Abstract
Iron is involved in many types of metabolism, including oxygen transport in hemoglobin. Iron deficiency (ID), ie a decrease in circulating iron, can have severe consequences. We provide an update on iron metabolism and ID, highlighting the particularities in older adults (OAs). There are three iron compartments in the human body: 1) the functional compartment, which consists of heme proteins including hemoglobin, myoglobin and respiratory enzymes; 2) iron reserves (IR), which consist mainly of liver stocks and are stored as ferritin; and 3) transferrin. There are two types of ID. Absolute ID is characterized by a decrease in IR. Its main pathophysiological mechanism is bleeding, which is often digestive and can be due to neoplasia, frequent in OAs. Biological assessment shows low serum ferritin and transferrin saturation (TS) levels. Furthermore, hypochromic microcytic anemia is frequent, and the serum-soluble transferrin receptor (sTfR) level is high. Functional ID, in which IR are high or normal, is due to inflammation, which is also frequent in OAs, particularly in its chronic form. Biological assessments show high serum ferritin, normal or low TS, and normal sTfR levels. Moreover, C-reactive protein is elevated, and there is moderate non-regenerative non-macrocytic anemia. The main characteristics of iron metabolism anomalies in the elderly are the high frequency of ID (20% of ID with anemia in adults ≥85 years) and the severity of its consequences, which include cognitive impairment in case of ID or iron overload and decrease of physical activity in case of ID. In conclusion, causes of ID are frequently intertwined in OAs as a result of the polymorbidity that characterizes them. ID can have dramatic consequences, especially in frail OAs. Thus, measuring the appropriate biological markers prevents errors in the positive diagnosis of ID type, clarifies etiology, and informs treatment-related decision-making.
Keywords: anemia, iron deficiency, iron metabolism, older adults
Introduction
Iron is the most represented trace metal in the human body.1
Iron deficiency (ID) is the most frequent nutritional deficiency disorder in the world, defined as a lack of human body iron reserves (IR), and it is generally due to an inadequate absorption and/or excessive loss of iron.2
ID has various consequences, and some effects have yet to be elucidated. ID consequences can be dramatic in older adults (OAs), especially those who are frail. The consequences include anemia, asthenia (even without anemia), occurrence of cognitive disorders, worsening of neurocognitive disorder (Alzheimer’s disease or other), decrease of immune defense causing infections, osteoporosis, and decrease in physical activity.3–9 In addition, ID is a marker of the severity of heart failure, which is a frequent condition in OAs.10
Although the role of iron in human body is well known, there have been recent advances in the understanding of iron metabolism, including its absorption pathways and precise regulation. The roles of hepcidin, ferroportin and transferrin receptors have also been clarified. These findings have led to the development of new diagnostic tools. However, the place of these tools in the diagnostic strategy of iron deficiency is not yet defined.
The objective of this article is to provide an update on the latest scientific data in the literature on iron metabolism and ID by highlighting specificities in OAs. Iron overload will be addressed briefly, and the main causes and consequences will be described.
Method
We performed a bibliographic search on the Medline database, including articles published from January 1990 to August 2020. The following terms were combined: iron with metabolism, anemia, disorders, deficiency, overload, causes, consequences, biological tests and/or aged, or their equivalents. We also looked at one book on dietary reference intakes. This first literature search was supplemented by a bibliographic search related to specific aspects that we wanted to clarify (for example, to explain the interest of physical activity, especially in the elderly).
The most relevant articles were retained for our bibliography.
Physiological Role of Iron in the Human Body
Iron participates in erythropoiesis. It is a component of heme, which combines with globins to form hemoglobin in the bone marrow.
Iron is the most abundant trace mineral, and it is involved in cell metabolism and the growth of organisms. A smaller fraction (2%) localized in some proteins containing heme and iron is present as iron-sulfur groups that contribute to physiological systems such as oxygen transport, deoxyribonucleic acid (DNA) synthesis, metabolic energy, cellular respiration and electron transport in mitochondria. Approximately 30% and 10% of body iron are stored as ferritin and hemosiderin in the liver, bone marrow, and muscle. In addition, iron can be used in erythropoiesis according to the demands of the body.1,3,11,12 Finally, iron is involved in the functioning of the immune system and cognition.13
IR and Location in the Human Body
IR in the human body are estimated at between 35 and 45 mg of iron/kg of body weight, or about 3 to 4 g total/person.2,14
In humans, there are two forms of iron, which depend on the state of oxidation. One bivalent, Fe2+, is reduced and soluble. The other trivalent, Fe3+, is oxidized and insoluble.15,16 Fe2+ is the intracellular storage form of iron, mainly in macrophages (of the liver and other organs) and enterocytes, in the ferritin form. In addition, it is a component of heme proteins. Fe3+ is the form of iron transport in blood by being linked to transferrin. In the Fe2+ form, iron is soluble and can therefore pass through the cell membrane, while it cannot in the Fe3+ form because it is not soluble.15,16
There are three iron compartments: the functional sector, the IR, and the transport compartment. The functional compartment consists of heme proteins including hemoglobin, myoglobin and respiratory enzymes.1,3,17 The second compartment (IR) consists mainly of liver stocks, but also of other tissues or cells (enterocytes), and is stored as ferritin.3 Finally, transferrin is the last sector (transport).3 Iron is always linked to a protein in each compartment, and there is very little iron in the free state. In clinical practice, the most effective available biomarkers for evaluating iron compartments are the hemoglobin level for functional iron, serum ferritin (SF) for IR, and the transferrin saturation (TS) for iron transport.17
In humans, iron is distributed as follows: about two-thirds, slightly less than 3 g, in hemoglobin, 0.5 to 1 g in the liver, 0.3 to 0.4 g in muscles and macrophages, and 0.1 g in the bone marrow and other cells.14,18 The amount of circulating iron, bound to transferrin in plasma, is less than 1%. With the largest quota of iron being in hemoglobin, blood loss is associated with a decrease in iron stocks at a rate of 500 mg per liter of blood lost.
Iron Metabolism
Our understanding of iron metabolism has improved considerably in recent years.
Iron sources
With the exception of medical treatment for a proven ID, iron is brought to the human body by two sources: a minor external source (6.5% to 8%) and a major internal source (≥92%).12,14,18
Food is the exogenous iron source. The dietary intake of iron is in the form of heme found in meats and fish or non-heme found in plants, egg yolk and dairy products. Heme iron (organic iron) is mainly ferrous (Fe2+) while non-heme iron (inorganic iron) is mainly ferric (Fe3+).16 Foods rich in heme iron include, among others, black pudding, liver (pork, lamb or beef), kidney of lamb, clams, oyster, octopus and mussels. Foods rich in non-heme iron include thyme, unrefined coconut blossom sugar, cocoa, soy, fruit, raw yellow beans, lentils, ginger, cereals and spinach.
Our main source of iron is endogenous. It is the iron resulting from phagocytosis of nonfunctional erythrocytes by macrophages.12
Women and adolescents have higher daily iron needs. The recommended dietary allowance for iron is 11 mg per day (/d) for teenage (14–18 years) boys, 15 mg/d for teenage girls, 8 mg/d for adult men (≥19 years) and postmenopausal women, including OAs, 18 mg/d for premenopausal women, and 27 mg/d for pregnant women.19
Intestinal Absorption, Transport and Storage of Iron
Iron is absorbed in the duodenum and proximal jejunum.20
In the digestive tract, non-heme iron, provided by plants, egg yolk and dairy products, is solubilized by stomach acid and it is transformed into Fe2+ by duodenal cytochrome B, a reductase present in the apical membrane of duodenal enterocytes. Then, the divalent metal transporter-1 (DMT1: iron and proton co-transporter) intervenes to transport Fe2+ into the cytoplasm of the enterocyte. That is why achlorhydria, associated with the consumption of proton pump inhibitors (PPIs), and pernicious anemia decrease the intestinal absorption of non-heme iron, similar to Helicobacter pylori infection and certain inflammatory conditions, celiac disease, for example.20–22 Once it is incorporated into the enterocyte cytoplasm, Fe2+ can be oriented: 1) either to apoferritin to form ferritin which is the IR of enterocytes (much less erythrocyte iron is not contained in ferritin), a part of which is eliminated during enterocyte desquamation; 2) or to the basolateral membrane of the enterocyte, then directed into the circulating blood by ferroportin and transformed into Fe3+ by hephaestin, a membrane ferroxidase functionally linked to ferroportin.15,16 Ferroportin is therefore much like a “front door” for intestinal iron to enter the body.
In the blood, Fe3+ binds to apotransferrin, forming holotransferrin (transferrin with two Fe3+ ions). Depending on the needs of the organs and in order to store iron, holotransferrin can bind to transferrin receptors (TfR) located on the surface of cells (including hepatocytes and macrophages). The TfR-holotransferrin complex thus formed is then incorporated into the cell and internalized in the endosome in which the two Fe3+ ions are released. The Fe3+ is then reduced to Fe2+, which is transported through the endosome membrane by DMT1. The Fe2+ released in the cytoplasm is either stored in ferritin or used for certain cellular functions.15,16 The acidification of endosomes facilitate iron release during the TfR-holotransferrin cycle.
The mechanism for the intestinal absorption of heme iron, provided by meats and fish, remains unclear.20 One hypothesis includes the transfer of heme iron from the intestinal lumen to the cytoplasm of the duodenal and jejunal enterocytes via the heme carrier protein-1, which may be a specific transporter of heme. Heme is released in the enterocyte cytoplasm and then metabolized by the heme oxygenase-1, allowing the release of iron in its ferrous form (Fe2+). Fe2+ from the heme iron then follows a similar process to that of non-heme iron. However, this mechanism has not been confirmed, and certain studies suggest that heme carrier protein-1 is a transporter not of heme, but of vitamin B9.16,20
When there is an increase in the need for circulating iron, in particular for erythropoiesis, the iron stocks contained in the liver, non-hepatic macrophages (of the spleen, for example), and enterocytes (mainly as ferritin) are mobilized to fuel the circulating blood sector. The transport of iron from cells into the blood circulation is made possible by the presence of ferroportin whose action is controlled by hepcidin (hepatic bactericidal protein).16
During the transfer of intracellular iron to the extracellular environment, Fe2+ (intracellular) is oxidized to create Fe3+, which attains the circulating blood. This oxidation is made possible by hephaestin, mainly located in the small intestine, and by exiting macrophages and hepatocytes by means of ceruloplasmin, another ferroxidase.16 Hephaestin and ceruloplasmin are “copper-dependent” ferroxidases.
Ferroportin is a membrane protein found on the surface of cells that store or transport iron, including duodenal enterocytes (at their basolateral pole, in contact with circulating blood), Kupffer cells, and macrophages.16 Ferroportin allows the passage of iron from the intracellular environment to the extracellular sector.3 It is considered to be a hepcidin receptor.14 Hepcidin can bind to ferroportin and cause it to internalize and degrade in the lysosome.14
Hepcidin is a hormone mainly produced in the liver.4,14,16 It is a peptide regulating the homeostasis of iron metabolism through ferroportin.18 The serum hepcidin rate fluctuates according to various conditions such as the variations in the body’s IR (including in the liver), inflammation, erythropoiesis, and hypoxia.18 Hepatocytes are sensitive to circulating total (bound and free) iron levels. A decrease in circulating iron inhibits the hepatic synthesis of hepcidin, and a lack of hepcidin will then stimulate the intestinal absorption of iron and its release from enterocytes and macrophages in the vascular system. Conversely, excess circulating iron stimulates the liver production of hepcidin. This results in iron sequestration in the enterocytes and the macrophages, a decrease in intestinal absorption, and therefore an inhibition of iron transfer to the blood.4,14,16 Hepcidin is eliminated in the urine, which means that its serum concentration increases during renal failure.3
The enterocytes are also sensitive to total iron stocks of the body, for which they play a regulatory role. If a decrease in the total IR is detected, the iron stored as ferritin in the enterocytes is released and discharged into the blood compartment, via ferroportin, and the intestinal absorption of iron increases.4,14,16 Conversely, when the IR in the body are normal or too high, iron remains sequestered in the enterocytes due to the inhibition of the ferroportin activity by hepcidin and the intestinal absorption of iron is greatly reduced.4,14,16 Schematically, ferroportin is a lock and hepcidin is the key. When the hepcidin level rises, it closes the lock, and vice versa.
The intestinal absorption of non-heme iron is highly dependent on diet.20 Tannins (polyphenols), found in tea, coffee or chocolate, inhibit the absorption of non-heme iron,3,20 as well as dairy products, cereals, dietary fiber, phosphate-containing carbonated beverages and certain foods (egg yolk), and multivitamin or dietary supplements containing calcium, zinc, manganese or copper.23 Phytates and oxalates contained in food (spinach, …), and peptides from partially digested vegetable proteins also reduce non-heme iron absorption.3,11,20 Finally, the consumption of certain drugs such as PPIs and other antacids, quinolones and tetracyclines also inhibits non-heme iron absorption.3,20,23
Vitamin C (ascorbic acid) increases non-heme iron absorption via the promotion of reduction and solubilization of dietary ferric iron.11,20 Non-heme iron absorption is also improved by diets rich in heme iron (meat, fish, shellfish, …), fermented food, black pepper and organic acids (malate or citrate).11
Heme iron absorption is generally not influenced by diet.20
Heme iron is better absorbed than non-heme iron.12,20 This is explained by the fact that heme iron (mostly Fe2+) is soluble and reduced, while non-heme iron (mostly Fe3+) is not soluble and not reduced.16,20 Another explanation is the fact that non-heme iron absorption is influenced by diet, contrary to heme iron absorption.3,20,23 Finally, to be absorbed, ferric iron must be reduced to ferrous iron in order to transport iron into enterocytes.20
Iron Elimination
Iron is eliminated through exfoliated intestinal epithelial cells, bile, urine, skin, hair, nails, sweat, semen and blood loss.18,24 There is no regulated way to eliminate iron.
Iron Homeostasis and Bioavailability
In physiological conditions, the iron input–output balance is zero with equivalent amounts absorbed and eliminated, ie one to two mg/d.18,25 However, to provide one to 2 mg of iron/d to the body, the recommended intake is 10 to 20 mg/d.14 Indeed, only 5% to 15% of the iron consumed is absorbed,14 ie low or middle bioavailability.11 Bioavailability of heme iron, whose absorption is almost uninfluenced by diet, is much better than that of non-heme iron, whose absorption is influenced by diet.11,20 Indeed, certain nutrients are non-heme absorption enhancers and others are inhibitors. Bioavailability also depends on IR; in case of ID, iron bioavailability can increase to 35%.11
Exogenous intake is far from covering the cumulative iron needs of all the body’s tissues or cells (including erythrocytes), which range from 15 to 25 mg/d. These needs are 90% to 95% covered by the action of macrophages, which recycle internal iron. Indeed, daily phagocytosis by the macrophages of senescent red cells recovers iron which is then delivered to the organism. This iron is then either sent to the blood, via ferroportin, or stored as ferritin.12 Thus, at least 92% of the iron comes from the internal channel and 8% at most is sourced from the exogenous intake.12,14,18,25 Iron balance is therefore maintained through meticulous control between intestinal absorption and recycling via phagocytosis by the macrophages of old erythrocytes.16
Iron absorption, transport and storage, ie iron homeostasis, are also accurately controlled at the translational level by iron regulatory proteins (IRPs), which exist in two forms, IRP1 and IRP2, and iron-responsive elements (IREs) signaling pathways.26 IRP1 and IRP2 are two known ribonucleic acid (RNA)-binding proteins, and their inducible interactions with IRE control the translation of ferritin and ferroportin messenger RNA (mRNA) and the stability of TfR mRNA.26 IREs are short conserved stem-loops that are bound by IRPs.26 IREs are found in untranslated regions (UTR), either the 3ʹ-UTR or 5ʹ-UTR, of various mRNAs whose products are involved in iron metabolism.26 IRPs can act as either a translational activator or a translational inhibitor.26 In case of ID cells, the interaction between IRPs and IRE motif in the 5ʹ-UTR of target mRNA can revoke translation by suspending important interactions between the mRNA and ribosomes for the initiation of translation.26 Yet, in iron-replete cells, iron can bind with IRPs to induce a conformational change, which furthers the separation of IRPs from the target mRNA, entailing facilitation of the translation of the target mRNA.26 In case of ID cells, the interaction between IRPs and the IRE motif in the 3ʹ-UTR of target mRNA can protect it from endonuclease cleavage.26 Thus, this interaction can prolong the half-life of transcripts and promote the translation of target mRNA.26 Nevertheless, in iron-replete cells, the separation of IRP from 3ʹ-UTR IRE exposes target transcripts to endonuclease attack and degradation, entailing downregulation of the translation of transcripts.26 Transcripts of ferritin and ferroportin have IRE in their 5ʹ-UTRs.26 Thus, in case of ID, the translation of ferritin and ferroportin can be suppressed by IRPs. The reduction of ferritin and ferroportin expression can decrease unnecessary iron binding by ferritin and iron export by ferroportin, entailing the augmentation of free iron available for cells.26 Nevertheless, both TfR and DMT1 mRNA have IRE motifs in the 3ʹ-UTR, which can bind to IRPs in case of ID, entailing the stabilization of transcripts and subsequent increased synthesis of TfR and DMT1 to encourage iron absorption into cells.26 However, an iron-replete state can disrupt the interaction between IRPs and IREs to further the translation of the ferritin and ferroportin transcripts, and dislocate TfR and DMT1 mRNA.26
Impact of Ageing on Iron Metabolism
Although not well documented, the effect of age on iron absorption and iron elimination seems minor, but IR increase with age.13
Frequency of ID without or with Anemia
The SU.VI.MAX study on a sample of 5,447 individuals found ID in 5.3% of postmenopausal women and in 1.8% of men, while the prevalence of ID with anemia was 0.7% in postmenopausal women and 0.4% in men.27
With ageing, the prevalence of ID anemia increases. More than 10% of people ≥65 years have ID anemia, and this rate doubles in people ≥85 years.8
Conditions That Can Affect the Amount of Iron in the Human Body
Causes of ID
There are two types of ID that reflect a decrease in circulating iron. One is absolute ID (AID), which is linked to reduced IR in the body, resulting in a very low SF and insufficient iron.3,25 The other type is functional ID (FID), caused by inflammation, in which iron stocks are increased. FID results in an increase in SF, while, with iron being sequestered in other tissues, the amount of available iron is no longer sufficient to ensure effective erythropoiesis.4,25
Causes of AID
Gastrointestinal or esophageal blood loss is the main cause of AID, especially in OAs.28 These can be secondary to neoplasia, which are more common in OAs,29 benign tumors, a gastric or duodenal ulcer, esophagitis, gastritis of autoimmune origin or linked to Helicobacter Pylori infection, through decreased intestinal absorption of non-heme iron or not, or hiatal hernia.20,28,30,31 Other causes of gastric, duodenal or esophageal bleeding include consumption, even occasional, of nonsteroidal anti-inflammatory drugs, platelet aggregation inhibitors, anticoagulants or corticosteroids.31–33 Chronic gastrointestinal conditions can also be complicated by AID.28 AID can be the result of malabsorption in inflammatory bowel disease and celiac disease, while it is due to bleeding in diverticulosis with or without diverticulitis, especially in OAs, or in case of hemorrhoids.2,20,31,34–36 Other intestinal lesions or abnormalities such as fistula, bowel obstruction, short bowel syndrome, colon angiodysplasia or restorative proctocolectomy may also be associated with AID.31 Finally, the chronic consumption of certain drugs such as PPIs and aging has also been implicated in AID through reduced stomach acid secretion resulting in a decrease in intestinal iron absorption.2,28,37 For PPIs, it is especially true for non-heme iron.20 This mechanism is also described in pernicious anemia and achlorhydria (reducing iron absorption due to the non-solubilization of non-heme iron) which are associated with gastric atrophy, causing vitamin B12 deficiency.22 It should be noted that the relationship between gastric atrophy, due in particular to aging, and AID is not currently established, and gastric atrophy does not actually seem to affect the absorption of iron.13
Some chronic hepatopathies can be complicated by AID anemia.31 This is the case for cirrhosis, which is accompanied by portal hypertension which can cause digestive hemorrhage.31 Nonalcoholic fatty liver disease can also cause AID with or without anemia, even though fatty liver can also cause an iron overload, as we will discuss further on.31,38
A malignant or benign lesion of the urinary or genital (in women) tract, surgery-related hemorrhage or hematoma are other possible causes of AID.13,39,40
In addition, AID can result from malnutrition, vegetarian diets (which cause AID but a priori with no anemia), or vegan diets (which are believed to cause AID with anemia).28,41,42 Blood donors are also exposed to AID, as are patients with kidney failure,18,43 which is particularly common in OAs. It has been shown that moderate chronic renal failure is present in almost all patients aged 80 and older hospitalized in a geriatric unit.44 In kidney failure, AID, due to excessive blood loss and an abnormality of iron reabsorption, worsens anemia secondary to a defect in the synthesis of erythropoietin.43 Finally, chronic alcohol abuse may be complicated by AID.28
It is imperative to keep in mind that OAs often develop AID for multiple and interrelated reasons.
Table 1 reports the main causes of AID.
Table 1.
Main Causes of AID
| Anatomical Location or Mechanism | Causes |
|---|---|
| Digestive blood loss | Cancer or benign tumor |
| Gastric or duodenal ulcer | |
| Esophagitis | |
| Gastritis (various origins) | |
| Hiatal hernia | |
| Drugs: Nonsteroidal anti-inflammatory Anticoagulants Platelet aggregation inhibitors Corticosteroids | |
| Inflammatory bowel disease | |
| Celiac disease | |
| Diverticulosis | |
| Colon angiodysplasia | |
| Portal hypertension | |
| Genito-urinary blood loss | Cancer or benign tumor |
| Traumatic blood loss | Hemorrhagic surgery (total hip prosthesis, etc.) |
| Hematoma | |
| Iron absorption reduction | Digestive tract aging |
| Drugs: Proton pump inhibitors | |
| Short bowel syndrome | |
| Pernicious anemia | |
| Iron intake decrease | Malnutrition |
| Vegetarian diet | |
| Vegan diet | |
| Chronic alcohol | |
| Blood extraction | Blood donors |
| Hepcidin elimination abnormality | Severe renal failure |
Causes of FID
Inflammatory conditions can cause FID at any age. These conditions include infections, acute or chronic systemic inflammatory diseases (particularly rheumatic), cancer, and thromboembolic disease.
Chronic inflammation is frequent in OAs.2 This is probably the result of the accumulation of various chronic conditions with an inflammatory background and regular inflammatory episodes.
Causes of Mixed Iron Deficiency (MID)
MID Associates AID and FID
Among the causes of MID, there are digestive cancers, which are particularly common in OAs, malignant lesions of urinary or genital (in women) tract, as well as rheumatic and systemic inflammatory diseases, with the consumption of nonsteroidal anti-inflammatory drugs or corticosteroids.
Causes of Iron Overload
The causes of iron overload (Table 2) include hemochromatosis, metabolic syndrome and chronic liver diseases, whatever their cause.14,45 Other causes of iron overload are myelodysplastic syndrome, which affects adults older than 65 years,46 hemophagocytic lymphohistiocytosis, iterative transfusions (especially in people with thalassemia),47 sideroblastic anemia, or homozygous sickle cell disease.14,48 Other situations can lead to the occurrence of secondary hemochromatosis. These are iron administration, either for supplementation in case of dialysis or in high-level athletes, and more rarely the excess of iron food intake.45,48,49 Hepatic steatosis, alcoholic or not, and porphyria cutanea tarda can also cause iron overload at any age.38,50 Finally, ferroportin mutation and hereditary aceruloplasminemia lead to iron overload, though rarely in OAs.14 Gaucher disease is not a common cause of iron overload but is sometimes found in cases of splenectomy.14
Table 2.
Causes of Iron Overload
| Anatomical Location or Mechanism | Causes | |
|---|---|---|
| Hereditary or acquired metabolic disorder | Hemochromatosis (hereditary or secondary) | |
| Porphyria cutanea tarda | ||
| Metabolic syndrome | ||
| Chronic hepatopathies | Hepatitis | Viral |
| Auto-immune | ||
| Toxic | Alcoholic | |
| Steatosis | Alcoholic | |
| Non-alcoholic | ||
| Hematological mechanism | Myelodysplastic syndrome | |
| Hemophagocytic lymphohistiocytosis | ||
| Excessive iron intake | Iterative transfusions | Thalassemia |
| Homozygous sickle cell disease | ||
| Sideroblastic anemia | ||
| Iron administration | Dialysis | |
| High level athletes | ||
| Other | Ferroportin mutation | |
| Hereditary aceruloplasminemia | ||
Consequences of Iron Metabolism Disorders
Negative Impact of ID on the Body
Besides anemia, whose frequency is particularly high in OAs (20% of people ≥85 years have ID with anemia),8 ID has the following potential consequences: asthenia (even without anemia), reduction of the immune defenses, aggravating immunosenescence leading to infections, and impairment of brain function including cognition, which could worsen neurocognitive disorders (Alzheimer’s disease or other) in OAs.3,4,6,9,13 The other consequences include worsening heart failure (for which it is a predictive factor of mortality), especially in the elderly, hair loss, delayed skin healing, deafness, asymptomatic bacteriuria in individuals with overweight, osteoporosis, Willis Ekbom disease (restless legs syndrome), or pruritus.5,13,17,51–56 Finally, ID with or without anemia can lead to a decrease in physical activity.7 This can be dramatic in OAs due to the preexisting negative impact of aging on physical activity. Indeed, it is known that physical activity decreases the risk of motor function decline, hence its importance in maintaining physical autonomy.57
ID is the leading cause of anemia worldwide.4 However, anemia is usually multifactorial, associating, for example, ID by occult gastrointestinal bleeding, chronic renal failure, anticoagulant or platelet aggregation inhibitor treatment (even nonsteroidal anti-inflammatory drugs, which are unfortunately sometimes still prescribed in the elderly) and ineffective erythropoiesis.4,58 In OAs, anemia can also be caused by various other chronic diseases, and a non-negligible proportion remains unexplained.4,59,60
Negative Impact of Iron Overload on the Body
In the free form, iron can be toxic and damage tissues by participating, directly or indirectly through hydrogen peroxide,61 in the production of free radicals that attack cell membranes, proteins and DNA.1,12 Another mechanism leading to cell death from excess iron is ferroptosis, which was recently described and was initially presented as non-apoptotic, non-necrotic and non-autophagic. Ferroptosis appears to be the result of the dysregulation of the antioxidant power of the cell and an increase in intracellular iron levels, resulting in lipid peroxidation of the plasma membrane leading to cell death.62
Iron overload can have a negative impact on the brain, and can even affect cognition.25 Some authors reported higher SF levels in individuals with Alzheimer’s disease, whose frequency increases with advancing age, than in those without.25 Liver dysfunction, including through hepatic sinusoidal obstruction syndrome, can also result from iron overload.63 In extreme cases, it can lead to cirrhosis.14 Cardiac consequences such as heart failure and supraventricular or ventricular rhythm disturbances are possible.48 Ultimately, excess iron can accumulate and cause damage in various organs or systems, including the liver, spleen, heart (cardiomyopathy), bone marrow, pituitary gland, pancreas and the central nervous system.48
Fortunately, the human body has defense mechanisms to prevent iron toxicity when it is possible. These are the previously described proteins involved in the transport of iron through cell membranes in order to store it initially in a nontoxic form, making it available to the body when needed.1,12
Laboratory Tests for a Suspected Decrease in the Level of Circulating Iron
There are two possible situations when exploring dysfunction in iron metabolism: a suspected decrease in the circulating iron, or, on the contrary, an iron overload. As mentioned in the introduction, here we are only addressing the first situation.
A decrease in circulating iron can be clinically suspected if the interview and physical examination of the patient suggests ID or anemia. A decrease in circulating iron can also be revealed by laboratory tests, including either a fortuitous serum hemoglobin exam completed by an assessment of iron status in case of anemia suggestive of an iron metabolism disorder, or directly with an assessment of iron status.
We should start by noting that when ID is suspected in a patient with anemia, bone marrow aspiration is the gold standard diagnostic test.64 Perls staining on bone marrow does not find iron in erythroblasts and macrophages, characterizing AID, while it shows that iron is present in macrophages and absent in erythroblasts, which is very suggestive of FID. However, the invasive nature of bone marrow aspiration means it is not appropriate for routine examinations.64 Therefore, the analysis of biomarkers is the most suitable means of gaining information about iron status.
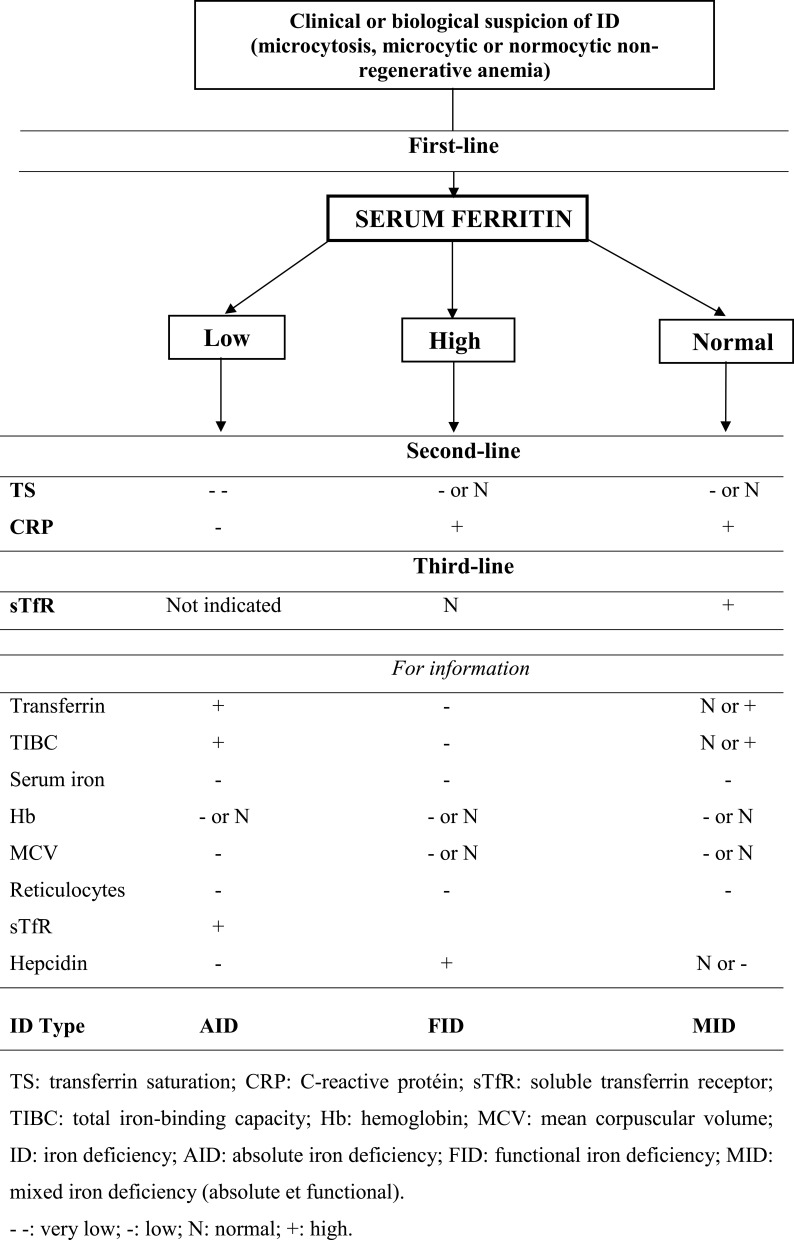
A suspected decrease in circulating iron, whether or not there is non-regenerative microcytic (or normocytic at the start of inflammation) anemia, suggests the need to measure SF, which is the first marker to quantify. It is important to clarify that the serum iron and transferrin assays are no longer useful in this situation, nor recommended because they are less reliable than ferritin.
In doubtful situations and as a second-line approach, assay of TS can be useful. In principle, normal ferritin and TS levels exclude AID. The assay of inflammatory markers can help at this stage. The third-line approach, and only if there is a doubt as to the inflammatory origin of anemia in the presence of normal SF, the assay of serum-soluble TfR (sTfR) may be useful for the positive diagnosis of the ID type.
In cases of a suspected decrease in circulating iron, SF can be low, normal or high.
Figure 1 summarizes the laboratory tests to be ordered in case of suspected ID with or without anemia.
Figure 1.
Laboratory tests recommended in case of suspicion of iron deficiency.
Low SF
Low SF indicates a decrease in IR, causing AID.3
The liver has the ability to detect AID and to inhibit the synthesis of hepcidin in order to increase the intestinal absorption of iron.12 Indeed, if the serum hepcidin level is lowered, the activity of ferroportin is no longer inhibited. Hence, the release of iron from enterocytes and macrophages is stimulated, as is the intestinal absorption of iron.
In case of deficiency, in addition to lowered SF, as well as a frequent hypochromic microcytic anemia, the remainder of the biological screening, though it has been proven useless and is no longer indicated, would show a low serum TS, high serum sTfR, low serum iron levels, low serum hepcidin concentrations, and finally high levels of serum transferrin and total iron-binding capacity (TIBC).3,64,65
High SF
In the event of clinical or biological suspicion of a decrease in circulating iron, elevated SF indicates inflammation. Anemia, which is often associated, is all or part of inflammatory origin. It is non-regenerative and initially normocytic before becoming microcytic.
Inflammation, whether acute or chronic and whatever its origin, therefore, induces an increase in SF. Indeed, in an inflammatory condition, the production of ferritin and hepcidin is stimulated by cytokines, in particular interleukin-6.65 Hepcidin inhibits the activity of ferroportin, thereby decreasing both the intestinal absorption of iron and its passage into the blood compartment while promoting the sequestration of iron in enterocytes and macrophages as ferritin.16 The result is an increase in ferritin in the body, therefore in iron stocks, while in parallel serum iron is low.4 This is what happens in FID.4,25
Inflammatory markers (such as C-reactive protein), which should be increased, and TS, which should be normal or decreased, are the second-line variables.65
If there is still a doubt, serum sTfR should be measured as a third-line. In FID, this marker will be not raised.64
The other biological assays, which are no longer considered relevant, would show low serum iron, high serum hepcidin concentrations and low levels of serum transferrin and TIBC.12,64,65
Normal SF
When ID is suspected while ferritin is within the normal range, two situations are possible: either a combination of AID and inflammation known as MID, which is both absolute and functional, or isolated inflammation (FID). For the latter, see the previous sub-paragraph.
In MID, the assays of inflammatory markers, serum TS and sTfR are indicated. Inflammatory markers are increased, TS is decreased or normal, and sTfR is increased.3,64 In addition, there is often non-regenerative microcytic or normocytic anemia.
The other biological assays, which are no longer considered relevant, would show a low serum iron level, normal or low serum hepcidin, and normal or slightly elevated levels of serum transferrin and TIBC.3,64
Frail OAs, characterized by polymorbidity, are particularly exposed to MID (associating absolute and FID).66
Conclusion
Iron is involved in various important mechanisms, including the synthesis of hemoglobin.
ID can be absolute if there is a decrease in both IR and circulating iron, or functional, resulting in a decrease in circulating iron while IR are increased or normal.
Causes of ID are frequently intertwined in OAs as a result of the polymorbidity that characterizes them.
ID can have dramatic consequences, especially in frail older individuals. It is therefore crucial to implement a careful approach to biological testing that targets and interprets the appropriate biological markers. These steps are important for avoiding errors in the positive diagnosis of AID or FID and for highlighting the one or more etiological diagnoses that can be used to tailor treatment.
Acknowledgments
The authors are grateful to Mrs. Suzanne RANKIN, a native English speaker, for editing and proofreading this article.
Funding Statement
There is no funding to report.
Abbreviations
ID, iron deficiency; OA(s), older adult(s); DNA, deoxyribonucleic acid; IR, iron reserves; SF, serum ferritin; TS, transferrin saturation; PPI(s), proton pump inhibitor(s); TfR, soluble transferrin receptor; d, day; AID, absolute iron deficiency; FID, functional iron deficiency; MID, mixed iron deficiency; sTfR, serum-soluble transferrin receptor; TIBC, total iron-binding capacity.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All the authors declare no conflict of interest.
References
- 1.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–1995. doi: 10.1056/NEJM199912233412607 [DOI] [PubMed] [Google Scholar]
- 2.Fairweather-Tait SJ, Wawer AA, Gillings R, Jennings A, Myint PK. Iron status in the elderly. Mech Ageing Dev. 2014;136–137:22–28. doi: 10.1016/j.mad.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavazzi G. Métabolisme du fer: physiopathologie et biomarqueurs chez le sujet âgé. Geriatr Psychol Neuropsychiatr Vieil. 2014;12(suppl 2):5–10. [DOI] [PubMed] [Google Scholar]
- 4.Randi ML, Bertozzi I, Santarossa C, et al. Prevalence and causes of anemia in hospitalized patients: impact on diseases outcome. J Clin Med. 2020;9:E950. doi: 10.3390/jcm9040950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toxqui L, Vaquero MP. Chronic iron deficiency as an emerging risk factor for osteoporosis: a hypothesis. Nutrients. 2015;7:2324–2344. doi: 10.3390/nu7042324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beard JL, Conner JR, Jones BC. Iron in the brain. Nutr Rev. 1993;51:157–170. doi: 10.1111/j.1753-4887.1993.tb03096.x [DOI] [PubMed] [Google Scholar]
- 7.Gallego-Narbón A, Zapatera B, Vaquero MP. Physiological and dietary determinants of iron status in Spanish vegetarians. Nutrients. 2019;11:1734. doi: 10.3390/nu11081734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812 [DOI] [PubMed] [Google Scholar]
- 9.Lasocki S, Chudeau N, Papet T, AtlanREA group; et al. Prevalence of iron deficiency on ICU discharge and its relation with fatigue: a multicenter prospective study. Crit Care. 18;2014:542. doi: 10.1186/s13054-014-0542-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138:80–98. doi: 10.1161/CIRCULATIONAHA.118.030099 [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Lázaro D, Mielgo-Ayuso J, Córdova Martínez A, Seco-Calvo J. Iron and physical activity: bioavailability enhancers, properties of black pepper (bioperine®) and potential applications. Nutrients. 2020;12:1886. doi: 10.3390/nu12061886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaumont C, Karim Z. Actualité du métabolisme du fer. Rev Med Interne. 2013;34:17–25. doi: 10.1016/j.revmed.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 13.Johnson MA, Fischer JG, Bowman BA, Gunter EW. Iron nutriture in elderly individuals. FASEB J. 1994;8:609–621. doi: 10.1096/fasebj.8.9.8005389 [DOI] [PubMed] [Google Scholar]
- 14.Lorcerie B, Audia S, Samson M, et al. Démarche diagnostique devant une hyperferritinémie. Rev Med Interne. 2015;36:522–529. doi: 10.1016/j.revmed.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 15.Beaumont C. Actualités du métabolisme du fer. Rev Med Interne. 2009;30:S307–S310. doi: 10.1016/j.revmed.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 16.Hamaï A, Mehrpour M. Homéostasie du fer et autophagie. Med Sci. 2017;33:260–267. doi: 10.1051/medsci/20173303012 [DOI] [PubMed] [Google Scholar]
- 17.Cacoub P. La carence martiale: nouvelles approches physiopathologiques et implications thérapeutiques. Rev Med Interne. 2018;39:381–385. doi: 10.1016/j.revmed.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 18.Chifman J, Laubenbacher R, Torti SV. A systems biology approach to iron metabolism. Adv Exp Med Biol. 2014;844:201–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for vitamin a, vitamin k, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, D.C: National Academy Press; 2001:290–393. [PubMed] [Google Scholar]
- 20.Gulec S, Anderson GJ, Collins JF. Mechanistic and regulatory aspects of intestinal iron absorption. Am J Physiol Gastrointest Liver Physiol. 2014;307:G397–G409. doi: 10.1152/ajpgi.00348.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annibale B, Capurso G, Delle Fave G. The stomach and iron deficiency anaemia: a forgotten link. Dig Liver Dis. 2003;35:288–295. doi: 10.1016/S1590-8658(03)00067-7 [DOI] [PubMed] [Google Scholar]
- 22.Demiroğlu H, Dündar S. Pernicious anaemia patients should be screened for iron deficiency during follow-up. N Z Med J. 1997;110:147–148. [PubMed] [Google Scholar]
- 23.Alleyne M, Horne MK, Miller JL. Individualized treatment for iron deficiency anemia in adults. Am J Med. 2008;121:943–948. doi: 10.1016/j.amjmed.2008.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028 [DOI] [PubMed] [Google Scholar]
- 25.Wawer AA, Jennings A, Susan J, Fairweather-Tait SJ. Iron status in the elderly: a review of recent evidence. Mech Ageing Dev. 2018;175:55–73. doi: 10.1016/j.mad.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 26.Zhou ZD, Tan E-K. Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol Neurodegener. 2017;12:75. doi: 10.1186/s13024-017-0218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galan P, Yoon HC, Preziosi P, et al. Determining factors in the iron status of adult women in the SU.VI.MAX study. SUpplementation en VItamines et Minéraux AntioXydants. Eur J Clin Nutr. 1998;52:383–388. doi: 10.1038/sj.ejcn.1600561 [DOI] [PubMed] [Google Scholar]
- 28.Park SH, Han SH, Chang KJ. Comparison of nutrient intakes by nutritional anemia and the association between nutritional anemia and chronic diseases in Korean elderly: based on the 2013–2015 Korea national health and nutrition examination survey data. Nutr Res Pract. 2019;13:543–554. doi: 10.4162/nrp.2019.13.6.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banks M, Graham D, Jansen M, et al. British society of gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545–1575. doi: 10.1136/gutjnl-2018-318126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockey DC, Cello JP. Evaluation of the gastrointestinal tract in patients with iron-deficiency anemia. N Engl J Med. 1993;329:1691–1695. doi: 10.1056/NEJM199312023292303 [DOI] [PubMed] [Google Scholar]
- 31.Stein J, Connor S, Virgin G, Ong DEH, Pereyra L. Anemia and iron deficiency in gastrointestinal and liver conditions. World J Gastroenterol. 2016;22:7908–7925. doi: 10.3748/wjg.v22.i35.7908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vetrano DL, Zucchelli A, Marconi E, et al. Predictors of iron-deficiency anemia in primary care older adults: a real-world European multi-country longitudinal study. Aging Clin Exp Res. 2020. doi: 10.1007/s40520-019-01454-6 [DOI] [PubMed] [Google Scholar]
- 33.Van De Bruaene A, Delcroix M, Pasquet A, et al. Iron deficiency is associated with adverse outcome in Eisenmenger patients. Eur Heart J. 2011;32:2790–2799. doi: 10.1093/eurheartj/ehr130 [DOI] [PubMed] [Google Scholar]
- 34.Schreiner P, Martinho-Grueber M, Studerus D, Vavricka SR, Tilg H, Biedermann L. Nutrition in inflammatory bowel disease. Digestion. 2020;1–16. doi: 10.1159/000505368 [DOI] [PubMed] [Google Scholar]
- 35.Peura DA, Lanza FL, Gostout CJ, Foutch PG. The American college of gastroenterology bleeding registry: preliminary findings. Am J Gastroenterol. 1997;92:924–928. [PubMed] [Google Scholar]
- 36.Fehr J, Favrat B, Schleiffenbaum B, Krayenbühl PA, Kapanci C, von Orelli F. Diagnostic et traitement de la carence en fer sans anémie. Rev Med Suisse. 2009;5:2229–2234. [PubMed] [Google Scholar]
- 37.Imai R, Higuchi T, Morimoto M, Koyamada R, Okada S. Iron deficiency anemia due to the long-term use of a proton pump inhibitor. Intern Med. 2018;57:899–901. doi: 10.2169/internalmedicine.9554-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kowdley KV, Brown KE, Ahn J, Sundaram V. ACG clinical guideline: hereditary hemochromatosis. Am J Gastroenterol. 2019;114:1202–1218. doi: 10.14309/ajg.0000000000000315 [DOI] [PubMed] [Google Scholar]
- 39.Quintana-Díaz M, Fabra-Cadenas S, Gómez-Ramírez S, Martínez-Virto A, García-Erce JA, Muñoz M. A fast-track anaemia clinic in the emergency department: feasibility and efficacy of intravenous iron administration for treating sub-acute iron deficiency anaemia. Blood Transfus. 2016;14:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinclair RC, Duffield KE, de Pennington JH. Improving preoperative haemoglobin using a quality improvement approach to treat iron deficiency anaemia. BMJ Open Qual. 2020;9:e000776. doi: 10.1136/bmjoq-2019-000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melina V, Craig W, Levin S. Position of the academy of nutrition and dietetics: vegetarian diets. J Acad Nutr Diet. 2016;116:1970–1980. doi: 10.1016/j.jand.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 42.Larpin C, Wozniak H, Genton L, Serratrice J. Alimentations végétariennes et véganes: quelles conséquences sur la santé ? Rev Med Suisse. 2019;15:1849–1853. [PubMed] [Google Scholar]
- 43.Batchelor EK, Kapitsinou P, Pergola PE, Kovesdy C, Jalal DI. Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis, and treatment. J Am Soc Nephrol. 2020;31:456–468. doi: 10.1681/ASN.2019020213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van den Noortgate NJ, Janssens WH, Afschrift MB, Lameire NH. Renal function in the oldest-old on an acute geriatric ward. Int Urol Nephrol. 2001;32:531–537. doi: 10.1023/A:1014454031451 [DOI] [PubMed] [Google Scholar]
- 45.Lorcerie B, Audia S, Samson M, et al. Diagnosis of hyperferritinemia in routine clinical practice. Presse Med. 2017;46:e329–e338. doi: 10.1016/j.lpm.2017.09.028 [DOI] [PubMed] [Google Scholar]
- 46.Syed K, Naguib S, Liu ZJ, Cimmino L, Yang FC. Novel combinations to improve hematopoiesis in myelodysplastic syndrome. Stem Cell Res Ther. 2020;11:132. doi: 10.1186/s13287-020-01647-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinto VM, Poggi M, Russo R, Giusti A, Forni GL. Management of the aging beta-thalassemia transfusion-dependent population - The Italian experience. Blood Rev. 2019;38:100594. doi: 10.1016/j.blre.2019.100594 [DOI] [PubMed] [Google Scholar]
- 48.Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron overload cardiomyopathy, better understanding of an increasing disorder. J Am Coll Cardiol. 2010;56:1001–1012. doi: 10.1016/j.jacc.2010.03.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kshirsagar AV, Li X. Long-term risks of intravenous iron in end-stage renal disease patients. Adv Chronic Kidney Dis. 2019;26:292–297. doi: 10.1053/j.ackd.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 50.Ryan Caballes F, Sendi H, Bonkovsky HL, Hepatitis C. Porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32:880–893. doi: 10.1111/j.1478-3231.2012.02794.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park SY, Na SY, Kim JH, Cho S, Lee JH. Iron plays a certain role in patterned hair loss. Dermatology J Korean Med Sci. 2013;28:934–938. doi: 10.3346/jkms.2013.28.6.934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. Front Pharmacol. 2014;5:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung SD, Chen PY, Lin HC, Hung SH. Sudden sensorineural hearing loss associated with iron-deficiency anemia: a population-based study. JAMA Otolaryngol Head Neck Surg. 2014;140:417–422. doi: 10.1001/jamaoto.2014.75 [DOI] [PubMed] [Google Scholar]
- 54.Cuttitta F, Torres D, Vogiatzis D, et al. Obesity and iron deficiency anemia as risk of asymptomatic bacteriuria. Eur J Intern Med. 2014;25:292–295. doi: 10.1016/j.ejim.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 55.Mehmood T, Auerbach M, Earley CJ, Allen RP. Response to intravenous iron in patients with iron deficiency anemia (IDA) and restless leg syndrome (Willis-Ekbom Disease). Sleep Med. 2014;15:1473–1476. doi: 10.1016/j.sleep.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 56.Lewiecki EM, Rahman F. Pruritus: a manifestation of iron deficiency. JAMA. 1976;236:2319–2320. doi: 10.1001/jama.1976.03270210045024 [DOI] [PubMed] [Google Scholar]
- 57.Manckoundia P, Barthélémy E, Bonnot R, d’Athis P. Impact of an ambulatory physical activity program on balance and motor abilities of retirees: a prospective study. Int J Clin Pract. 2020;74:e13474. doi: 10.1111/ijcp.13474 [DOI] [PubMed] [Google Scholar]
- 58.Girelli D, Marchi G, Camaschella C. Anemia in the elderly. Hemasphere. 2018;2:e40. doi: 10.1097/HS9.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–1750. doi: 10.1182/blood-2005-07-3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kassebaum NJ; GBD 2013 Anemia Collaborators. The global burden of anemia. Hematol Oncol Clin North Am. 2016;30:247–308. doi: 10.1016/j.hoc.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 61.Loréal O, Bardou-Jacquet E, Jouanolle A-M, et al. Métabolisme du fer et outils diagnostiques pour le clinicien. Rev Med Interne. 2012;33:S3–S9. doi: 10.1016/j.revmed.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 62.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y, Tang Z, An T, Zhao L. The impact of iron chelation therapy on patients with lower/intermediate IPSS MDS and the prognostic role of elevated serum ferritin in patients with MDS and AML: a meta-analysis. Medicine. 2019;98:e17406. doi: 10.1097/MD.0000000000017406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Celi J, Samii K, Perrier A, Reny JL. Anémie ferriprive, inflammatoire ou mixte: comment orienter le diagnostic? Rev Med Suisse. 2011;7:2018–2023. [PubMed] [Google Scholar]
- 65.Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med. 2012;366:348–359. doi: 10.1056/NEJMra1004967 [DOI] [PubMed] [Google Scholar]
- 66.Le Petitcorps H, Monti A, Pautas É. Anémie par carence ferrique du sujet âgé: utilisation pratique des biomarqueurs. Ann Biol Clin. 2015;73:639–642. [DOI] [PubMed] [Google Scholar]