ABSTRACT
Aging of society has increased the incidence of physical disability. The goal of this study was to examine the physical ability of elderly people classified as having sarcopenia, presarcopenia, or dynapenia (a low muscle function without low muscle mass) in a community in Japan. The subjects were volunteers aged >60 years who were participants in a health checkup in Yakumo, Hokkaido and were in good general health. Demographic data were collected and physical performance tests were performed to measure grip strength, walking speed, back muscle strength, maximum stride length, and 3-m timed-up-and-go (3m TUG) time. A measurement of skeletal muscle mass was used as a basis for calculating the appendicular skeletal muscle index (aSMI). The rates of sarcopenia, presarcopenia, and dynapenia were 10%, 22%, and 8% in males (n=101, age 69.7±5.4 years), and 19%, 23%, and 13% in females (n=112, 68.5±5.9 years). Body mass index in subjects with dynapenia was significantly higher compared to that in subjects with sarcopenia and presarcopenia (p<0.01). Back muscle strength, maximum stride length and 3m TUG were similar in dynapenia and sarcopenia, but differed significantly with those in presarcopenia in both males and females without the influence of age (p<0.05). Further studies are needed to evaluate the benefits of dynapenia intervention programs and to explore the underlying pathophysiology of dynapenia.
Key Words: dynapenia, muscle strength, physical performance, presarcopenia, sarcopenia
INTRODUCTION
Japanese society is aging rapidly and there is a need to lengthen healthy life expectancy.1 Limited physical ability occurs with age, and this increases the risk of fall, the need for institutionalization, comorbidities, and early death. A higher age-related prevalence of reduced physical ability, including impaired body function or structure, limited activity, and restricted participatory activities, is also likely to increase demands on the healthcare system.2 For these reasons, prevention of physical disability and treatment for physical limitations are important for public health and healthy aging.
The increase in life expectancy has made prevention of deterioration of muscular function and physical disability an important issue in elderly people. Decreases in muscle mass and muscle strength are strongly affected by age.3 A decrease in muscle mass starts at around 25 years of age, and further accelerates past the age of 50, with an average decrease of 40% at age 80 compared to age 20.3 In a longitudinal study of healthy elderly people over 12 years, muscle strength decreased by 1.4 to 25% per year.4 This decrease in muscle mass and function due to aging is referred to as sarcopenia (sarco meaning “muscle” in Greek and penia meaning “loss”), and was first proposed by Rosenberg.5 Epidemiological studies indicate a high prevalence of sarcopenia in older adults that may be related to high risks for mortality, disabilities, and dependence on others.6-8 Sarcopenia in elderly people has also been linked to a decrease in instrumental activities of daily living (IADL)9 and limited mobility.10 A preliminary stage, presarcopenia, which is defined only as a decrease of muscle mass, has also been described.11,12
Dynapenia was proposed by Clark et al in 2008 as an age-related decrease of muscle function.13 Dyna refers to “power, strength, or force” and penia to “poverty.” Dynapenia is related to an increased risk of functional decline and frailty in older adults independent of neurologic or muscular diseases, and has a high risk for mortality and physical disability.10,13 In a meta-analysis comparing subjects with dynapenia and a robust status, Manini et al found that dynapenia had a relative risk of 2.2 (95% CI: 1.5–3.1) for physical performance or disability.14 Development of dynapenia is thought to involve morphological factors such as reduced voluntary movements due to neurological factors such as transmission of cerebral excitability and age-related changes. It has also been suggested that muscle atrophy does not have a major effect on muscle weakness, and that muscle loss and muscle weakness are not necessarily related, which suggests that sarcopenia and dynapenia may be conceptually different.14,15
There have been few reports focusing on the effect of dynapenia on physical performance, and in particular, there are few studies of physical abilities in Japanese community-dwelling people. Therefore, the purpose of this study was to examine the physical ability of elderly people classified as having sarcopenia, presarcopenia, or dynapenia in a community in Japan, and to establish relationships among these conditions.
MATERIALS AND METHODS
Participants
The participants were recruited from people at a basic health checkup provided by a local government in 2016 in the town of Yakumo, which is located in Hokkaido in the north of Japan. This checkup has been held every year for 35 years and most residents attend at five-year intervals. All the participants were volunteers. Specialists in internal medicine, ophthalmology, otolaryngology, urology, psychology, and preventive medicine were present at the checkup, and physical performance tests were performed by orthopedic surgeons. In the current study, which began in 2011, we also examined locomotive syndrome based on examinations of motor function, spine and joint diseases, and osteoporosis, both for examination of health and for exercise guidance.11,16,17 The age, gender, and body mass index (BMI) of the participants are also recorded at the checkup. The inclusion criteria for this study were Japanese people (male and female) aged >60 years who received physical examinations (as described below) in the health checkup. Individuals with severe walking or standing disabilities or central or peripheral nervous system dysfunction were excluded. All participants gave written informed consent. The Institutional Review Board of our institution approved the study protocol and the procedures followed the principles of the Declaration of Helsinki.
Measurements of physical characteristics and performance
BMI was calculated as body weight (kg) divided by height2 (m2), as an indicator of obesity. Ultrasound was used to measure bone mineral density (BMD) in the calcaneus using a bone densitometer (A1000 Insight, Lunar Corp., Madison, WI, USA), and the % young adult mean (%YAM) was determined. Bioelectrical impedance analysis (BIA) was used for measurement of appendicular skeletal muscle mass (SMM) using an Inbody 770 BIA unit (Inbody Co., Ltd, Seoul, Korea), which uses differences in electric impedance among tissues such as fat, muscle, and bone to measure body composition.18 These data were used to calculate the appendicular skeletal muscle index (aSMI): aSMI = arm and leg SMM (kg) / height2 (m2),19 which indicates the muscle mass of the extremities. Bilateral grip strength was measured in the standing position as mainly an upper body strength indicator, using a Smedley handgrip dynamometer (Toei Light Co., Saitama, Japan), with the results for two hands (one test each) used to calculate the average grip strength. This parameter is also highly correlated with the strength of the whole body.20 The mobility of the participants was determined based on measurement of their walking speed5 on a 5-m straight course, on which participants walked once at their fastest pace as an indicator of walking ability and leg strength. The maximal isometric strength of the trunk muscles in a standing posture at 30° lumbar flexion was measured using a digital back muscle strength meter (T.K.K. 5002; Takei Co., Niigata, Japan) for evaluation of back muscle strength as an indicator of muscle strength of the whole body.21-23 Maximum stride length was measured while standing, with the participants asked to advance their right foot as far as possible, and then bring their left foot to the right foot without their hands touching the floor or their knees. This was repeated with the left foot forward, and the best value was used as the maximum stride as a reflection of lower limb strength.24 A 3-meter timed-up-and-go (3m TUG) test was used to measure the time required for participants to rise from a standard chair with a 46-cm seat height, walk 3 m, turn around, walk back to the chair, and sit down as indicators of lower limb strength, balance and walking ability.25 This test was performed twice by each subject, both at maximum pace, and the mean time was used for analysis. All physical measurements were made by 5 orthopedic surgeons.
Definition of sarcopenia, presarcopenia, and dynapenia
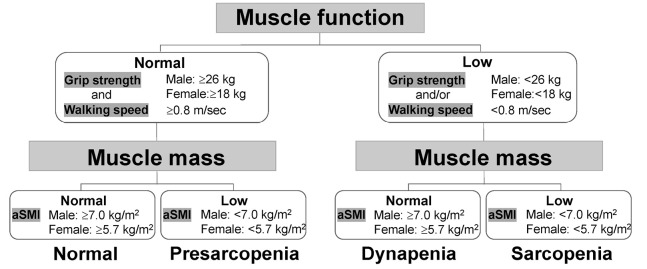
The diagnostic algorithm of the Asian Working Group for Sarcopenia (AWGS) was used to define sarcopenia, based on low muscle function (low physical ability or muscle strength) and low muscle mass. The cutoffs were <7.0 and <5.7 kg/m2 for low aSMI and <26 and <18 kg for low handgrip strength in males and females, respectively, and that for low gait speed was <0.8 m/s.26 Presarcopenia was defined as low muscle mass without low muscle function,8 and dynapenia as low muscle function without low muscle mass (Figure 1).27
Figure 1.
Algorithm for sarcopenia, presarcopenia, and dynapenia staging. Presarcopenia was defined as normal muscle function with low muscle mass
Dynapenia was defined as low muscle function with normal muscle mass, Sarcopenia was defined as low muscle mass with low muscle function.
Statistical analysis
Continuous variables are shown as mean ± standard deviation (SD), and categorical data as numbers (percentage). Intergroup comparison of continuous variables was performed by Mann-Whitney U test or Student t-test, and categorical data were evaluated by χ2-test or Fisher Exact test. A post hoc Bonferroni test was used to assess significant differences of one group from all other groups. SPSS ver. 23 (SPSS Inc., Chicago, IL, USA) was used for all calculations, and p<0.05 was considered to be significant in all analyses.
RESULTS
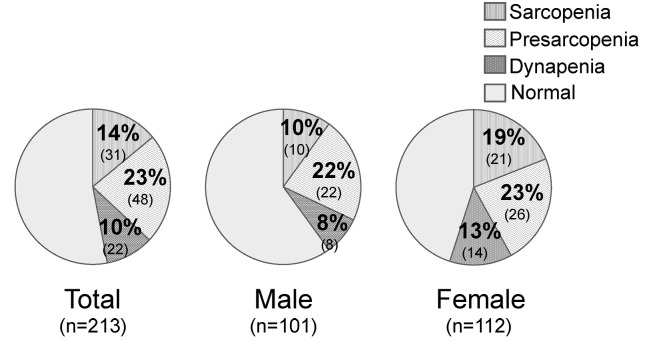
The participants characteristics and other data are shown in Table 1. There were significant differences between males (n=101) and females (n=112) for BMI, BMD, aSMI, grip strength, walking speed, back muscle strength, maximum stride length, and 3m TUG time. The mean ages were 69.7±5.4 years in males and 68.5±5.9 years in females. The rates of sarcopenia, presarcopenia, and dynapenia were 10% (n=10), 22% (n=22), and 8% (n=8) in males, and 19% (n=21), 23% (n=26), and 13% (n=14) in females, respectively (Figure 2).
Table 1.
Demographic data of the participants (n=213)
| Variable | Total
(n=213) |
Male
(n=101) |
Female
(n=112) |
P value |
| Demographic data | ||||
| Age (years) | 69.1±5.7 | 69.7±5.4 | 68.5±5.9 | n.s. |
| 60–69 years (n) | 120 (41%) | 54 (42%) | 66 (41%) | n.s. |
| 70–79 years (n) | 77 (26%) | 38 (29%) | 39 (25%) | n.s. |
| ≥80 years (n) | 16 (5%) | 9 (7%) | 7 (4%) | n.s. |
| Body mass index (kg/m2) | 23.6±3.3 | 24.0±3.0 | 23.2±3.5 | <0.05 |
| Bone mineral density (%YAM) | 77.1±13.9 | 80.7±15.2 | 73.5±11.7 | <0.01 |
| aSMI (kg/m2) | 6.7±1.0 | 7.5±0.8 | 6.1±0.7 | <0.01 |
| Physical performance | ||||
| Grip strength (kg) | 26.6±8.6 | 33.8±7.2 | 20.8±4.1 | <0.01 |
| Walking speed (m/s) | 1.26±0.22 | 1.25±0.23 | 1.27±0.22 | n.s. |
| Back muscle strength (kg) | 66.4±27.0 | 85.5±27.0 | 51.2±14.4 | <0.01 |
| Maximum stride length (cm) | 113.7±13.3 | 118.1±14.3 | 109.6±10.8 | <0.01 |
| 3m TUG (s) | 6.4±1.1 | 6.3±1.1 | 6.5±1.1 | n.s. |
Values are expressed as mean ± standard deviation or as a number (percentage)
aSMI: appendicular skeletal muscle index
3m TUG: 3m time-up-and-go
Figure 2.
Rates of sarcopenia, presarcopenia, and dynapenia by gender
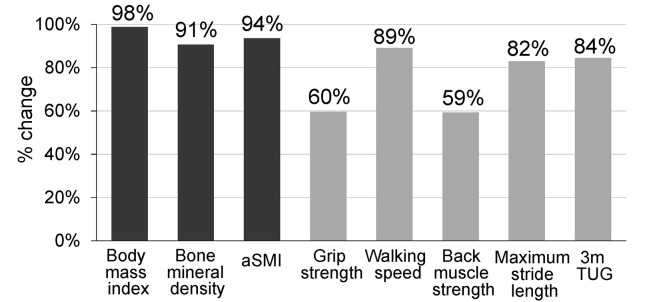
Among the four groups (sarcopenia, presarcopenia, dynapenia, and normal), participants with sarcopenia, presarcopenia, and dynapenia did not differ significantly in age. However, BMI in both male and females participants with dynapenia was significantly higher than that in participants with sarcopenia and presarcopenia (p<0.01) (Table 2). Back muscle strength, maximum stride length and 3mTUG were similar in participants with dynapenia and sarcopenia, but were significantly different to those with presarcopenia in both males and females without the influence of age (p<0.05) (Table 2). Details for dynapenia for each gender are shown in Table 3. In physical performance, grip strength and back muscle strength were significantly higher in males, but there were no significant differences in walking speed, maximum stride length, and 3mTUG (Table 3). Changes in parameters in the dynapenia group relative to those in the normal group are shown in Figure 3.
Table 2.
Characteristics of males and females in the sarcopenia, presarcopenia, dynapenia, and normal groups.
| Variables | Sarcopenia a | Presarcopenia b | Dynapenia c | Normal d | ANOVA
P-value |
Post hoc test |
| Male (n=101) | (n=10) | (n=22) | (n=8) | (n=61) | ||
| Demographic data | ||||||
| Age (years) | 72.1±5.9 | 70.8±7.1 | 71.3±4.7 | 68.7±5.1 | <0.05 | d < a, b, c |
| Body mass index (kg/m2) | 21.6±2.1 | 22.0±2.9 | 25.2±2.9 | 24.9±3.2 | <0.01 | a, b <c, d |
| Bone mineral density (%YAM) | 76.7±14.1 | 77.1±11.7 | 75.6±10.6 | 83.3±12.5 | <0.05 | a, b, c <d |
| aSMI (kg/m2) | 6.4±0.3 | 6.7±0.3 | 7.4±0.4 | 7.9±0.5 | <0.01 | a <b <c <d |
| Physical performance | ||||||
| Grip strength (kg) | 21.1±4.3 | 30.8±2.6 | 22.9±3.9 | 38.4±3.6 | <0.01 | a, c <b <d |
| Walking speed (m/s) | 1.07±0.23 | 1.27±0.18 | 1.15±0.17 | 1.29±0.19 | <0.05 | a, c <b, d |
| Back muscle strength (kg) | 55.1±16.4 | 75.3±18.3 | 57.9±9.1 | 97.7±12.4 | <0.05 | a, c <b <d |
| Maximum stride length (cm) | 104.5±12.1 | 114.5±12.7 | 102.5±9.8 | 123.6±10.1 | <0.05 | a, c <b <d |
| 3m TUG (s) | 7.3±1.4 | 6.4±1.0 | 7.1±1.1 | 6.0±1.2 | <0.05 | d <b <a, c |
| Female (n=112) | (n=21) | (n=26) | (n=14) | (n=51) | ||
| Demographic data | ||||||
| Age (years) | 71.3±6.4 | 70.4±7.0 | 70.9±4.6 | 65.7±5.6 | <0.05 | d < a, b, c |
| Body mass index (kg/m2) | 20.0±2.2 | 20.4±2.1 | 24.6±2.8 | 25.6±1.9 | <0.01 | a, b <c, d |
| Bone mineral density (%YAM) | 73.3±9.0 | 72.0±16.6 | 72.1±18.5 | 74.7±13.2 | n.s. | |
| aSMI (kg/m2) | 5.2±0.4 | 5.4±0.2 | 6.3±0.6 | 6.8±0.5 | <0.05 | a, b <c <d |
| Physical performance | ||||||
| Grip strength (kg) | 14.7±3.7 | 20.3±1.7 | 16.3±2.1 | 24.8±2.5 | <0.01 | a, c <b <d |
| Walking speed (m/s) | 1.15±0.25 | 1.31±0.23 | 1.20±0.19 | 1.32±0.24 | <0.05 | a, c <b, d |
| Back muscle strength (kg) | 39.4±11.0 | 50.8±11.0 | 42.5±11.3 | 57.7±13.6 | <0.05 | a, c <b< d |
| Maximum stride length (cm) | 103.5±14.0 | 109.7±10.2 | 101.2±12.3 | 111.2±11.5 | <0.05 | a, c <b, d |
| 3m TUG (s) | 6.9±1.2 | 6.5±0.9 | 6.8±0.9 | 6.3±1.0 | <0.05 | d, b <a, c |
Values are expressed as mean ± standard deviation or as a number (percentage)
aSMI: appendicular Skeletal Muscle Index
3m TUG: 3m time-up-and-go
Table 3.
Details for each gender in cases with dynapenia
| Variables | Dynapenia | P-value | |
| Male
(n=8) |
Female
(n=14) |
||
| Demographic data | |||
| Age (years) | 71.3±4.7 | 70.9±4.6 | n.s. |
| Body mass index (kg/m2) | 25.2±2.9 | 24.6±2.8 | <0.05 |
| Bone mineral density (%YAM) | 75.6±10.6 | 72.1±18.5 | <0.05 |
| aSMI (kg/m2) | 7.4±0.4 | 6.3±0.6 | <0.05 |
| Physical performance | |||
| Grip strength (kg) | 22.9±3.9 | 16.3±2.1 | <0.01 |
| Walking speed (m/s) | 1.15±0.17 | 1.20±0.19 | n.s. |
| Back muscle strength (kg) | 57.9±9.1 | 42.5±11.3 | <0.05 |
| Maximum stride length (cm) | 102.5±9.8 | 101.2±12.3 | n.s. |
| 3m TUG (s) | 7.1±1.1 | 6.8±0.9 | n.s. |
aSMI: appendicular skeletal muscle index
3m TUG: 3m time-up-and-go
Figure 3.
Percentage change in parameters in the dynapenia group compared to a normalized value of 1 in the normal group
DISCUSSION
Ethnicity and physical performance influence the incidence of sarcopenia in a population, with reported rates of 1–29% in community-dwelling people, 14–33% in patients in long-term care, 6–12% in studies with >1000 participants, and 7.5–8.2% in a cross-sectional survey in a Japanese population.1,28,29 Dynapenia is a significant measure of the decline in muscle strength with aging13 that is not related to neurologic or muscular diseases. Dynapenia affects movement and fall, and is associated with higher mortality.30 However, there are few reports on physical performance in participants with dynapenia in elderly Japanese people. In this study, we examined the physical ability of people classified as having sarcopenia, presarcopenia, or dynapenia (a decrease of muscle function due to aging) in elderly people in a community in Japan.
Toyoda et al reported that in patients with spinal disorders, the respective incidences of sarcopenia, dynapenia, and normal stages were 16.4%, 26.7%, and 56.9% in males, and 23.7%, 50.9%, and 25.4% in females.31 In Japanese community-dwelling older adults, Kim et al found rates of sarcopenia of 16.3% in males and 33% in females, and of dynapenia of 33.7% in males and 33.3% in females.32 In our study, the incidences of sarcopenia, presarcopenia, and dynapenia were 10%, 22%, and 8% in males, and 21%, 26%, and 14% in females, respectively, in elderly people in a community in Japan. The lower prevalence in the current study may be because participants lived in a relatively rural area, and most worked in agriculture or fishing. These demographics differ from those of people in urban environments, and the participants were relatively healthy and may have had more interest in their health compared with people from other areas.
Regarding dynapenia, it is understood that muscle weakness is associated with aging, although neuromuscular diseases are excluded; however, cerebral excitability transmission through the spinal cord and age-related changes in the nervous system may also be involved.1,2 The incidence of dynapenia was lower than that of sarcopenia in males and females, but without a significant difference. Regarding the relationship between sarcopenia and dynapenia, dynapenia is considered to be accompanied by a decrease in physical function and quality of life, similar to sarcopenia.1,32 However, an age-related study of weight loss and weight gain showed that muscle mass increased but muscle strength weakened in elderly patients with weight gain.14,15 In addition, the decrease in muscle mass and muscle strength associated with aging is not necessarily consistent.32 These findings suggest that loss of muscle mass and muscle weakness are not necessarily related. Therefore, it is likely that dynapenia will be used regularly and diagnostic criteria will be established. This will allow the relationship and differences with sarcopenia to be further clarified.
In our series, in dynapenia, BMIs of both males and females was significantly higher than those in sarcopenia and presarcopenia. Sarcopenia has been associated with thinness in elderly people, and a survey of elderly Japanese people showed that BMI and body fat percentage were significantly lower in those with sarcopenia than in non-sarcopenia cases.7,33 Delmonico et al reported that increases in intramuscular fat and intermuscular fat with age were related to muscle weakness.15 These previous studies are consistent with our results. In a comparison of dynapenia by gender, the only significant difference in physical performance between males and females was back muscle strength. Walking speed, maximum stride length, and 3mTUG did not differ significantly by gender. In other words, lower limb movement functions, such as lower limb walking speed and maximum stride, in dynapenia cases had no significant difference by gender. However, physical performance in participants with dynapenia was as low as that in sarcopenia, and was significantly different to that in presarcopenia. Diagnoses of sarcopenia, presarcopenia, and dynapenia were based on grip strength, walking speed, and aSMI. Grip strength is strongly related to the muscles of the lower limb, and in physical performance, grip strength is correlated with walking speed.34 Even with no loss of muscle mass, a case with only low grip strength could have dynapenia, and the prognosis may be poor. This suggests that intervention in community-dwelling elderly people with a focus on dynapenia and physical performance may be desirable.
In our series, the only significant difference between males and females was found for BMD (Table 2). Many of the male participants had jobs in agriculture or fishing, and this might have caused a significant difference in BMD compared to postmenopausal females. In males, dynapenia may have been associated with worsening BMD, whereas in females this association was not seen because the original BMD baseline was already low. This background may account for the significant difference between male and females. An examination of the change in parameters between the participants with dynapenia and normal subjects (Figure 3) showed that walking speed, 3mTUG and maximum stride length were not significantly worse in dynapenia. As well as reflecting lower limb muscle strength, rhese parameters are related to balance, coordination, and joint flexibility, which seem to be relatively well maintained in dynapenia.
There are several limitations in this study. First, there was a relatively small number of participants, and a study in more participants is needed for validation of the results. A larger study could identify different factors related to the three pathologies through further statistical analysis. Second, we defined dynapenia as low muscle function (low grip strength or walking speed) without low muscle mass; however, there are few reports on dynapenia and this condition has not been defined internationally. Third, this study mainly examined certain specific physical performances, but did not include physical abilities such as endurance and balance, and these should ideally be considered in conjunction with surveys on health and ADL. Fourth, BIA does not measure muscle mass directly, in contrast to MRI, and BMI does not consider intramuscular fat; thus, the accuracy of these data is limited. Despite these limitations, awareness of sarcopenia, presarcopenia, and dynapenia is important in an aging society, and dynapenia is especially important as an age-related decrease of muscle function. This condition is related to an increased risk of functional decline and frailty in older adults independent of neurologic or muscular disease, and has a high risk for mortality and physical disability. A longitudinal study is needed to establish the causal relationships among low muscle function, low muscle mass, and physical performance.
In summary, we examined dynapenia and physical performance in community-dwelling elderly people in Japan. Dynapenia is especially important as an age-related decrease of muscle function. Thus, we conclude that back muscle strength, maximum stride length and 3m TUG are similar in dynapenia and sarcopenia, but differ significantly from those in presarcopenia in both males and females, without the influence of age.
DISCLOSURE STATEMENT
None of the authors have a conflict of interest regarding the work in the manuscript.
REFERENCES
- 1.Iwamura M, Kanauchi M. A cross-sectional study of the association between dynapenia and higher-level functional capacity in daily living in community-dwelling older adults in Japan. BMC Geriatr. 2017;17:1. [DOI] [PMC free article] [PubMed]
- 2.Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9:3–19. [DOI] [PMC free article] [PubMed]
- 3.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. [DOI] [PubMed]
- 4.Frontera WR, Hughes VA, Fielding RA, et al. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–1326. [DOI] [PubMed]
- 5.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S–991S. [DOI] [PubMed]
- 6.Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017;12:e0169548. [DOI] [PMC free article] [PubMed]
- 7.Yamada M, Nishiguchi S, Fukutani N, et al. Prevalence of sarcopenia in community-dwelling Japanese older adults. J Am Med Dir Assoc. 2013;14:911–915. [DOI] [PubMed]
- 8.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. [DOI] [PMC free article] [PubMed]
- 9.Tanimoto Y, Watanabe M, Sun W, et al. Association between muscle mass and disability in performing instrumental activities of daily living (IADL) in community-dwelling elderly in Japan. Arch Gerontol Geriatr. 2012;54:e230–233. [DOI] [PubMed]
- 10.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. [DOI] [PubMed]
- 11.Kobayashi K, Ando K, Tsushima M, et al. Predictors of pre-sarcopenia in community-dwelling older adults: a 5-year longitudinal study. Mod Rheumatol. 2018;24:1–17. [DOI] [PubMed]
- 12.Di Monaco M, Castiglioni C, De Toma E, et al. Presarcopenia and sarcopenia in hip-fracture women: prevalence and association with ability to function in activities of daily living. Aging Clin Exp Res. 2015;27:465–472. [DOI] [PubMed]
- 13.Clark BC, Manini TM. Sarcopenia=/=dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834. [DOI] [PubMed]
- 14.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40. [DOI] [PMC free article] [PubMed]
- 15.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. [DOI] [PMC free article] [PubMed]
- 16.Imagama S, Hasegawa Y, Ando K, et al. Staged decrease of physical ability on the locomotive syndrome risk test is related to neuropathic pain, nociceptive pain, shoulder complaints, and quality of life in middle-aged and elderly people: the utility of the locomotive syndrome risk test. Mod Rheumatol. 2017;27:1051–1056. [DOI] [PubMed]
- 17.Kobayashi K, Ando K, Seki T, et al. Carotid artery plaque screening using abdominal aortic calcification on lumbar radiographs. PLoS One. 2019;14:e0209175. [DOI] [PMC free article] [PubMed]
- 18.Hoffer EC, Meador CK, Simpson DC. Correlation of whole-body impedance with total body water volume. J Appl Physiol. 1969;27:531–534. [DOI] [PubMed]
- 19.Heymsfield SB, Smith R, Aulet M, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–218. [DOI] [PubMed]
- 20.Imagama S, Ito Z, Wakao N, et al. Influence of spinal sagittal alignment, body balance, muscle strength, and physical ability on falling of middle-aged and elderly males. Eur Spine J. 2013;22:1346–1353. [DOI] [PMC free article] [PubMed]
- 21.Imagama S, Hasegawa Y, Wakao N, Hirano K, Hamajima N, Ishiguro N. Influence of lumbar kyphosis and back muscle strength on the symptoms of gastroesophageal reflux disease in middle-aged and elderly people. Eur Spine J. 2012;21:2149–2157. [DOI] [PMC free article] [PubMed]
- 22.Imagama S, Matsuyama Y, Hasegawa Y, et al. Back muscle strength and spinal mobility are predictors of quality of life in middle-aged and elderly males. Eur Spine J. 2011;20:954–961. [DOI] [PMC free article] [PubMed]
- 23.Imagama S, Hasegawa Y, Wakao N, Hirano K, Muramoto A, Ishiguro N. Impact of spinal alignment and back muscle strength on shoulder range of motion in middle-aged and elderly people in a prospective cohort study. Eur Spine J. 2014l;23:1414–1419. [DOI] [PubMed]
- 24.Muramoto A, Imagama S, Ito Z, Hirano K, Ishiguro N, Hasegawa Y. Physical performance tests are useful for evaluating and monitoring the severity of locomotive syndrome. J Orthop Sci. 2012;17:782–788. [DOI] [PubMed]
- 25.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. [DOI] [PMC free article] [PubMed]
- 26.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. [DOI] [PubMed]
- 27.Yamada M, Kimura Y, Ishiyama D, et al. Differential characteristics of skeletal muscle in community-dwelling older adults. J Am Med Dir Assoc. 2017;18:807.e9–16. [DOI] [PubMed]
- 28.Yoshida D, Suzuki T, Shimada H, et al. Using two different algorithms to determine the prevalence of sarcopenia. Geriatr Gerontol Int. 2014;14(Suppl 1):46–51. [DOI] [PubMed]
- 29.Yoshimura N, Muraki S, Oka H, et al. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int. 2017;28:189–199. [DOI] [PubMed]
- 30.Alexandre Tda S, Duarte YA, Santos JL, Wong R, Lebrão ML. Sarcopenia according to the European Working Group on Sarcopenia on Older People (EWGSOP) versus dynapenia as a risk factor for mortality in the elderly. J Nutr Health Aging. 2014;18:751–756. [DOI] [PubMed]
- 31.Toyoda H, Hoshino M, Ohyama S, et al. The association of back muscle strength and sarcopenia-related parameters in the patients with spinal disorders. Eur Spine J. 2019;28:241–249. [DOI] [PubMed]
- 32.Kim M, Tsuji T, Kttano N. Relationship between sarcopenia or dynapenia and physical function in community-dwelling older adults. Jpn J Test Eval Physical Ed Sports. 2016;15:1–10.
- 33.Sanada K, Miyachi M. A cross-sectional study of sarcopenia in Japanese men and women. Adv Exercise Sports Physiol. 2012;18:27–32.
- 34.Okuzumi H, Furuna T, Nishizawa S. Relationship between static balance scores and muscle strength in older adults. Equilib Res. 2000;59:574–578.