Abstract
Pregnancy is characterized by cumulative plasma volume expansion as a result of renal sodium retention, driven by activation of aldosterone. We previously reported that the abundance and activity of the aldosterone-responsive epithelial Na+ channel is increased, whereas the sodium–chloride cotransporter (NCC) is decreased in the kidney of the late-pregnant rat. The chloride–bicarbonate exchanger pendrin is also aldosterone responsive and has been shown to support activity of the aldosterone-responsive epithelial Na+ channel and compensate for the loss of NCC. Additionally, pendrin coupled to the sodium-dependent chloride–bicarbonate exchanger (NDCBE) mediates thiazide-sensitive sodium reabsorption in the cortical collecting duct. In this study, we investigated pendrin and NDCBE transcript expression, pendrin protein abundance, pendrin cellular localization and thiazide sensitivity in virgin, mid-pregnant and late-pregnant rats to test the hypothesis that increased pendrin activity might occur in pregnancy. By RT-PCR, NDCBE and pendrin mRNA expression was unchanged from virgins, whereas pendrin protein abundance determined by Western blotting was increased in both mid- and late-pregnant rats. The apical localization of pendrin was also increased in late-pregnant rats compared with virgins by immunohistochemistry. Pregnant rats displayed an increased natriuretic response to hydrochlorothiazide compared with virgins. Given that NCC expression is decreased in late pregnancy, an increased thiazide sensitivity may be due to inhibition of upregulated pendrin–NDCBE-coupled sodium reabsorption. Thus, increased pendrin in pregnant rats may compensate for the decreased NCC and aid in the renal sodium chloride reabsorption of pregnancy.
Introduction
A healthy pregnancy requires an increase in plasma volume of at least 40%, which is necessary to support the growing uterus and fetus. Insufficient volume expansion is associated with fetal growth restriction (Gibson, 1973; Salas et al. 2006), which increases the risk for adult-onset hypertension, metabolic syndrome (Barker et al. 1989) and reduced nephron numbers leading to renal disease (Brenner et al. 1988). The plasma volume expansion of pregnancy is driven by avid renal sodium retention supported by activation of the renin–angiotensin–aldosterone system.
Previous studies conducted in pregnant rats have demonstrated that aldosterone is critical for this mechanism. Pregnant rats that have undergone adrenalectomy and aldosynthase knockout mice have reduced plasma volume expansion, decreased blood pressure and restricted fetal growth (Barron et al. 1993; Todkar et al. 2012). It is likely that these effects are due to the loss of sodium reabsorption mediated by the epithelial sodium channel (ENaC), because in vivo ENaC activity is increased in pregnancy via a mineralocorticoid receptor-mediated pathway (West et al. 2010), and chronic ENaC inhibition prevents the pregnancy-mediated sodium retention, which results in growth-restricted pups (West et al. 2014).
We have recently examined the regulation of the sodium–chloride cotransporter (NCC) in pregnancy because it is also an aldosterone-responsive sodium transporter (Kim et al. 1998). However, unlike ENaC, we found that NCC abundance, phosphorylation and apical localization were all decreased in late-pregnant and unchanged in mid-pregnant rats compared with virgin rats (West et al. 2015). This was surprising because late pregnancy is the time when aldosterone concentrations and renal sodium reabsorption are highest (Alexander et al. 1980; Atherton et al. 1982; Brochu et al. 1997; Garland et al. 1987).
Recent studies have demonstrated that NCC and the chloride–bicarbonate exchanger pendrin may compensate for the loss of each other (Soleimani et al. 2012; Grimm et al. 2015). Pendrin is localized to type B and non-A, non-B intercalated cells and is an aldosterone-responsive protein that is coupled to sodium reabsorption via ENaC in principal cells (Wagner et al. 2002; Verlander et al. 2003). Pendrin has also been indicated to work in tandem with the sodium-driven chloride–bicarbonate exchanger (NDCBE) mediating thiazide-sensitive electroneutral sodium reabsorption in the collecting duct (Leviel et al. 2010). Given that pendrin supports sodium reabsorption in the collecting duct and compensates for loss of NCC, we have undertaken the present study to examine whether pendrin is increased in the pregnant rat and aids in the sodium reabsorption of pregnancy.
Methods
Animals
Animal experiments were carried out using a total of 50 female Sprague–Dawley rats (Harlan Laboratories, Indianapolis, IN, USA). Animals were maintained in the University of Florida animal facility in compliance with institutional guidelines and the National Institute of Health (NIH) Guide for Animal Use. All animal protocols were approved by the University of Florida Institutional Animal Care and Use Committee, and experiments were carried out according to institutional guidelines. Rats were maintained on a normal 12 h–12 h light–dark cycle and were given ad libitum access to water and Harlan Teklad LM-485 Mouse/Rat Sterilizable Diet (0.3% sodium, 0.8% potassium). Rats destined to become pregnant were placed with a fertile male, and day 1 of pregnancy was designated as the day that sperm was present in vaginal smears. Rat gestation is ~22 days. Group 1 rats [virgin (n = 6), mid-pregnant (day 14; n = 7) and late-pregnant animals (day 21; n = 7)] were used for determination of mRNA by qPCR and protein abundance by Western blotting; group 2 rats [virgin (n = 3), mid-pregnant (day 11–13; n = 4) and late-pregnant animals (day 18–20; n = 4)] were used for immunolocalization by immunohistochemistry; and group 3 rats [virgin (n = 6), mid-pregnant (day 12–14; n = 7) and late-pregnant animals (day 19–21; n = 6)] were used for baseline physical data and natriuretic response tests. Animals were killed by exsanguination under inhalation anaesthesia with 4% isoflurane (Baxter Healthcare Corp., Deerfield, IL, USA). Blood was taken from the abdominal aorta at harvest from group 3 rats for measurement of haematocrit. All animals were killed and tissue harvested between 14.00 and 18.00 h.
RNA isolation and qPCR
RNA was isolated using the Direct-zol RNA MiniPrep kit with on-column DNA digestion (Zymo Research Corp., Irvine, CA, USA) according to the manufacturer’s instructions. Samples of RNA (2 μg) were used as the template for reverse transcription with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The resulting cDNAs (20 ng) were then used as the template in real-time quantitative polymerase chain reactions (qPCRs; Applied Biosystems) to evaluate changes in pendrin (Rn01469208_m1) and NDCBE (Rn01532883_m1). Cycle threshold (CT) values were normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and relative quantification was performed using the ΔΔCT method (Livak & Schmittgen, 2001). Fold-change values were calculated as the change in mRNA expression levels relative to the control. Relative gene expression levels were then compared between groups using ANOVA (Gumz et al. 2009). TaqMan primer–probe sets were purchased from Applied Biosystems.
Homogenate preparation
Kidney cortex was dissected, snap frozen in liquid nitrogen and stored at −80°C until homogenization. Tissues were homogenized in ice-cold homogenization buffer [5% sorbitol containing 25 mm histidine-imidazole (pH 7.5), 100 mm Na2EDTA, 20 mg ml−1 aprotinin, 167 mm PMSF and a phosphatase inhibitor cocktail (Sigma P0044)]. After homogenization, samples were centrifuged at 2000g for removal of debris. The protein concentration of the homogenates was determined by BCA assay (Pierce Thermo, Rockford, IL, USA). All samples were solubilized at 60°C for 15 min in a Laemmli sample buffer and stored at −80°C.
Quantitative immunoblotting and reagents
Protein abundances were detected by Western blotting using 50 μg of kidney cortex for pendrin and 60 μg for NCC. Samples were loaded on 7.5% polyacrylamide gels and separated by gel electrophoresis at constant voltage, 100 V for 15 min followed by 140 V for 90 min. Membranes were incubated for 36 h with anti-pendrin primary antibody (1:1000 dilution; Knauf et al. 2001) or anti-NCC (1:1000 dilution; West et al. 2015). Blots were then incubated with a goat anti-rabbit IgG–HRP secondary antibody (1:15,000 dilution; sc-2004; Santa Cruz Biotechnology; this antibody does not give any IgG bands in rat kidney cortex when used alone). Bands of interest were visualized using enhanced chemiluminescence reagent (Supersignal West Pico; Thermo Scientific) and quantified by densitometry (VersaDoc imaging system and Quantity One Analysis software; Bio-Rad). Densitometry was normalized to Ponceau staining (Sigma) and virgin controls, with the mean for the virgin control group being set as 100%.
Tissue preparation for immunohistochemistry
Group 2 rats [virgins, 11–13 day mid-pregnant and 18–20 day late-pregnant rats (all n = 3)] were anaesthetized with inhalant isoflurane. Kidneys were perfused via an abdominal aortic cannula, first blood free with PBS (pH 7.4) followed by periodate–lysine–2% paraformaldehyde for 6 min, at a controlled pressure of 140 mmHg. The perfused kidneys were then cut transversely into several 2- to 4-mm-thick slices and immersed for ~24 h at 4°C in the same fixative, then placed in PBS at 4°C. Kidney samples from each animal were embedded in polyester wax [polyethylene glycol 400 distearate (Polysciences, Warrington, PA, USA) and 10% 1-hexadecanol], and 3-μm-thick sections were cut and mounted on triple chrome–alum–gelatin-coated glass slides (Verlander et al. 2013).
Immunohistochemistry
Immunolocalization was accomplished using immunoperoxidase, as follows. Sections were dewaxed in ethanol, rehydrated, heated in Trilogy (Cell Marque, Rocklin, CA, USA) to 88°C for 30 min and then to 96°C for 30 min, cooled for 30 min and rinsed in PBS. Endogenous peroxidase activity was inhibited by incubating the sections in 3% H2O2 in distilled water for 45 min. Sections were blocked for 15 min with Serum-Free Protein Block (DakoCytomation, Carpinteria, CA, USA) and then incubated at 4°C overnight with anti-pendrin primary antibody (1:20,000 dilution; Royaux et al. 2001). Sections were washed in PBS, incubated for 30 min with polymer-linked, peroxidase-conjugated goat anti-rabbit IgG (ImmPRESS; Vector Laboratories, Burlingame, CA, USA), washed again with PBS and then exposed to diaminobenzidine (DAB; Vector Elite) for 5 min. Sections were washed in distilled water, dehydrated with graded ethanols and xylene, mounted, and observed by light microscopy. Comparisons of labelling were made only between sections from the same immunohistochemistry experiment. Sections were examined on a Leica DM2000 microscope and photographed using a Leica DFC425 digital camera and Leica DFC Twain Software and LAS application suite (Leica Microsystems, Buffalo Grove, IL, USA). Colour adjustment was performed using Adobe Photoshop CS3 software (Adobe Systems, San Jose, CA, USA).
Quantitative analysis of immunohistochemisty
Quantification was performed as previously described (Kim HY et al. 2007; West et al. 2015). All tissues were prepared and stained using identical procedures and were viewed at the same time using identical microscope settings. Briefly, high-resolution digital micrographs were taken of all identifiable renal cortical collecting duct (CCD) segments in a single session using identical manual camera settings and a Leica DM2000 microscope equipped with a DFC425 digital camera and DFC Twain Software and LAS application suite. One to two cell profiles per CCD were selected for analysis using systematic random criteria. Raw images were analysed using freely available software (NIH ImageJ, version 1.48v) to quantify pixel intensity across a line drawn from the tubule lumen through the centre of an individual cell profile or adjacent to the nucleus in cell profiles with a visible nucleus. These data were then analysed using custom software. The pixel intensity at each point of the line was displayed graphically. The apical and basolateral edges were determined by the user. Total cellular expression was determined by integrating net pixel intensity measured along the line through the entire cell profile. Cell profile height was determined as the distance in pixels between the apical and basolateral edges of the cell profiles. The intensity of immunoreactivity in the apical 20% of the cell profile was determined by integrating the pixel intensity in this region of the cell profile. The individual performing the microscopy, photography and quantitative analysis was blinded to the treatment status of the animal.
Natriuretic response test
Sodium and potassium excreted in response to single subcutaneous (S.C.) injection of hydrochlorothiazide (HCTZ; 7.5 mg kg−1) was measured in virgin (n = 6), day 14 mid-pregnant (n = 7) and day 21 late-pregnant rats (n = 6), respectively. The collection time was selected based on a pilot test to determine the time course of the maximal diuretic effect. Urine was collected in metabolic cages for 0–4 h following HCTZ between 11.00 and 17.00 h. The day before the thiazide test, a baseline natriuretic response test to vehicle (water) S.C. was performed on each rat. Vehicle and thiazide response tests were conducted at the same time of day on consecutive days for each rat. Urine volume was determined gravimetrically, electrolyte concentrations by flame photometry, and pH was measured using a model UB-5 pH meter (Denver Instrument Company, Arvada, CO, USA) fitted with a semi-micro gel-filled Orion model 911600 electrode (Thermo Fisher Scientific, Beverly, MA, USA).
Statistics
Results are presented as means ± SEM. Statistical analyses were performed using Student’s unpaired t test or one-way ANOVA followed by Tukey’s post hoc test, and P < 0.05 was considered statistically significant.
Results
Baseline physical data
Body weight during pregnancy is directly correlated with plasma volume expansion (Rosso et al. 1992; Salas et al. 1993). As shown in Table 1, the pregnant rats in this study demonstrated the normal increase in body weight. Haematocrit decreased progressively in the pregnant rats, demonstrating the expected cumulative plasma volume expansion of normal pregnancy. The pregnant rats also had increases in kidney weight and uterine weight. By late gestation, a large proportion of the increased body weight (~60%) was composed of uterine weight and products of conception, and the majority of the increased uterine weight was pup weight (~67%).
Table 1.
Baseline physical data
| Parameter | Virgin rats (n = 6) | Mid-pregnant rats (n = 7) | Late-pregnant rats (n = 6) |
|---|---|---|---|
| Body weight (g) | 242 ± 2 | 276 ± 4* | 354 ± 8*† |
| Right kidney weight (g) | 0.72 ± 0.02 | 0.83 ± 0.01* | 0.81 ± 0.02* |
| Uterine weight (g) | 0.65 ± 0.12 | 7.90 ± 1.25 | 67.60 ± 7.45*† |
| Pup weight (g) | — | — | 45.52 ± 6.60 |
| Number of pups | — | 13 ± 2 | 14 ± 1 |
| Haematocrit (%) | 44 ± 0.5 | 41 ± 0.9* | 37 ± 0.4*† |
Data are presented as mean values ± SEM.
P < 0.05 versus virgin and
P < 0.05 versus mid-pregnant, by ANOVA.
Transcript expression of NDCBE and pendrin
The mRNA expression of NDCBE and pendrin was determined by real-time qPCR in renal cortical tissue of pregnant and virgin rats. Pendrin transcript expression (virgin CT = 26.9 ± 0.3) in the kidney cortex was ~32-fold higher than NDCBE (virgin CT = 32.0 ± 0.3). As shown in Table 2, there were no differences detected in the transcript expression of pendrin or NDCBE in pregnant rats compared with virgin control animals.
Table 2.
Relative gene expression
| Gene | Virgin rats (n = 6) | Mid-pregnant rats (n = 7) | Late-pregnant rats (n = 7) |
|---|---|---|---|
| Pendrin | 1.0 ± 0.12 | 1.09 ± 0.11 | 1.24 ± 0.15 |
| NDCBE | 1.0 ± 0.08 | 0.89 ± 0.06 | 1.11 ± 0.06 |
Data are presented as mean values ± SEM. Each sample was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Pendrin and GAPDH expression were run on a single qPCR plate for all rats; NDCBE and GAPDH were run on another qPCR plate for all rats.
Renal NCC and pendrin protein abundance
The kidney cortex protein abundance of pendrin was determined by Western blotting in virgin, mid- and late-pregnant rats. We also ran a confirmatory blot affirming the previously characterized changes in NCC (West et al. 2015) that occur in late pregnancy (Fig. 1). As summarized in Fig. 2, there was an increase in pendrin protein in mid-pregnant rats compared with virgin control animals (virgin, 100 ± 13%; mid-pregnant, 136 ± 10%, P < 0.05 by Student’s unpaired t test). There was also an increase in pendrin protein abundance in late-pregnant rats compared with virgins (virgin, 100 ± 11%; late-pregnant, 141 ± 14%, P < 0.05 by Student’s unpaired t test).
Figure 1. Confirmatory blot demonstrating previously characterized changes in the total protein abundance of the sodium–chloride cotransporter (NCC) in virgin, mid-pregnant (day 14) and late-pregnant rats (day 21); n = 2 per group.
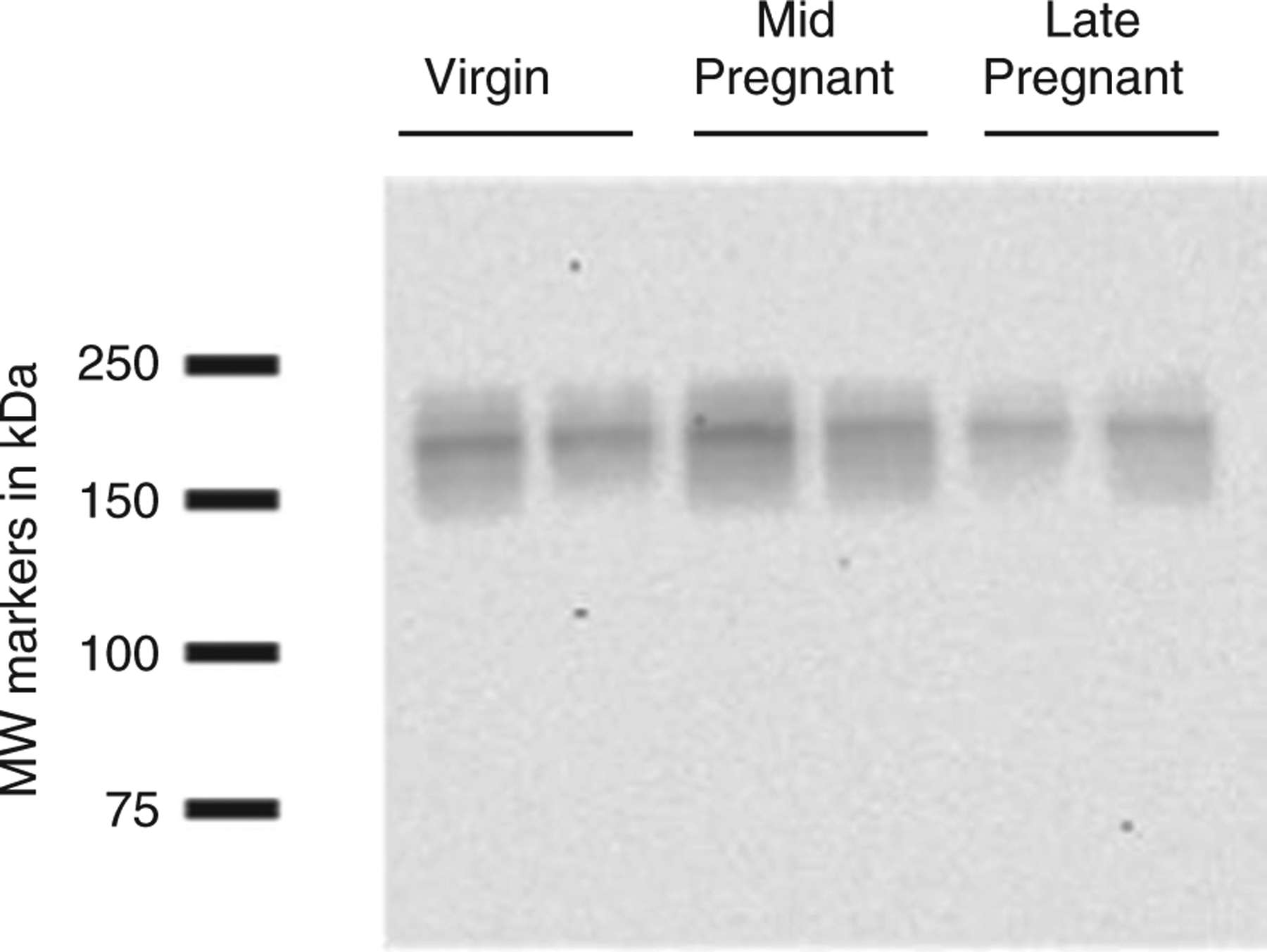
Figure 2. Protein abundance of pendrin in renal cortical tissue of virgin (n = 6), mid-pregnant (day 14; n = 7; A) and late-pregnant rats (day 21; n = 7; B).
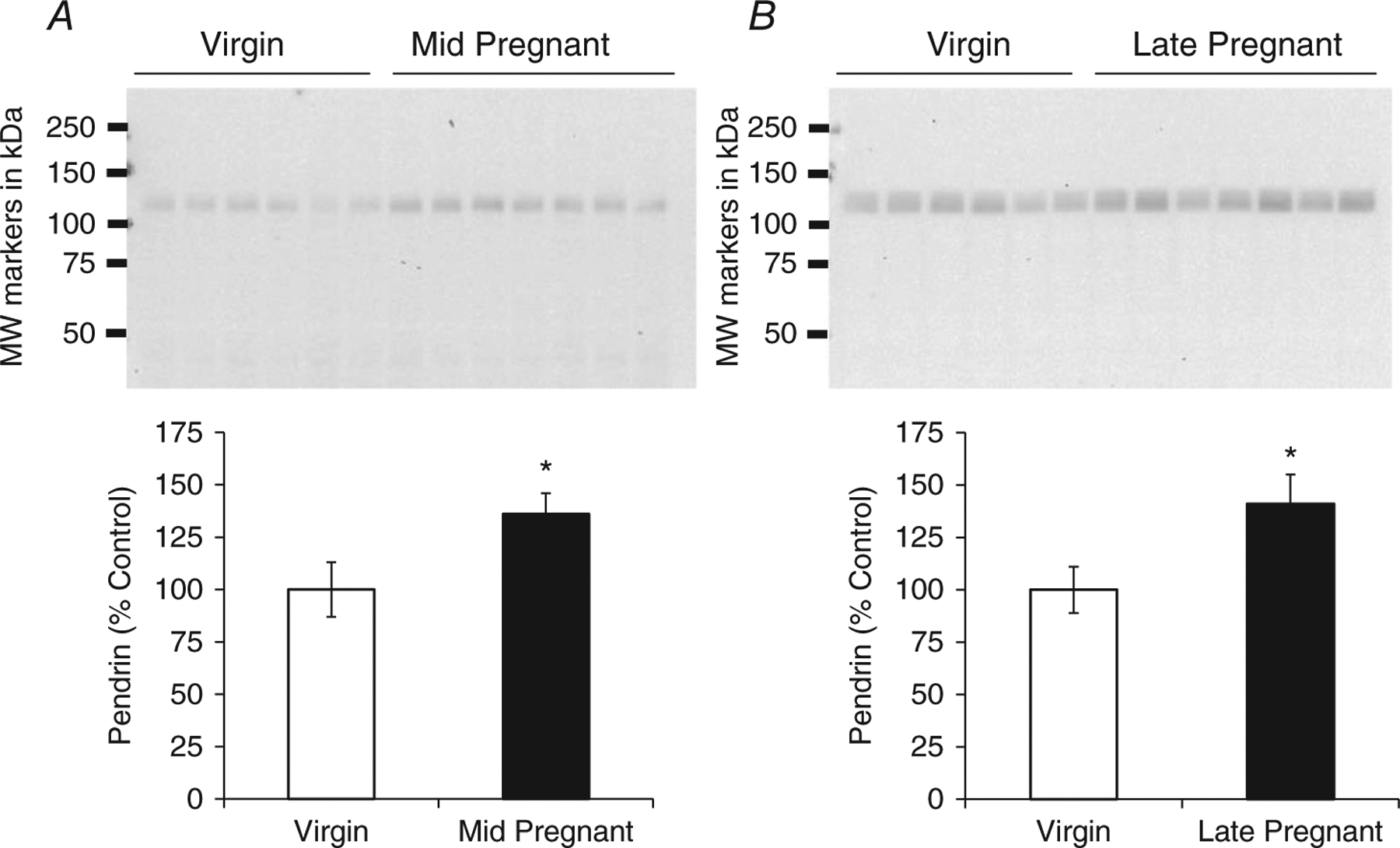
Band densities were normalized to virgin control animals, with virgins set at 100%, and summarized as bar graphs. One blot was run for mid-pregnant and one blot for late-pregnant rats versus virgins. Student's unpaired t test was performed and data are presented as means ± SEM. *P < 0.05 versus virgin.
Pendrin immunolocalization
As shown in Fig. 3, pendrin labelling was diffuse in virgin intercalated cells of renal CCD, whereas in mid-pregnant and late-pregnant CCD, pendrin immunoreactivity appeared more intense and more discretely apical in the majority of intercalated cells. Quantitative analyses confirmed that the apical expression of pendrin was increased in late-pregnant rats compared with virgin rats (pixel intensity in apical 20% of cell: virgin, 758 ± 107; mid-pregnant, 1155 ± 126, n.s.; late-pregnant, 1179 ± 43, P < 0.05 by ANOVA with Tukey’s post hoc test).
Figure 3. Immunolocalization of pendrin in virgin (n 3), mid-pregnant (day 11–13; n = 4) and late-pregnant rats (day 18–20; n = 4).
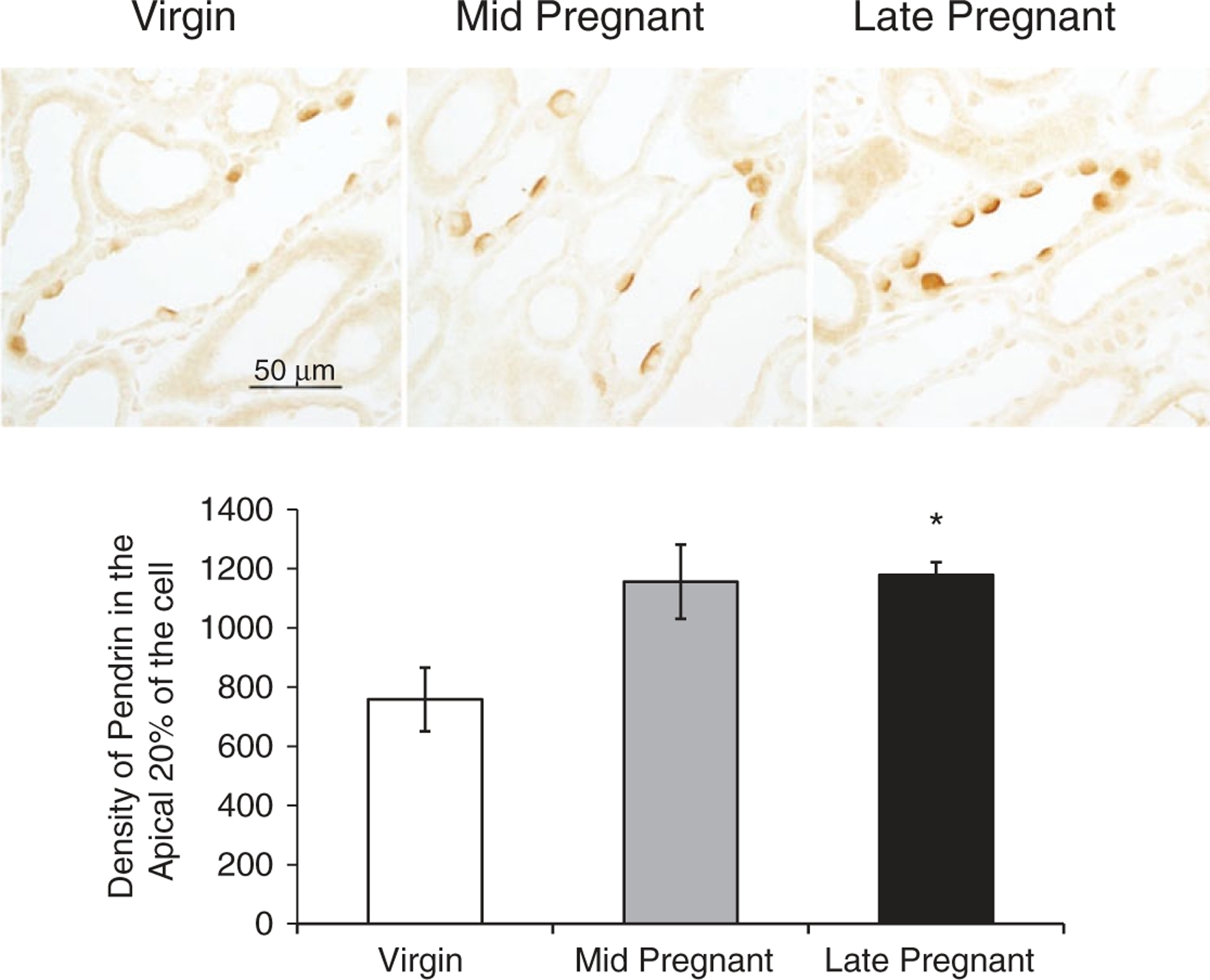
The expression of immunoreactivity as determined by pixel intensity was determined in the apical 20% of the cell and summarized as bar graphs. All slides were processed as one experiment. A one-way ANOVA with Tukey’s post hoc test was performed. Data are presented as means ± SEM. *P < 0.05 versus virgin.
Natriuretic response test
Given that NDCBE and pendrin mediate the thiazide-sensitive sodium transport of the CCD (Leviel et al. 2010) and we have recently found NCC to be unchanged in the mid-pregnant and decreased in the late-pregnant rat (West et al. 2015), we examined the natriuretic and kaliuretic responses to acute administration of HCTZ (7.5 mg kg−1, S.C.). As shown in Fig. 4A, there were no differences in sodium excretion between the virgin and pregnant rats following the administration of vehicle. Hydrochlorothiazide increased sodium excretion in both virgin and pregnant rats; however, the response was markedly enhanced in mid- and late-pregnant animals compared with virgins. Urinary K+ excretion increased during gestation, and increased further with HCTZ administration. However, the increment in K+ excretion observed with thiazide administration was similar in virgin and pregnant rats (Fig. 4B). Urine output was similar in virgin and pregnant rats after vehicle, and HCTZ increased urine output in all groups (Fig. 4C). There were no differences detected in the urine pH with pregnancy or after HCTZ treatment (Fig. 4D).
Figure 4. Sodium excretion (UNaV; A), potassium excretion (UKV; B), urine output (C) and urine pH (D) in response to hydrochlorothiazide (HCTZ) and vehicle (VEH) in virgin (n = 6), mid-pregnant (day 12–14; n = 7) and late-pregnant rats (day 19–21; n = 6).
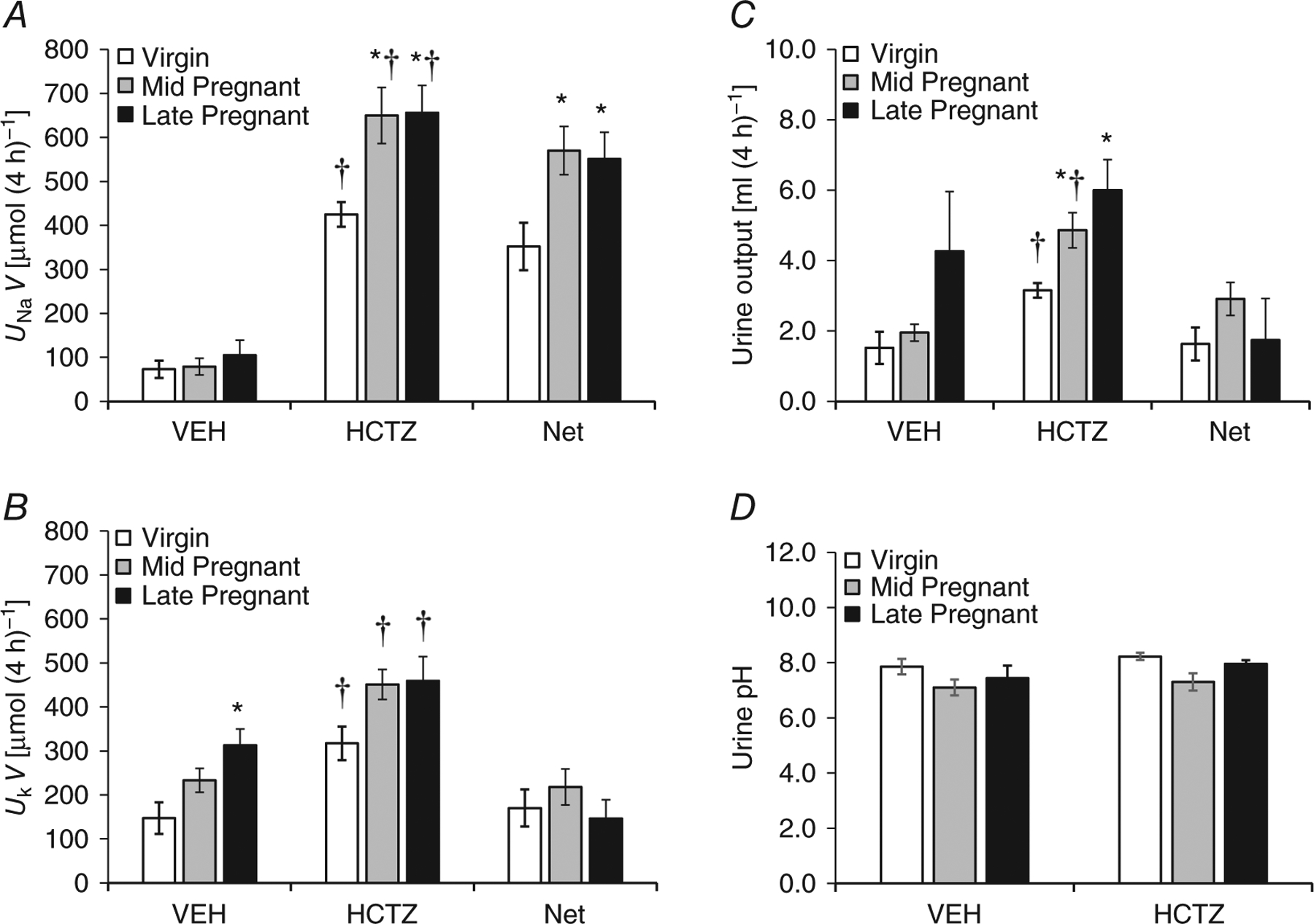
The thiazide-sensitive portion of the sodium excretion is the net UNaV, which is the difference between thiazide-mediated UNaV and vehicle-mediated UNaV, i.e. Net = HCTZ – VEH. A one-way ANOVA with Tukey’s post hoc test was performed. Data are presented as means ± SEM. *P < 0.05 versus virgin. †P < 0.05 versus VEH.
Discussion
The main findings of this study are as follows: (i) pendrin protein abundance and apical localization are increased in the pregnant rat; and (ii) thiazide sensitivity is increased in mid- and late pregnancy, despite our earlier finding of reduced NCC.
Previous work has demonstrated that pendrin expression is critical to volume homeostasis and maintenance of blood pressure. In baseline conditions, blood pressure is lower in pendrin-null versus wild-type mice (Pech et al. 2010). When placed on a NaCl-deficient diet (Wall et al. 2004), pendrin-null mice experienced a greater decrease in blood pressure compared with wild-type mice, indicating the physiological importance of pendrin expression in this model. Furthermore, pendrin-null mice in conditions of moderate dietary NaCl restriction had increased urine volume, urinary Cl− excretion, haematocrit and urea nitrogen values compared with wild-type, mice demonstrating the physiological importance of pendrin-mediated transport in fluid and volume homeostasis.
Angiotensin II and aldosterone regulate pendrin expression primarily by increasing apical plasma membrane protein abundance through subcellular redistribution (Verlander et al. 2003, 2011). Aldosterone analogues have also been shown to produce small increases in pendrin total protein abundance (Verlander et al. 2003). Here, we have demonstrated increases in both protein abundance and discrete apical localization of pendrin in CCD intercalated cells in the pregnant rat. This pregnancy-mediated increase in pendrin protein abundance is likely to occur by a post-transcriptional mechanism, because we did not detect any differences in pendrin mRNA expression. The more discrete apical localization in intercalated cells seen by light microscopy is likely to be correlated with increased apical plasma membrane pendrin expression, as we have demonstrated previously in studies using both light microscopic immunohistochemistry and immunogold cytochemistry to localize pendrin (Verlander, et al. 2003).
Although pendrin expression in renal homogenates of pregnant rats was only modestly upregulated, the contribution of pendrin to fluid homeostasis in pregnancy may be substantial. Recently, it was demonstrated that severe dietary NaCl restriction in mice causes only a 33% increase in pendrin protein abundance in renal homogenates (Lazo-Fernandez et al. 2015). With moderate dietary NaCl restriction (0.13 mequiv Na+ per day), apical plasma membrane pendrin abundance in type B intercalated cells doubles, although total pendrin protein abundance per cell is unchanged. This increment in apical plasma membrane pendrin abundance is important because this modest dietary NaCl restriction leads to marked apparent vascular volume contraction in the pendrin-null mouse (Wall et al. 2004). Mild dietary NaCl restriction, which probably elicited the same or less of an increase in pendrin protein in renal homogenates, caused a redistribution of pendrin immunogold label from the cytoplasmic vesicle compartment to the apical plasma membrane, resulting in a doubling of the density of pendrin immunolabel in the apical plasma membrane (Wall et al. 2004). Furthermore, these NaCl-restricted mice have no change in haematocrit and have decreased urine volume and urinary Cl− excretion compared with NaCl-replete mice, demonstrating the physiological importance of the modest increase in pendrin abundance (Wall et al. 2004). As such, it is likely that the modest increase in pendrin expression we observed in pregnant rats has a similarly important role in fluid and volume homeostasis.
The increase in thiazide sensitivity in pregnant rats is not due to changes in NCC, because NCC abundance, phosphorylation and apical localization are all decreased in late-pregnant and unchanged in mid-pregnant rats compared with virgins (West et al. 2015). The dissociation between thiazide sensitivity, which is widely used as an index of in vivo NCC activity, and NCC phosphorylation/localization in pregnant rats suggests that HCTZ is inhibiting another renal sodium chloride transport mechanism during pregnancy. Hydrochlorothiazide inhibits ~50% of renal sodium transport in the CCD (Terada & Knepper, 1990). However, NCC mRNA and protein expression is restricted to the distal convoluted tubule. Leviel et al. (2010) have shown that the thiazide sensitivity of the CCD is due to electroneutral transport through NDCBE, which works in tandem with pendrin at the apical plasma membrane of type B intercalated cells to mediate net reabsorption of sodium and chloride. This mechanism may be a candidate for the increased thiazide sensitivity that we have demonstrated in this study. Although we did not detect changes in the transcript expression of NDCBE, transcript expression is not always correlated with protein abundance, as is the case for pendrin in the present study. Unfortunately, there are no antibodies currently available that have been successful in detecting NDCBE in the rat kidney.
Pendrin modulates ENaC abundance, localization and function. Epithelial sodium channel-mediated sodium reabsorption is very low in CCD from pendrin-null mice compared with wild-type mice (Kim et al. 2007; Pech et al. 2010, 2015). Thus, functional pendrin is necessary to support ENaC activity. Given that ENaC activity is elevated in normal pregnancy and is required for the progression of a healthy pregnancy (West et al. 2010, 2014), the increased pendrin abundance and apical distribution may support the high ENaC activity.
A recent study indicated that pendrin and NCC may compensate for the loss of each other in baseline conditions. In the basal state, neither the pendrinnor the NCC-single knockout mice display any salt wasting or volume depletion (Soleimani et al. 2012). In the NCC knockout mouse, pendrin is increased in the kidneys (Vallet et al. 2006). Additionally, treatment of the pendrin knockout mice with HCTZ increased fluid loss in these mice relative to wild-types (Amlal et al. 2010). Furthermore, pendrin–NCC double knockout mice display severe salt wasting in basal conditions, resulting in volume depletion, hypotension, renal failure and metabolic alkalosis (Soleimani et al. 2012). In addition, a child with Pendred syndrome developed marked intravascular volume contraction, hypotension and metabolic alkalosis during thiazide administration (Pela et al. 2008). This suggests that pendrin and NCC play an important role in compensatory salt reabsorption in response to inactivation of each other. As NCC abundance, phosphorylation and localization are all decreased in the late-pregnant rat (West et al. 2015), we suggest that the increased pendrin may compensate for the decreased NCC.
Thiazides have been shown to inhibit carbonic anhydrases (Pickkers et al. 1999); however, their primary diuretic effect does not rely on carbonic anhydrase inhibition (Friedman & Berndt, 2004). Carbonic anhydrase inhibition results in sodium bicarbonate excretion (Friedman & Berndt, 2004); therefore, we measured the pH of the urine after HCTZ administration to determine whether the natriuretic effect was due to inhibition of carbonic anhydrase. Given that we did not find an alkalization of the urine with HCTZ compared with vehicle, we consider the effect of HCTZ on carbonic anhydrase to be minimal in this study.
In conclusion, we have demonstrated that pendrin protein abundance, pendrin apical localization and thiazide sensitivity are increased in the pregnant rat. This suggests that pendrin may be important in supporting the renal sodium chloride reabsorption and plasma volume expansion of pregnancy.
New Findings
• What is the central question of this study?
Pregnancy requires a robust plasma volume expansion driven by renal sodium retention. In the late-pregnant kidney, the aldosterone-responsive epithelial Na+ channel is increased, whereas the sodium–chloride cotransporter is decreased. Pendrin has been shown to support sodium reabsorption in the distal nephron and compensate for loss of the sodium–chloride cotransporter. We investigated the expression and abundance of pendrin in the pregnant kidney.
• What is the main finding and its importance?
Pendrin protein, apical localization and thiazide sensitivity are increased in pregnancy. This implicates a possible role for pendrin in supporting the renal sodium chloride reabsorption and plasma volume expansion of pregnancy.
Acknowledgements
The authors would like to thank Dr I. David Weiner for developing the software for the quantitative immunohisto-chemistry, Mr Richard Smith for his expert technical assistance and Dr Shyama Masilamani for providing the NCC antibody. We also thank Dr Sharon Matthews and Ms Tanisha Thomas in the University of Florida College of Medicine Electron Microscopy Core for preparing the tissues for immunohistochemistry.
Funding
This work was supported by the Robert and Mary Cade Professorship of Physiology and the National Institutes of Health grants T32-HL-093910, R01-HD-041571 and R01-DK-56843 to C.B. and R01-DK 46493 to S.M.W. C.A.W. was supported by NIH T32-HL-093910.
Footnotes
Competing interests
None declared.
References
- Alexander EA, Churchill S & Bengele HH (1980). Renal hemodynamics and volume homeostasis during pregnancy in the rat. Kidney Int 18, 173–178. [DOI] [PubMed] [Google Scholar]
- Amlal H, Barone SL, Xu J, & Soleimani M (2010). Pendrin confers partial resistance to the diuretic effect of loop and thiazide diuretics in mice. J Am Soc Nephrol 21, 480A. [Google Scholar]
- Atherton JC, Dark JM, Garland HO, Morgan MR, Pidgeon J & Soni S (1982). Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. J Physiol 330, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Osmond C & Law CM (1989). The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J Epidemiol Community Health 43, 237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron W, Brandt CN & Lindheimer MD (1993). Role of adrenal mineralocorticoid in volume homeostasis and pregnancy performance in the rat. Hypertens Pregnancy 12, 53–69. [Google Scholar]
- Brenner BM, Garcia DL & Anderson S (1988). Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1, 334–347. [DOI] [PubMed] [Google Scholar]
- Brochu M, Lehoux JG & Picard S (1997). Effects of gestation on enzymes controlling aldosterone synthesis in the rat adrenal. Endocrinology 138, 2354–2358. [DOI] [PubMed] [Google Scholar]
- Friedman PA & Berndt WO (2004). Diuretic drugs In Modern Pharmacology with Clinical Applications, 6th edn, ed. Craig & Stitzel RE, pp. 244–246. Lippincott Williams & Wilkins, Baltimore. [Google Scholar]
- Garland HO, Atherton JC, Baylis C, Morgan MR & Milne CM (1987). Hormone profiles for progesterone, oestradiol, prolactin, plasma renin activity, aldosterone and corticosterone during pregnancy and pseudopregnancy in two strains of rat: correlation with renal studies. J Endocrinol 113, 435–444. [DOI] [PubMed] [Google Scholar]
- Gibson HM (1973). Plasma volume and glomerular filtration rate in pregnancy and their relation to differences in fetal growth. J Obstet Gynaecol Br Commonw 80, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Grimm PR, Lazo-Fernandez Y, Delpire E, Wall SM, Dorsey SG, Weinman EJ, Coleman R, Wade JB & Welling PA (2015). Integrated compensatory network is activated in the absence of NCC phosphorylation. J Clin Invest 125, 2136–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR & Wingo CS (2009). The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119, 2423–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB & Knepper MA (1998). The thiazide-sensitive Na–Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA 95, 14552–14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME & Weiner ID (2007). Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293, F1238–F1247. [DOI] [PubMed] [Google Scholar]
- Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin W, Verlander JW, Sutliff RL & Wall SM (2007). Reduced ENaC expression contributes to the lower blood pressure observed in pendrin null mice. Am J Physiol Renal Physiol 293, F1314–F1324. [DOI] [PubMed] [Google Scholar]
- Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G & Aronson PS (2001). Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci USA 98, 9425–9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo-Fernandez Y, Aguilera G, Pham TD, Park AY, Beierwaltes WH, Sutliff RL, Verlander JW, Pacak K, Osunkoya AO, Ellis CL, Kim YH, Shipley GL, Wynne BM, Hoover RS, Sen SK, Plotsky PM & Wall SM (2015). Pendrin localizes to the adrenal medulla and modulates catecholamine release. Am J Physiol Endocrinol Metab, in press; DOI: 10.1152/ajpendo.00035.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R & Eladari D (2010). The Na+-dependent chloride bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120, 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ & Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC & Wall SM (2010). Pendrin modulates ENaC function by changing luminal HCO3−. J Am Soc Nephrol 21, 1928–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech V, Thumova M, Dikalov SI, Hummler E, Rossier BC, Harrison DG & Wall SM (2013). Nitric oxide reduces Cl− absorption in the mouse cortical collecting duct through an ENaC-dependent mechanism. Am J Physiol Renal Physiol 304, F1390–F1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech V, Wall SM, Nanami M, Bao HF, Kim YH, Lazo-Fernandez Y, Yue Q, Pham TD, Eaton DC & Verlander JW (2015). Pendrin gene ablation alters ENaC subcellular distribution and open probability. Am J Physiol Renal Physiol 309, F154–F163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pela I, Bigozzi M & Bianchi B (2008). Profound hypokalemia and hypochloremic metabolic alkalosis during thiazide therapy in a child with Pendred syndrome. Clin Nephrol 69, 450–453. [DOI] [PubMed] [Google Scholar]
- Pickkers P, Garcha RS, Schachter M, Smits P & Hughes AD (1999). Inhibition of carbonic anhydrase accounts for the direct vascular effects of hydrochlorothiazide. Hypertension 33, 1043–1048. [DOI] [PubMed] [Google Scholar]
- Rosso P, Donoso E, Braun S, Espinoza R & Salas S (1992). Hemodynamic changes in underweight pregnant women. Obstet Gynecol 79, 908–912. [PubMed] [Google Scholar]
- Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA & Green ED (2001). Pendrin, encoded by the pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98, 4221–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas SP, Marshall G, Gutierrez BL & Rosso P (2006). Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 47, 203–208. [DOI] [PubMed] [Google Scholar]
- Salas S, Rosso P, Espinoza R, Robert J, Valdes G & Donoso E (1993). Maternal plasma volume expansion and hormonal changes in women with idiopathic fetal growth retardation. Obstet Gynecol 81, 1029–1033. [PubMed] [Google Scholar]
- Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K & Amlal H (2012). Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci USA 109, 13368–13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y & Knepper MA (1990). Thiazide-sensitive NaCl absorption in rat cortical collecting duct. Am J Physiol Renal Physiol 259, F519–F528. [DOI] [PubMed] [Google Scholar]
- Todkar A, Chiara M, Loffing-Cueni D, Bettoni C, Mohaupt M, Loffing J & Wagner C (2012). Aldosterone deficiency adversely affects pregnancy outcome in mice. Eur J Physiol 464, 331–343. [DOI] [PubMed] [Google Scholar]
- Vallet M, Picard N, Loffing-Cueni D, Fysekidis M, Bloch-Faure M, Deschênes G, Breton S, Meneton P, Loffing J, Aronson PS, Chambrey R & Eladari D (2006). Pendrin regulation in mouse kidney primarily is chloride-dependent. J Am Soc Nephrol 17, 2153–2163. [DOI] [PubMed] [Google Scholar]
- Verlander JW, Chu D, Lee HW, Handlogten ME & Weiner ID (2013). Expression of glutamine synthetase in the mouse kidney: localization in multiple epithelial cell types and differential regulation by hypokalemia. Am J Physiol Renal Physiol 305, F701–F713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED & Wall SM (2003). Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension 179, 356–362. [DOI] [PubMed] [Google Scholar]
- Verlander JW, Hong S, Pech V, Bailey JL, Agazatian D, Matthews SW, Coffman TM, Le T, Inagami T, Whitehill FM, Weiner ID, Farley DB, Kim YH & Wall SM (2011). Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am J Physiol Renal Physiol 301, F1314–F1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS & Geibel JP (2002). Regulation of the expression of the Cl−/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int 62, 2109–2117. [DOI] [PubMed] [Google Scholar]
- Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED & Verlander JW (2004). NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl− conservation. Hypertension 44, 982–987. [DOI] [PubMed] [Google Scholar]
- West CA, Han W, Li N & Masilamani SM (2014). Renal epithelial sodium channel is critical for blood pressure maintenance and sodium balance in the normal late pregnant rat. Exp Physiol 99, 816–823. [DOI] [PubMed] [Google Scholar]
- West CA, McDonough AA, Masilamani SM, Verlander JW & Baylis C (2015). The renal NCC is unchanged in the midpregnant rat and decreased in the late pregnant rat despite avid renal Na+ retention. Am J Physiol Renal Physiol 309, F63–F70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C, Zhang Z, Ecker G & Masilamani S (2010). Increased renal α epithelial sodium channel (ENaC) protein and increased ENaC activity in normal pregnancy. Am J Physiol Regul Integr Comp Physiol 299, R1326–R1332. [DOI] [PMC free article] [PubMed] [Google Scholar]


