, 2009 [167] | 2004 | Costa Rica | CAMDI – San Jose, Costa Rica | Total prevalence: 7.9 (1.9 undiagnosed) 75.9 aware |
Oral meds: 57.2 Insulin: 24.8 Diet: 12.9 |
29.2 uncontrolled | NR | NR | NR |
| Brenes-Camacho, 2007 [42] | 2004–2006 | Costa Rica | Costa Rica: Estudio de Longevidad y Envejecimiento Saludable (CRELES) | Total prevalence: 23.4 (2.4 undiagnosed) 89.7 aware |
Oral meds: 69.4 Insulin: 31.0 |
HbA1c ≥ 7%: 37.0 |
SBP ≥ 130 mmHg: 78.0 DBP ≥ 80 mmHg: 66.0 |
LDL-C ≥ 100 mg/dL: 78.0 | NR |
| Brenes-Camacho, 2008 [43] | (Study described in Table 1) | ||||||||
| Wong-McClure, 2016 [44] | 2010 | San Jose, Costa Rica | Costa Rican National Cardiovascular Risk Factors Surveillance System (study described in Table 1) | Total prevalence: 10.8 (1.3 undiagnosed) 88.0 aware | NR | NR | NR | NR | NR |
| Organización Panamericana de la Salud [168] | 2004 | Honduras | CAMDI – Tegucigalpa (study described in Table 1) | Total prevalence 6.4 (3.1 undiagnosed) 50 aware |
On meds: 85.5 On no treatment: 3.2 |
BG < 130 mg/dL: 37.0 | 77.4 | LDL-C,130 mg/dL: 65.4 | NR |
| Montalván Sánchez, 2020 [51] | 2016–2017 | Honduras | Western Honduras (study described in Table 1.) | 68.1 aware | On treatment: 95.7 | NR | NR | NR | NR |
| Orellana-Pontaza, 2007 [169] | 2006 | Guatemala | CAMDI – Villa Nueva, Guatemala (study described in Table 1) | 51.2 aware |
Taking meds: 77.7 Oral meds: 58.3 Insulin: 8.2 Both: 2.0 None: 26.1 |
BG ≥ 130 mg/dL: 61.7 | HTN: 26.5 | LDL-C < 130 mg/dL: 69.6 | NR |
| Ministerio de Salud Pública y Asistencia Social de Guatemala, 2018 [49] | 2015 | Guatemala | National Survey – Urban region | NR |
Taking meds: 56.1 Insulin: 19.0 |
NR | NR | NR | NR |
| Amador Velazquez, 2010 [170] | 2004 | Managua, Nicaragua | CAMDI – Nicaragua (study described in Table 1) |
Total prevalence: 9.0 (3.9 undiagnosed, or 43.3 of those with diabetes) 56.7 aware |
Taking meds: 97.1 | BG < 130 mg/dL: 43.4 | HTN: 33.5 | LDL-C< 130 mg/dL: 46.1 | NR |
| Caribbean | |||||||||
| Ministerio de Salud Pública de Cuba [60] | 2010–2011 | Cuba | III Encuesta Nacional de Factores de Riesgo y Actividades Preventivas de Enfermedades No Transmisibles. Cuba 2010–2011 | 61 aware | On meds: 75.5 |
Control based on glycemia: On oral meds: 60.0 On insulin: 50.0 |
NR | NR | NR |
| Dethlefs, 2019 [171] | 2010–2012 | Dominican Republic |
Study of the implementation of diabetes and hypertension program in two rural clinics serving 30 communities in the Dominican Republic. The program was implemented 2010–2012, N = 1191 |
All patients had diabetes | NR | 50% of patients had A1c < 9% at baseline | NR | NR | NR |
| Ministerio de Salud Pública de la República Dominicana, 2014 [61] | 2013 | Dominican Republic |
National Survey “Encuesta Demográfica y de Salud - República Dominicana 2013” Study described in Table 1. |
NR |
On oral meds: women: 23.0 men: 51.0 On insulin: women: 12.0 men: 15.0 |
NR | NR | NR | NR |
| Carrère, 2017 [62] | 2014 | Guadeloupe | Cross-sectional multicenter study- Persons undergoing a periodic health examination on invitation from the general social security fund of Guadeloupe (CGSS). |
Aware Women: 84.5 Men: 67.3 |
Women: 97.7 Men: 97.0 |
HbA1c < 7% Women: 26.9 Men: 25.9 |
NR | NR | NR |
| Jean-Baptiste, 2006 [63] | 2002–2003 | Haiti |
Population-based survey PREDIAH (study described in Table 1) |
Aware Men: 77.3 Women: 69.2 |
On insulin: 10.0 No information on other medications |
NR | < 10.0 normal BP | NR | NR |
|
Ministère de la Santé Publique et de la Population, 2018 [65] |
2016–2017 | Haiti |
Enquête Mortalité, Morbidité et Utilisation des Services (EMMUS- VI) Random sampling, two-stage, stratified |
65.4% of women aged 35–64 years knew they had diabetes |
Women: 76.5 prescribed medications, but 54.2 taking them. Men: |
HbA1c > 6.5 Women: 64.5 Men: 62.7 |
NR | NR | NR |
| 71.1 prescribed medications, but 53.9 taking them. | |||||||||
| Pérez, 2012 [172] | 2005–2007 | Puerto Rico |
Three-stage cluster sampling design random selection N = 859 Secondary analysis of a previous epidemiologic study |
50 aware |
Oral meds only: 64.7 Insulin only: 8.1 Both: 12.3 |
HbA1c < 7.0%: 28.7 |
41.2 | 47.8 | 6.6 |
| Rodríguez-Vigil, 2014 [173] | 2010 | Puerto Rico | Descriptive study of patients with diabetes based on a random sampling throughout the five health regions. Age ≥ 18 years, N = 600 | All participants had diabetes |
Oral meds only: 64.5 Insulin only: 11.7 Both: 19.0 l |
HbA1c < 7.0%: 37.3 |
34.0 | 59.9 | 9.9 |
| South America | |||||||||
| Ministerio de Salud de Argentina, 2011 [70] | 2009 | Argentina (2da Encuesta Nacional de Factores de Riesgo para Enfermedades No Transmisibles) | Second National Survey on Risk Factors for Non-Communicable Diseases- 4-stage, probabilistic sampling of 24 jurisdictions | NR |
On treatment: 55.2 On “medical treatment: 39.5 No pharmacologic treatment: 16.9 Both: 43.65 |
NR | NR | NR | NR |
| Ministerio de Salud de Argentina, 2015 [71] | 2013 | Argentina (3ra Encuesta Nacional de Factores de Riesgo para Enfermedades No Transmisibles) |
Third National Survey on Risk Factors for Non-Communicable Diseases 4-stage probabilistic sampling |
NR |
On treatment: 61.3 (65.8% on insurance, 44.9% public system) Pharmacologic treatment: 34.1 No pharmacologic treatment: 14.4 Both: 51.5 |
NR | NR | NR | NR |
| Ministerio de Salud de Argentina, 2019 [72] | 2018 | Argentina (4ta Encuesta Nacional de Factores de Riesgo para |
Fourth National Survey National Survey of Risk Factors for Non- communicable Diseases - |
60.7 aware |
On treatment: 52.6 (59.3% men 47.4% women) Medications only: 24.8 Diet only: 22.5 |
31.4% had elevated CBG (≥ 110 mg/dL) | NR | NR | NR |
| Enfermedades No Transmisibles | 4-stage probabilistic sampling | Both: 52.7 | |||||||
| Gagliardino, 2019 [174] | 2006–2012 | Argentina | International Diabetes Management Practice Study (IDMPS) - an international, multicenter, prospective, observational study of patients with diabetes Sub-analysis of people with type 2 diabetes from Argentina (N = 2551) | All participants had type 2 diabetes. |
Oral meds only: 65.0 Insulin only: 13.0 Both: 22.0 |
Oral meds only: 61.6 Insulin only: 31.3 Both: 29.0 |
Oral meds only: 25.4 Insulin only: 25.1 Both: 18.3 |
Oral meds only: 40.4 Insulin only: 34.5 Both: 38.2 |
Oral meds only: 5.5 Insulin only: 2.7 Both: 1.5 |
| Santero, 2018 [175] | NR | Argentina |
Analysis of the implementation of an mHealth program in public primary clinics in the province of Corrientes. Quasi-experimental study with outcome measurements at baseline, 6 and 12 months N = 947 patients with diabetes (92.9% with Type 2) Age ≥ 18 years |
All participants had diabetes |
Oral meds only: 79.4 Insulin only: 5.8 Both: 8.9 No treatment: 5.9 |
HbA1c ≥ 8%: 44.4 | BP ≥ 140/90: 48.2 | NR | NR |
| Barceló, 2001 [73] | 1998 | Bolivia | Population-based survey of households in four cities (study described in Table 1) |
Aware Men: 73.6 Women: 69.8 |
NR | NR | BP 140–159/ 90–99 mmHg: 21.4 | NR | NR |
| Gomes, 2006 [176] | 2000–2001 | Brazil |
13 public endocrine clinics in 8 Brazilian cities Review of medical charts of patients with type 2 diabetes |
All patients had diabetes and received health care from the National Brazilian Health Care System |
Oral meds (monotherapy): 33.2 Insulin monotherapy or in combination: 55.2 |
HbA1c < 7%: 46.0 all (women 42.8%, men 50.9%) |
SBP (27.6 women, 29.8 men) DBP (19.8, women, 18.6 men) |
Women 17.2 Men 25.7 |
NR |
|
N = 2233 (60% women), Age ≥ 30 years |
Unknown treatment: 11.6 Diet only: 11.6 |
||||||||
| Busch Mendes, 2011 [75] | 2003 | Brazil | Study described in Table 1 | Awareness was not determined |
Oral meds: 60.8 Insulin: 15.1 |
NR | NR | NR | NR |
| Valverde Mendes, 2010 [177] | 2006–2007 | Brazil |
Health centers located in ten large cities in Brazil Cross-sectional study, nationwide survey Review of medical charts of 20 centers in 10 cities in four Brazilian regions; the largest cities in their regions and most populous. Sample of consecutive patients with diabetes attending each center during a 30-day period. N = 6671 patients with either type 1 or type 2 diabetes, Age ≥ 18 years |
All participants had diabetes | NR | Inadequate control: 76 (type 1: 90, type 2: 73) | NR | NR | NR |
| Moraes, 2020 [178] | 2008–2010 | Brazil |
Secondary analysis based on Estudo Longitudinal da Saude do Adulto (ELSA-Brasil) Prospective cohort study of active or retired civil servants from six public higher education institutions Analysis of sample of participants with previously diagnosed diabetes |
All participants had diabetes |
“Low/medium adherence to medications”: 60.2 Oral meds only: 86.5 Insulin only: 5.7 Both: 7.8 |
HbA1c ≥ 6.5%: 54.2 | NR | NR | NR |
| (N = 1242) | |||||||||
| Viana, 2013 [179] | 2006–2011 | Brazil | 5750 patients with type 2 diabetes from 14 centers in five regions of Brazil, including primary care units and outpatient clinics of university hospitals | All patients had type 2 diabetes |
On treatment: 99.0 Oral meds: 57.0 Insulin only: 13.0 Both: 22.0 Diet: 6.0 |
HbA1c < 7%: 26.0 -Those who performed more SBGM had lower A1c “non-white” had HbA1c |
NR | NR | NR |
| Baptista, 2015 [180] | 2012–2013 | Brazil |
Public outpatient clinic at a university hospital in Curitiba, Parana. Adults ≥ 18 years, and elderly adults ≥60 years with type 1 or type 2 diabetes; 1031 records: 299 type 1 (55.2% women) and 732 type 2 (68% women). All patients received care from the National Brazilian Health Care System and at the same endocrine clinic. |
All patients had either type 1 or type 2 diabetes |
Type 2 diabetes Oral meds and/or oral meds/insulin: 47.1 Diet alone: 2.1 Type 1 diabetes Insulin/oral meds: 12.1 |
HbA1c < 7%: 9.5 | NR | NR | NR |
|
Ministério da Saúde do Brasil, 2019 [80] |
2018 | Brazil | VIGITEL BRASIL 2018 study described in Table 1 | All self-reported | On meds: 88.7 (89.7 men, 88.0 women) | NR | NR | NR | NR |
|
Ministério da Saúde do Brasil, 2020 [81] |
2019 | Brazil | VIGITEL BRASIL 2019 study described in Table 1. | All self-reported | On meds: 89.3 (90.8 women, 87.4 men) | NR | NR | NR | NR |
|
Ministerio de Salud de Chile, 2010 [85] |
2009–2010 | Chile | Encuesta Nacional de Salud, ENS Chile 2009–2010 study described in Table 1. | Total aware: 78.49 (women 84.07, men 71.32) |
All: 52.05 (women 53.08, men 50.7) |
HbA1c < 7%: 34.32 of all with diabetes (women 38.52, men 29.33) (44% of those on pharmacological treatment had HbA1c < 7%) | NR | NR | NR |
|
Ministerio de Salud de Chile, 2017 [86] |
2016–2017 | Chile | Encuesta Nacional de Salud, ENS Chile 2016–2017 study described in Table 1 | NR | NR | 58.2 | NR | NR | NR |
| Ministerio de Salud y Protección Social de Colombia [87] | 2007 | Colombia | National survey “Encuesta Nacional de Salud (ENS) 2007” (study described in Table 1) | NR | 50.7 | NR | NR | NR | NR |
| Alba, 2009 [181] | 2008 | Colombia |
Cross-sectional study of a type 2 diabetes patient population attending a clinic associated with a university hospital in Bogotá N = 150 |
All participants had diabetes | On insulin: 54.0 | HbA1c < 7%: 49.0 | 47.0 | 52.6 | NR |
| Machado-Alba, 2009 [182] | 2006–2007 | Colombia |
Retrospective study, N = 19,704 patients treated by the national social security health system Age > 30 years |
All patients had type 2 diabetes | 45.8 | HbA1c < 7%: 42.9 | 66.2 | NR | NR |
|
Ministerio de Salud Pública y Bienestar Social de Paraguay, 2012 [96] |
2010–2011 |
Paraguay (Primera Encuesta Nacional de Factores de Riesgo de Enfermedades No Transmisibles en Población General) |
First National Health Survey Probabilistic, three-stage sampling Study described in Table 1. | All self-reported |
During the last 2 weeks: On oral meds only: 53.7 On insulin only: 5.3 On insulin or oral meds: 54.8 |
NR | NR | NR | NR |
| Ministerio de Salud de Perú, 2006 [98] | 2005 | Peru | National Survey 2005 (study described in Table 1) | Aware: Close to 50.0 | 65.4 | NR | NR | NR | NR |
| Seclen, 2015 [101] | 2010–2012 | Peru | PERUDIAB Study described in Table 1 | Aware: 60.0 | NR | NR | NR | NR | NR |
| Minderhoud, 2015 [104] | 2013–2014 | Republic of Suriname | The Rapid Assessment of Avoidable Blindness (RAAB) survey method (Study described in Table 1.) | Aware: 89.6 |
Oral meds only: 77.3 Insulin: 15.6 No medical treatment: 6.9 |
58.5% were considered well-controlled (based on random blood sugar ≥ 200 mg/dL) | NR | NR | NR |
| Krishnadath, 2016 [103] | 2013 | Suriname |
Secondary data analysis from the Suriname Health Study Stratified multistage cluster sample of households |
Aware: 60.0 | NR | NR | NR | NR | NR |
| Fort, 2012 [183] | 2008–2011 | Uruguay |
CVRF assessment of national health insurance card applicants Cross-sectional, electronic records |
Awareness: 50.3 (men: 39.3, women: 64.3) | NR | All: 14.4 (men: 6.0, women: 25.2) | NR | NR | NR |
| Multinational studies | |||||||||
| Gagliardino, 2001 [184] | 1999- |
Argentina Brazil Chile Colombia Paraguay Uruguay |
Analysis of 13,513 records from the diabetes network QUALIDIAB | All participants had diabetes. |
Type 2: Oral meds monotherapy: 42.0 Combination oral meds: 14.0 Insulin only: 14.0 |
HbA1c < 8.0%; 33.0 | BP < 140/90 mmHg: 38.0 | NR | NR |
| Barceló A, 2012 [111] | 2003–2006 |
Belize, Costa Rica El Salvador Guatemala Honduras Nicaragua |
Central America Diabetes Initiative (CAMDI) (study described in Table 1) |
Overall Undiagnosed: 40.0 Belize: 41.1 Honduras: 53.7 Costa Rica: 28.4 Nicaragua: 45.9 Guatemala: 39.7 El Salvador: 28.9 |
NR | NR | NR | NR | NR |
| Salas, 2016 [112] | 2003–2009 |
Cuba Dominican Republic Puerto Rico Venezuela Peru Mexico |
Sub-analysis of data from the 10/66 Dementia Research Group Population-based studies in 13 catchment areas in six Latin American countries (study described in Table 1) |
Undiagnosed Cuba: 31.1 (men > women) Dominican Rep: 24.3 (women > men) Urban Perú: 41.3 (men > women) Venezuela: 30.2 (men > women) Urban Mexico: 9.6 (no sex difference) Rural Mexico: 25 (men > women) Puerto Rico: 37.7 (men > women) |
On pharmacological treatment: Cuba: 62.0 Dominican Rep: 70.0 Urban Peru: 60.0 Rural Peru: 62.0 Venezuela: 65.0 Urban Mexico: 85.0 Rural Mexico: 85.0 Puerto Rico: 90.0 Puerto Rico with the largest proportion of insulin |
Among those with self-reported diabetes, with FG < 7 mmol/L Cuba: 61.4 Dominican Republic: 61.1 Urban Peru: 37.2 Venezuela: 56.9 Urban Mexico: 45.7 Rural Mexico: 40.0 Puerto Rico: 34.6 |
NR | NR | NR |
| Silva, 2010 [185] | 2003–2005 |
Argentina Chile Colombia Ecuador Mexico Uruguay Venezuela |
Sub-analysis from Cardiovascular Risk Factor Multiple Evaluation in Latin America study CARMELA (study described in Table 1) |
Awareness: 78.0 Highest in Bogota (87.5) and lowest in Lima (61.7) |
Not on treatment: 67.0 Adherence 63% among those on treatment |
Based on fasting plasma glucose ≥ 126 mg/dL: 16.0 | NR | NR | NR |
| Commendatore, 2013 [186] | NR | Argentina Colombia Peru |
Analysis of subsample from the Registry of medical data from patients with diabetes (QUALIDIAB). Six specialized diabetes centers in 3 countries N = 1118 Country data combined |
All patients had diabetes |
Oral meds only: 56.0 Insulin only: 13.0 Both: 26.0 Diet: 5.0 |
HbA1c < 7% Oral meds only: 54.0 Insulin only: 32.0 Both: 27.0 |
76% | 44% | NR |
| López Stewart, 2007 [187] | October 2004 |
Argentina Brazil Chile Costa Rica Ecuador Guatemala Mexico Peru Venezuela |
Multicenter, cross-sectional study, epidemiological study N = 3592 patients with type 2 diabetes Interviews with physicians Some country data combined |
All patients had diabetes | NR |
HbA1c < 7%: 43.2 HbA1c < 6.5%: 30.0 Costa Rica, Argentina, and Chile had the largest % of patients with HbA1c < 7% |
NR | NR | NR |
| Duarte, 2019 [188] | February 2006- June 2007 | Brazil and Venezuela |
Cross-sectional study based on nationwide survey on prevalence of glycemic control, 20 centers in Brazil and 32 in Venezuela Charts from consecutive patients attending the clinic during a 30-day period N = 5692 in Brazil, and N = 3726 in Venezuela Age ≥ 18 years |
All patients had diabetes | NR |
HbA1c ≥ 7.0%: (74.0 women and 73.0 men) Brazil: men: 72.0 women: 74.0 Venezuela: men: 75.0 women: 75.0 Higher education was associated with lower HbA1c. Private health insurance was associated with lower HbA1c. |
NR | NR | NR |
| Irazola, 2017 [189] | 2010–2011 | Argentina Chile Uruguay | Centro de Excelencia en Salud Cardiovascular para el Cono Sur I (CESCAS I) (study described in Table 1) |
Aware: 79.8 Marcos Paz 64.5, Bariloche 78.9, Temuco 81, Barros Blancos 85.2, Awareness slightly increased with educational attainment Awareness and control higher in women |
Treatment at the time of the home interview: 58.8 overall Marcos Paz 77.6 Bariloche 69.3 Temuco 78.9 Barros Blancos 60.9 |
Control defined as on pharmacologic treatment and FPG < 126 mg/dL: Controlled, all: 46.2 Marcos Paz 36.9 Bariloche 45.8 Temuco 51.6 Barros Blancos 50.3 |
NR | NR | NR |
“Undiagnosed” diabetes—a proxy for lack of diabetes awareness—ranged widely from 10.3 to 50% across studies and countries (Table 2). The prevalence of undiagnosed diabetes was higher in Guatemala (48.8%), Uruguay (48.7%), Puerto Rico (37.7–50%), Honduras (31.9–53.7% range), Mexico (29.9–50% range), and Nicaragua (43.3%) and lower in Colombia (Bogota) (23.5%), the southernmost countries of South America (20.2%), and Costa Rica (10.3–28.4%). Irazola et al. [189] described that diabetes awareness slightly increased with educational attainment. However, associations between undiagnosed diabetes with age, sex, educational attainment, SES, or geographic location were not published by most studies.
The observed range of undiagnosed diabetes suggests that the actual prevalence of diabetes across LatAm could exceed previous estimates [6, 124] and that a potentially significant proportion of persons with diabetes for whom both macro- and microvascular complications may be present but not assessed and treated. Therefore, current estimates of the prevalence of diabetes across continents may not fully account for the necessary resources to provide adequate health care for Latin Americans with diabetes [7, 8, 190, 191]. Considering the workforce and resources needed to screen the millions of persons across the region who are at risk of diabetes or have the disease and are not aware, experts have proposed diabetes predictive models requiring specific easily obtained clinical data points that could be readily used in primary care settings [192–194]. Also, the Finnish Diabetes Risk Score (FINDRISC) has been proposed, tested, or modified to screen and identify individuals at high risk of developing diabetes in Latin America [195–199]. Point-of-care tests for HbA1c and urine microalbumin have also been proposed as alternatives to identify persons with “undiagnosed diabetes” and/or those at risk of chronic kidney disease (CKD) in low-resource and remote settings in LatAm [200–203]. The standardization, reliability, and repeatability of some of these tests, as well as the clinical and public health benefit derived from their integration into the health care systems, may need to be determined [204]. However, these and other emerging diagnostic technologies [205, 206] are promising alternatives that could be incorporated to assess the prevalence of diabetes and implement timely interventions.
Treatment and Control of Diabetes, Blood Pressure, and LDL-C
The percent of persons with diabetes following any treatment for diabetes ranged from 52.6 to 99% across studies (Table 2). Prescription and/or use of antihyperglycemic medications was mostly assessed via interviews, although a few studies evaluated medical records. Most individuals reported taking oral antihyperglycemic medications either as monotherapy or as a combination of oral medications, while a smaller percent reported using insulin alone or in combination with oral medications. Five (5%) to 12.9% only followed diet/exercise prescription [161, 163, 167, 176, 179, 186], and 3.2 to 10.1% were not taking any medications [41, 104, 168, 169, 175]. Receiving or adhering to pharmacological treatment was positively associated with having health insurance [71], and receiving medical care in private rather than public health care settings [71, 187]. At least one study observed better pharmacologic treatment adherence with female sex [185].
Achievement of ADA/ALAD-recommended glycemic goals [23, 207] was assessed by multiple studies. The percentage of persons attaining HbA1c < 7% ranged from 3.5 to 54%. However, some studies defined glycemic control based on fasting or random blood glucose thresholds and reported attainment of glycemic control in the 31.4 to 61.4% range. Attainment of glycemic control was associated with higher socioeconomic status (SES) [160], having health insurance [160], and better access and services [208]. Not attaining glycemic control was associated with longer duration of diabetes [163, 187, 209], taking insulin (alone or in combination with oral antihyperglycemic medications) [176], forgetfulness (e.g., taking multiple medication for more than one condition) [185], complex therapeutic regimes [209], inadequate access to health care services [22], and availability or health insurance coverage of medications [187], among other factors.
In addition to glycemic control, a smaller number of studies examined the attainment of ADA/ALAD-recommended blood pressure and LDL-C—blood pressure < 130/80 mmHg and LDL-C < 100 mg/dL—for patients with diabetes [24, 207]. The percentage achieving blood pressure goals ranged from 25 to 67%, and the percent achieving LDL-C goals ranged from 12 to 52.6% (Table 2). The percent achieving optimal glycemic, blood pressure, and LDL-C levels altogether was reported by a handful of studies and up to 9.9% (Table 2).
The findings described above denote critical aspects of the state of diabetes care in Latin America. The achievement of glycemic goals reported by the studies included in our review is similar to previously published studies [164, 179, 180, 187, 210]. This implies seriously chronic and inadequate glycemic control at the population level across the region.
The inclusion of questions on treatment for glycemic control, medical, and self-care in some national surveys increases our understanding of health-seeking behaviors, both patients’ and clinicians’ adherence to recommended guidelines of care, and challenges related to the utilization of health care services and availability of medications. The smaller number of studies reporting on the attainment of blood pressure and LDL-C goals and the proportion of patients achieving those goals also poses questions about the prevention of macrovascular complications in persons with diabetes in Latin America, considering the raising prevalence of CVD in the region [16, 211]. Of note, most national surveys report prevalence and treatment and/or control of diabetes, hypertension, and blood cholesterol and the prevalence of tobacco use individually. Since diabetes involves multiple organs and deserves a holistic care approach, reporting on the co-existence of other CV risk factors with diabetes would enhance critical understanding of CV risk and health care needs. Also, some surveys collected biospecimens, but the test results were not included in the reports. It is possible that they are analyzed and published later. Yet including test results in the surveys would offer a more comprehensive picture of the status of diabetes prevention and care needs [180, 212, 213] to plan interventions accordingly.
Following Guidelines of Care for the Prevention of Microvascular Disease
Various studies included in our review reported on participants’ receiving or following ADA/ALAD-recommended guidelines of care [25] for early detection and prevention of microvascular disease—annual fundoscopic exam, examination for peripheral neuropathy and comprehensive foot examination, annual function/urine albumin excretion testing, and HbA1c tested at least 3 times per years [38–40, 72, 96, 159, 160, 165, 166, 168, 169, 171, 172, 175, 176, 180, 182, 186, 214] (Table 3). Some studies assessed the completion of several guidelines, whereas most studies focused on a few. The completion of the selected ADA guidelines varied, ranging from 14.7 to 97.5% for the foot exam, from 8.6 to 92% for the fundoscopic exam, and from 1.1 to 51.1% for the urine albumin excretion test. Most studies (especially national surveys) inquired about having HbA1c checked within the previous 12 months. The affirmative response ranged from 3.7 to 90.0%. In addition to inquiring about HbA1c testing, some surveys asked whether the participant’s blood glucose had been tested (by a health care professional). Having private health insurance was associated with a greater number of affirmative responses to the latter [70, 71, 91].
Table 3.
Completion of selected ADA-recommended guidelines of care across Latin America on reports published from 2005 to 2020
| Study (reference) | Study period | Place | Study type | A1c checked as recommended (ADA guidelines) (%) |
Foot exam at least once a year (%) | Annual fundoscopic Exam (%) |
Urinary albumin and renal function test at least once a year (%) |
|---|---|---|---|---|---|---|---|
| Mexico | |||||||
| López-López, 2012 [159] | 2000 and 2006 | Mexico, State of Hidalgo |
Based on the 2000 and 2006 National Survey (ENSANUT) data subsamples for the state of Hidalgo. (study described in Table 2.) |
35.6 | 97.5 | 92.0 | 1.1 |
| Flores-Hernández, 2015 [160] | 2006 and 2012 | Mexico (National Survey) |
Analysis based on two cycles of ENSANUT (Study described in Table 2.) |
2006: 3.7 2012: 7.7 |
2006: 9.4 2012: 14.7 |
2006: 12.3 2012: 8.6 |
2006: 6.6 2012: 12.6 |
| Secretaría de Salud de México, Instituto Nacional de Salud Pública (INSP), 2016 [40] | 2016 | Mexico | National Health and Nutrition Survey (ENSANUT-MC 2016) (Study described in Table 1.) | 15.2 within the last 12 months | 20.9 within the last 12 months | 13.1 within last 12 months | 4.7 within the last 12 months |
| Central America | |||||||
| Gough, 2009 [165] | 2006 | Belize |
CAMDI– Belize |
1.7% reported having it checked | NR | NR | NR |
| Dekker, 2017 [166] | 2014–2015 | Belize | Study described in Table 2. | NR | 41.0 | 41.0 |
39% serum creatinine 28% urinalysis |
| Orellana-Pontaza, 2007 [169] | 2006 | Guatemala |
CAMDI – Villa Nueva, Guatemala |
7.6 | 27.1 | 30.0 | NR |
| Organización Panamericana de la Salud [168] | 2004 | Tegucigalpa, Honduras |
CADMI – Honduras |
NR | NR | NR | NR |
| Caribbean | |||||||
| Dethlefs, 2019 [171] | 2010–2012 | Dominican Republic | Study described in Table 2. | 50.0 | 20.0 | NR | NR |
| Pérez, 2012 [172] | 2005–2007 | Puerto Rico | 136 adults who self-reported DM in Puerto Rico | 52.3 | 43.8 | 49.2 | NR |
| South America | |||||||
| Ministerio de Salud de Argentina, 2019 [72] | 2018 | Argentina | Fourth National Survey- Study described in Table 2. | NR | 30.0 | 40.0 | NR |
| Santero, 2018 [175] | -- | Argentina | Quasi-experimental study (Study described in Table 2.) | 16.9 | 69.1 | 29.0 | NR |
| Gomes, 2006 [176] | 2000–2001 | Brazil | Study described in Table 2. | 84.3 | 58.2 | 46.9 | 38.9 |
| Baptista, 2015 [180] | 2012–2013 | Brazil |
Public outpatient clinic at a university hospital in Curitiba, Parana. (Study described in Table 2.) |
NR | 59.9 | 43.2 | NR |
| Ministerio de Salud de Chile, 2010 [85] | 2009–2010 | Chile (Encuesta Nacional de Salud, ENS Chile 2009–2010) | Study described in Table 1. | NR | 6.7 in the last year | 34.8 (7.6 with retinopathy) | NR |
| Machado-Alba, 2009 [182] | 2006–2007 | Colombia | Study described in Table 2. | NR | 55.5 | 35.6 | 57.0 |
| Ministerio de Salud Pública y Bienestar Social de Paraguay, 2012 [96] | 2010–2011 | Paraguay | Study described in Table 1. | NR | 17% had their feet checked within the last year; 78.3% had not ever had their feet examined. | 31.5% had an exam within the last 2 years, and 55.8% had not ever had an eye exam | 51.1% within the last 2 years, and 36.2% had not ever had a 24-hr urine test done. |
| Multinational studies | |||||||
| Commendatore, 2013 [186] | -- | Argentina Colombia Peru | Analysis based on QUALIDIAB Registry. Study described in Table 2. | 90.0 | 60.0 | 62.0 | NR |
NR, not reported; HbA1c, hemoglobin A1c
Despite the smaller number of studies evaluating the completion of the ADA guidelines for foot care and prevention of microvascular disease, and the varied guideline completion rates previously described (Table 3), the prevalence of long-term microvascular complications associated with diabetes has been documented across LatAm. For instance, in the studies included on our review and others published during the same time frame, the rate of foot ulcers ranged from 1.2 to 14.8% [40, 214–216], and non-traumatic lower extremity amputations attributable to diabetes ranges from 1.2 to 7.3% [40, 184, 214, 215, 217–221], and the prevalence of diabetic retinopathy ranged from 11.2 to 48% [40, 184, 214, 222, 223]. CKD has become a major public health concern across Central America [224–226], and the increasing prevalence of diabetes could exacerbate the incidence of CKD—and eventually end-stage renal disease and its associated health complications—in the region [227–229].
Innovative Solutions: Emerging Research and Alternative Models of Care
The findings described above underline not just the urgent need to prevent diabetes but also to prevent complications among those with established disease, and the potentially underestimated burden on patients, societies, and health care systems across LatAm. In this regard, several innovative models of health care for patients with diabetes have been proposed and tested throughout LatAm. Combining care of diabetes and other chronic conditions would be expected to maximize time and resources and improve health outcome. Although combining diabetes and chronic pulmonary disease care did not demonstrate a difference in outcomes [230], this model could be revisited. Also, interventions at the health care system element of the chronic care model might need to be adapted to the local health care system [231] or synchronized with interventions at other levels. Improvement of health care system structure and processes [232] would assure timely access to patient information and enhance clinician decision-making. Integrating social determinants of health into diabetes care demonstrated objective improvements in patient knowledge and cardiometabolic parameters [233]. Enhancing medical continuing education [234], an intervention combining diabetes prevention and self-management [235], co-creating interventions with community stakeholders and other countries [141, 144] are other examples of alternatives to improve diabetes care throughout the region. Another major regional example of efforts to implement better care for patients with diabetes has been led by the Latin American Diabetes Association (ALAD in Spanish) to engage 17 medical associations and wrote a consensus statement on the treatment of type 2 diabetes in LatAm [207].
Kaselitz et al. published a scoping review of policies and interventions for diabetes in LatAm [147], telehealth, mobile clinics, and other non-traditional health care delivery models. In addition, a non-exhaustive list of examples of past or current interventions, policies, and initiatives is provided in Table 4 [184, 186, 234, 236–252]. Interventions in the list include tele-ophthalmology [249, 250, 253, 254], team-based foot self-care education [236], diabetic retinopathy education and screening at a community pharmacy [255], rapid assessment/diagnostic tools to screen for or detect retinopathy, nephropathy, and risk of developing foot ulcers [201, 256–262] and are examples of clinical research and/or implementation activities designed to strengthen the prevention and early detection of diabetes-associated complications and improve health outcomes throughout LatAm.
Table 4.
examples of past and ongoing diabetes care interventions initiated in Latin America from 2000 to 2020
| Author [reference] | Year of Study | Place | Key findings |
|---|---|---|---|
| Batista, 2010 [236] | 2000–2010 | São Paulo, Brazil | A multidisciplinary health care team was established aiming at increasing limb salvage |
| Barceló, 2010 [237] | 2002–2004 | Mexico | The VIDA project - RCT to test improvement of quality of diabetes care in primary health care centers using the chronic care model and the breakthrough series collaborative methodology. The proportion of patients attending the clinics under the intervention with HbA1c <7% increased from 28% to 39%, and the proportion of patients who achieved 3 or more quality improvement goals increased from 16.6% to 69.7%. |
| Lerario, 2010 [238] | 2009 | Brazil | The Brazilian Diabetes Society developed a new algorithm for the treatment of type 2 diabetes |
| Piette, 2013 [239] | 2009–2011 | Mexico, Honduras | Interactive Voice Response (IVR) support calls for chronic disease management for Spanish speakers- The investigators report cumulative findings in Honduras, Mexico, and Spanish-speakers in the U.S. Involvement of caregivers enhanced engagement. By self-report, there was improved medication adherence and self-management was similar across sites. |
| Piette, 2011 [240] | 2010 | Honduras | Cloud-Computing Model - The investigators tested a mobile phone-based intervention of weekly VoIP calls and IVR with patients with diabetes and automated emails to clinicians and voicemail reports to family caregivers for six weeks. Improved self-care and diabetes management and significant improvement in glycemic control were reported. |
| Piette, 2016 [241] | 2013 | Bolivia | Structured caregiver feedback – The investigators assessed whether automated telephone feedback to caregivers (“CarePartners”) increased engagement in mobile-health support among patients with diabetes and hypertension in Bolivia. Significantly greater engagement was observed. Patients who spoke indigenous languages at home were more than 3X as likely to complete the IVR calls. |
| Piette, 2014 [242] | 2013 | Bolivia | Mobile health program for chronic disease self-management in Bolivia – Assessment and implementation of IVR for 12 weeks. It was associated with improved medication adherence, self-reported health status, and satisfaction. |
| Prestes, 2017 [243] | 2016–2017 | Argentina | DIAPREM – integrated diabetes care program including systemic changes education, registry and disease management |
| Flood, 2016 [244] | 2010 | Guatemala | Implementation and outcomes of a comprehensive type 2 diabetes program in rural Guatemala through a non-government organization and involving nurse-directed care |
| Flood, 2017 [245] | 2012 | Rural Guatemala | Implementation of a multi-level quality improvement program for ambulatory diabetes care based on input from patients and other stakeholders |
| Flood, 2017 [246] | 2014–2016 | Rural Guatemala | Home-based type 2 diabetes self-management – intervention delivered by diabetes educator at home and synchronized with clinic follow-up. Mayan communities |
| Tapia-Conyer, 2016 [247] Gallardo-Rincón, 2017 [234] | 2012 to present | Mexico | CASALUD Model –is a comprehensive primary health care model implemented in Mexico that enables proactive prevention and disease management using innovative technologies and a patient-centered approach. The program was pilot tested in 2009 and implemented nationwide in 2012. |
| Salamanca, 2018 [248] | 2014–2017 | Peru | Implementation of a diabetic retinopathy referral and treatment network via collaboration and co-funding from a non-governmental organization. |
| Avendaño-Veloso, 2019 [249] | 2014–2015 | Chile | A non-experimental teleophthalmology program in the public health system to increase the reach of patients at risk of having diabetic retinopathy. The program allowed the evaluation of more than a quarter of the patients with diabetes. |
| Flores, 2019 [250] | 2016 | Chile | Implementation of a telemedicine model management network for diabetic retinopathy at a primary care level. |
| Cani, 2015 [251] | Not mentioned | Brazil | RCT to an intervention individualized pharmacotherapeutic care and diabetes education by a pharmacist. At 6 months the HbA1c improved and quality of life |
|
Gagliardino, 2013 [252] Gagliardino, 2001 [184] Commendatore, 2013 [186] |
2007-2009 |
Argentina Argentina and other countries |
PRODIACOR prospective intervention of diabetes education in primary care settings in the city of Corrientes QUALIDIAB Network– Registry of type 2 diabetes patients to measure the quality of care |
Many interventions on diabetes care have focused on patients and/or clinicians as the primary recipients or enablers of the interventions. Because of the complex nature of the disease and the multiple factors that mediate treatment effectiveness, interventions involving other levels or elements within the health care organization or system [155, 157, 232, 263–265] or the health care workforce [158, 263, 266] could be considered. Interventions involving other sectors (e.g., housing, infrastructure, national or local policies) could uncover very valuable and needed strategies to enhance treatment effectiveness and potentially reduce health care costs in the long-term. The feasibility and sustainability of such research efforts—and subsequent policies—would need to be demonstrated and supported locally [143].
Additional Observations
Women
Multiple studies in our review reported a higher prevalence of diabetes among women [36, 38, 40, 42, 44, 47, 49–52, 57, 60, 62, 63, 65, 70, 72, 75, 78, 79, 83–86, 90, 95, 96, 110, 113]. While the mediating factors for this sex difference need further study (e.g., history of GDM, which was outside of the scope of this review), the increased prevalence of diabetes among women in some LatAm countries would be expected to have implications for health and health care, and potentially future generations [267–269]. Since diabetes may increase women’s risk for CVD, including stroke [270], cognitive decline [271, 272], or some cancers [273, 274], timely and comprehensive preventive care for women of all ages would need to be prioritized.
Older Adults
Due to the epidemiologic transition already experienced by some countries throughout LatAm, the population pyramid is also shifting towards a greater proportion of older adults. Studies included in our review consistently reported an increased prevalence of diabetes with age. Diabetes care challenges specific to this age group include risk of obesity or undernutrition [42, 56], increased risk for disability [57], economic barriers to appropriate access to health care [42], disruption in funding of health insurance [275], disparate completion of diabetes care guidelines based on health insurance coverage [276], inequalities in access to and utilization of health care services [277–279], complex medical care needs and frailty [280], cultural beliefs, mental health, and lack of family or social support [281], among others. Prevention of diabetes and its complications and reliable continuity of care and social support [282] need to be especially tailored for this population across the region.
Indigenous and Other Ethnic Underserved Populations
A few studies in our review reported a low prevalence of diabetes among some indigenous populations in LatAm [55, 74, 82], in parallel to some previous reports [283–286] about other indigenous groups in the region and in contrast with the higher prevalence of diabetes among American Indians in the USA [287] and the First Nations in Canada [288]. However, other studies in our review and in the current literature have documented elevated diabetes prevalence or risk among indigenous and other socioeconomically disadvantaged ethnic groups [48, 50, 73, 76, 77, 83, 90, 91, 166, 289–293]. Some of the diabetes prevalence studies included in our review focused on or mentioned participants from indigenous groups [35, 48, 50, 74, 83] and other underrepresented groups (e.g., Garifuna, Afro-Panamanian, Afro-Peruvian, Afro-Ecuadorian) [55, 76, 90, 91, 166]. However, a few studies have evaluated diabetes care, prevalence, and/or prevention of macro- or microvascular complications, diabetes management interventions, other health care needs and access to health care among indigenous populations [241, 244, 246, 294–302], and none on the other groups (that we could identify through our search). Understanding the protective mechanisms (e.g., biochemical, immune, epigenetic) against diabetes experienced by some indigenous populations would be relevant to millions at high risk of developing diabetes. At the same time, the increased prevalence of the metabolic syndrome and diabetes experienced by some indigenous groups and other ethnic groups may increase their risk not only for CVD and other diabetes long-term complications but also for re-emerging infectious diseases, like tuberculosis [303–305]. Therefore, disease prevention and health care models that account and reach these populations need to be considered.
Conclusion
Through this review, we have highlighted the most current reports on prevalence, awareness, treatment, control, and adherence to recommended guidelines of care for diabetes mellitus across LatAm published from 2000 to 2020. During that time frame, a considerable number of surveys assessing the prevalence of the disease and an increasing body of reports on the achievement of treatment and care goals were identified. Such reports demonstrate the imperative need to garner a more comprehensive understanding of the extent of diabetes across countries, and both past and ongoing efforts to establish effective and sustainable models of prevention and high-quality care able to reach and serve all peoples across the region.
During the writing of this manuscript, Latin America had been recognized as the new epicenter of the SARS-CoV-2 (COVID-19) pandemic [306]. The effects of the disease in persons with diabetes in the region are beginning to be uncovered [307–309], while some solutions are proposed [310, 311]. The magnitude of the impact of the pandemic on the health and health care needs of persons with diabetes mellitus and other NCDs—let alone on the health care systems infrastructures—in the region are yet to be known. The task ahead is substantial and will require multidisciplinary and cross-sectoral strategies and collaborations to reduce diabetes burden and improve health outcomes across Latin America.
Acknowledgments
The authors would like to thank Ms. Jill Pope (Kaiser Permanente Center for Health Research) for her valuable review of the manuscript and the Ponce Health Sciences University for creating the opportunity for AM-R and AS-S to work in this review.
Authors’ Contributions
MLAS conceptualized the review. MLAS and NML co-led the literature search and the discussion of findings. AMR and ASS performed part of the literature search, summarized and discussed findings, and contributed to the writing. MLAS performed part of the literature search and wrote the manuscript. NML provided critical review to the manuscript. All authors approved the final version of the manuscript.
Data Availability
NA
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily reflect the official views of the National Institute on Minority Health and Health Disparities, the National Institutes of Health, or the U.S. federal government.
Code Availability
NA
Footnotes
This article is part of the Topical Collection on Diabetes Epidemiology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bukhman G, Bavuma C, Gishoma C, Gupta N, Kwan GF, Laing R, Beran D. Endemic diabetes in the world's poorest people. Lancet Diabetes Endocrinol. 2015;3(6):402–403. doi: 10.1016/s2213-8587(15)00138-2. [DOI] [PubMed] [Google Scholar]
- 2.Miranda JJ, Barrientos-Gutiérrez T, Corvalan C, Hyder AA, Lazo-Porras M, Oni T, et al. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nat Med. 2019;25(11):1667–1679. doi: 10.1038/s41591-019-0644-7. [DOI] [PubMed] [Google Scholar]
- 3.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 4.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/s0140-6736(11)60679-x. [DOI] [PubMed] [Google Scholar]
- 5.Aschner P, Aguilar-Salinas C, Aguirre L, Franco L, Gagliardino JJ, de Lapertosa SG, et al. Diabetes in South and Central America: an update. Diabetes Res Clin Pract. 2014;103(2):238–243. doi: 10.1016/j.diabres.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 7.Arredondo A. Type 2 diabetes and health care costs in Latin America: exploring the need for greater preventive medicine. BMC Med. 2014;12:136. doi: 10.1186/s12916-014-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barcelo A, Arredondo A, Gordillo-Tobar A, Segovia J, Qiang A. The cost of diabetes in Latin America and the Caribbean in 2015: evidence for decision and policy makers. J Glob Health. 2017;7(2):020410. doi: 10.7189/jogh.07.020410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colón-Ramos U, Rodríguez-Ayuso I, Gebrekristos HT, Roess A, Pérez CM, Simonsen L. Transnational mortality comparisons between archipelago and mainland Puerto Ricans. J Immigr Minor Health. 2017;19(5):1009–1017. doi: 10.1007/s10903-016-0448-5. [DOI] [PubMed] [Google Scholar]
- 10.Alegre-Díaz J, Herrington W, López-Cervantes M, Gnatiuc L, Ramirez R, Hill M, et al. Diabetes and cause-specific mortality in Mexico City. N Engl J Med. 2016;375(20):1961–1971. doi: 10.1056/NEJMoa1605368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bracco PA, Gregg EW, Rolka DB, Schmidt MI, Barreto SM, Lotufo PA, et al. A nationwide analysis of the excess death attributable to diabetes in Brazil. J Glob Health. 2020;10(1):010401. doi: 10.7189/jogh.10.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrillo-Larco RM, Barengo NC, Albitres-Flores L, Bernabe-Ortiz A. The risk of mortality among people with type 2 diabetes in Latin America: a systematic review and meta-analysis of population-based cohort studies. Diabetes Metab Res Rev. 2019;35(4):e3139. doi: 10.1002/dmrr.3139. [DOI] [PubMed] [Google Scholar]
- 13.Agudelo-Botero M, Dávila-Cervantes CA. Burden of mortality due to diabetes mellitus in Latin America 2000–2011: the case of Argentina, Chile, Colombia, and Mexico. Gac Sanit. 2015. 10.1016/j.gaceta.2015.01.015. [DOI] [PubMed]
- 14.Panamerican Health Organization. Mortality in the Americas (https://www.paho.org/salud-en-las-americas-2017/?tag=cardiovascular-diseases). Pan American Health Organization, Washington, D.C. 2017. [Last Accessed on August 6, 2020].
- 15.Aschner P. Diabetes trends in Latin America. Diabetes Metab Res Rev. 2002;18(Suppl 3):S27–S31. doi: 10.1002/dmrr.280. [DOI] [PubMed] [Google Scholar]
- 16.Fernando L, Pamela S, Alejandra L. Cardiovascular disease in Latin America: the growing epidemic. Prog Cardiovasc Dis. 2014;57(3):262–267. doi: 10.1016/j.pcad.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Conomos MP, Laurie CA, Stilp AM, Gogarten SM, McHugh CP, Nelson SC, et al. Genetic diversity and association studies in US Hispanic/Latino populations: qpplications in the Hispanic Community Health Study/Study of Latinos. Am J Hum Genet. 2016;98(1):165–184. doi: 10.1016/j.ajhg.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avilés-Santa ML, Colón-Ramos U, Lindberg NM, Mattei J, Pasquel FJ, Pérez CM. From Sea to Shining Sea and the Great Plains to Patagonia: A Review on Current Knowledge of Diabetes Mellitus in Hispanics/Latinos in the US and Latin America. Front Endocrinol (Lausanne) 2017;8:298. doi: 10.3389/fendo.2017.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Castelan C, Lopez-Calva LF, Lustig N, Valderrama D. Understanding the dynamics of labor income inequality in Latin America (http://repec.tulane.edu/RePEc/pdf/tul1608.pdf). Tulane Economics Working Paper Series 2016. Accessed 4 August 2020 and 5 October 2020
- 20.Abramo L, Cecchini S, Ullmann H. Addressing health inequalities in Latin America: the role of social protection. Cien Saude Colet. 2020;25(5):1587–1598. doi: 10.1590/1413-81232020255.32802019. [DOI] [PubMed] [Google Scholar]
- 21.Greene J, Guanais F. An examination of socioeconomic equity in health experiences in six Latin American and Caribbean countries. Rev Panam Salud Publica. 2018;42:e127. doi: 10.26633/rpsp.2018.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houghton N, Bascolo E, Del Riego A. Socioeconomic inequalities in access barriers to seeking health services in four Latin American countries. Rev Panam Salud Publica. 2020;44:e11. doi: 10.26633/rpsp.2020.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes Association. 6. Glycemic targets: <em>Standards of medical care in diabetes—2020</em>. Diabetes Care. 2020;43(Supplement 1):S66-S76. 10.2337/dc20-S006. [DOI] [PubMed]
- 24.American Diabetes Association. 10. Cardiovascular disease and risk management: <em>Standards of Medical Care in Diabetes—2020</em>. Diabetes Care. 2020;43(Supplement 1):S111-S34. 10.2337/dc20-S010. [DOI] [PubMed]
- 25.American Diabetes Association. 11. Microvascular complications and foot care: <em>Standards of Medical Care in Diabetes−2020</em>. Diabetes Care. 2020;43(Supplement 1):S135-S51. 10.2337/dc20-S011. [DOI] [PubMed]
- 26.Asociación Latino Americana de Diabetes (ALAD). Guias ALAD sobre Diagnostico, Control y Tratamiento de la Diabetes Mellitus Tipo 2 con Medicina Basada en Evidencia, Edicion 2019 (www.revistaalad.com). Revista de la Asociacion Latinomericana de Diabetes (ALAD). 2019.
- 27.Paniagua M, Vizcarrondo R. Diabetes mellitus in Puerto Rico; statistical survey of 700 cases. Diabetes. 1952;1(5):373–377. doi: 10.2337/diab.1.5.373. [DOI] [PubMed] [Google Scholar]
- 28.West KM, Kalbfleisch JM. Glucose tolerance, nutrition, and diabetes in Uruguay, Venezuela, Malaya, and East Pakistan. Diabetes. 1966;15(1):9–18. doi: 10.2337/diab.15.1.9. [DOI] [PubMed] [Google Scholar]
- 29.Neri R. Panorama of chronic disorders in the República Oriental de Uruguay. Salud Publica Mex. 1967;9(5):687–707. [PubMed] [Google Scholar]
- 30.Omran AR. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem Fund Q. 1971;49(4):509–538. doi: 10.2307/3349375. [DOI] [PubMed] [Google Scholar]
- 31.Frenk J, Lozano R, Bobadilla JL. The epidemiological transition in Latin America. Notas Poblacion. 1994;22(60):79–101. [PubMed] [Google Scholar]
- 32.Barceló A, Rajpathak S. Incidence and prevalence of diabetes mellitus in the Americas. Rev Panam Salud Publica. 2001;10(5):300–308. doi: 10.1590/s1020-49892001001100002. [DOI] [PubMed] [Google Scholar]
- 33.Olaiz-Fernandez G, Rojas R, Aguilar-Salinas CA, Rauda J, Villalpando S. Diabetes Mellitus en adultos mexicanos. Resultados de la Encuesta Nacional de Salud 2000. Salud Publica Mex. 2007;49:S331–S3S7. doi: 10.1590/S0036-36342007000900004. [DOI] [Google Scholar]
- 34.Meaney E, Lara-Esqueda A, Ceballos-Reyes GM, Asbun J, Vela A, Martínez-Marroquín Y, et al. Cardiovascular risk factors in the urban Mexican population: the FRIMEX study. Public Health. 2007;121(5):378–384. doi: 10.1016/j.puhe.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Stoddard P, Handley MA, Vargas Bustamante A, Schillinger D. The influence of indigenous status and community indigenous composition on obesity and diabetes among Mexican adults. Soc Sci Med. 2011;73(11):1635–1643. doi: 10.1016/j.socscimed.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Secretaría de Salud de México, Instituto Nacional de Salud Pública (INSP). Encuesta Nacional de Salud y Nutricion - 2006 (ENSANUT 2006) (https://ensanut.insp.mx/encuestas/ensanut2006/index.php). In: Instituto Nacional de Salud Publica SdSdM, editor. Mexico2006. [Last Accessed on August 6, 2020].
- 37.Kumar A, Wong R, Ottenbacher KJ, Al SS. Prediabetes, undiagnosed diabetes, and diabetes among Mexican adults: findings from the Mexican Health and Aging Study. Ann Epidemiol. 2016;26(3):163–170. doi: 10.1016/j.annepidem.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Secretaría de Salud de México, Instituto Nacional de Salud Pública (INSP). Encuesta Nacional de Salud y Nutricion - 2012 (ENSANUT 2012) (https://ensanut.insp.mx/encuestas/ensanut2012/index.php). In: Instituto Nacional de Salud Publica SdSdM, editor. Mexico2012. [Last Accessed on August 6, 2020].
- 39.Bello-Chavolla OY, Rojas-Martinez R, Aguilar-Salinas CA, Hernández-Avila M. Epidemiology of diabetes mellitus in Mexico. Nutr Rev. 2017;75(suppl 1):4–12. doi: 10.1093/nutrit/nuw030. [DOI] [PubMed] [Google Scholar]
- 40.Secretaría de Salud de México, Instituto Nacional de Salud Pública (INSP). Encuesta Nacional de Salud y Nutricion de Medio Camino 2016 (ENSANUT 2016) (https://ensanut.insp.mx/encuestas/ensanut2016/doctos/informes/ENSANUT2016ResultadosNacionales.pdf) (https://www.gob.mx/salud). Mexico, D. F., Mexico2016. [Last Accessed on August 6, 2020].
- 41.Basto-Abreu A, Barrientos-Gutiérrez T, Rojas-Martínez R, Aguilar-Salinas CA, López-Olmedo N, De la Cruz-Góngora V, et al. Prevalence of diabetes and poor glycemic control in Mexico: results from Ensanut 2016. Salud Publica Mex. 2020;62(1):50–59. doi: 10.21149/10752. [DOI] [PubMed] [Google Scholar]
- 42.Brenes-Camacho G, Rosero-Bixby L. Diabetes mellitus en adultos mayores costarricenses. Poblacion y Salud en Mesoamerica - Revista Electronica (https://revistas.ucr.ac.cr/index.php/psm) . 2007;5(1, Article number 2, jul-dic 2007):12. [Last Accessed on October 5, 2020].
- 43.Brenes-Camacho G, Rosero-Bixby L. Metabolic control in a nationally representative diabetic elderly sample in Costa Rica: patients at community health centers vs patients at other health care settings. BMC Int Health Hum Rights. 2008;8:5. doi: 10.1186/1472-698x-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong-McClure R, Gregg EW, Barcelo A, Sanabria-Lopez L, Lee K, Abarca-Gomez L, et al. Prevalence of diabetes and impaired fasting glucose in Costa Rica: Costa Rican National Cardiovascular Risk Factors Survey, 2010. J Diabetes. 2016;8(5):686–692. doi: 10.1111/1753-0407.12348. [DOI] [PubMed] [Google Scholar]
- 45.Orantes CM, Herrera R, Almaguer M, Brizuela EG, Núñez L, Alvarado NP, et al. Epidemiology of chronic kidney disease in adults of Salvadoran agricultural communities. MEDICC Rev. 2014;16(2):23–30. doi: 10.37757/MR2014.V16.N2.5. [DOI] [PubMed] [Google Scholar]
- 46.Orantes Navarro CM, Herrera Valdés R, López MA, Calero DJ. Fuentes de Morales J, Alvarado Ascencio NP et al. Epidemiological characteristics of chronic kidney disease of non-traditional causes in women of agricultural communities of El Salvador. Clin Nephrol. 2015;83(7 Suppl 1):24–31. doi: 10.5414/cnp83s024. [DOI] [PubMed] [Google Scholar]
- 47.Ministerio de Salud de El Salvador. Encuesta Nacional Sobre Enfermedades Cronicas No Transmisibles en la Poblacion Adulta de El Salvador (ENECA-ELS 2015) (https://www.salud.gob.sv/archivos/comunicaciones/archivos_comunicados2017/pdf/presentaciones_evento20032017/01-ENECA-ELS-2015.pdf) (https://www.salud.gob.sv/) . San Salvador, El Salvador2015. [Last Accessed on August 6, 2020].
- 48.Chen D, Rivera-Andrade Á, González J, Burt D, Mendoza-Montano C, Patrie J, et al. Prevalence of risk factors for noncommunicable diseases in an indigenous community in Santiago Atitlán, Guatemala. Rev Panam Salud Publica. 2017;41:e7. doi: 10.26633/rpsp.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ministerio de Salud Pública y Asistencia Social de Guatemala. Encuesta Nacional de Prevalencia de Enfermedades No Transmisibles y sus Factores de Riesgo en Poblacion de 18 anos o mas. Dominio I: Urbano Metropolitana, Departamento de Guatemala, diciembre 2015 (http://epidemiologia.mspas.gob.gt/files/Publicaciones%202019/ENT/STEPS%20ENT%20MSPAS%202018.pdf) (https://www.mspas.gob.gt/) . Guatemala, Guatemala2018. [Last Accessed on August 6, 2020].
- 50.Bream KDW, Breyre A, Garcia K, Calgua E, Chuc JM, Taylor L. Diabetes prevalence in rural Indigenous Guatemala: a geographic-randomized cross-sectional analysis of risk. PLoS One. 2018;13(8):e0200434. doi: 10.1371/journal.pone.0200434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montalvan Sanchez EE, Urrutia SA, Rodriguez AA, Duarte G, Murillo A, Rivera R, et al. Cardiovascular risk assessment in the resource limited setting of Western Honduras: an epidemiological perspective. Int J Cardiol Heart Vasc. 2020;27:100476. doi: 10.1016/j.ijcha.2020.100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laux TS, Bert PJ, González M, Unruh M, Aragon A, Lacourt CT. Prevalence of hypertension and associated risk factors in six Nicaraguan communities. Ethn Dis. 2012;22(2):129–135. [PMC free article] [PubMed] [Google Scholar]
- 53.Lebov JF, Valladares E, Peña R, Peña EM, Sanoff SL, Cisneros EC, et al. A population-based study of prevalence and risk factors of chronic kidney disease in León. Nicaragua Can J Kidney Health Dis. 2015;2:6. doi: 10.1186/s40697-015-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferguson R, Leatherman S, Fiore M, Minnings K, Mosco M, Kaufman J, et al. Prevalence and risk factors for CKD in the general population of southwestern Nicaragua. J Am Soc Nephrol. 2020;31(7):1585–1593. doi: 10.1681/asn.2019050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mc Donald PA, Montenegro GJ, Cruz GC, Moreno de Rivera AL, Cumbrera OA. Prevalence, sociodemographic distribution, treatment and control of diabetes mellitus in Panama. Diabetol Metab Syndr. 2013;5(1):69. doi: 10.1186/1758-5996-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Da Silva Coqueiro R, Rodrigues Barbosa A, Ferreti Borgatto A. Nutritional status, health conditions and socio-demographic factors in the elderly of Havana, Cuba: data from SABE survey. J Nutr Health Aging. 2010;14(10):803–808. doi: 10.1007/s12603-010-0126-6. [DOI] [PubMed] [Google Scholar]
- 57.Llibre Jde J, Valhuerdi A, Calvo M, García RM, Guerra M, Laucerique T, et al. Dementia and other chronic diseases in older adults in Havana and Matanzas: the 10/66 study in Cuba. MEDICC Rev. 2011;13(4):30–37. doi: 10.37757/MR2011V13.N4.7. [DOI] [PubMed] [Google Scholar]
- 58.Herrera-Valdés R, Almaguer M, Chipi J, Toirac X, Martínez O, Castellanos O, et al. Prevalence of obesity and its association with chronic kidney disease, hypertension and diabetes mellitus. Isle of Youth Study (ISYS), Cuba. MEDICC Rev. 2008;10(2):14–20. doi: 10.37757/MR2008.V10.N2.7. [DOI] [PubMed] [Google Scholar]
- 59.Armas NB, Hernández Yde L, Dueñas AF, García Rde L, Castillo A. Cardiovascular risk among older women in a Havana Health Area. MEDICC Rev. 2008;10(2):21–26. doi: 10.37757/MR2008.V10.N2.8. [DOI] [PubMed] [Google Scholar]
- 60.Bonet-Gorbea M, Varona P, Chang La Rosa M, Garcia Rocha RG, Suárez-Medina R, Montes de Oca M et al. III Encuesta Nacional de factores de riesgo y actividades preventivas de enfermedades no trasmisibles. Cuba 2010–2011 (https://www.researchgate.net/publication/325370475_III_Encuesta_Nacional_de_factores_de_riesgo_y_actividades_preventivas_de_enfermedades_no_trasmisibles_Cuba_2010-2011) . La Habana, Cuba: ECIMED - Editorial Ciencias Medicas; 2014. [Last Accessed on August 6, 2020].
- 61.Ministerio de Salud Pública de la República Dominicana. Encuesta Demografica y de Salud - Republica Dominicana, 2013. 2014. (https://dhsprogram.com/pubs/pdf/FR292/FR292.pdf) [Last Accessed on August 6, 2020].
- 62.Carrère P, Fagour C, Sportouch D, Gane-Troplent F, Hélène-Pelage J, Lang T, et al. Diabetes mellitus and obesity in the French Caribbean: a special vulnerability for women? Women Health. 2018;58(2):145–159. doi: 10.1080/03630242.2017.1282396. [DOI] [PubMed] [Google Scholar]
- 63.Jean-Baptiste ED, Larco P, Charles-Larco N, Vilgrain C, Simon D, Charles R. Glucose intolerance and other cardiovascular risk factors in Haiti. Prevalence of diabetes and hypertension in Haiti (PREDIAH) Diabetes Metab. 2006;32(5 Pt 1):443–451. doi: 10.1016/s1262-3636(07)70302-6. [DOI] [PubMed] [Google Scholar]
- 64.Burkhalter F, Sannon H, Mayr M, Dickenmann M, Ernst S. Prevalence and risk factors for chronic kidney disease in a rural region of Haiti. Swiss Med Wkly. 2014;144:w14067. doi: 10.4414/smw.2014.14067. [DOI] [PubMed] [Google Scholar]
- 65.Ministère de la Santé Publique et de la Population (MSPP) H. Haiti - Enquête Mortalité, Morbidité et Utilisation des Services (EMMUS-VI 2016–2017) (https://www.dhsprogram.com/pubs/pdf/FR326/FR326.pdf) (https://www.mspp.gouv.ht/documentation.php?start=0&debut=&fin=&categorie=0&mot=enquete) . In: (MSPP) MdlSPedlP, editor.2018. [Last Accessed on August 6, 2020].
- 66.Geiss L, Li Y, Kirtland K, Barker L, Burrows NR, Gregg EW. Increasing prevalence of diagnosed diabetes--United States and Puerto Rico, 1995–2010. MMWR Morb Mortal Wkly Rep. 2012;61(45):918–921. [PubMed] [Google Scholar]
- 67.Pérez CM, Soto-Salgado M, Suárez E, Guzmán M, Ortiz AP. High prevalence of diabetes and prediabetes and their coexistence with cardiovascular risk factors in a Hispanic Community. J Immigr Minor Health. 2015;17(4):1002–1009. doi: 10.1007/s10903-014-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pickens CM, Pierannunzi C, Garvin W, Town M. Surveillance for certain health behaviors and conditions among states and selected local areas - behavioral risk factor surveillance system, United States, 2015. MMWR Surveill Summ. 2018;67(9):1–90. doi: 10.15585/mmwr.ss6709a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cruz NI, Santiago E, Abdul-Hadi A. Prevalence of diabetes mellitus in the surgical population of the University of Puerto Rico Affiliated Hospitals: a study using the surgery database. P R Health Sci J. 2016;35(3):160–164. [PubMed] [Google Scholar]
- 70.Ministerio de Salud de Argentina. Segunda Encuesta Nacional de Factores de Riesgo Para Enfermedades No Transmisibles, Argentina 2009 (http://www.msal.gob.ar/images/stories/bes/graficos/0000001600cnt-2da-encuesta-nacional-factores-riesgo_2009_informe-completo.pdf) . Buenos Aires, Argentina2011. [Last Accessed on August 6, 2020].
- 71.Ministerio de Salud de Argentina. Tercera Encuesta Nacional de Factores de Riesgo Para Enfermedades No Transmisibles, Argentina 2013 (http://www.msal.gob.ar/images/stories/bes/graficos/0000000544cnt-3ra-encuesta-nacional-factores-riesgo_2013_informe-completo.pdf). Buenos Aires, Argentina: 2015. [Last Accessed on August 6, 2020].
- 72.Ministerio de Salud de Argentina. Cuarta Encuesta Nacional de Factores de Riesgo Para Enfermedades No Transmisibles, 2018 (http://www.msal.gob.ar/images/stories/bes/graficos/0000001622cnt-2019-10_4ta-encuesta-nacional-factores-riesgo.pdf). Buenos Aires, Argentina2019. [Last Accessed on August 6, 2020].
- 73.Barceló A, Daroca MC, Ribera R, Duarte E, Zapata A, Vohra M. Diabetes in Bolivia. Rev Panam Salud Publica. 2001;10(5):318–323. doi: 10.1590/s1020-49892001001100004. [DOI] [PubMed] [Google Scholar]
- 74.Kaplan H, Thompson RC, Trumble BC, Wann LS, Allam AH, Beheim B, et al. Coronary atherosclerosis in indigenous South American Tsimane: a cross-sectional cohort study. Lancet. 2017;389(10080):1730–1739. doi: 10.1016/s0140-6736(17)30752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mendes Tde A, Goldbaum M, Segri NJ, Barros MB, Cesar CL, Carandina L, et al. Diabetes mellitus: factors associated with prevalence in the elderly, control measures and practices, and health services utilization in São Paulo, Brazil. Cad Saude Publica. 2011;27(6):1233–1243. doi: 10.1590/s0102-311x2011000600020. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt MI, Hoffmann JF, de Fátima Sander Diniz M, Lotufo PA, Griep RH, Bensenor IM, et al. High prevalence of diabetes and intermediate hyperglycemia - The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Diabetol Metab Syndr. 2014;6:123. doi: 10.1186/1758-5996-6-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dal Fabbro AL, Franco LJ, da Silva AS, Sartorelli DS, Soares LP, Franco LF, et al. High prevalence of type 2 diabetes mellitus in Xavante Indians from Mato Grosso. Brazil Ethn Dis. 2014;24(1):35–40. [PubMed] [Google Scholar]
- 78.Ministério do Planejamento Orçamento e Gestão, -IBGE. Pesquisa Nacional de Saude 2013. Percepcao do estado de saude, estilos de vida e doencas cronicas. (https://www.saude.gov.br/vigilancia-em-saude/vigilancia-de-doencas-cronicas-nao-transmissiveis-dcnt/pesquisa-nacional-de-saude-pns). Rio de Janeiro, Brazil: Instituto Brasileiro de Geografia e Estatistica -IBGE; 2014. [Last Accessed on August 6, 2020].
- 79.de Oliveira AP, Maia EG, Silva FM, Martins APB, Claro RM. Needed Improvements in Diabetes Prevention and Management in Brazil. Prev Chronic Dis. 2018;15:E153. doi: 10.5888/pcd15.180269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ministério da Saúde do Brasil. Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico (VIGITEL BRASIL 2018) (https://portalarquivos2.saude.gov.br/images/pdf/2019/julho/25/vigitel-brasil-2018.pdf) (http://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2018_vigilancia_fatores_risco.pdf). Brasilia, Brazil: 2019. [Last Accessed on August 6, 2020].
- 81.Ministério da Saúde do Brasil. Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico (VIGITEL BRASIL 2019) (https://portalarquivos.saude.gov.br/images/pdf/2020/April/27/vigitel-brasil-2019-vigilancia-fatores-risco.pdf) (http://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2019_vigilancia_fatores_risco.pdf). Brasilia, Brazil: 2019. [Last Accessed on August 6, 2020].
- 82.Santos JL, Pérez-Bravo F, Carrasco E, Calvillán M, Albala C. Low prevalence of type 2 diabetes despite a high average body mass index in the Aymara natives from Chile. Nutrition. 2001;17(4):305–309. doi: 10.1016/s0899-9007(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 83.Carrasco EP, Pérez FB, Angel BB, Albala CB, Santos JL, Larenas GY, et al. Prevalence of type 2 diabetes and obesity in two Chilean aboriginal populations living in urban zones. Rev Med Chil. 2004;132(10):1189–1197. doi: 10.4067/s0034-98872004001000005. [DOI] [PubMed] [Google Scholar]
- 84.Cuevas A, Molina A, Rigotti A, Miquel JF, Marshall G, Reyes S, et al. Trends in obesity and diabetes prevalence in a Chilean urban population: 1993–2001. Metab Syndr Relat Disord. 2008;6(3):219–222. doi: 10.1089/met.2008.0018. [DOI] [PubMed] [Google Scholar]
- 85.Ministerio de Salud de Chile. Encuesta Nacional de Salud, ENS Chile 2009–2010 (https://cursoemergencia.files.wordpress.com/2013/04/encuesta-nacional-de-salud-2009-2010.pdf) (https://www.minsal.cl/) (http://epi.minsal.cl/resultados-encuestas/). Santiago, Chile2009–2010. [Last Accessed on August 6, 2020].
- 86.Ministerio de Salud de Chile. Encuesta Nacional de Salud 2016–2017 (https://www.minsal.cl/tercera-encuesta-nacional-de-salud-establece-prioridades-sanitarias-para-la-proxima-decada/) (https://www.minsal.cl/) (http://epi.minsal.cl/resultados-encuestas/). Santiago, Chile2016–2017. [Last Accessed on August 6, 2020].
- 87.Rodriguez J, Ruiz F, Penaloza E, Eslava J, Gomez LC, Sanchez H et al. Encuesta Nacional de Salud 2007. Resultados Nacionales (https://www.minsalud.gov.co/Documentos%20y%20Publicaciones/ENCUESTA%20NACIONAL.pdf). (https://www.minsalud.gov.co/Paginas/default.aspx). Bogota, Colombia: Fundacion Cultural Javeriana de Artes Graficas -JAVEGRAF-; 2009. [Last Accessed on August 6, 2020].
- 88.Camacho PA, Gomez-Arbelaez D, Otero J, González-Gómez S, Molina DI, Sanchez G, et al. Self-reported prevalence of chronic non-communicable diseases in relation to socioeconomic and educational factors in Colombia: a community-based study in 11 departments. Glob Heart. 2020;15(1):35. doi: 10.5334/gh.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Profamilia. Encuesta Nacional de Demografia y Salud (ENDS 2010). Bogota, Colombia: Printex Impresores, Ltda.; 2011.
- 90.Orces CH, Lorenzo C. Prevalence of prediabetes and diabetes among older adults in Ecuador: analysis of the SABE survey. Diabetes Metab Syndr. 2018;12(2):147–153. doi: 10.1016/j.dsx.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Ministerio de Salud Pública de Ecuador. Encuesta Nacional de Salud y Nutricion del Ecuador: ENSANUT-ECU 2012 (https://www.ecuadorencifras.gob.ec/documentos/web-inec/Estadisticas_Sociales/ENSANUT/MSP_ENSANUT-ECU_06-10-2014.pdf) [Last Accessed on August 6, 2020] (https://www.salud.gob.ec/). Quito, Ecuador2013.
- 92.Tufton N, Chowdhury T. Prevalence of diabetes on Santa Cruz Island in Galapagos Archipelago. Prev Chronic Dis. 2015;12:E94. doi: 10.5888/pcd12.150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alexander A, Florez H, Ladera N. Hyperglycemia and dyslipidemia of Isabela, Galápagos, Ecuador: a pilot study of cardiovascular risk factors in an Isolated Island community. Diabetes Res Clin Pract. 2017;130:108–112. doi: 10.1016/j.diabres.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 94.Bonilla-Sierra P, Vargas-Martínez AM, Davalos-Batallas V, Leon-Larios F, Lomas-Campos MD. Chronic diseases and associated factors among older adults in Loja, Ecuador. Int J Environ Res Public Health. 2020;17(11). 10.3390/ijerph17114009. [DOI] [PMC free article] [PubMed]
- 95.Chaves G, Brítez N, Maciel V, Klinkhof A, Mereles D. Prevalence of cardiovascular risk factors in an urban ambulatory adult population: AsuRiesgo study, Paraguay. Rev Panam Salud Publica. 2015;38(2):136–143. [PubMed] [Google Scholar]
- 96.Ministerio de Salud Pública y Bienestar Social de Paraguay. Primera Encuesta Nacional de Factores de Riesgo de Enfermedades No Transmisibles en Poblacion General (http://portal.mspbs.gov.py/dvent/encuesta-nacional-factores-de-riesgo-2011/) (www.mspbs.gov.py). Asuncion, Paraguay2012. [Last Accessed on August 6, 2020].
- 97.Segura-Vega L. Agusti, R., Parodi-Ramirez, J., e investigadores del estudio TORNASOL. Factores de Riesgo de las Enfermedades Cardiovasculares en el Peru (Estudio TORNASOL) Rev Peruana Cardiol. 2006;32(2):82–128. [Google Scholar]
- 98.Ministerio de Salud de Peru. Encuesta Nacional de Indicadores Nutricionales, Bioquimicos, Socioeconomicos y Culturales Relacionados con las Enfermedades Cronico Degenerativas (ENINBSC-ECNT 2005) (https://www.ins.gob.pe/insvirtual/BiblioDig/MISC/ENIN/IFENIN.pdf) (https://www.gob.pe/minsa/). In: Peru MoHo, editor. Lima, Peru2006. [Last Accessed on August 6, 2020].
- 99.Miranda JJ, Gilman RH, Smeeth L. Differences in cardiovascular risk factors in rural, urban and rural-to-urban migrants in Peru. Heart. 2011;97(10):787–796. doi: 10.1136/hrt.2010.218537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Segura-Vega L, Agusti R, Ruiz-Mori E, e investigadores del Estudio Factores de Riesgo de las enfermedades cardiovasculares en el Peru II. Estudio TORNASOL II comparado con TORNASOL I despues de cinco anos. Rev Peruana Cardiol. 2013;39(1):6–59. [Google Scholar]
- 101.Seclen SN, Rosas ME, Arias AJ, Huayta E, Medina CA. Prevalence of diabetes and impaired fasting glucose in Peru: report from PERUDIAB, a national urban population-based longitudinal study. BMJ Open Diabetes Res Care. 2015;3(1):e000110. doi: 10.1136/bmjdrc-2015-000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bernabé-Ortiz A, Carrillo-Larco RM, Gilman RH, Miele CH, Checkley W, Wells JC, et al. Geographical variation in the progression of type 2 diabetes in Peru: The CRONICAS Cohort Study. Diabetes Res Clin Pract. 2016;121:135–145. doi: 10.1016/j.diabres.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krishnadath IS, Smits CC, Jaddoe VW, Hofman A, Toelsie JR. A National surveillance survey on noncommunicable disease risk factors: suriname health study protocol. JMIR Res Protoc. 2015;4(2):e75. doi: 10.2196/resprot.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Minderhoud J, Pawiroredjo JC. Bueno de Mesquita-Voigt AM, Themen HC, Siban MR, Forster-Pawiroredjo CM et al. Diabetes and diabetic retinopathy in people aged 50 years and older in the Republic of Suriname. Br J Ophthalmol. 2016;100(6):814–818. doi: 10.1136/bjophthalmol-2015-307177. [DOI] [PubMed] [Google Scholar]
- 105.Ministerio de Salud Pública del Uruguay. Primera Encuesta Nacional de Factores de Riesgo de Enfermedades Cronicas No Transmisibles - ENFRECNT (https://www.gub.uy/ministerio-salud-publica/comunicacion/publicaciones/primera-encuesta-nacional-de-factores-de-riesgo-de-enfermedades-cronicas). 2006. [Last Accessed on August 6, 2020].
- 106.Fort MP, Alvarado-Molina N, Peña L, Mendoza Montano C, Murrillo S, Martínez H. Barriers and facilitating factors for disease self-management: a qualitative analysis of perceptions of patients receiving care for type 2 diabetes and/or hypertension in San José, Costa Rica and Tuxtla Gutiérrez. Mexico BMC Fam Pract. 2013;14:131. doi: 10.1186/1471-2296-14-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ministerio de Salud Pública del Uruguay. Segunda Encuesta Nacional de Factores de Riesgo de Enfermedades No Transmisibles – ENFRENT (https://www.gub.uy/ministerio-salud-publica/comunicacion/publicaciones/2da-encuesta-nacional-de-factores-de-riesgo-de-enfermedades-no). 2016. [Last Accessed on August 6, 2020].
- 108.Nieto-Martínez R, Mechanick JI, Brajkovich I, Ugel E, Risques A, Florez H, et al. Prevalence of diabetes in three regions of Venezuela. The VEMSOLS study results. Prim Care Diabetes. 2018;12(2):126–132. doi: 10.1016/j.pcd.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 109.Menéndez J, Guevara A, Arcia N, León Díaz EM, Marín C, Alfonso JC. Chronic diseases and functional limitation in older adults: a comparative study in seven cities of Latin America and the Caribbean. Rev Panam Salud Publica. 2005;17(5–6):353–361. doi: 10.1590/s1020-49892005000500007. [DOI] [PubMed] [Google Scholar]
- 110.Escobedo J, Buitrón LV, Velasco MF, Ramírez JC, Hernández R, Macchia A, et al. High prevalence of diabetes and impaired fasting glucose in urban Latin America: the CARMELA Study. Diabet Med. 2009;26(9):864–871. doi: 10.1111/j.1464-5491.2009.02795.x. [DOI] [PubMed] [Google Scholar]
- 111.Barcelo A, Gregg EW, Gerzoff RB, Wong R, Perez Flores E, Ramirez-Zea M, et al. Prevalence of diabetes and intermediate hyperglycemia among adults from the first multinational study of noncommunicable diseases in six Central American countries: the Central America Diabetes Initiative (CAMDI) Diabetes Care. 2012;35(4):738–740. doi: 10.2337/dc11-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salas A, Acosta D, Ferri CP, Guerra M, Huang Y, Jacob KS, et al. The Prevalence, correlates, detection and control of diabetes among older people in low and middle income countries. a 10/66 Dementia Research Group Population-Based survey. PLoS One. 2016;11(2):e0149616. doi: 10.1371/journal.pone.0149616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rubinstein AL, Irazola VE, Calandrelli M, Elorriaga N, Gutierrez L, Lanas F, et al. Multiple cardiometabolic risk factors in the Southern Cone of Latin America: a population-based study in Argentina, Chile, and Uruguay. Int J Cardiol. 2015;183:82–88. doi: 10.1016/j.ijcard.2015.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Macinko J, Andrade FCD, Nunes BP, Guanais FC. Primary care and multimorbidity in six Latin American and Caribbean countries. Rev Panam Salud Publica. 2019;43:e8. doi: 10.26633/rpsp.2019.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.American Diabetes Association. 2. Classification and diagnosis of diabetes: <em>Standards of Medical Care in Diabetes—2020</em>. Diabetes Care. 2020;43(Supplement 1):S14-S31. 10.2337/dc20-S002. [DOI] [PubMed]
- 116.Camejo M, Garcia A, Rodriguez E, Carrizales M. E., Chique, J. Vision Epidemiologica de la Diabetes Mellitus en Venezuela. Registro Epidemiologico y Propuesta de Registro. Programas de Deteccion Precoz. Rev Venez Endocrinol Metabol. 2012;10:2–6. [Google Scholar]
- 117.Rubinstein A, Gutierrez L, Beratarrechea A, Irazola VE. Increased prevalence of diabetes in Argentina is due to easier health care access rather than to an actual increase in prevalence. PLoS One. 2014;9(4):e92245. doi: 10.1371/journal.pone.0092245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Coutinho WF, Silva Júnior WS. Diabetes Care in Brazil. Ann Glob Health. 2015;81(6):735–741. doi: 10.1016/j.aogh.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 119.Nieto-Martínez R, González-Rivas JP, Lima-Martínez M, Stepenka V, Rísquez A, Mechanick JI. Diabetes Care in Venezuela. Ann Glob Health. 2015;81(6):776–791. doi: 10.1016/j.aogh.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 120.Mc Donald Posso AJ, Bradshaw Meza RA, Mendoza Morales EA, Jaen Y, Cumbrera Ortega A, Mendoza Posada EJ. Diabetes in Panama: epidemiology, risk factors, and clinical management. Ann Glob Health. 2015;81(6):754–764. doi: 10.1016/j.aogh.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 121.Villena JE. Diabetes Mellitus in Peru. Ann Glob Health. 2015;81(6):765–775. doi: 10.1016/j.aogh.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 122.Telo GH, Cureau FV, de Souza MS, Andrade TS, Copês F, Schaan BD. Prevalence of diabetes in Brazil over time: a systematic review with meta-analysis. Diabetol Metab Syndr. 2016;8(1):65. doi: 10.1186/s13098-016-0181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carrillo-Larco RM, Bernabé-Ortiz A. Type 2 diabetes mellitus in peru: a systematic review of prevalence and incidence in the general population. Rev Peru Med Exp Salud Publica. 2019;36(1):26–36. doi: 10.17843/rpmesp.2019.361.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barceló A. Diabetes in the Americas. Epidemiol Bull. 2001;22(2):1–3. [PubMed] [Google Scholar]
- 125.Bello-Chavolla OY, Aguilar-Salinas CA. Diabetes in Latin America. In: Dagogo-Jack S, editor. Diabetes mellitus in developing countries and underserved communities. Cham: Springer International Publishing; 2017. pp. 101–126. [Google Scholar]
- 126.Burroughs Pena MS, Bernabé-Ortiz A, Carrillo-Larco RM, Sánchez JF, Quispe R, Pillay TD, et al. Migration, urbanisation and mortality: 5-year longitudinal analysis of the PERU MIGRANT study. J Epidemiol Community Health. 2015;69(7):715–718. doi: 10.1136/jech-2015-205657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ruiz-Alejos A, Carrillo-Larco RM, Miranda JJ, Anderson CAM, Gilman RH, Smeeth L, et al. Addressing the impact of urban exposure on the incidence of type 2 diabetes mellitus: The PERU MIGRANT Study. Sci Rep. 2018;8(1):5512. doi: 10.1038/s41598-018-23812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carrera Boada CA, Martínez-Moreno JM. Pathophysiology of diabetes mellitus type 2: beyond the duo "insulin resistance-secretion deficit". Nutr Hosp. 2013;28(Suppl 2):78–87. doi: 10.3305/nh.2013.28.sup2.6717. [DOI] [PubMed] [Google Scholar]
- 129.Mendenhall E, Kohrt BA, Norris SA, Ndetei D, Prabhakaran D. Non-communicable disease syndemics: poverty, depression, and diabetes among low-income populations. Lancet. 2017;389(10072):951–963. doi: 10.1016/s0140-6736(17)30402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Albala C, Vio F, Kain J, Uauy R. Nutrition transition in Latin America: the case of Chile. Nutr Rev. 2001;59(6):170–176. doi: 10.1111/j.1753-4887.2001.tb07008.x. [DOI] [PubMed] [Google Scholar]
- 131.Carrillo-Larco RM, Pearson-Stuttard J, Bernabe-Ortiz A, Gregg EW. The Andean Latin-American burden of diabetes attributable to high body mass index: a comparative risk assessment. Diabetes Res Clin Pract. 2020;160:107978. doi: 10.1016/j.diabres.2019.107978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abu-Bakare A, Taylor R, Gill GV, Alberti KG. Tropical or malnutrition-related diabetes: a real syndrome? Lancet. 1986;1(8490):1135–1138. doi: 10.1016/s0140-6736(86)91846-5. [DOI] [PubMed] [Google Scholar]
- 133.Morrison EY, Ragoobirsingh D, Peter SA. The unitarian hypothesis for the aetiology of diabetes mellitus. Med Hypotheses. 2006;67(5):1115–1120. doi: 10.1016/j.mehy.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 134.Llanos G, Libman I. Diabetes in the Americas. Bull Pan Am Health Organ. 1994;28(4):285–301. [PubMed] [Google Scholar]
- 135.Wissow LS. Diabetes, poverty, and Latin America. Patient Educ Couns. 2006;61(2):169–170. doi: 10.1016/j.pec.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 136.McCurley JL, Mills PJ, Roesch SC, Carnethon M, Giacinto RE, Isasi CR, et al. Chronic stress, inflammation, and glucose regulation in U.S. Hispanics from the HCHS/SOL Sociocultural Ancillary Study. Psychophysiology. 2015;52(8):1071–1079. doi: 10.1111/psyp.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lopez-Jaramillo P, Lahera V, Lopez-Lopez J. Epidemic of cardiometabolic diseases: a Latin American point of view. Ther Adv Cardiovasc Dis. 2011;5(2):119–131. doi: 10.1177/1753944711403189. [DOI] [PubMed] [Google Scholar]
- 138.Frenk J, Frejka T, Bobadilla JL, Stern C, Lozano R, Sepúlveda J, et al. The epidemiologic transition in Latin America. Bol Oficina Sanit Panam. 1991;111(6):485–496. [PubMed] [Google Scholar]
- 139.Mendoza W, Miranda JJ. Global shifts in cardiovascular disease, the epidemiologic transition, and other contributing factors: toward a new practice of global health cardiology. Cardiol Clin. 2017;35(1):1–12. doi: 10.1016/j.ccl.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Meslé F, Vallin J. From epidemiological transition to health transition. Med Trop (Mars) 2007;67(6):545–551. [PubMed] [Google Scholar]
- 141.Barreto SM, Miranda JJ, Figueroa JP, Schmidt MI, Munoz S, Kuri-Morales PP, et al. Epidemiology in Latin America and the Caribbean: current situation and challenges. Int J Epidemiol. 2012;41(2):557–571. doi: 10.1093/ije/dys017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chomsky-Higgins K, Miclau TA, Mackechnie MC, Aguilar D, Avila JR, Dos Reis FB, et al. Barriers to Clinical Research in Latin America. Front Public Health. 2017;5:57. doi: 10.3389/fpubh.2017.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Owolabi M, Miranda JJ, Yaria J, Ovbiagele B. Controlling cardiovascular diseases in low and middle income countries by placing proof in pragmatism. BMJ Glob Health. 2016;1(3). 10.1136/bmjgh-2016-000105. [DOI] [PMC free article] [PubMed]
- 144.Lazo-Porras M, Perez-Leon S, Cardenas MK, Pesantes MA, Miranda JJ, Suggs LS, et al. Lessons learned about co-creation: developing a complex intervention in rural Peru. Glob Health Action. 2020;13(1):1754016. doi: 10.1080/16549716.2020.1754016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Afshin A, Micha R, Webb M, Capewell S, Whitsel L, Rubinstein A, et al. Effectiveness of dietary policies to reduce noncommunicable diseases. In: Prabhakaran D, Anand S, Gaziano TA, Mbanya JC, Wu Y, Nugent R, et al., editors. Cardiovascular, Respiratory, and Related Disorders. Washington: The International Bank for Reconstruction and Development / The World Bank © 2017 International Bank for Reconstruction and Development / The World Bank; 2017. [PubMed] [Google Scholar]
- 146.Franco M, Orduñez P, Caballero B, Tapia Granados JA, Lazo M, Bernal JL, et al. Impact of energy intake, physical activity, and population-wide weight loss on cardiovascular disease and diabetes mortality in Cuba, 1980–2005. Am J Epidemiol. 2007;166(12):1374–1380. doi: 10.1093/aje/kwm226. [DOI] [PubMed] [Google Scholar]
- 147.Kaselitz E, Rana GK, Heisler M. Public Policies and Interventions for Diabetes in Latin America: a Scoping Review. Curr Diab Rep. 2017;17(8):65. doi: 10.1007/s11892-017-0888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Posner BM, Quatromoni PA, Franz M. Nutrition policies and interventions for chronic disease risk reduction in international settings: the INTERHEALTH nutrition initiative. Nutr Rev. 1994;52(5):179–187. doi: 10.1111/j.1753-4887.1994.tb01417.x. [DOI] [PubMed] [Google Scholar]
- 149.Rubinstein AL, Irazola VE, Poggio R, Gulayin P, Nejamis A, Beratarrechea A. Challenges and opportunities for implementation of interventions to prevent and control CVD in low-resource settings: a report from CESCAS in Argentina. Glob Heart. 2015;10(1):21–29. doi: 10.1016/j.gheart.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Siqueira-Catania A, Cezaretto A, de Barros CR, Salvador EP, Dos Santos TC, Ferreira SR. Cardiometabolic risk reduction through lifestyle intervention programs in the Brazilian public health system. Diabetol Metab Syndr. 2013;5:21. doi: 10.1186/1758-5996-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Solomons NW. Programme and policy issues related to promoting positive early nutritional influences to prevent obesity, diabetes and cardiovascular disease in later life: a developing countries view. Matern Child Nutr. 2005;1(3):204–215. doi: 10.1111/j.1740-8709.2005.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fisberg M, Kovalskys I, Salas GG, Pareja Torres RG, Yépez García MC, Cortés Sanabria LY, et al. Developing a cooperative multicenter study in Latin America: lessons learned from the Latin American Study of Nutrition and Health Project. Rev Panam Salud Publica. 2017;41:e111. doi: 10.26633/rpsp.2017.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Werneck AO, Baldew SS, Miranda JJ, Díaz Arnesto O, Stubbs B, Silva DR. Physical activity and sedentary behavior patterns and sociodemographic correlates in 116,982 adults from six South American countries: the South American physical activity and sedentary behavior network (SAPASEN) Int J Behav Nutr Phys Act. 2019;16(1):68. doi: 10.1186/s12966-019-0839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Diez Roux AV, Slesinski SC, Alazraqui M, Caiaffa WT, Frenz P, Jordán Fuchs R, et al. A novel international partnership for actionable evidence on urban health in Latin America: LAC-Urban Health and SALURBAL. Global Chall. 2019;3(4):1800013. doi: 10.1002/gch2.201800013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Taype-Rondan A, Lazo-Porras M, Moscoso-Porras M, Moreano-Sáenz M, Miranda JJ. Inadequate glycaemic control in LMIC: health system failures in Peru. Br J Gen Pract. 2016;66(645):197. doi: 10.3399/bjgp16X684541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Beran D, Miranda JJ, Cardenas MK, Bigdeli M. Health systems research for policy change: lessons from the implementation of rapid assessment protocols for diabetes in low- and middle-income settings. Health Res Policy Syst. 2015;13:41. doi: 10.1186/s12961-015-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cardenas MK, Miranda JJ, Beran D. Delivery of Type 2 diabetes care in low- and middle-income countries: lessons from Lima. Peru Diabet Med. 2016;33(6):752–760. doi: 10.1111/dme.13099. [DOI] [PubMed] [Google Scholar]
- 158.Escalante M, Gagliardino JJ, Guzmán JR, Tschiedel B. Call-to-action: timely and appropriate treatment for people with type 2 diabetes in Latin America. Diabetes Res Clin Pract. 2014;104(3):343–352. doi: 10.1016/j.diabres.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 159.López-López E, Gutiérrez-Soria D, Idrovo AJ. Evaluation of a diabetes care program using the effective coverage framework. Int J Qual Health Care. 2012;24(6):619–625. doi: 10.1093/intqhc/mzs056. [DOI] [PubMed] [Google Scholar]
- 160.Flores-Hernández S, Saturno-Hernández PJ, Reyes-Morales H, Barrientos-Gutiérrez T, Villalpando S, Hernández-Ávila M. Quality of Diabetes Care: The challenges of an increasing epidemic in Mexico. Results from two national health surveys (2006 and 2012) PLoS One. 2015;10(7):e0133958. doi: 10.1371/journal.pone.0133958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fanghänel Salmón G, Sánchez-Reyes L, Chiquete Anaya E, de la Luz CJ, Escalante HA. Multicenter international registry to evaluate the clinical practice delivered to patients with type 2 diabetes mellitus: a sub-analysis of the experience in Mexico. Gac Med Mex. 2011;147(3):226–233. [PubMed] [Google Scholar]
- 162.Hernández-Romieu AC, Elnecavé-Olaiz A, Huerta-Uribe N, Reynoso-Noverón N. Analysis of population survey for determining the factors associated with the control diabetes mellitus in Mexico. Salud Publica Mex. 2011;53(1):34–39. doi: 10.1590/s0036-36342011000100006. [DOI] [PubMed] [Google Scholar]
- 163.Lavalle-González FJ, Chiquete E, de la Luz J, Ochoa-Guzmán A, Sánchez-Orozco LV, Godínez-Gutiérrez SA. Achievement of therapeutic targets in Mexican patients with diabetes mellitus. Endocrinol Nutr. 2012;59(10):591–598. doi: 10.1016/j.endonu.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 164.Wacher NH, Silva M, Valdez L, Cruz M, Gómez-Díaz RA. Poor metabolic control in primary care. Gac Med Mex. 2016;152(3):350–356. [PubMed] [Google Scholar]
- 165.Gough E, Emmanuel E, Jenkins V, Thompson L, Perez E, Barcelo A et al. Central America Diabetes Initiative (CAMDI): survey of diabetes, hyperntion and chronic disease risk factor, Belize (https://www.paho.org/hq/dmdocuments/2012/PAHO-CAMDI-Report-BELIZE-2009.pdf) (https://www.paho.org/hq/index.php?option=com_docman&view=list&format=html&layout=default&slug=reports-4402&Itemid=270&lang=fr). Belize City, Belize: Pan American Health Organization (PAHO); 2009. [Last Accessed on August 6, 2020].
- 166.Dekker AM, Amick AE, Scholcoff C, Doobay-Persaud A. A mixed-methods needs assessment of adult diabetes mellitus (type II) and hypertension care in Toledo. Belize BMC Health Serv Res. 2017;17(1):171. doi: 10.1186/s12913-017-2075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ministerio de Salud, Caja Costarricense del Seguro Social, Organización Panamericana de la Salud. Encuesta de Diabetes, Hipertension y Factores de Riesgo de Enfermedades Cronicas, San Jose, Costa Rica, 2004. (https://www.paho.org/hq/dmdocuments/2012/OPS-CAMDI-COSTA-RICA-2009.pdf) (https://www.paho.org/hq/index.php?option=com_docman&view=list&format=html&layout=default&slug=reports-4402&Itemid=270&lang=fr). 2009. [Last Accessed on August 6, 2020].
- 168.Organización Panamericana de la Salud. Iniciativa Centroamericana de Diabetes (CAMDI): Encuesta de Diabetes, Hipertension y Factores de Riesgo de Enfermedades Cronicas. Tegucigalpa, Honduras, 2004. (https://www.paho.org/hq/dmdocuments/2010/ENCUESTA_DE_DIABETES_HONDURAS.pdf) (https://www.paho.org/hq/index.php?option=com_content&view=article&id=3070:2010-survey-on-diabetes-hypertension-chronic-disease-risk-factors-central-america&Itemid=1353&lang=en#surveys). 2009. [Last Accessed on August 6, 2020].
- 169.Orellana Pontaza P, Ramirez-Zea M, Barcelo A, Gil E, Gregg EW, Meiners M et al. Iniciativa Centroamericana de Diabetes (CAMDI): Encuesta de Diabetes, Hipertension y Factores de Riesgo de Enfermedades Cronicas, Villa Nueva, Guatemala, 2006 (https://www.paho.org/hq/dmdocuments/2012/PAHO-CAMDI-Guatemala-2009.pdf) (https://www.paho.org/hq/index.php?option=com_docman&view=list&format=html&layout=default&slug=reports-4402&Itemid=270&lang=fr). Washington, D.C.: Organizacion Panamericana de la Salud; 2007. [Last Accessed on August 6, 2020].
- 170.Amador Velazquez JJ, Pastora Arostegui M, Barcelo A, Aldighieri S, Gregg EW, Pena R et al. Iniciativa Centromericana de Diabetes (CAMDI). Encuesta de Diabetes, Hipertension y Factores de Riesgo de Enfermedades Cronicas. Managua, Nicaragua, 2010. (https://www.paho.org/hq/index.php?option=com_content&view=article&id=3070:2010-survey-on-diabetes-hypertension-chronic-disease-risk-factors-central-america&Itemid=1353&lang=en#surveys) (https://www.paho.org/hq/index.php?option=com_content&view=article&id=3040:2010-encuesta-diabetes-factores-managua-nicaragua&Itemid=1353&lang=en). Washington, D. C.: Pan American Health Organization; 2010. [Last Accessed on August 6, 2020].
- 171.Dethlefs HJ, Walker EA, Schechter CB, Dowd R, Filipi L, Garcia JF, et al. Evaluation of a program to improve intermediate diabetes outcomes in rural communities in the Dominican Republic. Diabetes Res Clin Pract. 2019;148:212–221. doi: 10.1016/j.diabres.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pérez CM, Febo-Vázquez I, Guzmán M, Ortiz AP, Suárez E. Are adults diagnosed with diabetes achieving the American Diabetes Association clinical practice recommendations? P R Health Sci J. 2012;31(1):18–23. [PMC free article] [PubMed] [Google Scholar]
- 173.Rodríguez-Vigil E, Rodríguez-Chacón M, Trabanco C, Irizarry-Ramos J. Achievement of national clinical practice recommendations among those in the Puerto Rican population with diabetes mellitus. P R Health Sci J. 2014;33(4):157–162. [PubMed] [Google Scholar]
- 174.Gagliardino JJ, Elgart J, Forti L, Guaita MS, Chantelot JM. Treat-to-target HbA(1c) and lipid profile to prolong β-cell mass/function and optimize treatment goal attainment. Diabetes Metab Res Rev. 2019;35(6):e3166. doi: 10.1002/dmrr.3166. [DOI] [PubMed] [Google Scholar]
- 175.Santero M, Morelli D, Nejamis A, Gibbons L, Irazola V, Beratarrechea A. Using mHealth strategies in a Diabetes Management Program to improve the quality of care in Argentina: Study design and baseline data. Prim Care Diabetes. 2018;12(6):510–516. doi: 10.1016/j.pcd.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 176.Gomes MB, Gianella D, Faria M, Tambascia M, Fonseca RM, Réa R, et al. Prevalence of Type 2 diabetic patients within the targets of care guidelines in daily clinical practice: a multi-center study in Brazil. Rev Diabet Stud. 2006;3(2):82–87. doi: 10.1900/rds.2006.3.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mendes AB, Fittipaldi JA, Neves RC, Chacra AR, Moreira ED., Jr Prevalence and correlates of inadequate glycaemic control: results from a nationwide survey in 6,671 adults with diabetes in Brazil. Acta Diabetol. 2010;47(2):137–145. doi: 10.1007/s00592-009-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moraes HAB, Mengue SS, Molina M, Cade NV. Factors associated with glycemic control in a sample of individuals with Diabetes Mellitus taken from the Longitudinal Study of Adult Health, Brazil, 2008–2010. Epidemiol Serv Saude. 2020;29(3):e2018500. doi: 10.5123/s1679-49742020000300017. [DOI] [PubMed] [Google Scholar]
- 179.Viana LV, Leitão CB, Kramer CK, Zucatti AT, Jezini DL, Felício J, et al. Poor glycaemic control in Brazilian patients with type 2 diabetes attending the public healthcare system: a cross-sectional study. BMJ Open. 2013;3(9):e003336. doi: 10.1136/bmjopen-2013-003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Baptista DR, Thieme RD, Reis WC, Pontarolo R, Correr CJ. Proportion of Brazilian diabetes patients that achieve treatment goals: implications for better quality of care. Diabetol Metab Syndr. 2015;7:113. doi: 10.1186/s13098-015-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Alba LH, Bastidas C, Vivas JM, Gil F. Prevalence of glycemic control and associated factors in type 2 diabetes mellitus patients at the Hospital Universitario de San Ignacio, Bogotá-Colombia. Gac Med Mex. 2009;145(6):469–474. [PubMed] [Google Scholar]
- 182.Machado-Alba JE, Moncada-Escobar JC, Gaviria H. Quality and effectiveness of diabetes care for a group of patients in Colombia. Rev Panam Salud Publica. 2009;26(6):529–535. doi: 10.1590/s1020-49892009001200008. [DOI] [PubMed] [Google Scholar]
- 183.Fort Z. Factores de riesgo cardiovascular en 74.420 solicitantes de carne de salud. Rev Urug Cardiol. 2012;27:150–161. [Google Scholar]
- 184.Gagliardino JJ, de la Hera M, Siri F. Evaluation of the quality of care for diabetic patients in Latin America. Rev Panam Salud Publica. 2001;10(5):309–317. doi: 10.1590/s1020-49892001001100003. [DOI] [PubMed] [Google Scholar]
- 185.Silva H, Hernandez-Hernandez R, Vinueza R, Velasco M, Boissonnet CP, Escobedo J, et al. Cardiovascular risk awareness, treatment, and control in urban Latin America. Am J Ther. 2010;17(2):159–166. doi: 10.1097/MJT.0b013e3181a84ec5. [DOI] [PubMed] [Google Scholar]
- 186.Commendatore V, Dieuzeide G, Faingold C, Fuente G, Luján D, Aschner P, et al. Registry of people with diabetes in three Latin American countries: a suitable approach to evaluate the quality of health care provided to people with type 2 diabetes. Int J Clin Pract. 2013;67(12):1261–1266. doi: 10.1111/ijcp.12208. [DOI] [PubMed] [Google Scholar]
- 187.Lopez Stewart G, Tambascia M, Rosas Guzmán J, Etchegoyen F, Ortega Carrión J, Artemenko S. Control of type 2 diabetes mellitus among general practitioners in private practice in nine countries of Latin America. Rev Panam Salud Publica. 2007;22(1):12–20. doi: 10.1590/s1020-49892007000600002. [DOI] [PubMed] [Google Scholar]
- 188.F GD. da Silva Moreira S, Almeida M, de Souza Teles CA, Andrade CS, Reingold AL, et al. Sex differences and correlates of poor glycaemic control in type 2 diabetes: a cross-sectional study in Brazil and Venezuela. BMJ Open. 2019;9(3):e023401. doi: 10.1136/bmjopen-2018-023401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Irazola V, Rubinstein A, Bazzano L, Calandrelli M, Chung-Shiuan C, Elorriaga N, et al. Prevalence, awareness, treatment and control of diabetes and impaired fasting glucose in the Southern Cone of Latin America. PLoS One. 2017;12(9):e0183953. doi: 10.1371/journal.pone.0183953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Arredondo A, Azar A, Recamán AL. Diabetes, a global public health challenge with a high epidemiological and economic burden on health systems in Latin America. Glob Public Health. 2018;13(7):780–787. doi: 10.1080/17441692.2017.1316414. [DOI] [PubMed] [Google Scholar]
- 191.Gagliardino JJ, Olivera EM, Etchegoyen GS, González C, Guidi ML. Evaluation and cost of the health care process of diabetic patients. Medicina (B Aires) 2000;60(6):880–888. [PubMed] [Google Scholar]
- 192.Olivera AR, Roesler V, Iochpe C, Schmidt MI, Vigo Á, Barreto SM, et al. Comparison of machine-learning algorithms to build a predictive model for detecting undiagnosed diabetes - ELSA-Brasil: accuracy study. Sao Paulo Med J. 2017;135(3):234–246. doi: 10.1590/1516-3180.2016.0309010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Arellano-Campos O, Gómez-Velasco DV, Bello-Chavolla OY, Cruz-Bautista I, Melgarejo-Hernandez MA, Muñoz-Hernandez L, et al. Development and validation of a predictive model for incident type 2 diabetes in middle-aged Mexican adults: the metabolic syndrome cohort. BMC Endocr Disord. 2019;19(1):41. doi: 10.1186/s12902-019-0361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Milton EC, Herman WH, Aiello AE, Danielson KR, Mendoza-Avelarez MO, Piette JD. Validation of a type 2 diabetes screening tool in rural Honduras. Diabetes Care. 2010;33(2):275–277. doi: 10.2337/dc09-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bernabe-Ortiz A, Perel P, Miranda JJ, Smeeth L. Diagnostic accuracy of the Finnish Diabetes Risk Score (FINDRISC) for undiagnosed T2DM in Peruvian population. Prim Care Diabetes. 2018;12(6):517–525. doi: 10.1016/j.pcd.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Muñoz-González MC, Lima-Martínez MM, Nava A, Trerotola G, Paoli M, Cabrera-Rego JO, et al. FINDRISC modified for Latin America as a screening tool for persons with impaired glucose metabolism in Ciudad Bolívar. Venezuela Med Princ Pract. 2019;28(4):324–332. doi: 10.1159/000499468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nieto-Martínez R, González-Rivas JP, Ugel E, Marulanda MI, Durán M, Mechanick JI, et al. External validation of the Finnish diabetes risk score in Venezuela using a national sample: The EVESCAM. Prim Care Diabetes. 2019;13(6):574–582. doi: 10.1016/j.pcd.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 198.Carrillo-Larco RM, Aparcana-Granda DJ, Mejia JR, Bernabé-Ortiz A. FINDRISC in Latin America: a systematic review of diagnosis and prognosis models. BMJ Open Diabetes Res Care. 2020;8(1). 10.1136/bmjdrc-2019-001169. [DOI] [PMC free article] [PubMed]
- 199.Aschner PM, Muñoz OM, Girón D, García OM, Fernández-Ávila DG, Casas L, et al. Clinical practice guideline for the prevention, early detection, diagnosis, management and follow up of type 2 diabetes mellitus in adults. Colomb Med (Cali) 2016;47(2):109–131. doi: 10.25100/cm.v47i2.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Saxton AT, Miranda JJ, Ortiz EJ, Pan W. Assessment of two diabetes point-of-care analyzers measuring hemoglobin A1c in the Peruvian Amazon. Ann Glob Health. 2018;84(4):618–624. doi: 10.9204/aogh.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Valdez-González LA, Méndez-Padrón A, Gómez-Díaz RA, Valladares-Salgado A, Sánchez-Becerra MC, Mondragón-González R, et al. Agreement between the 'point of care' tests for microalbuminuria and HbA1c performed in mexican family medicine units and the results of standard laboratory tests. Scand J Clin Lab Invest. 2018;78(1–2):87–93. doi: 10.1080/00365513.2017.1416664. [DOI] [PubMed] [Google Scholar]
- 202.Piette JD, Milton EC, Aiello AE, Mendoza-Avelares MO, Herman WH. Comparison of three methods for diabetes screening in a rural clinic in Honduras. Rev Panam Salud Publica. 2010;28(1):49–57. doi: 10.1590/s1020-49892010000700008. [DOI] [PubMed] [Google Scholar]
- 203.Cortés-Sanabria L, Martínez-Ramírez HR, Hernández JL, Rojas-Campos E, Canales-Muñoz JL, Cueto-Manzano AM. Utility of the Dipstick Micraltest II in the screening of microalbuminuria of diabetes mellitus type 2 and essential hypertension. Rev Investig Clin. 2006;58(3):190–197. [PubMed] [Google Scholar]
- 204.Beran D, Chappuis F, Damasceno A, Jha N, Pesantes MA, Singh SB, et al. High-quality health systems: time for a revolution in research and research funding. Lancet Glob Health. 2019;7(3):e303–e3e4. doi: 10.1016/s2214-109x(18)30529-1. [DOI] [PubMed] [Google Scholar]
- 205.Plüddemann A, Heneghan C, Price CP, Wolstenholme J, Thompson M. Point-of-care blood test for ketones in patients with diabetes: primary care diagnostic technology update. Br J Gen Pract. 2011;61(589):530–531. doi: 10.3399/bjgp11X588600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Plüddemann A, Price CP, Thompson M, Wolstenholme J, Heneghan C. Primary care diagnostic technology update: point-of-care testing for glycosylated haemoglobin. Br J Gen Pract. 2011;61(583):139–140. doi: 10.3399/bjgp11X556290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guzmán JR, Lyra R, Aguilar-Salinas CA, Cavalcanti S, Escaño F, Tambasia M, et al. Treatment of type 2 diabetes in Latin America: a consensus statement by the medical associations of 17 Latin American countries. Latin American Diabetes Association. Rev Panam Salud Publica. 2010;28(6):463–471. doi: 10.1590/s1020-49892010001200008. [DOI] [PubMed] [Google Scholar]
- 208.Silva AD, Rosa T, Batista LE, Kalckmann S, Louvison MCP, Teixeira D, et al. Racial inequities and aging: analysis of the 2010 cohort of the Health, Welfare and Aging Study (SABE) Rev Bras Epidemiol. 2019;21Suppl 02(Suppl 02):e180004. doi: 10.1590/1980-549720180004.supl.2. [DOI] [PubMed] [Google Scholar]
- 209.Lima RF, Fontbonne A, Carvalho EM, Montarroyos UR, Barreto MN, Cesse E. Factors associated with glycemic control in people with diabetes at the Family Health Strategy in Pernambuco. Rev Esc Enferm USP. 2016;50(6):937–945. doi: 10.1590/s0080-623420160000700009. [DOI] [PubMed] [Google Scholar]
- 210.Aschner P, Gagliardino JJ, Ilkova H, Lavalle F, Ramachandran A, Mbanya JC, et al. Persistent poor glycaemic control in individuals with type 2 diabetes in developing countries: 12 years of real-world evidence of the International Diabetes Management Practices Study (IDMPS) Diabetologia. 2020;63(4):711–721. doi: 10.1007/s00125-019-05078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rivera-Andrade A, Luna MA. Trends and heterogeneity of cardiovascular disease and risk factors across Latin American and Caribbean countries. Prog Cardiovasc Dis. 2014;57(3):276–285. doi: 10.1016/j.pcad.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 212.Chan JC, Gagliardino JJ, Baik SH, Chantelot JM, Ferreira SR, Hancu N, et al. Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS) Diabetes Care. 2009;32(2):227–233. doi: 10.2337/dc08-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Belizan M, Alonso JP, Nejamis A, Caporale J, Copo MG, Sánchez M, et al. Barriers to hypertension and diabetes management in primary health care in Argentina: qualitative research based on a behavioral economics approach. Transl Behav Med. 2019. 10.1093/tbm/ibz040. [DOI] [PMC free article] [PubMed]
- 214.Rojas-Martínez R, Basto-Abreu A, Aguilar-Salinas CA, Zárate-Rojas E, Villalpando S, Barrientos-Gutiérrez T. Prevalence of previously diagnosed diabetes mellitus in Mexico. Salud Publica Mex. 2018;60(3):224–232. doi: 10.21149/8566. [DOI] [PubMed] [Google Scholar]
- 215.Carro GV, Saurral R, Sagüez FS, Witman EL. Diabetic foot among hospitalized patients in Latin America. Medicina (B Aires) 2018;78(4):243–251. [PubMed] [Google Scholar]
- 216.Chen-Ku CH, Gonzalez-Galvez G, Vásquez M, Fuente G, Nakazone MA, Silva Giordano AI, et al. Vascular Complications In Patients With Type 2 Diabetes: Prevalence And Comorbidities In 6 Countries of Latin America (a cohort of the discover study program) Endocr Pract. 2019;25(10):994–1002. doi: 10.4158/ep-2018-0473. [DOI] [PubMed] [Google Scholar]
- 217.Parisi MC, Moura Neto A, Menezes FH, Gomes MB, Teixeira RM, de Oliveira JE, et al. Baseline characteristics and risk factors for ulcer, amputation and severe neuropathy in diabetic foot at risk: the BRAZUPA study. Diabetol Metab Syndr. 2016;8:25. doi: 10.1186/s13098-016-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Laclé A, Valero-Juan LF. Diabetes-related lower-extremity amputation incidence and risk factors: a prospective seven-year study in Costa Rica. Rev Panam Salud Publica. 2012;32(3):192–198. doi: 10.1590/s1020-49892012000900004. [DOI] [PubMed] [Google Scholar]
- 219.Costa WJT, Penha-Silva N, Bezerra IMP, Paulo Dos Santos I, Ramos JLS, Castro JM, et al. Analysis of Diabetes Mellitus-Related Amputations in the State of Espírito Santo, Brazil. Medicina (Kaunas). 2020;56(6). 10.3390/medicina56060287. [DOI] [PMC free article] [PubMed]
- 220.Ascencio-Montiel IJ. 10 years analysis of diabetes-related major lower extremity amputations in Mexico. Arch Med Res. 2018;49(1):58–64. doi: 10.1016/j.arcmed.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 221.Bonilla GA, Hornsby PP, Pannone AF, Case SK, Aviles ES, Carrasco Apolinario ME, et al. Demographic and clinical characteristics of dominican adults admitted to a diabetic foot clinic in the Dominican Republic, 2015. Diabetes Metab Syndr. 2019;13(3):1727–1732. doi: 10.1016/j.dsx.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 222.Adrianzén RE, Rioja M, Manrique A. Frequency and severity of diabetic retinopathy in patients with type 2 diabetes mellitus at the Regional Institute of Ophthalmology. Rev Peru Med Exp Salud Publica. 2019;36(2):260–264. doi: 10.17843/rpmesp.2019.362.4076. [DOI] [PubMed] [Google Scholar]
- 223.Drummond KRG, Malerbi FK, Morales PH, Mattos TCL, Pinheiro AA, Mallmann F, et al. Regional differences in the prevalence of diabetic retinopathy: a multi center study in Brazil. Diabetol Metab Syndr. 2018;10:17. doi: 10.1186/s13098-018-0319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cusumano AM, González Bedat MC. Chronic kidney disease in Latin America: time to improve screening and detection. Clin J Am Soc Nephrol. 2008;3(2):594–600. doi: 10.2215/cjn.03420807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Correa-Rotter R, García-Trabanino R. Mesoamerican Nephropathy. Semin Nephrol. 2019;39(3):263–271. doi: 10.1016/j.semnephrol.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 226.González Villalpando C, Stern MP, Arredondo Pérez B, Martínez Díaz S, Islas Andrade S, Revilla C, et al. Nephropathy in low income diabetics: the Mexico City Diabetes Study. Arch Med Res. 1996;27(3):367–372. [PubMed] [Google Scholar]
- 227.Correa-Rotter R, González-Michaca L. Early detection and prevention of diabetic nephropathy: a challenge calling for mandatory action for Mexico and the developing world. Kidney Int Suppl. 2005;98:S69–S75. doi: 10.1111/j.1523-1755.2005.09813.x. [DOI] [PubMed] [Google Scholar]
- 228.Valdez-Ortiz R, Navarro-Reynoso F, Olvera-Soto MG, Martin-Alemañy G, Rodríguez-Matías A, Hernández-Arciniega CR, et al. Mortality in patients with chronic renal disease without health insurance in Mexico: opportunities for a national renal health policy. Kidney Int Rep. 2018;3(5):1171–1182. doi: 10.1016/j.ekir.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zenteno-Castillo P, Muñoz-López DB, Merino-Reyes B, Vega-Sánchez Á, Preciado-Puga M, González-Yebra AL, et al. Prevalence of diabetic nephropathy in Type 2 Diabetes Mellitus in rural communities of Guanajuato, Mexico. Effect after 6 months of Telmisartan treatment. J Clin Transl Endocrinol. 2015;2(4):125–128. doi: 10.1016/j.jcte.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bachmann MO, Bateman ED, Stelmach R, Cruz ÁA, Pacheco de Andrade M, Zonta R, et al. Integrating primary care of chronic respiratory disease, cardiovascular disease and diabetes in Brazil: Practical Approach to Care Kit (PACK Brazil): study protocol for randomised controlled trials. J Thorac Dis. 2018;10(7):4667–4677. doi: 10.21037/jtd.2018.07.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.da Silva Marinho MG, Fontbonne A, Vasconcelos Barbosa JM, de Melo Rodrigues H. Freese de Carvalho E, Vieira de Souza W et al. The impact of an intervention to improve diabetes management in primary healthcare professionals’ practices in Brazil. Prim Care Diabetes. 2017;11(6):538–545. doi: 10.1016/j.pcd.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 232.Simão C, Costa MB, Colugnati FAB, de Paula EA, Vanelli CP, de Paula RB. Quality of Care of patients with diabetes in primary health services in Southeast Brazil. J Environ Public Health. 2017;2017:1709807. doi: 10.1155/2017/1709807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Silva-Tinoco R, Cuatecontzi-Xochitiotzi T, De la Torre-Saldaña V, León-García E, Serna-Alvarado J, Guzmán-Olvera E, et al. Role of social and other determinants of health in the effect of a multicomponent integrated care strategy on type 2 diabetes mellitus. Int J Equity Health. 2020;19(1):75. doi: 10.1186/s12939-020-01188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gallardo-Rincón H, Saucedo-Martínez R, Mujica-Rosales R, Lee EM, Israel A, Torres-Beltran B, et al. Online continuing medical education as a key link for successful noncommunicable disease self-management: the CASALUD™ Model. Diabetes Metab Syndr Obes. 2017;10:443–455. doi: 10.2147/dmso.S137891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lew KN, McLean Y, Byers S, Taylor H, Braizat OM. Combined diabetes prevention and disease self-management intervention for Nicaraguan ethnic minorities: a pilot study. Prog Community Health Partnersh. 2017;11(4):357–366. doi: 10.1353/cpr.2017.0043. [DOI] [PubMed] [Google Scholar]
- 236.Batista F, Augusto Magalhães A, Gamba M, Nery C, Cardoso C. Ten years of a multidisciplinary diabetic foot team approach in Sao Paulo. Brazil Diabet Foot Ankle. 2010;1. 10.3402/dfa.v1i0.5203. [DOI] [PMC free article] [PubMed]
- 237.Barceló A, Cafiero E, de Boer M, Mesa AE, Lopez MG, Jiménez RA, et al. Using collaborative learning to improve diabetes care and outcomes: the VIDA project. Prim Care Diabetes. 2010;4(3):145–153. doi: 10.1016/j.pcd.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 238.Lerario AC, Chacra AR, Pimazoni-Netto A, Malerbi D, Gross JL, Oliveira JE, et al. Algorithm for the treatment of type 2 diabetes: a position statement of Brazilian Diabetes Society. Diabetol Metab Syndr. 2010;2(1):35. doi: 10.1186/1758-5996-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Piette JD, Marinec N, Gallegos-Cabriales EC, Gutierrez-Valverde JM, Rodriguez-Saldaña J, Mendoz-Alevares M, et al. Spanish-speaking patients' engagement in interactive voice response (IVR) support calls for chronic disease self-management: data from three countries. J Telemed Telecare. 2013;19(2):89–94. doi: 10.1177/1357633x13476234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Piette JD, Mendoza-Avelares MO, Ganser M, Mohamed M, Marinec N, Krishnan S. A preliminary study of a cloud-computing model for chronic illness self-care support in an underdeveloped country. Am J Prev Med. 2011;40(6):629–632. doi: 10.1016/j.amepre.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Piette JD, Marinec N, Janda K, Morgan E, Schantz K, Yujra AC, et al. Structured caregiver feedback enhances engagement and impact of mobile health support: a randomized trial in a lower-middle-income country. Telemed J E Health. 2016;22(4):261–268. doi: 10.1089/tmj.2015.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Piette JD, Valverde H, Marinec N, Jantz R, Kamis K, de la Vega CL, et al. Establishing an independent mobile health program for chronic disease self-management support in bolivia. Front Public Health. 2014;2:95. doi: 10.3389/fpubh.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Prestes M, Gayarre MA, Elgart JF, Gonzalez L, Rucci E, Paganini JM, et al. Improving diabetes care at primary care level with a multistrategic approach: results of the DIAPREM programme. Acta Diabetol. 2017;54(9):853–861. doi: 10.1007/s00592-017-1016-8. [DOI] [PubMed] [Google Scholar]
- 244.Flood D, Mux S, Martinez B, García P, Douglas K, Goldberg V, et al. Implementation and outcomes of a comprehensive type 2 diabetes program in rural Guatemala. PLoS One. 2016;11(9):e0161152. doi: 10.1371/journal.pone.0161152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Flood D, Douglas K, Goldberg V, Martinez B, Garcia P, Arbour M, et al. A quality improvement project using statistical process control methods for type 2 diabetes control in a resource-limited setting. Int J Qual Health Care. 2017;29(4):593–601. doi: 10.1093/intqhc/mzx051. [DOI] [PubMed] [Google Scholar]
- 246.Flood D, Hawkins J, Rohloff P. A home-based type 2 diabetes self-management intervention in rural Guatemala. Prev Chronic Dis. 2017;14:E65. doi: 10.5888/pcd14.170052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tapia-Conyer R, Gallardo-Rincón H, Saucedo-Martinez R. CASALUD: an innovative health-care system to control and prevent non-communicable diseases in Mexico. Perspect Public Health. 2015;135(4):180–190. doi: 10.1177/1757913913511423. [DOI] [PubMed] [Google Scholar]
- 248.Salamanca O, Geary A, Suárez N, Benavent S, Gonzalez M. Implementation of a diabetic retinopathy referral network. Peru Bull World Health Organ. 2018;96(10):674–681. doi: 10.2471/blt.18.212613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Avendaño-Veloso A, Parada-Hernández F, González-Ramos R, Dougnac-Osses C, Carrasco-Sáez JL, Scanlon PH. Teleophthalmology: a strategy for timely diagnosis of sight-threatening diabetic retinopathy in primary care, Concepción, Chile. Int J Ophthalmol. 2019;12(9):1474–1478. doi: 10.18240/ijo.2019.09.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Flores R, Donoso R, Anguita R. Management of diabetic retinopathy using telemedicine and network integration. Rev Med Chil. 2019;147(4):444–450. doi: 10.4067/s0034-98872019000400444. [DOI] [PubMed] [Google Scholar]
- 251.Cani CG, Lopes Lda S, Queiroz M, Nery M. Improvement in medication adherence and self-management of diabetes with a clinical pharmacy program: a randomized controlled trial in patients with type 2 diabetes undergoing insulin therapy at a teaching hospital. Clinics (Sao Paulo) 2015;70(2):102–106. doi: 10.6061/clinics/2015(02)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gagliardino JJ, Lapertosa S, Pfirter G, Villagra M, Caporale JE, Gonzalez CD, et al. Clinical, metabolic and psychological outcomes and treatment costs of a prospective randomized trial based on different educational strategies to improve diabetes care (PRODIACOR) Diabet Med. 2013;30(9):1102–1111. doi: 10.1111/dme.12230. [DOI] [PubMed] [Google Scholar]
- 253.Beauregard D, Schiffman JS, Tang R. Collaborative telemedicine between optometry and ophthalmology: an initiative from the University of Houston. Stud Health Technol Inform. 1999;64:173–178. [PubMed] [Google Scholar]
- 254.Villena JE, Yoshiyama CA, Sánchez JE, Hilario NL, Merin LM. Prevalence of diabetic retinopathy in Peruvian patients with type 2 diabetes: results of a hospital-based retinal telescreening program. Rev Panam Salud Publica. 2011;30(5):408–414. [PubMed] [Google Scholar]
- 255.Jiménez-Ramírez F, Pérez R. Diabetic retinopathy education and screening at the community pharmacy in Puerto Rico. P R Health Sci J. 2011;30(3):139–144. [PubMed] [Google Scholar]
- 256.Acevedo Castellón RI, Carranza Vargas E, Cortés Chavarría RE, Rodríguez Vargas GA. Rapid assessment of avoidable blindness and diabetic retinopathy in individuals aged 50 years or older in Costa Rica. PLoS One. 2019;14(2):e0212660. doi: 10.1371/journal.pone.0212660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ministerio de Salud de Argentina. Encuesta Rapida de Ceguera Evitable, Argentina, 2013 (http://www.msal.gob.ar/images/stories/bes/graficos/0000000603cnt-2015-01_encuesta-rapida-ceguera-evitable.pdf) Argentina2013. [Last Accessed on August 6, 2020].
- 258.Limburg H, Espinoza R, Lansingh VC, Silva JC. Functional low vision in adults from Latin America: findings from population-based surveys in 15 countries. Rev Panam Salud Publica. 2015;37(6):371–378. [PubMed] [Google Scholar]
- 259.Polack S, Yorston D, López-Ramos A, Lepe-Orta S, Baia RM, Alves L, et al. Rapid assessment of avoidable blindness and diabetic retinopathy in Chiapas. Mexico Ophthalmology. 2012;119(5):1033–1040. doi: 10.1016/j.ophtha.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 260.Martinez J, Hernandez-Bogantes E, Wu L. Diabetic retinopathy screening using single-field digital fundus photography at a district level in Costa Rica: a pilot study. Int Ophthalmol. 2011;31(2):83–88. doi: 10.1007/s10792-010-9413-9. [DOI] [PubMed] [Google Scholar]
- 261.Mendoza-Herrera K, Quezada AD, Pedroza-Tobías A, Hernández-Alcaraz C, Fromow-Guerra J, Barquera S. A Diabetic Retinopathy Screening Tool for Low-Income Adults in Mexico. Prev Chronic Dis. 2017;14:E95. doi: 10.5888/pcd14.170157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lazo-Porras M, Bernabe-Ortiz A, Sacksteder KA, Gilman RH, Malaga G, Armstrong DG, et al. Implementation of foot thermometry plus mHealth to prevent diabetic foot ulcers: study protocol for a randomized controlled trial. Trials. 2016;17(1):206. doi: 10.1186/s13063-016-1333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jimenez MM, Bui AL, Mantilla E, Miranda JJ. Human resources for health in Peru: recent trends (2007–2013) in the labour market for physicians, nurses and midwives. Hum Resour Health. 2017;15(1):69. doi: 10.1186/s12960-017-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Emmerick IC, Luiza VL, Camacho LA, Vialle-Valentin C, Ross-Degnan D. Barriers in household access to medicines for chronic conditions in three Latin American countries. Int J Equity Health. 2015;14:115. doi: 10.1186/s12939-015-0254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Esterson YB, Carey M, Piette JD, Thomas N, Hawkins M. A systematic review of innovative diabetes care models in low-and middle-income countries (LMICs) J Health Care Poor Underserved. 2014;25(1):72–93. doi: 10.1353/hpu.2014.0037. [DOI] [PubMed] [Google Scholar]
- 266.Maia LG, Silva LAD, Guimarães RA, Pelazza BB, Leite GR, Barbosa MA. The quality of primary care services, vocational training and the More Doctors Program in a health region of southwest Goiás. Rev Bras Epidemiol. 2020;23:e200014. doi: 10.1590/1980-549720200014. [DOI] [PubMed] [Google Scholar]
- 267.Butte NF, Cai G, Cole SA, Comuzzie AG. Viva la Familia Study: genetic and environmental contributions to childhood obesity and its comorbidities in the Hispanic population. Am J Clin Nutr. 2006;84(3):646–654. doi: 10.1093/ajcn/84.3.646. [DOI] [PubMed] [Google Scholar]
- 268.Pinney SE, Joshi A, Yin V, Min SW, Rashid C, Condon DE, et al. Exposure to gestational diabetes enriches immune related pathways in the transcriptome and methylome of human amniocytes. J Clin Endocrinol Metab. 2020. 10.1210/clinem/dgaa466. [DOI] [PMC free article] [PubMed]
- 269.Gómez Dantés H, Castro MV, Franco-Marina F, Bedregal P, Rodríguez García J, Espinoza A, et al. Burden of disease in Latin America. Salud Publica Mex. 2011;53(Suppl 2):s72–s77. [PubMed] [Google Scholar]
- 270.Tejero ME. Cardiovascular disease in Latin American women. Nutr Metab Cardiovasc Dis. 2010;20(6):405–411. doi: 10.1016/j.numecd.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 271.Bozanic A, Toro P, Formiga F. DIABDEM project: a pilot study of prevalence of cognitive impairment in diabetes mellitus in 2 Hispanic countries. Rev Esp Geriatr Gerontol. 2019;54(6):339–345. doi: 10.1016/j.regg.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 272.Goveas JS, Rapp SR, Hogan PE, Driscoll I, Tindle HA, Smith JC, et al. Predictors of optimal cognitive aging in 80+ women: the Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2016;71 Suppl 1(Suppl 1):S62–S71. doi: 10.1093/gerona/glv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Bernard L, Reix N, Benabu JC, Gabriele V, Mathelin C. Breast cancer and diabetes mellitus: complex interactions. Gynecol Obstet Fertil. 2016;44(12):701–711. doi: 10.1016/j.gyobfe.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 274.Overbeek JA, Kuiper JG, van der Heijden A, Labots M, Haug U, Herings RMC, et al. Sex- and site-specific differences in colorectal cancer risk among people with type 2 diabetes. Int J Color Dis. 2019;34(2):269–276. doi: 10.1007/s00384-018-3191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Pérez C, Ailshire JA. Aging in Puerto Rico: A Comparison of Health Status Among Island Puerto Rican and Mainland U.S. Older Adults. J Aging Health. 2017;29(6):1056–1078. doi: 10.1177/0898264317714144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lundeen EA, Wittenborn J, Benoit SR, Saaddine J. Disparities in receipt of eye exams among medicare part B fee-for-service beneficiaries with diabetes - United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(45):1020–1023. doi: 10.15585/mmwr.mm6845a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Louvison MC, Lebrão ML, Duarte YA, Santos JL, Malik AM, Almeida ES. Inequalities in access to health care services and utilization for the elderly in São Paulo, Brazil. Rev Saude Publica. 2008;42(4):733–740. doi: 10.1590/s0034-89102008000400021. [DOI] [PubMed] [Google Scholar]
- 278.Cataife G. Public versus private treatment of chronic diseases in seniors: Argentina, Brazil. Chile and Uruguay Glob Public Health. 2012;7(10):1157–1169. doi: 10.1080/17441692.2012.721894. [DOI] [PubMed] [Google Scholar]
- 279.Rodríguez-Vigil E, Kianes-Pérez Z. Quality of care provided to patients with diabetes mellitus in Puerto Rico; managed care versus fee-for-service experience. Endocr Pract. 2005;11(6):376–381. doi: 10.4158/ep.11.6.376. [DOI] [PubMed] [Google Scholar]
- 280.Hoogendijk EO, Rijnhart JJM, Kowal P, Pérez-Zepeda MU, Cesari M, Abizanda P, et al. Socioeconomic inequalities in frailty among older adults in six low- and middle-income countries: results from the WHO Study on global AGEing and adult health (SAGE) Maturitas. 2018;115:56–63. doi: 10.1016/j.maturitas.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 281.Whittemore R, Vilar-Compte M, De La Cerda S, Marron D, Conover R, Delvy R, et al. Challenges to diabetes self-management for adults with type 2 diabetes in low-resource settings in Mexico City: a qualitative descriptive study. Int J Equity Health. 2019;18(1):133. doi: 10.1186/s12939-019-1035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gallegos-Carrillo K, García-Peña C, Durán-Muñoz CA, Flores YN, Salmerón J. Relationship between social support and the physical and mental wellbeing of older Mexican adults with diabetes. Rev Investig Clin. 2009;61(5):383–391. [PubMed] [Google Scholar]
- 283.Briceño I, Barriocanal LA, Papiha SS, Ashworth LA, Gómez A, Bernal JE, et al. Lack of diabetes in rural Colombian Amerindians. Diabetes Care. 1996;19(8):900–901. doi: 10.2337/diacare.19.8.900. [DOI] [PubMed] [Google Scholar]
- 284.Alvarado-Osuna C, Milian-Suazo F, Valles-Sánchez V. Prevalence of diabetes mellitus and hyperlipidemia among Otomi indians. Salud Publica Mex. 2001;43(5):459–463. doi: 10.1590/S0036-36342001000500010. [DOI] [PubMed] [Google Scholar]
- 285.Guerrero-Romero F, Rodríguez-Morán M, Sandoval-Herrera F. Low prevalence of non-insulin-dependent diabetes mellitus in indigenous communities of Durango. Mexico Arch Med Res. 1997;28(1):137–140. [PubMed] [Google Scholar]
- 286.Barbosa CC, Sacuena ESR, Pinto AM, Cardoso-Costa GL, Guerreiro JF. Anthropometric and metabolic profile of a Brazilian Amerindian group: The Xikrin (Mebengôkre) Am J Hum Biol. 2019;31(4):e23255. doi: 10.1002/ajhb.23255. [DOI] [PubMed] [Google Scholar]
- 287.Breathett K, Sims M, Gross M, Jackson EA, Jones EJ, Navas-Acien A, et al. Cardiovascular health in American Indians and Alaska Natives: a scientific statement from the American Heart Association. Circulation. 2020;141(25):e948–ee59. doi: 10.1161/cir.0000000000000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Walker JD, Slater M, Jones CR, Shah BR, Frymire E, Khan S, et al. Diabetes prevalence, incidence and mortality in First Nations and other people in Ontario, 1995–2014: a population-based study using linked administrative data. Cmaj. 2020;192(6):E128–Ee35. doi: 10.1503/cmaj.190836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.de Souza Filho ZA, Ferreira AA, Dos Santos J, Meira KC, Pierin AMG. Cardiovascular risk factors with an emphasis on hypertension in the Mura Indians from Amazonia. BMC Public Health. 2018;18(1):1251. doi: 10.1186/s12889-018-6160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Rodríguez-Morán M, Guerrero-Romero F, Brito-Zurita O, Rascón-Pacheco RA, Pérez-Fuentes R, Sánchez-Guillén MC, et al. Cardiovascular risk factors and acculturation in Yaquis and Tepehuanos Indians from Mexico. Arch Med Res. 2008;39(3):352–357. doi: 10.1016/j.arcmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 291.Alvim Rde O, Mourao-Junior CA, de Oliveira CM, Krieger JE, Mill JG, Pereira AC. Body mass index, waist circumference, body adiposity index, and risk for type 2 diabetes in two populations in Brazil: general and Amerindian. PLoS One. 2014;9(6):e100223. doi: 10.1371/journal.pone.0100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lindgärde F, Widén I, Gebb M, Ahrén B. Traditional versus agricultural lifestyle among Shuar women of the Ecuadorian Amazon: effects on leptin levels. Metabolism. 2004;53(10):1355–1358. doi: 10.1016/j.metabol.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 293.Baldoni NR, Aquino JA, Alves GCS, Sartorelli DS, Franco LJ, Madeira SP, et al. Prevalence of overweight and obesity in the adult indigenous population in Brazil: a systematic review with meta-analysis. Diabetes Metab Syndr. 2019;13(3):1705–1715. doi: 10.1016/j.dsx.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 294.Araujo M, Moraga C, Chapman E, Barreto J, Illanes E. Interventions to improve access to health services by indigenous peoples in the Americas. Rev Panam Salud Publica. 2016;40(5):371–381. [PubMed] [Google Scholar]
- 295.Chary A, Greiner M, Bowers C, Rohloff P. Determining adult type 2 diabetes-related health care needs in an indigenous population from rural Guatemala: a mixed-methods preliminary study. BMC Health Serv Res. 2012;12:476. doi: 10.1186/1472-6963-12-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.de Alencar R, Galvao TF, Antonio BVR, Silva MT. Prevalence of Self-Reported Chronic Diseases and Health Services Utilization by Ethnic Minorities in Manaus Metropolitan Region. Ethn Dis. 2018;28(1):49–54. doi: 10.18865/ed.28.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Guevara PE, Andrade FC. Socioeconomic and lifestyle factors associated with chronic conditions among older adults in Ecuador. Rev Panam Salud Publica. 2015;38(3):226–232. [PubMed] [Google Scholar]
- 298.Harding T, Oetzel J. Implementation effectiveness of health interventions for indigenous communities: a systematic review. Implement Sci. 2019;14(1):76. doi: 10.1186/s13012-019-0920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Micikas M, Foster J, Weis A, Lopez-Salm A, Lungelow D, Mendez P, et al. A community health worker intervention for diabetes self-management among the Tz'utujil Maya of Guatemala. Health Promot Pract. 2015;16(4):601–608. doi: 10.1177/1524839914557033. [DOI] [PubMed] [Google Scholar]
- 300.Nieblas-Bedolla E, Bream KDW, Rollins A, Barg FK. Ongoing challenges in access to diabetes care among the indigenous population: perspectives of individuals living in rural Guatemala. Int J Equity Health. 2019;18(1):180. doi: 10.1186/s12939-019-1086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Coimbra CE, Jr, Santos RV, Welch JR, Cardoso AM, de Souza MC, Garnelo L, et al. The first national survey of indigenous people’s health and nutrition in Brazil: rationale, methodology, and overview of results. BMC Public Health. 2013;13:52. doi: 10.1186/1471-2458-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Mirassou CS. Health system and aboriginal communities in the province of Formosa, Argentina. Medicina (B Aires) 2013;73(5):453–456. [PubMed] [Google Scholar]
- 303.Rashak HA, Sánchez-Pérez HJ, Abdelbary BE, Bencomo-Alerm A, Enriquez-Ríos N, Gómez-Velasco A, et al. Diabetes, undernutrition, migration and indigenous communities: tuberculosis in Chiapas. Mexico Epidemiol Infect. 2019;147:e71. doi: 10.1017/s0950268818003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Céspedes C, López L, Aguirre S, Mendoza-Ticona A. Prevalence of comorbidity tuberculosis and diabetes mellitus in Paraguay, 2016 and 2017Prevalência de comorbidade tuberculose-diabetes mellitus no Paraguai, 2016 e 2017. Rev Panam Salud Publica. 2019;43:e105. doi: 10.26633/rpsp.2019.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Nascimento CV, Soares SM. Co-management of tuberculosis and diabetes: an integrative reviewManejo integrado de la tuberculosis y la diabetes: revisión integrativa. Rev Panam Salud Publica. 2019;43:e21. doi: 10.26633/rpsp.2019.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Pablos-Méndez A, Vega J, Aranguren FP, Tabish H, Raviglione MC. Covid-19 in Latin America. Bmj. 2020;370:m2939. doi: 10.1136/bmj.m2939. [DOI] [PubMed] [Google Scholar]
- 307.Barone MTU, Harnik SB, de Luca PV, Lima BLS, Wieselberg RJP, Ngongo B, et al. The impact of COVID-19 on people with diabetes in Brazil. Diabetes Res Clin Pract. 2020;166:108304. doi: 10.1016/j.diabres.2020.108304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Bello-Chavolla OY, González-Díaz A, Antonio-Villa NE, Fermín-Martínez CA, Márquez-Salinas A, Vargas-Vázquez A, et al. Unequal impact of structural health determinants and comorbidity on COVID-19 severity and lethality in older Mexican adults: Considerations beyond chronological aging. J Gerontol A Biol Sci Med Sci. 2020. 10.1093/gerona/glaa163. [DOI] [PMC free article] [PubMed]
- 309.Barone MTU, Villarroel D, de Luca PV, Harnik SB, Lima BLS, Wieselberg RJP, et al. COVID-19 impact on people with diabetes in South and Central America (SACA region) Diabetes Res Clin Pract. 2020;166:108301. doi: 10.1016/j.diabres.2020.108301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Díaz de León-Martínez L, de la Sierra-de la Vega L, Palacios-Ramírez A, Rodriguez-Aguilar M, Flores-Ramírez R. Critical review of social, environmental and health risk factors in the Mexican indigenous population and their capacity to respond to the COVID-19. Sci Total Environ. 2020;733:139357. doi: 10.1016/j.scitotenv.2020.139357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Teles M, Sacchetta T, Matsumoto Y. COVID-19 Pandemic Triggers Telemedicine Regulation and Intensifies Diabetes Management Technology Adoption in Brazil. J Diabetes Sci Technol. 2020;14(4):797–798. doi: 10.1177/1932296820930033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NA